Abstract
Background
Burkitt lymphoma (BL) is a relatively common cancer of childhood in tropical Africa, although its precise incidence and continent-wide geographic distribution have not been previously systematically studied.
Methods
Using the methods employed to produce national estimates of cancer incidence for the “Globocan” series of the International Agency for Research on Cancer, along with detailed information on cancer incidence by histological subtype from cancer registries in Africa, we estimate the numbers and rates of incidence by sex, age group, country and region of Africa.
Results
We estimate that the number of new cases that occurred in 2018 to be about 3900, two thirds in males, and 81% in children aged 0–14. On a national basis, the geographic distribution of incidence rates among children in sub-Saharan Africa resembles that of the prevalence of infection with Falciparum malaria. An estimated 81% of cases are associated with infection with Epstein Barr virus (EBV).
Conclusions
BL comprises almost 50% of childhood of non-Hodgkin lymphoma in Africa, almost all of which are associated with EBV, with the geographic distribution – at least in sub Saharan Africa - mediated by infection with malaria.
Keywords: Burkitt lymphoma, Africa, Epstein Barr virus, Epidemiology, Incidence
Background
Burkitt lymphoma (BL), an aggressive B cell lymphoma first recognized as a tumour of African children, occurs throughout the world, but has a markedly different incidence in different world regions, and even within regions [1]. By far the highest incidence rates of BL are found in tropical African countries, where it may account for up to half of all childhood cancers [2], and the tumour in these regions is consequently referred to as “endemic BL.” Other African countries outside the equatorial belt have much lower incidence rates, which are similar to those in high income countries. BL in these regions is consequently referred to as “sporadic BL”. Immunodeficiency-associated Burkitt lymphoma is primarily associated with HIV infection [3]. Unlike endemic BL, sporadic and HIV-associated BL occur in all age groups. Although there are differences in clinical features and prognosis of the endemic, sporadic and HIV-associated BL [3, 4], the unifying characteristic in all patients with BL is the unique morphology and the chromosomal translocation involving MYC oncogene, which is present in BL irrespective of geographical location, and immunodeficiency status [5]. Another distinguishing feature of BL is the association with Epstein-Barr virus (EBV) infection. The endemic form is almost always EBV-positive while the sporadic BL tumours are less than 30% EBV-positive.
There have been no recent systematic attempt to estimate the actual magnitude of the burden of BL where it is known to occur at a relatively high rate, in Africa. In this report, we estimate the incidence (number of cases, and rates) of BL that occurred in Africa in 2018, and the likely fraction attributable to EB.
Methods
Data sources
We used the sources of information and methods employed to make national estimates of incidence for Globocan 2018 [6]. Since the subtypes of non-Hodgkin lymphoma are not reported in Globocan, we used the original sources used in the estimations, to abstract information on BL. The sources were the cancer registries of Africa, listed in Annex A of Ferlay et al. (Cancer incidence and mortality data: sources and methods by country GLOBOCAN2018_Annex_A.xlsx (available at http://gco.iarc.fr)). From these datasets, we abstracted information on cases BL (ICD-O M9687/3). In addition to these registry data, we used information on the proportions of non-Hodgkin lymphomas that were Burkitt lymphoma in unpublished registry data from Yaoundé (Cameroon)1 and Gabon,2 from newly established national paediatric registries in Burkina Faso, Republic of Congo, and Cote d’Ivoire3, and in published data from the Democratic Republic of Congo [7] and northern Cameroon [8].
Method of estimation
Within the NHL catgory of Globocan 2018 (C82–86, C96 - Non-Hodgkin lymphoma) we take the proportion of BL within 5 broad age group and for each sex. These proportions were applied to the estimated number of NHL cases (by sex and age) in GLOBOCAN 2018. When the Globocan estimate derived from several cancer registries, the mean of the proportions (within age-sex groups) was used.
Incidence rates were calculated for recent periods, for males and females, for 5 broad age groups, and the age standardized incidence rates obtained (using the world standard population [9]. Registry data were available for 26 of the 48 countries of sub-Saharan Africa, and 5 of the 6 countries of Northern Africa (countries with populations < 150,000 were excluded from the analysis). For those countries for which no data were available, average incidence rates from selected neighbouring countries in the same region were used to derive national incidence within the country (method 9, [6]).
Results
Table 1 shows, for the 5 regions of Africa, the estimated numbers of cases and incidence rates per 100,000 population (age specific rate in children age 0–14, and crude and age standardized rate (ASR) at all ages).
Table 1.
Numbers and incidence rates (per 100,000 population) of Burkitt lymphoma by region and sex
| REGION | MALES | FEMALES | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Numbers | Incidence rate per 100,000 | Numbers | Incidence rate per 100,000 | |||||||
| 0–14 | TOTAL | 0–14 | CRUDE | ASR | 0–14 | TOTAL | 0–14 | CRUDE | ASR | |
| Eastern Africa | 1009 | 1191 | 1.09 | 0.51 | 0.38 | 401 | 487 | 0.44 | 0.22 | 0.19 |
| Middle Africa | 350 | 452 | 0.91 | 0.54 | 0.46 | 172 | 217 | 0.45 | 0.26 | 0.20 |
| Southern Africa | 13 | 56 | 0.13 | 0.17 | 0.18 | 5 | 46 | 0.05 | 0.14 | 0.14 |
| Western Africa | 568 | 719 | 0.67 | 0.37 | 0.29 | 350 | 451 | 0.43 | 0.24 | 0.21 |
| Sub-Saharan Africa | 1940 | 2320 | 0.86 | 0.44 | 0.35 | 928 | 1201 | 0.42 | 0.23 | 0.20 |
| Northern Africa | 233 | 292 | 0.59 | 0.24 | 0.23 | 74 | 87 | 0.20 | 0.07 | 0.07 |
| Africa | 2173 | 2612 | 0.82 | 0.41 | 0.33 | 1002 | 1288 | 0.39 | 0.20 | 0.17 |
The estimate is for a total of 3900 new cases of BL in Africa in 2018, with almost exactly two thirds in males (67%), and 81.4% of cases (3175) occurring in children age 0–14. This is almost half of all the estimated number (6474) of childhood cases of non-Hodgkin lymphoma. At all ages, incidence rates in sub-Saharan Africa do not show much variation by region (ASR’s between 0.18 and 0.46 per 100,000 in males, 0.14–0.26 per 100,000 in females), although in North Africa, the apparent rarity of BL in adult females results in a very low estimated ASR (0.07 per 100,000). In children, the highest incidence in boys is in East and Middle (Central) Africa, and in girls, the same two regions, plus West Africa. Estimated rates of childhood BL are lowest in Southern Africa.
Figure 1 shows the incidence rates, by 5-year age group, in children and young adults (ages 0–24), pooling the data from the 35 registries contributing to the national estimates for sub Saharan Africa (857 cases in males, 538 cases in females). Incidence rates peak in the 5–9 year age group, and – in children – are higher in boys that in girls.
Fig. 1.
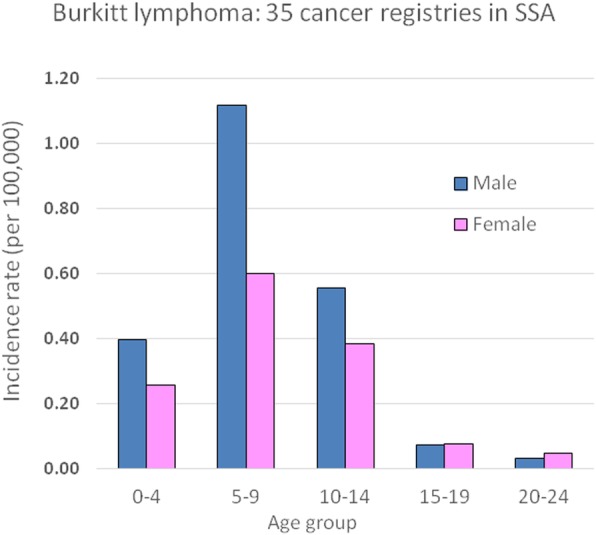
Age specific incidence rates of Burkitt lymphoma (1395 cases aged 0–24 from 35 cancer registries in sub Saharan Africa)
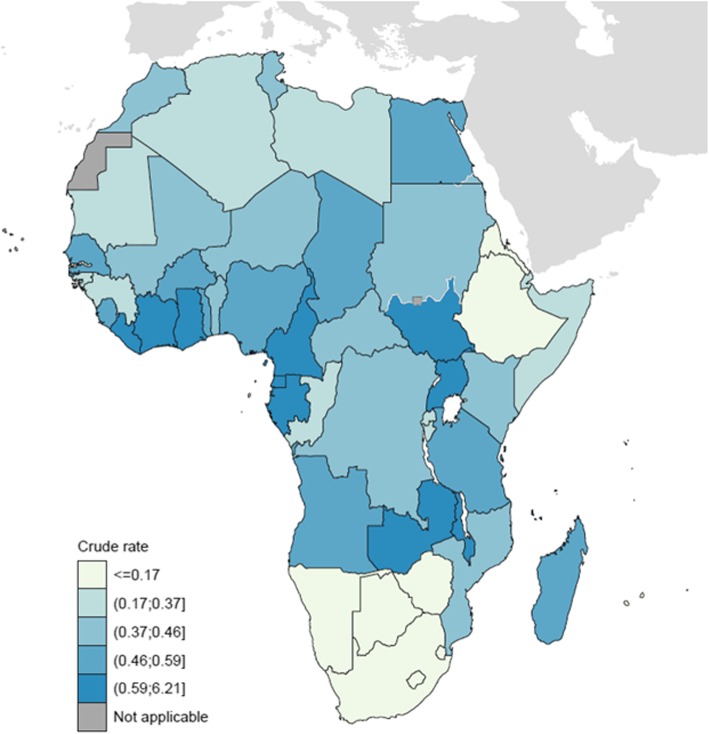
Figure 2 shows the distribution of estimated incidence rates of childhood BL at national level, as a map of Africa. The highest incidence rates are observed in Malawi (6.2 per 100,000), Cameroon (2.1), Uganda (1.4), Zambia (1.3) and Cote d’Ivoire (1.1). All of the countries of Southern Africa (as well as Ethiopia) have estimated rates of ≤0.17 per 100,000.
Fig. 2.
Burkitt lymphoma, patient age 0–14 years – both sexes (estimated incidence per 100,000 in 2018)
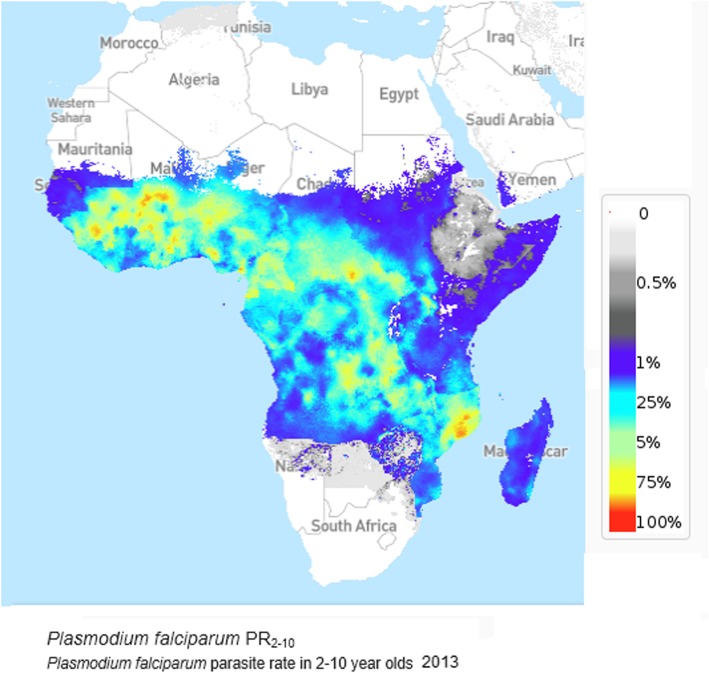
In the zone of high incidence of childhood BL in central Africa, almost all cases of endemic childhood BL are associated with EBV, as demonstrated by the presence of either EBV nuclear antigen (EBNA) or EBV DNA in the tumour cells [3]. This proportion is less in cases of sporadic and immunodeficiency associated Burkitt lymphoma [10, 11]. The peculiar distribution in sub Saharan Africa is not related to EBV exposure – which is ubiquitous, but has long been linked to the geographic occurrence on malaria, particularly where it is hyper- or holo-endemic [12]. Even today, the geographic occurrence of childhood BL bears a striking resemblance to that of endemicity of malaria due to P. falciparum (Fig. 3).
Fig. 3.
Plasmodium falciparum parasite rate in 2–10 year olds, Africa, 2013 [13]
Assuming that childhood BL in tropical Africa (Western, Middle, Eastern Africa) is of the endemic type (e-BL) with 100% EBV, while elsewhere, and among adolescents and adults, cases are of the sporadic type, with some 30% EBV associated [14], we can estimate that EBV is a causative factor in 3165 cases of BL in Africa (81% of the continental total).
Discussion
We have used cancer registry data from Africa to derive estimates of the numbers of cases of BL occurring on the continent in 2018, using the methods developed for 36 other cancer types in Globocan 2018. Although population based cancer registration has been slowly expanding in extent and quality in recent years, with some 30 registries in sub Saharan Africa meeting criteria rendering them suitable to contribute to the national estimates of Globocan (http://afcrn.org/index.php/membership/membership-list), the data they produce are not perfect. Most score between 4 and 7 on the quality (“q”) factor used to produce uncertainty estimates in Globocan, and only 6 of the countries of Africa have registries that aim to cover the entire national population – usually only a sample of 5–10% is involved. On the positive side, BL is relatively easy to diagnose – at least in childhood - because they present as rapidly growing tumours which may affect the face with specific histological characteristics, so that case ascertainment is likely to be much better than for other tumours. Indeed, by making our estimates based on the proportions of NHL cases that are BL, we assume that relatively few of the cancers allocated to the “Lymphoma not specified” codes in ICD-O (9590/9591) will actually have been cases of BL. Most cases of BL in the registry data had been diagnosed based on histology or pathology, indeed, for most registries, all BL registrations were based on morphological verification (MV) of diagnosis. There were some important exceptions – the percentage of MV cases in the series from Ibadan (Nigeria) was 87, 71% in Blantyre (Malawi), and 65% in Kampala (Uganda).
The observations provide more extensive data to confirm earlier studies on BL incidence, indicating an excess of cases in males v females and a peak incidence of childhood BL in the 5–9 year age group. They also confirm what has long been suspected concerning the geographic distribution, and illustrates how the geography is largely mediated by the endemicity of falciparum malaria; indeed, the importance of malarial infection as a co-factor in the occurrence of BL has been demonstrated at the individual level also [15]. Previous reports have shown high rates of incidence in Malawi, Uganda, and Ibadan (Nigeria) [4, 15], Cameroon [8], northern Tanzania [16] and western Kenya [1], while the incidence rates reported from Zimbabwe and South Africa have consistently been low [17], as was the frequency of BL among NHL cases in a large clinical series (487 cases) from South Africa and Zimbabwe [18].
Our estimates of incidence are based on data actually available from Africa, almost all of which are from population based cancer registries. Most of these cover urban populations, although malaria transmission intensity is highest in rural areas. It is therefore likely that incidence of BL is higher in rural that urban areas, and there is limited data to suggest that this is so [19]. It is thus possible that the calculated numbers of BL cases in Africa are an underestimate of the true burden of BL on the continent, although they are the best that can be made with data currently available.
It is rather surprising to note that the estimated incidence of childhood is moderately elevated in some North African countries, particularly in boys. Burkitt lymphoma has reported to be a common form of childhood NHL – especially in boys - in Egypt [20] and Algeria [21], with most cases being EBV positive, and BL was found to be a moderately common form of childhood cancer in case series from Tunisia, Sudan and Morocco [22]. The absence of holoendemic malaria suggests other pathogens or environmental factors are interacting with EBV to increase the risk for BL.
A recent study by Grande et al. [23] where both EBV+ and – BL tumours were sequenced, identified EBV infection as the BL driving phenotype not the geographic origin. However, because detection of EBV in tumours is not a diagnostic criterion, we were limited in this analysis of population based cancer registries to rely on geography and age to identify the EBV+ tumours. Importantly, in the Grande study, the majority of the EBV+ tumours sequenced were from childhood BL in tropical Africa.
Conclusions
In summary, using population based cancer registry data, we show that the burden of BL remains high in those parts of sub Saharan Africa where Falciparum malaria remains common. EBV is an etiological factor in more than 80% of cases (about 3200 in 2018).
Acknowledgements
We would like to thank all of the registries, members of the African Cancer Registry Network, for permission to access the AFCRN database to abstract the information on Burkitt Lymphoma presented in this paper. We also acknowledge the support of the Volkswagen Stiftung for financial support (Grant number: 94631) for the symposium “ Cancer Epidemiology meets Infectiology in Africa” held in Entebbe, Uganda, in October 2018, which provided a forum to bring together the researchers involved in this study. Our thanks are due to Jacques Ferlay (IARC) for help in the analysis of the data and Ms. Biying Liu (AFRCN) for administrative support.
Abbreviation
- ASR
Aged Standardlised Rate
- BL
Burkitt lymphoma
- eBL
Endemic type BL
- EBNA
EBV nuclear antigen
- EBV
Epstein Barr virus
- HIV
Human immunodeficiency virus
- IARC
International Agency for Research on Cancer
- ICD-O
International Classification of Diseases for Oncology
- NHL
Non Hodgkin lymphoma
- SSA
Sub-Saharan Africa
Authors’ contributions
LH analyzed the registry data regarding incidence of BL. MC abstracted the data on NHL and BL from the registry datasets contributing to Globocan 2018, RR and MO contributed to the Discussion of diagnosis of BL and EBV-related factors, DMP oversaw the analysis and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Funding
Lucia Hämmerl was supported by her home institute (Institute of Medical Epidemiology, Biometrics and Informatics, Medical Faculty of Martin Luther University Halle-Wittenberg Germany) to visit the University of Oxford to carry out the analytic work in this study.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the AFCRN database. Requests for access should be made via the AFCRN secretariat (https://afcrn.org/index.php/research/researches-and-collaborations).
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
No conflicts of interest.
Footnotes
Courtesy of Prof Georges Enow-Orok
Couretsy of Prof Ernest Belembaogo
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rainey JJ, Omenah D, Sumba PO, Moormann AM, Rochford R, Wilson ML. Spatial clustering of endemic Burkitt’s lymphoma in high-risk regions of Kenya. Int J Cancer. 2007;120:121–127. doi: 10.1002/ijc.22179. [DOI] [PubMed] [Google Scholar]
- 2.Stefan C, Bray F, Ferlay J, Liu B, Parkin DM. Cancer of childhood in sub-Saharan Africa. Ecancermedicalscience. 2017;11:755. 10.3332/ecancer.2017.755. [DOI] [PMC free article] [PubMed]
- 3.Molyneux EM, Rochford R, Griffin B, Newton R, Jackson G, Menon G, Harrison CJ, Israels T, Bailey S. Burkitt's lymphoma. Lancet. 2012;379:1234–1244. doi: 10.1016/S0140-6736(11)61177-X. [DOI] [PubMed] [Google Scholar]
- 4.Orem J, Mbidde EK, Lambert B, de Sanjose S, Weiderpass E. Burkitt's lymphoma in Africa, a review of the epidemiology and etiology. Afr Health Sci. 2007;7(3):166–175. doi: 10.5555/afhs.2007.7.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J. WHO classification of Tumours of Haematopoietic and lymphoid tissues 4th edition, vol. 2: International Agency for Research on Cancer Lyon; 2017. http://publications.iarc.fr/Book-And-Report-Series/Who-Iarc-Classification-Of-Tumours/Who-Classification-Of-Tumours-Of-Haematopoietic-And-Lymphoid-Tissues-2017.
- 6.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2018. 10.1002/ijc.31937. [DOI] [PubMed]
- 7.Budiongo AN, Ngiyulu RM, Lebwaze BM, Gini-Ehungu JL, Mafuta EM, Ekulu PM, Kabongo-Mpolesha JM, Aloni MN. Pediatric non-Hodgkin lymphomas: first report from Central Africa. Pediatr Hematol Oncol. 2015;32(4):239–249. doi: 10.3109/08880018.2015.1013231. [DOI] [PubMed] [Google Scholar]
- 8.Lewis N, Young J, Hesseling PB, McCormick P, Wright N. Epidemiology of Burkitt's lymphoma in Northwest Province, Cameroon, 2003-2010. Paediatr Int Child Health. 2012;32(2):82–85. doi: 10.1179/2046905511Y.0000000016. [DOI] [PubMed] [Google Scholar]
- 9.Doll R. & Smith PG. Comparison between registries: age standardised rates. In Waterhouse J, Muir C, Shanmugaratnam K & Powell J (eds.) Cancer incidence in five continents volume IV. IARC Scientific Publications 42. 1982, IARC, Lyon
- 10.Kelly G. L., Rickinson A. B. Burkitt Lymphoma: Revisiting the Pathogenesis of a Virus-Associated Malignancy. Hematology. 2007;2007(1):277–284. doi: 10.1182/asheducation-2007.1.277. [DOI] [PubMed] [Google Scholar]
- 11.Carbone A, Gloghini A, Dotti G. EBV-associated lymphoproliferative disorders: classification and treatment. Oncologist. 2008;13:577–585. doi: 10.1634/theoncologist.2008-0036. [DOI] [PubMed] [Google Scholar]
- 12.Rochford R, Cannon MJ, Moormann AM. Endemic Burkitt’s lymphoma: a polymicrobial disease? Nat Rev Microbiol. 2005;3:182–187. doi: 10.1038/nrmicro1089. [DOI] [PubMed] [Google Scholar]
- 13.The Malaria Atlas Project (https://map.ox.ac.uk/). Accessed 28 Feb 2019.
- 14.IARC Biological Agents. IARC Monogr Eval Carcinog Risks Hum. 2012;100(B):62–4. [PMC free article] [PubMed] [Google Scholar]
- 15.IARC Malaria and some polyomaviruses (SV40, BK, JC and Merkell cell viruses) IARC Monogr Eval Carcinog Risks Hum. 2013;104:41–120. [PMC free article] [PubMed] [Google Scholar]
- 16.Brubaker G, Geser A, Pike MC. Burkitt’s lymphoma in the North Mara District of Tanzania 1964–70: failure to find evidence of time-space clustering in a high risk isolated rural area. Br J Cancer. 1973;18:469–472. doi: 10.1038/bjc.1973.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stefan C, Bray F, Ferlay J, Liu B, Maxwell Parkin D. Cancer of childhood in sub-Saharan Africa. Ecancermedicalscience. 2017;11:755. 10.3332/ecancer.2017.755. eCollection 2017 [DOI] [PMC free article] [PubMed]
- 18.Perry AM, Perner Y, Diebold J, Nathwani BN, MacLennan KA, Müller-Hermelink HK, Bast M, Boilesen E, Armitage JO, Weisenburger DD. Non-Hodgkin lymphoma in southern Africa: review of 487 cases from the international non-Hodgkin lymphoma classification project. Br J Haematol. 2016;172(5):716–723. doi: 10.1111/bjh.13885. [DOI] [PubMed] [Google Scholar]
- 19.Biggar RJ, Nkrumah FK. Burkitt's lymphoma in Ghana: urban-rural distribution, time-space clustering and seasonality. Int J Cancer. 1979;23(3):330–336. doi: 10.1002/ijc.2910230310. [DOI] [PubMed] [Google Scholar]
- 20.Naresh KN, Advani S, Adde M, Aziz Z, Banavali S, Bhatia K, Belgaumi A, Ezzat A, Khaled H, Mokhtar N, Norton A, Rohatiner A, Sagar TG, Taciyliz N, Temmim L, Venkatesh C, Yan Tang J, Magrath I. Report of an international network of Cancer treatment and research workshop on non-Hodgkin's lymphoma in developing countries. Blood Cells Mol Dis. 2004;33(3):330–337. doi: 10.1016/j.bcmd.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Aboulola M, Boukheloua B, Ladjadj Y, Tazerout FZ. Burkitt's lymphoma in Algeria. In: Burkitt's Lymphoma: A Human Cancer Model (Eds: Lenoir GM, O'Conor GT, Olweny CLM) IARC Scientific Publication No. 60. pp 97-105. Lyon: International Agency for Research on Cancer; 1985. [PubMed]
- 22.Stiller CA, Parkin DM. International variations in the incidence of childhood lymphomas. Paediatr Perinat Epidemiol. 1990;4(3):303–324. doi: 10.1111/j.1365-3016.1990.tb00654.x. [DOI] [PubMed] [Google Scholar]
- 23.Grande BM, Gerhard DS, Jiang A, Griner NB, Abramson JS, Alexander TB, et al. Genome-wide discovery of somatic coding and non-coding mutations in pediatric endemic and sporadic Burkitt lymphoma. Blood. 2019;133(12):1313–24. doi: 10.1182/blood-2018-09-871418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the AFCRN database. Requests for access should be made via the AFCRN secretariat (https://afcrn.org/index.php/research/researches-and-collaborations).