A newly identified maize mitochondrial ribosomal protein that is highly conserved in monocots and only accumulates in kernels affects mitochondrial function and kernel development.
Abstract
Mitochondrial respiration depends on proteins encoded by the nuclear and mitochondrial genomes. Many respiratory chain-related proteins are encoded by the mitochondrial genome and undergo translation by mitochondrial ribosomes. The newly identified maize (Zea mays) defective kernel44 (dek44) mutant produces small kernels showing embryo-lethal phenotypes. We cloned Dek44 by isolating the Mutator tag that produced the mutation and identified it as encoding a putative 50S ribosomal protein L9. Subcellular fractionation by ultracentrifugation confirmed that DEK44 is a mitochondrial ribosomal protein. DEK44 is highly conserved in monocots and only accumulates in kernels. Transcriptome and reverse transcription quantitative PCR analyses revealed that loss of DEK44 function affects the expression of genes encoding respiratory chain-related proteins from the mitochondrial and nuclear genomes. Blue native-PAGE revealed significantly reduced assembly of respiratory chain complexes in dek44 mutant kernels. Transmission electron microscopy indicated that the biogenesis and morphology of mitochondria were strongly affected in dek44 mutant kernels. Furthermore, DEK44 might regulate cell growth and kernel development via cyclin/cyclin-dependent kinase-mediated activities. This study provides insight into the regulation of kernel development based on mitochondrial ribosomal protein function.
Mitochondria are the centers of cellular energy homeostasis and integrate numerous metabolic pathways (Sweetlove et al., 2007). Respiration is the core function of mitochondria for the release of free energy and ATP production. During respiration, electrons from NADPH and FADH2 are transferred to oxygen via the electron transport chain (ETC), generating ATP and oxidized NADP1 and FAD1 (Siedow and Day, 2000). The ETC is composed of five respiratory complexes. Depending on the substrate, electrons are transported from complex I (NADH dehydrogenase) and complex II (succinate dehydrogenase) through ubiquinone and complex III (cytochrome c reductase) to cytochrome c and to complex IV (cytochrome c oxidase [Cox]), which produces water, while ATP is generated by complex V (ATP synthase; Dudkina et al., 2006).
The mitochondrial genome contains genes encoding proteins involved in the respiratory chain and other biological processes that occur in mitochondria. In maize (Zea mays), the mitochondrial genome encodes 58 genes including three ribosomal RNAs, 21 transfer RNAs, and 33 proteins that include 22 proteins for respiratory chain function (18 subunits of complexes I, III, IV, and V and four subunits involved in cytochrome c biogenesis), nine ribosomal proteins, a transporter protein (MttB), and a maturase (MatR; Clifton et al., 2004). The primary mitochondrial genome-expressed pre-RNAs can be posttranscriptionally processed through RNA editing, RNA cis- and trans-splicing, RNA cleavage, and RNA maturation processes (Knoop, 2013; Barkan and Small, 2014; Hammani and Giegé, 2014).
The making of functional mitochondria requires coordination between the mitochondrial and nuclear genomes for transcription, posttranscriptional processing, translation, and posttranslational processing of thousands of genes (Kwasniak et al., 2013). Most mitochondrial ribosomal proteins are encoded by genes in the nucleus, imported into the mitochondrial matrix, and involved in mitochondrial ribosome assembly for translation (Unseld et al., 1997). A growing body of evidence shows that mutations in some nuclear genes encoding mitochondrially targeted proteins lead to specific developmental phenotypes (Skinner et al., 2001; Portereiko et al., 2006; Van Aken et al., 2007; Zhou et al., 2011; Kwasniak et al., 2013; Pineau et al., 2013; Deng et al., 2014; Pan et al., 2014; Zhang et al., 2015b). Some of these mutations occur in the genes encoding mitochondrial ribosomal proteins.
The ribosome filter hypothesis postulates that ribosomes are not simply translation machines but also function as regulatory elements that differentially affect or filter the translation of particular mRNAs (Mauro and Edelman, 2007). Recent data supporting ribosomal filtering come from plant mitochondria. It has been shown that the translation of mitochondrial transcripts can be differentially affected by alterations in mitochondrial ribosomes (Janska and Kwasniak, 2014). Besides mitochondrial protein synthesis and energy production, several mitochondrial ribosomal proteins were also implicated in cellular processes like cell cycle regulation (Li et al., 2016). Information on the function of mitochondrial ribosomes comes largely from the study of newly identified mutants with specific developmental phenotypes.
Maize is an excellent system for genetics research, partly because of its numerous, easily observable phenotypes (Neuffer and Sheridan, 1980). Defective kernel (dek) mutants are a major class of maize kernel mutants that are used to investigate seed development. More than 20 dek mutants have been characterized, and most of them are caused by mutations in pentatricopeptide repeat (PPR) proteins (Liu et al., 2013; Sun et al., 2015; Xiu et al., 2016; Cai et al., 2017; Chen et al., 2017; Qi et al., 2017a, 2017b; Dai et al., 2018). The PPR family members act specifically in mitochondria or plastids for RNA editing, cleavage, splicing, and stability as well as for translational initiation and regulation (Fujii and Small, 2011; Barkan and Small, 2014). Several non-PPR dek mutants were also reported. Dek1 encodes a calpain family protein affecting embryo and endosperm aleurone layer development (Becraft et al., 2002; Lid et al., 2002). Dek* encodes the ribosome biogenesis factor Rea1, and dek*, as a weak mutant allele, partly represses the maturation and export of the 60S ribosomal subunit, inducing pleiotropic cellular responses (Qi et al., 2016). Dek38 encodes a Tel2-interacting protein2 molecular cochaperone that regulates DNA damage response, playing an important role in male reproductive cell development in maize (Garcia et al., 2017). The dek mutants offer opportunities to investigate many basic biological processes during kernel development.
In this study, we characterized dek44, which has small kernels and delayed development. We report on the cloning of Dek44 and demonstrate that it encodes a mitochondrial ribosomal protein in maize. DEK44 only accumulates in the kernel. Loss of function of Dek44 affects the expression of mitochondrial respiration chain subunit genes, reduces the function of complexes in the ETC, and further inhibits cell proliferation. Consequently, loss of Dek44 arrests mitochondrial oxidative phosphorylation and embryo and endosperm development.
RESULTS
The dek44 Mutant Produces Small Kernels with Delayed Development
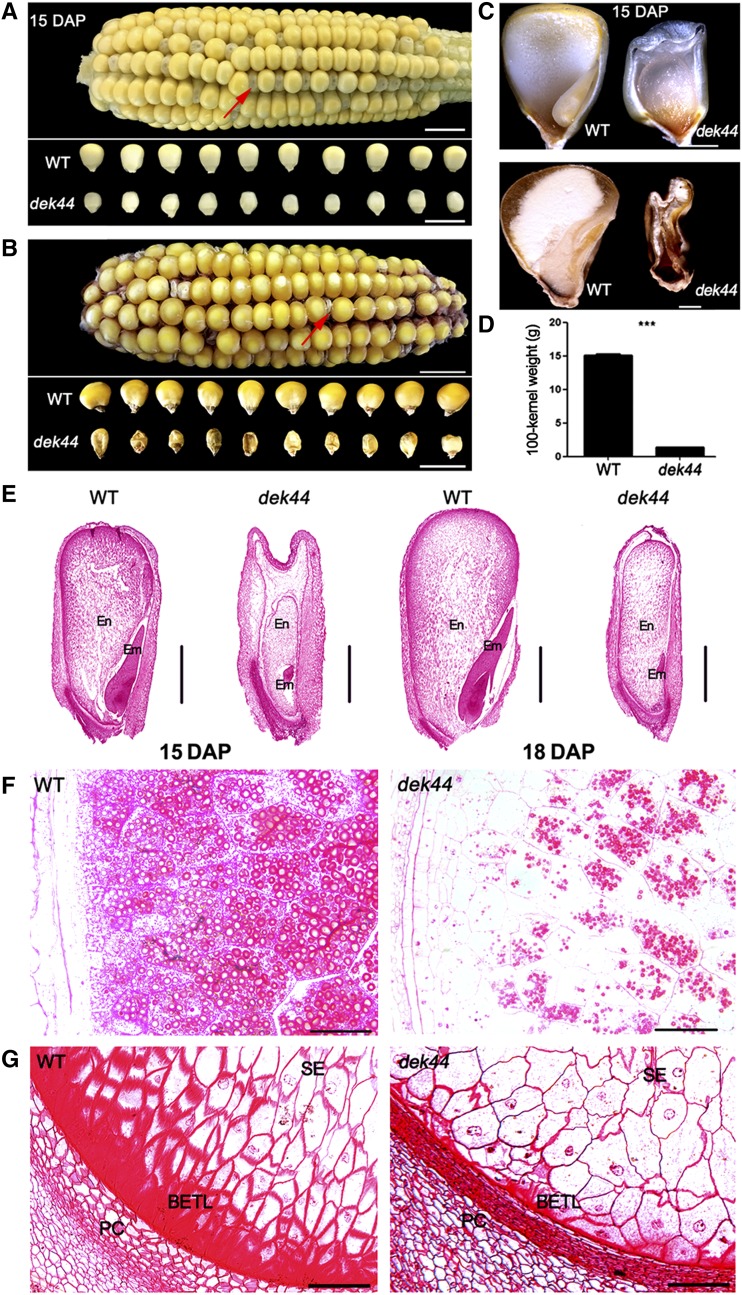
The maize dek44-Mu1 mutant is a dek mutant isolated from F2 populations containing active MuDR (see “Materials and Methods”). A plant from an F2 population that produced ears that displayed a 1:3 segregation of the dek (dek44/dek44) and wild-type (+/+ and +/dek44) kernel phenotypes was named the dek44 mutant. The dek44 mutant kernels were easily distinguished as smaller and emptier than the wild-type kernels during seed development (Fig. 1, A and C). At maturity, mutant kernels were much smaller and shrunken (Fig. 1, B and C). The 100-kernel weight of mutant kernels was less than 15% that of the wild-type kernels (Fig. 1D), and the mutant kernels were lethal and could not germinate.
Figure 1.
Phenotypic features of the maize dek44 mutant. A, F2 ear of dek44 × B73 15 d after pollination (DAP) and randomly selected dek44 and wild-type (WT) kernels in a segregating F2 population 15 DAP. The red arrow identifies a dek44 kernel. Bars = 5 mm. B, Mature F2 ear of dek44 × B73 population. The red arrow identifies a dek44 kernel. Bars = 10 mm. C, Longitudinal sections of dek44 and wild-type mature kernels at 15 DAP. Bars = 500 μm. D, Comparison of 100-grain weight of randomly selected mature dek44 and wild-type kernels in a segregating F2 population. Values are means with se, n = 3 individuals (***, P < 0.001, Student’s t test). E, Paraffin sections of 15- and 18-DAP dek44 and wild-type kernels. Em, Embryo; En, endosperm. Bars = 500 μm. F and G, Microstructure of developing starch endosperm (SE) and BETL of dek44 and wild-type kernels (15 DAP). PC, Placento-chalazal region. Bars = 100 μm.
To investigate the potential biochemical reason for the phenotype of the dek44 mutant, we measured the major components in the mature endosperm of dek44 and wild-type kernels. The total protein content and the nonzein protein content per weight of mature endosperm were higher in mutant seeds than in wild-type seeds (Supplemental Fig. S1, A and B). We also analyzed the total starch content and found that the amount of total starch was 24% lower per weight in dek44 mutant endosperm (Supplemental Fig. S1C).
We analyzed wild-type and dek44 kernels 15 and 18 DAP by light microscopy to compare their development. Longitudinal sections of the whole kernel indicated a severe developmental delay in dek44 mutant kernels compared with wild-type kernels (Fig. 1E). Embryo development was notably affected in the homozygous dek44/dek44 kernels, in which there was barely any differentiation of the plantule and radicle, so the homozygous mutant kernels contained dead embryos. For the endosperm, the starch endosperm cells were significantly less filled (Fig. 1F) and the basal endosperm transfer layer (BETL) was dramatically arrested in dek44 kernels (Fig. 1G).
Cloning of Dek44
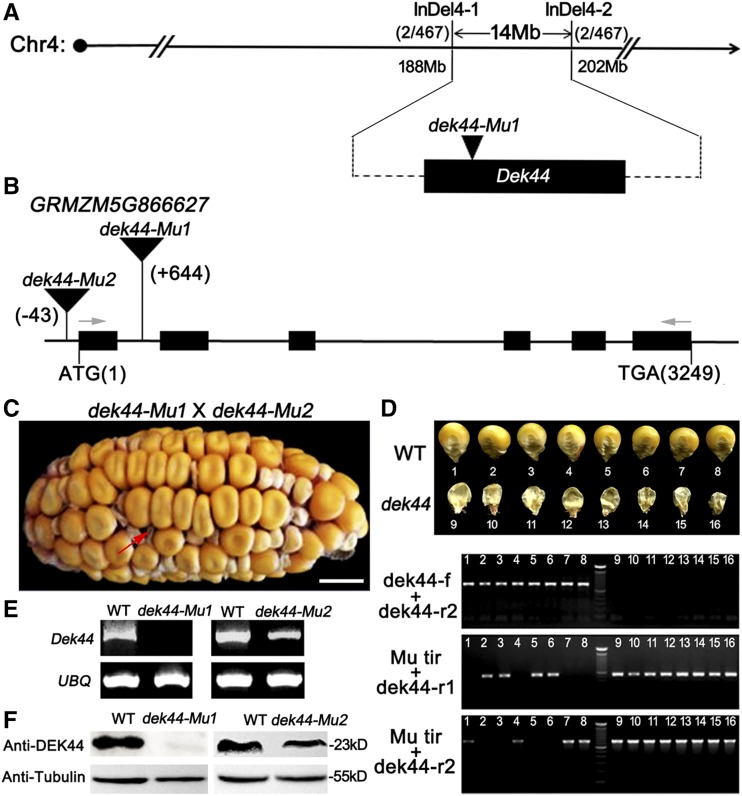
Genetic mapping of the Dek44 gene was carried out with an F2 mapping population. After we characterized 467 F2 individuals, Dek44 was mapped between the newly developed molecular markers InDel4-1 and InDel4-2, a region encompassing a physical distance of 14 Mb on chromosome 4 (Fig. 2A). Considering that the dek44 mutant was identified from an active Mutator (Mu) line, Mu tags were isolated and sequenced (Williams-Carrier et al., 2010). An insertion of a Mu element in gene GRMZM5G866627 was identified within our mapping interval. The Mu insertion is located in the first intron 644 bp downstream of the start codon. Thus, GRMZM5G866627 was considered the candidate gene.
Figure 2.
Cloning and identification of Dek44. A, The Dek44 locus was mapped to a 14-Mb region between molecular markers InDel4-1 (188 Mb) and InDel4-2 (202 Mb) on chromosome 4. See Supplemental Table S1 for primer information. B, Structure and mutation sites of the Dek44 gene. The lines represent introns, the black boxes represent exons, the black triangles represent Mu insertions, and the gray arrows identify the primers used for the mature Dek44 transcript amplification. C, Heterozygous dek44-Mu1 and dek44-Mu2 were used in an allelism test because homozygous dek44 mutants were lethal. Bar = 10 mm. D, Genotyping of allelism test kernels. Numbers 1 to 8, Kernels with wild-type (WT) phenotypes; numbers 9 to 16, kernels with mutant phenotypes. See Supplemental Figure S2 and Supplemental Table S1 for primer information. E, RT-PCR analysis of the full-length Dek44 transcript in dek44 and wild-type kernels. Ubiquitin (UBQ) served as an internal control. F, Immunoblot analysis with antibody against DEK44 in dek44 and wild-type kernels. Anti-tubulin was used as a sample loading control.
To confirm if the mutation in GRMZM5G866627 is the cause for the dek44 mutant phenotype, an allelism test was carried out with additional mutant alleles. Another allelic mutant (dek44-Mu2) was also recovered in our Mu lines, carrying a Mu insertion 43 bp upstream of the start codon in the 5′ untranslated region of GRMZM5G866627 (Fig. 2B; Supplemental Fig. S2). The dek44-Mu2 kernels also showed developmental delays during seed growth (Supplemental Fig. S3A), and the mutant kernels were smaller, shrunken, and lethal at maturity (Supplemental Fig. S3, B–D). The 100-kernel weight of mutant kernels was 23% that of the wild-type kernels (Supplemental Fig. S3E). The dek phenotype of the dek44-Mu2 mutant was slightly weaker than that of the dek44-Mu1 mutant. Allelism tests between the dek44-Mu1 and dek44-Mu2 mutants revealed a 1:3 segregation of dek and wild-type kernel phenotypes (Fig. 2, C and D). These results indicated that the dek44-Mu2 mutant cannot complement dek44-Mu1. Therefore, GRMZM5G866627 is indeed the Dek44 gene.
Sequence analysis of Dek44 revealed that it contains a 612-bp open reading frame (ORF) that encodes a protein with 203 amino acids. We examined the Dek44 transcript in immature seeds of dek44-Mu1, dek44-Mu2, and wild-type plants at 15 DAP by reverse transcription semiquantitative PCR (RT-PCR) analysis. Ubiquitin was used as an internal control. The mature Dek44 transcript (full-length ORF; see Fig. 2B for primer information) could not be detected in the dek44-Mu1 kernels, while some mature transcripts were detected in dek44-Mu2 kernels but their abundance was lower than that in the wild type (Fig. 2E). Using the antibody against DEK44 (Supplemental Fig. S4), we did not detect DEK44 in dek44-Mu1 kernels by immunoblot analysis, while DEK44 was detected in dek44-Mu2 kernels, but its abundance was also lower than that in the wild type (Fig. 2F). These results demonstrated that the Mu insertion at the first intron in the dek44-Mu1 mutant totally disrupts the expression of Dek44 and the Mu insertion at the 5′ untranslated region in the dek44-Mu2 mutant only down-regulates the expression of Dek44.
DEK44 Is Highly Conserved in Monocots and Only Accumulates in Kernels
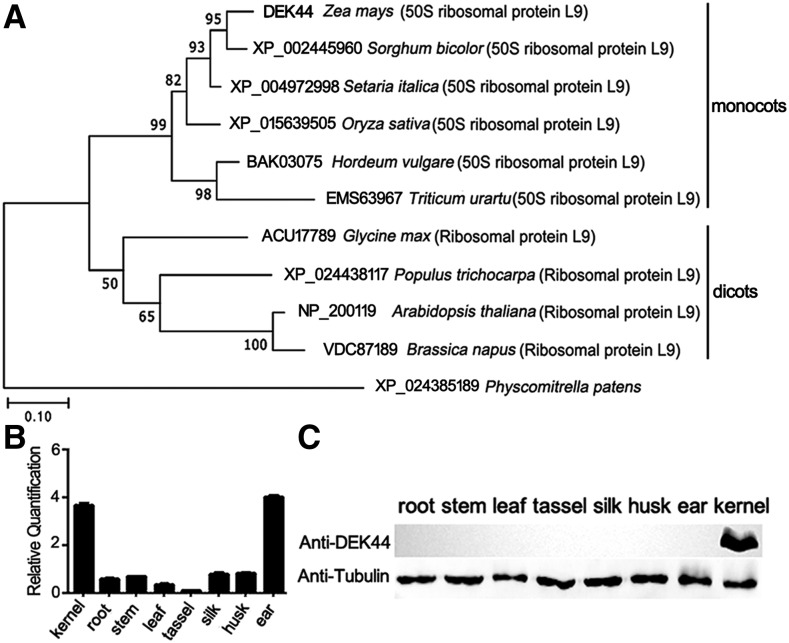
We constructed a phylogenetic tree on the basis of the full-length protein sequence of maize DEK44 and potential homologous protein sequences from other organisms. National Center for Biotechnology Information BLAST searches using the full-length DEK44 sequence revealed that DEK44 is highly conserved in monocots as a 50S ribosomal protein L9 (Fig. 3A; Supplemental Fig. S5). The sequence identities of DEK44 with its homologs in sorghum (Sorghum bicolor) and rice (Oryza sativa) were 93.2% and 83.6%, respectively, while the identity of DEK44 with its potential homolog in Arabidopsis (Arabidopsis thaliana) was only 55.4%. Furthermore, when we did BLAST of the potential homologs of DEK44 in dicots toward maize proteins, the best hit was not DEK44 (GRMZM5G866627 as 50S ribosomal protein L9) but another protein (GRMZM2G466947 as ribosomal protein L9).
Figure 3.
Phylogenetic and expression pattern analysis of DEK44. A, Phylogenetic relationships of DEK44 and its homologs. Numbers at the nodes represent percentages of 1,000 bootstraps. Bar = 0.1 substitutions per amino acid position. B, RNA expression level of Dek44 in various tissues. Ubiquitin was used as an internal control. Values are means with se, n = 6 individuals. C, Immunoblot analysis of DEK44 in various tissues. Anti-tubulin was used as a sample loading control.
Reverse transcription quantitative PCR (RT-qPCR) analysis revealed that Dek44 was expressed in a broad range of maize tissues, including silk, tassel, ear, root, husk, stem, leaf, and kernel, but it was highly expressed in only kernel and ear tissues (Fig. 3B). An immunoblot using DEK44-specific antibody revealed that the protein accumulation patterns of DEK44 were not identical to the accumulation patterns of its mRNAs, as DEK44 only accumulated in kernels and was not detected in other tissues (Fig. 3C).
DEK44 Is a Mitochondrial Ribosomal Protein
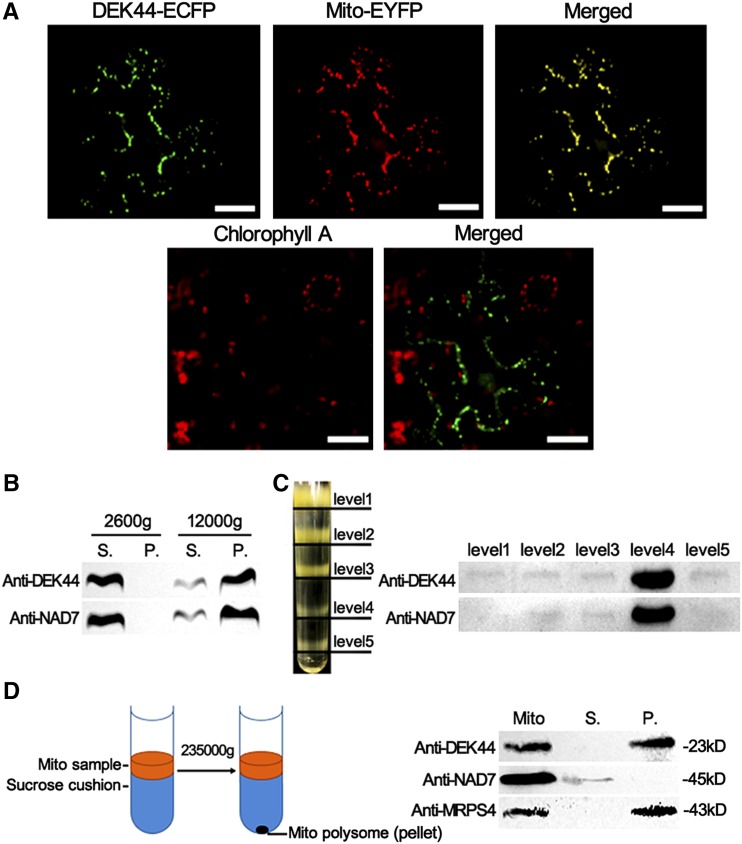
The 50S ribosomal proteins exist only in mitochondria or plastids in vascular plants. To determine the subcellular localization of DEK44, full-length DekK44 was fused to cyan fluorescent protein (CFP) in the binary vector pB7CWG. The fusion was transiently expressed in Nicotiana benthamiana leaf epidermal cells by particle bombarding, and the fluorescent signal was detected by confocal laser microscopy. The CFP signals colocalized with the red fluorescence of Mito-Tracker pBIN20-MT-YB used to label the mitochondria (Nelson et al., 2007; Fig. 4A). The chloroplast chlorophyll autofluorescence was also detected; however, DEK44 was not colocalized with chloroplasts (Fig. 4A).
Figure 4.
Subcellular localization analysis of DEK44. A, Subcellular localization of DEK44. The DEK44 fusion protein with CFP at the C terminus (green) and Mito-Tracker pBIN20-MT-RK (red) were transiently expressed in N. benthamiana leaf epidermal cells. Bars = 50 μm. B and C, Cellular fractionation assay detecting the mitochondrion localization of DEK44. The samples were centrifuged at 2,600g and 12,000g to pellet crude mitochondria. The pellet was resuspended and loaded on Suc density gradients (see “Materials and Methods”). Levels 1 to 5 identify the five sample layers in the Suc density gradient. Anti-NAD7 was used as a mitochondrial indicator. P, Pellet; S, supernatant. D, Isolation of mitochondrial polysome to confirm the mitochondrial ribosome localization of DEK44. Anti-NAD7 was used as a mitochondrion indicator. Anti-MRPS4 was used as a mitochondrial polysome indicator.
A cellular fractionation assay was then performed to confirm the mitochondrial distribution of DEK44 in maize kernels. The total proteins extracted from 15-DAP wild-type kernels were separated into five sample layers in a Suc density gradient after differential centrifugation (see “Materials and Methods”; Fig. 4, B and C; Qi et al., 2017a). The antibody against Nad7 was used as a mitochondrial indicator, and the fourth sample layer was detected as the mitochondrial fraction. The immunoblot with DEK44-specific antibody showed that DEK44 signal was present in the mitochondrial fraction (Fig. 4C). Thus, DEK44 is targeted to the mitochondria.
To investigate if DEK44 is a mitochondrial ribosomal protein, the mitochondrial fraction sample was further centrifuged (230,000g) through a Suc cushion to obtain a mitochondrial polysome pellet (Fig. 4D; Carroll, 2017). Supernatant and pellet fractions were subjected to immunoblot analysis with antibodies against NAD7 (a nonpolysome mitochondrial marker) and mitochondrial ribosomal protein S4 (MRPS4; a mitochondrial polysome marker). The immunoblot with DEK44-specific antibody showed that DEK44 signal was mainly present in the mitochondrial polysome fraction (Fig. 4D). Thus, DEK44 can target to the mitochondrial 50S ribosome as mitochondrial ribosomal protein L9 (MRPL9).
dek44 Affects Mitochondrial Function
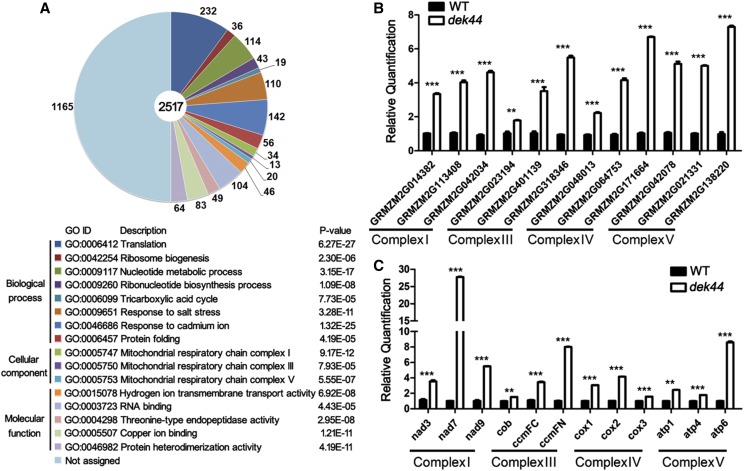
Mitochondrial ribosomal proteins are important for mitochondria-encoded protein synthesis and mitochondrial function (Li et al., 2016). The mutation in Dek44 might result in defects in mitochondrial function. We compared the transcript profiles of 15-DAP dek44 and wild-type kernels using RNA sequencing (RNA-seq). Among the 41,407 gene transcripts detected by RNA-seq, significantly differentially expressed genes (DEGs) were identified as those with a threshold fold change > 2 and q < 0.05. Based on this criterion, 3,880 genes showed significantly altered expression between dek44 and wild-type kernels. There were 3,397 (87.1%) genes with increased transcript levels, while 501 (12.9%) genes showed decreased transcript levels in dek44 compared with wild-type kernels. Within these DEGs, 2,517 genes could be functionally annotated (annotations were found using BLASTN and BLASTX analyses against the GenBank [http://www.ncbi.nlm.nih.gov/] database).
Gene Ontology (GO; http://bioinfo.cau.edu.cn/agriGO/) analysis indicated that DEGs were mostly related to 16 GO terms (Fig. 5A; Supplemental Data Set S1). Among them, three GO terms classified as cellular components were directly related to mitochondrial function: GO: 0005747 (mitochondrial respiratory chain complex I; P = 9.17E-12), GO: 0005750 (mitochondrial respiratory chain complex III; P = 7.93E-05), and GO: 0005753 (mitochondrial respiratory chain complex V; P = 5.55E-07). GO: 0006099 (tricarboxylic acid cycle; P = 7.73E-05), classified as biological process, and GO: 0015078 (hydrogen ion transmembrane transporter activity; P = 6.92E-08), classified as molecular function, were also involved in mitochondrial function.
Figure 5.
Genes with altered expression in dek44 kernels. A, The most significantly related GO terms of the 2,517 functionally annotated DEGs. The significance and number of genes classified within each GO term are shown. B, RT-qPCR analysis of nuclear genome-encoded genes associated with mitochondrial ETC function. Ubiquitin was used as an internal control. Values are means with se, n = 6 individuals (***, P < 0.001 and **, P < 0.01, Student’s t test). C, RT-qPCR analysis of mitochondrial genome-encoded genes associated with mitochondrial ETC function. Ubiquitin was used as an internal control. Values are means with se, n = 6 individuals (***, P < 0.001 and **, P < 0.01, Student’s t test). WT, Wild type.
The most enriched GO term was GO: 0006412 (translation; P = 6.27E-27), classified as biological process. Two more GO terms were protein translation related: GO: 0042254 (ribosome biogenesis; P = 2.30E-06), classified as biological process, and GO: 0003723 (RNA binding; P = 4.43E-05), classified as molecular function. One GO term (protein folding; GO: 0006457), classified as biological process, was related to protein posttranslational regulation.
Furthermore, four GO terms were related to stimulation response (GO: 0009651, response to salt stress; GO: 0046686, response to cadmium ion; GO: 0005507, copper ion binding; and GO: 0004298, Thr-type endopeptidase activity), two GO terms were related to nucleotide metabolism (GO: 0009117, nucleotide metabolic process; and GO: 0009260, ribonucleotide biosynthetic process), and one GO term was related to nucleosome assembly (GO: 0046982, protein heterodimerization activity).
Thirty-four DEGs were classified to mitochondrial respiratory chain complex I, 13 DEGs were classified to mitochondrial respiratory chain complex III, and 20 DEGs were classified to mitochondrial respiratory chain complex V. These genes are all nuclear genome-encoded ETC protein genes and were all up-regulated in the dek44 mutant kernels (Supplemental Data Set S1). To validate the differences observed in nuclear genome-encoded ETC proteins by RNA-seq, we performed RT-qPCR on three genes per complex, and the results confirmed similar differences of mRNA accumulation between the dek44 and wide-type seeds (Fig. 5B). Because DEK44 is a mitochondrial ribosome protein, the mutation in it might directly affect the translation and transcription of mitochondrial genome-encoded ETC proteins, and the up-regulation of nuclear genome-encoded ETC proteins might be a result of coordinating regulation. We further investigated the expression levels of three mitochondrial genome-encoded ETC proteins per complex, and the result indicated that these genes were also up-regulated in the dek44 mutant kernels (Fig. 5C).
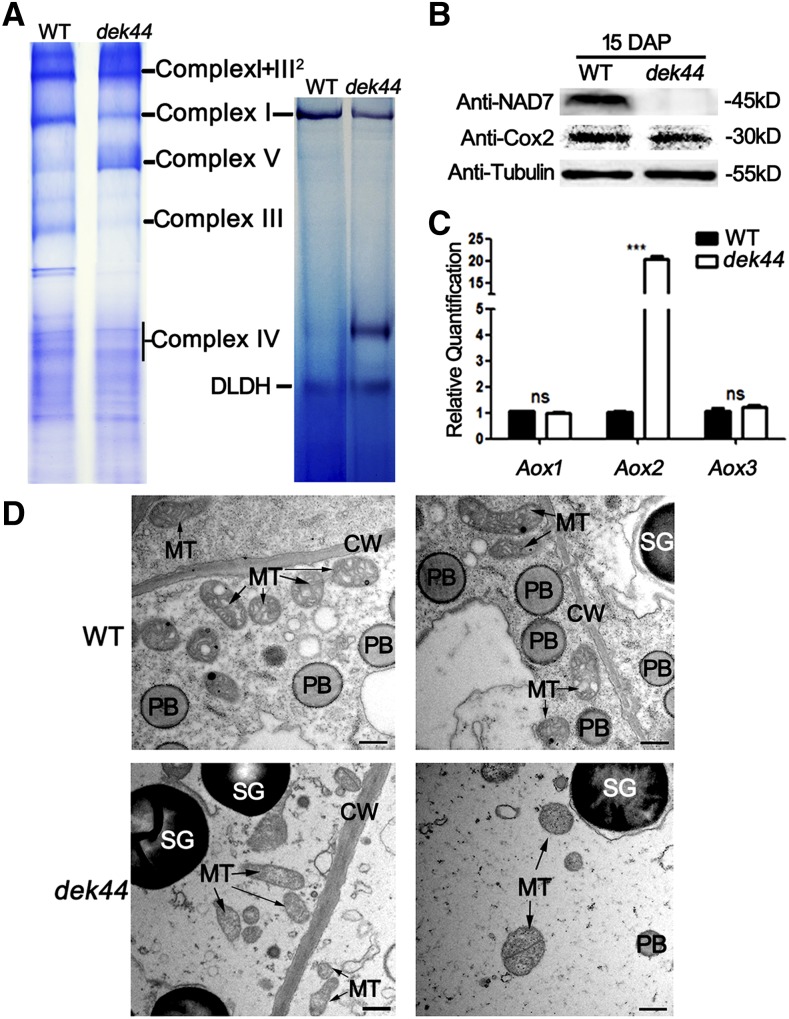
To further investigate the assembly and quantity of the respiratory complexes, mitochondrial proteins were isolated from dek44 and wild-type endosperm and analyzed by blue native-PAGE (BN-PAGE). The bands of different complexes were recognized according to Zsigmond et al. (2008). The dek44 mutant profile showed decreases of the complex I band, the complex III band, and the complex IV region but a slight increase in the complex V band compared with the wild-type profile (Fig. 6A). An in-gel NADH dehydrogenase activity test was further carried out to inspect the activity of complex I. A significant reduction of complex I activity was observed in the dek44 mutant kernel compared with the wild-type kernel (Fig. 6A). The protein levels of Nad7 (complex I) and Cox2 (complex IV) were examined by immunoblot analysis of dek44 and wild-type kernel proteins with specific antibodies. Nad7 was significantly decreased in dek44 kernels, and the protein level of Cox2 was slightly decreased in dek44 kernels (Cox2 level decreased approximately 15.7% in dek44 according to ImageJ grayscale analysis on the two bands; Supplemental Fig. S6), indicating that the accumulation of ETC components is affected in the dek44 mutant (Fig. 6B).
Figure 6.
Disrupted mitochondrial function in dek44 kernels. A, BN-PAGE of mitochondrial complexes and in-gel NADH dehydrogenase activity test of complex I activity. For BN-PAGE, the positions of supercomplex I+III2, complex I, complex III, complex IV, and complex V are indicated. For in-gel NADH dehydrogenase activity test, the activity of the dehydrolipoamide dehydrogenase (DLDH) was used as a sample loading control. B, Immunoblot analysis with antibodies against Nad7 and Cox2. Anti-tubulin was used as a sample loading control. C, RT-qPCR analysis of genes associated with the alternative respiratory pathway, including Aox1, Aox2, and Aox3. Ubiquitin was used as an internal control. Values are means with se, n = 6 individuals (***, P < 0.001 and ns, not significant, Student’s t test). D, Ultrastructure of developing endosperms of the wild type (WT) and dek44 (15 DAP) for mitochondria observation. CW, Cell wall; MT, mitochondrion; PB, protein body; SG, starch granule. Bars = 1 μm.
In plants, respiratory metabolism can also be fulfilled by alternative glycolytic, phosphorylating, and electron transport pathways. When there is an electron transport defect in the cytochrome c pathway, alternative oxidases (AOXs) are activated to maintain the tricarboxylic acid cycle and electron transport, even in the absence of oxidative phosphorylation (Vanlerberghe and Ordog, 2002). The expression of AOX genes was investigated in dek44 mutant and wild-type kernels, and Aox2 (GRMZM2G125669) was up-regulated 22-fold compared with the wild type (Fig. 6C), indicating that the alternative respiratory pathway was activated to compensate for the inefficient mitochondrial oxidative phosphorylation in the dek44 mutant.
dek44 Affects Mitochondria Morphology
The cytoplasm of 15-DAP dek44 endosperm was observed by transmission electron microscopy analysis. Normal activation of the ETC is required for the proper formation of the inner envelope cristae in mitochondria (Logan, 2006). The mitochondria in the wild-type endosperm formed distinct inner envelope cristae surrounded by a dense matrix, while the internal structure of mitochondria in the dek44 mutant lacked cristae and the mitochondria matrix was extremely light. Furthermore, the whole structure of mitochondria in the dek44 mutant was dilated and irregular (Fig. 6D). The functional reduction of the respiratory chain complexes causes abnormal morphology of mitochondria in the dek44 mutant.
dek44 Inhibits Cell Proliferation
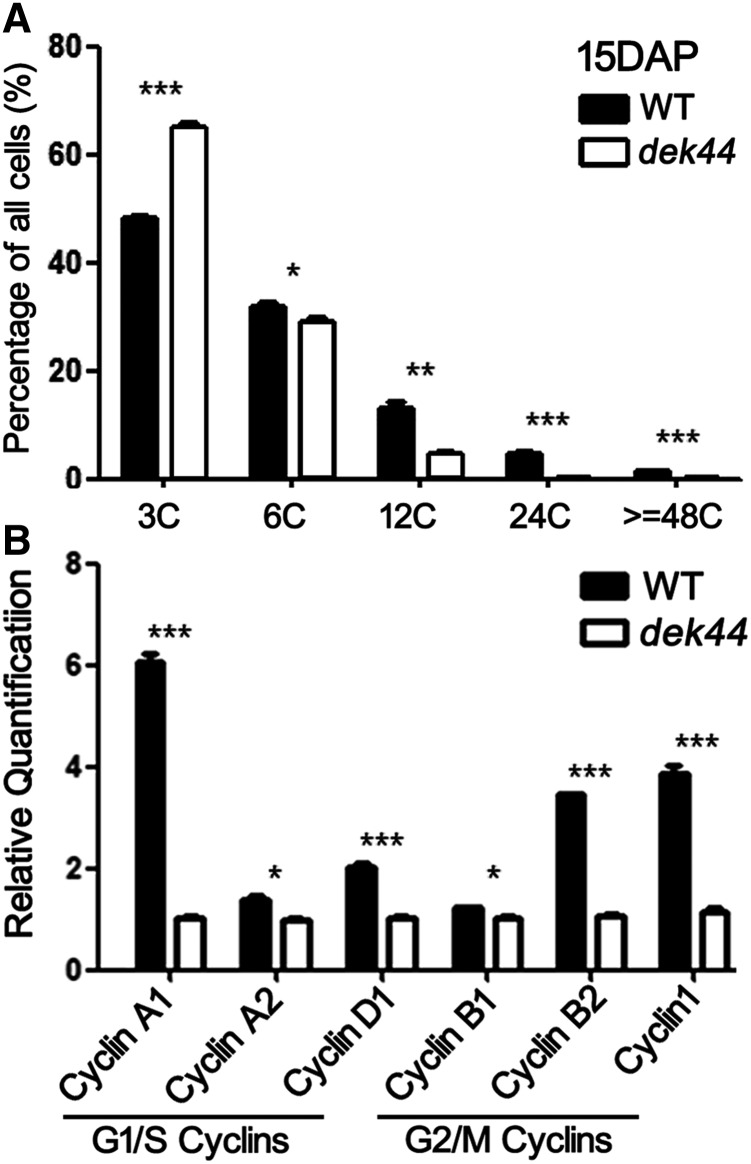
Endoreduplication is a general feature of endosperm development in maize, which involves replication of the nuclear genome without cell division, leading to elevated nucleic acid content (Sabelli and Larkins, 2009). Endoreduplication includes only the G1 and S phases, which is different from the mitotic cell cycle (G1-S-G2-M). Flow cytometry analysis of 15-DAP dek44 and wild-type endosperms showed endoreduplicated nuclei with C values of 12C or greater, accounting for 8.6% of the DNA in 15-DAP dek44 endosperm and 21.2% of the DNA in 15-DAP wild-type endosperm (Fig. 7A). This result demonstrated that the mutation of Dek44 affects cell proliferation.
Figure 7.
Inhibited cell proliferation in dek44 kernels. A, Cell proliferation analysis of 15-DAP endosperms of the wild type (WT) and dek44. 3C and 6C are DNA contents of the nuclei at G1 and S phases of 15-DAP endosperms. 12C and 24C are DNA contents of endoreduplicated nuclei at the S phase of 15-DAP endosperms. For each sample, three independent biological replicates were performed. Values are means with se, n = 3 individuals (*, P < 0.05; **, P < 0.01; and ***, P < 0.001, Student’s t test). B, RT-qPCR analysis of genes encoding cyclins. Ubiquitin was used as an internal control. Values are means with se, n = 6 individuals (*, P < 0.05 and ***, P < 0.001, Student’s t test).
Cyclins are a family of proteins that control the progression of cells through the cell cycle by activating cyclin-dependent kinase (CDK) enzymes. There are several cyclins that are active in different phases of the cell cycle and that cause the CDKs to phosphorylate different substrates. There are two main groups of cyclins: G1/S cyclins, essential for the control of the cell cycle at the G1/S transition, including Cyclin A and Cyclin D; and G2/M cyclins, essential for the control of the cell cycle at the G2/M transition, including Cyclin B and Cyclin F (Galderisi et al., 2003). The steady-state levels of transcripts of cyclin-encoding genes was markedly reduced in dek44 kernels (Fig. 7B), including Cyclin A1 (LOC103649996), Cyclin A2 (GRMZM2G060690), and Cyclin D1 (GRMZM2G140633) for the G1/S transition and Cyclin B1 (GRMZM2G025200), Cyclin B2 (GRMZM2G138886), and Cyclin 1 (Cyclin B-like; GRMZM2G034647) for the G2/M transition. This result illustrated that both the G1/S and G2/M transitions were affected in dek44. These results are consistent with the cell proliferation and endoreduplication defects found in the dek44 mutant, resulting in a developmental delay.
DISCUSSION
DEK44 Is a Newly Identified Mitochondrial Ribosomal Protein
The dek44 mutant is a severe dek mutant with abolished function of DEK44, which is a newly identified mitochondrial ribosomal protein (Figs. 1–4). Based on the phylogenetic tree constructed on the basis of the full-length protein sequence of maize DEK44 and potential homologous protein sequences from other organisms, DEK44 is highly conserved only in monocots as a mitochondrial ribosomal protein L9 (Fig. 3). Although Dek44 mRNA is expressed in a broad range of maize tissues and is highly expressed in the ear and kernels, DEK44 protein only accumulates in kernels and is not detected in other tissues (Fig. 3). The mRNA and protein expression levels of a great number of genes have been reported to differ (Qi et al., 2016). The expression patterns of Dek44 also show this feature.
Several maize dek mutants have been identified. Mutants of PPRs always have an obvious small-kernel phenotype with arrested development of the embryo, endosperm, and seedling; dek1 has severe growth and developmental defects, while dek* has only mild defects (Lid et al., 2002; Liu et al., 2013; Li et al., 2014; Sun et al., 2015; Qi et al., 2016). Most dek mutants possess mutations in housekeeping genes with essential but general functions (Lu et al., 2013; Zhan et al., 2015). These genes are simultaneously important but not specific. However, the housekeeping framework is not sufficient for understanding deks. DEK44 has a specific expression profile, as it is only accumulated in kernels (Fig. 3), and the dek44 mutant is a severe dek mutant with developmentally delayed endosperm and a lethal embryo (Fig. 1).
It is a widely held belief that posttranscriptional mechanisms dominate the regulation of mitochondrial gene expression in plants, but the experimental evidence is mostly limited to events affecting RNA quality and quantity (Binder and Brennicke, 2003). The basic process of translation involves the decoding of the mRNA-encoded information into proteins by ribosomes. Therefore, historically, ribosomes were considered to have a constitutive rather than a regulatory function. However, a number of observations indicated that ribosomes themselves could also differentially affect the translation of particular mRNAs, according to the ribosome filter hypothesis (Mauro and Edelman, 2007). Translation of mitochondrial gene transcripts encoding ETC complexes can be differentially affected by alterations in mitochondrial ribosomes (Janska and Kwasniak, 2014). The earliest evidence demonstrating the heterogeneity of ribosomes in a eukaryotic cell emerged in 1981 during studies on the social amoeba Dictyostelium discoideum (Ramagopal and Ennis, 1981). The first indication of a possible heterogeneity of plant mitochondrial ribosomes comes from a study on four paralogs of mitochondrial ribosome protein L12 in potato (Solanum tuberosum; Delage et al., 2007). This apparent ribosome heterogeneity forms the basis for the ribosome filter hypothesis. DEK44 is highly conserved only with its homologs in monocots as mitochondrial ribosomal protein L9 (Fig. 3) but has no highly conserved homologs in dicots. Therefore, in monocots, DEK44 might be a mitochondria-specific ribosomal protein that functions as a regulatory element that filters the translation of particular mRNAs, especially in maize kernels.
DEK44 Is Critical for Mitochondrial Function
Respiration, as the core process of mitochondrial metabolism, depends on the function of five ETC complexes on the substrate: complex I (NADH dehydrogenase), complex II (succinate dehydrogenase), complex III (cytochrome c reductase), complex IV (Cox), and complex V (ATP synthase; Dudkina et al., 2006). The making of a functional mitochondrial ETC requires coordination between the nuclear genome- and mitochondrial genome-encoded ETC proteins of each complex (Kwasniak et al., 2013). Most mitochondrial ETC proteins are encoded by nuclear genes, translated on cytoplasmic ribosomes, and imported into the mitochondrial matrix. Some mitochondrial ETC proteins are encoded by the mitochondrial genome and are translated on mitochondrial ribosomes (Unseld et al., 1997). Therefore, mitochondrial ribosome assembly and mitochondrial translation are involved in the proper accumulation of the mitochondrial genome-encoded ETC proteins and are further involved in their coordination with other nuclear genome-encoded ETC proteins.
The expression levels of mitochondrial genome-encoded ETC proteins were up-regulated in dek44 kernels, which might be a regulatory response to the defects in mitochondrial ribosome assembly and mitochondrial translation observed in this mutant (Fig. 5). Based on the DEGs of 15-DAP dek44 and wild-type kernels, three main GO terms were directly related to mitochondrial function: GO: 0005747 (mitochondrial respiratory chain complex I), GO: 0005750 (mitochondrial respiratory chain complex III), and GO: 0005753 (mitochondrial respiratory chain complex V). The expression levels of these nuclear genome-encoded ETC proteins were also up-regulated in dek44 kernels, which might result from their coordination with the up-regulated mitochondrial genome-encoded ETC proteins (Fig. 5).
Abolished synthesis of ETC proteins and ETC complex assembly resulted in a significant decrease of complex I, complex III, and complex IV in dek44 mutant kernels (Fig. 6), while there was a slight increase of complex V. The nuclear RPS10 gene encodes mitochondrial ribosomal protein S10. In the Arabidopsis rps10 mutant, the majority of the ETC transcripts were translated less efficiently, whereas most of the mitochondrial ribosome protein transcripts were translated with an enhanced efficiency relative to the wild type (Kwasniak et al., 2013), indicating that the translation of mitochondrial ribosome proteins is less sensitive to the loss of RPS10, just as is described by the ribosome filter hypothesis. In our study, the translation of mitochondrial ETC complex V subunits might also be less sensitive than other ETC transcripts to the loss of DEK44. Respiratory metabolism was blocked in dek44 kernels as Aox2 was dramatically up-regulated to rescue the electron flux and maintain a functional tricarboxylic acid cycle (Fig. 6). AOXs can reduce the reactive oxygen species levels in situations when complexes III and IV are unable to function properly for the maintenance of electron flux. Rapid activation of AOXs has also been reported in other PPR mutants (Sun et al., 2015; Xiu et al., 2016; Qi et al., 2017b).
Biogenesis of ETC complexes is required for the proper morphology of the cristae in mitochondria (Logan, 2006). The cristae formed by the inner membrane were first observed to be strongly reduced or completely missing in the maize ppr2263 mutant (Sosso et al., 2012). Abnormal morphology of mitochondria was also observed in the dek44 mutant (Fig. 6). The defect of biogenesis of ETC complexes affected not only respiratory metabolism of mitochondria but also their proper morphology. Sosso et al. (2012) hypothesized that the structurally altered mitochondria were likely nonfunctional or at least less functional than mitochondria with a normal ultrastructure.
The Defect in Mitochondrial Function in the dek44 Mutant Affects Cell Cycle Progression and Kernel Development
Mitochondrial function is essential for tissue development and cell cycle progression. Inactivation of cyclins/CDKs results in developmental arrest in multiple species (Lindqvist et al., 2007; Santamaría et al., 2007; Baffet et al., 2015). Proper regulation of CDKs or expression of cyclins may facilitate an adaptive metabolic response to mitochondrial dysfunction, allowing for cell survival. Recent studies have identified some components of the mitochondrial ETC as potential cyclin/CDK phosphorylation targets. Cyclin B1/Cdk1 can mediate the activation of mitochondrial complex I and enhance the generation of mitochondrial ATP via phosphorylation of its mitochondrial targets, which provides sufficient energy for cell cycle progression (Wang et al., 2014b). Furthermore, MRPL10 regulates Cyclin B1/CDK1 activity and mitochondrial protein synthesis in mammalian cells (Li et al., 2016). This study indicated that DEK44, as a component of the mitochondrial ribosome large subunit, might regulate cell growth via mediating cyclin/CDK activities (Fig. 7).
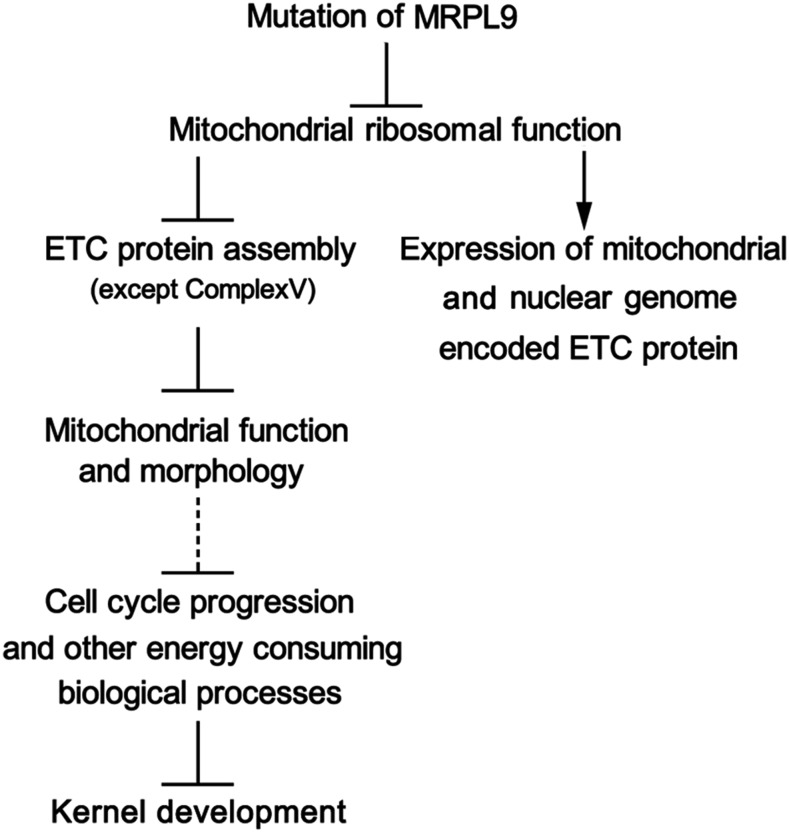
The development of the BETL was dramatically arrested in dek44 endosperm (Fig. 1). The BETL develops extensive cell wall ingrowths supporting an enlarged plasma membrane surface to promote nutrient (primarily Suc and amino acids) uptake by the endosperm (Pate and Gunning, 1972; Thompson et al., 2001). BETL cells allow rapid solute transport at the interface between maternal vascular tissue and the endosperm (Offler et al., 2003), which requires high metabolic rates. Therefore, transfer cells typically have a dense cytoplasm that is rich in small, spherical mitochondria. Embryo lethality is associated with several mitochondrial function-related mutants, highlighting the important functions of the mitochondrion in the regulation of embryo development (Lurin et al., 2004; Gutierrez et al., 2007). Dek44 is highly expressed in the embryo, and the growth of the embryo is abolished in the dek44 mutant (Fig. 1). The suppressed mitochondrial function in dek44 kernels also brings about changes in other important biological processes, including cytoplasmic translation processes, protein folding, nucleosome assembly, and some metabolic processes (Fig. 5; Supplemental Data Set S1). All these biological processes consume energy produced by mitochondria. Hence, the maize dek44 mutant has defects in kernel development due to defects in mitochondrial function and other secondary biological influences (Fig. 8).
Figure 8.
An explanation for the phenotypic consequences of DEK44 mutation.
MATERIALS AND METHODS
Plant Materials
The maize (Zea mays) MuDR stock (330I) was obtained from the Maize Genetics Cooperation Stock Center. The MuDR stock (330I) was crossed into the B73 inbred line as male parent to generate F1 population seeds and then self-crossed to generate the F2 population ears. Phenotypical screening for seed mutants was carried out on F2 ears. dek44-Mu1 and dek44-Mu2 were recovered as dek mutants from F2 ear screening, with progeny exhibiting 1:3 segregation of dek and wild-type seed phenotypes. All plants were cultivated in the field on the campus of Shanghai University. Immature seeds were harvested at 12, 15, 18, 21, 24, and 27 DAP.
Measurement of Protein, Lipids, and Starch
For the protein measurements, the endosperm of sgr1 and wild-type mature kernels was separated from the embryo and pericarp by dissection after soaking the kernels in water. The samples were dried to constant weight, pulverized with a mortar and pestle in liquid N2, and then measured according to a previously described protocol (Wang et al., 2014a). All of the measurements were replicated at least three times.
For the lipid measurements, 50 mature kernels of the wild type or sgr1 were collected from well-filled, mature ears. The kernels were pulverized with a mortar and pestle in liquid N2, and 100 mg of dried flour was used for lipid extraction and then measured according to a previously described protocol (Wang et al., 2014a). All of the measurements were replicated at least three times.
For the starch measurements, 50 mature kernels of the wild type and sgr1 were ground in liquid N2. The resulting powders were dried to a constant weight. Finally, the total starch was measured using an amyloglucosidase/α-amylase starch assay kit (Megazyme) according to the protocol cited by Wang et al. (2014a). All of the measurements were replicated at least three times.
Paraffin and Resin Sections
The 15- and 18-DAP kernels were fixed at 4°C overnight in FAA (50% [v/v] ethanol, 5% [v/v] acetic acid, and 3.7% [v/v] formaldehyde). After embedding in paraffin, 10-μm microtome sections on glass slides were dewaxed in xylene, rehydrated, and stained with fuchsin. The 15- and 18-DAP endosperm tissues were fixed at 4°C overnight in FAA. After embedding in Spurr’s epoxy resin, thin sections (1 μm) were heat fixed to glass slides and stained with fuchsin. Stained sections were rinsed in water three times and air dried. Bright-field photographs of the sections were taken using a Leica microscope.
Transmission Electron Microscopy
For transmission electron microscopy, immature kernels (15 DAP) of dek44 and the wild type were prepared as previously described (Wang et al., 2014a). The samples were observed with a Hitachi H7600 transmission electron microscope.
Mu Tag Isolation
The Mu tag isolation was performed according to Williams-Carrier et al. (2010), with some modifications. Genomic DNA was prepared for mechanical shearing at Majorbio Bio-Pharm Technology. Fragments of 200 to 500 bp were ligated to modified Illumina adapters to mark samples from different individuals. Mu-containing DNA fragments were enriched by hybridization to a biotinylated oligonucleotide corresponding to the end of the Mu terminal inverted repeat. Two successive hybrid enrichment steps were performed to ensure that the majority of the sequenced DNA fragments harbor Mu sequences. Using primers that bind to the ends of the adapters, 15 and 18 cycles of PCR were used to bulk up the recovered DNA after the first and second selection rounds, respectively. PCR products were cloned into pMD18-T (Takara). Effectiveness of the enrichment was tested by calculating the percentage of clones with the Mu fragment. DNA fragments with an enrichment rate that was above 30% were selected for further Mu Illumina sequencing at BerryGenomics.
Phylogenetic Analysis
Related sequences were identified in the National Center for Biotechnology Information nonredundant protein sequences database by performing a BLASTP search with maize DEK44 protein sequences. Amino acid sequences were aligned with the MUSCLE method in the MEGA5.2 software package using their default settings for multiple protein alignment. A rooted phylogenetic tree of DEK44 from maize, sorghum (Sorghum bicolor), rice (Oryza sativa), Setaria italica, Hordeum vulgare, Triticum urartu, Arabidopsis (Arabidopsis thaliana), Glycine max, Brassica napus, Populus trichocarpa, and Physcomitrella patens was constructed by the neighbor-joining method using the MEGA5.2 software package. The evolutionary distances were computed using Poisson correction analysis.
RNA Extraction and qPCR Analysis
Total RNA was extracted with TRIzol reagent (Tiangen), and DNA was removed by a treatment with RNase-free DNase I (Takara). Using ReverTra Ace reverse transcriptase (Toyobo), RNA was reverse transcribed to cDNA. qPCR was performed with SYBR Green Real-Time PCR Master Mix (Toyobo) using a Mastercycler ep realplex 2 (Eppendorf) according to the standard protocol of the manufacturer. Specific primers were designed (Supplemental Table S1), and the experiments were performed on two independent RNA sample sets with ubiquitin as the reference gene. From a pool of kernels collected from three individual plants, each RNA sample was extracted, for which three technical replicates were performed. A final volume of 20 mL contained 1 mL of reverse-transcribed cDNA (1–100 ng), 10 mL of 23 SYBR Green PCR buffer, and 1.8 mL of 10 mm L−1 forward and reverse primers for each sample. Relative quantifiable differences in gene expression were analyzed as described previously (Livak and Schmittgen, 2001).
Polyclonal Antibodies
For anti-DEK44 antibody production, the cDNA sequence encoding the full-length DEK44 (203 amino acids) was inserted into pGEX-4T-1 (Amersham Biosciences) at the EcoRI- and BamHI-digested sites, and GST-tagged fusion protein was purified with the AKTA purification system (GE Healthcare) using a GSTrap FF column. Protein expression and purification followed established procedures. Antibodies were produced in rabbits according to standard protocols of Shanghai ImmunoGen Biological Technology.
For the production of polyclonal antibody against NAD7, the first 21 amino acids were synthesized. Peptide synthesis, protein purification, and production of antibodies in rabbits were according to standard protocols of Shanghai ImmunoGen Biological Technology.
Immunoblot Analysis
Proteins extracted from dek44 and wild-type mature kernels were separated by SDS-PAGE. Separated protein samples were then transferred to polyvinylidene difluoride membrane (0.45 μm; Millipore). The membrane with protein sample attached on it was incubated with primary and secondary antibodies. Using the Super Signal West Pico chemiluminescent substrate kit (Pierce), the signal was visualized according to the manufacturer’s instructions. The purified anti-DEK44 antibody was used at 1:1,000, the antibody against NAD7 was used at 1:1,000, the antibody against MRPS4 (Agrisera) was used at 1:500, the antibody against Cox2 (Agrisera) was used at 1:500, and the anti-tubulin antibody (Sigma-Aldrich) was used at 1:1,000.
Subcellular Localization in Nicotiana benthamiana
The ORF sequences of Dek44 were amplified by PCR using KOD plus polymerase (Toyobo). Amplified fragments were subcloned into pENTR/D-TOPO with the Gateway TOPO cloning kit (Invitrogen) and sequenced. The right entry clone was introduced into a pB7WGY or pB7CWG plant expression vector through an LR reaction of the Gateway system (Invitrogen). The expression vectors were transformed into Agrobacterium tumefaciens strain GV3101. The agroinfiltration procedure was performed as previously described (Ahlquist, 2006).
Isolation of Mitochondria
The isolation of mitochondria was performed as described previously in de Longevialle et al. (2008). About 10 g of immature seeds at 15 DAP was harvested and ground with a mortar and pestle in liquid nitrogen, adding 20 mL of extraction buffer (100 mm Tricine, 300 mm Suc, 10 mm KCl, 1 mm MgCl2, 1 mm EDTA-K, 0.1% [w/v] BSA, and 5 mm DTT, pH 7.4) and 60 μL of plant protease inhibitor cocktail (Sigma-Aldrich). The samples were twice centrifuged at 2,600g for 15 min, after filtration through a Miracloth membrane (Calbiochem), retaining the supernatant, which was then centrifuged at 12,000g for 25 min to pellet crude mitochondria. The pellet was resuspended in wash buffer (100 mm Tricine, 300 mm Suc, 10 mm KCl, 1 mm MgCl2, 1 mm EDTA-K, and 0.1% [w/v] BSA, pH 7.4) and loaded on Suc density gradients of 1.5, 2.5, 2.5, 2, and 2 mL containing, respectively, 1.8, 1.45, 1.2, 0.9, and 0.6 m Suc diluted in wash buffer. After 90 min of centrifugation at 24,000 rpm (Beckman SW 41 Ti rotor) at 4°C, mitochondria were collected from the 1.2 m/1.45 m interface and diluted four times in wash buffer. The enriched mitochondria were collected after 20 min of centrifugation at 12,000 rpm (Beckman SW 41 Ti rotor) at 4°C.
Isolation of Mitochondrial Polysome
The mitochondria sample extracted from the mitochondrial fraction was hydrated in 2 volumes of polysome extraction buffer (200 mm Tris-HCl [pH 9], 200 mm KCl, 25 mm EGTA, 35 mm MgCl2, 1% [w/v] Brij-35, 1% [v/v] Triton X-100, 1% [v/v] Tween 20, 1% [v/v] Igepal CA-630, 1% [w/v] deoxycholic acid, 1% [v/v] polyethylene-10-tridecylether, 1 mm phenylmethylsulfonyl fluoride, 0.5 mg mL−1 heparin, 5 mm DTT, 50 mg mL−1 cycloheximide, and 50 mg mL−1 chloramphenicol; Kawaguchi et al., 2004), layered over a 1.75 m Suc cushion (400 mm Tris-HCl [pH 9], 200 mm KCl, 30 mm MgCl2, 1.75 m Suc, 5 mm DTT, 50 mg mL−1 chloramphenicol, and 50 mg mL−1 cycloheximide), and centrifuged at 230,000g at 4°C for 3 h (Fennoy and Bailey-Serres, 1995; modified from Carroll, 2017). The polysome pellet was washed with sterile water and resuspended in 700 μL of polysome extraction buffer lacking heparin and detergents.
RNA-Seq Analysis
Three dek44 or wild-type biological repeats were pooled together. Total RNA was extracted with TRIzol reagent (Tiangen), and DNA was removed by a treatment with RNase free DNase I (Takara). Using ReverTra Ace reverse transcriptase (Toyobo), nuclear RNA was reverse transcribed to cDNA using oligo(dT) primers. Library construction was performed according to Illumina standard instructions. Reads were aligned to the maize B73 genome using TopHat2 (Langmead et al., 2009). Data were normalized as fragments per kilobase of exon per million fragments mapped, for the sensitivity of RNA-seq depends on the transcript length. Significant DEGs were identified as genes with fold change in expression > 2 and q < 0.05. RNA-seq data are available from the National Center for Biotechnology Information Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo) under the series entry GSE126949.
BN-PAGE and Complex I Activity Assay
The enriched mitochondria were resuspended in 50 μL of B25G20 solution (25 mm Bis-Tris and 20% [w/v] glycerin, pH 7), adding 20% (w/v) N-Dodecyl-β-D-maltoside to the final concentration of 1% (w/v) N-Dodecyl-β-D-maltoside, and were gently mixed on ice for 1 h. After 15 min of centrifugation at 12,000 rpm (Beckman SW 41 Ti rotor) at 4°C, the supernatant was collected and was added to the loading buffer before BN-PAGE. The concentration of separation gel was from 4% to 13%. At first, electrophoresis was running at 50 V, adding 25 V every 20 min to the final 150 V until the loading dye migrated to the edge of the gel. The gel was stained by Coomassie Brilliant Blue (Zhang et al., 2015a). In-gel complex I activity assay were performed basically according to a previous report (Meyer et al., 2009).
Flow Cytometry Detection
For extraction of nuclei, endosperm and seedling tissues were finely chopped with a sharp razor blade in Beckman lysis buffer (C03551; Beckman Coulter). The resulting slurry was filtered through a 30-μm nylon filter to eliminate cell debris, and the suspension containing nuclei was immediately measured using an Accuri C6 (BD) flow cytometer equipped with an argon-ion laser tuned to a wavelength of 488 nm. For each sample, at least 15,000 nuclei were collected and analyzed using a logarithmic scale display. Each flow cytometric histogram was saved and analyzed using Accuri C6 (BD) software 1.0.264.21.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: Dek44, ACN31533, GRMZM5G866627; nad7, ABE98776. RNA-seq data are available from the National Center for Biotechnology Information Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo) under the series entry GSE126949.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Biochemical analysis of wild-type and dek44 endosperm.
Supplemental Figure S2. Primers used in the allelism test.
Supplemental Figure S3. Phenotypic features of dek44-Mu2.
Supplemental Figure S4. Full-scan image of immunoblot using the antibody against DEK44.
Supplemental Figure S5. Alignment of the full-length DEK44 protein sequence and homologous protein sequences from sorghum, rice, and Arabidopsis.
Supplemental Figure S6. Quantification of Cox2 levels by densitometry.
Supplemental Table S1. Primers used in this study.
Supplemental Data Set S1. GO classifications of DEGs with functional annotation.
Footnotes
This work was supported by the National Natural Sciences Foundation of China (31871629 to W.Q. and 91635303 and 31425019 to R.S.) and the Ministry of Science and Technology of China (2016YFD0100503 to W.Q. and 2016YFD0101003 to R.S.).
References
- Ahlquist P. (2006) Parallels among positive-strand RNA viruses, reverse-transcribing viruses and double-stranded RNA viruses. Nat Rev Microbiol 4: 371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baffet AD, Hu DJ, Vallee RB (2015) Cdk1 activates pre-mitotic nuclear envelope dynein recruitment and apical nuclear migration in neural stem cells. Dev Cell 33: 703–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A, Small I (2014) Pentatricopeptide repeat proteins in plants. Annu Rev Plant Biol 65: 415–442 [DOI] [PubMed] [Google Scholar]
- Becraft PW, Li K, Dey N, Asuncion-Crabb Y (2002) The maize dek1 gene functions in embryonic pattern formation and cell fate specification. Development 129: 5217–5225 [DOI] [PubMed] [Google Scholar]
- Binder S, Brennicke A (2003) Gene expression in plant mitochondria: Transcriptional and post-transcriptional control. Philos Trans R Soc Lond B Biol Sci 358: 181–188, discussion 188–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M, Li S, Sun F, Sun Q, Zhao H, Ren X, Zhao Y, Tan BC, Zhang Z, Qiu F (2017) Emp10 encodes a mitochondrial PPR protein that affects the cis-splicing of nad2 intron 1 and seed development in maize. Plant J 91: 132–144 [DOI] [PubMed] [Google Scholar]
- Carroll AJ. (2017) Isolation of mitochondrial ribosomes. Methods Mol Biol 1511: 267–280 [DOI] [PubMed] [Google Scholar]
- Chen X, Feng F, Qi W, Xu L, Yao D, Wang Q, Song R (2017) Dek35 encodes a PPR protein that affects cis-splicing of mitochondrial nad4 intron 1 and seed development in maize. Mol Plant 10: 427–441 [DOI] [PubMed] [Google Scholar]
- Clifton SW, Minx P, Fauron CM, Gibson M, Allen JO, Sun H, Thompson M, Barbazuk WB, Kanuganti S, Tayloe C, et al. (2004) Sequence and comparative analysis of the maize NB mitochondrial genome. Plant Physiol 136: 3486–3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D, Luan S, Chen X, Wang Q, Feng Y, Zhu C, Qi W, Song R (2018) Maize Dek37 encodes a P-type PPR protein that affects cis-splicing of mitochondrial nad2 intron 1 and seed development. Genetics 208: 1069–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delage L, Giegé P, Sakamoto M, Maréchal-Drouard L (2007) Four paralogues of RPL12 are differentially associated to ribosome in plant mitochondria. Biochimie 89: 658–668 [DOI] [PubMed] [Google Scholar]
- de Longevialle AF, Hendrickson L, Taylor NL, Delannoy E, Lurin C, Badger M, Millar AH, Small I (2008) The pentatricopeptide repeat gene OTP51 with two LAGLIDADG motifs is required for the cis-splicing of plastid ycf3 intron 2 in Arabidopsis thaliana. Plant J 56: 157–168 [DOI] [PubMed] [Google Scholar]
- Deng Y, Zou W, Li G, Zhao J (2014) TRANSLOCASE OF THE INNER MEMBRANE9 and 10 are essential for maintaining mitochondrial function during early embryo cell and endosperm free nucleus divisions in Arabidopsis. Plant Physiol 166: 853–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudkina NV, Heinemeyer J, Sunderhaus S, Boekema EJ, Braun HP (2006) Respiratory chain supercomplexes in the plant mitochondrial membrane. Trends Plant Sci 11: 232–240 [DOI] [PubMed] [Google Scholar]
- Fennoy SL, Bailey-Serres J (1995) Post-transcriptional regulation of gene expression in oxygen-deprived roots of maize. Plant J 7: 287–295 [DOI] [PubMed] [Google Scholar]
- Fujii S, Small I (2011) The evolution of RNA editing and pentatricopeptide repeat genes. New Phytol 191: 37–47 [DOI] [PubMed] [Google Scholar]
- Galderisi U, Jori FP, Giordano A (2003) Cell cycle regulation and neural differentiation. Oncogene 22: 5208–5219 [DOI] [PubMed] [Google Scholar]
- Garcia N, Li Y, Dooner HK, Messing J (2017) Maize defective kernel mutant generated by insertion of a Ds element in a gene encoding a highly conserved TTI2 cochaperone. Proc Natl Acad Sci USA 114: 5165–5170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez L, Van Wuytswinkel O, Castelain M, Bellini C (2007) Combined networks regulating seed maturation. Trends Plant Sci 12: 294–300 [DOI] [PubMed] [Google Scholar]
- Hammani K, Giegé P (2014) RNA metabolism in plant mitochondria. Trends Plant Sci 19: 380–389 [DOI] [PubMed] [Google Scholar]
- Janska H, Kwasniak M (2014) Mitoribosomal regulation of OXPHOS biogenesis in plants. Front Plant Sci 5: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi R, Girke T, Bray EA, Bailey-Serres J (2004) Differential mRNA translation contributes to gene regulation under non-stress and dehydration stress conditions in Arabidopsis thaliana. Plant J 38: 823–839 [DOI] [PubMed] [Google Scholar]
- Knoop V. (2013) Plant mitochondrial genome peculiarities evolving in the earliest vascular plant lineages. J Syst Evol 51: 1–12 [Google Scholar]
- Kwasniak M, Majewski P, Skibior R, Adamowicz A, Czarna M, Sliwinska E, Janska H (2013) Silencing of the nuclear RPS10 gene encoding mitochondrial ribosomal protein alters translation in Arabidopsis mitochondria. Plant Cell 25: 1855–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HB, Wang RX, Jiang HB, Zhang ED, Tan JQ, Xu HZ, Zhou RR, Xia XB (2016) Mitochondrial Ribosomal Protein L10 associates with cyclin B1/Cdk1 activity and mitochondrial function. DNA Cell Biol 35: 680–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Zhang YF, Hou M, Sun F, Shen Y, Xiu ZH, Wang X, Chen ZL, Sun SS, Small I, et al. (2014) Small kernel 1 encodes a pentatricopeptide repeat protein required for mitochondrial nad7 transcript editing and seed development in maize (Zea mays) and rice (Oryza sativa). Plant J 79: 797–809 [DOI] [PubMed] [Google Scholar]
- Lid SE, Gruis D, Jung R, Lorentzen JA, Ananiev E, Chamberlin M, Niu X, Meeley R, Nichols S, Olsen OA (2002) The defective kernel 1 (dek1) gene required for aleurone cell development in the endosperm of maize grains encodes a membrane protein of the calpain gene superfamily. Proc Natl Acad Sci USA 99: 5460–5465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A, van Zon W, Karlsson Rosenthal C, Wolthuis RM (2007) Cyclin B1-Cdk1 activation continues after centrosome separation to control mitotic progression. PLoS Biol 5: e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Xiu ZH, Meeley R, Tan BC (2013) Empty pericarp5 encodes a pentatricopeptide repeat protein that is required for mitochondrial RNA editing and seed development in maize. Plant Cell 25: 868–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Logan DC. (2006) The mitochondrial compartment. J Exp Bot 57: 1225–1243 [DOI] [PubMed] [Google Scholar]
- Lu X, Chen D, Shu D, Zhang Z, Wang W, Klukas C, Chen LL, Fan Y, Chen M, Zhang C (2013) The differential transcription network between embryo and endosperm in the early developing maize seed. Plant Physiol 162: 440–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurin C, Andrés C, Aubourg S, Bellaoui M, Bitton F, Bruyère C, Caboche M, Debast C, Gualberto J, Hoffmann B, et al. (2004) Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 16: 2089–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro VP, Edelman GM (2007) The ribosome filter redux. Cell Cycle 6: 2246–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EH, Tomaz T, Carroll AJ, Estavillo G, Delannoy E, Tanz SK, Small ID, Pogson BJ, Millar AH (2009) Remodeled respiration in ndufs4 with low phosphorylation efficiency suppresses Arabidopsis germination and growth and alters control of metabolism at night. Plant Physiol 151: 603–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BK, Cai X, Nebenführ A (2007) A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J 51: 1126–1136 [DOI] [PubMed] [Google Scholar]
- Neuffer MG, Sheridan WF (1980) Defective kernel mutants of maize. I. Genetic and lethality studies. Genetics 95: 929–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offler CE, McCurdy DW, Patrick JW, Talbot MJ (2003) Transfer cells: Cells specialized for a special purpose. Annu Rev Plant Biol 54: 431–454 [DOI] [PubMed] [Google Scholar]
- Pan X, Chen Z, Yang X, Liu G (2014) Arabidopsis voltage-dependent anion channel 1 (AtVDAC1) is required for female development and maintenance of mitochondrial functions related to energy-transaction. PLoS ONE 9: e106941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate JS, Gunning BES (1972) Transfer cells. Annu Rev Plant Physiol 23: 173–196 [Google Scholar]
- Pineau B, Bourge M, Marion J, Mauve C, Gilard F, Maneta-Peyret L, Moreau P, Satiat-Jeunemaître B, Brown SC, De Paepe R, et al. (2013) The importance of cardiolipin synthase for mitochondrial ultrastructure, respiratory function, plant development, and stress responses in Arabidopsis. Plant Cell 25: 4195–4208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portereiko MF, Sandaklie-Nikolova L, Lloyd A, Dever CA, Otsuga D, Drews GN (2006) NUCLEAR FUSION DEFECTIVE1 encodes the Arabidopsis RPL21M protein and is required for karyogamy during female gametophyte development and fertilization. Plant Physiol 141: 957–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W, Zhu J, Wu Q, Wang Q, Li X, Yao D, Jin Y, Wang G, Wang G, Song R (2016) Maize reas1 mutant stimulates ribosome use efficiency and triggers distinct transcriptional and translational responses. Plant Physiol 170: 971–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W, Tian Z, Lu L, Chen X, Chen X, Zhang W, Song R (2017a) Editing of mitochondrial transcripts nad3 and cox2 by Dek10 is essential for mitochondrial function and maize plant development. Genetics 205: 1489–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W, Yang Y, Feng X, Zhang M, Song R (2017b) Mitochondrial function and maize kernel development requires Dek2, a pentatricopeptide repeat protein involved in nad1 mRNA splicing. Genetics 205: 239–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramagopal S, Ennis HL (1981) Regulation of synthesis of cell-specific ribosomal proteins during differentiation of Dictyostelium discoideum. Proc Natl Acad Sci USA 78: 3083–3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabelli PA, Larkins BA (2009) The development of endosperm in grasses. Plant Physiol 149: 14–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaría D, Barrière C, Cerqueira A, Hunt S, Tardy C, Newton K, Cáceres JF, Dubus P, Malumbres M, Barbacid M (2007) Cdk1 is sufficient to drive the mammalian cell cycle. Nature 448: 811–815 [DOI] [PubMed] [Google Scholar]
- Siedow JA, Day DA (2000) Respiration and photorespiration. In Buchanan BB, Gruissem W, Jones RL, eds, Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD, pp 676–728 [Google Scholar]
- Skinner DJ, Baker SC, Meister RJ, Broadhvest J, Schneitz K, Gasser CS (2001) The Arabidopsis HUELLENLOS gene, which is essential for normal ovule development, encodes a mitochondrial ribosomal protein. Plant Cell 13: 2719–2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosso D, Mbelo S, Vernoud V, Gendrot G, Dedieu A, Chambrier P, Dauzat M, Heurtevin L, Guyon V, Takenaka M, et al. (2012) PPR2263, a DYW-subgroup pentatricopeptide repeat protein, is required for mitochondrial nad5 and cob transcript editing, mitochondrion biogenesis, and maize growth. Plant Cell 24: 676–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Wang X, Bonnard G, Shen Y, Xiu Z, Li X, Gao D, Zhang Z, Tan BC (2015) Empty pericarp7 encodes a mitochondrial E-subgroup pentatricopeptide repeat protein that is required for ccmFN editing, mitochondrial function and seed development in maize. Plant J 84: 283–295 [DOI] [PubMed] [Google Scholar]
- Sweetlove LJ, Fait A, Nunes-Nesi A, Williams T, Fernie AR (2007) The mitochondrion: An integration point of cellular metabolism and signalling. Crit Rev Plant Sci 26: 17–43 [Google Scholar]
- Thompson RD, Hueros G, Becker H, Maitz M (2001) Development and functions of seed transfer cells. Plant Sci 160: 775–783 [DOI] [PubMed] [Google Scholar]
- Unseld M, Marienfeld JR, Brandt P, Brennicke A (1997) The mitochondrial genome of Arabidopsis thaliana contains 57 genes in 366,924 nucleotides. Nat Genet 15: 57–61 [DOI] [PubMed] [Google Scholar]
- Van Aken O, Pecenková T, van de Cotte B, De Rycke R, Eeckhout D, Fromm H, De Jaeger G, Witters E, Beemster GTS, Inzé D, et al. (2007) Mitochondrial type-I prohibitins of Arabidopsis thaliana are required for supporting proficient meristem development. Plant J 52: 850–864 [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC, Ordog SH (2002) Alternative oxidase: Integrating carbon metabolism and electron transport in plant respiration. In Foyer GH, Noctor G, eds, Advances in Photosynthesis and Respiration, Photosynthetic Nitrogen Assimilation and Associated Carbon and Respiratory Metabolism, Vol 12 Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 173–191 [Google Scholar]
- Wang G, Qi W, Wu Q, Yao D, Zhang J, Zhu J, Wang G, Wang G, Tang Y, Song R (2014a) Identification and characterization of maize floury4 as a novel semidominant opaque mutant that disrupts protein body assembly. Plant Physiol 165: 582–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Fan M, Candas D, Zhang TQ, Qin L, Eldridge A, Wachsmann-Hogiu S, Ahmed KM, Chromy BA, Nantajit D, et al. (2014b) Cyclin B1/Cdk1 coordinates mitochondrial respiration for cell-cycle G2/M progression. Dev Cell 29: 217–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Carrier R, Stiffler N, Belcher S, Kroeger T, Stern DB, Monde RA, Coalter R, Barkan A (2010) Use of Illumina sequencing to identify transposon insertions underlying mutant phenotypes in high-copy Mutator lines of maize. Plant J 63: 167–177 [DOI] [PubMed] [Google Scholar]
- Xiu Z, Sun F, Shen Y, Zhang X, Jiang R, Bonnard G, Zhang J, Tan BC (2016) EMPTY PERICARP16 is required for mitochondrial nad2 intron 4 cis-splicing, complex I assembly and seed development in maize. Plant J 85: 507–519 [DOI] [PubMed] [Google Scholar]
- Zhan J, Thakare D, Ma C, Lloyd A, Nixon NM, Arakaki AM, Burnett WJ, Logan KO, Wang D, Wang X, et al. (2015) RNA sequencing of laser-capture microdissected compartments of the maize kernel identifies regulatory modules associated with endosperm cell differentiation. Plant Cell 27: 513–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HD, Cui YL, Huang C, Yin QQ, Qin XM, Xu T, He XF, Zhang Y, Li ZR, Yang ZN (2015b) PPR protein PDM1/SEL1 is involved in RNA editing and splicing of plastid genes in Arabidopsis thaliana. Photosynth Res 126: 311–321 [DOI] [PubMed] [Google Scholar]
- Zhang H, Luo M, Day RC, Talbot MJ, Ivanova A, Ashton AR, Chaudhury AM, Macknight RC, Hrmova M, Koltunow AM (2015a) Developmentally regulated HEART STOPPER, a mitochondrially targeted L18 ribosomal protein gene, is required for cell division, differentiation, and seed development in Arabidopsis. J Exp Bot 66: 5867–5880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Li Q, Chen X, Liu J, Zhang Q, Liu Y, Liu K, Xu J (2011) The Arabidopsis RETARDED ROOT GROWTH gene encodes a mitochondria localized protein that is required for cell division in the root meristem. Plant Physiol 157: 1793–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsigmond L, Rigó G, Szarka A, Székely G, Otvös K, Darula Z, Medzihradszky KF, Koncz C, Koncz Z, Szabados L (2008) Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport. Plant Physiol 146: 1721–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]