Abstract
We examined the possibility of a differential spatial control in the endogenous production of 3α5α-reduced steroids and its consequences on GABAA receptor-mediated miniature IPSCs (mIPSCs) in laminas II and III–IV of the rat spinal cord dorsal horn (DH). Early in postnatal development [younger than postnatal day 8 (P8)], mIPSCs displayed slow decay kinetics in laminas II and III–IV resulting from a continuous local production of 3α5α-reduced steroids. This was mediated by the tonic activity of the translocator protein of 18 kDa (TSPO), which controls neurosteroid synthesis by regulating the transport of cholesterol across the mitochondrial membrane system. TSPO activity disappeared in laminas III–IV after P8 and was functionally downregulated in lamina II after P15, resulting in a marked reduction of mIPSC duration in these laminas. TSPO-mediated synthesis of 3α5α-reduced steroids was spatially restricted, because, at P9–P15, when their production was maximal in lamina II, no sign of spillover to laminas III–IV was apparent. Interestingly, after P8, the enzymes necessary for the synthesis of 3α5α-reduced steroids remained functional in laminas III–IV and could produce such steroids from various precursors or after a single subcutaneous injection of progesterone. Moreover, induction of an acute peripheral inflammation by intraplantar injection of carrageenan, restored a maximal TSPO-mediated neurosteroidogenesis in laminas III–IV. Our results indicate that the decay kinetics of GABAA receptor-mediated mIPSCs in the DH of the spinal cord are primarily controlled by 3α5α-reduced steroids, which can be produced from circulating steroid precursors and/or in a spatially restricted manner by the modulation of the activity of TSPO.
Keywords: peripheral benzodiazepine receptor, synaptic transmission, synaptic inhibition, somatosensory, inflammation, neurosteroid
Introduction
Fast inhibitory synaptic transmission in the CNS is mainly mediated by the activation of GABAA receptors and plays a fundamental role in the integration and processing of sensory and motor messages. GABAA receptors are potently modulated by endogenous molecules such as neurosteroids (Lambert et al., 2003; Belelli and Lambert, 2005; Schlichter et al., 2006), which are synthesized in the nervous system by glial cells and/or neurons (Baulieu, 1997; Compagnone and Mellon, 2000). Neurosteroids modulate synaptic transmission in the CNS during development (Keller et al., 2004; Mameli et al., 2005) and in physiological and pathological situations (Reddy and Rogawski, 2002; Leroy et al., 2004; Sanna et al., 2004; Poisbeau et al., 2005; Mameli and Valenzuela, 2006). The 3α5α-reduced derivatives of progesterone [allopregnanolone (AP)] or of deoxycorticosterone (tetrahydrodeoxycorticosterone) represent the most powerful endogenous positive allosteric modulators of GABAA receptors (Lambert et al., 2003; Belelli et al., 2006; Schlichter et al., 2006). Recently, two distinct binding sites for 3α5α-reduced steroids have been identified at GABAA receptors and were shown to be responsible for a positive allosteric modulation of the receptors at low concentrations and a direct activation of the receptors at higher concentrations, respectively (Shu et al., 2004; Hosie et al., 2006). Therefore, the type and concentration/amount of neurosteroid present in the vicinity of synaptic GABAA receptors (Akk et al., 2005) will strongly influence the properties of the synaptic GABAA receptor-mediated currents.
In lamina II (lam II) of the dorsal horn (DH) of the spinal cord, we have shown recently that 3α5α-reduced neurosteroids (3α5α-NS) are synthesized locally and potentiate the function of synaptic GABAA receptors. The production of these 3α5α-NS was developmentally regulated (Keller et al., 2004), and in the adult, neurosteroidogenesis could be stimulated in lamina II after induction of a peripheral inflammation (Poisbeau et al., 2005). The control of neurosteroid production could therefore be implicated in the fine tuning of neuronal networks in the spinal cord as well as in other areas of the CNS (Belelli et al., 2006; Schlichter et al., 2006).
Most studies on the modulatory effects of 3α5α-reduced steroids on GABAA receptor function have been performed on recombinant GABAA receptors or in culture systems using exogenous applications of steroids (Belelli et al., 2002; Shu et al., 2004; Akk et al., 2005). These experimental models and situations give indications on the mechanism of action of such steroids, but issues such as that related to the synthesis and role of endogenous steroids, their possible local diffusion within the tissue, or the mechanisms regulating their production require more intact systems such as slice preparations. We addressed these points by investigating the consequences of manipulating endogenous neurosteroidogenesis on the properties of synaptic GABAA receptors in anatomically close but distinct laminas of the dorsal horn of the spinal cord.
Materials and Methods
Animals.
All experiments were performed on spinal cord slices from 6- to 30-d-old male Wistar or Sprague Dawley rats born in the animal house of the laboratory. Three groups were considered according to postnatal age: <P8, P9 ≤ Π ≤ P15, and >P21. All experiments were conducted in conformity with the rules of the European Communities Council Directive of November 24, 1986 (86/609/EEC) and the French Department of Agriculture (license #67-107 to R.S.).
Induction of inflammation.
Inflammation was induced in postnatal day 9 (P9)–P15 rats by a bilateral intraplantar injection of λ-carrageenan (10 μl of 3% solution in 0.9% NaCl; Sigma, St. Quentin Fallavier, France); control rats were injected with 0.9% NaCl only (10 μl). Inflammation developed overnight, and the animals were killed 15 h after the injection. Induction of inflammation in >P21 rats followed the same protocol, except that injected volume of carrageenan or 0.9% saline was 100 μl.
Subcutaneous injections.
In some experiments, rats were injected in the neck region with a single subcutaneous dose of progesterone (75 mg/kg) in olive oil. The effect of progesterone was assessed by comparing the results obtained with progesterone-injected animals to those obtained with animals injected with the same volume of vehicle.
Preparation of slices.
As described previously (Keller et al., 2001, 2004), rats were deeply anesthetized with ketamine (75 mg/kg, i.p.) and killed by decapitation. The spinal cord was removed by hydraulic extrusion and washed in ice-cold (≤4°C) sucrose–artificial CSF (ACSF) containing the following (in mm): 248 sucrose, 11 glucose, 26 NaHCO3, 2 KCl, 1.25 KH2PO4, 2 CaCl2, 1.3 MgSO4 (bubbled with 95% O2 and 5% CO2). The lumbar segment was embedded in 5% agarose, and 600-μm-thick transverse slices were cut with a vibratome (VT1000S; Leica, Nussloch, Germany). Slices were stored at room temperature in a chamber filled with normal ACSF containing the following (in mm): 126 NaCl, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, 2 MgCl2, 10 glucose (bubbled with 95% O2 and 5% CO2, pH 7.3; 310 mOsm measured).
Electrophysiological recordings.
Slices were transferred to a recording chamber and continuously superfused with oxygenated ACSF containing 0.5 μm tetrodotoxin (Latoxan, Rosans, France), 2 mm kynurenic acid (Fluka, St. Quentin Fallavier, France), and 1 μm strychnine (Sigma) to block glutamate and glycine-mediated fast synaptic currents and to record GABAA miniature IPSCs (mIPSCs) in isolation. Neurons from lamina II or laminas III–IV were recorded in the whole-cell configuration using a blind-patch approach. During electrophysiological experiments, lamina II was visually identified as a translucent crescent in the superficial dorsal horn. Patch pipettes were pulled from borosilicate glass capillaries (Harvard Apparatus, Edenbridge, UK) using a P-2000 puller (Sutter Instruments, Novato, CA). They were filled with a solution containing the following (in mm): 130 CsCl, 10 HEPES, 2 MgCl2, and 10 biocytin (Sigma) (pH 7.3, adjusted with CsOH; osmolarity 290 ± 10 mOsm adjusted with sucrose) (3–5 MΩ). Voltage-clamp recordings were performed with an Axopatch 200B amplifier (Molecular Devices, Union City, CA) at a holding potential fixed at −60 mV. The equilibrium potential for Cl− ions was set at 0 mV. Recordings were low-pass filtered (5 kHz) and acquired with the Fetchex module of pClamp 6 (Molecular Devices). Current traces were digitized and stored on the hard disk of a personal computer (10 kHz) and on videotape (20 kHz). All experiments were made at room temperature (20–25°C).
At the end of the experiments, slices were individually fixed with 4% paraformaldehyde. Neurons injected with biocytin were revealed with FITC-labeled extravidine (diluted 1:400; Sigma) or Marina blue-labeled streptavidin (diluted 1:100; Invitrogen, San Diego, CA), allowing the localization of the recorded neuron in either lamina II or laminas III–IV. Some slices were included in 5% agarose and cut again in 50-μm-thick transverse slices with a vibratome (Leica VT1000S). These slices were incubated overnight with Marina blue-labeled streptavidin and a rabbit antibody against protein kinase C type γ (PKCγ) (diluted 1:10,000; Santa Cruz Biotechnology, Santa Cruz, CA), followed by a 1 h incubation in secondary antibody (goat anti-rabbit IgG conjugated with FITC, diluted 1:400; Biosys, Compiègne, France). PKCγ is present in a subpopulation of neurons in the inner part of lamina II (Polgar et al., 1999) and allows a good localization of the border between laminas II and III. This staining was used to determine whether the recorded neuron was located in lamina II or in laminas III–IV (i.e., dorsal or ventral to the PKCγ-like immunoreactivity, respectively).
Pharmacological substances.
Blockade of GABAA receptors was achieved by adding bicuculline methiodide (10 μm; Sigma) to the ACSF. The short-term effects of AP (100 nm, 5α-pregnan-3α-ol-20-one; Sigma) or of diazepam (Diaz) (100 nm; Sigma) were determined after 20 min of general bath superfusion to be sure that the concentration of substance close to the recorded neuron had reached a steady state. For other drugs requiring longer times of exposure, slices were preincubated in ACSF containing the substance to be tested. The duration of incubation of the slices with a given substance was adjusted to obtain a stable steady-state effect produced by this substance at the concentration used. For example, in the case of substances blocking the synthesis of 3α5α-NS, 1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinoline carboxamide (PK11195) acts at the first step of synthesis, whereas finasteride acts at a late step. Therefore, it takes longer to reduce the concentration of 3α5α-NS with PK11195, because after blockade of the translocator protein of 18 kDa (TSPO), one has to wait until all previously synthesized precursors have been metabolized by the various downstream enzymes. In contrast, in the case of finasteride, the delay is much shorter, because only the 5α- and 3α5α-reduced compounds will have to be metabolized. Therefore, a time course of action was established for each substance in preliminary experiments by determining its effect on the deactivation kinetics of mIPSCs recorded in neurons from slices after various times of incubation with the substance. When the effect had reached a stable value, the duration of incubation was considered to be sufficient and was used as a minimal duration of incubation for subsequent experiments with this substance at the same concentration.
Diazepam (1 μm), pregnenolone (Preg) (100 nm; 3β-hydroxy-5-pregnen-20-one), progesterone (Prog) (100 nm), dihydro-progesterone (DHP) (100 nm; 5α-pregnan-3, 20-dione), 22(R)-hydroxy-cholesterol (22OH-Chol) (100 nm), indomethacin (Indo) (10 μm), RU486 (mifepristone) (1 μm; all from Sigma), flumazenil (Flu) (10 μm; Ro 15-1788; gift from Roche, Basel, Switzerland), PK11195 (PK) (10 μm; Tocris Cookson, Bristol, UK), and finasteride (Fina) (50 μm; Steraloids, Newport, RI) were prepared as 1000× concentrated stock solutions (in 95–99% ethanol) and stored at 0–5°C. All substances were diluted to their final concentrations in ACSF at the beginning of each experiment.
Data analysis.
Individual mIPSCs were analyzed off-line using the Strathclyde Electrophysiology software, WinEDR/WinWCP (courtesy of Dr. J. Dempster, University of Strathclyde, Glasgow, UK). Individual events were detected with a threshold method using a baseline-tracking amplitude threshold algorithm. The general principals for analysis of synaptic currents were similar to those described previously (Keller et al., 2004; Poisbeau et al., 2005). In this study, mIPSCs were fitted with the product of an error function and an exponential decay (enplate current fitting function of WinWCP) to determine the time constants of the rising and decaying phases of the miniature synaptic currents, respectively.
Statistics.
Data are expressed as arithmetic mean ± SEM. Mean values of the amplitude, rise time constant, decay time constant, and frequency of occurrence (Freq) of mIPSCs were compared between different laminas and after treatment with pharmacological substances. Comparison of group means was performed with Statistica 5.1 (Statsoft, Tulsa, OK) using one- or two-way ANOVA, with factors lamina, treatment, and/or age, depending on experiments. When the ANOVA test was significant, the Tukey's test was used for post hoc multiple comparisons between individual groups. Differences were considered significant for p < 0.05.
Results
We have shown previously that the endogenous production of 3α5α-NS is elevated in lamina II of the spinal cord at early stages of postnatal development (before P15) and is responsible for the slow decaying kinetics of GABAA receptor-mediated mIPSCs (Keller et al., 2004). The values of the decay time constants of GABAA receptor-mediated mIPSCs can therefore be used as very sensitive indicators for the local production of 3α5α-NS in a given anatomical region and in the vicinity of synapses. Here, we chose to record from P9–P15 animals to address two major and related questions: (1) is the high level of production of 3α5α-NS a general phenomenon in the DH of the spinal cord during early stages of postnatal development and/or (2) can 3α5α-NS produced in a given region of the spinal cord easily diffuse to adjacent anatomical regions and influence the characteristics of synaptic receptors in these regions?
Properties of GABAA receptor-mediated mIPSCs
As a first approach to the questions raised above, we characterized the properties of GABAA receptor-mediated mIPSCs recorded in neurons from laminas III–IV of the spinal cord dorsal horn of 9- to 15-d-old (P9–P15) rats and compared their properties to those observed in lamina II (Fig. 1A,B). In all cases tested (lam II, n = 21; lam III–IV, n = 19), superfusion with bicuculline (10 μm) totally blocked the mIPSCs (Fig. 1C,D), indicating that they were mediated by the activation of GABAA receptors.
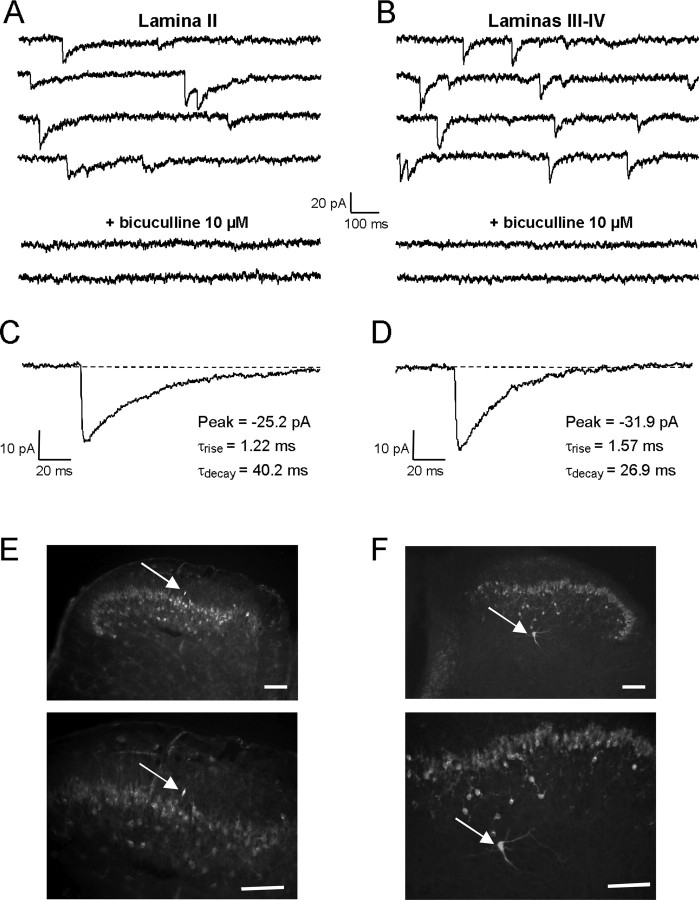
Figure 1.
Properties of GABAA receptor-mediated mIPSCs in the dorsal horn of P9–P15 rat spinal cord slices A, B, mIPSCs recorded in lamina II (A) and laminas III–IV (B) neurons in the presence of kynurenic acid (2 mm) and strychnine (1 μm) were blocked by the competitive GABAA receptor antagonist bicuculline (10 μm). C, D, Averaged traces of 10 individual mIPSCs recorded from the neurons illustrated in A and B, respectively. Decay kinetics of mIPSCs were slower in lamina II (C) than in laminas III–IV (D). The numbers next to the traces indicate the values of peak amplitude (Peak), rise time constant (τrise), and monoexponential decay time constant (τdecay) determined by the fitting procedure. E, F, Immunochemical labeling of the slices with an antibody against PKCγ was used to localize the border between lamina II and lamina III. The recorded biocytin-filled neuron (indicated by arrow) was judged to be localized in lamina II if situated within, or dorsal to, the PKCγ-like immunoreactivity (E) or in laminas III–IV if situated ventral to the PKCγ-like immunoreactivity (F). Scale bar, 100 μm.
GABAA receptor-mediated mIPSCs had significantly smaller decay time constants (τdecay) in laminas III–IV neurons (τdecay, 26.7 ± 1.7 ms; n = 14) than in lamina II neurons (τdecay, 41.1 ± 1.2 ms; n = 28; p < 0.001). In contrast, the amplitudes (A) (lam II: A, −23.0 ± 1.1 pA, n = 28; lam III–IV: A, −25.6 ± 2.6 pA, n = 14), rise time constants (lam II: τrise, 1.2 ± 0.1 ms, n = 28; lam III–IV: τrise, 1.3 ± 0.1 ms, n = 28), and frequencies of occurrence (lam II: Freq, 0.23 ± 0.003 Hz, n = 28; lam III–IV: Freq, 0.23 ± 0.003 Hz, n = 14) of GABA mIPSCs were not statistically different (p > 0.05) between lamina II and laminas III–IV.
Localization of the recorded neuron was confirmed at the end of the experiment by immunohistochemical revelation of biocytin injected via the patch pipette. In some cases, the limit between laminas II and III was verified using an immunohistochemical staining against PKCγ (Fig. 1E,F), which reveals a population of neurons in the inner part of lamina II and allows a relatively precise delineation of the border between laminas II and III (Polgar et al., 1999).
We observed no difference in the mean peak amplitude or the mean τrise values of the GABAA receptor-mediated mIPSCs between the different laminas or after the different pharmacological treatments described in the following sections. Therefore, we will only comment on the τdecay and frequency of occurrence values of mIPSCs in the results below.
Differential synthesis of 3α5α-NS in different laminas of the dorsal horn determines the kinetics of GABAA receptor-mediated mIPSCs
We next asked whether the difference in decay kinetics of mIPSCs recorded in lamina II and laminas III–IV neurons was caused by a difference in the local synthesis of 3α5α-NS. The first step in steroidogenesis or neurosteroidogenesis depends on the obligatory activation of a protein complex in the membrane of mitochondria (Papadopoulos et al., 2006a, 2007), which among different partners contains a protein, formerly termed peripheral benzodiazepine and recently renamed TSPO (Papadopoulos et al., 2006b). This protein participates in the transport of cholesterol across the mitochondrial membrane system, a process that allows the first step of steroidogenesis/neurosteroidogenesis (i.e., synthesis of pregnenolone) to take place inside the mitochondria. This protein is functionally antagonized by PK11195 (Fig. 4A) (Costa et al., 1994; Casellas et al., 2002; Keller et al., 2004).
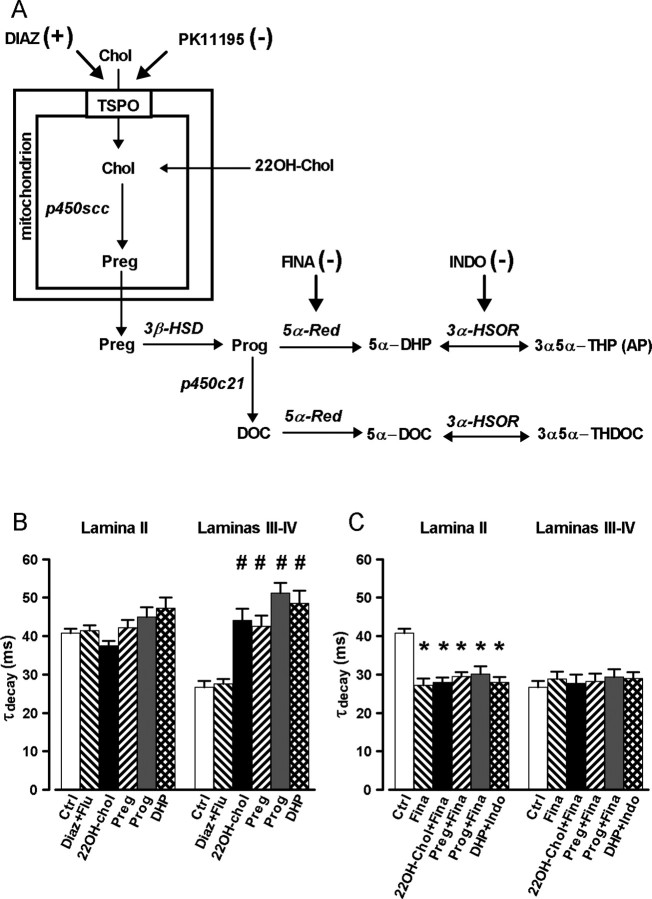
Figure 4.
Bypassing mitochondrial cholesterol transport mediated by TSPO allows to induce the synthesis of 3α5α-NS in laminas III–IV. A, Schematic representation of the biosynthetic pathways leading to the production of 3α5α-NS. The names of the different enzymes as well as the site of action of the different pharmacological agents used in our experiments are indicated. B, Histogram presenting the mean values (± SEM) of τdecay for lamina II (left) and laminas III–IV (right) neurons incubated with either diazepam (1 μm) plus flumazenil (10 μm) (Diaz + Flu), 22OH-Chol (100 nm), pregnenolone (100 nm), progesterone (100 nm), or DHP (100 nm). In lamina II, none of these treatments were able to increase significantly the value of τdecay with respect to its value in control conditions. In contrast, in laminas III–IV, all the treatments, except Diaz + Flu, significantly increased τdecay to values observed for mIPSCs recorded in lamina II. C, Protocol similar to that in B, but in the presence of finasteride (50 μm), an inhibitor of the 5α-reductase, or indomethacin (10 μm), an inhibitor of the 3α-HSOR. Note that, as indicated on the horizontal axis of the graph, Indo was tested only in the case of incubation with DHP, whereas Fina was tested in the case of all other precursors of 3α5α-NS. In lamina II, all treatments significantly reduced τdecay to the values of mIPSCs recorded in laminas III–IV neurons, whereas in laminas III–IV neurons, no change in τdecay was observed for any treatment. Two-way ANOVA with factors lamina and treatment showed a significant interaction (F(13,193) = 6.07; p < 0.001). DOC, Deoxycorticosterone; THDOC, tetrahydrodeoxycorticosterone.
As illustrated in Figure 2A, incubation of spinal cord slices with PK11195 (10 μm), for at least 5 h, decreased significantly τdecay values of mIPSCs recorded in lamina II neurons (τdecay, 30.4 ± 1.4 ms; n = 11; p < 0.001) but had no effect on the other properties of the mIPSCs (Fig. 2C). Similarly, incubation of the slices for at least 3 h with finasteride, an inhibitor of 5α-reductase involved in the synthesis of 3α5α-reduced steroids, decreased significantly the values of τdecay in lamina II neurons (τdecay, 27.2 ± 1.8 ms; n = 6; p < 0.001) (Fig. 2A), but finasteride did not affect the amplitude or the τrise of the mIPSCs (Fig. 2C). In laminas III–IV neurons, the decay time constants of GABAA receptor-mediated mIPSCs were not affected by PK11195 (τdecay, 29.0 ± 2.2 ms; n = 5; p = 0.97) or finasteride (τdecay, 28.8 ± 1.9 ms; n = 9; p = 0.95) when compared with control slices (τdecay, 26.6 ± 1.7 ms; n = 14).
Figure 2.
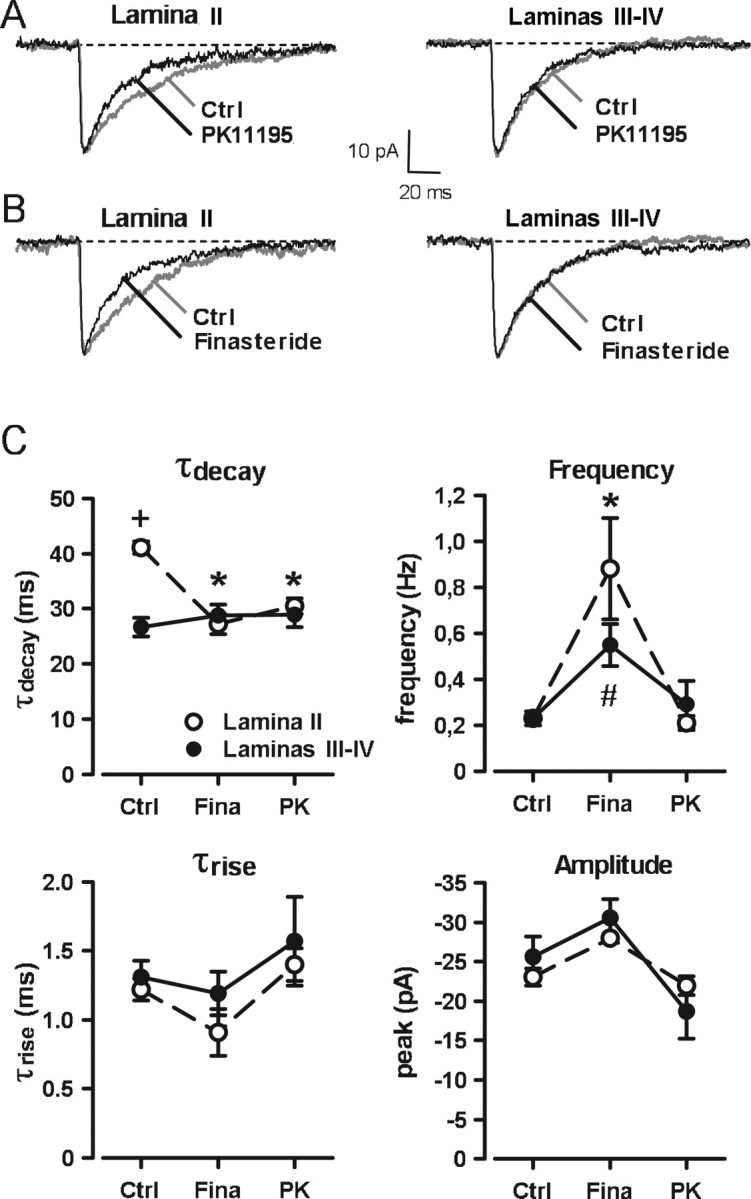
Pharmacological inhibition of the biosynthesis of 3α5α-NS accelerates the decay kinetics of GABAA receptor-mediated mIPSCs only in lamina II of P9–P15 animals. A, B, Averaged traces of 10 individual mIPSCs recorded from lamina II or laminas III–IV neurons after incubation of slices in normal ACSF (Ctrl; gray traces) or in the presence of either the TSPO inhibitor PK11195 (10 μm; A, black traces) or the 5α-reductase inhibitor finasteride (50 μm; B, black traces). C, PK11195 (PK) and finasteride (Fina) significantly reduced the value of the decay time constant (τdecay) of mIPSCs in lamina II (open circles), with respect to control, to a value that was no longer different from that in laminas III–IV (filled circles). Values of τdecay in laminas III–IV were not modified by PK or Fina. The frequency of mIPSCs was significantly increased by finasteride in both laminas, but their rise time constants (τrise) or their peak amplitudes were unchanged. Two-way ANOVA with factors lamina and treatment showed a significant interaction of lamina and treatment for τdecay (F(2,68) = 12.78; p < 0.001) and an effect of treatment for the frequency of occurrence mIPSCs (F(2,68) = 28.15; p < 0.001). In this and following figures, the symbols +, *, and # indicate statistically significant differences. The symbols represent the following comparisons: +, significant effect between lamina II and laminas III–IV for the same treatment; *, significant effect of treatment within lamina II; #, significant effect of treatment within laminas III–IV.
Together, these results indicated that, under basal conditions, mIPSCs in lamina II were tonically facilitated by endogenously and locally produced 3α5α-NS. However, this was not the case in laminas III–IV.
Note that we consistently observed that incubation of slices with finasteride resulted in a significant increase in the frequency of occurrence of GABAA mIPSCs both in lamina II (Freq, 0.88 ± 0.22 Hz; p < 0.001) and in laminas III–IV (Freq, 0.55 ± 0.09 Hz; p = 0.010) (Fig. 2C). Such increases in the frequency of the mIPSCs were noted in all experimental conditions in which finasteride (50 μm) was present and suggested that finasteride acted presynaptically to facilitate synaptic GABA release. In contrast, in slices of P9–P15 animals incubated with PK11195 (10 μm), no significant change in mIPSC frequency was detected, neither in lamina II nor in laminas III–IV neurons (Fig. 2C). This observation indicated that the facilitatory presynaptic action of finasteride was probably not directly related to its blocking effect on the synthesis of 3α5α-NS.
Sensitivity of synaptic GABAA receptors to exogenously applied 3α5α-NS
One possibility to explain the results presented above could be that, in contrast to lamina II, synaptic GABAA receptors in laminas III–IV were insensitive or less sensitive to 3α5α-NS. To check this point, we tested the effect of an exogenous application of AP. As shown in Figure 3, application of AP (100 nm) significantly increased τdecay in laminas III–IV neurons (before AP, τdecay, 30.4 ± 1.4 ms; during AP, τdecay, 42.1 ± 2.0 ms; n = 6; p < 0.001) but had no significant effect on peak amplitude, τrise, and frequency occurrence of mIPSCs (data not shown). In lamina II, AP (100 nm) had no apparent effect on τdecay (before AP, τdecay, 36.9 ± 2.2 ms; during AP, τdecay, 39.2 ± 3.1 ms; n = 5; p = 0.502), peak amplitude, τrise, and frequency occurrence of GABA mIPSCs. Interestingly, in the presence of AP, τdecay values of mIPSCs in laminas III–IV neurons were not significantly different from those of laminas II neurons under control conditions or in the presence of AP (100 nm; p = 0.253). We also attempted to test the effect of higher concentrations of AP (200 nm to 1 μm). However, at these concentrations, AP induced an inward current caused by direct activation of GABAA receptors (Shu et al., 2004), which was accompanied by an increase in membrane noise. This situation prevented the accurate detection and analysis of mIPSCs both in lamina II and laminas III–IV and therefore rendered impossible the evaluation of the effect of concentration of AP >100 nm on mIPSC kinetics.
Figure 3.
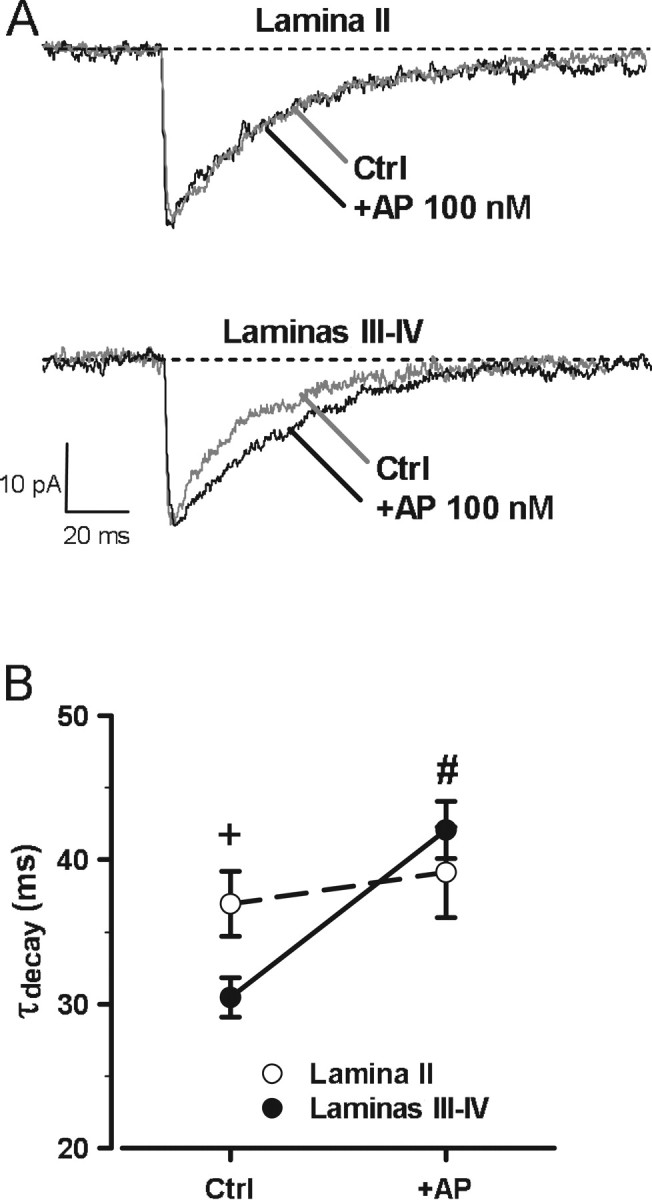
Differential effect of the 3α5α-NS AP on the decay kinetics of GABAA receptor-mediated mIPSCs in lamina II and laminas III–IV. A, Averaged traces of 10 individual mIPSCs recorded a lamina II or a laminas III–IV neuron, before (Ctrl; gray traces) and after (100 nm; black traces) bath application of AP for at least 20 min. B, Under control conditions, the value of the decay time constant (τdecay) of GABAA receptor-mediated mIPSCs was significantly smaller in laminas III–IV neurons (filled circles) than in lamina II neurons (white circles). AP significantly increased the value of τdecay of mIPSCs in laminas III–IV neurons but left that of lamina II neurons unaffected. Two-way ANOVA with factors lamina (between) and AP treatment (repeated measures) showed a significant interaction for lamina and treatment for τdecay (F(1,9) = 20.71; p < 0.01). Note also that, in the presence of AP, the values of τdecay of mIPSCs were similar in lamina II and laminas III–IV neurons.
Together, these results suggested that, although GABAA receptors in laminas III–IV were sensitive to 3α5α-NS, only synaptic GABAA receptors in lamina II neurons were under the tonic control of endogenously and locally produced 3α5α-NS in the spinal cord dorsal horn of immature (P9–P15) rats.
Pharmacological stimulation of the TSPO
One possibility for explaining the absence of a tonic production of 3α5α-NS in laminas III–IV could be the absence of an adequate signal to activate the TSPO. We therefore decided to stimulate TSPO activity by a pharmacological protocol, which has been validated in our previous studies on neurosteroidogenesis in the dorsal horn of the spinal cord (Keller et al., 2004; Poisbeau et al., 2005). TSPO can be activated by diazepam (Papadopoulos et al., 2006b), but this benzodiazepine also binds to the GABAA receptor to potentiate its activity (Barnard et al., 1998). However, the benzodiazepine binding to GABAA receptors is specifically antagonized by flumazenil (Barnard et al., 1998). Specific pharmacological stimulation of the TSPO was therefore achieved by incubating spinal cord slices for at least 5 h with diazepam (1 μm) in the presence of flumazenil (Diaz + Flu; 10 μm). In P9–P15 rats, Diaz + Flu had no significant effect on mIPSCs, neither in lamina II neurons (τdecay, 41.4 ± 1.4 ms; n = 9) nor in laminas III–IV neurons (τdecay, 27.5 ± 1.3 ms; n = 8.). We wondered whether this could be attributable to a problem with the stock solution of Diaz. To check for the validity of the protocol and of the substances used, we incubated slices from older animals (>P21) under the same conditions with Diaz + Flu from the same stock solutions as those used with slices from P9–P15 rats and evaluated its effects on the decay kinetics of mIPSCs in lamina II, in which Diaz + Flu was shown to prolong the duration of mIPSCs in previous studies (Keller et al., 2004; Poisbeau et al., 2005). Under these conditions, we observed a clear increase in the value of the decay time constant of mIPSCs in lamina II neurons incubated with Diaz + Flu (τdecay, 37.8 ± 2.4 ms; n = 5) compared with control slices (τdecay, 27.7 ± 1.8 ms; n = 6; p = 0.008). This result indicated that the stock solutions of Diaz and Flu used were valid, and that Diaz + Flu was able to induce the prolongation of mIPSCs in lamina II neurons of >P21 rats.
Incubation of slices from P9–P15 rats with flumazenil (10 μm) alone had no effect on the properties of the GABAA mIPSCs (lam II: τdecay, 40.8 ± 2.7 ms, n = 7; lam III–IV: τdecay, 29.5 ± 1.8 ms, n = 5). However, as outlined above, when in such slices neurosteroid production was globally inhibited with PK11195 (10 μm) (Fig. 2), the value of τdecay in lamina II neurons decreased (to ∼30 ms) and became similar to mIPSCs recorded in laminas III–IV neurons. When slices were incubated with diazepam and PK11195, the mean τdecay value was increased in lamina II and in laminas III–IV neurons (lam II: τdecay, 45.1 ± 1.3 ms, n = 4; lam III–IV: τdecay, 45.1 ± 3.4 ms, n = 4) with respect to the values observed in slices incubated with PK11195 alone. This effect of diazepam was completely antagonized by flumazenil (10 μm) in lamina II as well as in laminas III–IV neurons (lam II: τdecay, 30.9 ± 1.6, n = 8, p = 0.003; lam III–IV: τdecay, 32.0 ± 0.5 ms, n = 5, p = 0.028) and reflected an action of diazepam at the benzodiazepine binding site of synaptic GABAA receptors. Acute application of diazepam (100 nm) for at least 20 min on slices of P9–P15 rats also induced a significant prolongation of mIPSCs in lamina II neurons (control: τdecay, 43.1 ± 1.6 ms, n = 4; diazepam: τdecay, 62.6 ± 4.7 ms, n = 4; paired Student's t test, p = 0.012). This result indicated that, despite the presence of a tonic positive modulation of synaptic GABAA receptors in lamina II neurons by endogenously produced 3α5α-NS, it was possible to further prolong the duration of GABAA receptor-mediated mIPSCs by another positive allosteric modulator such as diazepam, which acts at the benzodiazepine site of GABAA receptors, distinct from the 3α5α-NS site.
Key role of TSPO in the synthesis of 3α5α-reduced neurosteroids
To study in more detail the steroidogenic potential of laminas III–IV, we evaluated the effect of incubating the slices with different precursors of allopregnanolone (Fig. 4A). We started with the closest precursor and progressively tested the effect of farther precursors. This protocol allowed us to test for the presence and the functionality of the different enzymes necessary for the complete synthesis of 3α5α-NS. The results obtained with each precursor and the effects of inhibitors of key enzymes governing the synthesis of 3α5α-NS are summarized in Figure 4, B and C.
As illustrated in Figure 4A, the closest precursor to allopregnanolone is DHP. Incubation of the slices with DHP for >1 h significantly increased the decay time constant of mIPSCs in laminas III–IV neurons (τdecay, 48.5 ± 3.3 ms; n = 5; p < 0.001) but had no effect in lamina II (τdecay, 47.2 ± 2.8 ms; n = 8; p = 0.623). The effect of DHP in laminas III–IV neurons was blocked (τdecay, 29.0 ± 1.6 ms; n = 4; p < 0.001) by coincubation of slices with indomethacin, an inhibitor of 3α-hydroxysteroid oxido-reductase (3α-HSOR) (Belelli et al., 2006), thus demonstrating that the effect of DHP was caused by its conversion to allopregnanolone and that DHP had no direct effect of mIPSC kinetics.
Incubation of slices with progesterone for >1 h increased the decay time constant of mIPSCs in laminas III–IV neurons (τdecay, 51.1 ± 2.6 ms; n = 7; p < 0.001) but had no supplemental effect in lamina II (τdecay, 45.0 ± 2.5 ms; n = 10; p = 0.98). This effect of Prog in laminas III–IV was blocked by coincubation with finasteride (τdecay, 29.3 ± 2.0 ms; n = 5; p < 0.001), indicating that in laminas III–IV, Prog was metabolized to 3α5α-NS, which prolonged the duration of GABAA receptor-mediated mIPSCs. In laminas III–IV, the effect of Prog on decay time constants of GABAA receptor-mediated mIPSCs was not affected by RU486 (1 μm), an antagonist of nuclear progesterone receptors (τdecay, 41.2 ± 2.6 ms; n = 7; p = 0.289). RU486 (1 μm) applied alone for >3 h had no effect on the decay time constants of mIPSCs in either lamina (lam II: τdecay, 38.6 ± 1.2 ms, n = 7, p = 1; lam III–IV: τdecay, 26.9 ± 1.9 ms, n = 7, p = 1).
Incubation of the slices with pregnenolone for >3 h also increased the value of the decay time constant in laminas III–IV neurons (τdecay, 42.5 ± 2.7 ms; n = 6; p < 0.001) but had no significant effect in lamina II (τdecay, 42.2 ± 2.1 ms; n = 6; p = 1). The effect of pregnenolone in laminas III-IV was completely blocked by coincubation with finasteride (τdecay, 28.2 ± 2.1 ms; n = 6; p = 0.011), indicating that pregnenolone was metabolized to a 3α5α-NS.
Finally, we tested the effect of 22OH-Chol. Indeed, once cholesterol has been transported across the mitochondrial membrane system, it is metabolized by P450scc (P450 side chain cleavage), which catalyzes three reactions leading successively to the production of 220H-Chol, 20,22OH-cholesterol, and pregnenolone (Papadopoulos et al., 2006a). Most interestingly, 22OH-Chol can freely cross the mitochondrial membrane system, because it is hydrosoluble and bypasses the TSPO-mediated transport process (Khanna et al., 1994; Espinosa-Garcia et al., 2000; Castillo et al., 2006). Therefore, incubation of cells (or isolated mitochondria) with 22OH-Chol can restore pregnenolone synthesis and full steroidogenesis under conditions where the TSPO-mediated cholesterol transport is inactive or blocked (Khanna et al., 1994; Espinosa-Garcia et al., 2000; Castillo et al., 2006). Incubation of slices with 22OH-Chol for >5 h increased the decay time constant of mIPSCs in laminas III–IV (τdecay, 44.1 ± 3.1 ms; n = 6; p < 0.001) but had no effect in lamina II (τdecay, 37.5 ± 1.3 ms; n = 6; p = 1). The effect of 22OH-Chol in laminas III–IV was totally blocked by coincubation with finasteride (τdecay, 27.6 ± 2.3 ms; n = 5; p = 0.002).
Together, these results indicate that all the enzymes necessary for the synthesis of 3α5α-NS from cholesterol are present and functional in laminas III–IV, and that the apparent limiting factor for the synthesis of 3α5α-NS in laminas III–IV is the transport of cholesterol across the mitochondrial membrane system. Moreover, our results provide evidence that the production of 3α5α-NS in lamina II was sufficient under basal conditions to induce a maximal potentiation of synaptic receptors via the modulatory site of 3α5α-NS at GABAA receptors. Indeed, incubating the slices with an excess of various precursors did not further increase the duration of mIPSCs in lamina II neurons, although a significant increase in mIPSC decay kinetics was still achieved during acute application of diazepam (see Materials and Methods). Therefore, it appeared that it was still possible to further potentiate synaptic GABAA receptors. However, such an additional potentiation was not observed during pharmacological stimulation of TSPO or addition of excess amounts of precursors of 3α5α-NS synthesis. Therefore, this finding strongly suggested that the potentiating effect induced via the 3α5α-NS-binding site on GABAA receptors had reached a maximum. This might also explain why addition of excess 3α5α-NS or other manipulations (see below, Effect of peripheral inflammation) prolonged the duration of mIPSCs recorded in lamina III–IV neurons but never beyond that in lamina II neurons under control conditions (i.e., a situation under which we suspect that the 3α5α-NS-induced potentiation at GABAA receptors was already maximal).
Developmental regulation of neurosteroidigenesis in lamina II and laminas III–IV
A previous study by our laboratory has shown that, in lamina II, the synthesis of 3α5α-NS is elevated during the first 15 d of postnatal life and subsequently decreases with development (Keller et al., 2004). Therefore, we decided to investigate and compare the properties of GABAA receptor-mediated mIPSCs properties in lamina II and laminas III–IV during postnatal development with special reference to the role of endogenously produced 3α5α-NS on mIPSC kinetics (Fig. 5). In young animals (<P8), τdecay was elevated and had similar values in lamina II (τdecay, 39.7 ± 2.5 ms; n = 6) and in laminas III–IV (τdecay, 37.5 ± 3.4 ms; n = 7) neurons (Fig. 5A). At later ages, τdecay showed a significant and rapid decrease in lamina III–IV between the first and the second week of postnatal life (P9–P15, τdecay, 26.6 ± 1.7 ms; n = 14; p = 0.004) and remained stable thereafter (>P21, τdecay, 22.0 ± 1.7 ms; n = 5; p < 0.001 with respect to <P8 and p = 0.699 with respect to P9–P15). In contrast, in lamina II, the τdecay value remained high, and similar to that observed for the <P8 group, until the end of the second postnatal week (P9–P15, τdecay, 41.1 ± 1.2 ms; n = 29; p = 0.998) and then decreased between the second and third postnatal weeks (>P21, τdecay, 27.7 ± 1.8 ms; n = 6; p = 0.015 with respect to <P8 and p < 0.001 with respect to P9–P15). Most importantly, at >P21, the values of decay time constants of mIPSCs were not significantly different in lamina II and laminas III–IV neurons (p > 0.05).
Figure 5.
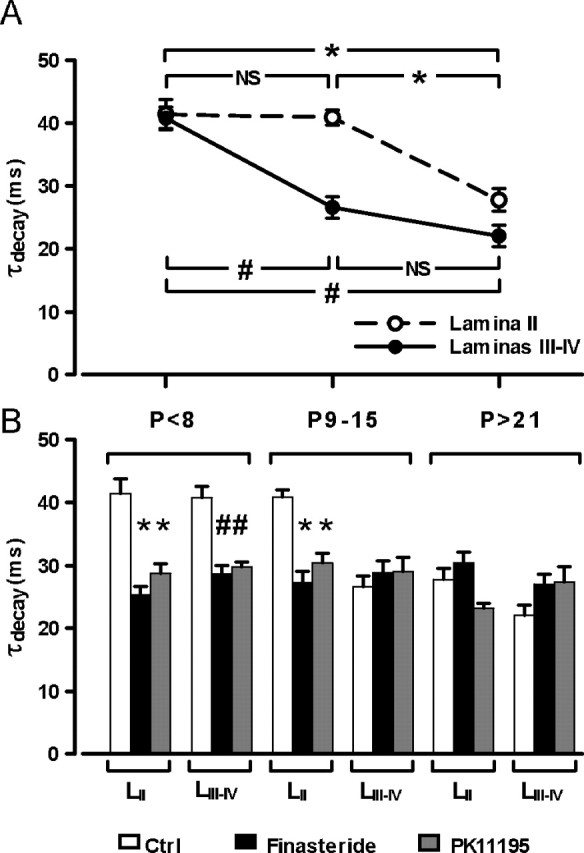
The tonic biosynthesis of 3α5α-NS is regulated during postnatal development. A, In young animals (<P8), τdecay values were elevated and similar in lamina II (open circles) and laminas III–IV (filled circles) neurons. At P9–P15, τdecay was significantly decreased in laminas III–IV but remained elevated in lamina II neurons. At >P21, τdecay was decreased in lamina II neurons reaching values similar to that in lamina III–IV neurons. Two-way ANOVA, with factors age and lamina, showed a significant interaction (F(2,62) = 5.63; p < 0.01). B, Incubation of spinal cord slices for >5 h with PK11195 (10 μm; gray bars) or >3 h with finasteride (50 μm; black bars) significantly reduced τdecay values (white bars) in both lamina II and laminas III–IV at <P8 but only in lamina II at P9–P15. Both substances had no significant effect at >P21, neither in lamina II nor in laminas III–IV. One-way ANOVA was used for factor treatment for each lamina at each age.
In laminas III–IV, at <P8, the high τdecay value was decreased when slices were incubated with PK11195 (τdecay, 29.7 ± 0.8 ms; n = 5; p = 0.028) or with finasteride (τdecay, 28.6 ± 1.4 ms; n = 7; p = 0.006) (Fig. 5B). In lamina II, the τdecay values were also significantly reduced at <P8 in PK11195 (τdecay, 28.7 ± 1.62 ms; n = 5; p = 0.004) or finasteride-incubated (τdecay, 25.3 ± 1.3 ms; n = 7; p < 0.001) slices compared with control slices. At >P21, incubation with PK11195 or finasteride had no significant effect in lamina II (PK11195: τdecay, 23.2 ± 0.7 ms, n = 5, p = 0.117; finasteride: τdecay, 30.4 ± 1.7 ms, n = 4, p = 0.465) or in laminas III–IV (PK11195: τdecay, 27.4 ± 2.4 ms, n = 5, p = 0.170; finasteride: τdecay, 27.0 ± 1.6 ms, n = 4, p = 0.238).
These results indicated that a tonic modulation by 3α5α-NS was detected at the synaptic level in laminas III–IV neurons of very young rats (<P8). Moreover, the synthesis of 3α5α-NS was placed under the control of the TSPO (because it was blocked by PK11195), indicating that, at <P8, TSPO is functional in laminas III–IV. Our results also suggest a differential temporal maturation of endogenous tonic 3α5α-NS production between lamina II and laminas III–IV, resulting in a differential modulation of synaptic GABAA receptor kinetics in these laminas.
Effect of peripheral inflammation
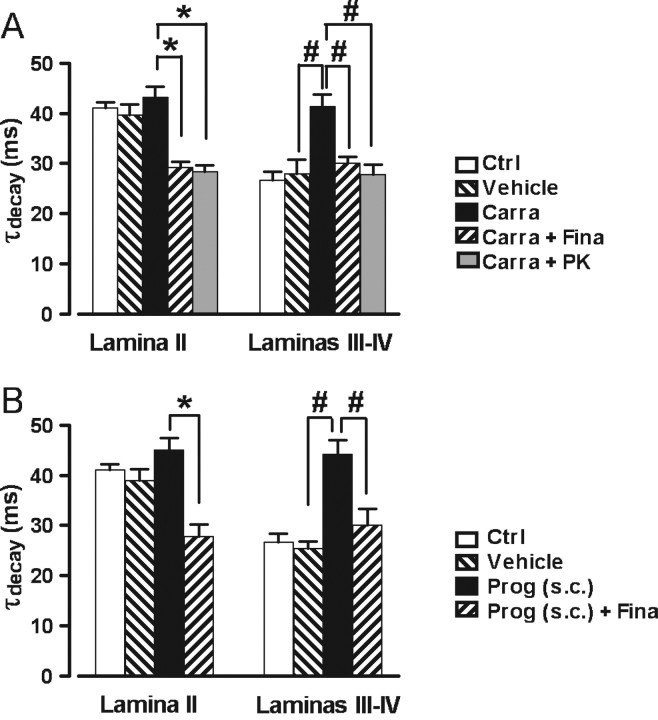
In lamina II of the adult rat, endogenous production of 3α5α-NS can be stimulated after induction of an inflammatory pain state by intraplantar injection of carrageenan (Poisbeau et al., 2005). Therefore, we tested the consequences of such a protocol on the mIPSC kinetics in lamina II and laminas III–IV in P9–P15 rats. In laminas III–IV neurons from inflamed animals, 15 h after the injection of carrageenan into the hindpaws, mIPSCs had significantly longer τdecay values (τdecay, 41.4 ± 2.3 ms; n = 9) than those of saline-injected controls (τdecay, 28.0 ± 2.8 ms; n = 7; p < 0.001) (Fig. 6A). This effect was totally blocked when the slices from carrageenan-injected animals were incubated in the presence of finasteride (50 μm) for >3 h (τdecay, 30.1 ± 1.2 ms; n = 6; p = 0.014) or in the presence of PK11195 (10 μm) for >6 h (τdecay, 27.9 ± 1.9 ms; n = 6; p = 0.001). In contrast, the carrageenan-induced inflammation had no effect on decay kinetics of mIPSCs in lamina II (carrageenan: τdecay, 43.1 ± 2.1 ms, n = 9, p = 0.999; saline: τdecay, 39.6 ± 2.2 ms, n = 5, p = 0.999). However, incubation of the slices with finasteride or PK11195 significantly reduced the τdecay values in lamina II neurons (finasteride: τdecay, 29.2 ± 1.1 ms, n = 6, p < 0.001; PK11195: τdecay, 28.4 ± 1.3 ms, n = 6, p < 0.001). These values were comparable with those observed in laminas III–IV neurons under control conditions (Fig. 6A).
Figure 6.
The biosynthesis of 3α5α-reduced neurosteroids can be stimulated in laminas III–IV after carrageenan-induced inflammation or after a subcutaneous injection of progesterone. A, Histogram showing the mean values (± SEM) of τdecay in lamina II and laminas III–IV neurons from naive control (Ctrl; white bars), saline-injected (vehicle; down-hatched bars), or carrageenan-injected (Carra; black bars) animals. Before recording, spinal cord slices from carrageenan-injected animals were incubated in normal ACSF, in ACSF containing finasteride (50 μm; Carra + Fina; up-hatched bars), or in ACSF containing PK11195 (10 μm; Carra + PK; gray bars). In lamina II, the high control values of τdecay were not affected by inflammation but were significantly reduced by finasteride or PK11195. In laminas III–IV, inflammation induced a significant increase in the τdecay value with respect to control, an effect that was abolished by finasteride or PK11195 treatment. Two-way ANOVA, with factors lamina and treatment, showed a significant interaction (F(4,90) = 7.88; p < 0.001). B, Histogram representing the mean values (± SEM) of τdecay in lamina II and laminas III–IV neurons from naive controls (Ctrl; white bars), oil-injected (vehicle; down-hatched bars), or progesterone-injected (Prog) (subcutaneously; black bars) animals. As in A, slices from progesterone-injected animals were incubated without or with finasteride [50 μm; Prog (subcutaneously) + Fina; up-hatched bars]. As observed in the case of inflammatory animals, progesterone injection in healthy animals induced a significant increase in τdecay values in laminas III–IV, an effect that was reversed by finasteride. Note that the progesterone injection had no effect on mIPSC kinetics in lamina II. Two-way ANOVA, with factors lamina and treatment, showed a significant interaction (F(3,75) = 8.37; p < 0.001).
These results indicated that peripheral inflammation was able to reactivate the synthesis of 3α5α-NS in laminas III–IV neurons from P9–P15 rats, and that this phenomenon involved the activation of the TSPO. However, peripheral inflammation was apparently unable to further prolong the duration of mIPSCs in lamina II, possibly indicating that the tonic production of 3α5α-NS in lamina II of these animals was already sufficient to produce a maximal modulatory effect on synaptic GABAA receptors via their 3α5α-NS binding site (see above, Key role of TSPO in the synthesis of 3α5α-reduced neurosteroids).
Another important question was whether this effect of inflammation on lamina III–IV neurons is also observed in more mature animals (i.e., at a developmental stage when the properties of synaptic networks in the dorsal horn are comparable with those in the adult) (Keller et al., 2004; Poisbeau et al., 2005). We therefore tested the effect of peripheral inflammation on >P21 rats (range, P21–P30). At these developmental stages, GABAA receptor-mediated mIPSCs have fast and similar decay kinetics in lamina II and in laminas III–IV because of the absence of tonic production of 3α5α-NS (Fig. 5). The decay time constant of GABAA receptor-mediated mIPSCs in laminas III–IV neurons was not affected by a saline injection into the hindpaws (control: τdecay, 20.2 ± 2.6 ms, n = 5; saline: τdecay, 23.2 ± 1.7 ms, n = 6; p = 0.957) but was significantly increased after injection of carrageenan (τdecay, 33.4 ± 2.3 ms; n = 7; p = 0.002 with respect to control and p = 0.016 with respect to saline). This increase in decay time constant was prevented when the slices from carrageenan-injected rats were incubated for >6 h with PK11195 (10 μm) (τdecay, 24.0 ± 1.9 ms; n = 6; p = 0.033 with respect to the carrageenan-injected group). These results indicate that the effect of inflammation was similar in immature (P9–P15) and in adult-like (p > 21) rats.
Effect of a subcutaneous injection of progesterone
We have shown that, if the step of cholesterol translocation across the mitochondrial membrane system mediated by TSPO was bypassed, a complete synthesis of 3α5α-NS was possible in laminas III–IV. We therefore wondered whether a peripherally circulating steroid or steroid precursor could gain access to laminas III–IV and serve locally as a precursor for 3α5α-reduced steroid synthesis. To test this hypothesis, we prepared slices from P9–P15 rats having received a subcutaneous injection of progesterone (75 mg/kg) 30–90 min before they were killed for slice preparation. In laminas III–IV, mIPSCs recorded in neurons from rats injected with progesterone had significantly longer τdecay values (τdecay, 44.2 ± 2.8 ms; n = 6; p < 0.001) than those of vehicle-injected controls (τdecay, 25.4 ± 1.4 ms; n = 6; p < 0.001) (Fig. 6B). This effect was totally reversed when slices were incubated for >3 h with 50 μm finasteride (τdecay, 30.1 ± 3.3 ms; n = 7; p = 0.005). The progesterone injection had no significant effect on decay kinetics of mIPSCs in lamina II neurons (Prog: τdecay, 45.0 ± 2.3 ms, n = 8; vehicle: τdecay, 39.0 ± 2.2 ms, n = 5). Incubation with finasteride reduced the decay time constant value of mIPSCs in lamina II neurons to levels observed in laminas III–IV under control conditions or after incubation of slices from progesterone-injected animals with finasteride (Fig. 6B).
These results indicated that steroids of peripheral origin, such as progesterone, can access the spinal cord and be locally metabolized to 3α5α-reduced neuroactive steroids, which in turn modulate the kinetics of GABAA receptor-mediated mIPSCs.
Discussion
We have shown previously that the decay kinetics of GABAA receptor-mediated mIPSCs in lamina II of the spinal cord were controlled by the tonic production of 3α5α-NS, which was downregulated during postnatal development (Keller et al., 2004) and reactivated after peripheral inflammation (Poisbeau et al., 2005). Here, we found that, in P9–P15 rats, the decay kinetics of GABAA receptor-mediated mIPSCs were clearly distinct in lamina II and laminas III–IV neurons (i.e., in neurons from two anatomically close regions of the DH of the spinal cord). This situation was caused by a difference in the tonic and local production of endogenous 3α5α-NS and indicated that 3α5α-NS synthesized in lamina II did not spill over to laminas III–IV. This difference in local production of 3α5α-NS was apparently based on a developmentally controlled downregulation of cholesterol transport across the mitochondrial membrane system involving an inhibition (lamina II) or an apparent disappearance (laminas III–IV) of the activity of TSPO. Interestingly, after its downregulation in laminas III–IV, the activity of TSPO could no longer be stimulated pharmacologically but was fully restored after the induction of a peripheral inflammation. Moreover, all steroidogenic enzymes remained functional in laminas III–IV, even after the apparent disappearance of TSPO activity, and allowed the synthesis of 3α5α-reduced steroids from circulating peripheral steroids such as progesterone.
In P9–P15 rat spinal cord slices, the decay time constants of pharmacologically isolated GABAA receptor-mediated mIPSCs were significantly shorter in laminas III–IV neurons (τdecay, ∼30 ms) than in lamina II neurons (τdecay, ∼40 ms), although their mean amplitudes or rise time constants were similar. However, after incubation of the slices with either finasteride, which selectively blocks the production of 3α5α-NS, or with PK11195, which antagonizes the activity of the TSPO and therefore acts as a general blocker of neurosteroidogenesis, the kinetics of mIPSCs in lamina II were accelerated to values characteristic of lamina III–IV neurons, whereas mIPSCs recorded in laminas III–IV neurons showed no change in decay kinetics. These observations clearly argued in favor of the existence of a tonic endogenous production of 3α5α-NS in lamina II, but not in laminas III–IV, of immature rats. Our results also indicate that in lamina II neurons of P9–P15 rats, the level of modulation of synaptic GABAA receptors by 3α5α-NS was probably maximal. Indeed, it was impossible to further increase the duration of mIPSCs in lamina II by pharmacological stimulation of TSPO or incubation of the spinal cord slices with an excess of precursors necessary for the synthesis of 3α5α-NS, whereas similar procedures led to the increase in mIPSC decay time constants in lamina III–IV neurons of P9–P15 rats or in lamina II neurons from older (>P21) rats. At the same time, it is important to emphasize that in lamina II neurons of P9–P15 rats, it was possible to further increase the duration of mIPSCs by an acute application of diazepam, which binds to the benzodiazepine site of GABAA receptors.
Despite this apparent high level of 3α5α-NS synthesis in lamina II, there was no sign of modulation of GABAA receptor-mediated mIPSCs in laminas III–IV, indicating that the 3α5α-NS produced in lamina II remained confined locally and did not spill over to laminas III–IV. It might be argued that 3α5α-NS did diffuse into laminas III–IV, but that the synaptic GABAA receptors in laminas III–IV were insensitive to these neurosteroids. This is unlikely, because the large majority of GABAA receptors are sensitive to 3α5α-NS (Belelli et al., 2002, 2006) and, indeed, we found that exogenous application of AP (100 nm) increased the τdecay values of mIPSCs in laminas III–IV neurons to values similar to that observed in lamina II. These results are consistent with the existence of a local synthesis of 3α5α-NS limited to lamina II.
Alternatively, laminas III–IV were perhaps unable to synthesize 3α5α-NS. This was not the case, because when the spinal cord slices were incubated with one of the precursors of AP, the values of τdecay of mIPSCs in laminas III–IV increased to values similar to that observed in lamina II, and this increase in τdecay values was blocked by pharmacological inhibitors of the synthesis of 3α5α-reduced steroids. Moreover, a single subcutaneous injection of progesterone mimicked the effect of incubating the spinal cord slices with precursors of AP, suggesting that peripheral fluctuations in steroid or steroid precursor levels might affect the production of neurosteroids in the spinal cord. Similar observations have been made in other areas of the CNS after peripheral injections of steroids (Reddy et al., 2004, 2005) or after endogenous fluctuations of steroid levels associated with ovarian cycle (Maguire and Mody, 2007) or stress (Reddy and Rogawski, 2002).
In fact, a tonic synthesis of 3α5α-NS was observed in laminas III–IV of very young (<P8) rats, but this production disappeared after the first week of postnatal life, whereas a similar phenomenon occurred in lamina II 1 week later. Interestingly, once this downregulation had occurred in laminas III–IV neurons, it was impossible to induce the production of 3α5α-NS by pharmacological stimulation of the TSPO with diazepam, whereas such a stimulation was still efficient in lamina II as shown in >P21 rats or in previous studies from our laboratory (Keller et al., 2004; Poisbeau et al., 2005). These results indicated that the TSPO played a key role in the control of neurosteroidogenesis and that the mechanisms controlling the functioning of the TSPO were distinct in lamina II and laminas III–IV. Interestingly, a marked finasteride- and PK11195-sensitive prolongation of mIPSCs was also observed in laminas III–IV of P9–P15 rats after the induction of a peripheral inflammation by an intraplantar injection of carrageenan, indicating that inflammatory conditions allowed to induce/restore the conditions necessary for the synthesis of 3α5α-NS in laminas III–IV.
How is it then possible to explain the apparently contradictory observations that pharmacological stimulation of TSPO was ineffective, whereas peripheral inflammation was able to stimulate 3α5α-NS synthesis in laminas III–IV? The activation of TSPO is an obligatory and fundamental step in the synthesis of neurosteroids, because it allows the translocation of cholesterol across the mitochondrial membrane system (Papadopoulos et al., 2006a, 2007). In non-neuronal systems, it has been shown that steroidogenesis requires the formation of a protein complex incorporating several partners, including TSPO and steroid acute regulatory protein (Hauet et al., 2002; Liu et al., 2006; Papadopoulos et al., 2007). This complex allows a constitutive synthesis of steroids/neurosteroids (Liu et al., 2006) that can be stimulated by diazepam or blocked by PK11195 via an action at the TSPO (Keller et al., 2004). It is therefore possible to speculate that, if one of the partners in the complex is missing or if the assembly of the complex is hindered, steroidogenesis is blocked at the earliest stage (i.e., the transport of cholesterol across the mitochondrial membrane). This was the case in laminas III–IV, because incubation of the slices with 22-OH cholesterol, which bypasses the transport via the TSPO, fully restored the synthesis of 3α5α-NS. The fact that it remained possible to stimulate pharmacologically the synthesis of 3α5α-NS in lamina II, but not in laminas III–IV, indicated that in lamina II, the complex was still functional but was expressed at a lower level or had a lower basal activity in the adult. In contrast, in laminas III–IV, the complex was probably dissociated or functionally blocked, but its association/functionality could be restored after peripheral inflammation. An alternative possibility could be that the absence of 3α5α-NS production was attributable to a deficit in cholesterol production. There are no objective arguments in favor of such a hypothesis. Indeed membrane excitability (data not shown) and the properties of glycine receptor-mediated mIPSCs (Inquimbert et al., 2007) were similar in lamina II and laminas III–IV. Moreover, it has been shown recently that a deficit in cholesterol increases the frequency of spontaneous and miniature synaptic currents, whereas it decreases transmitter release evoked by a K+-enriched extracellular solution (Wasser et al., 2007). Such changes were not observed in our preparation, suggesting that there was probably no deficit in cholesterol synthesis. Moreover, there is no evidence in the literature for a change in cholesterol synthesis in the CNS after peripheral inflammation, which could explain the restoration of 3α5α-NS production in our experiments.
In conclusion, our results show that cholesterol transport across the mitochondrial membrane system and the subsequent local production of 3α5α-NS play a crucial role in the shaping of GABAA receptor-mediated mIPSCs in the dorsal horn of the spinal cord, and that circulating steroids can gain access to the spinal cord and contribute to the local production of 3α5α-reduced steroids. It has been shown previously that the availability of 3α5α-NS at inhibitory synapses in the hippocampus can be regulated by the activity of 3α-hydroxisteroid reductase (Belelli and Herd, 2003). Here, we show the existence of another regulatory mechanism based on the modulation of the synthesis of neurosteroids via a control of mitochondrial cholesterol transport. It will be interesting to determine to which degree these mechanisms coexist and contribute to the regulation of inhibitory synaptic transmission in various regions of the CNS.
Footnotes
This work was supported by the Centre National de la Recherche Scientifique, Université Louis Pasteur, Institut UPSA de la Douleur, and Agence Nationale de la Recherche. We thank Francine Herzog for excellent technical assistance and Sylvain Hugel and Pierrick Poisbeau for helpful discussion and comments on this manuscript.
References
- Akk G, Shu HJ, Wang C, Steinbach JH, Zorumski CF, Covey DF, Mennerick S. Neurosteroid access to the GABAA receptor. J Neurosci. 2005;25:11605–11613. doi: 10.1523/JNEUROSCI.4173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International union of pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Prog Horm Res. 1997;52:1–32. [PubMed] [Google Scholar]
- Belelli D, Herd MB. The contraceptive agent Provera enhances GABAA receptor-mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids? J Neurosci. 2003;23:10013–10020. doi: 10.1523/JNEUROSCI.23-31-10013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABA(A) receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Belelli D, Herd MB, Mitchell EA, Peden DR, Vardy AW, Gentet L, Lambert JJ. Neuroactive steroids and inhibitory neurotransmission: mechanisms of action and physiological relevance. Neuroscience. 2006;138:821–829. doi: 10.1016/j.neuroscience.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Casellas P, Galiegue S, Basile AS. Peripheral benzodiazepine receptors and mitochondrial function. Neurochem Int. 2002;40:475–486. doi: 10.1016/s0197-0186(01)00118-8. [DOI] [PubMed] [Google Scholar]
- Castillo AF, Maciel FC, Castilla R, Duarte A, Maloberti P, Paz C, Podesta EJ. cAMP increases mitochondrial cholesterol transport through the induction of arachidonic acid release inside this organelle in Leydig cells. FEBS J. 2006;273:5011–5021. doi: 10.1111/j.1742-4658.2006.05496.x. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Costa E, Auta J, Guidotti A, Korneyev A, Romeo E. The pharmacology of neurosteroidogenesis. J Steroid Biochem Mol Biol. 1994;49:385–389. doi: 10.1016/0960-0760(94)90284-4. [DOI] [PubMed] [Google Scholar]
- Espinosa-Garcia MT, Strauss JF, 3rd, Martinez F. A trypsin-sensitive protein is required for utilization of exogenous cholesterol for pregnenolone synthesis by placental mitochondria. Placenta. 2000;21:654–660. doi: 10.1053/plac.2000.0562. [DOI] [PubMed] [Google Scholar]
- Hauet T, Liu J, Li H, Gazouli M, Culty M, Papadopoulos V. PBR, StAR, and PKA: partners in cholesterol transport in steroidogenic cells. Endocr Res. 2002;28:395–401. doi: 10.1081/erc-120016814. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Inquimbert P, Rodeau JL, Schlichter R. Differential contribution of GABAergic and glycinergic components to inhibitory synaptic transmission in lamina II and laminae III-IV of the young rat spinal cord. Eur J Neurosci. 2007;26:2940–2949. doi: 10.1111/j.1460-9568.2007.05919.x. [DOI] [PubMed] [Google Scholar]
- Keller AF, Coull JA, Chery N, Poisbeau P, De Koninck Y. Region-specific developmental specialization of GABA-glycine cosynapses in laminas I-II of the rat spinal dorsal horn. J Neurosci. 2001;21:7871–7880. doi: 10.1523/JNEUROSCI.21-20-07871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller AF, Breton JD, Schlichter R, Poisbeau P. Production of 5α-reduced neurosteroids is developmentally regulated and shapes GABAA miniature IPSCs in lamina II of the spinal cord. J Neurosci. 2004;24:907–915. doi: 10.1523/JNEUROSCI.4642-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna A, Aten RF, Behrman HR. Heat shock protein induction blocks hormone-sensitive steroidogenesis in rat luteal cells. Steroids. 1994;59:4–9. doi: 10.1016/0039-128x(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Prog Neurobiol. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Leroy C, Poisbeau P, Keller AF, Nehlig A. Pharmacological plasticity of GABA(A) receptors at dentate gyrus synapses in a rat model of temporal lobe epilepsy. J Physiol (Lond) 2004;557:473–487. doi: 10.1113/jphysiol.2003.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Rone MB, Papadopoulos V. Protein-protein interactions mediate mitochondrial cholesterol transport and steroid biosynthesis. J Biol Chem. 2006;281:38879–38893. doi: 10.1074/jbc.M608820200. [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABAA receptors: relevance to the ovarian cycle and stress. J Neurosci. 2007;27:2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Valenzuela CF. Alcohol increases efficacy of immature synapses in a neurosteroid-dependent manner. Eur J Neurosci. 2006;23:835–839. doi: 10.1111/j.1460-9568.2006.04597.x. [DOI] [PubMed] [Google Scholar]
- Mameli M, Carta M, Partridge LD, Valenzuela CF. Neurosteroid-induced plasticity of immature synapses via retrograde modulation of presynaptic NMDA receptors. J Neurosci. 2005;25:2285–2294. doi: 10.1523/JNEUROSCI.3877-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V, Lecanu L, Brown RC, Han Z, Yao ZX. Peripheral-type benzodiazepine receptor in neurosteroid biosynthesis, neuropathology and neurological disorders. Neuroscience. 2006a;138:749–756. doi: 10.1016/j.neuroscience.2005.05.063. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006b;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Liu J, Culty M. Is there a mitochondrial signaling complex facilitating cholesterol import? Mol Cell Endocrinol. 2007;265–266:59–64. doi: 10.1016/j.mce.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Poisbeau P, Patte-Mensah C, Keller AF, Barrot M, Breton JD, Luis-Delgado OE, Freund-Mercier MJ, Mensah-Nyagan AG, Schlichter R. Inflammatory pain upregulates spinal inhibition via endogenous neurosteroid production. J Neurosci. 2005;25:11768–11776. doi: 10.1523/JNEUROSCI.3841-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar E, Fowler JH, McGill MM, Todd AJ. The types of neuron which contain protein kinase C gamma in rat spinal cord. Brain Res. 1999;833:71–80. doi: 10.1016/s0006-8993(99)01500-0. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Stress-induced deoxycorticosterone-derived neurosteroids modulate GABAA receptor function and seizure susceptibility. J Neurosci. 2002;22:3795–3805. doi: 10.1523/JNEUROSCI.22-09-03795.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Castaneda DC, O'Malley BW, Rogawski MA. Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Ther. 2004;310:230–239. doi: 10.1124/jpet.104.065268. [DOI] [PubMed] [Google Scholar]
- Reddy DS, O'Malley BW, Rogawski MA. Anxiolytic activity of progesterone in progesterone receptor knockout mice. Neuropharmacology. 2005;48:14–24. doi: 10.1016/j.neuropharm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichter R, Keller AF, De Roo M, Breton JD, Inquimbert P, Poisbeau P. Fast nongenomic effects of steroids on synaptic transmission and role of endogenous neurosteroids in spinal pain pathways. J Mol Neurosci. 2006;28:33–51. doi: 10.1385/jmn:28:1:33. [DOI] [PubMed] [Google Scholar]
- Shu HJ, Eisenman LN, Jinadasa D, Covey DF, Zorumski CF, Mennerick S. Slow actions of neuroactive steroids at GABAA receptors. J Neurosci. 2004;24:6667–6675. doi: 10.1523/JNEUROSCI.1399-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser CR, Ertunc M, Liu X, Kavalali ET. Cholesterol-dependent balance between evoked and spontaneous synaptic vesicle recycling. J Physiol (Lond) 2007;579:413–429. doi: 10.1113/jphysiol.2006.123133. [DOI] [PMC free article] [PubMed] [Google Scholar]