Similar bell-shaped dose-dependent effects of water deficit (WD) and ABA on root development and hydraulics as well as analysis of ABA synthesis and response mutants suggest that ABA acts as a coordinator of root responses to WD.
Abstract
Root water uptake is influenced by root system architecture, which is determined by root growth and branching and the hydraulics of root cells and tissues. The phytohormone abscisic acid (ABA) plays a major role in the adaptation of plants to water deficit (WD). Here we addressed at the whole-root level in Arabidopsis (Arabidopsis thaliana) the regulatory role of ABA in mechanisms that determine root hydraulic architecture. Root system architecture and root hydraulic conductivity (Lpr) were analyzed in hydroponically grown plants subjected to varying degrees of WD induced by various polyethylene glycol (PEG) concentrations. The majority of root traits investigated, including first- and second-order lateral root production and elongation and whole-root hydraulics, had a bell-shaped dependency on WD, displaying stimulation under mild WD conditions (25 g PEG L−1) and repression under more severe conditions. These traits also showed a bell-shaped dependency on exogenous ABA, and their regulation by WD was attenuated in genotypes altered in ABA biosynthesis and response. Thus, we propose that ABA acts as a coordinator and an integrator of most root responses to mild and moderate WD, whereas responses to strong WD (150 g PEG L−1) are largely ABA independent. We also found that roots exhibit different growth responses to both WD and ABA depending on their rank and age. Taken together, our results give further insights into the coordinated water acquisition strategies of roots deployed in relation to WD intensity.
Water deficit (WD) is now recognized as the abiotic stress with the greatest effect on crop productivity (Comas et al., 2013). Thus, understanding how plants use water for optimal biomass production has become a fundamental issue worldwide (Koevoets et al., 2016). Plants are sessile organisms that cannot escape from environmental constraints and, as a result, have evolved numerous adaptive responses at molecular, cellular, and physiological levels. When exposed to WD, plants first respond by a strict regulation of stomatal aperture together with rapid changes in root hydraulic conductivity (Lpr; Maurel et al., 2010; Sutka et al., 2011; Rosales et al., 2012). Over a longer term, plants change both shoot growth (to restrict water loss) and root growth and differentiation to modulate their capacity to take up soil water (Deak and Malamy, 2005; Koevoets et al., 2016).
Root system architecture (RSA) refers to the three-dimensional organization of the root system. The model plant Arabidopsis has been widely used to unravel the molecular and genetic bases of root traits and adaptive responses. In this species, the root system is formed through a reiterative program in which lateral roots (LRs) are produced along the primary root (first-order LRs) or along LRs themselves (second-, third-, etc., order LRs). Despite this apparent simplicity, root development is highly flexible and can adjust to the environment to optimize soil foraging and nutrient and water uptake. More generally, the depth of rooting is an important parameter for foraging for water when deep water is available (Lynch, 1995; Uga et al., 2013), but the overall distribution of roots has received less attention because it displays a significant degree of plasticity in response to heterogeneous distribution of soil resources (Koevoets et al., 2016). When water availability is limited, plants first reduce shoot development leading to a shift in their allometry (metrics of root to shoot relationships) and then reduce root development depending on the constraint extent and duration (reviewed by Comas et al., 2013; Pierik and Testerink, 2014; Koevoets et al., 2016). However, several studies in maize (Zea mays; Sharp and Davies, 1979; Dowd et al., 2018) and Arabidopsis (van der Weele et al., 2000; Li et al., 2017) have reported that a mild WD can transiently stimulate root growth. There is, however, no consensus on the mechanisms underlying these responses.
Whereas long-distance water transport mostly occurs through xylem vessels, water uptake by roots first requires transport through living tissues that is mainly mediated by aquaporins, which are water channel proteins that facilitate water transport across cell membranes. Many reports show that Lpr is highly dependent on the content of the soil in water, nutrients, or oxygen and on biotic interactions. This control largely relies on the coordination of transcription, posttranslational modifications, and subcellular trafficking of aquaporins (Maurel et al., 2015). For instance, direct exposure of roots to water stress results in inhibition of aquaporin activity and hydraulic conductivity at the cell or root system levels (Boursiac et al., 2005; Sutka et al., 2011; Hachez et al., 2012). However, some plant species or Arabidopsis accessions showed an enhancement of Lpr under moderate stress (Sutka et al., 2011; Hachez et al., 2012).
Abscisic acid (ABA) has been recognized as the main plant stress hormone playing a major role during drought responses (Parent et al., 2009; Cutler et al., 2010; Wilkinson et al., 2012; Hong et al., 2013). Under WD conditions, ABA rapidly accumulates inducing stomatal closure to reduce water loss through transpiration (Zhang and Davies, 1987; Wilkinson et al., 2012) and, subsequently, a general inhibition of plant growth (Parent et al., 2009; Cutler et al., 2010; Rowe et al., 2016). Furthermore, high levels of exogenous ABA inhibit shoot and root growth under well-watered conditions (Ghassemian et al., 2000; van der Weele et al., 2000; De Smet et al., 2003; Deak and Malamy, 2005; Xiong et al., 2006; Harris, 2015; Rowe et al., 2016). Besides these well-characterized responses, analysis in Medicago truncatula suggested a positive role for ABA in the establishment and maintenance of root meristem function (Liang et al., 2007) and in stimulation of root elongation (Yang et al., 2014). Similarly, root development of maize plants with reduced endogenous ABA content was more repressed in response to drought than that in control plants, indicating that ABA plays a role in maintaining root elongation under low water potentials (Saab et al., 1990). Other studies have indicated a complex biphasic effect of exogenous ABA application on root growth under well-watered conditions where low concentrations of ABA stimulated root growth of Medicago (Gonzalez et al., 2015), rice (Oryza sativa), and Arabidopsis (Xu et al., 2013). Because this response was similar to the biphasic effect of increasing levels of WD, Xu et al. (2013) and Li et al. (2017) proposed that primary root adaptive response to WD was controlled by ABA. More recently, ABA has also been identified as a key component of several major adaptive responses to local WD (reviewed by Scharwies and Dinneny, 2019). First, Dietrich et al. (2017) have shown that local activation of ABA signaling in cortical cells of the elongation zone of Arabidopsis roots controls their hydrotropic response, i.e. their growth toward water. Second, Orman-Ligeza et al. (2018) reported that transient water deficit at the tip of cereal roots resulted in xerobranching, a local inhibition of lateral root formation that is dependent on the PYRABACTIN RESISTANCE/PYR-LIKE ABA signaling pathway. Finally, ABA and auxin signaling pathways have been shown to control hydropatterning, i.e. preferential positioning of lateral roots toward higher water availability (Bao et al., 2014; Orosa-Puente et al., 2018; Robbins and Dinneny, 2018).
ABA also exerts contrasting effects on root hydraulic conductance (Hose et al., 2000; Thompson et al., 2007; Parent et al., 2009). When applied in the micromolar range (0.1–1 µM), ABA rapidly enhances the hydraulic conductivity of both cortical cells (Hose et al., 2000) and whole roots of maize (Parent et al., 2009; Fan et al., 2015), whereas higher ABA concentrations (5–100 μM) inhibit the Lpr of maize (Aroca et al., 2003) and soybean (Glycine max; Markhart et al., 1979). The hydraulic effects of ABA are largely mediated through tissue-specific regulation of aquaporins. For instance, hydraulic conductance in maize roots closely follows the amount of aquaporin transcripts (Parent et al., 2009; Caldeira et al., 2014) and proteins (Fan et al., 2015) upon changes in ABA content following water deficit. A key role of phosphorylation in ABA-dependent regulation of aquaporins was also observed in Arabidopsis guard cells (Grondin et al., 2015).
In the present work, we investigated how WD alters the ability of the plant root system to acquire water, by connecting effects on root hydraulics and root growth and development. The latter effects were addressed from an elementary developmental process, LR formation, up to whole-root architecture in Arabidopsis adult plants grown in hydroponics under increasing PEG concentrations to induce a wide range of WD levels. This experimental set-up allowed us to observe a stimulation of both root development and hydraulics under mild WD, whereas severe WD had a repressive effect. Our results showed that ABA controls and coordinates both the developmental and physiological responses. These findings bring further insights into the mechanisms and significance of adaptive control of root hydraulic architecture depending on WD intensity.
RESULTS
Root Development Exhibits a Bell-Shaped Dose-Dependent Response to Water Deficit
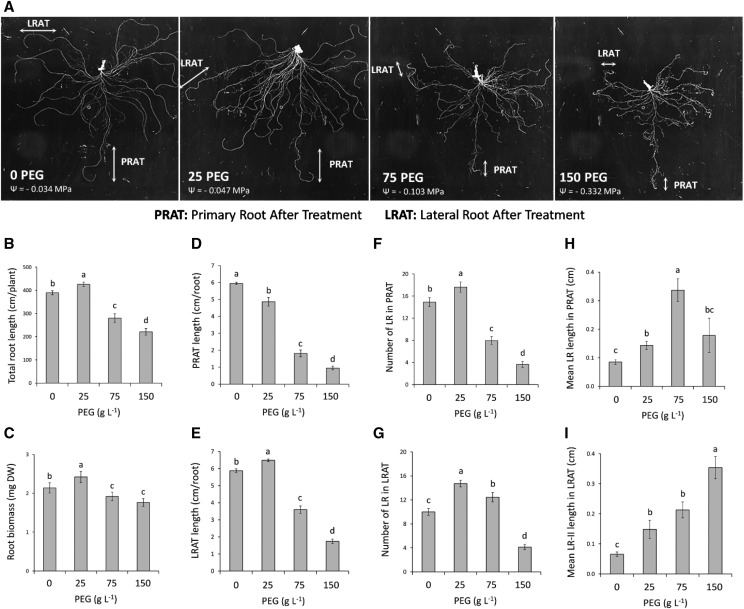
To analyze the adaptive responses of root development to WD, Arabidopsis plants were grown hydroponically under control conditions for 18 d after sowing (18 DAS) and also for an additional 5 d (23 DAS) in the presence of various concentrations of high molecular weight polyethylene glycol (PEG-8000). With respect to experiments in soil or agar plate, this procedure provides access to the RSA of fully developed plants and allows examining the effects of water stress, independently of any ion stress, mechanical impedance, or stress heterogeneity. In addition, the procedure allows a parallel monitoring of root water transport capacity. Whereas a control hydroponic solution shows a water potential of ‒0.034 MPa, three WD treatments were imposed by addition of 25, 75, or 150 g L−1 PEG yielding water potentials of ‒0.047 MPa (25PEG), ‒0.103 Mpa (75PEG), and ‒0.332 MPa (150PEG), respectively. Pronounced modifications in root growth and architecture were observed after 5 d of treatment (Fig. 1A). Total root length and root biomass (Fig. 1, B and C) showed a bell-shaped dose dependency, with an increase up to 10% in response to 25PEG, whereas a reduction was recorded after exposure to 75PEG or 150PEG. To explore the developmental mechanisms underlying this response, we analyzed in detail the morphology of root parts developed within the 5 d of PEG treatment, considering independently the primary root and the first-formed (oldest) LRs. These parts will be further referred to as Primary Root After Treatment (PRAT) and Lateral Root After Treatment (LRAT), respectively. The PRAT and LRAT were identified based on three criteria: (1) the length of the primary root and oldest LRs before the PEG treatments, (2) root morphological modifications (i.e. root curvature, root thickening, root hair length, etc.) induced by PEG and visualized under a microscope at the end of the experiment, and (3) changes in the slope between the position of the LRs and their distance to the root tip (Supplemental Fig. S1; see “Materials and Methods” for more details).
Figure 1.
Changes of RSA in response to WD. Plants were grown until 18 DAS in hydroponics under standard conditions and subjected for 5 additional d (23 DAS) to the indicated PEG-8000 treatments. A, Representative images showing the whole root system architecture, the parts of the PRAT, and the oldest LRAT used for specific analyses. B and C, Whole root systems were used to determine total root length (B) and biomass production (C) per plant. D to I, The length of the PRAT (D) and the LRAT (E), the number of LRs formed in the PRAT (F), the number of second-order LRs formed in the LRAT (G), the mean length of LRs formed in the PRAT (H), and the mean length of second-order LRs (LR-II) formed in the PRAT (I) per root are shown. Mean values ± se were obtained from plants grown in three independent experiments (n = 20–25). ‘Homogeneous group’ statistics were calculated through ANOVA, where mean values with different letters are significantly different according to lsd test at P ≤ 0.001.
The PRAT length showed a progressive reduction by 18% to 84% with increasing PEG concentrations (Fig. 1D). On the contrary, the LRAT length was stimulated by 12% at 25PEG and repressed by 38% and 67% at 75PEG and 150PEG, respectively (Fig. 1E). The number and mean length of LRs in both PRAT and LRAT parts also exhibited a bell-shaped dose-dependent response to WD (Fig. 1, F–I). Yet, we observed both a stronger stimulation and a lower sensitivity for inhibition of LR development in LRAT compared with that for PRAT. For instance, 75PEG caused 47% repression (Fig. 1F) and 20% stimulation (Fig. 1G) of LR number in PRAT and LRAT, respectively. Similarly, 150PEG had no significant effect on mean LR length in PRAT (Fig. 1H), whereas it induced stimulation by 450% in LRAT (Fig. 1I). We also investigated the LR development in sections of the primary root produced before the PEG treatment (Supplemental Fig. S2A). The 75PEG and 150PEG treatments stimulated the mean LR length (up to 86%) in the 0–2 cm section, whereas a repression of 60% was induced in the 2–4 cm section (Supplemental Fig. S2, B and C). On the contrary, the WD did not change the LR number in these sections (Supplemental Fig. S2, F and G). Taken together, our results indicate that RSA exhibits bell-shaped dose-dependent responses to WD, with overall stimulation responses at mild (25PEG) or moderate (75PEG) WD, and a general repression under severe WD (150PEG). In addition, a differential sensitivity to WD was observed between primary and oldest LRs, the former showing lower stimulatory responses associated with a higher sensitivity to WD.
ABA Accumulates under WD and Exerts both Stimulatory and Repressive Effects on RSA
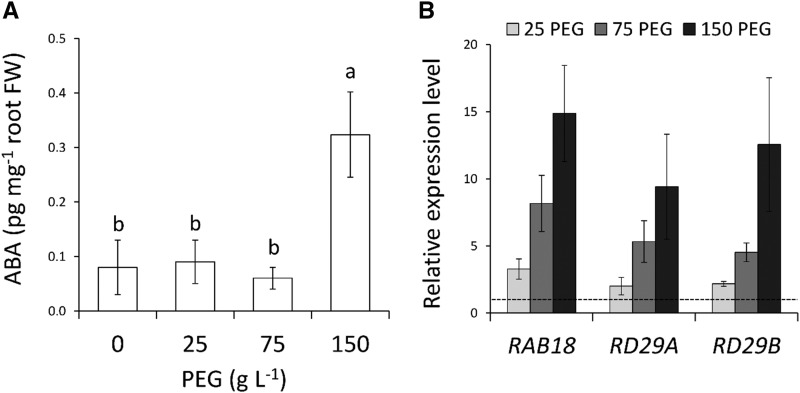
To investigate whether ABA participates in the root growth responses described above, we first quantified ABA accumulation in the same conditions. At the whole-root level, no significant alteration in ABA concentrations was induced by mild or moderate WD, whereas a 3-fold increase was detected at 150PEG (Fig. 2A). We next analyzed the expression of three ABA responsive genes: ras-related small GTPase homolog B18 (RAB18), responsive to desiccation 29A (RD29A), and RD29B (Leonhardt et al., 2004; Fujii et al., 2007). The mRNA abundance of these genes showed a gradual increase with increasing WD levels, up to 15-fold at 150PEG (Fig. 2B). Therefore, our results suggest that PEG treatments induce an accumulation and gradual response to ABA in relation to WD intensity.
Figure 2.
Effects of WD on ABA abundance and expression of ABA-regulated genes. A and B, ABA concentrations (A) and transcript abundance of ABA-regulated genes RAB18, RD29A, and RD29B in roots (B) of plants subjected from 18 DAS to a 5-d-long treatment with the indicated PEG concentrations. The dotted line in (B) represents the normalized expression level in control conditions. Mean values ± se were obtained from 15 plants grown in three independent experiments (n = 3). ‘Homogeneous group’ statistics were calculated through ANOVA, where mean values with different letters are significantly different according to lsd test at P ≤ 0.05 (A). FW, fresh weight.
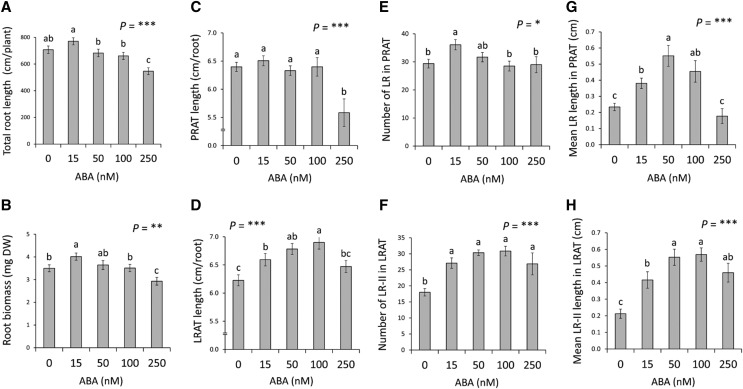
Several studies have shown that exogenous ABA, usually delivered at high concentrations, can dramatically modify root growth and architecture (for review, see Harris, 2015; Rowe et al., 2016). In order to thoroughly explore the role of ABA in shaping root developmental responses to WD, we investigated the long-term (5 d) effects of low exogenous ABA concentrations (up to 250 nM) in hydroponically grown plants. Reverse transcription quantitative PCR showed that the expression levels of RAB18, RD29A, and RD29B were gradually enhanced with increasing ABA concentrations to reach a 3- to 4-fold increase in mRNA abundance in response to the 250 nm ABA treatment (Supplemental Fig. S3). Exogenous ABA application revealed bell-shaped dose-dependent responses of growth, with an increase in the total root length and root biomass at the lowest ABA concentration (15 nM) and an inhibition by up to 25% at the highest ABA concentrations (Fig. 3, A and B). These responses were further dissected at the levels of PRAT and LRAT. Whereas PRAT length essentially showed an inhibition at 250 nm (Fig. 3C), a significant stimulation of LRAT length was recorded along the three lowest ABA treatments (15–100 nM; Fig. 3D). With respect to LR number, PRAT showed a mild (+22%) stimulation only at 15 nm ABA, whereas LRAT exhibited a more pronounced (up to 71%) stimulation at all ABA concentrations investigated (Fig. 3F). Mean LR length was stimulated by ABA in both PRAT and LRAT (Fig. 3, G and H), but with a distinct dose-dependency because a stimulation (+116%) or a lack of effect was observed at the highest ABA treatment for LRAT (Fig. 3H) and PRAT (Fig. 3G), respectively. Finally, when the primary root formed before ABA treatment was considered, a strong stimulation of mean LR length (up to 80%) was induced in the 0–2 cm section by the 50 nm ABA treatment, whereas no response was recorded in the 2–4 cm section (Supplemental Fig. S2, D and E). LR number was also insensitive to ABA in both sections (Supplemental Fig. S2, H and I). Taken together, our results demonstrate that RSA shows bell-shaped responses to exogenous ABA, with stimulation at low ABA concentrations (15–100 nM) and repression at higher concentrations (250 nM). In addition, dose-response curves point to a lower response amplitude but a higher sensitivity for LR formation and elongation in the primary root (PRAT) compared with that in the preformed LRs (LRAT).
Figure 3.
Effects of exogenous ABA on RSA. A to H, Plants grown in hydroponics were subjected at 18 DAS for 5 additional d (23 DAS) to the indicated concentrations of exogenous ABA. The figure shows the total root length (A) and the root biomass (B) per plant, the length of PRAT (C) and LRAT (D), the number of LRs formed in the PRAT (E), the number of second-order LRs (LR-II) formed in the LRAT (F), the mean length of LRs formed in the PRAT (G), and the mean length of second-order LRs formed in the LRAT (H) per root. Mean values ± se were obtained from plants grown in three independent experiments (n = 15–20). ‘Homogeneous group’ statistics were calculated through ANOVA, where mean values with different letters are significantly different according to lsd test at *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
ABA Mediates the Effects of WD on RSA
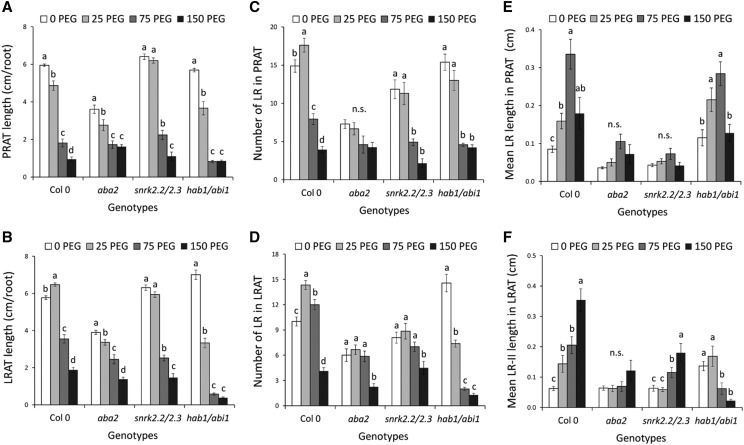
The responses of RSA to exogenous ABA, which largely recapitulate those observed in response to WD (Fig. 1), prompted us to investigate further the involvement of ABA in the root adaptive responses to WD. Our analyses were based on comparison of wild-type plants (Col-0) with mutants deficient in ABA synthesis (aba2-1; Schwartz et al., 1997) or with altered ABA signaling. In the latter case, we used snrk2.2 snrk2.3 (Fujii et al., 2007) and hab1-1 abi1-2 (Saez et al., 2006) double mutants, which show a lower and higher sensitivity to ABA, respectively. Under control conditions, the root and shoot growth of aba2 was strongly affected with respect to that in Col-0 (Supplemental Fig. S4, A–C). A significant but lower shoot and root growth reduction (by ∼25%) was also observed in snrk2.2 snrk2.3, whereas growth was unchanged in the hab1-1 abi1-2 hypersensitive mutant (Supplemental Fig. S4, A–C; Supplemental Table S1). Under mild WD (25PEG), all mutant lines lost the growth stimulation observed for Col-0 (Supplemental Fig. S4, A–C). On the contrary, under severe WD (150PEG), hab1-1 abi1-2 showed a higher growth reduction by up to 50% (under 150PEG) in comparison with that in Col-0, whereas growth of both aba2 and snrk2.2 snrk2.3 was not or only slightly altered (Supplemental Fig. S4, A–C). Root development was further dissected at the levels of PRAT and LRAT. When PRAT length is considered (Fig. 4A), all genotypes but snrk2.2 snrk2.3 showed qualitatively similar sensitivity to mild WD (Supplemental Table S1). In contrast, when LRAT length and LR number in PRAT and LRAT (Fig. 4, B–D) are considered, both aba2 and snrk2.2 snrk2.3 lacked the stimulation of LR growth and formation that was induced by mild WD (25PEG) in Col-0, whereas a significant LR inhibition was found in hab1-1 abi1-2 (Fig. 4, B–D). In response to moderate WD (PEG75), aba2 showed a lower inhibition of the LR growth and formation than that in Col-0, whereas hab1-1 abi1-2 had an enhanced repression of the root development (Fig. 4, B–D).
Figure 4.
Effects of WD on RSA of ABA biosynthesis (aba2) and response (snrk2.2 snrk2.3; hab1 abi1) mutants. A to F, Plants grown in hydroponics were subjected at 18 DAS for 5 additional d (23 DAS) to the indicated PEG concentrations. Three mutants including aba2 (an ABA biosynthesis deficient mutant), snrk2.2 snrk2.3 (an ABA signaling mutant), and hab1 abi1 (an ABA signaling hypersensitive mutant), were used in addition to the Col-0 wild-type control. The root system architecture was analyzed by several parameters: the length of the primary root grown after treatment (PRAT; A) and the oldest lateral root grown after treatment (LRAT; B), the number of lateral roots (LRs) formed in PRAT (C), the number of second-order LRs (LR-II) formed in the LRAT (D), the mean length of LRs formed in the PRAT (E), and the mean length of second-order LRs formed in the LRAT (F) per root. White, light gray, dark gray, and black boxes represent values for plants grown in PEG0, PEG25, PEG75, and PEG150 conditions, respectively. Mean values ± se were obtained from plants grown in three independent experiments (n = 10–20). ‘Homogeneous group’ statistics were calculated through ANOVA for each genotype, where mean values with different letters are significantly different according to lsd test at P ≤ 0.05. n.s., Not significant (P > 0.05).
To further investigate these differences in sensitivity, we performed a more detailed analysis of LR growth and development (Fig. 4, E and F). As described previously, increasing WD induced a stimulation of the LR elongation in Col-0 PRAT. This response was lost in aba2 and snrk2.2 snrk2.3 and strongly attenuated in hab1-1 abi1-2. However, the latter genotype showed a greater repression for highest WD levels (Fig. 4E). The most striking difference was observed in LRAT, where moderate and severe WD strongly stimulated the LR elongation in Col-0, whereas they dramatically repressed the LR elongation in hab1-1 abi1-2 (Fig. 4F). We also investigated the LR development in sections of the primary root that were produced before the PEG treatment (see Supplemental Fig. S2A for illustration). Whereas increasing WD stimulated the mean LR length in the 0–2 cm section of Col-0 as described above (Supplemental Fig. S2, B and C; Supplemental Table S1), this bell-shaped response was lost in ABA mutants (Supplemental Fig. S4E). By contrast, the WD-induced inhibition of the LR length observed in the 2–4 cm section of Col-0 (Supplemental Fig. S2F) was unaffected in the mutant genotypes (Supplemental Fig. S4F; Supplemental Table S1). Therefore, the distinct WD phenotypes of ABA biosynthesis and sensitivity mutants confirm that ABA plays a crucial role in most of the adaptive responses of RSA to WD.
Root Hydraulics Exhibits an ABA-Dependent Bell-shaped Dose Response Curve to WD
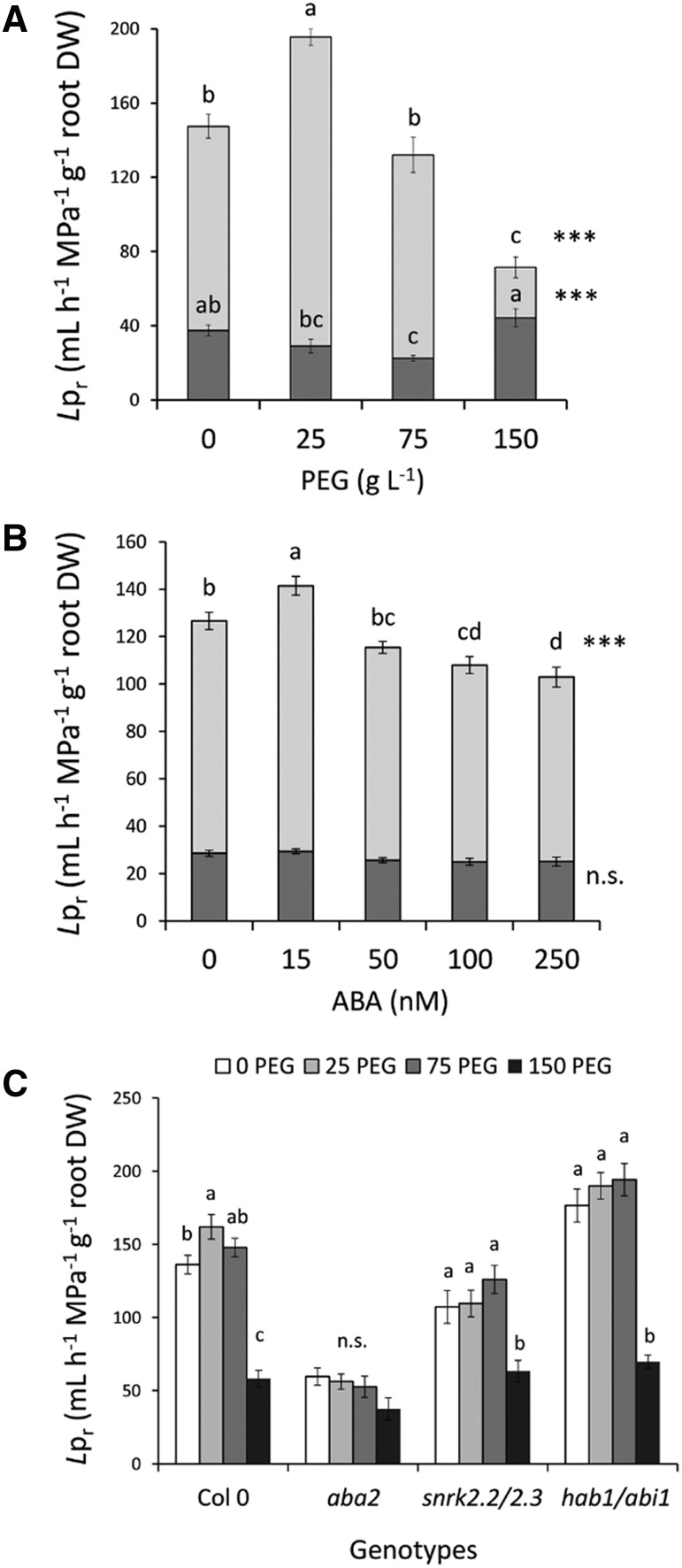
To investigate a putative coordination of root developmental and physiological responses to WD, we next investigated the root hydraulics of the same genotypes under PEG and ABA treatments as above. In agreement with these ideas, the whole-root hydraulic conductance (Lo) of Col-0 plants showed a bell-shaped response to increasing PEG concentrations (Supplemental Fig. S5A). The Lpr, as deduced by normalization of Lo to root size (dry weight), exhibited a similar dose-response curve with stimulation and inhibition by 30% and 51% at 25PEG and 150 PEG, respectively (Fig. 5A). When the measured water flow was normalized to the total root length instead of the dry weight, the maximal stimulation and inhibition of Lpr were of 40% and 36%, respectively (Supplemental Fig. S5B). Interestingly, Lpr also showed a bell-shaped dependency to exogenous ABA concentrations with a mild stimulation (12%) at 15 nm and an inhibition down to 20% at 100 and 250 nm ABA (Fig. 5B). Similar effects were observed when Lo (Supplemental Fig. S5C) or normalizing Lpr to the root length (Supplemental Fig. S5D) were considered.
Figure 5.
Effects of WD and exogenous ABA on root hydraulic properties of wild-type and ABA biosynthesis (aba2) and response (snrk2.2 snrk2.3; hab1 abi1) mutants. A to C, Plants grown in hydroponics were subjected at 18 DAS and for 5 additional d (23 DAS) to the indicated concentrations of PEG (A and C) and ABA (B), and different genotypes (C) were used as described previously. For A and B, the light and dark gray boxes represent the measured Lpr and residual Lpr after the aquaporin activity inhibition, respectively. For C, white, light gray, dark gray, and black boxes represent Lpr values for plants grown in PEG0, PEG25, PEG75, and PEG150 conditions, respectively. Mean values ± se were obtained from plants grown in three independent experiments (n = 20–25). ‘Homogeneous group’ statistics were calculated through ANOVA, where mean values with different letters are significantly different according to lsd test at P ≤ 0.05 (C). For A and B, levels of significance are represented as ***P ≤ 0.001; n.s. as not significant (P > 0.05). DW, dry weight.
Under control conditions, and by comparison with that in Col-0, aba2 and snrk2.2 snrk2.3 showed a reduction in Lpr by 56% and 21%, respectively, whereas hab1-1 abi1-2 showed stimulation by 30% (Fig. 5C; Supplemental Table S2). However, Lpr of the three mutant genotypes was insensitive to mild and moderate WD (25PEG and 75PEG), whereas it showed a strong repression in response to 150PEG (Fig. 5C). These combined physiological and genetic approaches indicate that ABA is involved in the stimulation of Lpr by mild WD (25PEG) and can reduce Lpr at the highest concentration investigated. In contrast, ABA plays a minor role, if any, in Lpr inhibition under severe WD (150PEG).
Water uptake in the Arabidopsis root is mediated in large part by aquaporin water channels. To estimate their contribution to the modulation of Lpr by WD and ABA, we applied azide, a well-characterized aquaporin blocker (Sutka et al., 2011), and measured the residual Lpr. Under control conditions, aquaporins accounted for more than 75% of Lpr (Fig. 5A) as described earlier (Sutka et al., 2011). Furthermore, we found a mild effect of WD on residual Lpr specifically at 75PEG (Fig. 5A), whereas none of the ABA treatments altered this parameter (Fig. 5B). Thus, the bell-shaped effects of PEG and ABA application on Lpr can be mostly accounted for by the modulation of root aquaporin function. To address this point further, we analyzed the mRNA abundance of all members of the Plasma membrane Intrinsic Protein (PIP) subfamily and of four highly expressed members of the Tonoplast Intrinsic Protein (TIP) subfamily. No significant variation was observed in response to either mild WD (PEG25; Supplemental Fig. S6A) or exogenous ABA (Supplemental Fig. S6B). In contrast, a moderate or severe WD (PEG75 or150) reduced the mRNA abundance of several aquaporin genes, with the strongest repression observed for PIP1;5, PIP2;4, PIP2;7, and two TIPs (TIP1;1, TIP1;2). Noticeably, PIP1;4 and PIP2;5 expression were induced 2- and 3-fold, respectively. Interestingly, expression of PIP1,4 but not PIP2,5 was significantly induced by the highest ABA concentration (Supplemental Fig. S6B).
Our results indicate that, similar to its effects on RSA, WD induces a double effect on root hydraulics, with a stimulation of aquaporin-dependent water transport at mild WD (PEG25) and a strong repression of the aquaporin pathway at severe WD (PEG150). Under mild or moderate WD, these responses seem to be largely mediated by ABA. In contrast, the inhibition of Lpr at 150 PEG is ABA independent and seems to involve an overall repression of aquaporin expression.
DISCUSSION
Understanding the mechanisms that underlie root development and hydraulics under drought is of major importance to maintain plant growth under suboptimal conditions. However, the analysis of RSA under drying soil conditions is extremely time consuming and not compatible with fine morphological or functional analyses. Thus, many studies have been conducted on Arabidopsis seedlings grown in agar plates and mostly subjected to severe osmotic stress, as induced by high mannitol, sorbitol, NaCl, or KCl concentrations. To have a more comprehensive view of adaptive and functional responses of mature plants to WD, we analyzed both root architecture and hydraulics of 23-d-old adult plants grown in hydroponics in the presence of a wide range of PEG concentrations. PEG is a nonpermeant molecule that can consistently reduce water potential in plates (van der Weele et al., 2000; Rowe et al., 2016). Because PEG can also induce anoxia (Verslues et al., 1998), the nutrient solution was constantly oxygenated and circulated around roots. These optimized culture conditions triggered a wide range of highly reproducible responses to WD.
WD Exerts Contrasting Dose-Dependent Effects on Root Development
Inhibition of plant growth and development is a common response to WD (Zhu et al., 1998; Deak and Malamy, 2005; Xiong et al., 2006; Claeys et al., 2014; Thalmann et al., 2016; reviewed in Comas et al., 2013). Accordingly, we observed here that a severe WD (150PEG) caused a significant reduction in both shoot and root growth (Fig. 1; Supplemental Fig. S7). Unlike in most fields experiments, no change in shoot:root ratio was observed (Supplemental Fig. S7) probably due to the fact that our culture conditions temperature and hygrometry are tightly controlled and nutrient availability is unchanged under WD, whereas it is dramatically reduced when soil water content drops. In addition, we observed that a mild WD (25PEG) significantly promoted both shoot and root development giving rise to bell-shaped dose-responses to WD. Fine analysis revealed that this type of response was observed for nearly all analyzed RSA parameters (Fig. 1; Supplemental Figs. S2 and S7). Such positive effects of mild WD on root development have been punctually reported for the primary root of Arabidopsis (van der Weele et al., 2000; Claeys et al., 2014), or roots of rice (Henry et al., 2011) or maize (Zhu et al., 2010; Dowd et al., 2018) in soil. Interestingly, our fine analysis showed that the adaptive responses to WD were shifted depending on root order, with the primary root being more sensitive than the oldest LRs (compare, for instance, root length or LR numbers in PRAT and LRAT in Fig. 1, D–I). Differential responses to WD were also recorded for LRs already initiated before WD treatment (Supplemental Fig. S2, B and C). Thus, elongation of nonemerged or juvenile LRs was stimulated by mild and moderate WD in the 0–2 cm part of the primary root, whereas it was repressed for older LRs in the 2-cm section above. We also note that growth stimulation of Col-0 roots by a 25PEG treatment was not unspecific, as this response was lost in ABA mutants (Fig. 4) and could be recapitulated in Col-0 seedlings grown in agar plates using small concentrations (25 mM) of sorbitol (Supplemental Fig. S8).
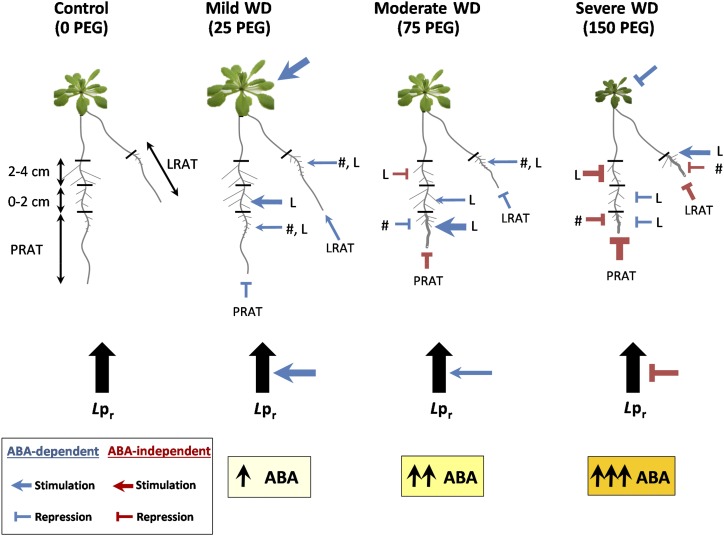
Taken together, these results highlight a complex response of the root system to WD with different sensitivity depending of root age and branching level (see model in Fig. 6). In brief, he primary root appears to be more sensitive than LRs to WD, first-order LRs are more sensitive than second-order LRs and, LR elongation is less repressed than LR emergence. Accordingly, combination of these WD responses gives rise to a more compact root system, which is highly branched and carries elongated LRs at its periphery (see PEG75 and PEG150 in Fig. 1A).
Figure 6.
Integrative model of root developmental and hydraulic responses to representative WD intensities. The different WD treatments are represented by four plants. The root system is schematically represented by a primary root, an old LR, and first and second-order LRs on the primary and the old LR. The root parameters analyzed are schematically represented, and their response to WD is represented by an arrow or a bar for stimulation or repression, respectively. The intensity of the responses is shown by the thickness of the line. L and #, mean LR length and LR number, respectively. The blue and red color codes represent ABA-dependent or -independent responses, respectively. The thickness of blue and red lines and arrows represent the intensity of response (thicker line represents stronger response). The three yellow boxes at the bottom of the model represent the root ABA content. Color intensity and number of arrows are a schematic representation of ABA concentrations.
A similar differential adaptive response of primary and LR growth has been reported by Julkowska et al. (2014) in Arabidopsis plants under salt stress. In contrast, studies in maize (Gao and Lynch, 2016), rice (Uga et al., 2013; Sandhu et al., 2016), and wheat (Awad et al., 2017) showed that WD dramatically reduced shoot-born roots and LR development, whereas it favored main root elongation. Such difference may reflect distinct adaptive strategies between monocots and dicots. Indeed, the fibrous root system of monocots, with postembryonic roots developing from the stem, provides a unique opportunity for plant foraging of late season precipitations, which is absent in dicots.
When common drought scenarios are considered, WD usually starts in the upper soil layers and progresses gradually in deeper layers. Our observation that LRs formed in both primary and oldest LRs are more tolerant to WD than their mother root fits with the idea that progression of the primary root in deep soil layers protects it from severe WD and that newly formed LRs experience stronger WD than their genitors as they develop in a soil area already foraged for water by the latter. Thus, when extrapolating our observations under homogenous and constant WD to a developing WD in soil, we can predict a massive stimulation of RSA mainly due to the progressive development of LRs from different orders to explore a larger volume of soil. The observed adaptive responses are also relevant to more severe WD. As surface soil dries, increase of root length and density in the deepest layers significantly contribute to improving the water and nutrient acquisition with a limited carbon investment. This adaptive response to WD agrees with traits found in crops. For instance, cultivars of rice and soybean with increased root length density (root length per soil volume) in the 35–45 cm layer appeared to have increased water uptake and improved resistance to drought (Carter et al., 1999; Henry et al., 2011).
WD also Exerts Contrasting Dose-Dependent Effects on Root Hydraulics
Root hydraulics (Lpr) is known to rapidly respond to environmental cues, such as water availability, supporting its role in plant adaptation to fluctuating environments (Steudle, 2000; Vandeleur et al., 2009). Accordingly, our measurements indicated that a mild WD enhances Arabidopsis Lpr, whereas a severe WD had a repressive effect (Fig. 5). The generally observed decline of Lpr under drought conditions can be interpreted as a means for hydraulically promoting stomatal closure or for limiting a counterflow of water from the root to the drying soil (for review, see Aroca et al., 2012). Drought-induced increases of Lpr have been reported under more specific conditions, generally under mild drought stress (Siemens and Zwiazek, 2004). This response is thought to promote water uptake as long as the water potential remains higher in the soil than in the root.
Previous work from our group has shown that aquaporins contribute to ∼75%–80% of Arabidopsis Lpr (Tournaire-Roux et al., 2003; Boursiac et al., 2005; Sutka et al., 2011; Li et al., 2015). Here, we provide pharmacological evidence that aquaporins can account for most of the stimulation and repression of Lpr under mild and severe WD, respectively (Fig. 5). Yet, no significant change in PIP or TIP gene expression was observed in response to mild or moderate WD (Supplemental Fig. S6). In contrast, severe WD reduced the mRNA abundance of most aquaporin genes, possibly explaining the parallel inhibition of Lpr. These contrasting results are consistent with other studies indicating that numerous posttranscriptional mechanisms can account for aquaporin regulation in roots under stress (for review, see Aroca et al., 2012; Li et al., 2015).
The observation that both root developmental and hydraulic parameters exhibited similar bell-shaped dose-dependent responses to WD points to an obvious coordination of these responses. Under severe WD (150PEG), for instance, both root development and hydraulics were dramatically repressed to restrict water exchange between roots and their harsh environment. Conversely, a mild WD (25PEG) induced a dual stimulation of root development and root hydraulics to optimize water uptake and support shoot biomass production during the onset of drought. Yet, we found a stimulation of total root length specifically under mild WD (Fig. 1B), whereas Lpr (as normalized by total root length instead of dry weight) was significantly stimulated under both mild and moderate (75PEG) WD (Supplemental Fig. S5B). Thus, moderate WD represses root development (Fig. 1B) while maintaining root hydraulics (Fig. 5A) to possibly maintain shoot hydration (Supplemental Fig. S4A). These responses illustrate different adaptation strategies to increasing levels of WD with differential carbon investment. Under mild WD, carbon is invested in the production of new and longer roots, whereas under moderate WD, carbon is invested in the production of second-order LRs and root thickening to prevent water loss. On the contrary, aquaporin-dependent water uptake that mainly recruits pre-existing proteins is stimulated to improve water uptake. Similar responses to moderate and severe WD were also proposed by Parent et al. (2009), Vandeleur et al. (2009), and Laur and Hacke (2013), who suggested that when soil water is scarce, increased aquaporin abundance and activity play a major role in compensating for reductions in root elongation and surface area production.
Another explanation of the increase in Lpr under moderate WD may account for the modification of root architecture that under these conditions present a significant increase in number and elongation of newly formed roots (mainly second-order LRs) that may be more active in water uptake. This hypothesis is supported by several reports suggesting that fine or LRs are the most active portion of root system in water uptake (for review, see Comas et al., 2013). Recently, Ahmed et al. (2016, 2018) used heavy water (D2O) labeling to show that, in maize, up to 80% of water absorption can be accounted for by laterals. Improved hydraulic measurements will have to be developed in the Arabidopsis root to directly test this hypothesis.
ABA Acts as an Integrator and Coordinator of Root Responses to WD
It well established that drought and osmotic stress induce an accumulation of ABA in both roots and shoots (Schachtman and Goodger, 2008; Wilkinson et al., 2012; Claeys and Inzé, 2013). Besides stomatal closure (Trejo and Davies, 1991; Schroeder et al., 2001), ABA can positively or negatively act on shoot and root growth depending on its concentration or the plant model investigated (Saab et al., 1990; Hooker and Thorpe, 1998; Barrero et al., 2005; Zhang et al., 2010; Leach et al., 2011; reviewed by Harris, 2015). Root hydraulics is also controlled by ABA but again stimulatory or inhibitory effects can be observed, depending on the experimental conditions and genotypes (reviewed by Aroca et al., 2012). Our aim here was to evaluate how ABA may coordinate the developmental and hydraulic responses of roots over a wide range of WD.
When ABA accumulation at the whole-root level is quantified, a significant increase was observed under severe WD only, whereas the expression level of ABA responsive genes was enhanced even under mild WD (Fig. 2B). Although this increase remained limited compared with related studies in, in vitro grown seedlings (Rowe et al., 2016), it potentially reflects a typical response to WD with possibly undetectable and local accumulations of low ABA amounts. These ideas prompted us to analyze the effect of exogenous ABA applications in the nanomolar range instead of the micromolar range as usually used. Interestingly, expression of the ABA reporter genes showed a slight stimulation that was comparable with that measured under mild WD (Supplemental Fig. S3).
When RSA is considered, low exogenous ABA concentrations revealed bell-shaped dose-response curves that were reminiscent of those observed under low and moderate WD. Moreover, all developmental parameters positively affected by mild WD were also positively regulated by low ABA concentrations (Fig. 3; Supplemental Figs. S2 and S4). Finally, the differential developmental responses of roots to WD, with young tissues being more responsive than older ones (Fig. 1; Supplemental Fig. S4), were also observed in the context of exogenous ABA application (Fig. 3; Supplemental Fig. S4).
The idea that ABA mediates a large range of RSA responses to WD was further supported by the analysis of ABA mutants with aba2 and snrk2.2 snrk2.3 having an attenuated response of most root developmental parameters to WD, whereas the hypersensitive hab1 abi1 mutant showed enhanced responsiveness.
We note, however, that some root responses to WD seem to be ABA independent (Fig. 6). When the primary root (PRAT) is considered, its elongation was dramatically repressed by increasing levels of WD (Figs. 1D and 3A), but to a similar extent in the different ABA mutants (Fig. 4A). Accordingly, exogenous ABA application had little effect on PRAT (Fig. 3C), suggesting that this WD adaptive response is mainly ABA independent. A similar conclusion can be drawn for LRs formed in the 2–4 cm part of the primary root present before transfer (see Supplemental Fig. S2A). On the contrary, elongation of roots in the 0–2 cm part was stimulated by WD (Supplemental Fig. S2C), and this response was typically dependent on exogenous ABA (Supplemental Fig. S2E) and dramatically modified in the ABA mutants (Supplemental Fig. S4E). Taken together, these results suggest that ABA-dependent responses to WD are mainly observed in young roots, whereas the adaptive responses of older tissues become ABA independent.
Similar to the adaptive response of RSA to WD, we showed that the response of root hydraulics to WD is in large part mediated by ABA. In brief, Lpr of Col-0 showed a bell-shaped dose-dependent response to both WD and exogenous ABA (Fig. 5, A and B), and Lpr response to WD was dramatically attenuated in ABA mutants (Fig. 5C). These results establish that ABA coordinates both root developmental and hydraulic response to mild and moderate WD. The complex interactions between RSA, hydraulics, and ABA are summarized in the integrative model presented in Figure 6. When severe WDs were considered, both the RSA and hydraulic responses were ABA independent (as illustrated on Figure 6 by a large number of red symbols). We speculate that, due to the severity of the stress, strong inhibitory responses are governed by local signals, independent of the overall hormonal status of the plant.
CONCLUSION
In the current study, we showed that mild and somewhat moderate WD stimulate both root growth and root hydraulics. In addition, the fine analysis of root architecture revealed that mild WD mainly stimulates elongation and to a lower extent production of LRs, this response being markedly enhanced in pre-existing LRs. Concomitantly, mild WD significantly stimulates root hydraulics in an aquaporin-dependent manner. Both responses are mediated by ABA. Since under WD ABA is mainly synthetized in shoots and transported to roots via the phloem (review by Osakabe et al., 2014), we assume that the differential adaptive responses observed in our experiments can be accounted for by either a differential accumulation of ABA due to local regulation of ABA transporters or by differential ABA responses depending on WD level and root tissue types. This nonlinear response is well documented for phytohormones and has been extensively studied for auxin. Identifying the molecular basis of these local ABA responses will be critical to better understand how the plant coordinates its RSA and root hydraulic responses, and how these responses can be reshaped to possibly improve the tolerance of crops to water stress. Our work also shows that a severe WD dramatically modifies both RSA and hydraulics and, again, the molecular bases of these ABA-independent regulations remain to be identified. Overall, the experimental framework established in the present work will be crucial to explore the adaptive significance of the multiple responses of roots to WD.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The Arabidopsis (Arabidopsis thaliana) accession Columbia-0 (Col-0 N60000) and the aba2-1 (Léon-Kloosterziel et al., 1996), snrk2.2 snrk2.3 (Fujii et al., 2007), and hab1-1 abi1-2 (Saez et al., 2006) mutants in Col-0 background were used in this work. Seeds were surface sterilized and sown on 1/2 MS agar vertical plates (2.2 g L−1 MS, 1% [w/v] Suc, 0.05% [w/v] MES, and 0.8% [w/v] agar, pH 5.7). Plates were incubated for 2 d at 4°C in dark for stratification. Plants were germinated and further grown on these plates for 9–11 d in a growth chamber at 70% relative humidity and 20°C, with 16 h/8 h light/dark cycles (250 µE m−2 s−1). Subsequently, seedlings were transferred to a hydroponic medium containing 1.25 mm KNO3, 0.75 mm MgSO4, 1.5 mm Ca(NO3)2, 0.5 mm KH2PO4, 0.1 mm MgCl2, 50 µM Fe-EDTA, 50 µM H3BO3, 12 µM MnSO4, 0.7 µM CuSO4, 1 µM ZnSO4, 0.24 µM MoO4Na2, 0.01 µM CoCl2, 100 µM Na2SiO3, and 1 mm MES, pH 5.7 adjusted using KOH. At 18 DAS, seedlings were transferred for 5 d to a fresh medium containing different concentrations (25, 75, or 150 g L−1) of PEG-8000 to reduce the water potential (Ψ) of the nutrient solution. To avoid anoxia, culture solutions were constantly bubbled with air and recirculated in each basin. The solution was replaced at the middle of the treatment period (at 2.5 d) to avoid bacterial contamination. The Ψ was measured with a vapor pressure osmometer WESCOR 5520 as described by Turner (1981). For ABA treatments, 18-d-old plants were transferred for 5 d to a fresh hydroponic solution containing different concentrations of ABA (15, 50, 100, and 250 nM), obtained using ABA stock solutions in ethanol. Control plants grown in the absence of ABA were mock treated with a same amount of ethanol (final concentration of 0.00033% [v/v]). The hydroponic solution was replaced once during the treatment period (at 2.5 d). All experiments were performed at least three independent times. All chemicals were provided by Sigma-Aldrich.
Analysis of RSA
Root systems were excised at 23 DAS from plants grown in hydroponics (growth was performed for 18 d under control conditions followed by 5 d in the presence of PEG or ABA, as described above), and immediately used for root hydraulic measurements (see below in section "Measurement of Lpr"). Subsequently, roots were washed and stored in 20% (v/v) ethanol before analysis of RSA. For imaging, each root system was manually separated on a 240 × 240 mm petri dish containing water, and scanned with an Epson Perfection V850 Pro scanner (Epson Europe BV). RSA was analyzed using the OPTIMAS image analysis software (Adept Turnkey Pty Ltd). Several parameters were measured. Total root length was quantified on the whole-root system. The PRAT and LRAT correspond to the parts of primary and lateral roots that were produced during the 5 d treatment. The PRAT and LRAT were precisely observed at the end of the culture using a binocular microscope. Root length measured before transplanting was combined to these microscopic observations in order to identify at the cellular level the morphological changes induced by the PEG or ABA treatment (Supplemental Fig. S1). Microscopic morphological changes (curvature of the root, changes in cell size, and a transient increase in distance between lateral roots) were not only observed in response to PEG or ABA but also, though at a much lower extend, in plants grown on a control medium. These morphological changes were found to be very reproducible, probably due to a transient stress induced by manipulating the plants. Finally, plants with no clear differentiation between the initially formed root parts and the PRAT and/or LRAT were discarded for the RSA analysis. Overall, the length of PRAT and LRAT as well as the number and mean length of lateral roots formed in these parts during the treatment were measured. The number and mean length of lateral roots were also determined in the 0–2 cm and 2–4 cm parts of the primary root that were produced before treatment application (Fig. 1A; Supplemental Fig. S2A).
ABA Quantification
For ABA quantification, root and shoot tissues were frozen in liquid nitrogen and ABA was extracted and quantified by the Metatoul platform (https://www6.toulouse.inra.fr/metatoul/). Three biological replicates from three independent experiments were used.
Measurement of Lpr
A freshly detopped root system was inserted into a pressure chamber filled with the same hydroponic medium as used for each treatment. The hypocotyl was tightly sealed inside a combination of plastic and metallic seals using a low-viscosity dental paste (President Light). The rate of pressure (P)-induced sap flow (Jv) exuded from the hypocotyl section was recorded using high-accuracy flow meters in an automated manner using a LabVIEW-derived application. In practice, roots excised at 23 DAS from plants grown in standard conditions were subjected to a pretreatment at 350 kPa for 10 min to attain flow equilibration, and Jv was measured successively at 320, 160, and 240 kPa for about 5 min. For PEG treatments, pressure increments corresponding to the Ψ of the hydroponic solution were applied in order to counteract osmotically induced water efflux from the root. Root dry weight (DWr) was analyzed after measurement of Jv and RSA. The Lpr (mL g−1 h−1 MPa−1) of an individual root system was calculated using the following equation:
In NaN3 experiments, Lpr was derived from continuous Jv measurement at 320 kPa as described in Sutka et al. (2011).
Total RNA Isolation and Analysis
Root samples were frozen in liquid nitrogen and disrupted for 1 min at 30 oscillations s−1 in a Retch mixer mill MM301 homogenizer. Total RNA was extracted using TRI REAGENT (Molecular Research Center Inc), and DNA was eliminated by RQ1 RNase-Free DNase (Promega) according to the manufacturer’s instructions. RNA was purified by ethanol precipitation. The 2 µg of total RNA were used as a template for first strand complementary DNA (cDNA) synthesis, which was performed using Moloney Murine Leukemia Virus Reverse Transcriptase, RNase H Minus, Point Mutant (Promega) and Oligo(dT)15 Primer (Promega) in a final volume of 20 µL, according to the manufacturer’s instructions.
First strand cDNA was diluted 10 times, and 2 µL of diluted cDNA solution were used as template for gene expression level quantification by reverse transcription quantitative PCR. The latter was performed in 384-well plates with a LightCycler 480 Real-Time PCR System (Hoffmann-La Roche AG). SYBR Premix Ex Taq (TaKaRa) was used to monitor cDNA amplification at an annealing temperature of 57°C according to the manufacturer’s instructions. SYBR Green Primer efficiencies for each pair were evaluated from the analysis of dilution series of 1, 1:5, 1:10, 1:100, and 1:1000 of a mix of all the diluted first strand cDNA samples and derived from Cp values calculated according to the second derivative maximum method (LightCycler LC480 II, Hoffmann-La Roche AG). Three to six biological replicates from three independent plant cultures and two technical repeats were used per treatment in every run. Expression data were normalized to expression of three housekeeping genes, TIP41-like protein (At4g34270), CLATHRIN (At4g24550), and SAND family protein (At2g28390), which were selected as reference genes according to Czechowski et al., 2005. Data from mutant and control plants were compared using a two-tailed Student’s t test. The primer sequences used are listed in Supplemental Table S3.
Statistical Analyses
Statistical analysis was performed using the STATGRAPHICS Centurion XVI software (StatPoint Technologies). One-way ANOVA was performed to determine significant differences between groups of samples, as indicated by different letters. Levels of significance are indicated in the figures by asterisks: *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. Not significant (n.s.) differences correspond to P > 0.05. Multiple range comparisons of means were determined by the lsd (lsd) test included in the above-mentioned software. Values represent the mean of 10–40 Arabidopsis plants in each treatment and come from at least 3 independent plant cultures.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers ABA 2: AT1G52340; SnRK2.2, At3g50500; SnRK2.3, At5g66880; ABI1: AT4G2542, and HAB1: AT1G72770
SUPPLEMENTAL DATA
The following supplemental materials are available.
Supplemental Figure S1. Distribution of LRs along the primary root in response to various WD levels.
Supplemental Figure S2. LR elongation and number in response to WD or exogenous ABA.
Supplemental Figure S3. Expression of ABA-regulated genes in response to exogenous ABA.
Supplemental Figure S4. Effects of WD on biomass production and RSA of ABA biosynthesis and response mutants.
Supplemental Figure S5. Effects of WD and exogenous ABA on root hydraulic properties of wild-type and ABA biosynthesis and response mutants.
Supplemental Figure S6. Expression of aquaporin genes in response to WD and exogenous ABA.
Supplemental Figure S7. Shoot and root biomass production in response to WD or exogenous ABA.
Supplemental Figure S8. Changes of RSA of seedlings in response to sorbitol (WD).
Supplemental Table S1. Statistical analysis of the effects of WD on RSA of wild-type and ABA 2 biosynthesis (aba2) and response (snrk2.2 snrk2.3; hab1 abi1) mutants.
Supplemental Table S2. Statistical analysis of the effects of WD on root hydraulic properties of wild-8 type and ABA biosynthesis (aba2) and response (snrk2.2 snrk2.3; hab1 abi1) mutants.
Supplemental Table S3. Sequences of gene primer pairs used for RT-qPCR.
Acknowledgments
We thank Pedro L. Rodriguez (IBMCP-UPV-CSIC, Spain) for providing the ABA mutant lines. We fully acknowledged all former and current members of Aquaporins’ team at Biochimie & Physiologie Moléculaire des Plantes (Centre national de la recherche scientifique/ Institut national de recherche agronomique/SupAgro/Université de Montpellier, France) for the exciting discussions and inputs on this work. We want to thank X. Dumont, H. Baudot, T. Dessup, J. Garcia, R. Picaud, and F. Lecocq for technical and logistical assistance; P. Rudinger, C. Dufour, J. Fernandes, V. Rafin, C. Abauzit, and C. Baracco for administrative and financial support; and H. Afonso for informatics assistance.
Footnotes
This work was supported by the European Commission within the AgreenSkills (FP7-26719) and Marie Sklodowska-Curie programs (H2020-657374 to M.A.R.), as well as by the Institut National de la Recherche Agronomique and the Centre National de la Recherche Scientifique.
Articles can be viewed without a subscription.
References
- Ahmed MA, Zarebanadkouki M, Kaestner A, Carminati A (2016) Measurements of water uptake of maize roots: The key function of lateral roots. Plant Soil 398: 59–77 [Google Scholar]
- Ahmed MA, Zarebanadkouki M, Meunier F, Javaux M, Kaestner A, Carminati A (2018) Root type matters: Measurement of water uptake by seminal, crown, and lateral roots in maize. J Exp Bot 69: 1199–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroca R, Vernieri P, Irigoyen JJ, Sánchez-Díaz M, Tognoni F, Pardossi A (2003) Involvement of abscisic acid in leaf and root of maize (Zea mays L.) in avoiding chilling-induced water stress. Plant Sci 165: 671–679 [Google Scholar]
- Aroca R, Porcel R, Ruiz-Lozano JM (2012) Regulation of root water uptake under abiotic stress conditions. J Exp Bot 63: 43–57 [DOI] [PubMed] [Google Scholar]
- Awad W, Byrne PF, Reid SD, Comas LH, Haley SD (2017) Great plains winter wheat varies for root length and diameter under drought stress. Agron J 110: 1–10 [Google Scholar]
- Bao Y, Aggarwal P, Robbins NE II, Sturrock CJ, Thompson MC, Tan HQ, Tham C, Duan L, Rodriguez PL, Vernoux T, et al. (2014) Plant roots use a patterning mechanism to position lateral root branches toward available water. Proc Natl Acad Sci USA 111: 9319–9324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrero JM, Piqueras P, González-Guzmán M, Serrano R, Rodríguez PL, Ponce MR, Micol JL (2005) A mutational analysis of the ABA1 gene of Arabidopsis thaliana highlights the involvement of ABA in vegetative development. J Exp Bot 56: 2071–2083 [DOI] [PubMed] [Google Scholar]
- Boursiac Y, Chen S, Luu D-T, Sorieul M, van den Dries N, Maurel C (2005) Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol 139: 790–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeira CF, Bosio M, Parent B, Jeanguenin L, Chaumont F, Tardieu F (2014) A hydraulic model is compatible with rapid changes in leaf elongation under fluctuating evaporative demand and soil water status. Plant Physiol 164: 1718–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T, DeSouza P, Purcell L (1999) Recent advances in breeding for drought and aluminium resistance in soybean. Proceedings of the World Soybean Research Conference VI. Champaign, IL. 106-125 [Google Scholar]
- Claeys H, Inzé D (2013) The agony of choice: How plants balance growth and survival under water-limiting conditions. Plant Physiol 162: 1768–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claeys H, Van Landeghem S, Dubois M, Maleux K, Inzé D (2014) What is stress? Dose-response effects in commonly used in vitro stress assays. Plant Physiol 165: 519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comas LH, Becker SR, Cruz VMV, Byrne PF, Dierig DA (2013) Root traits contributing to plant productivity under drought. Front Plant Sci 4: 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: Emergence of a core signaling network. Annu Rev Plant Biol 61: 651–679 [DOI] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak KI, Malamy J (2005) Osmotic regulation of root system architecture. Plant J 43: 17–28 [DOI] [PubMed] [Google Scholar]
- De Smet I, Signora L, Beeckman T, Inzé D, Foyer CH, Zhang H (2003) An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J 33: 543–555 [DOI] [PubMed] [Google Scholar]
- Dietrich D, Pang L, Kobayashi A, Fozard JA, Boudolf V, Bhosale R, Antoni R, Nguyen T, Hiratsuka S, Fujii N, et al. (2017) Root hydrotropism is controlled via a cortex-specific growth mechanism. Nat Plants 3: 17057. [DOI] [PubMed] [Google Scholar]
- Dowd TG, Braun DM, Sharp RE (2018) Maize lateral root developmental plasticity induced by mild water stress. I: Genotypic variation across a high-resolution series of water potentials. Plant Cell Environ 1–15 [DOI] [PubMed] [Google Scholar]
- Fan W, Li J, Jia J, Wang F, Cao C, Hu J, Mu Z (2015) Pyrabactin regulates root hydraulic properties in maize seedlings by affecting PIP aquaporins in a phosphorylation-dependent manner. Plant Physiol Biochem 94: 28–34 [DOI] [PubMed] [Google Scholar]
- Fujii H, Verslues PE, Zhu J-K (2007) Identification of two protein kinases required for abscisic acid regulation of seed germination, root growth, and gene expression in Arabidopsis. Plant Cell 19: 485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Lynch JP (2016) Reduced crown root number improves water acquisition under water deficit stress in maize (Zea mays L.). J Exp Bot 67: 4545–4557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P (2000) Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 12: 1117–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AA, Agbévénou K, Herrbach V, Gough C, Bensmihen S (2015) Abscisic acid promotes pre-emergence stages of lateral root development in Medicago truncatula. Plant Signal Behav 10: e977741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondin A, Rodrigues O, Verdoucq L, Merlot S, Leonhardt N, Maurel C (2015) Aquaporins contribute to ABA-triggered stomatal closure through OST1-mediated phosphorylation. Plant Cell 27: 1945–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachez C, Veselov D, Ye Q, Reinhardt H, Knipfer T, Fricke W, Chaumont F (2012) Short-term control of maize cell and root water permeability through plasma membrane aquaporin isoforms. Plant Cell Environ 35: 185–198 [DOI] [PubMed] [Google Scholar]
- Harris JM. (2015) Abscisic acid: Hidden architect of root system structure. Plants (Basel) 4: 548–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry A, Gowda VRP, Torres RO, McNally KL, Serraj R (2011) Variation in root system architecture and drought response in rice (Oryza sativa): Phenotyping of OryzaSNP panel in rainfed lowland fields. Field Crops Res 120: 205–214 [Google Scholar]
- Hong JH, Seah SW, Xu J (2013) The root of ABA action in environmental stress response. Plant Cell Rep 32: 971–983 [DOI] [PubMed] [Google Scholar]
- Hooker TS, Thorpe TA (1998) Effects of fluridone and abscisic acid on lateral root initiation and root elongation of excised tomato roots cultured in vitro. Plant Cell Organ Culture 52: 199–203 [Google Scholar]
- Hose E, Steudle E, Hartung W (2000) Abscisic acid and hydraulic conductivity of maize roots: A study using cell- and root-pressure probes. Planta 211: 874–882 [DOI] [PubMed] [Google Scholar]
- Julkowska MM, Hoefsloot HCJ, Mol S, Feron R, de Boer G-J, Haring MA, Testerink C (2014) Capturing Arabidopsis root architecture dynamics with ROOT-FIT reveals diversity in responses to salinity. Plant Physiol 166: 1387–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koevoets IT, Venema JH, Elzenga JTM, Testerink C (2016) Roots withstanding their environment: Exploiting root system architecture responses to abiotic stress to improve crop tolerance. Front Plant Sci 7: 1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laur J, Hacke UG (2013) Transpirational demand affects aquaporin expression in poplar roots. J Exp Bot 64: 2283–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach HA, Hejlek LG, Hearme LB, Nguyen HT, Sharp RE, Davis GL (2011) Primary root elongation rate and abscisic acid levels of maize in response to water stress. Crop Sci 51: 157–172 [Google Scholar]
- Leonhardt N, Kwak JM, Robert N, Waner D, Leonhardt G, Schroeder JI (2004) Microarray expression analyses of Arabidopsis guard cells and isolation of a recessive abscisic acid hypersensitive protein phosphatase 2C mutant. Plant Cell 16: 596–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léon-Kloosterziel KM, Gil MA, Ruijs GJ, Jacobsen SE, Olszewski NE, Schwartz SH, Zeevaart JAD, Koornneef M (1996) Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. Plant J 10: 655–661 [DOI] [PubMed] [Google Scholar]
- Li G, Boudsocq M, Hem S, Vialaret J, Rossignol M, Maurel C, Santoni V (2015) The calcium-dependent protein kinase CPK7 acts on root hydraulic conductivity. Plant Cell Environ 38: 1312–1320 [DOI] [PubMed] [Google Scholar]
- Li X, Chen L, Forde BG, Davies WJ (2017) The biphasic root growth response to abscisic acid in Arabidopsis involves interaction with ethylene and auxin signalling pathways. Front Plant Sci 8: 1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Mitchell DM, Harris JM (2007) Abscisic acid rescues the root meristem defects of the Medicago truncatula latd mutant. Dev Biol 304: 297–307 [DOI] [PubMed] [Google Scholar]
- Lynch J. (1995) Root architecture and plant productivity. Plant Physiol 109: 7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markhart AH, Fiscus EL, Naylor AW, Kramer PJ (1979) Effect of abscisic Acid on root hydraulic conductivity. Plant Physiol 64: 611–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C, Simonneau T, Sutka M (2010) The significance of roots as hydraulic rheostats. J Exp Bot 61: 3191–3198 [DOI] [PubMed] [Google Scholar]
- Maurel C, Boursiac Y, Luu D-T, Santoni V, Shahzad Z, Verdoucq L (2015) Aquaporins in Plants. Physiol Rev 95: 1321–1358 [DOI] [PubMed] [Google Scholar]
- Orman-Ligeza B, Morris EC, Parizot B, Lavigne T, Babé A, Ligeza A, Klein S, Sturrock C, Xuan W, Novák O, et al. (2018) The xerobranching response represses lateral root formation when roots are not in contact with water. Curr Biol 28: 3165–3173.e5 [DOI] [PubMed] [Google Scholar]
- Orosa-Puente B, Leftley N, von Wangenheim D, Banda J, Srivastava AK, Hill K, Truskina J, Bhosale R, Morris E, Srivastava M, et al. (2018) Root branching toward water involves posttranslational modification of transcription factor ARF7. Science 362: 1407–1410 [DOI] [PubMed] [Google Scholar]
- Osakabe Y, Yamaguchi-Shinozaki K, Shinozaki K, Tran LS (2014) ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. New Phytol 202: 35–49 [DOI] [PubMed] [Google Scholar]
- Parent B, Hachez C, Redondo E, Simonneau T, Chaumont F, Tardieu F (2009) Drought and abscisic acid effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: A trans-scale approach. Plant Physiol 149: 2000–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Testerink C (2014) The art of being flexible: How to escape from shade, salt, and drought. Plant Physiol 166: 5–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins NE II, Dinneny JR (2018) Growth is required for perception of water availability to pattern root branches in plants. Proc Natl Acad Sci USA 115: E822–E831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales MA, Ocampo E, Rodríguez-Valentín R, Olvera-Carrillo Y, Acosta-Gallegos J, Covarrubias AA (2012) Physiological analysis of common bean (Phaseolus vulgaris L.) cultivars uncovers characteristics related to terminal drought resistance. Plant Physiol Biochem 56: 24–34 [DOI] [PubMed] [Google Scholar]
- Rowe JH, Topping JF, Liu J, Lindsey K (2016) Abscisic acid regulates root growth under osmotic stress conditions via an interacting hormonal network with cytokinin, ethylene and auxin. New Phytol 211: 225–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab IN, Sharp RE, Pritchard J, Voetberg GS (1990) Increased endogenous abscisic acid maintains primary root growth and inhibits shoot growth of maize seedlings at low water potentials. Plant Physiol 93: 1329–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez A, Robert N, Maktabi MH, Schroeder JI, Serrano R, Rodríguez PL (2006) Enhancement of abscisic acid sensitivity and reduction of water consumption in Arabidopsis by combined inactivation of the protein phosphatases type 2C ABI1 and HAB1. Plant Physiol 141: 1389–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu N, Raman KA, Torres RO, Audebert A, Dardou A, Kumar A, Henry A (2016) Rice root architectural plasticity traits and genetic regions for adaptability to variable cultivation and stress conditions. Plant Physiol 171: 2562–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Goodger JQ (2008) Chemical root to shoot signaling under drought. Trends Plant Sci 13: 281–287 [DOI] [PubMed] [Google Scholar]
- Scharwies JD, Dinneny JR (2019) Water transport, perception, and response in plants. J Plant Res 132: 311–324 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Kwak JM, Allen GJ (2001) Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature 410: 327–330 [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Léon-Kloosterziel KM, Koornneef M, Zeevaart JAD (1997) Biochemical characterization of the aba2 and aba3 mutants in Arabidopsis thaliana. Plant Physiol 114: 161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp RE, Davies WJ (1979) Solute regulation and growth by roots and shoots of water-stressed maize plants. Planta 147: 43–49 [DOI] [PubMed] [Google Scholar]
- Siemens JA, Zwiazek JJ (2004) Changes in root water flow properties of solution culture-grown trembling aspen (Populus tremuloides) seedlings under different intensities of water-deficit stress. Physiol Plant 121: 44–49 [DOI] [PubMed] [Google Scholar]
- Steudle E. (2000) Water uptake by plant roots: an integration of views. Plant Soil 226: 45–56 [Google Scholar]
- Sutka M, Li G, Boudet J, Boursiac Y, Doumas P, Maurel C (2011) Natural variation of root hydraulics in Arabidopsis grown in normal and salt-stressed conditions. Plant Physiol 155: 1264–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalmann M, Pazmino D, Seung D, Horrer D, Nigro A, Meier T, Kölling K, Pfeifhofer HW, Zeeman SC, Santelia D (2016) Regulation of leaf starch degradation by abscisic acid is important for osmotic stress tolerance in plants. Plant Cell 28: 1860–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Andrews J, Mulholland BJ, McKee JMT, Hilton HW, Horridge JS, Farquhar GD, Smeeton RC, Smillie IRA, Black CR, Taylor IB (2007) Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion. Plant Physiol 143: 1905–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu D-T, Bligny R, Maurel C (2003) Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 425: 393–397 [DOI] [PubMed] [Google Scholar]
- Trejo CL, Davies WJ (1991) Drought-induced closure of Phaseolus vulgaris L. stomata precedes leaf water deficit and any increase in xylem ABA concentration. J Exp Bot 42: 1507–1516 [Google Scholar]
- Turner NC. (1981) Techniques and experimental approaches for the measurement of plant water status. Plant Soil 58: 339–366 [Google Scholar]
- Uga Y, Sugimoto K, Ogawa S, Rane J, Ishitani M, Hara N, Kitomi Y, Inukai Y, Ono K, Kanno N, et al. (2013) Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat Genet 45: 1097–1102 [DOI] [PubMed] [Google Scholar]
- Vandeleur RK, Mayo G, Shelden MC, Gilliham M, Kaiser BN, Tyerman SD (2009) The role of plasma membrane intrinsic protein aquaporins in water transport through roots: Diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol 149: 445–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Weele CM, Spollen WG, Sharp RE, Baskin TI (2000) Growth of Arabidopsis thaliana seedlings under water deficit studied by control of water potential in nutrient-agar media. J Exp Bot 51: 1555–1562 [DOI] [PubMed] [Google Scholar]
- Verslues PE, Ober ES, Sharp RE (1998) Root growth and oxygen relations at low water potentials. Impact of oxygen availability in polyethylene glycol solutions. Plant Physiol 116: 1403–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S, Kudoyarova GR, Veselov DS, Arkhipova TN, Davies WJ (2012) Plant hormone interactions: Innovative targets for crop breeding and management. J Exp Bot 63: 3499–3509 [DOI] [PubMed] [Google Scholar]
- Xiong L, Wang R-G, Mao G, Koczan JM (2006) Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic Acid. Plant Physiol 142: 1065–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Jia L, Shi W, Liang J, Zhou F, Li Q, Zhang J (2013) Abscisic acid accumulation modulates auxin transport in the root tip to enhance proton secretion for maintaining root growth under moderate water stress. New Phytol 197: 139–150 [DOI] [PubMed] [Google Scholar]
- Yang L, Zhang J, He J, Qin Y, Hua D, Duan Y, Chen Z, Gong Z (2014) ABA-mediated ROS in mitochondria regulate root meristem activity by controlling PLETHORA expression in Arabidopsis. PLoS Genet 10: e1004791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Davies WJ (1987) Increased synthesis of ABA in partially dehydrated root-tips and ABA transport from roots to leaves. J Exp Bot 38: 2015–2023 [Google Scholar]
- Zhang H, Han W, De Smet I, Talboys P, Loya R, Hassan A, Rong H, Jürgens G, Paul Knox J, Wang MH (2010) ABA promotes quiescence of the quiescent centre and suppresses stem cell differentiation in the Arabidopsis primary root meristem. Plant J 64: 764–774 [DOI] [PubMed] [Google Scholar]
- Zhu B, Su J, Chang M, Verma DPS, Fan YL, Wu R (1998) Overexpression of a Δ1-pyrroline-5-carboxylate synthetase gene and analysis of tolerance to water- and salt-stress in transgenic rice. Plant Sci 139: 41–48 [Google Scholar]
- Zhu J, Brown KM, Lynch JP (2010) Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant Cell Environ 33: 740–749 [DOI] [PubMed] [Google Scholar]