Squash nectaries break down starch to synthesize nectar sugars, with some contribution coming directly from the phloem, whereas trehalose metabolism appears to be important for nectar secretion.
Abstract
Floral nectar is a sugary solution produced by plants to entice pollinator visitation. A general mechanism for nectar secretion has been established from genetic studies in Arabidopsis (Arabidopsis thaliana); however, supporting metabolic and biochemical evidence for this model is scarce in other plant species. We used squash (Cucurbita pepo) to test whether the genetic model of nectar secretion in Arabidopsis is supported at the metabolic level in other species. As such, we analyzed the expression and activity of key enzymes involved in carbohydrate metabolism in squash nectaries throughout floral maturation and the associated starch and soluble sugars, as well as nectar volume and sugar under different growth conditions. Here we show that the steps that are important for nectar secretion in Arabidopsis, including nectary starch degradation, Suc synthesis, and Suc export, are supported by metabolic and biochemical data in C. pepo. Additionally, our findings suggest that sugars imported from the phloem during nectar secretion, without prior storage as starch, are important for generating C. pepo nectar. Finally, we predict that trehalose and trehalose 6-P play important regulatory roles in nectary starch degradation and nectar secretion. These data improve our understanding of how nectar is produced in an agronomically relevant species with the potential for use as a model to help us gain insight into the biochemistry and metabolism of nectar secretion in flowering plants.
Floral nectar is a plant secretion made predominantly of sugars that serves as a reward for pollinators and is essential for efficient reproduction in many plants. Nectar is secreted from specialized glands called nectaries and involves a number of steps that are tightly regulated in order to increase pollination success while conserving precious carbon resources (Roy et al., 2017). The composition of sugars in nectar varies greatly depending on the species. Some species, such as Arabidopsis (Arabidopsis thaliana), produce nectar rich in hexoses (Davis et al., 1998). Other species, such as tobacco (Nicotiana tabacum; Ren et al., 2007) and squash (Nepi et al., 2001; Solhaug et al., 2019), produce nectar rich in Suc. Previous research has shown that squash (Cucurbita pepo) cultivars with a higher Suc/hexose ratio in their nectar have increased pollinator visits compared with cultivars with lower Suc relative to hexoses (Roldán-Serrano and Guerra-Sanz, 2005), suggesting that composition of nectar sugar can influence pollinator visitation, which has also been shown to have a direct impact on fruit yield (Motzke et al., 2015; Pereira et al., 2015; Zou et al., 2017).
Early stages of nectary maturation are typically associated with build-up of starch in the nectary. Indeed, presecretory accumulation of starch in nectaries has been reported for many flowering species (Nepi et al., 1996; Peng et al., 2004; Ren et al., 2007; Lin et al., 2014; Solhaug et al., 2019). As secretion proceeds, nectary starch is degraded and nectar sugar is produced concurrently (Solhaug et al., 2019).
In Arabidopsis, Suc synthesis during secretion is important for efflux of sugar from nectar-secreting cells. Plants silenced for SUCROSE PHOSPHATE SYNTHASE 1F and 2F (sps1f/2f) fail to secrete nectar and accumulate more starch in their nectaries than wild type, suggesting synthesis of Suc from starch breakdown products is essential for nectar secretion in plants that accumulate starch in nectariferous tissues (Lin et al., 2014).
Although the connection between starch degradation and nectar secretion has been well established in a number of species (Peng et al., 2004; Ren et al., 2007; Lin et al., 2014; Solhaug et al., 2019), the proportion of nectar sugar coming from starch versus sugar derived directly from the phloem is not well understood. In some species, such as tobacco (Ren et al., 2007) and Anigozanthos flavidus (Wenzler et al., 2008), phloem sugar can be directly transported into nectar (bypassing starch). It is likely that the source of nectar sugar (i.e. nectary starch or direct phloem sugar) varies between species and may partially depend on coevolution of specific plant-pollinator interactions (Heil, 2011).
During secretion in Arabidopsis nectaries, Suc is exported predominantly through SUGARS WILL EVENTUALLY BE EXPORTED TRANSPORTERS9 (AtSWEET9), a nectary-specific plasma membrane-localized Suc uniporter (Lin et al., 2014). Similar to atsps1f/2f plants, atsweet9 mutants lack nectar and accumulate large amounts of starch in nectar-secreting cells (Lin et al., 2014), suggesting that Suc synthesis and export are essential for maintenance of nectary starch degradation and nectar secretion in Arabidopsis. Because AtSWEET9 is a bidirectional transporter (Lin et al., 2014), the transport of Suc from nectar-secreting cells is contingent on the development and maintenance of a concentration gradient of Suc between the inside and outside of the cells. One way that this gradient can be generated is by cleavage of Suc in the extracellular space by nectary-specific CELL WALL INVERTASE4 (AtCWINV4). atcwinv4 mutants do not make any nectar, but still accumulate starch in the nectary (Ruhlmann et al., 2010), suggesting that extracellular hydrolysis of Suc by AtCWINV4 is essential for nectar secretion, but is not necessary for import of phloem sugar into Arabidopsis nectaries. Although CWINV4 is essential for nectar secretion in Arabidopsis, other species, particularly those that utilize vesicles via a granulocrine model of nectar secretion (such as Echinacea purpurea; Wist and Davis, 2008) or those that produce a Suc-rich nectar (such as tobacco; Ren et al., 2007), may secrete nectar via a mechanism alternative to CWINV4.
In summary, the most supported model of nectar secretion in Arabidopsis involves build-up and degradation of nectary starch, resynthesis of Suc from starch-derived hexoses, export of Suc by AtSWEET9, and extracellular hydrolysis of Suc, which maintains a high intracellular-to-extracellular concentration gradient of Suc while also increasing the solute potential, causing water to flow out into the developing nectar droplet (Lin et al., 2014; Roy et al., 2017). Although this model has been highly useful in improving our understanding of the genes and proteins that are important for nectar secretion in starch-accumulating nectaries, it has not yet been fully tested from a biochemical and metabolic perspective. The small size of Arabidopsis nectary tissue makes such studies nearly impossible. In order to biochemically test the primary model of nectar secretion in Arabidopsis, we used C. pepo. C. pepo is an ideal system for studying metabolism in nectaries before, during, and after nectar secretion because the plants make many flowers with a typical flower producing over 1000-fold more nectar sugar than a typical Arabidopsis flower (∼25 mg sugar/flower for C. pepo [Nepi et al., 2001]; 1.5 µg sugar/flower in Arabidopsis [Davis et al., 1998]).
Transcriptomic studies have suggested that the mechanism of nectar secretion in C. pepo is similar to that in Arabidopsis (Solhaug et al., 2019). However, additional experiments are warranted to test whether this genetic model is reflected by the enzymatic patterns and flux of metabolites through the nectary system. In this study, we sought to examine how carbohydrates are trafficked, partitioned, and secreted into the nectar of C. pepo flowers. Here we show that the genetic model of nectar secretion in Arabidopsis is supported by physiological and biochemical experiments in C. pepo. We next show numerous pieces of evidence that phloem-derived sugars play an important role in nectar secretion in C. pepo. Finally, we examine the role of trehalose metabolism in regulation of starch degradation and production of nectar sugar. These data represent an important step in improving our understanding of how floral nectar is produced from a metabolic and biochemical perspective.
RESULTS
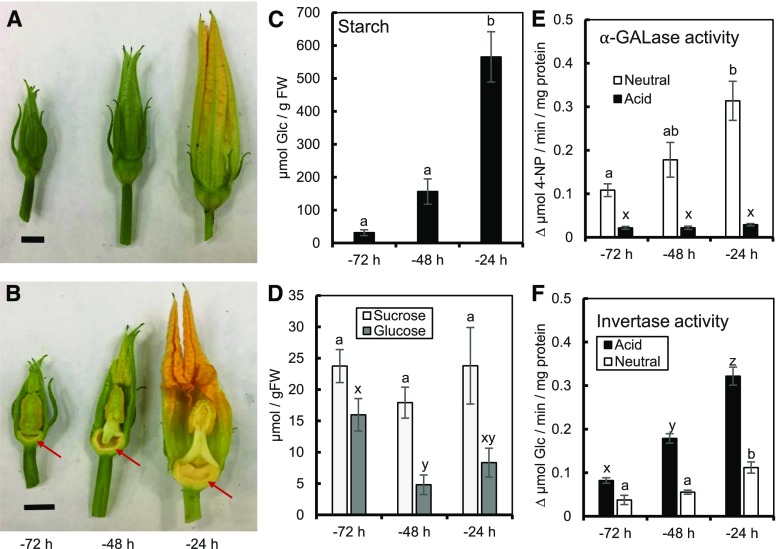
Most floral nectaries are nonphotosynthetic, being fully dependent on phloem-derived sugars, primarily stored as starch, as the precursors to nectar (Roy et al., 2017). As such, we first examined starch and soluble sugar accumulation from 3 d (−72 h) to 1 d (−24 h) before secretion (see Fig. 1, A and B for developmental series) in order to determine how nectaries store energy throughout development. Nectary starch increased over 10-fold from −72 to −24 h (Fig. 1C). There was no change in levels of nectary Suc, although nectary glucose decreased about 2-fold from −72 to −24 h (Fig. 1D). Over the same time course, we found a 3-fold increase in nectary mass (Supplemental Fig. S1C). These data suggest that most of the carbohydrates entering the nectaries between −72 and −24 h, henceforth termed the starch-filling stage, are broken down and either used to synthesize starch or metabolized to produce ATP for other processes essential for growth (e.g., cell wall acidification and loosening via H+-ATPase; Majda and Robert, 2018).
Figure 1.
Carbohydrate partitioning during the starch-filling stage in C. pepo nectaries. A and B, Example images of three developmental stages for whole (A) and dissected (B) flowers. Scale bars = 1 cm. Nectaries are denoted by an arrow in (B). C, Starch content in nectaries from −72 to −24 h before peak secretion. D, Levels of Suc and Glc in nectaries at the same time points. E, Neutral (pH 7.4) and acid (pH 5.5) ⍺-galactosidase (α-GALase) activity. F, Acid (pH 4.8) and neutral (pH 7) invertase activity (micromole Glc produced). For the activity assays, activity was normalized to total protein added (milligram protein). Bars that share a letter are not significantly different from one another (a, b, c were tested together; x, y, z were tested together; P < 0.05, Tukey post hoc test). Error bars represent SE (n = 4). 4-NP, 4-nitrophenol.
Because C. pepo uses raffinose-family oligosaccharides (RFOs), such as stachyose, as its primary transport sugars (Zhang et al., 2010), we used α-GALase as a measure of sink strength. α-GALase hydrolyses galactosyl units from stachyose (tetrasaccharide) and raffinose (trisaccharide) to form Suc (Carmi et al., 2003). We sought to determine whether there was a temporal similarity between α-GALase activity and starch-filling in nectaries at different time points. Total neutral α-GALase activity (cytosolic) in nectaries increased nearly 3-fold from −72 to −24 h (Fig. 1E). However, over the same time period, the acid α-GALase activity (vacuolar) was not significantly different. At each time point measured, the neutral α-GALase activity was higher than the acid activity (Fig. 1E). In order to determine whether α-GALase activity induction is specific to the nectary, we examined α-GALase activity in nectary, receptacle, and peduncle tissues (diagrammed in Supplemental Fig. S2A). The neutral α-GALase activity was over 2-fold higher in the nectary when compared with the peduncle or the receptacle at all three time points measured (Supplemental Fig. S2B). In contrast, acid α-GALase activity was not significantly different throughout the starch-filling stage (Supplemental Fig. S2C).
In order to further dissect the breakdown and utilization of soluble sugars during nectary development, we looked at invertase activity from −72 to −24 h. Acid (vacuolar) invertase (vINV) activity increased nearly 4-fold from −72 to −24 h, whereas neutral (cytosolic) invertase (cINV) increased 3-fold over the same time (Fig. 1F). vINV activity was consistently higher than cINV activity at all time points measured (Fig. 1F). Taken together, these data show that the starch-filling stage is accompanied by increases in cytosolic α-GALase and cINV, as well as vINV.
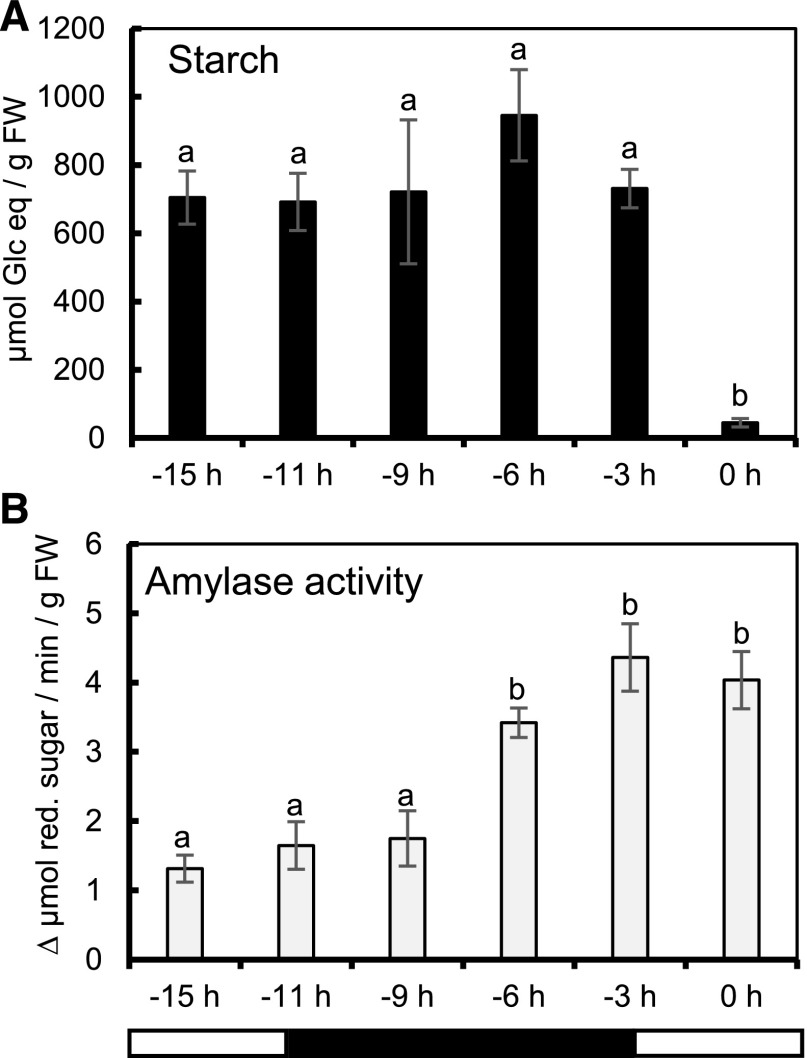
Previous studies have shown that degradation of nectary starch occurs simultaneously with the production of nectar sugar in many species (Nepi et al., 1996; Peng et al., 2004; Ren et al., 2007; Lin et al., 2014; Solhaug et al., 2019). In order to further examine starch metabolism in C. pepo nectaries, we first asked whether the starch was degraded during the dark period immediately preceding nectar secretion. Starch content in nectaries remained relatively constant from −15 h until −3 h (dawn = −3 h; Fig. 2A). However, from −3 to 0 h, the starch was degraded rapidly (P < 0.005; Fig. 2A). Because we saw an apparent dawn-induced degradation of starch beginning at −3 h, we wondered whether amylase (a group of major starch degradative enzymes) activity showed the same pattern. Amylase activity was not significantly different from −15 to −9 h (Fig. 2B). However, from −9 to −6 h, there was a significant increase in amylase activity (P = 0.039), which then remained constant until 0 h (Fig. 2B). These data show that amylase activity is strongly induced 3 h before the onset of dawn, suggesting the regulation of starch degradative enzymes is at least partially light independent, although it may be controlled via entrained circadian rhythms.
Figure 2.
Starch and sugar metabolism as nectaries progress toward nectar secretion. Starch (A) and total amylase activity [B; micromole reducing (red.) sugar produced] in nectaries are shown. Error bars represent SE (n = 8). Columns that share a letter are not significantly different from one another (Tukey post hoc test, P < 0.05). The bottom bar indicates light and dark periods.
Sink tissues, such as maize kernels or potato tubers, often contain a portion of their starch that is more resistant to degradation than transitory leaf starch (McCleary and Monaghan, 2002). In order to confirm that we were detecting all of the starch with our analytical method, we used an additional KOH treatment to measure starch that is resistant to hydrolysis by boiling with thermo-stable amylolytic enzymes (Megazyme, resistant starch protocol). There was no detectable difference when analyzing starch using a resistant starch protocol at −24 h, (Supplemental Fig. S3) indicating all of the starch is fully hydrolyzed during the normal starch assay.
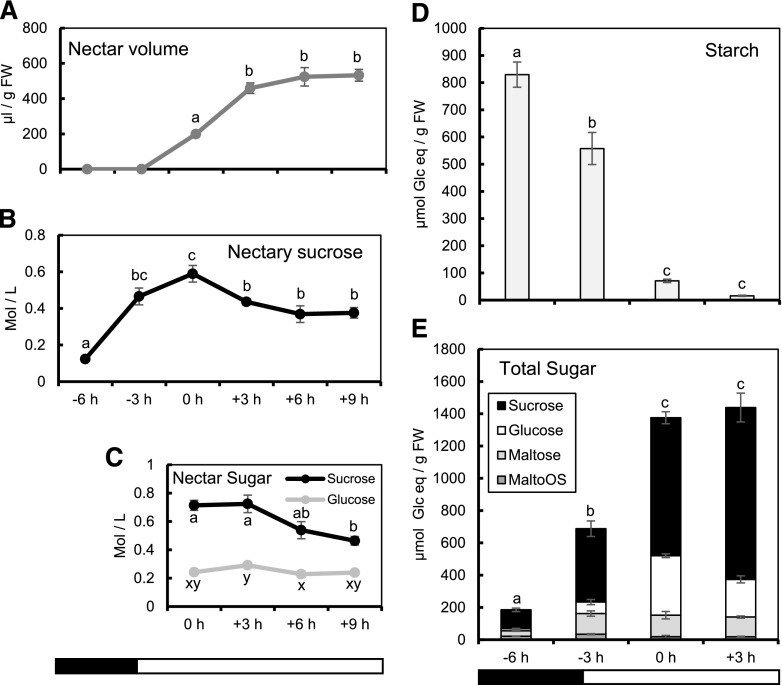
To further examine the role of starch degradation in nectar production, we analyzed starch, nectar volume and sugar, nectary sugar, and total combined sugar over a 9-h period starting from 3 h before dawn (Fig. 3; −6 h relative to peak nectar secretion). There was consistently no nectar present at dawn (−3 h; Fig. 3A), when starch is still present in nectaries (Fig. 2A; Fig. 3D). Nectar volume increased more than 2-fold from 0 to +3 h, but after +3 h the nectar volume did not significantly change (Fig. 3A). The period of greatest change in nectar volume was from −3 to +3 h (Fig. 3A), which coincided with near complete degradation of nectary starch (Fig. 3D), supporting previous results showing that nectary starch degradation is intimately connected to nectar secretion (Nepi et al., 1996; Peng et al., 2004; Ren et al., 2007; Lin et al., 2014; Solhaug et al., 2019). Our nectar volume data were also supported by previous data showing that most C. pepo nectar is produced in the early morning (Nepi et al., 2011).
Figure 3.
Carbohydrate partitioning during nectar secretion in C. pepo. A, Nectar volume corrected for nectary size (microliter per gram FW). B, Nectary Suc concentration (mole per liter). C, Nectar Suc and Glc concentrations (mole per liter) throughout the day. D and E, Total nectary starch (D) and total sugar (E, nectary+nectar) throughout secretion. Error bars represent SE [n = 8 for all data presented, except for (D) and (E), where n = 7 for −3 and +3 h and (A), where n = 9 for 0 h]. Columns that share a letter are not significantly different from one another (a, b, c were tested together; x, y, z were tested together; P < 0.05, Tukey post hoc test). The bottom bars indicates light and dark periods.
Previous studies in Arabidopsis suggested that extracellular hydrolysis of Suc by AtCWINV4 is required for the generation of a concentration gradient to drive Suc export (Ruhlmann et al., 2010). However, our recent study suggested there was low expression of CpCWINV4 in C. pepo nectaries at 0 h (Solhaug et al., 2019). In order to examine the mechanism of nectar secretion in squash, we sought to compare concentrations of sugar in the nectar and nectary throughout the process of nectar secretion. At 0 h (peak nectar secretion), the concentration of nectar Suc was 3-fold higher than Glc (Fig. 3C). From 0 to +3 h, nectar Suc concentration ([Suc]) stayed constant, whereas nectar Glc concentration ([Glc]) increased by 20% (Fig. 3C). From +3 to +9 h, nectar [Suc] decreased by 36%, whereas nectar [Glc] decreased by only 18% (Fig. 3C). However, an additional experiment testing the nectar sugar from a +24 h flower showed that [Suc] and [Glc] both decrease equally from 0 to +24 h (Supplemental Fig. S4), suggesting that resorption of Suc and Glc may occur simultaneously in C. pepo nectaries.
Based on a mass-flow model of nectar secretion predicted by Suc export via SWEET9 (Lin et al., 2014), two scenarios are possible for the export of nectar sugar. One possibility involves complete or near complete hydrolysis of Suc by CWINV4 in the extracellular space to create a concentration gradient facilitating continued export of nectary Suc, generating a hexose-rich nectar. Alternatively, the nectary could maintain a similarly high [Suc] as present in the nectar, allowing newly synthesized (or phloem-derived) Suc to be transported down the concentration gradient into the nectar without complete hydrolysis, leading to a nectar rich in Suc.
In order to compare the [Suc] between the nectary and the nectar, we estimated nectary volume using a two-half-sphere volume equation (Supplemental Fig. S5). At 0 h, during the highest rate of increase in nectar volume, the [Suc] in the nectary was ∼0.6 M (Fig. 3B), which was only 17% lower than the [Suc] in the nectar at 0h (∼0.7 M), suggesting that similar [Suc] in the nectary and nectar may be important to allow SWEET9-facilitated Suc export into the nectar. The nectary [Suc] decreased 36% from 0 to +9 h (Fig. 3B), almost perfectly matching the decrease in nectar [Suc], which also decreased 36% from +3 to +9 h (Fig. 3C). The striking similarity between nectary (cytosolic) and nectar (apoplastic) [Suc] further suggests that the creation and maintenance of a high cytosolic [Suc] in C. pepo nectaries is required for the initiation and continued flow of Suc into the nectar.
The close temporal alignment of nectary starch degradation with the accumulation of nectary Suc and nectar secretion led us to question what proportion of the total system (nectary+nectar) sugar comes from starch breakdown versus sugars supplied directly from the phloem without prior storage. In order to answer this question, we calculated total carbon balance comparing the total starch, Suc, Glc, maltose, and malto-oligosaccharides (maltoOS) in the nectary and nectar throughout the process of nectar secretion (Table 1). We found a maximum of ∼830 µmol of Glc equivalents per gram fresh weight (µmol Glc eq/g FW) existing as nectary starch (−6 h; Fig. 3D). At this time, there was ∼185 µmol Glc eq/g FW in total soluble sugar (Fig. 3E; Table 1). Because we did not find substantial degradation of starch before −6 h (Fig. 2A), we assumed that any soluble sugar present at −6 h did not come from nectary starch degradation. From −6 to −3 h, the starch decreased by ∼270 µmol Glc eq/g FW (Fig. 3E; Table 1), whereas the total sugar in the nectary increased by nearly 2-fold more (∼502 µmol Glc eq/g FW; Fig. 3E), suggesting at least some of this sugar does not come from starch degradation (Table 1). The total system (nectary+nectar, summed together) sugar increased by ∼1190 µmol Glc eq/g FW from −6 to 0 h, whereas the starch only decreased by ∼758 µmol Glc eq/g FW (Table 1). Additionally, our estimate of the total system sugar at +3 h was 1454 µmol Glc eq/g FW (Table 1), which is nearly 2-fold higher than the maximum starch we estimated to be in the nectary at −6 h (Table 1). Taken together, these data suggest that a majority (∼59%) of the total sugar in the nectary/nectar system at +3 h (time of peak Suc content in nectary+nectar system; Supplemental Fig. S6) comes from starch.
Table 1. Total Glc eq in the nectary plus nectar system during the secretory processa.
| Stage | Total Sucb | Total Glcb | Total Maltosec | Total MaltoOSc | Total Sugard | Total Starch | Total GLC Eqe |
|---|---|---|---|---|---|---|---|
| −6 h (n = 8) | 117 ± 11 | 13 ± 2 | 34 ± 4 | 21 ± 1 | 185 ± 16 | 829 ± 46 | 1,014 ± 57 |
| −3 h (n = 7) | 455 ± 48 | 71 ± 16 | 128 ± 17 | 34 ± 4 | 688 ± 78 | 558 ± 59 | 1,246 ± 76 |
| 0 h (n = 8) | 855 ± 37 | 369 ± 11 | 133 ± 23 | 19 ± 8 | 1,375 ± 43 | 71 ± 6 | 1,446 ± 45 |
| +3 h (n = 7) | 1,065 ± 89 | 233 ± 22 | 122 ± 7 | 18 ± 3 | 1,439 ± 103 | 16 ± 6 | 1,455 ± 103 |
Content of different sugars and starch in micromole of Glc eq per gram FW.
Sum of nectary and nectar (micromole Glc eq per grams FW). Total Suc (in Glc eq) was calculated as two times the total Suc measured.
Obtained from nectaries alone. Total Glc equivalents in maltose was calculated as two times the total maltose measured.
Sum of Suc, Glc, maltose, and maltoOS at individual time points.
Sum of total sugars and starch in the nectary and nectar system (in micromole Glc eq per grams FW).
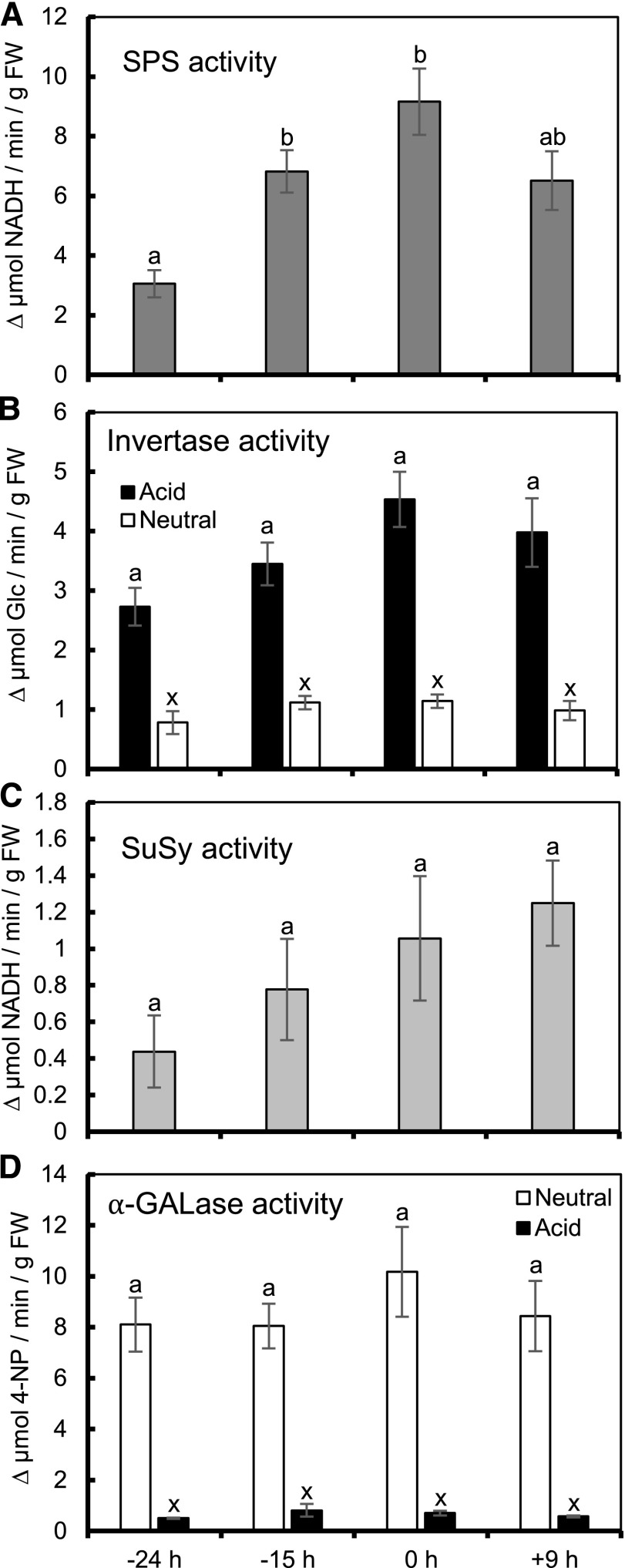
Previous data has suggested that Suc is synthesized from starch-derived hexoses via AtSPS1F/2F during nectar production in Arabidopsis (Lin et al., 2014). In order to determine whether Suc synthesis is also activated during secretion in C. pepo, we analyzed SPS activity at four stages of nectary maturation (−24, −15, 0, and +9 h; examples in Supplemental Fig. S1A). SPS activity was nearly 3-fold higher at 0 h compared to −24 h (P = 0.003; Fig. 4A), suggesting that de novo synthesis of Suc from hexoses plays an important role during nectar secretion. SPS activity decreased from 0 h to +9 h by nearly 20%; however, this difference was not statistically significant (Fig. 4A).
Figure 4.
Activity of sugar metabolism enzymes during nectary maturation. A, SPS activity is induced during secretion. B, Acid (pH 4.8) invertase increases during secretion (P < 0.1), whereas neutral (pH 7) invertase activity stays constant throughout secretion. C, SuSy activity increases throughout secretion. D, Acid (pH 5.5) and neutral (pH 7.4) α-GALase assays show no change in activity throughout secretion. Error bars represent SE (n = 4, except for SuSy activity at −15 h, where n = 3). Columns that share a letter are not significantly different from one another ([Tukey post hoc test, P < 0.05 for (A), (C), and (D); P < 0.1 for (B)]. 4-NP, 4-nitrophenol.
Because we found evidence that the maintenance of a high cytosolic Suc concentration is important for generating C. pepo nectar (Fig. 3), we wanted to see whether Suc degradation by intracellular invertase (Suc hydrolase) and Suc synthase (SuSy; important for Suc catabolism and sink strength; Angeles-Núñez and Tiessen, 2010; Ferreira and Sonnewald, 2012) were decreased in order to maintain intracellular [Suc]. Activity of acid invertase (vacuolar, vINV) increased by nearly 66% from −24 to 0 h (Fig. 4B), suggesting vacuolar hydrolysis of stored Suc increases during secretion. However, neutral invertase (cytosolic, cINV) activity did not change throughout secretion (Fig. 4B). Additionally, SuSy activity increased gradually from −24 to +9 h, but these differences were not statistically significant (P > 0.18; Fig. 4C).
In order to further test whether sugar imported from the phloem is a substantial portion of the total carbon balance in nectaries during nectar secretion, we examined whether the activity of α-GALase, as a measure of sink capacity, varied throughout the same stages. Neither neutral (cytosolic) nor acid (vacuolar) α-GALase activity were significantly different at any of the four stages measured (Fig. 4D), suggesting that import and breakdown of phloem-derived RFOs by C. pepo nectaries is unchanged during nectar secretion.
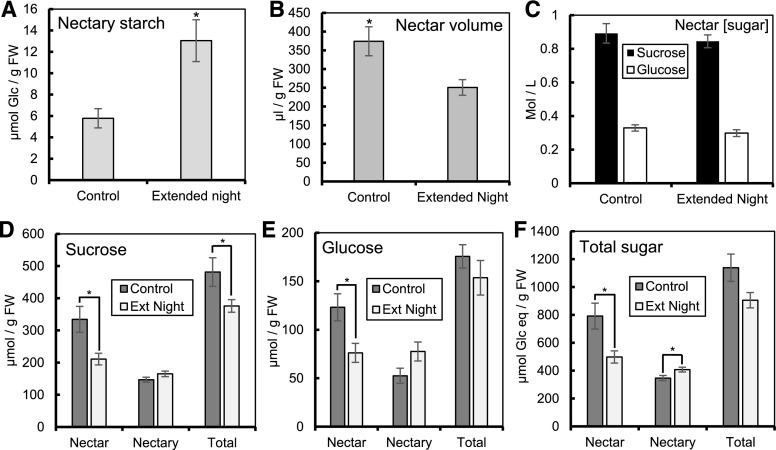
We next attempted to reduce the amount of sugar coming from phloem-derived photoassimilates in order to determine the extent to which phloem sugars (without prior storage as starch) contribute to C. pepo nectar sugar production. Toward this end, we exposed whole plants to a 5-h extended night to induce starvation in source tissues (and nectaries) before analyzing nectar(y) carbohydrates. Nectary starch was 2.5-fold higher in plants exposed to an extended night compared with control nectaries harvested the previous morning (P = 0.0045; Fig. 5A); however, this difference represented less than 2% of the total starch present in the nectary (assuming ∼830 µmol Glc eq/g FW at peak of nectary starch), suggesting most of the starch is still degraded in the absence of the light stimulus. Total nectar volume was 49% higher in the control nectaries compared with the extended night (Fig. 5B), but there was no significant difference in nectar [Suc] or [Glc] (Fig. 5C). We next examined total Suc, Glc (in micromole per gram FW), and total sugar (Suc+Glc, in Glc eq) in nectar, nectary, and total system (nectary+nectar, summed together) in order to determine whether reduction of phloem-derived sugar led to differences in carbohydrate partitioning. The total nectar Suc was 58% higher in the control versus the extended night samples (Fig. 5D), whereas the total system Suc was 28% higher in the control compared with extended night (Fig. 5D). However, the total nectary Suc was not significantly reduced upon treating the plants with an extended night (Fig. 5D). Similar to nectar Suc, the total nectar Glc was 62% higher in the control versus the extended night samples (Fig. 5E), but there was no difference in nectary or total system Glc (Fig. 5E). Additionally, we found 59% more total nectar sugar in control versus extended night samples (Fig. 5F). Although there was more nectar sugar in control samples, total system sugar did not vary between control versus extended night samples (Fig. 5F). Interestingly, there was actually 15% more total nectary sugar in extended night samples compared with control (P = 0.032; Fig. 5F). In summary, exposing plants to an extended night causes flowers to store more sugar in their nectaries rather than secreting sugar into the nectar.
Figure 5.
Carbohydrate partitioning during an artificially extended night. Dawn was delayed for 5 h before harvesting nectar and nectary tissue and analyzing sugar and starch. A and B, Nectary starch (A) and nectar volume (B) corrected for grams FW of nectary. C, Concentration (mole per liter) of Suc and Glc in the nectar. D to F, Total Suc (D), Glc (E), and total sugar (Suc+Glc) in Glc equivalents (F) of nectary and nectar, corrected for FW of nectary. *P ≤ 0.05 (2-sample t test). Error bars represent SE (n = 8). Ext Night, Extended Night.
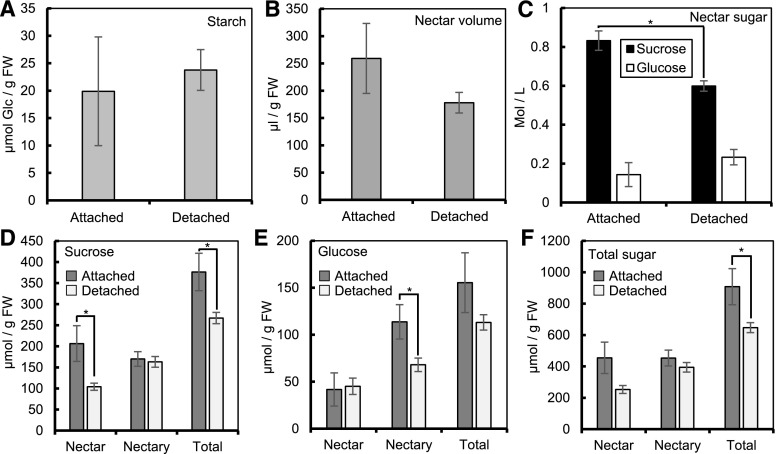
We next sought to alter the amount of phloem sugars entering the nectaries by removing flowers from plants at −15 h (the evening before secretion) and placing their peduncles in water. We compared carbon balance at +1 h (total 16-h starvation) in flowers left on the plant for the night (attached) to flowers removed and put into water (detached). Removing the flowers from the plants led to no change in nectary starch (Fig. 6A) or nectar volume (Fig. 6B). However, the nectar [Suc] was 39% higher in the attached flowers compared with the detached flowers (P = 0.0008; Fig. 6C), whereas the nectar [Glc] was actually higher in the detached flowers, although this difference was not statistically significant (Fig. 6C). The total nectar Suc was nearly 2-fold higher in the attached samples compared with detached (Fig. 6D), whereas total system Suc was also higher in attached flowers, but to a lesser extent (41%; Fig. 6D). Similar to the response seen when extending the night, nectary Suc did not change as a result of removing the flowers from the plants (Fig. 6D). The total nectar Glc and total system Glc (micromole per gram FW) were not significantly different between detached and attached samples, but nectary Glc was nearly 67% higher in the attached samples compared with the detached samples (Fig. 6E). Total sugar in the nectar or nectary was not statistically different between attached and detached flowers (Fig. 6F). However, there was more total system sugar in attached flowers compared with detached flowers (P = 0.037; Fig. 6F). Taken together, these data suggest that removing the flowers from plants and treating them with water overnight causes a highly similar response to exposing them to an extended night, in that the flowers retain more sugar in their nectaries rather than secreting the sugar into the nectar.
Figure 6.
Carbohydrate partitioning after removing flowers from plants. A and B, Nectary starch (A) and nectar volume (B) corrected for grams FW of nectary. C, Concentration (mole per liter) of Suc and Glc in the nectar. D to F, Total Suc (D), Glc (E), and total sugar (Suc+Glc) in Glc equivalents (F) content of nectary and nectar, corrected for FW of nectary. Detached, removed at −15 h and placed in water; Attached, removed from the plant the following morning. *P ≤ 0.05 (2-sample t test). Error bars represent SE (n = 8 for Detached, n = 7 for Attached).
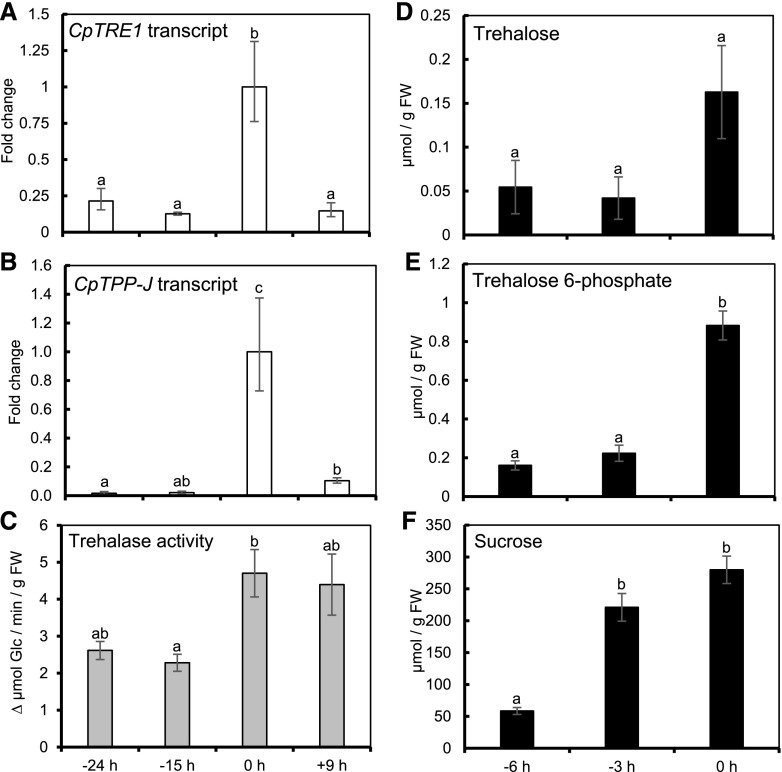
Because Suc metabolism plays a central role in nectar secretion, we were interested in whether sugar signals play regulatory roles in essential secretory processes such as starch degradation and Suc synthesis. In particular, we previously found up-regulation of transcripts for genes encoding enzymes involved in trehalose metabolism during secretion (Solhaug et al., 2019). In order to validate these data, we re-examined expression using reverse transcription-quantitative PCR (RT-qPCR) for Trehalase1 (CpTRE1) and Trehalose-phosphate phosphatase-J (CpTPP-J), which code for important enzymes in the catabolism of trehalose and Tre-6P, respectively (Figueroa and Lunn, 2016). Expression of CpTRE1 was nearly 5-fold higher during secretion (0 h) compared with −24 and −15 h before secretion, and were nearly 6-fold higher at 0 h compared with +9 h (Fig. 7A). Additionally, CpTPP-J transcripts were over 5-fold induced during secretion compared with −24 and −15 h, then decreased slightly from 0 to +9 h (not significant; Fig. 7B). We next examined whether the increase in CpTRE1 expression led to a difference in enzymatic activity for trehalase. Trehalase activity was nearly 2-fold higher at 0 h relative to −15 h (P = 0.039; Fig. 7C), further suggesting induction of trehalose-related catabolism may be an important step in C. pepo nectar secretion. Given the induction of trehalose metabolic enzymes during secretion, we wondered whether trehalose and trehalose-6P (Tre6P) levels changed in the lead up to secretion. Both trehalose (Fig. 7D) and Tre6P (Fig. 7E) remained constant from −6 to −3 h, then increased by nearly 3-fold from −3 to 0 h (Fig. 7, D and E). Numerous studies have demonstrated that Tre6P levels are directly proportional to Suc across different species, growth conditions, and tissues (Wingler et al., 2000; Debast et al., 2011; Martins et al., 2013; Nuccio et al., 2015; Figueroa et al., 2016; Figueroa and Lunn, 2016), so we also included a reanalysis of nectary Suc accumulation (same data as in Fig. 3B, except expressed in micromole per gram FW) during the same time points. The nectary Suc increased nearly 4-fold from −6 to −3 h (Fig. 7F), then increased further from −3 to 0 h, but to a much lesser extent (not significant; Fig. 7F). These data show that Tre, Tre6P, and trehalose catabolic enzymes accumulate along with Suc in nectaries during secretion (0 h), although Suc accumulation precedes that of Tre and Tre6P (−3 h in Fig. 7).
Figure 7.
Trehalose metabolism in nectaries. A and B, Gene expression of Trehalase1 (CpTRE1; A) and Trehalose-phosphate phosphatase J (CpTPP-J; B) measured in nectaries by RT-qPCR throughout maturation; normalized to CpRING (XM _008439865.1) and presented as relative expression (0 h = 1; n = 3). C, Trehalase activity throughout nectary maturation (n = 4). D to F, Accumulation of trehalose (D), Tre6P (E), and Suc (F) throughout nectary maturation (n = 7 for trehalose; n = 3 for Tre6P; n = 8 for Suc). Bars that share a letter are not statistically significantly different from one another (Tukey post hoc test, P < 0.05). Error bars represent SE.
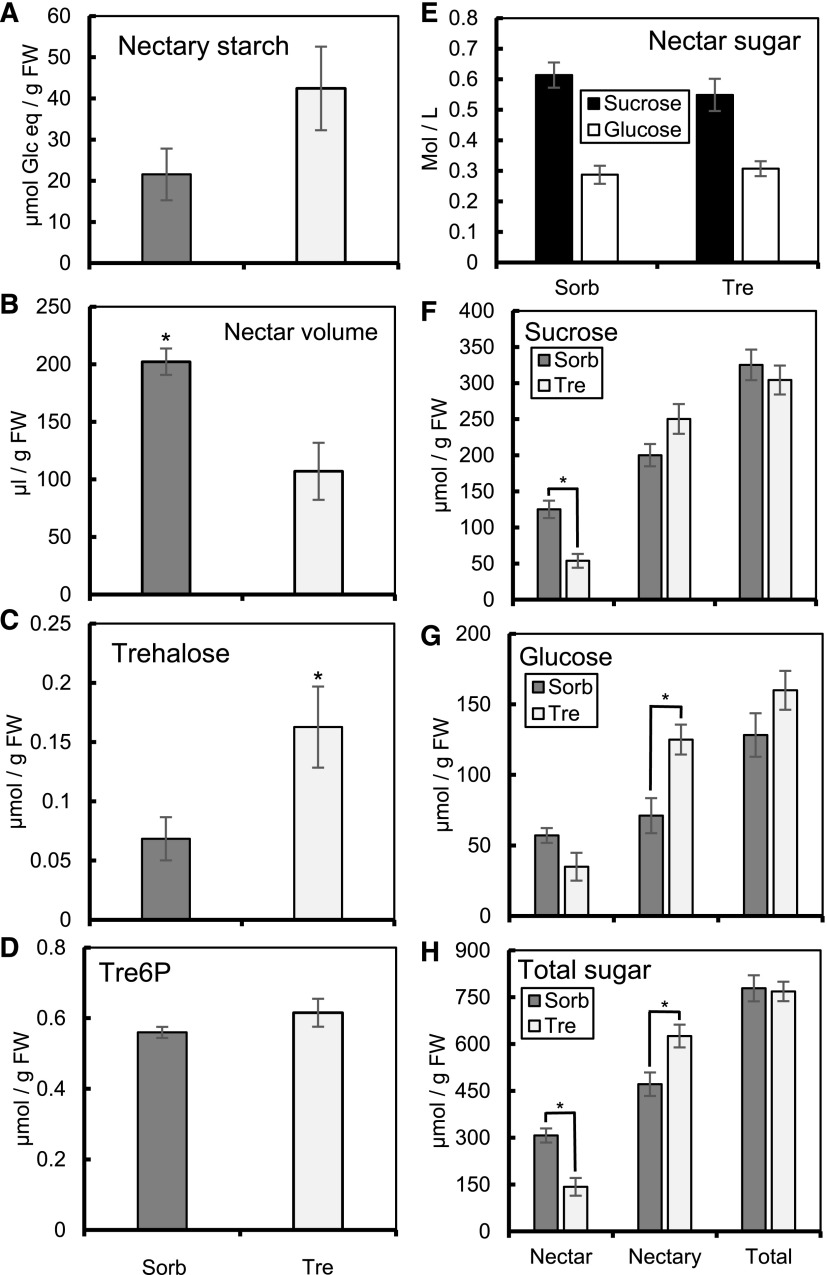
In order to further examine the role of trehalose in regulation of nectar production, we treated detached flowers (−15 h) overnight with either 10 mm trehalose (Tre) or 10 mm sorbitol (Sorb). Tre treatment led to 2-fold higher nectary starch at +1 h when compared with the sorbitol treatment; however, the difference was not statistically significant (P = 0.122; Fig. 8A). Tre-treated flowers also produced nearly 2-fold lower nectar volume compared with the sorbitol control (Fig. 8B). There was no significant difference in nectar [Suc] or [Glc] of trehalose-treated flowers compared with sorbitol (Fig. 8E). The total nectar Suc was over 2-fold lower in the trehalose-treated flowers compared with sorbitol controls, whereas the nectary Suc and total system Suc was not significantly different between the two treatments (Fig. 8F). The total nectary Glc was nearly 2-fold higher in trehalose-treated samples compared with sorbitol; however, neither nectar Glc nor total system Glc were significantly different (Fig. 8G). Finally, the total sugar in the nectar was more than 2-fold lower in the trehalose-treated samples compared with control, whereas the total nectary sugar was 33% higher in the trehalose-treated samples compared with control (Fig. 8H). The total system sugar was remarkably similar between the sorbitol and trehalose-treated samples (Fig. 8H). In order to determine whether the inhibited starch degradation and nectar secretion was due to alterations in either trehalose or Tre6P, we analyzed Tre6P and trehalose content in trehalose- and sorbitol-treated samples. There was nearly 3-fold more trehalose in the trehalose-treated samples compared with sorbitol control (P = 0.041; Fig. 8C), but there was only slightly more Tre6P (Fig. 8D). Taken together, these data show that trehalose treatment inhibits nectary starch degradation and production of nectar sugar in C. pepo nectaries, possibly via a Tre6P-independent mechanism.
Figure 8.
Exogenous trehalose inhibits nectar production. To examine a potential role for trehalose in regulating nectar production, the peduncles of excised flowers were placed in solutions containing either 10 mm sorbitol (Sorb; osmotic control) or 10 mm trehalose (Tre). A to D, Nectary starch (A), nectar volume (B), nectary trehalose (C), and nectary Tre6P (D), all corrected for grams FW of nectary, are shown. E, Concentration (mole per liter) of Suc and Glc in the nectar. F to H, Total Suc (F), Glc (G), and total sugar (Suc+Glc) in Glc equivalents (H) of nectary and nectar, corrected for FW of nectary. *P ≤ 0.05 (2-tailed t test). Error bars represent SE (n = 3 for Tre6P; n = 6 for trehalose; n = 6 for Sorb samples in starch data; n = 7 for all other samples).
DISCUSSION
This work provides substantial insight into how carbohydrates are trafficked and partitioned by nectaries during nectary maturation and nectar secretion in C. pepo. We have presented metabolic evidence supporting the eccrine model of nectar secretion discovered in Arabidopsis, suggesting the mechanism of nectar secretion is conserved between these two species. It appears that phloem-derived sugars, without prior storage as starch, are important for the generation of nectar, but that nectar can still be produced in their absence. Finally, trehalose metabolism is likely important for regulation of starch degradation and nectar secretion in C. pepo.
An Updated Model of Secretion for a Suc-Rich Nectar
In Arabidopsis, previous genetic experiments have identified a number of steps that are important for nectar secretion, including starch accumulation and degradation, Suc synthesis by AtSPS1F/2F, Suc export by AtSWEET9, and extracellular hydrolysis of Suc by AtCWINV4 (Ruhlmann et al., 2010; Lin et al., 2014). In this study, we tested various steps of this mechanism to see if they are conserved in C. pepo.
Starch Accumulation and Degradation
In C. pepo nectaries from −72 until −24 h (before secretion), the nectaries accumulate starch whereas the levels of soluble sugars do not change (Fig. 1). During this same time period, the nectaries grow drastically, although they continue to grow throughout secretion to a lesser extent (Supplemental Fig. S1). These findings suggest that most of the carbohydrate coming into the nectary is converted into starch or metabolized to fuel other energetically demanding biosynthetic processes essential for continued growth of nectaries. During the starch-filling stage (−72 to −24 h), nectaries also increase in both acid (vacuolar) invertase and neutral (cytosolic) α-galactosidase activity (Fig. 1), indicating a mechanism by which sink strength of the nectary increases throughout the starch-filling stage.
The close temporal relationship between degradation of nectary starch and production of nectar sugar has been demonstrated in a number of species (e.g. Ren et al., 2007; Lin et al., 2014), but very little is known about the mechanism behind this process. Previous data has shown that the expression of β-amylase1 (CpBAM1) is induced during secretion in C. pepo (Solhaug et al., 2019). In Arabidopsis, AtBAM1 plays an important role in light-induced starch degradation in leaf guard cells (Horrer et al., 2016). Although AtBAM1 is regulated by light in Arabidopsis leaves, and CpBAM1 likely plays a crucial role in nectary starch degradation, we have shown evidence that nectary starch degradation in C. pepo is regulated independently of light. First, total amylase activity (α and β) is induced 3 h before dawn and the beginning of nectar secretion (Fig. 2B). Second, transcripts coding for other enzymes crucial for starch degradation, such as α-glucan phosphorylase 2 (CpPHS2) and disproportionating enzyme 2 (CpDPE2; Streb and Zeeman, 2012), are expressed at high levels in the evening before nectar secretion (Supplemental Fig. S7). Finally, a large portion of the starch is still degraded even if the night is extended, although it is likely degraded at a slower rate (Fig. 5A). Taken together, these data indicate that nectary starch degradation probably occurs in response to developmental and/or circadian signals, but is mostly light independent.
Suc Synthesis
Previous research has shown that Suc synthesis by AtSPS1F/2F is important for nectar secretion in Arabidopsis (Lin et al., 2014). In C. pepo, CpSPS3F mRNA is high at −24 h and remains high throughout secretion, before decreasing at +9 h (Solhaug et al., 2019). Although CpSPS3F transcript does not change much from −24 to 0 h, we show here that the SPS activity is significantly higher at 0 h compared to −24 h (Fig. 4A), suggesting SPS may be regulated post-transcriptionally and/or post-translationally. SPS has been shown to be regulated by reversible phosphorylation in leaves (Winter and Huber, 2000), altering affinity for substrates without impacting maximum catalytic activity (McMichael et al., 1995).
Whereas Suc synthesis activity via SPS is induced during secretion, the activities of Suc degradation enzymes do not substantially change (cINV and SuSy; Fig. 4, B and C). Because SPS typically exists in the cytosol (Champigny and Foyer, 1992), it is possible that an increase in SPS activity without a concomitant increase in cINV and SuSy could favor Suc synthesis (rather than degradation), leading to high cytosolic [Suc] and passive transport of newly synthesized Suc out of the cell via SWEET9. While cytosolic Suc degradation (relative to synthesis) is likely lower during secretion, activity of vacuolar invertase was induced slightly (Fig. 4B). vINV may be important for liberating hexoses from stored vacuolar Suc during secretion, as transcripts encoding two predicted vacuolar hexose transporters (Poschet et al., 2011), ERD6-like 6 (CpEL6) and ERD6-like 16 (CpEL16), are highly expressed in nectaries during the build-up to secretion (Supplemental Fig. S7).
Suc export via SWEET9
SWEET9 is a uniporter (Lin et al., 2014; Eom et al., 2015); therefore, transport of Suc into nectar is contingent on the generation of a concentration gradient between the nectar-secreting cells and the nectar (analogous to the apoplast). In species that produce a hexose-rich nectar, like Arabidopsis, this intracellular:apoplastic Suc gradient is likely generated by extracellular hydrolysis of Suc by CWINV4 (Ruhlmann et al., 2010); however, species that produce a Suc-rich nectar cannot rely on complete hydrolysis of Suc to generate a Suc gradient. Our estimate of intracellular Suc concentration ([Suc]; Fig. 3B) at the time of maximum nectar secretion (0 h; Fig. 3A) was remarkably similar to the [Suc] in the nectar (Fig. 3, B and C). If nectary and nectar [Suc] are highly similar, any additional Suc produced from Suc synthesis in the nectary (or degradation of phloem-derived RFOs) during secretion would be transported down the Suc gradient and into the nectar. Taken together, these data indicate that maintaining an intracellular [Suc] that is similar to the nectar may be important for sustaining Suc export in species that produce a Suc-rich nectar.
Even though the [Suc] in the nectar and nectary are similar, we did see a slightly lower [Suc] in the nectary compared with the nectar (Fig. 3), which seemingly detracts from our hypothesis of mass-flow transport via SWEET9. However, it is possible that our estimate of [Suc] may be different than what is actually present in the nectar-secreting cells. In determining nectar [Suc], we calculated cytosolic volume based on estimates from scanning electron microscopy imaging data (Nepi et al., 1996) that reported the relative volumes of vacuole, mitochondria, and plastids in C. pepo nectary parenchyma cells (Supplemental Table S1). Our calculations are thus partially dependent on the accuracy of those estimates. Additionally, different parts of nectaries likely play different roles in nectar secretion. Nepi and others found that epidermal cells are predominantly composed of vacuoles (which may be important for sugar storage), whereas parenchyma cells stored most of the nectary starch (Nepi et al., 1996). Data from Nepi and others suggest that epidermal cells in C. pepo nectaries may be important for storing and eventually producing the sugar destined for secretion in the nectar. Our estimate of nectary sugar concentration is based on whole nectaries (epidermis and parenchyma). Because parenchyma cells near the epidermis (or possibly epidermal cells themselves) are likely the ones secreting the nectar, it is possible that the [Suc] near or at the epidermis is actually much higher than what we measured merely because we measured all parenchyma cells (some of which may have a lower [Suc]), thereby diluting the [Suc] of the cells that are actively secreting nectar.
In Arabidopsis, the expression and activity of AtCWINV4 is induced during secretion and is required for the eccrine model of nectar secretion via SWEET9 (Ruhlmann et al., 2010). Previous studies have shown that CpCWINV4 is also highly expressed in C. pepo nectaries, although it shows a slightly different expression pattern than in Arabidopsis. CpCWINV4 expression is decreased during secretion in C. pepo nectaries (Solhaug et al., 2019), suggesting that down-regulation of extracellular hydrolysis may be important for generating a Suc-rich nectar. However, previous results have shown that C. pepo nectar likely contains endogenous invertase activity (Nepi et al., 2012), suggesting Suc hydrolysis may partially drive export of nectar sugar. Our data suggest that from 0 h to +3 h, the levels of Glc in the nectar increase whereas the levels of Suc stay the same (Fig. 3C). This increase in Glc is not accompanied by a change in Suc (Fig. 3C), suggesting apoplastic (or nectar) Suc hydrolysis may be occurring. If extracellular hydrolysis by CpCWINV4 (or nectar-derived invertases) is important for nectar production in C. pepo, it is surely to a lesser extent than in species producing a hexose-rich nectar.
Recovery of Nectar Sugar by Resorption
Previous research has suggested that nectar sugar can be resorbed from C. pepo flowers (Nepi et al., 2001). Recovery and re-utilization of nectar sugar may partially ameliorate the energetic cost of producing nectar. We show here that the [Suc] in both the nectar and nectary falls drastically throughout the day (Fig. 3C), and [Glc] may also decrease to a similar extent based on our measurements of nectar sugar in flowers at +24 h after secretion (Supplemental Fig. S4A). We see sustained activity of SuSy and INV in the nectary post-secretion (+9h; Fig. 4), suggesting these enzymes may play a role in breaking down nectar-derived Suc after secretion. Hexoses produced from Suc breakdown can then be used for biosynthesis of new RFOs, or to produce energy for senescence-related processes or growth of seeds in the case of female flowers. The exact mechanism of nectar resorption and the role it plays in overall plant health is currently unknown and requires further testing.
The Role of Nonstarch Sugar in Nectar Production
Here we present numerous pieces of evidence to suggest that direct transport of phloem sugar, without prior storage as starch, plays an important role in the production of nectar in C. pepo. First, the total sugar in the nectar and nectary system is substantially more than exists in nectary starch at the peak of starch accumulation. Second, nectary sink strength is maintained throughout nectar secretion, showing that import of phloem sugars into nectaries likely remains constant during secretion. Finally, reducing the amount of phloem sugar coming to the nectary results in lower nectar sugar, further suggesting phloem sugars play an important role in nectar production.
Total Sugar in Nectary and Nectar Exceed Total Starch
The similar timing of nectar sugar production and breakdown of nectary starch (Fig. 3D) suggests that nectar sugar may come from hexoses derived from starch. Conversely, Nicotiana nectaries have shown the ability to uptake Suc from media during secretion, suggesting a bypass of nectary starch and direct transport of phloem sugar into the nectar is possible (Ren et al., 2007). We have presented evidence that the total sugar in the nectar and nectary system is substantially more than exists only in starch (Table 1).
Although these data suggest that at least some of the nectar and nectary sugar comes from sources other than starch, it is also possible that there are other forms of carbon that come from the starch that we did not detect. For instance, it is possible that other long-chain maltoOS (Critchley et al., 2001) derived from starch are present, but were not measured in this study. Additionally, it is possible that we may be underestimating the level of sugar in the nectary. For example, we did not measure Fru, which likely represents a large portion of the total soluble sugar present in the tissue and nectar. However, assuming that at least some Fru is present, this would only increase the estimate of total sugar (which is already higher than starch; Fig. 3E), lending more evidence to support the hypothesis that phloem-derived sugar makes up a substantial portion of total system sugar.
Sink Strength Is Maintained in Nectaries during Nectar Secretion
We have presented evidence that activity of enzymes important for sink strength, including INV, α-GALase, and SuSy, do not decrease during secretion (Fig. 4), suggesting that sink status and sugar import is maintained. In Arabidopsis, the import and degradation of Suc from phloem during secretion is difficult to reconcile with the mounting evidence that nectaries actively synthesize Suc during secretion (Lin et al., 2014; Solhaug et al., 2019). However, the breakdown products of starch (hexose-phosphates) can be incorporated into Suc directly, preventing futile degradation and synthesis of Suc in the nectary. From an energetics perspective, the current eccrine model in Arabidopsis may require a reduced role of direct phloem sugars (and an increased role of degraded nectary starch) in producing nectar sugar.
Although direct phloem sugars may be less important for generating nectar sugar in species that transport Suc in their phloem, the story may be different for species that transport RFOs in their phloem, such as C. pepo (Beebe and Turgeon, 1992). Stachyose, the predominant phloem sugar in C. pepo (Zhang et al., 2010), contains two galactosyl moieties attached to Suc (Holthaus and Schmitz, 1991). Stachyose degradation by α-GALase produces one Suc and two Gal, which can be converted into UDP-Glc (UDP-glc) by a number of enzymes, of which UDP-Glc/UDP-Gal-4-epimerase (UGE) is an important step. CpUGE5 expression is high during the build-up to secretion in C. pepo nectaries (Supplemental Fig. S7). Because the degradation of RFOs liberates hexoses without degrading Suc, import and degradation of phloem sugar can coexist with nectary Suc synthesis without futile synthesis/degradation cycles. Even though our data suggest that import of phloem-derived sugars during secretion is important for nectar production in C. pepo, it is possible that other species (particularly those that transport Suc via the phloem) might rely more on nectary starch to generate nectar sugar due to the conflict between breaking down incoming phloem Suc and synthesizing Suc from starch-derived hexoses.
Reducing Phloem Sugar Leads to a Reduction in Nectar Sugar
We have shown that two methods of reducing phloem sugar lead to decreases in nectar sugar. Exposing the plants to prolonged darkness led to decreased Suc and total sugar in the nectar (Fig. 5, D and F), likely due to reduced photosynthate from leaves and thus lower transport of phloem sugars to the nectary. Interestingly, extended night samples also had more nectary sugar than the control (Fig. 5F), suggesting that sugars were being retained more by the nectaries in response to darkness. Removing the flowers from the plants led to somewhat similar responses, namely a reduction in total system sugar (Fig. 6F) and a reduced [Suc] in the nectar (Fig. 6C). The removed (detached) flowers likely had a lower amount of phloem sugar available to them during secretion compared with the attached flowers, which likely caused the production of nectar to be inhibited. Both of these results show that reduction or removal of phloem-derived sugar negatively impacts nectar secretion, lending further support to our hypothesis that import of phloem-derived sugar during secretion is important for nectar production.
One interesting finding from the flower removal study is that the total sugar in the system is reduced by ∼200 µmol Glc eq in detached flowers versus attached (Fig. 6F), which is remarkably similar to the difference in starch content (also ∼200 µmol Glc eq; Fig. 2A) between −15 h (when the flowers were excised) and −6 h, the time of maximum nectary starch accumulation based on our data. It is, therefore, not out of the realm of possibility that the detached nectaries were deprived of full starch accumulation, which may have led to the reduction in total system sugar and [Suc] in the nectar. However, the difference of 200 µmol Glc eq also exists between control and extended night samples (Fig. 5F). In this case, the flowers had been attached to the plant for the whole night and were likely able to fully fill their nectaries with starch. Therefore, even though it is possible that the flower removal experiment was also affecting the ability of nectaries to accumulate starch, the fact that we saw the same response in extended night experiment supports the important role of phloem Suc in maintaining high intracellular [Suc] and Suc export by SWEET9.
Although these data clearly suggest that sugar produced from starch is supplemented by sugar derived from phloem in C. pepo nectaries during secretion, the fact remains that most of the sugar (∼59%) in the nectar comes from starch. The role of starch in nectar secretion may be to facilitate rapid production of high concentrations of intracellular sugar, which likely would not be possible if C. pepo nectar was produced via only export of phloem-derived sugar. Future experiments, possibly incorporating mutation of key genes such as BAM1 (Solhaug et al., 2019), may allow us to better understand the extent to which nectary starch facilitates secretion of nectar sugar in C. pepo.
A Role for Trehalose and Sugar Signaling during Nectar Secretion
Trehalose-6 phosphate (Tre6P) is a well-studied signal of Suc status in plants (Figueroa and Lunn, 2016). Here we analyzed the accumulation and catabolism of trehalose and Tre6P throughout secretion in order to determine their role(s) in C. pepo nectar secretion. Expression and activity of Tre6P- and trehalose-degradation enzymes is induced during secretion, whereas trehalose and Tre6P levels are increased at the same time (Fig. 7). Additionally, trehalose treatment leads to an inhibition of starch degradation and secretion of nectar sugar (Fig. 8), suggesting trehalose may play an inhibitory role in nectar sugar production and secretion.
Previous research has implicated Tre6P in regulation of starch degradation and maintenance of Suc homeostasis in both source and sink tissues (Wingler et al., 2000; Debast et al., 2011; Martins et al., 2013; Nuccio et al., 2015; Figueroa et al., 2016; Figueroa and Lunn, 2016). The rosette leaves of soil-grown Arabidopsis accumulate trehalose and Tre6P up to ∼20 nmol/gFW and ∼0.5 nmol/gFW, respectively (Carillo et al., 2013), with potato tubers accumulating Tre6P to similar levels (nearly 1 nmol/gFW; Debast et al., 2011); however, the endosperm of developing wheat grains have among the highest reported values for Tre6P (up to 119 nmol/g FW; Martínez-Barajas et al., 2011). Our measurements for trehalose and Tre6P in C. pepo nectaries were considerably higher than these previous studies (∼1 µmol/gFW for Tre6P, ∼0.2 µmol/gFW for Tre; Fig. 7). However, nectaries also accumulate substantially more Suc (270 µmol/gFW; Fig. 7) than tubers (17 µmol/gFW; Debast et al., 2011), Arabidopsis rosette leaves (∼1-10 µmol/gFW; Carillo et al., 2013), and developing wheat endosperm (∼74 µmol/gFW; Martínez-Barajas et al., 2011), which may account for the relatively high levels of trehalose and Tre6P in squash nectaries. Alternatively, the threshold at which trehalose and Tre6P impact metabolic processes may be higher in nectaries compared with other plant tissues, which is currently unknown because the role of trehalose and Tre6P metabolism in nectaries had not yet been investigated before this study.
Our findings from carbohydrate analyses of C. pepo nectaries support the predicted role of Tre6P in regulation of starch degradation and Suc homeostasis from previous studies (Debast et al., 2011; Nuccio et al., 2015). Starch degradation occurs predominantly from −3 to 0 h (Figs. 2 and 3), and accumulation of nectary Suc occurs mostly from −6 to −3 h (Figs. 4 and 7). Although Tre6P increases drastically from −3 h to 0 h (Fig. 7E), this increase occurs after both starch degradation (Figs. 2 and 3) and Suc accumulation (Fig. 3; Supplemental Fig. S6) are mostly complete; any potential inhibitory effects from increased Tre6P likely would not impact these processes. At 0 h, C. pepo nectaries are likely transitioning from a “Suc-accumulating state” (a combination of Suc synthesis and breakdown of phloem-derived RFOs) to a “Suc-export state” in which Suc is exported into the nectar. One way that Tre6P (or possibly Tre) can reduce intracellular Suc levels is by positively regulating Suc export (Lunn et al., 2014), although there is currently no evidence to support this hypothesis. It would be interesting to examine the role of Tre or Tre6P on regulation of the Suc exporter SWEET9 in future studies in order to test the hypothesis that accumulation of Tre6P at 0 h leads to induction of Suc export in order to reduce the levels of intracellular Suc in nectaries.
Although previous studies have shown that Tre6P, not trehalose, is the true signal for Suc-mediated changes in morphology and carbohydrate partitioning (Schluepmann et al., 2003), it is possible that Tre6P-independent signaling may also occur. In our study, exogenous trehalose treatment leads to inhibition of nectary starch degradation (Fig. 8A) and lower nectar sugar (Fig. 8E), and only slightly higher Tre6P in nectaries (Fig. 8D). However, there was significantly more nectary trehalose in the trehalose-treated samples compared with the sorbitol control (Fig. 8C). Because we did not see a significant difference in Tre6P in the trehalose-treated sample compared with sorbitol (Fig. 8D), it is unlikely that increased Tre6P is causing the reduced nectar in the trehalose-treated samples. It should also be noted that trehalose plays a highly conserved role in the regulation of cellular responses to osmotic stress, ranging from bacteria to plants and animals (Iturriaga et al., 2009; Chen et al., 2017). Although a molecular mechanism for the involvement of trehalose within the context of our study is unclear, secretory nectaries are exposed to a tremendous amount of osmotic stress due to a high intracellular concentration of Suc (Fig. 3B). It is thus possible that exogenously applied trehalose could cause nectaries to have an altered response in nectary starch degradation and nectar secretion via a mechanism related to osmotic potential. Finally, AtTRE1 (encoding Arabidopsis trehalase) has been shown to be induced during carbon starvation conditions (Garapati et al., 2015; Sun et al., 2019), which leads to activation of catabolic processes such as starch breakdown (Garapati et al., 2015). However, AtTRE1 expression is unaffected by feeding of Suc (Schluepmann et al., 2004), suggesting that AtTRE1 may be specifically important for initiating low-carbon metabolic responses (such as starch degradation).
While the simultaneous accumulation of both Tre/Tre6P and their catabolic enzymes may seem counterintuitive, there is a well-known positive relationship between [Suc] and [Tre6P/Tre] (Yadav et al., 2014; Figueroa et al., 2016). Thus, the extremely high levels of Suc in nectaries may necessitate the expression of Tre/Tre6P catabolic enzymes in order to prevent overaccumulation of Tre6P and Tre. It is possible that catabolism of Tre/Tre6P in C. pepo nectaries may partially lessen the inhibitory effects that Tre6P and Tre have on starch degradation (Martins et al., 2013; Garapati et al., 2015) and osmotic stress tolerance (Van Houtte et al., 2013), respectively, during starch degradation and sugar accumulation in C. pepo nectar-producing cells (Lin et al., 2014; Solhaug et al., 2019). Taken together, these data suggest a conserved role for trehalose and Tre6P in inhibiting breakdown of starch and keeping Suc levels constant, linking carbohydrate partitioning and energy storage to the amount of Suc available. The exact mechanism of this response in C. pepo nectaries is currently being researched.
CONCLUSION
These results represent an important examination of how nectar is produced from a biochemical and metabolic perspective in squash. We have presented evidence that nectary starch accumulation during the starch-filling stage coincides with an increase in sink strength and sink activity. Nectary starch degradation is an important step in the secretion of nectar, and the regulation of this process may be mostly light independent in squash. We have shown that Suc synthesis is active and induced during secretion; however, direct phloem sugar also plays an important role in determining the content and composition of nectar sugar in C. pepo. Finally, we have shown that trehalose metabolism plays an important role in C. pepo nectar production and carbon partitioning. Our predicted model of nectar secretion in C. pepo (summarized in Supplemental Fig. S8) opens up numerous avenues for future research that will continue to improve our understanding of how nectar is produced, which may lead to important agronomic advancements to improve yields in crops that rely on pollinator visitation.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Staminate (male) flowers from Crookneck Yellow Squash (Cucurbita pepo var torticollia) plants were used for all studies. Plants were grown either under greenhouse conditions at >200 µmol/m2s and 28°C to 30°C or on a growth rack at ∼250 µmol/m2s and 24°C.
Description of Experimental Design and Replication
Unless otherwise stated, biological replicates were defined as flowers taken from a group of four plants. All four plants were at the same developmental stage and grown under the same conditions. For each figure, the data represent results from one experiment with indicated biological replicates.
Excised Flower Treatment Experiments
For all excised flower treatment experiments, flowers were excised from plants at −15 h and placed in 1 to 2 mL of solution overnight. After the initial cut, the peduncle was fully immersed in deionized (DI) water and a second cut was made on the peduncle of the flower. This was done in order to avoid embolism on xylem vessels from the initial removal from the plant. Nectar and nectaries were harvested the following morning at ∼4 h after dawn (+1 h, total ∼16-h treatment). For figures using excised flowers (Figs. 6 and 8), the flowers were taken from a total of six plants.
Starch Assays
Total starch was quantitatively determined using a kit from Megazyme (Total Starch Assay Kit, K-TSTA). Samples were ground in 0.25 mL of 80% (v/v) ethanol, then an additional 0.25 mL of 80% (v/v) ethanol was added. Samples were incubated at 80°C for 5 min, and then an additional 0.5 mL of 80% (v/v) ethanol was added. Samples were centrifuged for 10 min at 1,800g, and supernatants were removed. The samples were resuspended in 1 mL 80% (v/v) ethanol followed by repeating centrifugation at 1,800g for 10 min and removal of supernatant. From each wash, 0.5 mL was saved for soluble sugar analyses (1 mL total). After removal of soluble sugars, 300 µL of α-amylase (100 U/mL in 100 mm sodium acetate buffer, pH 5.0) was added to each sample, followed by incubation at 100°C for 6 min with vortexing at 2-min intervals. Then 10 µL of amyloglucosidase (AMG; 3,300 U/mL) was added to each sample, and samples were incubated at 50°C for 30 min. Samples were made up to 1 mL final volume and then centrifuged at 1,800g. Supernatants were then assayed for d-Glc using the Glc oxidase/peroxidase (GOPOD) method. To get the concentration of d-Glc in the range of detection for the GOPOD assay, −48 through −3 h samples were diluted 10-fold. Supernatant (33 µLl) was added to 1 mL of GOPOD solution (made according to kit protocol) in duplicate, and tubes were incubated at 50°C for 20 min. Absorbance was read at 510 nm (∆A510) using a visible spectrophotometer. Starch content (percentage, w/w) was calculated using the equation for solid samples provided in the Megazyme protocol supplied with the kit. These values were then converted to micromole of Glc eq per grams FW using the ∆A510 for 100 µg of d-Glc standard and the molar mass of d-Glc.
Resistant starch was analyzed by protocol “c” in the total starch assay protocol procedure for the Megazyme Total Starch Assay Kit (K-TSTA). Briefly, soluble sugars were removed as with the normal starch assay. A magnetic stir bar and 0.2 mL of 2 m KOH were added to each tube, and samples were incubated for 20 min in an ice/water bath over a stirrer. Then 0.8 mL of 1.2 m sodium acetate (pH 3.8), 0.3 mL of α-amylase, and 0.01 mL of AMG were added to each sample, followed by incubation for 30 min at 50°C. Samples were made up to a final volume of 10 mL, before Glc produced from the resistant starch was analyzed by using the same method as in the normal starch assay (GOPOD method, described above).
Soluble Sugar Assays
Nectar and nectary sugars were quantified using an AmpRed/Glc oxidase/horseradish peroxidase method as described previously by Bethke and Busse (2008). Suc was measured by pretreatment of samples with invertase. Briefly, two 25 µL aliquots of sugar were prepared. To one (Atotal), 25 µL of invertase [38 U/mL in 80 mm sodium acetate (pH 4.8)] was added; to the other sugar aliquot (Aglc), 80 mm sodium acetate (pH 4.8) was added. All samples were then incubated at 22° to 23°C for 15 min. Then 25 µL of sodium phosphate buffer (150 mM, pH 7.4) was added to each sample. Finally, 25 µL of Glc assay mix was added to each sample consisting of 400 µM Amp-red (stock solution dissolved in dimethyl sulfoxide), 0.1 U of horseradish peroxidase and 0.1 U of Glc oxidase, and 34 mm sodium phosphate buffer (pH 7.4). Samples were incubated at 22° to 23°C for 25 min before reading A570. Asuc was determined by (Atotal − Aglc). Absorbance readings were compared with a standard curve of Glc (25–500 µM), and Suc standards were also included to ensure that Suc hydrolysis by invertase was proceeding adequately.
Maltose was assayed by the same method as with Suc, but 25 µL maltase [20 U/mL in 25 mm NaPO4 (pH 7)] was included instead of invertase. Nectary sugar was assayed from ethanol extracts prepared as described above for the starch assay. The concentration of nectary sugar in the ethanol extract was multiplied by the volume of the extract (2 mL) to obtain the micromoles of sugar present in each sample, before normalization to the amount of FW added. The total amount of sugar in the nectar was determined by multiplying the concentration by the nectar volume (microliter) to get amount of sugar present, then dividing by FW to get the total amount of sugar in micromole per grams FW. All samples were tested before being assayed to determine the dilution that would yield absorbances that were in the linear range of the sugar assay (between 25 and 500 µM).
Trehalose Assay
Trehalose was assayed using a Trehalose assay kit (Megazyme, K-TREH). Briefly, 20 µL of sample was added to 20 µL of alkaline borohydride solution (10 mg/mL NaBH4 in 50 mm NaOH). Samples were then incubated at 40°C for 30 min to remove reducing sugars from samples. To the samples, 50 µL of acetic acid (200 mM) was added and then 20 µL of buffer 1 (supplied with kit). This solution was used in the detection of trehalose. Due to the low concentrations of trehalose in our samples, the method of the trehalose assay was modified slightly. Sugar sample (50 µL; from NaBH4 treatment) was added to 50 µl of reaction mix containing 20 µL solution 1, 10 µL solution 2 (NADP+/ATP), 2 µL suspension 3 (HK/G-6-PDH), 2–4 µL suspension 4 (trehalase), and 14–16 µL DI water. Trehalase (2 µL) was added when assaying trehalose-treated samples (Fig. 8C), and 4 µL of trehalase was added when assaying overnight samples (Fig. 7D) because −6 and −3 h had a much lower level of soluble sugars. Both methods produced similar results for secretory (0 h) flowers (∼0.15 µmol/gFW). A negative (Glc) control was included with 2 µL of water added instead of trehalase. The A340 was measured after 20 min, and the amount of trehalose produced was determined using a standard curve of trehalose (5–250 µM).
Assay for Malto-Oligosaccharides
Malto-oligosaccharides were assayed in ethanol extracts using enzymes supplied with the Megazyme starch assay kit described above. α-Amylase [100 U/mL in 100 mm sodium acetate (pH 5.0)] and AMG (3,300 U/mL) were combined 1:1 and diluted 10-fold. Sugar (25 µL; diluted to <500 µM Glc) was added to 25 µL of α-amylase/AMG enzyme mix; then samples were incubated at 50°C for 30 min. Next, 25 µL of 150 mm NaPO4 (pH 7.4) was added, and then 25 µL of AmpRed/GlcOx/HRP was added (components described above in the “Soluble Sugar Assays” section) and Glc produced from degradation of maltoOS was assayed as described above (AmpRed/GlcOx/HRP method; A570).
Determination of Nectary Sugar Concentration
Nectary sugar concentration was determined by calculating nectary volume and dividing the total micromoles in each sample by the calculated volume. Volume was estimated by first determining radius of inner cup (ri) and the radius of the entire nectary (ro) and using these values to determine the volume of the nectary (VN) based on the equation provided in Supplemental Fig. S5. We also measured the mass of the same nectaries and used the volume to calculate the density of the nectary. Using this density value, we estimated nectary volume based on FW values that we had measured for normalization of nectary sugar data.
We performed an additional correction in order to estimate the relative volume of the cytosol, based on percentage of cell component estimates from imaging studies done by Nepi et al. (1996). Briefly, we set a cell radius of 10 µm, and then calculated the volume based on estimates of 30% vacuole (r = 3 µm), 25% mitochondria (r = 2.5 µm), and 25% plastid (r = 2.5 µm) and subtracted the volume of each from the total cell volume to get volume of cytosol (Supplemental Table S1).
Enzyme Activity Assays
All absorbances were measured on a BioTek PowerWave HT Microplate Spectrophotometer. All enzymatic activity assays were normalized per grams FW. For each enzyme activity assay, mass of tissue varied from 50 to 300 mg. For nectaries that were drastically (>2-fold) different in weight, heavier protein samples were prediluted accordingly before adding to the assay.
Amylase Assay
Total amylase activity was assayed as described previously by Laby et al. (2001), with a few alterations. Samples were ground in 150 µL Tris-HCl, pH 8.0, on ice and then centrifuged at ∼17,000g for 20 min at 4°C. Supernatant (10 µL) was added to a new tube containing 150 µL of 0.1 m sodium acetate (pH 4.6) and 75 µL of amylopectin (20 mg/mL in 0.2 m KOH). Each reaction was then made up to 300 µL with DI water then incubated at 37°C for 60 min. The reactions were stopped by incubation at 100°C for 3 min. Ten microliters of each reaction was added to 750 µL of p-hydroxybenzoic acid hydrazide solution, and DI water was added to a final volume of 1 mL. Samples were incubated at 100°C for 5 min, and then A410 was read for each sample blanked against a reagent blank with Tris-HCl grinding buffer added in place of crude protein. A standard curve of maltose (20–2500 µM) was used to express amylase activity as µmol of reducing sugar produced (representing maltose and Glc) per minute per gram FW. Amylopectin stocks were prepared by boiling amylopectin/KOH solution for 10 min until the solution became clear, and then aliquots were stored at −20°C before use. p-hydroxybenzoic acid hydrazide solution was prepared by mixing one part 5% (w/v) p-hydroxybenzoic acid hydrazide in 0.5 m HCl with four parts 0.5 m NaOH.
α-Galactosidase Assay
α-Galactosidase activity was assayed as described previously in Miao et al. (2007), with some changes. Samples were ground in extraction buffer containing 50 mm HEPES-NaOH, 2 mm MgCl2, 1 mm EDTA, and 5 mm dithiothreitol (DTT). Samples were then centrifuged at 17,000g for 30 min at 4°C. Protein extracts were diluted 5-fold in extraction buffer for the neutral activity assay, and 2-fold for the acid activity assay. For the neutral assay, we used HEPES-NaOH (pH 7.4), whereas for the acid assay, we used HEPES-NaOH (pH 5.5). Reaction mixtures contained 240 µL of 125 mm HEPES NaOH (pH 7.4 or pH 5.5), 30 µL of 20 mm paranitrophenyl galactopyranoside, and 30 µL of diluted protein extract (300 µL total). Samples were then incubated at 37°C for 20 min, and then reactions were stopped by addition of 1 mL of 5% (w/v) Na2CO3, which also caused color to develop. Absorbance was measured at 410 nm and compared against a standard curve of 4-nitrophenol. Standards were prepared by adding 1 mL of 5% (w/v) Na2CO3 to 300 µL of 4-nitrophenol at concentrations ranging from 25 to 5000 µM.
Invertase Assay
Invertase activity was assayed as described previously in Ruhlmann et al. (2010), with a few alterations. Protein was extracted by grinding nectary tissue (100–200 mg) in 250 µL of buffer containing 50 mm HEPES-NaOH (pH 8.0), 5 mm MgCl2, 2 mm EDTA, 1 mm MnCl2, 1 mm CaCl2, 1 mm DTT, and 0.1 mm phenyl-methyl-sulfonyl fluoride. Extracts were centrifuged at 17,000g at 4°C, and the supernatant was used in the assay. Soluble sugars were removed from protein extracts using a SpinOUT GT-600 (3 mL) desalting column (G-Biosciences). Briefly, columns were centrifuged for 1 min at 1000g to compact the resin, and then buffer was removed by centrifuging again at the same speed for 2 min. The column was washed with five columns of protein extraction buffer, centrifuging for 2 min at 1000g. Extracts were applied to the columns, and then the columns were centrifuged at 1,000g for 6 min to collect the desalted extracts. Ten microliters of desalted extract was added to 90 µL of either acid [80 mM sodium acetate (pH 4.8), 5 mm Suc] or neutral [75 mm NaPO4 buffer (pH 7.4), 5 mm Suc] assay solution. Samples were incubated at 30°C for 30 min, and then Glc produced was quantified as described above (AmpRed/GlcOx/HRP method; A570). Acid assays were diluted 1:5, and neutral assays were measured undiluted for the Glc assays.
SPS Assay
SPS activity was measured as described previously in Stitt et al. (1988), with a few modifications. Nectaries were ground in 250 µL extraction buffer containing 50 mm HEPES-NaOH (pH 7.4), 4 mm MgCl2, 1 mm EDTA, 10% (v/v) glycerol, and 0.1% (v/v) Triton X-100. Extracts were then centrifuged at 17,000g at 4°C and then desalted as described in the “Invertase Assay” section to remove soluble sugars. Assay mixture contained 10 µL desalted protein, 50 mm HEPES-NaOH (pH 7.4), 4 mm MgCl2, 1 mm EDTA, 10 mm Glc-6-P, 2 mm Fru-6-P (Fru6P), and 2 mm UDP-Glc in a final volume of 100 µL. The reactions were incubated at 22° to 23°C for 15 min before boiling the samples for 3 min to stop the reaction. UDP formed from Suc-6-P synthesis was then quantified by an additional reaction. This reaction contained (final volume 100 µL) 50 mm HEPES (pH 7.4), 5 mm MgCl2, 0.3 mm NADH, 0.8 mm phosphoenolpyruvate, 10 µL of initial SPS reaction, and 2 µL of pyruvate kinase/lactate dehydrogenase enzyme mix (900-1400 U/mL; Sigma). Reactions were incubated at 22° to 23°C for 30 min, and ∆A340 (end point) was measured and calculated from a standard curve of NADH (50–500 µM).
SuSy Assay
SuSy activity was assayed as described previously by Sun et al. (1992), with some minor differences as outlined below. Nectary protein was extracted by grinding in 300 µL extraction buffer containing 200 mm HEPES-KOH (pH 7.0), 3 mm Mg acetate, 0.5 mm EDTA, 0.5 mm phenyl methyl sulphonyl fluoride, 5 mm DTT, and 20 mm 2-mercaptoethanol. Extracts were centrifuged for 15 min at 17,000g and at 4°C, before being desalted as described above in the “Invertase Assay” section. SuSy was assayed in the synthesis direction in a 100 µL reaction containing 100 mm Tris HCl (pH 8.5), 25 mm Mg acetate, 75 mm KCl, 0.2 mm UDP-Glc, 4 mm phosphoenolpyruvate, 15 mm Fru, 0.3 µL of pyruvate kinase/lactate dehydrogenase mix (900–1400 U/mL, Sigma), and 10 µL of protein. Disappearance of NADH was monitored at ∆A340 per min for 5 min, and the rate was calculated from a standard curve of NADH (50–500 µM).
Trehalase Activity Assay
Trehalase activity was assayed as described previously by Garapati et al. (2015), with some differences. Nectary tissue was ground in 400 µL of protein extraction buffer containing 0.1 m MES-KOH (pH 6), 1 mm EDTA, 1 mm phenyl methyl sulphonyl fluoride, 1% (w/v) polyvinylpolypyrrolidone, and 1 mm DTT. Protein extracts were desalted as described in the “Invertase Assay” section to remove Glc in the extract. Desalted protein extract (10 µL) was added to 100 µL of reaction buffer containing 62.5 mm MES-KOH (pH 7), 125 µM CaCl2, and 100 mm trehalose. The reaction was brought up to 250 µL with DI water, and then samples were incubated at 30°C for 30 min. Reactions were stopped by boiling for 3 min before assaying Glc produced from trehalase reaction using AmpRed/GlcOx/HRP method described above in the “Soluble Sugar Assays” section. Reaction solution (25 µL) was added to 25 µL of 150 mm NaPO4 (pH 7.4), 25 µl of DI water, and 25 µl of Glc assay solution. Glc was quantified spectrophotometrically as described above in the “Soluble Sugar Assays” section (AmpRed/GlcOx/HRP method; A570) and used to determine trehalase activity.
Analysis of Gene Expression
Nectary RNA was extracted by Trizol method (Catalog #15596026), and complementary DNA was prepared using the Promega GoScript Reverse Transcription System (Catalog #A5000), with 1 µg of RNA used for complementary DNA preparation. Expression of key genes in trehalose metabolism was analyzed via RT-qPCR using Agilent Brilliant III Ultra-fast SYBR Green QPCR Master Mix (Catalog #600882). Expression values are expressed as fold change relative to the 0 h time point and are based on the ∆∆Ct values obtained from the normalized Ct values for each gene. Gene expression was normalized to a gene encoding a RING (Really Interesting New Gene)/U-Box ligase superfamily protein (CpRING; C. melo hit = gi|65907532 8|ref|XM _008439865.1|). This gene was chosen as the internal reference based on its stable expression level in all nectary samples in our RNA-seq dataset (Solhaug et al., 2019). Primer sequences for each gene are provided below: CpRING, F = GGGGAAGCCCAAAGCAAAGCCATGA, R = GCCTTCGAGGAGGGGCTTGGC; CpTRE1, F = ACCCTTGTGAGAGCCATTATC, R = GGAGGCTTCACTAGTCAGAAAG; CpTPP-J, F = TCCCACTCTTTCTCTCTCTACTT, R = CCTTCCGAGGTTATGCACTT.
Measurement of Tre6P by Liquid Chromatography-Tandem Mass Spectrometry
Samples were extracted in 80% (v/v) ethanol as described in the “Starch Assays” section for soluble sugar analyses. Samples were diluted in 80% (v/v) acetonitrile at a 1:10 dilution. Samples (10 µL) for Selective Reaction Monitoring (SRM) analysis were subjected to separation using a Shimadzu guard column followed an analytical SeQuant ZIC-pHILIC column (150×4.6mm; Millipore Sigma) at 30°C connected to the Applied Biosystem 5500 iontrap fitted with a turbo V electrospray source run in negative mode with declustering potential and collision energies in Supplemental Table S2. The samples were subjected to a linear gradient of 80% B to 25% B over an 18 min gradient [A: 10 mm ammonium acetate (pH 7), 20% acetonitrile; B: 100% acetonitrile] at a column flow rate of 400 µL/min. The column was cleared with 25% B for 2 min and then equilibrated to 80% B for 10 min. Transitions monitored as in Supplemental Table S2 were established using the instrument’s compound optimization mode with direct injection for each compound. The data were analyzed using MultiQuant (ABI Sciex Framingham) providing the peak area. A standard curve was constructed using 10 µL. Samples were run in triplicate and concentrations determined from the standard curve. Sample chromatograms of standards and a secretory stage (0 h) nectary are shown in Supplemental Figure S9.
Statistical Analysis
All statistical analyses were performed using R (R Core Team, 2014). For each experiment, a 1-way ANOVA was conducted in addition to a Tukey post hoc comparison of means. Statistical difference was determined at the P ≤ 0.05 level unless otherwise stated.
Accession Numbers
The accession numbers for genes analyzed in this study were derived from the recently published C. pepo genome (Montero-Pau et al., 2018), including: Trehalase 1 (CpTRE1), XM_023678639.1; Trehalose phosphate phosphatase-J (CpTPP-J), XM_023657393.1; Maltose excess protein 1 (CpMEX1), XM_023678269.1; α-glucan phosphorylase (CpPHS2), XM_023656828.1; ERD6-like transporter 6 (CpEL6), XM_023690129.1; Disproportionating enzyme 2 (CpDPE2), XM_023687667.1; ERD6-like transporter 16 (CpEL16), XM_023670748.1; Hexokinase 1 (CpHXK1), XM_023690103.1; UDP-Glc/UDP-Gal-4-epimerase 5 (CpUGE5), XM_023697437.1.
Additional accession numbers for genes mentioned but not analyzed in this paper are SWEET9 (CpSWEET9), XM_023681010.1; β-amylase 1 (CpBAM1), XM_023671176.1; Cell wall invertase 4 (CpCWINV4), XM_023689815.1; Suc phosphate synthase 3F (CpSPS3F), XM_023658084.1.
SUPPLEMENTAL DATA
The following supplemental materials are available.
Supplemental Figure S1. Representative floral stages and nectary masses.
Supplemental Figure S2. α-Galactosidase activity in floral tissues.
Supplemental Figure S3. Comparison of soluble and resistant starch in C. pepo nectaries.
Supplemental Figure S4. Nectar sugar composition at 0 and +24 h.
Supplemental Figure S5. Method for calculation of nectary volume and density.
Supplemental Figure S6. Accumulation of total Suc (nectary + nectar) during secretion in C. pepo.
Supplemental Figure S7. Gene expression for select genes involved in carbohydrate metabolism during nectar secretion in C. pepo.
Supplemental Figure S8. Predicted model of enzymes and pathways involved in carbohydrate metabolism in C. pepo nectaries.
Supplemental Figure S9. Sample chromatogram of Tre6P and Suc6P.
Supplemental Table S1. Estimation of relative cytosolic volume for C. pepo nectary cells.
Supplemental Table S2. Transitions for Tre6P in LC MS/MS analysis.
Acknowledgments
The authors thank Neil Olszewski, Adrian Hegeman, and John Ward of the University of Minnesota for helpful comments on this work and Bruce Witthuhn and the University of Minnesota Center for Mass Spectrometry and Proteomics for assistance with Tre6P analyses.
Footnotes
This work was supported by the U.S. National Science Foundation (grant 1339246 to C.J.C.).
Articles can be viewed without a subscription.
References
- Angeles-Núñez JG, Tiessen A (2010) Arabidopsis sucrose synthase 2 and 3 modulate metabolic homeostasis and direct carbon towards starch synthesis in developing seeds. Planta 232: 701–718 [DOI] [PubMed] [Google Scholar]
- Beebe DU, Turgeon R (1992) Localization of galactinol, raffinose, and stachyose synthesis in Cucurbita pepo leaves. Planta 188: 354–361 [DOI] [PubMed] [Google Scholar]
- Bethke PC, Busse JC (2008) Validation of a simple, colorimetric, microplate assay using amplex red for the determination of glucose and sucrose in potato tubers and other vegetables. Am J Potato Res 85: 414–421 [Google Scholar]
- Carillo P, Feil R, Gibon Y, Satoh-Nagasawa N, Jackson D, Bläsing OE, Stitt M, Lunn JE (2013) A fluorometric assay for trehalose in the picomole range. Plant Methods 9: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmi N, Zhang G, Petreikov M, Gao Z, Eyal Y, Granot D, Schaffer AA (2003) Cloning and functional expression of alkaline alpha-galactosidase from melon fruit: Similarity to plant SIP proteins uncovers a novel family of plant glycosyl hydrolases. Plant J 33: 97–106 [DOI] [PubMed] [Google Scholar]
- Champigny ML, Foyer C (1992) Nitrate activation of cytosolic protein kinases diverts photosynthetic carbon from sucrose to amino Acid biosynthesis: Basis for a new concept. Plant Physiol 100: 7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, An L, Fan X, Ju F, Zhang B, Sun H, Xiao J, Hu W, Qu T, Guan L, et al. (2017) A trehalose biosynthetic enzyme doubles as an osmotic stress sensor to regulate bacterial morphogenesis. PLoS Genet 13: e1007062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley JH, Zeeman SC, Takaha T, Smith AM, Smith SM (2001) A critical role for disproportionating enzyme in starch breakdown is revealed by a knock-out mutation in Arabidopsis. Plant J 26: 89–100 [DOI] [PubMed] [Google Scholar]
- Davis AR, Pylatuik JD, Paradis JC, Low NH (1998) Nectar-carbohydrate production and composition vary in relation to nectary anatomy and location within individual flowers of several species of Brassicaceae. Planta 205: 305–318 [DOI] [PubMed] [Google Scholar]
- Debast S, Nunes-Nesi A, Hajirezaei MR, Hofmann J, Sonnewald U, Fernie AR, Börnke F (2011) Altering trehalose-6-phosphate content in transgenic potato tubers affects tuber growth and alters responsiveness to hormones during sprouting. Plant Physiol 156: 1754–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom JS, Chen LQ, Sosso D, Julius BT, Lin IW, Qu XQ, Braun DM, Frommer WB (2015) SWEETs, transporters for intracellular and intercellular sugar translocation. Curr Opin Plant Biol 25: 53–62 [DOI] [PubMed] [Google Scholar]
- Ferreira SJ, Sonnewald U (2012) The mode of sucrose degradation in potato tubers determines the fate of assimilate utilization. Front Plant Sci 3: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa CM, Lunn JE (2016) A tale of two sugars: Trehalose 6-phosphate and sucrose. Plant Physiol 172: 7–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa CM, Feil R, Ishihara H, Watanabe M, Kölling K, Krause U, Höhne M, Encke B, Plaxton WC, Zeeman SC, et al. (2016) Trehalose 6-phosphate coordinates organic and amino acid metabolism with carbon availability. Plant J 85: 410–423 [DOI] [PubMed] [Google Scholar]
- Garapati P, Feil R, Lunn JE, Van Dijck P, Balazadeh S, Mueller-Roeber B (2015) Transcription factor arabidopsis activating factor1 integrates carbon starvation responses with trehalose metabolism. Plant Physiol 169: 379–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M. (2011) Nectar: Generation, regulation and ecological functions. Trends Plant Sci 16: 191–200 [DOI] [PubMed] [Google Scholar]
- Holthaus U, Schmitz K (1991) Distribution and immunolocalization of stachyose synthase in Cucumis melo L. Planta 185: 479–486 [DOI] [PubMed] [Google Scholar]
- Horrer D, Flütsch S, Pazmino D, Matthews JS, Thalmann M, Nigro A, Leonhardt N, Lawson T, Santelia D (2016) Blue light induces a distinct starch degradation pathway in guard cells for stomatal opening. Curr Biol 26: 362–370 [DOI] [PubMed] [Google Scholar]
- Iturriaga G, Suárez R, Nova-Franco B (2009) Trehalose metabolism: From osmoprotection to signaling. Int J Mol Sci. 10: 3793–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laby RJ, Kim D, Gibson SI (2001) The ram1 mutant of Arabidopsis exhibits severely decreased beta-amylase activity. Plant Physiol 127: 1798–1807 [PMC free article] [PubMed] [Google Scholar]
- Lin IW, Sosso D, Chen L-Q, Gase K, Kim S-G, Kessler D, Klinkenberg PM, Gorder MK, Hou B-H, Qu X-Q, et al. (2014) Nectar secretion requires sucrose phosphate synthases and the sugar transporter SWEET9. Nature 508: 546–549 [DOI] [PubMed] [Google Scholar]
- Lunn JE, Delorge I, Figueroa CM, Van Dijck P, Stitt M (2014) Trehalose metabolism in plants. Plant J 79: 544–567 [DOI] [PubMed] [Google Scholar]
- Majda M, Robert S (2018) The role of auxin in cell wall expansion. Int J Mol Sci 19: 951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Barajas E, Delatte T, Schluepmann H, de Jong GJ, Somsen GW, Nunes C, Primavesi LF, Coello P, Mitchell RA, Paul MJ (2011) Wheat grain development is characterized by remarkable trehalose 6-phosphate accumulation pregrain filling: Tissue distribution and relationship to SNF1-related protein kinase1 activity. Plant Physiol 156: 373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins MCM, Hejazi M, Fettke J, Steup M, Feil R, Krause U, Arrivault S, Vosloh D, Figueroa CM, Ivakov A, et al. (2013) Feedback inhibition of starch degradation in Arabidopsis leaves mediated by trehalose 6-phosphate. Plant Physiol 163: 1142–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleary BV, Monaghan DA (2002) Measurement of resistant starch. J AOAC Int 85: 665–675 [PubMed] [Google Scholar]
- McMichael RW Jr., Bachmann M, Huber SC (1995) Spinach leaf sucrose-phosphate synthase and nitrate reductase are phosphorylated/inactivated by multiple protein kinases in vitro. Plant Physiol 108: 1077–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao M, Xu X, Chen X, Xue L, Cao B (2007) Cucumber carbohydrate metabolism and translocation under chilling night temperature. J Plant Physiol 164: 621–628 [DOI] [PubMed] [Google Scholar]
- Montero-Pau J, Blanca J, Bombarely A, Ziarsolo P, Esteras C, Martí-Gómez C, Ferriol M, Gómez P, Jamilena M, Mueller L, Picó B, Cañizares J (2018) De novo assembly of the zucchini genome reveals a whole-genome duplication associated with the origin of the Cucurbita genus. Plant Biotechnol J 16: 1161–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzke I, Tscharntke T, Wanger TC, Klein A-M (2015) Pollination mitigates cucumber yield gaps more than pesticide and fertilizer use in tropical smallholder gardens. J Appl Ecol 52: 261–269 [Google Scholar]
- Nepi M, Ciampolini F, Pacini E (1996) Development and ultrastructure of Cucurbita pepo nectaries of male flowers. Ann Bot (Lond) 78: 95–104 [Google Scholar]
- Nepi M, Guarnieri M, Pacini E (2001) Nectar secretion, reabsorption, and sugar composition in male and female flowers of Cucurbita pepo. Int J Plant Sci 162: 353–358 [Google Scholar]
- Nepi M, Cresti L, Guarnieri M, Pacini E (2011) Dynamics of nectar production and nectar homeostasis in male flowers of Cucurbita pepo L. Int J Plant Sci 172: 183–190 [Google Scholar]
- Nepi M, Soligo C, Nocentini D, Abate M, Guarnieri M, Cai G, Bini L, Puglia M, Bianchi L, Pacini E (2012) Amino acids and protein profile in floral nectar: Much more than a simple reward. Flora 207: 475–481 [Google Scholar]
- Nuccio ML, Wu J, Mowers R, Zhou H-P, Meghji M, Primavesi LF, Paul MJ, Chen X, Gao Y, Haque E, Basu SS, Lagrimini LM (2015) Expression of trehalose-6-phosphate phosphatase in maize ears improves yield in well-watered and drought conditions. Nat Biotechnol 33: 862–869 [DOI] [PubMed] [Google Scholar]
- Peng YB, Li YQ, Hao YJ, Xu ZH, Bai SN (2004) Nectar production and transportation in the nectaries of the female Cucumis sativus L. flower during anthesis. Protoplasma 224: 71–78 [DOI] [PubMed] [Google Scholar]
- Pereira ALC, Taques TC, Valim JOS, Madureira AP, Campos WG (2015) The management of bee communities by intercropping with flowering basil (Ocimum basilicum) enhances pollination and yield of bell pepper (Capsicum annuum). J Insect Conserv 19: 479–486 [Google Scholar]
- Poschet G, Hannich B, Raab S, Jungkunz I, Klemens PAW, Krueger S, Wic S, Neuhaus HE, Büttner M (2011) A novel Arabidopsis vacuolar glucose exporter is involved in cellular sugar homeostasis and affects the composition of seed storage compounds. Plant Physiol 157: 1664–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R-project.org/ (February 10, 2015)
- Ren G, Healy RA, Klyne AM, Horner HT, James MG, Thornburg RW (2007) Transient starch metabolism in ornamental tobacco floral nectaries regulates nectar composition and release. Plant Sci 173: 277–290 [Google Scholar]
- Roldán-Serrano AS, Guerra-Sanz JM (2005) Reward attractions of zucchini flowers (Cucurbita pepo L.) to bumblebees (Bombus terrestris L.). Eur J Hortic Sci 70: 23–28 [Google Scholar]
- Roy R, Schmitt AJ, Thomas JB, Carter CJ (2017) Review: Nectar biology: From molecules to ecosystems. Plant Sci 262: 148–164 [DOI] [PubMed] [Google Scholar]
- Ruhlmann JM, Kram BW, Carter CJ (2010) CELL WALL INVERTASE 4 is required for nectar production in Arabidopsis. J Exp Bot 61: 395–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluepmann H, Pellny T, van Dijken A, Smeekens S, Paul M (2003) Trehalose 6-phosphate is indispensable for carbohydrate utilization and growth in Arabidopsis thaliana. Proc Natl Acad Sci USA 100: 6849–6854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluepmann H, van Dijken A, Aghdasi M, Wobbes B, Paul M, Smeekens S (2004) Trehalose mediated growth inhibition of Arabidopsis seedlings is due to trehalose-6-phosphate accumulation. Plant Physiol 135: 879–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solhaug EM, Roy R, Chatt EC, Klinkenberg PM, Mohd-Fadzil N-A, Hampton M, Nikolau BJ, Carter CJ (2019) An integrated transcriptomics and metabolomics analysis of the Cucurbita pepo nectary implicates key modules of primary metabolism involved in nectar synthesis and secretion. Plant Direct 3: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Wilke I, Feil R, Heldt HW (1988) Coarse control of sucrose-phosphate synthase in leaves: Alterations of the kinetic properties in response to the rate of photosynthesis and the accumulation of sucrose. Planta 174: 217–230 [DOI] [PubMed] [Google Scholar]
- Streb S, Zeeman SC (2012) Starch metabolism in Arabidopsis. The Arabidopsis Book 10: e0160, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Loboda T, Sung SJ, Black CC (1992) Sucrose synthase in wild tomato, Lycopersicon chmielewskii, and tomato fruit sink strength. Plant Physiol 98: 1163–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Zhang P, Wang R, Wan J, Ju Q, Rothstein SJ, Xu J (2019) The SNAC-A transcription factor ANAC032 reprograms metabolism in Arabidopsis. Plant Cell Physiol 60: 999–1010 [DOI] [PubMed] [Google Scholar]
- Van Houtte H, Vandesteene L, López-Galvis L, Lemmens L, Kissel E, Carpentier S, Feil R, Avonce N, Beeckman T, Lunn JE, Van Dijck P (2013) Overexpression of the trehalase gene AtTRE1 leads to increased drought stress tolerance in Arabidopsis and is involved in abscisic acid-induced stomatal closure. Plant Physiol 161: 1158–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzler M, Hölscher D, Oerther T, Schneider B (2008) Nectar formation and floral nectary anatomy of Anigozanthos flavidus: A combined magnetic resonance imaging and spectroscopy study. J Exp Bot 59: 3425–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler A, Fritzius T, Wiemken A, Boller T, Aeschbacher RA (2000) Trehalose induces the ADP-glucose pyrophosphorylase gene, ApL3, and starch synthesis in Arabidopsis. Plant Physiol 124: 105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H, Huber SC (2000) Regulation of sucrose metabolism in higher plants: Localization and regulation of activity of key enzymes. Crit Rev Biochem Mol Biol 35: 253–289 [DOI] [PubMed] [Google Scholar]
- Wist T, Davis A (2008) Floral structure and dynamics of nectar production in Echinacea pallida var. angustifolia (Asteraceae). Int J Plant Sci 168: 708–722 [Google Scholar]
- Yadav UP, Ivakov A, Feil R, Duan GY, Walther D, Giavalisco P, Piques M, Carillo P, Hubberten HM, Stitt M, Lunn JE (2014) The sucrose-trehalose 6-phosphate (Tre6P) nexus: Specificity and mechanisms of sucrose signalling by Tre6P. J Exp Bot 65: 1051–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Tolstikov V, Turnbull C, Hicks LM, Fiehn O (2010) Divergent metabolome and proteome suggest functional independence of dual phloem transport systems in cucurbits. Proc Natl Acad Sci USA 107: 13532–13537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Xiao H, Bianchi FJJA, Jauker F, Luo S, van der Werf W (2017) Wild pollinators enhance oilseed rape yield in small-holder farming systems in China. BMC Ecol 17: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]