Abstract
Background
In this study, the association between high-risk human papillomavirus (hrHPV) infection and the vaginal microbiome in pregnant women was evaluated in Chinese cohorts.
Methods
The vaginal bacterial composition of four groups, 38 hrHPV-infected pregnant women (PHR, n = 38), pregnant women without HPV infection (PN, n = 48), nonpregnant women with hrHPV infection (NPHR, n = 19) and nonpregnant women without HPV infection (NPN, n = 30), was characterized by deep sequencing of barcoded 16S rRNA gene fragments (V3–4) using Illumina MiSeq.
Results
The results revealed that both pregnancy and HPV infection can increase vaginal bacterial microbial richness and diversity, with the bacterial composition being most influenced by pregnancy. Lactobacillus was the most dominant genus among all samples. NPN samples were dominated by CST (community state type) III, mainly composed of Lactobacillus iners. Both pregnancy and hrHPV infection were accompanied by an increased proportion of CST I (dominated by Lactobacillus crispatus), as opposed to CST III. Bifidobacterium, Bacillus, Megasphaera, Sneathia, Prevotella, Gardnerella, Fastidiosipila and Dialister were found to be biomarkers for hrHPV-infected women, though different genera (Bifidobacterium, Megasphaera, Bacillus, Acidovorax, Oceanobacillus and Lactococcus) were associated with hrHPV-infected pregnant women.
Conclusions
This work uncovered a probable synergistic effect of hrHPV infection and pregnancy on the vaginal microbial composition. HPV infection in pregnant women was associated with a more complex and diverse microbial environment.
Electronic supplementary material
The online version of this article (10.1186/s12879-019-4279-6) contains supplementary material, which is available to authorized users.
Keywords: Pregnancy, Vaginal microbiome, High-risk human papillomavirus
Background
Human papillomavirus (HPV), a type of DNA virus, is associated with cervical intraepithelial neoplasia (CIN) and cervical adenocarcinoma. Indeed, HPV infection is closely related with cervical cancer and it is also present in other anogenital, head and neck cancers. According to their oncogenic potential, more than 100 types of HPV were classified as high-risk, probable high-risk, and low-risk types [1]. More than 40 types are sexually transmitted and infect the anus and genitals. Compared to warts caused by low-risk HPV types, high-risk HPV types, which account for 99% of cervical neoplasias, are usually asymptomatic [1], and as a result, these latter types are more difficult to detect. Many factors have been proven to increase the risk of genital HPV infection, e.g., sexual behaviors, number of sexual partners, early onset of sexual activity and coinfection with other sexually transmitted infections [2–4].
A large number of microorganisms inhabit the female genital tract, and hundreds of studies have revealed that a dominance of Lactobacillus lowers bacterial richness and diversity, indicating a healthy vaginal status. A healthy microbial biofilm may prevent or hinder many urogenital diseases, such as Candida infection [5, 6], sexually transmitted diseases [7], urinary tract infections [8, 9] and human immunodeficiency virus (HIV) infection [10]. Some studies to date have also reported that changes in the vaginal microbial structure have a close connection with HPV infection [11–14] and CIN progression [15–17].
Pregnancy is a unique physiological state, and the composition of the vaginal microbiota changes when women become pregnant due to fluctuations in hormone levels [18, 19]. It has been claimed that a vaginal bacterial composition dominated by one or two species of Lactobacillus is especially present during pregnancy [18–20]. However, dysbiosis of the vaginal microbiota during pregnancy has been reported to be associated with many complications of pregnancy, such as an increased risk of miscarriage, preterm birth and endometritis [18, 21, 22], yet few studies have investigated the relationship between HPV infection and the vaginal microbiome in pregnant women. It has been reported that children born to HPV-positive mothers have a significantly higher risk for developing infantile anal and genital warts [23], though the occurrence rate of cervical precancerous lesions during pregnancy is comparable to that of nonpregnant women [24]. Overall, the prevalence of HPV in pregnant women remains controversial in the literature, varying from 16.8 to 34.2% [25–28]. Hence, it is still under debate whether pregnant women are predisposed toward HPV infection, and the factors that may influence susceptibility are unclear. Because the vaginal microbiome plays an important role in HPV infection in nonpregnant women, we hypothesized that a different vaginal microbial environment during pregnancy might facilitate HPV infection.
Therefore, the aim of this study is to investigate the association between the vaginal microbial composition and high-risk HPV infection in pregnant women. We attempt to distinguish a different microbial profile of HPV-infected pregnant women from that of nonpregnant women.
Methods
Study population and sample collection
Between May 2016 and September 2016, 38 hrHPV (high-risk human papillomavirus)-infected pregnant women (PHR, n = 38) and 48 pregnant women without HPV infection (PN, n = 48) and with an uncomplicated pregnancy were recruited for this study at their first antenatal care visit at the Department of Obstetrics, Renji Hospital of Shanghai, Jiao Tong University School of Medicine. The inclusion criteria for this cohort study were as follows: age ranging from 25 to 40 years; gestational age between 16 and 30 weeks; and no obvious medical problems or adverse outcomes during any previous pregnancy, such as preterm delivery, diabetes, autoimmune disease or malignant tumors. Participants who had participated in sexual activity or vaginal lavage within 72 h of sampling, reported cervical disease or genital HPV infection, reported vaginal bleeding in the preceding weeks, or used probiotics, antibiotics or corticoids in the preceding 2 weeks were excluded. Two other nonpregnant groups with the same age range and a normal medical history were established as control groups: nonpregnant women with hrHPV infection (NPHR, n = 19) and nonpregnant women without HPV infection (NPN, n = 30).
All enrolled women were preliminarily screened by both an HPV genotyping test and the ThinPrep cytology test (TCT). HPV detection and genotyping were performed using a commercial HPV genotyping kit for 21 HPV types (Hybribio®, Guangdong): 15 high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68) and 6 low-risk HPV types (6, 11, 42, 43,44 and 81). Pap smears of cervical exfoliated cells were evaluated by two experienced pathologists. Cytological cell samples were categorized according to Bethesda System criteria [29].
Samples for vaginal microbial analysis were collected from the lateral and posterior fornix using a sterile swab under direct visualization during a speculum examination. The vaginal swab samples were immediately frozen and stored at − 80 °C until extraction.
Total bacterial genomic DNA extraction and MiSeq sequencing
The swabs obtained were thawed on ice, and cells were suspended in transport buffer by vortexing and transferred to a sterile DNase/RNase-free 2.0 mL tube for enzymatic lysis. The lysate was purified using a QIAamp DNA Mini Kit (Qiagen®) according to the manufacturer’s recommendations. The total genomic DNA quality was assessed by 1% agarose gel electrophoresis, and the DNA concentration was measured using a Nanodrop ND-2000 (Nanodrop®).
The V3–4 hypervariable regions of the 16S rRNA gene were amplified using primers 338F (ACTCCTACGGGAGGCAGCA) and 806R (GGACTACHVGGGTWTCTAAT). An 8-bp barcode sequence was added to the ends of both the forward and reverse primers. Amplification was performed in 50-μl reactions with TransStart Fast Pfu DNA Polymerase (TransGen Biotech®), 200 nM of each primer and 2 μl of template. The reactions were performed using a GeneAmp PCR System 9700 (Applied Biosystems®) under the following thermal profile: 94 °C for 2 min, followed by 30 cycles of 94 °C for 30 s, 57 °C for 30 s, and 72 °C for 30 s, and one cycle of 72 °C for 10 min and a 4 °C hold. Three PCR products per sample were pooled to reduce reaction-level PCR bias. PCR products were examined by 2% agarose gel electrophoresis and then purified using an AxyPrep DNA Gel Extraction Kit (AXYGEN®). Amplicons were quantified using the QuantiFluor-ST™ system (Promega®). All sequencing was performed using the Illumina MiSeq platform at Majorbio Biopharm Technology Company (Shanghai).
Sequence analysis
Low-quality sequences with an average quality score less than 20, a length shorter than 50 bp, or any mismatches to the primers or barcode containing chimeras were removed by Trimmomatic [30]. Operational taxonomic units (OTUs) were defined using a cutoff value of 97% by QIIME. The taxonomy of OTUs (from phylum to species) was determined using the Ribosomal Database Project (RDP) classifier script (version 2.2). A Silva database was also used in this study (Release 128 http://www.arb-silva.de). Chimeric sequences and singletons were removed prior to taxonomic assignments.
The Chao richness estimator and Shannon alpha-diversity index were calculated using mothur (version v.1.30.1) [31]. Comparisons between different groups were assessed by Student’s t-test. Based on unweighted UniFrac distances calculated by the vegan package implemented in R [32], principal coordinates analysis (PCoA) was used to assess the difference in overall microbial community composition for beta-diversity assessment among four groups using R and tested by ANOSIM test. Taxonomic differences between the four groups were analyzed using the nonparametric Kruskal-Wallis test at different levels. Microbiological markers were detected using the linear discriminant analysis (LDA) effect size (LEfSe) algorithm [33]. To conform to vaginal community state types (CSTs) [34], hierarchical clustering analysis was conducted based on Jensen–Shannon distances between all pairs of community states and Ward linkage methods, as previously published [35]. Redundancy analysis (RDA) was applied to evaluate correlation between specific taxa and HPV infection or pregnancy using Canoco [36]. Heatmaps were generated and statistical analyses performed using R. A p-value < 0.05 was considered significant in all statistical analyses mentioned above.
Results
Characteristics of the study population
The average ages of each group (PHR, PN, NPHR, NPN) were 30.13 ± were, 29.77 ± 29., 33.53 ± 364 and 34.90 ± 4.17 years, respectively. The main genotype of HPV in both pregnant and nonpregnant women was HPV-16 (Additional file 1: Table S1 and Additional file 2: Table S2). All cytological tests were normal, and no lesions were detected. The demographic characteristics of each group are presented in the Additional file 3: Table S3).
Sequencing results
After filtering low-quality reads, 3,968,879 assembled clean reads were obtained from 135 samples, with a mean read length of 447.37 ± 7n57 bp. For normalization, the reads in each sample were randomly subsampled to the lowest number of 20,308 in sample NP_24 (PN group). After removing singletons (the OTUs contained less than 2 reads), 320 OTUs were identified, ranging from 10 OTUs in sample 4398 (NPN group) to 199 OTUs in sample 142_HH (PHR group) (Additional file 4: Table S4).
Vaginal microbiota richness and diversity
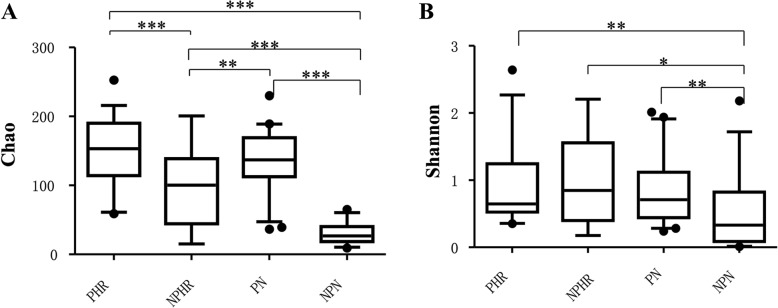
At the OTU level, microbial richness and diversity were estimated using Chao and Shannon indices, respectively, as shown in Fig. 1. These two indices revealed that both pregnancy and HPV infection increased vaginal bacterial richness and diversity. The means of Chao and Shannon indices were much higher in groups PN (135.97 ± 7ere and 0.82 ± and, respectively) and PHR (151.24 ± (151. and 0.96 ± and, respectively) than in NPHR (94.20 ± 094sp and 0.91 ± and, respectively) and NPN (29.94 ± (29. and 0.51 ± 1nd, respectively). Furthermore, the influence of pregnancy on bacterial richness was greater than that of HPV infection (Chao index, PN vs. NPHR: 135.97 ± N vs. > 94.20 ± 4.20., p = 0.002 < 0.01).
Fig. 1.
Vaginal bacterial richness and diversity in four groups. a Chao index; b Shannon index; Student’s t-test was used to compare differences between two groups; data are presented as the mean ± SD; ***: p ≤ 0.001; **: 0.001 < p < 0.01; *: p < 0.05
Vaginal bacterial structure and beta-diversity in different groups
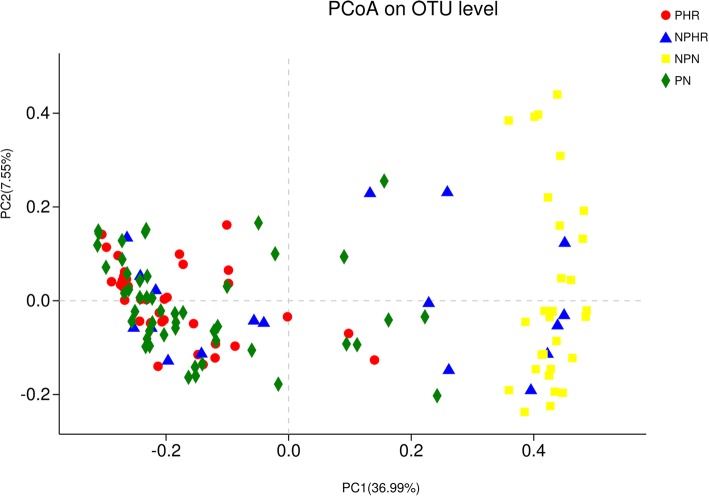
In PCoA, the first two principal components explained 36.99 and 7.55%, respectively, of the variance along the first and second axes, with the PHR, PN and NPHR samples visually separated from the NPN sample (Fig. 2). Comparison between two groups based on the ANOSIM test revealed that the bacterial structure of groups PHR, PN, NPHR and NPN were significantly different from each other, except for PHR vs. PN (R = − 0.0062, p = 0.545) (Additional file 7: Table S5).
Fig. 2.
Unweighted UniFrac principal coordinates analysis (PCoA) plot comparing sample distribution for the different groups
Taxonomy of the vaginal microbiota in different groups
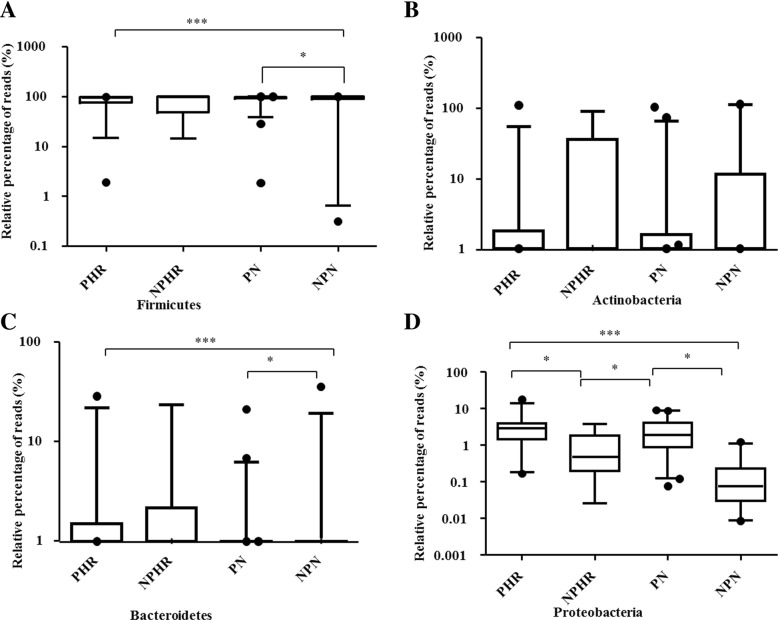
Overall, 22 bacterial phyla were recovered across all samples (Additional file 5: Figure S1), and 99% of the samples were dominated by Firmicutes, Actinobacteria, Bacteroidetes and Proteobacteria (Additional file 5: Figure S1). Firmicutes was the most abundant phylum, accounting for 85.57, 78.32, 88.73, and 82.94% of the NPN, NPHR, PN and PHR groups, respectively (Fig. 3a). Furthermore, pregnancy tended to increase the proportion of Firmicutes (PHR > NPN, PN > NPN, p < 0.05), but no influence was found for HPV infection (NPN vs. NPHR, p > 0.05). There were no differences among the different groups with regard to the proportion of Actinobacteria (Fig. 3b). The percentage of Bacteroidetes was significantly higher in the PHR group (3.06%) than in the NPN group (1.40%) but was lower in the PN group (1.10%) (Fig. 3c). Moreover, the proportions of Proteobacteria (Fig. 3d) were significantly increased after pregnancy, from 0.19% in the NPN group to 3.04% in the PHR group and 2.61% in the PN group (p < 0.05). HPV infection also decreased the relative percentage of Proteobacteria, from 2.61% in the PN group to 0.98% in the NPHR group (p < 0.05), though HPV infection did not change the dynamics of Proteobacteria during pregnancy (PHR vs. PN, p > 0.05).
Fig. 3.
Relative abundance counts of Firmicutes (a), Actinobacteria (b), Bacteroidetes (c) and Proteobacteria (d), which were found to be the most abundant phyla across all samples. The Wilcoxon test was used to compare differences in the abundance of each phylum between two groups. ***: p ≤ 0.001; **: 0.001 < p < 0.01; *: p < 0.05
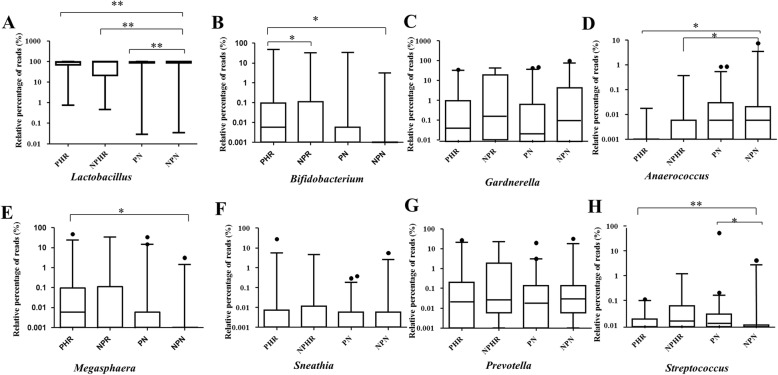
At the genus level, a total of 192 taxa were found across all samples (Additional file 6: Figure S2), with Lactobacillus being the most dominant genus overall. In addition, the proportion of Lactobacillus was significantly reduced during pregnancy and HPV infection compared to the NPN group (PHR: 75.27%, PN: 81.30%, NPHR: 70.10%, NPN: 84.58%) (Fig. 4a). In addition, pregnancy had a negative effect on the abundance of Bifidobacterium, which sharply decreased from 4.37% in the NPHR group to 0.02% in the PHR group (p < 0.05) (Fig. 4b). Pregnancy, however, increased the relative percentage of Streptococcus (Fig. 4h) from 0.19% in the NPN group to 1.11% in the PN group (p < 0.05). It was surprising to find that the abundance of Bifidobacterium was increased in HPV-infected patients (from 1.72% in the NPN group to 4.37% in the NPHR group, p < 0.01) (Fig. 4b). Conversely, HPV infection had a negative effect on the abundance of Anaerococcus, though pregnancy did not influence its proportions (NPHR: 0.04%, PHR: 0.002% vs. NPN: 0.27%, p < 0.05) (Fig. 4d). Megasphaera was significantly more abundant in the PHR group (3.74%) than in the NPN group (0.10%) (Fig. 4e), which revealed that the association between pregnancy and HPV infection increased the proportion of Megasphaera. The dual effect of pregnancy and HPV infection (PHR: 0.01% vs. NPN: 0.19%) also influenced the abundance of Streptococcus (Fig. 4h).
Fig. 4.
Relative abundance counts of Lactobacillus (a), Bifidobacterium (b), Gardnerella (c), Anaerococcus (d), Megasphaera (e), Sneathia (f), Prevotella (g) and Streptococcus (h), which were found to be the most abundant genera across all samples. The Wilcoxon test was used to compare differences in the abundance of each phylum between two groups. ***: p ≤ 0.001; **: 0.001 < p < 0.01; *: p < 0.05
Identification of vaginal microbiological markers in different groups
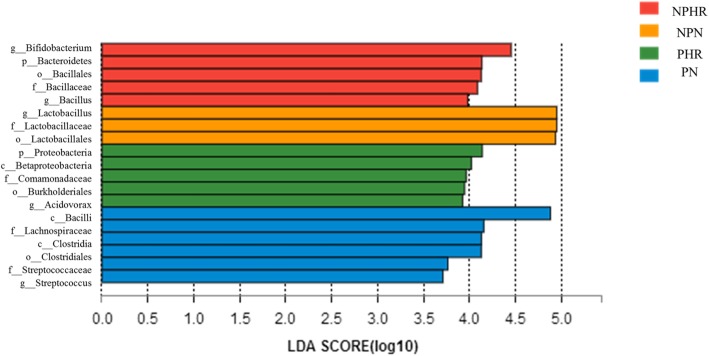
LEfSe modeling was employed to identify microbiological markers related to HPV infection and pregnancy (Fig. 5). The threshold for the logarithmic LDA model score for discriminative features in this study was 3.5. The most abundant genus in the NPN group was Lactobacillus. HPV infection was strongly associated with two genera, Bifidobacterium and Bacillus. Streptococcus at the genus level, Lachnospiraceae at the family level, and Clostridiales at the order level were three taxa related to pregnancy. Nevertheless, the PHR group was associated with Acidovorax and members of the families Comamonadaceae, both from the phylum Proteobacteria.
Fig. 5.
The unique taxa and microbiomarkers for different groups. Shown is a histogram of LDA scores computed for features differentially abundant in the four groups
Characteristics of vaginal community state types (CSTs) for different groups
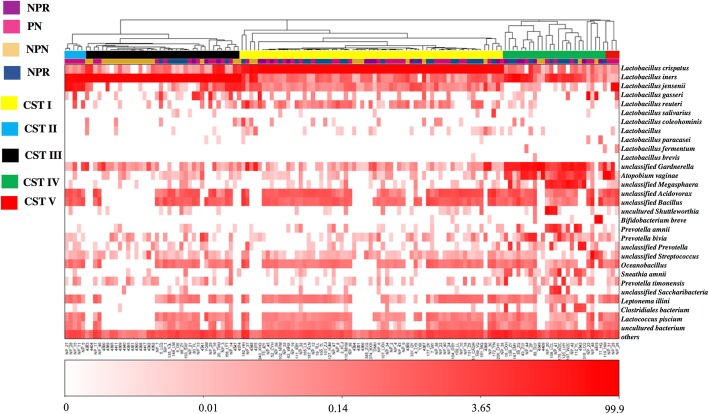
The vaginal bacterial CST analysis visualized by hierarchical clustering revealed that all samples clustered into five major groups: CST I, CST II, CSTII, CST IV and CST V (Fig. 6). The most commonly observed community was CST I (64/135, 47.4%), followed by CST III (38/135, 28.1%), CST IV (26/135, 19.3%), CST II (2/135, 1.5%) and CST V (5/135, 3.7%). The proportions of CSTs in different groups are shown in Table 1. Samples in the NPN group were assigned to CST III (18/30, 60.0%). Pregnancy converted the vaginal bacterial community structure from CST III to CST I, as the PN group was dominated by CST I (24/48, 50.0%) and presented less CST III (10/48, 20.8%). Similarly, the proportion of CST I increased to 52.6% (10/19) in the NPHR group. CST I was also dominant in hrHPV-infected pregnant women (group PHR) (22/38, 57.9%), and hrHPV infection increased the proportions of CST IV in both pregnant and nonpregnant women, as NPHR (31.6%) > PHR (23.7%) > PN (14.6%) > NPN (13.3%).
Fig. 6.
Heat map of the relative abundance of the 31 most abundant bacterial taxa found in the vaginal bacterial communities of all participants in the study. Ward linkage clustering was used to cluster samples based on their Jensen-Shannon distance. Identified CSTs are labeled as I, II, III, IV and V, according to a previous naming convention
Table 1.
The distribution of community state types (CSTs) in different groups
| PN N (%) |
PHR N (%) |
NPN N (%) |
NPHR N (%) |
|
|---|---|---|---|---|
| CST I | 24 (50.00) | 22 (57.90) | 8 (26.70) | 10 (52.60) |
| CST II | 2 (4.20) | 0 (0) | 0 (0) | 0 (0) |
| CST III | 10 (20.80) | 7 (18.40) | 18 (60.00) | 3 (15.80) |
| CST IV | 7 (14.60) | 9 (23.70) | 4 (13.30) | 6 (31.60) |
| CST V | 5 (10.40) | 0 (0) | 0 (0) | 0 (0) |
Redundancy analysis of samples
The results of RDA (Fig. 7) showed that the abundances of Megasphaera, Sneathia, Prevotella, Gardnerella, Fastidiosipila and Dialister correlated positively with HPV infection; in contrast, the abundances of Lactobacillus, Streptococcus and Shuttleworthia correlated negatively with HPV infection. In addition, the abundance of Shuttleworthia correlated positively with pregnancy, and that of Gardnerella correlated negatively with pregnancy. The abundances of Bacillus, Acidovorax, Oceanobacillus, Lactococcus and Bifidobacterium correlated positively with a dual influence of pregnancy and HPV infection.
Fig. 7.
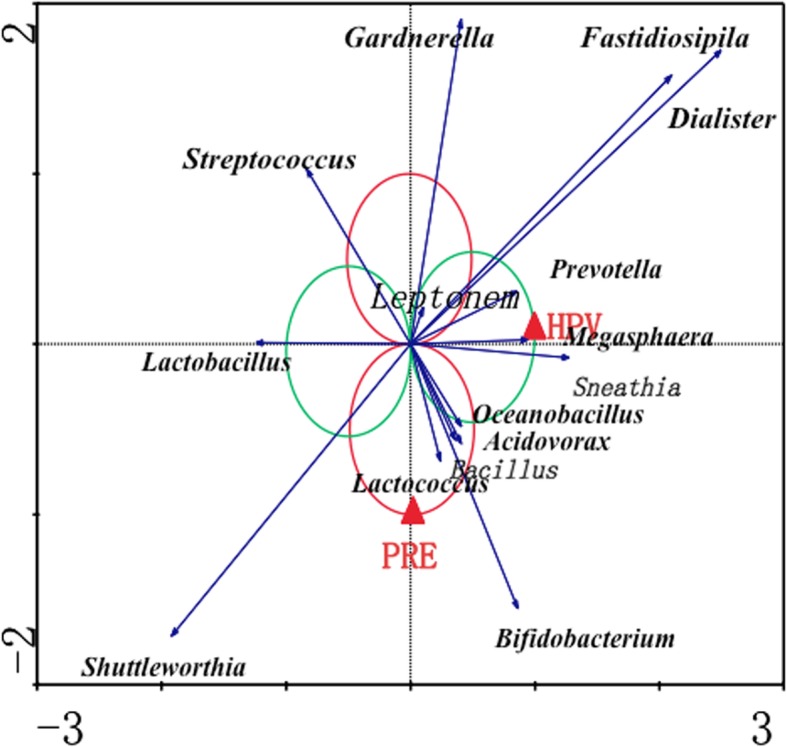
Redundancy analysis of correlations between different specific genera and HPV infection or pregnancy
Discussion
Our study addressed an undetermined topic about the association between the vaginal microbiota and hrHPV infection in pregnant women. We observed that both pregnancy and hrHPV infection were accompanied by increased vaginal bacterial richness and diversity. Lactobacillus was still the most abundant genus in all groups; however, both hrHPV infection and pregnancy had a negative influence on its abundance. Pregnancy and hrHPV infection were also accompanied by an increased proportion of CST I (dominated by Lactobacillus crispatus), as opposed to CST III. The abundances of various genera were differentially influenced by hrHPV infection and pregnancy. Overall, more anaerobic bacteria were associated with hrHPV infection and pregnancy.
According to our results, pregnancy increased vaginal bacterial richness and diversity, though some previous studies have reported lower vaginal microbial diversity during pregnancy [19, 20, 37]. A possible reason for the different findings might be that higher estrogen concentrations during pregnancy resulting in an accumulation of glycogen metabolized to lactic acid by Lactobacillus spp. on the upper layer of the vaginal epithelium, and leading to an increased abundance of Lactobacillus spp. in the vagina, thus decreasing overall bacterial richness and diversity. In this study, we observed a reduced abundance of Lactobacillus during pregnancy, which might lead to the growth of other bacteria, increasing bacterial diversity and richness in pregnant women. A similar result was reported in another study of a Chinese cohort, whereby Huang et al. found higher vaginal bacterial diversity in pregnant women, especially in the first trimester [38]. A reasonable explanation might be that fluctuation in the vaginal microbiota differs between Asian populations and Western populations and is heavily influenced by ethnicity. Regardless, the intrinsic causes need to be further studied by investigations of larger scope.
HPV infection can increase vaginal bacterial richness and diversity and lower the percentage of Lactobacillus [11, 12], and our results are in agreement with these previous studies. HPV infection is thought to alter the acidic environment of the vagina, which might promote outbreaks of bacteria [12]. In addition, the mucosal immunity and inflammation induced by HPV infection, including induction of pro-inflammatory cytokines, production of reactive oxygen species and activation of immune cells, might lead to changes in the vaginal microbiota [39]. However, other studies have indicated that hrHPV is not necessarily sufficient to induce changes in the cervicovaginal microbiota [17, 40], even though enrichment of certain anaerobic bacteria was found in patients with CIN lesions [17]. As we did not conduct cervical biopsy for high-risk HPV-infected women in our study, the presence of CIN was not assessed. Hence, we cannot exclude the possibility that the vaginal microbiota was also influenced by CIN lesions [17]. Interestingly, we found that the influence of pregnancy on bacterial richness was greater than that of HPV infection. To date, Tuominen H. et al. has also reported an altered microbial composition in cervix and placenta of HPV positive pregnant women [14]. However, it remains uncertain whether the changes result from HPV or other factors. Although both pregnancy and hrHPV infection reduced the abundance of Lactobacillus, which may contribute to increased bacterial richness and diversity, it is clear that the underlying mechanisms responsible are different. Changes in physiological hormones and the immunosuppressive state that occurs during pregnancy might lead to a fragile balance of the vaginal microbiome, which should be further explored in future studies.
Regarding community state types, the most abundant CST among nonpregnant reproductive-age women (NPN) was CST III, which was in accordance with the results from an Asian population in the study by Ravel et al. [34]. The most dominant CST in the hrHPV infection and pregnancy groups (PN, PHR and NPHR) was CST I. The predominant CSTs during pregnancy reported in previous studies varied from CST III to CST I [19, 37, 41]. Several previous studies have shown that L. iners and L. crispatus are the two most abundant Lactobacillus species found in pregnant women [18, 19, 37, 38, 41–43]. In the vagina, a bacterial community change from CST III to CST IV is commonly observed, thus indicating that a colonization of anaerobes is more frequent in an L. iners-dominant VMB (vaginal microbiome). Conversely, an L. crispatus-dominant VMB has been associated with a low-stress environment and an adequate level of autophagy by vaginal epithelial cells to remove harmful cytoplasmic components, as well as bacteria, in pregnant women [41]. Similarly, the predominant CSTs in cases of HPV infection are controversial. Lee et al. reported that the prevalence of HPV infection did not differ between CST III and CST I [12], whereas another study found a higher HPV infection rate for an L. iners-dominant VMB [13]. It should be noted that these studies were performed using cohorts of different ethnicities, which might be one of the reasons for the differences. We also observed HPV infection to be associated with an increased proportion of CST IV in both nonpregnant and pregnant women. In two longitudinal studies, CST IV dominated by anaerobic bacteria comprised the greatest proportion of HPV-positive samples, and CST IV was associated with an increased risk of transitioning to an HPV-positive state [13, 44].
HPV infection and pregnancy equally influenced the vaginal microbial composition, but different specific genera were enriched in hrHPV-infected or pregnant women. Bifidobacterium, Bacillus, Megasphaera, Sneathia, Prevotella, Gardnerella, Fastidiosipila and Dialister were identified as significant taxa in nonpregnant hrHPV-infected populations; in previous studies, anaerobic bacteria such as Bacillus, Megasphaera, Sneathia, Prevotella, Gardnerella and Dialister have been associated with HPV infection [12, 13, 45, 46]. In general, a microenvironment with a high proportion of anaerobic bacteria and a lower proportion of Lactobacillus spp. is more susceptible to HPV infection. A surprising finding was that Bifidobacterium, a type of lactic acid-producing probiotic [47], was enriched in HPV-positive women. Although participants who used probiotics in the preceding 2 weeks were excluded from the study, we cannot rule out the influence of probiotics taken prior to that time, and they might have some persistent effects on the vaginal microbiota [48]. It has been hypothesized that Bifidobacterium might be able to guarantee a healthy vaginal balance by the production of lactic acid, in the lack of Lactobacillus. However, there are several cases reporting Bifidobacterium species as pathogens in various infectious conditions [49, 50], and a high level of stress inducers has been detected in vaginal epithelial cells when Bifidobacterium predominated the VMB [38]. Hence, the role of members of this genus should be further investigated. However, as both HPV infection and pregnancy are able to influence the vaginal microbiome, an inconsistent profiling of significant bacteria was found in hrHPV-infected pregnant women compared to nonpregnant women. Anaerobic bacteria (Megasphaera and Bacillus) and lactic acid-producing bacteria (Bifidobacterium and Lactococcus) were identified in these samples. Moreover, we found novel vaginal bacterial taxa, i.e., Acidovorax and Oceanobacillus, which were also associated with HPV infection in pregnant women. Nevertheless, the roles of these microorganisms are not clear and should be investigated further. These findings support our hypothesis that hrHPV infection and pregnancy have a dual effect on increasing vaginal bacterial diversity, leading to a differentially altered vaginal microbiota in HPV-infected pregnant women compared to nonpregnant HPV-infected women.
The strength of this study is that it addressed still-unresolved issues regarding the association between the VMB and HPV infection in pregnant women. We found high diversity and richness for the vaginal microbiome during pregnancy in a Chinese cohort and uncovered that pregnancy and HPV infection have a probable synergistic effect on altering the VMB, which caused a more complicated vaginal bacterial environment in pregnant women with hrHPV infection. The limitations of this study were that it was a small, cross-sectional study, and thus we could not determine any causation between the VMB and HPV infection or pregnancy. Further studies with longitudinal sampling are needed to assess correlations between the dynamics of the VMB microbiome and the transition of HPV at early, mild, and late gestational stages as well as postpartum and to investigate its relationship with different subtypes of HPV.
Conclusion
This work uncovered a probable synergistic effect of hrHPV infection and pregnancy on the vaginal microbial composition. Despite not having measured hormone level in our patients, pregnancy status seems to be a bigger driver of the vaginal diversity in our cohort, as other studies demonstrated that HPV alone does not lead to changes in it [17, 40]. HPV infection in pregnant women was associated with a more complex and diverse microbial environment.
Additional files
Table S1. HPV genotyping and distribution in pregnant women. (DOCX 18 kb)
Table S2. HPV genotyping and distribution in not pregnant women. (DOCX 17 kb)
Table S3. Characteristics of study population. (DOCX 20 kb)
Table S4. Sequences information. (DOCX 24 kb)
Figure S1. Heat map of relative abundance for the 22 most abundant bacterial phyla found in the vaginal bacterial communities of 4 groups. (PDF 173 kb)
Figure S2. Heat map of relative abundance for the 20 most abundant bacterial genus found in the vaginal bacterial communities of 4 groups. (PDF 142 kb)
Table S5. The comparison of the bacterial structure between two groups tested by ANOSIM test. (DOCX 34 kb)
Acknowledgements
None
Abbreviations
- CIN
Cervical intraepithelial neoplasia
- CST
Community state type
- HPV
Human papillomavirus
- hrHPV
High-risk human papillomavirus
- LEfSe
Linear discriminant analysis (LDA) effect size
- OTUs
Operational taxonomic units
- PCoA
Principal coordinates analysis
- RDA
Redundancy analysis
- RDP
Ribosomal Database Project
- TCT
ThinPrep cytology test
Authors’ contributions
YC, ZH, WD and LQ contributed to the conception and design of the study. YC, ZH, WW, LG and HG organized the database. YC, ZH and WW performed the data analysis. YC and ZH wrote the first draft of manuscript. YC and ZH contributed equally to this work. All authors contributed to manuscript revision and read and approved the submitted version.
Funding
This study was supported by National Key R&D Program of China (2016YFC1302900), Shanghai Municipal Commission of Health and Family Planning (2017ZZ02016), the Health and Family Planning Commission of Shanghai PUDONG New Area (PW2016E-2), Shanghai Municipal Education Commission (Gaofeng Clinical Medicine Grant Support, 20161412), Shanghai Science and Technology Commission (18411963100) and Shanghai Municipal Commission of Health and Family Planning (20134205). The funders did not have any other role in the design of the study, collection, analysis, and interpretation of data and in writing of this manuscript.
Availability of data and materials
All reads obtained were submitted to NCBI Sequence Read Archive (SRA) under the accession number SRP126438 (http://www.ncbi.nlm.nih.gov/bioproject/421398).
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Renji Hospital, School of Medicine, Shanghai Jiao Tong University (registration number: 2018–026), and all experiments were performed in accordance with relevant guidelines and regulations. All participants provided written informed consent. Upon enrollment, some of the participants were asked to complete a questionnaire detailing sexual and reproductive health history and hygiene practices with demographic information.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yulian Chen and Zubei Hong contributed equally to this work.
Contributor Information
Lihua Qiu, Email: lilyqiulh@126.com.
Wen Di, Email: diwen163@163.com.
References
- 1.Muñoz N, Bosch FX, De SS, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 2.Erickson BK, Alvarez RD, Huh WK. Human papillomavirus: what every provider should know. Am J Obstet Gynecol. 2013;208(3):169–175. doi: 10.1016/j.ajog.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hariri S, Unger ER, Sternberg M, Dunne EF, Swan D, Patel S, et al. Prevalence of genital human papillomavirus among females in the United States, the national health and nutrition examination survey, 2003-2006. J Infect Dis. 2011;204(4):566–573. doi: 10.1093/infdis/jir341. [DOI] [PubMed] [Google Scholar]
- 4.Hernández-Girón C, Smith JS, Lorincz A, Lazcano E, Hernández-Avila M, Salmerón J. High-risk human papillomavirus detection and related risk factors among pregnant and nonpregnant women in Mexico. Sex Transm Dis. 2005;32(32):613–618. doi: 10.1097/01.olq.0000179888.47309.db. [DOI] [PubMed] [Google Scholar]
- 5.Deidda F, Amoruso A, Nicola S, Graziano T, Pane M, Allesina S, et al. The in vitro effectiveness of Lactobacillus fermentum against different Candida species compared with broadly used azoles. J Clin Gastroenterol. 2016;50:S171. doi: 10.1097/MCG.0000000000000686. [DOI] [PubMed] [Google Scholar]
- 6.Prieto D, Pla J. Distinct stages during colonization of the mouse gastrointestinal tract by Candida albicans. Front Microbiol. 2015;6(1):792. doi: 10.3389/fmicb.2015.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borgdorff H, Tsivtsivadze E, Verhelst R, Marzorati M, Jurriaans S, Ndayisaba GF, et al. Lactobacillus-dominated cervicovaginal microbiota associated with reduced HIV/STI prevalence and genital HIV viral load in African women. ISME J. 2014;8(9):1781–1793. doi: 10.1038/ismej.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirjavainen PV, Pautler S, Baroja ML, Anukam K, Crowley K, Carter K, et al. Abnormal immunological profile and vaginal microbiota in women prone to urinary tract infections. Clin Vaccine Immunol. 2009;16(1):29–36. doi: 10.1128/CVI.00323-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messano GA. Inadequate antibiotic therapy of genitourinary tract infections could be responsible for viral sexually transmitted diseases. Ann Ig. 2013;25(6):553–554. [PubMed] [Google Scholar]
- 10.Chehoud C, Stieh DJ, Bailey AG, Laughlin AL, Allen SA, McCotter KL, et al. Associations of the vaginal microbiota with HIV infection, bacterial vaginosis, and demographic factors. AIDS. 2017;31(7):895–904. doi: 10.1097/QAD.0000000000001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao W, Weng J, Gao Y, Chen X. Comparison of the vaginal microbiota diversity of women with and without human papillomavirus infection: a cross-sectional study. BMC Infect Dis. 2013;13(1):271. doi: 10.1186/1471-2334-13-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JE, Lee S, Lee H, Song YM, Lee K, Han MJ, et al. Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS One. 2013;8(5):e63514. doi: 10.1371/journal.pone.0063514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brotman RM, Shardell MD, Gajer P, Tracy JK, Zenilman JM, Ravel J, et al. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J Infect Dis. 2014;210(11):1723. doi: 10.1093/infdis/jiu330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuominen H, Rautava S, Syrjänen S, Collado MC, Rautava J. HPV infection and bacterial microbiota in the placenta, uterine cervix and oral mucosa. Sci Rep. 2018;8:9787. doi: 10.1038/s41598-018-27980-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitra A, Macintyre DA, Lee YS, Smith A, Marchesi JR, Lehne B, et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci Rep. 2015;5:16865. doi: 10.1038/srep16865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh HY, Kim BS, Seo SS, Kong JS, Lee JK, Park SY, et al. The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clin Microbiol Infect. 2015;21(7):674.e1–674.e9. doi: 10.1016/j.cmi.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 17.Godoy-Vitorino F, Romaguera J, Zhao C, Vargas-Robles D, Ortiz-Morales G, Vázquez-Sánchez F, et al. Cervicovaginal fungi and bacteria associated with cervical intraepithelial neoplasia and high-risk human papillomavirus infections in a Hispanic population. Front Microbiol. 2018;9:2533. doi: 10.3389/fmicb.2018.02533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Bieda J, et al. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome. 2014;2:18. doi: 10.1186/2049-2618-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacIntyre DA, Chandiramani M, Lee YS, Kindinger L, Smith A, Angelopoulos N, et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci Rep. 2015;5:8988. doi: 10.1038/srep08988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aagaard K, Riehle K, Ma J, Segata N, Mistretta TA, Coarfa C, et al. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS One. 2012;7(6):e36466. doi: 10.1371/journal.pone.0036466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Son K-A, Kim M, Kim YM, Kim SH, Choi S-J, Oh S-y, et al. Prevalence of vaginal microorganisms among pregnant women according to trimester and association with preterm birth. Obstet Gynecol Sci. 2018;61(1):38–47. doi: 10.5468/ogs.2018.61.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Sun C, Liu C, Yang Y, Lu W. Comparison of vaginal microbial community structure in healthy and endometritis dairy cows by PCR-DGGE and real-time PCR. Anaerobe. 2016;38:1–6. doi: 10.1016/j.anaerobe.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Merckx M, Liesbeth WV, Arbyn M, Meys J, Weyers S, Temmerman M, et al. Transmission of carcinogenic human papillomavirus types from mother to child: a meta-analysis of published studies. Eur J Cancer Prev. 2013;22(3):277–285. doi: 10.1097/CEJ.0b013e3283592c46. [DOI] [PubMed] [Google Scholar]
- 24.Palle C, Bangsbøll S, Andreasson B. Cervical intraepithelial neoplasia in pregnancy. Int J Gynaecol Obstet. 1982;20(2):111–118. [Google Scholar]
- 25.Liu P, Xu L, Sun Y, Wang Z. The prevalence and risk of human papillomavirus infection in pregnant women. Epidemiol Infect. 2014;142(8):1567–1578. doi: 10.1017/S0950268814000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison EA, Gammon MD, Goldberg GL, Vermund SH, Burk RD. Pregnancy and cervical infection with human papillomaviruses. Int J Gynecol Obstet. 1996;54(2):125–130. doi: 10.1016/0020-7292(96)02694-x. [DOI] [PubMed] [Google Scholar]
- 27.Schmeink CE, Melchers WJ, Hendriks JC, Quint WG, Massuger LF, Bekkers RL. Human papillomavirus detection in pregnant women: a prospective matched cohort study. J Womens Health. 2012;21(12):1295. doi: 10.1089/jwh.2012.3502. [DOI] [PubMed] [Google Scholar]
- 28.Trottier H, Mayrand MH, Baggio ML, Galan L, Ferenczy A, Villa LL, et al. Risk of human papillomavirus (HPV) infection and cervical neoplasia after pregnancy. BMC Pregnancy Childbirth. 2015;15(1):244. doi: 10.1186/s12884-015-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solomon D, Davey D, Kurman R. Bethesda system\terminology for reporting results of cervical cytology. JAMA. 2002;287(287):2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 30.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75(23):7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.R. Core Team . R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; R. Core Team; 2018. [Google Scholar]
- 33.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108:4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gajer P, Brotman RM, Bai GY, Sakamoto J, Schuette UME, Zhong X, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012;4(132):132ra52. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braak TCJF. CANOCO-an extension of DECORANA to analyze species-environment relationships. Hydrobiologia. 1989;184(3):169–170. [Google Scholar]
- 37.Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014;2(1):4. doi: 10.1186/2049-2618-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang YE, Wang Y, He Y, Ji Y, Wang LP, Sheng HF, et al. Homogeneity of the vaginal microbiome at the cervix, posterior fornix, and vaginal canal in pregnant Chinese women. Microb Ecol. 2015;69(2):407–414. doi: 10.1007/s00248-014-0487-1. [DOI] [PubMed] [Google Scholar]
- 39.Woodworth CD. HPV innate immunity. Front Biosci. 2002;7(7):d2058–d2071. doi: 10.2741/A898. [DOI] [PubMed] [Google Scholar]
- 40.Malgorzata BH, Wioleta L, Myers JE, Keiffer TR, Stephen DG, Paula P, et al. A new cell culture model to genetically dissect the complete human papillomavirus life cycle. PLoS Pathog. 2018;14(3):e1006846. doi: 10.1371/journal.ppat.1006846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nasioudis D, Forney LJ, Schneider GM, Gliniewicz K, France MT, Boester A, et al. The composition of the vaginal microbiome in first trimester pregnant women influences the level of autophagy and stress in vaginal epithelial cells. J Reprod Immunol. 2017;123:35. doi: 10.1016/j.jri.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Hyman RW, Fukushima M, Jiang H, Fung E, Rand L, Johnson B, et al. Diversity of the vaginal microbiome correlates with preterm birth. Reprod Sci. 2014;21(1):32–40. doi: 10.1177/1933719113488838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salinas AM, Osorio VG, Endara PF, Salazar ER, Vasco GP, Vivero SG, et al. Bacterial identification of the vaginal microbiota in Ecuadorian pregnant teenagers: an exploratory analysis. PeerJ. 2018;6:e4317. doi: 10.7717/peerj.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jalil EM, Bastos FI, Melli PP, Duarte G, Simoes RT, Yamamoto AY, et al. HPV clearance in postpartum period of HIV-positive and negative women: a prospective follow-up study. BMC Infect Dis. 2013;13(1):1–9. doi: 10.1186/1471-2334-13-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Łaniewski P, Barnes D, Goulder A, Cui H, Roe DJ, Chase DM, et al. Linking cervicovaginal immune signatures, HPV and microbiota composition in cervical carcinogenesis in non-Hispanic and Hispanic women. Sci Rep. 2018;8(1):7593. doi: 10.1038/s41598-018-25879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paola MD, Sani C, Clemente AM, Iossa A, Perissi E, Castronovo G, et al. Characterization of cervico-vaginal microbiota in women developing persistent high-risk human papillomavirus infection. Sci Rep. 2017;7(1):10200. doi: 10.1038/s41598-017-09842-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayo B, Sinderen DV, Ventura M. Genome analysis of food grade lactic acid-producing Bacteria: from basics to applications. Curr Genomics. 2008;9(3):169–183. doi: 10.2174/138920208784340731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verdenelli MC, Cecchini C, Coman MM, Silvi S, Orpianesi C, Coata G, et al. Impact of probiotic synbio® administered by vaginal suppositories in promoting vaginal health of apparently healthy women. Curr Microbiol. 2016;73(4):483–490. doi: 10.1007/s00284-016-1085-x. [DOI] [PubMed] [Google Scholar]
- 49.Bhaskar MM, Sistla S, Kumaravel S. A case of pyometrocolpos with Bifidobacterium, species. Anaerobe. 2017;44:48–50. doi: 10.1016/j.anaerobe.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 50.Pathak P., Trilligan C., Rapose A. Bifidobacterium--friend or foe? A case of urinary tract infection with Bifidobacterium species. Case Reports. 2014;2014(sep24 1):bcr2014205122–bcr2014205122. doi: 10.1136/bcr-2014-205122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. HPV genotyping and distribution in pregnant women. (DOCX 18 kb)
Table S2. HPV genotyping and distribution in not pregnant women. (DOCX 17 kb)
Table S3. Characteristics of study population. (DOCX 20 kb)
Table S4. Sequences information. (DOCX 24 kb)
Figure S1. Heat map of relative abundance for the 22 most abundant bacterial phyla found in the vaginal bacterial communities of 4 groups. (PDF 173 kb)
Figure S2. Heat map of relative abundance for the 20 most abundant bacterial genus found in the vaginal bacterial communities of 4 groups. (PDF 142 kb)
Table S5. The comparison of the bacterial structure between two groups tested by ANOSIM test. (DOCX 34 kb)
Data Availability Statement
All reads obtained were submitted to NCBI Sequence Read Archive (SRA) under the accession number SRP126438 (http://www.ncbi.nlm.nih.gov/bioproject/421398).