Abstract
Complete understanding of the neural mechanisms by which stimulants such as methylphenidate ameliorate attention deficit hyperactivity disorder is lacking. Theories of catecholamine function predict that the neural effects of stimulant drugs will vary according to task requirements. We used event-related, pharmacological functional magnetic resonance imaging to investigate the effects of 60 mg of methylphenidate, alone and in combination with 400 mg of sulpiride, on blood oxygenation level-dependent (BOLD) signal in a group of 20 healthy participants during probabilistic reversal learning, in a placebo-controlled design. In a whole-brain analysis, methylphenidate attenuated BOLD signal in the ventral striatum during response switching after negative feedback but modulated activity in the prefrontal cortex when subjects maintained their current response set. The results show that the precise neural site of modulation by methylphenidate depends on the nature of the cognitive subprocess recruited.
Keywords: methylphenidate, dopamine, striatum, prefrontal cortex, reversal learning, fMRI
Introduction
Methylphenidate, a stimulant that blocks the transporters of the catecholamines dopamine and noradrenaline (Seeman and Madras, 1998), is used to treat attention deficit hyperactivity disorder (ADHD), a disorder characterized by inattention, impulsivity, and hyperactivity (Barkley, 1997; Nigg, 2001). Methylphenidate has been shown to modulate neural activity in the striatum during response inhibition (Vaidya et al., 1998) and divided attention (Shafritz et al., 2004) but parietofrontal regions during working memory (Mehta et al., 2000; Szobot et al., 2003). Thus, the precise neural site of modulation by methylphenidate appears to depend on the cognitive requirements of the task being performed (Cools and Robbins, 2004). Specifically, according to recent theorizing and empirical work, dopamine has been proposed to facilitate the flexible updating of task representations by acting at the level of the striatum while promoting the maintenance of stable task representations by acting at the level of the prefrontal cortex (PFC) (Frank et al., 2001; Bilder et al., 2004; Seamans and Yang, 2004; Cools et al., 2007a).
In the present study, we tested these hypotheses by examining the effects of the catecholamine enhancer methylphenidate on blood oxygenation level-dependent (BOLD) signal during distinct subcomponent processes of probabilistic reversal learning. We used a well established two-choice visual discrimination task (Cools et al., 2002), in which subjects receive immediate feedback after their choice of one of two abstract patterns presented in the left and right visual fields. On each trial, participants must choose the correct stimulus from two options and, when the stimulus-outcome contingencies reverse, select the previously incorrect stimulus instead. Presentation of occasional spurious negative feedback also allowed the examination of neural activity associated with the receipt of misleading negative feedback that was not followed by behavioral switching. The hypothesis that striatal dopamine is important in the flexible updating of task representations leads to the prediction that methylphenidate modulates BOLD signal in the striatum during response switching, i.e., the requirement to adapt responding to the new stimulus-outcome rule. In contrast, the hypothesis that prefrontal dopamine is important in the maintenance of stable task representations leads to the prediction that methylphenidate modulates BOLD signal in the prefrontal cortex during the receipt of misleading negative feedback because of the requirement to maintain the current response set in the face of distraction.
This investigation was performed as part of a larger design that also examined the possible opposing effects of sulpiride, a dopamine D2 receptor antagonist, to test whether the effects of methylphenidate are mediated specifically by dopamine D2 receptor signaling. Healthy participants were scanned while performing the reversal learning task after receiving either placebo, 60 mg of methylphenidate, 400 mg of sulpiride, or 60 mg of methylphenidate and 400 mg of sulpiride together. A possible confound in pharmacological functional magnetic resonance imaging (fMRI) studies arises from the fact that neurotransmitters such as dopamine exert direct effects on regional vasoactivity (Krimer et al., 1998). To account for this possibility, participants also performed a passive-viewing checkerboard task. If any modulation of BOLD signal in the reversal learning task is attributable to such neurovascular effects, then similar modulations in the checkerboard task would be expected.
Materials and Methods
Participants.
Twenty healthy volunteers (mean age, 22.2 years; range, 19–33 years; 14 male and 6 female; all right-handed) completed four testing sessions each. Data from three participants were excluded from the final analysis attributable to problems with fMRI data acquisition in at least one session. Mean NART (National Adult Reading Test) value was 121, with a range of 116–126. Participants were recruited among students and staff of University of Cambridge and Addenbrooke's Hospital. All participants entered the study after screening by a research psychiatrist (U.M.) and had no major psychiatric, neurological, or medical illness, including alcohol and drug abuse. They were asked to abstain from alcohol for 12 h, as well as from caffeine and nicotine for 3 h, before the testing sessions. A light breakfast or snack was allowed before, but not during, the experimental session. All participants were questioned about compliance with alcohol and caffeine restrictions before inclusion into the study. All participants gave written informed consent before testing and received monetary compensation of £200. The protocol was approved by the Local Research Ethics Committee Cambridge (LREC number 03/266) and formally exempted from clinical trial regulations by Medicines and Healthcare Products Regulatory Agency.
Pharmacological design and procedures.
All 20 participants completed the protocol and were tested in four pharmacological conditions using a counterbalanced, placebo-controlled, double-blind design. The four conditions were placebo, methylphenidate, sulpiride, and methylphenidate and sulpiride combined. The minimum time between testing sessions was 3 d. Single oral doses of 60 mg of methylphenidate (Ritalin; Novartis), 400 mg of sulpiride (Dolmatil; Sanofi-Synthelabo), or placebo (lactose with microcrystalline cellulose) contained in identical opaque gelatin capsules were administered 2 h (sulpiride) or 1.5 h (methylphenidate) before fMRI scanning. Because of the different pharmacokinetics of the two drugs, a double dummy procedure was used, whereby in every testing session participants were given two capsules 2 h before testing, containing either sulpiride or placebo, and then an additional three capsules 1.5 h before testing, containing either methylphenidate or placebo. Dose selection was based on previous, similar studies showing behavioral effects at similar (and lower) doses using the same drugs in healthy volunteers (Mehta et al., 1999, 2000;Volkow et al., 2002). The timing of fMRI testing was performed at approximately the time of maximal plasma levels of both drugs based on pharmacokinetic data (Sugnaux et al., 1983; Wagstaff et al., 1994; Kimko et al., 1999).
Between dosing and scanning, all volunteers were asked to spend the waiting time with low arousing activities (reading, watching television, etc.) in a day room and were monitored by research nurses.
Before scanning on the first session, participants were trained on the task to reduce practice effects that are common in pharmacological designs with repeated testing. If the gap between sessions was long, i.e., more than 1 week, participants were retrained on subsequent sessions to refamiliarize them with the task.
Unblinding was performed after the analysis of behavioral data and the first-level analysis of fMRI data.
Probabilistic reversal task.
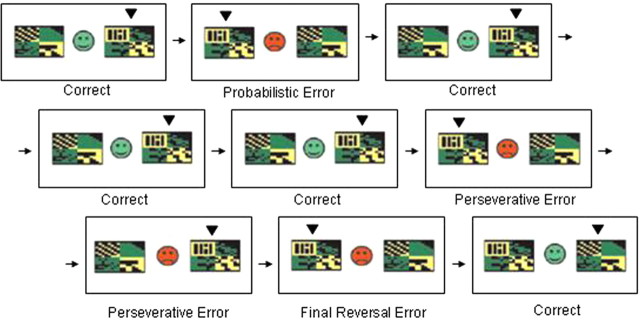
The task used here was similar to that used by Cools et al. (2002)). In each experimental session, each subject was scanned while performing the behavioral task in three successive echo planar imaging (EPI) runs, each lasting ∼8.5 min. Before entering the scanner on the first day, subjects performed a 30 trial training session. This was a simple probabilistic discrimination task (i.e., without reversal stages) designed to introduce the subject to the concept of a probabilistic error without the need to reverse responding (Fig. 1).
Figure 1.
The probabilistic reversal-learning task. On each trial, subjects are presented with two abstract visual patterns. Using trial-and-error feedback, subjects must discover which of the two patterns is correct (the subject's choice is indicated here with a small, black arrowhead). Feedback (a green smiley face or red sad face) is presented as soon as the subject has chosen one of the patterns with a left or right button press.
In the main task, on each trial the same two patterns were presented. One of the patterns was correct and the other pattern was incorrect, and subjects had to choose the correct pattern on each trial. During the task, the rule changed intermittently so that the other pattern was correct. On each block, the rule changed after a total of between 10 and 15 correct responses (pseudorandomly varied from block to block). Subjects were instructed to start choosing the other pattern only when they were sure that the rule had changed. To prevent subjects from adopting a strategy such as always reversing after two consecutive errors, probabilistic negative feedback was given on two consecutive trials once during each task block.
The task was programmed in Microsoft Visual Basic 6.0, and stimuli were presented on a computer display projected onto a mirror in the MRI scanner. Responses were made using the left or right button on a button box positioned on the stomach of the subject. Different stimuli were used in each of the three task blocks (and training stage), and the order of presentation of the blocks was counterbalanced across subjects. Each block consisted of 10 discrimination stages and, therefore, nine reversal stages. Reversal of the stimulus–reward contingency occurred after between 10 and 15 correct responses (including probabilistic errors). The two stimuli in each block were abstract colored patterns presented simultaneously in the left and right visual fields (location randomized). Different pairs of stimuli were used in each block of the task, with order randomized across subjects. Responses were made using the left or right button on a button box positioned on the stomach of the subject.
On each individual trial, the stimuli were presented for 2000 ms within which the response had to be made (or else a “too late” message was presented). Feedback, consisting of a green smiley face for correct responses or a red sad face for incorrect responses, was presented immediately after the response. The feedback faces were presented centrally, between the two stimuli, for 500 ms during which the stimuli also remained on the screen. After feedback, the stimuli were removed and the face was replaced by a fixation cross for a variable interval so that the overall interstimulus interval was 3253 ms.
Checkerboard task.
Subjects viewed an 8 × 8 black and white checkerboard pattern that flashed at a frequency of 8 Hz. The visual presentation alternated between 20 s of flashing checkerboard and 20 s of blank white screen for a total of 4 min (six blocks of checkerboard and six blocks of rest), allowing a block-related contrast of visual activity − crosshair fixation. There was no response requirement.
fMRI data acquisition and analyses.
Participants were scanned at the Wolfson Brain Imaging Centre (University of Cambridge, Cambridge, UK) on a 3 T Bruker scanner using a head coil. Functional images were collected using 21 slices covering the whole brain (slice thickness, 4 mm; interslice gap, 1 mm; in-plane resolution, 1.56 × 1.56 mm) with an EPI sequence (repetition time, 1.6 s; echo time, 27 ms; matrix size, 128 × 128). The number of volumes acquired per run varied for each run from ∼320 to ∼380 because the number of correct responses before a rule change varied pseudorandomly between 10 and 15 from block to block. The first 12 volumes were discarded to allow for T1-equilibrium effects. Structural and functional images were collected in the axial oblique plane.
All fMRI data were preprocessed (transformed) using SPM2 (statistical parametric mapping software) and analyzed using SPM5 software (Wellcome Department of Cognitive Neurology, London, UK). During preprocessing before analysis, all images were corrected for slice timing, subject motion corrected, and geometrically undistorted using phase maps (Cusack et al., 2003). Using the mean realigned image, all images were coregistered to a skull-stripped [using the Brain Extraction Tool (Smith, 2002)] high-resolution structural scan (voxel size, 1 × 1 × 1 mm) that was acquired on the first scanning day. Images were then normalized, using affine and smoothly nonlinear transformations, to an EPI template in Montreal Neurological Institute (MNI) space. Finally, all normalized images were spatially smoothed with a 10 mm full-width, half-maximum Gaussian kernel.
The time series were high-pass filtered (128 s), and a canonical hemodynamic response function was modeled to the onset of the responses, which occurred at the same time as the presentation of the feedback. The following events were modeled: (1) correct responses; (2) final reversal errors (negative feedback followed by a response shift); (3) probabilistic errors (on which erroneous negative feedback was given and the subject did not shift their response); and (4) perseverative errors (errors generated by a change in contingency but on which the participant did not shift their response). For the purposes of the analysis, probabilistic and perseverative errors were collapsed and are referred to as nonswitch errors: on both types of errors, participants received negative feedback but did not switch their response. In each testing session, the average number of trials for each condition (across the three runs of the task) were as follows: final reversal errors, 27; perseverative errors, 29; probabilistic errors, 45; correct responses, 374.
To delineate the general network of areas involved in performance of the task independent of any drug effects, we computed a contrast, final reversal errors − correct responses, at the subject-specific level only on scans from the placebo condition. The contrast images from this comparison were taken to a second-level analysis involving a one-sample t test to test for effects at the group level.
The preceding contrast is useful to reveal areas generally involved in task performance. However, our central predictions were that dopaminergic drugs would modulate BOLD signal in different areas of this task network during different cognitive subprocesses, specifically, that methylphenidate would modulate striatal BOLD signal during switching and prefrontal BOLD signal during maintenance of the current response “set.” Accordingly, we tested these hypotheses by examining the interaction of the drugs with two more restricted contrasts designed to reveal BOLD signal specifically related to the subprocesses of interest.
The first of these contrasts, final reversal errors − nonswitch errors, investigated areas that were modulated by drug specifically during the process of switching (subtracting out any activity related to receiving negative feedback when there was no response switch). The second of these contrasts, nonswitch errors − correct responses, investigated areas that were modulated by drug specifically during receipt of negative feedback when there was no response switch, i.e., when participants maintained the current mode of responding in the face of misleading negative feedback.
We examined the drug effects by performing these two critical contrasts at the subject-specific level and taking the contrast images from these comparisons to second-level group analyses involving separate 2 × 2 repeated-measures ANOVAs, with the factors methylphenidate (on vs off) and sulpiride (on vs off). This enabled an examination of both the main effects of drugs on process-specific activity and of the interaction between the two drugs in their effects on this activity.
For all comparisons, the threshold set for statistical significance was p < 0.05, corrected for false discovery rate (FDR). All analyses were performed on the whole-brain group data.
Results
Behavioral data
Two separate 2 × 2 repeated-measures ANOVAs were performed on the behavioral data, with methylphenidate (on or off) and sulpiride (on or off) as the two factors. The two ANOVAs examined the effects of these factors on two dependent measures: (1) number of consecutive errors preceding a switch (perseverative errors), and (2) probability of switching after erroneous feedback [the number of probabilistic errors on which participants switched response divided by the total number of probabilistic errors (prob switch)]. There was no main effect of methylphenidate (perseverative errors, F(1,16) = 0.36, p = 0.55; prob switch, F(1,16) = 0.26, p = 0.62), no main effect of sulpiride (perseverative errors, F(1,16) = 0.12, p = 0.73; prob switch, F(1,16) = 0.24, p = 0.63), and no methylphenidate × sulpiride interaction (perseverative errors, F(1,16) = 1.48, p = 0.24; prob switch, F(1,16) = 2.4, p = 0.14) .
fMRI data
Task-related activity
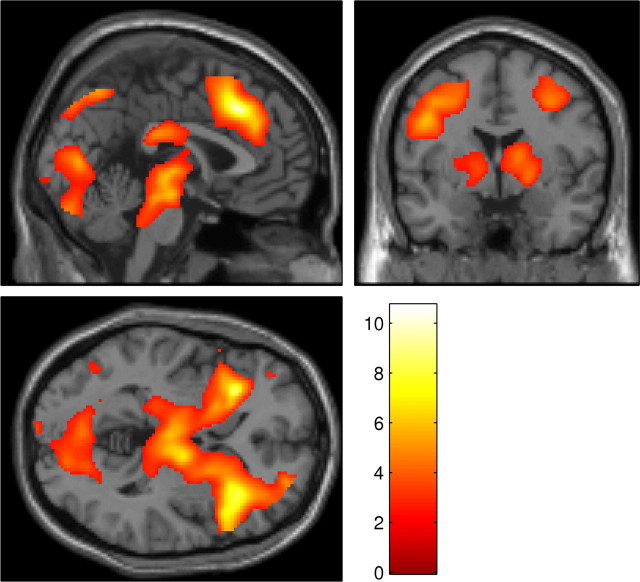
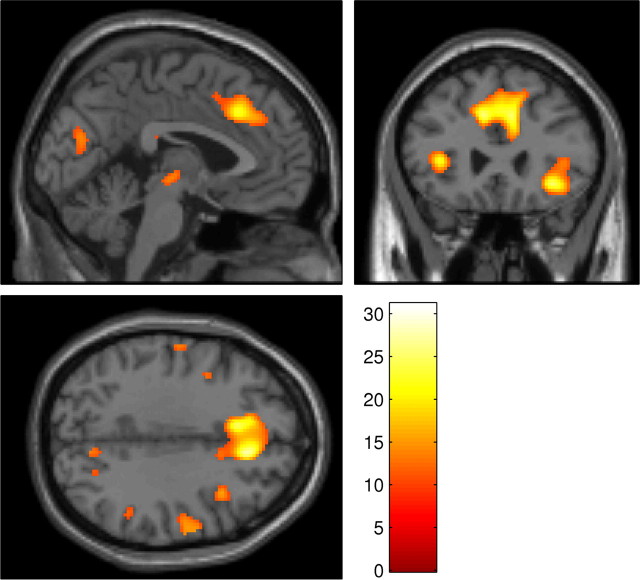
To investigate task-related activations, an initial contrast was performed at the random-effects level, only including scans from the placebo session. This contrast, final reversal errors − correct responses, revealed an extensive network of activated areas (Fig. 2, Table 1). This included a large cluster extending from the anterior cingulate cortex (ACC), into ventrolateral prefrontal cortex (VLPFC), striatum (including the putamen), and thalamus.
Figure 2.
Whole-brain SPM showing the results of the contrast final reversal errors − correct responses performed at the random-effects level on scans from the placebo condition, superimposed on the MNI template brain. Results are corrected for FDR (p < 0.05). Color scale represents magnitude of t values.
Table 1.
Areas of activation from the whole-brain contrast final reversal errors − correct responses in the placebo condition
| Anatomical location | Talairach coordinates |
t value | ||
|---|---|---|---|---|
| x | y | z | ||
| Medial frontal gyrus | 2 | 26 | 40 | 10.7 |
| Inferior frontal gyrus | 38 | 22 | −4 | 9.25 |
| Insula | −30 | 22 | 0 | 9.21 |
| Middle frontal gyrus | 38 | 12 | 52 | 8.82 |
| 46 | 28 | 26 | 8.51 | |
| Precuneus | 32 | −64 | 42 | 8.56 |
| Superior frontal gyrus | 26 | 58 | −8 | 8.01 |
| Inferior parietal lobule | −32 | −54 | 46 | 7.98 |
| Thalamus | 14 | −12 | 6 | 7.75 |
To provide precise coordinates of activated clusters, only clusters that survive correction for FWE (p < 0.05) and contain at least 20 voxels are reported.
Drug effects on process-specific BOLD responses
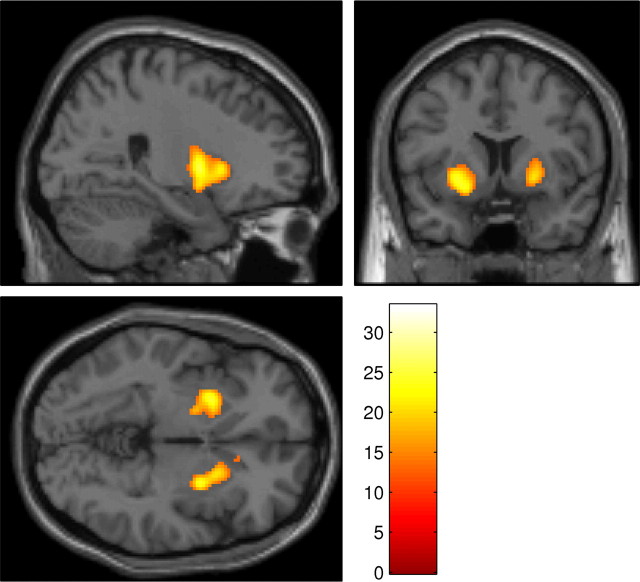
The first of the contrasts examining drug effects on process-specific BOLD response was performed on the contrast images from the comparison final reversal errors − nonswitch errors. This revealed a highly selective, significant effect of methylphenidate bilaterally in the ventral putamen (Fig. 3), as well as smaller peaks in the right precentral gyrus and right cuneus. The activation in the right putamen remained significant even at the more conservative familywise error rate (FWE) threshold of p < 0.05. As shown in Figure 4 and supplemental Figure 1 (available at www.jneurosci.org as supplemental material), this region showed a reduced BOLD response on reversal trials in the methylphenidate and combined drug conditions relative to the sulpiride and placebo conditions. No areas showed significant modulation by sulpiride, and no areas showed a significant methylphenidate × sulpiride interaction.
Figure 3.
Whole-brain SPM showing areas in which there was a significant interaction between methylphenidate and condition in the random-effects contrast final reversal errors − nonswitch errors, superimposed on the MNI template brain. Methylphenidate reduced switch-related activity bilaterally in the ventral putamen, with small peaks also in the right cuneus and right precentral gyrus. In this figure, higher-intensity values represent a greater methylphenidate-induced decrease in BOLD response. The figure displays all significantly activated clusters below a threshold of p < 0.05, FDR corrected, containing at least 20 voxels. Color scale represents magnitude of F values. The right putamen was also activated at the more conservative threshold of p < 0.05, FWE corrected.
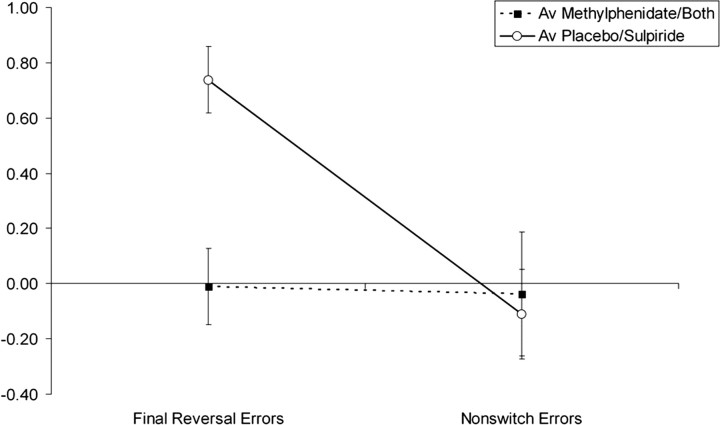
Figure 4.
Mean parameter estimates for final reversal errors and nonswitch errors in the right putamen. For each subject, beta values were extracted from a region of interest (ROI) based on the activated cluster from the second-level contrast, which showed a main effect of methylphenidate on BOLD signal in the contrast final reversal errors − nonswitch errors. This region is shown in Figure 3. Beta values were then averaged across the three task sessions and across all subjects to obtain a mean beta value for each event in each drug condition. These values were then averaged across the methylphenidate and combined drug conditions and across the sulpiride and placebo drug conditions to show the main effect of methylphenidate on switch-related BOLD signal. ROI definition and beta value extraction were performed with the ROI toolbox Marsbar (Brett et al., 2002). Error bars represent SEM.
To confirm that there was no drug effect on BOLD signal in the PFC during switching, the statistical threshold was dropped to the more liberal p < 0.001 uncorrected for multiple comparisons. Even at this more liberal threshold, there was no significant drug effect on BOLD signal in any prefrontal or parietal cortical regions.
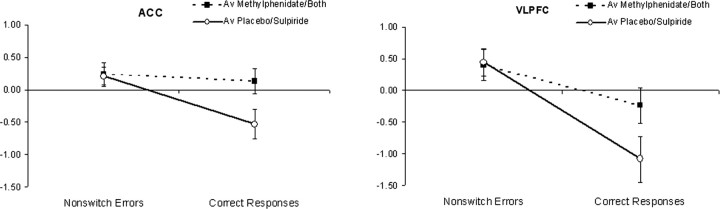
The second of these contrasts, performed on the contrast images from the comparison nonswitch errors − correct responses, revealed significant effects of methylphenidate in bilateral VLPFC, ACC, right dorsolateral prefrontal cortex, right precentral gyrus, and bilateral inferior parietal lobe (Fig. 5). As shown in Figure 6 and supplemental Figure 2 (available at www.jneurosci.org as supplemental material), these effects in VLPFC and ACC are actually driven by an attenuation of deactivation during correct responses in the methylphenidate condition relative to the placebo condition or, in other words, a methylphenidate-induced increase in BOLD response during correct responses. There was no effect of sulpiride and no interaction between methylphenidate and sulpiride in this contrast.
Figure 5.
Whole-brain SPM showing areas in which there was a significant interaction between methylphenidate and condition in the random-effects contrast nonswitch errors − correct responses, superimposed on the MNI template brain. There was a significant interaction in several different areas. Prominent peaks were observed in ACC and bilateral VLPFC. In this figure, higher-intensity values represent a greater methylphenidate-induced decrease in BOLD response. However, as Figure 6 shows, this effect is driven by a methylphenidate-induced increase in activation for correct responses. Color scale represents magnitude of F values. The figure displays all significantly activated clusters below a threshold of p < 0.05, corrected for false discovery rate, containing at least 20 voxels.
Figure 6.
Mean parameter estimates for nonswitch errors and correct responses in the ACC and VLPFC. For each subject, beta values were extracted from ROIs based on the activated clusters from the second-level contrast, which showed an interaction between methylphenidate and the contrast nonswitch errors − correct responses. These regions are shown in Figure 5. Beta values were then averaged across the three task sessions and across all subjects to obtain a mean beta value for each event in each drug condition. These values were then averaged across the methylphenidate and combined drug conditions and across the sulpiride and placebo drug conditions to show the main effect of methylphenidate on feedback-related BOLD signal. ROI definition and beta value extraction were performed with the ROI toolbox Marsbar (Brett et al., 2002). Error bars represent SEM.
Finally, despite the absence of a significant interaction between methylphenidate and sulpiride, our previous hypothesis that sulpiride would reverse the effects of methylphenidate justified performing simple effects analyses to see whether activity in the combined condition was significantly different from activity in the methylphenidate condition. Student's t tests performed on both of the contrasts described above comparing the methylphenidate and combined conditions revealed no regions in which activity varied significantly between these two conditions.
Checkerboard task
Analysis of the fMRI data from the passive viewing task revealed, as expected, a widespread increase in activation in visual cortex during visual stimulation relative to the rest condition. The peak of the most highly activated cluster was at coordinates (10, −96, −4), with an F value of 375, significant at p < 0.05 corrected for FDR. The remaining activated clusters were all in a similar region of visual cortex. Comparison of the activation maps from the four different drug conditions revealed no effects of methylphenidate or sulpiride on brain activity. To confirm the absence of a drug effect, the threshold for statistical significance was dropped to a more liberal 0.001 uncorrected for multiple comparisons. Even at this liberal threshold, there were no significant drug effects in any voxel.
Discussion
We found that the precise neural site of modulation by methylphenidate depends on the cognitive process being performed; methylphenidate modulated BOLD signal in the putamen during response switching but modulated BOLD signal in the PFC when subjects maintained their current response set. There was no effect of methylphenidate on switch-related BOLD signal in any prefrontal cortical regions, and there was no effect of methylphenidate on BOLD response during nonswitch trials in the putamen. This is the first study to show that methylphenidate exerts differential effects at cortical and striatal sites to affect distinct cognitive components within a single task, in an event-related fMRI paradigm.
The modulation of BOLD signal in the putamen during switching may be related to the cognitive inhibitory component of the task. When the stimulus–reward contingencies change, participants must inhibit responding to the previously rewarded stimulus and shift their response to the previously incorrect stimulus. Thus, the modulation of putamen activity cannot be related to a simple inhibition of a prepotent motor response, as in the important study by Vaidya et al. (1998). Effects on motor inhibition per se are precluded by an important feature of the probabilistic reversal learning task, that the location of each stimulus, and consequently the stimulus–response mapping, is randomized on each trial such that participants do not know in advance of stimulus presentation which button is associated with the presently correct stimulus. Thus, the present finding shows that methylphenidate modulates striatal activity not only during simple motor inhibition but also during inhibition of more abstract stimulus–reward associations.
These data indicate a putative mechanism of stimulant action, which could in principle be mediated by either dopamine or noradrenaline (Gatley et al., 1996; Spencer et al., 1996; Kuczenski and Segal, 1997). However, the relative paucity of noradrenaline compared with dopamine in the striatum makes noradrenergic mediation an unlikely explanation (Herregodts et al., 1991). Furthermore, positron emission tomography (PET) imaging data show that methylphenidate is very effective in blocking dopamine transporters (Volkow et al., 1998), and studies involving patients with Parkinson's disease provide support for the important role for striatal dopamine in behavioral flexibility (Cools et al., 2001, 2007).
Thus, assuming that any effect of methylphenidate on noradrenergic function within the striatum would be minimal, the present data support and extend previous findings that dopaminergic signaling in the striatum plays an important role in executive control. Dopamine neurons in the striatum encode reward prediction errors, facilitating learning by providing information about the difference between obtained and expected rewards (Schultz et al., 1997). The present data suggest that dopaminergic modulation of neural activity in the putamen is especially important when subjects change their behavior on the basis of negative feedback. These findings support a model of executive control in which dopaminergic signals originating in the striatum facilitate the flexible updating of new goal-related representations (Frank et al., 2001; Cools et al., 2007b).
In the absence of any behavioral effect of the drug, the reduction in BOLD signal in the striatum may reflect an increase in the efficiency of executive control. This interpretation is consistent with previous findings that amphetamine increases the efficiency of prefrontal working memory processing in lower performing subjects (Mattay et al., 2003) and also with the results of a previous PET study showing that methylphenidate-related improvements in spatial working memory are associated with task-related reductions in regional cerebral blood flow in the dorsolateral prefrontal cortex (Mehta et al., 2000). However, an alternative explanation is that the relatively high dose of methylphenidate used in the present study placed our participants on the right side of an inverted U-shaped dose–response function relating dopamine levels to behavioral performance. This is consistent with the results of the Vaidya et al. (1998) study, which revealed that methylphenidate reduced striatal BOLD signal in healthy controls but increased BOLD signal in ADHD patients, suggesting that the precise effects of methylphenidate may depend on baseline catecholamine levels.
The absence of any effect of the D2/D3 receptor antagonist sulpiride on brain activity or behavior makes it difficult to make any definitive conclusion about the role of specific dopamine receptors in these effects. However, it is likely that too small a dose of sulpiride was used, resulting in the absence of any observable effect on brain activity. Although previous studies that used a 400 mg dose found subtle effects of neurocognitive testing (Mehta et al., 1999), a recent PET study by Mehta et al. (2007) found that a single 400 mg dose of sulpiride (the same dose as used in the present study because of ethical constraints in testing higher doses) leads to only 28% occupancy of dopamine D2 receptors in the striatum. In contrast, 60 mg of methylphenidate (the same dose as used in the present study) has been shown to lead to a much higher percentage occupancy (74%) of the dopamine transporter (Volkow et al., 1998). Thus, 400 mg of sulpiride may not have been a sufficiently large dose to cause observable changes in BOLD signal in the striatum.
The results of the task-specific analysis replicated the findings of Cools et al. (2002), who, using exactly the same task, found an increase in activation in the VLPFC for final reversal errors relative to correct responses. However, despite the widespread increases in PFC activity associated with reversals when examined independently of any drug effects, we did not observe any modulation of PFC activity by methylphenidate during response switching. In fact, methylphenidate modulated activity in the ACC and lateral PFC during receipt of correct feedback that was not followed by a subsequent response switch.
Given the widespread distribution of both dopamine and noradrenaline projections to the PFC, we cannot rule out the possibility that the observed drug effects in the PFC reflect modulation of noradrenergic signaling. Indeed, therapeutic doses of methylphenidate with cognitive enhancing effects increase the efflux of both dopamine and noradrenaline in the rat PFC (Berridge et al., 2006). However, the present modulation of PFC activity by methylphenidate appears to be consistent with the hypothesized role of dopamine D1 receptors in stabilizing goal representations in the face of distracting information (Durstewitz and Seamans, 2002).
The effects of methylphenidate observed in the present study contrast with the effects of tryptophan depletion (which normally reduces central 5-HT levels) on BOLD signal during reversal learning observed by Evers et al. (2005). In that study, tryptophan depletion increased BOLD signal during negative feedback in a region of medial PFC, which was more dorsal than the ACC region modulated by methylphenidate in the present study. There was also no modulation in the study by Evers et al. (2005) of BOLD signal in VLPFC during performance monitoring or striatal BOLD signal during switching. The differential neural effects of tryptophan depletion and methylphenidate during performance monitoring may reflect different functional roles of the indoleamine serotonin and the catecholamines, respectively, during reversal learning. Indeed, tryptophan depletion modulated medial PFC activity during negative feedback, whereas methylphenidate modulated activity in a nearby medial PFC region during positive feedback. This suggests that serotonin and the catecholamines may play complementary roles in reversal learning, with serotoninergic modulation of PFC activity mediating the processing of aversive signals and catecholaminergic modulation of PFC activity mediating the processing of positive signals (cf. Clarke et al., 2007).
Interestingly, the putaminal region in which methylphenidate modulated BOLD signal in the present study is different from the ventral striatal region in which levodopa modulated BOLD signal in patients with Parkinson's disease during reversal learning (Cools et al., 2007b). One possible reason for this discrepancy is that the study by Cools et al., 2007b involved patients with presumably abnormal dorsal striatal dopamine levels. It is likely that any effects of dopaminergic drugs on dorsal striatal BOLD signal in healthy volunteers will be abnormal in such patients.
In summary, we observed different effects of methylphenidate on cortical and subcortical BOLD signal related to distinct behavioral processes. Methylphenidate reduced activity in the ventral putamen during switching after negative feedback and increased activity in the PFC during maintenance of the current response set. These data suggest that the precise neural effects of methylphenidate during the performance of a cognitive task will depend on the extent to which that task recruits the separate neural systems involved in the stabilization and flexible updating of goal-related representations.
Footnotes
This work was supported by Programme Grant 076274/4/Z/04/Z awarded by the Wellcome Trust (T.W.R., B. J. Everitt, A. C. Roberts, and B. J. Sahakian) and completed within the University of Cambridge Behavioural and Clinical Neuroscience Institute supported by a joint award from the Medical Research Council and the Wellcome Trust. U.M. was supported by a Feodoy–Lynen Fellowship awarded by the Alexander von Humboldt Foundation and the Isaac Newton Trust, University of Cambridge. R.C. was a University Research fellow of the Royal Society. We thank all participants, nurses, and administrative staff at the Wellcome Trust Clinical Research Facility and Vicky Lupton and her team at the Wolfson Brain Imaging Centre.
References
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue V, Poline JB. Region of interest analysis using an SPM toolbox. Presented at the Eighth International Conference on Functional Mapping of the Human Brain; June; Sendai, Japan. 2002. [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- Cools R, Robbins TW. Chemistry of the adaptive mind. Philos Transact A Math Phys Eng Sci. 2004;362:2871–2888. doi: 10.1098/rsta.2004.1468. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001;11:1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci. 2002;22:4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Sheridan M, Jacobs E, D'Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J Neurosci. 2007a;27:5506–5514. doi: 10.1523/JNEUROSCI.0601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Lewis SJG, Clark L, Barker RA, Robbins TW. l-DOPA disrupts activity in the nucleus accumbens during reversal learning in parkinson's disease. Neuropsychopharmacology. 2007b;32:180–189. doi: 10.1038/sj.npp.1301153. [DOI] [PubMed] [Google Scholar]
- Cusack R, Brett M, Osswald K. An evaluation of the use of magnetic field maps to undistort echo-planar images. NeuroImage. 2003;18:127–142. doi: 10.1006/nimg.2002.1281. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. The computational role of dopamine D1 receptors in working memory. Neural Netw. 2002;15:561–572. doi: 10.1016/s0893-6080(02)00049-7. [DOI] [PubMed] [Google Scholar]
- Evers EA, Cools R, Clark L, van der Veen FM, Jolles J, Sahakian BJ, Robbins TW. Serotonergic modulation of prefrontal cortex during negative feedback in probabilistic reversal learning. Neuropsychopharmacology. 2005;30:1138–1147. doi: 10.1038/sj.npp.1300663. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Loughry B, O'Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn Affect Behav Neurosci. 2001;1:137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- Gatley SJ, Pan D, Chen R, Chaturvedi G, Ding YS. Affinities of methylphenidate derivatives for dopamine, norepinephrine and serotonin transporters. Life Sci. 1996;58:231–239. doi: 10.1016/0024-3205(96)00052-5. [DOI] [PubMed] [Google Scholar]
- Herregodts P, Ebinger G, Michotte Y. Distribution of monoamines in human brain: evidence for neurochemical heterogeneity in subcortical as well as in cortical areas. Brain Res. 1991;542:300–306. doi: 10.1016/0006-8993(91)91582-l. [DOI] [PubMed] [Google Scholar]
- Kimko HC, Cross JT, Abernethy DR. Pharmacokinetics and clinical effectiveness of methylphenidate. Clin Pharmacokinet. 1999;37:457–470. doi: 10.2165/00003088-199937060-00002. [DOI] [PubMed] [Google Scholar]
- Krimer LS, Muly EC, III, Williams GV, Goldman-Rakic PS. Dopaminergic regulation of cerebral cortical microcirculation. Nat Neurosci. 1998;1:286–289. doi: 10.1038/1099. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem. 1997;68:2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Sahakian BJ, McKenna PJ, Robbins TW. Systemic sulpiride in young adult volunteers simulates the profile of cognitive deficits in Parkinson's disease. Psychopharmacology (Berl) 1999;146:162–174. doi: 10.1007/s002130051102. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20(RC65):1–6. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Montgomery AJ, Kitamura Y, Grasby PM. Dopamine D2 receptor occupancy levels of acute sulpiride challenges that produce working memory and learning impairments in healthy volunteers. Psychopharmacology (Berl) 2007;196:157–165. doi: 10.1007/s00213-007-0947-0. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Is ADHD a disinhibitory disorder? Psychol Bull. 2001;127:571–598. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Seeman P, Madras BK. Anti-hyperactivity medication: methylphenidate and amphetamine. Mol Psychiatry. 1998;3:386–396. doi: 10.1038/sj.mp.4000421. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Marchione KE, Gore JC, Shaywitz SE, Shaywitz BA. The effects of methylphenidate on neural systems of attention in attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161:1990–1997. doi: 10.1176/appi.ajp.161.11.1990. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Wilens T, Harding M, O'Donnell D, Griffin S. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35:409–432. doi: 10.1097/00004583-199604000-00008. [DOI] [PubMed] [Google Scholar]
- Sugnaux FR, Benakis A, Fonzo D, Di Carol R. Dose-dependent pharmacokinetics of sulpiride and sulpiride-induced prolactin secretion in man. Eur J Drug Metab Pharmacokient. 1983;8:189–200. [Google Scholar]
- Szobot CM, Ketzer C, Cunha RD, Parente MA, Langleben DD, Acton PD, Kapczinski F, Rohde LA. The acute effect of methylphenidate on cerebral blood flow in boys with attention-deficit/hyperactivity disorder. Eur J Nucl Med Mol Imaging. 2003;30:423–426. doi: 10.1007/s00259-002-1082-0. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JD. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci USA. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, Hitzemann R, Pappas N. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155:1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Ding YS, Gatley SJ. Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: results from imaging studies. Eur Neuropsychopharmacol. 2002;12:557–566. doi: 10.1016/s0924-977x(02)00104-9. [DOI] [PubMed] [Google Scholar]
- Wagstaff AJ, Fitton A, Benfield P. Sulpiride: a review if its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in schizophrenia. CNS Drugs. 1994;2:313–333. [Google Scholar]