An analysis of a root-sucker–repressing technology for poplar plants that reduces root cytokinin contents.
Abstract
Root sprouts—the formation of new shoots from roots—is an important mechanism for local gene flow from poplar (Populus spp). An effective strategy to reduce root sprout formation could therefore help to ensure containment during field research and commercial deployment of poplar when grown as exotic or transgenic forms. We used a flavonoid glycosyltransferase gene promoter from Scutellaria barbata (SbUGT) to drive the expression of AtCKX2, a cytokinin oxidase from Arabidopsis that converts active to inactive cytokinins in roots of poplar. In the greenhouse, SbUGT::AtCKX2 transgenic plants exhibited a similar shoot growth habit, but had enhanced root growth and fewer root sprouts, compared to the wild-type control and transgenic events with low transgene expression in roots. Under field conditions, the transgenic trees also had similar growth habits and stem growth rates that were not statistically different from wild-type trees over 3 years. Removal of trunks generally induced high rates of root sprouting; however, in selected SbUGT::AtCKX2 transgenic poplar events there was an absence or fewer root sprouts compared to wild-type trees, consistent with the greenhouse results. Our study demonstrates that the SbUGT::AtCKX2 gene can effectively inhibit root sprouting of poplar trees under field conditions, and thus may provide a useful tool to address concerns associated with root-sprouting-mediated transgene spread.
Poplar (Populus spp) includes woody crops that are important for a variety of uses and products, including pulp, bioenergy, wood, bioremediation, wind protection, and agroforestry. Although transgene-free methods have been developed using the clustered regularly interspersed short palindromic repeat/Cas system to improve perennial plants such as poplar, transgenic technologies are still powerful means to create new traits (Chen et al., 2018). Transgenic technology has been effectively used to produce poplar trees with a variety of useful traits, including herbicide resistance, biotic and abiotic tolerance, improved growth rate, higher nutrient use efficiency, and improved processing and end-use characteristics (Yang et al., 2009; Chang et al., 2018). Although many transgenic field trials have been carried out in the United States, China, and elsewhere, no transgenic trees have been commercially adopted to our knowledge (Li et al., 2016; Klocko et al., 2018).
For most tree species, gene flow primarily occurs through seed and pollen (DiFazio et al., 2012). A number of molecular confinement strategies have been reported that may block seed and pollen-mediated transgene flow in tree species (Klocko et al., 2018). We have also recently demonstrated that an AGAMOUS intron-driven expression of a cytotoxin gene can be used to produce flowerless tobacco (Nicotiana benthamiana), and this technique may also be effective in reducing pollen- and seed-mediated transgene flow in poplar (Li et al., 2016). Further, we have reported a transgene deletion technology called the “Gene Deletor” that might be useful in eliminating transgenes from pollen and seeds of poplar, possibly obviating the need for containment (Luo et al., 2007).
For many plant species, including poplar trees, root sprouts are another vehicle for spread of transgenes. In general, root sprouting is common in poplar in both natural and commercial plantings (Jean et al., 2019). When the trunk of a poplar tree is removed or damaged, sprout bud primordia from roots will develop and grow to produce shoots. The ability to grow new ramets via root sprouting can be extensive and repetitive, making poplar difficult to eliminate from field plantings. Also, after many years, poplar root sprouts can spread to form extensive clonal colonies, extending from many meters to kilometers (Wiehle et al., 2009; Stener et al., 2018). Further, traits such as faster growth, improved water use efficiency and pest resistance, could create additional concerns if these genes were to spread to the native flora (Strauss et al., 2015). For small-scale plantings, sprouts can be controlled by hand pruning and/or by repeated use of herbicides. However, for large-scale plantations, it can be difficult and expensive to eliminate root sprouts entirely, making full compliance with regulatory requirements for transgenic field trials difficult.
The plant hormone cytokinins play a role in root sprouting. Exogenous cytokinins may stimulate root sprouting in poplar (Frey et al., 2003). Overproduction of cytokinins in transgenic plants using the Agrobacterium ipt gene resulted in enhanced adventitious shoot development from both callus tissues and unwounded plants (Smigocki and Owens, 1988). A high ratio of exogenously applied cytokinin/auxin enhances root sprouts in Emmenopterys henryi (Guo et al., 2017), and the positive effects of auxin depletion on shoot initiation in citrus was shown to be mediated by cytokinins (Hu et al., 2017). Overexpression of a cytokinin oxidase (CKX) gene has also been shown to affect shoot development. Cytokinin-deficient transgenic Arabidopsis (Arabidopsis thaliana) and tobacco that overexpressed CKXs showed improved root growth but reduced shoot initiation and growth (Werner et al., 2003, 2010). CKX overexpression also led to fewer shoots and flowers (Werner et al., 2003), while overexpression of a CKX gene driven by a root-predominant promoter caused few observable effects on shoot growth and development in both Arabidopsis and tobacco (Werner et al., 2010; Li et al., 2017).
In this study, we first used the root-dominant promoter SbUGT (Chiou et al., 2010; Li et al., 2017) to control the expression of the GUS reporter gene and CKX2-coding sequence. The SbUGT::GUS fusion gene was predominantly expressed in roots of transgenic poplar trees (Supplemental Fig. S1A). We were able to detect CKX2 gene expression in roots for all 121 SbUGT::CKX2 (CKX2) transgenic poplar events produced, of which 92 insertion events (76%) had no detectable CKX2 expression in shoots (Supplemental Fig. S1B); the remaining 29 events (24%) exhibited modest but visible CKX2 expression in shoots. More than 80% (98 events) showed visibly similar shoot growth compared to wild type. The remaining 20% showed reduced shoot growth similar to constitutive overexpression of a CKX gene, presumably due to leaky expression of the CKX2 gene in leaf and stem tissues (Werner et al., 2003). The root-predominant SbUGT promoter sequence has been used previously in our lab to control the expression of GUS reporter gene and iaaM and CKX2 genes in roots of tobacco (Li et al., 2017). Here, we further demonstrate that the SbUGT promoter is predominantly expressed in roots of poplar. The SbUGT promoter appears to be a novel root-predominant promoter sequence in poplar and thus may provide a useful tool for transgene-mediated improvement of root growth and other characteristics in poplar and related species.
Auxin has been proposed as a negative regulator, and cytokinin as a positive regulator, of root sprout development (Jean et al., 2019). We analyzed cytokinin and auxin concentrations in roots of wild type and two representative events, 117 and 97. Cytokinin (zeatin riboside) levels in both transgenic events were reduced by 46% and 88%, respectively, relative to the levels in wild-type control tissues (Supplemental Fig. S1C). Also, indole acetic acid levels in both transgenic poplar events 117 and 97 were significantly reduced by 51% and 30% of the wild-type poplar roots, respectively (Supplemental Fig. S1D). These results suggest that the reduction in cytokinin level in root tissues is caused by root-predominant CKX overexpression, and thus contributes to the inhibition of sprout development. It has also been proposed that the auxin to cytokinin ratio may play an important role in regulating root sprouting (Frey et al., 2003; Guo et al., 2017). For event 97 (which had no sprouts observed), we found a significant increase in the auxin to cytokinin ratio when compared to that of the wild-type roots (i.e. 242 versus 42). On the other hand, for event 117 that also had no sprouts, the ratio of root auxin to cytokinin did not appear to increase compared to wild type (i.e. 38 versus 42). It is therefore unclear whether the auxin to cytokinin ratio plays a significant role in regulating root sprout development. Further analyses of the concentrations of the different types of active cytokinins would improve our understanding of their effects in regulating root sprout formation of poplar trees, and should be a priority for further studies.
After three months of growth in a greenhouse, the trunks of wild-type controls and 98 CKX2 transgenic events with similar shoot growth were removed at 2" above the soil surface and the number of root sprouts of each tree was recorded six months later. We observed that 57% (56 events) of the CKX2 transgenic poplar trees produced no root sprouts, 26% (25 events) produced a reduced number of sprouts, and 17% (17 events) produced a similar number of sprouts as the wild-type poplar trees (Supplemental Fig. S2; Supplemental Table S1). We performed reverse transcription quantitative PCR analyses for representative transgenic poplar events: three events with no sprouts (Events 97, 28, and 117); four events with fewer sprouts (Events 75, 27, 51, and 101); and three events with numbers of sprouts similar to those of wild-type plants (Events 9, 49, and 125). Supplemental Table S2 shows that relatively high CKX expression was detected in the roots of three events with no sprouts, medium expression levels were detected in four events with reduced numbers of sprouts, and events that produced sprouts similar to that from wild-type poplar plants had <0.5% of the expression levels of the UBQ gene in roots. It therefore appears that the expression level of the CKX gene in roots is negatively correlated with the number of root sprouts.
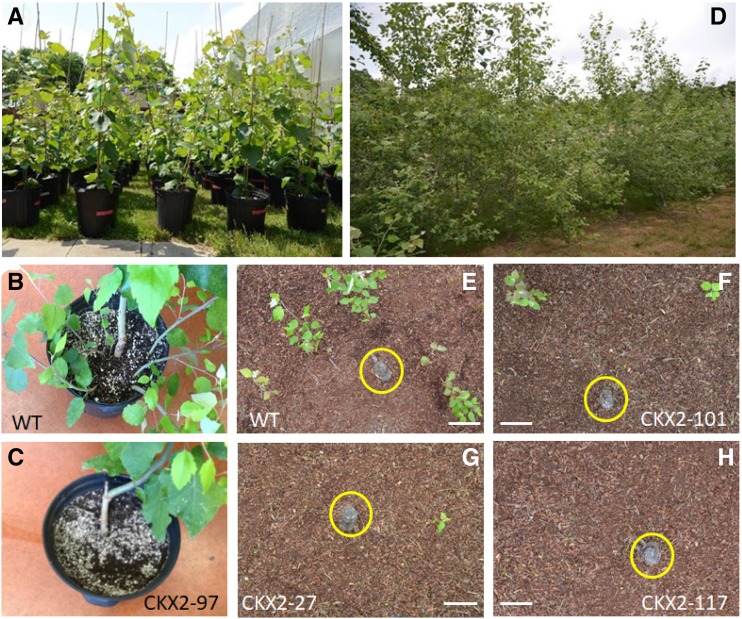
We then selected five representative CKX2 transgenic poplar events to retest the response to trunk removal under both greenhouse and field conditions. These CKX2 transgenic poplar events included three (97, 28, and 117) that produced no sprout shoots and two (27 and 101) that produced fewer sprout shoots relative to the wild type. In the greenhouse, each event was propagated and grown for one year before removal of their trunk (Fig. 1A). Wild-type trees produced 2.6 sprout shoots per plant on average. The propagated replicates of CKX2 transgenic events 27 and 101 produced fewer root sprouts than the wild-type plants under the same conditions, 1.0 and 1.2 per plant on average, respectively (Table 1). Further, all vegetatively propagated replicates of CKX2 transgenic events 28, 97, and 117 failed to produce any root sprouts under greenhouse conditions (Fig. 1, B and C). The sprouting abilities of these propagated poplar plants were consistent with those of their parental plants. Plants evaluated under field conditions were allowed to grow for three seasons before trunk removal (Fig. 1D). One growth season after the removal of their trunks, wild-type poplar trees produced 5.0 root sprouts per plant on average, while events 27 and 101 produced 1.8 and 2.7 sprouts, respectively. Further, all replicate plants of events 28, 97, and 117 produced no sprouts (Table 1). These results are consistent with the observations in the greenhouse studies (Fig. 1, E–H). Our results demonstrate that the SbUGT::AtCKX2 gene can effectively inhibit root sprouting of poplar trees under both greenhouse and field conditions.
Figure 1.
An absence of, or reduced, sprouting in transgenic poplars in the greenhouse and field. A, Four-month–old potted, greenhouse-grown plants. B and C, In the greenhouse, repressed root sprouting was observed in CKX2 transgenic event 97 (C) when compared to wild type (WT; B). D, Images of the field test, the photo was taken in July 2016. E–H, CKX2 transgenic events 101 (F), 27 (G), and 117 (H) exhibited no or fewer root sprouts than wild-type (E) plants one growth season after the excision of the trunk in the field. Photos were taken in November of 2017. The trunks are inside the yellow colored circles. Bars = 12 cm.
Table 1. Rate of root sprouting for CKX transgenic and wild-type poplar in the greenhouse and field.
Data in the table represent average number of suckers per event. At least six replicates were used for wild-type and each transgenic event. Differences determined by pairwise, two-sided χ2 tests on the data before averaging. Asterisks (*) represent significant differences between wild-type and CKX2 transgenic event for either greenhouse or field evaluation (P < 0.05).
| Genotype | In Greenhouse | In Field |
|---|---|---|
| Wild type | 2.6 ± 0.4 | 5.0 ± 0.9 |
| Event 27 | 1.0 ± 0.3 | 1.8 ± 0.5 |
| Event 101 | 1.2 ± 0.3 | 2.7 ± 0.3 |
| Event 28 | 0* | 0* |
| Event 97 | 0* | 0* |
| Event 117 | 0* | 0* |
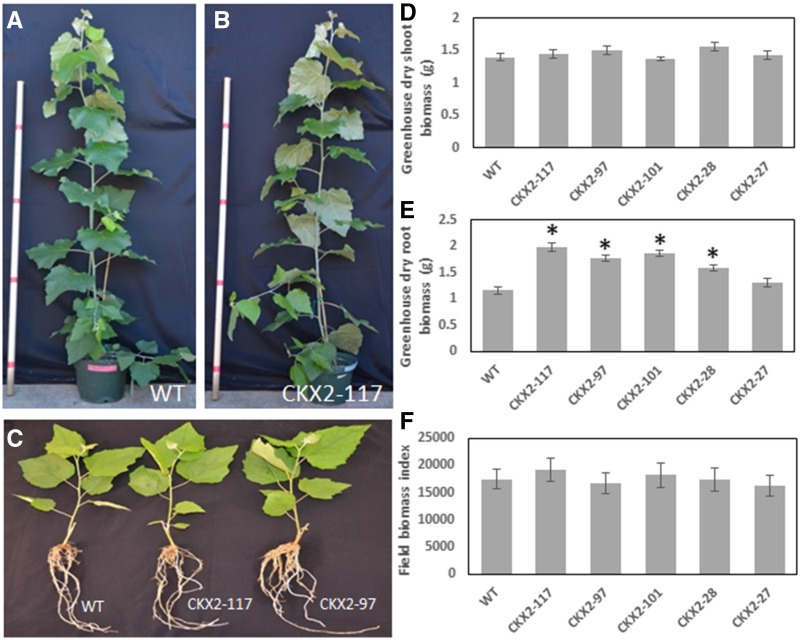
Five selected representative transgenic events exhibited similar shoot growth as the wild-type controls in the greenhouse (Fig. 2, A–D). Events 28, 97, 101, and 117 had significantly enhanced root biomass when compared to wild type, while event 27 had similar root biomass as wild type (Fig. 2E). Under field conditions, no significant differences were observed in the biomass index (estimated using height × diameter2) between the average of all tested CKX2 transgenic events and wild-type poplar after 3 years (Fig. 2F). The effects of CKX2 overexpression in roots on tree physiology warrant further study before commercial use. The enhanced root growth observed may be advantageous or cause unintended effects, for example, on drought tolerance or long-term stem productivity. These effects are likely to be highly soil- and environment-dependent, and thus are difficult to judge from a single field study. Further, we have not studied whether CKX2 overexpression also affects propagation from stem cuttings—the main method by which poplars are amplified for commercial use. Hopefully, by selecting events with optimal root expression, or ultimately using a more cell-specific root-dominant promoter, a system can be developed that strongly suppresses root sprouts without any undesired physiological effects.
Figure 2.
Shoot and root growth in CKX2 compared to wild-type (WT) poplar. A and B, No morphological differences were observed between wild-type (A) and transgenic poplar plants (B) upon visual inspection after 3 months of growth in the greenhouse. C–E, In greenhouse, overexpression of the CKX2 gene in roots of poplar gave similar shoot growth (C and D) but significantly enhanced root growth (E) when compared to wild type. Asterisks (*) represent significant differences in dry root biomass between transgenic and wild-type plants in greenhouse according to Student's t test with the pooled variance at P < 0.05. F, No statistically significant differences in stem biomass index (estimated using height × diameter2) were observed between transgenic and wild-type plants after 3 years of growth (2013–2016) in the field according to Student’s t test with the pooled variance at P = 0.05. Bars = ses.
In summary, we have shown that overexpression of CKX2 driven by a root-predominant promoter SbUGT resulted in reduced levels of cytokinins in roots of poplar. We have further shown that under both greenhouse and field conditions some SbUGT::CKX2 transgenic poplar events exhibited no visible alterations of shoot growth, but did exhibit enhanced root growth, compared to wild-type trees. Finally, we have demonstrated that some of the same SbUGT::CKX2 poplar events did not produce root sprouts under both greenhouse and field conditions. These results suggest that this technology may be useful to eliminate or reduce sprout shoot development in poplar, as well as other root-sprouting plant species. The root-sprout–repressing approach described here may provide a useful tool to address sprout-mediated gene flow and related problems for transgenic poplar and other woody plant species.
Supplemental Data
The following supplemental materials are available.
Supplemental Materials and Methods. Detailed information on experimental materials and methods.
Supplemental Figure S1. Root-dominant expression of CKX2 resulted in reduced cytokinins content in roots.
Supplemental Figure S2. Sprouts number of nine wild-type ramets and 98 independent transgenic events.
Supplemental Table S1. Data of sprouts number of wild-type ramets and independent transgenic events.
Supplemental Table S2. Relative expression levels of the CKX gene in representative transgenic poplar plants.
Acknowledgments
We thank Mr. Frederick Pettit and his crew at the University of Connecticut, Greenhouse Plant Growth Facility, for their help in growing and maintaining plants.
Footnotes
The work was supported by the U.S. Department of Agriculture, National Institute of Food and Agriculture Biotechnology Risk Assessment Grants Program (grant no. 2010-33522-21697), the Storrs Agricultural Experiment Station, and 111 Project (no. B17043).
Articles can be viewed without a subscription.
References
- Chang S, Mahon EL, MacKay HA, Rottmann WH, Strauss SH, Pijut PM, Powell WA, Coffey V, Lu H, Mansfield SD, et al. (2018) Genetic engineering of trees: Progress and new horizons. In Vitro Cell Dev Biol Plant 54: 341–376 [Google Scholar]
- Chen L, Li W, Katin-Grazzini L, Ding J, Gu X, Li Y, Gu T, Wang R, Lin X, Deng Z, et al. (2018) A method for the production and expedient screening of CRISPR/Cas9-mediated non-transgenic mutant plants. Hortic Res 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou SJ, Liu WY, Fang CL, Lin TY (2010) Characterization of the Scutellaria barbata glycosyltransferase gene and its promoter. Planta 232: 963–974 [DOI] [PubMed] [Google Scholar]
- DiFazio SP, Leonardi S, Slavov GT, Garman SL, Adams WT, Strauss SH (2012) Gene flow and simulation of transgene dispersal from hybrid poplar plantations. New Phytol 193: 903–915 [DOI] [PubMed] [Google Scholar]
- Frey BR, Lieffers VJ, Landhäusser SM, Comeau PG, Greenway KJ (2003) An analysis of sucker regeneration of trembling aspen. Can J For Res 33: 1169–1179 [Google Scholar]
- Guo L, Shao X, Xue P, Tian Y, Xiao Z, Wu Y (2017) Root sprouting ability and growth dynamics of the root suckers of Emmenopterys henryi, a rare and endangered plant endemic to China. For Ecol Manage 389: 35–45 [Google Scholar]
- Hu W, Fagundez S, Katin-Grazzini L, Li Y, Li W, Chen Y, Wang X, Deng Z, Xie S, McAvoy RJ, et al. (2017) Endogenous auxin and its manipulation influence in vitro shoot organogenesis of citrus epicotyl explants. Hortic Res 4: 17071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean SA, Pinno BD, Nielsen SE (2019) Trembling aspen root suckering and stump sprouting response to above ground disturbance on a reclaimed boreal oil sands site in Alberta, Canada. New For doi: 10.1007/s11056-018-09698-2 [Google Scholar]
- Klocko AL, Lu H, Magnuson A, Brunner AM, Ma C, Strauss SH (2018) Phenotypic expression and stability in a large-scale field study of genetically engineered poplar containing sexual containment transgenes. Front Bioeng Biotechnol 6: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Hu W, Fang C, Chen L, Zhuang W, Katin-Grazzini L, McAvoy RJ, Guillard K, Li Y (2016) An AGAMOUS intron-driven cytotoxin leads to flowerless tobacco and produces no detrimental effects on vegetative growth of either tobacco or poplar. Plant Biotechnol J 14: 2276–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Fang C, Krishnan S, Chen J, Yu H, Murphy AS, Merewitz E, Katin-Grazzini L, McAvoy RJ, Deng Z, et al. (2017) Elevated auxin and reduced cytokinin contents in rootstocks improve their performance and grafting success. Plant Biotechnol J 15: 1556–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo K, Duan H, Zhao D, Zheng X, Deng W, Chen Y, Stewart CN Jr., McAvoy R, Jiang X, Wu Y, et al. (2007) ‘GM-gene-deletor’: Fused loxP-FRT recognition sequences dramatically improve the efficiency of FLP or CRE recombinase on transgene excision from pollen and seed of tobacco plants. Plant Biotechnol J 5: 263–274 [DOI] [PubMed] [Google Scholar]
- Smigocki AC, Owens LD (1988) Cytokinin gene fused with a strong promoter enhances shoot organogenesis and zeatin levels in transformed plant cells. Proc Natl Acad Sci USA 85: 5131–5135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stener LG, Rungis D, Belevich V, Malm J (2018) Change of clonal frequency in the second root sucker generation of hybrid aspen. For Ecol Manage 408: 174–182 [Google Scholar]
- Strauss SH, Costanza A, Séguin A (2015) BIOTECHNOLOGY. Genetically engineered trees: Paralysis from good intentions. Science 349: 794–795 [DOI] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T (2003) Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15: 2532–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Nehnevajova E, Köllmer I, Novák O, Strnad M, Krämer U, Schmülling T (2010) Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 22: 3905–3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiehle M, Eusemann P, Thevs N, Schnittler M (2009) Root suckering patterns in Populus euphratica (Euphrates poplar, Salicaceae). Trees (Berl) 23: 991–1001 [Google Scholar]
- Yang W, Kalluri UC, Difazio SP, Wullschleger SD, Tschaplinski TJ, Cheng ZM, Tuskan GA (2009) Poplar genomics: State of the science. Crit Rev Plant Sci 28: 285–308 [Google Scholar]