Abstract
The Gluteus Maximus (GM) muscle is the largest and most powerful in the human body. It plays an important role in optimal functioning of the human movement system as well as athletic performance. It is however, prone to inhibition and weakness which contributes to chronic pain, injury and athletic under-performance. As such, understanding how to assess and treat GM dysfunction is an important aspect of sports science and medicine, as it has relevance for injury prevention, rehabilitation and performance enhancement. Despite GMs considerable importance there is little research attempting to translate evidence into practice to support practitioners when faced with ‘sleepy glutes’. This clinical commentary discusses the importance of GM for athletic performance and injury risk; factors which contribute to GM dysfunction and then provides evidenced informed approaches to assess and treat GM dysfunction. This can be used as part of rehabilitation or injury prevention practices as well as athletic performance training.
Level of Evidence
5
Keywords: Gluteus maximus, muscle imbalances, movement system, performance training, rehabilitation
INTRODUCTION
In a normal functioning human, the gluteus maximus (GM) is the strongest and biggest muscle in the body. It is an important muscle, which has developed throughout human evolution to allow us to be able to maintain an upright erect posture. GM is an important muscle for activities of daily living, displays of explosive athletic performance, and stability of certain joints in the body. Unfortunately, GM is prone to weakness and inhibition, which negatively affects athletic performance and has been identified as the mechanism responsible (or linked to depending on strength of evidence) for numerous injury types and chronic pain.1-4 GM dysfunction could be a key factor in the increased injury risk apparent in those with previous injury. This clinical commentary will review the role of GM in injury risk, its importance for optimal biomechanics and athletic performance and provide practical recommendations on how to assess and treat GM weakness and dysfunction.
ANATOMY AND FUNCTION OF GLUTEUS MAXIMUS MUSCLE AND ITS IMPLICATIONS IN INJURY RISK AND CHRONIC PAIN
GM is the largest muscle in the human body,5 accounting for 16% of the total cross-sectional area.6 Traditionally, GM was thought to originate at the posterior quarter of the iliac crest, the posterior surface of the sacrum and coccyx, and to the fascia of the lumbar spine.7,8 Recent authors have also suggested attachments originating from the gluteus medius fascia, ilium, thoracolumbar fascia, erector spinae aponeurosis, dorsal sacroiliac and sacrotuberous ligaments, as well as the more traditionally known attachments at the sacrum and coccyx.9 The muscle runs inferiorly and laterally, splitting into two portions, with the superior portion inserting into the iliotibial tract of the fascia lata and the inferior portion inserting at the gluteal tuberosity of the femur.10
GM assumes three basic functions, to act as both a local and global stabilizer and to exert force (to perform global movement at the lumbopelvic region) as a global mobilizer. As a local stabilizer, the GM roles include segmental stabilization, of the a) lower back via its connection with the erector spinae and thoraco-lumbar fascia;11,12 b) sacroiliac joint (SIJ) by bracing and compression;13,14 c) in the lumbo-sacral region via co-contraction with the psoas major15,16 as well as d) femoral head stabilization in the acetabulum via control of femoral head translation17 and e) due to its attachment into the iliotibial band, superior fibers of the GM may play a role in stabilizing the knee joint in extension.
As a global stabilizer, the GM functions through eccentric and/or isometric actions to control range of motion across three planes of motion. Acting as a tri-planar stabilizer in movement, it acts to prevent trunk forward lean; trunk rotation (via working in conjunction with the contralateral latissimus dorsi as part of the posterior oblique system), stabilization of the pelvis during single leg stance, preventing adduction and internal rotation of the femur. Collectively, the GM functions in conjunction with the other gluteal muscles (gluteus medius and gluteus minimus) to stabilize the hip by counteracting gravity's hip adduction torque and maintain proper leg alignment by eccentrically controlling adduction and internal rotation of the thigh.18,19
As a global mobilizer, GM produces large amounts of force and power to contribute to hip extension and external rotation of the femur, while the superior fibers act to produce hip abduction torque, and the inferior fibers act to produce hip adduction torque.
The human body is a linked mechanical system and the GM functions as part of a muscle system in conjunction with other muscles and muscle groups. GM has an array of functions which contribute to optimal movement and athletic performance. The neuromuscular system is designed to compensate to allow for movement in the presence of certain muscle dysfunction. As such, when this muscle is dysfunctional it does not stop movement or necessarily elicit symptoms of injury as compensation occurs. However, the resultant altered intrinsic muscle coordination and kinematics because of GM dysfunction may ultimately contribute to the numerous chronic ‘biomechanical overload’ type injuries20 or to certain acute injuries when certain joints may be overcome through excessive force acting upon a compromised neuromuscular system (e.g., ACL injuries).21
Weakness of GM has been implicated in numerous injury types such as anterior knee pain,22,23 anterior cruciate ligament injuries21 low back pain24,25 hamstring strains,26 femoral acetabular impingement syndrome17 and ankle sprains,27,28 and its weakness/dysfunction may be a contributing risk factor to or the result of injury.
WHY MAY GLUTEUS MAXIMUS BECOME DYSFUNCTIONAL?
Activity status and posture
Understanding why GM becomes dysfunctional is important to understanding how to correct the underlying dysfunction and potentially reduce injury risk. Firstly, lifestyle is thought to be a major contributor to reduced activity of GM. It is thought prolonged sitting reduces the activation of GM and over time these muscles become atrophied and weak.10,29 This weakness of GM is thought to increased reliance on the secondary hip extensor muscles, such as the hamstrings and hip adductors to produce hip extension torque,30,31 clinically referred to as ‘synergistic dominance’.30 This is due to the human body utilizing the path of least resistance, which refers to utilizing the most energy efficient motor pattern regardless whether this uses what would be considered the primary agonist for that role.30 This would increase the relative demands placed upon the synergist muscles and potentially contribute to pain and strain injuries associated with these muscles.
Furthermore, altered posture of the pelvis can influence the length-tension relationship of GM, as such, reducing its stabilizing capacity.32 Associated with hip flexor tightness and local core weakness is an anterior tilted pelvis, which elongates the GM and places the muscle in a mechanically disadvantaged position.8
Reciprocal inhibition and synergistic dominance
Reciprocal inhibition of the GM, secondary to over-activity of the hip flexor muscle group has been implicated to occur and lead to lower extremity injury.33-35 It has reported that those with reduced hip extension range of motion, and as such, tightness of the hip flexor muscles as measured via the modified Thomas Test, exhibited less GM activation and lower GM: biceps femoris co-activity during a bilateral squat, i.e. ‘synergistic dominance’, despite producing similar hip and knee extension moments.34
Pain and/or swelling
Pain is considered a potent inhibitor of GM resulting in delayed and reduced GM activation with concurrent hamstring and low back compensation.15,27,36-38 This includes pain from local areas such as the back25,37,38 and pain at body regions located away from the hip and pelvic region, such as the ankle.27 This inhibitory effect is thought to act as a protective mechanism to preserve short term musculoskeletal health through limiting the ability for powerful movements. Hodges and Tucker39 proposed that pain may trigger neuromuscular changes with the intent of protecting the injured region of the body and minimizing the experience of pain. The authors proposed these adaptations to include: redistribution of activity within or between muscles and changes in mechanical behavior including stiffness or modified movement patterns. The authors further suggested that changes occur at multiple levels of the nervous system and may be additive, complementary or competitive. While these changes may provide short term pain relief and protection from further damage, they may have long-term implications on musculoskeletal health, re–injury risk and athletic performance.
Swelling/joint inflammation is also thought to result in arthrogenic muscle inhibition.40 Freeman et al40 mimicked the effects of arthrogenic inhibition following injury using fluid injection into the hip capsule and showed clinically significant reductions in GM activation.
ASSESSING GLUTEUS MAXIMUS FUNCTION
Assessing muscle strength
Weak muscles have limited capacity to produce force in functional situations, which would be expected to result in synergistic dominance (adductor magnus and hamstrings in hip extension, biceps femoris and local hip external rotators, in external rotation).30 As such, it is important to understand the muscle's strength capacity. GM strength is typically assessed using a prone hip extension task, with the knee flexed to 90 ° (to minimize force contribution of the hamstrings, through active insufficiency).34 This is typically performed via manual testing for muscle strength, but we encourage the use where possible with a hand-held dynamometer.34 Other methods may include isometric or isokinetic assessment of hip extension strength using an isokinetic dynamometer.41 A short lever bridge, performed either isometrically (timed) or dynamically (number of repetitions) can also provide an indication of GM muscle strength endurance capacity, which can be used clinically with ease and for regular monitoring (e.g., monitoring as part of a activation exercises or training session).42
Movement analysis
Qualitative assessment of movement performance during sporting type tasks can provide an indication of GM function. The GM supports many functions of movement, and so, understanding its anatomy and function can support an understanding of its ability to optimally contribute to neuromuscular control. Inability to maintain limb control, evidenced by hip adduction and internal rotation may indicate the gluteal muscles are not functioning optimally (Figure 1).43 Subsequent assessment to delineate if the movement issues are motor control driven (e.g., due to altered motor patterns) or due to underlying muscle dysfunction (e.g., a small weak muscle which does not sufficiently activate/generate force) is needed. Movement should be assessed during foundational motor pattern tasks such as the squat, deadlift, step up/down as well as during sporting type tasks such as landing, jumping and change of direction to get a more complete understanding of an individual's movement performance.
Figure 1.
Qualitative assessment of movement patterns can allow for indication of specific muscle function. The femoral internal rotation and adduction (1a), and so medial knee displacement (1b) may indicate insufficient function of gluteal muscles, presented here by two young male athletes during a jumping and a deceleration task.
Assessing muscle activation
Often GM can be strong but may present with reduced activation or delayed onset during functional tasks.34 For example, those with short hip flexors (defined as less than zero degrees measured during the modified Thomas test), had two-fold less gluteal activation (assessed using surface electromyography [sEMG]) during a bilateral squat than those with normal length hip flexors (defined as greater than 15 ° during the modified Thomas test), despite similar levels of GM strength under isolated testing situations (using prone hip extension strength with hand-held dynamometer as described above).34 Having an understanding of both neuromuscular activation (ability to recruit the muscle versus a reference contraction) and muscle onset timing (e.g., is there a time delay versus other recruited muscles) during particular tasks can provide important information on GM's function.
Most research studies utilize sEMG to describe and understand voluntary activation of muscle.44-47 Clinically however, this is not always possible (due to skill and/or budgets) or plausible (using sEMG in an applied clinical environment for athletes/patients is expensive and time consuming). Additionally, sEMG use has many limitations (e.g., cross-talk, reliability, signal interference, electrode movement/displacement, assessing a specific portion of the muscle to refer to the whole muscle, normalization/reference tasks).48 As part of the assessment and treatment process, it is important to understand how active GM is during various tasks and if it is working as the primary agonist during GM dominant tasks. One approach the authors use clinically, is to take the exercise task (e.g., single leg bridge) to the point of fatigue, which enables understanding of time/repetitions to fatigue (as such the muscle strength/endurance capacity) and which muscle (if any) is the first muscle to fatigue (as such typically the most active muscle during the exercise). This supports player/patient education, increased buy-in (a sense of an individualized approach) and optimized self-management (e.g., not reliant on external technology but internal perceptions). One particular test advised to utilize is the single leg bridge to fatigue, examining if the GM is the muscle to fatigue (e.g., primary agonist), and when it fatigues (e.g., muscle endurance capabilities, e.g., number of repetitions or time to failure). As the hamstrings are in a shortened position during a short lever bridge, synergistic dominance is typically indicated by cramping of the hamstring muscle31 (e.g., active insufficiency).
Pelvic alignment, mobility and stability
As support to understanding why the muscle may be dysfunctional, assessing pelvic alignment and stability and hip flexibility is important. Attaining data on standing and functional pelvic positioning (e.g., standing pelvic alignment) and control of the pelvis during movements such as bilateral squat, can provide an indication on mobility and dynamic stability of the pelvis. The authors advise the use of 1) the modified Thomas test for hip flexibility;34 2) a standing posture analysis; 3) active straight leg raise for assessing hamstring flexibility and 4) assessment of pelvic alignment and control in functional movements, specifically a bilateral squat/overhead squat.49
CORRECTING GLUTEAL WEAKNESS/DYSFUNCTION – A HOLISTIC APPROACH
As discussed, GM dysfunction can be the result of many factors, and as such, it is advised to incorporate a holistic treatment approach to correct its dysfunction, ideally supported by the assessment techniques to ascertain the patient's profile (e.g., weak GM or not, tight hip flexors or not etc.). Below, a proposed holistic treatment approach to correct GM dysfunction is presented. Although it is holistic in nature, the degree of time and attention spent on each factor will depend upon the athlete's profile ascertained during the screening process.
1. Restore optimal lumbopelvic stability and balance
Corrective work may be needed to address an anterior pelvic tilt due to both weakness of the postural control muscles (e.g., GM, hamstrings, transversus abdominis, rectus abdominus) as well as tightness of the muscles which may result in an anterior tilt (e.g., rectus femoris, psoas, erector spinae). Additionally, over-activity of the hip-flexor muscles can result in reciprocal muscle inhibition of GM.34 Therefore, these tight and/or short muscles should be relaxed, released and lengthened using a combination of manual release and flexibility techniques.50 Self-massage techniques such foam rolling can be an effective method for increasing joint range of motion and performance in subsequent functional movement tasks.50 Additionally, education of the patient maintaining static and dynamic pelvic control (e.g., ability to maintain pelvic alignment and stability in motion) is needed as part of this treatment strategy.
Often following injury, the local stability system can be inhibited (e.g., Transversus Abdominis inhibition as discussed above),51 which may require the global stabilizers or mobilizers to function in a local stability role.7,51 This altered coordination can have influence on the ability to recruit the GM. Therefore, it is essential that for optimal GM function, there should also be good core stability with optimal coordinative control between the core stabilizing musculature. This program should focus on re-activating and integrating the local stability system and developing muscular strength and endurance capabilities of the global stabilizers (Figure 2).
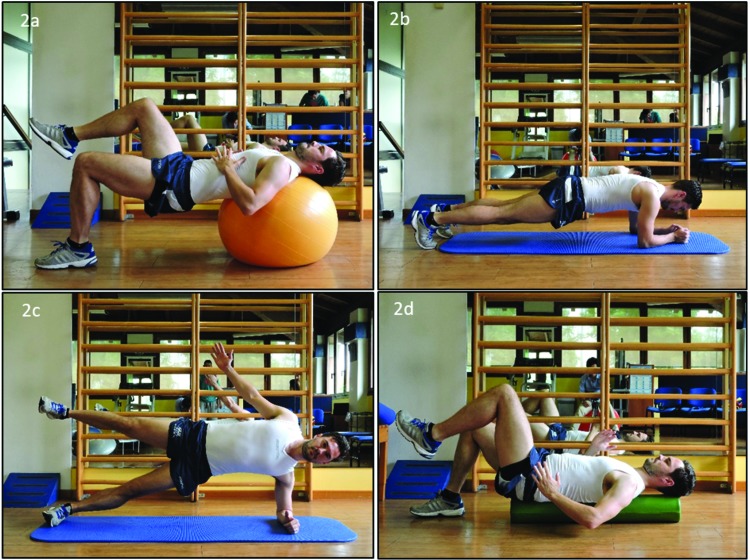
Figure 2.
Example of some local and global core stability exercises which should be included as part of the holistic programme, including single leg bridge with trunk on swiss ball (2a); front plank (2b); side plank (2c) and alternating leg lifts whilst stabilising trunk and pelvis on long foam roller (2d).
2. Strengthen the gluteus maximus muscle and integrate into motor pattern
As described previously, a weak GM muscle has limited capacity to produce force in functional situations, which would be expected to result in synergistic dominance.30 So, developing the strength and endurance capabilities of GM is essential for optimal functioning. Strengthening of any muscle group requires careful planning and well-designed progressions from less challenging to more challenging exercise. Ensuring a sufficient stimulus to bring about hypertrophy and improved strength is essential. Strength adaptations have been observed in training studies where the intensity of exercise ranged between 40% and 95% of maximal intensity.52 Neuromuscular activation of 40-60% is recommended as a minimum for a strengthening effect,53 although it is apparent there is a dose-response relationship with greater gains in strength from exercise which elicit higher neuromuscular activation values.54-56 Around 70% of activation is thought to elicit an optimal ‘strengthening’ effect and achieve desired adaptations in muscle morphology, such as hypertrophy.45,52,53,57 Traditionally, it was believed that very high loads were necessary to bring about activation of all type II motor units based on the Henneman size principle58 and achieve full and complete muscle hypertrophy (targeted at all motor units). However, it is suggested that more low-load training also recruits fast-twitch muscle fibers and can achieve muscle hypertrophy and strength gains, provided the working set is continued close to volitional fatigue.59 This would result in activation and fatigue of the respective motor units, with the addition of progressively larger motor units with time. The efficacy of low to moderate load strengthening exercises (≤70% MVC) for developing maximal eccentric strength and RFD is however, questionable.60-63 It is now becoming accepted that adaptations to moderate resistance training are specific to the high force aspect of the isometric force time curve63 do not enhance the slope of the force time61-63 and also do not result in significant gains in maximal eccentric strength.60 Thus, balancing the use of load to failure and high load resistance training (e.g. > 85% max force or ≤ 5 repetition maximum [RM]) may appear important to provide all round neuromuscular optimization, highlighting the need for effective training programming/periodization.
Recently, there has been a body of research investigating the GM recruitment during various exercises.28,45-47,64-67 This research has reported GM activation across various exercises including non-weight bearing (NWB) exercises (such as the clam shell, bridge, planks), and more functional weight-bearing (WB) exercises (e.g., single leg squat, Romanian deadlift).
Weight-bearing strengthening exercises
Weakness and dysfunction of GM may bring about changes in biomechanics and altered muscle coordination. It is known that enhanced muscle strength does not directly transfer to enhanced functional performance (kinetics and kinematics).68-70 Instead, coordinative changes are required to be able make full use of the enhanced muscle strength.68 As such, motor pattern re-training is needed following/alongside a corrective program to practice and re-learn the desired motor pattern using the ‘new’ muscles. This should involve WB foundational exercises with biofeedback (to teach and correct compensation strategies). In addition, presuming appropriate technique, WB exercises such as single leg squats and deadlifts can produce higher levels of activation than isolated exercises such as the clam,71 thus been potentially more effective more muscle strengthening. The higher activation in WB movements is thought to be due to these exercises imposing greater movement demands on GM.72 In addition these single-leg stance exercises also require the gluteus medius, minimus and upper part of the GM to resist gravity's hip adduction torque. As well as involving concentric and/or eccentric hip extension throughout a large range of motion, frontal plane pelvic stability, together with a control of the stance leg in the frontal and transverse plane, which results in a high neural drive to the gluteus maximus, medius and other muscles of the lateral system and targets all roles of the muscle in a functional manner.
One consideration with the use of WB exercises is in the presence of GM dysfunction (due to either pain, swelling, reciprocal muscle inhibition, synergistic dominance as discussed). In this situation GM activation may be reduced, so that the synergists are the muscles most active,34 thereby reducing the work load of GM and minimizing the stimulus for neuromuscular adaptation. Most studies have utilised healthy injury free participants when examining the typical voluntary activation of GM.
The most common and typically adopted WB exercises for the GM include the squat, deadlift, step up and lunge, as well as their numerous variations e.g., split squat, single leg Romanian deadlift, lateral step up as shown in Figure 3. Understanding GM function and the use of appropriate programming and strategies can maximize the benefits of these foundational strength exercises. These strategies will be discussed below.
Figure 3.
Examples of functional weight bearing exercises to train gluteus maximus. The appropriate technique is crucial to load the whole kinetic chain. Exercises included bilateral squat (3a), single leg Romanian deadlift (3b), single leg squat (3c), split squat/lunge (3d), frontal step up (3e), lateral step up (3f). Each exercise can be loaded with additional weight to provide the necessary loading to develop strength.
Ensure correct technique
During functional tasks, the GM must support body weight and pelvis in stance and prevent adduction and internal rotation of the femur.18,19 During WB exercises it is important to ensure optimal technique to 1) maximize GM activation and 2) maximize the motor pattern retraining benefit. Powers73 discusses this relationship where trunk position, either in the sagittal or frontal plane, that moves the body center of mass (and resultant ground reaction force vector) away from the hip joint will increase the demand placed on the hip muscles. A knee or quadriceps dominant motor pattern (Figure 4) is thought to be the result of weak or under-active hip extensors and preferential over-reliance on knee extensors. Greater hip muscle activation during jumping and running tasks occur with the trunk in a more flexed position.74,75 As such, coaching the athlete in optimal movement strategies (e.g., optimal sagittal plane motor strategy and no presence of dynamic knee valgus or limb rotation) and providing biomechanical feedback are important to optimise motor patterning during WB tasks and accelerate the motor learning process (Figure 5).
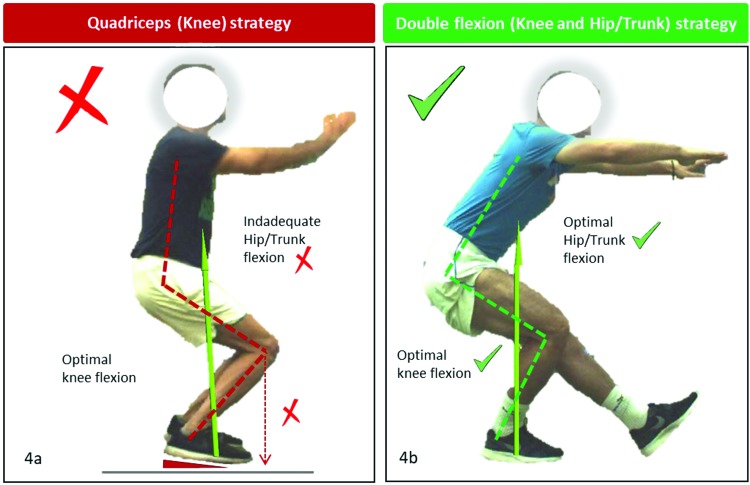
Figure 4.
An example of a knee/quadriceps dominant movement strategy with upright trunk, resulting in greater knee load. This is most commonly associated with the knee excessively positioned anterior to toes (4a). An optimal movement strategy balancing hip and knee contributions, with the knee slightly but not excessively over the toe and similar hip and knee flexions (4b).
Figure 5.
Use of real-time feedback for motor patterning technique on the sagittal plane using a system of high-speed cameras. Use of video-analysis techniques can facilitate different forms of feedback (from real time to delayed feedback) to the patient as part of movement education, as well as assessing motor performance.
Use of load
Most research examining GM activation in WB tasks have used body weight only. However, most studies typically do not quantify the extent of the maximal strength, and thus may work at lower than optimal intensities in certain WB exercises, thus resulting in lower than maximal activation values. Optimizing GM activation can at times be achieved by ensuring the correct load/exercise is used to minimize compensations (due to being too heavy) or to elevate GM activation (e.g., addition of load to make harder). The addition of load and typical type of load can modulate activity. For example, in trained athletes, Foley et al.76 showed that body weight bilateral squat had GM activity of 78% during the eccentric phase, but 120% during the eccentric phase when using a 3RM load. Additionally, Contreras et al.66 reported peak EMG of 85 and 130% EMG maximum of superior and inferior GM during the 10RM back squat.
Use of bands
Use of bands can support elevated GM activation. It has been shown that performing a bilateral squat with a band around the knee can elevate activation of GM and gluteus medius,76 due to maximizing the roles of the muscle (due to the need to perform extra work) (Figure 6).
Figure 6.
Example exercises of using a band during a bilateral squat (6a) or cable around the knee during a split squat (6b). The added resistance will force increased activation of gluteal muscles to prevent adduction and/or internal rotation of the femur. It also acts as a cue to train control of the limb and avoidance of dynamic knee valgus in functional movement tasks.
Exercise variation
Variations of the foundation movements through incorporating an opposing resistance in the opposite limb, for example, including the single leg RDL and pull (Figure 7) involves loading the hand opposite to the stance leg. The added rotary force stimulates the external rotator capability of the GM and medius and gives these exercises a multi-planar character. GM need to stabilize the hip in the frontal (resisting gravity's hip adduction torque) and transverse plane (preventing internal rotation of the thigh) and generate movement in the sagittal plane (concentric/eccentric hip extension). Finally, these exercises also train the posterior oblique system in that force is transmitted forces from the ground through the leg and hip, across the SIJ via the thoracodorsal fascia, into the opposite latissimus dorsi.
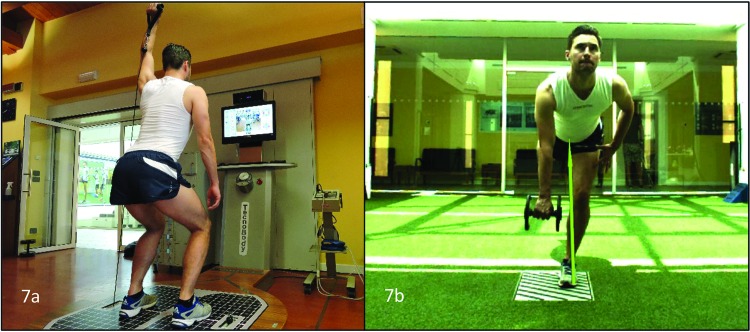
Figure 7.
Example of specific exercises. Double leg squat with one arm overhead resistance (7a) single leg Romanian deadlift with weight in opposite had to the stance limb (7b). The exercise challenges the rotation and load transfer requirements of the task and also challenges gluteus maximus as part of the posterior oblique system.
Non-weight bearing exercises
NWB exercises involve performing specific exercises on the ground. They have both strengths and weaknesses. The weaknesses include a lack of specificity and transference to functional exercises/movement,70 as well as being time in-efficient (a body of time devoted to each specific muscle group, as opposed to targeting multiple muscle groups at once). The strengths of these exercises are that you can often target a specific muscle group in isolation, which can be highly effective in the presence of specific muscle weakness or inhibition, or in load compromised athletes, during the early periods of rehabilitation after injury.
These NWB exercises may also serve as an effective pre-activation stimulus, supporting elevated force output of the muscle in functional and/sport-specific tasks,77 thus optimizing coordination and work/load distribution in subsequent exercises.77
NWB exercises should overload the muscle group using a specific action. As a mobilizer GM functions as a hip extensor (posterior and superior fibers), an external rotator of the femur and a secondary hip abductor. It is thought that maximizing the number of roles required for the muscle may support elevated activation (e.g., exercises using external rotation, abduction and hip extension).72 Additionally, it is important to note that even during NWB isolated exercises, other muscle groups than GM will contribute (e.g., hamstrings and adductor magnus as hip extensors or biceps femoris during external rotation). These muscles could potentially compensate (e.g., synergistic dominance). Particular techniques which can minimize the activation of other agonists, antagonists and/or synergists may maximize GM activation during these NWB exercises. For example, the single leg bridge is a commonly utilized NWB exercise for GM and can be performed with differing knee angles. Performing the bridge with a straighter knee angle will result in greater hamstring: GM activation ratios.47 In this situation, the hamstring will perform the most work and fatigue prior to GM, therefore, resulting in limited GM work and stimulus for adaptation. Altering the knee angle and using a short lever bridge (e.g., < 60%) has been shown to reduce activation of the hamstrings (due to active insufficiency), thereby reducing their ability to contribute to hip extension torques, resulting in GM becoming the primary activated agonist.47
Isolated exercises have minimal degrees of freedom, skill or motor learning. As such, their aim is to maximize muscle activation and endurance capacity of the muscle. The common exercises and images can be seen in Figure 8. Of note, one specific exercise with emerging research as well as excellent use anecdotally in clinical practice is the barbell hip thrust. Contreras et al.66 reported peak EMG values of 172 and 216% maximum for superior and inferior GM respectively during the 10RM hip thrust which was significantly greater than that recorded during the back squat (85 vs 130% peak EMG, respectively). Thus, this has been an exercise of choice in those with GM dysfunction.
Figure 8.
Non-weight bearing exercises for the gluteus maximus muscle including clam (8a), side leg raise in hip extension (8b), single leg bridge (8c), bilateral glute bridge (8d), side plank with abduction (8e), bird dog exercise (8f).
3. Develop explosive neuromuscular performance and optimize sport-specific motor control
Improved biomechanics in sports-related tasks does not necessarily optimise biomechanics during actual sporting performance.78 Additionally, although optimizing biomechanics during sporting tasks is important to potentially reduce injury risk,79,80 athletic success is not just about quality of movement, but also dependent upon the ability to produce high levels of force and power during sporting tasks. The biomechanical characteristics of sporting movement are quite different from that of NWB and WB strength tasks discussed. Sporting movements such as changing direction, sprint running and jumping, as well as the stabilisation of the lower limb and pelvis in these high load actions, requires differ functional demands. Differences include that sporting type movements often involve high velocity (sprint running), high loads (such as landing from a jump at 1.5 times body weight)81 fast stretch-shortening cycle actions and minimal ground contact times (50-200 ms).82 As such, components of neuromuscular function including power, rate force development, optimal muscle pre-activation, optimal coordination at high movement speeds, and in unconscious sport-specific situations are important factors for optimal ‘sport specific’ neuromuscular function.
Ballistic exercise and plyometric type exercises as well as agility type drills are important and typically mimic the velocity and/or time characteristics of sporting movement. Additionally, these exercises can produce high levels of eccentric RFD which may support the optimization of control in sporting tasks.81 There is limited research on activation of GM during certain sporting movements, however, of the available research, it is clear that the activations can be high, and substantially higher than other tasks, with the added benefit of task-specificity. Plyometric exercises such as change of direction and land and cut maneuvers were shown to result in very high activation values ( > 100% EMG at maximum voluntary isometric contraction), with GM the most active of the lower limb muscles at between 200-300% EMG maximum (reference was EMG during a maximal prone hip extension task).83 Furthermore, sprint running is a good stimulus to train the activation of GM and this movement involves high levels of coordination at rapid speeds. Activation values of GM have been reported to range between 100-300% EMG maximum, depending on phase of running gait.26 Those with low GM recruitment during sprint running were at heightened risk of sustaining a hamstring muscle injury.26 So, optimizing GM recruitment in sprinting could support a reduction in sprint related hamstring muscle injury.
Clearly, these movements are the most complex and specific, and as such, a reduction in activation due to inhibition may facilitate compensation (altered kinematics and/or internal muscle coordination). Recognizing the high activation values and potential benefit from sporting type movements is important and often under-appreciated.
SUMMARY AND IMPLEMENTATION CONSIDERATIONS
Above the roles of GM, its contribution to injury, and factors which are thought to inhibit it have been discussed. Strategies to assess GM function, including assessment of strength and activation, movement quality and lumbopelvic alignment, mobility and stability were then presented. Finally, treatment approaches based on utilizing a holistic approach to GM treatment were presented including releasing and lengthening the antagonists, realigning the pelvis, developing lumbopelvic stability, strengthening GM and integration into basic motor patterns, as well as then optimizing its function, through restoring explosive neuromuscular and sporting performance. The strategies will depend in part of the profile of the individual, thus requiring the need for optimal assessment and appropriate clinical decision making on the part of the rehabilitation professional. In those patients with severe dysfunction and limited strength capacity, a greater focus on corrective exercise may be needed, before the muscle can be strengthened and trained to work optimally in functional tasks. This corrective program should begin with NWB to develop the strength capacity of the muscle and address other factors causing its inhibition. Following this corrective programme, it is essential to retrain optimal motor patterns by integrating GM into normal foundation motor tasks. Optimal and consistent performance in foundational exercise such as squats is a pre-requisite for progression to high load (e.g., use of additional weight) foundational exercise tasks.84 Thus, a movement re-integration and patterning programme is needed prior to progressing to a strength, power and sports based exercise program (e.g., optimization of explosive neuromuscular performance) (Figure 9).
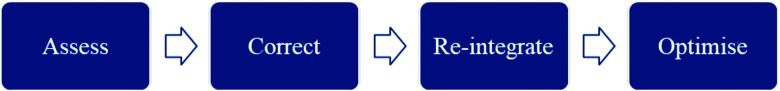
Figure 9.
Simple model of GM treatment strategy, beginning with a thorough assessment, tailored corrective programme followed by motor pattern retraining to re-integrate GM into normal function and then training to optimise GM function in sport-specific tasks.
This clinical commentary aims to enhance practice and facilitate more optimal outcomes after injury by providing clinicians with a resource to support practice in a common but often difficult problem, correction of GM dysfunction.
REFERENCES
- 1.Ireland ML Willson JD Ballantyne BT, et al. Hip strength in females with and without patellofemoral pain. J Orthop Sports Phys Ther. 2003;33:671–676. [DOI] [PubMed] [Google Scholar]
- 2.Powers CM Flynn T. Research Forum. Presented at: Combined Sections Meeting of the American Physical Therapy Association; February 2003, Tampa.
- 3.Sahrmann S. Diagnosis and treatment of movement impairment syndromes, Mosby, 2002. [DOI] [PMC free article] [PubMed]
- 4.Tyler TF Nicholas SJ Mullaney MJ McHugh MP. The role of hip muscle function in the treatment of patellofemoral pain syndrome. Am J Sports Med. 2006;34(4):630–636. [DOI] [PubMed] [Google Scholar]
- 5.Ito J Moriyama H Inokuchi S Goto N. Human lower limb muscles: an evaluation of weight and fiber size. Okajimas Folia Anat Jpn. 2003;80(2-3): 47-55. [DOI] [PubMed] [Google Scholar]
- 6.Winter DA. Biomechanics and motor control of human movement. Hoboken, NJ, John Wiley & Sons; 2005. [Google Scholar]
- 7.Gibbons SGT Mottram SL. The anatomy of the deep sacral part of the gluteus maximus and the psoas muscle: a clinical perspective. Proceedings of: The 5th Interdisciplinary World Congress on Low Back Pain. 2004;November 7-11, Melbourne, Australia.
- 8.Neumann DA. Kinesiology of the hip: a focus on muscular actions. J Orthop Sports Phys Ther. 2010;40(2):82-94. [DOI] [PubMed] [Google Scholar]
- 9.Barker PJ Hapuarachchi KS Ross JA Sambaiew E Ranger TA Briggs CA. Anatomy and biomechanics of gluteus maximus and the thoracolumbar fascia at the sacroiliac joint. Clin Anat. 2014;27(2):234-240. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins D. Hollinshead's functional anatomy of the limbs and back. (7th ed) Philadelphia; London; Boston: WB Saunders; 1998. [Google Scholar]
- 11.Snijders CJ Vleeming A Stoeckart R, et al. Biomechanics of the interface between the spine and pelvis in different postures. In: Vleeming A, Mooney V, Dorman T, et al. eds: Movement, Stability and Low Back Pain Edinburgh, Churchill Livingstone, 103-113, 1997.
- 12.Vleeming A Pool-Goudzwaad AJ Stoeckart R, et al. : The posterior layer of the thoracolumbar fascia: its function in load transfer from spine to legs. Spine. 1995;20:753-758. [PubMed] [Google Scholar]
- 13.Ireland ML. The female ACL: why is it more prone to injury? Orthop Clin North Am. 2002;33:637-651. [DOI] [PubMed] [Google Scholar]
- 14.Friel K McLean N Myers C Caceres M. Ipsilateral hip abductor weakness after inversion ankle sprain. J Athl Train. 2006;41:74-78. [PMC free article] [PubMed] [Google Scholar]
- 15.Burger H Valencic V Marincek C Kogovsek N. Properties of musculus gluteus maximus in above-knee amputees. Clin Biomech. 1996;11(1):35-38. [DOI] [PubMed] [Google Scholar]
- 16.Gibbons SGT Comerford MJ. Strength versus stability Part 1; Concepts and terms. Orthopaedic Division Review. March/April 2011;21-27.
- 17.Lewis CL Sahrmann SA Moran DW, Anterior hip joint force increases with hip extension, decreased gluteal force, or decreased iliopsoas force. J Biomech. 2007;40(16):3725-3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lafond D Normand MC Gosselin G. Rapport force, J Canadian Chiropractic Assoc. 1998;42(2):90-100. [Google Scholar]
- 19.Vakos JP Nitz AJ Threlkeld AJ, et al. Electromyographic activity of selected trunk and hip muscles during a squat lift. Spine. 1994;19(6):687-695. [DOI] [PubMed] [Google Scholar]
- 20.Franklyn-Miller A Roberts A Hulse D, et al. Biomechanical overload syndrome: defining a new diagnosis. Br J Sports Med. 2014;48:415–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khayambashi K Ghoddosi N Straub RK Powers CM. Hip muscle strength predicts non-contact anterior cruciate ligament injury in male and female athletes: a prospective study. Am J Sports Med. 2016;44(2):355-361. [DOI] [PubMed] [Google Scholar]
- 22.Lee TQ Anzel SH Bennett KA, et al. The influence of fixed rotational deformities of the femur on the patellofemoral contact pressures in human cadaver knees. Clin Orthop. 1994;302:69-74. [PubMed] [Google Scholar]
- 23.Powers CM Ward SR Fredericson M, et al. Patellofemoral kinematics during weightbearing and non-weightbearing knee extension in persons with patellar subluxation: A preliminary study. J Orthop Sports Phys Ther. 2003;33:677-685. [DOI] [PubMed] [Google Scholar]
- 24.Kankaanpää M Taimela S Laaksonen D, et al. Back and hip extensor fatigability in chronic low back pain patients and controls. Arch Phys Med Rehabil 1998;79(4):412-417. [DOI] [PubMed] [Google Scholar]
- 25.Nelson-Wong E Alex B Csepe D Lancaster D Callaghan JP. Altered muscle recruitment during extension from trunk flexion in low back pain developers. Clin Biomech. 2012;27(10):994-998. [DOI] [PubMed] [Google Scholar]
- 26.Schuermans J Danneels L Tiggelen DV et al. Proximal neuromuscular control protects against hamstring injuries in male soccer players. Am J Sports Med. 2017;45:1315-1325. [DOI] [PubMed] [Google Scholar]
- 27.Bullock-Saxton JE Janda V Bullock MI. The influence of ankle sprain injury on muscle activation during hip extension. Int J Sports Med. 1994;15:130-134. [DOI] [PubMed] [Google Scholar]
- 28.Webster KA Gribble PA. A comparison of electromyography of gluteus medius and maximus in subjects with and without chronic ankle instability during two functional exercises. Phys Ther Sport . 2013;14(1):17-22. [DOI] [PubMed] [Google Scholar]
- 29.Marzke MW Longhill JM Rasmussen SA. Gluteus maximus muscle function and the origin of hominid bipedality. Am J Phys Anthropol. 1988;77:519-528. [DOI] [PubMed] [Google Scholar]
- 30.Sahrmann S. Diagnosis and Treatment of Movement Impairment Syndromes. Oxford, UK: Elsevier Health Sciences; 2013. [Google Scholar]
- 31.Wagner T Behnia N Ancheta W-KL, et al. Strengthening and neuromuscular reeducation of the gluteus maximus in a triathlete with exercise associated cramping of the hamstrings. J Orthop Sports Phys Ther. 2010;2:112-119. [DOI] [PubMed] [Google Scholar]
- 32.Richardson C Sims K. An inner range holding contraction as an objective measure of stabilizing function of an antigravity muscle. 11th International congress of the World Confederation of Physical Therapy, London. 1991.
- 33.Liebenson C. Rehabilitation of the Spine: a Pracitioner's Manual. 2nd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 34.Mills M Frank B Goto S. Effects of restricted hip flexor muscle length on hip extensor muscle activity and lower extremity biomechanics in college-aged female soccer players. Int J Sports Phys Ther. 2015;10(7):946-954. [PMC free article] [PubMed] [Google Scholar]
- 35.Opar D Williams M Shield A. Hamstring strain injuries: Factors that lead to injury and re-injury. Sport Med. 2012;1;42(3):209-226. [DOI] [PubMed] [Google Scholar]
- 36.Freeman MAR Dean MRE Hanham IWF. The aetiology and prevention of functional instability of the foot. J Bone Joint Surg Br. 1965;47:578-685. [PubMed] [Google Scholar]
- 37.Leinonen V Kankaapää M Airaksinen O Hanninen O. Back and hip extensor activities during trunk flexion/extension: effects of low back pain and rehabilitation. Arch Phys Med Rehabil. 2000;81:32-37. [DOI] [PubMed] [Google Scholar]
- 38.Vogt L Pfeifer K Banzer W. Neuromuscular control of walking with chronic low-back pain. Man Ther. 2003;8(1):21-28. [DOI] [PubMed] [Google Scholar]
- 39.Hodges PW Tucker K. Moving differently in pain: a new theory to explain the adaptation to pain. Pain. 2011;152:S90-98. [DOI] [PubMed] [Google Scholar]
- 40.Freeman S Mascia A McGill S. Arthrogenic neuromusculature inhibition: a foundational investigation of existence in the hip joint. Clin Biomech. 2013;28(2):171-177. [DOI] [PubMed] [Google Scholar]
- 41.Meyer C Corten K Wesseling M, et al. Test-Retest Reliability of Innovated Strength Tests for Hip Muscles. PLoS One. 2013;8(11):e81149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buckthorpe M Wright S Bruce-Low S, et al. Recommendations for hamstring injury prevention in elite football: translating research into practice. Br J Sports Med. 2019;53:449-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levangie P. The Hip complex. In: Levangie P Norkin C. Joint Structure and Function: A Comprehensive Analysis, 4th ed Philadelphia, PA: F.A. Davis Company; 2005:368-370. [Google Scholar]
- 44.Ayotte NW Stetts DM Keenan G Greenway EH. Electromyographical analysis of selected lower extremity muscles during 5 unilateral weight-bearing exercises. J Orthop Sports Phys Ther. 2007;37(2): 48-55. [DOI] [PubMed] [Google Scholar]
- 45.Boren K Conrey C Le Coguic J, et al. Electromyographic analysis of gluteus medius and gluteus maximus during rehabilitation exercises. Int J Sports Phys Ther. 2011;6(3):206-223. [PMC free article] [PubMed] [Google Scholar]
- 46.Distefano LJ Blackburn JT Marshall SW Padua DA, Gluteal muscle activation during common therapeutic exercises. J Orthop Sports Phys Ther. 2009;39(7):532-540. [DOI] [PubMed] [Google Scholar]
- 47.Lehecka BJ Edwards M Haverkamp R, et al. Building a better gluteal bridge: electromyographic analysis of hip muscle activity during modified single-leg bridges. Int J Sports Phys Ther. 2017;12(4):543-549. [PMC free article] [PubMed] [Google Scholar]
- 48.De Luca CJ. The use of surface electromyography in biomechanics. J Appl Biomech. 1997;13:135-163. [Google Scholar]
- 49.Cook G Burton L Hoogenboom BJ Voight M. Functional movement screening: The use of fundamental movements as an assessment of function - Part 1. Int J Sports Phys Ther. 2014;9(3):396-409. [PMC free article] [PubMed] [Google Scholar]
- 50.Monteiro ER Vigotsky AD Novaes JDS Škarabot J. Acute effects of different anterior thigh self-massage on hip range-of-motion in trained men. Int J Sports Phys Ther. 2018;13(1):104-113. [PMC free article] [PubMed] [Google Scholar]
- 51.Comerford MJ Mottram SL. Movement and stability dysfunction--contemporary developments. Man Ther. 2001;6(1):15-26. [DOI] [PubMed] [Google Scholar]
- 52.Fry A. The Role of Resistance Exercise Intensity on Muscle Fibre Adaptations. Sports Med. 2004;34:663–679. [DOI] [PubMed] [Google Scholar]
- 53.Anderson L Magnusson S Nielsen M, et al. Neuromuscular Activation in Conventional Therapeutic Exercises and Heavy Resistance Exercises: Implications for Rehabilitation. Phys Ther. 2006;86:683–697. [PubMed] [Google Scholar]
- 54.Anderson T Kearney JT. Effects of three resistance training programs on muscular strength and absolute and relative endurance. Res Q Exerc Sport. 1982;53:1–7. [DOI] [PubMed] [Google Scholar]
- 55.Campos GE Luecke TJ Wendeln HK, et al. Muscular adaptations in response to three different resistance-training regimens: specificity of repetition maximum training zones. Eur J Appl Physiol. 2002;88:50–60. [DOI] [PubMed] [Google Scholar]
- 56.Harber MP Fry AC Rubin MR, et al. Skeletal muscle and hormonal adaptations to circuit weight training in untrained men. Scand J Med Sci Sports. 2004;14:176–185. [DOI] [PubMed] [Google Scholar]
- 57.Visser J Mans E van den Berg-Vos RM, et al. Comparison of maximal voluntary isometric contraction and hand-held dynamometry in measuring muscle strength of patients with progressive lower motor neuron syndrome. Neuromuscul Disord. 2003;13:744–750. [DOI] [PubMed] [Google Scholar]
- 58.Henneman E Clamann HP Gillies JD Skinner RD. Rank order of motoneurons within a pool: law of combination. J Neurophysiol. 1974;37:1338-1349. [DOI] [PubMed] [Google Scholar]
- 59.Burd NA West DW Staples AW, et al. Low-load high volume resistance exercise stimulates muscle protein synthesis more than low volume resistance exercise in young men. PLoS One. 2010;5:e12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aagaard P Simonsen E Andersen JL, et al. Neural inhibition during maximal eccentric and concentric quadricep contraction: effects of resistance training. J Appl Physiol. 2000;89:2249, 2257. [DOI] [PubMed] [Google Scholar]
- 61.Andersen LL Andersen JL Kebis MK Aagaard P. Early and late rate of force development: differential adaptive responses to resistance training? Scand J Med Sci Sports. 2010;20(1):e162-e169. [DOI] [PubMed] [Google Scholar]
- 62.Mangine GT Hoffman JR Wang R, et al. Resistance training intensity and volume affect changes in rate of force development in resistance‑trained men. Eur J Appl Physiol. 2016;116:2367–2374. [DOI] [PubMed] [Google Scholar]
- 63.Tillin NA Pain MTG Folland JP. Short-term unilateral resistance training affects the agonist-antagonist but not the force-agonist activation relationship. Muscle Nerve. 2011;43:375-384. [DOI] [PubMed] [Google Scholar]
- 64.Bouillon LE Wilhelm J Eisel P Wiesner J Rachow M Hatteberg L. Electromyographic assessment of muscle activity between genders during unilateral weight-bearing tasks using adjusted distances. Int J Sports Phys Ther. 2012;7(6):595-605. [PMC free article] [PubMed] [Google Scholar]
- 65.Cambridge ED Sidorkewicz N Ikeda DM McGill SM. Progressive hip rehabilitation: the effects of resistance band placement on gluteal activation during two common exercises. Clin Biomech. 2012;27:719-724. [DOI] [PubMed] [Google Scholar]
- 66.Contreras B Vigotsky AD Schoenfeld BJ Beardsley C Cronin J. A Comparison of gluteus maximus, biceps femoris, and vastus lateralis electromyographic activity in the back squat and barbell hip thrust exercises. J Appl Biomech. 2015;31(6):452-458. [DOI] [PubMed] [Google Scholar]
- 67.Rekstrom RA Donatelli RA Carp KC. Electromyographic analysis of core trunk, hip, and thigh muscles during 9 rehabilitation exercises. J Orthop Sports Phys Ther. 2007;37(12):754-762. [DOI] [PubMed] [Google Scholar]
- 68.Bobbert MF Van Soest AJ. Effects of muscle strengthening on vertical jump height: a simulation study. Med Sci Sports Ex. 1994;26(8):1012-1020. [PubMed] [Google Scholar]
- 69.Nagano A Gerritsen KGM. Effects of neuromuscular strength training on vertical jumping – A computer simulation study. J Appl Biomech. 2001;17(2):113-128. [Google Scholar]
- 70.Herman DC Weinhold PS Guskiewicz KM Garrett WE Yu B Padua DA. The effects of strength training on the lower extremity biomechanics of female recreational athletes during a stop-jump task. Am J Sports Med. 2008;36(4):733–740. [DOI] [PubMed] [Google Scholar]
- 71.Macadam P Cronin J Contreras B. An examination of the gluteal muscle activity associated with dynamic hip abduction and hip external rotation exercise: a systematic review. Int J Sports Phys Ther. 2015;10(5):573-591. [PMC free article] [PubMed] [Google Scholar]
- 72.Reiman MP Bolgla LA Loudon JK. A literature review of studies evaluating gluteus maximus and gluteus medius activation during rehabilitation exercises. Physiother Theory Pract. 2012;28(4):257-68. [DOI] [PubMed] [Google Scholar]
- 73.Powers CM. The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther. 2010;40(2):42-51. [DOI] [PubMed] [Google Scholar]
- 74.Kopper B Ureczky D Tihanyi J. Trunk position influences joint activation pattern and physical performance during vertical jumping. Acta Physiol Hung. 2012;99(2):194–205. [DOI] [PubMed] [Google Scholar]
- 75.Teng HL Powers CM. Influence of trunk posture on lower extremity energetics during running. Med Sci Sports Exerc. 2015;47(3):625–630. [DOI] [PubMed] [Google Scholar]
- 76.Foley RCA Bulbrook BD Button DC Holmes MWR. Effects of a band loop on lower extremity muscle activity and kinematics during the barbell squat. Int J Sports Phys Ther. 2017;12(4):550-559. [PMC free article] [PubMed] [Google Scholar]
- 77.Parr M Price PDB Cleather DJ. Effect of gluteal activation warm-up on explosive exercise performance. BMJ Open Sport Exerc Med. 2017;3:e000245. 10.1136/bmjsem-2017-000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Willy RW Davis IS. The effect of a hip strengthening program on mechanics during running and during a single leg squat. J Orthop Sports Phys Ther. 2011;41(9):625–632. [DOI] [PubMed] [Google Scholar]
- 79.Paterno MV Schmitt LC Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010;38(10):1968–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paterno MV Kiefer AW Bonnette S, et al. Prospectively identified deficits in sagittal plane hip-ankle coordination in female athletes who sustain a second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Clin Biomech. 2015;30(10):1094-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cleather D Goodwin J Bull A. Hip and knee joint loading during vertical jumping and push jerking. Clin Biomech. 2013;28:98-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beneke R Taylor MJ. What gives Bolt the edge-A.V. Hill knew it already! J Biomech. 2010;43(11):2241-2243. [DOI] [PubMed] [Google Scholar]
- 83.Hanson A Padua D Blackburn T, et al. , Muscle Activation During Side-Step Cutting Maneuvers in Male and Female Soccer Athletes. J Athl Train. 2008;43:133-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Myer GD Faigenbaum AD Chu DA, et al. Integrative training for children and adolescents: techniques and practices for reducing sports-related injuries and enhancing athletic performance. Phys Sportsmed. 2011;39(1):74-84. [DOI] [PubMed] [Google Scholar]