Abstract
We showed previously that genetic absence epilepsy rats from Strasbourg (GAERS) resist secondary generalization of focal limbic seizures after electrical kindling. We now investigate the effect of intra-amygdaloid injection of kainic acid, as another model of temporal lobe epilepsy, focusing on epileptogenesis, spike-and-wave discharges (SWDs), and the transition from basal to SWD states in GAERS. The EEG was recorded from the hippocampus and cortex of adult GAERS and Wistar rats before kainic acid injections into the basolateral amygdala and for 3 months thereafter. EEG and video recordings monitored SWDs and convulsive seizures. We analyzed spectral changes of the EEG during kainic acid-induced status epilepticus, SWDs, for 10 s before (silent period) and for 2 s before (transition period) SWDs. After the injection of kainic acid, all animals experienced convulsive seizures for at least 3 h. The first convulsive seizure was significantly delayed in GAERS compared with Wistar rats. SWDs and increases in power of the delta, alpha, and beta frequency ranges during the transition period disappeared after the kainic acid injection for 1–3 d and gradually reappeared. Power increases in the delta and alpha ranges were significantly correlated with the number of SWDs, in the beta and alpha ranges with their mean duration. Neo-Timm's staining at the end of experiments demonstrated that mossy fiber sprouting in GAERS is less pronounced than in Wistar rats. Our findings show that mechanisms underlying absence epilepsy and temporal lobe epilepsy interact with each other, although a site of this interaction remains to be defined.
Keywords: spike-and-wave discharges in EEG, power spectrum, fast Fourier transformation, interictal to ictal transition, cortico-thalamo-cortical circuitry, mossy fiber sprouting
Introduction
This study is concerned with a recently demonstrated relationship between temporal lobe epilepsy and typical absence epilepsy. Previous studies of kindling in models of genetic absence epilepsy have shown a resistance to secondary generalization of focal limbic seizures and an increase in number and duration of spike-and-wave discharges after electrical kindling stimulations (Eşkazan et al., 2002; Onat et al., 2005; Aker et al., 2006). The duration of spike-and-wave discharges in adult genetic absence epilepsy rats from Strasbourg (GAERS) or Wistar Albino Glaxo Rats from Rijswijk (WAG/Rij) rats, both well validated genetic models of absence epilepsy (Marescaux et al., 1992; Coenen and van Luijtelaar, 2003), correlates negatively with kindling rates (Onat et al., 2007). This demonstrates that a high level of spike-and-wave discharge activity in absence epilepsy reduces the effectiveness of amygdaloid kindling. The increase in spike-and-wave discharges immediately after kindling stimulations suggests an involvement of the thalamic nuclei responsible for the spike-and-wave discharges (Onat et al., 2007). We have further evaluated the interplay between absence epilepsy and limbic epilepsy by using intra-amygdaloid injection of kainic acid in adult GAERS as another model of temporal lobe epilepsy (Sperk, 1994) to compare with intra-amygdaloid kindling.
A second aim of our study was to determine whether kainic acid has any effect on spike-and-wave discharges or on the transition from basal activity to the spike-and-wave discharges. Spontaneous spike-and-wave discharges are a characteristic feature of the electroencephalogram (EEG) in typical absence epilepsy (Huguenard, 2000). They consist of high-voltage spike and subsequent wave components. The mechanisms that are responsible for transition to spike-and-wave discharges are not clearly understood. Steriade and Amzica (1994) demonstrated that cortical neurons in cats show rhythmic spike trains that become progressively more synchronized at the beginning of the spike-and-wave discharges. Furthermore, in GAERS, precursor activity was typically recorded in cortical units, concomitant with “embryonic” spike-and-wave seizures on the EEG, before a paroxysm was evident on the gross EEG (Seidenbecher et al., 1998). We put special emphasis on the spectral changes in the EEG of the transition period shortly before the onset of spike-and-wave discharge to be able to observe such precursor activity in the EEG.
To determine whether the effect of kainic acid injection on the induction of temporal lobe epilepsy is modified in GAERS and whether kainic acid has any effect on spontaneous spike-and-wave discharges, we examined the following features: (1) behavioral and EEG changes during status epilepticus in Wistar controls and GAERS; (2) spontaneous convulsive seizures after status epilepticus in Wistar controls and GAERS; (3) duration and number of spike-and-wave discharges in GAERS before kainic acid injection and for 3 months thereafter; (4) power spectra related to spike-and-wave discharges and to the transition from the interictal to the spike-and-wave discharge state in GAERS before kainic acid injection and for 3 months thereafter; and (5) synaptic reorganization of mossy fibers of the dentate gyrus 3 months after a kainic acid injection into the amygdaloid region in Wistar controls and GAERS. The latter serve as a marker of epileptogenesis (Cavazos et al., 2003).
Materials and Methods
Animals
Male Wistar rats (n = 11) and GAERS (n = 18) were used in the experiments for short-term EEG analysis during and after kainic acid injection-induced status epilepticus and for Neo-Timm's staining. The GAERS had been bred on a Wistar background (Marescaux et al., 1992). Naive (Wistar, n = 5; GAERS, n = 5) and sham-operated (Wistar, n = 5; GAERS, n = 5) male animals were used as controls without kainic acid injection for the Neo-Timm's staining. Male Wistar rats (n = 6) and GAERS (n = 6) were used to monitor the occurrence of spontaneous convulsive seizures after kainic acid-induced status epilepticus in the long-term. The age of the animals was 5–12 months, and body weights were 200–310 g. All rats were born and raised under standard laboratory conditions in the animal unit of Marmara University. The animals were maintained on a 12 h light/dark cycle with unlimited access to food and water. All experiments were performed with the approval of Marmara University Ethical Committee for Experimental Animals (60.2006.Mar).
Surgery
Animals were anesthetized with ketamine (100 mg/kg, i.p.; Ketasol; Richterpharma) and chlorpromazine (0.5 mg/kg, i.p.; Largactil; Eczacıbaşı). All coordinates for surgery were obtained from the stereotaxic atlas of Paxinos and Watson, 1998) with bregma as a reference point. A guide cannula (C315G; Plastics One) was stereotaxically implanted into the right basolateral amygdala [anteroposterior (AP), −2.3 mm; lateral (L), −4.5 mm from bregma; ventral (V), −7.2 mm from the surface of the skull] for the kainic acid injection. The ventral coordinate was 1 mm dorsal to the target area for the injection. For cortical EEG recordings, stainless-steel screws with soldered insulated wires were bilaterally placed on the frontal (AP, +2 mm; L, ±3.5 mm from bregma) and occipital cortex (AP, −6 mm; L, ±4 mm from bregma). For the hippocampal EEG recordings, a bipolar twisted electrode was positioned into the right CA1 (AP, −4.0 mm; L, −2.0 mm from bregma; V, −2.8 mm from the surface of the skull). A microconnector was soldered to the tips of cortical and hippocampal electrodes and was fixed together with the cannula to the skull with dental acrylic. Animals were monitored for vital functions during a 1 week recovery period. Wounds were treated with iodine solution to prevent infections, and warm 0.9% NaCl (2.5 ml/100 g body weight) was injected subcutaneously to compensate for dehydration.
Experimental protocol
EEG and video recording was performed during a 3 month experimental period, animals were put into a Plexiglas cage, and a microconnector was plugged to the EEG recording system via cables with swivel junctions (SL6C; Plastics One). EEG and video recordings (EMKA Technologies) were started after an adaptation period of 30 min. The EEG signals were amplified, filtered between 0.3 and 500 Hz, and digitized at 1000 samples/s. The duration of the recordings was 12 h each day. Animals had access to food and water ad libitum during the experiments.
On the day before the kainic acid injection, the baseline EEG was recorded for 12 h. On the morning of the first day thereafter, the animals were given a single intra-amygdaloid injection of kainic acid (750 ng) (Ben-Ari et al., 1980; Berger et al., 1986) during the period of EEG and video recording. An infusion cannula (C315I; Plastics One) with a polyethylene tube extension (PE 20) was put into the guide cannula and fixed by a captive collar of the external tube to allow injections to be made in freely moving rats. The polyethylene tube extension was connected to a Hamilton syringe with a capacity of 1 μl. Injections were made at a flow rate of 500 nl/min. Both Wistar rats and GAERS received 750 ng of kainic acid (K0250; Sigma-Aldrich) in 300 nl of artificial CSF (in mm: 2.5 KCl, 125 NaCl, 1.26 CaCl2, 1.18 MgCl2, and 0.2 NaH2PO4, pH 7.0). The infusion cannula assembly was removed 5 min after the completion of the injection to prevent damage of the tubes during the subsequent status epilepticus.
The behavior of rats during the kainic acid-induced status epilepticus was evaluated on the basis of a six-stage scale (Veliskova, 2006): stage 1, staring with mouth clonus; stage 2, automatisms (scratching and wet-dog shake); stage 3, unilateral forelimb clonus; stage 4, bilateral forelimb clonus; stage 5, bilateral forelimb clonus with rearing and falling; and stage 6, generalized tonic-clonic seizures. Stage 1 and 2 seizures are nonconvulsive limbic seizures, whereas stages 3–6 are convulsive.
According to Dudek et al. (2006), the duration of status epilepticus with repetitive convulsive seizures induced by the kainic acid injection should be at least 3 h for the model to be fully established. In our experiments, the status epilepticus was stopped by an injection of zolazepam (10 mg/kg, i.p.; Zoletil 50; Virbac) at the end of 3 h of intense convulsive seizures. In cases of extreme agitation or complete immobility of the rats, which indicated severe neurotoxic effects of the kainic acid, the animals received injections of zolazepam (10 mg/kg, i.p.) before the end of 3 h. For several days after the kainic acid injections, the body weights of the animals were monitored, and warm 0.9% NaCl (2.5 ml/100 g body weight) was injected subcutaneously to prevent dehydration. If the condition of the animals did not improve, they were killed by an injection of thiopental sodium (0.2 mg/kg, i.p.; Pental; I. E. Ulagay) and not included in the data analysis
After the status epilepticus, all surviving animals were monitored for EEG activity and concomitant behavior for continuous 12 h periods on alternate days during an additional 3 months. The data were evaluated for the following periods only: the preinjection day, injection day, the third day, the end of the first, second, and third weeks, and the end of the first, second and third months.
Data analysis
Evaluation of the spike-and-wave discharges.
Spike-and-wave discharges were detected visually in the EEG before and after kainic acid injection. Criteria for spike-and-wave discharges were high-amplitude asymmetric synchronized rhythmic activity expressed as spike-and-wave complexes lasting at least 1 s (Midzianovskaia et al., 2001). The spike-and-wave discharges were analyzed over a 12 h period divided into 12 1-h intervals. The number, mean duration, and cumulative total duration of the spike-and-wave discharges for each 1 h interval were evaluated. The mean duration of the spike-and-wave discharges was calculated as the ratio of cumulative total duration to the number of spike-and-wave discharges.
Evaluation of the nonconvulsive and convulsive limbic seizures.
Short-term observations included the following. The number of nonconvulsive and convulsive seizures per hour during kainic acid-induced status epilepticus was obtained from video recordings. The times to the first nonconvulsive and convulsive seizures during and after the kainic acid-induced status epilepticus were calculated. Long-term observations included the following. The occurrence of the first convulsive seizures and the number of convulsive seizures per hour after kainic acid injection were obtained from video recordings. The results were expressed as mean ± SEM and statistically evaluated by ANOVA (GraphPad Software Prism 4.0). Two-way ANOVA followed by the post hoc Bonferroni's test was used to compare the number and duration of (1) spike-and-wave discharges before and after kainic acid injection in GAERS and (2) the nonconvulsive and convulsive seizures in Wistar controls and GAERS for both the short- and the long-term observation data.
Evaluation of the spectral characteristics of the EEG.
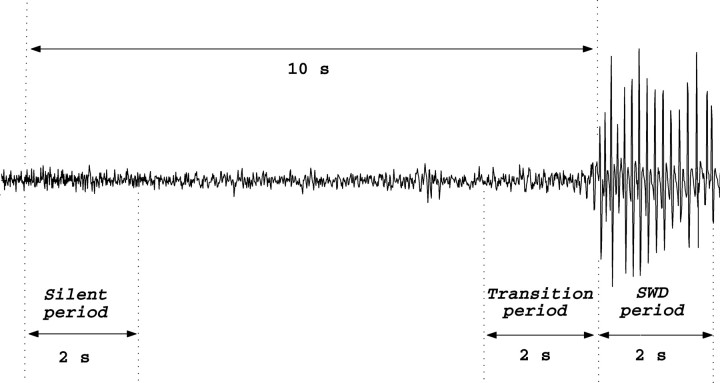
The spectral changes during the 3 h period after completion of kainic acid injection were displayed in the form of spectrograms based on the power spectrum for each 2 s epoch. Kainic acid-induced changes in the frequency spectrum of the EEG during spike-and-wave discharges and during the transition period from the interictal to the ictal state of spike-and-wave discharges were analyzed by computing the power spectrum with the fast Fourier transform (MathWorks Matlab 6.5). The first spike of a spike-and-wave discharge complex with an amplitude at least twofold higher than the basal EEG was accepted as the spike-and-wave discharge onset. The 2 s periods chosen for a spectral analysis are shown in Figure 1: that is, the first (“silent period”) and the last (“transition period”) 2 s epochs of the 10 s before the first spike of the spike-and-wave discharge and also 2 s epoch after the first spike of the spike-and-wave discharge (“spike-and-wave discharge period”) were computed (Fig. 1). Thus, we picked 10 segments of 2 s length out of the whole 12 h EEG recording of each evaluation time point for spectral estimation. The mean power spectrum of 10 spike-and-wave discharge complexes was computed for each animal and each evaluation period. Because the number and length of EEG segments used were the same across all evaluation time points and across all animals, we assume that the results are comparable without an additional normalization on a per spike-and wave discharge or per hour basis. The area under the curve for each frequency range of interest was calculated to represent the signal energy in that frequency band. A one-way ANOVA test was used to compare the total power of each frequency band between the transition period and the silent period. The change in the power was calculated by subtracting the power of the silent period from that of the transition period for the frequency bands that showed significant differences. Correlations between these power differences and either the number or the mean duration of spike-and-wave discharges were tested using the Pearson's correlation analysis.
Figure 1.
The 2 s epochs 10 s before (silent period), immediately before (transition period), and after the first spike of the spike-and-wave discharge (SWD period) in EEG of untreated GAERS.
Histology
Neo-Timm's staining was used to demonstrate the extent of hippocampal synaptic reorganization. Synaptic reorganization of mossy fiber terminals has been recognized as an indicator of increased hippocampal excitability in temporal lobe epilepsy (Cavazos et al., 2003; Cavazos and Cross, 2006). Mossy fiber synaptic terminals contain large amounts of zinc, which can be stained by Timm's method (Danscher, 1981). In view of the correlation of Timm's staining and the degree of hippocampal synaptic reorganization (Cavazos and Cross, 2006), we have compared the Timm's staining in GAERS relative to Wistar controls for naive, sham-operated, and kainic acid-injected groups.
Three months after the kainic acid injections, all animals were killed and perfused for neo-Timm's staining. The perfusion method was modified after Shetty and Turner (1999) and Wenzel et al. (1997). Rats were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine hydrochloride (20 mg/kg, i.p.; Alfazyne; Alfasan) and perfused through the aorta with an infusion pump (50 ml/min) as follows (Wenzel et al., 1997; Shetty and Turner, 1999): (1) 150 ml of 0.09% NaCl; (2) 100 ml of 1% sodium sulfide (30854; Lachema) in 0.1 m PBS (P4417; Sigma-Aldrich); (3) 100 ml of fixative containing 4% paraformaldehyde (P6148; Sigma), 0.1% glutaraldehyde (104239; Merck), and 0.1% picric acid (324 K2636721; Merck) in 0.1 m PBS; and (4) 50 ml of 1% sodium sulfide in 0.1 m PBS. After decapitation, brains were removed and incubated overnight in the same fixative.
Coronal serial sections (40 μm) were cut on a Leica VT 1000S vibratome, and every fifth section was collected on 1% gelatin-coated slides and dried at room temperature overnight. For the visualization of mossy fiber sprouting, sections were developed using the following solution: 60 ml of 50% gum arabic (G9752; Sigma), 10 ml of 2 m sodium citrate buffer [sodium citrate: S4641 (Sigma); citric acid monohydrate: 645 K2656443 (Merck), pH 3.7], 15 ml of 5.67% hydroquinone (H7148; Sigma), and 15 ml of 0.73% silver lactate (L7771; Sigma) (Wenzel et al., 1997). The physical development was performed in the dark at room temperature for 120 min with continuous gentle agitation. The reaction was stopped by using 5% sodium thiosulphate (106512; Merck) for 12 min, followed by a rinse in tap water and then in distilled water. The sections were then stained with thionin, dehydrated, cleared, and coverslipped in Entellan (107961.0100; Merck), observed and photographed with an Olympus BX51TF photomicroscope.
The assessment of mossy fiber sprouting was made using a semiquantitative method according to the criteria used by Cavazos et al. (1991) as follows: 0, no granules between the tip and crests of the dentate gyrus; 1, sparse granules in the supragranular region in a patchy distribution between the tip and crests of the dentate gyrus; 2, more numerous granules in the supragranular region in a continuous distribution between the tip and crests of the dentate gyrus; 3, prominent granules in the supragranular region in a continuous pattern between the tip and crests, with occasional patches of confluent granules between the tip and crests; 4, prominent granules in the supragranular region that form a confluent dense laminar band between the tip and crests; and 5, a confluent dense laminar band of granules in the supragranular region that extends into the inner molecular layer. These scores were recorded for the dentate gyrus in coronal sections from the ipsilateral dorsal hippocampus by an observer who was blind to the groups. The scores of the individual sections for each animal in the groups were averaged, and the differences between group mean scores were analyzed with a two-way ANOVA, followed by the post hoc Bonferroni's test.
Results
Short-term behavioral and EEG changes during status epilepticus in Wistar controls and GAERS
After the kainic acid injection, a status epilepticus developed, and convulsive seizures lasted at least for 3 h in all animals in the Wistar and GAERS groups. Alternating periods of irregular spikes and baseline activity in the EEG were observed during the first h after the kainic acid injections. After the first hour, the amplitude and rhythmicity of spikes increased and developed into a continuous high-voltage epileptiform activity. These changes in the EEG were accompanied by the following signs: masticatory movements, orofacial automatisms, wet-dog shakes, head nodding, salivation, rearing, running, jumping, circling, barrel rotation, falling with subsequent tonic-clonic seizures, and complete immobility. Five of the 11 Wistar rats (45%) and 9 of the 18 GAERS (50%) survived after the kainic acid-induced status epilepticus.
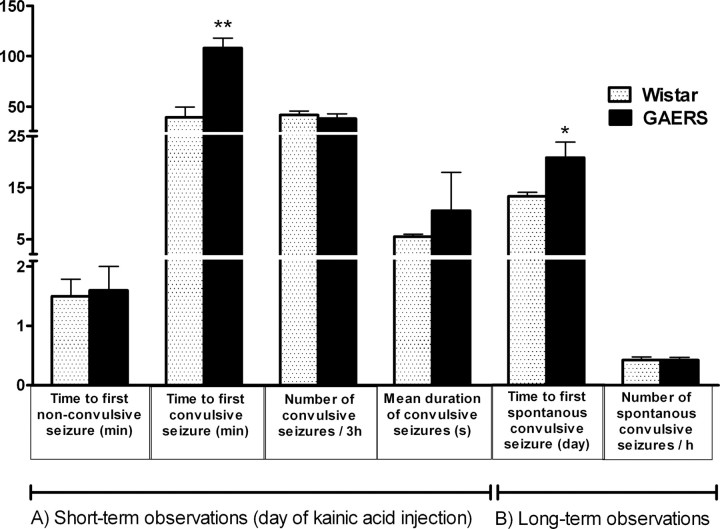
Although there was no significant difference between Wistar control rats and GAERS in the time to first nonconvulsive seizure after the kainic acid injection (1.4 ± 0.2 and 1.6 ± 0.4 min, respectively), convulsive seizures were significantly (p < 0.01) delayed in GAERS (100.4 ± 13.8 min) compared with the Wistar group (39.6 ± 10.1 min). The number and mean duration of convulsive seizures during the 3 h were not significantly different for the two groups (Fig. 2A). The spectral changes of the EEG during the first 3 h after the kainic acid injection were analyzed using spectrograms based on Fourier analysis of 2 s epochs for the 3 h period (Fig. 3A). In the spectrograms, a steep amplitude increase in the gamma range (25–45 Hz) was present within the first 40 min in both the Wistar and GAERS groups. In the Wistar group, this was the point at which convulsive seizures first occurred.
Figure 2.
A, Short-term observations: nonconvulsive and convulsive limbic seizures during kainic acid-induced status epilepticus in Wistar rats (n = 5) and GAERS (n = 9). **p < 0.01, the first convulsive seizure was significantly delayed in GAERS compared with the Wistar group. B, Long-term observations: time to the first spontaneous convulsive seizures and number of spontaneous convulsive seizures after kainic acid injection in the long-term period in Wistar rats (n = 6) and GAERS (n = 6). *p < 0.05, the first convulsive seizure was significantly delayed in GAERS compared with the Wistar group.
Figure 3.
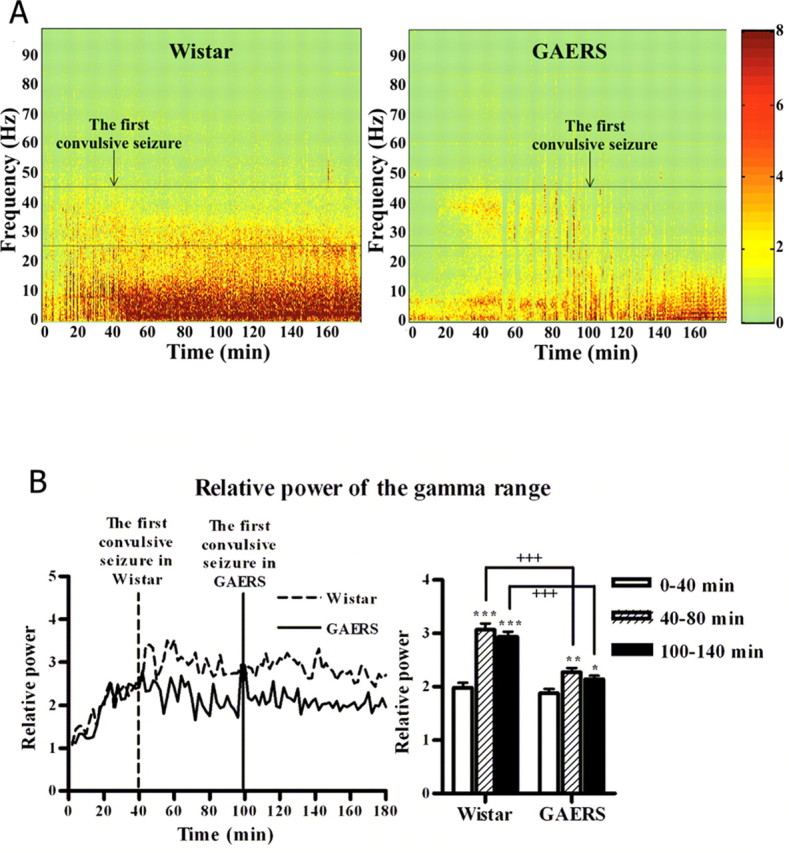
A, Spectral characteristics of the EEG during kainic acid-induced status epilepticus in Wistar rats and GAERS. The color scales represent the power of the signal (square millivolts). B, Time-dependent changes of relative power of the gamma range (25–45 Hz) in Wistar rats (n = 5) and GAERS (n = 9). *p < 0.05; **p < 0.01; ***p < 0.001, significant increase in power during 40–80 and 100–140 min compared with 0–40 min. +++p < 0.001, power of the gamma range is significantly lower in GAERS than in the Wistar group.
Figure 3A shows that this increase continued for an additional 40 min period in the Wistar but not in the GAERS group. The increase in the gamma range remained almost constant for the rest of the 3 h period for the Wistar rats. In the GAERS group, even after the first convulsive seizure occurred, the activity in the gamma range remained below the Wistar level (Fig. 3A,B). To analyze this pattern quantitatively, the power of the gamma range (25–45 Hz) after the kainic acid injection relative to the power before the injection was calculated. Figure 3B shows the mean values for this ratio for the first 40 min after the kainic acid injection and for 40 min intervals after the start of the convulsive seizures in the Wistar and GAERS groups (40–80 and 100–140 min, respectively). An ANOVA showed that, although initially the gamma range power increased equally rapidly in both groups, a significant difference appeared between the groups: there was a significantly lower power in the GAERS group after the start of the convulsive seizures in the Wistar group at ∼40 min (p < 0.001). Even after the start of the convulsive seizures, the power in the gamma range did not increase further in the GAERS group and remained below the Wistar level for the rest of the period recorded (p < 0.001).
Long-term behavioral changes after the kainic acid injection in Wistar controls and GAERS
The time interval between the kainic acid injection and the first spontaneous convulsive seizure is shown in Figure 2B. The first spontaneous convulsive seizure was observed on the 13.33 ± 0.76 d in the Wistar group in contrast to the GAERS in which the first seizure occurred on the 20.14 ± 2.61 d. This difference was significant (p < 0.05). After the occurrence of the first spontaneous convulsive seizure, there was no difference between the Wistar and the GAERS groups in terms of the number of convulsive seizures per hour (Fig. 2B). Thus, the animals in both groups became epileptic, although this was delayed for the GAERS relative to the Wistar group.
Spike-and-wave discharges before and after kainic acid injection in GAERS
All GAERS showed spike-and-wave discharges with the previously defined pattern in their preinjection baseline EEG (Fig. 1). After the kainic acid-induced changes had appeared in the EEG, the intensive spike-and-wave discharge activity was completely suppressed. The duration of this disappearance of spike-and-wave discharges ranged from 1 to 3 d. The cumulative total duration of spike-and-wave discharges per hour was 656 ± 140 s during the preinjection period as opposed to 23.8 ± 13.9 s on the third day and 177.3 ± 50.6 s by the end of first week (Fig. 4A). By the end of second month after the kainic acid injections, the cumulative total duration of the spike-and-wave discharges per hour returned to approximately the baseline level (564.3 ± 224.4 s) but declined again in the third month (116.5 ± 114.5 s) (Fig. 4A). Both the number and mean duration of the spike-and-wave discharges showed significant decreases after the kainic acid injection, but they showed different temporal patterns. The number of spike-and-wave discharges was 41.5 ± 5.9 during the preinjection baseline and dropped to zero immediately after the kainic acid injection (Fig. 4B). Thereafter, the number of spike-and-wave discharges gradually increased; it was 5.8 ± 3.4 on the third day and 14.6 ± 3.9 on the first week but did not reach the baseline levels even after 2 months (31.8 ± 6.8) (Fig. 4B). The decrease of the mean duration of spike-and-wave discharges was only significant for the first (p < 0.001) and third (p < 0.05) days after the injection (Fig. 4C).
Figure 4.
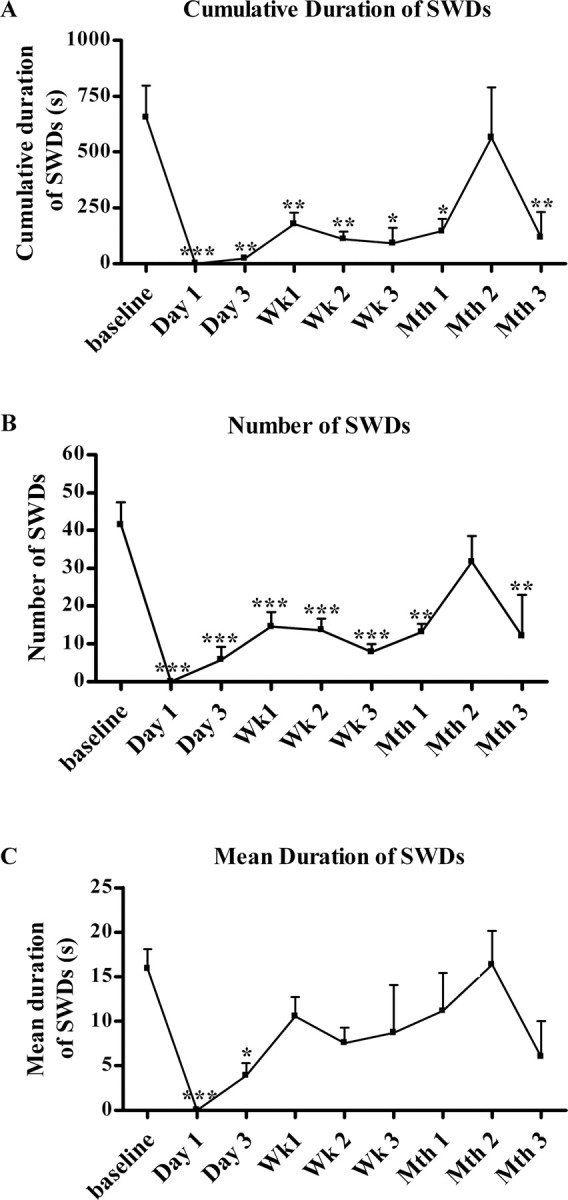
Cumulative duration (A), number (B), and mean duration (C) of spike-and-wave discharges (SWDs) per hour before and after kainic acid injection in GAERS (n = 9). *p < 0.05; **p < 0.01; ***p < 0.001, there was a significant decrease in the number and duration of the spike-and-wave discharges compared with the preinjection baseline.
The power spectrum of the spike-and-wave discharge period before and after kainic acid injections in GAERS
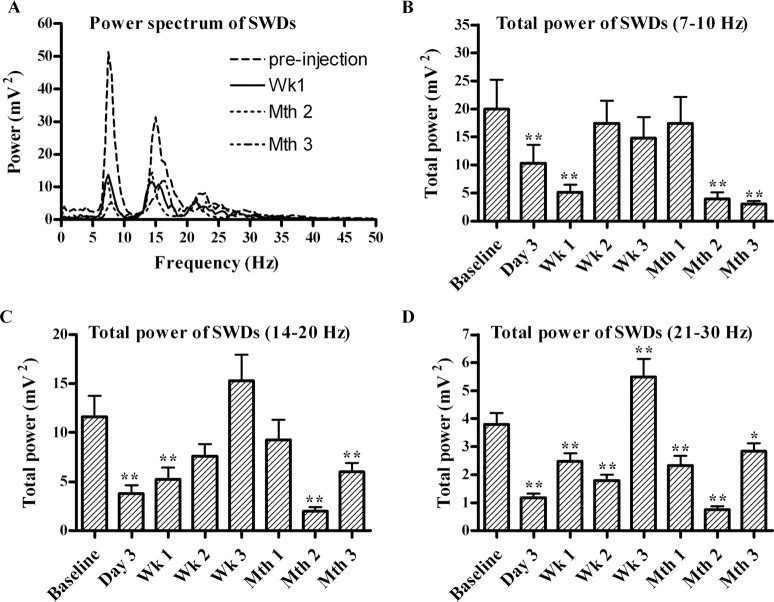
The power spectrum of the spike-and-wave discharge period before the kainic acid injection consisted mainly of a peak at 7–9 Hz and its first and second harmonics at 14–18 and 21–27 Hz, respectively (Fig. 5A). This is the typical spectral pattern of the spike-and-wave discharge complex (Drinkenburg et al., 1993). The total power of the frequency spectrum decreased clearly after the kainic acid injection without significant changes in the spectral characteristics of the power spectrum (Fig. 5A). Because the power of specific peaks changed across the different evaluation time points, the power in the peaks of 7–10, 14–20, and 21–30 Hz frequency ranges (Fig. 5B–D) was quantified to test the significance of the power changes after the kainic acid injection. Figure 5, B and C, shows that the decrease after the kainic acid injection relative to the preinjection baseline in the power of the 7–10 and 14–20 Hz frequency ranges was significant in the records for GAERS for the third day, first week, second month, and third month (p < 0.01). The power of the 21–30 Hz frequency range significantly decreased on the third day, first week, second week, first month, second month, and third month after kainic acid injection (p < 0.01) (Fig. 5D).
Figure 5.
A, The total power spectrum of the spike-and-wave discharges (SWDs) before kainic acid injections (preinjection) and after 1 week (Wk 1), 2 months (Mth 2), and 3 months (Mth 3) in GAERS (n = 9). The power of 7–10 Hz (B), 14–20 Hz (C), and 21–30 Hz (D) frequency bands of the SWD periods before and after kainic acid injections. *p < 0.05, **p < 0.01, significant decrease of total power compared with preinjection baseline.
The power spectrum of the silent and transition periods before and after kainic acid injections in GAERS
Figure 6A shows that, in GAERS before the kainic acid injections (preinjection baseline), the power spectrum of the transition period clearly differed from that of the silent period in the delta (1–4 Hz), alpha (8–12 Hz), and beta (14–20 Hz) frequency ranges. The area under the curve for each of these three frequency ranges was calculated. During the transition period, the power of these frequency ranges significantly increased relative to the earlier silent period (ANOVA: delta, p < 0.05; alpha, p < 0.05; beta, p < 0.01). To follow the spectral changes after the kainic acid injections in GAERS, the same analysis was performed for the third day, first, second, and third weeks, and first, second, and third months after the injection (see Materials and Methods). The spike-and-wave discharges partially recovered on the third day after injection with a progressive increase in their number and mean duration until the second month. In line with this, the power difference between the transition and the silent periods disappeared after the injection and progressively increased with time until the second month. The power difference between the transition and the silent periods in the alpha and beta bands reached significance in the second month as for the preinjection values (p < 0.05) (Fig. 6B–H).
Figure 6.
The power spectrum of the silent and transition periods before (A) and after (B–H) kainic acid injection in GAERS (n = 9).
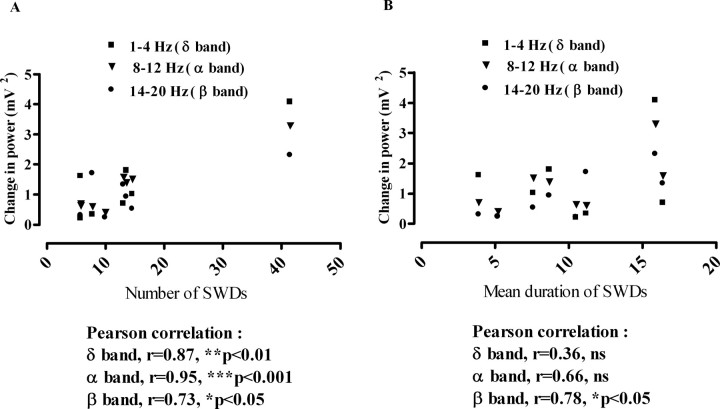
We tested whether this power difference between the transition and the silent periods is correlated with the number and mean duration of spike-and-wave discharge complexes at the defined times (preinjection day, third day, first, second, and third weeks, and first, second, and third months after the injection). The Pearson's correlation analysis showed that the power increases in the delta and alpha frequency ranges in the transition period were significantly correlated with the number of spike-and-wave discharge complexes (Fig. 7A) (r = 0.84, p < 0.01; r = 0.76, p < 0.05, respectively), whereas the beta and alpha frequency ranges were significantly correlated with their mean duration (Fig. 7B) (r = 0.85, p < 0.01; r = 0.80, p < 0.05, respectively).
Figure 7.
Correlation of number (A) and mean duration (B) of spike-and-wave discharges (SWDs) with the change in power of the delta (1–4 Hz), alpha (8–12 Hz), and beta (14–20 Hz) frequency ranges before and after the kainic acid injections in GAERS (n = 9).
Synaptic reorganization of mossy fibers of the dentate gyrus in Wistar controls and GAERS
The neo-Timm's sulfide/silver method demonstrated the distribution of the zinc reaction product in the hippocampus of naive, sham-operated, and kainic acid-injected Wistar rats and GAERS (Fig. 8A). Scoring of mossy fiber sprouting in the supragranular region of kainic acid-injected Wistar rats and GAERS showed significantly (p < 0.001) increased sulfide/silver staining compared with their controls (Fig. 8A,B). However, this increase was less pronounced in kainic acid-injected GAERS than in kainic acid-injected Wistar rats (p < 0.001) (Fig. 8B).
Figure 8.
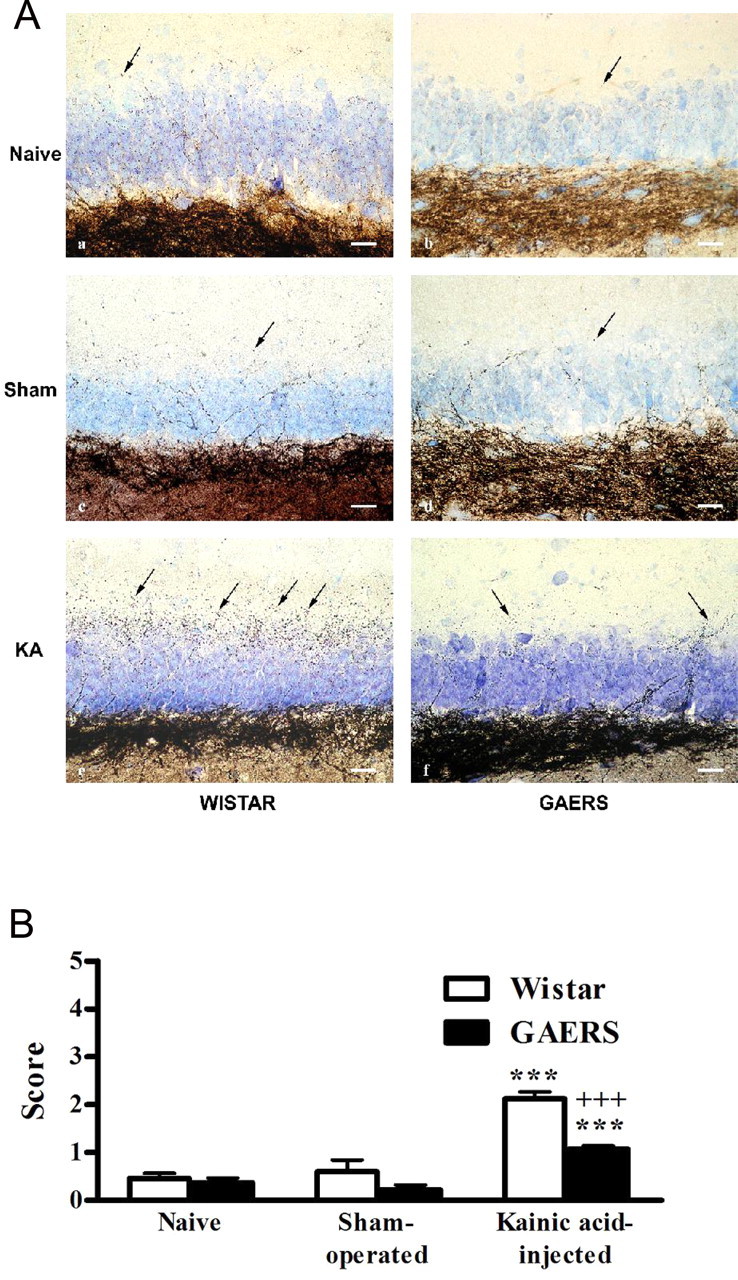
A, Light micrographs from neo-Timm's-stained sections, demonstrating the density of mossy fiber sprouting (arrows) in the supragranular region of naive (a, b), sham-operated (c, d), and kainic acid-injected (KA; e, f) Wistar rats (n = 5, 5, and 5, respectively) and GAERS (n = 5, 5, and 9, respectively) hippocampus. Neo-Timm's and thionin staining. Scale bars, 25 μm. B, Scoring of mossy fiber sprouting in the supragranular region of naive, sham-operated, and kainic acid-injected Wistar rats (n = 5) and GAERS (n = 9). ***p < 0.001, significantly increased sulfide/silver staining in kainic acid-injected Wistar rats and GAERS compared with their controls. +++p < 0.001, less-pronounced increase in kainic acid-injected GAERS than in kainic acid-injected Wistar rats.
Discussion
The induction of status epilepticus in Wistar controls and GAERS
Our results show that, after kainic acid injections, status epilepticus and, over the long term, spontaneous convulsive seizures developed in both groups. The similarity in onset of the first nonconvulsive seizures for the two strains indicates comparable activation of local events and circuits at the injection site and suggests that equivalent convulsant doses were obtained in each group. However, the first convulsive seizure was significantly delayed in GAERS compared with Wistar rats. Furthermore, the delay to the first convulsive seizure in the long-term period was greater in GAERS than Wistar animals. Vergnes et al. (2000) showed that the first seizure induced by low kainic acid doses (5 mg/kg, i.p.) was less severe and had a longer latency in GAERS than in nonepileptic controls. Kindling studies by Eşkazan et al. (2002) and Aker et al., 2006) showed that, when the animals are stimulated at their afterdischarge thresholds, the transition from stage 2 to stage 3 is absent or delayed in GAERS and WAG/Rij models. The resistance to secondary generalization in response to kindling and the increase in the spike-and-wave discharges after each kindling stimulation in the genetic absence epilepsy models show an interplay between limbic and absence epilepsy mechanisms (Onat et al., 2007). Although GAERS showed convulsive seizures after kainic acid injections, which are not seen after kindling, the time to the first convulsive seizure was significantly longer than for the Wistar group. Although the two models of epileptogenesis are not readily comparable, because kainic acid produces more severe neuronal losses than kindling (Morimoto et al., 2004; Cavazos and Cross, 2006), the present study strengthens the hypothesis that absence epilepsy rats are resistant to secondary generalization of focal limbic seizures, no matter how induced. The resistance to secondary generalization of focal limbic seizures in GAERS is not absolute and can be broken down. This breakdown may depend on the greater severity of the insult with kainic acid. Kainic acid or kindling models in rats with absence epilepsy provide an opportunity for understanding and exploring the fact that it is extremely uncommon for idiopathic generalized and temporal lobe epilepsy to coexist in the same patient (Koutroumanidis et al., 1999; Sofue et al., 2003).
The power spectrum of the EEG during status epilepticus in Wistar controls and GAERS
The power of the gamma frequency range (25–45 Hz) was increased during the first 3 h after the kainic acid injection in both groups, but it was lower in GAERS than Wistar rats. This lower increase of gamma activity in GAERS corresponded to the delay of the convulsive seizures in this group. In Wistar rats, an additional increase in the power of the gamma frequency range started after the convulsive seizures, but this was not seen in GAERS. Medvedev et al., 2000) reported that an injection of kainic acid increases the gamma activity in rats. An increased level of gamma activity before epileptic seizures has been described in animals and humans (Herrmann and Demiralp, 2005). In vivo and in vitro studies (Whittington et al., 1995; Martin, 2001) suggest that gamma activity is produced by glutamatergic cells in rat hippocampus and neocortex. Although a conclusive interpretation of the significance of the gamma oscillations in the generation of epileptic seizures is not possible at present, the increase of gamma activity seems to reflect the increase of synchronized excitation in cortex, leading to convulsive seizures (Herrmann and Demiralp, 2005). Brenner et al. (2005) showed that, in the dentate gyrus, calcium-activated potassium channels act as low-pass filters blocking gamma activity and thus protecting against the hyperexcitability that leads to temporal lobe seizures. Calcium-activated potassium currents are also involved in the pathogenesis of absence epilepsy (Lorenz et al., 2007) and are one of the targets for anti-absence drugs (Broicher et al., 2007). These results suggest that the gamma oscillations after the kainic acid injection reflect the level of synchronization in the cortex, which probably is one of the important determinants of the occurrence of convulsive seizures, and that this synchronization is less marked in GAERS than in Wistar rats.
Spike-and-wave discharges in GAERS
We demonstrated that, in GAERS, spike-and-wave discharges are significantly suppressed after kainic acid-induced EEG changes and gradually reappear. Although the spectral pattern of the spike-and-wave discharges seen after the injections does not change, the total power decreased significantly during the first week and again in the second and third months. All these results demonstrate a breakdown of mechanisms underlying absence seizures and show that there is an interaction between the limbic circuits stimulated by intra-amygdaloid kainic acid injection and cortico-thalamo-cortical networks involved in spike-and-wave discharges. Velazquez et al. (2007) demonstrated bilateral synchronization of the hippocampi during spike-and-wave discharges in a typical absence epilepsy, showing an involvement of the hippocampus in absence seizures and interplay between the two circuits.
The reappearance of the spike-and-wave discharges after kainic acid injection may reflect the capacity of the cortico-thalamo-cortical circuitry to reorganize after the changes produced by the kainic acid, indicating either that the genetically determined cortico-thalamo-cortical oscillatory circuits recover over time or become free from extrinsic influences modified by the kainic acid.
The power spectrum of the EEG during the transition period in GAERS
We found increases in power of delta (1–4 Hz), alpha (8–12 Hz), and beta (14–20 Hz) activity during the transition period preceding the abrupt initiation of spike-and-wave discharges in the macroscopic EEG of untreated GAERS. Recent studies have also shown that there are changes in the spectral characteristics of the EEG during this transition period. Inouye et al. (1990) reported that the EEG changes occurring just before the spike-and-wave discharges in human absence epilepsy last 1.5–4.5 s and generally show a gradual increase in the power of the alpha frequency. Pinault et al. (2001) suggested that spike-and-wave discharges in GAERS were generated from medium-voltage 5–9 Hz oscillations and that these spectral changes may correspond to a transition state from the basal activity to the spike-and-wave discharges. Polack et al. (2007) showed that 9–11 Hz cortical oscillations preceding spike-and-wave discharges can generate these discharges in GAERS. The present and previous studies confirm that the increases in these frequency ranges are critical for the spike-and-wave discharge onset.
A kainic acid-induced decrease and a gradual recovery in the power of the transition period preceding spike-and-wave discharges were in parallel with the changes in the mean duration and the number of spike-and-wave discharges. This suggests that the kainic acid-induced status epilepticus may alter the mechanisms that produce the transition from the interictal to the spike-and-wave state of absence epilepsy. Additional analysis of the spectral changes that precede the spike-and-wave discharges shows that the delta activity is correlated with the number of spike-and-wave complexes, whereas the beta activity is correlated with the mean duration of spike-and-wave complexes. The alpha band displays an intermediate character and correlates with both the number and the duration of the spike-and-wave discharge complexes. Considering that the delta oscillations are mainly produced in thalamocortical loops whereas fast oscillations in the beta and gamma ranges are produced by the internal circuitry of the cortex through the rhythmic activation of inhibitory interneurons and their interaction with the pyramidal cells (Steriade et al., 1990; Whittington et al., 1995), these findings could shed light on the generation and maintenance of spike-and-wave discharge complexes. Thus, the cortico-thalamo-cortical circuit may trigger the spike-and-wave discharge generation, whereas the duration of the complexes would be modulated by the cortical circuitry. Kainic acid-induced status epilepticus can impair either the initiation or the maintenance mechanisms (or both) of spike-and-wave discharges.
Synaptic reorganization of mossy fibers of the dentate gyrus in Wistar controls and GAERS
GAERS showed a delay in secondary generalization of the focal limbic seizures after kainic acid injections, and, 3 months thereafter, mossy fiber sprouting occurred in these animals comparable with that seen in human temporal lobe epilepsy and in other experimental models (Isokawa et al., 1993; De Biasi and Bendotti, 1998; Cavazos and Cross, 2006). The reduced amount of mossy fiber sprouting seen in GAERS relative to the Wistar controls is thus in accord with the reduced effectiveness of the kainic acid injections in producing secondarily generalized convulsive seizures and confirms that the responses of the hippocampal formation of GAERS animals differ from those in normal Wistar animals. However, it is relevant that there have been reports indicating that mossy fiber sprouting is not necessary for the production of spontaneous seizures (Elmer et al., 1997; Longo and Mello 1998).
Conclusion
Our findings suggest an interplay between mechanisms underlying absence epilepsy and temporal lobe epilepsy. Additional evidence for a mutual cross-inhibition of the circuits involved in limbic versus generalized absence epilepsy is demonstrated by our observations. These include particularly the delayed convulsive seizures in the short-term (during kainic acid-induced status epilepticus) and long-term periods, the loss and reappearance of the spike-and-wave discharges, the changes that occur in the power spectrum of the EEG activity during the transition period, and the spike-and-wave discharges as well as the reduced sprouting of mossy fibers in GAERS.
Footnotes
This study was supported by Marmara University Scientific Research Committee Grant SAG-TUS-140607-0110. We thank Ray Guillery for his critical help in the preparation of the manuscript. We also thank one of the referees for suggesting the following useful phrase: “mutual cross-inhibition.”
References
- Aker RG, Yananli HR, Gurbanova AA, Ozkaynakçi AE, Ateş N, van Luijtelaar G, Onat FY. Amygdala kindling in the WAG/Rij rat model of absence epilepsy. Epilepsia. 2006;47:33–40. doi: 10.1111/j.1528-1167.2006.00367.x. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Tremblay E, Ottersen OP. Injections of kainic acid into the amygdaloid complex of the rat: an electrographic, clinical and histological study in relation to the pathology of epilepsy. Neuroscience. 1980;5:515–528. doi: 10.1016/0306-4522(80)90049-4. [DOI] [PubMed] [Google Scholar]
- Berger ML, Charton G, Ben-Ari Y. Effects of seizure-induced by intra-amygdaloid kainic acid on kainic acid binding sites in rat hippocampus and amygdala. J Neurochem. 1986;47:720–727. doi: 10.1111/j.1471-4159.1986.tb00671.x. [DOI] [PubMed] [Google Scholar]
- Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci. 2005;8:1752–1759. doi: 10.1038/nn1573. [DOI] [PubMed] [Google Scholar]
- Broicher T, Seidenbecher T, Meuth P, Munsch T, Meuth SG, Kanyshkova T, Pape HC, Budde T. T-current related effects of antiepileptic drugs and a Ca2+ channel antagonist on thalamic relay and local circuit interneurons in a rat model of absence epilepsy. Neuropharmacology. 2007;53:431–446. doi: 10.1016/j.neuropharm.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Cavazos JE, Cross DJ. The role of synaptic reorganization in mesial temporal lobe epilepsy. Epilepsy Behav. 2006;8:483–493. doi: 10.1016/j.yebeh.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazos JE, Golarai G, Sutula TP. Mossy fiber synaptic reorganization induced by kindling: time course of development, progression, and permanence. J Neurosci. 1991;11:2795–2803. doi: 10.1523/JNEUROSCI.11-09-02795.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazos JE, Zhang P, Qazi R, Sutula TP. Ultrastructural features of sprouted mossy fiber synapses in kindled and kainic acid-treated rats. J Comp Neurol. 2003;458:272–292. doi: 10.1002/cne.10581. [DOI] [PubMed] [Google Scholar]
- Coenen AM, Van Luijtelaar EL. Genetic animal models for absence epilepsy: a review of the WAG/Rij strain of rats. Behav Genet. 2003;33:635–655. doi: 10.1023/a:1026179013847. [DOI] [PubMed] [Google Scholar]
- Danscher G. Histochemical demonstration of heavy metals—a revised version of the silver sulphide method suitable for both light and electron microscopy. Histochemistry. 1981;71:1–16. doi: 10.1007/BF00592566. [DOI] [PubMed] [Google Scholar]
- De Biasi S, Bendotti C. A simplified procedure for the physical development of the sulphide silver method to reveal synaptic zinc in combination with immunocytochemistry at light and electron microscopy. J Neurosci Methods. 1998;79:87–96. doi: 10.1016/s0165-0270(97)00169-6. [DOI] [PubMed] [Google Scholar]
- Drinkenburg WH, van Luijtelaar EL, van Schaijk WJ, Coenen AML. Aberrant transients in the EEG of epileptic rats: a spectral analytical approach. Physiol Behav. 1993;54:779–783. doi: 10.1016/0031-9384(93)90092-t. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Clark S, Williams PA, Grabenstatter HL. Kainate-induced status epilepticus: a chronic model of aquired epilepsy. In: Pitkanen A, Schwartzkroin PA, Moshe SL, editors. Models of seizures and epilepsy. New York: Elsevier Academic; 2006. pp. 415–432. [Google Scholar]
- Elmer E, Kokaia Z, Kokaia M., Lindvall O, McIntyre DC. Mossy fiber sprouting: evidence against a facilitatory role in epileptogenesis. Neuroreport. 1997;8:1193–1196. doi: 10.1097/00001756-199703240-00027. [DOI] [PubMed] [Google Scholar]
- Eşkazan E, Onat FY, Aker R, Oner G, Onat FY. Resistance to propagation of amygdaloid kindling seizures in rats with genetic absence epilepsy. Epilepsia. 2002;43:1115–1119. doi: 10.1046/j.1528-1157.2002.35601.x. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neurophysiol. 2005;116:2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Huguenard JR. Circuit mechanisms of spike-wave discharge: are there similar underpinnings for centrotemporal spikes? Epilepsia. 2000;41:1076–1077. doi: 10.1111/j.1528-1157.2000.tb00306.x. [DOI] [PubMed] [Google Scholar]
- Inouye T, Sakamoto H, Shinosaki K, Toi S, Ukai S. Analysis of rapidly changing EEGs before generalized spike and wave complexes. Electroencephalogr Clin Neurophysiol. 1990;76:205–221. doi: 10.1016/0013-4694(90)90016-d. [DOI] [PubMed] [Google Scholar]
- Isokawa M, Levesque MF, Babb TL, Engel J., Jr Single mossy fiber axonal systems of human dentate granule cells studied in hippocampal slices from patients with temporal lobe epilepsy. J Neurosci. 1993;13:1511–1522. doi: 10.1523/JNEUROSCI.13-04-01511.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutroumanidis M, Hennessy MJ, Elwes RD, Binnie CD, Polkey CE. Coexistence of temporal lobe and idiopathic generalized epilepsies. Neurology. 1999;53:490–495. doi: 10.1212/wnl.53.3.490. [DOI] [PubMed] [Google Scholar]
- Longo BM, Mello LE. Supragranular mossy fiber sprouting is not necessary for spontaneous seizures in the intrahippocampal kainite model of epilepsy in the rat. Epilepsy Res. 1998;32:172–182. doi: 10.1016/s0920-1211(98)00049-7. [DOI] [PubMed] [Google Scholar]
- Lorenz S, Heils A, Kasper JM, Sander T. Allelic association of a truncation mutation of the KCNMB3 gene with idiopathic generalized epilepsy. Am J Med Genet Part B. 2007;144B:10–13. doi: 10.1002/ajmg.b.30369. [DOI] [PubMed] [Google Scholar]
- Marescaux C, Vergnes M, Depaulis A. Genetic absence epilepsy rats from Strasbourg. J Neural Trans Suppl. 1992;35:37–69. doi: 10.1007/978-3-7091-9206-1_4. [DOI] [PubMed] [Google Scholar]
- Martin SJ. Activation of metabotropic glutamate receptors induces gamma frequency oscillations in the rat dentate gyrus in vivo. Neuropharmacology. 2001;40:634–637. doi: 10.1016/s0028-3908(00)00190-8. [DOI] [PubMed] [Google Scholar]
- Medvedev A, Mackenzie L, Hiscock JJ, Willoughby JO. Kainic acid induces distinct types of epileptiform discharge with differential involvement of hippocampus and neocortex. Brain Res Bull. 2000;52:89–98. doi: 10.1016/s0361-9230(00)00239-2. [DOI] [PubMed] [Google Scholar]
- Midzianovskaia IS, Kuznetsova GD, Coenen AM, Spiridonov AM, van Luijtelaar EL. Electrophysiological and pharmacological characteristics of two types of spike-wave discharges in WAG/Rij rats. Brain Res. 2001;911:62–70. doi: 10.1016/s0006-8993(01)02705-6. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Onat FY, Eskazan E, Aker R. Experimental absence versus amygdaloid kindling. In: Moshe S, Corcoran M, editors. Kindling 6. New York: Springer; 2005. pp. 37–47. [Google Scholar]
- Onat FY, Aker RG, Gurbanova AA, Ateş N, van Luijtelaar G. The effect of generalized absence seizures on the progression of kindling in the rat. Epilepsia. 2007;48(Suppl 5):150–156. doi: 10.1111/j.1528-1167.2007.01303.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 4. San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Pinault D, Vergnes M, Marescaux C. Medium-voltage 5–9 Hz oscillations give rise to spike-and-wave discharges in a genetic model of absence epilepsy: in vivo dual extracellular recording of thalamic relay and reticular neurons. Neuroscience. 2001;105:181–201. doi: 10.1016/s0306-4522(01)00182-8. [DOI] [PubMed] [Google Scholar]
- Polack PO, Guillemain I, Hu E, Deransart C, Depaulis A, Charpier S. Deep layer somatosensory cortical neurons initiate spike-and-wave discharges in a genetic model of absence seizures. J Neurosci. 2007;27:6590–6599. doi: 10.1523/JNEUROSCI.0753-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenbecher T, Staak R, Pape HC. Relations between cortical and thalamic cellular activities during absence seizures in rats. Eur J Neurosci. 1998;10:1103–1112. doi: 10.1046/j.1460-9568.1998.00123.x. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Aging impairs axonal sprouting response of dentate granule cells following target loss and partial deafferentation. J Comp Neurol. 1999;414:238–254. doi: 10.1002/(sici)1096-9861(19991115)414:2<238::aid-cne7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Sofue A, Okumura A, Negoro T, Hayakawa F, Nakai Y, Toyota N, Watanabe K. Absence seizures in patients with localization-related epilepsy. Brain Dev. 2003;25:422–426. doi: 10.1016/s0387-7604(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Sperk G. Kainic acid seizures in the rat. Prog Neurobiol. 1994;42:1–32. doi: 10.1016/0301-0082(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Steriade M, Amzica F. Dynamic coupling among neocortical neurons during evoked and spontaneous spike-wave seizure activity. J Neurophysiol. 1994;72:2051–2069. doi: 10.1152/jn.1994.72.5.2051. [DOI] [PubMed] [Google Scholar]
- Steriade M, Gloor P, Llinas RR, Lopes da Silva FH, Mesulam MM. Basic mechanisms of cerebral rhvthmic activities. Electroencephalogr Clin Neurophysiol. 1990;76:481–508. doi: 10.1016/0013-4694(90)90001-z. [DOI] [PubMed] [Google Scholar]
- Velazquez JL, Huo JZ, Dominguez LG, Leshchenko Y, Snead OC., 3rd Typical versus atypical absence seizures: network mechanisms of the spread of paroxysms. Epilepsia. 2007;48:1585–1593. doi: 10.1111/j.1528-1167.2007.01120.x. [DOI] [PubMed] [Google Scholar]
- Veliskova J. Behavioral characterization of seizures in rats. In: Pitkanen A, Schwartzkroin PA, Moshe SL, editors. Models of seizures and epilepsy. New York: Elsevier Academic; 2006. pp. 601–610. [Google Scholar]
- Vergnes M, Boehrer A, Reibel S, Simler S, Marescaux C. Selective susceptibility to inhibitors of GABA synthesis and antagonists of GABAA receptor in rats with genetic absence epilepsy. Exp Neurol. 2000;161:714–723. doi: 10.1006/exnr.1999.7302. [DOI] [PubMed] [Google Scholar]
- Wenzel HJ, Cole TB, Born DE, Schwartzkroin PA, Palmiter RD. Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc Natl Acad Sci USA. 1997;94:12676–12681. doi: 10.1073/pnas.94.23.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]