Abstract
Phencyclidine (PCP) and ketamine are dissociative anesthetics capable of inducing analgesia, psychomimetic behavior, and a catatonic state of unconsciousness. Despite broad similarities, there are notable differences between the clinical actions of ketamine and PCP. Ketamine has a lower incidence of adverse effects and generally produces greater CNS depression than PCP. Both noncompetitively inhibit NMDA receptors, yet there is little evidence that these drugs affect GABAA receptors, the primary target of most anesthetics. α6β2/3δ receptors are subtypes of the GABAA receptor family and are abundantly expressed in granular neurons within the adult cerebellum. Here, using an oocyte expression system, we show that at anesthetically relevant concentrations, ketamine, but not PCP, modulates α6β2δ and α6β3δ receptors. Additionally, at higher concentrations, ketamine directly activates these GABAA receptors. Comparatively, dizocilpine (MK-801 [(+)-5-methyl-10,11-dihydro-5H-dibenzo [a,d] cyclohepten-5,10-imine maleate]), a potent noncompetitive antagonist of NMDA receptors that is structurally unrelated to PCP, did not produce any effect on α6β2δ receptors. Of the recombinant GABAA receptor subtypes examined (α1β2, α1β2γ2, α1β2δ, α4β2γ2, α4β2δ, α6β2γ2, α6β2δ, and α6β3δ), the actions of ketamine were unique to α6β2δ and α6β3δ receptors. In dissociated granule neurons and cerebellar slice recordings, ketamine potentiated the GABAergic conductance arising from α6-containing GABAA receptors, whereas PCP showed no effect. Furthermore, ketamine potentiation was absent in cerebellar granule neurons from transgenic functionally null α6−/− and δ−/−mice. These findings suggest that the higher CNS depressant level achieved by ketamine may be the result of its selective actions on α6β2/3δ receptors.
Keywords: ketamine, α6β2/3δ GABAA receptors, granule neurons, slice recording, cerebellum, transgenic mice, tonic conductance
Introduction
Ketamine and phencyclidine (PCP) are chemical congeners belonging to a class of pharmacological agents known as dissociative anesthetics. These drugs produce similar clinical actions, although the impact of ketamine on CNS functioning differs from that of PCP and its analogues (Greifenstein et al., 1958; Johnstone et al., 1959; Collins et al., 1960; Chen, 1965; Domino et al., 1965; Corssen and Domino, 1966). Ketamine has a lower potency, a shorter duration of action, a faster rate of induction, results in a lower incidence of adverse emergence reactions, and is an effective anesthetic across different animal species (PCP shows species selectivity). Furthermore, ketamine lacks the convulsive side effects that PCP produces at higher doses. Collectively, these studies suggest that ketamine possesses a higher CNS depressant activity and produces a better quality of anesthesia than PCP (McCarthy et al., 1965).
It has been established that dissociative anesthetics are noncompetitive inhibitors of NMDA excitatory ligand-gated ion channels (Lodge and Anis, 1982; Anis et al., 1983; MacDonald et al., 1987, 1991; ffrench-Mullen and Rogawski, 1989; Rogawski and Wenk, 2003). These drugs also modulate the activity of nicotinic acetylcholine, muscarinic, and opioid receptors, and voltage-gated ion channels, but at significantly higher concentrations than needed to block NMDA receptors (Finck and Ngai, 1982; Ramoa et al., 1990; Hustveit et al., 1995; Hirota and Lambert, 1996; Scheller et al., 1996; Brau et al., 1997; Furuya et al., 1999; Flood and Krasowski, 2000; Yamakura et al., 2000; Schnoebel et al., 2005). Studies conducted at low doses of ketamine suggest that the inhibition of NMDA receptors results in its psychomimetic and analgesic properties (Tricklebank et al., 1987; Klepstad et al., 1990; Oye et al., 1992). However, the mechanism by which ketamine produces a higher CNS depression than PCP at high doses has remained an enigma (Kress, 1997).
GABAA receptors are the prime target for the hypnotic class of anesthetic compounds (Franks and Lieb, 1994; Macdonald and Olsen, 1994). A number of studies also implicated GABAA receptors in ketamine anesthesia (Scholfield, 1980; Gage and Robertson, 1985; Bennett et al., 1988; Irifune et al., 1992, 2000; Lin et al., 1992; Wakasugi et al., 1999), although these provided either indirect evidence for the action of ketamine on GABAA receptors, or the concentration of ketamine used was significantly higher than the anesthetically relevant concentration.
The GABAA receptor subtypes α6β2/3δ are expressed at high levels exclusively within mature cerebellar granule neurons (Laurie et al., 1992; Wisden et al., 1996) and show high sensitivity to GABA (Saxena and Macdonald, 1996; Storustovu and Ebert, 2006; Hadley and Amin, 2007).
We investigated the effects of ketamine, PCP, and MK-801 [(+)-5-methyl-10,11-dihydro-5H-dibenzo [a,d] cyclohepten-5,10-imine maleate] (a potent noncompetitive inhibitor of NMDA receptor) on α6- and δ-containing GABAA receptors. We show that ketamine, but not PCP or MK-801, within an anesthetically relevant concentration range, potentiated the GABA current arising from α6- and δ-containing GABAA receptors in oocytes, dissociated granule neurons, and cerebellar slices isolated from rodents. The potential role of GABAA α6β2/3δ receptors in ketamine anesthesia is discussed.
Materials and Methods
cRNA quantitation and oocyte injection.
Xenopus laevis frogs were anesthetized by bathing in a solution containing 0.1% MS-222 (tricaine methane sulfonate; Sigma-Aldrich). Before ovariectomy, the toe of the frog was pinched to assess the state of anesthesia. After surgery, the frog was killed by decapitation according to the protocol approved by the Institutional Animal Care and Use Committee. Oocyte preparation, in vitro transcription of complementary rat RNA (cRNA), and the drug perfusion system have been described previously (Walters et al., 2000). The quality of cRNA was determined by electrophoresis of the cRNA on a 1% formaldehyde-containing agarose gel and then quantified spectrophotometrically. Two to four preparations of cRNAs were tested for each subunit. The cRNA mixture (in diethylpyrocarbonate-treated water) was injected into Xenopus laevis oocytes using a Picospritzer II (General Valve Corporation). The ratio of the coinjected subunits α:β:γ/δ was1:1:1.8 except for expression of α6β2δ receptors in the low-affinity state where the amount of β2 was relatively decreased to one-tenth (1:0.1:1.8) (Hadley and Amin, 2007). Five to ten nanograms of α6, β2, and δ cRNA mixture per oocyte was used for the ketamine studies.
Oocytes electrophysiology.
Recording microelectrodes were fabricated with a Narishige PP-83 puller filled with 3 m KCl, and had input resistances of 0.7–1.6 MΩ. A Turbo TEC-05 (NPI Electronic) two-electrode voltage-clamp amplifier (Adams and List) was used to record the currents. In all oocyte recordings, membrane potential was clamped to −70 mV. Data were visualized on a Gould TA-240 chart recorder during the experiments and stored online using Pulse Fit (HEKA).
Cerebellar preparation.
Sprague Dawley rats, C57BL/6 mice, δ−/−mice (provided by G. Homanics, University of Pittsburgh, Pittsburgh, PA) (Mihalek et al., 1999), and α6−/−mice [provided by W. Wisden (University of Aberdeen, Aberdeen, UK) and E. Korpi (University of Turku, Turku, Finland)] (Jones et al., 1997) were reared in the central animal facilities of the University of Mainz and the University of Leipzig and transferred to the local adaptation facilities several days before experiments. All procedures followed the German guidelines for animal care. Male animals between postnatal day 20 (P20) and P48 were decapitated and their brains rapidly removed into ice-cold carboxygenated (5% CO2/95% O2) cutting solution (see below, Solutions and drugs). The cerebellar vermis was trimmed and then glued to the stage of an IntegraSlice 7550MM (cyanoacrylate glue; Campden Instruments). Sagittal slices of ∼200 or ∼500 μm thickness were prepared and maintained in a continuously carboxygenated perfusion chamber at room temperature (21°C) containing artificial CSF (ACSF) (see below, Solutions and drugs) for 1 h. Single slices (∼200 μm) were transferred to a recording chamber (RC-26GLP; Warner Instruments), placed under an upright fixed stage microscope (Axioskop FS; Zeiss), and perfused at ∼4 ml/min with carboxygenated ACSF. Using Nomarski optics and an enhanced infrared video system (Luigs and Neumann), whole-cell patch-clamp recordings of individual cells within the second or third layer of the inner granule cell area were obtained. To account for possible regional differences in the expression of the α6-subunit (Mellor et al., 1998), we restricted slice studies to lobes VIII to X of the cerebellar cortex.
Preparation of dissociated granule neurons.
To obtain dissociated cells, slices of ∼500 μm thickness were transferred to preheated cutting solution (35°C, bubbled with carbogen) supplemented with 0.5–1 mg/ml pronase. After 20–40 min, slices were transferred to ice-cold HEPES-buffered cutting solution and carefully triturated with fire-polished glass pipettes of decreasing tip diameter. An aliquot (∼ 20 μl) of dissociated-cell suspension was plated onto poly-l-lysine-coated coverslips and allowed to settle in ACSF for ∼20 min before recordings.
Granule neurons electrophysiology.
Whole-cell recordings were obtained with thick-walled borosilicate electrodes (1.5 mm outer diameter, 0.5 mm inner diameter; 8–12 MΩ resistances; Vitrex 11394; Science Products) using standard procedures (Hamill et al., 1981). Recordings were routinely filtered at 1 kHz, amplified and recorded on a personal computer using an EPC-9 amplifier and Pulse 8 software (HEKA). Current responses were recorded under voltage-clamp conditions at a holding potential of −60 mV and series resistances within 20–35 MΩ were compensated (70–80%; Pulse software; HEKA). Adequate clamp conditions (error <5 mV) (Rossi et al., 1994) were verified by the fast activation of routinely recorded voltage-gated Na+ currents (300 μs time to peak).
Solutions and drugs.
For oocyte recording, the extracellular oocyte ringer (OR2) solution contained (in mm) 83.5 NaCl, 2.5 KCl, 10 HEPES, 1 CaCl2, and 1 MgCl2, pH 7.5. For cerebellar recording, the ACSF was bubbled with carbogen and contained (in mm) 135 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, 30 Na2HCO3, and 1.5 NaHPO4, pH 7.4. For tissue preparation and cutting, the solution contained (in mm) 60 NaCl, 140 sucrose, 5 KCl, 0.3 CaCl2, 6 MgCl2, 10 glucose, 30 Na2HCO3, and 1.5 NaHPO4, pH 7.4. For cell dissociation, Na2HCO3 and NaHPO4 were replaced by HEPES. For dissociated granule neurons or slice recordings, drugs were diluted in (in mm) 150 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES, with pH 7.4 (NaOH). All solutions were adjusted to ∼305 mOsmol with sucrose or H2O. Patch electrodes were filled with (in mm) 125 CsCl, 2 MgCl2, 10 EGTA, 10 tetra-ethyl-ammonium chloride (TEA), 4–5 Na2-ATP, 0.5 Na-GTP, and 10 HEPES, pH 7.3 (CsOH), adjusted to ∼295 mOsmol with sucrose or H2O.
All solutions were made from analytical grade salts. TEA, picrotoxinin, furosemide, ketamine, and bicuculline were purchased from Sigma-Aldrich. For some experiments, a premade ketamine solution (ketalar, 100 mg/ml) was used without noticeable differences. GABA was diluted from a 100 mm fresh or frozen stock solution to the final concentrations, furosemide from a 100 mm stock solution in 200 mm NaOH, picrotoxinin from a 10 mm stock solution in ethanol, bicucculine from a 100 mm in DMSO, and ketamine from a 100 mm in water. DMSO or ethanol at their highest concentrations used (0.1 and 0.5%, respectively, for oocytes) did not alter the responses. For oocyte recordings, stock solutions were diluted in the recording OR2 solution.
For slice recordings, drugs were applied focally using a gravity-fed Y-tube system described previously (Hevers and Luddens, 2002). In short, a 5-mm-long fused-silica tube (MicroFil; World Precision Instruments) was inserted into a sharply bent Teflon tube connected on one side to a vacuum source and on the other to the drug reservoirs. Focal drug application was initiated by interrupting the vacuum line with the tip, positioned in close proximity (<50 μm) to the cell, allowing a solution exchange within ∼150 ms. For dissociated cells, a parallel three-barrel system was used in which the middle barrel contained buffer and the outside barrels contained different drugs. The middle barrel outlet was positioned in close proximity to the cells and switched to one of the drug lines with a stepping motor (Warner SF-77B; Warner Instruments) or a piezo translator (Burleigh LSS-3200; NPI Electronic).
Data analysis.
We estimated the EC50 value for GABA and ketamine direct action by fitting the data from concentration–response relationships to the logistic equation according to the following formula (Sigmaplot 2000 or origin 6.0; OriginLab): I = Imax/(1+ (EC50/[A])n).
The data for the GABA concentration response relationship of α6β2δ receptors was fitted with a sum of two logistic equations: I = Imax1/(1+ (EC501/[A])n1 + Imax2/(1+ (EC502/[A])n2), where I is the peak current at a given concentration of agonist A, Imax, Imax1 and Imax2 are the maximum currents, EC50, EC501, and EC502 are the concentrations of agonist yielding the half maximal currents, and n, n1, and n2 are the Hill coefficients of the curves.
Determinations of the statistical significant were performed either using the student t test or ANOVA as appropriate, requiring a p value of <0.05 for statistical significance. All statistical calculations are presented as means ± SE.
Results
α6β2δ receptors show high sensitivity to ketamine
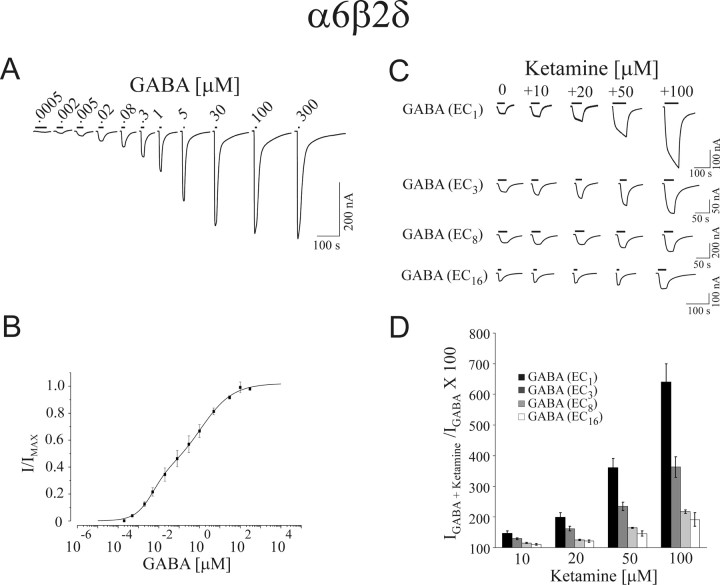
The degree a drug inhibits or potentiates the activity of a GABAA receptor depends markedly on the level of receptor channel activation by GABA (Morris et al., 1999; Walters et al., 2000). To test the modulatory activity of ketamine on α6β2δ receptors, we determined the GABA concentration–response relationship for α6β2δ receptors to establish the concentrations eliciting 1, 3, 8, and 16% of the maximal current (EC1,GABA EC3,GABA, EC8,GABA, and EC16,GABA, respectively). Three to four days after cRNA injection, GABA-activated currents were recorded (Fig. 1 A,B). The GABA data for α6β2δ receptors were fitted with a logistic equation (see Materials and Methods) and the extrapolated EC values were tested and adjusted empirically. These experiments revealed both a high-affinity and a low-affinity state of the α6β2δ receptors [the detailed characterization of GABA concentration–response relationship for these receptors has recently been described further (Hadley and Amin, 2007)]. A significant level of spontaneous channel activity accompanies the high-affinity state.
Figure 1.
Ketamine potentiation of GABA-induced currents in α6β2δ receptors. A, B, GABA concentration–response relationship of α6β2δ receptors. A, GABA-induced current traces for α6β2δ receptors. B, Data points for GABA concentration–response relationship. The α6β2δ receptors show two distinct and separable states of agonist affinity, one exhibiting μm and the other nm affinities for GABA. The high-affinity state is associated with a significant level of constitutive channel activity. C, Representative current traces for ketamine-dependent potentiation of EC1,GABA, EC3,GABA, EC8,GABA, and EC16,GABA for α6β2δ receptors. D, Bar graph depicting the average of 10, 20, 50, and 100 μm ketamine potentiation at EC1,GABA, EC3,GABA, EC8,GABA, and EC16,GABA. Error bars indicate SEM.
The sensitivity of α6β2δ receptors to ketamine was examined next at the presence of EC1,GABA EC3,GABA, EC8,GABA, and EC16,GABA values (Fig. 1C,D). Ketamine at 10 μm increased the EC1,GABA current by 46 ± 8%, whereas at 20, 50, and 100 μm the increases in GABA currents by ketamine were 100 ± 14%, 261 ± 30%, and 540 ± 60%, respectively. Ketamine at 10 to 100 μm augmented the EC3,GABA currents from ∼30–260% (Fig. 1). At EC8,GABA and EC16,GABA, ketamine produced a moderate increase in the GABA currents; i.e., for 10 and 100 μm ketamine, the increase of the EC16,GABA currents ranged from 10 to 91%, respectively. After ketamine application, the subsequent GABA currents were similar in magnitude to the preceding GABA control. Therefore ketamine exhibited little to no residual effects (data not shown).
The high sensitivity of ketamine is unique to α6β2δ receptors
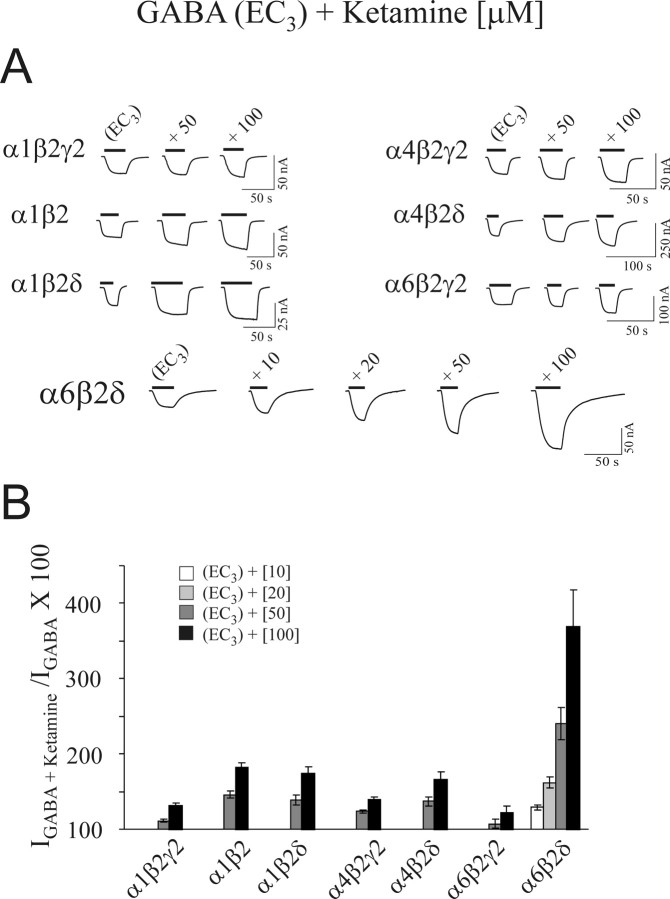
We compared the sensitivity of ketamine to α1β2γ2, α6β2γ2, α1β2δ, α4β2δ, α4β2γ2, α1β2, and α6β2δ GABAA receptors at their respective EC3,GABA value (Fig. 2). Ketamine produced diminutive effects on α1β2γ2, α4β2γ2, and α6β2γ2 receptors. Ketamine (100 μm) increased the respective EC3,GABA currents between 22 and 39%, the values being significantly below those induced by 20 μm ketamine on α6β2δ receptors. The increases by 50 and 100 μm ketamine for α4β2δ receptors were 37 ± 6% and 66 ± 10%, respectively, whereas the corresponding values for α1β2δ receptors were 39 ± 7% and 74 ± 9%. Thus, ketamine produced a modest potentiation at the EC3,GABA currents for α1β2δ and α4β2δ receptors. The α1β2 GABA current (EC3) increased by 46 ± 5% and 81 ± 7% at 50 and 100 μm ketamine, respectively.
Figure 2.
High sensitivity of α6β2δ receptors to ketamine is unique among different GABAA receptor subtypes. A, Representative current traces for ketamine-dependent potentiation of GABA currents arising from α1β2γ2, α1β2, α1β2δ, α4β2γ2, α4β2δ, α6β2γ2, and α6β2δ GABAA receptors. B, Bar graph representing the average of ketamine-dependent potentiation of the respective EC3,GABA current for the above GABAA receptor subtypes. Ketamine produced a low level of potentiation for the three GABA receptor subtypes containing γ2 subunit. In comparison, ketamine induces a marked potentiation of GABA currents arising from α6β2δ receptors. Error bars indicate SEM.
In summary, the presence of the γ2 subunit within GABAA receptors reduced the effects of ketamine, whereas those receptors containing the δ subunits (or α1β2 alone) exhibited relatively higher sensitivity to ketamine. However, both α6 and δ subunits were needed to confer the highest sensitivity to ketamine. Thus, among the tested GABAA receptor subtypes, the high sensitivity to ketamine is unique to α6β2δ receptors.
Ketamine directly activates α6β2δ and α6β3δ receptors
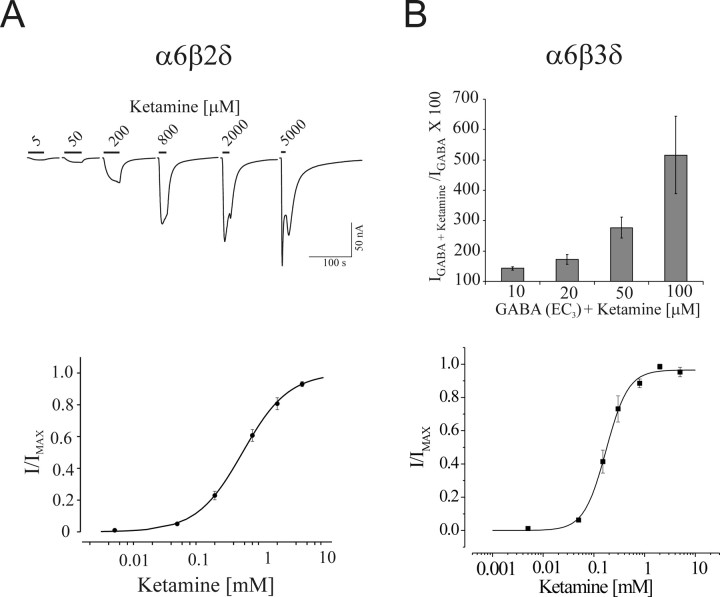
Intravenous anesthetics such as pentobarbital modulate and directly activate most, if not all, GABAA receptor subtypes. To examine whether ketamine can also act as an agonist, we bath applied ketamine in concentrations ranging from 5 to 5000 μm on oocytes expressing α6β2δ receptors (Fig. 3A). Ketamine directly activated α6β2δ receptors at concentrations as low as 50 μm, with an EC50 of 577 ± 63 μm and a Hill slope of 1.20 ± 0.09 (n = 4). When higher concentrations of ketamine were removed, the residual current increased before returning to the baseline, suggesting that ketamine possessed a low-affinity blocking action (Fig. 3A). This apparent block by ketamine at higher concentrations resembled the direct action of pentobarbital on GABAA receptors.
Figure 3.
Ketamine modulates and can directly activate α6β2δ or α6β3δ receptors. A, Ketamine-induced current traces for α6β2δ receptors and ketamine concentration–response relationship. Ketamine directly activates the α6β2δ receptors with an EC50 of ∼570 μm. B, Bar graph represents the average ketamine potentiation at EC3,GABA current and ketamine concentration–response relationship for α6β3δ receptors. Similar to α6β2δ receptors, ketamine produces a marked potentiation of GABA current arising from α6β3δ receptors. The EC50 for ketamine direct activation was ∼245 μm for α6β3δ receptors. Error bars indicate SEM.
For α6β2δ receptors, the relative maximal current of ketamine (1.8 mm) to GABA (50 μm) was 0.51 ± 0.06 (n = 10), indicating that ketamine is a partial agonist on α6β2δ receptors. In contrast, ketamine did not directly activate α1β2δ, α6β2γ2, α4β2δ, and α4β2γ2 receptors, with the relative efficacies ranging from 0.01 to 0.02 for ketamine (1.8 mm) to GABA (1.2 mm).
In addition to the β2 subunit, the β3 subunit is abundantly expressed in adult cerebellar granule neurons. Thus, we examined the modulatory and direct actions of ketamine on α6β3δ receptors as well (Fig. 3B). At 10, 20, 50, and 100 μm ketamine potentiated the EC3,GABA by 43 ± 6%, 72 ± 16%, 177 ± 35%, and 416 ± 128%, respectively, similar to those of α6β2δ receptors. Ketamine also directly activated α6β3δ receptors with an EC50 of 245 ± 26 μm and slope of 1.9 ± 0.1 (n = 11). Thus, ketamine at low concentrations (10–20 μm) significantly potentiated the GABA current of α6β3δ receptors and at higher concentration directly activated these receptors.
In summary, ketamine directly activated α6β2δ and α6β3δ receptors. The direct and modulatory actions of ketamine resemble those of the classical intravenous anesthetic pentobarbital. However, direct activation of ketamine appeared to be selective to α6β2/3δ receptors, whereas pentobarbital directly activates most GABAA receptors, including α1β2/3γ2 receptors.
MK-801 or PCP do not modulate α6β2/3δ receptors
PCP blocks NMDA receptors by an order of magnitude higher than that of ketamine. MK-801, a dissociative anesthetic chemically unrelated to PCP, possesses an even greater affinity to, and inhibits NMDA receptors with higher potency than PCP (Wong et al., 1986; Huettner and Bean, 1988; MacDonald and Nowak, 1990; MacDonald et al., 1991). Furthermore, the blocking action of MK-801 on the NMDA receptor is less dependent on the membrane potential than ketamine or PCP (Halliwell et al., 1989; Dravid et al., 2007). We examined whether the sensitivity of α6β2δ and α6β3δ receptors to ketamine extends to PCP or MK-801. First, the modulatory effect of PCP at the EC3,GABA value for α6β2δ and α6β3δ receptors was examined (Fig. 4A,B). PCP produced no significant potentiation of the GABA current arising from α6β2δ receptors even at 50 μm, a concentration significantly higher than the anesthetically relevant concentrations. Second, the effect of MK-801 up to 50 μm was tested on α6β2δ receptors (Fig. 4C). As with PCP, MK-801 did not affect the EC3,GABA currents arising from α6β2δ receptors. Thus, among the three dissociative anesthetics tested, α6β2δ receptors are uniquely sensitive to ketamine.
Figure 4.
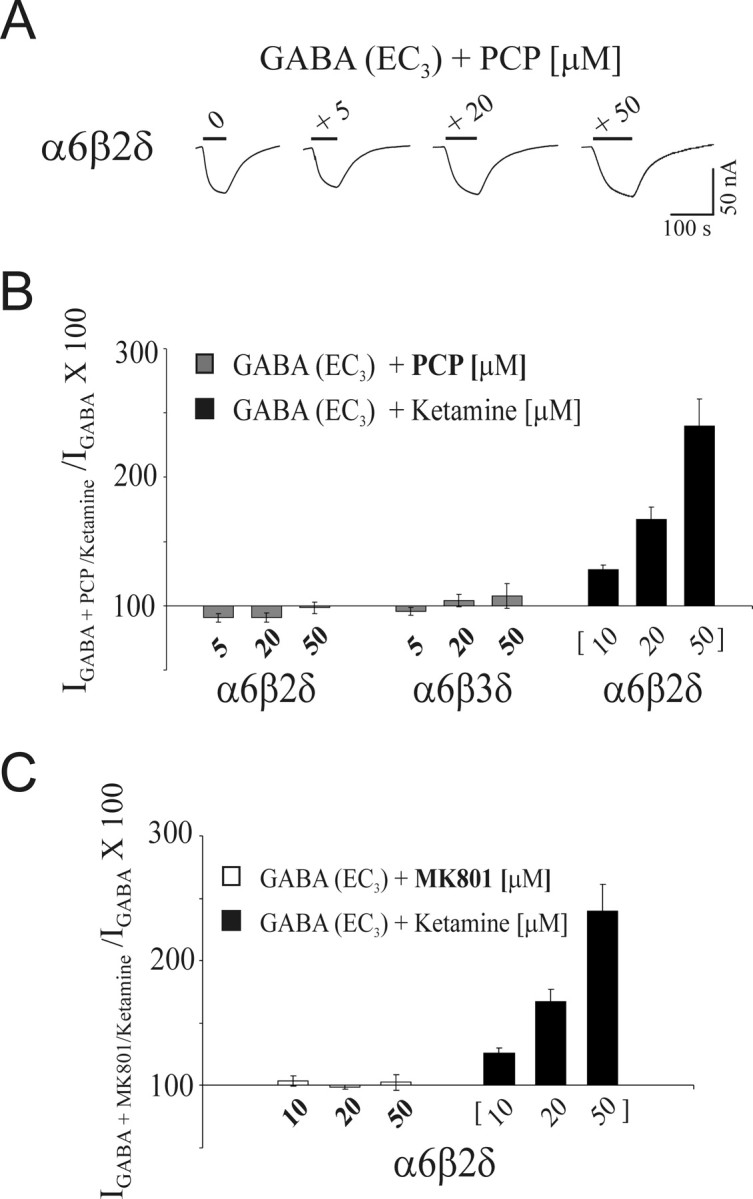
Comparison of PCP, MK-801, and ketamine action on α6β2δ receptors. A, Representative current traces depicting the effect of coapplication of 5, 20, and 50 μm PCP with GABA (EC3) on α6β2δ receptors. B, Bar graph depicting the effect of 5, 20, and 50 μm PCP on GABA (EC3) current arising from α6β2δ and α6β3δ receptors. The ketamine potentiation of the EC3,GABA current from α6β2δ is included. PCP shows no significant effect on α6β2δ and α6β3δ receptors. C, The bar graph representing the effect of 10, 20, and 50 μm MK-801 on GABA (EC3) current arising from α6β2δ receptors. The ketamine potentiation of the EC3,GABA current from α6β2δ is included. Similar to PCP, MK-801 produced no significant change in the magnitude of GABA-evoked currents of the α6β2δ receptors. Error bars indicate SEM.
Action of ketamine on the α6β2δ receptors within a low- or high-affinity state
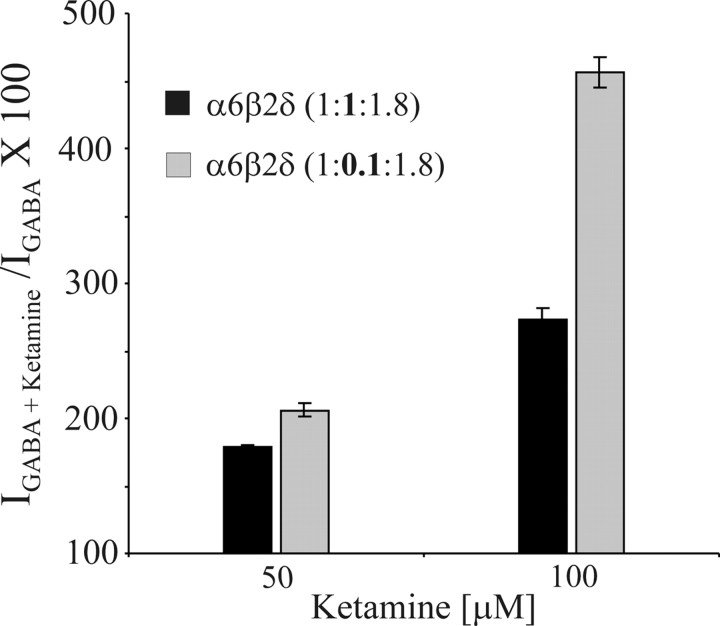
The affinity state of the α6β2δ receptors toward GABA expressed in Xenopus oocytes can be controlled by (1) the expression level and (2) the alteration of the ratio of β2 to α6 and δ subunits (Hadley and Amin, 2007). The ketamine data for α6β2δ receptors shown up to here was obtained using a cRNA injection ratio for α6:β2:δ of 1:1:1.8 (5–10 ng of cRNA per injection), where both the high- and low-affinity states of the receptor coexist. The high-affinity state exhibits constitutive activity. Previous studies have shown that injection using a lower ratio of β subunit cRNA, i.e., 1:0.1:1.8, results in a preponderance of α6β2δ receptors in the low-affinity state (Hadley and Amin, 2007). To investigate the sensitivity of α6β2δ receptors to ketamine in the high- versus low-affinity state, we injected oocytes with either the standard ratio of 1:1:1.8 with 10–15 ng of cRNA per injection (see Materials and Methods) or with a low β2 subunit ratio of 1:0.1:1.8 to express the α6β2δ receptors in predominantly the high- or low-affinity state, respectively. For the α6β2δ receptor in the low-affinity state, the percentage increases of the GABA current at EC3,GABA by 50 and 100 μm ketamine were 106 ± 5% and 356 ± 11%, respectively (Fig. 5), compared with the α6β2δ receptor in the high-affinity state, whereas the increase was 79 ± 1% and 173 ± 8% for 50 and 100 μm ketamine, respectively. Furthermore, ketamine on its own showed low efficacy (Imax ketamine/Imax GABA ∼ 0.1) for α6β2δ receptors in the low-affinity state. Collectively, ketamine can significantly potentiate the GABA currents arising from α6β2δ receptors in either the low or the high-affinity states yet the direct activation by ketamine appears to be selective for α6β2δ receptors in the high-affinity state.
Figure 5.
Ketamine modulation of the high-affinity versus low-affinity state of α6β2δ receptors. Oocytes were injected with either 10–15 ng of cRNA per injection of the standard ratio α6:β2:δ (1:1:1.8) or with a low β2 subunit ratio of 1:0.1:δ1.8 to express the α6β2δ receptors in predominantly the high- or low-affinity state, respectively. Bar graphs show ketamine potentiation (50 and 100 μm) of EC3,GABA current arising from α6β2δ receptors in the predominantly low versus high-affinity state. Ketamine produced a marked potentiation of α6β2δ receptors GABA current in either low or high-affinity states. Error bars indicate SEM.
Action of ketamine on cerebellar granule neurons
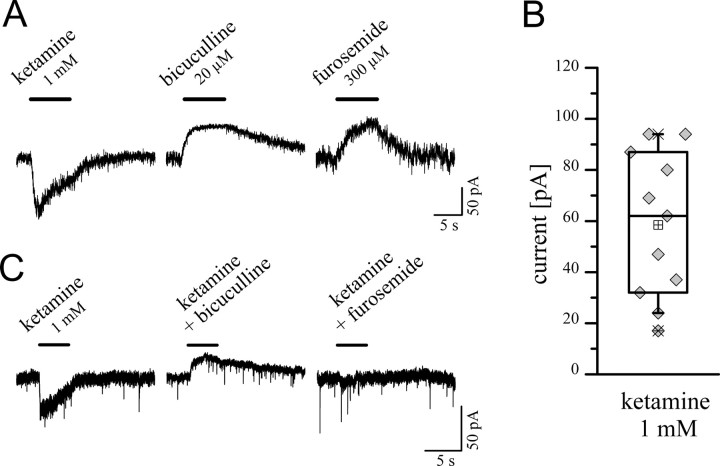
We investigated the action of ketamine on granule neurons in rat cerebellar slices from P20–P48 when the expression of α6 and δ subunit reaches high levels (Laurie et al., 1992). Individual granule neurons from the inner granule layer were voltage clamped (−60 mV) in whole-cell recording configurations using equimolar Cl− concentrations. In these recordings, granule neurons showed a tonic current that could be blocked by adding the GABAA receptor antagonist bicuculline (20 μm), or the specific α6-containing GABAA receptor antagonist furosemide (300 μm) (Korpi et al., 1995; Korpi and Luddens, 1997) (Fig. 6A,B). Ketamine (1 mm) evoked currents that ranged in amplitude from 15 to over 100 pA (58.5 ± 8.6 pA; n = 11). Bicuculline (20 μm) and furosemide (300 μm) blocked the ketamine-induced currents within the granule neurons, indicating that these currents arose from α6-containing GABAA receptors (Fig. 6C).
Figure 6.
Ketamine-induced currents in rat cerebellar granule cells. A, Whole-cell patch-clamp recordings of granule cells were obtained in adult cerebellar slices (here P30). At a holding potential of −60 mV, using equimolar Cl− concentrations, ketamine evoked currents with a rapid time course. Granule neurons showed a tonic current that could be blocked by adding GABAA receptor antagonists, i.e., bicuculline or the specific α6-containing GABAA receptor antagonist furosemide. B, Ketamine (1 mm) induced currents that ranged from ∼15–100 pA (mean 58.5 ± 8.6 pA; n = 11; P21–P34). C, Ketamine-induced currents were blocked by bicuculline or furosemide coapplication indicating that the ketamine effects were mediated through α6-containing GABAA receptors. Error bars indicate SEM.
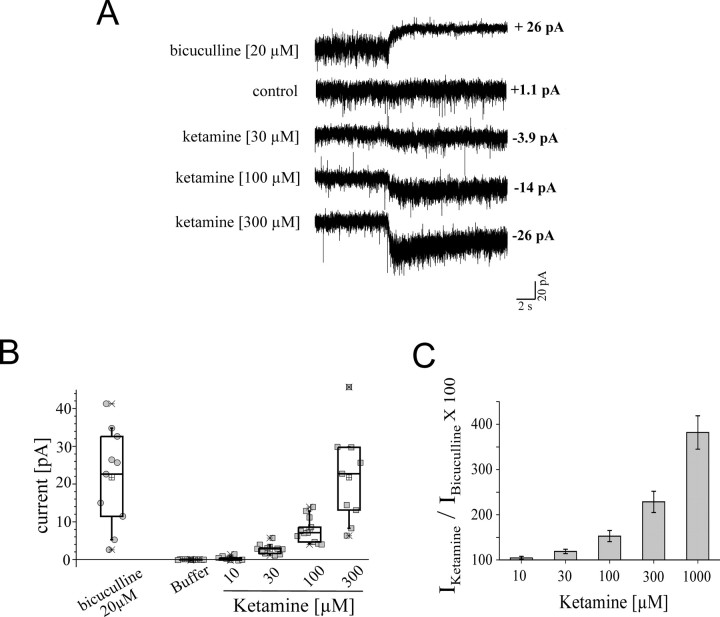
We next examined the potentiation of the tonic current by 10, 30, 100, 300, and 1000 μm ketamine (Fig. 7). The level of bicuculline-dependent block of the tonic current was used as the denominator to determine the ketamine potentiation. Ketamine, at a concentration as low as 30 μm, produced a significant increase in the tonic current, resulting in 20 ± 15% potentiation (p = 0.0003; n = 10). Furthermore, 100, 300, and 1000 μm ketamine increased the tonic current by 50, 130, and 280%, respectively. Thus, ketamine at clinically relevant concentrations (see Discussion) of 30 or 100 μm can increase the tonic current significantly.
Figure 7.
Potentiation of ketamine at 30, 100, 300, and 1000 μm tonic current in cerebellar slice recordings. A, Current traces representing block of the tonic current by bicuculline and potentiation of the tonic current by 30, 100, and 300 μm ketamine. The drugs were applied for 20 s. The current amplitudes shown were determined from all point histographs of the current traces. B, The range of current amplitudes for bicuculline-dependent block, and ketamine (10, 30, 100, and 300 μm)-induced potentiation, of the tonic current. C, Ketamine, at a concentration as low as 30 μm, produced a significant increase in the tonic current (p = 0.0003). The level of bicuculline-dependent block of the tonic current was used as the denominator to determine the ketamine potentiation. Error bars indicate SEM.
Ketamine does not induce currents in α6−/−or δ−/− transgenic mice
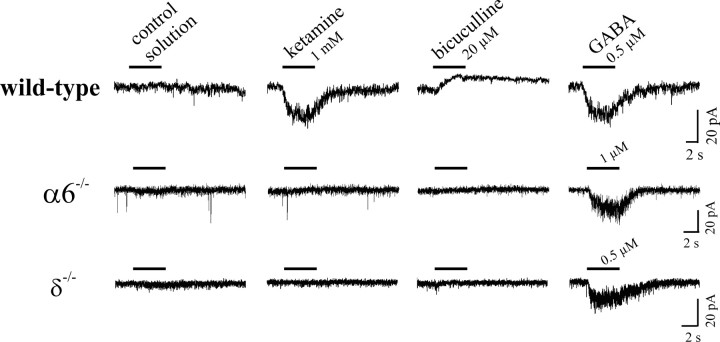
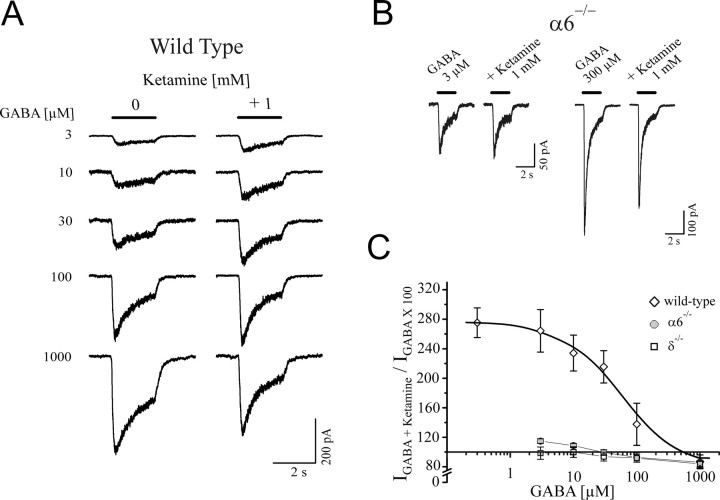
To further elucidate the involvement of α6 and test whether δ subunits play a role in the ketamine-induced currents recorded in cerebellar slices, we used mice in which the functional expression of α6- and δ- (α6−/−) or δ-subunits (δ−/−) alone was abolished (Jones et al., 1997). The GABAergic tonic background conductance was found to be absent or nearly absent within the cerebellar slice recordings from granule neurons of α6−/− or δ−/−mice, respectively (Fig. 8). In contrast to experiments from wild-type animals, ketamine (1 mm) did not induce a current in granule cells from either α6−/− or δ−/−mice. These experiments demonstrate that the δ subunit and, likely, the α6 subunit are needed to confer ketamine sensitivity to cerebellar granule neurons.
Figure 8.
Absence of the effect of ketamine in cerebellar slice recordings from α6−/− or δ−/−mice. Ketamine-induced currents in cerebellar granule cells from wild-type mice, similar to the experiments using rats. Bicuculline verified the presence of a tonic background conductance in these experiments (here, P31). In α6−/−mice, where there is also a lack of expression of the δ subunit, bicuculline failed to reveal any background conductance. Ketamine (1 mm) produced no effect in cerebellar slice recording from granule neurons of α6−/−mice (here, P32). In δ−/−mice, in which only the δ subunits are missing, ketamine modulation was also absent indicating the importance of the δ subunit in ketamine-dependent modulation of granule neurons (shown for P36).
Ketamine's modulation of dissociated granule neurons
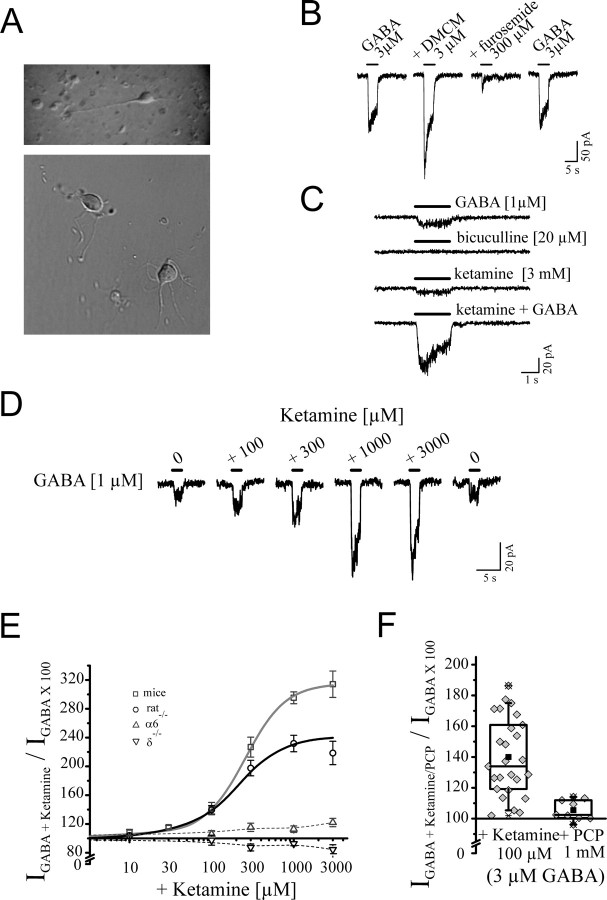
The tonic activation of extrasynaptically localized α6β2/3δ receptors may indicate the omnipresence of low concentrations of GABA at these receptor sites. To differentiate the modulatory effects in the presence of GABA versus a direct effect of ketamine on granule neurons, we developed a preparation of dissociated granule neurons from mice and rats up to an age of P28. In this procedure, neurons resembled granule neurons with small globular shape with several offshoots and dendrites (Fig. 9A). The cell capacitance was not significantly (p = 0.29) altered a few hours after dissociation (2.76 ± 0.27 pF; n = 6) when compared with granule neurons within the slice (2.89 ± 0.16 pF; n = 15). We verified presence of α6- and δ-containing GABAA receptors within the dissociated neuron using furosemide, 3-carbomethoxy-4-ethyl-6,7-dimethoxy-β-carboline (DMCM), and 4,5,6,7-tetrahydrois-oxazolo[5,4-c]-pyridin-3-ol (THIP). Furosemide inhibited, whereas DMCM potentiated, the GABA-induced current in these cells (Fig. 9B). Furthermore, 1 μm THIP, a concentration that can selectively activate α6- and δ-containing GABAA receptors (Storustovu and Ebert, 2006; Saarelainen et al., 2008), evoked 37.5 ± 4.8 pA compared with 38.3 ± 5.2 pA (n = 11) of current for 1 μm GABA within these dissociated granule neurons. These data suggest the presence of α6- and δ-containing GABAA receptors within the dissociated granule neurons. Ketamine produced a marked potentiation of the GABA current (1 μm), whereas, in contrast to slice recordings, bicuculline did not change the control current (Fig. 9C).
Figure 9.
Ketamine action on acutely dissociated granule cells. A, These neurons (P20 to P30) resembled granule neurons with their small globular shape and several offshoot dendrites. B, The pharmacological characteristics of these dissociated cells, such as the inhibitory effect by furosemide or potentiation by DMCM, were similar to granule neuron recording within slices. C, Bicuculline (20 μm) alone did not produce an effect indicating the absence of tonic background conductance within these dissociated neurons. Ketamine (1 mm) produced a pronounced potentiation of GABA (3 μm) currents, whereas ketamine (3 mm) alone induced a small current in the absence of GABA. D, Current traces for ketamine-dependent modulation of GABA-evoked currents (1 μm) for mice. E, Concentration–response relationship of the ketamine modulatory action for wild-type mice yielded EC50 values of ∼260 and 200 μm ketamine and a maximal potentiation of 310 and 240% for mice and rat, respectively. Ketamine did not produce a significant effect on GABA current (1 μm) from dissociated neurons isolated from α6−/− or δ−/−mice, when compared with rats or wild-type mice. F, Ketamine at 100 μm showed pronounced potentiation of GABA currents (3 μm) for mice and rats (140 ± 5%; n = 26, rat and mice pooled) within these neurons. PCP did not modulate GABA (3 μm) currents arising from dissociated granule neurons isolated from rat or mice (mean 105 ± 3%; n = 8). Error bars indicate SEM.
We tested the ketamine concentration response relationship in the presence of a low concentration of GABA (1 μm) for the dissociated granule neurons isolated from rats or mice (Fig. 9D,E). The half maximal potentiation was ∼300 μm for the two species (mice: 260 ± 16 μm, maximal potentiation 310 ± 50%; for rats: 198 ± 32 μm, maximal potentiation 240 ± 80%). Within these experiments, 100 μm ketamine induced a significant potentiation of the GABA current (Fig. 9F). In the presence of 3 μm GABA, 100 μm ketamine produced a 140 ± 5% potentiation (n = 26, rats and mice pooled because there were little species-dependent difference between the mice and rats in terms of ketamine modulation). Under equivalent conditions, PCP at 1 and 3 mm was ineffective in modulating the GABA current (Fig. 9F) (1 mm PCP, 106 ± 2.7%, n = 8, p = 0.78; 3 mm PCP, 112 ± 3.5%, n = 8, p = 0.67). Thus, similar to the oocyte experiments, ketamine, but not PCP, modulated the α6- and δ-containing GABAA receptors.
Ketamine evoked a relatively small current on its own with a mean value of 4.2 pA (Fig. 9C), which could be blocked by bicuculline (data not shown). These experiments suggest that α6β2/3δ receptors may be present predominately in a low-affinity state and the α6β2/3δ receptors in the high-affinity state could constitute a small fraction of the total receptor population in these dissociated granule neurons from preadolescent p28 or younger animals.
The effect of increasing concentrations of GABA on the ketamine response (1 mm) was also examined within wild-type and α6−/− or δ−/−mice dissociated granule cells (Fig. 10). For wild-type mice, the effect of ketamine was most pronounced at concentrations of GABA <100 μm with an EC50 of 57 ± 18 μm (slope = 0.98; n = 6). In contrast, ketamine potentiation was absent in the mutant α6−/− or δ−/−mice regardless of the GABA concentration, indicating the importance of α6 or δ subunits in the action of ketamine.
Figure 10.
The degree of Ketamine potentiation depends on the concentration of GABA for granule neurons isolated from mice. A, B, Current traces and GABA-concentration response in the presence of ketamine (1 mm) for wild-type and α6−/−mice. The ketamine potentiation is most pronounced at concentrations of GABA <100 μm. B, Current traces representing GABA (3 or 300 μm) and GABA plus 1 mm ketamine within α6−/−mice dissociated granule neuron. Ketamine did not produce a significant change in the current induced by 3 μm GABA and at higher concentration of GABA (300 μm) caused a moderate inhibition. C, Potentiation by ketamine (1 mm) was dependent on GABA concentration. Half-maximal potentiation by ketamine occurred at ∼60 μm GABA. Ketamine did not produce a significant potentiation at any GABA concentration in dissociated granule neuron derived from α6−/− and δ−/−mice. Furthermore, at 30 μm GABA or higher, ketamine produced a moderate inhibition in granule cells of α6−/− and δ−/−mice. Error bars indicate SEM.
Collectively, these data support the findings from the oocyte and slice recording experiments, demonstrating the unique action of ketamine on the α6- and δ-containing GABAA receptors, whereas PCP had no effect.
Discussion
We demonstrated that ketamine selectively modulates rat α6β2/3δ GABAA receptors within the oocyte expression system. These receptors were highly sensitive to ketamine, but showed no response to PCP or MK-801. Ketamine-induced currents were observed in the recordings of cerebellar granule neurons from rodents, where they were confirmed to arise from α6- and δ-containing GABAA receptors. Furthermore, the ketamine-evoked currents were absent within cerebellar granule neurons of transgenic functionally null α6−/− or δ−/−mice.
The cerebellum has a primary function in motor control and learning. Previous anatomical, neuroimaging, and pyschological studies suggest that the cerebellum may also participate in higher cognitive functions. Patients with physical-induced lesions of the cerebellum exhibit both motor and cognitive disabilities. Cerebellum anomalies have been noted in patients suffering from schizophrenia, dyslexia, and autism (Martin and Albers, 1995; Timmann and Daum, 2007). Furthermore, neuroimaging recordings of volunteers performing cognitive tasks suggest that the cerebellum is involved in memory, learning, language and attention (Bellebaum and Daum, 2007; Haarmeier and Thier, 2007; Sultan and Glickstein, 2007; Thach, 2007; Timmann and Daum, 2007). Collectively, the cerebellum appears to coordinate not only motor, but also cognitive activity.
Cerebellar granule cells are the most abundant neurons in the CNS and play a pivotal role in storing motor commands (Tyrrell and Willshaw, 1992). A key characteristic of these neurons is the postnatal development of a tonic chloride current, known as tonic inhibition, which arises from GABAA receptors containing α6 subunits (Kaneda et al., 1995; Brickley et al., 1996; Tia et al., 1996; Wall and Usowicz, 1997; Hamann et al., 2002). The tonic inhibition plays an important role in tuning the indirect transmission of information from mossy fibers to the Purkinje neurons (via granule neurons) by reducing the overall gain of information transmitted through the cerebellar cortex. It is calculated that at the adult stage, contribution of the tonic inhibition to overall inhibitory conductance is at least threefold larger than phasic inhibition. Moreover, >90% of the tonic inhibition of granule neurons is mediated by α6- and δ-containing GABAA receptors (Hamann et al., 2002). Thus, the high sensitivity of α6β2/3δ receptors to ketamine has the potential to impact the informational transfer from mossy fibers to the Purkinje neurons. Ketamine/xylazine anesthesia has been used to study cerebellar circuitry in vivo. In these studies, ketamine/xylazine significantly suppresses the activity within the parallel fiber synapses, and the states of the membrane potential of the Purkinje neurons are significantly affected as well (Schonewille et al., 2006; Bengtsson and Jorntell, 2007). In addition to the α6β2/3δ GABAA receptors, there are also NMDA and AMPA receptors expressed within the granule neurons (Garthwaite and Brodbelt, 1990). Compared with the action of ketamine on the α6β2/3δ GABAA receptors, however, block of these receptors by ketamine occurs at markedly higher concentrations (Arenz et al., 2006). Given the relatively higher sensitivity of α6β2/3δ GABAA receptors to ketamine and the abundance of these receptors at the adult stage, the ketamine potentiation of the tonic current arising from α6β2/3δ GABAA receptors may have a profoundly greater impact on the overall suppression of the parallel fiber synapses than the blocking action of ketamine on the excitatory pathway during ketamine anesthesia.
The action of ketamine on cerebellar α6β2/3δ receptors occurred at anesthetically relevant concentrations. The steady-state ketamine concentration in the plasma of anesthetized rats is ∼100 μm after the distribution phase. In the brain, the ketamine concentration has been shown to exceed 300 μm (Cohen and Trevor, 1974). Here, we demonstrate that 10–100 μm ketamine significantly potentiated GABA currents arising from α6β2/3δ receptors. Thus, the concentrations of ketamine effective at α6β2/3δ receptors are within the realm of anesthetically relevant concentrations.
The anesthetic efficacies of ketamine, PCP, and MK-801 measured by CNS depressive action do not show a strong correlation to their actions on NMDA receptors. Binding and electrophysiological studies have demonstrated that PCP and MK-801 are superior noncompetitive inhibitors of, and have markedly higher affinity for, NMDA receptors than ketamine (Chen et al., 1959; Johnstone et al., 1959; Chen, 1965; McCarthy et al., 1965; Wong et al., 1986; MacDonald et al., 1991; Rogawski and Wenk, 2003). In addition, MK-801 block can occur across different membrane potentials, whereas PCP and ketamine inhibit NMDA receptors predominately at depolarized potentials (Halliwell et al., 1989; Dravid et al., 2007). However, a number of studies have shown that PCP and MK-801 are not effective anesthetics in rats, mice or pigeons, even at near lethal doses (Chen et al., 1959; Koek et al., 1987a,b, 1988; Kelland et al., 1993; Irifune et al., 2007). Therefore, contrary to the order of their impact on NMDA receptors, ketamine is a more effective anesthetic across different animal species and appears to possess a higher CNS depressant activity than PCP and MK-801 (Chen et al., 1959; Johnstone et al., 1959; Chen, 1965; Domino et al., 1965; McCarthy et al., 1965).
In human studies, a number of patients placed under catatonic state using PCP are arousable (Johnstone et al., 1959), indicating suboptimal CNS depression, whereas patients under ketamine anesthesia do not respond to verbal commands. Furthermore, with increasing doses, PCP can induce surgically unrelated excitation (Greifenstein et al., 1958), an adverse effect absent with ketamine administration. Ketamine also produces a lower rate and a shorter duration of emergence reactions, such as vivid dreams and hallucinations, when compared with PCP.
Collectively, these observations have led to the withdrawal of PCP from further clinical trails and veterinary practice despite the higher potency of PCP in producing dissociation and having a significantly higher affinity to NMDA receptors. Although MK-801 has been extensively characterized for two decades and possesses a nearly 1000 times greater affinity for the NMDA receptors than ketamine, MK-801 has not been approved for clinical or veterinary practice. Our findings suggest the distinguishing factor is the alternative action of ketamine on the inhibitory pathway via GABAA α6β2/3δ receptors.
We have shown that ketamine, but not PCP, can increase the activity of GABAA receptors containing α6 and δ subunits within an anesthetically relevant concentration range. This is the first demonstration of two anesthetics, highly analogous in chemical structure, molecular action, and clinical effect, in which each exhibits differentiating all or none action on cerebellar α6β2/3δ GABAA receptors. The actions of ketamine are disproportionate regarding their impact on excitatory (NMDA) versus inhibitory (GABAA) pathways, with the former showing higher potency. These findings have the potential to lead to the development of PCP analogues that exhibit more balanced actions on the inhibitory versus excitatory pathways. Such anesthetics would offer a broader spectrum for desired effects, lower the incidence of adverse effects, and provide a higher margin of safety.
Footnotes
This work was supported by grants from the German Science Foundation (Deutsche Forschungsgemeinschaft Lu 319/2–1) (H.L.), the MAIFOR program of the University of Mainz (W.H.), and American Heart Established Investigator Awards (J.A.). We thank Blake Singletary for help with editing, Stacey Kolar for help with Figures, and Ingo Bohme for genotyping the mouse lines.
References
- Anis NA, Berry SC, Burton NR, Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983;79:565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenz A, Margrie R, Silver R. The effects of barbiturate and ketamine anesthesia on excitatory synaptic transmission at the mossy fiber-granule cell synapse in the cerebellum. Soc Neurosci Abstr. 2006;32:795.4/D19. [Google Scholar]
- Bellebaum C, Daum I. Cerebellar involvement in executive control. Cerebellum. 2007;6:184–192. doi: 10.1080/14734220601169707. [DOI] [PubMed] [Google Scholar]
- Bengtsson F, Jorntell H. Ketamine and xylazine depress sensory-evoked parallel fiber and climbing fiber responses. J Neurophysiol. 2007;98:1697–1705. doi: 10.1152/jn.00057.2007. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Bernard PS, Amrick CL. A comparison of PCP-like compounds for NMDA antagonism in two in vivo models. Life Sci. 1988;42:447–454. doi: 10.1016/0024-3205(88)90083-5. [DOI] [PubMed] [Google Scholar]
- Brau ME, Sander F, Vogel W, Hempelmann G. Blocking mechanisms of ketamine and its enantiomers in enzymatically demyelinated peripheral nerve as revealed by single-channel experiments. Anesthesiology. 1997;86:394–404. doi: 10.1097/00000542-199702000-00014. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol (Lond) 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. Evaluation of phencyclidine-type cataleptic activity. Arch Int Pharmacodyn Ther. 1965;157:193–201. [PubMed] [Google Scholar]
- Chen G, Ensor CR, Russell D, Bohner B. The pharmacology of 1-(1-phenylcyclohexyl) piperidine-HCl. J Pharmacol Exp Ther. 1959;127:241–250. [PubMed] [Google Scholar]
- Cohen ML, Trevor AJ. On the cerebral accumulation of ketamine and the relationship between metabolism of the drug and its pharmacological effects. J Pharmacol Exp Ther. 1974;189:351–358. [PubMed] [Google Scholar]
- Collins VJ, Gorospe CA, Rovenstine EA. Intravenous nonbarbiturate, nonnarcotic analgesics: preliminary studies. 1. Cyclohexylamines. Anesth Analg. 1960;39:302–306. [PubMed] [Google Scholar]
- Corssen G, Domino EF. Dissociative anesthesia: further pharmacologic studies and first clinical experience with the phencyclidine derivative CI-581. Anesth Analg. 1966;45:29–40. [PubMed] [Google Scholar]
- Domino EF, Chodoff P, Corssen G. Pharmacologic effects of Ci-581, a new dissociative anesthetic, in man. Clin Pharmacol Ther. 1965;6:279–291. doi: 10.1002/cpt196563279. [DOI] [PubMed] [Google Scholar]
- Dravid SM, Erreger K, Yuan H, Nicholson K, Le P, Lyuboslavsky P, Almonte A, Murray E, Mosely C, Barber J, French A, Balster R, Murray TF, Traynelis SF. Subunit-specific mechanisms and proton sensitivity of NMDA receptor channel block. J Physiol (Lond) 2007;581:107–128. doi: 10.1113/jphysiol.2006.124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ffrench-Mullen JM, Rogawski MA. Interaction of phencyclidine with voltage-dependent potassium channels in cultured rat hippocampal neurons: comparison with block of the NMDA receptor-ionophore complex. J Neurosci. 1989;9:4051–4061. doi: 10.1523/JNEUROSCI.09-11-04051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck AD, Ngai SH. Opiate receptor mediation of ketamine analgesia. Anesthesiology. 1982;56:291–297. doi: 10.1097/00000542-198204000-00011. [DOI] [PubMed] [Google Scholar]
- Flood P, Krasowski MD. Intravenous anesthetics differentially modulate ligand-gated ion channels. Anesthesiology. 2000;92:1418–1425. doi: 10.1097/00000542-200005000-00033. [DOI] [PubMed] [Google Scholar]
- Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- Furuya R, Oka K, Watanabe I, Kamiya Y, Itoh H, Andoh T. The effects of ketamine and propofol on neuronal nicotinic acetylcholine receptors and P2x purinoceptors in PC12 cells. Anesth Analg. 1999;88:174–180. doi: 10.1097/00000539-199901000-00033. [DOI] [PubMed] [Google Scholar]
- Gage PW, Robertson B. Prolongation of inhibitory postsynaptic currents by pentobarbitone, halothane and ketamine in CA1 pyramidal cells in rat hippocampus. Br J Pharmacol. 1985;85:675–681. doi: 10.1111/j.1476-5381.1985.tb10563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J, Brodbelt AR. Glutamate as the principal mossy fibre transmitter in rat cerebellum: pharmacological evidence. Eur J Neurosci. 1990;2:177–180. doi: 10.1111/j.1460-9568.1990.tb00410.x. [DOI] [PubMed] [Google Scholar]
- Greifenstein FE, Devault M, Yoshitake J, Gajewski JE. A study of a 1-aryl cyclo hexyl amine for anesthesia. Anesth Analg. 1958;37:283–294. [PubMed] [Google Scholar]
- Haarmeier T, Thier P. The attentive cerebellum—myth or reality? Cerebellum. 2007;6:177–183. doi: 10.1080/14734220701286187. [DOI] [PubMed] [Google Scholar]
- Hadley SH, Amin J. Rat α6β2δ GABAA receptors exhibit two distinct and separable agonist affinities. J Physiol (Lond) 2007;581:1001–1018. doi: 10.1113/jphysiol.2007.132886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell RF, Peters JA, Lambert JJ. The mechanism of action and pharmacological specificity of the anticonvulsant NMDA antagonist MK-801: a voltage clamp study on neuronal cells in culture. Br J Pharmacol. 1989;96:480–494. doi: 10.1111/j.1476-5381.1989.tb11841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hevers W, Luddens H. Pharmacological heterogeneity of gamma-aminobutyric acid receptors during development suggests distinct classes of rat cerebellar granule cells in situ. Neuropharmacology. 2002;42:34–47. doi: 10.1016/s0028-3908(01)00158-7. [DOI] [PubMed] [Google Scholar]
- Hirota K, Lambert DG. Ketamine: its mechanism(s) of action and unusual clinical uses. Br J Anaesth. 1996;77:441–444. doi: 10.1093/bja/77.4.441. [DOI] [PubMed] [Google Scholar]
- Huettner JE, Bean BP. Block of N-methyl-d-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci USA. 1988;85:1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hustveit O, Maurset A, Oye I. Interaction of the chiral forms of ketamine with opioid, phencyclidine, sigma and muscarinic receptors. Pharmacol Toxicol. 1995;77:355–359. doi: 10.1111/j.1600-0773.1995.tb01041.x. [DOI] [PubMed] [Google Scholar]
- Irifune M, Shimizu T, Nomoto M, Fukuda T. Ketamine-induced anesthesia involves the N-methyl-d-aspartate receptor-channel complex in mice. Brain Res. 1992;596:1–9. doi: 10.1016/0006-8993(92)91525-j. [DOI] [PubMed] [Google Scholar]
- Irifune M, Sato T, Kamata Y, Nishikawa T, Dohi T, Kawahara M. Evidence for GABAA receptor agonistic properties of ketamine: convulsive and anesthetic behavioral models in mice. Anesth Analg. 2000;91:230–236. doi: 10.1097/00000539-200007000-00043. [DOI] [PubMed] [Google Scholar]
- Irifune M, Katayama S, Takarada T, Shimizu Y, Endo C, Takata T, Morita K, Dohi T, Sato T, Kawahara M. MK-801 enhances gabaculine-induced loss of the righting reflex in mice, but not immobility. Can J Anaesth. 2007;54:998–1005. doi: 10.1007/BF03016634. [DOI] [PubMed] [Google Scholar]
- Johnstone M, Evans V, Baigel S. Sernyl (CI-395) in clinical anaesthesia. Br J Anaesth. 1959;31:433–439. doi: 10.1093/bja/31.10.433. [DOI] [PubMed] [Google Scholar]
- Jones A, Korpi ER, McKernan RM, Pelz R, Nusser Z, Makela R, Mellor JR, Pollard S, Bahn S, Stephenson FA, Randall AD, Sieghart W, Somogyi P, Smith AJ, Wisden WS. Ligand-gated ion channel subunit partnerships: GABAA receptor alpha6 subunit gene inactivation inhibits delta subunit expression. J Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M, Farrant M, Cull-Candy SG. Whole-cell and single-channel currents activated by GABA and glycine in granule cells of the rat cerebellum. J Physiol (Lond) 1995;485:419–435. doi: 10.1113/jphysiol.1995.sp020739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelland MD, Soltis RP, Boldry RC, Walters JR. Behavioral and electrophysiological comparison of ketamine with dizocilpine in the rat. Physiol Behav. 1993;54:547–554. doi: 10.1016/0031-9384(93)90248-e. [DOI] [PubMed] [Google Scholar]
- Klepstad P, Maurset A, Moberg ER, Oye I. Evidence of a role for NMDA receptors in pain perception. Eur J Pharmacol. 1990;187:513–518. doi: 10.1016/0014-2999(90)90379-k. [DOI] [PubMed] [Google Scholar]
- Koek W, Woods JH, Jacobson AE, Rice KC. Phencyclidine (PCP)-like discriminative stimulus effects of metaphit and of 2-amino-5-phosphonovalerate in pigeons: generality across different training doses of PCP. Psychopharmacology (Berl) 1987a;93:437–442. doi: 10.1007/BF00207232. [DOI] [PubMed] [Google Scholar]
- Koek W, Woods JH, Mattson MV, Jacobson AE, Mudar PJ. Excitatory amino acid antagonists induce a phencyclidine-like catalepsy in pigeons: structure-activity studies. Neuropharmacology. 1987b;26:1261–1265. doi: 10.1016/0028-3908(87)90085-2. [DOI] [PubMed] [Google Scholar]
- Koek W, Woods JH, Winger GD. MK-801, a proposed noncompetitive antagonist of excitatory amino acid neurotransmission, produces phencyclidine-like behavioral effects in pigeons, rats and rhesus monkeys. J Pharmacol Exp Ther. 1988;245:969–974. [PubMed] [Google Scholar]
- Korpi ER, Luddens H. Furosemide interactions with brain GABAA receptors. Br J Pharmacol. 1997;120:741–748. doi: 10.1038/sj.bjp.0700922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Kuner T, Seeburg PH, Luddens H. Selective antagonist for the cerebellar granule cell-specific gamma-aminobutyric acid type A receptor. Mol Pharmacol. 1995;47:283–289. [PubMed] [Google Scholar]
- Kress HG. [Mechanisms of action of ketamine] Anaesthesist. 1997;46(Suppl 1):S8–S19. doi: 10.1007/pl00002469. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LH, Chen LL, Zirrolli JA, Harris RA. General anesthetics potentiate gamma-aminobutyric acid actions on gamma-aminobutyric acidA receptors expressed by Xenopus oocytes: lack of involvement of intracellular calcium. J Pharmacol Exp Ther. 1992;263:569–578. [PubMed] [Google Scholar]
- Lodge D, Anis NA. Effects of phencyclidine on excitatory amino acid activation of spinal interneurones in the cat. Eur J Pharmacol. 1982;77:203–204. doi: 10.1016/0014-2999(82)90022-x. [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Nowak LM. Mechanisms of blockade of excitatory amino acid receptor channels. Trends Pharmacol Sci. 1990;11:167–172. doi: 10.1016/0165-6147(90)90070-O. [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Miljkovic Z, Pennefather P. Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J Neurophysiol. 1987;58:251–266. doi: 10.1152/jn.1987.58.2.251. [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Bartlett MC, Mody I, Pahapill P, Reynolds JN, Salter MW, Schneiderman JH, Pennefather PS. Actions of ketamine, phencyclidine and MK-801 on NMDA receptor currents in cultured mouse hippocampal neurones. J Physiol (Lond) 1991;432:483–508. doi: 10.1113/jphysiol.1991.sp018396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Martin P, Albers M. Cerebellum and schizophrenia: a selective review. Schizophr Bull. 1995;21:241–250. doi: 10.1093/schbul/21.2.241. [DOI] [PubMed] [Google Scholar]
- McCarthy DA, Chen G, Kaump DH, Ensor C. General anesthetic and other pharmacological properties of 2-(O-chlorophenyl)-2-methylamino cyclohexanone HCl (Ci-58l) J New Drugs. 1965;28:21–33. doi: 10.1002/j.1552-4604.1965.tb00219.x. [DOI] [PubMed] [Google Scholar]
- Mellor JR, Merlo D, Jones A, Wisden W, Randall AD. Mouse cerebellar granule cell differentiation: electrical activity regulates the GABAA receptor alpha 6 subunit gene. J Neurosci. 1998;18:2822–2833. doi: 10.1523/JNEUROSCI.18-08-02822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KD, Moorefield CN, Amin J. Differential modulation of the gamma-aminobutyric acid type C receptor by neuroactive steroids. Mol Pharmacol. 1999;56:752–759. [PubMed] [Google Scholar]
- Oye I, Paulsen O, Maurset A. Effects of ketamine on sensory perception: evidence for a role of N-methyl-d-aspartate receptors. J Pharmacol Exp Ther. 1992;260:1209–1213. [PubMed] [Google Scholar]
- Ramoa AS, Alkondon M, Aracava Y, Irons J, Lunt GG, Deshpande SS, Wonnacott S, Aronstam RS, Albuquerque EX. The anticonvulsant MK-801 interacts with peripheral and central nicotinic acetylcholine receptor ion channels. J Pharmacol Exp Ther. 1990;254:71–82. [PubMed] [Google Scholar]
- Rogawski MA, Wenk GL. The neuropharmacological basis for the use of memantine in the treatment of Alzheimer's disease. CNS Drug Rev. 2003;9:275–308. doi: 10.1111/j.1527-3458.2003.tb00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi P, D'Angelo E, Magistretti J, Toselli M, Taglietti V. Age-dependent expression of high-voltage activated calcium currents during cerebellar granule cell development in situ. Pflügers Arch. 1994;429:107–116. doi: 10.1007/BF02584036. [DOI] [PubMed] [Google Scholar]
- Saarelainen KS, Ranna M, Rabe H, Sinkkonen ST, Möykkynen T, Uusi-Oukari M, Linden AM, Lüddens H, Korpi ER. Enhanced behavioral sensitivity to the competitive GABA agonist, gaboxadol, in transgenic mice over-expressing hippocampal extrasynaptic alpha6beta GABA(A) receptors. J Neurochem. 2008;105:338–350. doi: 10.1111/j.1471-4159.2007.05136.x. [DOI] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Properties of putative cerebellar gamma-aminobutyric acid A receptor isoforms. Mol Pharmacol. 1996;49:567–579. [PubMed] [Google Scholar]
- Scheller M, Bufler J, Hertle I, Schneck HJ, Franke C, Kochs E. Ketamine blocks currents through mammalian nicotinic acetylcholine receptor channels by interaction with both the open and the closed state. Anesth Analg. 1996;83:830–836. doi: 10.1097/00000539-199610000-00031. [DOI] [PubMed] [Google Scholar]
- Schnoebel R, Wolff M, Peters SC, Brau ME, Scholz A, Hempelmann G, Olschewski H, Olschewski A. Ketamine impairs excitability in superficial dorsal horn neurones by blocking sodium and voltage-gated potassium currents. Br J Pharmacol. 2005;146:826–833. doi: 10.1038/sj.bjp.0706385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholfield CN. Potentiation of inhibition by general anaesthetics in neurones of the olfactory cortex in vitro. Pflügers Arch. 1980;383:249–255. doi: 10.1007/BF00587527. [DOI] [PubMed] [Google Scholar]
- Schonewille M, Khosrovani S, Winkelman BH, Hoebeek FE, De Jeu MT, Larsen IM, Van der Burg J, Schmolesky MT, Frens MA, De Zeeuw CI. Purkinje cells in awake behaving animals operate at the upstate membrane potential. Nat Neurosci. 2006;9:459–461. doi: 10.1038/nn0406-459. [DOI] [PubMed] [Google Scholar]
- Storustovu SI, Ebert B. Pharmacological characterization of agonists at delta-containing GABAA receptors: functional selectivity for extrasynaptic receptors is dependent on the absence of gamma2. J Pharmacol Exp Ther. 2006;316:1351–1359. doi: 10.1124/jpet.105.092403. [DOI] [PubMed] [Google Scholar]
- Sultan F, Glickstein M. The cerebellum: comparative and animal studies. Cerebellum. 2007;6:168–176. doi: 10.1080/14734220701332486. [DOI] [PubMed] [Google Scholar]
- Thach WT. On the mechanism of cerebellar contributions to cognition. Cerebellum. 2007;6:163–167. doi: 10.1080/14734220701373530. [DOI] [PubMed] [Google Scholar]
- Tia S, Wang JF, Kotchabhakdi N, Vicini S. Developmental changes of inhibitory synaptic currents in cerebellar granule neurons: role of GABAA receptor alpha 6 subunit. J Neurosci. 1996;16:3630–3640. doi: 10.1523/JNEUROSCI.16-11-03630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmann D, Daum I. Cerebellar contributions to cognitive functions: a progress report after two decades of research. Cerebellum. 2007;6:159–162. doi: 10.1080/14734220701496448. [DOI] [PubMed] [Google Scholar]
- Tricklebank MD, Singh L, Oles RJ, Wong EH, Iversen SD. A role for receptors of N-methyl-d-aspartic acid in the discriminative stimulus properties of phencyclidine. Eur J Pharmacol. 1987;141:497–501. doi: 10.1016/0014-2999(87)90573-5. [DOI] [PubMed] [Google Scholar]
- Tyrrell T, Willshaw D. Cerebellar cortex: its simulation and the relevance of Marr's theory. Philos Trans R Soc Lond B Biol Sci. 1992;336:239–257. doi: 10.1098/rstb.1992.0059. [DOI] [PubMed] [Google Scholar]
- Wakasugi M, Hirota K, Roth SH, Ito Y. The effects of general anesthetics on excitatory and inhibitory synaptic transmission in area CA1 of the rat hippocampus in vitro. Anesth Analg. 1999;88:676–680. doi: 10.1097/00000539-199903000-00039. [DOI] [PubMed] [Google Scholar]
- Wall MJ, Usowicz MM. Development of action potential-dependent and independent spontaneous GABAA receptor-mediated currents in granule cells of postnatal rat cerebellum. Eur J Neurosci. 1997;9:533–548. doi: 10.1111/j.1460-9568.1997.tb01630.x. [DOI] [PubMed] [Google Scholar]
- Walters RJ, Hadley SH, Morris KD, Amin J. Benzodiazepines act on GABAA receptors via two distinct and separable mechanisms. Nat Neurosci. 2000;3:1274–1281. doi: 10.1038/81800. [DOI] [PubMed] [Google Scholar]
- Wisden W, Korpi ER, Bahn S. The cerebellum: a model system for studying GABAA receptor diversity. Neuropharmacology. 1996;35:1139–1160. doi: 10.1016/s0028-3908(96)00076-7. [DOI] [PubMed] [Google Scholar]
- Wong EH, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL. The anticonvulsant MK-801 is a potent N-methyl-d-aspartate antagonist. Proc Natl Acad Sci USA. 1986;83:7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakura T, Chavez-Noriega LE, Harris RA. Subunit-dependent inhibition of human neuronal nicotinic acetylcholine receptors and other ligand-gated ion channels by dissociative anesthetics ketamine and dizocilpine. Anesthesiology. 2000;92:1144–1153. doi: 10.1097/00000542-200004000-00033. [DOI] [PubMed] [Google Scholar]