Abstract
The “club drug” 3,4-methylenedioxymethamphetamine (MDMA; also known as ecstasy) binds preferentially to and reverses the activity of the serotonin transporter, causing release of serotonin [5-hydroxytryptamine (5-HT)] stores from nerve terminals. Subsequent activation of postsynaptic 5-HT receptors by released 5-HT has been shown to be critical for the unique psychostimulatory effects of MDMA. In contrast, the effects of direct activation of presynaptic and/or postsynaptic receptors by MDMA have received far less attention, despite the agonist actions of the drug itself at 5-HT2 receptors, in particular the 5-HT2B receptor. Here we show that acute pharmacological inhibition or genetic ablation of the 5-HT2B receptor in mice completely abolishes MDMA-induced hyperlocomotion and 5-HT release in nucleus accumbens and ventral tegmental area. Furthermore, the 5-HT2B receptor dependence of MDMA-stimulated release of endogenous 5-HT from superfused midbrain synaptosomes suggests that 5-HT2B receptors act, unlike any other 5-HT receptor, presynaptically to affect MDMA-stimulated 5-HT release. Thus, our findings reveal a novel regulatory component in the actions of MDMA and represent the first demonstration that 5-HT2B receptors play an important role in the brain, i.e., modulation of 5-HT release. As such, 5-HT2B receptor antagonists may serve as promising therapeutic drugs for MDMA abuse.
Keywords: MDMA, 5-HT2B, serotonin release, microdialysis, behavior, synaptosomes
Introduction
Despite a widespread distribution in the CNS (Choi and Maroteaux, 1996; Schmuck et al., 1996; Duxon et al., 1997; Bonaventure et al., 2002), 5-HT2B receptor function in the brain is mainly unknown. Few studies have attributed a direct effect of the 5-HT2B receptor in the CNS. For example, α-methyl-5-(2-thienylmethoxy)-1H-indole-3-ethanamine (BW723C86), a preferential 5-HT2B agonist, was shown to increase food consumption, reduce grooming, and after microinjection in the medial amygdala to elicit anxiolysis in the social interaction model (Kennett et al., 1998). Evidence indicates that 5-HT2B receptors are also involved in non-rapid eye movement sleep regulation (Popa et al., 2005) and neonatal respiratory rhythms (Gunther et al., 2006). Moreover, a recent study showed that 5-HT2B receptors govern the overall 5-HT transport system by promoting phosphorylation of serotonin transporter (SERT) in primary neurons from raphe nuclei (Launay et al., 2006).
However, no empirical evidence exists in support of a role for either central or peripheral 5-HT2B receptors in psychostimulant-evoked hyperactivity. Several reasons could explain this lack of investigation. Only modest levels of 5-HT2B receptors were commonly detected in brain, and most available pharmacological agents do not discriminate 5-HT2B and 5-HT2C receptors. It remains possible that some of the psychostimulant effects attributed to the 5-HT2C receptor are 5-HT2B receptor dependent (McCreary and Cunningham, 1999).
The mechanism of action of the amphetamine derivative 3,4-methylenedioxymethamphetamine (MDMA; also known as ecstasy) is based on its ability to reverse monoamine reuptake transporters, resulting in monoamine release from nerve terminals, especially 5-HT and dopamine (DA) (Rudnick and Wall, 1992). MDMA is thus classically considered to act as an indirect agonist of several 5-HT and DA receptor subtypes. MDMA binds to SERT with high affinity and inhibits 5-HT uptake more potently than DA uptake (Crespi et al., 1997). Thus, a unique contribution of 5-HT has been proposed to underlie the behavioral responses to MDMA. Indeed, 5-HT reuptake inhibitors such as fluoxetine, as well as genetic ablation of SERT, prevent the effects of MDMA in mice (Bengel et al., 1998; Trigo et al., 2007). However, MDMA and its N-demethylated metabolite 3,4-methylenedioxyamphetamine (MDA) each preferentially bind to and activate human recombinant 5-HT2B receptors at concentrations close to those reported in plasma after a single recreational dose. MDMA and MDA elicit also a prolonged mitogenic responses in human valvular interstitial cells, via direct activation of 5-HT2B receptors (Setola et al., 2003). Thus, the behavioral effect of MDMA may be mediated, in part, through direct action on 5-HT2B receptors or other central and/or peripheral recognition sites, including α2-adrenergic receptors, for which these compounds exhibit relatively high affinity (Battaglia et al., 1988).
To evaluate the role of 5-HT2B receptors in mediating the actions of MDMA, we used two parallel approaches: a pharmacological approach using the most selective 5-HT2B receptor antagonist currently known, 2-amino-4-(4-fluoronaphth-1-yl)-6-isopropylpyrimidine (RS127445) (Bonhaus et al., 1999), and a genetic approach using mice lacking the 5-HT2B receptor (Nebigil et al., 2000). Our results demonstrate that functional 5-HT2B receptors are required for MDMA-induced behavioral effects and 5-HT release in vivo and in vitro.
Materials and Methods
Animals.
5-HT2B−/− mice used in these experiments have a pure 129SV/PAS background. Wild-type (WT) 129SV/PAS mice (8–10 weeks of age) were used as a control group (Charles River Laboratories, L'Arbresle, France). No locomotor differences were seen between males and females, or between WT and 5-HT2B−/− mice, in response to MDMA or saline injection. Groups were composed of 50% male and 50% female for each experiment.
Reagents.
MDMA (Sigma-Aldrich, Saint-Quentin Fallavier, France) was dissolved in 0.9% (w/v) NaCl solution (saline); RS127445 (Bonhaus et al., 1999) was kindly provided by Roche Bioscience (Indianapolis, IN) and slowly dissolved in saline solution. BW723C86 (Sigma-Aldrich) was dissolved in saline solution. 1-[2-(Diphenylmethoxy)ethyl]-4-(3-phenylpropyl)-piperazine (GBR12935) (Sigma-Aldrich) was dissolved in water.
Locomotor activity.
Locomotor activity was measured in a circular corridor with four infrared beams placed at every 90° (Imetronic, Passac, France). Counts were incremented by consecutive interruption of two adjacent beams (i.e., mice moving through one-quarter of the corridor). Mice were injected with a saline solution and individually placed in the activity box for 30 min during 3 d consecutively for habituation before experiments.
Microdialysis.
Anesthetized animals were placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA), and microdialysis probes were implanted in the raphe nuclei, ventral tegmental area (VTA), or nucleus accumbens (NAcc). Dialysis probes were equipped with a Cuprophan membrane (membrane length, 2 mm; diameter, 0.24 mm; cutoff, 5000 Da; CMA Microdialysis, Solna, Sweden). Stereotaxic coordinates (in mm) were as follows: for raphe, anteroposterior (AP) −4.5, lateral (L) −1.0, and dorsoventral (DV) −4.4 with a lateral angle of 20° from bregma and top of the skull; for NAcc, AP +1.2, L +0.6, DV −4.2; for VTA, AP −3.0, L +0.4, DV −4.0 both to bregma and dura surface. Probes were perfused at a constant rate of 5 μl/min with artificial CSF containing 154.1 mm Cl−, 147 mm Na+, 2.7 mm K+, 1 mm Mg2+, and 1.2 mm Ca2+, adjusted to pH 7.4 with 2 mm sodium phosphate buffer. Dialysates were collected every 10 min. All measurements were performed 150 min after the beginning of perfusion, by which time a steady state was achieved. Mice were injected with RS127445 (0.5 mg/kg, i.p.) 1 h before MDMA injection (10 mg/kg). Absolute basal levels of 5-HT and DA in dialysate collected from the raphe were taken as the mean ± SEM of four to five values. At the end of the experiment, all brains were fixed into a formaldehyde solution, and serial coronal slices were made on a microtome. Histological examination of cannula tip placement was subsequently made on 100 μm safranine-stained coronal sections.
Dialysate samples were injected without any purification into an HPLC system that consists of a pump linked to an automatic injector (Agilent 1100; Agilent Technologies, Palo Alto, CA), a reverse-phase column (Zorbax SB C18; 3.5 lm, 150 × 4.6 mm; Agilent Technologies), and a coulometric detector (Coulochem II; ESA, Chelmsford, MA) with a 5011 analytical cell to quantify DA or 5-HT. The first electrode was fixed at −100 mV and the second electrode at +300 mV. The gain of the detector was set at 50 nA. The signal of the second electrode was connected to an HP Chemstation for HPLC. The composition of the mobile phase was 50 mm NaH2PO4, 0.1 mm Na2 EDTA, 0.65 mm octyl sodium sulfate, and 14% (v/v) methanol, pH 3.5. The flow rate was set at 1 ml/min.
Synaptosomes.
Crude synaptosomes were prepared as described previously (Gray and Whittaker, 1962). This preparation was used to measure MDMA-induced release of endogenously stored 5-HT (without [3H]5-HT preloading). 5-HT measurement was performed as for dialysate samples. Crude synaptosomes (0.25 mg of protein) were distributed onto cellulose mixed ester filters (0.65 μm pore size; Millipore, Molsheim, France) in a four-chamber superfusion apparatus held thermostatically at 37°C. The synaptosomes were layered onto the filters by aspiration from the bottom under moderate vacuum. Synaptosomes were preincubated at 37°C for 5 min with Krebs–Henseleit buffer with the following composition (in mm): 125 NaCl, 3 KCl, 1.2 CaCl2, 1.2 MgSO4, 1 NaH2PO4, 22 NaHCO3, and 10 glucose, gassed with 95% O2 and 5% CO2, pH 7.4. Then, MDMA (10 μm) or buffer was added and incubated at 37°C for 30 min followed by rapid filtration under vacuum (GF/B filters; Whatman, Clifton, NJ).
Reverse transcription-PCR.
Isolation of total RNA from raphe nucleus was performed with the RNAsolv reagent (Omega-Biotek, Norcross, GA). Raphe nucleus was dissected from midbrain and homogenized in 2 ml of RNAsolv using a Polytron homogenizer. Genomic DNA was removed by digestion with RNase-free DNase I (Sigma-Aldrich) for 10 min at 25°C. First-strand cDNA was synthesized by reverse transcription of 1 μg of total RNA with Superscript-II reverse transcriptase (Invitrogen, Carlsbad, CA) according to standard protocols. Reverse transcriptase was omitted in some samples as negative control. The sequences of the upstream and downstream oligonucleotides were as follows: 5′-CAGAAGACATGTGATCACCTGATC-3′ and 5′-TGTAATCTTGATGAATGCAGTAGCC-3′ for the mouse 5-HT2B; 5′-TGGCGTGAGGGAGAGCATAGC-3′ and 5′-GATGGTGGGAATGGGTCAGAA-3′ for β-actin; 5′-AGGCAGAGCCTGGACAAATATC-3′ and 5′-GCCCGATTTTCAAAGGCATTGG-3′ for SERT cDNA. PCR amplifications were performed with 1–2 U of TaqDNA polymerase, 1 mm MgSO4, and 1 pg/μl selective primers for 30 cycles (1 min at 94°C, 1 min at 58°C, and 45 s at 72°C). The identity of the PCR products has been verified by direct sequence analysis.
Monoamine uptake and SERT/DA transporter binding in synaptosomes.
Whole-brain synaptosomes from three 5-HT2B−/− and WT mice used in three experiments for [3H]5-HT (124 Ci/mmol; GE Healthcare, Velizy, France) and [3H]DA (34.4 Ci/mmol; PerkinElmer, Courtaboeuf, France) uptake. The 5-HT or DA uptake was measured using six concentrations of [3H]5-HT (6–200 nm) and [3H]DA (7.8–250 nm). Brain synaptosomes were added to tubes containing 120 mm NaCl, 20 mm Tris·HCl, 5 mm KCl, 1.2 mm MgSO4, 2.5 mm CaCl2, 10 mm glucose, 1 mm ascorbic acid, and 0.1 mm pargyline plus [3H]5-HT or [3H]DA, and uptake was allowed to occur for 10 min at 37°. Nonspecific [3H]5-HT uptake was defined as the accumulation in the presence of 100 nm paroxetine and was subtracted from total uptake. Nonspecific [3H]DA uptake was defined as the accumulation in the presence of 10 μm GBR12935 and was subtracted from total uptake. The process was terminated by immersing the tubes in ice-cold buffer followed by rapid filtration through Whatman GF/B filters. Radioactivity was measured using liquid scintillation counting. For saturation binding assay on synaptosomes membrane fraction, [3H]citalopram varied from 0.6 to 20 nm. Nonspecific binding was determined in the presence of 100 nm paroxetine and was subtracted from total binding. For [3H]GBR12935 (40 Ci/mmol; PerkinElmer) binding, whole brain was homogenized with 25 ml of ice-cold buffer containing 50 mm Tris, 120 mm NaCl, and 5 mm MgCl2, pH 7.4. The homogenate was centrifuged for 20 min at 15,000 × g. The pellet was resuspended and centrifuged under the same condition three times. To the final suspension (0.6 mg/ml) was added [3H]GBR12935 (2 nm) and GBR12935 (1 nm to 10 μm). Binding data were analyzed using the iterative nonlinear fitting software GraphPad (San Diego, CA) Prism 4.0 to estimate dissociation constants (KD) and maximum number of sites (Bmax).
Statistics.
Locomotor activity and microdialysis data were analyzed with two-way ANOVA repeated measures over time (mixed model) with drug treatment or genotype as factors and locomotor behavior intervals as the repeated measure. Post hoc analysis was done with Bonferroni test. p < 0.05 was the statistical criterion for null hypothesis rejection in these t test comparisons.
Results
We first examined the effect of the highly selective and potent 5-HT2B receptor antagonist RS127445 (0.5, 0.1, and 0.05 mg/kg) on MDMA (10 mg/kg)-induced locomotion in WT mice (Fig. 1a,b). In agreement with previous studies (Bengel et al., 1998), MDMA induced a marked locomotion in WT mice (Fig. 1a). However, pretreatment with RS127445 completely (0.5 and 0.1 mg/kg) or partially (0.05 mg/kg) blocked MDMA-induced locomotion in WT mice (Fig. 1b). To rule out any nonspecific basal locomotor effect of RS127445, we compared locomotor activity in mice injected with either RS127445 (0.5 mg/kg) or saline solution (see supplemental Fig. 1, available at www.jneurosci.org as supplemental material). RS127445 had no effect on basal locomotor activity. These data demonstrate that pharmacological blockade of the 5-HT2B receptor abolishes MDMA-induced locomotion.
Figure 1.
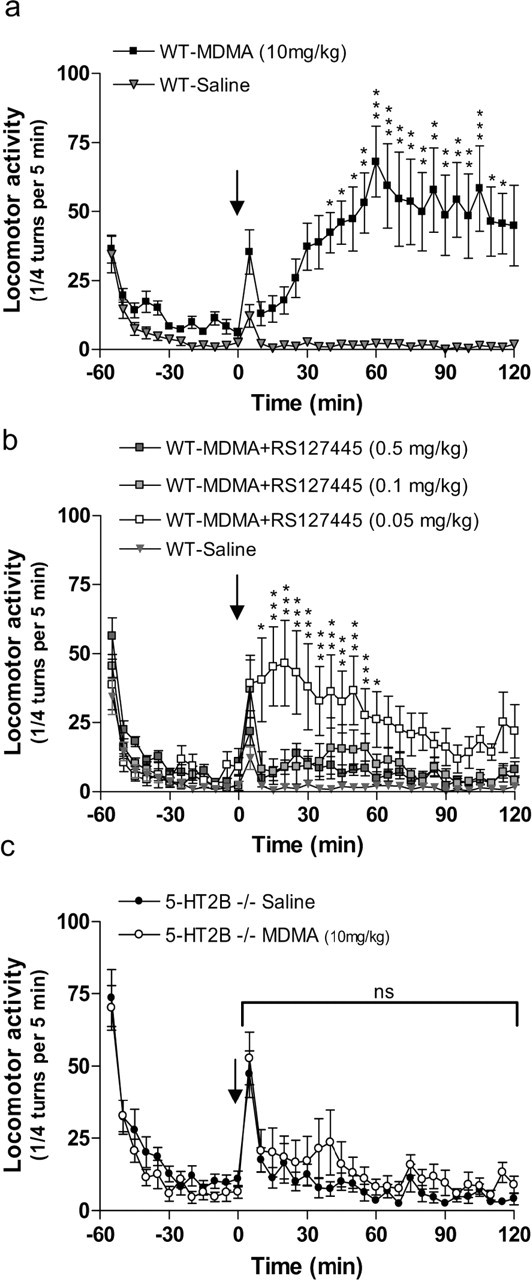
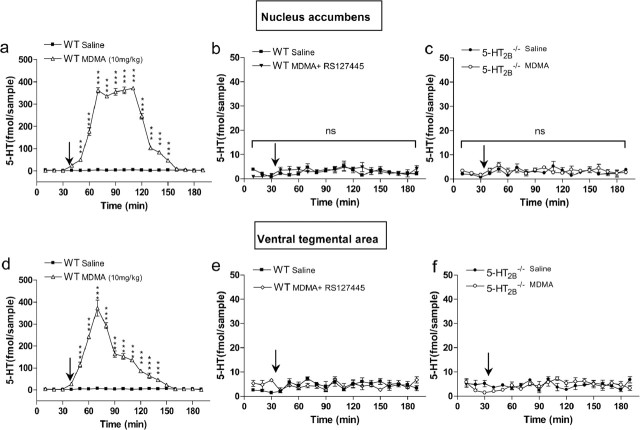
Effect of 5-HT2B receptor inhibition on MDMA-induced hyperlocomotion. a, MDMA-induced locomotion in WT mice. WT mice were injected (i.p.) with either MDMA (10 mg/kg; squares) or saline solution (triangles) (arrow) after 1 h of habituation. Data, between 0 and 120 min, were analyzed using two-way ANOVA (means ± SEM; n = 12 per group): effect of MDMA, F(1,480) = 22.25; p = 0.0002. b, RS127445 abolishes MDMA-induced locomotion in WT mice. WT mice were injected with RS127445 [0.5 mg/kg (dark squares), 0.1 mg/kg (light gray squares), or 0.05 mg/kg (white squares)] solution 1 h before MDMA (10 mg/kg) injection (arrow). Data, between 0 and 120 min, were analyzed using two-way ANOVA (means ± SEM; n = 12 per group): effect of RS127445 compared with saline injection, 0.5 mg/kg, F(1,480) = 10; p = 0.005; 0.1 mg/kg, F(1,480) = 6.7; p = 0.02; 0.005 mg/kg, F(1,480) = 12.5; p = 0.003. c, MDMA-induced locomotion failed in 5-HT2B−/− mice. MDMA (10 mg/kg) or saline solutions were injected after 1 h of habituation (arrow). Effect of MDMA on 5-HT2B−/− mice (n = 12), F(1,528) = 1.31; p = 0.265. A Bonferroni posttest was also applied on each graph. *p < 0.05; **p < 0.01; ***p < 0.001; ns, nonsignificant.
To confirm that MDMA-induced locomotion is 5-HT2B receptor dependent, 5-HT2B−/− mice were injected with either saline or MDMA. Despite an increase in novelty-induced locomotion in 5-HT2B−/− mice compared with WT mice (see supplemental Figs. 2, 3, available at www.jneurosci.org as supplemental material), there was no significant difference in the locomotor activity of saline- or MDMA-treated 5-HT2B−/− mice (F(1,528) = 1.31; p = 0.265) (Fig. 1c). To preclude any interfering behavior that could affect the interpretation of the results, general activity (single beam activation), rearing, and putative MDMA-induced stereotypy were recorded. In particular, analysis of RS127445-treated or 5-HT2B−/− mice did not reveal any significant increase in MDMA-induced stereotypy (data not shown), which could be an explanation for the inhibition of MDMA-induced locomotion in those mice. Together, these results confirm the involvement of 5-HT2B receptors in MDMA-induced hyperlocomotion.
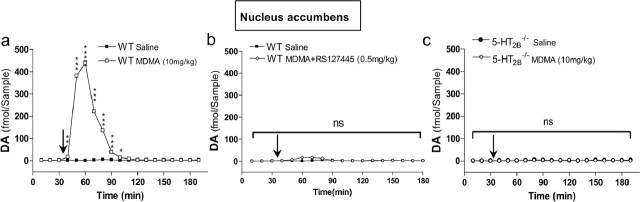
Serotonin and DA extracellular concentrations in the NAcc and VTA are key components of MDMA-induced hyperactivity (Kankaanpaa et al., 1998; Bankson and Yamamoto, 2004). We thus investigated the effect of genetic ablation or pharmacological inhibition of 5-HT2B receptors on the release of these monoamines by in vivo microdialysis in awake mice. Administration of MDMA increased extracellular 5-HT concentration in WT mice ∼80-fold within 70 min in both the NAcc (Fig. 2a) and the VTA (Fig. 2d). In 5-HT2B−/− mice as well as in mice pretreated with RS127445, MDMA caused no increase in extracellular 5-HT concentration in the NAcc (Fig. 2b,c) and the VTA (Fig. 2e,f). In WT mice, MDMA caused an 80-fold increase in extracellular DA levels in the NAcc within 50 min (Fig. 3a). However, in RS127445-treated (Fig. 3b) or 5-HT2B−/− mice (Fig. 3c), MDMA caused no increase in extracellular DA concentration in the NAcc. Thus, our data demonstrate that functional 5-HT2B receptors are necessary for MDMA-evoked 5-HT and DA release in the NAcc and VTA.
Figure 2.
Effect of 5-HT2B receptor inhibition on MDMA-induced 5-HT release as measured by in vivo microdialysis. a, Effect of MDMA (10 mg/kg; triangles) or saline injection (squares) on 5-HT concentrations in dialysates from NAcc of WT mice. b, c, Effect of RS127445 (5-HT2B receptor antagonist; b) or 5-HT2B receptor genetic ablation (c) on MDMA-induced 5-HT release in NAcc. d, Effect of saline (squares) or MDMA (triangles) in WT mice on 5-HT level in the VTA. e, f, Effect of MDMA in RS127445-treated mice (diamonds; e) or in 5-HT2B−/− mice (open circles; f) on 5-HT level in the VTA. MDMA or saline solutions were injected 35 min after testing began (arrow). Data (means ± SEM; n = 5 per group) were analyzed using two-way ANOVA: effect of MDMA on 5-HT level in the NAcc WT mice, F(1,144) = 6822.46; p < 0.0001; effect of MDMA on 5-HT level in 5-HT2B−/− NAcc or VTA mice, ns; effect of MDMA on 5-HT level in RS127445-treated mice NAcc or VTA mice, ns; effect of MDMA on 5-HT level in the VTA, F(18,162) = 110.29; p < 0.0001. A Bonferroni posttest was also applied on each graph. ***p < 0.001; ns: nonsignificant.
Figure 3.
Effect of 5-HT2B receptor inhibition on MDMA-induced DA release as measured by in vivo microdialysis in NAcc. a, Effect of MDMA (10 mg/kg; open squares) or saline injection (filled squares) on DA concentrations in dialysates from NAcc of WT mice. b, Effect of RS127445 (0.5 mg/kg; diamonds) on MDMA-induced DA release in NAcc. c, Effect of 5-HT2B receptor genetic ablation (open circles) on MDMA-induced DA release in NAcc. MDMA or saline solutions were injected 35 min after testing began (arrow). Data (means ± SEM; n = 5 per group) were analyzed using two-way ANOVA: effect of MDMA on DA level in the NAcc WT mice, F(1,144) = 9162.79; p < 0.0001; effect of MDMA on DA level in RS127445-treated mice, ns; effect of MDMA on DA level in NAcc 5-HT2B−/− mice, ns. A Bonferroni posttest was also applied on each graph. *p < 0.05; **p < 0.01; ***p < 0.001; ns, nonsignificant.
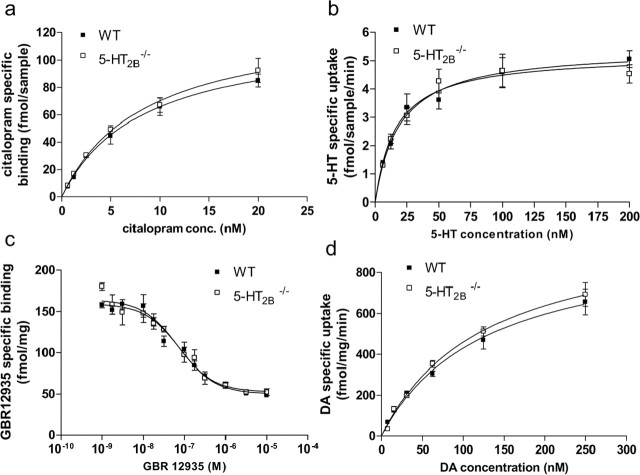
To confirm that SERT expression in 5-HT2B−/− and WT mice is comparable, radioligand saturation binding assays with [3H]citalopram were performed on synaptosomal membranes prepared from whole brain (Fig. 4a). Neither Bmax (WT: 120.2 ± 10.6 fmol/sample; 5-HT2B−/−: 129.8 ± 11.8) nor KD (WT: 8.2 ± 1.6 nm; 5-HT2B−/−: 8.4 ± 1.6) was altered in 5-HT2B−/− mice compared with wild-type mice. SERT function was assessed in wild-type and 5-HT2B−/− mice by measuring [3H]5-HT uptake by synaptosomes prepared from whole brain (Fig. 4b). The resulting saturation isotherms revealed no differences in either Vmax (WT: 5.22 ± 0.3; 5-HT2B−/−: 5.44 ± 0.3 fmol/sample/min) or Km (WT: 16.29 ± 3.8; 5-HT2B−/−: 19.13 ± 4.2 fmol/sample), demonstrating that SERT uptake activity is not altered in 5-HT2B−/− mice compared with wild-type mice. Similarly, DA transporter (DAT) expression (Fig. 4c) and function (Fig. 4d) were compared in WT and 5-HT2B−/− mice. Radioligand binding assays with [3H]GBR12935 (selective ligand for DAT) were performed on membranes prepared from whole brain (Fig. 4c). Neither Bmax (WT: 11688 ± 1459 fmol/mg; 5-HT2B−/−: 10,484 ± 1787) nor KD (WT: 7.12 ± 0.08 nm; 5-HT2B−/−: 7.17 ± 0.11) was altered in 5-HT2B−/− mice compared with wild-type mice. DAT function was assessed in wild-type and 5-HT2B−/− mice by measuring [3H]DA uptake by synaptosomes prepared from whole brain (Fig. 4d). The resulting saturation isotherms revealed no differences in either Vmax (WT: 972 ± 101; 5-HT2B−/−: 1043 ± 86 fmol/mg/min) or Km (WT: 126 ± 27; 5-HT2B−/−: 127 ± 21 fmol/mg), demonstrating that DAT uptake activity is not altered in 5-HT2B−/− mice compared with wild-type mice. Together these data rule out the possibility that the observed effects on MDMA-induced behavior and 5-HT/DA release in 5-HT2B−/− mice could be caused by decreased SERT/DAT expression or uptake activity.
Figure 4.
SERT and DAT binding site and uptake analysis. a, SERT expression in 5-HT2B−/− and WT mice using radioligand saturation binding assays with [3H]citalopram on synaptosome membranes prepared from whole brain. [3H]citalopram binding analysis did not reveal differences in the Bmax. b, [3H] 5-HT uptake in 5-HT2B−/− and WT mice synaptosomal preparation from whole brain. Saturation isotherms of [3H]5-HT uptake were similar for the WT and 5-HT2B−/− mice, and nonlinear regression analysis did not reveal differences in the Vmax. c, Radioligand binding assays with [3H]GBR12935 (selective ligand for DAT) on membranes prepared from whole brain. Neither Bmax nor KD was altered in 5-HT2B−/− mice compared with wild-type mice. d, DAT function was assessed in wild-type and 5-HT2B−/− mice by measuring [3H]DA uptake in synaptosomes prepared from whole brain. The resulting saturation isotherms revealed no differences in either Vmax or Km, demonstrating that DAT uptake function is not altered in 5-HT2B−/− mice compared with wild-type mice.
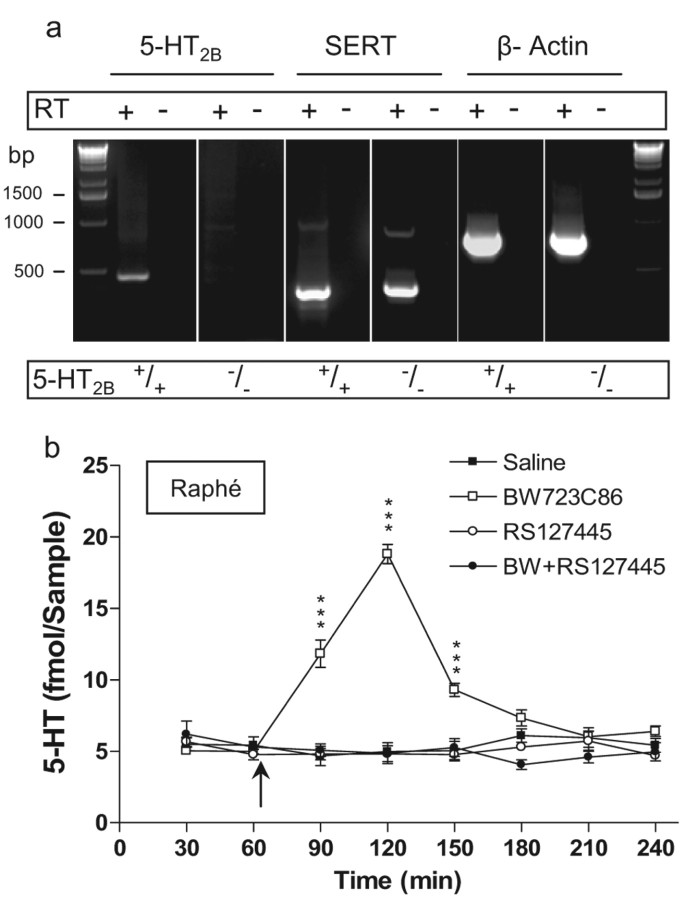
A previous study showed that 5-HT2B receptor mRNA and protein are coexpressed in SERT-expressing primary neurons from mice raphe nuclei (Launay et al., 2006). Given the normal expression and uptake activity of SERT in 5-HT2B−/− mice, we hypothesized that the 5-HT2B receptor acts presynaptically in raphe neurons to permit MDMA-induced SERT-dependant 5-HT release. Using reverse transcription (RT)-PCR, we first confirmed that 5-HT2B receptor mRNA is expressed in mouse raphe nucleus, as previously observed in rats by DNA microarray and in situ hybridization (Bonaventure et al., 2002). As shown in Figure 5a, samples of raphe nucleus from WT mice expressed both 5-HT2B receptor and SERT mRNA. In contrast, samples from 5-HT2B−/− mice expressed only SERT mRNA, confirming the expression of the 5-HT2B receptor mRNA in raphe nucleus of WT mice. To evaluate the expression of functional 5-HT2B receptor proteins in the raphe nucleus, we next studied the effect of BW723C86, a preferential 5-HT2B receptor agonist, on 5-HT extracellular concentration by in vivo microdialysis (Fig. 5b). Local infusion of BW723C86 (10 nmol) through the microdialysis probe produced a fourfold increase in 5-HT concentration. Pretreatment with RS127445 (100 nm) completely blocked the BW723C86-induced increase in 5-HT, whereas RS127445 alone had no effect on basal 5-HT concentration. Thus, we confirmed in vivo the expression of 5-HT2B receptors in the murine raphe nucleus and demonstrated a functional coupling of the receptor to extracellular 5-HT levels.
Figure 5.
5-HT2B receptor mRNA and protein expression in raphe nucleus. a, Total RNA were isolated from 5-HT2B+/+ or 5-HT2B−/− mice raphe nucleus. Putative contaminating genomic DNA was removed by digestion with RNase-free DNase. RT was omitted in samples indicated RT(−) as a control. On these samples, we used β-actin mRNA amplification, as positive control. We found 5-HT2B receptor mRNA only in WT mice raphe nucleus (first panel). b, BW723C86 (preferential 5-HT2B receptor agonist) injection (arrow) through microdialysis probe (10 nmol) in the raphe nucleus produced an increase in 5-HT extracellular concentration (open squares). Pretreatment with RS127445 (100 nm; selective 5-HT2B receptor antagonist) completely blocked BW723C86-induced 5-HT increase (filled circles), whereas RS127445 alone (open circles) had no effect on basal 5-HT concentration (filled squares). Data (means ± SEM; n = 5 per group) were analyzed using two-way ANOVA (repeated measures); each drug's effects were compared with saline. Effect of BW723C86 on 5-HT level, F(3,70) = 52.31; p < 0.0001. A Bonferroni posttest was also applied. ***p < 0.001.
Using a superfused mouse midbrain synaptosome preparation, we next sought to assess whether the MDMA-induced SERT-dependent 5-HT release in vivo (i.e., microdialysis studies) was similarly 5-HT2B receptor dependent in vitro in serotoninergic nerve ending. As shown in Figure 6, MDMA (10 μm) caused a fivefold greater synaptosomal 5-HT release than saline in WT synaptosomes. In contrast, MDMA did not increase 5-HT release over baseline levels from 5-HT2B−/− synaptosomes. Notably, basal synaptosomal 5-HT release was similar for WT and 5-HT2B−/− synaptosomes. Thus, our data show that activation of 5-HT2B receptors in serotoninergic nerve ending particles is required for MDMA-induced SERT-dependent 5-HT release.
Figure 6.
MDMA-induced 5-HT release in vitro is 5-HT2B receptor dependent. MDMA (10 μm) induced 5-HT release from a superfused midbrain synaptosome preparation of WT mice (75 ± 5.7 fmol/sample), whereas it had no effect in 5-HT2B−/− mice (15 ± 1.8 fmol/sample). Data (means ± SEM) were analyzed using unpaired t test (two tailed): t = 9.9; df, 6. ***p < 0.001; ns, nonsignificant.
Discussion
Here we show that the most selective 5-HT2B receptor antagonist currently known, RS127445 (0.5 and 0.1 mg/kg), potently and completely reverses the hyperactivity caused by a “conventional” dose (10 mg/kg) of MDMA in mice. RS-127445 was found to have subnanomolar affinity for the 5-HT2B receptor (pKi = 9.5 ± 0.1) and at least 1000-fold selectivity for this receptor compared with numerous other receptors and monoamine uptake sites (Bonhaus et al., 1999). Some of these receptors are of special interest in MDMA-induced locomotion, especially 5-HT2A, 5-HT2C, and 5-HT1B receptors, but also 5-HT and DA transporters (Bankson and Cunningham, 2001). Thus, the results obtained at such a low dose of RS127445 are unlikely caused by an interaction of this compound with other receptors or transporters involved in MDMA-induced locomotion. Rather, the effect can safely be attributed to the acute pharmacological inhibition of the 5-HT2B receptor, because genetic deletion of the 5-HT2B receptor in 5-HT2B−/− mice gives rise to an identical phenotype vis-à-vis MDMA-induced hyperactivity.
Previous studies showed that systemic injection of MDMA increased extracellular levels of 5-HT in the NAcc and VTA, as measured by microdialysis (Gudelsky and Nash, 1996; Crespi et al., 1997; Kankaanpaa et al., 1998). The psychomotor stimulant effect of MDMA is considered subsequent to an extracellular increase of 5-HT in these nuclei, as demonstrated by its elimination by 5-HT-specific reuptake inhibitors (SSRIs) or in SERT−/− mice (Bengel et al., 1998; Bankson and Cunningham, 2001; Trigo et al., 2007). Here we show that blockade of the 5-HT2B receptor also completely prevents the MDMA-induced increase of 5-HT in the NAcc and VTA. Moreover, using RT-PCR and in vivo microdialysis, we show that mRNA and functional 5-HT2B receptor protein are expressed in the mouse raphe nucleus. Because the NAcc and VTA ascending serotonergic projections arise from the dorsal raphe nuclei and given that raphe neurons are the principal source of 5-HT release in the brain, this result supports the notion that the 5-HT2B receptor acts presynaptically in serotoninergic raphe neurons to permit MDMA-induced SERT-dependent 5-HT release. However, 5-HT release from serotonergic neurons is controlled through complex and multiple neural inputs to 5-HT cell bodies and nerve terminals (Adell et al., 2002; Sharp et al., 2007). Thus, indirect regulation of MDMA-induced 5-HT release, by other afferent inputs expressing 5-HT2B receptor, could also explain the lack of 5-HT release seen in vivo in 5-HT2B−/− or RS127445-treated mice. Nevertheless, that MDMA-stimulated endogenous 5-HT release from superfused midbrain synaptosome preparation is similarly 5-HT2B receptor dependent strongly argues against any indirect influence of nerve inputs and reinforces the hypothesis that 5-HT2B receptor acts presynaptically in serotoninergic raphe neurons to permit MDMA-induced 5-HT release. To our knowledge, a contribution of presynaptic 5-HT autoreceptors in MDMA-induced behavioral effect or 5-HT release, such as the one revealed here for 5-HT2B receptors, has not yet been described. Along these lines, with respect to the 5-HT1B and 5-HT1A autoreceptors, 5-HT1B receptors have been shown to mediate their effect postsynaptically vis-à-vis MDMA-induced hyperlocomotion (Bankson and Cunningham, 2001), whereas the 5-HT1A receptor had no effect (Muller et al., 2007).
That MDMA-evoked 5-HT release is completely abolished in 5-HT2B−/− mice and in RS127445-treated WT mice suggests that MDMA acting directly on SERT is not sufficient to induce 5-HT release. Also, MDMA exhibits moderate agonist potency (100 nm) and relative efficacy (50%) at 5-HT2B receptors (Setola et al., 2003). Thus, it is conceivable that activation of 5-HT2B receptors by MDMA somehow renders SERT capable of MDMA-induced 5-HT release. Alternatively, simple coexpression of 5-HT2B receptors and SERT in 5-HT-releasing terminals may suffice for the former protein to render the latter molecule able to release 5-HT. Along these lines, recent in vitro and ex vivo findings indicate that monoamine transporters are highly regulated at the cellular and molecular levels (Torres et al., 2003). SERT reuptake activity is controlled by several intracellular signaling pathways (Blakely et al., 1998), including histamine and adenosine A3 receptor-associated PKG signaling pathways (Launay et al., 1994; Miller and Hoffman, 1994; Zhu et al., 2007). Control of SERT by 5-HT2B receptors has been recently detailed: in embryonic raphe neurons, the 5-HT2B receptor coupling to PKG-dependent NO production ensures SERT phosphorylation to basal level (Launay et al., 2006). Such a basal level of SERT phosphorylation, associated with a maximal transport capacity in cell line, is likely to result from the 5-HT2B receptor intrinsic activity (Manivet et al., 2000). Indeed, PKG-mediated control of 5-HT uptake by SERT was recently confirmed independently (Ramamoorthy et al., 2007). In the presence of 5-HT, however, the 5-HT2B receptor–PKC coupling promotes additional phosphorylations of both SERT and Na+,K+-ATPase α-subunit, impairing the electrochemical gradient necessary for 5-HT uptake (Launay et al., 2006). However, the control of SERT-induced MDMA or amphetamine analog-dependent 5-HT release has received far less attention. Acute in vivo stimulation of 5-HT2B receptors by norfenfluramine (the main metabolite of the anorexigen fenfluramine, a 5-HT releaser and 5-HT2B receptor agonist) or BW723C86 triggers a transient SERT-dependent increase in plasma 5-HT that is blocked by RS127445 or genetic ablation. This increase in plasma 5-HT concentration is induced by SERT-mediated 5-HT release instead of uptake inhibition, because paroxetine (an SSRI) had no effect on basal 5-HT plasma concentration (Callebert et al., 2006). An in vivo monoamine transporter regulation by phosphorylation has been proposed for amphetamine-triggered DA efflux through DAT (Fog et al., 2006). Amphetamine stimulates PKC activity that is associated with the outward transport of DA (Mortensen and Amara, 2003), and phosphorylation of serines in the N terminus of the human DAT is essential for amphetamine-induced, DAT-mediated DA efflux with no alterations of DAT expression or uptake activity (Khoshbouei et al., 2004). Our findings support the hypothesis that 5-HT2B receptor genetic ablation or pharmacological inhibition impairs also SERT release activity in such a way that MDMA-induced 5-HT release is abolished without alterations of SERT expression or uptake activity.
When access to SERT is blocked by fluoxetine (an SSRI), the MDMA-evoked DA efflux in the striatum is reduced. Moreover, tetrodotoxin infusion in the striatum reduces the extracellular concentration of DA evoked by MDMA (Gudelsky and Nash, 1996). In other words, MDMA-induced DA release involves stimulation of postsynaptic 5-HT receptors after SERT-dependent 5-HT release (Gudelsky and Nash, 1996; Bankson and Cunningham, 2001). These data are supportive of a stimulatory role of 5-HT in the regulation of dopamine release. Moreover, DAT expression and function are not altered in 5-HT2B−/− mice. Thus, inhibition of DA release in NAcc seen in 5-HT2B−/− and in RS127445-treated mice after MDMA administration (Fig. 3b,c) may be attributed to the absence of 5-HT release in VTA and/or NAcc, where postsynaptic 5-HT2A (Bankson and Yamamoto, 2004) and 5-HT3 (De Deurwaerdere et al., 1998) receptors have stimulatory effect, respectively. Whether non-5-HT-selective releasers such as d-amphetamine may also be affected by 5-HT2B receptor activity is currently under investigation in our laboratory. Additional studies are also underway to ascertain the direct or indirect role of 5-HT2B receptor in the action of MDMA on DA release.
There are currently no pharmacological treatments for the wide range of symptoms associated with MDMA abuse (Morton, 2005). DA increase in the NAcc plays a critical role in reward and drug dependence and is a common response generated by all drugs of abuse, including MDMA (Nestler, 2005). The absence of DA release indicates that mice lacking functional 5-HT2B receptor should not exhibit conditioned place preference or self administration with a “conventional” dose of MDMA, i.e., 10 mg/kg. The identification of single nucleotide polymorphisms in the 5-HT2B receptor gene among drug abusers supports the notion that this receptor could be involved in drug-reinforcing mechanisms in humans (Lin et al., 2004). Moreover, although still a matter of debate, repeated administration of MDMA to animals produces 5-HT and DA release-dependent neurotoxicity. Unlike SSRIs (fluoxetine, paroxetine, or citalopram), RS127445 has no effect on basal 5-HT extracellular concentration, suggesting that 5-HT2B receptor antagonists could serve as a promising therapeutic drug for the prevention of the acute and long-term effects associated with MDMA abuse.
Footnotes
This work has been supported by the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Université Pierre et Marie Curie, and grants from the Fondation de France, the Fondation pour la Recherche Médicale, the Association pour la Recherche contre le Cancer, the French Ministry of Research (Agence Nationale pour la Recherche), and the European community. L.M.'s team is an “Equipe FRM.” We thank A. Sturny and K. Boutourlinsky for excellent technical assistance.
References
- Adell A, Celada P, Abellan MT, Artigas F. Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res Brain Res Rev. 2002;39:154–180. doi: 10.1016/s0165-0173(02)00182-0. [DOI] [PubMed] [Google Scholar]
- Bankson MG, Cunningham KA. 3,4-Methylenedioxymethamphetamine (MDMA) as a unique model of serotonin receptor function and serotonin-dopamine interactions. J Pharmacol Exp Ther. 2001;297:846–852. [PubMed] [Google Scholar]
- Bankson MG, Yamamoto BK. Serotonin-GABA interactions modulate MDMA-induced mesolimbic dopamine release. J Neurochem. 2004;91:852–859. doi: 10.1111/j.1471-4159.2004.02763.x. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Brooks BP, Kulsakdinun C, De Souza EB. Pharmacologic profile of MDMA (3,4-methylenedioxymethamphetamine) at various brain recognition sites. Eur J Pharmacol. 1988;149:159–163. doi: 10.1016/0014-2999(88)90056-8. [DOI] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Ramamoorthy S, Schroeter S, Qian Y, Apparsundaram S, Galli A, DeFelice LJ. Regulated phosphorylation and trafficking of antidepressant-sensitive serotonin transporter proteins. Biol Psychiatry. 1998;44:169–178. doi: 10.1016/s0006-3223(98)00124-3. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Guo H, Tian B, Liu X, Bittner A, Roland B, Salunga R, Ma XJ, Kamme F, Meurers B, Bakker M, Jurzak M, Leysen JE, Erlander MG. Nuclei and subnuclei gene expression profiling in mammalian brain. Brain Res. 2002;943:38–47. doi: 10.1016/s0006-8993(02)02504-0. [DOI] [PubMed] [Google Scholar]
- Bonhaus DW, Flippin LA, Greenhouse RJ, Jaime S, Rocha C, Dawson M, Van Natta K, Chang LK, Pulido-Rios T, Webber A, Leung E, Eglen RM, Martin GR. RS-127445: a selective, high affinity, orally bioavailable 5-HT2B receptor antagonist. Br J Pharmacol. 1999;127:1075–1082. doi: 10.1038/sj.bjp.0702632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callebert J, Esteve JM, Herve P, Peoc'h K, Tournois C, Drouet L, Launay JM, Maroteaux L. Evidence for a control of plasma serotonin levels by 5-hydroxytryptamine(2B) receptors in mice. J Pharmacol Exp Ther. 2006;317:724–731. doi: 10.1124/jpet.105.098269. [DOI] [PubMed] [Google Scholar]
- Choi DS, Maroteaux L. Immunohistochemical localisation of the serotonin 5-HT2B receptor in mouse gut, cardiovascular system, and brain. FEBS Lett. 1996;391:45–51. doi: 10.1016/0014-5793(96)00695-3. [DOI] [PubMed] [Google Scholar]
- Crespi D, Mennini T, Gobbi M. Carrier-dependent and Ca(2+)-dependent 5-HT and dopamine release induced by (+)-amphetamine, 3,4-methylendioxymethamphetamine, p-chloroamphetamine and (+)-fenfluramine. Br J Pharmacol. 1997;121:1735–1743. doi: 10.1038/sj.bjp.0701325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deurwaerdere P, Stinus L, Spampinato U. Opposite change of in vivo dopamine release in the rat nucleus accumbens and striatum that follows electrical stimulation of dorsal raphe nucleus: role of 5-HT3 receptors. J Neurosci. 1998;18:6528–6538. doi: 10.1523/JNEUROSCI.18-16-06528.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duxon MS, Flanigan TP, Reavley AC, Baxter GS, Blackburn TP, Fone KC. Evidence for expression of the 5-hydroxytryptamine-2B receptor protein in the rat central nervous system. Neuroscience. 1997;76:323–329. doi: 10.1016/s0306-4522(96)00480-0. [DOI] [PubMed] [Google Scholar]
- Fog JU, Khoshbouei H, Holy M, Owens WA, Vaegter CB, Sen N, Nikandrova Y, Bowton E, McMahon DG, Colbran RJ, Daws LC, Sitte HH, Javitch JA, Galli A, Gether U. Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron. 2006;51:417–429. doi: 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Gray EG, Whittaker VP. The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J Anat. 1962;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- Gudelsky GA, Nash JF. Carrier-mediated release of serotonin by 3,4-methylenedioxymethamphetamine: implications for serotonin-dopamine interactions. J Neurochem. 1996;66:243–249. doi: 10.1046/j.1471-4159.1996.66010243.x. [DOI] [PubMed] [Google Scholar]
- Gunther S, Maroteaux L, Schwarzacher SW. Endogenous 5-HT2B receptor activation regulates neonatal respiratory activity in vitro. J Neurobiol. 2006;66:949–961. doi: 10.1002/neu.20253. [DOI] [PubMed] [Google Scholar]
- Kankaanpaa A, Meririnne E, Lillsunde P, Seppala T. The acute effects of amphetamine derivatives on extracellular serotonin and dopamine levels in rat nucleus accumbens. Pharmacol Biochem Behav. 1998;59:1003–1009. doi: 10.1016/s0091-3057(97)00527-3. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Trail B, Bright F. Anxiolytic-like actions of BW 723C86 in the rat Vogel conflict test are 5-HT2B receptor mediated. Neuropharmacology. 1998;37:1603–1610. doi: 10.1016/s0028-3908(98)00115-4. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, Galli A, Javitch JA. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launay JM, Bondoux D, Oset-Gasque MJ, Emami S, Mutel V, Haimart M, Gespach C. Increase of human platelet serotonin uptake by atypical histamine receptors. Am J Physiol. 1994;266:R526–R536. doi: 10.1152/ajpregu.1994.266.2.R526. [DOI] [PubMed] [Google Scholar]
- Launay JM, Schneider B, Loric S, Da Prada M, Kellermann O. Serotonin transport and serotonin transporter-mediated antidepressant recognition are controlled by 5-HT2B receptor signaling in serotonergic neuronal cells. FASEB J. 2006;20:1843–1854. doi: 10.1096/fj.06-5724com. [DOI] [PubMed] [Google Scholar]
- Lin Z, Walther D, Yu XY, Drgon T, Uhl GR. The human serotonin receptor 2B: coding region polymorphisms and association with vulnerability to illegal drug abuse. Pharmacogenetics. 2004;14:805–811. doi: 10.1097/00008571-200412000-00003. [DOI] [PubMed] [Google Scholar]
- Manivet P, Mouillet-Richard S, Callebert J, Nebigil CG, Maroteaux L, Hosoda S, Kellermann O, Launay JM. PDZ-dependent activation of nitric-oxide synthases by the serotonin 2B receptor. J Biol Chem. 2000;275:9324–9331. doi: 10.1074/jbc.275.13.9324. [DOI] [PubMed] [Google Scholar]
- McCreary AC, Cunningham KA. Effects of the 5-HT2C/2B antagonist SB 206553 on hyperactivity induced by cocaine. Neuropsychopharmacology. 1999;20:556–564. doi: 10.1016/S0893-133X(98)00087-6. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Hoffman BJ. Adenosine A3 receptors regulate serotonin transport via nitric oxide and cGMP. J Biol Chem. 1994;269:27351–27356. [PubMed] [Google Scholar]
- Mortensen OV, Amara SG. Dynamic regulation of the dopamine transporter. Eur J Pharmacol. 2003;479:159–170. doi: 10.1016/j.ejphar.2003.08.066. [DOI] [PubMed] [Google Scholar]
- Morton J. Ecstasy: pharmacology and neurotoxicity. Curr Opin Pharmacol. 2005;5:79–86. doi: 10.1016/j.coph.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Muller CP, Carey RJ, Huston JP, Souza Silva MA. Serotonin and psychostimulant addiction: focus on 5-HT1A-receptors. Prog Neurobiol. 2007;81:133–178. doi: 10.1016/j.pneurobio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Nebigil CG, Choi DS, Dierich A, Hickel P, Le Meur M, Messaddeq N, Launay JM, Maroteaux L. Serotonin 2B receptor is required for heart development. Proc Natl Acad Sci USA. 2000;97:9508–9513. doi: 10.1073/pnas.97.17.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Popa D, Lena C, Fabre V, Prenat C, Gingrich J, Escourrou P, Hamon M, Adrien J. Contribution of 5-HT2 receptor subtypes to sleep-wakefulness and respiratory control, and functional adaptations in knock-out mice lacking 5-HT2A receptors. J Neurosci. 2005;25:11231–11238. doi: 10.1523/JNEUROSCI.1724-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, Samuvel DJ, Buck ER, Rudnick G, Jayanthi LD. Phosphorylation of threonine residue 276 is required for acute regulation of serotonin transporter by cyclic GMP. J Biol Chem. 2007;282:11639–11647. doi: 10.1074/jbc.M611353200. [DOI] [PubMed] [Google Scholar]
- Rudnick G, Wall SC. The molecular mechanism of “ecstasy” [3,4-methylenedioxy-methamphetamine (MDMA)]: serotonin transporters are targets for MDMA-induced serotonin release. Proc Natl Acad Sci USA. 1992;89:1817–1821. doi: 10.1073/pnas.89.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuck K, Ullmer C, Kalkman HO, Probst A, Lubbert H. Activation of meningeal 5-HT2B receptors: an early step in the generation of migraine headache? Eur J Neurosci. 1996;8:959–967. doi: 10.1111/j.1460-9568.1996.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Setola V, Hufeisen SJ, Grande-Allen KJ, Vesely I, Glennon RA, Blough B, Rothman RB, Roth BL. 3,4-Methylenedioxymethamphetamine (MDMA, “ecstasy”) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol Pharmacol. 2003;63:1223–1229. doi: 10.1124/mol.63.6.1223. [DOI] [PubMed] [Google Scholar]
- Sharp T, Boothman L, Raley J, Queree P. Important messages in the “post”: recent discoveries in 5-HT neurone feedback control. Trends Pharmacol Sci. 2007;28:629–636. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- Trigo JM, Renoir T, Lanfumey L, Hamon M, Lesch KP, Robledo P, Maldonado R. 3,4-methylenedioxymethamphetamine self-administration is abolished in serotonin transporter knockout mice. Biol Psychiatry. 2007;62:669–679. doi: 10.1016/j.biopsych.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Steiner JA, Munn JL, Daws LC, Hewlett WA, Blakely RD. Rapid stimulation of presynaptic serotonin transport by a3 adenosine receptors. J Pharmacol Exp Ther. 2007;322:332–340. doi: 10.1124/jpet.107.121665. [DOI] [PubMed] [Google Scholar]