Abstract
Background
For brain metastases from non-specific primary tumors, the most frequently used and validated clinical prognostic assessment tool is Karnofsky performance status (KPS). Given the lack of prognostic factors of brain metastases from colorectal cancer (CRC) other than KPS, this study aimed to identify new prognostic factors.
Methods
This retrospective cohort study was conducted at a tertiary care cancer center. Subjects were patients with brain metastases from CRC among all patients who received initial treatment for CRC at the National Cancer Center Hospital from 1997 to 2015 (n = 7147). Prognostic clinicopathological variables for overall survival (OS) were investigated.
Results
There were 68 consecutive patients with brain metastases from CRC, corresponding to 1.0% of all patients with CRC during the study period. Median survival time was 6.8 months. One-year and 3-year OS rates were 28.0 and 10.1%, respectively. Among the six covariates tested (age, KPS, presence of extracranial metastases, control of primary lesion, number of brain metastases, and history of chemotherapy), multivariate analysis revealed KPS (score ≥ 70), number of brain metastases (1–3), and no history of chemotherapy to be independent factors associated with better prognosis.
Conclusions
In addition to KPS, the number of brain lesions and history of chemotherapy were independent prognostic factors for OS in patients with brain metastases from CRC. An awareness of these factors may help gastrointestinal surgeons make appropriate choices in the treatment of these patients.
Keywords: Brain metastases, Colorectal cancer, Karnofsky performance status
Background
Brain metastases, the most common intracranial tumors in adults, accounts for more than half of all brain tumors [1, 2]. The incidence of brain metastases is on the rise, likely due to improved detection of small metastases by magnetic resonance imaging and improved management of extracerebral disease by progress of systemic therapy. The incidence of brain metastases by origin was reported as follows [1, 2]; lung cancer – 16 to 20%, melanoma – 7%, renal cell cancer – 7 to 10%, breast cancer – 5%, and colorectal cancer (CRC) – 1 to 2%.
Regarding prognostic factors of brain metastases, the Radiation Therapy Oncology Group (RTOG) proposed and validated recursive partitioning analysis (RPA) using Karnofsky performance status (KPS) score, which reflects a patient’s functional status [3, 4]. RPA consists of the following three classes: Class 1: patients with a KPS score ≥ 70 (score of 70: cares for self) [5] and age < 65 years with controlled primary tumor and no extracranial metastases; Class 3: KPS score < 70; and Class 2: all others [3]. KPS score, age, adequate control of primary tumor, and presence of extracranial metastases are currently considered reliable prognostic factors of brain metastases from non-specific primary cancer [3, 4, 6–9]. With respect to the establishment of criteria by origin, the diagnosis-specific graded prognostic assessment (DS-GPA), which adds the number of brain metastases to the four prognostic factors reported by RPA as covariates for multivariate analysis, was proposed and validated for non-small cell lung cancer, breast cancer, renal cell carcinoma, melanoma, and gastrointestinal cancer [10, 11]. In the DS-GPA model, KPS was the only significant prognostic factor for gastrointestinal cancer, which includes CRC, gastric cancer, and esophageal cancer [10, 12, 13].
Since survival following the treatment of brain metastases is highly variable and partly dependent on the clinical course of the primary tumor, investigating prognostic factors of brain metastases from CRC is important. While a number of reports have examined prognostic factors of brain metastases from CRC [14–18], these studies did not adopt KPS as a covariate, which as mentioned above is a significant prognostic factor in the DS-GPA model. To this end, the present study aimed to investigate prognostic factors of brain metastases from CRC, including KPS as an essential covariate, in order to provide insight that could help in the development of appropriate treatment strategies for brain metastases.
Methods
Patient selection and data
The present study was retrospective in design. Inclusion criteria were patients with synchronous or metachronous brain metastases of colorectal adenocarcinoma among all patients who received initial treatment for CRC at the National Cancer Center Hospital from January 1997 to December 2015. During the study period, there were 5894 patients with Stage I/II/III CRC and 1153 patients with Stage IV CRC (Fig. 1). Targeted molecular agents were introduced in Japan for use in systemic chemotherapy for metastatic CRC during the study period (i.e., in 2007). Thus, we divided the study period into two halves (first half, 1997–2005; second half: 2006–2015).
Fig. 1.
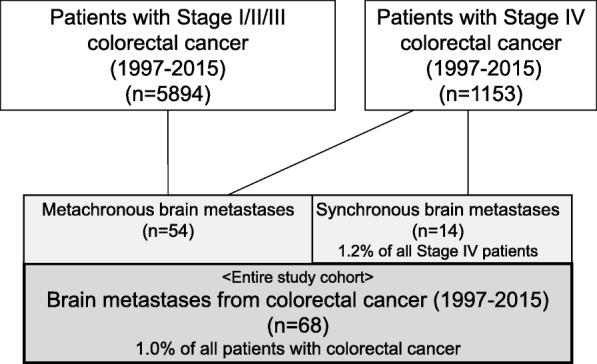
The study cohort. The final study population consisted of 68 patients with brain metastases who underwent initial treatment for colorectal cancer at the National Cancer Center Hospital from 1997 to 2015. The 68 patients account for 1.0% of all patients with colorectal cancer during the study period
The following parameters at the time of diagnosis of brain metastases were assessed retrospectively: sex, age, KPS score, number of brain metastases, maximum diameter of brain metastases, location of brain lesions (supratentorial or infratentorial), treatment procedure for brain metastases and parameters related to the primary tumor such as initial treatment period, time interval between diagnosis of primary tumor and brain metastases, location of primary tumor [19], presence of extracranial metastases at the time of diagnosis of brain metastases, control of primary tumor, and history of systemic chemotherapy before the development of brain metastases.
This study was approved by the Institutional Review Board (IRB) of the National Cancer Center Hospital (IRB code: 2017–437).
Definition of Karnofsky performance status
KPS is a standard tool for measuring the ability of cancer patients to perform ordinary tasks [20]. It provides a comprehensive score that reflects a patient’s functional status, rated on a 11-point scale from 0 (death) to 100 (normal: no complaints, no evidence of disease). A higher score reflects a better ability to carry out daily activities. The cut-off identified in the RPA by the RTOG was between a score of 70 (cares for self but unable to carry on normal activity or to do active work) and 60 (requires occasional assistance but is able to care for most of personal needs).
Treatment of brain metastases from colorectal cancer
Current treatment options for brain metastases from CRC include surgical resection, whole-brain radiotherapy (WBRT), stereotactic radiotherapy (SRT), which includes stereotactic radiosurgery and fractionated stereotactic radiotherapy, and chemotherapy, alone or in combination. In general, surgical resection is performed for a single large (typically > 3 cm) brain metastasis with massive edema or when the metastasis is located in the eloquent area. Stereotactic radiosurgery using the Gamma Knife® or CyberKnife® is usually indicated for oligometastases of small sizes up to 3 cm. In fractionated stereotactic radiotherapy, which is typically performed for larger-sized brain metastases that cannot be handled by stereotactic radiosurgery, patients are treated with the CyberKnife® using a prescribed dose of 27–35 Gy delivered in 3–5 fractions in the tumor periphery. WBRT is selected for patients with multiple metastases or large-sized oligometastases with uncontrolled extracranial metastases, and/or those with poor performance status. Typical WBRT consists of 30 Gy in 10 fractions to the isocenter, delivered five times weekly with a linear accelerator.
Statistical analysis
Estimation of overall survival (OS) and multivariate analysis were performed as described previously [21, 22]. Briefly, OS was defined as the interval between the date of diagnosis of brain metastases and the date of death from all causes or date of last follow-up for survivors. The study cut-off date was September 2018, and patients who were alive at the end of follow-up were censored. OS was estimated by using the Kaplan-Meier method, and differences in survival were assessed with the log-rank test. Multivariate Cox proportional hazards regression models were subsequently fitted to evaluate the relationship between brain metastases from CRC and OS, while controlling for potential confounding covariates.
Data are presented as numbers of patients, ratios (%), or hazard ratios (HR) and 95% confidence intervals (CI). P < 0.05 was considered statistically significant. All statistical analyses were performed using the JMP14 software program (SAS Institute Japan Ltd., Tokyo, Japan).
Results
Characteristics of the study cohort
Details of the study cohort are summarized in Fig. 1. During the study period, 5894 patients with Stage I/II/III CRC underwent tumor resection, and 1153 patients with Stage IV CRC received treatment, for example, by tumor resection and/or systemic chemotherapy. Of the 1153 Stage IV patients, 14 (1.2% of all Stage IV patients) had synchronous brain metastases. Fifty-four patients had metachronous brain metastases. Accordingly, the final study population consisted of 68 consecutive patients (43 males and 25 females), corresponding to 1.0% of all patients with CRC during the study period (Fig. 1). The primary tumor site was the colon for 42 patients (0.9% among 4844 colon cancers) and rectum for 26 patients (1.2% among 2203 rectal cancers).
Patient characteristics are shown in Table 1. The median age at the time of brain metastases was 65 years (age range, 32–84 years). Twenty-seven patients (40%) had a KPS score ≥ 70 and 41 patients (60%) had a score ≤ 60. Fifty-four patients (79%) had extracranial disease. Eleven patients (16%) had uncontrolled primary tumors. Before the occurrence of brain metastases, 46 patients (68%) had received systemic chemotherapy. Twenty-one patients (31%) were treated with both oxaliplatin-containing and irinotecan-containing regimens. For brain metastases, 42 patients (62%) had limited (1–3) lesions, whereas 26 patients (38%) had multiple (≥4) lesions. Twenty-eight patients (41%) had brain metastases > 3 cm in maximal diameter. In terms of location of brain lesions, 39 patients (57%) had supratentorial lesions, 11 patients (16%) had infratentorial lesions, and 18 patients (27%) had both types of lesions.
Table 1.
Patient characteristics (n = 68)
| Characteristic | Category | No. of patients (%) |
|---|---|---|
| Sex | Male | 43 (63%) |
| Female | 25 (37%) | |
| Age at the time of brain metastases (years) | < 65 | 33 (49%) |
| ≥65 | 35 (51%) | |
| Location of primary tumor | Colon | 42 (62%) |
| Rectum | 26 (38%) | |
| Time interval from diagnosis of primary tumor to brain metastases | Synchronous | 14 (21%) |
| Metachronous | 54 (79%) | |
| Karnofsky performance status score | ≥70 | 27 (40%) |
| < 70 (≤60) | 41 (60%) | |
| Presence of extracranial metastases | Absent (Brain only) | 14 (21%) |
| Present (Brain and other sites) | 54 (79%) | |
| Control of primary tumor | Controlled | 57 (84%) |
| Uncontrolled | 11 (16%) | |
| Maximum diameter of brain metastases | < 3 cm | 40 (59%) |
| ≥3 cm | 28 (41%) | |
| Number of brain metastases | Limited: 1–3 | 42 (62%) |
| Multiple: ≥4 | 26 (38%) | |
| Interval from diagnosis of primary tumor to brain metastases | < 12 months | 17 (25%) |
| ≥12 months | 51 (75%) | |
| Location of brain lesions | Supratentorial | 39 (57%) |
| Infratentorial | 11 (16%) | |
| Both | 18 (27%) | |
| History of systemic chemotherapy before diagnosis of brain metastases | None | 22 (32%) |
| Fluoropyrimidine only | 13 (19%) | |
| Fluoropyrimidine + oxaliplatin | 6 (9%) | |
| Fluoropyrimidine + irinotecan | 6 (9%) | |
| Fluoropyrimidine + oxaliplatin + irinotecan | 21 (31%) |
Long-term outcomes after diagnosis of brain metastases and causes of death
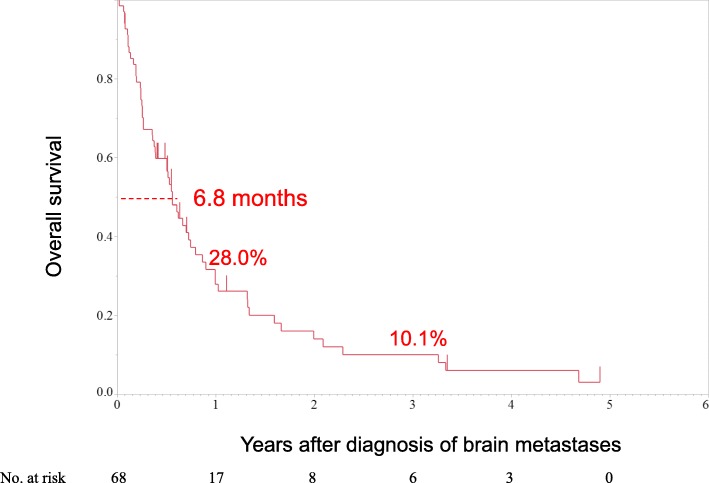
Median survival time was 6.8 months from the diagnosis of brain metastases. One-year and 3-year OS rates were 28.0 and 10.1%, respectively. Notably, no patient survived for more than 5 years (Fig. 2). During the study period, 57 of 68 patients died. Of these, 43 (75%) died of extracranial disease related to the primary tumor, 10 (18%) died of intracranial lesions due to brain metastases (seven died due to an intracranial mass and three died due to leptomeningeal metastases), and four (7%) died of unknown causes.
Fig. 2.
Overall survival curve of patients with brain metastases from colorectal cancer (n = 68). Median survival time was 6.8 months. One-year and 3-year overall survival rates were 28.0 and 10.1%, respectively
Factors affecting prognosis after diagnosis of brain metastases
According to univariate analysis, sex, timing of brain metastases (synchronous versus metachronous), primary tumor site (colon versus rectum), and brain lesion site (infratentorial versus supratentorial versus both) were not associated with prognosis (p = 0.320, 0.405, 0.893, and 0.878, respectively). In contrast, history of systemic chemotherapy before the development of brain metastases was significantly associated with worse OS (p = 0.028). Thus, we performed multivariate analyses to control for history of systemic chemotherapy before the development of brain metastases as a covariate, in addition to the five well-known prognostic factors of brain metastases from non-specific primary tumors suggested by RPA and DS-GPA (i.e., age, KPS, presence of extracranial metastases, number of brain metastases, and control of primary tumor).
Of the above-mentioned six factors, multivariate Cox proportional hazards regression models revealed that KPS score ≥ 70 [HR of KPS score < 70, 1.88 (95% CI: 1.02–3.59); p = 0.045], number of brain lesions ≤3 [HR of number of brain lesions > 3, 2.04 (95% CI: 1.06–3.97); p = 0.033], and no history of systemic chemotherapy before the development of brain metastases [HR of past history of systemic chemotherapy, 2.39 (95% CI: 1.28–4.68); p = 0.006] were independent factors associated with a better prognosis (Table 2). Age, presence of extracranial metastases, and control of primary tumor were not prognostic factors.
Table 2.
Univariate and multivariate analyses of factors affecting survival in patients with brain metastases from colorectal cancer
| Variable | Category | Median overall survival (months) | Univariate analysis p value |
Multivariate analysis | ||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p value | ||||
| Age at the time of brain metastases (years) | < 65 | 6.1 (3.1–10.5) | 0.913 | ref | 0.963 | |
| ≥65 | 7.3 (4.6–10.9) | 1.01 | 0.57–1.82 | |||
| Karnofsky performance status score | ≥70 | 6.8 (2.9–12.1) | 0.384 | ref | 0.045 | |
| < 70 | 6.8 (4.4–10.5) | 1.88 | 1.02–3.59 | |||
| Presence of extracranial metastases | Absent (Brain only) | 9.7 (3.2–16.3) | 0.507 | ref | 0.943 | |
| Present (Brain and other sites) | 6.7 (3.2–8.8) | 1.03 | 0.43–2.05 | |||
| Control of primary tumor | Controlled | 6.8 (4.6–9.7) | 0.941 | ref | 0.979 | |
| Uncontrolled | 6.1 (1.3–39.6) | 1.01 | 0.44–2.18 | |||
| Number of brain metastases | Limited: 1–3 | 8.8 (6.1–12.1) | 0.003 | ref | 0.033 | |
| Multiple: ≥4 | 3.1 (1.4–7.3) | 2.04 | 1.06–3.97 | |||
| History of systemic chemotherapy before brain metastases | No | 10.9 (6.1–25.4) | 0.003 | ref | 0.006 | |
| Yes | 6.8 (3.1–7.3) | 2.39 | 1.28–4.68 | |||
Data are presented as median (95% CI) or hazard ratio (95% CI)
CI Confidence interval
Treatments for brain metastases from colorectal cancer
As shown in Table 3, treatments for brain metastases significantly differed between the first half (1997–2005) and second half (2006–2015) of the study period (p = 0.004). Notably, whereas WBRT alone was performed in 19 of 36 patients (53%) in the first half, it was performed in only three of 31 patients (10%) in the second half. In contrast, whereas SRT alone was performed in 13 of 32 patients (41%) in the second half, it was performed in only four of 36 patients (11%) in the first half.
Table 3.
Treatment of brain metastases from colorectal cancer
| Initial treatment period | First half 1997–2005 | Second half 2006–2015 | p value |
|---|---|---|---|
| Treatment of brain metastases | |||
| WBRT or WBRT + SRT | 21 (58%) | 3 (9%) | 0.004 |
| SRT | 4 (11%) | 13 (41%) | |
| Surgery + SRT | 3 (8%) | 4 (13%) | |
| Surgery or Surgery + WBRT | 4 (11%) | 9 (28%) | |
| Supportive care | 4 (11%) | 3 (9%) | |
| Regimen before diagnosis of brain metastases | |||
| None | 13 (36%) | 9 (28%) | 0.011 |
| Fluoropyrimidine only | 9 (25%) | 4 (13%) | |
| Fluoropyrimidine + oxaliplatin | 2 (6%) | 4 (13%) | |
| Fluoropyrimidine + irinotecan | 6 (17%) | 0 (0%) | |
| Fluoropyrimidine + oxaliplatin + irinotecan | 6 (17%) | 15 (46%) | |
| Targeted agents | |||
| Not used | 34 (94%) | 11 (34%) | <.0001 |
| Used | 2 (6%) | 21 (66%) | |
WBRT Whole brain radiotherapy, SRT Stereotactic radiotherapy
Discussion
In this study, we demonstrated that KPS, the number of brain lesions, and history of chemotherapy are independent prognostic factors for OS in patients with brain metastases from CRC. Our finding that KPS is an independent prognostic factor is consistent with what was suggested by DS-GPA for patients with brain metastases from gastrointestinal cancers (i.e., CRC, gastric cancer, esophageal cancer), non-small cell lung cancer, breast cancer, renal cell cancer, and malignant melanoma [10, 11]. Similarly, our finding that the number of brain metastases is a prognostic factor is consistent with the results of another study of brain metastases from gastrointestinal cancers [23]. History of systemic chemotherapy before brain metastases was found to be the strongest prognostic factor (HR: 2.39; p = 0.006), and patients with this history had poor survival. Although the presence of extracranial metastases and control of primary tumor were not prognostic factors, the cause of death was systemic progression of primary CRC rather than neurologic death for the majority of patients (75% versus 18%) in this study. Taken together, these results suggest the possibility that patients who have more chemotherapy regimens available to try have opportunities to undergo various regimens against systemic progression and thus can achieve a better prognosis. This is consistent with two studies reporting that patients who underwent less chemotherapy prior to the development of brain metastases from CRC survived longer [24, 25].
WBRT, the standard treatment for brain metastases, is associated with late adverse events, such as leukoencephalopathy, hydrocephalus, and cerebral atrophy, which can lead to cognitive dysfunction in 10 to 20% of mpatients who undergo the procedure [26]. Since many patients now live longer after the diagnosis and treatment of brain metastases, there have been increasing concerns regarding treatment-related toxicities associated with WBRT. In the last few decades, SRT, less neurotoxic than and not as invasive as WBRT, is gaining wide acceptance in clinical settings [27]. In a recent phase III randomized controlled trial (JCOG0504) that compared the effects of salvage stereotactic radiosurgery versus surgery with WBRT in patients with 1–4 brain metastases, the median OS was 15.6 months in both the WBRT arm and salvage stereotactic radiosurgery arm; 16% of patients in the WBRT arm experienced grade 2 to 4 cognitive dysfunction, whereas the rate was only 8% in the salvage stereotactic radiosurgery arm (p = 0.048) [28]. The JCOG0504 study concluded that ‘salvage stereotactic radiosurgery is noninferior to WBRT and can be established as a standard therapy for patients with ≤4 brain metastases’ [28]. In the present study, treatments performed for brain metastases significantly differed between the first half (1997–2005) and second half (2006–2015) of the study period (Table 3). Notably, WBRT alone was rarely performed in the second half (3%). In contrast, SRT alone was performed in 41% of patients in the second half, but in only 11% of patients in the first half.
In the present study, 1.2% of all Stage IV patients had synchronous brain metastases, which corresponds to 0.20% (14 of 7047 patients) of the entire patient cohort at the time of CRC diagnosis. These results are similar to those of a recent large population-based study [29], in which roughly 1.0% of all patients with CRC were found to have brain metastases, as well as those reported from the Metropolitan Detroit Cancer Surveillance System [1] and a Dutch series [2]. Thus, our study population is considered representative of the global CRC patient population.
This study has potential limitations. First, biases may exist given the retrospective design. Second, the sample size was relatively small. However, to our knowledge, the number of patients with brain metastases from CRC with sufficient background records, including KPS, was one of the largest reported to date. Third, the study period spanned 1997 to 2015. During this long period, treatment strategies as well as the detectability of brain metastases have dramatically changed. Thus, these data will be difficult to compare with other studies focused on novel approaches. Despite these limitations, our findings warrant further studies in a larger patient series of CRC with brain metastases.
Conclusion
In addition to KPS, the number of brain lesions and history of chemotherapy were found to be independent prognostic factors for OS in patients with brain metastases from CRC. An awareness of these factors may help gastrointestinal surgeons make appropriate choices in the treatment of these patients.
Acknowledgements
The authors thank all colleagues and nurses who provided patient care.
Abbreviations
- CI
confidence interval
- CRC
colorectal cancer
- DS-GPA
diagnosis-specific graded prognostic assessment
- HR
hazard ratio
- KPS
Karnofsky performance status
- OS
overall survival
- RPA
recursive partitioning analysis
- RTOG
Radiation Therapy Oncology Group
- SRI
Score Index for Radiosurgery in Brain Metastases
- SRT
stereotactic radiotherapy
- WBRT
whole-brain radiotherapy
Authors’ contributions
JIm designed the study, collected the data, analyzed and interpreted the data, and prepared the manuscript. DS participated in the design and coordination of the study, analyzed and interpreted the data, and was responsible for writing the manuscript. YN, YM, TT, AT, NB, HI, JIt, and YK collected the data, performed the treatments, interpreted the data, and edited the manuscript. All authors read and approved the final manuscript.
Authors’ information
JIm, DS, TT, YK: Department of Colorectal Surgery, National Cancer Center Hospital. YN, YM: Department of Neurosurgery and Neuro-Oncology, National Cancer Center Hospital. AT, NB: Gastrointestinal Medical Oncology Division, National Cancer Center Hospital. HI, JIt: Department of Radiation Oncology, National Cancer Center Hospital.
Funding
None.
Availability of data and materials
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This retrospective study was approved by the Institutional Review Board (IRB) of the National Cancer Center Hospital (IRB code: 2017–437). It was determined to be a retrospective analysis of de-identified data, and thus was determined to be exempt from requiring written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jun Imaizumi, Email: jimaizum@ncc.go.jp.
Dai Shida, Phone: +81-3-3542-2511, Email: dshida@ncc.go.jp.
Yoshitaka Narita, Email: yonarita@ncc.go.jp.
Yasuji Miyakita, Email: ymiyakit@ncc.go.jp.
Taro Tanabe, Email: tatanabe1209@outlook.jp.
Atsuo Takashima, Email: atakashi@ncc.go.jp.
Narikazu Boku, Email: nboku@ncc.go.jp.
Hiroshi Igaki, Email: hirigaki@ncc.go.jp.
Jun Itami, Email: jitami@ncc.go.jp.
Yukihide Kanemitsu, Email: ykanemit@ncc.go.jp.
References
- 1.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan Detroit Cancer surveillance system. J Clin Oncol. 2004;22(14):2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 2.Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 3.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R. Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745–751. doi: 10.1016/S0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 4.Gaspar LE, Scott C, Murray K, Curran W. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000;47(4):1001–1006. doi: 10.1016/S0360-3016(00)00547-2. [DOI] [PubMed] [Google Scholar]
- 5.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2(3):187–193. doi: 10.1200/JCO.1984.2.3.187. [DOI] [PubMed] [Google Scholar]
- 6.Diener-West M, Dobbins TW, Phillips TL, Nelson DF. Identification of an optimal subgroup for treatment evaluation of patients with brain metastases using RTOG study 7916. Int J Radiat Oncol Biol Phys. 1989;16(3):669–673. doi: 10.1016/0360-3016(89)90483-5. [DOI] [PubMed] [Google Scholar]
- 7.Caballero JA, Sneed PK, Lamborn KR, Ma L, Denduluri S, Nakamura JL, Barani IJ, McDermott MW. Prognostic factors for survival in patients treated with stereotactic radiosurgery for recurrent brain metastases after prior whole brain radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83(1):303–309. doi: 10.1016/j.ijrobp.2011.06.1987. [DOI] [PubMed] [Google Scholar]
- 8.Likhacheva A, Pinnix CC, Parikh N, Allen PK, Guha-Thakurta N, McAleer M, Sulman EP, Mahajan A, Shiu A, Luo D, et al. Validation of recursive partitioning analysis and diagnosis-specific graded prognostic assessment in patients treated initially with radiosurgery alone. J Neurosurg. 2012;117:38–44. doi: 10.3171/2012.3.GKS1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nieder C, Marienhagen K, Dalhaug A, Aandahl G, Haukland E, Pawinski A. Prognostic models predicting survival of patients with brain metastases: integration of lactate dehydrogenase, albumin and extracranial organ involvement. Clin Oncol (R Coll Radiol) 2014;26(8):447–452. doi: 10.1016/j.clon.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D, Bhatt A, Jensen AW, Brown PD, Shih H, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655–661. doi: 10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Nieder C, Andratschke NH, Geinitz H, Grosu AL. Diagnosis-specific graded prognostic assessment score is valid in patients with brain metastases treated in routine clinical practice in two European countries. Med Sci Monit. 2012;18(7):CR450–CR455. doi: 10.12659/MSM.883213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joshi RS, Hirshman BR, Ali MA, Alattar A, Carroll K, Nagano O, Aiyama H, Serizawa T, Yamamoto M, Chen CC. Prognostic importance of cumulative intracranial tumor volume in patients with gastrointestinal brain metastasis treated with stereotactic radiosurgery. World Neurosurg. 2019;121:e747–e754. doi: 10.1016/j.wneu.2018.09.209. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki Y, Yamaguchi T, Matsumoto H, Nakano D, Honda G, Shinoura N, Karasawa K, Takahashi K. Prognostic factors and treatment effects in patients with curatively resected brain metastasis from colorectal cancer. Dis Colon Rectum. 2014;57(1):56–63. doi: 10.1097/01.dcr.0000436998.30504.98. [DOI] [PubMed] [Google Scholar]
- 15.Noura S, Ohue M, Shingai T, Fujiwara A, Imada S, Sueda T, Yamada T, Fujiwara Y, Ohigashi H, Yano M, et al. Brain metastasis from colorectal cancer: prognostic factors and survival. J Surg Oncol. 2012;106(2):144–148. doi: 10.1002/jso.23055. [DOI] [PubMed] [Google Scholar]
- 16.Mongan JP, Fadul CE, Cole BF, Zaki BI, Suriawinata AA, Ripple GH, Tosteson TD, Pipas JM. Brain metastases from colorectal Cancer: risk factors, incidence, and the possible role of chemokines. Clin Colorectal Cancer. 2009;8(2):100–105. doi: 10.3816/CCC.2009.n.016. [DOI] [PubMed] [Google Scholar]
- 17.Michl M, Thurmaier J, Schubert-Fritschle G, Wiedemann M, Laubender RP, Nussler NC, Ruppert R, Kleeff J, Schepp W, Reuter C, et al. Brain metastasis in colorectal Cancer patients: survival and analysis of prognostic factors. Clin Colorectal Cancer. 2015;14(4):281–290. doi: 10.1016/j.clcc.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Nozawa H, Ishihara S, Kawai K, Sasaki K, Murono K, Otani K, Nishikawa T, Tanaka T, Kiyomatsu T, Hata K, et al. Brain metastasis from colorectal Cancer: predictors and treatment outcomes. Oncology. 2017;93(5):309–314. doi: 10.1159/000478661. [DOI] [PubMed] [Google Scholar]
- 19.Shida D, Tanabe T, Boku N, Takashima A, Yoshida T, Tsukamoto S, Kanemitsu Y. Prognostic value of primary tumor sidedness for Unresectable stage IV colorectal Cancer: a retrospective study. Ann Surg Oncol. 2019;26(5):1358–1365. doi: 10.1245/s10434-019-07209-x. [DOI] [PubMed] [Google Scholar]
- 20.Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky performance status scale. An examination of its reliability and validity in a research setting. Cancer. 1984;53(9):2002–2007. doi: 10.1002/1097-0142(19840501)53:9<2002::AID-CNCR2820530933>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 21.Shida D, Ahiko Y, Tanabe T, Yoshida T, Tsukamoto S, Ochiai H, Takashima A, Boku N, Kanemitsu Y. Shorter survival in adolescent and young adult patients, compared to adult patients, with stage IV colorectal cancer in Japan. BMC Cancer. 2018;18(1):334. doi: 10.1186/s12885-018-4241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shida D, Yoshida T, Tanabe T, Tsukamoto S, Ochiai H, Kanemitsu Y. Prognostic impact of R0 resection and targeted therapy for colorectal Cancer with synchronous peritoneal metastasis. Ann Surg Oncol. 2018;25(6):1646–1653. doi: 10.1245/s10434-018-6436-3. [DOI] [PubMed] [Google Scholar]
- 23.Bartelt S, Momm F, Weissenberger C, Lutterbach J. Patients with brain metastases from gastrointestinal tract cancer treated with whole brain radiation therapy: prognostic factors and survival. World J Gastroenterol. 2004;10(22):3345–3348. doi: 10.3748/wjg.v10.i22.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung M, Ahn JB, Chang JH, Suh CO, Hong S, Roh JK, Shin SJ, Rha SY. Brain metastases from colorectal carcinoma: prognostic factors and outcome. J Neuro-Oncol. 2011;101(1):49–55. doi: 10.1007/s11060-010-0214-9. [DOI] [PubMed] [Google Scholar]
- 25.Aprile G, Zanon E, Tuniz F, Iaiza E, De Pauli F, Pella N, Pizzolitto S, Buffoli A, Piga A, Skrap M, et al. Neurosurgical management and postoperative whole-brain radiotherapy for colorectal cancer patients with symptomatic brain metastases. J Cancer Res Clin Oncol. 2009;135(3):451–457. doi: 10.1007/s00432-008-0468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown PD, Ahluwalia MS, Khan OH, Asher AL, Wefel JS, Gondi V. Whole-brain radiotherapy for brain metastases: evolution or revolution? J Clin Oncol. 2018;36(5):483–491. doi: 10.1200/JCO.2017.75.9589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, Yamanaka K, Sato Y, Jokura H. Stereotactic radiosurgery for patients with brain metastases – authors’ reply. Lancet Oncol. 2014;15(7):e248. doi: 10.1016/S1470-2045(14)70221-9. [DOI] [PubMed] [Google Scholar]
- 28.Kayama Takamasa, Sato Shinya, Sakurada Kaori, Mizusawa Junki, Nishikawa Ryo, Narita Yoshitaka, Sumi Minako, Miyakita Yasuji, Kumabe Toshihiro, Sonoda Yukihiko, Arakawa Yoshiki, Miyamoto Susumu, Beppu Takaaki, Sugiyama Kazuhiko, Nakamura Hirohiko, Nagane Motoo, Nakasu Yoko, Hashimoto Naoya, Terasaki Mizuhiko, Matsumura Akira, Ishikawa Eiichi, Wakabayashi Toshihiko, Iwadate Yasuo, Ohue Shiro, Kobayashi Hiroyuki, Kinoshita Manabu, Asano Kenichiro, Mukasa Akitake, Tanaka Katsuyuki, Asai Akio, Nakamura Hideo, Abe Tatsuya, Muragaki Yoshihiro, Iwasaki Koichi, Aoki Tomokazu, Watanabe Takao, Sasaki Hikaru, Izumoto Shuichi, Mizoguchi Masahiro, Matsuo Takayuki, Takeshima Hideo, Hayashi Motohiro, Jokura Hidefumi, Mizowaki Takashi, Shimizu Eiji, Shirato Hiroki, Tago Masao, Katayama Hiroshi, Fukuda Haruhiko, Shibui Soichiro. Effects of Surgery With Salvage Stereotactic Radiosurgery Versus Surgery With Whole-Brain Radiation Therapy in Patients With One to Four Brain Metastases (JCOG0504): A Phase III, Noninferiority, Randomized Controlled Trial. Journal of Clinical Oncology. 2018;36(33):3282–3289. doi: 10.1200/JCO.2018.78.6186. [DOI] [PubMed] [Google Scholar]
- 29.Yang L, He W, Xie Q, Liu S, Kong P, Jiang C, Zhang B, Xia L. Brain metastases in newly diagnosed colorectal cancer: a population-based study. Cancer Manag Res. 2018;10:5649–5658. doi: 10.2147/CMAR.S180173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.