To the Editor:
Primary graft dysfunction (PGD), a form of acute lung injury that occurs within 72 hours of lung transplantation, is associated with increased early mortality and an increased risk of bronchiolitis obliterans syndrome (BOS) (1, 2). Obesity is a risk factor for PGD, but pathways linking obesity and PGD are unknown (1). PTX3 (long pentraxin-3) is overexpressed in intrathoracic visceral adipose tissue (ITAT) during lung transplant surgery (3), and increased serum PTX3 is associated with PGD (4), suggesting that ITAT inflammation may link obesity with PGD. We sought to examine differences in ITAT gene expression among lung transplant recipients with and without PGD.
ITAT biopsies (1 cm × 1 cm) were obtained before native lung explantation and within 1 hour of allograft reperfusion. Samples were cut into 3-mm cubes and centrifuged at 500 g for 5 minutes. Four cubes were formalin fixed and paraffin embedded. Approximately 100 μl of tissue were snap-frozen with liquid nitrogen or dry ice and stored in liquid nitrogen.
Total RNA was extracted using a Qiagen RNeasy Lipid Tissue kit (catalog number 74804). Libraries were prepared from polyadenylated mRNA enriched from total RNA (>100 ng and RNA integrity number > 6.5 by Agilent Bioanalyzer 2100) using the Illumina TruSeq RNA prep kit. The libraries were sequenced in multiplex, targeting 30 million single-end 100-bp reads per sample on an Illumina HiSeq2000 at the Columbia University Genome Center. Illumina Real Time Analysis software was used for base calling, followed by bcl2fastq (v.1.8.4) for adaptor trimming. Reads were aligned with STAR (version 2.4.0c) and genes annotated in Gencode v18 were quantified with FeatureCounts (v1.4.3-p1). Normalization and differential expression were conducted using the Bioconductor package DESeq. Differences in expression are reported as fold change, indicating 2(log2(expression with PGD) − log2(expression without PGD). A Benjamini-Hochberg false discovery rate with an adjusted P value of <0.05 determined statistical significance.
Formalin-fixed, paraffin-embedded samples were sectioned and stained with primary antibody for AHRR (aryl-hydrocarbon receptor repressor) (rabbit polyclonal, ab108518; Abcam), followed by fluorescently labeled goat anti-rabbit secondary antibody. Mean corrected total cell fluorescence was measured using ImageJ software (National Institutes of Health) (5).
Chest radiographs were reviewed by two independent observers (1). Grade 3 PGD at 48 or 72 hours after lung transplantation was defined as an arterial oxygen tension/fraction of inspired oxygen ratio of <200 with parenchymal opacities consistent with diffuse pulmonary edema (1).
Thirty-one subjects had at least one biopsy (47 samples [27 explantation and 20 reperfusion]). Only one explantation sample came from a subject with PGD, so explantation samples were excluded. One reperfusion sample was excluded because it had an RNA integrity number of <6. The remaining 19 reperfusion samples were collected from subjects with a mean ± SD age of 56.2 ± 11.9, mean body mass index of 25.5 ± 4.2 kg/m2, and mean lung allocation score of 46.9 ± 19.9; 79% were men, 58% had interstitial lung disease, 21% had chronic obstructive pulmonary disease, 21% had cystic fibrosis, and 10% had donors with a smoking history. Four subjects (21%) developed grade 3 PGD at 48 or 72 hours. Subjects with and without PGD were similar with regard to age, sex, body mass index, lung allocation score, use of intraoperative extracorporeal support, and allograft ischemic time (Table E1 in the data supplement). Subjects with PGD were more likely to have interstitial lung disease and use preoperative corticosteroids, and less likely to undergo double lung transplantation.
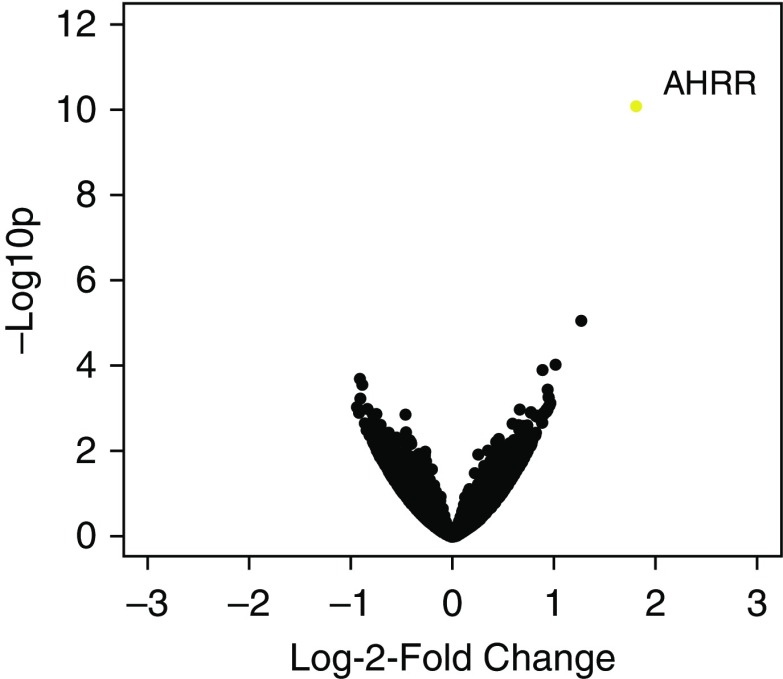
AHRR was the only significantly upregulated gene in reperfusion adipose (3.5-fold change, adjusted P value <0.001; Figure 1) of subjects who developed PGD compared with those who did not.
Figure 1.
Volcano plot of log2 fold change in expression versus log10 P values in reperfusion adipose for subjects who developed grade 3 primary graft dysfunction at 48 or 72 hours compared with those who did not. Orange indicates adjusted P value < 0.05. AHRR = aryl-hydrocarbon receptor repressor.
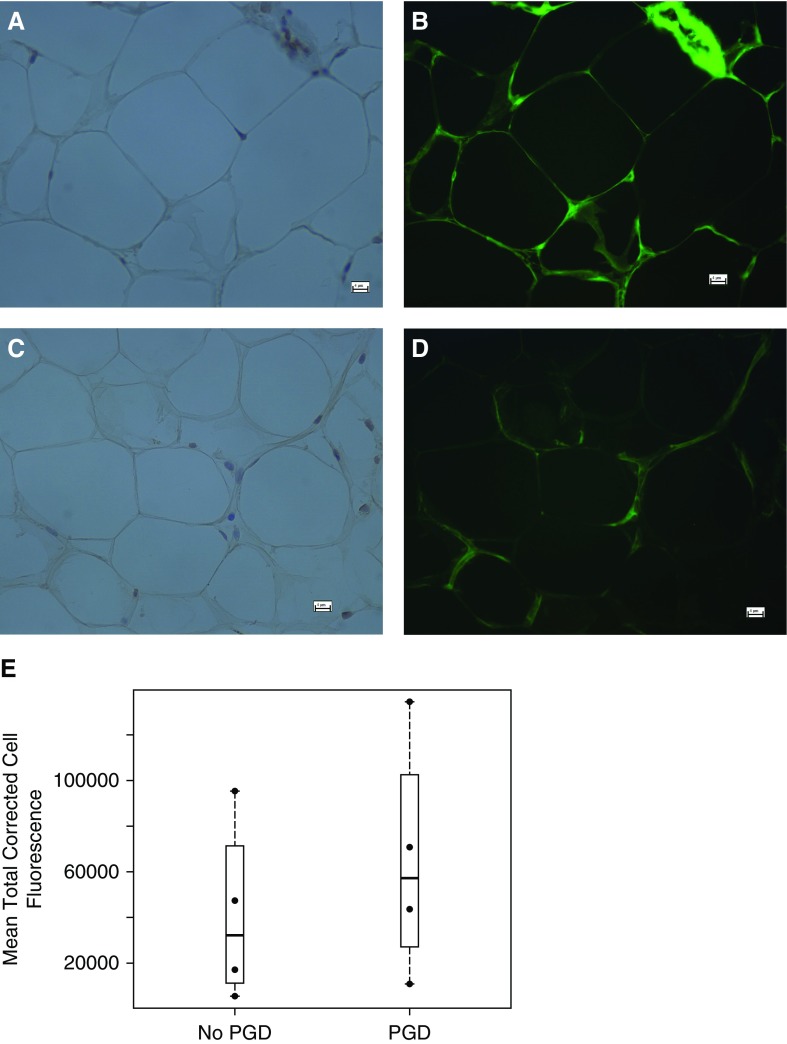
Staining for AHRR using immunofluorescent antibodies was performed on eight reperfusion samples. Staining confirmed the presence of AHRR in the cytoplasm of adipocytes and stromal vascular cells (Figures 2A–2D). Compared with subjects without PGD, subjects with PGD may have increased mean total corrected cell fluorescence for AHRR (Figure 2E).
Figure 2.
(A and B) Adipose samples from a subject with primary graft dysfunction (PGD) stained for hematoxylin (A), and immunofluorescently stained for AHRR (B). (C and D) Adipose sample from a subject without PGD stained for hematoxylin (C), and immunofluorescently stained for AHRR (D). (E) Bee-swarm plot of the mean total corrected cell fluorescence for subjects with and without PGD. Scale bars: 4 μm.
We identified increased expression of the AHRR gene in reperfusion adipose among subjects with grade 3 PGD at 48 or 72 hours. Aryl-hydrocarbon receptor (AHR) is involved in detoxification of reactive oxygen species (ROS), environmental toxins, and drugs. After a ligand displaces stabilizing proteins on cytosolic AHR, the complex translocates to the nucleus, where it binds a nuclear translocator, and promoter sites for IL-1β, TNF-α, CXCL chemokines, IL-6, IL-8 (6), and the xenobiotic response elements CYP1A1 (cytochrome P450-1A1), CYP1B1 (cytochrome p450-1B1), and NQO1 (NAD(P)H-quinone reductase-1) (7). AHRR competitively binds the nuclear translocator and transcription factors, preventing gene transcription (6). Decreased expression of the AHR-mediated genes CYP1A1, NQO1, and SOD1 (superoxide-dismutase 1) impairs ROS neutralization, leading to increased lipid peroxidation and cell death (8), and increased risk of hyperoxia-induced lung injury in newborn mice (9). Increased fraction of inspired oxygen at allograft reperfusion, increased ROS, and increased lipid peroxidation are associated with an increased risk of PGD (1, 10), suggesting that AHRR suppression of these enzymes may link adiposity and PGD. Whether total body adiposity alters local or systemic AHRR expression and ROS is unknown. Future work should evaluate the association between ROS and adiposity in PGD.
AHRR hypomethylation is associated with cigarette smoking, chronic bronchitis, and a faster decline in lung function in the general population (11). PGD is a risk factor for BOS, a process characterized by a progressive decline in lung function (2). Future investigations should evaluate whether AHRR hypomethylation or changes in AHRR expression in various tissues (including circulating lymphocytes, lung parenchyma, endothelial cells, and adipose tissue) are present in BOS, and whether these changes reflect a mechanistic link or shared susceptibility to DNA injury.
There are several limitations to this study. Given the small number of cases and differences in diagnoses between subjects with and without PGD, these findings cannot be generalized to the larger population of lung transplant recipients. In addition, we have not adjusted for potential confounders, although, reassuringly, there does not appear to be a consistent direction of bias in the baseline characteristics. Cigarette smoking is associated with increased AHRR hypomethylation; however, smoking cessation is required for transplantation and hypomethylation is partially reversed with smoking cessation, suggesting that AHRR expression changes are unlikely to reflect smoking by the recipient (11).
This is the first study to evaluate ITAT gene expression associated with PGD after lung transplantation. Further studies should clarify the role of AHRR in PGD, including changes in AHR-mediated gene products and the association between ROS and adiposity in PGD.
Supplementary Material
Footnotes
Supported by National Institutes of Health/NHLBI grants R01 HL114626, R01 HL087115, K24 HL115354, K24 HL 131937, K23 HL121406, T32 HL105323, K23 HL 116656, R03 HL135227, R01 DK066525, and T32 DK07328.
Author Contributions: Conception and design: M.R.A., E.A.E., J.M.D., A.F., J.D.C., and D.J.L. Acquisition, analysis, and interpretation of data: all authors. First draft: M.R.A. Drafting or revising the manuscript for important intellectual content: M.R.A., E.A.E., J.M.D., A.F., J.D.C., and D.J.L. Final approval of the version to be published: all authors.
This letter has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Diamond JM, Lee JC, Kawut SM, Shah RJ, Localio AR, Bellamy SL, et al. Lung Transplant Outcomes Group. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013;187:527–534. doi: 10.1164/rccm.201210-1865OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175:507–513. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 3.Diamond JM, Arcasoy S, McDonnough JA, Sonett JR, Bacchetta M, D’Ovidio F, et al. Adipose gene expression profile changes with lung allograft reperfusion. Am J Transplant. 2017;17:239–245. doi: 10.1111/ajt.13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamond JM, Lederer DJ, Kawut SM, Lee J, Ahya VN, Bellamy S, et al. Lung Transplant Outcomes Group. Elevated plasma long pentraxin-3 levels and primary graft dysfunction after lung transplantation for idiopathic pulmonary fibrosis. Am J Transplant. 2011;11:2517–2522. doi: 10.1111/j.1600-6143.2011.03702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen EC. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat Rec (Hoboken) 2013;296:378–381. doi: 10.1002/ar.22641. [DOI] [PubMed] [Google Scholar]
- 6.Vogel CF, Chang WL, Kado S, McCulloh K, Vogel H, Wu D, et al. Transgenic overexpression of aryl hydrocarbon receptor repressor (AhRR) and AhR-mediated induction of CYP1A1, cytokines, and acute toxicity. Environ Health Perspect. 2016;124:1071–1083. doi: 10.1289/ehp.1510194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Favreau LV, Pickett CB. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene: identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J Biol Chem. 1991;266:4556–4561. [PubMed] [Google Scholar]
- 8.Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, et al. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- 9.Shivanna B, Zhang W, Jiang W, Welty SE, Couroucli XI, Wang L, et al. Functional deficiency of aryl hydrocarbon receptor augments oxygen toxicity-induced alveolar simplification in newborn mice. Toxicol Appl Pharmacol. 2013;267:209–217. doi: 10.1016/j.taap.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diamond JM, Porteous MK, Roberts LJ, II, Wickersham N, Rushefski M, Kawut SM, et al. Lung Transplant Outcomes Group. The relationship between plasma lipid peroxidation products and primary graft dysfunction after lung transplantation is modified by donor smoking and reperfusion hyperoxia. J Heart Lung Transplant. 2016;35:500–507. doi: 10.1016/j.healun.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kodal JB, Kobylecki CJ, Vedel-Krogh S, Nordestgaard BG, Bojesen SE. AHRR hypomethylation, lung function, lung function decline and respiratory symptoms. Eur Respir J. 2018;51:1701512. doi: 10.1183/13993003.01512-2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.