Abstract
Soluble amyloid-β (Aβ) peptide is likely to play a key role during early stages of Alzheimer's disease (AD) by perturbing synaptic function and cognitive processes. Receptor for advanced glycation end products (RAGE) has been identified as a receptor involved in Aβ-induced neuronal dysfunction. We investigated the role of neuronal RAGE in Aβ-induced synaptic dysfunction in the entorhinal cortex, an area of the brain important in memory processes that is affected early in AD. We found that soluble oligomeric Aβ peptide (Aβ42) blocked long-term potentiation (LTP), but did not affect long-term depression, paired-pulse facilitation, or basal synaptic transmission. In contrast, Aβ did not inhibit LTP in slices from RAGE-null mutant mice or in slices from wild-type mice treated with anti-RAGE IgG. Similarly, transgenic mice expressing a dominant-negative form of RAGE targeted to neurons showed normal LTP in the presence of Aβ, suggesting that neuronal RAGE functions as a signal transducer for Aβ-mediated LTP impairment. To investigate intracellular pathway transducing RAGE activation by Aβ, we used inhibitors of stress activated kinases. We found that inhibiting p38 mitogen-activated protein kinase (p38 MAPK), but not blocking c-Jun N-terminal kinase activation, was capable of maintaining LTP in Aβ-treated slices. Moreover, Aβ-mediated enhancement of p38 MAPK phosphorylation in cortical neurons was reduced by blocking antibodies to RAGE. Together, our results indicate that Aβ impairs LTP in the entorhinal cortex through neuronal RAGE-mediated activation of p38 MAPK.
Keywords: amyloid-β, protein, LTP, RAGE, p38 MAPK, entorhinal cortex, Alzheimer's disease
Introduction
Overproduction and accumulation of amyloid-β (Aβ) is a pathologic feature of Alzheimer's disease (AD). Aβ, particularly Aβ42, has been ascribed a crucial role in neuronal stress (Eisenhauer et al., 2000; Johnson et al., 2002). Studies in AD animal models have highlighted a dichotomy between behavioral deficits and neuropathologic findings. Impaired memory and synaptic loss occur before extensive deposition of amyloid in the brains of AD-type murine models and AD patients (D'Hooge et al., 1996; Hsia et al., 1999; Li et al., 1999; Chapman et al., 1999; Larson et al., 1999; Giacchino et al., 2000; Wirths et al., 2001; Oddo et al., 2003; Ingelsson et al., 2004). These observations suggest that early in AD, when levels of Aβ are low, mechanisms amplifying and focusing the effects of Aβ on cellular targets contribute to neuronal dysfunction.
Previous studies showed that Aβ affects synaptic plasticity in the hippocampus (for a review, see Selkoe, 2002). In particular, oligomeric Aβ42 was capable of acutely inhibiting long-term potentiation (LTP) (Lambert et al., 1998; Chen et al., 2002; Walsh et al., 2002, 2005; Wang et al., 2004; Zhao et al., 2004), one form of synaptic plasticity that is thought to underlie learning and memory (Whitlock et al., 2006). Remarkably, LTP in the hippocampus was inhibited by naturally secreted human Aβ oligomers when administered in the nanomolar range (Walsh et al., 2002; Wang et al., 2004). Previously, a few reports showed that administration of Aβ at low micromolar concentrations depressed synaptic transmission by interacting with glutamate receptors (Snyder et al., 2005; Tyszkiewicz and Yan, 2005; Hsieh et al., 2006; Parameshwaran et al., 2007). Together, these results indicate the relevance of identifying cell surface acceptor sites mediating the interaction with oligomeric Aβ and, as a consequence, activating signal transduction mechanisms contributing to synaptic dysfunction.
The receptor for advanced glycation end products (RAGE), a multiligand receptor in the Ig superfamily, functions as a cell surface binding site for Aβ (Yan et al., 1996). Experimental evidence has shown that RAGE is a receptor that directs the effects of Aβ to target cells (neurons, glia, endothelial cells) (Yan et al., 1995; Hofmann et al., 1999; Lue et al., 2001; Schmidt et al., 2001; Deane et al., 2003; Lue et al., 2005). Furthermore, introduction of a wild-type (WT) RAGE transgene in neurons in the AD-type transgenic (Tg) mouse model expressing mutant human amyloid precursor protein (mAPP) accelerated Aβ-mediated neuronal perturbation (Arancio et al., 2004).
In the present work, we investigated the effect of oligomeric Aβ42 at a concentration of 200 nm on LTP, long-term depression (LTD), paired-pulse facilitation, and basal synaptic transmission in entorhinal cortex, an area affected at an early stage of AD (Braak and Braak, 1991). In addition, we investigated the role of RAGE and signal transduction pathways, p38 and c-Jun N-terminal kinase (JNK) mitogen-activated protein kinases (MAPKs) in Aβ-induced synaptic dysfunction. Finally, we have used a cell culture model to investigate RAGE-triggered signal transduction mechanisms underlying the effects of pathophysiologically relevant concentrations of Aβ (200 nm), focusing on these key intracellular kinases.
Materials and Methods
Homozygous RAGE-null and transgenic DN-RAGE mice.
Homozygous RAGE-null mice were generated and characterized as described previously (Sakaguchi et al., 2003; Wendt et al., 2003). Homozygous RAGE-null mice were backcrossed for >10 generations into the C57BL/6 background. In addition we used transgenic mice with signal transduction deficient mutants of RAGE in which the cytosolic domain of the receptor has been deleted, thereby imparting a dominant-negative (DN)-RAGE effect, targeted to neurons (DN-RAGE) driven by the platelet-derived growth factor-B (PDGF-B) chain promoter. The PDGF-B chain promoter has been successfully used to drive expression of transgene targeted to neurons (Sasahara et al., 1991; Mucke et al., 2000; Lustbader et al., 2004; Takuma et al., 2005). Furthermore, transgenic mice expressing neuronal DN-RAGE driven by the PDGF-B chain promoter have been previously characterized, demonstrating localization of DN-RAGE in cortical neurons (Arancio et al., 2004). Male RAGE-null, male transgenic DN-RAGE and littermate control mice were used for in vitro electrophysiology (age range, 2–3 months) (see below, Slice preparation).
Pharmacologic agents.
Aβ42 and the reversed peptide Aβ(42–1) were purchased from Biosource (Camarillo, CA). Oligomeric Aβ42 peptide was prepared as described previously, and characterized by atomic force microscopy (Yan et al., 2007) and mass spectrometry (Voyager DE PRO MALDI; Applied Biosystems, Warrington, UK). Aliquots were stored at −20°C in DMSO as a 200 μm stock solution and diluted to the desired final concentration in artificial CSF [ACSF; containing (in mm) 119 NaCl, 2.5 KCl, 2 CaCl2, 1.2 MgSO4, 1 NaH2PO4, 26.2 NaHCO3, 11 glucose], immediately before application (Simmons et al., 1994). 4-(4-Fluorophenyl)-2-(4-metylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole (SB203580) and anthra(1–9-cd) pyrazol-6(2H)-one (SP600125) were purchased from Alexis (Grünberg, Germany) and prepared in DMSO stock solutions. These drugs were also diluted to the desired final concentration in ACSF. Specific antibodies to RAGE have been described in our previous studies (Yan et al., 1996) and purified rabbit IgG was used as a control (Bethyl, Montgomery, TX).
Slice preparation.
RAGE-null mice, transgenic DN-RAGE mice, and littermate control mice (age range, 2–3 months) were used. Animals were deeply anesthetized using urethane (20% solution, 0.1 ml/100 g body weight) via intraperitoneal injection and then decapitated after disappearance of the tail pinch reflex. The brain was rapidly removed and thick horizontal sections (400 μm) containing the entorhinal area were made on a Vibratome. All steps were performed in ice-cold ACSF solution bubbled with 95% O2/5% CO2. Before recording, slices were stored for at least 1 h in a recovery chamber containing oxygenated ACSF solution at room temperature. During electrophysiological recordings, slices were perfused at a rate of 2.5–3 ml/min with oxygenated ACSF at 33 ± 1°C.
Electrophysiological recordings.
Extracellular field potentials (FPs) were evoked in layer III in response to electrical stimulation of layer II (see Fig. 1A, schematic diagram). The amplitude of the FPs was used as a measure of the evoked population excitatory current (Domenici et al., 1995; Pesavento et al., 2002). All FPs had peak latency between 4.5 and 6 ms. Amplitudes of FPs were calculated at different stimulus intensities to obtain input–output curves. Experiments to assess paired-pulse stimulation (PP) were performed as well; two consecutive stimuli were applied at different interstimulus intervals (30–200 ms). PP-induced change was calculated as the ratio of the peak amplitude of the second FP to the first one. Baseline responses were obtained with a stimulation intensity that yielded 50–60% of maximal amplitude. FP amplitude was monitored every 20 s and averaged every three responses by online data acquisition software (Anderson and Collingridge, 2001). After 15 min of stable baseline recording, high-frequency stimulation (HFS; three trains of 100 pulses at 100 Hz, 10 s interval) or low-frequency stimulation (LFS; 900 paired pulses at 1Hz, the interval between paired pulses was 30 ms) (Kourrich and Chapman, 2003) was used to induce LTP or LTD, respectively. LTP and LTD magnitude were measured as the average of FP amplitudes between the 40th and the 50th min after termination of induction protocol. Values were expressed as mean ± SEM percentage change relative to their mean baseline amplitude. Aβ peptides were applied by general perfusion for 10 min starting 5 min before HFS and LFS. In experiments using the P38 MAPK inhibitor (SB203580) or JNK inhibitor (SP600125), compounds were continuously perfused over slices starting at least 30 min before HFS or LFS.
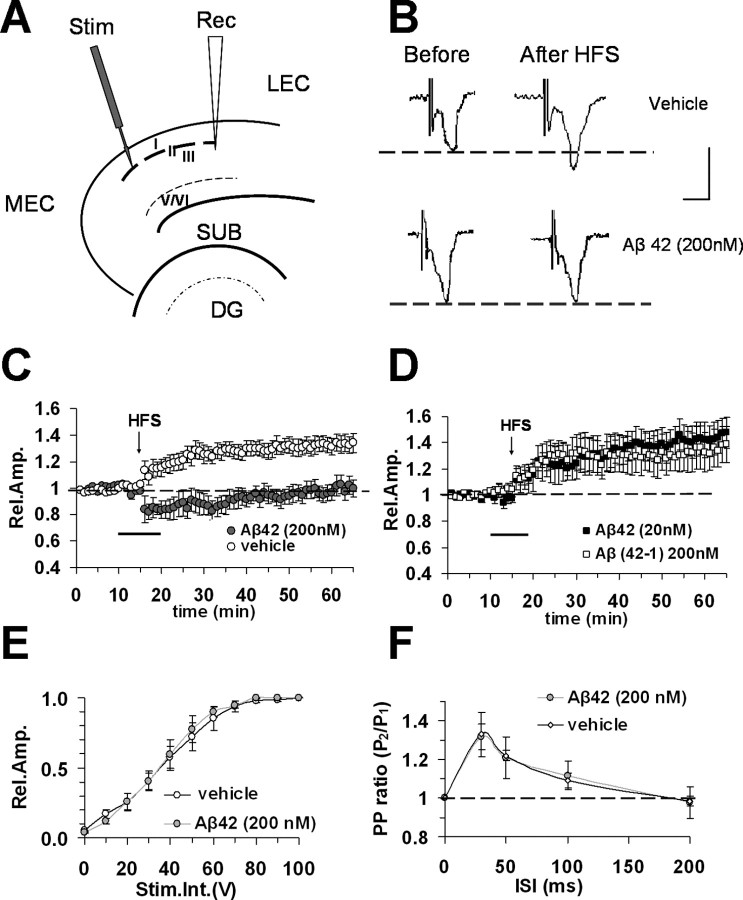
Figure 1.
Inhibitory effect of Aβ42 on LTP in entorhinal cortex slices. A, Schematic drawing of entorhinal cortex slices and position of electrodes (stimulus position in layer II, recording pipette in layer II/III). MEC, Medial entorhinal cortex; LEC, lateral entorhinal cortex; DG, dentate gyrus; SUB, subiculum. B, Representative field potentials recorded before and 50 min after HFS in vehicle-treated slices and in slices treated with Aβ42 (200 nm). Calibration: 1 mV, 5 ms. Under control conditions LTP expression is induced by HFS, applied after 15 min of baseline recording. C, LTP was induced by HFS stimulation in vehicle-treated slices (open circles), whereas LTP expression was blocked by bath application of Aβ42 (200 nm, gray circles) for 10 min (dark bar). D, No effect on LTP expression was observed in the presence of reversed peptide Aβ (42–1, 200 nm, open squares) or a low concentration of Aβ42 (20 nm, filled squares). E, Input–output curves. The relative amplitude (Rel. Amp.) as a function of stimulus intensity (Stim. Int.) measured in volts (V) did not show significant differences between vehicle-treated (open circles) and Aβ42-treated slices (gray circles). F, Paired-pulse facilitation. Paired-pulse-induced change was calculated as the ratio of the peak amplitude of the second evoked field potential to the first one (PP ratio) for different interstimulus intervals (25–200 ms). PP ratio was not modified in Aβ42 (200 nm, gray circles) treated slices respect to vehicle-treated slices (open circles). Error bars indicate SEM.
Cell culture experiments.
Primary cell cultures were prepared as follows: cortex from 0- to 3-d-old mice was dissected. Dissections were performed in 200 μm kynurenic acid (Sigma, St. Louis, MO) and 25 μm 2-amino-5-phosphonovalerate (Tocris Cookson, Bristol, UK) on ice. Tissue slices were digested with trypsin in the presence of DNAase, blocked with a trypsin inhibitor on ice, and dissociated in medium containing DNAase. Cells were recovered, washed by two successive centrifugations, and plated on glass coverslips coated with 50 μg/ml polyornithine and 2% Matrigel (Collaborative Research, Bedford, MA) in 35 mm Nunc (Naperville, IL) Petri dishes. Cells were maintained for 7–10 d in a 5% CO2 humidified incubator at 37°C, in minimum essential medium with Earle's salts and Glutamax I (Invitrogen, Carlsbad, CA) to which 5–10% fetal bovine serum, 6 mg/ml d-glucose, 3.6 mg/ml HEPES, 0.1 μg/ml biotin, 1.5 μg/ml vitamin B12, 30 μg/ml insulin, and 100 μg/ml bovine transferrin (Sigma) were added. Proliferation of non-neuronal cells was prevented by the addition of 2.5–5.0 μm cytosine β-d-arabinofuranoside (Sigma) from the second day in culture. To assess viability, cells were incubated with Hoechst 33342 (0.3 ng/ml) and propidium iodide (PI) (1 μg/ml) in culture medium for 1 h in a CO2 incubator at 37°C, washed in PBS and mounted on microscope slides; double staining was analyzed with a fluorescence microscope (BC 1083 INV 11722; Nikon, Tokyo, Japan). PI is a polar compound which only enters damaged cell membranes; inside cells, it binds to nucleic acids and becomes brightly red fluorescent. In a separate set of experiments, cell viability was assayed using CytoTox 96 Non-Radioactive Cytotoxicity Assay kit (Promega, Madison, WI) according to the manufacturer's protocol. This assay measures lactate dehydrogenase (LDH) activity through the conversion of a tetrazolium salt into a red formazan product. Relative absorbance was measured at 490 nm using a microplate reader (Bio-Rad, Hercules, CA). Background LDH release, determined in nontreated/control cultures, was subtracted from all experimental values. Finally, we used an antibody recognizing the active form of Caspase-3 (Promega), as a marker of apoptosis, followed by a secondary anti-rabbit antibody conjugated with Alexa Fluor 488. Apoptotic neurons appeared as green fluorescent cells.
ELISA assay.
Quantification of phosphorylated [pTpY180/182] and total p38 MAPK was detected in protein extracts from neuronal cell cultures using two different ELISA kits (Biosource, Camarillo, CA) according to the manufacturer's protocol. Cell pellets were collected, immediately frozen at −80°C, and subsequently lysed in cell extraction buffer (10 mm Tris, pH 7.4, 100 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1 mm NaF, 20 mm Na4P2O7, 2 mm Na3VO4, 1% Triton X-100, 10% glycerol, 0.1% SDS, 0.5% deoxycholate, and 1 mm PMSF; Sigma protease mixture inhibitor). The extract was then centrifuged (13,000 rpm, 10 min at 4°C) to obtain a clear lysate that was used for the assay. Samples were pipetted into wells coated with a specific monoclonal antibody against p38 MAPK (regardless of phosphorylation state); after washing, the detection antibody (rabbit anti-p38 MAPK [pTpY180/182] or rabbit anti-total-p38 MAPK) was added to wells. The sandwich was completed by adding anti-rabbit IgG-HRP conjugated. After completing the reaction with stabilized chromogen (tetramethylbenzidine), absorbance was read at 450 nm using a microplate reader (Bio-Rad). Values are expressed as p38 MAPK [pTpY180/182] units normalized for the content of total p38 MAPK.
Statistical analysis.
Statistical comparisons between experimental groups or between FP amplitudes measured during baseline and after the induction protocol were performed by applying a two-way repeated-measures ANOVA with pairwise multiple comparison procedures (Holm–Sidak method). Comparisons between different groups in cell culture experiments were performed using one-way ANOVA. Differences were considered significant when p < 0.05.
Results
Application of oligomeric Aβ42 affects LTP in the entorhinal cortex
We focused our studies on the entorhinal cortex, an area of the brain crucially involved in cognitive and memory processes that is reciprocally connected to the hippocampus and associative cortical areas. In particular, the distribution of its afferent, efferent, and associational projections suggests that different rostrocaudal strips of entorhinal cortex may operate independently in processing neocortex- and hippocampus-originated information (Witter et al., 1989; Dolorfo and Amaral, 1998). We recorded FPs in horizontal sections of entorhinal slices from superficial layer II/III after stimulation of layer II (Fig. 1A,B). Regarding laminar differentiation, superficial layers (I–III) receive projections from unimodal and polymodal association areas via the perirhinal cortex and then provide the majority of input projections to the hippocampus (Witter et al., 1989). Intrinsic associational fibers originating from superficial layers are confined within the same layers (Dolorfo and Amaral, 1998). Previous studies have shown that in different layers, including superficial layer II/III of the entorhinal cortex, LTP can be elicited (Alonso et al., 1990; Yun et al., 2000) using various stimulus patterns (Yun et al., 2002).
LTP was reliably elicited after HFS in layer II. The mean LTP was 133% (SEM, 6%) of baseline amplitude at 50 min after HFS (n = 11 slices, 6 mice) (Fig. 1C). When Aβ42 at 200 nm (a concentration shown to not cause cell death; see results in cell culture below) was applied for 10 min, starting 5 min before HFS delivery, in interleaved experiments, LTP expression was completely inhibited (98 ± 6%, n = 6, 4 mice; p < 0.05) (Fig. 1C). A lower concentration of Aβ42 (20 nm) did not affect LTP (143 ± 11%; n = 6, 3 mice) (Fig. 1D). LTP amplitude was not affected in slices treated with the reversed peptide Aβ(42–1) (200 nm; 134 ± 14%; n = 8, 4 mice) (Fig. 1D). Impairment of synaptic transmission by Aβ42 was evaluated by measuring the amplitude of FPs as a function of stimulus intensity. Aβ42 did not change the input–output curve with respect to vehicle-treated slices (Fig. 1E). The latter result, together with absence of baseline modifications by Aβ42 (Fig. 1C), suggests that synaptic transmission is not impaired by Aβ42 in accordance with previous reports in hippocampal slices (Chen et al., 2002; Vitolo et al., 2002; Raymond et al., 2003; Wang et al., 2004). Finally, effects of Aβ42 on short-term plasticity were assayed using two consecutive electrical stimuli (PP) applied at different interstimulus intervals (30–200 ms). The PP ratio was unaffected by Aβ42 (Fig. 1F). Therefore, there was no indication that Aβ42 (200 nm) affects mechanisms of PP facilitation.
RAGE deficiency rescues Aβ42-induced inhibition of LTP in the entorhinal cortex
RAGE is a key cofactor mediating Aβ-induced synaptic plasticity impairment in the hippocampus (Arancio et al., 2004). To test whether RAGE was involved in Aβ42 entorhinal cortex impairment of LTP, we examined the effect of a brief application of Aβ42 on LTP in cortical slices from RAGE-null mice and in slices from WT mice pretreated with anti-RAGE IgG. Input–output curves (Fig. 2A, left), as well as short-term plasticity properties (PP ratio) (Fig. 2A, right), did not differ between RAGE-null slices and anti-RAGE IgG-treated slices from WT mice. The latter two demonstrated the same results as cortical slices from age-matched WT mice exposed to vehicle alone. Moreover, deficiency of RAGE (RAGE-null mice) and blockade of RAGE by specific antibodies to the receptor (WT slices incubated with anti-RAGE IgG, 2.5 μg/ml for at least 2 h before recordings) did not affect LTP (Fig. 2B). The mean LTP amplitude in RAGE-null mice (135 ± 11%; n = 8, 4 mice) and in slices from WT mice treated with anti-RAGE IgG (131 ± 6%; n = 5, 3 mice) was not significantly different with respect to vehicle-treated WT slices, suggesting that blockade of RAGE does not affect LTP in entorhinal cortex. Remarkably, when Aβ42 (200 nm) was bath applied to RAGE-null and anti-RAGE IgG-treated WT slices, LTP was normal (Fig. 2C). The mean LTP amplitude measured in RAGE-null (126 ± 2%; n = 8, 5 mice) and anti-RAGE IgG-incubated (136 ± 7%; n = 6, 4 mice) WT slices treated with Aβ42 was significantly increased (p < 0.05) with respect to LTP in slices treated with Aβ42 alone (control) (Fig. 2C). In contrast, values of LTP amplitude were similar in RAGE-null slices or anti-RAGE IgG-treated WT slices without Aβ.
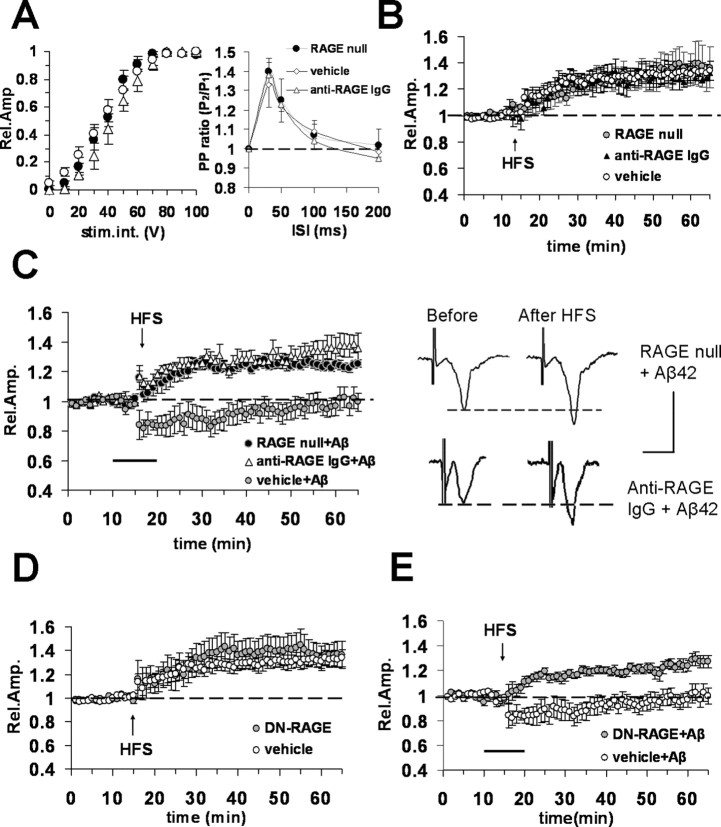
Figure 2.
Blockade of RAGE protects against Aβ42 (200 nm)-induced reduction of LTP in entorhinal cortex slices. A, Left, Input–output curves did not show significant differences between vehicle-treated WT slices (open circles) and RAGE-null slices (filled circles) or WT slices treated with anti-RAGE IgG (open triangles). Right, Paired-pulse facilitation, PP ratio was not modified in RAGE-null slices and in slices from WT mice treated with anti-RAGE IgG with respect to vehicle-treated slices. B, LTP expression was unaffected in slices from RAGE-null mice (open circles) or in slices from WT mice preincubated with anti-RAGE IgG (2.5 μg/ml for 2 h, filled triangles) with respect to vehicle-treated slices (open circles). C, Left, Bath application (dark bar) of Aβ42 (200 nm) did not inhibit LTP in slices from RAGE-null mice (filled circles) or in slices from WT mice preincubated with anti-RAGE IgG (2.5 μg/ml for 2 h, open triangles) with respect to vehicle-treated slices (gray circles). Right, Representative field potentials recorded before and 50 min after HFS in RAGE-null and anti-RAGE IgG-treated slices from WT mice; bath application of Aβ was unable to block LTP. Calibration: 1 mV, 5 ms. D, LTP expression was comparable in vehicle-treated slices from WT mice (open circles) and in slices from Tg DN-RAGE (gray circles). E, Aβ42 (200 nm, bath applied, dark bar) failed to inhibit LTP in DN-RAGE slices (gray circles). Error bars indicate SEM.
Because RAGE is expressed in neurons as well as in other cells (glial cells, endothelial cells) (Schmidt et al., 2001; Lue et al., 2005), we determined whether neuronal RAGE is responsible for Aβ effects on LTP in entorhinal cortex. We investigated transgenic mice with signal transduction deficient mutants of RAGE in which the cytosolic domain of the receptor has been deleted, thereby imparting a dominant-negative phenotype, targeted to neurons (DN-RAGE) by the PDGF-B chain promoter. The PDGF-B chain promoter has been successfully used to drive expression of transgene targeted to neurons (Sasahara et al., 1991; Mucke et al., 2000; Lustbader et al., 2004; Takuma et al., 2005). Furthermore, transgenic mice expressing neuronal DN-RAGE driven by the PDGF-B chain promoter have been previously characterized, demonstrating localization of DN-RAGE in cortical neurons (Arancio et al., 2004). Previous studies revealed that these transgenic mice displayed preservation of spatial learning and memory and decreased neuropathological changes when bred into transgenic mice expressing mutant APP (Arancio et al., 2004). Similar to what was observed in slices from RAGE-null mice, LTP was unaffected in slices from transgenic mice with defective RAGE targeted to neurons (DN-RAGE slices: 136 ± 10%, n = 9, 5 mice; not significantly different from vehicle-treated WT slices) (Fig. 2D). Most importantly, defective RAGE signaling targeted to neurons protected against Aβ-mediated reduction of LTP (DN-RAGE) (Fig. 2E); LTP values in DN-RAGE slices (127 ± 5%; n = 8, 5 mice) in the presence of Aβ42 (200 nm) were significantly increased (p < 0.05) with respect to slices from WT mice treated with 200 nm Aβ. Thus, functionless RAGE in neurons was able to abolish Aβ inhibition of LTP.
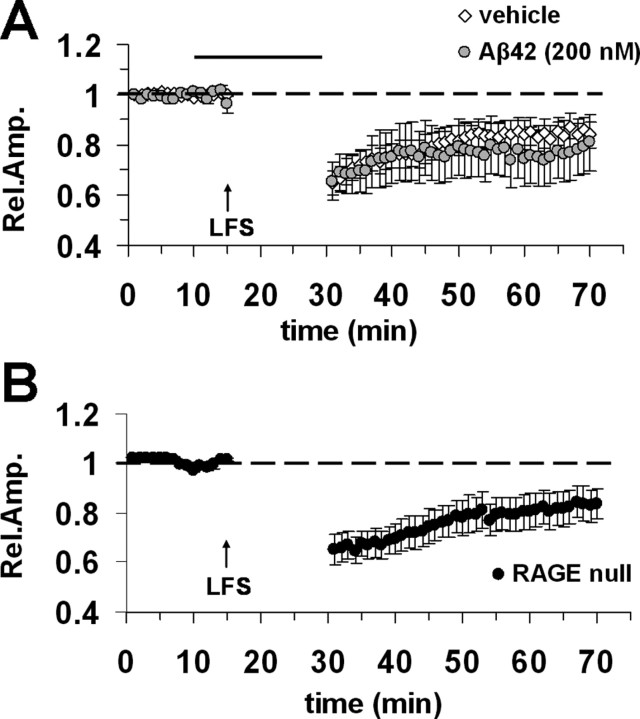
Based on a previous observations in double knock-in mice carrying human mutations in the genes for APP and presenilin-1 showing that LTD was impaired, in addition to LTP (Chang et al., 2006; but see Wang et al., 2002, showing no effect of exogenous synthetic Aβ42 on hippocampal LTD), we conducted a complementary study on the effects of Aβ42 on LTD in entorhinal cortex slices. LTD was reliably induced using paired-pulse LFS in agreement with the report by Kourrich and Chapman (2003). After this induction protocol, LTD magnitude was 84 ± 5% (n = 10 slices, 4 mice) (Fig. 3A). Consistent with data obtained by Wang et al. (2002) in hippocampus, Aβ42 (200 nm) did not affect LTD in entorhinal cortex (77 ± 10%; n = 9 slices, 4 mice) (Fig. 3A) (p > 0.05 vs control vehicle-treated slices). Moreover, when we tested LTD in RAGE-null slices, we found LTD to be normal (Fig. 3B) indicating that RAGE deficiency does not affect LTD expression in entorhinal cortex. Thus, LTD induced by LFS is not impaired by Aβ42-RAGE signaling.
Figure 3.
LTD is not affected in entorhinal cortex slices treated with Aβ42. A, LTD was reliably inducible by LFS (arrow) in vehicle-treated slices (open circles) and Aβ42-treated slices from WT mice (Aβ42, 200 nm; gray circles). The top horizontal dark bar corresponds to bath applied Aβ42. B, LTD was unimpaired in slices from RAGE-null mice. Error bars indicate SEM.
Aβ42 impairment of LTP is prevented by inhibition of p38 MAPK but not JNK
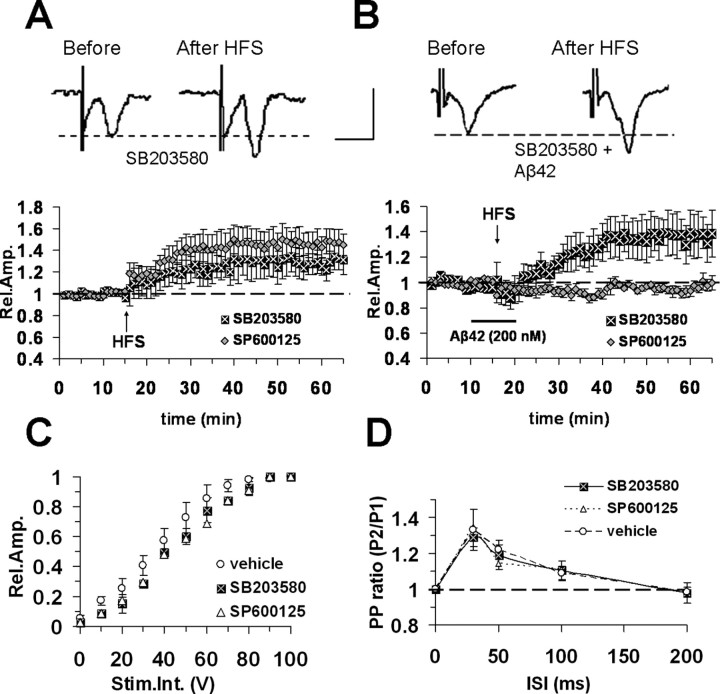
Stress-related protein kinases, such as JNK and p38 MAPK, are activated in several pathologic processes, including AD neurodegeneration and Aβ42 cytotoxicity (Pei et al., 2001; Troy et al., 2001; Zhu et al., 2005). It is noteworthy that both JNK and p38 MAPK inhibition prevented Aβ-induced LTP impairment in hippocampal slices (Wang et al., 2004). These observations suggested the hypothesis that RAGE/Aβ interaction induced impairment of LTP through activation of specific kinase signaling cascades. To evaluate this hypothesis, we tested the effect of MAPK inhibitors on development of LTP in cortical slices. In particular, we used a JNK inhibitor (SP600125) and a p38 MAPK inhibitor (SB203580). SB203580 is a cell permeable inhibitor of p38 MAPK (IC50, 0.3 μm) showing a high selectivity at concentrations <2 μm (Lali et al., 2000). SP600125 is a potent selective and reversible JNK inhibitor (IC50, 0.19 μm) (Bennett et al., 2001). Perfusion with either compound did not induce significant change in FP amplitude during baseline recordings in entorhinal cortex slices (Fig. 4A). When entorhinal cortex slices were continuously perfused with ACSF containing SB203580 (1 μm) and SP600125 (20 μm), LTP was normally expressed after HFS stimulation (131 ± 10%, n = 6, 4 mice, and 146 ± 11%, n = 6, 4 mice, respectively; not significantly different with respect to control LTP in vehicle-treated slices) (Fig. 4A). Perfusion of the p38 inhibitor (SB203580, 1 μm) in the presence of Aβ42 (200 nm, for 10 min starting from 5 min before HFS delivery) restored normal LTP (136 ± 13%; n = 7, 4 mice; p < 0.05 vs Aβ42 alone) (Fig. 4B). LTP amplitude was not significantly different between SB203580-treated and vehicle-treated slices (p > 0.05). In contrast, a specific inhibitor of JNK (SP600125, 20 μm) failed to rescue LTP in Aβ42-treated slices (95 ± 3%; n = 6, 3 mice) (Fig. 4B). Input–output curves (Fig. 4C), as well as short-term plasticity properties (PP ratio) (Fig. 4D), did not differ between SB203580- (1 μm) or SP600125 (20 μm)-treated slices and age-matched vehicle-treated WT slices.
Figure 4.
Aβ42 impairment of LTP is prevented by inhibition of p38 MAPK, but not JNK in entorhinal cortex slices from WT mice. A, LTP induction and maintenance was not affected by continuous perfusion with 1 μm SB203580 (p38 MAPK inhibitor) (filled square) or 20 μm SP600125 (JNK inhibitor) (gray diamonds). Top, Representative field potentials recorded before and 50 min after HFS slices treated with SB203580. B, SB203580 (1 μm), but not SP600125 (20 μm), perfusion prevented LTP blockade in Aβ42-treated slices (bath application at 200 nm corresponding to dark bar). Top, Representative field potentials recorded before and 50 min after HFS in slices treated with SB203580 and Aβ42. Calibration: A, B, 1 mV, 5 ms. C, Input–output curves. Slice perfusion with ACSF containing 1 μm SB203580 (filled squares) or 20 μm SP600125 (open triangles) did not change the input–output curves with respect to vehicle-treated slices (open circles). D, Paired-pulse facilitation, PP ratio was not modified in 1 μm SB203580- and 20 μm SP600125-treated slices with respect to vehicle-treated slices; other conventions are as in C. Error bars indicate SEM.
Aβ42 increases p38 MAPK phosphorylation in cultured neurons
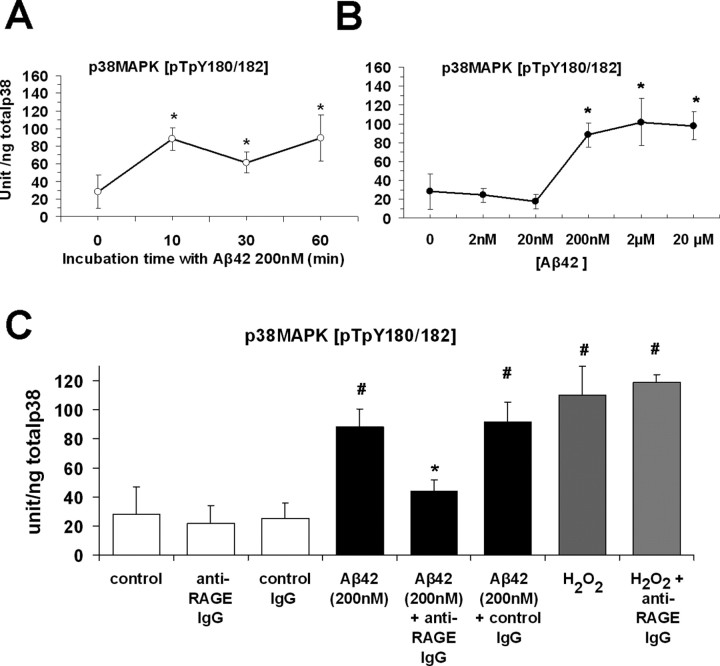
To further investigate the effect of RAGE-dependent activation of p38 MAPK on Aβ-mediated neuronal dysfunction, we measured levels of p38 MAPK in primary cortical cultures. p38 MAPK is activated by a variety of extracellular stimuli, including Aβ peptides, and is modulated by several upstream MAPK kinases (MKK3, MKK4, MKK6), which phosphorylate p38 at threonine 180 and tyrosine 182 in the TGY motif (Ono and Han, 2000). Phospho-p38[pTpY180/182] was measured in primary cultures of mouse cortical neurons after exposure to Aβ42 after different incubation times and a range of concentrations. The kinetic profile of p38 activation under these conditions (Aβ42, 200 nm) demonstrated an apparent maximum level of phosphorylation after 10 min. Increasing incubation time to 1 h did not produce any additional change in phospho-p38 (Fig. 5A). When neurons were incubated with different concentrations of Aβ42 for 10 min (2 nm–20 μm), we found a significant increase in phospho-p38 starting from a concentration of 200 nm, compared with vehicle-treated control cultures (88 ± 12.7 vs 28.1 ± 18.7 U/ng total p38; p < 0.001) (Fig. 5B,C). Additional increases in Aβ42 (2 and 20 μm) did not induce significantly higher phosphorylation of p38 (Fig. 5B). Low concentrations of Aβ (2 and 20 nm) did not produce any increase in phospho-p38 (Fig. 5B). Thus, Aβ is able to activate p38 MAPK at a concentration and with application times in culture comparable with conditions in LTP experiments.
Figure 5.
p38 MAPK phosphorylation increased in cultured neurons exposed to a nontoxic concentration of Aβ42. A, Effect of Aβ42 (200 nm) on p38 phosphorylation at different incubation times (n = 6 experiments for each group; *p < 0.05 vs control untreated cells). B, Effect of increasing concentrations of Aβ42 on p38 phosphorylation; 200 nm Aβ42 significantly increased phospho-p38 levels (n = 6 experiments in each group, *p < 0.001 vs control untreated). No additional increase was induced by higher concentrations Aβ42 (2 and 20 μm). C, Aβ42 (200 nm, 10 min incubation) or H2O2 (100 μm, 10 min) increased p38 phosphorylation compared with anti-RAGE IgG. Levels of p38 phosphorylation in cultures exposed to Aβ42 in the presence of anti-RAGE IgG were similar to those observed in cultures not exposed to Aβ42 and incubated with control IgG or in control cells (#p < 0.001). Anti-RAGE IgG pretreatment of neuronal cultures significantly reduced Aβ-, but not H2O2-induced increase in phospho-p38 (*p < 0.05 vs Aβ42 200 nm; n = 6 experiments for each group). Error bars indicate SEM.
To determine the effect of RAGE on activation of p38 induced by Aβ, we have performed experiments with blocking anti-RAGE antibodies in cell culture. When cells were pretreated with anti-RAGE IgG (2.5 μg/ml) for 2 h before exposure to Aβ, levels of phosho-p38 were significantly reduced compared with neurons incubated with Aβ alone (44.3 ± 7.4 vs 88 ± 12.7 U/ng total p38; p < 0.05, Fig. 5C). However, when anti-RAGE IgG was replaced by nonimmune IgG (control, rabbit IgG), levels of phospho-p38 in Aβ-treated cells were not decreased and remained significantly higher in comparison with those of vehicle-treated control cells (91.9 ± 13 vs 28 ± 18.7 U/ng total p38, Fig. 5C). These results suggest that blocking RAGE by administration of anti-RAGE IgG suppressed Aβ-mediated activation of p38. Furthermore, we used hydrogen peroxide (H2O2) in the incubation medium (10 min at 100 μm) to activate p38 to estimate maximal levels of phospho-p38 and to examine whether H2O2-induced activation of p38 might also be mediated by RAGE. Under the latter conditions (dose and time of exposure to H2O2), cultured cortical neurons displayed activation of p38 MAPK in the absence of cytotoxicity (Wang et al., 2003). Indeed, we found that H2O2 significantly increased phospho-p38 with respect to control cultures (110 ± 20.2 U/ng total p38, p < 0.001 vs vehicle-treated cultures; p = 0.3 vs Aβ-treated cultures) (Fig. 5C). Importantly, anti-RAGE IgG did not block increased levels of phospho-p38 induced by H2O2 (118.6 ± 5.4 vs 110 ± 20.2 in cultures incubated with H2O2, Fig. 5C). Administration of nonimmune IgG alone did not affect baseline levels of phospho-p38 (Fig. 5C). These results further support the concept that Aβ-mediated activation of p38 MAPK occurred as a downstream consequence of RAGE-Aβ interaction.
In additional experiments, we assessed whether Aβ42 (200 nm) induced cell death using two different methods: 1 h incubation with PI and a biochemical assay measuring LDH activity (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). No evidence of cell death was observed with 200 nm Aβ42 using either of these methods. In addition, immunocytochemical analysis with an antibody to the activated form of caspase-3 (a marker of apoptosis) showed that Aβ42 (200 nm) did not significantly increase the number of apoptotic cells even with prolonged Aβ incubation (supplemental Fig. 2, available at www.jneurosci.org as supplemental material).
Together, these results obtained with cultured neurons revealed that (1) Aβ is able to activate p38 MAPK when applied at nm concentrations, and (2) the increase in phospho-p38 can be prevented by blocking RAGE. Furthermore, it is plausible that RAGE-mediated activation of p38 MAPK in neurons after exposure to Aβ42 might represent a cellular mechanism accounting for LTP impairment.
Discussion
Oligomeric forms of Aβ play a critical role in the pathogenesis of AD. In particular, Aβ is believed to contribute to early impairment of cognitive functions such as learning and memory (Cleary et al., 2005; Lesne et al., 2006). Such early signs of functional impairment precede extensive neurodegeneration characteristic of the middle/late phase of AD, which includes extensive deposition of amyloid in the form of plaques. Although not completely clear, the mechanism underlying early functional impairment is likely to involve perturbation of synapse efficacy in different brain areas in the presence of an environment with increased extracellular levels of Aβ. Consistent with this hypothesis, oligomeric Aβ affects long-term forms of synaptic plasticity, such as LTP in the hippocampus (Lambert et al., 1998; Chen et al., 2002; Vitolo et al., 2002; Wang et al., 2004; Puzzo et al., 2005), a brain area fundamentally involved in learning and memory. We have focused our work on entorhinal cortex slices, because this region is a major locus of information exchange between the hippocampus and other cortical areas (Witter et al., 1989; Braak and Braak, 1993; Suzuki and Amaral, 2004) that is also affected at an early stage of AD (Braak and Braak, 1991). In addition, synaptic transmission and long-term synaptic plasticity in the forms of LTP and LTD are well characterized in the entorhinal cortex layers (Alonso et al., 1990; Yun et al., 2000, 2002; Kourrich and Chapman, 2003) representing a suitable assay to probe neuronal function.
Our experiments indicate that oligomeric Aβ42, at a concentration of 200 nm, is capable of blocking LTP, but not LTD or PP, at cortical synapses in the entorhinal cortex. The present results in the entorhinal cortex confirm previous data obtained in hippocampus, which showed that oligomeric Aβ impairs LTP (Walsh et al., 2002, 2005; Wang et al., 2004). Also, our results are in agreement with Wang et al. (2002), who showed that soluble Aβ inhibited LTP in the hippocampus without affecting LTD. Thus, it is likely that even brief exposure to 200 nm Aβ42 specifically perturbs LTP in the entorhinal cortex. In contrast, Aβ42 at the same concentration did not induce a concomitant impairment of synaptic transmission as evaluated by input–output functions and baseline neurotransmission. A few reports have shown that administration of Aβ at low μm concentrations (i.e., at concentrations higher than that used in the present work) can depress synaptic transmission by regulating glutamate receptors trafficking (Snyder et al., 2005; Tyszkiewicz and Yan, 2005; Hsieh et al., 2006; Parameshwaran et al., 2007). Moreover, higher levels of Aβ, thought to be present in intermediate/late stages of neurodegeneration, may affect synaptic transmission and LTD in addition to LTP by regulating trafficking of AMPA- and/or NMDA-receptors (Hsia et al., 1999; Oddo et al., 2003; Chang et al., 2006; Shemer et al., 2006). Interestingly, studies conducted in animal models of AD characterized by progressive accumulation of Aβ42 have shown that LTP disruption occurs before deficits in basal transmission (Trinchese et al., 2004; Chang et al., 2006) and LTD (Chang et al., 2006). Thus, these results in animal models of AD and synthetic Aβ lend support to the concept that impaired LTP characterizes an early stage in AD progression corresponding to a low Aβ load. Later stages in AD progression are associated with a greater Aβ load and also affect basal synaptic transmission and LTD.
Concerning the possible cell surface receptors that are able to bind Aβ and activate downstream intracellular cascades leading to LTP impairment, a number of studies have identified RAGE as a receptor capable of binding to Aβ, in monomeric, fibrillized, and oligomeric form (for review, see Ding and Keller, 2005) (S. D. Yan, unpublished observation) at the cell surface (Lue et al., 2001). RAGE immunoreactivity is present at sites of Aβ deposition and binding of Aβ is considered an important event, increasing Aβ aggregation at the cell membrane (Yan et al., 1996, 1998). This viewpoint assigns an important role for Aβ engagement of RAGE in downstream signaling and effector mechanisms leading to oxidative stress, inflammation, and neurotoxicity. Moreover, RAGE has been implicated as an important cofactor for Aβ-induced neuronal dysfunction in a murine model of AD (Tg APP mice). Indeed, hippocampal LTP was altered only in double-Tg mice (expressing RAGE/mutant APP transgenes) and not in single Tg mice (mutant APP or RAGE), suggesting that overexpression of RAGE in an Aβ-rich environment perturbs synaptic efficacy (Arancio et al., 2004). In the present study, we found that RAGE deficiency or functional blockade of RAGE by specific antibodies counteracts LTP dysfunction induced by Aβ42. Because RAGE is expressed in neurons, as well as other cells critical for neurodegenerative pathology (glial cells, endothelial cells and pericytes of the blood–brain barrier) (Schmidt et al., 2001; Lue et al., 2005), we sought to determine whether neuronal RAGE was responsible for Aβ-dependent LTP impairment in entorhinal cortex. To shed light on this issue, we made use of transgenic mice with a signal transduction deficient mutant of RAGE in which the cytosolic domain of the receptor had been deleted, thereby imparting a DN-RAGE effect. The latter transgene was targeted to neurons (DN-RAGE) by the PDGF-B chain promoter. Previous data reported that expression of DN-RAGE, even in cells expressing wild-type RAGE, blocked RAGE-dependent cellular activation (Hofmann et al., 1999; Arancio et al., 2004). Our findings clearly showed that entorhinal cortex slices from DN-RAGE transgenic mice were resistant to disruption of LTP by 200 nm Aβ, suggesting the involvement of neuronal RAGE in mechanisms leading to Aβ-induced cortical inhibition of LTP. Our results do not exclude that non-neuronal RAGE (RAGE expressed in glial cells, endothelial cells and pericytes of the blood–brain barrier) might be involved in synaptic dysfunction associated with oxidative stress, inflammation, and neurotoxicity characterizing AD progression, as suggested by a previous study (Lue et al., 2001). In perspective, it will be interesting to investigate the role of non-neuronal RAGE in synaptic failure induced by Aβ.
Signaling cascades activated after ligand–RAGE interaction include pathways such as p21ras, extracellular signal-regulated kinase 1/2 (p44/p42) MAP kinases, p38 MAPK and SAPK (stress-activated protein kinase)/JNK, Rho GTPases, phosphoinositol-3 kinase, and the JAK/STAT (signal transducer and activator of transcription) pathway. Downstream consequences, such as the activation of the key transcription factors, nuclear factor-κB and CREB (cAMP response element binding protein), have also been reported previously (Lander et al., 1997; Deora et al., 1998; Hofmann et al., 1999; Huttunen et al., 1999; Kislinger et al., 1999; Huang et al., 2001). Activation of different protein kinase cascades is a principal target of RAGE activation in the pathway controlling synaptic plasticity. In particular, we considered two different kinases, namely JNK and p38 MAPK as potentially involved in inhibition of LTP. Recent results showed that p38 MAPK and JNK activation are required for LTP inhibition induced by proinflammatory agents such as interleukin-1β and lipopolysaccharide (Curran et al., 2003; Kelly et al., 2003). These results prompted us to test whether p38 MAPK and JNK were involved in LTP inhibition by Aβ. In contrast to inflammatory stimuli, Aβ induced LTP impairment could be prevented by a p38 MAPK inhibitor alone, suggesting that activation of this downstream kinase is a key event in the Aβ-initiated signaling pathway. Our results are in agreement with previous data obtained in the RAGE/mutant APP double-transgenic mice that showed a role for RAGE in the activation of p38 MAPK in the hippocampus during AD-like neurodegeneration (Arancio et al., 2004). Although, the role of p38 MAPK in the pathogenesis of AD has been well established (Hensley et al., 1999; Zhu et al., 2005), we also investigated the role of another major stress-activated protein kinase, namely, JNK. However, inhibition of JNK with SP600125 was ineffective in rescuing cortical LTP after treatment of entorhinal cortex slices from WT mice with Aβ. According to the work of others, inhibition of JNK only partially rescued LTP after the application of synthetic Aβ42 peptide in the hippocampus (Wang et al., 2004), leading us to conclude that Aβ-induced inhibition of LTP may occur via a proinflammatory pathway that is principally controlled by activation of p38 MAPK. These observations are further supported by our results showing that Aβ42 at nontoxic concentrations was able to phosphorylate p38 MAPK in cultured cortical neurons. In contrast, addition of anti-RAGE IgG to neuronal cultures prevented Aβ42-mediated activation of p38 MAPK. However, delineation of the precise route from p38 MAPK phosphorylation to the key substrate(s) leading to Aβ-dependent LTP impairment has yet to be identified.
Together, our results indicate that neuronal RAGE is involved in mechanisms underlying Aβ-induced inhibition of LTP, at least in part, through activation of p38 MAPK in mouse cortex. This work contributes to an increasing body of evidence that activation of RAGE by oligomeric Aβ represents an important early step in neuronal dysfunction before amyloid plaque deposition. Thus, RAGE activation by Aβ may lead to an early impairment of cognitive functions in brain areas critically affected in AD.
Footnotes
This work was supported by National Institutes of Health Grant PO1AG17490 and the Alzheimer's Association (S.D.Y.), and Fondazione Cassa di Risparmio di Pisa (05/140) and Italian Ministero Università e Ricerca (PRIN, 2006) (L.D.). S.D.Y. is a co-senior author. We thank Giulio Cappagli and Carlo Orsini for their technical help and assistance.
References
- Alonso A, de Curtis M, Llinas R. Postsynaptic Hebbian and non-Hebbian long-term potentiation of synaptic efficacy in the entorhinal cortex in slices and in the isolated adult guinea pig brain. Proc Natl Acad Sci USA. 1990;87:9280–9284. doi: 10.1073/pnas.87.23.9280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson WW, Collingridge GL. The LTP program: a data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J Neurosci Methods. 2001;108:71–83. doi: 10.1016/s0165-0270(01)00374-0. [DOI] [PubMed] [Google Scholar]
- Arancio O, Zhang HP, Chen X, Lin C, Trinchese F, Puzzo D, Liu S, Hegde A, Yan SF, Stern A, Luddy JS, Lue LF, Walker DG, Roher A, Buttini M, Mucke L, Li W, Schmidt AM, Kindy M, Hyslop PA, et al. RAGE potentiates Aβ-induced perturbation of neuronal function in transgenic mice. EMBO J. 2004;23:4096–4105. doi: 10.1038/sj.emboj.7600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1991;3:213–216. doi: 10.1111/j.1750-3639.1991.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Entorhinal-hippocampal interaction in mnestic disorders. Hippocampus. 1993;3:239–246. [PubMed] [Google Scholar]
- Chang EH, Savage MJ, Flood DG, Thomas JM, Levy RB, Mahadomrongkul V, Shirao T, Aoki C, Huerta PT. AMPA receptor downscaling at the onset of Alzheimer's disease pathology in double knock-in mice. Proc Natl Acad Sci USA. 2006;103:3410–3415. doi: 10.1073/pnas.0507313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- Chen QS, Wei WZ, Shimahara T, Xie CW. Alzheimer amyloid beta-peptide inhibits the late phase of long-term potentiation through calcineurin-dependent mechanisms in the hippocampal dentate gyrus. Neurobiol Learn Mem. 2002;77:354–371. doi: 10.1006/nlme.2001.4034. [DOI] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Curran BP, Murray HJ, O'Connor JJ. A role for c-Jun N-terminal kinase in the inhibition of long-term potentiation by interleukin-1β and long-term depression in the rat dentate gyrus in vitro. Neuroscience. 2003;118:347–357. doi: 10.1016/s0306-4522(02)00941-7. [DOI] [PubMed] [Google Scholar]
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- Deora A, Win T, Vanhaesebroeck B, Lander H. A redox triggered Ras-effector interaction: recruitment of phosphatidyli-nositol 30-kinase to Ras by redox stress. J Biol Chem. 1998;273:29923–29928. doi: 10.1074/jbc.273.45.29923. [DOI] [PubMed] [Google Scholar]
- D'Hooge R, Nagels G, Westland CE, Mucke L, De Deyn PP. Spatial learning deficit in mice expressing human 751-amino acid beta-amyloid precursor protein. NeuroReport. 1996;7:2807–2811. doi: 10.1097/00001756-199611040-00080. [DOI] [PubMed] [Google Scholar]
- Ding Q, Keller JN. Evaluation of rage isoforms, ligands, and signaling in the brain. Biochim Biophys Acta. 2005;1746:18–27. doi: 10.1016/j.bbamcr.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Dolorfo CL, Amaral DG. Entorhinal cortex of the rat: organization of intrinsic connections. J Comp Neurol. 1998;398:49–82. doi: 10.1002/(sici)1096-9861(19980817)398:1<49::aid-cne4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Domenici L, Harding GW, Burkhalter A. Patterns of synaptic activity in forward and feedback pathways within rat visual cortex. J Neurophysiol. 1995;74:2649–2664. doi: 10.1152/jn.1995.74.6.2649. [DOI] [PubMed] [Google Scholar]
- Eisenhauer PB, Johnson RJ, Wells JM, Davies TA, Fine RE. Toxicity of various amyloid beta peptide species in cultured human blood-brain barrier endothelial cells: increased toxicity of dutch-type mutant. J Neurosci Res. 2000;60:804–810. doi: 10.1002/1097-4547(20000615)60:6<804::AID-JNR13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Giacchino J, Criado JR, Games D, Henriksen S. In vivo synaptic transmission in young and aged amyloid precursor protein transgenic mice. Brain Res. 2000;876:185–190. doi: 10.1016/s0006-8993(00)02615-9. [DOI] [PubMed] [Google Scholar]
- Hensley K, Floyd RA, Zheng NY, Nael R, Robinson KA, Nguyen X, Pye QN, Stewart CA, Geddes J, Markesbery WR, Patel E, Johnson GV, Bing G. p38 kinase is activated in the Alzheimer's disease brain. J Neurochem. 1999;72:2053–2058. doi: 10.1046/j.1471-4159.1999.0722053.x. [DOI] [PubMed] [Google Scholar]
- Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proc Natl Acad Sci USA. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Aβ-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Guh J, Chen H, Hung W, Lai Y, Chuang L. Role of RAGE and the JAK/STAT-signaling pathway in AGE-induced collagen production in NRK-49F cells. J Cell Biochem. 2001;81:102–113. doi: 10.1002/1097-4644(20010401)81:1<102::aid-jcb1027>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Huttunen H, Fages C, Rauvala H. RAGE-mediated neurite outgrowth and activation of NF-κB require the cytoplasmic domain of the receptor but different downstream signaling pathways. J Biol Chem. 1999;274:19919–19924. doi: 10.1074/jbc.274.28.19919. [DOI] [PubMed] [Google Scholar]
- Ingelsson M, Fukumoto H, Newell KL, Growdon JH, Hedley-Whyte ET, Frosch MP, Albert MS, Hyman BT, Irizarry MC. Early Aβ accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology. 2004;62:925–931. doi: 10.1212/01.wnl.0000115115.98960.37. [DOI] [PubMed] [Google Scholar]
- Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH. The Alzheimer's A β-peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc Natl Acad Sci USA. 2002;18:11830–11835. doi: 10.1073/pnas.192203399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A, Vereker E, Nolan Y, Brady M, Barry C, Loscher CE, Mills KH, Lynch MA. Activation of p38 plays a pivotal role in the inhibitory effect of lipopolysaccharide and interleukin-1 beta on long term potentiation in rat dentate gyrus. J Biol Chem. 2003;278:19453–19462. doi: 10.1074/jbc.M301938200. [DOI] [PubMed] [Google Scholar]
- Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Yan SD, Hofmann M, Yan SF, Pischetsrider M, Stern D, Schmidt AM. Nε (carboxymethyl) lysine modifications of proteins are ligands for RAGE that activate cell signaling pathways and modulate gene expression. J Biol Chem. 1999;274:31740–31749. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Chapman CA. NMDA receptor-dependent long-term synaptic depression in the entorhinal cortex in vitro. J Neurophysiol. 2003;89:2112–2119. doi: 10.1152/jn.00714.2002. [DOI] [PubMed] [Google Scholar]
- Lali FV, Hunt AE, Turner SJ, Foxwell BM. The pyridinyl imidazole inhibitor SB203580 blocks phosphoinositide-dependent protein kinase activity, protein kinase B phosphorylation, and retinoblastoma hyperphosphorylation in interleukin-2-stimulated T cells independently of p38 mitogen-activated protein kinase. J Biol Chem. 2000;275:7395–7402. doi: 10.1074/jbc.275.10.7395. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander H, Tauras J, Ogiste J, Moss R, Schmidt AM. Activation of RAGE triggers a MAP kinase pathway regulated by oxidant stress. J Biol Chem. 1997;272:17810–17814. doi: 10.1074/jbc.272.28.17810. [DOI] [PubMed] [Google Scholar]
- Larson J, Lynch G, Games D, Seubert P. Alterations in synaptic transmission and long-term potentiation in hippocampal slices from young and aged PDAPP mice. Brain Res. 1999;840:23–35. doi: 10.1016/s0006-8993(99)01698-4. [DOI] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Li QX, Maynard C, Cappai R, McLean CA, Cherny RA, Lynch T, Culvenor JG, Trevaskis J, Tanner JE, Bailey KA, Czech C, Bush AI, Beyreuther K, Masters CL. Intracellular accumulation of detergent-soluble amyloidogenic A beta fragment of Alzheimer's disease precursor protein in the hippocampus of aged transgenic mice. J Neurochem. 1999;72:2479–2487. doi: 10.1046/j.1471-4159.1999.0722479.x. [DOI] [PubMed] [Google Scholar]
- Lue LF, Walker DG, Brachova L, Beach TG, Rogers J, Schmidt AM, Stern DM, Yan SD. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer's disease: identification of a cellular activation mechanism. Exp Neurol. 2001;171:29–45. doi: 10.1006/exnr.2001.7732. [DOI] [PubMed] [Google Scholar]
- Lue LF, Yan SD, Stern DM, Walker DG. Preventing activation of receptor for advanced glycation endproducts in Alzheimer's disease. Curr Drug Targets CNS Neurol Disord. 2005;4:249–266. doi: 10.2174/1568007054038210. [DOI] [PubMed] [Google Scholar]
- Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SD, Wu H. ABAD directly links Aβ to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of Aβ1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, Laferla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Ab and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Parameshwaran K, Sims C, Kanju P, Vaithianathan T, Shonesy BC, Dhanasekaran M, Bahr BA, Suppiramaniam V. Amyloid β-peptide Aβ(1–42) but not Aβ(1–40) attenuates synaptic AMPA receptor function. Synapse. 2007;61:367–374. doi: 10.1002/syn.20386. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, Cowburn RF. Localization of active forms of C-jun kinase (JNK) and p38 kinase in Alzheimer's disease brains at different stages of neurofibrillary degeneration. J Alzheimers Dis. 2001;3:41–48. doi: 10.3233/jad-2001-3107. [DOI] [PubMed] [Google Scholar]
- Pesavento E, Capsoni S, Domenici L, Cattaneo A. Acute cholinergic rescue of synaptic plasticity in the neurodegenerating cortex of anti-nerve growth factor mice. Eur J Neurosci. 2002;15:1030–1036. doi: 10.1046/j.1460-9568.2002.01937.x. [DOI] [PubMed] [Google Scholar]
- Puzzo D, Vitolo O, Trinchese F, Jacob JP, Palmeri A, Arancio O. Amyloid-beta peptide inhibits activation of the nitric oxide/cGMP/cAMP-responsive element-binding protein pathway during hippocampal synaptic plasticity. J Neurosci. 2005;25:6887–6897. doi: 10.1523/JNEUROSCI.5291-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CD, Ireland DR, Abraham WC. NMDA receptor regulation by amyloid-b does not account for its inhibition of LTP in rat hippocampus. Brain Res. 2003;968:263–272. doi: 10.1016/s0006-8993(03)02269-8. [DOI] [PubMed] [Google Scholar]
- Sakaguchi T, Yan SF, Yan SD, Belov D, Rong LL, Sousa M, Andrassy M, Marso SP, Duda S, Arnold B, Liliensiek B, Nawroth PP, Stern DM, Schmidt AM, Naka Y. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest Apr. 2003;111:959–972. doi: 10.1172/JCI17115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasahara M, Fries JWU, Raines EW, Gown AM, Westrum LE, Frosch MP, Bonthron DT, Ross R, Collins T. PDGF B-chain in neurons of the central nervous system, posterior pituitary, and in a transgenic model. Cell. 1991;64:217–227. doi: 10.1016/0092-8674(91)90223-l. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Shemer I, Holmgren C, Min R, Fulop L, Zilberter M, Sousa KM, Farkas T, Hartig W, Penke B, Burnashev N, Tanila H, Zilberter Y, Harkany T. Non-fibrillar beta-amyloid abates spike-timing-dependent synaptic potentiation at excitatory synapses in layer 2/3 of the neocortex by targeting postsynaptic AMPA receptors. Eur J Neurosci. 2006;23:2035–2047. doi: 10.1111/j.1460-9568.2006.04733.x. [DOI] [PubMed] [Google Scholar]
- Simmons LK, May PC, Tomaselli KJ, Rydel RE, Fuson KS, Brigham EF, Wright S, Lieberburg I, Becker GW, Brems DN. Secondary structure of amyloid beta peptide correlates with neurotoxic activity in vitro. Mol Pharmacol. 1994;45:373–379. [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Functional neuroanatomy of the medial temporal lobe memory system. Cortex. 2004;40:220–222. doi: 10.1016/s0010-9452(08)70958-4. [DOI] [PubMed] [Google Scholar]
- Takuma K, Yao J, Huang J, Xu H, Chen X, Luddy J, Trillat AC, Stern DM, Arancio O, Yan SD. ABAD enhances Aβ-induced cell stress via mitochondrial dysfunction. FASEB J. 2005;19:597–598. doi: 10.1096/fj.04-2582fje. [DOI] [PubMed] [Google Scholar]
- Trinchese F, Liu S, Battaglia F, Walter S, Mathews PM, Arancio O. Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Ann Neurol. 2004;55:801–814. doi: 10.1002/ana.20101. [DOI] [PubMed] [Google Scholar]
- Troy CM, Rabacchi SA, Xu Z, Maroney AC, Connors TJ, Shelanski ML, Greene LA. beta-Amyloid-induced neuronal apoptosis requires c-Jun N-terminal kinase activation. J Neurochem. 2001;77:157–164. doi: 10.1046/j.1471-4159.2001.t01-1-00218.x. [DOI] [PubMed] [Google Scholar]
- Tyszkiewicz JP, Yan Z. Beta-Amyloid peptides impair PKC-dependent functions of metabotropic glutamate receptors in prefrontal cortical neurons. J Neurophysiol. 2005;93:3102–3111. doi: 10.1152/jn.00939.2004. [DOI] [PubMed] [Google Scholar]
- Vitolo OV, Sant'Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid beta-peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci USA. 2002;99:13217–13221. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Townsend M, Podlisny MB, Shankar GM, Fadeeva JV, El Agnaf O, Hartley DM, Selkoe DJ. Certain inhibitors of synthetic amyloid beta-peptide (Aβ) fibrillogenesis block oligomerization of natural Abeta and thereby rescue long-term potentiation. J Neurosci. 2005;25:2455–2462. doi: 10.1523/JNEUROSCI.4391-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HW, Pasternak JF, Kuo H, Ristic H, Lambert MP, Chromy B, Viola KL, Klein WL, Stine WB, Krafft GA, Trommer BL. Soluble oligomers of beta amyloid (1–42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res. 2002;924:133–140. doi: 10.1016/s0006-8993(01)03058-x. [DOI] [PubMed] [Google Scholar]
- Wang JY, Shum A, Ho YJ, Wang JY. Oxidative neurotoxicity in rat cerebral cortex neurons: synergistic effects of H2O2 and NO on apoptosis involving activation of p38 mitogen-activated protein kinase and caspase-3. J Neurosci Res. 2003;72:508–519. doi: 10.1002/jnr.10597. [DOI] [PubMed] [Google Scholar]
- Wang Q, Walsh DM, Rowan MJ, Selkoe DJ, Anwyl R. Block of long-term potentiation by naturally secreted and synthetic amyloid beta-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. J Neurosci. 2004;24:3370–3378. doi: 10.1523/JNEUROSCI.1633-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt TM, Tanji N, Guo J, Kislinger TR, Qu W, Lu Y, Bucciarelli LG, Rong LL, Moser B, Markowitz GS, Stein G, Bierhaus A, Liliensiek B, Arnold B, Nawroth PP, Stern DM, D'Agati VD, Schmidt AM. RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol. 2003;162:1123–1137. doi: 10.1016/S0002-9440(10)63909-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Wirths O, Multhaup G, Czech C, Blanchard V, Moussaoui S, Tremp G, Pradier L, Beyreuther K, Bayer TA. Intraneuronal Abeta accumulation precedes plaque formation in beta-amyloid precursor protein and presenilin-1 double-transgenic mice. Neurosci Lett. 2001;306:116–120. doi: 10.1016/s0304-3940(01)01876-6. [DOI] [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AH. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol. 1989;33:161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Yan SD, Yan SF, Chen X, Fu J, Chen M, Kuppusamy P, Smith MA, Perry G, Godman GC, Nawroth P, Zweier J, Stern DM. Non-enzymatically glycated tau in Alzheimer's disease induces neuronal oxidant stress resulting in cytokine gene expression and release of amyloid beta-peptide. Nat Med. 1995;1:693–699. doi: 10.1038/nm0795-693. [DOI] [PubMed] [Google Scholar]
- Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- Yan SD, Stern D, Kane MD, Kuo YM, Lampert HC, Roher AE. RAGE-Abeta interactions in the pathophysiology of Alzheimer's disease. Restor Neurol Neurosci. 1998;12:167–173. [PubMed] [Google Scholar]
- Yan Y, Liu Y, Sorci M, Belfort G, Lustbader JW, Yan SD, Wang C. Surface plasmon resonance and nuclear magnetic resonance studies of ABAD-Aβ interaction. Biochemistry. 2007;46:1724–1731. doi: 10.1021/bi061314n. [DOI] [PubMed] [Google Scholar]
- Yun SH, Huh K, Jung MW. Selective enhancement of non-NMDA receptor-mediated responses following induction of long-term potentiation in entorhinal cortex. Synapse. 2000;35:1–7. doi: 10.1002/(SICI)1098-2396(200001)35:1<1::AID-SYN1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Yun SH, Jung IM, Jung MW. Variation in effective stimulus pattern for induction of long-term potentiation across different layers of rat entorhinal cortex. J Neurosci. 2002;22:RC214. doi: 10.1523/JNEUROSCI.22-05-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Watson JB, Xie CW. Amyloid beta prevents activation of calcium/calmodulin-dependent protein kinase II and AMPA receptor phosphorylation during hippocampal long-term potentiation. J Neurophysiol. 2004;92:2853–2858. doi: 10.1152/jn.00485.2004. [DOI] [PubMed] [Google Scholar]
- Zhu X, Mei M, Lee HG, Wang Y, Han J, Perry G, Smith MAP38 activation mediates amyloid-beta cytotoxicity. Neurochem Res. 2005;30:791–796. doi: 10.1007/s11064-005-6872-x. [DOI] [PubMed] [Google Scholar]