Abstract
Background
Doxorubicin is currently the most effective chemotherapeutic drug used to treat breast cancer. It has, however, been shown that doxorubicin can induce drug resistance resulting in poor patient prognosis and survival. Studies reported that the interaction between signalling pathways can promote drug resistance through the induction of proliferation, cell cycle progression and prevention of apoptosis. The aim of this study was therefore to determine the effects of doxorubicin on apoptosis signalling, autophagy, the mitogen-activated protein kinase (MAPK)- and phosphoinositide 3-kinase (PI3K)/Akt signalling pathway, cell cycle control, and regulators of the epithelial-mesenchymal transition (EMT) process in murine breast cancer tumours.
Methods
A tumour-bearing mouse model was established by injecting murine E0771 breast cancer cells, suspended in Hank’s Balances Salt Solution and Corning® Matrigel® Basement Membrane Matrix, into female C57BL/6 mice. Fourty-seven mice were randomly divided into three groups, namely tumour control (received Hank’s Balances Salt Solution), low dose doxorubicin (received total of 6 mg/ml doxorubicin) and high dose doxorubicin (received total of 15 mg/ml doxorubicin) groups. A higher tumour growth rate was, however, observed in doxorubicin-treated mice compared to the untreated controls. We therefore compared the expression levels of markers involved in cell death and survival signalling pathways, by means of western blotting and fluorescence-based immunohistochemistry.
Results
Doxorubicin failed to induce cell death, by means of apoptosis or autophagy, and cell cycle arrest, indicating the occurrence of drug resistance and uncontrolled proliferation. Activation of the MAPK/ extracellular-signal-regulated kinase (ERK) pathway contributed to the resistance observed in treated mice, while no significant changes were found with the PI3K/Akt pathway and other MAPK pathways. Significant changes were also observed in cell cycle p21 and DNA replication minichromosome maintenance 2 proteins. No significant changes in EMT markers were observed after doxorubicin treatment.
Conclusions
Our results suggest that doxorubicin-induced drug resistance and tumour growth can occur through the adaptive role of the MAPK/ERK pathway in an effort to protect tumour cells. Previous studies have shown that the efficacy of doxorubicin can be improved by inhibition of the ERK signalling pathway and thereby treatment failure can be overcome.
Electronic supplementary material
The online version of this article (10.1186/s12885-019-5939-z) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, Doxorubicin, Drug resistance, Tumour growth, Signalling pathways, ERK
Background
Cancer is a major disease burden worldwide and the occurrence of cancer is expected to increase due to the increasing prevalence of lifestyle risk factors and the growth and aging of the population [1]. Based on Global Cancer Incidence, Mortality and Prevalence (GLOBOCAN) estimates, breast cancer was the most frequently diagnosed cancer and the leading cause of cancer deaths among females in 2012 worldwide [1]. Although significant progress has been made regarding treatment options for cancer patients, therapeutic resistance and toxicity of these drugs to normal tissue still remains a major problem. Resistance to chemotherapeutic drugs can cause treatment failure in over 90% of patients with metastatic cancer [2].
Doxorubicin (DXR) is part of the anthracycline family and is currently the most effective chemotherapeutic drug used to treat breast cancer [3, 4]. It has, however, been shown that DXR can induce drug resistance and even tumour growth resulting in poor patient prognosis and survival [5–7]. Although several mechanisms have been investigated, DXR resistance still remains a major unresolved issue in the treatment of cancer patients [8]. Studies reported that the interaction between signalling pathways can promote DXR resistance through the induction of proliferation, cell cycle progression and prevention of apoptosis [5, 9, 10].
The mitogen-activated protein kinase (MAPK)/ extracellular-signal-regulated kinase (ERK) and phosphoinositide 3-kinase (PI3K)/Akt pathways play an essential role in the regulation of proliferation, cell cycle progression, and apoptosis [9, 11]. The MAPK/ERK pathway has been shown to promote DXR resistance through its adaptive role in protecting cancer cells from oxidative stress [12]. Reactive oxygen species (ROS) generation following DXR treatment can also activate other MAPK pathways, including the c-jun N-terminal kinases (JNK) and p38 pathways [12]. The PI3K/Akt pathway can also induce chemo-resistance and promote tumorigenesis by phosphorylating various downstream substrates involved in cell survival, cell cycle, cell metabolism, gene transcription and protein synthesis [13].
In addition to the mechanism of DXR to induce apoptosis, DXR-mediated DNA damage can also induce cell cycle arrest [14]. This can occur through the activation of the tumour suppressor p53, which regulates the transcription of various genes, including p21 and p16, that are involved in cell cycle control, DNA repair and apoptosis [14, 15]. Defects in these regulators can lead to the failure of DXR to induce cell cycle arrest and thereby promote DXR resistance [16, 17].
Besides the effects of proliferation markers and cell cycle regulators on drug resistance, the epithelial-mesenchymal transition (EMT) process has also been shown to play a role in drug resistance by inhibiting apoptosis and preventing senescence [18]. Biomarkers of the EMT process include the cell surface protein, E-cadherin, cytoskeletal proteins, such as alpha smooth muscle actin (α-SMA) and Vimentin, and the transcription factor, Snail [19]. This process can be activated by the MAPK/ERK and PI3K/Akt signalling pathways, which further emphasises the interaction between various pathways and drug resistance [20].
Another process that can be involved in drug resistance is autophagy [21, 22]. Autophagy, also known as macroautophagy, plays a role in maintaining intracellular homeostasis and survival by degrading damaged proteins and organelles and recycling their components to regenerate metabolic precursors [21]. It has been shown that autophagy can drive cancer cells to acquired chemo-resistance by preventing cellular damage and protecting cancer cells against apoptosis [10, 23]. However, when autophagy is induced by excessive cellular stress it can lead to the upregulation of apoptosis and autophagy dependent cell death (ADCD) [10, 23].
Elucidating the mechanism by which these pathways promote drug resistance can improve the efficacy of chemotherapeutic drugs and overcome treatment failure. The aim of this study was therefore to determine the effects of doxorubicin on apoptosis signalling, autophagy, the MAPK- and PI3K/Akt signalling pathway, cell cycle control and regulators of the EMT process in murine breast cancer tumours. We compared the expression levels of markers involved in cell death and survival signalling pathways, by means of western blotting and fluorescence-based immunohistochemistry, in the breast tumours of mice treated with low and high doses of doxorubicin. Our results suggest that DXR-induced drug resistance and tumour growth can occur through the adaptive role of the MAPK/ERK pathway in an effort to protect tumour cells. Previous studies have shown that the efficacy of doxorubicin can be improved by inhibition of the ERK signalling pathway and thereby treatment failure can be overcome [6, 24–26].
Methods
Animal model
Ethical clearance was obtained from the Stellenbosch University Ethical Committee (no. SU-ACUM13–00027). Six-week old female wildtype C57BL/6 mice were obtained from the Tygerberg animal facility. The mice were kept under temperature controlled conditions, underwent a reverse dark-light cycle and were provided standard mouse pellets and tap-water ad libitum in individually ventilated cages (IVC) with autoclaved bedding at the Stellenbosch University animal facility.
Murine E0771 breast cancer cells, syngeneic to C57BL/6 mice, suspended in Hank’s Balances Salt Solution (HBSS, Sigma-Aldrich, MO, USA) were added to Corning® Matrigel® Basement Membrane Matrix (9.2 mg/ml protein, BD Bio-sciences, CA, USA) in a 1:1 ratio. When the mice reached an age of 10 weeks (21.9 g ± 0.25 g), 100 μl was injected subcutaneously at the fourth mammary fat pad of the mice to initiate tumour growth. Tumour growth was monitored every second day and length and width measurements were taken to calculate tumour volume (mm3) with the following equation: (length x width2)/2.
DXR treatment were initiated once the tumours were palpable (between day 6 and day 9). Forty-seven mice were randomly divided into three groups, namely TC (n = 15), LD-DXR (n = 16) and HD-DXR (n = 16) groups. The sample size were based on previously unpublished studies. Three doses of DXR were administered three days apart by means of intraperitoneal injection. The TC group received HBSS, the LD-DXR group received 2 mg/kg DXR (total of 6 mg/kg) and the HD-DXR group received 5 mg/kg DXR (total of 15 mg/kg). The dosages were based on previously unpublished studies.
The endpoint of the study was reached when the tumours reached a volume of 400 mm3. Mice were anaesthetised with Isofluorane (Isofor, Safeline Pharmaceuticals, RSA) before exsanguination. The tumours were then excised and cut into two parts to be used for western blotting and immunohistochemistry. The samples used for western blotting were snap-frozen in liquid nitrogen and stored at − 80 °C. The samples used for immunohistochemistry were mounted with tissue freezing medium (Leica Biosystems, Germany, UK) and frozen in ice-cold isopentane and stored at − 80 °C.
Western blots
Standard Radioimmunoprecipitation assay (RIPA) buffer was used to harvest protein lysates from tumour tissues while a Bradford assay was performed to determine protein concentration. Protein samples (50 μg), prepared with Laemmli’s sample buffer, were loaded onto 4–20% Criterion™ TGX Stain-Free™ Precast Gels (BioRad, CA, USA). Proteins were separated at 100 V for 10 min and 120 V for 60 min in Tris/Glycine/SDS running buffer (BioRad, CA, USA). Proteins were transferred onto Polyvinylidene difluoride (PVDF) membranes (Trans-Blot® Turbo RTA Midi PVDF transfer kit, BioRad, CA, USA) with the Trans-Blot® Turbo Transfer System (BioRad, CA, USA) with the conditions of 25 V, 1.0A, 30 min. The Stain-Free™ properties of the membranes were utilized on the Chemidoc™ MP System (BioRad, CA, USA) to determine the total protein intensities of each membrane. Membranes were blocked in 5% milk for 1 h and incubated in primary antibody, prepared in tris-buffered saline with tween 20 (TBS-T), at 4 °C overnight. On the following day membranes were incubated in secondary antibody, prepared in TBS-T, for 1 h at RT. Antibody details are listed in the Additional file 1. After incubation, membranes were developed on the ChemiDoc™ MP System with Clarity™ Electrochemiluminescence (ECL) Substrate (BioRad, CA, USA).
Fluorescence-based immunohistochemistry
Tumour tissues were sectioned into 8 μm sections with a Leica CM110 Cryostat (Leica Biosystems, Germany, UK). The sections were fixed in 100% ice-cold methanol for 5 min and blocked in 2.5% goat serum, prepared in phosphate-buffered saline (PBS), for 1 h. Sections were covered with p-ERK primary antibody, prepared in PBS, and incubated at 4 °C overnight or with p21 primary antibody, prepared in 2.5% goat serum, and incubated for 1 h at RT. The sections for the secondary antibody control were covered in PBS. On the following day sections were covered in fluorescein isothiocyanate (FITC) secondary antibody, prepared in PBS or 2.5% goat serum, and incubated for 1 h at RT. Antibody details are listed in the Additional file 1. The sections were stained with 10 μg/ml Hoechst 33342 (Sigma-Aldrich, MO, USA), prepared in PBS (1:200), for 15 min at RT. Coverslips were mounted onto the slides with Dako Fluorescence Mounting Medium (Agilent Technologies, CA, USA). Slides were protected from light and stored at − 20 °C until imaging. Five images per section were obtained with the Nikon NIS-Element BR v4.10.00 imaging software on the Nikon Eclipse E400 microscope using the 40x objective. The 405 nm and 488 nm excitation lasers for Hoechst and FITC, detected in the emission ranges of 450–490 nm and 520–540 nm, respectively, were used.
Statistical analyses
An analysis of covariance (ANCOVA) (p < 0.05) was performed to compare the tumour volume between the TC (n = 15), LD-DXR (n = 16) and HD-DXR (n = 16) groups. The western blot experiments were conducted with technical repeats of n = 2 and biological repeats of n = 8. Bio-Rad Image Lab™ software v5.1 was used for normalization of the protein specific intensities against total protein intensities. Results were analysed in GraphPad Prism v7.0 by performing a one-way analysis of variance (ANOVA) with Bonferonni post hoc test (p < 0.05). The mean values ± standard error of the mean was reported. The fluorescent-based immunohistochemistry experiments were conducted with biological repeats of n = 8. Five images per section were analysed in Image J software v1.52a. The following equation was used to calculate the corrected total cryosection fluorescence: mean of integrated density – (mean of area of selected cell x mean fluorescence of background readings).
Results
Higher tumour growth rate was observed after DXR treatment
A tumour-bearing mouse model was established by injecting murine breast cancer cells, suspended in Corning® Matrigel®, into female mice. When the tumours were palpable, the mice either received a vehicle treatment, a low dose of DXR or a high dose of DXR treatment. The tumours grew rapidly over the study period and showed resistance to both DXR doses. Analyses revealed that both low dose (LD)-DXR and high dose (HD)-DXR groups has a significantly increased tumour volume when compared to the tumour control (TC) group (Fig. 1). No significant differences in tumour volume were observed between the LD-DXR and HD-DXR groups (Fig. 1).
Fig. 1.
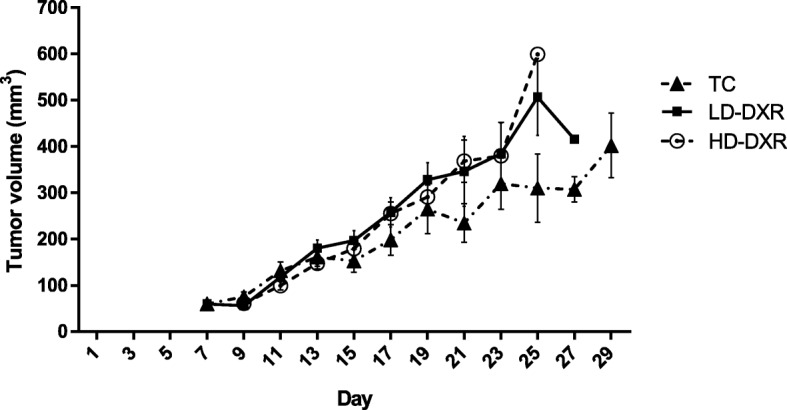
Effects of DXR treatment on the average tumour volume (mm3). DXR treatment were initiated between day 6 and day 9. Three doses of DXR were administered three days apart. Error bars indicate the standard error of the mean (n = 15 (TC group), n = 16 (LD-DXR and HD-DXR groups)). The slope of the regression lines from the LD-DXR and HD-DXR groups were significantly different compared to the TC group. [31]
Inability of DXR to induce apoptosis or autophagy, indicating the occurrence of DXR resistance
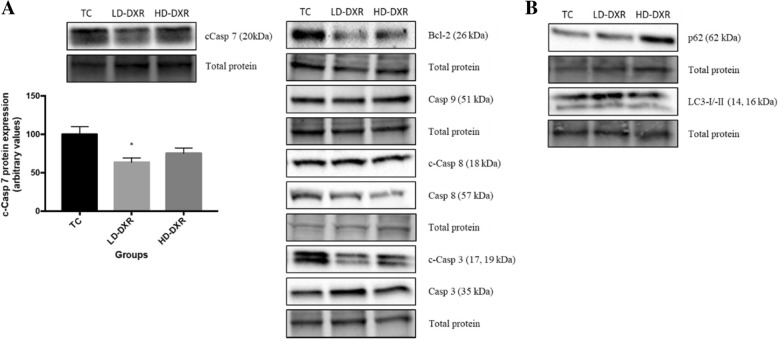
To determine whether apoptosis was induced after DXR treatment, western blot experiments were performed to compare the protein expression levels of different apoptotic markers, including B-cell lymphoma 2 (Bcl-2), caspase (Casp)-9, Casp-8, Casp-3 and Casp-7 between the different groups. c-Casp 7 protein expression decreased significantly in tumour-bearing mice treated with a low dose of DXR, whereas a non-significant decrease was observed in mice treated with a high dose of DXR (Fig. 2a). No significant differences in the other apoptotic markers were observed between the different groups. Bcl-2 protein expression showed a slight decrease in the LD-DXR and HD-DXR groups compared to the TC group (Fig. 2a). Casp-8 showed a greater decrease in protein expression levels after DXR treatment, than Casp-9 (Fig. 2a). The ratio between cleaved Casp-3 and Casp-3 showed a decrease in both treatment groups compared to the control, with the LD-DXR group showing the lowest protein expression (results not shown). To determine whether autophagy was induced after DXR treatment, western blot experiments were performed to compare the protein expression levels of markers, p62 and microtubule-associated protein light chain 3 (LC3)-I/−II, between the TC, LD-DXR and HD-DXR groups. No significant differences were again observed between the different groups (Fig. 2b).
Fig. 2.
a Protein expression of c-Casp 7 between the TC, LD-DXR and HD-DXR groups (n = 8). * - significantly different compared to TC group (p < 0.05). Representative images of Bcl-2, Casp 9, c-Casp 8, Casp 8, c-Casp 3 and Casp 3 protein expression between the TC, LD-DXR and HD-DXR groups (n = 8). b Representative images of p62 and LC3-I/−II protein expression between the TC, LD-DXR and HD-DXR groups (n = 8)
The MAPK/ERK pathway had a greater effect on DXR resistance than the PI3K/Akt pathway and other MAPK pathways
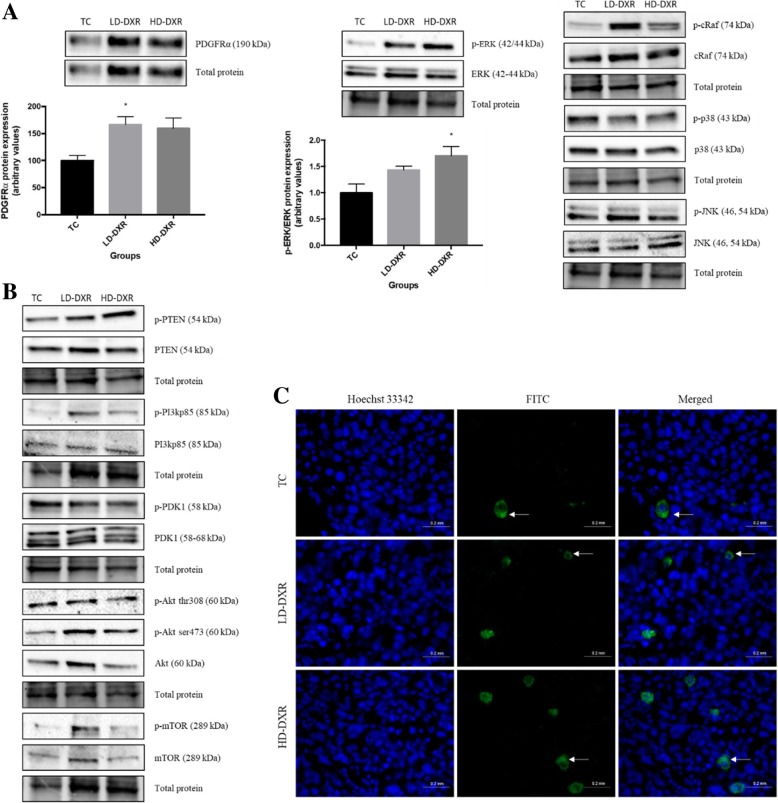
To determine the effects of the MAPK/ERK signalling pathway on DXR resistance, we performed western blot experiments to compare the protein expression levels of markers, platelet-derived growth factor receptor alpha (PDGFRα), c-Raf and ERK, between the different groups. We also assessed other MAPK pathways, including the p38 and JNK pathways. PDGFRα protein expression increased significantly in tumour-bearing mice treated with a low dose of DXR, whereas a non-significant increase was observed in mice treated with a high dose of DXR (Fig. 3a). The ratio between phosphorylated ERK (p-ERK) and total ERK showed a gradual increase in protein expression levels as the dosage of DXR increased, with the HD-DXR group showing a significant increase compared to the TC group (Fig. 3a). Fluorescence-based immunohistochemistry was performed to support the western blot results of p-ERK. However, no significant differences in p-ERK signal was observed between the different groups (Fig. 3c). No significant differences were observed in the protein expression levels of c-Raf, p38 and JNK between the different groups (Fig. 3a). To determine the effects of the PI3K/Akt/mammalian target of rapamycin (mTOR) pathway on DXR resistance, western blot experiments were performed to compare the protein expression level of markers, phosphatase and tensin homolog (PTEN), PI3Kp85, phosphoinositide-dependent kinase-1 (PDK1), Akt and mTOR, between the different groups. There were, however, no significant differences in protein expression observed between the different groups (Fig. 3b).
Fig. 3.
a Protein expression of PDGFRα and p-ERK/ERK between the TC, LD-DXR and HD-DXR groups (n = 8). * - significantly different compared to TC group (p < 0.05). Representative images of p-cRaf, cRaf, p-p38, p38, p-JNK and JNK protein expressions between the TC, LD-DXR and HD-DXR groups (n = 8). b Representative images of p-PTEN, PTEN, p-PI3Kp85, PI3Kp85, p-PDK1, PDK1, p-Akt thr308, p-Akt ser473, Akt, p-mTOR and mTOR protein expressions between the TC, LD-DXR and HD-DXR groups (n = 8). c Representative images of p-ERK-FITC signal in tumour tissues following DXR treatment (n = 8). Hoechst 33342 – nuclei; FITC – p-ERK; solid arrows – localised areas of intense signal in cytoplasm; scale = 20 μm, 40x objective
Changes observed in cell cycle regulators after DXR treatment
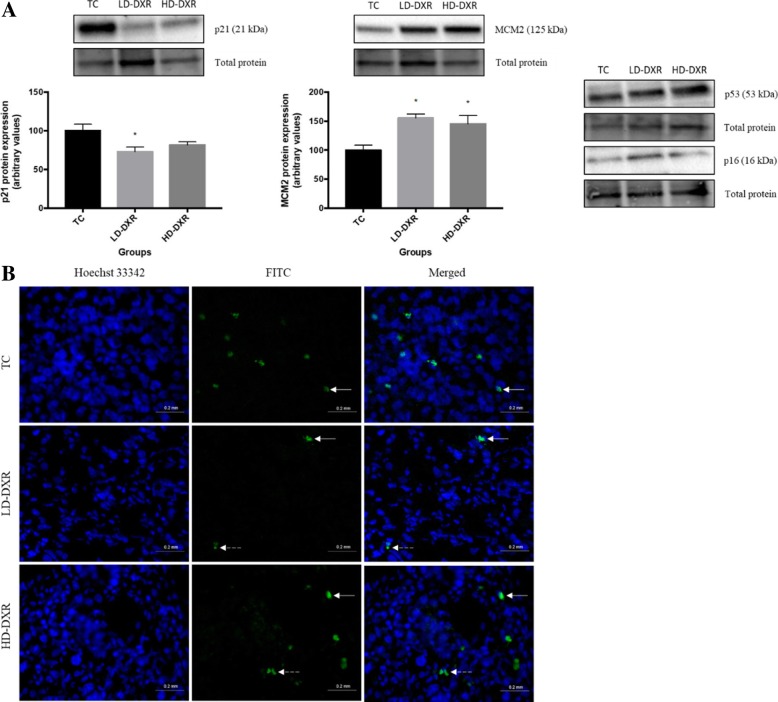
Western blot experiments were performed to compare the protein expression levels of different markers involved in the cell cycle and DNA replication between the TC, LD-DXR and HD-DXR groups. These markers included the tumour suppressor p53, cyclin-dependent kinase (CDK) inhibitors, p21 and p16, and the proliferation marker, minichromosome maintenance 2 (MCM2). A significant reduction in p21 expression was observed in the LD-DXR group compared to the TC group, while a non-significant reduction in p21 expression was observed in the HD-DXR group (Fig. 4a). Fluorescence-based immunohistochemistry was performed to support the western blot results of p21 qualitatively (Fig. 4b). Cytoplasmic signal of inactive p21 was also observed in the LD-DXR and HD-DXR groups (Fig. 4b). MCM2 protein expression increased significantly in both treatment groups compared to the control group (Fig. 4a). No significant differences were observed in the protein expression levels of p53 and p16 between the different groups (Fig. 4a).
Fig. 4.
a Protein expression of p21 and MCM2 between the TC, LD-DXR and HD-DXR groups (n = 8). * - significantly different compared to TC group (p < 0.05). Representative images of p16 and p53 protein expressions between the TC, LD-DXR and HD-DXR groups (n = 8). b Representative images of p21-FITC signal and localisation in tumour tissues following DXR treatment (n = 8). Hoechst 33342 – nuclei; FITC – p21; solid arrows – localised areas of intense signal in nuclei; dashed arrows- localised areas of intense signal in cytoplasm; scale = 20 μm, 40x objective
EMT did not occur during DXR-induced drug resistance and tumour growth
Western blot experiments were performed to compare the protein expression levels of the EMT markers, α-SMA, E-cadherin, Snail and Vimentin, between the TC, LD-DXR and HD-DXR groups. No significant differences were however observed between the different groups (Fig. 5).
Fig. 5.
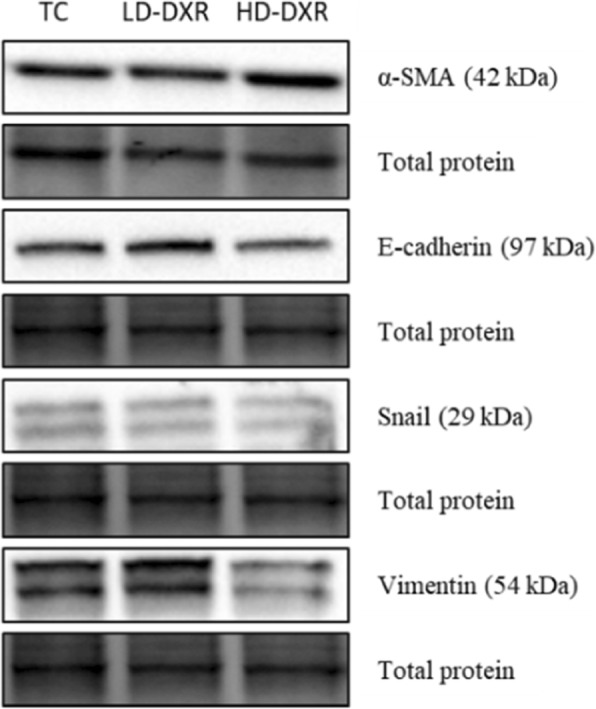
Representative images of α-SMA, E-cadherin, Snail and Vimentin protein expressions between the TC, LD-DXR and HD-DXR groups (n = 8)
Discussion
DXR can induce drug resistance and even tumour growth resulting in poor patient prognosis and survival [5–7]. Although several mechanisms have been investigated, DXR resistance still remains a major unresolved issue in the treatment of cancer patients [8]. Studies have shown that the interaction between signalling pathways can promote drug resistance through the induction of proliferation, cell cycle progression and prevention of apoptosis [5, 9, 10].
The aim of this study was therefore to determine the effects of doxorubicin on apoptosis signalling, autophagy, the MAPK- and PI3K/Akt signalling pathway, cell cycle control, and regulators of the EMT process in murine breast cancer tumours. We compared the expression levels of markers involved in cell death and survival signalling pathways, by means of western blotting and fluorescence-based immunohistochemistry, in the breast tumours of mice treated with low and high doses of doxorubicin.
To determine whether cell death was induced after DXR treatment we assessed the expression levels of different markers involved in apoptosis and autophagy. A significant decrease in c-Casp 7 protein expression was observed in tumour-bearing mice treated with a low dose of DXR, whereas a non-significant decrease was observed in mice treated with a high dose of DXR (Fig. 2a). An increase in p62 protein expression was observed in the HD-DXR group, however, not significant (Fig. 2b). No significant changes in LC3 protein expression was observed after DXR treatment (Fig. 2b). We therefore showed that DXR failed to induce cell death through apoptosis or ADCD, indicating the occurrence of drug resistance. It is well known that chemo-resistance is associated with the inability of chemotherapeutic drugs to induce cell death [10, 23].
Interactions between MAPK/ERK and PI3K/Akt signalling pathways play an essential role in the regulation of proliferation, cell cycle progression and apoptosis [9, 11]. The mechanisms by which the MAPK/ERK and PI3K/Akt signalling pathways regulate DXR-induced cell death and drug resistance are, however, controversial [5]. Some studies have shown that sustained ERK activation contributes to DXR-induced cell death and can be negatively regulated by the PI3K/Akt pathway [5, 6].
In contrast, other studies have reported that ERK activation can protect cells from DXR-induced cell death [6, 12]. The MAPK/ERK pathway has been shown to promote DXR resistance through its adaptive role in protecting cells from oxidative stress [12]. ROS generation following DXR treatment can activate PDGFRα, thereby increasing the activation of the MAPK/ERK pathway as well as other MAPK pathways, including the JNK and p38 pathways [12].
A study done by Jin et al. showed that the PI3K/Akt pathway had a greater effect on chemo-resistance than the MAPK pathway in breast cancer cells [27]. We assessed the expression levels of markers associated with the MAPK pathway, such as PDGFRα, cRaf, ERK, p38 and JNK, and markers associated with the PI3K/Akt pathway, such as PTEN, PI3Kp85, PDK1, Akt, and mTOR, to determine which pathway was responsible for DXR-induced drug resistance and tumour growth in the tumour-bearing mouse model. We observed increased protein expression for both PDGFRα and p-ERK and no significant changes in markers of the PI3K/Akt pathway and other MAPK pathways, indicating that the MAPK/ERK pathway had a greater effect on DXR resistance (Fig. 3).
In addition to the mechanism of DXR to induce apoptosis, DXR-mediated DNA damage can also induce cell cycle arrest [14]. This can occur through the activation of the tumour suppressor p53, which regulates the transcription of various genes involved in cell cycle control, DNA repair and apoptosis [14]. CDK inhibitors, such as p21 and p16, are major targets of p53 and is known to function as mediators of DXR-induced cell cycle arrest [14–17].
We therefore assessed the expression level of different cell cycle regulators, such as p53, p21 and p16, to determine whether DXR was able to induce cell cycle arrest. A significant reduction in p21 expression was observed in the LD-DXR group compared to the TC group, while a non-significant reduction in p21 expression was observed in the HD-DXR group (Fig. 4a). p21 is an inhibitor of CDKs and activation of this protein can induce cell cycle arrest. Downregulation of this protein may therefore indicate that DXR was unable to induce cell cycle arrest. Hwang et al. showed that sustained activation of ERK2 can downregulate p21, resulting in cell cycle progression [28]. We therefore suggest that the significant increase observed in ERK expression after DXR treatment (Fig. 3a), contributed to the reduction in p21 expression (Fig. 4a).
MCM2 is a marker of proliferation and plays an essential role in DNA replication [29]. The increase in MCM2 expression observed after DXR treatment indicated that cell cycle arrest did not occur and that the cancer cells were actively proliferating (Fig. 3a). This increase in proliferation supports the increased tumour growth observed in both low and high doses of DXR.
It has been suggested that EMT can also promote chemo-resistance in breast cancer tumours [18, 30]. Since we did not observe any significant changes in the expression levels of the cell surface protein, E-cadherin, the cytoskeletal proteins, such as α-SMA and Vimentin, and the transcription factor, Snail, we propose that EMT did not contribute to the DXR-resistance and tumour growth observed in our study (Fig. 5).
Conclusions
In conclusion, our results suggest that DXR-induced drug resistance and tumour growth can occur through the adaptive role of the MAPK/ERK pathway in an effort to protect tumour cells. Previous studies have shown that the efficacy of doxorubicin can be improved by inhibition of the ERK signalling pathway and thereby treatment failure can be overcome [6, 24–26].
Additional file
Table S1. Primary and secondary antibody details. Table S2. Characteristics of mice. (DOCX 28 kb)
Acknowledgements
Not applicable
Abbreviations
- ADCD
Autophagy dependent cell death
- ANCOVA
Analysis of covariance
- ANOVA
Analysis of variance
- Bcl-2
B-cell lymphoma 2
- Casp
Caspase
- CDK
Cyclin-dependent kinase
- DXR
Doxorubicin
- ECL
Electrochemiluminescence
- EMT
Epithelial-mesenchymal transition
- ERK
Extracellular-signal-regulated kinase
- FITC
Fluorescein isothiocyanate
- GLOBOCAN
Global cancer incidence, mortality and prevalence
- HBSS
Hank’s balanced salt solution
- HD-DXR
High dose DXR
- JNK
c-jun N-terminal kinases
- LC3
microtubule-associated protein light chain 3
- LD-DXR
Low dose DXR
- MAPK
Mitogen-activated protein kinase
- MCM2
Minichromosome maintenance 2
- mTOR
mammalian target of rapamycin
- PBS
Phosphate-buffered saline
- PDGFRα
Platelet-derived growth factor receptor alpha
- PDK1
Phosphoinositide-dependent kinase-1
- PI3K
Phosphoinositide 3-kinase
- PTEN
Phosphatase and tensin homolog
- PVDF
Polyvinylidene difluoride
- RIPA
Radioimmunoprecipitation assay
- ROS
Reactive oxygen species
- TBS-T
Tris-buffered saline with tween 20
- TC
Tumour control
- α-SMA
Alpha smooth muscle actin
Authors’ contributions
All authors have read and approved the manuscript. CC performed the western blot and fluorescence-based immunohistochemistry techniques and analysed and interpreted the results. CC was also a major contributor in writing the manuscript. TD co-supervised this study, performed the animal model and reviewed the manuscript. AI helped with the analysis of the fluorescence-based immunohistochemistry results. GVN helped with the animal model. SH co-supervised this study. AME supervised this study and reviewed the manuscript.
Funding
This study was supported financially by the Harry Crossley Foundation (HCF); National Research Foundation (NRF) [Grant No: 99093]; and the South African Medical Research Council (SAMRC). No specific funding was received for this study.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Ethical clearance for the in vivo study was obtained from the Stellenbosch University Ethical Committee (no. SU-ACUM13–00027 and no. ACU-2017-1758). Institutional and international ethical guidelines were applied with respect to the handling of experimental animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Claudia Christowitz, Email: claudiac@sun.ac.za.
Tanja Davis, Email: tadavis@sun.ac.za.
Ashwin Isaacs, Email: awisaacs@sun.ac.za.
Gustav van Niekerk, Email: gustavvn@sun.ac.za.
Suzel Hattingh, Email: smhatt@sun.ac.za.
Anna-Mart Engelbrecht, Email: ame@sun.ac.za.
References
- 1.Torre Lindsey A., Bray Freddie, Siegel Rebecca L., Ferlay Jacques, Lortet-Tieulent Joannie, Jemal Ahmedin. Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205(2):275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- 3.Barrett-Lee P. J., Dixon J. M., Farrell C., Jones A., Leonard R., Murray N., Palmieri C., Plummer C. J., Stanley A., Verrill M. W. Expert opinion on the use of anthracyclines in patients with advanced breast cancer at cardiac risk. Annals of Oncology. 2009;20(5):816–827. doi: 10.1093/annonc/mdn728. [DOI] [PubMed] [Google Scholar]
- 4.Shi Y, Bieerkehazhi S, Ma H. Next-generation proteasome inhibitor oprozomib enhances sensitivity to doxorubicin in triple-negative breast cancer cells. Int J Clin Exp Pathol. 2018;11(5):2347–2355. [PMC free article] [PubMed] [Google Scholar]
- 5.Lee ER, Kim JY, Kang YJ, Ahn JY, Kim JH, Kim BW, et al. Interplay between PI3K/Akt and MAPK signaling pathways in DNA-damaging drug-induced apoptosis. Biochim Biophys Acta - Mol Cell Res. 2006;1763(9):958–968. doi: 10.1016/j.bbamcr.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Shukla A, Hillegass JM, MacPherson MB, Beuschel SL, Vacek PM, Pass HI, et al. Blocking of ERK1 and ERK2 sensitizes human mesothelioma cells to doxorubicin. Mol Cancer [Internet] BioMed Central Ltd. 2010;9(314):1–13. doi: 10.1186/1476-4598-9-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Lu Y, Liang K, Liu B, Fan Z. Differential responses to doxorubicin-induced phosphorylation and activation of Akt in human breast cancer cells. Breast Cancer Res. 2005;7(5):589–597. doi: 10.1186/bcr1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith L, Watson MB, O’Kane SL, Drew PJ, Lind MJ, Cawkwell L. The analysis of doxorubicin resistance in human breast cancer cells using antibody microarrays. Mol Cancer Ther [Internet] 2006;5(8):2115–2120. doi: 10.1158/1535-7163.MCT-06-0190. [DOI] [PubMed] [Google Scholar]
- 9.Abrams SL, Steelman LS, Shelton JG, Wong W, Chappell WH, Bäsecke J, et al. The Raf/MEK/ERK pathway can govern drug resistance , apoptosis and sensitivity to targeted therapy. Cell Cycle. 2010;9(9):1781–1791. doi: 10.4161/cc.9.9.11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohammad RM, Muqbil I, Lowe L, Yedjou C, Hsu HY, Lin LT, et al. Broad targeting of resistance to apoptosis in cancer. Semin Cancer Biol [Internet] Elsevier Ltd. 2015;35:S78–103. doi: 10.1016/j.semcancer.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, et al. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17(3):590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 12.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EWT, Chang F, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta - Mol Cell Res. 2007;1773(8):1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West KA, Castillo SS, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat. 2002;5(6):234–248. doi: 10.1016/S1368-7646(02)00120-6. [DOI] [PubMed] [Google Scholar]
- 14.Lüpertz R, Wätjen W, Kahl R, Chovolou Y. Dose- and time-dependent effects of doxorubicin on cytotoxicity, cell cycle and apoptotic cell death in human colon cancer cells. Toxicology. 2010;271(3):115–121. doi: 10.1016/j.tox.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Park M-T, Lee S-J. Cell cycle and cancer J Biochem Mol Biol. 2003;36(1):60–65. doi: 10.5483/bmbrep.2003.36.1.060. [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Bishop WR, Liu M. Differential effects of cell cycle regulatory protein p21WAF1/Cip1on apoptosis and sensitivity to cancer chemotherapy. Drug Resist Updat. 2003;6(4):183–195. doi: 10.1016/S1368-7646(03)00044-X. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt CA, Fridman JS, Yang M, Lee S, Baranov E, Hoffman RM, et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell. 2002;109(3):335–346. doi: 10.1016/S0092-8674(02)00734-1. [DOI] [PubMed] [Google Scholar]
- 18.Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta - Rev Cancer [Internet] Elsevier BV. 2009;1796(2):75–90. doi: 10.1016/j.bbcan.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Scanlon CS, Van Tubergen EA, Inglehart RC, Silva NJ. Biomarkers of epithelial- mesenchymal transition in squamous cell carcinoma. J Dent Res. 2013;92(2):114–121. doi: 10.1177/0022034512467352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalluri R, Weinberg R. a. the basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun K, Deng W, Zhang S, Cai N, Jiao S, Song J, et al. Paradoxical roles of autophagy in different stages of tumorigenesis: Protector for normal or cancer cells. Cell Biosci [Internet]. Cell & Bioscience; 2013;3(1):1. Available from: Cell & Bioscience. [DOI] [PMC free article] [PubMed]
- 22.Choi KS. Autophagy and cancer. Exp Mol Med. 2012;44(2):109–120. doi: 10.3858/emm.2012.44.2.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, et al. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 2013;4:10):1–10)12. doi: 10.1038/cddis.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Franklin RA, Montalto G, et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR Cascade inhibitors: how mutations can result in therapy resistance and how to overcome resistance. Oncotarget [Internet] 2012;3(10):1068–1111. doi: 10.18632/oncotarget.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheng Y, You Y, Chen Y. Dual-targeting hybrid peptide-conjugated doxorubicin for drug resistance reversal in breast cancer. Int J Pharm [Internet] Elsevier BV. 2016;512:1):1–1)13. doi: 10.1016/j.ijpharm.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Chen T, Wang C, Liu Q, Meng Q, Sun H, Huo X, et al. Dasatinib reverses the multidrug resistance of breast cancer MCF-7 cells to doxorubicin by downregulating P-gp expression via inhibiting the activation of ERK signaling pathway. Cancer Biol Ther. 2015;16(1):106–114. doi: 10.4161/15384047.2014.987062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin W, Wu L, Liang K, Liu B, Lu Y, Fan Z. Roles of the PI-3K and MEK pathways in Ras-mediated chemoresistance in breast cancer cells. Br J Cancer. 2003;89(1):185–191. doi: 10.1038/sj.bjc.6601048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang CY, Lee C, Kwon K. Extracellular Signal-Regulated Kinase 2-Dependent Phosphorylation Induces Cytoplasmic Localization and Degradation of p21 Cip1. 2009;29(12):3379–3389. [DOI] [PMC free article] [PubMed]
- 29.Abe S, Yamamoto K, Kurata M, Abe-Suzuki S, Horii R, Akiyama F, et al. Targeting MCM2 function as a novel strategy for the treatment of highly malignant breast tumors. Oncotarget [Internet]. 2015;6(33). Available from: http://www.oncotarget.com/index.php?journal=oncotarget&page=article&op=view&path%5B%5D=5408&path%5B%5D=14102. [DOI] [PMC free article] [PubMed]
- 30.Drasin DJ, Robin TP, Ford HL. Breast cancer epithelial-to-mesenchymal transition: examining the functional consequences of plasticity. Breast Cancer Res. 2011;13(6):226. doi: 10.1186/bcr3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis Tanja, van Niekerk Gustav, Peres Jade, Prince Sharon, Loos Ben, Engelbrecht Anna-Mart. Doxorubicin resistance in breast cancer: A novel role for the human protein AHNAK. Biochemical Pharmacology. 2018;148:174–183. doi: 10.1016/j.bcp.2018.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primary and secondary antibody details. Table S2. Characteristics of mice. (DOCX 28 kb)
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.