Abstract
Clotrimazole (CLT) is a widely used drug for the topical treatment of yeast infections of skin, vagina, and mouth. Common side effects of topical CLT application include irritation and burning pain of the skin and mucous membranes. Here, we provide evidence that transient receptor potential (TRP) channels in primary sensory neurons underlie these unwanted effects of CLT. We found that clinically relevant CLT concentrations activate heterologously expressed TRPV1 and TRPA1, two TRP channels that act as receptors of irritant chemical and/or thermal stimuli in nociceptive neurons. In line herewith, CLT stimulated a subset of capsaicin-sensitive and mustard oil-sensitive trigeminal neurons, and evoked nocifensive behavior and thermal hypersensitivity with intraplantar injection in mice. Notably, CLT-induced pain behavior was suppressed by the TRPV1-antagonist BCTC [(N-(-4-tertiarybutylphenyl)-4-(3-cholorpyridin-2-yl)tetrahydropyrazine-1(2H)-carboxamide)] and absent in TRPV1-deficient mice. In addition, CLT inhibited the cold and menthol receptor TRPM8, and blocked menthol-induced responses in capsaicin- and mustard oil-insensitive trigeminal neurons. The concentration for 50% inhibition (IC50) of inward TRPM8 current was ∼200 nm, making CLT the most potent known TRPM8 antagonist and a useful tool to discriminate between TRPM8- and TRPA1-mediated responses. Together, our results identify TRP channels in sensory neurons as molecular targets of CLT, and offer means to develop novel CLT preparations with fewer unwanted sensory side effects.
Keywords: TRP channels, sensory neurons, clotrimazole, temperature sensing, pain, chemoreceptor
Introduction
Clotrimazole (CLT) is an antifungal compound commonly used in over-the-counter medications for the topical treatment of fungal infections of the skin, vagina, and mouth (Sawyer et al., 1975). CLT exerts its antifungal actions by inhibiting P450-dependent enzymes (Hitchcock et al., 1990). Other well known molecular targets of CLT are intermediate conductance Ca2+-activated potassium (IKCa) channels, including the erythrocyte Gardos channel (KCNN4) (Alvarez et al., 1992); CLT-induced inhibition of the Gardos channel, leading to reduced erythrocyte dehydratation, is a promising therapy in sickle cell disease (Brugnara et al., 1996). In addition, CLT can inhibit cell proliferation in vitro and in vivo, which has been ascribed to a CLT-induced depletion of intracellular Ca2+ stores and blockade of store-dependent Ca2+ influx (Benzaquen et al., 1995). As such, CLT and related substances have potential as antiproliferative and antimetastatic agents for the treatment of various human diseases.
Although mostly well tolerated, a significant fraction of patients using topical CLT experience side effects such as irritation and burning of the skin and mucous membranes (Binet et al., 1994; del Palacio et al., 2001). Moreover, oral CLT has been reported to induce nausea, gastrointestinal disturbances, and altered taste sensations in some patients (Sawyer et al., 1975; Koletar et al., 1990). The mechanisms underlying these unwanted side effects of CLT are still not well understood.
The transient receptor potential (TRP) superfamily is a large group of cation channels that play a general role as cellular sensors (Clapham, 2003; Voets et al., 2005; Nilius et al., 2007). Several TRP channels members are involved in the detection of thermal, mechanical and chemical stimuli, and in the initiation of irritation and pain caused by such stimuli (Julius and Basbaum, 2001; Voets et al., 2005; Tominaga, 2007). In this study, we identify TRP channels in sensory neurons as novel sensitive targets of CLT. We demonstrate that CLT is an agonist of TRPV1 and TRPA1, two TRP channels involved in the detection of noxious physical and chemical stimuli by nociceptors (Caterina et al., 1997; Caterina et al., 2000; Davis et al., 2000; Story et al., 2003; Bautista et al., 2005; Nagata et al., 2005; Bautista et al., 2006; Kwan et al., 2006), a potent antagonist of TRPM8, an important sensor of environmental cold and of cooling substances such as menthol (McKemy et al., 2002; Peier et al., 2002a; Bautista et al., 2007; Colburn et al., 2007; Dhaka et al., 2007; Voets et al., 2007b). Moreover, we show that intraplantar injection of CLT evokes nocifensive behavior and thermal hyperalgesia in mice, which can be attenuated by pharmacological inhibition or genetic ablation of TRPV1.
Materials and Methods
Cell cultures and gene expression.
Human embryonic kidney 293 (HEK293) cells were grown in DMEM containing 10% (v/v) fetal calf serum, 4 mm l-alanyl-l-glutamine, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin at 37°C in a humidity-controlled incubator with 10% CO2. Cells were transiently transfected with human TRPM8, human TRPV1 or mouse TRPA1 cloned in the bicistronic pCAGGS-IRES-GFP vector using TransIT-293 transfection reagent (Mirus, Madison, WI). In some experiments, we also used a tetracycline-inducible system for expression of mouse TRPA1 in Chinese hamster ovary (CHO) cells, as described previously (Story et al., 2003).
Trigeminal ganglion neurons from adult mice (postnatal months 1–3) were cultured as described previously (Madrid et al., 2006). In brief, mice were killed by inhalation of 100% CO2 followed by rapid decapitation. After removal, ganglia were cut in small pieces and incubated for 45 min at 37°C in a dissociation solution containing (in mm) 155 NaCl, 1.5 K2HPO4, 5.6 HEPES, 4.8 Na-HEPES, and 5 glucose. The solution also contained 0.07% collagenase type XI (Sigma, St. Louis, MO) and 0.3% dispase (Invitrogen, Carlsbad, CA). After incubation, tissue fragments were gently triturated with a fire-polished glass pipette, and the resultant suspension was centrifuged at 1700 rpm for 10 min. The pellet obtained was resuspended and cultured in a medium containing: 89% minimum essential medium (MEM), 10% fetal calf serum supplemented with 1% MEM vitamins (Invitrogen), 100 μg/ml penicillin/streptomycin, and nerve growth factor (NGF; mouse 7S, 100 ng/ml; Sigma). Cells were plated on poly-l-Lysine-coated glass coverslips and used after 1 d in culture. TRPV1−/− mice (stock number 003770) (see Caterina et al., 2000) were purchased from The Jackson Laboratory (Bar Harbor, ME).
Measurement of the intracellular [Ca2+]i.
Cells were incubated with 2 μm Fura-2 AM ester for 30 min at 37°C. Intracellular Ca2+ concentration ([Ca2+]i) was measured on a monochromator-based imaging system consisting of a Polychome IV monochromator (Till Photonics, Martinsried, Germany) and a Roper Scientific (Tucson, AZ) CCD camera connected to a Zeiss (Oberkochen, Germany) Axiovert 200M inverted microscope, or on an Olympus (Tokyo, Japan) CellM̂ system. Fluorescence was measured during excitation at 340 and 380 nm, and after correction for the individual background fluorescence signals, the ratio of the fluorescence at both excitation wavelengths (F340/F380) was monitored. The extracellular solution used in [Ca2+]i measurements contained (in mm) 140 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 5 glucose, and 10 HEPES, pH 7.4.
Electrophysiological recordings.
Currents were recorded in the whole-cell configuration of the patch-clamp technique using an EPC-7 or EPC-9 amplifier and Pulse (HEKA Elektronik, Lambrecht/Pfalz, Germany) or pClamp 9 (Molecular Devices, Sunnyvale, CA) software. Data were sampled at 5–20 kHz and filtered off-line at 1–5 kHz. Between 40 and 60% of the series resistance was compensated to minimize voltage errors. Most experiments were performed using identical intracellular and extracellular Ca2+-free solutions containing (in mm) 150 NaCl, 5 MgCl2, 5 EGTA, and 10 HEPES, pH 7.4. Indicated experiments were performed using a Ca2+-containing extracellular solution with (in mm) 150 NaCl, 5 CsCl, 5 CaCl2, 10 glucose, and 10 HEPES, pH 7.4.
Reagents.
Clotrimazole [1-(chloro-α, α-diphenylbenzyl)-imidazole], capsaicin, and mustard oil (allyl isothiocyanate; MO) were purchased from Sigma. Menthol (dl-menthol) was from Merck (Darmstadt, Germany). N-(-4-tertiarybutylphenyl)-4-(3-cholorpyridin-2-yl)tetrahydropyrazine-1(2H)-carboxamide (BCTC) was a kind gift from Grünenthal (Aachen, Germany). Clotrimazole and mustard oil were dissolved in DMSO, and menthol and capsaicin in ethanol.
Behavioral analysis.
Thirty-three adult mice were used in the behavioral experiments [16 wild-type (WT) and 17 TRPV1−/−]. All procedures described were approved by the local ethics committee and followed guidelines of the International Association for the Study of Pain (Zimmermann, 1983). Animals were housed maximum six per cage on a 12 h light/dark cycle with food and water ad libitum.
Mice were placed in individual transparent plastic chambers and allowed to acclimatize for at least 1 h before testing. CLT (0.5%) and CLT plus BCTC (0.5% + 1 μm) were prepared in a solution of the following composition: 10% DMSO, 10% Tween 80, and 80% PBS. Drugs were delivered in a volume of 10 μl via intraplantar injection to the left hind paw using a 30-gauge needle coupled to a Hamilton syringe. In control experiments, mice were injected with vehicle alone.
Nocifensive behavior (licking, biting, flinching, lifting, or guarding of the injected hind paw) was monitored for 10 min after injection and expressed as the total number of seconds during which these behaviors were exhibited over the 10 min period.
The hot plate (Letica Scientific Instruments, Barcelona, Spain) was maintained at 55 ± 0.5°C with a cutoff latency of 30 s to avoid tissue damage. Endpoints for withdrawal were licking, biting, and flinching of the hind paws, and jumping. The mean of two baseline tests determined the preinjection withdrawal latency. Postinjection hot plate tests were performed immediately after the evaluation of nocifensive behavior and the results were expressed as a percentage of preinjection withdrawal latency.
Data analysis.
Data analysis was performed using Origin 7.0 (OriginLab, Northampton, MA). Group data are expressed as mean ± SEM from n independent experiments. Significance between groups was tested using the unpaired or paired Student's t tests or the Kolmogorov–Smirnov test for comparison of non-normally distributed data sets.
Plots of steady-state currents (Iss) in function of V were fitted using a modified Boltzmann function as follows:
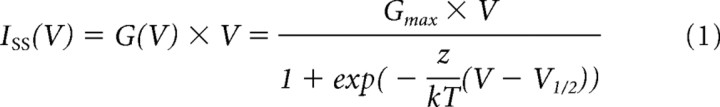 |
where Gmax is the maximal conductance (kept constant for a given cell), z the gating charge (in elementary charge units: eo = 1.6 × 10−19 C), V1/2 the voltage for half-maximal activation, k the Boltzmann constant (1.38 × 10−23 JK−1), and T the absolute temperature. Apparent Popen was determined as G/Gmax, where G was obtained from steady-state currents as Iss/V. At 0 mV, G was taken as the value obtained from the fit using Equation 1. As an alternative approach, voltage-dependent activation curves were also estimated from peak tail currents measured at a fixed potential (+60 mV for TRPM8 and TRPV1; −150 mV for TRPA1) following steps to different test voltages. Outward tail currents (at +60 mV) were used in the case of TRPM8 and TRPV1, because inward currents at negative voltages inactivate too fast (time constant <1 ms) to be reliably measured (Voets et al., 2004). In general, both approaches yielded V1/2 estimates that differed by <10 mV.
Results
Screening the effects of clotrimazole on sensory TRP channels
To evaluate the possible role of TRP channels in mediating the sensory effects of CLT, we performed intracellular Ca2+ imaging experiments on HEK293 cells expressing TRP channels present in sensory neurons and skin keratinocytes, namely TRPV1–4, TRPM8, and TRPA1 (Voets et al., 2005). Note that a previous report already described irreversible inhibition by CLT of TRPM2 (Hill et al., 2004), an ADP-ribose-gated, heat-activated channel expressed in brain and blood cells but not in sensory neurons (Perraud et al., 2001; Togashi et al., 2006).
Application of 10 μm CLT to HEK293 cells expressing the heat-activated TRPV1 produced a robust [Ca2+]i increase, which was of comparable magnitude to the response to 100 nm capsaicin (Fig. 1A). In contrast, CLT (10 μm) did not evoke a Ca2+ increase in control HEK293 cells or cells expressing the closely related heat-activated TRPV2, TRPV3, and TRPV4 channels (Caterina et al., 1999; Peier et al., 2002b; Smith et al., 2002; Xu et al., 2002; Nilius et al., 2004) (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Application of CLT led to a reduction of the basal [Ca2+]i in cells expressing TRPV4, suggesting a partial inhibition of basal channel activity (supplemental Fig. 1C, available at www.jneurosci.org as supplemental material), which is in line with previous studies showing an indirect inhibition of TRPV4 activity by cytochrome P450 inhibitors (Watanabe et al., 2003).
Figure 1.
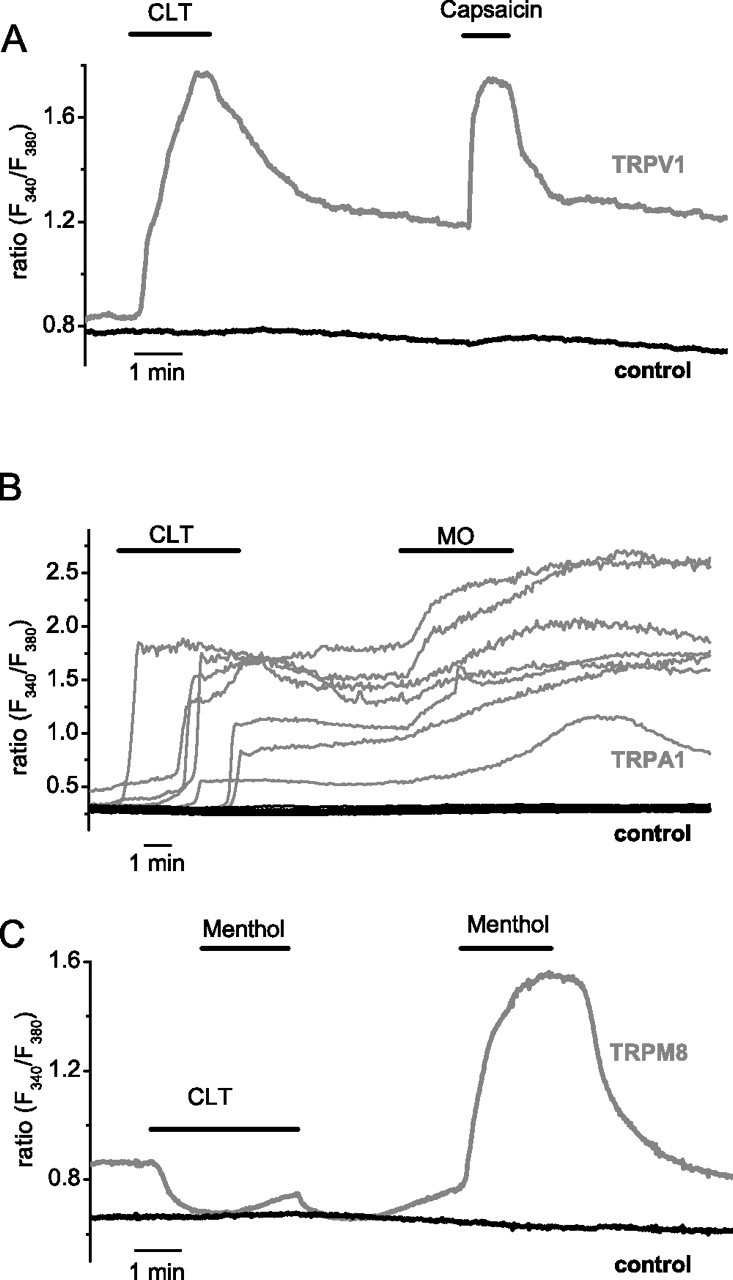
Effects of clotrimazole on calcium responses mediated by heterologously expressed TRPV1, TRPA1 and TRPM8. A, Average ratiometric [Ca2+]i responses to 5 μm CLT and 100 nm capsaicin in TRPV1-transfected HEK cells loaded with Fura-2 AM (red trace; n = 8). In the same field, nontransfected cells did not respond to either stimuli (black trace; n = 10). B, Ratiometric [Ca2+]i responses to 5 μm CLT and 20 μm MO in TRPA1-transfected HEK cells. Recordings from seven individual cells (red traces) are shown to illustrate the variability in the time course of the CLT responses. In the same field, nontransfected cells did not respond to either stimuli (black traces). C, Average ratiometric [Ca2+]i responses to 100 μm menthol in the presence and absence of 10 μm CLT in TRPM8-transfected HEK cells (red trace; n = 10) and nontransfected cells in the same field (black trace; n = 5).
HEK293 cells expressing TRPA1, a sensor of various irritant chemicals, also displayed a clear [Ca2+]i increase in response to 10 μm CLT (Fig. 1B). However, whereas [Ca2+]i responses to CLT in TRPV1-expressing cells were fast, reaching maximal amplitude in <1 min (Fig. 1A), TRPA1-expressing cells responded significantly slower, with variable delays of up to 3 min (Fig. 1B).
HEK293 cells expressing the cold-activated TRPM8 exhibit an increased basal intracellular Ca2+ concentration ([Ca2+]i) caused by significant inward TRPM8 current at room temperature (McKemy et al., 2002; Peier et al., 2002a; Voets et al., 2004). In these cells, application of 10 μm CLT rapidly decreased the basal [Ca2+]i and strongly impaired the response to 100 μm menthol (Fig. 1C).
With respect to the unwanted sensory side effects of CLT, activation of TRPV1 and TRPA1 appears most relevant. Moreover, because activation of TRPM8 in sensory neurons by cooling or menthol elicits analgesia, inhibition of this channel may also contribute to the irritation evoked by CLT. We therefore set out to evaluate the effects of CLT on these three channels in more detail.
Clotrimazole activates TRPV1
In whole-cell patch-clamp experiments, application of 5 μm CLT activated robust inward and outward whole-cell currents in HEK293 cells expressing TRPV1 (Fig. 2A). This activation was never observed in nontransfected cells (data not shown). The current–voltage relationship of the CLT-activated current showed outward rectification and a reversal potential close to 0 mV, in line with the previously described properties of TRPV1 (Caterina et al., 1997; Voets et al., 2004) (Fig. 2B). CLT also activated outwardly rectifying cation currents when applied to the intracellular face of inside-out patches from TRPV1-expressing cells (supplemental Fig. 2, available at www.jneurosci.org as supplemental material), indicating that CLT activates the channel in a membrane-delimited manner. As shown in Figure 2, A and C, currents evoked in the continuous presence of CLT at concentrations 5 μm or higher exhibited desensitization, which was not evident at lower concentrations. The effect of CLT was voltage dependent (Fig. 2D), suggesting that this drug affects the mechanism of TRPV1 activation rather than then number of active TRPV1 channels. Previous studies have shown that several temperature-sensitive TRP channels (TRPV1, TRPV3, TRPM8, TRPM4, and TRPM5) are voltage-gated, and that thermal and chemical stimuli act on these channels by shifting the voltage dependence of activation (Voets et al., 2004, 2007a; Chung et al., 2005; Talavera et al., 2005; Malkia et al., 2007). To investigate the voltage dependence of the effect of CLT on TRPV1 in more detail, we applied voltage steps ranging from −120 to +160 mV (Fig. 2D,E), which allows determining the voltage-dependent activation curves from the steady-state current–voltage relationships (see Materials and Methods). Analysis of steady-state currents measured during voltage steps at 25°C revealed a strong leftward shift of the activation curve, with a change in the voltage for half-maximal activation (V1/2) from 127 ± 23 mV in control to 41 ± 13 mV in the presence of 3 μm CLT (n = 4) (Fig. 2E,F). Even larger shifts of the activation curves were evident at higher concentrations, but these were difficult to quantify because of the rapid current desensitization (Fig. 2C). From these data we can conclude that CLT activates TRPV1 by shifting the voltage dependence of activation, highly similar to the effects of capsaicin (Voets et al., 2004).
Figure 2.
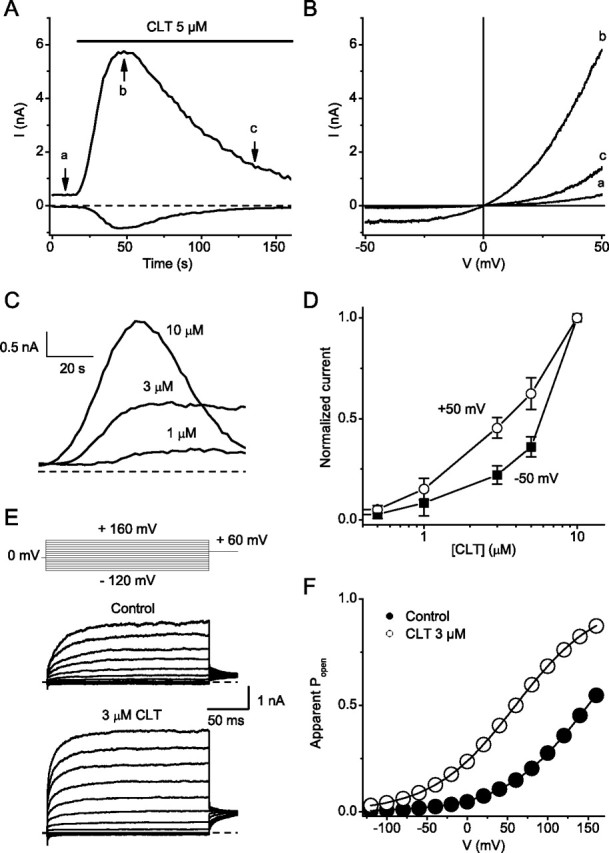
Clotrimazole activates TRPV1-mediated whole-cell currents in HEK cells. A, Time course of the development of inward (at −50 mV) and outward (at +50 mV) TRPV1 current after application of 5 μm CLT. B, Current–voltage relations obtained during 200-ms voltage ramps from −50 to +50 mV applied at the time points indicated in (A). C, Comparison of the time course of TRPV1 current development after application of 1, 3, and 10 μm clotrimazole. D, Normalized dose–response curve for the CLT-induced TRPV1 current development at the indicated voltages. E, Currents elicited by 200 ms voltage steps ranging from −120 to +160 mV before (left) and during (right) addition of 3 μm CLT. F, Activation curves obtained from steady-state currents in the absence (filled circles) and presence of 3 μm CLT (open circles).
Clotrimazole activates TRPA1
Next, we investigated the effects of CLT on heterologously expressed TRPA1 channels. Note that most initial experiments were performed using tetracycline-inducible expression of TRPA1 in CHO cells; highly similar results were later obtained in transiently expressed HEK293 cells (see Figs. 1, 6) (and data not shown). In control (Ca2+-free) extracellular solution and in the absence of agonist, voltage ramps from −150 to +150 mV elicited sizable, strongly outwardly rectifying currents (0.12 ± 0.03 nA at +75 mV; −0.025 ± 0.005 nA at −75 mV; n = 12), indicative of some basal TRPA1 channel activity (Fig. 3A,B). Application of CLT at a concentration of 5 μm evoked a modest and reversible increase of this outwardly rectifying current (Fig. 3A,B). CLT had no measurable effect on whole-cell currents in naive CHO cells (data not shown). The effect of CLT on TRPA1 current was dose-dependent, and did not saturate at concentrations up to 30 μm, which is close to the solubility limit of CLT in extracellular solution (Fig. 3F).
Figure 6.
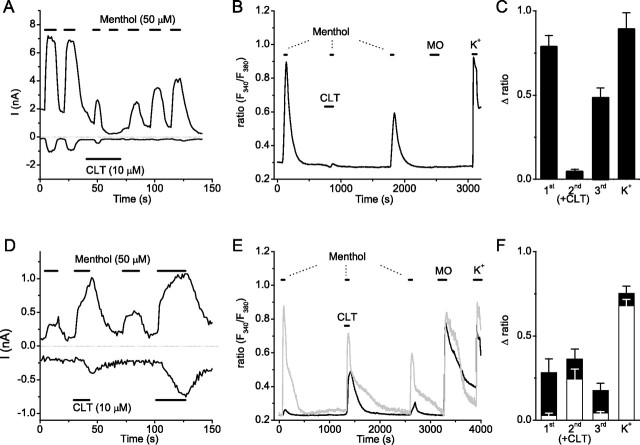
Clotrimazole distinguishes between TRPM8- and TRPA1-mediated menthol-responses. A, Whole-cell currents in HEK cells transfected with TRPM8 illustrating the reversible inhibition of responses to menthol (100 μm) by CLT(10 μm). B, Ratiometric [Ca2+]i responses to menthol (100 μm) in an MO-insensitive sensory neuron in the absence and presence of CLT (10 μm). C, Mean responses in menthol-sensitive, MO-insensitive sensory neurons (n = 15) to a stimulation protocol as in B, illustrating the strong inhibition (95 ± 2% block) of the menthol response by CLT. D, Same as in A, but for HEK cells transfected with TRPA1, illustrating the reversible potentiation of menthol responses by 10 CLT. E, Same as in B, but in MO-sensitive TRPV1−/− sensory neurons. Two traces are shown, illustrating two distinct patterns of responses in these cells. A cell with a small response to a first menthol application (black trace; Δratio, 0.05) exhibited a strongly potentiated response to the combination menthol plus CLT. In contrast, CLT-induced potentiation of the menthol response was not observed in a cell with a robust response to a first menthol application (gray trace; Δratio, 0.65). F, Mean responses in MO-sensitive TRPV1−/− sensory neurons (n = 15) to a stimulation protocol as in E.
Figure 3.
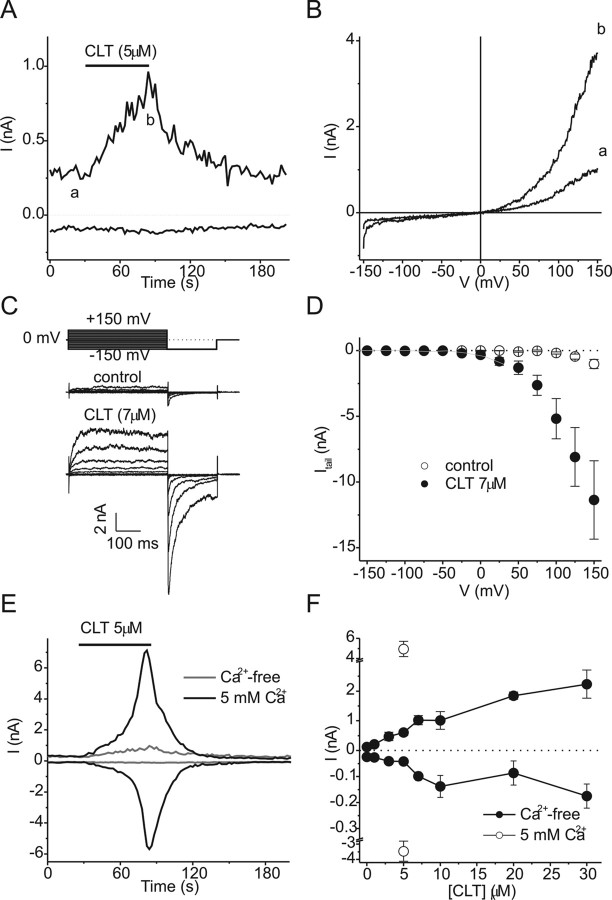
Clotrimazole activates TRPA1-mediated whole-cell currents in CHO cells. A, Time course of the development of inward (at −75 mV) and outward (at +75 mV) TRPA1 currents after application of 5 μm CLT in control (Ca2+-free) extracellular solution. B, Current–voltage relations obtained during 500 ms voltage ramps from −150 to +150 mV at the time points indicated in A. C, Currents evoked in response to 400 ms voltage steps ranging from −150 to 150 mV followed by an invariant step to −150 mV before and during application of 7 μm CLT. D, Peak inward tail currents at −150 mV in the absence (filled circles) and presence of 7 μm CLT (open circles). The gray line represents the data obtained in the absence of CLT, shifted to the left by 110 mV. E, Time course of TRPA1 current development and desensitization after application of 5 μm clotrimazole in the presence of extracellular Ca2+ (5 mm; black line). A time course obtained in Ca2+-free solution (gray; same data as in Fig. 3A) is shown for comparison. F, Dose–response curve for the CLT-induced inward and outward TRPA1 current.
As TRPA1 shows voltage dependence (Karashima et al., 2007; Macpherson et al., 2007; Zurborg et al., 2007), we analyzed the effects of CLT using a voltage step protocol consisting of 400-ms voltage steps to test potentials ranging from −150 to +150 mV followed by an invariant step to −150 mV (Fig. 3C). In the absence of CLT, significant inward tail currents at −150 mV were only measured following test potentials ≥ +100 mV (Fig. 3C,D). After application of 7 μm CLT, inward tail currents were already significant at test potentials ≥0 mV, and the peak amplitude of the tail current following a test potential of +150 mV increased 10-fold (Fig. 3C,D). Even in the presence of CLT, tail current amplitudes did not show saturation for test potentials up to +150 mV (Fig. 3D). As a result, reliable estimation of Gmax and determination of V1/2 from Boltzmann was not feasible. Yet, a comparison of the voltage dependence of tail current amplitudes indicates that 7 μm CLT causes a leftward shift of the voltage-dependent activation curve of >100 mV (Fig. 3D).
Intracellular Ca2+ directly activates TRPA1, and permeating Ca2+ exerts a strong positive feedback on TRPA1 activity (Nagata et al., 2005; Doerner et al., 2007; Zurborg et al., 2007). Moreover, entering Ca2+ can induce rapid desensitization of TRPA1 currents activated by agonists such as mustard oil (Nagata et al., 2005; Doerner et al., 2007). Therefore, we tested the effects of CLT on TRPA1 in Ca2+-containing extracellular solution and found a striking potentiation of the agonist effect of CLT. In the presence of 5 mm Ca2+, outward TRPA1 currents activated by 5 μm CLT were increased ∼10-fold and inward currents ∼100-fold in comparison with Ca2+ free conditions (Fig. 3E,F). Moreover, current activation in the presence of extracellular Ca2+ was followed by pronounced desensitization, despite the continued presence of CLT (Fig. 3E,data not shown). Thus, the efficacy of CLT to activate TRPA1 is strongly dependent on extracellular Ca2+ and Ca2+ influx through the channel. It should also be noted that the maximal CLT-induced inward current amplitude in Ca2+-containing solution (−3.47 ± 0.65 nA; n = 10) was only half of that induced by 20 μm MO (−6.45 ± 0.7 nA; n = 8; p < 0.01), indicating that CLT is a partial TRPA1 agonist.
Clotrimazole inhibits TRPM8
Application of 100 μm menthol at 25°C activates a current in TRPM8 transfected HEK293 cells that reverses near 0 mV and exhibits outward rectification (Fig. 2B), in line with previous reports (McKemy et al., 2002; Peier et al., 2002a; Voets et al., 2004). CLT (10 μm) produced a pronounced suppression of the menthol-induced current, which recovered to a large extent after washout (Fig. 4A). Detailed analysis of the dose dependence revealed that CLT is a more potent inhibitor of inward current (IC50 = 200 nm at −50 mV) than outward current (IC50 = 1.2 μm at +50 mV). Analysis of the voltage dependence of TRPM8 using voltage steps revealed that application of 1 μm CLT induced a significant rightward shift of the voltage-dependent activation curve of TRPM8. V1/2 increased by 89 ± 7 mV (n = 5), resulting in an almost complete inhibition of channel activity at negative voltages. The change in V1/2 (ΔV1/2) produced by CLT was dose-dependent (Fig. 4F); fitting a Hill function to the data yielded a concentration for half-maximal effect of 3.0 ± 1.6 μm, a maximal shift of 340 ± 70 mV and a Hill coefficient of 0.9 ± 0.2 (n = 5). Together, these results indicate that CLT inhibits TRPM8 currents by causing a positive shift of the voltage dependence of channel activation.
Figure 4.
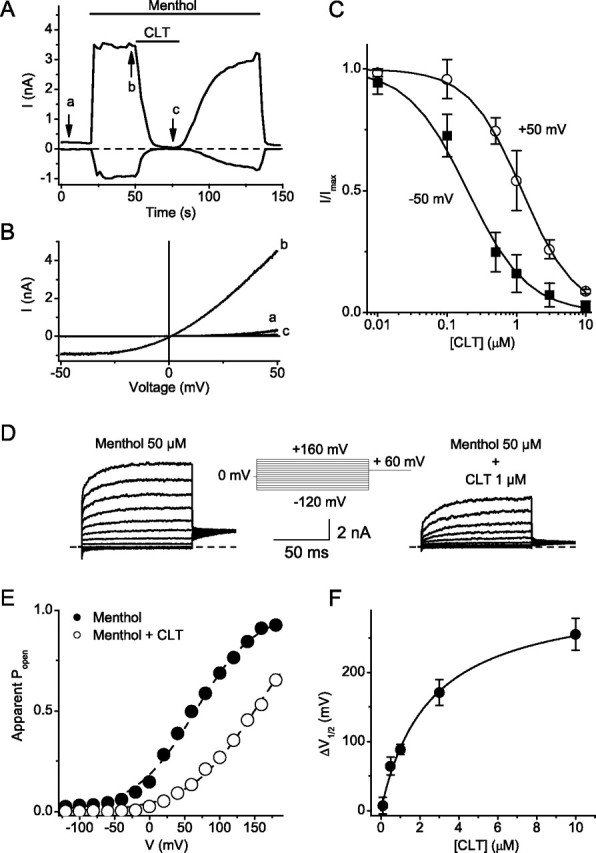
Clotrimazole inhibits TRPM8-mediated whole-cell current in transfected HEK cells. A, Time course of inward (at −50 mV) and outward (at +50 mV) TRPM8 current activation by 100 μm menthol and its reversible inhibition by 10 μm CLT. B, Current–voltage relations obtained during 200 ms voltage ramps from −50 to + 50 mV at the time points indicated in A. C, Dose–response curve for the inhibition of menthol induced TRPM8 current by CLT at −50 mV (filled square) and +50 mV (open circles). D, Currents elicited in response to 100 ms voltage steps ranging from −120 to +160 mV in presence of 100 μm menthol, before (left) and during (right) addition of CLT. E, Activation curves obtained from steady-state currents for menthol-induced currents in the absence (filled circles) and presence (open circles) of 1 μm clotrimazole. F, Change in V1/2 as a function of CLT concentration.
CLT excites a subset of capsaicin- and mustard oil-sensitive sensory neurons
Next, we tested the effects of CLT on cultured mouse trigeminal sensory neurons. TRPV1, TRPA1 and TRPM8 are expressed in subsets of primary sensory neurons where they function as thermosensors and/or chemosensors. Although the exact distribution and colocalization of these channels in these neurons is somewhat controversial, the consensus is that TRPA1 and TRPV1 have a partially overlapping expression pattern in capsaicin-sensitive nociceptive neurons, whereas TRPM8 is expressed in a clearly distinct subpopulation of sensory neurons (Story et al., 2003; Kobayashi et al., 2005).
Application of 10 μm CLT evoked an increase in [Ca2+]i in a subset of cells that corresponded to 21% of viable neurons (Fig. 5A,C). Importantly, all CLT-activated neurons were also sensitive to 1 μm capsaicin, indicating that CLT-induced activation is restricted to TRPV1-expressing neurons (Fig. 5A, inset). It should be noted, however, that only approximately half of the capsaicin-sensitive neurons, which corresponded to 49% of the total cell population, responded to 10 μm CLT (Fig. 5A).
Figure 5.
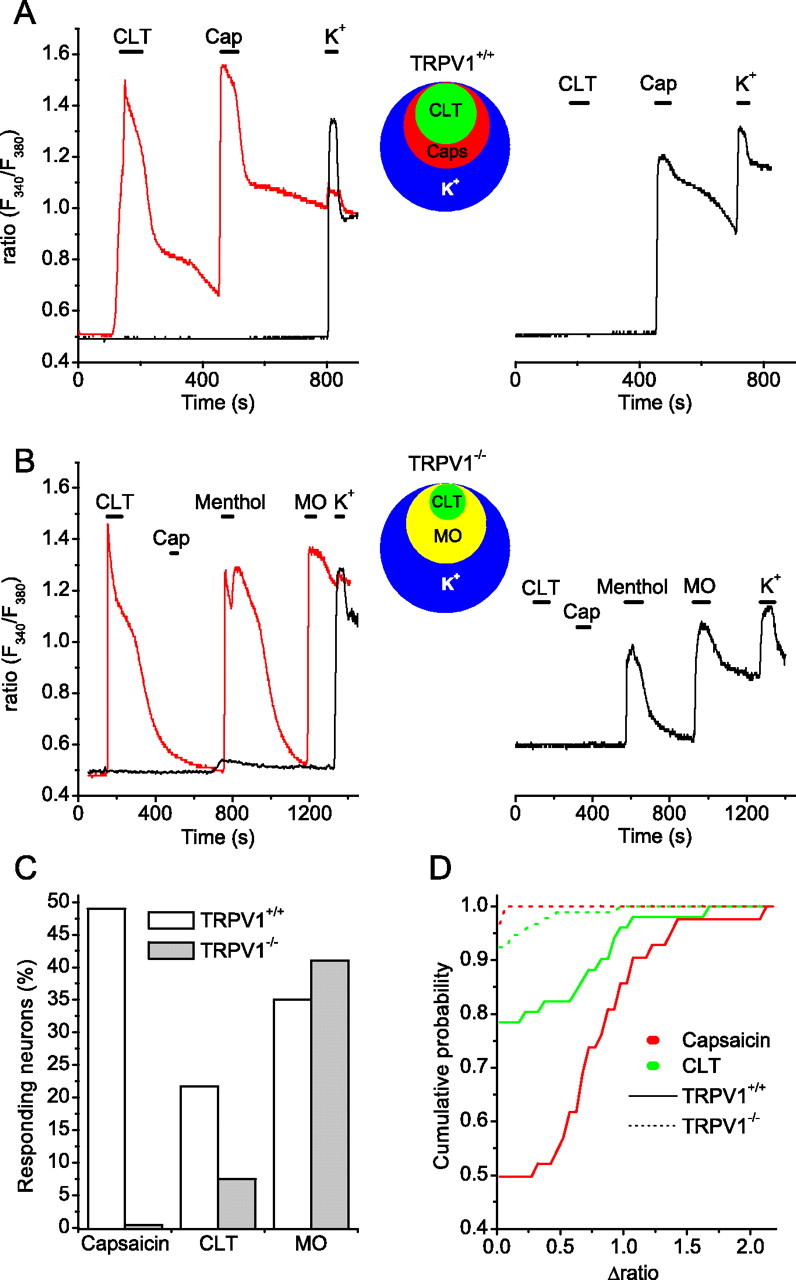
Clotrimazole responses in mouse trigeminal neurons. A, Ratiometric [Ca2+]i measurements from Fura-2 AM-loaded neurons from wild type mice showing different types of responses to 10 μm CLT, 1 μm capsaicin (Caps) and 60 mm K+. As depicted by circles (inset), CLT responses were only observed in capsaicin-sensitive neurons. B, Representative ratiometric [Ca2+]i responses to 10 μm CLT, 100 μm capsaicin, 100 μm menthol (Men), 100 μm MO, and 60 mm K + in neurons (n = 83) from TRPV1−/− mice. As depicted by circles (inset), CLT responses were only observed in mustard oil sensitive neurons. C, Percentage of wild-type and TRPV1−/− neurons responding to capsaicin, CLT, and mustard oil. D, Cumulative probability plot of the responses to 1 μm capsaicin (red) and 10 μm CLT (green) in wild-type (solid lines) and TRPV1−/− (dashed lines) neurons.
To evaluate the relative contribution of TRPV1 and TRPA1 channels to the CLT-induced responses, we tested the effects of CLT on trigeminal neurons from TRPV1−/− mice. As expected from previous work (Caterina et al., 2000), all TRPV1-deficient neurons were fully unresponsive to capsaicin (Fig. 5B,C). Notably, the responsiveness to CLT was significantly reduced compared with wild type (p < 0.001, Kolmogorov–Smirnov test): only 7% of the cells showed a detectable response to 10 μm CLT (Fig. 5C,D), and the average amplitude of the [Ca2+]i increase in responsive cells was lower than in wild-type cells (Δratio, 0.72 ± 0.12 for WT vs 0.31 ± 0.12 for TRPV1−/− neurons; p < 0.05). Importantly, all CLT-responsive TRPV1-deficient neurons were also stimulated by MO (100 μm), indicating that CLT sensitivity in the TRPV1−/− neurons is restricted to TRPA1-expressing neurons (Fig. 5B). Together, these data indicate that CLT excites nociceptive neurons through activation of TRPV1 and to a lesser extent TRPA1 channels.
CLT differentiates TRPM8- and TRPA1-mediated responses to menthol
Previously, menthol-induced responses in sensory neurons were considered strong evidence for the expression of TRPM8. However, this vision needs to be revised after our recent findings that menthol also has an agonistic effect on TRPA1 channels (Karashima et al., 2007). Given its opposite effects on TRPA1 and TRPM8, we investigated whether CLT could be used to distinguish between TRPA1- and TRPM8-mediated menthol responses. As illustrated in Figure 6, A and D, repeated application of 50 μm menthol leads to rapid and reversible activation of TRPM8 and TRPA1 heterologously expressed in HEK cells. Coapplication of 10 μm CLT leads to a strong inhibition of the menthol-response in TRPM8-expressing cells, whereas the menthol response in TRPA1-expressing cells is clearly potentiated by CLT (Fig. 6A,D).
To isolate the effects of CLT on TRPM8-mediated menthol responses in sensory neurons, we studied its effects in mustard oil-insensitive, menthol-sensitive trigeminal neurons (Karashima et al., 2007). In this subpopulation, which corresponds to ∼10% of viable neurons (Karashima et al., 2007), 10 μm CLT caused a decrease of basal [Ca2+]i and produced a strong and reversible inhibition of the [Ca2+]i rise evoked by applying 100 μm menthol (Fig. 6B,C). These results indicate that the inhibitory effect of CLT on basal and menthol-induced TRPM8 activity is preserved in sensory neurons. Then, to dissect the effects of CLT on TRPA1-mediated menthol responses in sensory neurons, we examined the effect of coapplication of menthol and CLT in MO-sensitive trigeminal neurons from TRPV1−/− mice (Karashima et al., 2007). We observed two types of responses in this subset of neurons. Neurons that showed only a small response to a first menthol application (Δratio, <0.3) displayed a significantly enhanced response to a subsequent application of menthol in the presence of CLT (Fig. 6E,F). In contrast, neurons that showed a more robust response to a first menthol application (Δratio, >0.3) did not show significant CLT-induced enhancement (Fig. 6E,F). Possibly, CLT-induced TRPA1 potentiation in these cells is masked by Ca2+-induced desensitization.
Together, these properties demonstrate that CLT can be used as pharmacological tool to discriminate between TRPM8- and TRPA1-mediated chemosensory responses.
CLT causes TRPV1-mediated nocifensive behavior and thermal hyperalgesia
Finally, we tested to what extent the effects of CLT on TRP channels in sensory neurons results in pain-related behavior in mice. Intra-plantar injection of CLT evoked clear nocifensive behavior in wild type mice, as evidenced by strongly increased flinching and licking behavior compared with vehicle-injected mice (Fig. 7A). Coinjection of BCTC, a potent TRPV1 antagonist, significantly attenuated CLT-induced pain behavior (Fig. 7A). Moreover, TRPV1−/− mice did not exhibit significant nocifensive behavior in response to CLT injection (Fig. 7A).
Figure 7.
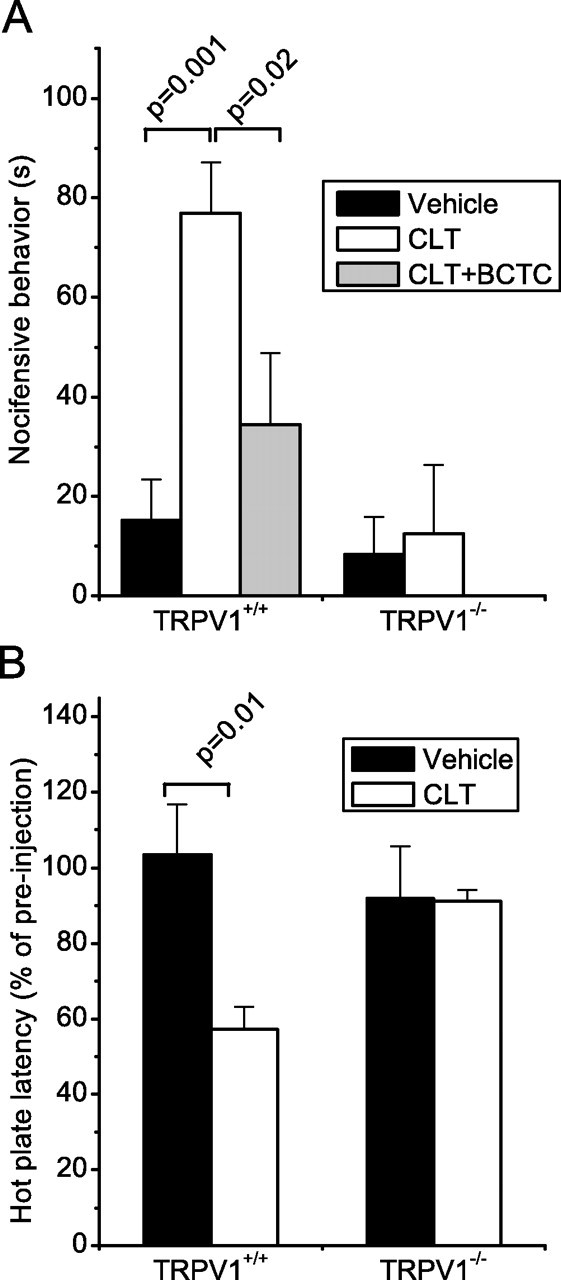
Intraplantar injection of Clotrimazole evokes pain and thermal hyperalgesia in mice. A, Duration of nocifensive behavior in the first 10 min after intraplantar injection of vehicle, CLT or CLT plus BCTC in wild-type and TRPV1−/− mice. B, Change in response latency on a hot plate (55°C) 10–15 min after intraplantar injection of vehicle or CLT wild-type and TRPV1−/− mice.
TRPV1 is involved in detecting noxious heat, and previous studies have shown that several endogenous and exogenous chemicals can sensitize TRPV1 to heat resulting in thermal hyperalgesia in vivo. To investigate whether CLT affects the sensitivity to heat, we assayed the effect of the intraplantar CLT injection on the thermoresponsive behavior using a hot plate assay at 55°C. These experiments were performed 10 min after CLT injection, at which time the acute CLT-induced nocifensive behavior had fully ceased. In line with a previous study (Davis et al., 2000; but see Caterina et al., 2000), hot plate latencies in noninjected or vehicle-injected mice were not significantly different between WT (15.4 ± 2.2 s) and TRPV1−/− mice (13.7 ± 1.1 s; p = 0.50). Importantly, intraplantar injection of CLT caused a significant reduction in hot plate latency in WT but not in the TRPV1−/− mice (Fig. 7B). Together, these behavioral experiments demonstrate that CLT evokes nocifensive behavior and thermal hyperalgesia in mice in a TRPV1-dependent manner.
Discussion
Despite its wide use in various over-the-counter medications, the biological actions of CLT are only partly understood. In particular, the origin of the sensory side effects of oral or topical CLT were not known. Our present results identify TRP channels involved in thermosensation and chemosensation in sensory neurons as novel CLT targets. We demonstrate that CLT is an agonist of TRPV1 and TRPA1, two excitatory TRP channels expressed in nociceptors (Caterina et al., 1997; Caterina et al., 2000; Davis et al., 2000; Story et al., 2003; Jordt et al., 2004; Bautista et al., 2005; Nagata et al., 2005; Bautista et al., 2006; Kwan et al., 2006), and a potent antagonist of TRPM8, a cold- and menthol sensor in cold-sensitive sensory neurons (McKemy et al., 2002; Peier et al., 2002a; Bautista et al., 2007; Colburn et al., 2007; Dhaka et al., 2007; Voets et al., 2007b). Moreover, we show that intraplantar injection of CLT evokes nocifensive behavior and induces hypersensitivity to heat in mice, which can be attenuated by pharmacological inhibition or genetic ablation of TRPV1. These results point at TRPV1 as a major culprit for the irritation and burning that can be experienced after topical application of CLT.
Previous studies have shown that thermal stimuli as well as a variety of chemical agonists and antagonists can modulate activity of certain TRP channels, including TRPV1, TRPM8, and TRPA1, by shifting the voltage dependence of activation (Piper et al., 1999; Brauchi et al., 2004; Voets et al., 2004; Chung et al., 2005; Talavera et al., 2005; Karashima et al., 2007; Malkia et al., 2007; Voets et al., 2007a). Our present results show that CLT also changes the voltage dependence of TRPV1, TRPA1, and TRPM8. In the case of TRPV1 and TRPA1, CLT shifts the voltage dependence toward more negative voltages, which promotes channel opening in the physiological voltage range. In contrast, CLT shifts the voltage dependence of TRPM8 toward more positive voltages, thereby counteracting the effects of menthol and cold on the channel. As such, CLT can also be considered as a gating modifier rather than as an (ant)agonist of these channels.
Two previous reports have provided compelling evidence that activation of TRPA1 by several of its agonists occurs through covalent modification of cysteine residues on the channel (Hinman et al., 2006; Macpherson et al., 2007). The authors of these studies realized that known TRPA1 agonists such as mustard oil, cinnamaldehyde or acrolein are electrophiles that can react with the thiol group of cysteine residues on proteins. One study also provided direct evidence for a covalent modification of TRPA1 by these agonists (Macpherson et al., 2007). Moreover, mutation of specific cysteine residues in the N terminus strongly reduced its sensitivity to cysteine-reactive agonists, but not to icilin, an agonist that exhibits no obvious reactivity to cysteines (Hinman et al., 2006; Macpherson et al., 2007). It is unlikely that CLT activation of TRPA1 involves covalent binding to the channel. First, CLT is neither electrophilic nor known to react with cysteine or other amino acids. Second, the effect of CLT on TRPA1 was readily reversible on washout and could be repeated several times in the same cell. In contrast, covalent modification of cysteine residues can persist for minutes to hours (Macpherson et al., 2007). Thus, we consider it most likely that CLT acts on TRPA1 through a “classical,” noncovalent interaction with the channel, similar to the effects of menthol on TRPA1 (Karashima et al., 2007).
In previous studies it has proven difficult to unambiguously separate TRPM8 and TRPA1 responses in sensory neurons. This is mainly because of the substantial overlap in stimuli that activate TRPM8 and TRPA1: both channels were shown to be activated by icilin, cold and menthol (McKemy et al., 2002; Peier et al., 2002a; Story et al., 2003; Karashima et al., 2007). It should be noted that whether TRPA1 plays a role as a cold sensor in vivo remains a matter of strong debate (Story et al., 2003; Jordt et al., 2004; Bautista et al., 2006; Kwan et al., 2006; Sawada et al., 2007; Zurborg et al., 2007). Previous studies have often used TRPA1-selective agonists such as MO to discriminate between TRPA1- and TRPM8-mediated responses. However, because of their covalent binding to TRPA1 (and possibly other cellular targets) the effects of these agonists are only poorly reversible. Our present findings identify CLT as probably a more useful tool to distinguish between TRPM8- and TRPA1-mediated responses in sensory neurons. CLT can be used as a potent inhibitor of TRPM8, while activating TRPA1 and potentiating TRPA1-mediated responses to agonists such as menthol. Importantly, these effects of CLT are rapidly and almost completely reversible.
Only a subset of capsaicin-sensitive and/or MO-sensitive trigeminal neurons showed a Ca2+ response to 10 μm CLT, whereas 100% of TRPV1- or TRPA1-expressing HEK293 cells responded to the same dose of CLT in Ca2+ imaging or patch-clamp experiments. This indicates that CLT-induced activation of TRPA1 and/or TRPV1 is not always sufficient to provoke a detectable Ca2+ signal in trigeminal neurons, which can be attributed to the lower agonist potency of CLT compared with capsaicin (TRPV1) or MO (TRPA1). Moreover, both TRPV1 and TRPA1 are voltage-dependent, and their sensitivity to activating stimuli such as CLT varies depending on the membrane potential of the neuron. In general, it is well known that the excitability of thermosensory neurons is determined by the relative activity of excitatory and inhibitory ionic channels (Viana et al., 2002). Finally, it cannot be excluded that the some neurons have mechanisms that mediate rapid extrusion or breakdown of CLT and thereby prevent it from significantly activating TRPV1/TRPA1. For example, it has been shown in yeast that high expression of drug extrusion pumps such as multidrug resistance 1 (MDR1) can lead to resistance to CLT (White et al., 2002; Looi et al., 2005). Independent of the mechanism, the lower cellular sensitivity to CLT compared with capsaicin or MO matches with the milder perceived sensory effects of CLT when applied to the tongue or mucous membranes.
It is known that topical application of CLT preparations in humans can evoke unwanted side effects such as irritation and burning of the skin and mucous membranes (Binet et al., 1994; del Palacio et al., 2001). Our present results provide a straightforward explanation for such sensory side-effects, namely activation of TRPV1- and TRPA1-containing nociceptive neurons. Most importantly, we found that intraplantar injection of CLT evoked robust nocifensive behavior in mice and increased the sensitivity to noxious heat, similar to what has been observed after intraplantar injection of selective TRPV1 agonists such as capsaicin or resiniferatoxin (Caterina et al., 2000). As for capsaicin and resiniferatoxin (Caterina et al., 2000), we found that both the acute nocifensive behavior and the thermal hyperalgesia evoked by CLT were completely absent in TRPV1−/− mice. The absence of obvious signs of pain-related behavior in the TRPV1−/− mice could indicate that TRPA1 plays only a minor role in the initiation of CLT-induced pain. Our results obtained in heterologous systems and isolated sensory neurons suggest, however, that activation of TRPA1 may become more relevant to nociception at higher CLT concentrations or when CLT is coapplied with other TRPA1-agonists such as menthol, as is the case in many CLT-containing creams.
In conclusion, we have identified thermosensory and chemosensory TRP channels in sensory neurons as novel targets of the widely used drug CLT. CLT is, to our knowledge, the most sensitive known inhibitor of TRPM8, and as such CLT may have therapeutic potential for the treatment of TRPM8-related conditions such as cold allodynia or even certain types of malignancies (Bidaux et al., 2007; Colburn et al., 2007; Voets et al., 2007b). In addition, we demonstrated that CLT represents a useful tool to discriminate between TRPM8- and TRPA1-mediated responses. Finally, knowledge of the agonist effect of CLT on TRP channels in nociceptors may form the basis for the development of new CLT preparations with reduced sensory side effects.
Footnotes
This work was supported by grants from the Human Frontiers Science Program (RGP32/2004), the Belgian Federal Government (IUAP P5/05), the Research Foundation-Flanders (G.0172.03; G.0565.07), the Research Council of the KU Leuven (GOA 2004/07; EF/95/010), the Spanish Minsitry of Education (SAF2004-01011), the Generalitat Valenciana Predoctoral Fellowship Program (CTBPRB/2003/151), and the Fundación Marcelino Botín. We thank the members of our laboratories for helpful discussions, and J. Prenen and A. Janssens for technical assistance.
References
- Alvarez J, Montero M, Garcia-Sancho J. High affinity inhibition of Ca2+-dependent K+ channels by cytochrome P-450 inhibitors. J Biol Chem. 1992;267:11789–11793. [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Benzaquen LR, Brugnara C, Byers HR, Gatton-Celli S, Halperin JA. Clotrimazole inhibits cell proliferation in vitro and in vivo. Nat Med. 1995;1:534–540. doi: 10.1038/nm0695-534. [DOI] [PubMed] [Google Scholar]
- Bidaux G, Flourakis M, Thebault S, Zholos A, Beck B, Gkika D, Roudbaraki M, Bonnal JL, Mauroy B, Shuba Y, Skryma R, Prevarskaya N. Prostate cell differentiation status determines transient receptor potential melastatin member 8 channel subcellular localization and function. J Clin Invest. 2007;117:1647–1657. doi: 10.1172/JCI30168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binet O, Soto-Melo J, Delgadillo J, Videla S, Izquierdo I, Forn J. Flutrimazole 1% dermal cream in the treatment of dermatomycoses: a randomized, multicentre, double-blind, comparative clinical trial with 1% clotrimazole cream. Flutrimazole Study Group. Mycoses. 1994;37:455–459. doi: 10.1111/j.1439-0507.1994.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Brauchi S, Orio P, Latorre R. Clues to understanding cold sensation: thermodynamics and electrophysiological analysis of the cold receptor TRPM8. Proc Natl Acad Sci USA. 2004;101:15494–15499. doi: 10.1073/pnas.0406773101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnara C, Gee B, Armsby CC, Kurth S, Sakamoto M, Rifai N, Alper SL, Platt OS. Therapy with oral clotrimazole induces inhibition of the Gardos channel and reduction of erythrocyte dehydration in patients with sickle cell disease. J Clin Invest. 1996;97:1227–1234. doi: 10.1172/JCI118537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Chung MK, Guler AD, Caterina MJ. Biphasic currents evoked by chemical or thermal activation of the heat-gated ion channel, TRPV3. J Biol Chem. 2005;280:15928–15941. doi: 10.1074/jbc.M500596200. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D'Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- del Palacio A, Ortiz FJ, Perez A, Pazos C, Garau M, Font E. A double-blind randomized comparative trial: eberconazole 1% cream versus clotrimazole 1% cream twice daily in Candida and dermatophyte skin infections. Mycoses. 2001;44:173–180. doi: 10.1046/j.1439-0507.2001.00632.x. [DOI] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007;282:13180–13189. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- Hill K, McNulty S, Randall AD. Inhibition of TRPM2 channels by the antifungal agents clotrimazole and econazole. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:227–237. doi: 10.1007/s00210-004-0981-y. [DOI] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock CA, Dickinson K, Brown SB, Evans EG, Adams DJ. Interaction of azole antifungal antibiotics with cytochrome P-450-dependent 14 alpha-sterol demethylase purified from Candida albicans. Biochem J. 1990;266:475–480. doi: 10.1042/bj2660475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, Nilius B. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci. 2007;27:9874–9884. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with aδ/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- Koletar SL, Russell JA, Fass RJ, Plouffe JF. Comparison of oral fluconazole and clotrimazole troches as treatment for oral candidiasis in patients infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1990;34:2267–2268. doi: 10.1128/aac.34.11.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Looi CY, EC DS, Seow HF, Rosli R, Ng KP, Chong PP. Increased expression and hotspot mutations of the multidrug efflux transporter, CDR1 in azole-resistant Candida albicans isolates from vaginitis patients. FEMS Microbiol Lett. 2005;249:283–289. doi: 10.1016/j.femsle.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- Madrid R, Donovan-Rodriguez T, Meseguer V, Acosta MC, Belmonte C, Viana F. Contribution of TRPM8 channels to cold transduction in primary sensory neurons and peripheral nerve terminals. J Neurosci. 2006;26:12512–12525. doi: 10.1523/JNEUROSCI.3752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkia A, Madrid R, Meseguer V, de la Pena E, Valero M, Belmonte C, Viana F. Bidirectional shifts of TRPM8 channel gating by temperature and chemical agents modulate the cold sensitivity of mammalian thermoreceptors. J Physiol (Lond) 2007;581:155–174. doi: 10.1113/jphysiol.2006.123059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Nilius B, Vriens J, Prenen J, Droogmans G, Voets T. TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol Cell Physiol. 2004;286:C195–C205. doi: 10.1152/ajpcell.00365.2003. [DOI] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002a;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Patapoutian A. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002b;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, Kinet JP, Scharenberg AM. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–599. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- Piper AS, Yeats JC, Bevan S, Docherty RJ. A study of the voltage dependence of capsaicin-activated membrane currents in rat sensory neurones before and after acute desensitization. J Physiol (Lond) 1999;518:721–733. doi: 10.1111/j.1469-7793.1999.0721p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y, Hosokawa H, Hori A, Matsumura K, Kobayashi S. Cold sensitivity of recombinant TRPA1 channels. Brain Res. 2007;1160:39–46. doi: 10.1016/j.brainres.2007.05.047. [DOI] [PubMed] [Google Scholar]
- Sawyer PR, Brogden RN, Pinder RM, Speight TM, Avery Clotrimazole: a review of its antifungal activity and therapeutic efficacy. Drugs. 1975;9:424–447. doi: 10.2165/00003495-197509060-00003. [DOI] [PubMed] [Google Scholar]
- Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 2005;438:1022–1025. doi: 10.1038/nature04248. [DOI] [PubMed] [Google Scholar]
- Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y, Tominaga M. TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J. 2006;25:1804–1815. doi: 10.1038/sj.emboj.7601083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M. Nociception and TRP channels. Handb Exp Pharmacol. 2007;179:489–505. doi: 10.1007/978-3-540-34891-7_29. [DOI] [PubMed] [Google Scholar]
- Viana F, de la Pena E, Belmonte C. Specificity of cold thermotransduction is determined by differential ionic channel expression. Nat Neurosci. 2002;5:254–260. doi: 10.1038/nn809. [DOI] [PubMed] [Google Scholar]
- Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- Voets T, Talavera K, Owsianik G, Nilius B. Sensing with TRP channels. Nat Chem Biol. 2005;1:85–92. doi: 10.1038/nchembio0705-85. [DOI] [PubMed] [Google Scholar]
- Voets T, Owsianik G, Janssens A, Talavera K, Nilius B. TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat Chem Biol. 2007a;3:174–182. doi: 10.1038/nchembio862. [DOI] [PubMed] [Google Scholar]
- Voets T, Owsianik G, Nilius B. Trpm8. Handb Exp Pharmacol. 2007b;179:329–344. doi: 10.1007/978-3-540-34891-7_20. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- White TC, Holleman S, Dy F, Mirels LF, Stevens DA. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob Agents Chemother. 2002;46:1704–1713. doi: 10.1128/AAC.46.6.1704-1713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nat Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]