Abstract
There is an unmet need for blood biomarkers in diagnosis and prognosis of chronic obstructive pulmonary disease (COPD). The search for these biomarkers has been revolutionized by high-throughput sequencing techniques and multiplex platforms that can measure thousands of gene transcripts, proteins, or metabolites. We review COPDGene (Genetic Epidemiology of COPD) project publications that include DNA methylation, transcriptomic, proteomic, and metabolomic blood biomarkers and discuss their impact on COPD. Key contributions from COPDGene include identification of DNA methylation effects from smoking and genetic variation, new transcriptomic signatures in the blood, identification of protein biomarkers associated with severity and progression (e.g., sRAGE [soluble receptor for advanced glycosylation end products], inflammatory cytokines IL-6 and IL-8), and identification of small molecules (ceramides and sphingomyelin) that may be pathogenic. COPDGene studies have revealed that some of the COPD genome-wide association study polymorphisms are strongly associated with blood biomarkers (e.g., rs2070600 in AGER is a pQTL [protein quantitative trait locus] for sRAGE), underscoring the importance of combining omics results. Investigators have developed molecular networks identifying lower CD4+ resting memory cells associated with COPD. Genes, proteins, and metabolite networks are particularly important because the explanatory value of any single molecule is small (1–10%) compared with panels of multiple markers. COPDGene has been a useful resource in the identification and validation of multiple biomarkers for COPD. These biomarkers, either combined in multiple biomarker panels or integrated with other omics data types, may lead to novel diagnostic and prognostic tests for COPD phenotypes and may be relevant for assessing novel therapies.
Keywords: chronic obstructive pulmonary disease, biomarkers, metabolomics, epigenetics, gene expression
Although the lung is the primary target organ in chronic obstructive pulmonary disease (COPD), there is substantial evidence that COPD has systemic manifestations. For instance, patients with COPD commonly have bone and muscle loss, depression, anxiety, and reduced exercise tolerance beyond that explained by lung function. Patients with COPD are also more likely to have comorbid diseases such as coronary artery disease, congestive heart failure, pulmonary hypertension, interstitial lung disease, cancer, weight loss, metabolic syndrome, diabetes, hypertension, and hyperlipidemia (1). Although smoke exposure is a prominent factor linking COPD to other smoking-associated conditions, the increased risk of these systemic diseases persists even after adjusting for smoking intensity (2).
The blood compartment may be the best site to assess these systemic effects, and blood biomarkers have been proposed as useful assessments of the systemic nature of smoking and COPD (3). Compared with sampling from the lung, blood has the advantage of minimal risk and the potential for repeated sampling to monitor disease progression. In addition, blood sampling has more widely available expertise and is more reproducible and less time consuming than other minimally invasive biospecimen sampling, such as for urine, sputum, and exhaled breath condensate. These features make blood sampling practical in large research cohorts such as COPDGene (Genetic Epidemiology of COPD).
Although blood may be the most common biofluid used for omics studies, it has limitations. It can be affected by processing issues (time, tubes, coordinator), leading to unwanted measurement variation. Biological factors such as sex, age, and race and behaviors such as smoking and eating can have large effects on blood biomarkers. Furthermore, comorbid conditions associated with COPD may affect interpretation of biomarkers (e.g., diabetes and sRAGE [soluble receptor for advanced glycosylation end products]), and biomarkers may not be reproducible across different panels. Recognition of and proper adjustment for these factors are essential in the discovery and validation phases of biomarker studies.
Two factors have facilitated the identification of blood biomarkers of COPD. First is the development of several well-phenotyped longitudinal COPD cohorts with blood sampling (e.g., COPDGene, ECLIPSE [Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points], SPIROMICS [Subpopulations and Intermediate Outcomes in COPD Study]) (3–6). Second, there have been substantial technical advances in the large-scale measurement of omics data types, including DNA, RNA, proteins, and metabolites. For example, multiplex proteomic platforms can now interrogate thousands of proteins, hundreds of thousands of transcripts, and millions of SNPs in small sample volumes (<1 ml).
This perspective article summarizes evidence from COPDGene that supports four types of blood biomarkers: 1) DNA methylation, which has been assessed using array-based technologies and pyrosequencing; 2) RNA transcriptome, which has been quantified using arrays (e.g., Affymetrix or OpenArray [Thermo Fisher Scientific]) or RNA sequencing (RNA-Seq); 3) proteins, which are typically measured with targeted high-affinity approaches such as antibodies (e.g., ELISAs, multiplex bead assays) or aptamers (e.g., SOMAmers) or low-affinity approaches using mass spectrometry (targeted or untargeted); and 4) metabolites, which are typically lower-molecular-weight compounds (e.g., lipids, amino acids, nucleotides, antioxidants) that are measured most commonly using mass spectrometry or enzymatic assays.
Overview of the COPDGene Study
The COPDGene study enrolled 10,198 non-Hispanic white or African American smokers (aged 45–80 yr) with and without COPD between 2007 and 2011 (5). Five-year follow-up visits occurred from 2013 to 2017, and 10-year follow-up visits were begun in 2018. An additional 456 never smokers (<100 lifetime cigarettes) were enrolled from 2008 to 2017. Subjects were phenotyped with spirometry, computed tomographic (CT) imaging of the chest, and questionnaires, and blood samples (whole blood, plasma, and serum) were obtained for omic analyses.
Epigenomic Biomarkers
“Epigenomics” refers to the study of DNA changes beyond the DNA sequence, such as chromatin modification or, more commonly in large population studies such as COPDGene, DNA methylation. Like gene expression, variability in DNA methylation is impacted by subject age, cell type and cellular heterogeneity of samples, environmental exposures, and disease status. DNA methylation marks may set transcriptomic trajectories at presymptomatic states, thus representing a potential biomarker to leverage for targeted prevention.
Both internal (e.g., genetic, hormonal) and external (e.g., smoking) factors are major drivers of variable DNA methylation. Qiu and colleagues (7) examined 27,000 methylation sites in whole blood from 85 current and former smokers from COPDGene to study the impact of genetic variation and cigarette smoking on DNA methylation. They identified 1,287 methylation sites at 1,242 unique genes associated with smoking; many of these significantly associated methylation sites were related to differences in cell type composition within the blood samples, underscoring the importance of adjusting for cell type in whole-blood assays. The study also identified 770 methylation sites in 708 unique genes that were significantly associated with genotype; however, the overlap between the differentially methylated and genetically influenced gene sets was not greater than expected by chance, suggesting that the impact of smoking on DNA methylation is independent from the genetic impact on methylation.
To address the influence of cell type on methylation signatures, Wan and colleagues studied DNA methylation in buccal brushings from 82 COPDGene subjects using a DNA methylation array that assayed approximately 450,000 methylation sites (8). They identified seven genes with methylation sites associated with current smoking. Significant methylation sites that annotated to the CYP1B1 and PARVA genes were validated using pyrosequencing in 130 independent subjects. Target genes were subsequently tested for association with lung function and CT scan measures of emphysema, with the main finding being differential methylation of CYP1B1 associated with reduced lung function and increased emphysema in women. Their conclusions were that site-specific methylation in buccal source DNA could provide insights into sex-specific impacts of cigarette smoking and COPD.
Transcriptomic Biomarkers
The largest gene expression (transcriptome) studies in COPD have been on blood. Similar to the epigenome, the transcriptome can be influenced by factors such as age, sex, cell type, environmental exposures, and disease status. Bahr and colleagues performed microarray gene expression profiling in peripheral blood mononuclear cells collected using cell preparation tubes (BD Biosciences) from 136 COPDGene subjects (9). They found 1,090 transcripts associated with FEV1 percent predicted and 1,745 associated with FEV1/FVC (with much overlap). These genes overrepresented pathways related to immunity, inflammatory response, and sphingolipid (ceramide) metabolism and signaling; however, subsequent reanalysis of these data by Halper-Stromberg and colleagues (10) revealed that cell composition accounted for a significant amount of transcriptome variation and that COPD was associated with a lower proportion of specific transcriptomic signatures for CD4+ resting memory cells and naive B cells.
A significant limitation of transcriptomic studies is that the number of potential transcript variables typically vastly outnumbers the total subjects, which leads to large multiple testing penalties. One strategy to address the dimensionality problem has been to use weighted gene co-expression network analysis to assign genes to a smaller number of related groups (11). Reinhold and colleagues (12) performed weighted gene co-expression network analysis using the COPDGene microarray dataset from Bahr and colleagues (9) as well as whole-blood PAXGene RNA microarray (PreAnalytiX) data from ECLIPSE (13); results were validated using transcriptome data from the TESRA (Treatment of Emphysema with a γ-Selective Retinoid Agonist) study (14). The study found several network modules of coexpressed genes that were more strongly associated with lung function than emphysema; further analysis determined that the module signatures represented natural killer cells, dendritic cells, and neutrophils.
Another approach to reducing dimensionality is clustering. For instance, Chang and colleagues (15) leveraged preexisting gene interaction networks to guide unsupervised clustering of blood microarray expression data from ECLIPSE and COPDGene. The resultant clusters were more stable and more strongly associated with measures of lung function than clusters derived from a network-naive approach. One example of a pathway that was identified was the IL6–JAK–STAT3 signaling network.
A more recent approach to transcriptomic analysis is RNA-Seq, which offers advantages over array-based expression technologies, such as the ability to resolve expression changes at the subgenic level. Using whole-blood PAXGene RNA and RNA-Seq from 515 COPDGene subjects, Parker and colleagues (16) identified 171 differentially expressed genes associated with current smoking exposure. As expected from blood samples, the most enriched pathways were related to immune activation. In addition to protein coding genes, seven long noncoding RNAs were differentially associated with current smoking. Leveraging the ability of RNA-Seq to characterize exon-specific expression, these authors identified nine instances of differential exon use (i.e., exon-level expression that differs significantly from the expression of other exons within the same gene) in eight genes. Interestingly, the bulk of these exons were either the first or last exons in a transcript, suggesting that transcriptomic changes associated with current smoking may involve alternative transcriptions start sites or processes related to transcription termination or other splicing-related control mechanisms.
Protein Biomarkers
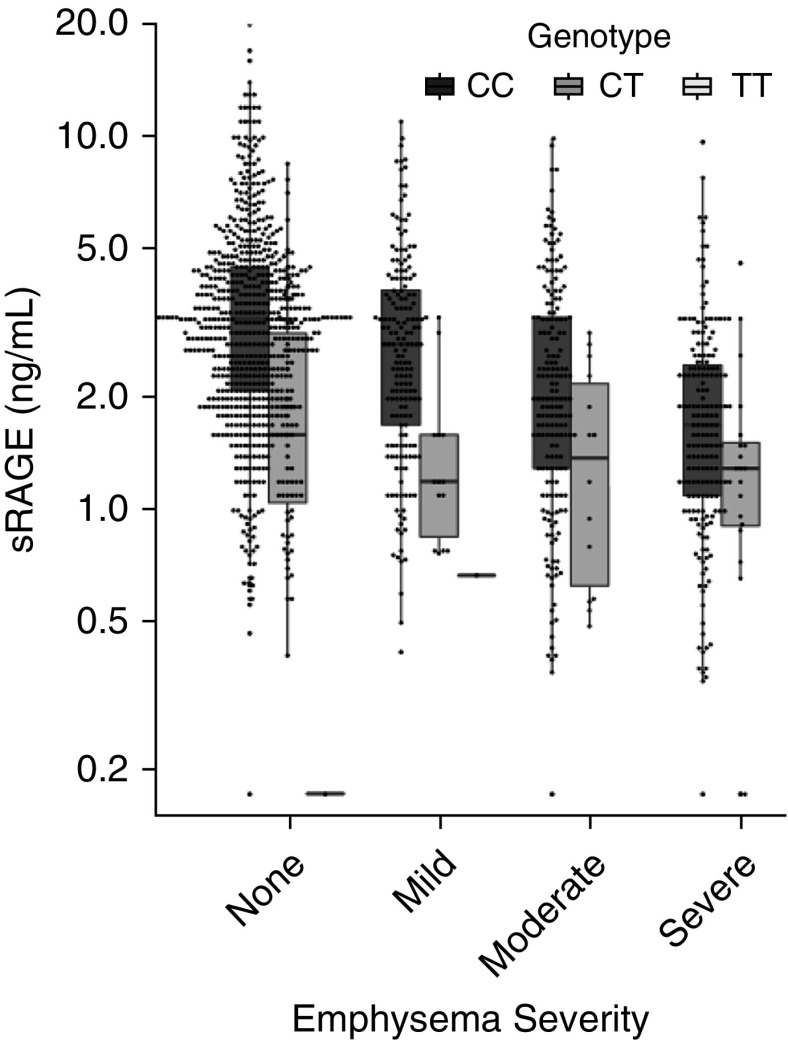
Currently, the most promising blood COPD biomarker is sRAGE. sRAGE is an isoform of the transmembrane receptor for advanced glycosylation end products (RAGE) that lacks the transmembrane domain through proteolytic cleavage (see review [17]). RAGE is coded by the AGER gene, and SNPs in AGER are associated with COPD and emphysema in targeted and genome-wide association studies (18–20). RAGE binds damage-associated molecular pattern molecules to perpetuate inflammation in lung epithelial cells. In COPDGene, subjects with more severe emphysema had lower plasma sRAGE (Figure 1) (21). A SNP in AGER (rs2070600) is associated with lower plasma levels of sRAGE in COPDGene and other cohorts (18, 22). Plasma sRAGE is a predictor of emphysema progression (23), and sRAGE will be the first blood biomarker of emphysema to be submitted to the Food and Drug Administration and the European Medicines Agency in the Biomarker Qualification Program (24).
Figure 1.
Plasma sRAGE (soluble receptor for advanced glycosylation end products) is lower in COPDGene (Genetic Epidemiology of COPD) subjects with more severe emphysema (P < 0.0001) but is dependent on their genotype at rs2070600. COPD = chronic obstructive pulmonary disease. Adapted from Reference 30.
Adiponectin and Other Biomarkers
In a study of 633 COPDGene subjects, Carolan and colleagues demonstrated that plasma adiponectin was higher in subjects with emphysema, even after adjusting for body mass index (25). Additional analyses by Suh and colleagues found that subjects with emphysema who had osteoporosis had the highest adiponectin levels (26). This study also found that adjusting for T-cadherin, a receptor for adiponectin, strengthened the statistical association. Other plasma biomarkers of COPD and emphysema include sICAM1 (soluble intracellular adhesion molecule 1), cadherin 1, cadherin 13 (21), CRP (C-reactive protein), SP-D (surfactant protein D), and CC16 (club cell secretory protein 16) (27).
Inflammatory Protein Biomarkers
Although sRAGE is currently the best biomarker of emphysema, blood markers of inflammation are also associated with severity and progression of COPD (Table 1). For instance, in a study of 2,123 subjects from COPDGene and 1,117 subjects from SPIROMICS, Bradford and colleagues found that plasma IL-6 and IL-8 were positively associated with emphysema progression independent of COPD severity and smoking status (28). IL-6 was also associated with progressive decline in FEV1 over 5 years.
Table 1.
Protein Biomarker Associations with Major Chronic Obstructive Pulmonary Disease Phenotypes in COPDGene
| Proteins |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenotype | sRAGE | IL-2 | IL-6 | IL-8 | IL-10 | TNF-α | IFN-γ | Fibrinogen | SP-D | CRP | sICAM | CC-16 | Eotaxin | ||
| Airflow obstruction | FEV1 (severity) | ↓ | — | ↑ | ↑ | ↑ |
↑ | ↑ | ↑ | ↑ | ↑ | — | ↓ | — | |
| FEV1/FVC | ↓ | — | — | — | — |
— | — | — | — | — | — | — | — | ||
| Progressive decline | — | — | ↑ | — | — | — |
— | — | — | — | — | — | — | ||
| Emphysema | Severity | ↓ | — | — | — | — |
— | — | — | — | — | — | — | — | |
| Progression | ↓ | — | ↑ | ↑ | |
— | — | — | — | — | — | — | — | ||
| Chronic bronchitis | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||
Definition of abbreviations: ↓ = phenotype was significantly associated with lower biomarker in plasma; ↑ = phenotype was significantly associated with higher biomarker in plasma; COPD = chronic obstructive pulmonary disease; COPDGene = Genetic Epidemiology of COPD; CRP = C-reactive protein; FEV1/FVC = forced expiratory volume in 1 second/forced vital capacity; NS = phenotype was not significantly associated biomarker in plasma; sICAM = soluble intracellular adhesion molecule; SP-D = surfactant protein D; sRAGE = soluble receptor for advanced glycosylation end products.
Cells with dashes indicate not yet reported for COPDGene.
Protein Biomarkers Associated with Exacerbations
The COPDGene study was also instrumental in assessing the role of plasma protein biomarkers in predicting COPD exacerbations. Keene and colleagues (29) investigated 90 candidate COPD plasma biomarkers in 602 subjects from COPDGene and 1,554 subjects from SPIROMICS to determine whether biomarkers could add to clinical variables in predicting future COPD exacerbations. Although the study identified multiple biomarkers predictive of future exacerbations, there was little overlap between the biomarkers identified by COPDGene and those identified in SPIROMICS, suggesting that replication of protein biomarkers for predicting COPD exacerbations is challenging.
Protein Quantitative Trait Loci
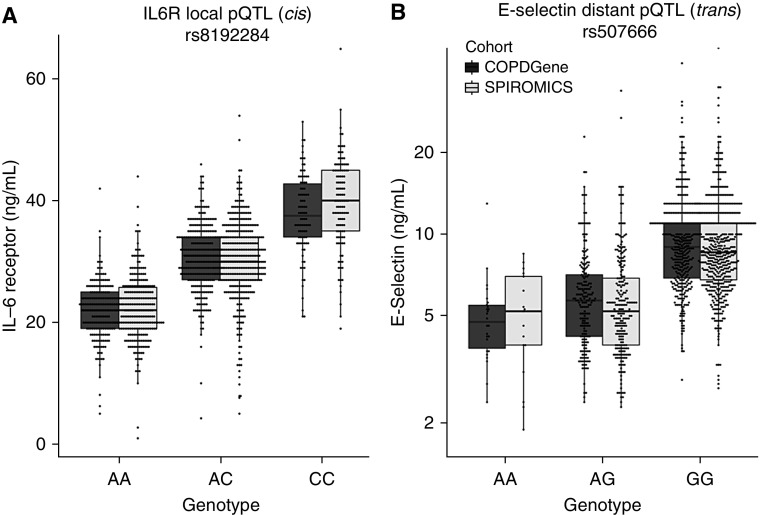
One of the major strengths of COPDGene is its abundance of omics data. Integrating these data in COPDGene and SPIROMICS subjects allowed Sun and colleagues to use the same proteomic platform to identify 478 unique SNPs associated with 88 different protein biomarkers (90% novel) (30). For nine of these proteins, one pQTL (protein quantitative trait locus) accounted for more than 10% of the variance in plasma levels (Figure 2). These pQTLs are distinct from eQTLs (transcriptome quantitative trait loci) and are enriched for missense mutations, which alter the amino acid sequence of the resulting protein. Although many of the strongest pQTL SNPs were within or near the gene that encoded their respective protein, investigators from COPDGene identified several genetic “hot spots” that were QTLs for multiple proteins. For instance, the ABO locus on chromosome 9, which encodes a glycosyltransferase, was a pQTL for six different proteins, all of which had coding genes on a different chromosome. Subsequent work has shown that more than two-thirds of proteins have pQTL SNPs, suggesting that future biomarker analyses should consider genetic variation (31); A second and compelling reason for including pQTLs is that it improves clinical prediction. For instance, when adjusting sRAGE plasma levels for the sRAGE pQTL SNP rs2070600 as discussed above, the explanation of variance for emphysema improved from 20% to 40%.
Figure 2.
Examples of biomarker pQTL (protein quantitative trait locus) SNPs. Plasma levels of (A) IL6R and (B) E-selectin are strongly influenced by pQTL SNPs (P = 10−193 and P = 4 × 10−104, respectively). Biomarkers were transformed to standard normal distribution, and statistical testing was performed using PLINK version 1.9 as described elsewhere (30). The pQTL SNP for IL6R (IL-6 receptor) is on chromosome 4, which is local (cis) to IL6R, the gene coding for its protein. The pQTL SNP for E-selectin protein is on chromosome 9, which is distant (trans) from SELE (chromosome 1), the gene coding for its protein. This pQTL SNP is in the ABO locus, which encodes α-1-3-N-acetylgalactosaminyltransferase. SPIROMICS = Subpopulations and Intermediate Outcomes in COPD Study.
The study by Sun and colleagues (30) characterized the significant pQTL SNPs further by examining all pQTL–biomarker–COPD phenotype associations. For instance, if a SNP is associated with a COPD phenotype and the COPD phenotype is independently associated with changes in biomarker measurement, this would be considered a “reactive” pQTL. In contrast, if a SNP alters the biomarker level and this change in biomarker level mediates the change in COPD phenotype, this would be considered a “casual” pQTL SNP. Other types of pQTL SNPs include “independent,” “complete,” and “collide,” which can be defined using their statistical associations between SNP, biomarker, and COPD phenotype (see Reference 30 for more details). The Sun and colleagues study also illustrated a potential caution in assuming that an association with a protein measurement is equivalent to that protein being differentially expressed. For instance, the rs7041 SNP in GC (VDBP [vitamin D–binding protein]) explained 75% of the variance in plasma VDBP measurements using an assay with a monoclonal antibody (most protein assays use monoclonal antibodies for detection); however, when a polyclonal antibody was used to measure VDBP, this relationship was lost (30). Because the rs7041 SNP minor allele has significantly higher prevalence in African populations, if a monoclonal antibody–based assay were used, one might erroneously conclude that those of African ancestry have lower plasma VDBP than those of European ancestry (32).
Lessons from Protein Biomarker Studies of COPD
A phenomenon rarely mentioned in biomarker literature is that biomarker studies often use hundreds or thousands of subjects to achieve statistical significance. These large sample sizes are needed because the additional phenotypic variance explained by a single COPD biomarker is often only 2–3% when included in models with clinical predictors. A strategy that is likely to be more successful is using a panel of biomarkers rather than individual biomarkers. In a study by Zemans and colleagues using 1,465 COPDGene subjects and 2,746 ECLIPSE subjects (27), a combination of five plasma biomarkers (CC16, CRP, fibrinogen, SP-D, and sRAGE) explained 13% of the variance of FEV1 in COPDGene and 24% of the variance in ECLIPSE, which was substantially higher than individual biomarkers (1–11%).
Metabolomics
Metabolomic Signatures in Plasma
Smoking results in increased levels of nicotine and its metabolites but also has a strong influence on systemic metabolism of amino acids, lipids, and other small molecules (33). Bahr and colleagues, using 211 COPDGene subjects, found that peripheral blood mononuclear cell sphingolipid pathway enzyme expression and plasma small molecules such as ceramides were biomarkers of COPD and emphysema, even after adjusting for smoking (9). A subsequent targeted plasma metabolomic study of 129 COPDGene subjects further identified five sphingomyelins that were associated with emphysema (Table 2), as well as four trihexosylceramides and three dihexosylceramides that were associated with COPD exacerbations (34). Siska and colleagues (35) combined the gene expression and sphingolipid data to show that subjects with COPD had discordant relationships between sphingolipid gene expression and sphingolipid metabolites, suggesting that altered regulation of sphingolipids in COPD was not due solely to altered gene expression. Because sphingolipid pathways have also been successfully targeted in experimental COPD models (36), these findings support sphingolipids as potential novel therapeutic targets for emphysema.
Table 2.
Meta-Analysis of Sphingolipids Associated with Emphysema in COPDGene Subjects
| |
Targeted Sphingolipid Platform |
|
Untargeted Metabolomics Platform |
|
Meta-Analysis |
|---|---|---|---|---|---|
| Sphingolipid | β-Slope | P Value | β-Slope | P Value | P Value |
| Ganglioside GM3 (d18:1/16:0) | −0.7654 | 0.0004 | −0.1277 | 0.3768 | 0.0018* |
| Sphingomyelin (d18:0/24:1[15Z]) | −0.4534 | 0.0057 | −0.0623 | 0.5349 | 0.0167* |
| Sphingomyelin (d18:1/14:0) | −0.4335 | 0.0040 | −0.1070 | 0.3650 | 0.0075* |
| Sphingomyelin (d18:1/16:0) | −0.8964 | 0.0010 | −0.1867 | 0.4258 | 0.0037* |
| Sphingomyelin (d18:1/16:1) | −0.5198 | 0.0054 | −0.1459 | 0.3621 | 0.0090* |
| Sphingomyelin (d18:1/24:1[15Z]) | −0.6062 | 0.0009 | −0.2182 | 0.0829 | 0.0004* |
| Sphingomyelin (d18:2/14:0) | −0.3520 | 0.0237 | −0.0673 | 0.5444 | 0.0425 |
The targeted sphingolipid platform included assays for 69 sphingolipids with standards in 129 subjects, and the untargeted metabolomics platform was used for replication using 131 subjects, but it did not include labeled standards (see Reference 34 for more details). The β-slope represents the regression coefficient for emphysema quantitated by high-resolution computed tomography.
False discovery rate less than 0.10.
Overlap Between Plasma and BAL Fluid Metabolome
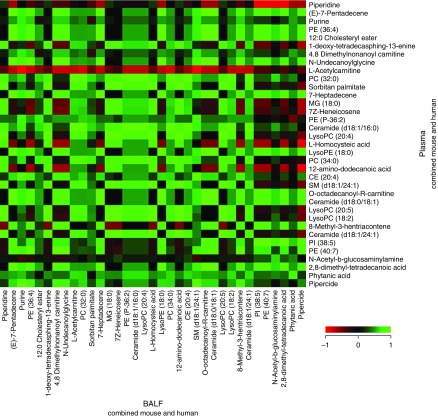
COPDGene has also been useful to establish the overlap between the plasma metabolome and the BAL fluid (BALF) metabolome (37). In a study of five COPDGene subjects, Cruickshank-Quinn and colleagues (38) found that most of 298 annotated metabolites in BALF and plasma were positively correlated. Examples include l-homocysteic acid, octadecanoylcarnitine, N-undecanoylglycine, LysoPE (lysophosphatidylethanolamine) (18:0), LysoPC (lysophosphatidycholine) (20:4), MG (monogylcerides) (18:0), PC (phosphotidylcholines) (32:0), PC (34:0), PE (phosphotidylcholines) (40:7), and PI (phosphotidylinositol) (38:5) (Figure 3). This suggests that plasma could serve as a surrogate for BALF.
Figure 3.
Metabolite correlations across BAL fluid (BALF) and plasma. Data from the mouse and human BALF and plasma samples were combined to identify metabolites that correlated across both biofluids using Spearman rank correlation. From among 298 annotated metabolites, a subset of 35 metabolites was selected on the basis of their detected high abundances in BALF and plasma. Red indicates a negative correlation (r = −1) across BALF and plasma; green indicates a positive correlation (r = +1) between BALF and plasma; and black indicates no correlation (r = 0) between BALF and plasma. CE = cholesterol ester; LysoPC = lysophosphatidycholine; LysoPE = lysophosphatidylethanolamine; MG = monoglycerides; PC = phosphotidylcholines; PE = phosphotidylethanolamines; PI = phosphotidylinositol; SM = sphingomyelins.
Conclusions
COPDGene is a large, well-characterized cohort of smokers in which there have been many genetic, transcriptomic, proteomic, and metabolomic studies. Although the lung is the site of primary exposure to tobacco smoke, COPDGene studies have identified many blood biomarkers even after adjusting for smoking, confirming that COPD has systemic manifestations. Although many of these biomarkers have statistically significant associations with COPD phenotypes, many are not specific to COPD and are more related to inflammation (e.g., IL-6) or associated with other chronic diseases such as diabetes (sRAGE). Other biomarkers may be more lung specific (e.g., CC16, SP-D) but are biomarkers of other pulmonary diseases such as asthma and interstitial lung disease. Indeed, the work described in this perspective article suggests that no single individual molecule can fully explain the pathogenesis of COPD. Although individual molecules may explain only 1–5% of the variance in clinical phenotypes, combinations of multiple biomarkers in combination can explain more than 15% of the variance for some COPD phenotypes, suggesting that further work should focus on multi-omics panels rather than individual markers and that subsets of biomarkers could be used to define subsets of subjects with COPD rather than just lumping subjects together on the basis of clinical phenotyping. Furthermore, studies in COPDGene have revealed that there are interactions between genetic variants and transcriptome/proteome/metabolome and support the concept that including these multi-omic interactions can improve the statistical understanding of the relationship between COPD phenotypes and molecular signatures. We encourage future researchers to take both of these concepts into account and report how much additional explanation of variance is gained by the biomarker measurement and whether it adds to other biomarker measurements.
Supplementary Material
Footnotes
Supported by awards U01 HL089897 and U01 HL089856 from the National Heart, Lung, and Blood Institute, and grant R01 HL137995 (R.P.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. The COPDGene (Genetic Epidemiology of COPD) project is also supported by the COPD Foundation through contributions made to an industry advisory board comprised of AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Siemens, and Sunovion.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0245PS on March 15, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Agusti A, Soriano JB. COPD as a systemic disease. COPD. 2008;5:133–138. doi: 10.1080/15412550801941349. [DOI] [PubMed] [Google Scholar]
- 2.Müllerova H, Agusti A, Erqou S, Mapel DW. Cardiovascular comorbidity in COPD: systematic literature review. Chest. 2013;144:1163–1178. doi: 10.1378/chest.12-2847. [DOI] [PubMed] [Google Scholar]
- 3.Faner R, Tal-Singer R, Riley JH, Celli B, Vestbo J, MacNee W, et al. ECLIPSE Study Investigators. Lessons from ECLIPSE: a review of COPD biomarkers. Thorax. 2014;69:666–672. doi: 10.1136/thoraxjnl-2013-204778. [DOI] [PubMed] [Google Scholar]
- 4.Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, et al. SPIROMICS Research Group. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69:491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rennard SI. The promise of observational studies (ECLIPSE, SPIROMICS, and COPDGene) in achieving the goal of personalized treatment of chronic obstructive pulmonary disease. Semin Respir Crit Care Med. 2015;36:478–490. doi: 10.1055/s-0035-1555609. [DOI] [PubMed] [Google Scholar]
- 7.Qiu W, Wan E, Morrow J, Cho MH, Crapo JD, Silverman EK, et al. The impact of genetic variation and cigarette smoke on DNA methylation in current and former smokers from the COPDGene study. Epigenetics. 2015;10:1064–1073. doi: 10.1080/15592294.2015.1106672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan ES, Qiu W, Carey VJ, Morrow J, Bacherman H, Foreman MG, et al. Smoking-associated site-specific differential methylation in buccal mucosa in the COPDGene study. Am J Respir Cell Mol Biol. 2015;53:246–254. doi: 10.1165/rcmb.2014-0103OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bahr TM, Hughes GJ, Armstrong M, Reisdorph R, Coldren CD, Edwards MG, et al. Peripheral blood mononuclear cell gene expression in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2013;49:316–323. doi: 10.1165/rcmb.2012-0230OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halper-Stromberg E, Yun JH, Parker MM, Singer RT, Gaggar A, Silverman EK, et al. Systemic markers of adaptive and innate immunity are associated with chronic obstructive pulmonary disease severity and spirometric disease progression. Am J Respir Cell Mol Biol. 2018;58:500–509. doi: 10.1165/rcmb.2017-0373OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol. 2005;4:Article17. doi: 10.2202/1544-6115.1128. [DOI] [PubMed] [Google Scholar]
- 12.Reinhold D, Morrow JD, Jacobson S, Hu J, Ringel B, Seibold MA, et al. Meta-analysis of peripheral blood gene expression modules for COPD phenotypes. PLoS One. 2017;12:e0185682. doi: 10.1371/journal.pone.0185682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh D, Fox SM, Tal-Singer R, Bates S, Riley JH, Celli B. Altered gene expression in blood and sputum in COPD frequent exacerbators in the ECLIPSE cohort. PLoS One. 2014;9:e107381. doi: 10.1371/journal.pone.0107381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrow JD, Qiu W, Chhabra D, Rennard SI, Belloni P, Belousov A, et al. Identifying a gene expression signature of frequent COPD exacerbations in peripheral blood using network methods. BMC Med Genomics. 2015;8:1. doi: 10.1186/s12920-014-0072-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang Y, Glass K, Liu YY, Silverman EK, Crapo JD, Tal-Singer R, et al. COPD subtypes identified by network-based clustering of blood gene expression. Genomics. 2016;107:51–58. doi: 10.1016/j.ygeno.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker MM, Chase RP, Lamb A, Reyes A, Saferali A, Yun JH, et al. RNA sequencing identifies novel non-coding RNA and exon-specific effects associated with cigarette smoking. BMC Med Genomics. 2017;10:58. doi: 10.1186/s12920-017-0295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yonchuk JG, Silverman EK, Bowler RP, Agustí A, Lomas DA, Miller BE, et al. Circulating soluble receptor for advanced glycation end products (sRAGE) as a biomarker of emphysema and the RAGE axis in the lung. Am J Respir Crit Care Med. 2015;192:785–792. doi: 10.1164/rccm.201501-0137PP. [DOI] [PubMed] [Google Scholar]
- 18.Cheng DT, Kim DK, Cockayne DA, Belousov A, Bitter H, Cho MH, et al. TESRA and ECLIPSE Investigators. Systemic soluble receptor for advanced glycation endproducts is a biomarker of emphysema and associated with AGER genetic variants in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188:948–957. doi: 10.1164/rccm.201302-0247OC. [DOI] [PubMed] [Google Scholar]
- 19.Hobbs BD, de Jong K, Lamontagne M, Bossé Y, Shrine N, Artigas MS, et al. COPDGene Investigators; ECLIPSE Investigators; LifeLines Investigators; SPIROMICS Research Group; International COPD Genetics Network Investigators; UK BiLEVE Investigators; International COPD Genetics Consortium. Genetic loci associated with chronic obstructive pulmonary disease overlap with loci for lung function and pulmonary fibrosis. Nat Genet. 2017;49:426–432. doi: 10.1038/ng.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller S, Henry AP, Hodge E, Kheirallah AK, Billington CK, Rimington TL, et al. The Ser82 RAGE variant affects lung function and serum RAGE in smokers and sRAGE production in vitro. PLoS One. 2016;11:e0164041. doi: 10.1371/journal.pone.0164041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carolan BJ, Hughes G, Morrow J, Hersh CP, O’Neal WK, Rennard S, et al. The association of plasma biomarkers with computed tomography-assessed emphysema phenotypes. Respir Res. 2014;15:127. doi: 10.1186/s12931-014-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoonhorst SJ, Lo Tam Loi AT, Pouwels SD, Faiz A, Telenga ED, van den Berge M, et al. Advanced glycation endproducts and their receptor in different body compartments in COPD. Respir Res. 2016;17:46. doi: 10.1186/s12931-016-0363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coxson HO, Dirksen A, Edwards LD, Yates JC, Agusti A, Bakke P, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. The presence and progression of emphysema in COPD as determined by CT scanning and biomarker expression: a prospective analysis from the ECLIPSE study. Lancet Respir Med. 2013;1:129–136. doi: 10.1016/S2213-2600(13)70006-7. [DOI] [PubMed] [Google Scholar]
- 24.Casaburi R, Celli B, Crapo J, Criner G, Croxton T, Gaw A, et al. The COPD Biomarker Qualification Consortium (CBQC) COPD. 2013;10:367–377. doi: 10.3109/15412555.2012.752807. [DOI] [PubMed] [Google Scholar]
- 25.Carolan BJ, Kim YI, Williams AA, Kechris K, Lutz S, Reisdorph N, et al. The association of adiponectin with computed tomography phenotypes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188:561–566. doi: 10.1164/rccm.201212-2299OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suh YJ, McDonald MN, Washko GR, Carolan BJ, Bowler RP, Lynch DA, et al. COPDGene Investigators. Lung, fat and bone: increased adiponectin associates with the combination of smoking-related lung disease and osteoporosis. Chronic Obstr Pulm Dis. 2018;5:134–143. doi: 10.15326/jcopdf.5.2.2016.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zemans RL, Jacobson S, Keene J, Kechris K, Miller BE, Tal-Singer R, et al. Multiple biomarkers predict disease severity, progression and mortality in COPD. Respir Res. 2017;18:117. doi: 10.1186/s12931-017-0597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradford E, Jacobson S, Varasteh J, Comellas AP, Woodruff P, O’Neal W, et al. The value of blood cytokines and chemokines in assessing COPD. Respir Res. 2017;18:180. doi: 10.1186/s12931-017-0662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keene JD, Jacobson S, Kechris K, Kinney GL, Foreman MG, Doerschuk CM, et al. COPDGene and SPIROMICS Investigators. Biomarkers predictive of exacerbations in the SPIROMICS and COPDGene cohorts. Am J Respir Crit Care Med. 2017;195:473–481. doi: 10.1164/rccm.201607-1330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun W, Kechris K, Jacobson S, Drummond MB, Hawkins GA, Yang J, et al. SPIROMICS Research Group; COPDGene Investigators. Common genetic polymorphisms influence blood biomarker measurements in COPD. PLoS Genet. 2016;12:e1006011. doi: 10.1371/journal.pgen.1006011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao C, Chen G, Song C, Keefe J, Mendelson M, Huan T, et al. Genome-wide mapping of plasma protein QTLs identifies putatively causal genes and pathways for cardiovascular disease. Nat Commun. 2018;9:3268. doi: 10.1038/s41467-018-05512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369:1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu F, Derkach A, Freedman ND, Landi MT, Albanes D, Weinstein SJ, et al. Cigarette smoking behaviour and blood metabolomics. Int J Epidemiol. 2016;45:1421–1432. doi: 10.1093/ije/dyv330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowler RP, Jacobson S, Cruickshank C, Hughes GJ, Siska C, Ory DS, et al. Plasma sphingolipids associated with chronic obstructive pulmonary disease phenotypes. Am J Respir Crit Care Med. 2015;191:275–284. doi: 10.1164/rccm.201410-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siska C, Bowler R, Kechris K. The discordant method: a novel approach for differential correlation. Bioinformatics. 2016;32:690–696. doi: 10.1093/bioinformatics/btv633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tibboel J, Reiss I, de Jongste JC, Post M. Ceramides: a potential therapeutic target in pulmonary emphysema. Respir Res. 2013;14:96. doi: 10.1186/1465-9921-14-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.degli Uberti EC, Trasforini G, Salvadori S, Margutti A, Teodori V, Rotola C, et al. Effect of somatostatin on growth hormone and prolactin response to dermorphin in man. Acta Endocrinol (Copenh) 1985;108:20–25. doi: 10.1530/acta.0.1080020. [DOI] [PubMed] [Google Scholar]
- 38.Cruickshank-Quinn C, Powell R, Jacobson S, Kechris K, Bowler RP, Petrache I, et al. Metabolomic similarities between bronchoalveolar lavage fluid and plasma in humans and mice. Sci Rep. 2017;7:5108. doi: 10.1038/s41598-017-05374-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.