Asthma is a chronic airway inflammatory disease of the airways characterized by reversible airflow obstruction and symptoms such as chest tightness, wheezing, and cough (1). The current gold standard treatment for bronchoconstriction in asthma is the use of β2-adrenoceptor agonists (β2-agonists), which are potent and effective bronchodilators that relax airway smooth muscle contraction regardless of stimulus (2). However, some patients with severe asthma have shown increased resistance to the effects of β2-agonists (3), and interestingly, it also has been demonstrated that β2-agonist therapy is less effective the higher the severity of airway hyperresponsiveness, or bronchoconstriction (4). The mechanisms behind this are not yet fully elucidated, and further understanding may help in the treatment of airway obstruction in patients with severe asthma.
In this issue of the Journal (pp. 209–218), Ojiaku and colleagues demonstrate a new way in which transforming growth factor (TGF)-β1, a profibrotic cytokine, may cause an attenuation of β2-agonist–induced relaxation of airway smooth muscle (5). TGF-β1 has previously been implicated in the pathogenesis of asthma, with increased levels in the BAL fluid (6) together with increased expression in bronchial tissue samples of patients with asthma (7). In the context of airway smooth muscle function, Ojiaku and colleagues have previously shown that preincubation with TGF-β1 can increase muscarinic agonist–induced contractile responses in human airway smooth muscle (HASM) (8). It has also been shown that bronchoconstriction caused by the contractile agent methacholine can cause the release of TGF-β1 (9). This interplay highlights the key role that TGF-β1 may play in asthma pathogenesis.
In the context of asthma therapy rather than pathogenesis, TGF-β1 has also been shown to decrease β2-adrenoreceptor agonist function via a reduction in receptor expression (10), suggesting a role for TGF-β1 in the development of resistance to the mainstay asthma therapeutic, such as that seen in patients with severe asthma. The key message of the article by Ojiaku and colleagues (9) is that there may be an additional mechanism for the reduction in β2-agonist efficacy caused by TGF-β1, whereby the downstream cAMP signaling induced by β2-agonists is attenuated by increased PDE4D expression.
In their study, the authors used magnetic twist cytometry, which measures dynamic changes in cell stiffness as a surrogate for contraction, and demonstrated that pretreatment with TGF-β1 decreased the β2-adrenoreceptor agonist isoproterenol–induced relaxation in HASM cells precontracted with the muscarinic agonist carbachol. Although isoproterenol is a partial agonist of both β1- and β2-adrenoreceptors, HASM cells have been shown to express only the β2-subtype (11), indicating a potential role for TGF-β1 in the β2-agonist tolerance seen in many patients with asthma (5).
β2-Agonists induce relaxation though activation of adenylyl cyclase and increases in cAMP to inhibit contractile stimuli. HASM cells that were precontracted with carbachol and then treated with isoproterenol demonstrated an increase in cAMP, which was inhibited after pretreatment with TGF-β1. This has also been indicated in previous publications in which TGF-β1 was shown to inhibit cAMP accumulation in response to isoproterenol in HASM through downregulation of β2-receptor number and function (10). However, TGF-β1 had no effect on forskolin-induced cAMP elevation, indicating that TGF-β1 is not impacting the function of adenylyl cyclase itself (5).
The actions of cyclic nucleotides are counteracted by a group of PDE enzymes, which hydrolyze cAMP (or cGMP) to the inactive 5′-AMP (or 5′-GMP) (12). Ojiaku and colleagues demonstrate that the inhibitory effect of TGF-β1 is reversed after pretreatment with the pan-PDE inhibitor 3-isobutyl-1-methylxanthine, implicating PDEs as drivers of the response. Further investigation revealed that treatment with the selective PDE4 antagonist roflumilast reversed the blunted isoproterenol cAMP responses, as well as that TGF-β1 increased the expression of an isoform of PDE4 (PDE4D) in HASM cells (5). PDE4D5, a splice variant of PDE4D, has previously been shown using targeted siRNA knockdown to be the key physiological regulator of β2-cAMP turnover within HASM (13).
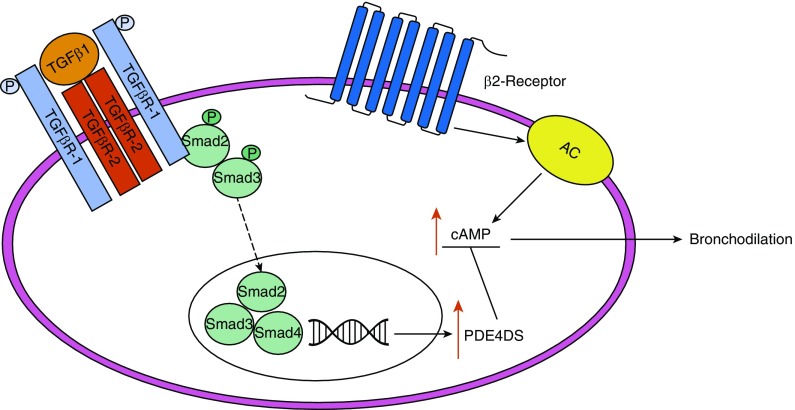
The canonical TGF-β1 pathway involves phosphorylation of the intracellular signal transducers Smad2 and Smad3 after activation by TGF-β1 (14). Phosphorylated Smad2 and Smad3 then integrate with Smad4 and translocate to the nucleus, where the complex regulates gene transcription (15). Ojiaku and colleagues have shown that Smad2 and Smad3 knockdown in HASM cells reduced the increased PDE4D gene expression induced by TGF-β1, leading to the conclusion that TGF-β1 decreases β2-agonist–induced relaxation in a Smad2/3-dependent manner (5) (Figure 1). This is a novel observation and may help in the therapeutic use of β2-agonists in patients with severe asthma.
Figure 1.
β2-Agonists cause relaxation through activation of the β2-adrenoceptor, which subsequently activates adenylyl cyclase (AC) to increase cAMP and cause bronchodilation. Ojiaku and colleagues suggest that the increased resistance to β2-agonist–induced bronchodilation in asthmatics may be mediated by the effects of transforming growth factor (TGF)-β1. Activation of the TGF-β receptor by TGF-β1 causes phosphorylation of the transcription factors Smad2 and Smad3, which then translocate to the nucleus, where they form a complex with Smad4. This complex increases expression of the PDE isomer PDE4DS, which leads to greater breakdown of cAMP and thereby reduces the level of bronchodilation. P = phosphorylation.
There are, of course, a number of questions that this work raises that we look forward to seeing examined in future studies. This includes determining whether TGF-β1 pretreatment affects relaxation responses in whole human tracheal tissues and whether TGF-β1 also attenuates the effectiveness of more clinically relevant β2-agonists such as formoterol or salbutamol. Furthermore, it would be interesting to determine if this mechanism plays a role in the reduced β2-agonist effectiveness in tracheal tissues from patients with severe asthma.
In summary, this highly interesting and novel study reveals a mechanism by which resistance to the effects of bronchodilators may develop in disease, implicating TGF-β1 as a key driver in the resistance to β2-agonist bronchodilator effects. If future research confirms these findings in the context of severe asthma, this mechanism could lead to the development of novel therapeutics to increase bronchodilator sensitivity.
Supplementary Material
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald JM, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 2.Giembycz MA, Newton R. Beyond the dogma: novel β2-adrenoceptor signalling in the airways. Eur Respir J. 2006;27:1286–1306. doi: 10.1183/09031936.06.00112605. [DOI] [PubMed] [Google Scholar]
- 3.Yim RP, Koumbouris AC. Tolerance & resistance to β₂-agonist bronchodilators. Paediatr Respir Rev. 2013;14:195–198. doi: 10.1016/j.prrv.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Wraight JM, Hancox RJ, Herbison GP, Cowan JO, Flannery EM, Taylor DR. Bronchodilator tolerance: the impact of increasing bronchoconstriction. Eur Respir J. 2003;21:810–815. doi: 10.1183/09031936.03.00067503. [DOI] [PubMed] [Google Scholar]
- 5.Ojiaku CA, Chung E, Parikh V, Williams JK, Schwab A, Fuentes AL, et al. Transforming growth factor-β1 decreases β2-agonist–induced relaxation in human airway smooth muscle. Am J Respir Cell Mol Biol. 2019;61:209–218. doi: 10.1165/rcmb.2018-0301OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redington AE, Roche WR, Holgate ST, Howarth PH. Co-localization of immunoreactive transforming growth factor-beta 1 and decorin in bronchial biopsies from asthmatic and normal subjects. J Pathol. 1998;186:410–415. doi: 10.1002/(SICI)1096-9896(199812)186:4<410::AID-PATH198>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Vignola AM, Chanez P, Chiappara G, Merendino A, Pace E, Rizzo A, et al. Transforming growth factor-beta expression in mucosal biopsies in asthma and chronic bronchitis. Am J Respir Crit Care Med. 1997;156:591–599. doi: 10.1164/ajrccm.156.2.9609066. [DOI] [PubMed] [Google Scholar]
- 8.Ojiaku CA, Yoo EJ, Panettieri RA., Jr Transforming growth factor β1 function in airway remodeling and hyperresponsiveness: the missing link? Am J Respir Cell Mol Biol. 2017;56:432–442. doi: 10.1165/rcmb.2016-0307TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oenema TA, Maarsingh H, Smit M, Groothuis GMM, Meurs H, Gosens R. Bronchoconstriction induces TGF-β release and airway remodelling in Guinea pig lung slices. PLoS One. 2013;8:e65580. doi: 10.1371/journal.pone.0065580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nogami M, Romberger DJ, Rennard SI, Toews ML. TGF-beta 1 modulates beta-adrenergic receptor number and function in cultured human tracheal smooth muscle cells. Am J Physiol. 1994;266:L187–L191. doi: 10.1152/ajplung.1994.266.2.L187. [DOI] [PubMed] [Google Scholar]
- 11.Carstairs JR, Nimmo AJ, Barnes PJ. Autoradiographic visualization of beta-adrenoceptor subtypes in human lung. Am Rev Respir Dis. 1985;132:541–547. doi: 10.1164/arrd.1985.132.3.541. [DOI] [PubMed] [Google Scholar]
- 12.Maurice DH, Ke H, Ahmad F, Wang Y, Chung J, Manganiello VC. Advances in targeting cyclic nucleotide phosphodiesterases. Nat Rev Drug Discov. 2014;13:290–314. doi: 10.1038/nrd4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Billington CK, Le Jeune IR, Young KW, Hall IP. A major functional role for phosphodiesterase 4D5 in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2008;38:1–7. doi: 10.1165/rcmb.2007-0171OC. [DOI] [PubMed] [Google Scholar]
- 14.Saito A, Horie M, Nagase T. TGF-β signaling in lung health and disease. Int J Mol Sci. 2018;19:E2460. doi: 10.3390/ijms19082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.