Abstract
Frequency facilitation, elicited by low-frequency stimulation (LFS) is a specific property of mossy fiber–CA3 synapses. Although it has been widely described in vitro, no evidence as yet exists as to whether this phenomenon occurs in vivo. Here, we show that, in freely behaving rats, frequency facilitation at mossy fiber–CA3 synapses consistently occurs in response to LFS (1 Hz). Extracellular adenosine regulates presynaptic neurotransmitter release via action on adenosine A1 receptors and contributes to frequency facilitation in vitro. We investigated whether adenosine A1 receptors mediate frequency facilitation in freely behaving animals. The adenosine A1 receptor antagonists, DPCPX (8-cyclopentyl-1,3-dipropylxanthine) and phenylxanthine, markedly enhanced mossy fiber synaptic transmission and significantly occluded frequency facilitation. Evoked responses were suppressed by application of the group II metabotropic glutamate receptor agonist (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV), in line with the known sensitivity of mossy fiber–CA3 synapses to this agent. By comparison, neither frequency facilitation, effects of the adenosine A1 antagonists, nor effects of DCG-IV were evident at either perforant path–dentate gyrus synapses or commissural/associational CA3 synapses in vivo. These data support that frequency facilitation is an intrinsic property of information storage processes at mossy fiber–CA3 synapses in the intact animal and that frequency facilitation in vivo may be mediated by regulation of the adenosine A1 receptor.
Keywords: CA3, long-term depression, frequency facilitation, in vivo, adenosine, mossy fiber
Introduction
Hippocampal synaptic plasticity is intrinsically involved in formation of memory in the mammalian brain (Bear, 1996). It exists in a multitude of forms, ranging from long-term potentiation to long-term depression, but also encompasses a broad range of short-term plasticities. Different hippocampal subregions respond with distinct types of synaptic plasticity to novel spatial experience (Kemp and Manahan-Vaughan, 2007). In addition, hippocampal subregions subserve different aspects of information processing: the dentate gyrus may engage in pattern completion, whereas the CA1 region integrates information. The CA3 region may, however, function as a pattern separator or pattern integrator depending on the nature of spatial information it receives (Guzowski et al., 2004; Gilbert and Kesner, 2006). More recently it has emerged that the CA3 region may engage in processing of working (i.e., short-term) memory (Kesner, 2007).
One possibility for holding memories “online” comprises short-term plasticity. In vitro studies have demonstrated that the CA3 region responds to low-frequency stimulation (LFS) with a potent enhancement of evoked responses at mossy fiber–CA3 synapses (Salin et al., 1996; Moore et al., 2003). Here, changing the frequency of stimulation from a very low rate (e.g., 0.05 Hz) to a low rate (e.g., 1 Hz) results in a striking increase in the amplitude of evoked potentials. The potentiation endures only for as long as LFS occurs, thus fulfilling the role of holding synaptic information “online.” In rodents, the synaptic potentiation that occurs in response to LFS in the range of 1–4 Hz has been reported to achieve levels of 300–1000% in rats (Salin et al., 1996; Toth et al., 2000; Nicoll and Schmitz, 2005). This suggests that mossy fiber synaptic transmission is normally subjected to a high degree of tonic control. This is achieved in part by tonic activation of adenosine A1 receptors, which normally serve to maintain low transmitter release probability at mossy fiber synapses (Moore et al., 2003). Removal of this tonic regulation by adenosine A1 receptors also occludes frequency facilitation (FF) (Moore et al., 2003), suggesting that the adenosine A1 receptors play an important role in enabling these characteristic properties of the mossy fiber synapse.
However, no evidence exists that FF exists in the intact behaving animal, and the role of the adenosine A1 receptor has not yet been studied in vivo. Our objective therefore was to examine whether FF occurs in the freely behaving rat, and to examine whether under conditions of a fully functional, fully intact hippocampus, this phenomenon might be regulated by adenosine A1 receptors.
Materials and Methods
Electrophysiology.
Male Wistar rats (7–8 weeks; Charles River, Sulzfeld, Germany) were anesthetized (pentobarbital, 52 mg/kg) and underwent chronic implantation of a bipolar stimulation electrode and a monopolar recording electrode to enable monitoring of evoked potentials at mossy fiber–CA3, commissural/associational CA3, or perforant path–dentate gyrus synapses.
For mossy fiber–CA3 recordings, the recording electrode was placed in the CA3 stratum lucidum [anteroposterior (AP), −2.7 mm; mediolateral (ML), 2.4 mm] and the stimulation electrode was implanted in the perforant path (AP, −6.9 mm; ML, 4.1 mm) to enable activation of mossy fibers via the dentate gyrus. We adopted this approach because of the high risk of commissural/associational contamination of the responses if we placed our stimulating electrode in the CA3 region. In addition, by placing the stimulating electrode in the perforant pathway, we were certain to activate a large portion of mossy fibers which would improve the chances of obtaining substantially detectable potentials.
For commissural/associational CA3 recordings, the recording electrode was placed in the CA3 stratum radiatum (AP, −3.1 mm; ML, 3.1 mm) and the stimulation electrode was implanted in the commissural/associational fibers (AP, −3.6 mm; ML, 4.1 mm). Where perforant path–dentate gyrus responses were assessed, we implanted a recording electrode into the dentate gyrus granule cell layer (AP, −3.1 mm; ML, −1.9 mm) and a stimulation electrode in the perforant path (AP, −6.9 mm; ML, 4.1 mm).
To enable injections, a guide cannula was placed in the ipsilateral cerebral ventricle, as described previously (Manahan-Vaughan,1997). Experiments were commenced 7–10 d after surgery. During all experiments, the animals could move freely in the recording chamber (40 × 40 × 40 cm) and had ad libitum access to food and water. To allow the animals to acclimatize, they were transferred to the experiment room the day before the experiment took place.
The head stage was connected to an amplifier and stimulator via a flexible cable with a swivel connector. Recordings were analyzed and stored on computer and the EEG was monitored throughout the experiments.
Measurement of evoked potentials and data analysis.
To evoke field EPSPs (fEPSPs), a biphasic pulse was given with a half-wave duration of 0.2 ms. For recordings, the stimulation intensity was set to produce an fEPSP that was 40% of the maximal obtainable. The intensity was found on the basis of an input–output curve (maximal stimulation, 900 μA). Each recording consisted of an average of five consecutive pulses at 0.025 Hz. To ensure stability of the recordings, all animals were first tested in a baseline experiment over the same time period as subsequent experiments.
For each time point, five consecutively evoked responses at 40 s intervals were averaged. These first 30 min of recording (six time points) served as baseline, and the data obtained subsequently were expressed as the mean percentage ±SEM of this average baseline value. After monitoring of basal synaptic transmission for 30–45 min, drug or vehicle injection was applied. The stability of basal synaptic transmission was then followed for 2.5 h, with additional recordings conducted 24 h after injection.
For analysis of differences between groups, a two-way ANOVA was applied. Data correlation was assessed using Spearman's rho. The level of significance was set at p < 0.05.
Investigations of frequency facilitation.
FF was elicited using LFS consisting of 30 pulses at 1 Hz. The effects of FF were evaluated during the course of the baseline experiments described above. Thus, after drug or vehicle injection, 30 pulses at 1 Hz were applied to afferent fibers. The change in fEPSP magnitude was calculated relative to the mean of the four data points obtained immediately before application of LFS. This was done so that we could make a comparison of the relative change in fEPSP that occurred under vehicle and drug conditions. The data obtained during the 30 LFS pulses were thus expressed as the mean percentage ±SEM of these four averaged time points. The data in Figures 1C, 2B, and 3B show, in addition to the responses to LFS, the four test-pulse values obtained (at 5 min intervals) immediately before LFS and the two test-pulse values obtained immediately afterward.
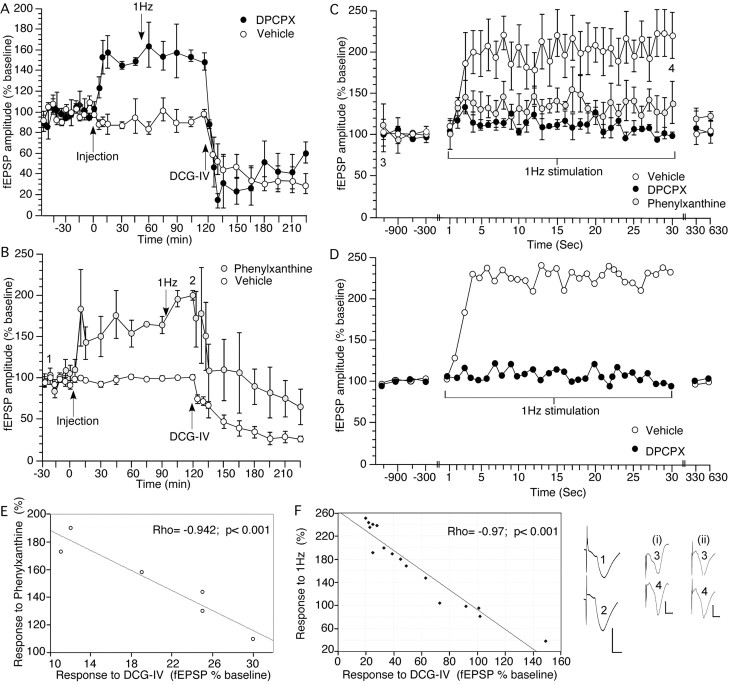
Figure 1.
Antagonism of adenosine A1 receptors causes a significant increase in mossy fiber transmission that is suppressed by the group II mGluR agonist DCG-IV. Low-frequency stimulation of mossy fiber–CA3 synapses induces frequency facilitation that is prevented by antagonism of adenosine A1 receptors. A, B, Application of the adenosine A1 receptor antagonists DPCPX (76 μg, n = 6; A) or phenylxanthine (12.5 μg, n = 6; B) at the time point indicated in the graph (Injection) significantly increase the amplitude of synaptic responses evoked at mossy fiber–CA3 synapses in the intact freely behaving rat compared with controls (n = 6). Effects are significantly inhibited by application of the group II mGluR agonist DCG-IV (20 ng). Analogs represent treatment with phenylxanthine at the time points noted. Calibration: 3 mV, 5 ms. C, This graph shows the evoked responses obtained during 1 Hz stimulation (30 pulses) given at the time point indicated in A. The graph shows, in addition to the responses to LFS, the four test-pulse values obtained (at 5 min intervals) immediately before, and the two test-pulse values obtained immediately after LFS. Low-frequency stimulation at 1 Hz results in a marked increase in the amplitude of synaptic responses evoked at mossy fiber–CA3 synapses in the intact freely behaving rat (n = 6). Treatment with DPCPX (76 μg; n = 6) or phenylxanthine (12.5 μg; n = 6) significantly impairs the amplitude of frequency facilitation. D, Representative trace of the frequency facilitation response obtained after 1 Hz stimulation of one animal. E, The amount of synaptic potentiation achieved with phenylxanthine (12.5 μg; n = 6) correlated significantly with the relative amount of depression observed 1 h after application of DCG-IV (20 ng) to the same animals. F, In control animals (including those that responded poorly to DCG-IV application), the amount of frequency facilitation (at the 30th pulse at 1 Hz) correlated significantly with the relative amount of depression observed 1 h after application of DCG-IV (20 ng; n = 16) to the same animals. A strong DCG-IV-mediated depression correlated with potent FF. A weak response to DCG-IV, or absence of DCG-IV depression correlated with poor, or no, FF. Analogs represent treatment with vehicle (i) or DPCPX (ii) at the time points noted. Calibration: 3 mV, 5 ms.
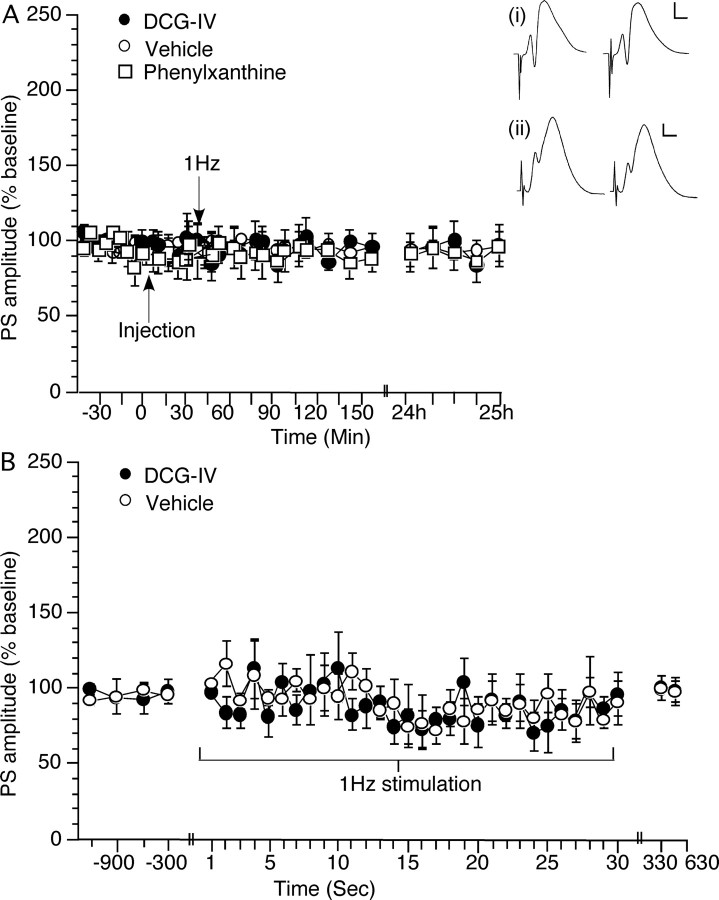
Figure 2.
Antagonism of adenosine A1 receptors or agonist activation of group II mGluRs has no effect on synaptic responses evoked at perforant path–dentate gyrus synapses of freely behaving rats. Frequency facilitation is also absent. A, Acute application of phenylxanthine (12.5 μg; n = 9) at the time point indicated in the graph (Injection) has no effect on synaptic transmission evoked by test-pulse stimulation of perforant path–dentate gyrus synapses monitored over a 24 h period (compared with controls; n = 6). Acute application of DCG-IV (20 ng; n = 6) at the time point indicated in the graph (Injection) has no effect on the profile of responses obtained. B, Evoked responses obtained during 1 Hz stimulation (30 pulses) given at the time point indicated in A. The graph shows, in addition to the responses to LFS, the four test-pulse values obtained (at 5 min intervals) immediately before and the two test-pulse values obtained immediately after LFS. FF does not occur after 1 Hz stimulation of perforant path–dentate gyrus synapses (n = 6) in the presence of either vehicle or DCG-IV (20 ng; n = 6). Analogs represent treatment with vehicle (i) or DCG-IV (ii) preinjection (left trace) or ∼2.5 h (right trace) after injection in the presence of test-pulse stimulation of perforant path–dentate gyrus synapses. Calibration: 3 mV, 2.5 ms.
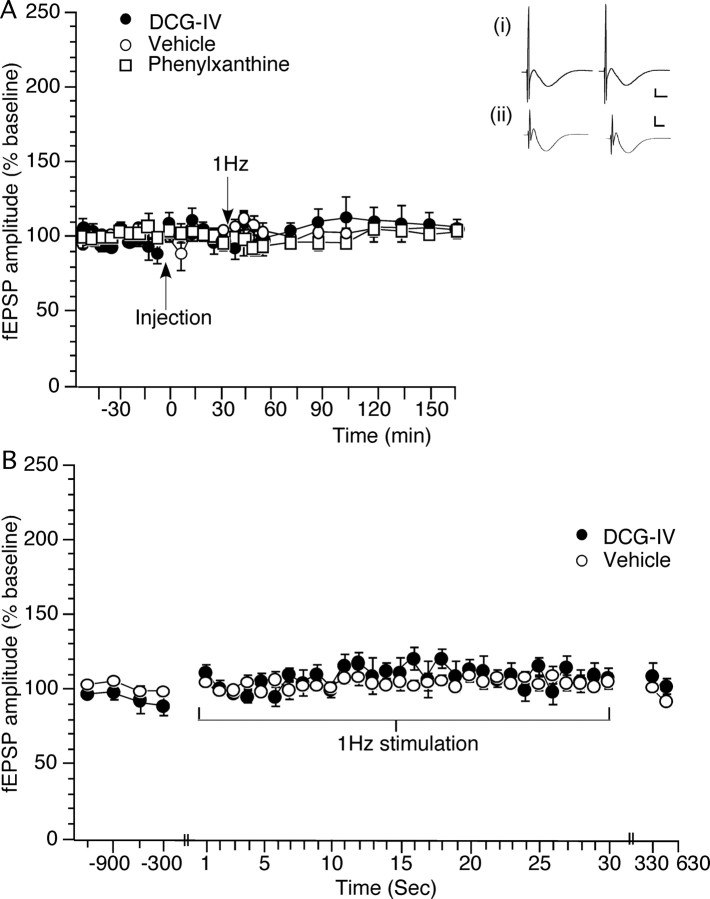
Figure 3.
Basal synaptic transmission at commissural/associational CA3 synapses of freely behaving rats is unaffected by antagonism of adenosine A1 receptors or agonist activation of group II mGluRs. Frequency facilitation is also absent. A, Application of phenylxanthine (12.5 μg; n = 6), at the time point indicated in the graph (Injection), or DCG-IV (20 ng; n = 5) does not affect synaptic transmission evoked by test-pulse stimulation of commissural/associational synapses in the CA3 region (compared with controls; n = 8). B, Evoked responses obtained during 1 Hz stimulation (30 pulses) given at the time point indicated in A. The graph shows, in addition to the responses to LFS, the four test-pulse values obtained (at 5 min intervals) immediately before, and the two test-pulse values obtained immediately after LFS. FF does not occur after 1 Hz stimulation of commissural/associational CA3 synapses in the presence of either vehicle (n = 8) or DCG-IV (20 ng; n = 5). Analogs represent treatment with vehicle (i) or DCG-IV (ii) preinjection (left trace) or ∼2.5 h (right trace) after injection in the presence of test-pulse stimulation of commissural/associational CA3 synapses. Calibration: 3 mV, 2.5 ms.
Drugs.
The adenosine A1 receptor antagonist, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) was dissolved in 10 mm DMSO. The adenosine A1 receptor antagonist, 1,3-dipropyl-8-(4-acrylate)phenylxanthine (phenylxanthine), was dissolved in a solution containing 5% 1N NaOH and 95% distilled water, with HCl added to correct for pH. The group II metabotropic glutamate receptor (mGluR) agonist, (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV; Tocris Bioscience, Bristol, UK), was dissolved in isotonic saline (0.9% NaCl) solution. The concentration of DCG-IV that was used was chosen because it has no effect on evoked responses in the dentate gyrus (Manahan-Vaughan, 1998), whereas higher concentrations depress basal synaptic transmission in this structure. Drugs were injected in a 5 μl volume as an acute, single injection into the ipsilateral lateral ventricle (i.c.v.), via the implanted cannula, in the following amounts: DCG-IV, 20 ng; DPCPX, 57, 76, or 95 μg; phenylxanthine, 6.25, 12.5, 18.75, 25, 50, or 125 μg.
Verification of mossy fiber–CA3 recordings.
The mossy fiber synapse is very sensitive to agonism of group II mGluRs by DCG-IV that selectively inhibits mossy fiber but not associational/commissural EPSPs (Kamiya et al., 1996; Yeckel et al., 1999; Goussakov et al., 2000). Animals were excluded from the mossy fiber–CA3 study when the fEPSP responses evoked in the stratum lucidum failed to show strong sensitivity (i.e., a reduction of test-pulse-evoked responses by 60% or greater) to DCG-IV, 1 h after DCG-IV application. Postmortem histological analysis of the electrode localizations was also conducted.
Results
Antagonism of adenosine A1 receptors causes a significant increase in mossy fiber transmission, and evoked responses are suppressed by the group II mGluR agonist DCG-IV
Application of the adenosine A1 receptor antagonist DPCPX (76 μg; n = 6) significantly increased the amplitude of synaptic responses evoked at mossy fiber–CA3 synapses in the intact freely behaving rat compared with controls (n = 6) (Fig. 1A) (ANOVA, F(1,29) = 18.232; p = 0.0001). The lower amount of 57 μg (n = 4) did not significantly alter responses, and raising the amount to 95 μg (n = 4) did not improve the facilitation seen (data not shown).
Phenylxanthine (12.5 μg; n = 6) elicited similar effects (Fig. 1B) (ANOVA, F(1,29) = 13.766; p = 0.0001). The lower amount of 6.25 μg (n = 3) was ineffective, and the higher amounts of 18.75 (n = 8), 25 (n = 9), 50 (n = 4), and 125 μg (n = 4) did not significantly improve the facilitation seen (data not shown).
Evoked responses were significantly inhibited by application of the group II mGluR agonist DCG-IV (20 ng) (Fig. 1A,B), indicating that responses were predominantly evoked from mossy fiber–CA3 synapses (Kamiya et al., 1996; Yeckel et al., 1999; Goussakov et al., 2000).
A significant correlation was seen between the degree of synaptic potentiation evoked in the presence of either DPCPX or phenylxanthine (Fig. 1E), compared with subsequent responses evoked during the same experiment 1 h after application of DCG-IV (DPCPX, Spearman's rho, −0.943; p < 0.001; phenylxanthine, rho, −0.942; p < 0.001).
Low-frequency stimulation of mossy fiber–CA3 synapses induces frequency facilitation that is prevented by antagonism of adenosine A1 receptors
LFS at 1 Hz resulted in a significant increase in the amplitude of synaptic responses evoked at mossy fiber–CA3 synapses in the intact freely behaving rat (n = 6) (Fig. 1C,D). The facilitation rapidly reached a maximum level of ∼200% and stabilized at this level until stimulation ceased. Thirty pulses at 1 Hz had no effect on the subsequent stability of responses evoked with test-pulse (0.025 Hz) stimulation (Fig. 1A,B).
Treatment with DPCPX (76 μg; n = 6) or phenylxanthine (12.5 μg; n = 6) significantly impaired the amplitude of FF (DPCPX, ANOVA, F(1,29) = 2.549, p = 0.0004; phenylxanthine, ANOVA, F(1,29) = 2.117, p = 0.0027) (Fig. 1C).
The amount of FF seen in control animals correlated with the relative depression elicited when DCG-IV was later applied (during the same experiment) to these animals (n = 6). The response to the 30th pulse given at 1 Hz to elicit FF was compared with the amount of synaptic depression seen 1 h after application of DCG-IV. A significant effect was observed (rho, −0.986; p = 0.0003). This correlation was also sustained when animals that responded poorly to DCG-IV (presumed mixed afferent CA3 group) were analyzed together with animals that achieved a reduction of test-pulse-evoked responses by 60% or greater after DCG-IV application (mossy fiber responders) (Fig. 1F) (n = 16; rho, −0.97; p < 0.0001).
Antagonism of adenosine A1 receptors and agonist activation of group II mGluRs has no effect on synaptic responses evoked at perforant path–dentate gyrus synapses; frequency facilitation is absent
Animals were preselected in this study on the basis of the sensitivity to DCG-IV of their fEPSP responses in stratum lucidum (20 ng) (Materials and Methods). By these means we could be sure that the synaptic responses we measured from the stratum lucidum of the CA3 region were derived predominantly by means of the mossy fiber afferent pathway to CA3 (Fig. 1). However, we cannot exclude that the changes in synaptic responsiveness we observed may derive from alterations in synaptic transmission or synaptic strength in the dentate gyrus, that were then relayed to the CA3 region via the mossy fibers. We therefore examined if adenosine antagonism affects synaptic transmission at perforant path–dentate gyrus synapses. Application of phenylxanthine (12.5 μg; n = 9) had no effect over the 24 h monitoring period (Fig. 2A) (ANOVA, F(1,30) = 0.543; p = 0.976, compared with vehicle-injected controls; n = 6). DCG-IV (20 ng; n = 6) was also ineffective (ANOVA, F(1,30) = 0.668; p = 0.91).
LFS, in the presence of vehicle, elicited no significant change in synaptic responses in the dentate gyrus (Fig. 2B) (n = 5). When LFS was given in the presence of DCG-IV (20 ng; n = 5) no effect was observed (Fig. 2B) (ANOVA, F(1,30) = 1.373; p = 0.11). Thirty pulses at 1 Hz also had no effect on the subsequent stability of responses evoked with test-pulse (0.025 Hz) stimulation (Fig. 2A,B).
We can therefore conclude that the effects observed in the CA3 region occurred independently of “upstream” synaptic processing in the dentate gyrus.
Antagonism of adenosine A1 receptors and agonist activation of group II mGluRs has no effect on synaptic responses evoked at commissural/associational CA3 synapses; frequency facilitation is also absent
Test-pulse stimulation given in the presence of vehicle (n = 8) or DCG-IV (20 ng; n = 5) had no effect on basal synaptic transmission at commissural/associational CA3 synapses (Fig. 3A) (ANOVA, F(1,25) = 0.593; p = 0.9395).
Application of phenylxanthine (12.5 μg; n = 6) also had no effect on basal synaptic transmission compared with controls (Fig. 3C) (ANOVA, F(1,25) = 1.259; p = 0.187).
The possible occurrence of FF at commissural/associational synapses in the CA3 region has not been investigated in freely behaving rats. LFS at 1 Hz (30 pulses) did not alter the profile of evoked responses (Fig. 3B) either in controls (n = 8) or in the presence of DCG-IV (20 ng; n = 5). Thus, FF was not observed at commissural/associational CA3 synapses in freely behaving adult rats. Thirty pulses at 1 Hz also had no effect on the subsequent stability of responses evoked with test-pulse (0.025 Hz) stimulation (Fig. 3A).
Discussion
These data show for the first time that FF occurs at mossy fiber–CA3 synapses in the intact freely behaving rat. Effects are mediated by modulation of the adenosine A1 receptor, and presumably by subsequent changes in the transmitter release probability at the synapse (Moore et al., 2003).
It is striking that the amplitude of FF in the intact animal is less than that reported in rats in vitro (Salin et al., 1996; Toth et al., 2000; Nicoll and Schmitz, 2005), where response increases of 300–600% have been described during 1 Hz LFS, and increases of up to 1000% have been described during 2–4 Hz stimulation (Toth et al., 2000). In vivo, FF at mossy fiber synapses of ∼200% was evident. This suggests that in the intact animal, mechanisms regulating synaptic transmission and potentiation may prevent very large increases in synaptic strength. This is perhaps not so unreasonable: as on a background of very low release probability and corresponding low synaptic activity (low noise, so to speak), mossy fiber synapses may only need very moderate changes in responsiveness to enable effective and high-resolution information processing. The relatively low level of FF may relate, on the one hand, to lower levels of ambient adenosine in the intact animal, or to a low level of activation of adenosine A1 receptors by ambient adenosine, as reported in vitro (Kukley et al., 2005). Another mechanism that may regulate synaptic transmission and potentiation is the presynaptic metabotropic glutamate receptor (Scanziani et al., 1997), which under conditions of prolonged glutamate release may become activated and mediate a negative feedback of transmitter release. Age may also be a factor: our animals were at least 9 weeks old at the time of investigation. Most in vitro studies are conducted in 2- to 7-week-old rodents. FF becomes increasingly less with age (Mori-Kawakami et al., 2003). An additional factor to consider is that although slice equilibration in vitro may enable a naive synaptic state, an intact fully functional animal is unlikely to possess a large population of naive synapses, thus possibly restricting the degree of FF that can occur. Finally, although we were careful to verify that our mossy fiber responses were sensitive to DCG-IV (Kamiya et al., 1996), we cannot exclude that contamination from commissural/associational fibers occurred. Because commissural/associational CA3 synapses do not display FF, contamination of responses by these synapses would reduce the relative degree of FF seen at the mossy fiber synapses.
Adenosine appears to play a crucial role in synaptic efficacy at hippocampal synapses. Extracellular adenosine acting on presynaptic adenosine A1 receptors regulates neurotransmitter release (Hessler et al., 1993; Masino and Dunwiddie,1999). Furthermore, the low release probability that is characteristic of mossy fiber synapses results from tonic activation of presynaptic adenosine A1 receptor by adenosine (Moore et al., 2003). Effects are mediated by inhibition of presynaptic calcium channels (Gundlfinger et al., 2007), large increases in intraterminal calcium concentrations, and activation of calcium calmodulin kinase II (Salin et al., 1996). In vitro, reductions of basal adenosine tonus at mossy fiber synapses, enhances synaptic transmission, occludes FF, and impairs LTP (Fedele et al., 2005; Moore et al., 2003; Nicoll and Schmitz, 2005).
The increases in synaptic strength that occurred in vivo as a result of application of adenosine A1 antagonists were very similar to the levels of FF seen. Applying LFS in the presence of the adenosine A1 antagonists resulted in a very small FF. This suggests that occlusion occurred, and supports that regulation of A1 receptors may critically contribute to FF at mossy fiber synapses. Consistent with reports that FF is mostly a unique property of the mossy fiber–CA3 synapse (Salin et al., 1996), the same stimulus protocol (1 Hz) that was effective at the mossy fiber synapse in vivo elicited no change in the responses evoked either in the dentate gyrus or at commissural/associational CA3 synapses. This finding adds to reports that FF is either absent or of very modest proportions at non-mossy fiber synapses in the hippocampus (Salin et al., 1996). DCG-IV at the concentration used had no effect on basal synaptic transmission at perforant path–dentate gyrus synapses or at commissural/associational CA3 synapses, but effectively suppressed synaptic transmission at mossy fiber–CA3 synapses. This finding is in agreement with reports that the mossy fiber–CA3 synapse is highly sensitive to activation of group II mGluRs (Kamiya et al., 1996; Yeckel et al., 1999), which regulate glutamate release from mossy fiber terminals. A significant correlation between the amount of FF and the responsiveness of the individual animals to DCG-IV was observed, which suggests that the FF seen was genuinely mediated by mossy fibers.
Conclusion
This study demonstrates for the first time that FF occurs at the mossy fiber synapse of freely behaving rats. Effects are less potent than those reported in vitro, and are sensitive to pharmacological activation of group II mGluRs and to antagonism of adenosine A1 receptors. These findings support that regulation of the adenosine A1 receptor may comprise an important mechanism underlying FF. Furthermore, the observation that FF occurs in vivo suggests that this form of synaptic plasticity contributes to information storage in the intact animal.
Footnotes
This work was supported by a Deutsche Forschungsgemeinschaft Grant to D.M.V. We thank A. Bikbaev for help with statistics.
References
- Bear MF. A synaptic basis for memory storage in the cerebral cortex. Proc Natl Acad Sci USA. 1996;93:13453–13459. doi: 10.1073/pnas.93.24.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedele DE, Gouder N, Güttinger M, Gabernet L, Scheurer L, Rülicke T, Crestani F, Boison D. Astrogliosis in epilepsy leads to overexpression of adenosine kinase, resulting in seizure aggravation. Brain. 2005;128:2383–2395. doi: 10.1093/brain/awh555. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. The role of the dorsal CA3 hippocampal subregion in spatial working memory and pattern separation. Behav Brain Res. 2006;169:142–149. doi: 10.1016/j.bbr.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Goussakov IV, Fink K, Elger CE, Beck H. Metaplasticity of mossy fiber synaptic transmission involves altered release probability. J Neurosci. 2000;20:3434–3441. doi: 10.1523/JNEUROSCI.20-09-03434.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlfinger A, Bischofberger J, Johenning FW, Torvinen M, Schmitz D, Breustedt J. Adenosine modulates transmission at the hippocampal mossy fibre synapse via direct inhibition of presynaptic calcium channels. J Physiol (Lond) 2007;582:263–277. doi: 10.1113/jphysiol.2007.132613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44:581–584. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Hessler NA, Shirke AM, Malinow R. The probability of transmitter release at a mammalian central synapse. Nature. 1993;366:569–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Shinozaki H, Yamamoto C. Activation of metabotropic glutamate receptor type 2/3 suppresses transmission at rat hippocampal mossy fibre synapses. J Physiol (Lond) 1996;493:447–455. doi: 10.1113/jphysiol.1996.sp021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci. 2007;30:111–118. doi: 10.1016/j.tins.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kesner RP. Behavioral functions of the CA3 subregion of the hippocampus. Learn Mem. 2007;14:771–781. doi: 10.1101/lm.688207. [DOI] [PubMed] [Google Scholar]
- Kukley M, Schwan M, Fredholm BB, Dietrich D. The role of extracellular adenosine in regulating mossy fiber synaptic plasticity. J Neurosci. 2005;25:2832–2837. doi: 10.1523/JNEUROSCI.4260-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D. Priming of group 2 metabotropic glutamate receptors facilitates induction of long-term depression in the dentate gyrus of freely moving rats. Neuropharmacology. 1998;37:1459–1464. doi: 10.1016/s0028-3908(98)00150-6. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D. Group 1 and 2 metabotropic glutamate receptors play differential roles in hippocampal long-term depression and long-term potentiation in freely moving rats. J Neurosci. 1997;17:3303–3311. doi: 10.1523/JNEUROSCI.17-09-03303.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori-Kawakami F, Kobayashi K, Takahashi T. Eevelopmental decrease in synaptic facilitation at the mouse hippocampal mossy fibre synapse. J Physiol (Lond) 2003;553:37–48. doi: 10.1113/jphysiol.2003.045948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Dunwiddie TV. Temperature-dependent modulation of excitatory transmission in hippocampal slices is mediated by extracellular adenosine. J Neurosci. 1999;19:1932–1939. doi: 10.1523/JNEUROSCI.19-06-01932.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Nicoll RA, Schmitz D. Adenosine gates synaptic plasticity at hippocampal mossy fiber synapses. Proc Natl Acad Sci USA. 2003;100:14397–14402. doi: 10.1073/pnas.1835831100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci USA. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani M, Salin PA, Vogt KE, Malenka RC, Nicoll RA. Use-dependent increases in glutamate concentration activate presynaptic metabotropic glutamate receptors. Nature. 1997;385:630–634. doi: 10.1038/385630a0. [DOI] [PubMed] [Google Scholar]
- Toth K, Suares G, Lawrence JJ, Philips-Tansey E, McBain CJ. Differential mechanisms of transmission at three types of mossy fiber synapse. J Neurosci. 2000;20:8279–8289. doi: 10.1523/JNEUROSCI.20-22-08279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeckel MF, Kapur A, Johnston D. Multiple forms of LTP in hippocampal CA3 neurons use a common postsynaptic mechanism. Nat. Neurosci. 1999;2:625–633. doi: 10.1038/10180. [DOI] [PMC free article] [PubMed] [Google Scholar]