Abstract
Background
The recycling of citrulline by argininosuccinate synthase 1 (ASS1) and argininosuccinate lyase (ASL) is crucial to maintain arginine availability and nitric oxide (NO) production. Pegylated arginine deiminase (ADI-PEG20) is a bacterial enzyme used to deplete circulating arginine.
Objective
The goal of this research was to test the hypothesis that citrulline is able to sustain intracellular arginine availability for NO production in ADI-PEG20 arginine–depleted mice.
Methods
Six- to 8-wk-old male C57BL/6J mice injected with ADI-PEG20 (5 IU) or saline (control) were used in 4 different studies. Arginine, citrulline, and NO kinetics were determined by using stable isotopes in unchallenged (study 1) and endotoxin-challenged (study 2) mice. Blood pressure was determined by telemetry for 6 d after ADI-PEG20 administration (study 3), and vasomotor activity and ASS1 and ASL gene expression were determined in mesenteric arteries collected from additional mice (study 4).
Results
ADI-PEG20 administration resulted in arginine depletion (<1 compared with 111 ± 37 µmol/L) but in greater plasma citrulline concentrations (900 ± 123 compared with 76 ± 8 µmol/L; P < 0.001) and fluxes (402 ± 17 compared with 126 ± 4 µmol ⋅ kg−1 ⋅ h−1; P < 0.001) compared with controls. Endotoxin-challenged ADI-PEG20–treated mice produced less NO than controls (13 ± 1 compared with 27 ± 2 µmol ⋅ kg−1 ⋅ h−1; P < 0.001). No differences (P > 0.50) were observed for cardiovascular variables (heart rate, blood pressure) between ADI-PEG20–treated and control mice. Furthermore, no ex vivo vasomotor differences were observed between the 2 treatments. ADI-PEG20 administration resulted in greater gene expression of ASS1 (∼3-fold) but lower expression of ASL (–30%).
Conclusion
ADI-PEG20 successfully depleted circulating arginine without any effect on cardiovascular endpoints in healthy mice but limited NO production after endotoxin challenge. Therefore, the citrulline recycling pathway can sustain local arginine availability independently from circulating arginine, satisfying the demand of arginine for endothelial NO production; however, it is unable to do so when a high demand for arginine is elicited by endotoxin.
Keywords: ADI-PEG20, arginine, argininosuccinate, citrulline, mouse, recycling
Introduction
Arginine is a conditionally indispensable amino acid that serves as a precursor for the synthesis of numerous compounds. Among these, the signaling molecule NO is especially important because it is involved in many physiologic and pathophysiologic processes. NO has a central role in the cardiovascular system (1) and in the regulation of blood pressure (2); in addition, NO is a powerful bactericide and first line of defense of the immune response (3). The synthesis of NO is catalyzed by the oxidation of the imino group of arginine by intracellular NO synthase (NOS), generating citrulline as a coproduct of this reaction (4) (Figure 1). The availability of arginine seems to limit the synthesis of NO, because local arginase activity (5) or arginine supplementation (6) affects the rate of production of NO. Citrulline has the potential to be recycled intracellularly through the action of argininosuccinate synthase 1 (ASS1) and argininosuccinate lyase (ASL) to regenerate a new arginine molecule and thus sustain NO production (7, 8). Furthermore, extracellular citrulline can also be transported into the cell and utilized to produce arginine locally (9, 10).
FIGURE 1.
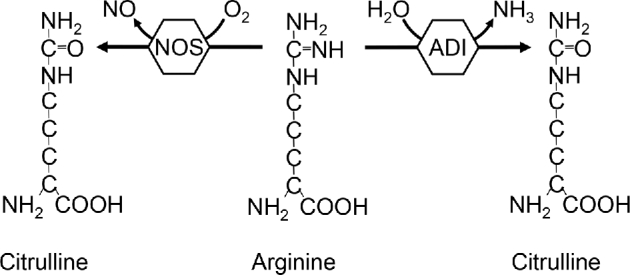
Utilization of arginine by NOS and ADI to produce NO and ammonia, respectively. Both reactions generate citrulline as a coproduct. ADI, arginine deiminase; NOS, nitric oxide synthase.
Arginine deiminase (ADI) is a bacterial enzyme that catalyzes the reduction of the imino group of arginine to generate NH3 and citrulline (Figure 1). This enzyme has been pegylated (ADI-PEG20) to increase its half-life and is currently used in the treatment of certain cancer types that lack ASS1 activity (11–13). The arginine depletion that follows ADI-PEG20 treatment, and the inability of these cancer cells to utilize citrulline to generate local arginine, results in impaired growth and eventual apoptotic cancer cell death (14) with minimal adverse effect in patients (15). Furthermore, arginine depletion by ADI-PEG20 seems to have no cardiovascular effects in humans (16) or mice (17).
We have shown that citrulline supplementation is a more effective way to increase global arginine availability than arginine supplementation itself, because a substantial amount of arginine is metabolized during first-pass metabolism (18). In addition, due to the widespread localization of ASS1 and ASL (19, 20), citrulline supplementation also has the potential to increase local (intracellular) arginine availability through the enzymes of the citrulline recycling pathway. The present work was conducted to test the hypothesis that citrulline is able to sustain NO production in ADI-PEG20 arginine–depleted mice.
Methods
Animals and husbandry
Six- to 8-wk-old C57BL/6J male mice were used for the 4 experiments described below. Mice were ad libitum–fed regular rodent feed pellets (LabDiet 5V5R chow; fat, 59 g/kg; fiber, 24 g/kg; carbohydrates, 570 g/kg; crude protein, 180 g/kg; arginine, 7.5 g/kg; ash, 50 g/kg; LabDiet). Mice were under a 12-h light cycle (0600–1800) in a temperature- (22°C) and humidity- (55%) controlled environment. Autoclaved reverse osmosis water was available at all times, and the bedding used was corn cobs. All animal procedures were authorized by the Baylor College of Medicine Institutional Animal Care and Use Committee.
Determination of arginine and citrulline fluxes in ADI-PEG20–treated mice (study 1)
Mice were injected intramuscularly with 50 µL (5 IU) ADI-PEG20 (n = 8) or saline (control; n = 8). The route of delivery and dose used were determined according to the manufacturer's instructions (Polaris Pharmaceutical, Inc.). Because the half-life of ADI-PEG20 is ∼6–8 d (17), mice were studied 6 d later to allow for possible adaptation to arginine depletion. On the day of the infusion, after 3 h of feed deprivation, a catheter was placed in the lateral tail vein (21) and mice were infused for 4 h with (guanidino)[15N2]l-arginine (prime: 45 µmol/kg; continuous infusion: 45 µmol ⋅ kg−1 ⋅ h−1) and 1,2,3,4,5[13C5]l-citrulline (25 µmol/kg; 25 µmol ⋅ kg−1 ⋅ h−1) to determine arginine and citrulline fluxes and with (ring)[2H5]l-phenylalanine (16 µmol/kg; 16 µmol ⋅ kg−1 ⋅ h−1) and 3,3[2H2]l-tyrosine (10 µmol/kg; 10 µmol ⋅ kg−1 ⋅ h−1) to determine whole-body protein turnover. All labeled tracers were obtained from Cambridge Isotope Laboratories, Inc., unless otherwise indicated. At the end of the infusion, a blood sample was collected from the submandibular bundle. We have previously shown that plateau enrichments are achieved within the 4-h infusion (21).
Endotoxin challenge in arginine-depleted mice (study 2)
A similar protocol as in the previous experiment was followed, with the exception that mice received an intravenous endotoxin challenge (7 mg LPS/kg, E. coli O111:B4 endotoxin; Sigma Aldrich) at the beginning of the infusion (ADI-PEG20+LPS; n = 10) or saline (saline+LPS; n = 10). In addition, a 15N18O3 tracer (K15N18O3; Isotec) was infused (prime: 0.2 µmol/kg; continuous infusion: 0.2 µmol ⋅ kg−1 ⋅ h−1) to determine the flux of nitrate.
Blood pressure in arginine-depleted mice (study 3)
Mice underwent surgery for the implantation of a pressure catheter in the carotid artery attached to a telemetry device (HDX-10; Data Sciences International). Mice were housed individually, and after a 4-d recovery period, blood pressure was measured for 24 h before injecting ADI-PEG20 (n = 8) or saline (n = 9), as previously described. Blood pressure was then continuously monitored for 6 d. A blood sample was taken at the end of the experiment to verify arginine depletion in the ADI-PEG20–treated mice.
Ex vivo arterial vasomotor response and arterial ASS1 and ASL expression in arginine-depleted mice (study 4)
Six days after ADI-PEG20 (n = 7) or saline (control; n = 4) administration, mesentery arteries were collected from mice. Smaller arteries make a greater contribution to vascular resistance than larger arteries (e.g., aorta) and thus are more relevant to the goal of this study. Vascular reactivity was measured in 2-mm segments of mesenteric artery mounted in a wire myograph (model 610; JP Trading I/S) with 25-µm tungsten wires. The preparations were bathed in Krebs solution that was maintained at 37°C (pH 7.4). A mixture of 95% O2 and 5% CO2 was bubbled continuously through the solution. The force was recorded continuously by an isometric force transducer and analyzed with Power Lab system and Chart 8 data acquisition and playback software (AD Instruments). The optimal diameter and the passive tension that were applied to the vessels (1.5 mN) and the protocol and concentrations that were used in the vascular reactivity studies were determined in previous experiments (22). After stabilization of the tone, the vessels were contracted twice with 60 mmol KCl/L for 10 min to enhance reproducibility of responses. The second response to potassium chloride was used as a reference contraction in the final calculations. To evaluate endothelial function, a dose-response of acetylcholine (10–10 to 10–6 mol/L) in vessels that were precontracted with norepinephrine (3 × 10–6 mol/L) was determined. Only experiments with intact endothelium were used in our final analysis. After 1 h of equilibration, relaxant responses to l-arginine and l-citrulline (10–10 to 10–5 mol/L) in the presence or absence of the NOS inhibitor l-N-Nitroarginine methyl ester (l-NAME) were obtained after precontraction of the vessels with norepinephrine (3 × 10–6 mol/L) to produce matching contractions in the study groups. After each agent was tested, the vessels were washed with Krebs solution and left to recover for 30 min until they returned to their basal passive tension. In an additional set of mice, mesenteric arteries were collected to determine ASS1 and ASL gene expression in control (n = 5) and ADI-PEG20–treated (n = 5) mice.
Sample analysis
Amino acid concentrations and enrichments were determined by Heated Electrospray Ionization LC–tandem MS after their dansyl derivatization, as previously described (23). A U-[13C15N]amino acid mixture and 2,3,3,4,4,5,5-[2H7]citrulline (CDN Isotopes) were used as internal standard to quantify amino acid concentrations. Nitrate enrichment was determined as its pentafluorobenzyl derivative by GC-MS (24).
Arteries were homogenized in guanidinium thiocyanate-phenol-chloroform extraction solution (TRIzol; Life Technologies) and RNA was isolated by using Qiagen RNeasy Mini elute columns (Qiagen). After cDNA synthesis, PCR amplification and gene differentiation expression for ASS1 (forward 5′-CTGTTCGCCACTGTATCCAGAAGTC -3′; reverse 5′-CCGCTCCTCTTTGTCAGGGTCTA -3′) and ASL (forward 5′-CGGACTCCTGATGACCCTCAAG -3′; reverse 5′-TGTACTGTTCCACGCTGTGACTGT -3′) were performed with SYBR Green1 on a multicolor real-time PCR detection system (CFX96; BioRad Laboratories). GAPDH expression (forward 5′-CGACCCCTTCATTGACCTCAACT -3′; reverse 5′-GGCCTCACCCCATTTGATGTTAG-3′) was used to normalize the expression of the genes of interest.
Calculations
Fluxes of amino acids and nitrate were calculated on the basis of the dilution of the corresponding infused tracer. Rates of conversion of arginine into citrulline and phenylalanine into tyrosine (rate of hydroxylation) were calculated on the basis of the transfer of the label from the precursor to the product (25). The conversion of arginine to citrulline has been used as a proxy to determine NO synthesis, because equimolar amounts of NO and citrulline are generated by NOS. Because the conversion of arginine into citrulline cannot be determined in arginine-depleted mice, we also measured nitrate flux (a stable product of NO oxidation) as a proxy for NO production rate. Whole-protein turnover was estimated on the basis of the model of Thompson et al. (26); briefly, phenylalanine flux represents protein breakdown and the nonhydroxylative disposal of phenylalanine, protein synthesis.
The maximal relaxations of mesenteric arteries induced by either citrulline or arginine were determined from individual concentration response curves. Gene expression data are shown as 2(–ΔΔCt) and normalized to the control treatment.
Data analysis
Fluxes, plasma concentrations, and gene expression data were analyzed statistically utilizing the Proc Mixed procedure of SAS (version 9.2; SAS Institute), with treatment (ADI-PEG20 or control) as the fixed effect. Cardiovascular variables and vasomotor response data were analyzed as repeated measurements including mouse (and arterial segment) as the random effect of the model. The validation between the rate of conversion of arginine to citrulline and nitrate flux for the estimation of NO was determined by using a paired t test. All data are reported as means ± SEMs, and differences were considered significant at P < 0.05.
Results
Determination of arginine and citrulline fluxes in ADI-PEG20–treated mice
ADI-PEG20 resulted in the depletion of plasma arginine and a greater (P < 0.001) plasma citrulline concentration (Figure 2A). The flux of arginine could not be determined in the ADI-PEG20–treated mice due to the lack of circulating arginine. Furthermore, arginine deiminase activity was present in the plasma samples, which was detected by the conversion of the arginine internal standard into citrulline. As expected, the administration of ADI-PEG20 resulted in a greater (P < 0.001) flux of citrulline than in the control mice (Figure 2B). No differences in the fluxes of phenylalanine or tyrosine, or the conversion of phenylalanine into tyrosine, between the 2 treatments were detected, indicating no differences in protein turnover (P > 0.38; Figure 2C).
FIGURE 2.
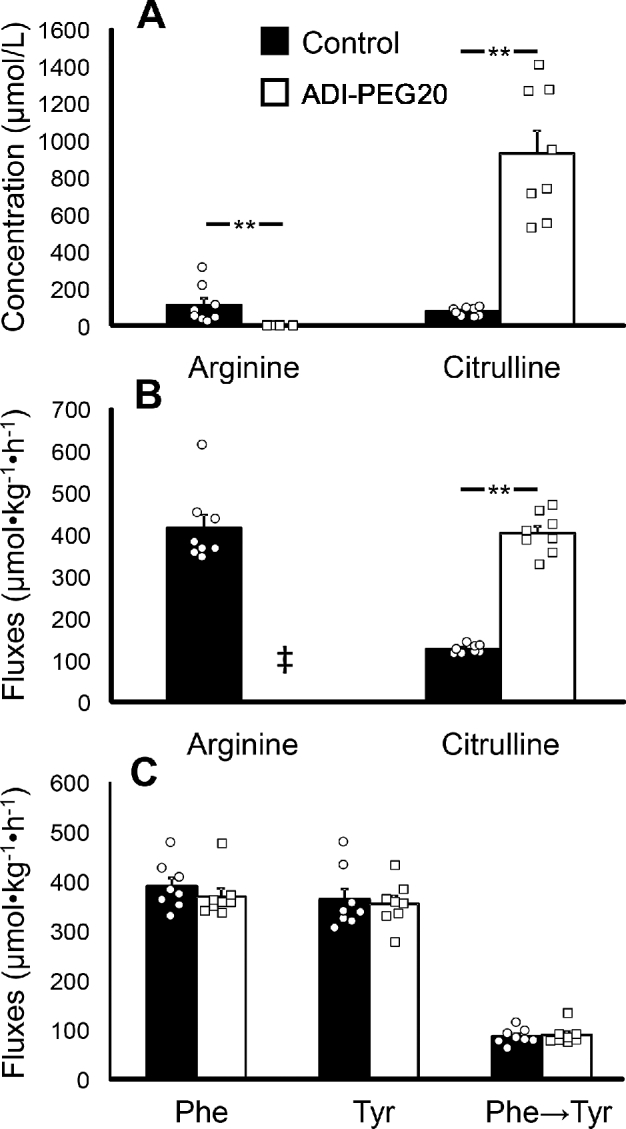
Study 1: plasma arginine and citrulline concentrations (A) and fluxes (B) and phenylalanine and tyrosine fluxes and the phenylalanine-to-tyrosine conversion (C) in male C57BL/6J mice treated with ADI-PEG20 (n = 8, represented by squares) or saline (control; n = 8, represented by circles). Values are means ± SEs. Individual values are also shown. ‡Arginine flux was not determined in ADI-PEG20 mice due to arginine depletion. **P < 0.01. ADI-PEG20, pegylated arginine deiminase.
Endotoxin challenge in arginine-depleted mice
LPS-challenged ADI-PEG20–treated mice had virtually no circulating arginine, but had greater (P = 0.001) plasma citrulline concentrations and fluxes than did the saline+LPS group (Figure 3A, B). The determination of NO production in saline+LPS mice using the arginine-to-citrulline conversion or the flux of nitrate yielded similar values (P = 0.40; Figure 3C). Nitrate flux was greater (P = 0.001) in mice that received the saline+LPS treatment than in ADI-PEG20+LPS-treated animals (Figure 3C). No differences in protein turnover, as determined by phenylalanine and tyrosine fluxes (P > 0.20) and conversion of phenylalanine to tyrosine (P = 0.09), between the ADI-PEG20– and saline LPS–challenged mice were found (Figure 3D).
FIGURE 3.
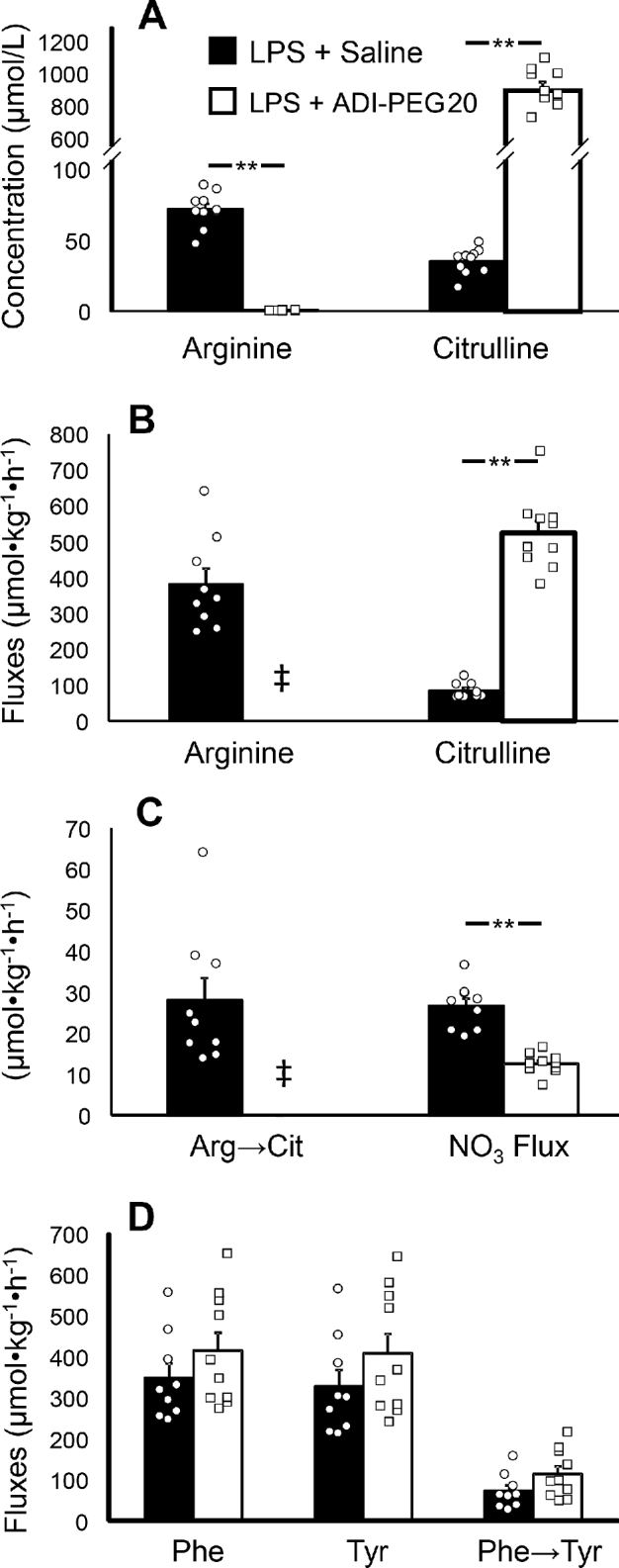
Study 2: plasma arginine and citrulline concentrations (A) and fluxes (B), NO production (C), and phenylalanine and tyrosine fluxes and the phenylalanine-to-tyrosine conversion (D) in male mice treated with ADI-PEG20 (n = 10, represented by squares) or saline (n = 9, represented by circles) after endotoxin (LPS) challenge. Values are means ± SEs. Individual values are also shown. ‡Arginine flux was not determined in ADI-PEG20 mice due to arginine depletion. **P < 0.01. ADI-PEG20, pegylated arginine deiminase.
Blood pressure in arginine-depleted mice
No differences between ADI-PEG20– and saline-treated mice were observed for heart rate (541 ± 12 compared with 537 ± 11 beats/min; P = 0.75), systolic blood pressure (116 ± 3 compared with 124 ± 7 mm Hg; P = 0.33), diastolic blood pressure (91 ± 5 compared with 95 ± 7 mm Hg; P = 0.62), or mean arterial blood pressure (104 ± 3 compared with 109 ± 7 mm Hg; P = 0.58). The mean arterial blood pressure time line shows that ADI-PEG20 treatment had no effect on this variable over the week-long study (Figure 4). Likewise, there were no differences in blood pressure immediately after the administration of ADI-PEG20 (results not shown). The blood sample taken at the end of the trial confirmed that the ADI-PEG20–treated mice were arginine depleted.
FIGURE 4.
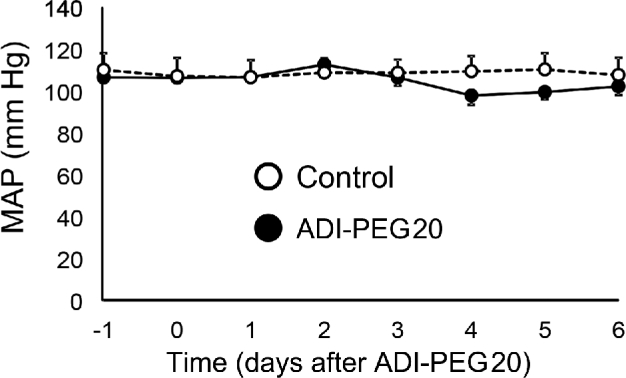
Study 3: MAP time course from 1 d before through 6 d after ADI-PEG20 administration (day 0) in control (n = 9) and ADI-PEG20–treated (n = 8) mice. Values are means ± SEs. ADI-PEG20, pegylated arginine deiminase; MAP, mean arterial blood pressure.
Ex vivo arterial vasomotor response and arterial ASS1 and ASL gene expression in arginine-depleted mice
No differences in mesenteric artery relaxation were found between control and ADI-PEG20–treated mice after the addition of either citrulline (Figure 5A) or arginine (Figure 5B). Likewise, there were no differences in relaxation when the NOS inhibitor l-NAME was added to the solution (Figure 5C, D). There were no differences between citrulline and arginine in their ability to vasodilate the mesenteric artery preparation when given alone or combined with l-NAME. The gene expression of ASS1 was greater (P < 0.001) in ADI-PEG20–treated mice than in the control mice; in contrast, ASL gene expression was lower (P < 0.01; Figure 6A, B).
FIGURE 5.
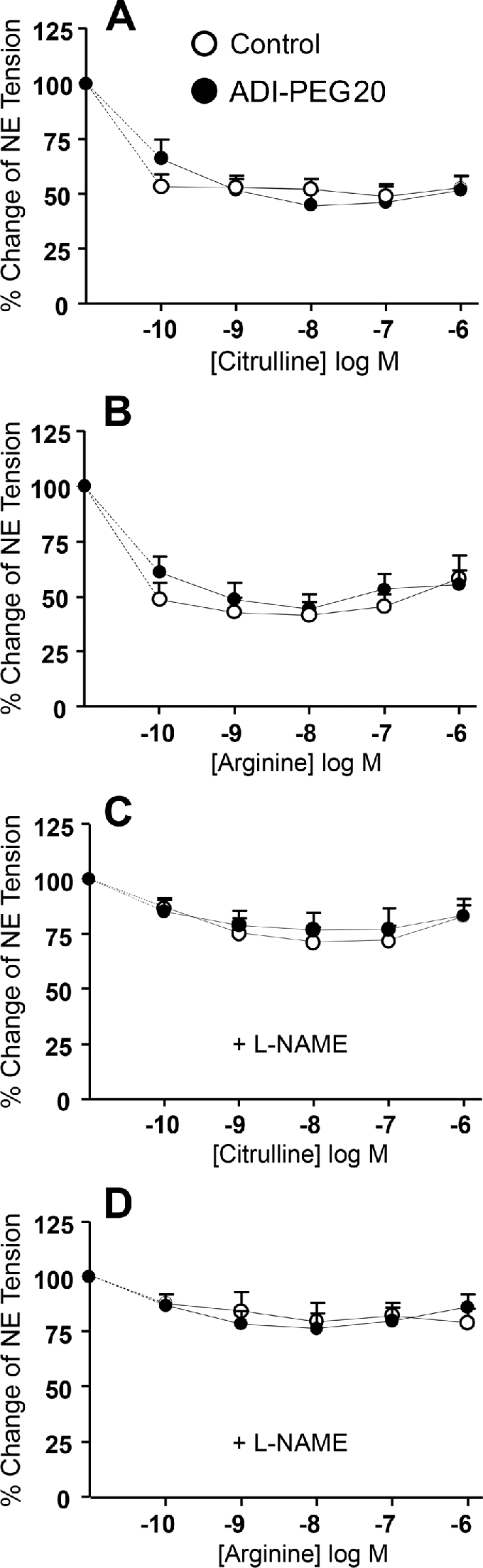
Study 4: vasomotor response of preconstricted mesenteric arteries collected from control (n = 4) or ADI-PEG20–treated (n = 7) mice after the addition of citrulline (A) or arginine (B) and in combination with the NOS inhibitor l-NAME (C and D, respectively). Values are means ± SEs. ADI-PEG20, pegylated arginine deiminase; l-NAME, l-N-nitroarginine methyl ester; NE, norepinephrine; NOS, nitric oxide synthase.
FIGURE 6.
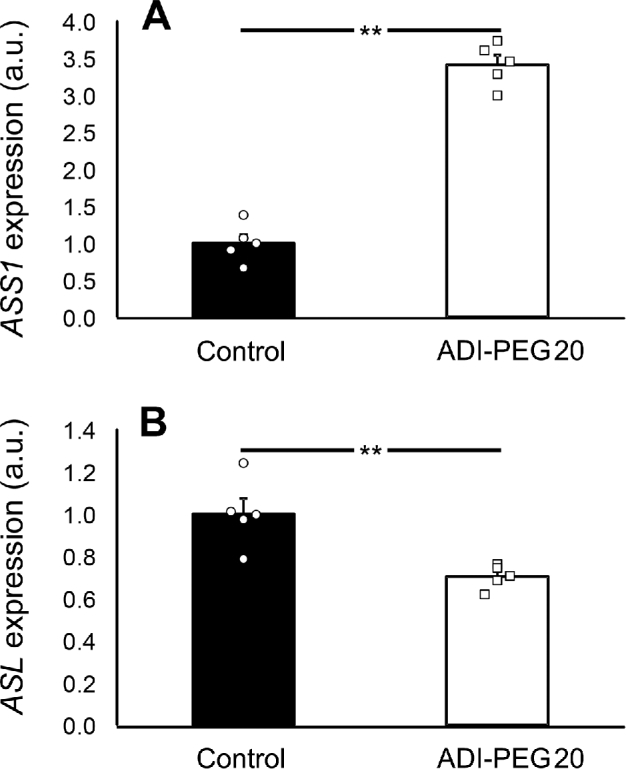
Study 4: ASS1 (A) and ASL (B) expression in mesenteric arteries collected from control and ADI-PEG20–treated mice. Values are means ± SEs. Individual values are also shown. **P < 0.01. ADI-PEG20, pegylated arginine deiminase; ASL, argininosuccinate lyase; ASS1, argininosuccinate synthase 1; a.u., arbitrary units.
Discussion
Arginine is considered conditionally indispensable because under certain physiologic [e.g., growth (27)] or pathophysiologic [e.g., endotoxemia, sepsis (28)] conditions the endogenous synthesis is insufficient to meet the increased demand for this amino acid. The endogenous synthesis of arginine relies on the production of citrulline by the small intestine, which is then utilized by the kidney (where ASS1 and ASL are highly expressed) to produce arginine (29). This enteral-renal axis for arginine synthesis, however, only accounts for the utilization of ∼60–70% of the citrulline produced in humans (30), pigs (31), and mice (18). Due to the widespread localization of ASS1 and ASL (19, 20), it is possible for other cell types and tissues to utilize citrulline directly to meet their local arginine needs. In fact, we and others have shown in vivo that citrulline disposal and utilization continue even after kidney ligation (10, 32), suggesting that a substantial citrulline utilization also takes place in extrarenal tissue. Furthermore, we also showed that young-adult mice were able to sustain protein synthesis despite having virtually no circulating arginine, which indicates extensive local arginine production (9).
The administration of ADI-PEG20 disrupts the interorgan metabolism of arginine, because all circulating arginine is converted into citrulline and any arginine synthesized and exported by the kidney is then converted back to citrulline upon entering the circulation. Although circulating arginine is considered essential for blood pressure maintenance (33), the depletion of arginine per se has little consequence on the metabolism of animals and humans when citrulline is available for local arginine synthesis (9, 16, 17). This is best exemplified by arginine depletion by arginase, which results in weight loss and death, but that can be prevented by citrulline supplementation (34). Although it is relatively simple to study the contribution of the intracellular citrulline recycling pathway in in vitro systems (35), its study in vivo is more challenging. For this reason, arginine depletion by ADI-PEG20 is a useful tool to study the ability of different tissues and organs to produce local arginine to meet their arginine needs.
In this study, ADI-PEG20 resulted in the depletion of circulating arginine as previously reported (9, 17). As a consequence, plasma citrulline concentrations and fluxes were greater in these mice. The flux of arginine in the control mice was similar to previous reports in C57BL/6 mice (27). Because there was no circulating arginine, it was not possible to determine arginine fluxes in the ADI-PEG20–treated mice. Endotoxin challenge resulted in lower arginine and citrulline fluxes in control mice, which agrees with previous reports in mice (36) and in septic humans (37). ADI-PEG20–treated mice had an NO3 flux ∼50% smaller than control mice, corroborating previous work that reported lower plasma nitrate and nitrite concentrations in mice after endotoxin challenge (17).
Citrulline recycling was first described in macrophages >20 y ago (8) and recycling activity is required for controlling Mycobacterium bovis infection (38). Here we have shown that local arginine synthesis was unable to sustain NO production in vivo when the inducible NOS isoform is activated by endotoxin (4). Similar results have been reported in in vitro studies (35); when isomolar concentrations of arginine and citrulline were compared, the NO response to citrulline was only ∼13% of that achieved by arginine. However, when we recalculated this value utilizing the plasma concentrations observed in our mice, the in vitro NO response to citrulline was ∼61% of that achieved by arginine and similar to that determined in the arginine-depleted mice.
No differences in heart rate or blood pressure were evident in arginine-depleted healthy mice when compared with control mice. In vitro studies have shown that in endothelial cells the recycling of citrulline is substantial (39) and thus likely to support function during arginine depletion. The deletion of ASL (40), but not ASS1 (33), in endothelial cells results in hypertension, which indicates the importance of the recycling pathway even under normal conditions. These observations recapitulate the persistent hypertension seen in ASL-deficient patients (40). However, it is unclear to what extent the hypertension seen in ASL knockout mice (40) is due to the lack of enzymatic activity or to the failure to the enzymes related to NO production to assemble into a functional unit (41).
The ability of endothelial cells to utilize the citrulline recycling pathway for local arginine synthesis was further corroborated by the vasomotor activity data, which showed no relaxation difference between citrulline and arginine. The ∼3-fold greater expression of ASS1 observed in ADI-PEG20–treated mice is consistent with previous reports in normal cells of induced transcriptional activation of ASS1 due to arginine depletion (42). However, it is not clear what effect the ∼30% reduction in ASL may have on arginine synthesis, because ASS1 is considered the rate-limiting step in the conversion of citrulline into arginine (19).
In conclusion, arginine depletion by ADI-PEG20 had no effect on cardiovascular endpoints in healthy mice, but limited NO production after endotoxin challenge. Therefore, the citrulline recycling pathway can sustain by itself and independently from circulating arginine the demand of arginine for endothelial NO production, but it is unable to do so when a high demand for arginine is elicited by endotoxin. This may be advantageous because ADI-PEG20 may be used to modulate NO produced by inducible NOS without affecting the cardiovascular system. The better outcome of citrulline, compared with arginine, supplementation (43–45) may not only be due to a more efficient use of citrulline for arginine synthesis (due to its negligible first-pass extraction) but also to the ability of different cell types to produce arginine locally by means of the citrulline recycling pathway.
Acknowledgments
The authors’ responsibilities were as follows—YY: conducted the surgeries, measured blood pressure, and determined gene expression; MAM: analyzed plasma samples and data and reviewed the manuscript; AB and CY: measured arterial vasomotor response and reviewed the manuscript; ICD: conducted the research and prepared samples for analysis; JCM: designed and conducted the research, performed surgeries, analyzed the data, wrote the final manuscript, and had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
Supported by federal funds from the USDA, Agricultural Research Service, under Cooperative Agreement 58-6250-6-001, and the NIH (R01 GM108940). Polaris Pharmaceutical, Inc. (San Diego, California), provided the ADI-PEG20.
Author disclosures: YY, MAM, AB, ICD, CY, and JCM, no conflicts of interest.
Abbreviations used: ADI, arginine deiminase; ADI-PEG20, pegylated arginine deiminase; ASL, argininosuccinate lyase; ASS1, argininosuccinate synthase 1; l-NAME, l-N-Nitroarginine methyl ester; NOS, nitric oxide synthase.
References
- 1. Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med 2005;26:33–65. [DOI] [PubMed] [Google Scholar]
- 2. Rees DD, Palmer RMJ, Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci USA 1989;86:3375–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bogdan C. Nitric oxide and the immune response. Nat Immunol 2001;2:907–16. [DOI] [PubMed] [Google Scholar]
- 4. Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 2012;33:829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson FK, Peyton KJ, Liu XM, Azam MA, Shebib AR, Johnson RA, Durante W. Arginase promotes endothelial dysfunction and hypertension in obese rats. Obesity 2015;23:383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kohli R, Meininger CJ, Haynes TE, Yan W, Self JT, Wu GY. Dietary L-arginine supplementation enhances endothelial nitric oxide synthesis in streptozotocin-induced diabetic rats. J Nutr 2004;134:600–8. [DOI] [PubMed] [Google Scholar]
- 7. Hecker M, Sessa WC, Harris HJ, Anggard EE, Vane JR. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothelial cells recycle L-citrulline to L-arginine. Proc Natl Acad Sci USA 1990;87:8612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu GY, Brosnan JT. Macrophages can convert citrulline into arginine. Biochem J 1992;281:45–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marini JC, Didelija IC. Arginine depletion by arginine deiminase does not affect whole protein metabolism or muscle fractional protein synthesis rate in mice. PLoS One 2015;10:e0119801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marini JC, Didelija IC, Fiorotto ML. Extrarenal citrulline disposal in mice with impaired renal function. Am J Physiol 2014;307:F660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walts AE, Bomalaski JS, Ines D, Orsulic S. Argininosuccinate synthetase (ASS) deficiency in high-grade pulmonary neuroendocrine carcinoma: an opportunity for personalized targeted therapy. J Cancer Res Clin Oncol 2015;141:1363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tomlinson BK, Thomson JA, Bomalaski JS, Diaz M, Akande T, Mahaffey N, Li T, Dutia MP, Kelly K, Gong IY et al. Phase I trial of arginine deprivation therapy with ADI-PEG 20 plus docetaxel in patients with advanced malignant solid tumors. Clin Cancer Res 2015;21:2480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Phillips M, Sheaff MT, Szlosarek PW. Targeting arginine-dependent cancers with arginine-degrading enzymes: opportunities and challenges. Cancer Res Treat 2013;45:251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Delage B, Fennell DA, Nicholson L, McNeish I, Lemoine NR, Crook T, Szlosarek PW. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int J Cancer 2010;126:2762–72. [DOI] [PubMed] [Google Scholar]
- 15. Izzo F, Marra P, Beneduce G, Castello G, Vallone P, De Rosa V, Cremona F, Ensor CM, Holtsberg FW, Bomalaski JS et al. Pegylated arginine deiminase treatment of patients with unresectable hepatocellular carcinoma: results from phase I/II studies. J Clin Oncol 2004;22:1815–22. [DOI] [PubMed] [Google Scholar]
- 16. Ascierto PA, Scala S, Castello G, Daponte A, Simeone E, Ottaiano A, Beneduce G, De Rosa V, Izzo F, Melucci MT et al. Pegylated arginine deiminase treatment of patients with metastatic melanoma: results from phase I and II studies. J Clin Oncol 2005;23:7660–8. [DOI] [PubMed] [Google Scholar]
- 17. Thomas JB, Holtsberg FW, Ensor CM, Bomalaski JS, Clark MA. Enzymic degradation of plasma arginine using arginine deiminase inhibits nitric oxide production and protects mice from the lethal effects of tumour necrosis factor α and endotoxin. Biochem J 2002;363:581–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Agarwal U, Didelija IC, Yuan Y, Wang X, Marini JC. Supplemental citrulline is more efficient than arginine in increasing systemic arginine availability in mice. J Nutr 2017;147:596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Husson A, Brasse-Lagnel C, Fairand A, Renouf S, Lavoinne A. Argininosuccinate synthetase from the urea cycle to the citrulline-NO cycle. Eur J Biochem 2003;270:1887–99. [DOI] [PubMed] [Google Scholar]
- 20. Yu Y, Terada K, Nagasaki A, Takiguchi M, Mori M. Preparation of recombinant argininosuccinate synthetase and argininosuccinate lyase: expression of the enzymes in rat tissues. J Biochem (Tokyo) 1995;117:952–7. [DOI] [PubMed] [Google Scholar]
- 21. Marini JC, Didelija IC, Castillo L, Lee B. Glutamine: precursor or nitrogen donor for citrulline synthesis? Am J Physiol 2010;299:E69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mateus J, Bytautiene E, Lu F, Tamayo EH, Betancourt A, Hankins GDV, Longo M, Saade GR. Endothelial growth factor therapy improves preeclampsia-like manifestations in a murine model induced by overexpression of sVEGFR-1. Am J Physiol 2011;301:H1781–7. [DOI] [PubMed] [Google Scholar]
- 23. Marini JC. Quantitative analysis of 15N-labeled positional isomers of glutamine and citrulline via electrospray ionization tandem mass spectrometry of their dansyl derivatives. Rapid Commun Mass Spectrom 2011;25:1291–6. [DOI] [PubMed] [Google Scholar]
- 24. Tsikas D. Simultaneous derivatization and quantification of the nitric oxide metabolites nitrite and nitrate in biological fluids by gas chromatography/mass spectrometry. Anal Chem 2000;72:4064–72. [DOI] [PubMed] [Google Scholar]
- 25. Marini JC. Arginine and ornithine are the main precursors for citrulline synthesis in mice. J Nutr 2012;142:572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thompson GN, Pacy PJ, Merritt H, Ford GC, Read MA, Cheng KN, Halliday D. Rapid measurement of whole body and forearm protein turnover using a [2H5]phenylalanine model. Am J Physiol 1989;256:E631–639. [DOI] [PubMed] [Google Scholar]
- 27. Marini JC, Agarwal U, Didelija IC. Dietary arginine requirements for growth are dependent on the rate of citrulline production in mice. J Nutr 2015;145:1227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luiking YC, Poeze M, Dejong CH, Ramsay G, Deutz NE. Sepsis: an arginine deficiency state? Crit Care Med 2004;32:2135–45. [DOI] [PubMed] [Google Scholar]
- 29. Marini JC, Agarwal U, Robinson JL, Yuan Y, Didelija IC, Stoll B, Burrin DG. The intestinal-renal axis for arginine synthesis is present and functional in the neonatal pig. Am J Physiol 2017;313:E233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Castillo L, Chapman TE, Sanchez M, Yu YM, Burke JF, Ajami AM, Vogt J, Young VR. Plasma arginine and citrulline kinetics in adults given adequate and arginine-free diets. Proc Natl Acad Sci USA 1993;90:7749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marini JC, Stoll B, Didelija IC, Burrin DG. De novo synthesis is the main source of ornithine for citrulline production in neonatal pigs. Am J Physiol 2012;303:E1348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Featherston WR, Rogers QR, Freedland RA. Relative importance of kidney and liver in synthesis of arginine by the rat. Am J Physiol 1973;224:127–9. [DOI] [PubMed] [Google Scholar]
- 33. Chennupati R, Meens MJPMT, Marion V, Janssen BJ, Lamers WH, De Mey JGR, Eleonore Köhler S. Endothelial arginine resynthesis contributes to the maintenance of vasomotor function in male diabetic mice. PLoS One 2014;9:e102264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mauldin JP, Zeinali I, Kleypas K, Woo JH, Blackwood RS, Jo CH, Stone EM, Georgiou G, Frankel AE. Recombinant human arginase toxicity in mice is reduced by citrulline supplementation. Transl Oncol 2012;5:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baydoun AR, Bogle RG, Pearson JD, Mann GE. Discrimination between citrulline and arginine transport in activated murine macrophages: inefficient synthesis of NO from recycling of citrulline to arginine. Br J Pharmacol 1994;112:487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luiking YC, Poeze M, Ramsay G, Deutz NEP. Reduced citrulline production in sepsis is related to diminished de novo arginine and nitric oxide production. Am J Clin Nutr 2009;89:142–52. [DOI] [PubMed] [Google Scholar]
- 37. Kao CC, Bandi V, Guntupalli KK, Wu M, Castillo L, Jahoor F. Arginine, citrulline and nitric oxide metabolism in sepsis. Clin Sci 2009;117:23–30. [DOI] [PubMed] [Google Scholar]
- 38. Qualls JE, Subramanian C, Rafi W, Smith AM, Balouzian L, Defreitas AA, Shirey KA, Reutterer B, Kernbauer E, Stockinger S et al. Sustained generation of nitric oxide and control of mycobacterial infection requires argininosuccinate synthase 1. Cell Host Microbe 2012;12:313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Flam BR, Eichler DC, Solomonson LP. Endothelial nitric oxide production is tightly coupled to the citrulline-NO cycle. Nitric Oxide 2007;17:115–21. [DOI] [PubMed] [Google Scholar]
- 40. Nagamani SCS, Campeau PM, Shchelochkov OA, Premkumar MH, Guse K, Brunetti-Pierri N, Chen Y, Sun Q, Tang Y, Palmer D et al. Nitric-oxide supplementation for treatment of long-term complications in argininosuccinic aciduria. Am J Hum Genet 2012;90:836–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Erez A, Nagamani SCS, Shchelochkov OA, Premkumar MH, Campeau PM, Chen Y, Garg HK, Li L, Mian A, Bertin TK et al. Requirement of argininosuccinate lyase for systemic nitric oxide production. Nat Med 2011;17:1619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bowles TL, Kim R, Galante J, Parsons CM, Virudachalam S, Kung HJ, Bold RJ. Pancreatic cancer cell lines deficient in argininosuccinate synthetase are sensitive to arginine deprivation by arginine deiminase. Int J Cancer 2008;123:1950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wijnands KAP, Vink H, Briedé JJ, van Faassen EE, Lamers WH, Buurman WA, Poeze M. Citrulline a more suitable substrate than arginine to restore NO production and the microcirculation during endotoxemia. PLoS One 2012;7:e37439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. El-Hattab AW, Hsu JW, Emrick LT, Wong LJC, Craigen WJ, Jahoor F, Scaglia F. Restoration of impaired nitric oxide production in MELAS syndrome with citrulline and arginine supplementation. Mol Genet Metab 2012;105:607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Asgeirsson T, Zhang S, Nunoo R, Mascarenas C, Dujovny N, Luchtefeld M, Cavey GS, Senagore A. Citrulline: a potential immunomodulator in sepsis. Surgery 2011;150:744–51. [DOI] [PubMed] [Google Scholar]


