Methyl salicylate glucosylation negatively modulates salicylate homeostasis and plant systemic defense responses.
Abstract
Plant systemic acquired resistance (SAR) provides an efficient broad-spectrum immune response to pathogens. SAR involves mobile signal molecules that are generated by infected tissues and transported to systemic tissues. Methyl salicylate (MeSA), a molecule that can be converted to salicylic acid (SA), is an essential signal for establishing SAR, particularly under a short period of exposure to light after pathogen infection. Thus, the control of MeSA homeostasis is important for an optimal SAR response. Here, we characterized a uridine diphosphate-glycosyltransferase, UGT71C3, in Arabidopsis (Arabidopsis thaliana), which was induced mainly in leaf tissue by pathogens including Pst DC3000/avrRpt2 (Pseudomonas syringae pv tomato strain DC3000 expressing avrRpt2). Biochemical analysis indicated that UGT71C3 exhibited strong enzymatic activity toward MeSA to form MeSA glucosides in vitro and in vivo. After primary pathogen infection by Pst DC3000/avrRpt2, ugt71c3 knockout mutants exhibited more powerful systemic resistance to secondary pathogen infection than that of wild-type plants, whereas systemic resistance in UGT71C3 overexpression lines was compromised. In agreement, after primary infection of local leaves, ugt71c3 knockout mutants accumulated significantly more systemic MeSA and SA than that in wild-type plants. whereas UGT71C3 overexpression lines accumulated less. Our results suggest that MeSA glucosylation by UGT71C3 facilitates negative regulation of the SAR response by modulating homeostasis of MeSA and SA. This study unveils further SAR regulation mechanisms and highlights the role of glucosylation of MeSA and potentially other systemic signals in negatively modulating plant systemic defense.
Plants have evolved multiple lines of inducible defense responses to protect themselves against pathogen invasion. The inherent immune system can offer a rapid response to infection by activating pathogen-associated molecular pattern-triggered immunity (PTI) upon recognition of conserved pathogen- or microbe-associated molecular patterns (Jones and Takemoto, 2004). Successful pathogens, however, secrete effectors to inhibit PTI. Correspondingly, plant-resistance proteins have evolved to recognize these specific effectors directly or indirectly and induce an immune response known as “effector-triggered immunity” (Chisholm et al., 2006; Jones and Dangl, 2006). PTI and effector-triggered immunity result in quick and robust responses to pathogen attacks (Conrath, 2006, 2011).
Systemic-acquired resistance (SAR) is an inducible defense mechanism, the activation of which is accompanied by the accumulation of salicylic acid (SA) and expression of pathogenesis-related (PR) genes (Ross, 1961; Durrant and Dong, 2004; Dempsey and Klessig, 2012; Fu and Dong, 2013). SAR can enhance resistance-protein–mediated immunity in distal (systemic) tissues of a plant in response to pathogen infection in local tissues providing continuous and smart resistance to broad-spectrum secondary infections (Ryals et al., 1996; Shah et al., 2009; Ahmad et al., 2010). Previous studies have determined that SAR can be activated by the addition of SA or its bioactive analogs 2,6-dichloroisonicotinc acid and benzothiadiazole (Mou et al., 2003). Therefore, SA was initially believed to be a long-distance signal associated with SAR (Gaffney et al., 1993). However, grafting studies using SA-deficient tobacco (Nicotiana tabacum) expressing the salicylate hydroxylase gene showed that SA is unlikely to be the mobile signal (Vernooij et al., 1994; Pallas et al., 1996). Subsequent studies implicated methyl salicylate (MeSA), a methylated derivative of SA, in phloem-based mobile signaling involved in SAR (Park et al., 2007). It was observed that once SA methyltransferase, an enzyme that converts SA to MeSA, was silenced locally in tobacco leaves, SAR was inhibited. Similarly, SAR was abolished in tobacco if SA-Binding Protein2, which mediates the hydrolysis of MeSA to SA via MeSA esterase activity, was silenced in the distal leaves (Park et al., 2007). These studies suggest that MeSA is required in long-distance communication and the regeneration of SA for SAR development in distal tissues. Several studies in non-tobacco species such as Arabidopsis (Arabidopsis thaliana) and potato (Solanum tuberosum) further suggest that MeSA plays a pivotal role during SAR (Vlot et al., 2008; Park et al., 2009; Manosalva et al., 2010; Liu et al., 2011a). A different study, however, found that transfer DNA insertion lines that are defective in the expression of the SA methyltransferase gene are completely devoid of induced MeSA production, but develop SAR upon local inoculation with P. syringae, raising questions about the validity of MeSA as a mobile signal for SAR (Attaran et al., 2009). Liu et al. (2011b) carried out further investigations and demonstrated that the length of light exposure of plants after primary infection determines the extent to which MeSA is required for SAR. Thus, the conflicting results concerning MeSA and SAR in the different studies were resolved.
In addition to MeSA, several other molecules have been proposed as candidate mobile signals that initiate SAR, including azelaic acid (Jung et al., 2009), glycerol-3-P (Chanda et al., 2011), dehydroabietinal (Chaturvedi et al., 2012), and pipecolic acid (Pip; Návarová et al., 2012). The identification of these key components of the SAR pathway provides critical insight into the mechanisms controlling long-lasting and broad-spectrum disease resistance in plants (Dempsey and Klessig, 2012; Fu and Dong, 2013). Some compounds work synergistically to activate SAR and/or regulate MeSA metabolism (Maldonado et al., 2002; Truman et al., 2007; Chaturvedi et al., 2008; Jung et al., 2009; Shah et al., 2009; Chanda et al., 2011). Recently, two groups independently reported the role of n-hydroxy-pipecolic acid (NHP or n-OH-Pip), rather than Pip, in the SAR pathway (Chen et al., 2018; Hartmann et al., 2018). These researchers provide evidence that the plant-derived metabolite NHP plays a key role in SAR signal transduction in Arabidopsis. Future investigations of the role of NHP in long-distance communication may offer insight into the relationship between NHP and other mobile signals such as MeSA.
Given that MeSA is proposed as being a critical, phloem-based mobile SAR signal, its homeostasis and concentration is likely to be controlled as part of a SA-mediated systemic immune process. As mentioned above, SA methyltransferase and MeSA esterase have been proven critical for maintaining MeSA levels and establishing SA-associated SAR (Park et al., 2007, 2009; Manosalva et al., 2010). However, it is still unclear as to whether other mechanisms controlling MeSA and SA homeostasis exist in plants. In this study, we identified and characterized a pathogen-induced uridine diphosphate (UDP)-glycosyltransferase, UGT71C3, in Arabidopsis. We found that UGT71C3 could use MeSA but not SA as the sugar acceptor in glucosylation reactions in vitro. Using knockout mutants and transgenic plants, the involvement of UGT71C3 in controlling endogenous MeSA homeostasis by glucosylation was also verified. Moreover, we demonstrated that UGT71C3 could negatively modulate the establishment of SA-associated SAR. Our data suggest that MeSA glucosylation functions in negative modulation of MeSA homeostasis and SA-associated plant systemic defense.
RESULTS
Expression of UGT71C3 Is Induced by Pst DC3000/avrRpt2
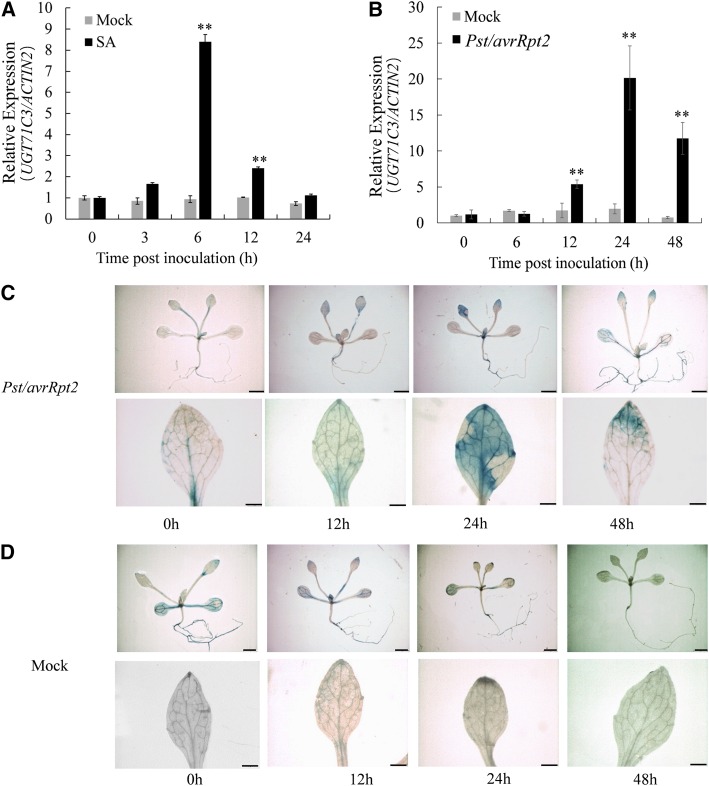
By searching the publicly available microarray data (https://genevestigator.com) for Arabidopsis UDP-glycosyltransferase (UGT) genes associated with plant secondary metabolism, we found that the expression of UGT71C3 could be induced by several pathogens. This gene inspired us to investigate whether the enzyme UGT71C3 is involved in the disease defense response. To verify if expression of UGT71C3 is induced by pathogen attack, Arabidopsis seedlings were exposed to time-course treatments with Pseudomonas syringae pv tomato strain DC3000 expressing avrRpt2 (Pst DC3000/avrRpt2) and SA. Then, reverse transcription quantitative PCR (RT-qPCR) was used to analyze the relative expression levels of UGT71C3. As shown in Figure 1, A and B, we found that UGT71C3 was significantly induced by both SA and Pst DC3000/avrRpt2. In addition, 2-week–old UGT71C3Pro::GUS transgenic seedlings were investigated for inducibility of UGT71C3 expression under pathogen infection. We found that UGT71C3 expression was clearly induced in young leaves of seedlings 24 h after infection with Pst DC3000/avrRpt2 compared to that in control plants treated with MgCl2 (Fig. 1, C and D). For 4-week–old adult UGT71C3Pro::GUS transgenic plants, the inducibility of UGT71C3 expression under pathogen infection in leaves was also clearly observed (Supplemental Fig. S1). However, UGT71C3 expression in roots appeared to be constitutive (Fig. 1; Supplemental Fig. S1). These results indicated that UGT71C3 is a pathogen-responsive gene mainly in leave tissues and likely plays a role in plant immunity.
Figure 1.
Expression of UGT71C3 in wild type Arabidopsis is induced by SA and Pst DC3000/avrRpt2. A and B, RT-qPCR analysis of UGT71C3 expression induced by SA and Pst DC3000/avrRpt2 treatments. Mock treatments are DMSO solvent (A) and 10-mm MgCl2 solution (B). The value for wild type under mock treatment for 0 h was set at “1.” UGT71C3 levels were detected and normalized to ACTIIN2. Data are means ≤ sd of three biological replicates (**P < 0.01, *P < 0.05, Student’s t test). C and D, GUS staining of UGT71C3Pro:GUS transgenic seedlings after Pst DC3000/avrRpt2 (C) and MgCl2 (Mock, D) inoculation. Bars = 1 mm (upper) and 200 μm (lower).
UGT71C3 Catalyzes MeSA Glucosylation In Vitro
To explore the physiological role of glycosyltransferases, it is important to investigate the sugar acceptor(s) for these enzymes. UGT71C3 is a member of the UDP-dependent glycosyltransferase family, most members of which use secondary metabolites as sugar acceptors and UDP-Glc as the sugar donor. Therefore, we tested a variety of natural secondary metabolites as potential substrate(s) of UGT71C3, including SA and its derivatives, phenylpropanoids, flavonoids, anthocyanins, and plant hormones (Supplemental Table S1). The recombinant glutathione-s-transferase (GST)-tagged UGT71C3 fusion protein was expressed, purified, and used in this experiment (Fig. 2A). We found that UGT71C3 can only catalyze the glucosylation of MeSA but not SA itself in vitro, producing potential MeSA glucoside (MeSAG) with the same retention time in high performance-liquid chromatography (HPLC) analysis as the authentic standard of MeSAG (Fig. 2, B, C, and E). For the structure analogs of SA and MeSA such as benzoic acid and methyl benzoate, no reaction products were observed in the assays (Supplemental Fig. S2, A and B). Only trace levels of reaction products were detectable for some other compounds tested here (Supplemental Table S1). Therefore, we hypothesized that UGT71C3 is mainly responsible for the MeSA glucosylation.
Figure 2.
The glucosylating activity of UGT71C3 toward MeSA but not SA. A, SDS-PAGE detection of the purified recombinant UGT71C3-GST fusion protein. Proteins were visualized by Coomassie blue staining. UGT71C3: UGT71C3 fusion protein. B, No reaction product from SA was found by HPLC analysis. C, Potential reaction product MeSAG from MeSA was found by HPLC analysis. UDP-Glc was used as the sugar donor. Heat-inactivated UGT71C3 and GST were used as the negative controls in these enzymatic reactions. D, Identity of reaction products from MeSA was further confirmed by LC-MS analysis under positive ion mode. E, Retention time of authentic standard MeSAG in HPLC analysis. F, Ion peaks of authentic standard MeSAG in LC-MS analysis under positive ion mode.
To further confirm the identity of MeSA reaction products, potential glucosylation products were analyzed by liquid chromatography-mass spectrometry (LC-MS). It is known that the Mr (M) of MeSAG is 314. We found that, in the positive ionization mode, MeSA reaction products showed dominant ion peaks at m/z 153 (M + H+ − Glc), 315 (M + H+ ), 337 (M + Na+), and 353 (M + K+ ), which correspond well to the authentic standard of MeSAG (Fig. 2, D and F). We therefore conclude that UGT71C3 catalyzes MeSA glucosylation.
UGT71C3 Exhibits MeSAG Activity In Vivo
Usually, glycosyltransferase genes can be induced transcriptionally by its substrates (Dean and Delaney, 2008; Husar et al., 2011; Huang et al., 2018). To determine the inducibility of UGT71C3 by MeSA, wild-type Arabidopsis seedlings were exposed to time-course treatments with MeSA and RT-qPCR was used to analyze the relative expression levels of UGT71C3. As shown in Figure 3A, UGT71C3 was significantly induced by exogenously applied MeSA. Similar results were acquired using UGT71C3Pro:GUS transgenic plants and the GUS staining method (Fig. 3B).
Figure 3.
Induced expression of UGT71C3 by MeSA and the assays of UGT71C3 enzyme activity in vivo. A, RT-qPCR analysis of UGT71C3 expression induced by MeSA treatment. Mock treatment is DMSO solvent. The value of the wild type under mock treatment for 0 h was set at 1. Data are means ± sd of three biological replicates (**P < 0.01, Student’s t test). B, GUS staining of UGT71C3Pro:GUS expression after MeSA inoculation. Bars = 1 mm (upper) and 200 μm (lower). C, The glucosyltransferase activities of the crude protein extracts for the MeSAG (MeSAG) formation from 2-week–old UGT71C3 transgenic plants, wild type, and mutant lines. A quantity of 0.1 mg of crude proteins from each sample was used in these assays. OE-6 and ko-1 were used as representatives for overexpression lines and mutant lines, respectively. D and E, HPLC profiling of MeSAGs from wild type, overexpression lines (OE-6, OE-10), and mutant lines (ko-1 used as the representative). “St” in (C), (D), and (E) represents the authentic standard of MeSAG. For (D) and (E), the plant tissues were incubated with MeSA before the extraction process. To monitor the recovery rate, 2-methoxybenzoic acid was used as a reference in these assays. WT, wild type.
To determine the in vivo function of UGT71C3 in MeSA catabolism, we generated UGT71C3 knockout mutants and overexpression lines. The term ugt71c3ko-1 stands for a deletion mutant created by the clustered regularly interspaced short palindromic repeats (CRISPR)/ CRISPR associated protein 9 (Cas9; Supplemental Fig. S3A), which is a transfer DNA insertion mutant devoid of UGT71C3 expression. In contrast, OE-6 and OE-10 are two overexpression lines with much higher transcript levels of UGT71C3 than that in wild-type plants (Supplemental Fig. S3, B and C). Soluble proteins were extracted from 2-week–old rosette leaves of UGT71C3 overexpression lines and mutants for MeSA glucosylation analysis. The enzyme assay indicated that the overexpression lines produced much more MeSAG than that in wild-type plants. In contrast, much less MeSAG was produced by UGT71C3 knockout mutants compared to that in wild-type plants (Fig. 3C).
Importantly, MeSAGs were extracted from overexpression lines, mutants, and wild-type plants. Usually, MeSA is not synthesized in healthy leaves and MeSA is only present in trace amounts in plants (Shulaev et al., 1997); therefore, the endogenous level of MeSAGs was lower than the detection limit of the HPLC system. Thus, we incubated Arabidopsis leaves in exogenous MeSA for the purpose of detecting endogenous MeSAGs. After 48 h of incubation, the level of MeSAG in the mutants was found to be much lower than that in the wild type. In comparison, the transgenic overexpression lines of UGT71C3 accumulated much more MeSAG than the wild type (Fig. 3, D and E). The potential MeSAGs were compared with authentic standards using HPLC and LC-MS, further confirming the identity of the MeSAGs extracted from the plants (Fig. 3, D and E; Supplemental Fig. S4). These results showed a good correlation between the expression levels of UGT71C3 and the accumulation of glucosylated MeSA in vivo, indicating the activity of UGT71C3 was responsible for MeSA glucosylation.
UGT71C3 Negatively Modulates the MeSA-Associated SAR Response
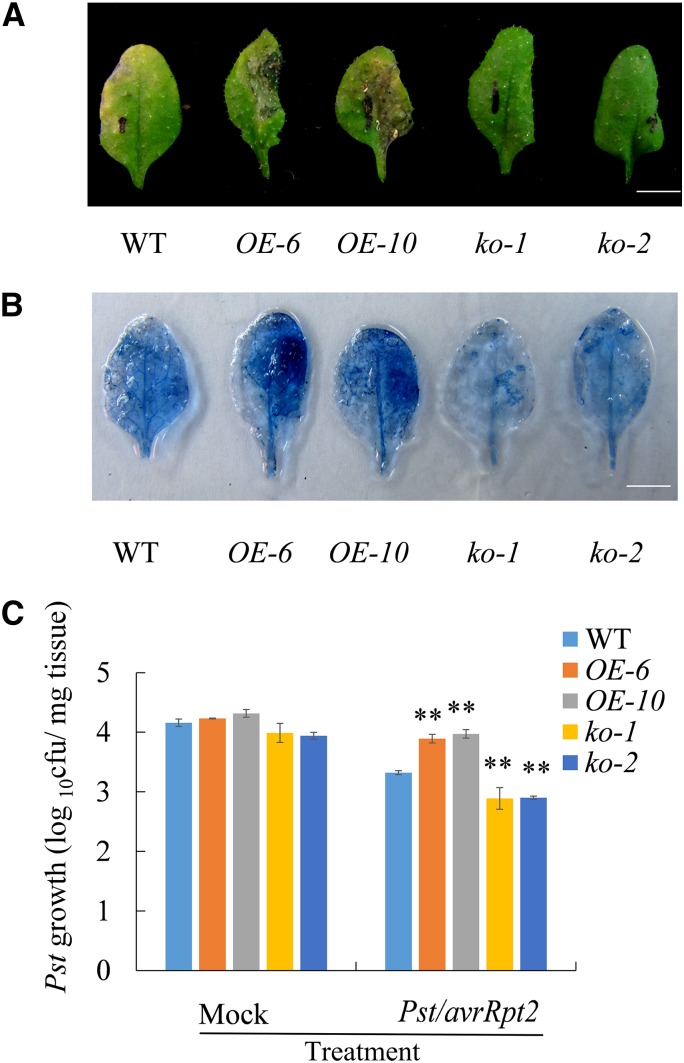
Given that UGT71C3 could glucosylate MeSA to form MeSA-glucosyl conjugates in vivo, the next question was whether UGT71C3 is involved in MeSA-associated SAR. We investigated the SAR responses in upper leaves of wild-type plants, UGT71C3 overexpression lines, and ugt71c3 mutants after primary inoculation of lower leaves with 10-mm MgCl2 containing 1 × 106 colony forming units (CFU)/mL suspension of Pst DC3000/avrRpt2. Under secondary infection of the pathogen Pst DC3000 on upper leaves, UGT71C3 overexpression lines showed more severe disease symptom development than that in the wild type (i.e. leaf yellowing and tissue collapse), whereas ugt71c3 mutants showed substantially reduced symptom development (Fig. 4A). Necrosis in the upper leaves (indicated by Trypan blue staining) was demonstrated, which was consistent with the disease symptom phenotype (Fig. 4B).
Figure 4.
Defense phenotypes of the 35S::UGT71C3 transgenic plants and ugt71c3 mutants. A, SAR response to avirulent pathogen infection in wild type, 35S::UGT71C3 transgenic plants, and ugt71c3 mutants. The lower leaves of 5–6-week–old plants were primarily inoculated with avirulent bacteria (Pst DC3000/avrRpt2 suspended in 10-mm MgCl2). At 2 d after primary infection, upper leaves were inoculated with virulent bacteria (Pst DC3000). Photographs of disease resistance phenotypes of upper leaves were taken 5 d after infection of upper leaves with pathogens. Bars = 0.5 cm. B, Trypan blue staining showing the necrosis in upper leaves infected with pathogens as described in (A). Bars = 0.5 cm. C, Growth of Pst DC3000 in upper leaves of wild type, 35S::UGT71C3 transgenic plants, and ugt71c3 mutants infected with pathogens. At first, the lower leaves of 5–6-week–old plants were primarily inoculated with avirulent bacteria (Pst DC3000/avrRpt2 suspended in 10-mm MgCl2) or with 10-mm MgCl2 as mock treatment. At 2 d after primary infection, upper leaves were inoculated with virulent bacteria (Pst DC3000). The in planta bacterial titers were determined 3 d postinoculation of upper leaves. Data represent the mean of 10 independent samples with sd of three biological replicates (**P < 0.01, Student’s t test). WT, wild type.
Moreover, the growth of virulent Pst DC3000 was determined 3 d after inoculation of upper leaves on wild-type plants, overexpression lines, and mutant plants previously inoculated with 10-mm MgCI2 (mock) or infected with 10-mm MgCl2 containing Pst DC3000/avrRpt2 (SAR induction). The number of pathogen colonies that formed after secondary infection of wild-type plants that were initially inoculated with the pathogen was reduced by ∼16% compared with that in plants that initially received a mock infection, indicating the development of SAR (Fig. 4C; Table 1). However, overexpression lines displayed only a 6% to 8% reduction in the number of pathogen colonies after secondary infection. In contrast, ugt71c3 mutants exhibited a 20% reduction in pathogen growth, indicating clearly enhanced systemic resistance compared with that in the wild type (Fig. 4C; Table 1). These data suggest that UGT71C3 is involved in SAR and negatively modulates the SAR response.
Table 1. SAR development was assessed by monitoring the pst growth in the systemic leaves.
At first, the lower leaves of 5–6-week–old plants were primarily inoculated with avirulent bacteria (Pst DC3000/avrRpt2 suspended in 10-mm MgCl2) or with 10-mm MgCl2 as mock treatment. At 2 d after primary infection, upper leaves were inoculated with virulent bacteria (Pst DC3000). The in planta bacterial titers were determined 3-d postinoculation of upper leaves. The experiment was done three times with similar results. “**” = significant differences relative to the wild type (**P < 0.01, Student’s t test). N/A, Not applicable.
| Local infection | Wild Type | OE-6 | OE-10 | ko-1 | ko-2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mock | Pst | Mock | Pst | Mock | Pst | Mock | Pst | Mock | Pst | |
| Pst growth in systemic leaves (log10 cfu) | 4.16 ± 0.46 | 3.47 ± 0.45 | 4.23 ± 0.33 | 3.89 ± 0.37** | 4.23 ± 0.28 | 3.97 ± 0.27** | 3.99 ± 0.28 | 3.18 ± 0.20** | 3.94 ± 0.30 | 3.13 ± 0.19** |
| Reduction(%) in Pst growth to Mock | N/A | 16.58 | N/A | 8.16 | N/A | 6.11 | N/A | 20.30 | N/A | 20.47 |
| SAR relative to wild type | N/A | N/A | N/A | — | N/A | — | N/A | + | N/A | + |
In addition to Pst DC3000 carrying avrRpt2, P. syringae pv maculicola (Psm) ES4326 and Psm ES4326/avrRpt2 were also used to investigate the possible induction of UGT71C3 expression and SAR response. We found that UGT71C3 expression can also be induced by Psm ES4326 and Psm ES4326/avrRpt2 (Supplemental Fig. S5, A and B). After primary inoculation of lower leaves with Psm ES4326/avrRpt2, the disease symptom development and Psm ES4326 growth were reduced in systemic leaves of ugt71c3 mutants. Conversely, UGT71C3 overexpression lines showed more severe symptom development and pathogen growth (Supplemental Fig. S5, C and D).
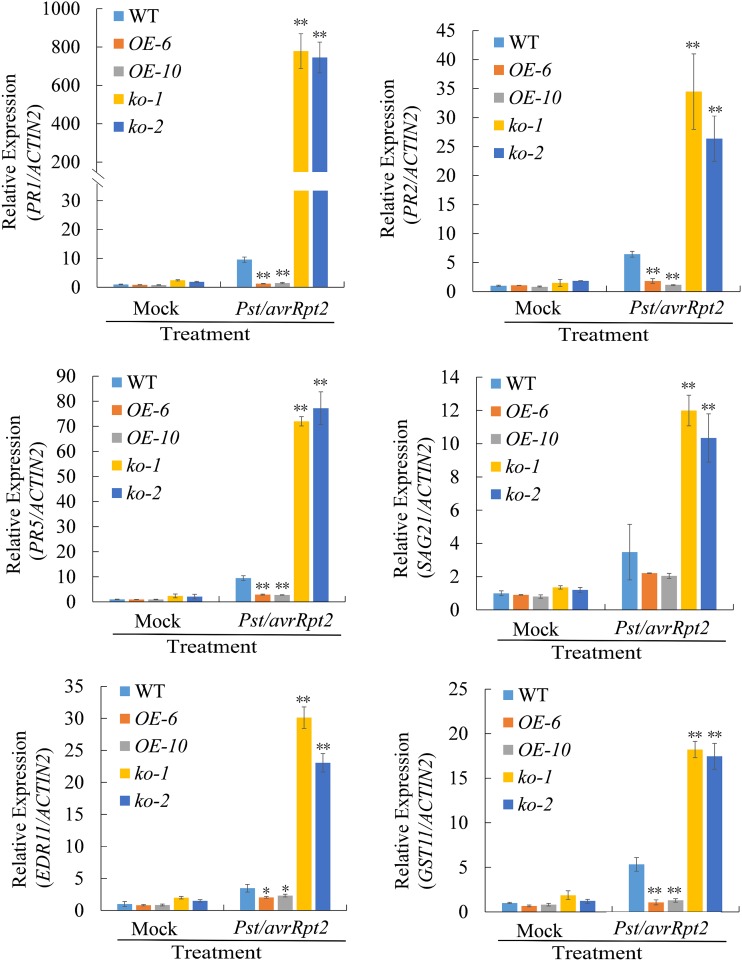
Further, the expression of several genes related to SAR was analyzed in overexpression lines and mutant lines. It is known that PR1, PR2, PR5, SA 2-o-β-d-glucoside 21 (SAG21), Enhanced disease resistance 11, and GST11 are induced in systemic leaves during SAR (Maleck et al., 2000). Thus, these six genes were selected and their expression levels were examined with ACTIN2 used as reference gene. Three lower leaves per plant of wild type, overexpression lines, and mutant lines were inoculated with 10-mm MgCl2 (mock treatment) or 10-mm MgCl2 containing Pst DC3000/avrRpt2 (SAR induction). Two d later, we examined SAR-induced gene expression in the upper, untreated distal leaves. We found that expression levels of the six genes in systemic leaves were much higher in the ugt71c3 mutant plants, whereas their expression levels were much lower in the overexpression lines than that in the wild-type plants after primary infection with the pathogen (Fig. 5), suggesting that UGT71C3 represses defense gene expression in systemic leaves during SAR.
Figure 5.
The induced expression of SAR-related genes in systemic leaves of UGT71C3 overexpression lines, ugt71c3 mutants, and wild type. Three lower leaves on each plant were inoculated with Pst/avrRpt2 (1 × 106 CFU/mL in 10-mm MgCl2) or mock-treated with 10-mm MgCl2. After 2 d, total RNA was extracted from the upper untreated systemic leaves and analyzed for the expression of indicated genes using RT-qPCR. Expression was normalized against constitutively expressed ACTIN2. The value of wild-type plants under mock-treated condition was set at 1.0. Data = means ± sd of three biological replicates (Student’s t test, *P < 0.05, **P < 0.01). WT, wild type.
To validate the effectiveness of ACTIN2 as a reference gene for gene expression analyses under the conditions of plant-pathogen interactions in this study, we used UBQTIN5 as an alternative reference gene to investigate several gene expressions. Our analyses produced similar results as that generated using ACTIN2 (Supplemental Fig. S6).
UGT71C3 Perturbs MeSA Homeostasis Via the Glucosylation Pathway
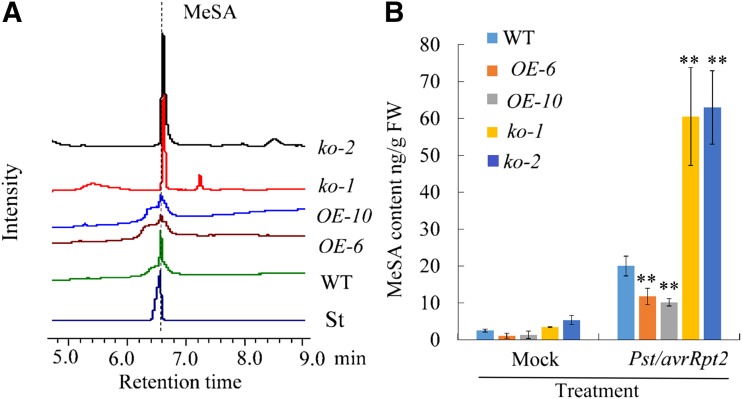
Due to the MeSA glucosyltransferase activity of UGT71C3, the altered SAR response in UGT71C3 mutants and overexpression lines may be the result of the perturbation of MeSA homeostasis. To validate this hypothesis, we determined and compared the relative levels of MeSA in UGT71C3 transgenic plants and mutants to that in the wild type. Tissues for MeSA measurements were collected from upper leaves 24 h after primary inoculation of lower leaves with MgCl2 (mock) or Pst DC3000/avrRpt2 (SAR induction). Under mock treatment, the amounts of MeSA were very low and nearly at the same level in distal leaves of wild type, overexpression lines, and mutant lines (Supplemental Fig. S7). After the primary infection of lower leaves with pathogen, however, the amounts of MeSA in distal leaves showed substantial difference among the three genotypes. UGT71C3 overexpression lines produced much less MeSA than that in the wild type, whereas the ugt71c3 knockout mutants accumulated much more MeSA than that in the wild type (Fig. 6, A and B). These results suggest that UGT71C3 perturbs MeSA homeostasis via the glucosylation pathway during SAR development.
Figure 6.
Profiling of MeSA contents in mutant lines and overexpression lines. A, Profiling of MeSA levels using a GC system for upper leaves of wild type, mutant lines, and overexpression lines 24 h after inoculation of lower leaves with Pst/avrRpt2. “St” represents the authentic standard of MeSA. B, MeSA contents in upper leaves of wild type, mutant line, and overexpression lines after 10-mm MgCl2 (mock) or Pst/avrRpt2 treatment for 24 h. Asterisks denote statistically significant difference of a particular line compared with that in the wild type. Data = means ± sd. All experiments were repeated three times with similar results (**P < 0.01, Student’s t test). WT, wild type.
MeSA Glucosylation Influences SA Homeostasis during the SAR Response
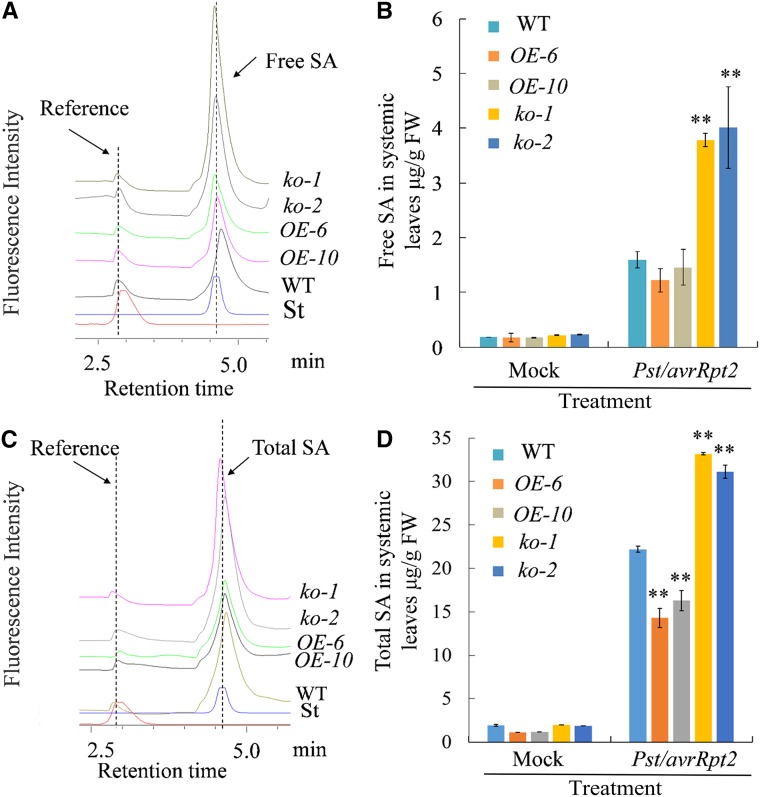
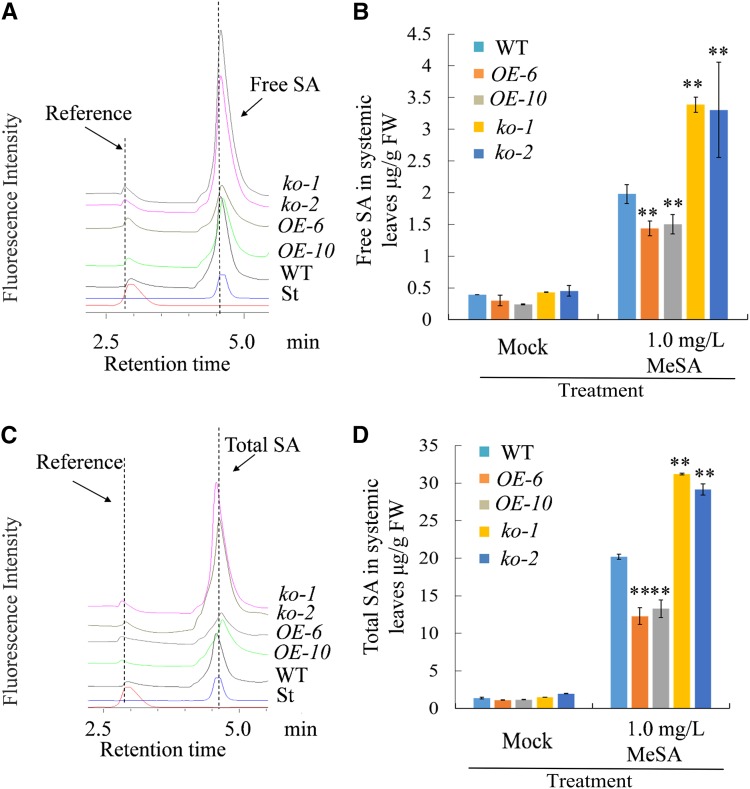
Studies have shown that SA is required for the expression of defense genes and the establishment of SAR (Wildermuth et al., 2001; Mou et al., 2003; Fu and Dong, 2013). In addition, it is proposed that MeSA can be converted to SA and take a part in the SAR response (Park et al., 2007; Liu et al., 2011a; Fu and Dong, 2013). To determine whether MeSA glucosylation catalyzed by UGT71C3 influences SA content, we monitored both free and total SA of 5–6-week-old plants of overexpression lines, mutant lines, and the wild type using a fluorescence detector, because fluorescence detection is much more selective than UV. Plant tissues were collected from unchallenged upper leaves 2 d after the primary infection of lower leaves with a mock treatment or Pst DC3000/avrRpt2. Under mock treatment, we found that the levels of free and total SA in upper leaves were comparable among plants of overexpression lines, mutant lines, and the wild type. However, after the infection of lower leaves with Pst DC3000/avrRpt2, the levels of free and total SA in upper leaves of mutant lines were much more than that in the wild type, whereas they were less in overexpression lines than that in the wild type (Fig. 7). In addition, we examined the influence of MeSA glucosylation on SA levels when MeSA was applied exogenously to plants. Similar results for free and total SA were observed in upper leaves 48 h after treatment with 1 mg/L MeSA on the lower leaves (Fig. 8). The changes in SA homeostasis determined here were consistent with the expression levels of genes that are involved in the defense response and SA inducibility, including genes encoding PR proteins examined earlier in this study. Altogether, we conclude that MeSA glucosylation catalyzed by UGT71C3 influences SA homeostasis during plant systemic immunity.
Figure 7.
Profiling of SA contents in mutant lines and overexpression lines during SAR. A, HPLC profiling of free SA levels in upper leaves of wild type, mutant lines (ko-1 and ko-2), and overexpression lines (OE-6 and OE-10) 48 h after inoculation of lower leaves with Pst /avrRpt2. B, Quantification of free SA levels in upper leaves of wild type, mutant lines, and overexpression lines 48 h after inoculation of lower leaves with 10-mm MgCl2 (Mock) or Pst/avrRpt2. C, HPLC profiling of total SA levels in upper leaves of wild type, mutant lines (ko-1 and ko-2), and overexpression lines (OE-6 and OE-10) 48 h after inoculation of lower leaves with Pst /avrRpt2. D, Quantification of total SA levels in upper leaves of wild type, mutant lines, and overexpression lines 48 h after inoculation of lower leaves with 10 mm MgCl2 (Mock) or Pst /avrRpt2. “St” represents the authentic standard of SA. All experiments were repeated three times with similar results. Data are means ≥ sd (Student’s t test, **P < 0.01). WT, wild type.
Figure 8.
Profiling of SA contents in mutant lines and overexpression lines after treatment with MeSA. A, HPLC profiling of free SA levels in upper leaves of wild type, mutant lines (ko-1 and ko-2), and overexpression lines (OE-6 and OE-10) 48 h after inoculation of lower leaves with 1 mg/L MeSA. B, Quantification of free SA levels in upper leaves of wild type, mutant lines, and overexpression lines 48 h after inoculation of lower leaves with 1 mg/L MeSA or DMSO solvent (Mock). C, HPLC profiling of total SA levels in upper leaves of wild type, mutant lines (ko-1 and ko-2), and overexpression lines (OE-6 and OE-10) 48 h after inoculation of lower leaves with 1 mg/L MeSA. D, Quantification of total SA levels in upper leaves of wild type, mutant lines, and overexpression lines 48 h after treatment of lower leaves with 1 mg/L MeSA or DMSO solvent (Mock). “St” represents the authentic standard of SA. All experiments were repeated three times with similar results. Data = means ≥ sd (Student’s t test, **P < 0.01). WT, wild type.
DISCUSSION
MeSA Glucosylation Pathway Is Present in Plants
Glycosylation of secondary metabolites and hormones commonly occurs in the plant kingdom, suggesting that UDP-glycosyltransferases play a key role in maintaining cellular homeostasis. As a major defense signal, SA biosynthesis and metabolism has always attracted a lot of attention (An and Mou, 2011; Dempsey et al., 2011; Dempsey and Klessig, 2012; Fu and Dong, 2013). Thus, the glycosylation of SA has previously been studied. For example, it was reported that SA can be converted to SAG by the glucosyltransferases UGT74F1 and UGT74F2 (Lim et al., 2002). Loss of UGT74F1 and UGT76B1 induces disease resistance in plants (Dean and Delaney, 2008; Noutoshi et al., 2012). The methylated derivative of SA, MeSA, was proposed as essential for SAR under primary infection in the late afternoon (Liu et al., 2011a). So far, beside SA glucosyltransferases, only the MeSA esterase and SA methyltransferase are known to preserve the dynamic balance between SA and MeSA. However, whether an enzyme responsible for the formation of MeSA glycosides exists in plants remained unknown. In this study, UGT71C3 was characterized and identified as a MeSA glucosyltransferase in Arabidopsis. This molecule showed strong enzyme activity in MeSA glucosylation. Furthermore, the level of MeSAG in line overexpressing UGT71C3 was much higher than that in wild-type plants. However, ugt71c3 mutant lines contained much lower concentrations of MeSAG after pathogen challenge, suggesting that UGT71C3 has an in vivo function in glucosylating MeSA. In addition, we found that the accumulation of free MeSA in overexpression and mutant lines was logically consistent with UGT71C3 function. Therefore, this work demonstrated that the glucosylation of the systemic signal molecule MeSA by UGT71C3 does exist in plants.
Acting as a MeSA glucosyltransferase, UGT71C3 belongs to group E in the UGT super family, whereas SA glucosyltransferase UGT74F1 and UGT74F2 fall to group L (see the published trees of UGTs in Arabidopsis; Ross et al., 2001). Group E usually shows activity toward scopoletin and esculetin, implying that the UGT71 family can recognize substrates with features common to hydroxycoumarins (Lim et al., 2003a). With the evolution of plants, vastly diverse secondary metabolites were successively produced to protect plants from various challenges. Correspondingly, new members of the UGT family evolved to recognize structurally related metabolites, including the monolignols, hydroxycinnamates, benzoates, and auxins (Jackson et al., 2001; Lim et al., 2001, 2002). For example, UGT71C1 has been shown to exhibit glucosyltransferase activity toward phenylpropanoids such as caffeic acid, o-coumaric acid, p-coumaric acid, and flavonoids, affecting secondary metabolites in response to oxidative stress (Lim et al., 2003b, 2008; Okazawa et al., 2014). Biochemical analyses have demonstrated in vitro and in vivo that UGT71C5 glucosylates ABA and is involved in drought stress resistance (Liu et al., 2015). Here, our analysis shows that UGT71C3 uses MeSA as a substrate for glycosylation as part of SAR. We presume that the diverse biochemical functions of different members in the same UGT group are determinants for plant evolution. Diversified enzymatic activities toward secondary metabolites offer a wide degree of versatility and flexibility for plants to enable survival in changing environments. In evolutionary terms, the substrate for each glycosyltransferase would become increasingly specific and each enzyme would retain its irreplaceability through natural selection so as to adapt to the evolutionary diversity of secondary metabolites.
MeSA Glucosylation Provides an Additional Layer of Modulation for MeSA and SA Homeostasis
MeSA and SA homeostasis is crucial to the plant defense response. Thus, their levels are normally tightly regulated in plants. The synthetase and hydroxylase usually acts as the first line of control in MeSA and SA homeostasis. For instance, MeSA is produced from SA via SA carboxyl methyltransferase in local leaves after primary infection. Then, MeSA is hydrolyzed to SA by MeSA esterase as a signal in the systemic leaves to stimulate SAR (Park et al., 2009). For the biosynthesis and metabolism of SA itself, several enzymes and regulating factors such as isochorismate synthase1, enhanced disease susceptibility5, phytoalexin deficient4, SA 3-hydroxylase, SA 5-hydroxylase, and others constitute the most important part of SA dynamic balance in plant cells (Fu and Dong, 2013; Zhang et al., 2013, 2017). On the other hand, glycosylation is an indispensable step in the modification of multiple secondary metabolites to produce stable, soluble, and inactive forms via the transfer of sugars (Lim and Bowles, 2004). It is known that the great majority of pathogen-induced SA is modified by glucosylation to form nontoxic SA Glc conjugates (Dean et al., 2005). Previous studies have identified UGT74F1 and UGT74F2 as SA glucosyltransferases responsible for SA glucosylation in Arabidopsis (Lim et al., 2002; Dean and Delaney, 2008). It was found that the loss of UGT74F1 increased the endogenous levels of free SA and induced disease resistance in plants (Dean and Delaney, 2008; Noutoshi et al., 2012). However, a different result was demonstrated in another study. It was found that, although both ugt74f1 and ugt74f2 mutants have altered levels of SAGs and SA Glc ester compared to that in wild-type plants, ugt74f2 accumulates higher levels of free SA whereas ugt74f1 accumulates lower levels of SA in response to pathogen infection (Boachon et al., 2014). Both mutants were affected in their basal resistance against Pst. The mutant ugt74f1 showed enhanced susceptibility, whereas ugt74f2 showed enhanced resistance against the same pathogen (Boachon et al., 2014). Actually, besides SA Glc conjugates, there are also possibly SA amino acid conjugates present in plants. Although there is lack of research on SA amino acid conjugates, we cannot exclude the possibility of SA amino acid conjugates to regulate free SA levels as well.
MeSA was previously shown to accumulate during SAR and suggested to induce defense via its conversion to SA (Shulaev et al., 1997). In this study, we found that MeSA glucosylation catalyzed by UGT71C3 could influence levels of both MeSA itself and free SA. ugt71c3 mutant lines contained much higher concentrations of MeSA and SA than that in the wild type after a pathogen challenge. However, overexpression lines of UGT71C3 accumulated much lower levels of MeSA and SA than that in the wild type. We propose that the accumulation of free SA in ugt71c3 mutants after pathogen inoculation is likely to be due to MeSA accumulation, which converts to SA under these circumstances. Thus, altered levels of SA may be an indirect result of changed levels of MeSA due to the loss of UGT71C3 in the mutant lines.
It has been determined that the duration of light exposure that plants receive after primary infection determines the extent to which MeSA is required for SAR signaling (Liu et al., 2011a). In this study, we found that UGT71C3 expression was up-regulated after pathogen infection under dark conditions compared with that in light conditions (Supplemental Fig. S8), which was in contrast to the expression pattern of UGT74F1, another UGT involved in the defense response, and the glycosylation of SA, as mentioned above. Therefore, this finding suggests that the function of MeSA and SA glycosylation may be of varying significance in the plant SAR response depending on the light conditions. Plants may respond to the environment and maintain the required levels of SA by selectively using MeSA or SA glycosylation. This strategy would offer greater flexibility to plants responding to pathogen infection under different light conditions. Therefore, the data in this study may suggest that MeSA glucosylation provides an additional layer of regulation to MeSA and SA homeostasis in plant SAR.
MeSA Glucosylation Is a Negative Regulatory Pathway for SAR
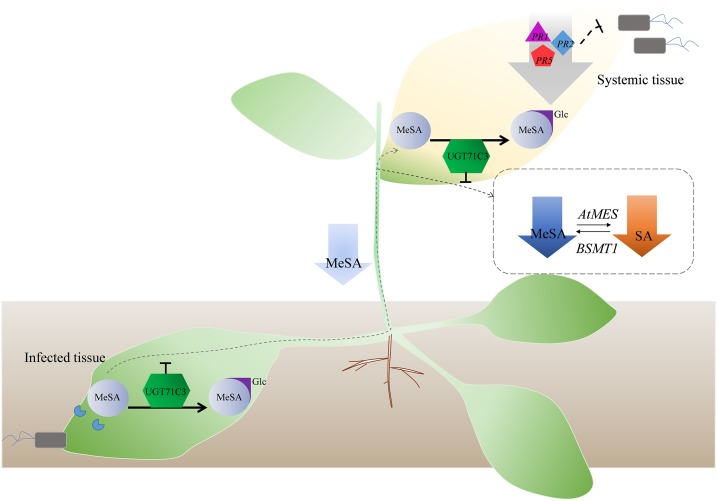
MeSA is proposed to be an important systemic signal for the establishment of SAR, and it is believed to act by being converted back to SA (Shulaev et al., 1997). Because the accumulation of MeSA in plants is essential for SAR, the precise control of MeSA levels is important to maintaining SA levels and a proper immune response. In this study, our results suggest that the glucosylation of MeSA catalyzed by UGT71C3 is involved in SA-dependent SAR and exerts a negative effect on the SAR response in Arabidopsis. Based on these findings present in this study, a working model may be proposed to better understand the regulation of SA accumulation during SAR through MeSA glucosylation catalyzed by UGT71C3 (Fig. 9). When plants are infected by some pathogens, MeSA levels rise rapidly in the primary infected tissues. UGT71C3 expression is up-regulated due to induction by pathogens and MeSA. MeSA glucosylation is thus accelerated, resulting in less MeSA being transported to the uninoculated systemic tissues. In systemic tissues, MeSA is further glucosylated by UGT71C3, resulting in a further reduced level of MeSA and hence a reduced level of SA, which is converted from MeSA. Thereby, the expression levels of pathogen-related proteins are decreased and defense responses are attenuated (Fig. 9).
Figure 9.
Working model for the negative regulation of plant systemic defense through MeSA glucosylation. When plants are infected by some pathogens, MeSA levels rise rapidly in the primary infected plant tissues. UGT71C3 is up-regulated due to the induction of pathogens and MeSA. MeSA glucosylation is thus accelerated, resulting in less MeSA being transported to the uninoculated systemic tissues. In the systemic tissues, MeSA is further glucosylated by UGT71C3, resulting in further reduced MeSA levels and thus a reduced level of SA, which is converted from MeSA. Thereby, the expression of pathogen-related proteins decreases and defense responses are attenuated. The downward arrows mean the decrease of MeSA and SA levels or down-regulation of PR genes. AtMES, Arabidopsis methylesterases; BSMT1, SA methyl transferase 1.
This work provides an opportunity to address fundamental questions about SAR biology; for example: What is the mechanism of signal attenuation once a heightened defense response is no longer required? To activate SAR, a mobile signal such as MeSA must be generated in the locally inoculated tissues and then transported systemically to the distal tissues via the vasculature. On the other hand, presumably UGT71C3-mediated down-regulation of SA by MeSA glucosylation occurs once resistance responses have been successfully activated to prevent a continued increase in SA levels. This may be a new way for the regulation of SAR, which helps to maintain the dynamic balance of MeSA and SA and avoid excessive resistance responses in plants. Our findings have determined another layer of SAR regulation and highlight the role of glucosylation of MeSA and potentially other systemic signals in modulating plant systemic defense.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Columbia 0 (Col-0) was used in this study. The mutant line ugt71c3 (SALK_042564C) was obtained from the Nottingham Arabidopsis Stock Center (http://nasc.life.nott.ac.uk/) and confirmed by PCR and RT-PCR (the primers are listed in Supplemental Table S2). All the plants were grown on Murashige and Skoog medium (Sigma-Aldrich) or soil mix (Pindstrup) in a controlled growth chamber at 22°C under long-day conditions (16-h light/8-h dark, 80 μmol·m−2 s−1) and a relative humidity of 70%. For pathogen infection experiments, plant materials were grown in short-day conditions (8 h light/16 h dark).
Preparation for Transgenic and Knock-Out Lines of UGT71C3
To generate the 35S::UGT71C3 construct, the coding region of this gene was cloned into the pBI121 binary vector driven by the CaMV 35S promoter. To prepare the knock-out construct of UGT71C3, CRISPR/Cas9 strategy was applied by the Hangzhou Biogle Vector Construction Kit (http://www.biogle.cn/). All the constructs were transformed into Arabidopsis Col-0 though Agrobacterium-mediated floral dip (Clough and Bent, 1998). The generated transformants were selected on Murashige and Skoog medium supplemented with 50 mg/L of kanamycin. Independent T1 kanamycin-resistant lines were selected and homozygous T3 progeny lines were isolated. The expression level of UGT71C3 in overexpression lines was determined by RT-PCR. ACTIN2 was used as the internal control for RT-PCR analysis. For the ugt71c3 mutants generated by CRISPR/Cas9, BASTA (glufosinate) was added into Murashige and Skoog medium to select the resistant seedlings.
Pathogen Experiments
Pathogen inoculation experiments were carried out using 5–6-week–old plants. Plants were infected by inoculating the abaxial leaf surface with pathogens using a 1-mL syringe. For SAR assays, induction in lower leaves was carried out as described in Attaran et al. (2009) and Liu et al. (2010, 2011b). In brief, the lower leaves were primarily inoculated with 10-mm MgCl2 or avirulent bacteria (Pst DC3000/avrRpt2 or Psm ES4326/avrRpt2) at a concentration of 1 × 106 CFU/mL in 10-mm MgCl2. Two d after primary infection, upper leaves were inoculated with virulent bacteria (Pst DC3000 or Psm ES4326) at a density of 1 × 105 CFU/mL in 10-mm MgCl2. Primary and secondary infections of Arabidopsis were carried out between 5:30 and 6:00 pm. The infected plants were immediately moved to a dark room and grown under short-day conditions (8-h light/16-h dark). Photographs of the disease resistance phenotype of the upper leaves were taken 5 d after infection of the upper leaves with pathogens. At least 30 leaves from different plants were assessed for each genotype during each experiment.
Bacterial growth was determined 3 d after infection of upper leaves with Pst DC3000 or Psm ES4326. Approximately 0.02 g of upper leaf disks from different plants were homogenized and shaken in 10-mm MgCl2 supplemented with 0.1-m Suc at room temperature for 4 h. The resulting bacterial suspensions were serially diluted and spots of 20 μL per dilution were grown on Kings’ B medium with rifampicin at 20 μg/mL, kanamycin at 50 μg/mL, and 1.7% (w/v) agar for 48 h at 28°C. After this, the number of colonies was counted. Each experiment contained at least three replicates.
Purification of UGT71C3 Enzyme and Assays of Glucosyltransferase Activity
For the construction of the prokaryotic expression vector, the full-length cDNA of UGT71C3 was cloned into a pGEX-2T vector fused with a GST tag. The expression of soluble recombinant proteins was induced by isopropyl-b-d-thiogalactoside in Escherichia coli XL1-Blue. The fusion protein was purified according to the method described by Hou et al. (2004). The assay of glucosyltransferase activity of UGT71C3 was executed as previously described with some modifications (Lim et al., 2008). A quantity of 2 μg of purified protein was added to the enzyme reaction mix containing 100-mm Tris-HCl at pH 7.0, 2.5-mm MgSO4, 10-mm KCl, 5-mm UDP-Glc, and 1-mm substrate. All reactions were performed at 30°C for 2–3 h and then analyzed on a reverse-phase HPLC system (Shimadzu; http://www.shimadzu.com) equipped with a UV light detector and a 5-μM C18 column (150 × 4.6 mm; Agilent; http://www.agilent.com).
For the enzyme activity assays where enzymes were isolated from plants, total crude protein was extracted from 14-d–old seedlings of different lines and the glucosyltransferase activity toward MeSA was investigated. The same amount (0.1 mg) of crude proteins isolated from different lines was added into the reaction mixture for the comparison of enzyme activities. Reaction conditions and reaction product analyses followed the method described above.
Quantification of SA, MeSA, and MeSAGs
Free SA and total SA (including free SA and the SA portion released from its glucosides) were extracted and measured using methods described in Bowling et al. (1994) and Nawrath and Métraux (1999). Lower leaves of 5–6-week–old plants were inoculated with a suspension of Pst/avrRpt2 at a concentration of 1 × 106 CFU/mL in 10-mm MgCl2 (10-mm MgCl2 used as mock in this case), or inoculated with 1 mg/L of MeSA (water used as mock in this case). At 2 d after the primary inoculation, nontreated upper leaves (0.2 g from different plants) were pooled for SA extraction. Briefly, the leave tissue was frozen in liquid nitrogen and homogenized in 600 µL of 90% (v/v) methanol. Here, 2,6-dichloroisonicotinic acid was used as the internal reference to estimate the loss rates during extraction. After centrifugation, the pellet was re-extracted with 100% (v/v) methanol. The combined supernatants were dried in a speed vacuum with heat (∼65°C). The residue was resuspended in 500 µL of 5% (v/v) trichloroacetic acid and sonicated for 10 min (for total SA measurement, each sample was added in this step with 0.4 mg of β-glucosidase in 30 µL of 0.1-m sodium acetate and incubated for 1.5 h at 30°C; for free SA measurement, no addition of β-glucosidase). The SA was then extracted with 2 vol of ethylacetate-cyclopentane-isopropanol (50:50:1). The organic phase containing the SA was then dried in a speed vacuum with heat (∼65°C). The residue was dissolved in a solvent (H2O/C2H5OH/H3PO4; 45:55:0.025). SA was detected with a 295-nm excitation wavelength and a 405-nm emission wavelength using a fluorescence detector in HPLC analysis.
MeSA was extracted and measured by the protocol described previously with modifications (Park et al., 2007). Briefly, lower leaves of 5–6-week–old plants were first infiltrated with a suspension of Pst/avrRpt2 at a concentration of 1 × 106 CFU/mL in 10-mm MgCl2, or with 10-mm MgCl2 as a control. At 1 d after the primary inoculation, nontreated upper leaves were harvested for MeSA extraction and determination. Frozen leaf tissue (150 mg) was homogenized with 200 μL of extraction buffer (water/1-propanol/HCl = 1:2:0.005). To avoid loss of the sample during the extraction process, the tubes containing samples were not replaced during the operation and the samples were guaranteed to have the same weight as at the beginning. Because the same tube was used throughout the soluble compound extraction, the recovery rate should be the same between different samples, and thus the internal control was omitted in this extraction. After adding 500 μL of methylene chloride into the homogenate, the mixture was shaken thoroughly and centrifuged at 18,800g for phase separation. The lower, organic phase was removed. After that, aliquots (3 μL) of the supernatants were separated using a gas chromatography (GC) system (GC2010ATF; Shimadzu) equipped with a split/splitless injector (in splitless mode) and a fused silica capillary column (Rtx-1701; 30 m × 0.32 mm ID, 0.25-μm film thickness). A flame ionization detector and thermal conductivity detector were used.
MeSAGs were quantified with minor modifications to the protocol described in Lin et al. (2016) and Huang et al. (2018). Because at its base level, MeSA is only present in trace amounts in plants (Shulaev et al., 1997), the resulting endogenous levels of MeSAGes were lower than the HPLC detection limit. Thus, we used exogenous MeSA to incubate Arabidopsis leaves for the purpose of detecting endogenous MeSAGs. 2-methoxybenzoic acid was used as a reference in these assays to monitor the recovery rate. Leaves of 2-week–old plants were incubated and enclosed with MeSA in a tube with cover for 48 h, then leaf tissues were frozen in liquid nitrogen, ground to a powder, and homogenized in 80% (v/v) methanol overnight at 4°C. After centrifugation at 15,000g for 10 min, supernatants containing MeSAGs were obtained. A linear gradient was used for MeSAG analysis by HPLC with increasing methanol (“solvent A”) against double-distilled water (“solvent B”) at a flow rate of 0.8 mL/min over 40 min. Both solutions contained 0.1% formic acid (v/v). The detailed HPLC conditions were as follows: 0 min, 90% B; 25 min, 40% B; 27 min, 90% B; 40 min, stop. Photodiode array was used for the detection of UV-visible absorption at 280 nm. The MeSAG was further confirmed by the LC-MS system (Thermo Fisher Scientific). The methods and mobile phases were the same as the HPLC conditions. The mass spectrometer was operated in positive electrospray ionization mode. Authentic MeSAG (Beijing Chemsynlab) was used as standard in the HPLC and LC-MS analyses for confirmation of the MeSAG.
GUS Assays and Trypan Blue Staining
Sequences of ∼1.5 kb upstream of the start site (ATG) of UGT71C3 (At1g07260) were amplified from Arabidopsis genomic DNA by PCR using UGT71C3 promoter primers (Supplemental Table S2). The promoter sequences were inserted into the pBI121 binary vector to replace the CaMV35S promoter. Then the wild-type Arabidopsis was transformed with the UGT71C3 Pro:GUS vector to generate transgenic plants using the floral dip method (Clough and Bent, 1998). For GUS staining assays, leaves of 2-week–old transgenic seedlings of UGT71C3 Pro:GUS were soaked in 10-mm MgCl2 (mock) or 10-mm MgCl2 containing Pst/avrRpt2 (OD600 = 0.01). At each time point, GUS activity was measured in at least 20 individual plants and photographs were taken. Two independent transgenic lines showing similar results were used as subjects for the photos. To detect necrosis in leaves after pathogen infection, Trypan blue staining was carried out according to the method described in Yang et al. (2015).
RNA Extraction and Gene Expression Analysis
Induced expression of UGT71C3 was analyzed in 2-week–old wild-type seedlings. Systemic leaves of 5–6-week–old mutants and wild-type plants were collected for analysis of SAR-related gene expression 2 d after primary infection of local (lower) leaves. Total RNA was extracted using the Trizol reagent (TaKaRa). Reverse transcription was performed using a Prime Script RT reagent kit (TaKaRa). RT-qPCR was performed with a CFX Connect Thermal-Cycling System (Bio-Rad) using a SYBR Green PCR Master Mix kit (TaKaRa). Expression levels were normalized with the reference gene ACTIN2 or UBQTIN5 using the ΔΔCt method. Primer information for the RT-qPCR assay is included in Supplemental Table S2.
Statistical Analysis
All experiments were carried out with at least three independent biological replicates. Each measurement was carried out in triplicate. Data are presented as mean ± sd. Data were statistically analyzed using the Student’s t test. Asterisks indicate significant differences relative to the wild type or control (*P < 0.05, **P < 0.01).
Accession Numbers
Sequence data from this article can be found in the GenBank data libraries under accession numbers UGT71C3 (At1g07260), PR1 (At2g14610), PR2 (At3g57260), PR5 (At1g75040), GST11 (At1g02920), EDR11 (At1g02930), SAG21 (At4g02380), UGT74F1(AT2G43840), ACTIN2 (At3g18780), UBQTIN5 (At3g62250).
Supplemental Data
The following supplemental information is available.
Supplemental Figure S1. GUS staining of 4-week–old adult leaves and roots after 12 h of inoculation of UGT71C3Pro:GUS transgenic plants with Pst DC3000/avrRpt2 or MgCl2 (Mock).
Supplemental Figure S2. HPLC analysis of UGT71C3 activity toward methyl benzoate and benzoic acid.
Supplemental Figure S3. Generation and identification of UGT71C3 mutant lines and overexpression lines.
Supplemental Figure S4. Ion peaks of MeSAGs extracted from overexpression lines are consistent with that of the authentic standard MeSAGs in LC-MS analysis under positive ion mode.
Supplemental Figure S5. The induction of UGT71C3 expression by Psm ES4326 and the defense phenotypes of the 35S::UGT71C3 transgenic plants and ugt71c3 mutants.
Supplemental Figure S6. RT-qPCR analyses of several representative gene expressions using UBQTIN5 as an alternative reference gene produced similar results with that using ACTIN2.
Supplemental Figure S7. GC profiling of MeSA levels under mock conditions.
Supplemental Figure S8. The different expression patterns of MeSA glucosyltransferase gene UGT71C3 and SA glucosyltransferase gene UGT74F1.
Supplemental Table S1. Substrates screened for enzyme activity of UGT71C3.
Supplemental Table S2. Primers used in this research.
Acknowledgments
We thank Dr. Shuhua Yang (China Agricultural University) for providing the pathogenic strains Pst DC3000 and Pst DC3000/avrRpt2, and Dr. Lijing Liu (Shandong University) for providing the pathogenic strains Psm ES4326 and Psm ES4326/avrRpt2. The ugt71c3 mutant line (SALK_042564C) was obtained from the Nottingham Arabidopsis Stock Centre (http://nasc.life.nott.ac.uk/).
Footnotes
This work was supported by the National Natural Science Foundation of China (grant nos. 91217301, 31570299, and 31770313 to B.K.H.).
References
- Ahmad S, Gordon-Weeks R, Pickett J, Ton J (2010) Natural variation in priming of basal resistance: From evolutionary origin to agricultural exploitation. Mol Plant Pathol 11: 817–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An C, Mou Z (2011) Salicylic acid and its function in plant immunity. J Integr Plant Biol 53: 412–428 [DOI] [PubMed] [Google Scholar]
- Attaran E, Zeier TE, Griebel T, Zeier J (2009) Methyl salicylate production and jasmonate signaling are not essential for systemic acquired resistance in Arabidopsis. Plant Cell 21: 954–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boachon B, Gamir J, Pastor V, Erb M, Dean JV, Flors V, Mauch-Mani B (2014) Role of two UDP-Glycosyltransferases from the L group of Arabidopsis in resistance against Pseudomonas syringae. Eur J Plant Pathol 139: 707–720 [Google Scholar]
- Bowling SA, Guo A, Cao H, Gordon AS, Klessig DF, Dong X (1994) A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell 6: 1845–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda B, Xia Y, Mandal MK, Yu K, Sekine KT, Gao QM, Selote D, Hu Y, Stromberg A, Navarre D, et al. (2011) Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants. Nat Genet 43: 421–427 [DOI] [PubMed] [Google Scholar]
- Chaturvedi R, Krothapalli K, Makandar R, Nandi A, Sparks AA, Roth MR, Welti R, Shah J (2008) Plastid omega3-fatty acid desaturase-dependent accumulation of a systemic acquired resistance inducing activity in petiole exudates of Arabidopsis thaliana is independent of jasmonic acid. Plant J 54: 106–117 [DOI] [PubMed] [Google Scholar]
- Chaturvedi R, Venables B, Petros RA, Nalam V, Li M, Wang X, Takemoto LJ, Shah J (2012) An abietane diterpenoid is a potent activator of systemic acquired resistance. Plant J 71: 161–172 [DOI] [PubMed] [Google Scholar]
- Chen YC, Holmes EC, Rajniak J, Kim JG, Tang S, Fischer CR, Mudgett MB, Sattely ES (2018) n-hydroxy-pipecolic acid is a mobile metabolite that induces systemic disease resistance in Arabidopsis. Proc Natl Acad Sci USA 115: E4920–E4929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm ST, Coaker G, Day B, Staskawicz BJ (2006) Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 124: 803–814 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Conrath U. (2006) Systemic acquired resistance. Plant Signal Behav 1: 179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrath U. (2011) Molecular aspects of defence priming. Trends Plant Sci 16: 524–531 [DOI] [PubMed] [Google Scholar]
- Dean JV, Delaney SP (2008) Metabolism of salicylic acid in wild-type, ugt74f1 and ugt74f2 glucosyltransferase mutants of Arabidopsis thaliana. Physiol Plant 132: 417–425 [DOI] [PubMed] [Google Scholar]
- Dean JV, Mohammed LA, Fitzpatrick T (2005) The formation, vacuolar localization, and tonoplast transport of salicylic acid glucose conjugates in tobacco cell suspension cultures. Planta 221: 287–296 [DOI] [PubMed] [Google Scholar]
- Dempsey DA, Klessig DF (2012) SOS—too many signals for systemic acquired resistance? Trends Plant Sci 17: 538–545 [DOI] [PubMed] [Google Scholar]
- Dempsey DA, Vlot AC, Wildermuth MC, Klessig DF (2011) Salicylic acid biosynthesis and metabolism. Arabidopsis Book 9: e0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42: 185–209 [DOI] [PubMed] [Google Scholar]
- Fu ZQ, Dong X (2013) Systemic acquired resistance: Turning local infection into global defense. Annu Rev Plant Biol 64: 839–863 [DOI] [PubMed] [Google Scholar]
- Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J (1993) Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261: 754–756 [DOI] [PubMed] [Google Scholar]
- Hartmann M, Zeier T, Bernsdorff F, Reichel-Deland V, Kim D, Hohmann M, Scholten N, Schuck S, Bräutigam A, Hölzel T, et al. (2018) Flavin monooxygenase-generated n-hydroxypipecolic acid is a critical element of plant systemic immunity. Cell 173: 456–469.e16 [DOI] [PubMed] [Google Scholar]
- Hou B, Lim EK, Higgins GS, Bowles DJ (2004) N-glucosylation of cytokinins by glycosyltransferases of Arabidopsis thaliana. J Biol Chem 279: 47822–47832 [DOI] [PubMed] [Google Scholar]
- Huang XX, Zhu GQ, Liu Q, Chen L, Li YJ, Hou BK (2018) Modulation of plant salicylic acid-associated immune responses via glycosylation of dihydroxybenzoic acids. Plant Physiol 176: 3103–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husar S, Berthiller F, Fujioka S, Rozhon W, Khan M, Kalaivanan F, Elias L, Higgins GS, Li Y, Schuhmacher R, et al. (2011) Overexpression of the UGT73C6 alters brassinosteroid glucoside formation in Arabidopsis thaliana. BMC Plant Biol 11: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RG, Lim EK, Li Y, Kowalczyk M, Sandberg G, Hoggett J, Ashford DA, Bowles DJ (2001) Identification and biochemical characterization of an Arabidopsis indole-3-acetic acid glucosyltransferase. J Biol Chem 276: 4350–4356 [DOI] [PubMed] [Google Scholar]
- Jones DA, Takemoto D (2004) Plant innate immunity—direct and indirect recognition of general and specific pathogen-associated molecules. Curr Opin Immunol 16: 48–62 [DOI] [PubMed] [Google Scholar]
- Jones JD, Dangl JL (2006) The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Jung HW, Tschaplinski TJ, Wang L, Glazebrook J, Greenberg JT (2009) Priming in systemic plant immunity. Science 324: 89–91 [DOI] [PubMed] [Google Scholar]
- Lim EK, Bowles DJ (2004) A class of plant glycosyltransferases involved in cellular homeostasis. EMBO J 23: 2915–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CE, Choi JN, Kim IA, Lee SA, Hwang YS, Lee CH, Lim J (2008) Improved resistance to oxidative stress by a loss-of-function mutation in the Arabidopsis UGT71C1 gene. Mol Cells 25: 368–375 [PubMed] [Google Scholar]
- Lim EK, Li Y, Parr A, Jackson R, Ashford DA, Bowles DJ (2001) Identification of glucosyltransferase genes involved in sinapate metabolism and lignin synthesis in Arabidopsis. J Biol Chem 276: 4344–4349 [DOI] [PubMed] [Google Scholar]
- Lim EK, Doucet CJ, Li Y, Elias L, Worrall D, Spencer SP, Ross J, Bowles DJ (2002) The activity of Arabidopsis glycosyltransferases toward salicylic acid, 4-hydroxybenzoic acid, and other benzoates. J Biol Chem 277: 586–592 [DOI] [PubMed] [Google Scholar]
- Lim EK, Baldauf S, Li Y, Elias L, Worrall D, Spencer SP, Jackson RG, Taguchi G, Ross J, Bowles DJ (2003a) Evolution of substrate recognition across a multigene family of glycosyltransferases in Arabidopsis. Glycobiology 13: 139–145 [DOI] [PubMed] [Google Scholar]
- Lim EK, Higgins GS, Li Y, Bowles DJ (2003b) Regioselectivity of glucosylation of caffeic acid by a UDP-glucose:glucosyltransferase is maintained in planta. Biochem J 373: 987–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JS, Huang XX, Li Q, Cao Y, Bao Y, Meng XF, Li YJ, Fu C, Hou BK (2016) UDP-glycosyltransferase 72B1 catalyzes the glucose conjugation of monolignols and is essential for the normal cell wall lignification in Arabidopsis thaliana. Plant J 88: 26–42 [DOI] [PubMed] [Google Scholar]
- Liu PP, Yang Y, Pichersky E, Klessig DF (2010) Altering expression of benzoic acid/salicylic acid carboxyl methyltransferase 1 compromises systemic acquired resistance and PAMP-triggered immunity in Arabidopsis. Mol Plant Microbe Interact 23: 82–90 [DOI] [PubMed] [Google Scholar]
- Liu PP, von Dahl CC, Klessig DF (2011a) The extent to which methyl salicylate is required for signaling systemic acquired resistance is dependent on exposure to light after infection. Plant Physiol 157: 2216–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PP, von Dahl CC, Park SW, Klessig DF (2011b) Interconnection between methyl salicylate and lipid-based long-distance signaling during the development of systemic acquired resistance in Arabidopsis and tobacco. Plant Physiol 155: 1762–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Yan JP, Li DK, Luo Q, Yan Q, Liu ZB, Ye LM, Wang JM, Li XF, Yang Y (2015) UDP-glucosyltransferase71c5, a major glucosyltransferase, mediates abscisic acid homeostasis in Arabidopsis. Plant Physiol 167: 1659–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado AM, Doerner P, Dixon RA, Lamb CJ, Cameron RK (2002) A putative lipid transfer protein involved in systemic resistance signalling in Arabidopsis. Nature 419: 399–403 [DOI] [PubMed] [Google Scholar]
- Maleck K, Levine A, Eulgem T, Morgan A, Schmid J, Lawton KA, Dangl JL, Dietrich RA (2000) The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat Genet 26: 403–410 [DOI] [PubMed] [Google Scholar]
- Manosalva PM, Park SW, Forouhar F, Tong L, Fry WE, Klessig DF (2010) Methyl esterase 1 (StMES1) is required for systemic acquired resistance in potato. Mol Plant Microbe Interact 23: 1151–1163 [DOI] [PubMed] [Google Scholar]
- Mou Z, Fan W, Dong X (2003) Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 113: 935–944 [DOI] [PubMed] [Google Scholar]
- Návarová H, Bernsdorff F, Döring AC, Zeier J (2012) Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 24: 5123–5141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath C, Métraux JP (1999) Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11: 1393–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noutoshi Y, Okazaki M, Kida T, Nishina Y, Morishita Y, Ogawa T, Suzuki H, Shibata D, Jikumaru Y, Hanada A, et al. (2012) Novel plant immune-priming compounds identified via high-throughput chemical screening target salicylic acid glucosyltransferases in Arabidopsis. Plant Cell 24: 3795–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazawa A, Kusunose T, Ono E, Kim HJ, Satake H, Shimizu BI, Mizutani M, Seki H, Muranaka T (2014) Glucosyltransferase activity of Arabidopsis UGT71C1 towards pinoresinol and lariciresinol. Plant Biotechnol 31: 561–566 [Google Scholar]
- Pallas JA, Paiva NL, Lamb C, Dixon RA (1996) Tobacco plants epigenetically suppressed in phenylalanine ammonia‐lyase expression do not develop systemic acquired resistance in response to infection by tobacco mosaic virus. Plant J 10: 281–293 [Google Scholar]
- Park SW, Kaimoyo E, Kumar D, Mosher S, Klessig DF (2007) Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318: 113–116 [DOI] [PubMed] [Google Scholar]
- Park SW, Liu PP, Forouhar F, Vlot AC, Tong L, Tietjen K, Klessig DF (2009) Use of a synthetic salicylic acid analog to investigate the roles of methyl salicylate and its esterases in plant disease resistance. J Biol Chem 284: 7307–7317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AF. (1961) Systemic acquired resistance induced by localized virus infections in plants. Virology 14: 340–358 [DOI] [PubMed] [Google Scholar]
- Ross J, Li Y, Lim E, Bowles DJ (2001) Higher plant glycosyltransferases. Genome Biol 2: S3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8: 1809–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah BA, Kaur R, Gupta P, Kumar A, Sethi VK, Andotra SS, Singh J, Saxena AK, Taneja SC (2009) Structure-activity relationship (SAR) of parthenin analogues with pro-apoptotic activity: Development of novel anti-cancer leads. Bioorg Med Chem Lett 19: 4394–4398 [DOI] [PubMed] [Google Scholar]
- Shulaev V, Silverman P, Raskin I (1997) Airborne signaling by methyl salicylate in plant pathogen resistance. Nature 385: 718–721 [Google Scholar]
- Truman W, Bennett MH, Kubigsteltig I, Turnbull C, Grant M (2007) Arabidopsis systemic immunity uses conserved defense signaling pathways and is mediated by jasmonates. Proc Natl Acad Sci USA 104: 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij B, Friedrich L, Morse A, Reist R, Kolditz-Jawhar R, Ward E, Uknes S, Kessmann H, Ryals J (1994) Salicylic acid is not the translocated signal responsible for inducing systemic acquired resistance but is required in signal transduction. Plant Cell 6: 959–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlot AC, Klessig DF, Park SW (2008) Systemic acquired resistance: The elusive signal(s). Curr Opin Plant Biol 11: 436–442 [DOI] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414: 562–565 [DOI] [PubMed] [Google Scholar]
- Yang L, Li B, Zheng XY, Li J, Yang M, Dong X, He G, An C, Deng XW (2015) Salicylic acid biosynthesis is enhanced and contributes to increased biotrophic pathogen resistance in Arabidopsis hybrids. Nat Commun 6: 7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Halitschke R, Yin C, Liu CJ, Gan SS (2013) Salicylic acid 3-hydroxylase regulates Arabidopsis leaf longevity by mediating salicylic acid catabolism. Proc Natl Acad Sci USA 110: 14807–14812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhao L, Zhao J, Li Y, Wang J, Guo R, Gan S, Liu CJ, Zhang K (2017) S5H/DMR6 encodes a salicylic acid 5-hydroxylase that fine-tunes salicylic acid homeostasis. Plant Physiol 175: 1082–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]