Abstract
Background
In the absence of treatment options, the WHO emphasizes the identification of effective prevention strategies as a key element to counteract the dementia epidemic. Regarding the complex nature of dementia, trials simultaneously targeting multiple risk factors should be particularly effective for prevention. So far, however, only few such multi-component trials have been launched, but yielding promising results. In Germany, comparable initiatives are lacking, and translation of these complex interventions into routine care was not yet done. Therefore, AgeWell.de will be conducted as the first multi-component prevention trial in Germany which is closely linked to the primary care setting.
Methods
AgeWell.de will be designed as a multi-centric, cluster-randomized controlled multi-component prevention trial. Participants will be older community-dwelling general practitioner (GP) patients (60–77 years; n = 1,152) with increased dementia risk according to CAIDE (Cardiovascular Risk Factors, Aging, and Incidence of Dementia) Dementia Risk Score. Recruitment will take place at 5 study sites across Germany. GP practices will be randomized to either intervention A (advanced) or B (basic). GPs will be blinded to their respective group assignment, as will be the statistician conducting the randomization. The multi-component intervention (A) includes nutritional counseling, physical activity, cognitive training, optimization of medication, management of vascular risk factors, social activity, and, if necessary, further specific interventions targeting grief and depression. Intervention B includes general health advice on the intervention components and GP treatment as usual. We hypothesize that over the 2-year follow-up period the intervention group A will benefit significantly from the intervention program in terms of preserved cognitive function/delayed cognitive decline (primary outcome), and other relevant (secondary) outcomes (e.g. quality of life, social activities, depressive symptomatology, cost-effectiveness).
Discussion
AgeWell.de will be the first multi-component trial targeting risk of cognitive decline in older adults in Germany. Compared to previous trials, AgeWell.de covers an even broader set of interventions suggested to be beneficial for the intended outcomes. The findings will add substantial knowledge on modifiable lifestyle factors to prevent or delay cognitive decline.
Trial registration
German Clinical Trials Register (reference number: DRKS00013555).
Keywords: Prevention, Multi-component intervention, Lifestyle, Cognition, Mental health, Dementia, Primary care, Cluster-randomized controlled trial, Late life
Background
Relevance
Dementia is not only burdensome for the individuals affected and their relatives, it is also a major public health concern [1]. A substantial increase in the absolute number of people affected is to be expected due to dramatic demographic changes [1, 2]. So far, most types of dementia, particularly Alzheimer’s dementia (AD) as the most common type (60–70% of cases; [3]), cannot be cured. Therefore, the identification of effective strategies for prevention has been emphasized as a key element to counteract the dementia epidemic [4, 5].
Numerous vascular and lifestyle factors have been linked to dementia and AD [6, 7], suggesting that 35% of current dementia cases could be attributed to nine modifiable risk factors (midlife hypertension and obesity, diabetes mellitus, depression, physical inactivity, smoking, low educational attainment, hearing loss, and social isolation [8–11]. Similar results were found for Germany, suggesting a tremendous potential for prevention [10]. Indeed, recent studies indicated trends towards lower dementia incidence in Western high-income countries, likely being the result of improvements in such modifiable lifestyle factors [12]. Further modifiable risk factors may include the use of anticholinergic drugs [13] and atrial fibrillation [14]. Previous randomized controlled trials (RCTs) on interventions aiming at preventing cognitive decline, however, mainly focused on the management of single such risk factors [7]. Regarding the complex nature of dementia as well as the concurrence and interaction of the underlying risk factors, RCTs simultaneously targeting multiple risk factors should be more effective [7]. Large international multi-component trials [15–17] yielded very promising results in this regard.
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) was the first large RCT providing evidence that a multi-component intervention can be effective in improving or maintaining cognitive functioning in older individuals [5, 16]. Individuals aged 60–77 years (n = 1,200) with an increased dementia risk were randomly assigned to receive either a 2-year multi-component intervention (nutritional counseling, physical activity enhancement, cognitive training, vascular risk monitoring) or regular health advice (control group). The primary outcome was change in cognition as measured through a comprehensive neuropsychological test battery (NTB) [16]. During the 2-year follow-up period, cognition improved significantly in the intervention group compared to the control group [5].
In Germany, comparable initiatives have been lacking so far. Thus, the AgeWell.de study is designed to first-time investigate prevention potential for cognitive decline through a multi-component intervention, with a focus on modifiable risk factors such as, for example, lifestyle interventions, in older primary care patients in Germany.
Objectives
The primary objective of AgeWell.de is to evaluate the effectiveness of a multi-component intervention in preventing or delaying cognitive decline in older adults at increased risk for dementia, specifically tailored to the German health care context. To do so, a pragmatic multi-centric, cluster-randomized controlled prevention trial will be conducted in primary care. The secondary objective is to assess effects of the multi-component intervention regarding (i) mortality, (ii) nursing home placement, (iii) functioning in everyday activities, (iv) quality of life, (v) depressive symptoms, (vi) social inclusion, and (vii) the cost-effectiveness of the intervention. A detailed overview of the study aims and associated hypotheses is provided in Fig. 1.
Fig. 1.
Study aims and hypotheses of AgeWell.de
On the one hand, our study will be guided by the FINGER trial by including the components nutritional counseling, physical activity enhancement, cognitive training, and vascular risk monitoring in the 2-year intervention program. On the other hand, we aim to additionally include components of social lifestyle and recommendations on medication overuse and underuse. Encouraging participants to be socially active should also be a beneficial component of the intervention as even in old age an active lifestyle is protective against dementia [6, 18–20]. Likewise, we will provide specific recommendations on the medications of the study participants to their respective GP, if needed. German health claims data suggested that 22–25% of individuals aged 64 years and older receive at least one prescription of a potentially inappropriate medication (PIM) drug within one year [21, 22]. Primary data from a sample of community-dwelling people with dementia indicate that 22% receive at least one PIM with the highest prevalence being antidepressants, benzodiazepines and analgetics [23]. PIM constitutes a major public health concern especially in older age leading, for example, to an increased risk of adverse drug reactions, hospitalization, and mortality [24, 25]. Moreover, PIM is of particular importance as studies strongly suggest that specific drugs (e.g. with anticholinergic properties) can also increase the risk for dementia [13, 26, 27]. On the other hand, medication underuse can modify cardiovascular and other risks [28] and thus outcomes relevant for dementia development.
Finally, we will address further known risk factors for dementia by providing specific interventions in case of bereavement, grief, and depressive symptoms [29, 30], if necessary. The broad range of outcomes will additionally enable the estimation of total benefit and cost-effectiveness of the intervention suggested to be more beneficial for the intended outcomes. The study protocol describes the rationale and the study design of the AgeWell.de trial in adherence to the SPIRIT 2013 statement (Standard Protocol Items: Recommendations for Interventional Trials; [31]).
Methods
Study design
To evaluate the effectiveness of a multi-component intervention in preventing or delaying cognitive decline in older adults at risk, a multi-centric cluster-randomized controlled trial with primary care patients will be conducted (Fig. 2).
Fig. 2.
AgeWell.de study design
Inclusion and exclusion criteria
To target individuals at risk for dementia and suitable for the preventive intervention program, we will include community-dwelling GP patients who are 60–77 years old and have an increased CAIDE Dementia Risk Score (Cardiovascular Risk Factors, Aging, and Incidence of Dementia; [16, 32]). We will use a CAIDE Dementia Risk Score ≥ 9 for inclusion. The risk score predicted dementia risk with a sensitivity of 0.77 and a specificity of 0.63, when a cut-off ≥9 was applied [32]. CAIDE is based on information that is easy to assess (age, education, gender, blood pressure, body mass index, total cholesterol, and physical activity). Therefore, case finding of eligible participants for AgeWell.de can be easily conducted in the GP practices. Moreover, the procedure could be transferred into daily care later on.
Exclusion criteria are conditions affecting safe engagement in the intervention (malignant disease/fatal illness, severe clinical depression, symptomatic cardiovascular disease, revascularization within the previous year) as judged by the GP; severe loss of vision, hearing, or communicative ability/insufficient ability to speak and read German; severe mobility impairment; concurrent participation in another intervention trial, and previously diagnosed dementia or dementia suspected by the GP.
Interventions
During the 2-year follow-up period, participants of intervention group A (advanced) will receive a systematic comprehensive multi-component intervention program (Table 1). All intervention components will be delivered face-to-face by the study nurses during a visit at the participants’ homes. The intervention will include
advice on healthy nutrition, based on the guidelines of the German Nutrition Society (DGE)
exercises for strength, balance and flexibility on two days per week; aerobic training (3–5 days per week for 20–30 min) planned individually with the participant
cognitive training with tablet computers, using the cognitive training software “NeuroNation”, three times per week
enhancement of social engagement, planned individually with the participant
if necessary, feedback on vascular risk factors (e.g. smoking, medical history) and ways to reduce the respective risk
assessment of depressive symptoms and underlying risk factors (e.g. bereavement, grief); if necessary, patients will be encouraged to contact their GP who will provide adequate support and care (e.g. referring participants to groups, psychiatrists, psychotherapists, psychiatric hospitals); written information on addresses and helplines which can be contacted in case of grief and/or depressive symptoms.
optimization of medication: see Table 1 for a detailed description.
Table 1.
Components of the AgeWell.de intervention program (intervention group A)
| Intervention Component | Description |
|---|---|
| Nutritional counseling |
- Based on the guidelines of the German Nutrition Society [33] including recommendations e.g. on intake of cereal products, fruit and vegetables, fish, sugar, salt, and of fluid. - Anthropometric measurements (height, weight, BMI) |
| Physical activity enhancement |
- Combined training program including (i) muscle-strengthening activity, (ii) flexibility activity/balance exercise, on 2 days/week, and (iii) aerobic activity (3–5 days a week for 20–30 min). Strength and flexibility/balance training can be conducted at home. Aerobic training will be planned with each participant individually - Participants will receive a pedometer to record the number of steps walked daily |
| Cognitive training |
- Information on cognitive functioning and the impact of cognitive activity/training on cognitive performance and dementia risk; information on strategies to train cognition in everyday life - Cognitive training at home on a regular basis (3 times/week, 15 min per session) with tablet computers (software: NeuroNation; https://www.neuronation.com/) |
| Optimization of medication |
- Collection of baseline information (i) from the GP on participants’ medication, diagnoses, and lab values (creatinine, hemoglobin A1c) and (ii) from the participants on their actual medication - Electronically supported data evaluation to identify potentially inappropriate medication (e.g. anticholinergics, using a list based on Gray and coworkers [13], potentially missing drugs (using START criteria A1 to A8; [34]), contraindicated drug combinations, and contraindications in patients with renal impairment (using databases from the electronic drug information system AiDKlinik). Discrepancies in the information on medication collected from the participant and the GP will be identified. - Reports including recommendations on the medication of a participant will be transmitted to the GP. For recommended drugs, this will include information on potentially serious drug-drug interactions and on drug dose adjustment in patients with renal impairment. A procedure is established in case of emergencies, e.g. if an important drug for a serious condition is not administered by the patient. If a patient reports difficulties with drug administration (difficulties swallowing tablets or capsules, tablet splitting, subcutaneous injections, use of inhalers, transdermal patch application, or with the administration of eye drops, nose drops, ear drops, or rectal or vaginal drug administration), specific information is provided to the patient. |
| Management of vascular risk factors |
- Assessment of medication and diagnoses, lab values, health parameters, lifestyle factors, blood pressure and anthropometric measurements (height, weight) - Information on further vascular risk factors (e.g. smoking, medical history) - Feedback on vascular risk, importance of reducing the risk, and possible ways of achieving such a reduction - Nutritional counseling, recommendation of weight loss (if necessary), physical activity enhancement, and optimization of medication as described above |
| Enhancement of social engagement |
- Assessment of level of social activity and risk of social isolation - Information on the importance of an active lifestyle including high social engagement for dementia risk - Enhancement of social engagement will be planned together with each study participant individually |
| Bereavement, grief and depressive symptoms |
- Assessment of depressive symptoms and underlying risk factors (e.g. bereavement, grief) - If necessary, participants will be encouraged to contact their GP and will be provided with adequate support and care (e.g. referring participants to groups, psychiatrists, psychotherapists, psychiatric hospitals). Additionally, participants will receive written information on addresses and helplines which can be contacted in case of grief and/or depressive symptoms. - If applicable, encouragement to use MoodGym – a scientifically developed and evaluated free web-based training program to prevent and reduce depressive symptoms (http://www.moodgym-deutschland.de/). |
| Motivational tasks across all components |
- Personnel continuity regarding the contact person for the participants - Study nurses will be trained in motivational interviewing techniques and participants will be strongly encouraged to contact their contact person, whenever necessary - Birthday/Christmas/holiday cards originally signed by trial authorities/principal investigators - Participants of intervention A will receive a brochure including recommendations (additional tips and suggestions each week for an active lifestyle, e.g. recipes, suggestions for physical activity, and information on healthy ageing) as well as a weekly diary to track their activities in the intervention components. |
Participants of intervention group B (basic) will receive GP treatment as usual (GPTAU) and general health advice (GHA) related to the components of intervention A. We hypothesize that the multi-component intervention program of intervention A will be superior to GPTAU and GHA (intervention B) regarding trial outcomes.
Outcomes
Primary endpoint
In accordance with previous trials [2, 5, 7], we will assess change in cognitive performance as the primary endpoint. Cognitive performance will be assessed with a neuropsychological test battery covering the six cognitive domains for diagnosing mild and/or major neurocognitive disorder according to DSM-5 (attention, executive function, learning/memory, language, perceptual-motor abilities, and social cognition). Composite cognitive z-scores based on the results from all single tests will be calculated. Single z-scores will be calculated using baseline mean and standard deviations and then, for the composite score, the single z-scores will be averaged.
Secondary endpoints
An overview of secondary endpoints (mortality, nursing home placement, instrumental activities of daily living/activities of daily living, quality of life, depressive symptoms, social inclusion, motivation for behavior change, cost-effectiveness) is provided in Fig. 1. Corresponding assessments are detailed in Table 2. Furthermore, we will assess readiness for behavior change and explore mediating and moderating factors for the effectiveness of the intervention.
Table 2.
Instruments used in AgeWell.de
| Measures of cognitive performance (primary endpoint) | |
|---|---|
| Construct | Instrument |
| Cognitive performance a |
• Trail Making Test A and B [35] • Word List Memory - CERAD subtest [36–38] • Verbal Fluency Test - Animals - CERAD subtest [37–40] • Constructional Practice – CERAD subtest [37, 38, 41] • Reading the Mind in the Eyes Test - Revised version [42, 43]b • Montreal Cognitive Assessment (MoCA; [44]). |
| Instruments to assess secondary endpoints in AgeWell.de | |
| Construct | Instrument |
| Mortality | Information obtained from the GP or confidant elected by the participant [self-constructed itemsc] |
| Nursing home placement | Information from the participant or – if the participant is unavailable or dead – from the GP or a contact person elected by the participant |
| ADL/IADL | Barthel Index [45], Amsterdam-IADL scale [46]a |
| Quality of life | EQ-5D and visual analogue scale (EQ VAS scale) [47]; WHOQOL-Bref [48], WHOQOL-Old [49] |
| Depressive symptoms a | Geriatric Depression Scale (GDS; [50, 51]) |
| Social inclusion | Lubben Social Network Scale (LSNS; [52] in combination with standardized questionnaire on social activity [self-constructed itemsc] |
| Cost effectiveness | Fragebogen zur Inanspruchnahme medizinischer und nicht-medizinischer Versorgungsleistungen im Alter (FIMA)-Questionnaire for Health-Related Resource Use in an Elderly Population [53]; unit costs for monetary valuation of resource use [54]; EQ-5D [47] |
| Motivation for behavior change | Stage assessment in the adoption and maintenance of physical activity and fruit and vegetable consumption [55–57] |
| Instruments to assess further information relevant for the AgeWell.de-trial | |
| Construct | Instrument |
| Sociodemographic information | Standardized questionnaire (age, sex, educational level/professional life/activity, living situation/marital status, socio-economic status) |
| Subjective cognitive decline a | Standardized questionnaire on subjective cognitive decline [58] |
| Self-reported impairments and symptoms |
Standardized questionnaire on self-reported impairment in walking, vision, or hearing [self-constructed itemsc] Standardized questionnaire on self-reported anticholinergic symptoms [self-constructed itemsc] |
| Anthropometry, blood pressure | Measurement of height, weight, blood pressure; calculation of body-mass-index (BMI) |
| Nutrition | Standardized questionnaire on food consumption (food frequency questionnaire/FFQ [59]) |
| Physical activity I | Standardized questionnaire on physical activity [self-constructed itemsc] |
| Bereavement, grief | Standardized questionnaire on bereavement [60] |
| Medication I | (i) Information from the attending GP on participants’ medication (“GP-list”) and diagnosesb, lab values using GP practice records and (ii) information from participants on their actual medication (“brown-bag review”), adherence, and difficulties with drug administration using standardized questionnaires [self-constructed itemsc] |
| additional vascular risks | Standardized questionnaires on additional vascular risk factors (e.g. smoking, medical history, familial medical history; [self-constructed itemsc]) |
| Instruments to assess further information in intervention group A | |
| Construct | Instrument |
| Physical activity II | Weekly records on conduct of the physical activity intervention component, training, and pedometer results [self-constructed itemsc] |
| Cognitive training | Weekly records on conduct of the cognitive training [self-constructed itemsc] |
| Medication II | Standardized feedback questionnaires on potential changes in a participant’s medication or reasons for not following the recommendations, completed by the attending GP [self-constructed itemsc] |
| Social activity | Weekly records on social activity, in addition to the information collected on social inclusion (see above) |
| Motivation and readiness for change | Standardized questionnaire on motivation and readiness for behavior change [55–57] |
| Instruments to conduct the process evaluation | |
| Construct | Instrument |
| Success rate of recruitment and quality of the study population | Standardized questionnaires filled out by the GP practice personnel (number of eligible GP patients, number of participants and non-participants, (baseline) characteristics of participants and non-participants, reasons for non-participation) [self-constructed itemsc] |
| Standardized telephone interview on reasons for leaving the study with intervention A-participants, if applicable [self-constructed itemsc] | |
| Quality of the execution of the intervention, burden for GP patients and GPs | Standardized interviews with all intervention A-participants on adherence to the intervention components and potential burdens [self-constructed itemsc] |
| Standardized GP questionnaires on barriers and facilitators for adherence to intervention components in the GPs’ view and on own potential burdens [self-constructed itemsc] | |
GP general practitioner, ADL Activities of Daily Living, IADL Instrumental Activities of Daily Living
aInformation should be also used to diagnose DSM-5 Mild and Major Neurocognitive Disorder/dementia
bThe composite cognitive z-score for the primary endpoint change in cognitive performance will be calculated based on the test results regarding these six domains
cQuestionnaires can be obtained from the corresponding author upon request
Sample size
Sample size calculations are based on a composite z-score of cognitive performance from previous findings in mild AD [61], i.e., a mean decrease in the composite z-score of cognitive performance of − 0.21 with a common SD of 0.5 is assumed in intervention group B during the two year follow-up period. Accordingly, the required sample size is estimated to be 475 participants per group in order to detect a 50% difference in change in the composite z-score between intervention groups (2-sided t-test for equal variances; with 5% significance level and 90% power). We assume an inflation factor of 1.1 (corresponding to an intra-cluster correlation coefficient of 0.02 and a cluster size of 6). In view of the findings from the FINGER trial, we further assume a dropout rate of no higher than 10% [5]. Therefore, a total sample size of n = 1,152 individuals (n = 576 per group) seems sufficient.
Recruitment procedure
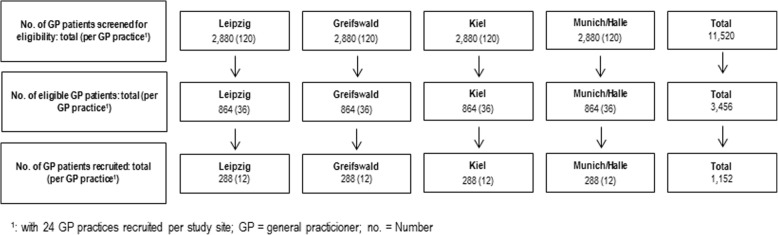
To ensure the inclusion of 1,152 patients, recruitment will take place at five study sites across Germany (Greifswald, Kiel, Leipzig, and Munich/Halle; Fig. 3). We estimate that (i) 30% of the GP patients between 60 and 77 years are eligible for the study (according to the FINGER trial, approximately 40% have the required CAIDE Dementia Risk Score ≥ 9 [30]; among these another 25% are estimated to be excluded according to the AgeWell.de exclusion criteria.). Based on previous experiences from other multi-centric trials [62, 63] and regarding the demanding nature of this prevention trial, we assume a rather conservative response rate of approximately 33%. Thus, every study site will screen n = 2,880 GP patients to identify n = 864 potentially eligible GP patients (except for Munich/Halle: half numbers due to shared recruitment between the two study sites). To ensure these numbers, we suggest a number of n = 24 GP practices per study site (n = 96 GP practices in total) as sufficient, leading to a total number of n = 12 patients that have to be recruited per GP practice. GP practices will receive monetary incentives for recruitment and provision of patients’ data.
Fig. 3.
AgeWell.de recruitment of participants per study site
Randomization
To ensure a balance in sample size across groups over time, block-randomization of the GP practices will be used with a targeted assignment ratio of participants to intervention A vs. B of 1:1. Participating GP practices (clusters) will be randomized either to intervention A or B. Randomization will be conducted at the data management center at the Institute for General Practice at the Hannover Medical School, using a randomization list that is concealed to the recruiting study sites.
Blinding
Whereas blinding of interviewers and study nurses cannot be realized in AgeWell.de, GPs will be blinded towards their respective group allocation. Eligible participants will receive all necessary information about the study in an information sheet provided by the GP, including information on the components of both interventions and on the random assignment of participants to either intervention A or B. After signing an informed consent at the GP’s practice, participants will receive a letter from the study sites with information on their respective group allocation and on next steps of the study.
Data collection
Each study center will be recruiting GP practices using an invitation letter with information on study design and aims as well as GPs’ duties during the trial. GPs interested in study participation can reply per fax or telephone. The recruiting study sites will then schedule a personal appointment at the GP practice to explain the recruitment procedure and to provide all necessary documents (informed consent form, patient information, screening sheets etc.).
Case finding and recruitment will be conducted by the GP practices. Data of regular GP patients will be screened by trained practice personnel in association with the AgeWell.de-study personnel, according to the inclusion and exclusion criteria. The GP will then provide all necessary information about the study to eligible patients. Patients who are interested in study participation will sign an informed consent. Contact information of the patients will be sent to the local recruiting centers. Next, patients who have consented to participate will receive a letter from the study personnel, informing them about their respective group allocation. Home visits for baseline assessment will be scheduled.
At baseline and at follow-up assessment after 24 months, fully structured interviews will be conducted with all participants, assessing the primary and secondary endpoints. Other relevant information assessed include sociodemographic information, subjective cognitive decline, self-reported impairments and symptoms, anthropometry, blood pressure, nutrition, physical activity, bereavement/grief, medication, vascular risks). Moreover, GPs will provide data on participants’ health characteristics (diagnoses, lab values, medication) using standardized questionnaires. To conduct the process evaluation, the success rate of recruitment (number of eligible GP patients, number of participants and non-participants, (baseline) characteristics of participants and non-participants, reasons for non-participation) will be provided by the GP practice personnel. Standardized telephone interviews on reasons for dropout will be held with intervention A-participants, if applicable. For participants in intervention A, additional interim sessions will be scheduled: one face-to-face assessment after 12 months at the participants’ home and five telephone calls after 2, 4, 8, 16, and 20 months, respectively (see Table 3). These fully structured interviews serve the purpose of monitoring adherence to the intervention and motivation (monitoring & booster sessions). Participants in intervention group A will be asked to track their activities in the intervention components using weekly diaries. To reduce non-response as well as dropout in AgeWell.de, interviewers will undergo training regarding all procedures and assessments, including motivational interviewing techniques [64]. Likewise, motivational tasks will also be carried out by the principal investigators (Table 1).
Table 3.
Schedule of enrolment, interventions and assessments in the course of AgeWell.de

1: Telephone interim monitoring and booster sessions 2, 4, 8, 16, and 20 months after baseline-assessment in intervention group A; 2: Face-to-face intervention visit in intervention group A after 12 months; 3: limited assessment of the actual medication via telephone
Data management
Each local recruiting center will enter collected data via an internet-based electronic data capture system which complies with the FDA requirements (21 CFR Part 11) as well as the guidelines for Good Clinical Practice (GCP). Data will be stored in a central Oracle-database. For statistical analyses, data can be exported via a web-based export-tool into SPSS, SAS, CDISC ODM, or similar.
The technical infrastructure includes the internet-based remote data entry system and the central database server. Data will not be stored locally. For transfers, data will be secured using 128 bit SSL encryption. Access to the database and webserver is controlled by two consecutive firewall systems. Data will only be stored using a pseudonym generated automatically upon first entry of a patient’s data into the database. Access to the electronic data entry system will be provided to members of the AgeWell.de study group according to a detailed concept of roles and rights.
Statistical analyses
Preliminary analyses involve three parts. First, data will be cleaned and checked for inconsistencies by statisticians at the central data management center to ensure data accuracy and coherence. Second, outcome variables will be examined to identify potential outliers and leverage points. Third, missing data will be inspected and handled according to patterns of data missingness, e.g., by multiple imputation [65].
A dropout analysis will be performed to test whether complete and incomplete cases differ according to relevant sociodemographic variables, potentially introducing selection bias.
Descriptive analyses will be carried out to examine differences in baseline measurements regarding the composite cognitive z-score (primary endpoint) and secondary endpoints between the two intervention groups. Additionally, single scores for the six neurocognitive domains will be calculated and analyzed. Suitable balancing techniques, such as entropy balancing, will be used for baseline measurements to warrant a high degree of comparability between study groups [66]. In order to analyze changes in primary and secondary endpoints of the two groups over time (treatment effect), latent growth curve modeling (LGM) will be utilized. Group membership (A vs. B) as well as other time-invariant characteristics will be assessed as predictors of change in cognitive performance in the models. Model estimates will be weighted using, for example, calculated entropy balancing weights to equalize possible baseline differences in covariates and outcomes. The models will further be stratified by age and gender to evaluate whether treatment effects on primary and secondary outcomes differ by subgroups.
Secondary analyses include, among others, investigation of effects of drug-associated risk factors. Effects of the intervention in patients with or without such factors will be analyzed by comparing primary and secondary endpoints in the respective subgroups. Plans for stratified analyses by overall cognitive performance as expressed by MoCA scores are effective. Cost-effectiveness will be analyzed based on both direct costs and quality-adjusted life-years (QALYs). To derive direct costs, health care utilization during 24 months follow-up will be valued using specific German unit costs. Intervention costs will be calculated using accounting principles. QALYs will be calculated using preference-based utilities as derived from the EQ-5D-5 L. Additionally, incremental cost-effectiveness ratios (ICER) will be calculated and net benefit regressions will be conducted to determine the uncertainty of the point estimates of the ICER [67].
Monitoring
Data monitoring
A data monitoring committee will not be established as the overall risk associated with the trial is considered low, and therefore, the likelihood for the need to modify or discontinue the trial is considered insignificant. However, precautions have been taken to minimize the potential of any harms, as detailed below. Moreover, a detailed process evaluation will be conducted throughout the entire trial to produce in-depth insight into the delivery of the interventions, (i) to prevent drawing inappropriate conclusions on the efficacy or effectiveness of the interventions, and (ii) to formulate recommendations for future studies [68]. Most importantly, process evaluation should be used to identify and avoid potential burdens for study participants and GPs. The process evaluation will comprise three dimensions:
Evaluation of the success rate of recruitment: Standardized questionnaires filled in by the GP personnel collecting number of eligible patients, number of participants and non-participants, baseline-characteristics of both groups, and reasons for non-participation. Loss of participants during the follow-up-period as well as reasons for leaving the study will be assessed to evaluate attrition rate as well as barriers and facilitators for staying in the trial [5].
Evaluation of the quality of the execution of the intervention components: At every face-to-face or telephone interim session, adherence to the intervention will be assessed using standardized questionnaires with intervention A participants in order to identify potential barriers and facilitators for adherence to the intervention. Likewise, GPs will be asked to fill out a standardized questionnaire to identify potential burdens for participating GPs.
Evaluation of the process of data acquisition regarding completeness and validity: Documentation of reasons for discontinuing an assessment before completion as well as the motivation of the participants and the GPs for assessment. Number and characteristics of missing data will be analyzed. Information will be used to evaluate barriers and facilitators for data collection.
Harms
Trial-related adverse events (AEs) will be assessed without regard towards a causal relation to the intervention. AEs will be recorded at each contact with participants after the baseline assessment. AEs in AgeWell.de include loss experiences and grief, depressive symptoms, severe injuries, hospitalization, and private or occupational stress reported by participants. Specific AEs (depressive symptoms; severe injuries; hospitalization) will be considered serious adverse events (SAEs) if they result in a life-threatening condition (immediate risk or death), hospitalization/prolongation thereof, or any lasting impairment. Occurrence of AEs/SAEs will be documented in the electronic database after each contact with participants. In case of a SAE, an automatic case-report form will notify the coordinating study center at the University of Leipzig, which, in turn, will contact the respective study center. A shared decision will then be made between the coordinating study center and non-elective principal investigator (PI) at the respective study center, including one of the following options: informing the participants’ GP about the SAE, interruption or discontinuation of trial participation, or none of the above.
Auditing
Auditing will take place in form of reviews of the data collection across key assessment waves for each recruitment center. Specifically, 2 % of the questionnaires at baseline, at the face-to-face interim assessment (for intervention group A only), and at follow-up, respectively, will be randomly drawn and inspected regarding their degree of matching with the database input. Source data verification will be performed independently from investigators by the Hannover Medical School that is the entrusted site performing all data management tasks throughout the whole trial.
Protocol amendments
Possible modifications to the protocol will be tracked in the German Clinical Trials Register (DRKS, registry number: DRKS00013555).
Dissemination policy
AgeWell.de results will be published in scientific international, peer-reviewed journals, if possible with open access. Moreover, results will be presented at national and international scientific conferences as well as during seminars for GPs, regional care networks, and public events for seniors. Furthermore, publication results will be disseminated to the wider research community via press releases.
Organizational structure and responsibilities
The Institute for Social Medicine, Occupational Health and Public Health (ISAP) in Leipzig acts as the coordinating center in AgeWell.de. This implies the organization of regular telephone-conferences and meetings with all participating centers, the compensation of all participating GP practices, preparation of documents needed for the conduct of the trial as well as reports to the funder of the study. Moreover, the study center in Leipzig also acts as one of the recruiting centers. As a subcontractor, the Institute for General Practice at the Hannover Medical School acts as the responsible study site for the task of data management, including the setup of the internet-based remote data capture system. The study centers in Greifswald, Kiel and Munich/Halle act as further recruiting study sites. Evaluation of cost-effectiveness of the trial will be conducted by the Department of Health Economics and Health Service Research at the University Medical Center Hamburg-Eppendorf. The intervention component “optimization of medication” will be realized by the Department of Clinical Pharmacology and Pharmacoepidemiology at the University Hospital Heidelberg, including the programming of algorithms to evaluate electronic data for optimization of medication and provision of documents for corresponding feedback to and from the GP.
Discussion
Multi-domain interventions targeting lifestyle factors have been pointed out as a promising prevention strategy against dementia in international trials [5]. AgeWell.de will be the first such multi-component trial in older adults in Germany. We hypothesize that the multi-component intervention will be superior to general health advice and GPTAU in maintaining cognitive functioning in older adults at risk for dementia (primary hypothesis). Moreover, we assume beneficial effects in reducing mortality, nursing home placement, depressive symptoms, and in maintaining quality of life as well as functioning in everyday life (secondary hypotheses). Subsequently, this should also reduce direct health costs.
The AgeWell.de-study will add valuable insights regarding the role of modifiable risk and lifestyle factors to prevent or delay cognitive decline. A specifically useful asset should be findings from the intervention components that address potentially inappropriate medication [13, 26] and depression [8, 9, 69], which, to our knowledge, have not been examined in comparable RCTs so far.
Beyond that, our intervention will possibly also reduce risk for other diseases such as hypertension, stroke, cardiovascular disease, overweight etc. by addressing common risk factors for the respective diseases [2].
Limitations
Participation in our prevention trial is rather demanding since the intervention components address various lifestyle factors and require e.g. cognitive training and physical activity on several days a week. The follow-up period is longer than in most previous trials testing single interventions. Therefore, adherence to the intervention might be lower compared to trials targeting only single components for a shorter time period. These factors also raise the chance of dropping out. We will address these potential problems by putting a strong focus on continuous motivation of participants and monitoring of adherence to the intervention, e.g. by regular telephone contacts and the use of motivational interviewing techniques. For ethical reasons, general health advice and feedback on known risk factors for dementia will also be provided to intervention group B. On these grounds, estimates of the effects of our intervention should be considered to be conservative.
Outlook
Since the domains of our multi-component intervention address behaviors common in the general population, the intervention, if effective, will be implemented in real world settings. Thereby, AgeWell.de could add to targeted and cost-effective strategies in preventing dementia in older adults at risk. Most interventions tested (e.g. physical activity and a wholesome diet) are easily available to the majority of older adults [70]. Likewise, AgeWell.de addresses lifestyle factors that are common in general practice. Therefore, even a moderate decrease in exposure to the targeted lifestyle factors could lead to a significant reduction in incident dementia cases on a population level [5, 70]. Concerning the projected increase in number of dementia cases due to ageing populations, there is an evident need for effective prevention strategies. This becomes particularly relevant in regard to the absence of effective treatment options. To date, lifestyle interventions might constitute the most cost-effective and sustainable option for dementia prevention [71]. Against this background, the results of AgeWell.de will make a highly relevant contribution to the growing body of knowledge on modifiable risk factors for dementia.
Acknowledgements
Members of the AgeWell.de-study group: Principal Investigator: Steffi G. Riedel-Heller; Co-Principal Investigators: Wolfgang Hoffmann, Jochen Gensichen, Walter E. Haefeli, Hanna Kaduszkiewicz, Hans-Helmut König, Thomas Frese, Jochen René Thyrian and David Czock; Franziska Berg, Andrea Bischhoff, Christian Brettschneider, Juliane Döhring, Alexander Eßer, Corinna Gräble, Caroline Jung-Sievers, Kerstin Klauer-Tiedtke, Kerstin Krebs-Hein, Dagmar Lochmann, Tobias Luck, Silke Mamone, Andreas Meid, Michael Metzner, Lydia Neubert, Anke Oey, Susanne Röhr, Franziska Samos, Karin Schumacher, Theresa Terstegen, Sandy Thieme, Lars Wamsiedler, Tanja Wehran, Birgitt Wiese, Ines Winkler, Andrea Zülke, Ina Zwingmann.
Further, the authors want to thank all participating GP-practices and study participants of the AgeWell.de-trial in advance.
We acknowledge support from the German Research Foundation (DFG) and the University Leipzig within the program of Open Access Publishing.
Abbreviations
- AD
Alzheimer’s disease
- ADL
Activities of daily living
- AE
Adverse event
- BMBF
Bundesministerium für Bildung und Forschung (German Federal Ministry for Education and Research)
- CAIDE score
“Cardiovascular Risk Factors, Aging, and Incidence of Dementia” Dementia Risk Score
- CERAD
Consortium to Establish a Registry for Alzheimer’s Disease
- DSM-5
Diagnostic and statistical manual of mental disorders, 5th edition
- FINGER
Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability
- GCP
Good clinical practice
- GHA
General health advice
- GP
General practitioner
- GPTAU
General practitioner treatment as usual
- IADL
Instrumental activities of daily living
- ICER
Incremental cost-effectiveness ratios
- LGM
Latent growth curve modeling
- MoCA
Montreal Cognitive Assessment
- NTB
Neuropsychological test battery
- PI
Principal investigator
- PIM
Potentially inappropriate medication
- RCT
Randomized controlled trial
- SAE
Serious adverse event
- SD
Standard deviation
Authors’ contributions
SRH conceptualized and designed the study and was supported by TL, WH, JRT, JG, HK, HHK, WEH, DC, AP and BW. AZ, TL, SR and SRH drafted the manuscript. WH, JRT, JG, HK, HHK, WEH, DC, BW, AP and TF revised the manuscript for intellectual content. All authors read and approved the final version of the manuscript.
Funding
AgeWell.de is funded by the German Federal Ministry for Education and Research (BMBF; reference numbers: 01GL1704A, 01GL1704B, 01GL1704C, 01GL1704D, 01GL1704E, 01GL1704F). The BMBF had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Availability of data and materials
After publication of the final AgeWell.de trial results, electronic research data will be made accessible free of charge for third parties/further interested researchers upon request from the central data management center (work group Medical Statistics and IT-infrastructure at the Institute for General Practice, Hannover Medical School).
Ethics approval and consent to participate
AgeWell.de was approved by the responsible ethics boards of all participating study sites (Ethical Committee at the Medical Faculty, Leipzig University; Ethical Committee at the Medical Faculty, Christian-Albrechts-University, Kiel; Ethical Committee at Universitätsmedizin Greifswald; Ethical Committee at the Medical Faculty, Ludwig-Maximilian-University, Munich; Ethical Committee at the Medical Faculty, Martin-Luther-University Halle-Wittenberg; Ruprecht-Karls-University, Heidelberg). The study design was discussed with GPs and seniors organized in a senior interest group (Seniorenbeirat der Stadt Leipzig).
Written consent to participate will be obtained at the GP practice after the GP has provided all necessary information about the study. The signed informed consent form will be sent to the recruiting study site by the GP, along with participants’ contact information and data on participants’ health characteristics. Participants will then be contacted by the recruiting study center, informing them about the next steps of the study.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Andrea Zülke, Tobias Luck, Susanne Röhr and Steffi G. Riedel-Heller contributed equally to this work.
Susanne Röhr and Steffi G. Riedel-Heller shared last authorship
Contributor Information
Andrea Zülke, Phone: +49 341 97 15483, Email: Andrea.Zuelke@medizin.uni-leipzig.de.
Tobias Luck, Email: tobias.luck@hs-nordhausen.de.
Alexander Pabst, Email: Alexander.Pabst@medizin.uni-leipzig.de.
Wolfgang Hoffmann, Email: wolfgang.hoffmann@uni-greifswald.de.
Jochen René Thyrian, Email: Rene.Thyrian@dzne.de.
Jochen Gensichen, Email: Jochen.Gensichen@med.uni-muenchen.de.
Hanna Kaduszkiewicz, Email: kaduszkiewicz@allgemeinmedizin.uni-kiel.de.
Hans-Helmut König, Email: h.koenig@uke.uni-hamburg.de.
Walter E. Haefeli, Email: walter.emil.haefeli@med.uni-heidelberg.de
David Czock, Email: david.czock@med.uni-heidelberg.de.
Birgitt Wiese, Email: Wiese.Birgitt@MH-Hannover.de.
Thomas Frese, Email: Thomas.Frese@uk-halle.de.
Susanne Röhr, Email: Susanne.Roehr@medizin.uni-leipzig.de.
Steffi G. Riedel-Heller, Email: Steffi.Riedel-Heller@medizin.uni-leipzig.de
References
- 1.Prince M, Wimo A, Guerchet M, Ali GC, Wu YT, Prina M. World Alzheimer Report 2015: the global impact of dementia: an analysis of prevalence, incidence, cost and trends. London: Alzheimer's Disease International (ADI); 2015.
- 2.Andrieu S, Coley N, Lovestone S, Aisen PS, Vellas B. Prevention of sporadic Alzheimer's disease. Lessons learned from clinical trials and future directions. Lancet Neurol. 2015;14:926–944. doi: 10.1016/S1474-4422(15)00153-2. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . Fact sheet N 362: dementia. Geneva: WHO Press; 2015. [Google Scholar]
- 4.World Health Organization. Dementia: a public health priority. Geneva: World Health Organization; 2012
- 5.Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, Bäckman L, Hänninen T, Jula A, Laatikainen T, Lindström J, Mangialasche F, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Soininen H, Kivipelto M. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER). A randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 6.Mangialasche F, Kivipelto M, Solomon A, Fratiglioni L. Dementia prevention: current epidemiological evidence and future perspective. Alzheimers Res Ther. 2012;4:6. doi: 10.1186/alzrt104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sindi S, Mangialasche F, Kivipelto M. Advances in the prevention of Alzheimer's Disease. F1000prime Rep. 2015;7:50. doi: 10.12703/P7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 10.Luck T, Riedel-Heller SG. Prävention von Alzheimer-Demenz in Deutschland. Eine Hochrechnung des möglichen Potenzials der Reduktion ausgewählter Risikofaktoren. Nervenarzt. 2016;87:1194–1200. doi: 10.1007/s00115-015-0045-1. [DOI] [PubMed] [Google Scholar]
- 11.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 12.Roehr S, Pabst A, Luck T, Riedel-Heller SG. Is dementia incidence declining in high-income countries? A systematic review and meta-analysis. Clin Epidemiol. 2018;10:1233. doi: 10.2147/CLEP.S163649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, Yu O, Crane PK, Larson EB. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med. 2015;175:401–407. doi: 10.1001/jamainternmed.2014.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs V, Cutler MJ, Day JD, Bunch TJ. Atrial fibrillation and dementia. Trends Cardiovasc Med. 2015;25:44–51. doi: 10.1016/j.tcm.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Richard E, van den Heuvel E, Moll van Charante EP, Achthoven L, Vermeulen M, Bindels PJ, van Gool WA. Prevention of dementia by intensive vascular care (PreDIVA): a cluster-randomized trial in progress. Alzheimer Dis Assoc Disord. 2009;23:198–204. doi: 10.1097/WAD.0b013e31819783a4. [DOI] [PubMed] [Google Scholar]
- 16.Kivipelto M, Solomon A, Ahtiluoto S, Ngandu T, Lehtisalo J, Antikainen R, Backman L, Hanninen T, Jula A, Laatikainen T, Lindstrom J, Mangialasche F, Nissinen A, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Soininen H. The Finnish geriatric intervention study to prevent cognitive impairment and disability (FINGER): study design and progress. Alzheimers Dement. 2013;9:657–665. doi: 10.1016/j.jalz.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Vellas B, Carrie I, Gillette-Guyonnet S, Touchon J, Dantoine T, Dartigues JF, Cuffi MN, Bordes S, Gasnier Y, Robert P. MAPT study: a multidomain approach for preventing Alzheimer’s disease: design and baseline data. J Prev Alzheimers Dis. 2014;1:13. [PMC free article] [PubMed] [Google Scholar]
- 18.Karp A, Paillard-Borg S, Wang H, Silverstein M, Winblad B, Fratiglioni L. Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dement Geriatr Cogn Disord. 2006;21:65–73. doi: 10.1159/000089919. [DOI] [PubMed] [Google Scholar]
- 19.Paillard-Borg S, Fratiglioni L, Winblad B, Wang H. Leisure activities in late life in relation to dementia risk: principal component analysis. Dement Geriatr Cogn Disord. 2009;28:136–144. doi: 10.1159/000235576. [DOI] [PubMed] [Google Scholar]
- 20.Paillard-Borg S, Fratiglioni L, Xu W, Winblad B, Wang H. An active lifestyle postpones dementia onset by more than one year in very old adults. J Alzheimers Dis. 2012;31:835–842. doi: 10.3233/JAD-2012-120724. [DOI] [PubMed] [Google Scholar]
- 21.Amann U, Schmedt N, Garbe E. Prescribing of potentially inappropriate medications for the elderly: an analysis based on the PRISCUS list. Dtsch Arztebl Int. 2012;109:69. doi: 10.3238/arztebl.2012.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schubert I, Küpper-Nybelen J, Ihle P, Thürmann P. Prescribing potentially inappropriate medication (PIM) in Germany's elderly as indicated by the PRISCUS list. An analysis based on regional claims data. Pharmacoepidemiol Drug Saf. 2013;22:719–727. doi: 10.1002/pds.3429. [DOI] [PubMed] [Google Scholar]
- 23.Wucherer D, Eichler T, Hertel J, Kilimann I, Richter S, Michalowsky B, Thyrian JR, Teipel S, Hoffmann W. Potentially inappropriate medication in community-dwelling primary care patients who were screened positive for dementia. J Alzheimers Dis. 2017;55:691–701. doi: 10.3233/JAD-160581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau DT, Kasper JD, Potter DE, Lyles A, Bennett RG. Hospitalization and death associated with potentially inappropriate medication prescriptions among elderly nursing home residents. Arch Intern Med. 2005;165:68–74. doi: 10.1001/archinte.165.1.68. [DOI] [PubMed] [Google Scholar]
- 25.Dormann H, Sonst A, Müller F, Vogler R, Patapovas A, Pfistermeister B, Plank-Kiegele B, Kirchner M, Hartmann N, Bürkle T. Adverse drug events in older patients admitted as an emergency: the role of potentially inappropriate medication in elderly people (PRISCUS) Dtsch Arztebl Int. 2013;110:213. doi: 10.3238/arztebl.2013.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jessen Frank, Kaduszkiewicz Hanna, Daerr Moritz, Bickel Horst, Pentzek Michael, Riedel-Heller Steffi, Wagner Michael, Weyerer Siegfried, Wiese Birgitt, van den Bussche Hendrik, Broich Karl, Maier Wolfgang. Anticholinergic drug use and risk for dementia: target for dementia prevention. European Archives of Psychiatry and Clinical Neuroscience. 2010;260(S2):111–115. doi: 10.1007/s00406-010-0156-4. [DOI] [PubMed] [Google Scholar]
- 27.Heser K, Luck T, Röhr S, Wiese B, Kaduszkiewicz H, Oey A, Bickel H, Mösch E, Weyerer S, Werle J, Brettschneider C, König H, Fuchs A, Pentzek M, van den Bussche H, Scherer M, Maier W, Riedel-Heller SG, Wagner M. Potentially inappropriate medication: association between the use of antidepressant drugs and the subsequent risk for dementia. J Affect Disord. 2018;226:28–35. doi: 10.1016/j.jad.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Meid AD, Groll A, Heider D, Mächler S, Adler J, Günster C, König H, Haefeli WE. Prediction of drug-related risks using clinical context information in longitudinal claims data. Value Health. 2018;21:1390–1398. doi: 10.1016/j.jval.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Prince M, Albanese E, Guerchet M, Prina M. World Alzheimer report 2014: dementia and risk reduction: an analysis of protective and modifiable risk factors. London: Alzheimer Disease International; 2014. [Google Scholar]
- 30.Sikorski C, Luppa M, Heser K, Ernst A, Lange C, Werle J, Bickel H, Mösch E, Wiese B, Prokein J. The role of spousal loss in the development of depressive symptoms in the elderly—implications for diagnostic systems. J Affect Disord. 2014;161:97–103. doi: 10.1016/j.jad.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 31.Moher D, Chan A. SPIRIT (standard protocol items: recommendations for interventional trials) 2014. pp. 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5:735–741. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 33.German Nutrition Society: 10 guidelines of the German Nutrition Society (DGE) for a wholesome diet. [https://www.dge.de/fileadmin/public/doc/fm/10-guidelines-for-a-wholesome-diet.pdf]. Accessed 17 Nov 2018.
- 34.O'mahony D, O'sullivan D, Byrne S, O'connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44:213–218. doi: 10.1093/ageing/afu145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reitan RM. Trail Making Test: Manual for administration and scoring. Tempe, AZ: Reitan neuropsychology laboratory; 1992
- 36.Satzger W, Hampel H, Padberg F, Bürger K, Nolde T, Ingrassia G, Engel RR. Practical application of the CERAD test battery as a neuropsychological dementia screening test. Nervenarzt. 2001;72:196–203. doi: 10.1007/s001150050739. [DOI] [PubMed] [Google Scholar]
- 37.Morris JC, Mohs RC, Rogers H, Fillenbaum G, Heyman A. Consortium to establish a registry for Alzheimer's disease (CERAD) clinical and neuropsychological assessment of Alzheimer's disease. Psychopharmacol Bull. 1988;24:641–652. [PubMed] [Google Scholar]
- 38.Moms J. C., Heyman A., Mohs R. C., Hughes J. P., van Belle G., Fillenbaum G., Mellits E. D., Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assesment of Alzheimer's disease. Neurology. 1989;39(9):1159–1159. doi: 10.1212/WNL.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 39.Isaacs B, Kennie AT. The set test as an aid to the detection of dementia in old people. Br J Psychiatry. 1973;123:467–470. doi: 10.1192/bjp.123.4.467. [DOI] [PubMed] [Google Scholar]
- 40.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14:167–177. [PubMed] [Google Scholar]
- 41.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141(11):1356–64. [DOI] [PubMed]
- 42.Bölte S. Reading Mind in the Eyes Test für Erwachsene (dt. Fassung) von S. Frankfurt: JW Goethe Universität Frankfurt/M; 2005. [Google Scholar]
- 43.Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the mind in the eyes” test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry Allied Discip. 2001;42:241–251. doi: 10.1111/1469-7610.00715. [DOI] [PubMed] [Google Scholar]
- 44.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 45.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index: a simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Maryland state medical journal. 1965;14:56–61. [PubMed]
- 46.Sikkes Sietske A.M., Knol Dirk L., Pijnenburg Yolande A.L., de Lange-de Klerk Elly S.M., Uitdehaag Bernard M.J., Scheltens Philip. Validation of the Amsterdam IADL Questionnaire©, a New Tool to Measure Instrumental Activities of Daily Living in Dementia. Neuroepidemiology. 2013;41(1):35–41. doi: 10.1159/000346277. [DOI] [PubMed] [Google Scholar]
- 47.EuroQol G. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 48.Skevington SM, Lotfy M, O'Connell KA. The World Health Organization's WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual Life Res. 2004;13:299–310. doi: 10.1023/B:QURE.0000018486.91360.00. [DOI] [PubMed] [Google Scholar]
- 49.Winkler I, Matschinger H, Angermeyer MC. Der WHOQOL-OLD -- Ein Fragebogen zur interkulturellen Erfassung der Lebensqualität im Alter. Psychother Psychosom Med Psychol. 2006;56:63–69. doi: 10.1055/s-2005-915334. [DOI] [PubMed] [Google Scholar]
- 50.Gauggel S, Birkner B. Validity and reliability of a German version of the geriatric depression scale (GDS) Zeitschrift fur Klinische Psychologie-Forschung und Praxis. 1999;28:18–27. doi: 10.1026//0084-5345.28.1.18. [DOI] [Google Scholar]
- 51.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. The Journal of Aging and Mental Health. 1986;5(1-2):165–173.
- 52.Lubben J, Blozik E, Gillmann G, Iliffe S, von Renteln Kruse W, Beck JC, Stuck AE. Performance of an abbreviated version of the Lubben social network scale among three European community-dwelling older adult populations. Gerontologist. 2006;46:503–513. doi: 10.1093/geront/46.4.503. [DOI] [PubMed] [Google Scholar]
- 53.Seidl H, Bowles D, Bock J, Brettschneider C, Greiner W, König H, Holle R. FIMA–Fragebogen zur Erhebung von Gesundheitsleistungen im Alter: Entwicklung und Pilotstudie. Das Gesundheitswesen. 2015;77:46–52. doi: 10.1055/s-0034-1372618. [DOI] [PubMed] [Google Scholar]
- 54.Bock J, Brettschneider C, Seidl H, Bowles D, Holle R, Greiner W, König HH. Ermittlung standardisierter Bewertungssätze aus gesellschaftlicher Perspektive für die gesundheitsökonomische Evaluation. Das Gesundheitswesen. 2015;77:53–61. doi: 10.1055/s-0034-1374621. [DOI] [PubMed] [Google Scholar]
- 55.Schwarzer R, Renner B. Health-specific self-efficacy scales. Freie Universität Berlin. 2009;14:2009. [Google Scholar]
- 56.Lippke S, Fleig L, Pomp S, Schwarzer R. Validity of a stage algorithm for physical activity in participants recruited from orthopedic and cardiac rehabilitation clinics. Rehabil Psychol. 2010;55:398. doi: 10.1037/a0021563. [DOI] [PubMed] [Google Scholar]
- 57.Jerusalem M, Schwarzer R. Skalen zur Erfassung von Lehrer-und Schülermerkmalen. Dokumentation der psychometrischen Verfahren im Rahmen der Wissenschaftlichen Begleitung des Modellversuchs Selbstwirksame Schulen. Berlin: Freie Universität Berlin; 1999. Skala zur allgemeinen Selbstwirksamkeitserwartung. [Google Scholar]
- 58.Rami L, Mollica MA, García-Sanchez C, Saldaña J, Sanchez B, Sala I, Valls-Pedret C, Castellví M, Olives J, Molinuevo JL. The subjective cognitive decline questionnaire (SCD-Q): a validation study. J Alzheimers Dis. 2014;41:453–466. doi: 10.3233/JAD-132027. [DOI] [PubMed] [Google Scholar]
- 59.Haftenberger M, Heuer T, Heidemann C, Kube F, Krems C, Mensink GBM. Relative validation of a food frequency questionnaire for national health and nutrition monitoring. Nutr J. 2010;9:36. doi: 10.1186/1475-2891-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Forstmeier S, Maercker A. Comparison of two diagnostic systems for complicated grief. J Affect Disord. 2007;99:203–211. doi: 10.1016/j.jad.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 61.Harrison J, Minassian SL, Jenkins L, Black RS, Koller M, Grundman M. A neuropsychological test battery for use in Alzheimer disease clinical trials. Arch Neurol. 2007;64:1323–1329. doi: 10.1001/archneur.64.9.1323. [DOI] [PubMed] [Google Scholar]
- 62.Schwarzbach M, Luppa M, Hansen H, König H, Gensichen J, Petersen JJ, Schön G, Wiese B, Weyerer S, Bickel H. A comparison of GP and GDS diagnosis of depression in late life among multimorbid patients–results of the MultiCare study. J Affect Disord. 2014;168:276–283. doi: 10.1016/j.jad.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 63.Luck T, Riedel-Heller SG, Kaduszkiewicz H, Bickel H, Jessen F, Pentzek M, Wiese B, Koelsch H, van den Bussche H, Abholz H. Mild cognitive impairment in general practice: age-specific prevalence and correlate results from the German study on ageing, cognition and dementia in primary care patients (AgeCoDe) Dement Geriatr Cogn Disord. 2007;24:307–316. doi: 10.1159/000108099. [DOI] [PubMed] [Google Scholar]
- 64.Miller WR, Rollnick S. Motivational interviewing: Helping people change. New York: Guilford press; 2012.
- 65.van Buuren S. Flexible imputation of missing data. Boca Raton, FL: Chapman and Hall/CRC press; 2012.
- 66.Hainmueller J. Entropy balancing for causal effects: a multivariate reweighting method to produce balanced samples in observational studies. Polit Anal. 2012;20:25–46. doi: 10.1093/pan/mpr025. [DOI] [Google Scholar]
- 67.Briggs AH, O'Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health. 2002;23:377–401. doi: 10.1146/annurev.publhealth.23.100901.140534. [DOI] [PubMed] [Google Scholar]
- 68.Prick A, de Lange J, van’t Leven N, Pot AM. Process evaluation of a multicomponent dyadic intervention study with exercise and support for people with dementia and their family caregivers. Trials. 2014;15:401. doi: 10.1186/1745-6215-15-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202:329–335. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vellas B, Carrie I, Guyonnet S, Touchon J, Dantoine T, Dartigues J, Cufi MN, Bordes S, Gasnier Y, Robert P, Bories L, Rouaud O, Desclaux F, Sudres K, Bonnefoy M, Pesce A, Fougere B, Delrieu J, Faisant C, Lala F, Dupuy C, Cantet C, Coley N, Belleville S, Willis SL, Weiner MW, Ousset PJ, Andrieu S. MAPT (multi-domain Alzheimer’s prevention trial). Results at 36 months. Alzheimers Dement. 2015;11:P331. doi: 10.1016/j.jalz.2015.08.151. [DOI] [Google Scholar]
- 71.Hussenoeder FS, Riedel-Heller SG. Primary prevention of dementia: from modifiable risk factors to a public brain health agenda? Soc Psychiatry Psychiatr Epidemiol. 2018;53:1289–1301. doi: 10.1007/s00127-018-1598-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
After publication of the final AgeWell.de trial results, electronic research data will be made accessible free of charge for third parties/further interested researchers upon request from the central data management center (work group Medical Statistics and IT-infrastructure at the Institute for General Practice, Hannover Medical School).