Multiple WRKY transcription factors activate the expression of sugar-responsive genes by physically interacting with HISTONE ACETYLTRANSFERASE1 and promoting acetylation of histone 3 lysine 27.
Abstract
Sugars provide a source of energy; they also function as signaling molecules that regulate gene expression, affect metabolism, and alter growth in plants. Rapid responses to sugar signaling and metabolism are essential for optimal growth and fitness, but the regulatory mechanisms underlying these are largely unknown. In this study, we found that the rapid induction of sugar responses in Arabidopsis (Arabidopsis thaliana) requires the W-box cis-elements in the promoter region of GLC 6-PHOSPHATE/PHOSPHATE TRANSLOCATOR2, a well-studied sugar response marker gene. The transcription factors WRKY18 and WRKY53 directly bind to the W-Box cis-elements in the promoter region of sugar response genes and activate their expression. In addition, HISTONE ACETYLTRANSFERASE 1 (HAC1) is recruited to the WRKY18 and WRKY53 complex that resides on the promoters. In this complex, HAC1 facilitates the acetylation of histone 3 Lys 27 (H3K27ac) on the sugar-responsive genes. Taken together, our findings demonstrate a mechanism by which sugar regulates chromatin modification and gene expression, thus helping plants to adjust their growth in response to environmental changes.
Sugars are energy sources, the primary storage form for carbohydrates, and structural components of the cell wall; therefore, sugars have many crucial roles in plant metabolism and growth (Rolland and Sheen, 2005). Glc, Fru, Suc, and trehalose also function as signaling molecules to directly regulate plant metabolism and growth (Sheen et al., 1999; Rolland and Sheen, 2005; Wind et al., 2010). In Arabidopsis (Arabidopsis thaliana), nearly 10% of genes are transcriptionally regulated by sugars (Price et al., 2004; Bläsing et al., 2005). For example, a group of sugar and starch metabolism-related genes, e.g. ADP-GLC PYROPHOSPHORYLASE, GLC 6-PHOSPHATE/PHOSPHATE TRANSLOCATOR2 (GPT2), and BETA-AMYLASE 3 (BAM3), are rapidly induced by sugar, whereas catabolism-related genes, e.g. DARK INDUCIBLE1 (DIN1), DIN6, and SUGAR TRANSPORTER PROTEIN1, are repressed by sugar treatment (Price et al., 2004; Bläsing et al., 2005; Baena-González et al., 2007; Cordoba et al., 2015).
Three Glc signaling pathways, glycolysis-dependent, hexokinase (HXK) dependent, and HXK independent, have been proposed to act in eukaryote cells (Rolland and Sheen, 2005). The glycolysis-dependent pathway has been found to be related to pathogen-stimulated responses (Sheen et al., 1999). HXKs directly participate in sugar metabolism via the phosphorylation of Glc; it can also act as an evolutionarily conserved Glc sensor to mediate Glc signal transduction in eukaryotic cells (Lastdrager et al., 2014). In Arabidopsis, HXK1 integrates multiple signals including sugar, light, abscisic acid (ABA), and ethylene to control plant metabolism and growth (Moore et al., 2003). Target-of-rapamycin, SNF1-related Protein Kinase 1, and Regulator of G-protein function in HXK1-independent pathways (Baena-González et al., 2007; Grigston et al., 2008; Cho et al., 2010; Xiong et al., 2013).
In the past decades, multiple types of transcription factors, including basic leucine-zippers (bZIPs), MYB domain proteins, WRKYs, and APETALA2s, have been identified as major players in sugar responses (Lu et al., 2002; Teng et al., 2005; Baena-González et al., 2007; Kang et al., 2010). The WRKY transcription factor Sugar Signaling in Barley 2 (SUSIBA2) is specifically expressed in the barley (Hordeum vulgare) endosperm where it mediates sugar responses through regulating the transcription of the starch debranching enzyme gene Isoamylase1 (Sun et al., 2003). Consistent with this, overexpression of barley SUSIBA2 in rice (Oryza sativa) significantly increased starch content in the seeds (Su et al., 2015). MYB transcription factors and their partners, i.e. basic helix-loop-helix (bHLH) transcription factors and WD40-repeat proteins, form MYB-bHLH-WD40 complexes to regulate the biosynthesis of anthocyanin induced by sugars (Solfanelli et al., 2006; Lloyd et al., 2017). In contrast with the functions of WRKYs and MYBs in sugar responses, some bZIP transcription factors, e.g. bZIP1, bZIP10, bZIP11, and bZIP63, mediate sugar starvation responses (Kang et al., 2010; Matiolli et al., 2011; Kunz et al., 2015). In addition to these transcription factors, the regulation of transcription in plants involves epigenetic modulations, e.g. histone methylation and acetylation of chromatin (Kouzarides, 2007; Feng et al., 2010). However, whether epigenetic regulation is involved in sugar responses is largely unknown.
Most prior efforts aiming to screen for sugar insensitive and hypersensitive mutants used high concentrations of Glc or Suc in the growth medium and focused on long-term (e.g. days) effects (Moore et al., 2003; Gibson, 2005). High concentrations (∼6% to 3%) of sugar affect plant growth and development via signaling mechanisms directly associated with sugar, but also indirectly impact physiological processes by osmotic effects (Li and Sheen, 2016; Sami et al., 2019). To avoid secondary osmotic effects caused by high concentrations of sugar, in this study we used physiological levels of Glc (15 mm, about 0.27%) in most of our experiments to mimic the endogenous sugar responses.
Here, to demonstrate the mechanism of rapidly regulated sugar responses, we focused on the elucidation of components involved in the transcription of GPT2, a well-studied sugar response gene. Arabidopsis GPT2 and its homolog GPT1 localize on the inner envelope membrane of chloroplasts and are responsible for the transport of Glc-6-P, glyceraldehyde 3-phosphate, and 3-phosphoglycerate across the membrane (Fischer, 2011; Lee et al., 2017). Arabidopsis GPT1 is essential for the development of male and female gametophytes, embryo development, and seed maturation, but these processes are not directly induced by sugar (Niewiadomski et al., 2005). However, GPT2 is rapidly induced by both Glc and Suc and thus is essential for leaf growth and acclimation of metabolism to daily environmental changes (Gonzali et al., 2006; Athanasiou et al., 2010; Dyson et al., 2014;, 2015; Van Dingenen et al., 2016). In this study, we discovered that two WRKY transcription factors (WRKY18 and WRKY53) are required for the induction of GPT2 in response to a low concentration of Glc. In addition, we found that HAC1 mediated histone acetylation further improved the transcription efficiency of the downstream sugar responsive genes.
RESULTS
Transcription of GPT2 Is Rapidly Induced by Physiological Levels of Glc
An earlier study showed that, in response to a high concentration of sugar, increased expression of GPT2 could be detected as early as 30 min, although a much stronger effect was found at 3 h (h; Gonzali et al., 2006). To investigate whether GPT2 has a similar response to physiological levels of Glc, 7-d-old Arabidopsis wild-type Col-0 seedlings were first transferred into dark conditions for 24 h (-C treatment) to reduce intracellular sugar contents; however, the senescence associated genes expression were not induced in this condition (Supplemental Fig. S1A). These plants were then transferred to medium containing 15 mm Glc or 15 mm Mannitol (Mtl) for various periods of time. In response to Glc treatment, the transcript levels of DIN1 and DIN6 were dramatically repressed (Supplemental Fig. S1C; Baena-González et al., 2007), whereas the transcript levels of GPT2, OEP16, PFK, PGD3, ISA2, BAM3, and ApL3 were significantly induced (Supplemental Fig. S1B). The expression of GPT2 was induced by ∼20-fold at 1 h and 50-fold at 3 h, but other phosphate-related transporter genes, such as GPT1 and TPT, were not induced (Supplemental Fig. S1C). The induction of GPT2 by Glc is dependent on the concentration of Glc and treatment time, and does not occur in response to light, abscisic acid, or other indirect signaling pathways (Supplemental Fig. S1D–G). The much higher induction of GPT2 than other representative sugar-inducible genes shows that GPT2 rapidly responds to physiological levels of Glc.
To further demonstrate the effect of low-level sugar on the temporal and spatial expression of GPT2 in vivo, we generated transgenic reporter lines harboring GPT2pro::GUS or GPT2pro::LUC (Luciferase). GPT2pro::GUS signals were detectable in most photoautotrophic tissues and organs, and the intensities were significantly increased after Glc treatment. The induction of GUS signals in the Mtl control plants was much lower than that in the Glc treatment, indicating a sugar signaling specific effect, rather than an osmotic effect associated with the increased expression of GPT2 (Fig. 1A; Supplemental Figs. S1D and S2, A and B).
Figure 1.
Sugar-induced expression of GPT2 depends on the W-Box cis-element. A, Histochemical analysis of the expression of GPT2pro::GUS in response to 15 mm mannitol (Mtl; left) or Glc (Glc; right) treatment for 3 h. Scale bars = 0.2 mm. B, Bioluminescence assays of the expression of GPT2pro::LUC over time in response to 15 mm Glc or Mtl. Images at right show the LUC signals after 3 h treatment, with Glc at the top, Mtl below. CPS: counts per second. C and D, Transient expression analysis of the response of various cis-elements to Glc in the P3 region of the GPT2 promoter. The fragments of P3 without mutation (P3wt) or with various mutated cis-elements (P3m1–P3m7, named m1–m7) were fused in front of the minimal region of 35Spro (mini) and used as reporters. P3wt (P3wt-mini::LUC/35S::REN) and mini (mini::LUC/35S::REN) were used as positive and negative controls, respectively. Mean ± sd (n = 5). **P < 0.01. E, Bioluminescence assays showing the LUC activity resulting from expression of P3wt-mini::LUC and the same construct carrying a mutation in the W-Box cis-element (P3m1-mini::LUC) in response to 15 mm Glc in Arabidopsis. Five independent transgenic lines of P3wt-mini::LUC or P3m1-mini::LUC were used in the assay. F and G, Histochemical assays in cotyledons, showing GUS activity from the expression of 3xW-Box-mini::GUS and 3xSURE-mini::GUS, in response to treatment with 15 mm Glc or Mtl for 3 h. Scale bars = 0.2 mm.
We also conducted a time course observation of the induction effect of Glc on GPT2 using the GPT2pro::LUC reporter construct. Bioluminescence signals acquired every 10 min on the GPT2pro::LUC reporter lines showed that the initiation of the induction by Glc occurred at 10 min and peaked at 3 to 4 h after treament (Fig. 1B; Supplemental Fig. S1D).
The W-Box cis-Element Is Required for Sugar-Induced GPT2 Expression
To identify the essential regions responsive to sugar signals in the promoter of GPT2, a series of truncated fragments within the 2.36 kb upstream of the GPT2 start codon were fused in front of the LUC gene as reporters for transient expression assays in Arabidopsis protoplasts. As shown in Supplemental Figure S3, only construct P3, which contained the region from -1075 to -1400 nt upstream of the start codon of GPT2, led to over 3-fold induction of bioluminescence signals after Glc treatment. This demonstrated that the 326-bp fragment located in this region was essential for the response to Glc treatment.
Promoter sequence analysis revealed that the 326-bp fragment harbors multiple cis-elements, including three Suc-Responsive Elements (SURE), one W-Box, and the other three types of Glc-responsive associated cis-elements (Fig. 1C; Supplemental Table S1). To verify the functionality of these cis-elements in response to sugar, a 326-bp promoter fragment harboring a point mutation on each of these cis-elements was generated (P3m1–P3m7) and fused in front of LUC for transient expression assays in Arabidopsis protoplasts (Supplemental Table S2). Arabidopsis protoplasts transformed with one W-Box mutant construct (P3m1) or two SURE mutants (P3m6 and P3m7) showed no difference in bioluminescence intensity between the Mtl control and Glc treatment; this demonstrated that these three cis-elements may be involved in mediating sugar signaling (Fig. 1D).
To confirm whether these cis-elements can induce transcription in vivo in response to Glc signaling, we generated P3wt-mini::LUC and P3m1-mini::LUC stable transgenic lines to study the “real time” participation of the W-Box cis-element in sugar responsive gene regulation. The P3m1-mini::LUC reporter line showed a diminished response to Glc in the bioluminescence assay, compared with P3wt-mini::LUC, further verifying that the W-Box is indispensable for mediating sugar signaling (Fig. 1E).
Further, we generated stable transgenic lines harboring multiple copies of the cis-elements fused to the minimal 35S promoter (3x W-Box-mini::GUS or 3 x SURE-mini::GUS). In the 3x W-Box-mini::GUS transgenic plants, GUS was dramatically induced by Glc, but only in the leaves (Fig. 1F; Supplemental Fig. S2C), verifying the role of the W-Box cis-element in mediating sugar signaling. By contrast, in the 3x SURE-mini::GUS transgenic plants, increased GUS staining induced by Glc was only observed in the roots, not in the leaves (Fig. 1G; Supplemental Fig. S2D).
WRKY18 and WRKY53 Regulate the Glc-Induced Expression of GPT2
WRKY type transcription factors specifically recognize W-Box cis-elements (Eulgem et al., 2000; Jiang et al., 2017). To identify the WRKY transcription factors involved in the transcriptional regulation of GPT2, we performed a yeast one-hybrid assay to detect the direct binding of 56 WRKY transcription factors (Ou et al., 2011), to the W-Box cis-element in the P3 region of the GPT2 promoter. Our initial screening identified seven WRKY type transcription factors, i.e. WRKY11, WRKY18, WRKY26, WRKY27, WRKY39, WRKY45, and WRKY53, that bound to the GPT2 promoter (Supplemental Fig. S4A). Real-time quantitative PCR (RT-qPCR) assays analyzing the expression of the seven WRKY transcription factors in response to Glc showed that transcript abundances of WRKY11, WRKY18, WRKY27, and WRKY53 rapidly increased in response to Glc treatment (Supplemental Fig. S4B). Among these four transcription factors, transient assays in protoplasts showed that WRKY18 and WRKY53 were able to activate the expression of GPT2 (Fig. 2A; Supplemental Fig. S4), indicating they act upsteam of GPT2 in the sugar signaling cascade.
Figure 2.
WRKY transcription factors mediate the induction of GPT2 in response to sugar. A, Transient expression assays showing the LUC activity resulting from transcription of GPT2pro-P3wt-mini::LUC as induced by various WRKY (W) transcription factors. B and C, Transient expression assays of GPT2pro-P1.4K (B), P3wt-mini::LUC (C; maroon points), and P3m1-mini::LUC (C; gray points), which were cotransformed with 35S::WRKY18 or 35S::WRKY53 in Arabidopsis protoplasts. CK: empty vector without effectors was used as the negative control in A–C. Mean ± sd (n = 5). D and E, Relative expression level of GPT2 in the wrky18 or wrky53 mutants (w18, w53), complemented lines (W18 C13, W18 C21, W53 C6, and W53 C15; D), or overexpression lines (W18 OE6, W18 OE7, W53 OE4, and W53 OE8; E). Multiple seedlings treated with Mtl or Glc were used to perform RT-qPCR assays. UBQ1 was used as an internal control. Mean ± sd (n = 3). *P < 0.05, **P < 0.01 in A–E. F, Bioluminescence assay showing LUC activity resulting from expression of GPT2pro::LUC in the w18 and w53 mutants as compared with wild-type Col-0, in response to 15 mm Glc. Five independent transgenic lines of GPT2pro::LUC in different backgrounds were used in the assay.
To test whether the expression of GPT2 mediated by WRKY18 and WRKY53 depends on the W-Box cis-element, we transformed WRKY18 or WRKY53 effectors with a reporter containing the P3 region driving LUC. The reporters contained either wild-type (P3wt-mini::LUC) or mutated W-Box cis-elements (P3m1-mini::LUC). In the construct with the mutated W-Box cis-element, WRKY18 and WRKY53 failed to activate the expression of GPT2, indicating their essential role in sugar signaling (Fig. 2, B and C).
To explore whether the direct regulation of GPT2 by WRKY18 and WRKY53 occurs in vivo, we measured GPT2 expression in the wrky18 and wrky53 null mutants, complementation, and overexpression lines (Supplemental Fig. S5A–C). RT-qPCR analyses revealed that Glc-induced GPT2 expression was significantly repressed in the wrky18 (Salk_093916C, hereafter referred as w18) or wrky53 (Salk_034157C, hereafter referred as w53) single mutants, and dramatically repressed in the w18 w53 double mutant (Fig. 2D). Consistent with this, the induction of GPT2 by Glc was restored in the WRKY18pro::WRKY18-GFP/w18 (W18-C13 and C21) and WRKY53pro::WRKY53-GFP/w53 (W53-C6 and C15) complementation lines, and significantly enhanced in the 35S::WRKY18-GFP/Col-0 (W18-OE7 and OE8) and 35S::WRKY53-GFP/Col-0 lines (W53-OE4 and OE8) compared with Col-0 (Fig. 2E). Moreover, we found that the bioluminescence response was impaired in lines carrying GPT2pro::LUC or GPT2pro-P3wt::LUC in the w18 or w53 mutant background (Fig. 2F; Supplemental Fig. S6). These results collectively verified that WRKY18 and WRKY53 are key regulators mediating rapidly regulated sugar responses to trigger GPT2 expression.
WRKY18 and WRKY53 Are Essential for Rapidly Regulated Sugar Responses
When sugar signaling is enhanced in plants, one of the classical physiological alterations is the accumulation of anthocyanin (Solfanelli et al., 2006; Hu et al., 2016). To study whether WRKY18 and WRKY53 play physiological roles in sugar signaling, we treated their mutant plants and complementation lines with 6% (about 333 mm) Glc for 3 d. The accumulation of anthocyanin in w18, w53, or w18 w53 was significantly decreased (Fig. 3, A and B), but was restored in the complementation lines (Supplemental Fig. S7, A and B), compared with wild-type controls. RT-qPCR assays further revealed that the expression of multiple anthocyanin biosynthetic genes, such as DFR, and CHS, was significantly decreased in w18 w53 double mutants but recovered in the complementation lines after either short- (3 h) or long-term (3 d) Glc treatment (Fig. 3C; Supplemental Figs. S5, D and E and S7, C and D). These results indicated that WRKY18 and WRKY53 play essential roles in mediating sugar signaling.
Figure 3.
WRKY18 and WRKY53 directly bind to the promoters of GPT2, DFR, and CHS. A and B, Phenotype (A) and measurement (B) of anthocyanin accumulation in the seedlings of wrky18, wrky53, and w18 w53. 7-d-old seedling plants grown on MS medium with 1% (w/v) Suc were treated with 6% (∼333 mm) Glc or Mtl for 3 d. Scale bar = 1 mm. C, Relative expression of DFR and CHS in w18 w53 as compared with Col-0 in response to Glc. Col-0 and w18 w53 seedlings were treated with 15 mm Glc for 3 h. UBQ1 was used as an internal control. Mean ± sd (n = 3). D and E, ChIP-qPCR assay of fragments containing the putative W-Box cis-elements in the promoter regions of GPT2, DFR, and CHS using anti-GFP to precipitate the WRKY18-GFP and WRKY53-GFP fusion proteins expressed in Arabidopsis overexpression lines treated with 15 mm Glc or Mtl for 3 h. The positions of the various cis-elements and fragments used for ChIP-qPCR are shown in (D): black lines represent the W-Box (including TTGACT/C and the pure W-Box: TGAC). The red line indicates the P3 region of the GPT2 promoter. The ChIP results, as percent of input, are shown in (E), and the promoter region of ACTIN12 (ACT) was used as the negative control. Mean ± sd (n = 3). *P < 0.05, **P < 0.01 in (B) to (E).
To study whether WRKY18 or 53 direct binds to the promoter regions of GPT2, DFR, and CHS to mediate sugar signaling, we performed chromatin immunoprecipitation (ChIP)-qPCR assays in 35S::WRKY18-GFP/Col-0 (W18 OE7) and 35S::WRKY53-GFP/Col-0 (W53 OE8) transgenic plants. ChIP-qPCR assays revealed that the F3 fragment of GPT2 promoter (which is included in the GPT2pro-P3 fragment), the F1 fragment of the DFR promoter, and the F1 fragment of the CHS promoter were specifically enriched by immunoprecipitation of WRKY18-GFP or WRKY53-GFP, particularly under Glc treatment conditions (Fig. 3E).
In addition, we conducted electrophoretic mobility shift assays (EMSAs) to investigate whether WRKY18 and WRKY53 bind to GPT2, CHS, and DFR in vitro. His-WRKY18 and His-WRKY53 were expressed in Escherichia coli and purified. As shown in Supplemental Figure S8, B and C, both WRKY18 and WRKY53 could bind to the fragments of the GPT2, CHS, and DFR promoters. The fragments used in EMSA are included in the F3 fragment of the GPT2 promoter (GPT2 probe), the F1 fragment of the DFR promoter (DFR probe), and the F1 fragment of the CHS promoter (CHS probe), which contain at least one W-Box. These results suggested that sugar signaling can be realized via the direct binding of WRKY18 and WRKY53 to the promoters of sugar response genes.
WRKY18 and WRKY53 Directly Interact with HAC1 In Vitro and In Vivo
To demonstrate the mechanism by which WRKY18 and WRKY53 mediate sugar signaling, we performed yeast two-hybrid assays to identify their possible interacting proteins. Yeast two-hybrid assays showed that WRKY18 and WRKY53 physically interact with the N terminus of HAC1, a well-studied histone acetyltransferase (Fig. 4, A and B).
Figure 4.
WRKY18 and WRKY53 physically interact with HAC1 in vitro and vivo. A, The position of various fragments of HAC1 used for yeast two-hybrid assays (Y2H). TAZ: transcription adaptor putative zinc finger domain; ZZ: Zinc DNA-binding domains; PHD: plant homeodomain. The number indicates the amino acid position. B, Y2H assays showing the interaction of the HAC1 fragments (shown in A) with WRKY18 (W18) and WRKY53 (W53). The full-length, N-, or C terminus of HAC1 was fused with the DNA binding domain of GAL4 (BD); full-length WRKY18 and WRKY53 were fused with the activation domain of GAL4 (AD). C and D, BiFC (C) and LCI (D) assays for the physical interaction of HAC1 with WRKY18 and WRKY53 in planta. The full-length, N-, or C terminus of HAC1 was fused with N terminus of YFP or LUC; WRKY18 or WRKY53 was fused with the C terminus of YFP or LUC, respectively. Empty vectors were used as negative controls. E, Co-IP assay of the interaction of HAC1 with WRKY18 and WRKY53 in planta. Proteins were extracted from the leaves of N. benthamiana infiltrated with a construct expressing HAC1-myc alone or coinfiltrated with constructs expressing GFP, WRKY18-GFP, or WRKY53-GFP. The red arrowhead indicates the HAC1-specific band.
Bimolecular fluorescence complementation (BiFC) and LUC complementation imaging (LCI) assays were used to verify the interaction of WRKY18 or WRKY53 with HAC1. Strong yellow fluorescent protein (YFP) fluorescence signals were clearly detected in the nuclei of epidermal cells after cotransformation of the C terminus of YFP (cYFP) fused to WRKY18, or WRKY53, with N terminus of YFP (nYFP) fused to the full-length, or just N terminus of HAC1, in the leaves of Nicotiana benthamiana (Fig. 4C). Similarly, cotransformation of an nLUC-HAC1 fusion protein (harboring the N terminus of LUC and the HAC1) and cLUC-WRKY18/WRKY53 (harboring the C terminus of LUC and WRKY18 or WRKY53) into N. benthamiana leaves produced strong LUC activity (Fig. 4D).
Next, we coexpressed HAC1-myc, GFP, WRKY18-GFP, and WRKY53-GFP in N. benthamiana to perform coimmunoprecipitation (Co-IP) assays. WRKY18-GFP and WRKY53-GFP were successfully detected in the samples immunoprecipitated for HAC1-myc (Fig. 4E). Taken together, these results demonstrated that WRKY18 and WRKY53 interact with HAC1 in vivo.
WRKY18, WRKY53, and HAC1 coordinately regulate rapid sugar responses
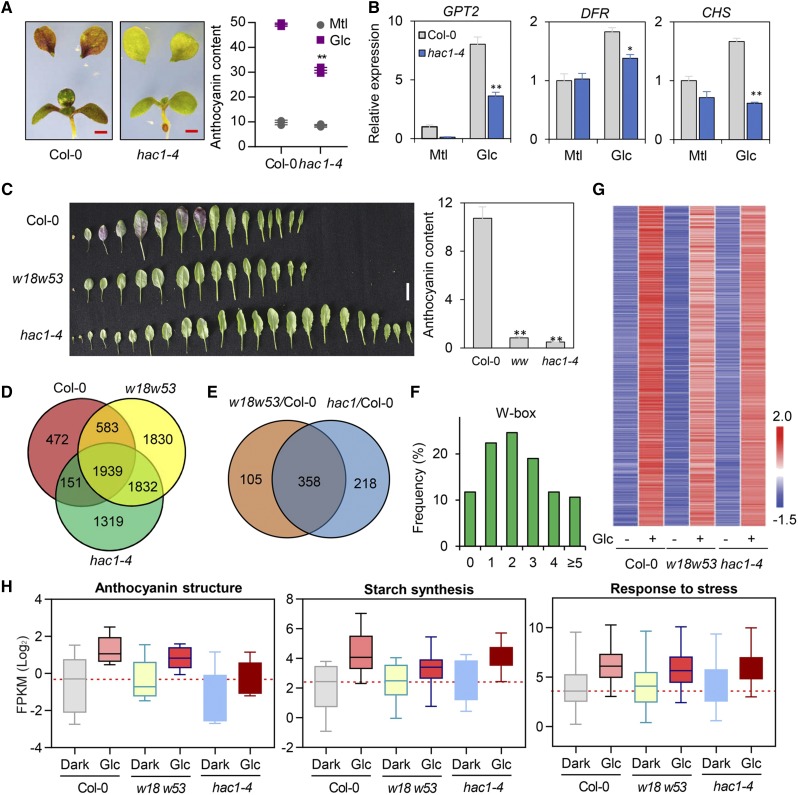
HAC1 acetylates Lys on histone H3, thus regulating transcription (Deng et al., 2007; An et al., 2017). To investigate whether there is a physiological role associated with HAC1 in sugar responses, we checked the expression of sugar response genes and the accumulation of anthocyanin in the hac1-4 mutant. As shown in Fig. 5, A and B, the expression levels of representative genes (i.e. GPT2, DFR, and CHS) in response to short-term (3 h) Glc treatment and anthocyanin accumulation in response to long-term (3 d) Glc treatment were significantly repressed in the mutant. In line with this observation, the accumulation of anthocyanin in the adult hac1-4 and w18 w53 plants was also extremely reduced (Fig. 5C). All these results indicated that HAC1 is involved in sugar signaling.
Figure 5.
WRKY18, WRKY53, and HAC1 coordinately regulate the sugar response. A, Phenotype (leaf) and measurement of anthocyanin contents (right) in 7-d-old wild-type and hac1-4 mutant seedlings after 6% (about 333 mm) Glc or Mtl treatment for 3 d. Scale bars = 1 mm. B, RT-qPCR assays showing Glc-induced expression of GPT2, DFR, and CHS in the hac1 mutant after Glc treatment. Mean ± sd (n = 3). C, Reduced anthocyanin accumulation in the 4-week-old plants of w18 w53 (ww) and hac1-4 under short day conditions (8 h light/16 h dark). The contents of anthocyanin of leaves 6–8 are indicated on the right. Scale bars = 1 cm. *P < 0.05, **P < 0.01 in (A) to (C). D, Venn diagram showing the overlap of WRKY18, WRKY53, and HAC1 coregulated genes after 15 mm Glc treatment compared with dark treatment. E, The diagram shows the numbers of genes with significantly repressed induction in w18 w53, and hac1-4 as compared with Col-0 in the Glc-induced group. F, Distribution of W-Box abundances in the promoters of the 358 overlapping genes shown in (E). G, Heatmap representing the Log2FPKM of 358 Glc-induced genes in Col-0, w18 w53, and hac1-4 treated with Glc or not. H, Box graphs representing the expression of sugar-responsive genes in w18 w53 and hac1-4 as compared with Col-0. Center lines are the medians; box limits indicate the 25th and 75th percentiles; whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles.
To get a view of the global transcriptomic regulation by WRKY18/53 and HAC1, RNA-sequencing (RNA-seq) was used to analyze Col-0, w18 w53, and hac1-4 seedlings in response to short-term Glc (3 h, 15 mm) treatment. In total, 1,939 genes were coregulated by WRKY18, WRKY53, and HAC1 after Glc treatment (Fig. 5D; Supplemental Dataset 1). Gene ontology analysis indicated that these genes were significantly enriched in pathways associated with abiotic stimulus, nucleoside metabolic process, carbohydrate metabolism, and stimulus and stress response (Supplemental Fig. S9A). Among these Glc-upregulated genes, the expression levels of 463 genes were significantly reduced in w18 w53, and 576 genes were reduced in hac1-4. Comparing these two set of genes led to the identification of 358 genes with reduced expression in both w18 w53 and hac1-4 mutants (Fig. 5E; Supplemental Dataset 2). Furthermore, we performed the conserved cis-elements analyses in the promoters of these WRKY18/53 and HAC1 coactivated genes and found that approximately 90% of the promoters contained at least one W-Box cis-element, and more than 65% contained two or more (Fig. 5F).
Further exploration of the expression patterns of these 358 coactivated genes suggested that WRKY18/WRKY53 and HAC1 might use different regulatory mechanisms to control the transcription of sugar response genes. A subset of these 358 genes showed dramatically reduced induction in the w18 w53 and hac1-4 mutants compared with the wild-type Col-0, and part showed expression patterns that differed between w18 w53 and hac1-4 (Fig. 5G; Supplemental Dataset 2). These results suggested that WRKY transcription factors might specifically mediate transcriptional activation in response to Glc, but HAC1 might have broader functions in addition to its role in Glc responses.
To understand the functions of these coactivated 358 genes, we performed gene ontology analysis. This showed that a number of pathways were enriched among these genes, including ribosome biogenesis, ribosomal RNA processing, pyrimidine ribonucleotide metabolic and biosynthetic process, and other processes (Supplemental Fig. S9B). Further detailed analysis of a few sugar-regulated pathways, i.e. anthocyanin biosynthesis and starch synthesis, revealed that the average gene expression induction levels associated with these pathways were significantly reduced in both w18 w53 and hac1-4 mutants compared with the wild type (Fig. 5H; Supplemental Dataset 3). This informatics analysis result is consistent with the phenotypic alteration in the mutants in which a reduced sugar response was observed.
HAC1 Mediates Sugar Responses by Facilitating H3K27 Acetylation
Because acetylation is the main function associated with HAC1, we first treated wild-type seedlings with the histone deacetylase inhibitor trichostatin A to test whether histone acetylation is involved in the sugar response. As expected, trichostatin A treatment led to rapid induction of the sugar response genes GPT2 and DFR, even without addition of Glc (Supplemental Fig. S10A). In addition, global acetylation levels of histone 3 Lys 27 (H3K27ac) but not H3K9ac, H3K14ac, or H3ac increased significantly after Glc treatment (Fig. 6A). ChIP-qPCR assays revealed that H3K27ac was significantly enriched on the promoters of GPT2, DFR, and CHS, and Glc treatment further increased their enrichment (Fig. 6B). Acetylation of H3K27 increases the accessibility of DNA inside chromatin and promotes gene expression (Lin et al., 2016; Xu et al., 2016). Increased enrichment of H3K27ac on the promoters of sugar response genes revealed that sugar-induced gene expression was dependent on H3K27ac levels.
Figure 6.
HAC1 mediates sugar responses by facilitating the acetylation of H3K27. A, Immunoblot analysis showing changes in the levels of H3K27ac, H3K9ac, H3K14ac, and H3ac in response to treatment with 15 mm Glc or Mtl in the 7-d-old wild-type seedlings. H3 proteins were used as loading controls. B, ChIP-qPCR assays of histone acetylation levels in the promoter regions of GPT2, DFR, and CHS in response to treatment with 15 mm Glc or Mtl for the indicated times. C, Immunoblot analysis showing the accumulation of H3K27ac in wild-type and hac1-4 mutant plants. Bands were quantified with ImageJ in (A) and (C). D, ChIP-qPCR assays for enrichment of H3K27ac in the promoter regions of GPT2, DFR, and CHS in hac1-4 and w18 w53 (w w) mutants in response to Glc treatment. The fragments used for ChIP-qPCR assays in (B) and (D) are shown in Figure 3D. The ACT12 promoter was used as a negative control. *P < 0.05; **P < 0.01 in (B) and (D). E, A working model for the involvement of WRKYs and HAC1 in the response to Glc. After the plant perceives the increased Glc signal, WRKY18 and WRKY53 bind to the W-Box cis-elements and then recruit HAC1 into the promoter regions of sugar-responsive genes through physical interactions. The subsequent acetylation of H3K27 further promotes the transcription of downstream targets.
The global H3ac, H3K9ac, and H3K27ac levels were slightly reduced in the hac1-4 mutant compared with Col-0 before Glc was added (Supplemental Fig. S10B). After Glc treatment, the global H3K27ac increased less in hac1-4 than in the wild type (Fig. 6C). To elucidate whether the reduced sugar response in the hac1-4 and w18 w53 was caused by the reduced H3K27ac, we performed ChIP-qPCR, which showed that the association of H3K27ac with the promoter of GPT2, DFR, or CHS in response to Glc treatment was significantly reduced in both hac1-4 and w18 w53 mutants compared with Col-0 (Fig. 6D), whereas the Mtl control showed no change in enrichment (Supplemental Fig. S11).
We also used transient assays in mutant protoplasts to test the effect of lack of HAC1. The reduced LUC signal from GPT2pro::LUC in the hac1-4 protoplasts in which either W18, W53, or W18+53 was coexpressed demonstrated that the acetylation of H3K27 via HAC1 is essential for W18 and W53 to activate GPT2 (Supplemental Fig. S12, A and B). Taken together, these results suggested that WRKY18 and WRKY53 interact with HAC1 to form a complex to coactivate their expression by mediating histone acetylation of the target loci.
To further clarify the physiological function of the interaction between WRKY18, WRKY53, and HAC1, we generated lines with different HAC1 and WRKY genetic backgrounds (Supplemental Fig. S12C). The anthocyanin accumulation in w18 w53 HAC1 OE was higher than w18 w53, but significantly lower than in HAC1 OE lines (Supplemental Fig. S12, D and E). Consistent with this, the expression of sugar-induced genes (i.e. GPT2, CHS, and DFR) in w18 w53 HAC1 OE was lower than in HAC1 OE lines after Glc treatment (Supplemental Fig. S12H). Furthermore, the w18 hac1-4 double mutant showed extremely reduced anthocyanin levels after 6% Glc treatment compared with Col-0, w18, and hac1-4 seedlings (Supplemental Fig. S12, F and G). Expression of sugar-induced genes was also decreased in w18 hac1-4 after Glc treatment (Supplemental Fig. S12I). Taken together, these results indicated that the role of WRKY18 and WRKY53 on Glc response partially depends on HAC1 function.
DISCUSSION
Sugars are major storage and metabolism molecules in cells and their biosynthesis, signaling, and turnover are under stringent regulation (Ruan, 2014; Li and Sheen, 2016). Plants need to rapidly respond to their endogenous sugar status to optimize their fitness and survival during development and environmental changes (Baena-González et al., 2007; Chen et al., 2010; Bruggeman et al., 2015). GPT2 is a marker gene whose transcription is rapidly altered in response to sugar. Increased expression of this gene and the associated protein abundance can promote the transport of Glc-6-P from the cytosol to chloroplasts, thus affecting sugar homeostasis, starch synthesis, and plant growth (Athanasiou et al., 2010; Dyson et al., 2014, 2015; Van Dingenen et al., 2016). Although the importance of GPT2 in rapid sugar responses has been well documented, components regulating the transcription of GPT2 or other sugar-responsive genes have largely remained unknown.
In this study, we demonstrated that two transcription factors, WRKY18 and WRKY53, are required to activate the expression of a series of sugar-responsive genes such as GPT2, DFR, and CHS (Figs. 1–3). In addition, we discovered that acetylation of H3K27 mediated by HAC1, an epigenetic level of regulation, could improve activation of their downstream sugar response genes by WRKY18 and WRKY53 (Figs. 4–6).
WRKY18 and WRKY53 Are Novel Regulators of Sugar Responses
Multiple WRKY transcription factors play essential roles in immunity, leaf senescence, and stress responses (Eulgem et al., 2000; Rushton et al., 2010; Jiang et al., 2017). In the immunity response, WRKY18, WRKY33, and WRKY40 function as negative regulators to prevent exaggerated defense responses (Birkenbihl et al., 2017; Abeysinghe et al., 2019). WRKY53 is a positive regulator of leaf senescence, whereas WRKY18 negatively regulates leaf senescence probably by repressing WRKY53 expression (Miao et al., 2004; Potschin et al., 2014). WRKY18 interacts with WRKY53 to form a heterodimer and regulate leaf senescence (Potschin et al., 2014). However, whether WRKYs are involved in the sugar response is still unclear.
In this study, we identified that both WRKY18 and WRKY53 positively regulate a large proportion of sugar response genes, including GPT2, DFR, and CHS (Figs. 2 and 3). The single or double w18 and w53 mutants had reduced sugar-inducible gene expression and decreased accumulation of anthocyanin in response to Glc (Figs. 2, 3, and 5C; Supplemental Fig. S7). It should be noted that both WRKY53 and GPT2 are highly expressed in the senescing leaf (Zentgraf et al., 2010), which suggests that the regulation of GPT2 by WRKYs is not only active in the seedling stage, but might also function in senescing leaves.
In our study, we discovered that various W-Box cis-elements are distributed in the promoter regions of a large number of sugar-responsive genes, including GPT2, DFR, and CHS (Fig. 3D). We further found that WRKY18 and WRKY53 can bind to these W-Box cis-elements to regulate gene expression in vivo and vitro (Fig. 3D; Supplemental Fig. S8). Interestingly, the W-Box cis-elements in the promoter of GPT2 are required for rapid sugar-induced expression in the cotyledon (Fig. 1), but the GPT2 SURE cis-elements mediated rapid sugar-induced expression in the root (Supplemental Fig. S2D). This is consistent with a previous study showing that the barley WRKY transcription factor SUSBIA2 binds to the SURE cis-elements in the promoter of Isoamylase1 and regulates endosperm starch synthesis and accumulation (Sun et al., 2003). These results further indicated that WRKY-mediated sugar signaling via the W-Box cis-element might be mainly active in the photoautotrophic tissues (e.g. cotyledon and mature leaves), but SURE cis-elements might play a dominant role in the photoheterotrophic tissues, such as root and endosperm.
Histone Modification Participates in Sugar Responses
Although hac1-4 was previously identified as a Glc and Suc insensitive mutant (Heisel et al., 2013), to the best of our knowledge, the mechanism by which HAC1 participates in sugar signaling has not been explored in any plant species. In our study, HAC1-mediated acetylation of H3K27 was discovered to be one of the mechanisms associated with rapid sugar signaling (Fig. 6). Our results showed that HAC1 is part of the WRKY18/53 complex binding to the promoters of sugar-responsive genes. Moreover, the lack of HAC1 led to physiological dysfunction, i.e. decreased sugar-induced gene expression and anthocyanin accumulation in the mutant plants (Figs. 4 and 5). In mammals, high Glc exposure increases H3 acetylation, including H3K9ac, H3K14ac, H3K27ac, and H3K56ac (Yu et al., 2016). Discoveries from our lab and others suggested that Glc signaling and downstream regulation through histone modification might be evolutionarily conserved among different organisms (Abate et al., 2012; Li et al., 2012; Honma et al., 2014).
Although HAC1 forms a complex with WRKY18 and 53, different regulatory mechanisms might be associated with these proteins to control the transcription of sugar response genes. We noticed that the basal abundances and Glc induction of a group of transcripts differ in w18 w53 or hac1-4 mutant. For instance, the FPKM (Fragments Per Kilobase of transcript per million mapped reads) values of GPT2 were induced from 3.96 to 194.85 in wild type, 3.42 to 92.54 in w18 w53, and 0.95 to 41.30 in hac1-4 (Fig. 5G; Supplemental Dataset 2). The reduced induction level of GPT2 in the w18 w53 double mutant indicated that WRKY18 and WRKY53 specifically mediate transcriptional activation in response to Glc. For HAC1, although the induction fold changes were similar in wild type and hac1-4, the dramatic reduction (70%) of the FPKM value of GPT2 in the mutant is consistent with the fundamental role of HAC1 in the epigenetic activation of transcription. Our work suggested that WRKYs or other transcription factors bind to the promoter regions of sugar-responsive genes to regulate transcription, and the recruitment of HAC1 to the WRKY complex may make the transcription even more efficient. Although the important role of sugar, WRKY18, WRKY53, or HAC1 in plant immunity has been well recognized (Singh et al., 2014; Birkenbihl et al., 2017; Jiang et al., 2017), whether complex formation is involved in regulating immunity is worth exploring.
The importance of HAC1 and its interacting protein have been indicated in other physiological processes such as flowering time and hormonal responses (Deng et al., 2007; Singh et al., 2014). For example, HAC1 interacts with MEDIATOR 25 (MED25) to regulate jasmonate signaling by acetylating H3K9 (An et al., 2017). MED25 is also required for sugar-mediated transcriptional regulation, starch synthesis, and anthocyanin biosynthesis (Seguela-Arnaud et al., 2015). We did not detect a direct physical interaction between WRKYs and MED25, indicating that HAC1 might serve as a bridge to bring the Mediator complex to the WRKY18/53-HAC1 for coordinately regulating sugar responses. The weakened sugar responses in w18 w53 HAC1 OE compared with HAC1 OE further indicate the modulations of GPT2, DFR, and CHS by WRKY18/53 and HAC1 are two independent mechanisms (Supplemental Fig. S12). WRKY53 physically interacts with HISTONE DEACETYLASE 9 (HDA9) and the reduced deacetylation in the hda9 mutant led to the increased expression of GPT2, pointing out the importance of acetylation in sugar signaling (Chen et al., 2016). Besides histone acetylation, other histone modifications (e.g. methylation) may also be involved in sugar responses. CURLY LEAF and SWINGER are two subunits of the Polycomb Repressive Complex 2, which promotes H3K27 trimethylation (Köhler et al., 2012). Reduced trimethylation of H3K27 in the clf-29 swn-21 mutant led to higher expression of sugar-induced genes including GPT2 and ApL3 (Zhou et al., 2018), indicating the methylation of H3K27 might also be essential for sugar responses. Whether deacetylation and methylation are involved in sugar signaling remains interesting to pursue.
In summary, our results suggested that when sugars are increased in the cytosol, the expression levels of sugar-responsive genes were increased by the coordinate actions of WRKY18, WRKY53, and HAC1 (Fig. 6E). WRKY transcription factors are significantly enriched on the promoters of Glc-induced genes through binding to the W-Box cis-elements. WRKY transcription factors recruit HAC1 to facilitate the acetylation of H3K27 on the loci of Glc-induced genes, thus loosening the chromatin and activating transcription. The increased cytosolic sugar content could then be lowered by more active sugar import into cellular compartments (e.g. chloroplast, mitochondria, and vaculoe) or enhanced metabolism (e.g. anthocyanin biosynthesis).
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) wild-type and transgenic lines are in the Col-0 ecotype. The mutant seeds of wrky18 (Salk_093916C, w18), wrky53 (Salk_034157C, w53), and hac1-4 (Salk_070277) were obtained from the Arabidopsis Biological Resource Center (The Ohio State University, Columbus), and the wrky18 wrky53 (w18 w53) and wrky18 hac1-4 (w18 hac1-4) double mutants were generated from wrky18 crossed with wrky53 or hac1-4, respectively. The seeds were sterilized and grown on Murashige and Skoog (MS) medium with 0.05% (w/v) MES and 1% (w/v) Suc unless noted. For exogenous sugar treatment, 7-d-old seedlings vertically grown under long-day (16-h light/8-h dark) conditions were transferred to filter paper with liquid MS medium (without Suc) and incubated for 24 h under constant darkness. After that, the seedlings were then treated with 15 mm Glc or Mtl (or other indicated concentration) for the indicated times.
Plasmid Construction and Transgenic Plants
To generate GPT2pro::GUS and GPT2pro::LUC, a 2,360-bp fragment upstream the ATG start code of the GPT2 was amplified by PCR from Col-0 genomic DNA, and then inserted into KpnI and SalI digested pPZP211-GUS or pPZP211-LUC to produce pPZP211-GPT2pro::GUS or pPZP211-GPT2pro::LUC, respectively.
For the luciferase reporters used in the transient expression assay, various promoter-deleted fragments were PCR amplified with different pairs of primers (Supplemental Table S3) and then inserted into KpnI and SalI digested pGreen-0800 vector (Hellens et al., 2005) to produce various LUC reporters. To produce the plasmids of the pGreen-0800- (P1–P4)-LUC, a 91-bp 35S minimal promoter was first inserted into SalI and BamHI digested pGreen-0800 to produce the pGreen-0800-35Smini construct. Then, a series of fragments between −583 and −2,360 of the GPT2 promoter were ligated to pGreen-0800-35mini to produce pGreen-0800- (P1–P4)-LUC reporters, respectively. Finally, pGreen-0800-P3 was used as a template to produce the mutated P3-LUC (m1–m7) reporter genes by DpnI-mediated site-directed mutagenesis. To generate the 3xW-Box-mini::GUS and 3xSURE-mini::GUS, 3xW-Box and 3xSURE fragments were ligated into pGreen-0800-35mini first, and then the fragments containing 3xW-Box-mini and 3xSURE-mini were released and inserted into KpnI and BamHI digested pPZP211 -GUS to produce 3xW-Box-mini::GUS and 3xSURE-mini::GUS, respectively.
To generate 35S::WRKY18-GFP and 35S::WRKY53-GFP, the full-length coding sequences of WRKY18 or WRKY53 were inserted into pPZP211-35S::GFP. The promoter sequence of WRKY18 or WRKY53 was then replaced with the 35S promoter to generate WRKY18pro::WRKY18-GFP or WRKY53pro::WRKY53-GFP, respectively. To generate 35S::HAC1-myc, the full-length coding region of HAC1 was cloned into pCAMBIA1301-35S::myc to produce the 35S::HAC1-myc construct using the one step cloning kit (Vazyme, C112). Various constructs were transferred into Agrobacterium tumefaciens GV3101 and then transformed into wild-type Col-0 or mutant plants by floral dipping. Transgenic lines were selected in MS medium with kanamycin or hygromycin B. At least fifteen independent lines were selected and used for further analyses. The w18 w53 HAC1 OE seedlings was generated from HAC1 OE crossed with w18 w53. For all the transgenic lines, both RT-qPCR and immunoblotting were used to identify plants with high expression of mRNA and protein. The primers used are listed in Supplemental Table S3.
Transient Expression Assays in Arabidopsis Protoplasts
Three-week-old Arabidopsis Col-0 plants grown under short-day conditions were used to prepare mesophyll protoplasts. The procedure for protoplast transformation was modified based on a previous report (Yoo et al., 2007). For promoter deletion analysis and subcellular localization assays, 0.5 m mannitol was replaced with 0.015 m Glc and 0.485 m mannitol in the WI incubation buffer. For protoplast transformation, 10 μg of the total luciferase reporter and 5 μg effectors were introduced into protoplasts. After 16 h incubation under dim light, the Dual-Luciferase Report Assay system (Promega) and a GloMax 20/20 luminometer were used to detect the luciferase signal. Reporter gene expression was calculated from the ratio of LUC/Renilla luciferase (REN).
Yeast One-Hybrid and Two-Hybrid Assays
Yeast one-hybrid for transcription factor library screening was performed following the procedure described previously (Ou et al., 2011). The P3 region of GPT2 promoter was ligated to pLacZi-2μ (Lin et al., 2007) to produce the P3::LacZ reporter. Then, the P3::LacZ plasmid was integrated into yeast strain YM4271 and mated with AD-WRKY-TF in yeast strain Y187 (Clontech) to detect the interaction.
To generate the yeast two-hybrid constructs, the full-length coding sequence of WRKY53 and WRKY18 were inserted into pGBKT7 and pGADT7, respectively. The full-length, N- (1-2130 bp) and C terminus (2661-5091 bp) of HAC1, respectively, were ligated into pGBKT7. The yeast two-hybrid was performed according to the procedure described previously (Shi et al., 2018).
Anthocyanin measurement
The 7-d-old seedlings were transferred to 6% Glc medium for 3 d and then harvested for anthocyanin measurement according to procedures described previously. The Arabidopsis leaves were extracted with buffer [acidic methanol, 1% (v/v) HCl] at 4°C in the dark. The homogenate was cleared by centrifugation, and the supernatant was used for photometric measurements. To quantify anthocyanins, the following equation was used: (A530 - 0.25 × A657) × M-1 [g] = relative units of anthocyanin (with A530 and A657 = absorption at the indicated wavelengths, M = plant fresh weight). Each experiment was performed in triplicate, and measurements from 18 plants were pooled for one replicate. Three independent experiments were performed.
BiFC Assays
The full-length coding regions of HAC1, WRKY18, or WRKY53 were individually subcloned into the pTOPO vector and then recombined into the pSITE-BIFC-nEYFP (nYFP) or pSITE-BIFC-cEYFP (cYFP) vectors (Martin et al., 2009). The nYFP and cYFP vectors were separately transformed into Agrobacterium C58C1, and then coinfiltrated into the leaves of N. benthamiana. After 2 d incubation, fluorescence signals of YFP in the transfected epidermal cells were imaged by confocal microscopy (LSCM; Zeiss).
LCI Assays
The full-length coding regions of HAC1, WRKY18, or WRKY53 were individually cloned into pCAMBIA1300-nLUC or pCAMBIA1300-cLUC vectors (Chen et al., 2008). Then, various kinds of nLUC- and cLUC-fused plasmids were separately transformed into Agrobacterium C58C1, and then coinfiltrated into the leaves of N. benthamiana. At 2 d after the incubation, the leaves were injected with 0.5 mm luciferin and placed in darkness for 2 min before detection of luminescence.
Co-IP Assays
Agrobacteria C58C1 carrying different construct combinations were coinfiltrated into the leaves of N. benthamiana and then harvested after 3 d incubation under low-intensity light conditions. The cotransformation and Co-IP procedures were described in a previous study (Li et al., 2011). Anti-HAC1 antibodies were used to perform IP and detect the protein expression of HAC1, and anti-GFP antibodies (TransGene, HT801) were used to detect the expression of GFP, WRKY18-GFP, and WRKY53-GFP fusion proteins. Anti-HAC1 polyclonal antibodies were generated by Sangon biotech in rabbit using the peptide AYDNLQRSGMQGDGY.
RNA-Seq and Data Analysis
After 24 h darkness carbon starvation, the 8-d-old seedlings were treated with, or without, 15 mm Glc for 3 h. Two biological replicates were collected for RNA-seq analysis. Total RNA of each sample was extracted using a RNeasy plant Mini Kit (Qiagen, 74904). The RNA-seq analysis was performed by Annoroad Gene Tech. Co., Ltd. The reads were mapped to Arabidopsis genome TAIR10 using HISAT2 software. Differentially expressed genes were identified when the comparison was made on Normalized Reads Count between Glc + and Glc− treatment with P < 0.05 and fold change > 2. Comparison analysis and plots were performed by the TBtools software. The abundance of W-Box cis-elements in the upstream of the coding region of differentially expressed genes was identified by R.
Immunoblot and ChIP Assay
Arabidopsis seedlings were harvested after treatment with 15 mm Mlt or Glc for 3 h, unless noted otherwise. The Western blot and ChIP-qPCR were performed following the procedures described previously (Li et al., 2011). Anti-GFP (TransGene, HT801), Anti-H3 (Beyotime, AF0009), Anti-H3K27ac (Abcam, ab4729), Anti-H3k9ac (Abcam, ab10812), Anti-H3k14ac (Abcam, ab52946), and Anti-H3ac (Abcam, ab47915) were used to perform the immunoblot or ChIP assay. The F1–F4 fragments of the GPT2 promoter, the F1–F2 fragments of the DFR promoter, the F1–F3 fragments of the CHS promoter, and a fragment of the ACTIN12 promoter were used for the ChIP-qPCR assay (Supplemental Table S3).
Protein Expression and EMSA
The coding sequences of WRKY18 and WRKY53 were amplified and cloned into the pET28a vector. The primer sequences used are listed in Supplemental Table S3. The recombinant plasmid was introduced to Escherichia coli strain BL21. E. coli cells were induced with 0.2 mm isopropylthio-β-galactoside overnight at 18°C and collected by centrifugation. The His-WRKY18 and His-WRKY53 protein was purified using BeaverBeads IDA-Nickel kit (Beaver, 70501-5).
For EMSA, the probes were obtained by PCR using biotin-labeled or unlabeled primers (Supplemental Table S3). The biotin-unlabeled fragments of the same sequences were used as competitors. The reaction mixture (10 μL) for EMSA contained 2 μL purified protein, 1 μL 50 μg/mL biotin-labeled annealed oligonucleotide, 2 μL 5×binding buffer, and ultrapure water. The reactions were incubated at 22°C for 30 min. The reactions were fractionated on a 6% native polyacrylamide gel in 0.5×Tris-borate/EDTA buffer. The detection of biotin-labeled DNA by chemiluminescence was performed using a Chemiluminescent EMSA Kit (Beyotime, GS009) following the manufacturer’s protocol.
Statistical Analysis
Samples were analyzed in triplicate, and the data were expressed as the mean ± sd unless noted otherwise. Statistical significance was determined using Student’s t test. A difference at P < 0.05 was considered significant, and P < 0.01 was considered extremely significant.
Others
The histochemical analyses of GUS and RT-qPCR were performed as described previously (Niewiadomski et al., 2005; Li et al., 2011). All the primers used in this study are listed in Supplemental Table S3.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative database under the following accession numbers: UBQ10 (AT4G05320), ACTIN12 (AT3G46520), GPT1 (AT5G54800), GPT2 (AT1G61800), TPT (AT5G46110), WRKY18 (AT4G31800), WRKY53 (AT4G23810), DIN1 (AT4G35770), DIN6 (AT3G47340), CHS (AT5G13930), DFR (AT5G42800), HAC1 (AT1G79000).
SUPPLEMENTAL DATA
The following supplemental materials are available.
Supplemental Figure S1. GPT2 is one of the markers for sugar rapid response.
Supplemental Figure S2. Expression pattern of GPT2pro::GUS.
Supplemental Figure S3. Identification of the core-regions responding to Glc in GPT2 promoter region.
Supplemental Figure S4. Multiple WRKY-type transcription factors mediated Glc induced expression of GPT2.
Supplemental Figure S5. Identification of WRKY18 and WRKY53 double mutant and their transgenic lines.
Supplemental Figure S6. WRKY18 and WRKY53 mediate the induction of GPT2 by sugar.
Supplemental Figure S7. The sugar response is restored by the complementation of wrky18 or wrky53.
Supplemental Figure S8. WRKY18 and WRKY53 bind to the promoters of GPT2, DFR and CHS in vitro.
Supplemental Figure S9. Summary of GO analysis.
Supplemental Figure S10. Histone acetylation regulated by HAC1 affects the expression of Glc-induced genes.
Supplemental Figure S11. Sugar-induced enrichment of histone acetylation is independent of osmotic stress.
Supplemental Figure S12. WRKY18, WRKY53, and HAC1 coordinately regulate sugar-responsive genes expression.
Supplemental Table S1. The locations of various sugar response cis-elements in the GPT2 promoter region.
Supplemental Table S2. The sequences of mutated cis-elements shown in Figure 1C.
Supplemental Table S3. A list of the primers used in this work.
Supplemental Dataset 1. Genes whose expression is regulated by WRKY18, WRKY53 and HAC1 after Glc treatment.
Supplemental Dataset 2. Genes whose FC is lower in w18w53 and hac1-4 compared with Col-0 in the Glc upregulated group.
Supplemental Dataset 3. A list of genes expression used in Figure 5H.
Acknowledgments
We thank Professor Li-Jia Qu for providing the yeast one-hybrid transcription factor library.
Footnotes
This work was supported by the National Natural Science Foundation of China (NSFC) (grant nos. 31870266 and 31670249).
Articles can be viewed without a subscription.
References
- Abate G, Bastonini E, Braun KA, Verdone L, Young ET, Caserta M (2012) Snf1/AMPK regulates Gcn5 occupancy, H3 acetylation and chromatin remodelling at S. cerevisiae ADY2 promoter. Biochim Biophys Acta 1819: 419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeysinghe JK, Lam KM, Ng DW (2019) Differential regulation and interaction of homoeologous WRKY18 and WRKY40 in Arabidopsis allotetraploids and biotic stress responses. Plant J 97:352–367 [DOI] [PubMed] [Google Scholar]
- An C, Li L, Zhai Q, You Y, Deng L, Wu F, Chen R, Jiang H, Wang H, Chen Q, Li C (2017) Mediator subunit MED25 links the jasmonate receptor to transcriptionally active chromatin. Proc Natl Acad Sci USA 114: E8930–E8939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiou K, Dyson BC, Webster RE, Johnson GN (2010) Dynamic acclimation of photosynthesis increases plant fitness in changing environments. Plant Physiol 152: 366–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena-González E, Rolland F, Thevelein JM, Sheen J (2007) A central integrator of transcription networks in plant stress and energy signalling. Nature 448: 938–942 [DOI] [PubMed] [Google Scholar]
- Birkenbihl RP, Kracher B, Roccaro M, Somssich IE (2017) Induced genome-wide binding of three Arabidopsis WRKY transcription factors during early MAMP-triggered immunity. Plant Cell 29: 20–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bläsing OE, Gibon Y, Günther M, Höhne M, Morcuende R, Osuna D, Thimm O, Usadel B, Scheible WR, Stitt M (2005) Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell 17: 3257–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggeman Q, Prunier F, Mazubert C, de Bont L, Garmier M, Lugan R, Benhamed M, Bergounioux C, Raynaud C, Delarue M (2015) Involvement of Arabidopsis hexokinase1 in cell death mediated by myo-inositol accumulation. Plant Cell 27: 1801–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zou Y, Shang Y, Lin H, Wang Y, Cai R, Tang X, Zhou JM (2008) Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol 146: 368–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LQ, Hou BH, Lalonde S, Takanaga H, Hartung ML, Qu XQ, Guo WJ, Kim JG, Underwood W, Chaudhuri B, et al. (2010) Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468: 527–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Lu L, Mayer KS, Scalf M, Qian S, Lomax A, Smith LM, Zhong X (2016) POWERDRESS interacts with HISTONE DEACETYLASE 9 to promote aging in Arabidopsis. eLife 5: e17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YH, Sheen J, Yoo SD (2010) Low glucose uncouples hexokinase1-dependent sugar signaling from stress and defense hormone abscisic acid and C2H4 responses in Arabidopsis. Plant Physiol 152: 1180–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoba E, Aceves-Zamudio DL, Hernández-Bernal AF, Ramos-Vega M, León P (2015) Sugar regulation of SUGAR TRANSPORTER PROTEIN 1 (STP1) expression in Arabidopsis thaliana. J Exp Bot 66: 147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Liu C, Pei Y, Deng X, Niu L, Cao X (2007) Involvement of the histone acetyltransferase AtHAC1 in the regulation of flowering time via repression of FLOWERING LOCUS C in Arabidopsis. Plant Physiol 143: 1660–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson BC, Webster RE, Johnson GN (2014) GPT2: A glucose 6-phosphate/phosphate translocator with a novel role in the regulation of sugar signalling during seedling development. Ann Bot 113: 643–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson BC, Allwood JW, Feil R, Xu Y, Miller M, Bowsher CG, Goodacre R, Lunn JE, Johnson GN (2015) Acclimation of metabolism to light in Arabidopsis thaliana: The glucose 6-phosphate/phosphate translocator GPT2 directs metabolic acclimation. Plant Cell Environ 38: 1404–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Robatzek S, Somssich IE (2000) The WRKY superfamily of plant transcription factors. Trends Plant Sci 5: 199–206 [DOI] [PubMed] [Google Scholar]
- Feng S, Jacobsen SE, Reik W (2010) Epigenetic reprogramming in plant and animal development. Science 330: 622–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K. (2011) The import and export business in plastids: transport processes across the inner envelope membrane. Plant Physiol 155: 1511–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson SI. (2005) Control of plant development and gene expression by sugar signaling. Curr Opin Plant Biol 8: 93–102 [DOI] [PubMed] [Google Scholar]
- Gonzali S, Loreti E, Solfanelli C, Novi G, Alpi A, Perata P (2006) Identification of sugar-modulated genes and evidence for in vivo sugar sensing in Arabidopsis. J Plant Res 119: 115–123 [DOI] [PubMed] [Google Scholar]
- Grigston JC, Osuna D, Scheible WR, Liu C, Stitt M, Jones AM (2008) D-Glucose sensing by a plasma membrane regulator of G signaling protein, AtRGS1. FEBS Lett 582: 3577–3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisel TJ, Li CY, Grey KM, Gibson SI (2013) Mutations in HISTONE ACETYLTRANSFERASE1 affect sugar response and gene expression in Arabidopsis. Front Plant Sci 4: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens RP, Allan AC, Friel EN, Bolitho K, Grafton K, Templeton MD, Karunairetnam S, Gleave AP, Laing WA (2005) Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma K, Masuda Y, Mochizuki K, Goda T (2014) Re-feeding rats a high-sucrose diet after 3 days of starvation enhances histone H3 acetylation in transcribed region and expression of jejunal GLUT5 gene. Biosci Biotechnol Biochem 78: 1071–1073 [DOI] [PubMed] [Google Scholar]
- Hu DG, Sun CH, Zhang QY, An JP, You CX, Hao YJ (2016) Glucose Sensor MdHXK1 phosphorylates and stabilizes MdbHLH3 to promote anthocyanin biosynthesis in apple. PLoS Genet 12: e1006273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Ma S, Ye N, Jiang M, Cao J, Zhang J (2017) WRKY transcription factors in plant responses to stresses. J Integr Plant Biol 59: 86–101 [DOI] [PubMed] [Google Scholar]
- Kang SG, Price J, Lin PC, Hong JC, Jang JC (2010) The arabidopsis bZIP1 transcription factor is involved in sugar signaling, protein networking, and DNA binding. Mol Plant 3: 361–373 [DOI] [PubMed] [Google Scholar]
- Köhler C, Wolff P, Spillane C (2012) Epigenetic mechanisms underlying genomic imprinting in plants. Annu Rev Plant Biol 63: 331–352 [DOI] [PubMed] [Google Scholar]
- Kouzarides T. (2007) Chromatin modifications and their function. Cell 128: 693–705 [DOI] [PubMed] [Google Scholar]
- Kunz S, Gardeström P, Pesquet E, Kleczkowski LA (2015) Hexokinase 1 is required for glucose-induced repression of bZIP63, At5g22920, and BT2 in Arabidopsis. Front Plant Sci 6: 525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastdrager J, Hanson J, Smeekens S (2014) Sugar signals and the control of plant growth and development. J Exp Bot 65: 799–807 [DOI] [PubMed] [Google Scholar]
- Lee Y, Nishizawa T, Takemoto M, Kumazaki K, Yamashita K, Hirata K, Minoda A, Nagatoishi S, Tsumoto K, Ishitani R, Nureki O (2017) Structure of the triose-phosphate/phosphate translocator reveals the basis of substrate specificity. Nat Plants 3: 825–832 [DOI] [PubMed] [Google Scholar]
- Li L, Sheen J (2016) Dynamic and diverse sugar signaling. Curr Opin Plant Biol 33: 116–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Siddiqui H, Teng Y, Lin R, Wan XY, Li J, Lau OS, Ouyang X, Dai M, Wan J, et al. (2011) Coordinated transcriptional regulation underlying the circadian clock in Arabidopsis. Nat Cell Biol 13: 616–622 [DOI] [PubMed] [Google Scholar]
- Li H, Gao Z, Zhang J, Ye X, Xu A, Ye J, Jia W (2012) Sodium butyrate stimulates expression of fibroblast growth factor 21 in liver by inhibition of histone deacetylase 3. Diabetes 61: 797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Erkek S, Tong Y, Yin L, Federation AJ, Zapatka M, Haldipur P, Kawauchi D, Risch T, Warnatz HJ, et al. (2016) Active medulloblastoma enhancers reveal subgroup-specific cellular origins. Nature 530: 57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Ding L, Casola C, Ripoll DR, Feschotte C, Wang H (2007) Transposase-derived transcription factors regulate light signaling in Arabidopsis. Science 318: 1302–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A, Brockman A, Aguirre L, Campbell A, Bean A, Cantero A, Gonzalez A (2017) Advances in the MYB-bHLH-WD Repeat (MBW) pigment regulatory model: addition of a WRKY factor and co-option of an anthocyanin MYB for betalain regulation. Plant Cell Physiol 58: 1431–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CA, Ho TH, Ho SL, Yu SM (2002) Three novel MYB proteins with one DNA binding repeat mediate sugar and hormone regulation of alpha-amylase gene expression. Plant Cell 14: 1963–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K, Kopperud K, Chakrabarty R, Banerjee R, Brooks R, Goodin MM (2009) Transient expression in Nicotiana benthamiana fluorescent marker lines provides enhanced definition of protein localization, movement and interactions in planta. Plant J 59: 150–162 [DOI] [PubMed] [Google Scholar]
- Matiolli CC, Tomaz JP, Duarte GT, Prado FM, Del Bem LE, Silveira AB, Gauer L, Corrêa LG, Drumond RD, Viana AJ, et al. (2011) The Arabidopsis bZIP gene AtbZIP63 is a sensitive integrator of transient abscisic acid and glucose signals. Plant Physiol 157: 692–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Laun T, Zimmermann P, Zentgraf U (2004) Targets of the WRKY53 transcription factor and its role during leaf senescence in Arabidopsis. Plant Mol Biol 55: 853–867 [DOI] [PubMed] [Google Scholar]
- Moore B, Zhou L, Rolland F, Hall Q, Cheng WH, Liu YX, Hwang I, Jones T, Sheen J (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300: 332–336 [DOI] [PubMed] [Google Scholar]
- Niewiadomski P, Knappe S, Geimer S, Fischer K, Schulz B, Unte US, Rosso MG, Ache P, Flügge UI, Schneider A (2005) The Arabidopsis plastidic glucose 6-phosphate/phosphate translocator GPT1 is essential for pollen maturation and embryo sac development. Plant Cell 17: 760–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou B, Yin KQ, Liu SN, Yang Y, Gu T, Wing Hui JM, Zhang L, Miao J, Kondou Y, Matsui M, et al. (2011) A high-throughput screening system for Arabidopsis transcription factors and its application to Med25-dependent transcriptional regulation. Mol Plant 4: 546–555 [DOI] [PubMed] [Google Scholar]
- Potschin M, Schlienger S, Bieker S, Zentgraf U (2014) Senescence networking: WRKY18 is an upstream regulator, a downstream target gene, and a protein interaction partner of WRKY53. J Plant Growth Regul 33: 106–118 [Google Scholar]
- Price J, Laxmi A, St Martin SK, Jang JC (2004) Global transcription profiling reveals multiple sugar signal transduction mechanisms in Arabidopsis. Plant Cell 16: 2128–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland F, Sheen J (2005) Sugar sensing and signalling networks in plants. Biochem Soc Trans 33: 269–271 [DOI] [PubMed] [Google Scholar]
- Ruan YL. (2014) Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu Rev Plant Biol 65: 33–67 [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Somssich IE, Ringler P, Shen QJ (2010) WRKY transcription factors. Trends Plant Sci 15: 247–258 [DOI] [PubMed] [Google Scholar]
- Sami F, Siddiqui H, Hayat S (2019) Interaction of glucose and phytohormone signaling in plants. Plant Physiol Biochem 135:119–126 [DOI] [PubMed] [Google Scholar]
- Seguela-Arnaud M, Smith C, Uribe MC, May S, Fischl H, McKenzie N, Bevan MW (2015) The Mediator complex subunits MED25/PFT1 and MED8 are required for transcriptional responses to changes in cell wall arabinose composition and glucose treatment in Arabidopsis thaliana. BMC Plant Biol 15: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J, Zhou L, Jang JC (1999) Sugars as signaling molecules. Curr Opin Plant Biol 2: 410–418 [DOI] [PubMed] [Google Scholar]
- Shi Q, Zhang H, Song X, Jiang Y, Liang R, Li G (2018) Functional characterization of the maize phytochrome-interacting factors PIF4 and PIF5. Front Plant Sci 8: 2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Yekondi S, Chen PW, Tsai CH, Yu CW, Wu K, Zimmerli L (2014) Environmental history modulates Arabidopsis pattern-triggered immunity in a HISTONE ACETYLTRANSFERASE1-dependent manner. Plant Cell 26: 2676–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P (2006) Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 140: 637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Hu C, Yan X, Jin Y, Chen Z, Guan Q, Wang Y, Zhong D, Jansson C, Wang F, et al. (2015) Expression of barley SUSIBA2 transcription factor yields high-starch low-methane rice. Nature 523: 602–606 [DOI] [PubMed] [Google Scholar]
- Sun C, Palmqvist S, Olsson H, Borén M, Ahlandsberg S, Jansson C (2003) A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar-responsive elements of the iso1 promoter. Plant Cell 15: 2076–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng S, Keurentjes J, Bentsink L, Koornneef M, Smeekens S (2005) Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol 139: 1840–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dingenen J, De Milde L, Vermeersch M, Maleux K, De Rycke R, De Bruyne M, Storme V, Gonzalez N, Dhondt S, Inzé D (2016) Chloroplasts are central players in sugar-induced leaf growth. Plant Physiol 171: 590–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wind J, Smeekens S, Hanson J (2010) Sucrose: Metabolite and signaling molecule. Phytochemistry 71: 1610–1614 [DOI] [PubMed] [Google Scholar]
- Xiong Y, McCormack M, Li L, Hall Q, Xiang C, Sheen J (2013) Glucose-TOR signalling reprograms the transcriptome and activates meristems. Nature 496: 181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Hu T, Smith MR, Poethig RS (2016) Epigenetic regulation of vegetative phase change in Arabidopsis. Plant Cell 28: 28–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Yu J, Wu Y, Yang P (2016) High glucose-induced oxidative stress represses sirtuin deacetylase expression and increases histone acetylation leading to neural tube defects. J Neurochem 137: 371–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentgraf U, Laun T, Miao Y (2010) The complex regulation of WRKY53 during leaf senescence of Arabidopsis thaliana. Eur J Cell Biol 89: 133–137 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wang Y, Krause K, Yang T, Dongus JA, Zhang Y, Turck F (2018) Telobox motifs recruit CLF/SWN-PRC2 for H3K27me3 deposition via TRB factors in Arabidopsis. Nat Genet 50: 638–644 [DOI] [PubMed] [Google Scholar]