Abstract
Microglia in the spinal cord may play an important role in the development and maintenance of neuropathic pain. A metabotropic ATP receptor, P2Y12, has been shown to be expressed in spinal microglia constitutively and be involved in chemotaxis. Activation of p38 mitogen-activated protein kinase (MAPK) occurs in spinal microglia after nerve injury and may be related to the production of cytokines and other mediators, resulting in neuropathic pain. However, it remains unknown whether any type of P2Y receptor in microglia is involved in the activation of p38 MAPK and the pain behaviors after nerve injury.
Using the partial sciatic nerve ligation (PSNL) model in the rat, we found that P2Y12 mRNA and protein increased in the spinal cord and peaked at 3 d after PSNL. Double labeling studies revealed that cells expressing increased P2Y12 mRNA and protein after nerve injury were exclusively microglia. Both pharmacological blockades by intrathecal administration of P2Y12 antagonist and antisense knockdown of P2Y12 expression suppressed the development of pain behaviors and the phosphorylation of p38 MAPK in spinal microglia after PSNL. The intrathecal infusion of the P2Y12 agonist 2-(methythio) adenosine 5′-diphosphate trisodium salt into naive rats mimicked the nerve injury-induced activation of p38 in microglia and elevated pain behaviors.
These data suggest a new mechanism of neuropathic pain, in which the increased P2Y12 works as a gateway of the following events in microglia after nerve injury. Activation of this receptor by released ATP or the hydrolyzed products activate p38 MAPK pathway and may play a crucial role in the generation of neuropathic pain.
Keywords: P2Y12, ATP, ADP, microglia, p38 MAPK, neuropathic pain, spinal cord
Introduction
Injury to the peripheral nerve is known to induce the variety of changes in intracellular signaling molecules and gene expression in the spinal cord (Woolf and Salter, 2000; Scholz and Woolf, 2002; Obata and Noguchi, 2004; Zhuang et al., 2006). Recent evidence shows that glial cells in the spinal cord have important roles in the mechanisms of neuropathic pain (Marchand et al., 2005; Tsuda et al., 2005). The glial activation in the spinal cord after peripheral nerve injury is considered to trigger the production and release of proinflammatory cytokines, such as interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and neurotrophins (DeLeo and Yezierski, 2001; Watkins et al., 2001a,b; Watkins and Maier, 2003), that then may affect and augment the nociceptive signals in the spinal cord.
Two fundamentally distinct classes of receptors for ATP exist: the P2X ionotropic ligand-gated ion channel receptors (P2X1–P2X7) and the P2Y metabotropic G-protein-coupled receptors (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14). These receptors subtypes have specific distributions in vivo and different binding properties against ATP and its metabolites such as ADP. The functional significance of P2X receptors in the sensory nervous system has been widely investigated (for review, see Khakh and North, 2006). Findings about two ionotropic P2X receptors in glial cells have collected much interest. P2X4 in microglia was upregulated in spinal microglia, and blocking P2X4 resulted in decreased neuropathic pain (Tsuda et al., 2003). Moreover, P2X7 deletion or antagonism also produced significant inhibition of neuropathic pain behaviors (Chessell et al., 2005; McGaraughty et al., 2007).
One of most important questions about neuro-glial communication is that how glial cells in the spinal cord are activated, in other words, what signal is transmitted from neuron to glial cells after peripheral nerve injury. Because ATP is one of the most abundant neurotransmitters in the sensory nervous system, ATP and its metabolites might be released from several sources after peripheral nerve injury, such as central terminals of primary afferent, postsynaptic dorsal horn neurons, and astrocytes (for review, see Hansson and Ronnback, 2003; Burnstock, 2007).
There is increasing evidence that metabotropic P2Y receptors also contribute to neurotransmission and modulation in the sensory nervous system (for review, see Burnstock, 2007). P2Y receptors appear to potentiate pain induced by chemical or physical stimuli via capsaicin-sensitive transient receptor potential vanilloid receptor channels (Tominaga et al., 2001; Lakshmi and Joshi, 2005). On the contrary, some P2Y receptors, such as P2Y1, P2Y2, and P2Y6, produce inhibitory effects in spinal pain transmission (Okada et al., 2002; Borvendeg et al., 2003; Gerevich et al., 2004). It has been suggested that functional metabotropic P2Y receptors, including the P2Y12 receptor, mediate the chemotaxis that occurs during activation (Honda et al., 2001).
In the present study, we show that the gene expression of P2Y12 in spinal microglia increases dramatically in a neuropathic pain model, and this P2Y12 increase in microglia significantly contributes to the development of mechanical and thermal pain hypersensitivity through the p38 mitogen-activated protein kinase (MAPK) pathway. These data suggest the G-protein-coupled P2Y12 receptor has an important role as a gate to activate p38 signaling and neuropathic pain behaviors.
Materials and Methods
Animal treatment.
Male Sprague Dawley rats weighing 200–250 g were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and received partial sciatic nerve transection (PSNL) (Seltzer et al., 1990). Briefly, a tight ligature was made around the dorsal half of the left sciatic nerve using 8-0 nylon such that one-half to one-third of the sciatic nerve was ligated. The wounds were then closed, and the rats were allowed to recover. At several time points (1, 3, 7, 14, and 30 d) after the PSNL, groups of rats were processed for histological analysis (n = 4 in each time point). Every effort was made to minimize animal suffering and reduce the number of animals used. All animal experimental procedures were approved by the Hyogo College of Medicine Committee on Animal Research and were performed in accordance with the National Institutes of Health guidelines on animal care.
Reverse transcription-PCR and in situ hybridization histochemistry.
The rats were killed by decapitation under deep ether anesthesia. The rats were transcardially perfused with PBS, and their spinal cords (L4–L5) were removed and rapidly frozen with powdered dry ice and stored at −80°C until used. The extraction of total RNA was performed using the RNA extraction reagent ISOGEN (Nippon Gene, Tokyo, Japan), and the PCR reaction was performed as described previously (Kobayashi et al., 2006).
For in situ hybridization histochemistry (ISHH), the rats were killed by decapitation under deep ether anesthesia. The bilateral L4–L5 spinal cord was dissected out, rapidly frozen in powdered dry ice, and cut on a cryostat at a 12 μm thickness. Sections were thaw mounted onto MAS-coated glass slides (Matsunami, Osaka, Japan). These sections were processed for ISHH, as described previously (Kobayashi et al., 2005, 2006). For the quantification of P2Y12 mRNA signals in the dorsal horn of the L4–L5 segment, 10 randomly selected sections per each rat (each time point, n = 4) were measured with a computer-assisted imaging analysis system (NIH Image, version 1.61). To know the P2Y12 mRNA signal intensity in each cell, the relative intensity of silver grains on and around positively labeled cells (90–110 cells per rat, n = 4 each group) were measured with the same method above. Data are expressed throughout as mean ± SEM (percentage). Differences in changes of values over time of each group were tested using one-way ANOVA, followed by individual post hoc comparisons (Fisher's test). A difference was accepted as significant at p < 0.05.
Immunohistochemistry.
Rats that received the PSNL were used for immunohistochemistry (IHC). These rats were deeply anesthetized with sodium pentobarbital and perfused transcardially with 250 ml of 1% formaldehyde in 0.1 m phosphate buffer, pH 7.4, followed by 500 ml of 4% formaldehyde in 0.1 m phosphate buffer. The spinal cords were dissected out and postfixed in the same fixative at 4°C overnight, followed by immersion in 20% sucrose in 0.1 m phosphate buffer at 4°C for 2 d. The tissue was frozen in powdered dry ice and cut on a cryostat at a 25 μm thickness for the spinal cord. The sections were processed for IHC using the ABC method (Yamanaka et al., 2004).
The following antibodies were used: rabbit anti-P2Y12 polyclonal antiserum (1: 1000; gift from Prof. David Julius, University of California, San Francisco, CA), rabbit anti-ionized calcium-binding adapter molecule 1 (Iba1) polyclonal antiserum (1:500; Wako Chemicals, Tokyo, Japan), goat anti-Iba1 polyclonal antiserum (1:1000; Abcam, Cambridge, MA), rabbit anti-phospho-p38 MAPK (p-p38) polyclonal antiserum (1:500; Cell Signaling Technology, Beverly, MA), mouse anti-neuronal-specific nuclear protein (NeuN) monoclonal antiserum (1:2000; Chemicon, Temecula, CA), and rabbit anti-glial fibrillary acidic protein (GFAP) polyclonal antiserum (1:1000; DakoCytomation, Glostrup, Denmark). The P2Y12 antibody showed immunoreactivity for mouse, rat, and human P2Y12 (Haynes et al., 2006). P2Y12 immunoreactivity localized in the surface of CNS microglia in adult wild-type mice and was not detectable in P2Y12-deficient mice.
In brief, spinal cord sections were incubated with a primary antibody overnight at 4°C, followed by biotinylated secondary antibodies (1: 200; Vector Laboratories, Vector Laboratories, CA) overnight at 4°C. Antibodies were visualized by 0.05% 3,3-diaminobenzidine tetrahydrochloride (Wako Chemicals). Double-immunofluorescent staining was performed with anti-rabbit Alexa Fluor 594 IgG (1:1000; Invitrogen, San Diego, CA), anti-goat Alexa Fluor 488 IgG (1:1000), and anti-mouse Alexa Fluor 488 IgG (1:1000) after incubation with respective primary antibodies.
To quantify positive cell profiles in the spinal cord, four to six sections from the L5 spinal cord segments were randomly selected. An image in a square (400 × 400 μm) centered on the medial two-thirds of the superficial dorsal horn (laminas I–III) was captured under a 100× objective, and all of the positively stained cells in the area were counted. To quantify p-p38 expression in the dorsal horn of the L4–L5 segment, 10 randomly selected sections per rat (each time point, n = 4) were measured with a computer-assisted imaging analysis system (NIH Image, version 1.61). Data are expressed throughout as mean ± SEM (percentage). Changes of values over time were tested using one-way ANOVA, followed by individual post hoc comparisons (Fisher's exact test). Pairwise comparisons (t test) were used to assess differences of values between the intrathecal groups. A difference was accepted as significant if p < 0.05.
Double-labeling study.
To examine the distribution of mRNAs for P2Y12 in neurons versus glial cells, we used a combined IHC with ISHH. The cryostat sections were fixed in 4% formaldehyde in 0.1 m phosphate buffer for 20 min. The treatment of sections and methods of double labeling with IHC and ISHH were described previously (Kobayashi et al., 2006).
Drug treatments.
Two days before or 4 d after the PSNL, the L5 vertebra was laminectomized under adequate anesthesia with sodium pentobarbital, and a soft tube (Silascon, Kaneka Medix Company, Osaka, Japan; outer diameter, 0.64 mm) filled with 5 μl of saline was inserted into the subarachnoid space for an ∼0.5 cm length. After the muscle incision was closed, the mini-osmotic pumps (model 2001; 7d pump, 1 μl/h; Alzet, Cupertino, CA) filled with P2Y12 antisense oligodeoxynucleotide (AS-ODN) or mismatch ODN (MM-ODN) (0.5 nmol/μl) diluted by saline or the P2Y12 antagonist MRS2395 [2,2-dimethyl-propionic acid 3-(2-chloro-6-methylaminopurin-9-yl)-2-(2,2-dimethyl-propionyloxymethyl)-propyl ester] (10 and 100 pmol/μl; Sigma, Poole, UK) diluted by 5%DMSO saline were connected to the tube. AS-ODN (5′-AAAAACAGGACAGTGTAG-3′) and MM-ODN (5′-ACTACTACACTAGACTAC-3′) were designed and manufactured by Biognostik (Göttingen, Germany). FITC-labeled ODN injected intrathecally was used to confirm the uptake of ODN by almost all cells in the spinal cord. The intrathecal delivery of the p38 MAPK inhibitor SB203580 [4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole] was performed as described previously (Obata et al., 2004). To obtain a sustained drug infusion, an Alzet osmotic pump (3d pump, 1 μl/h; Durect, Cupertino, CA) was filled with the MAP kinase kinase 1/2 inhibitor SB203580 (0.5 μg/μl; Calbiochem, La Jolla, CA) in 10% DMSO, and the associated catheter was implanted intrathecally before PSNL. A potent P2Y12 agonist, 2-(methythio) adenosine 5′-diphosphate trisodium salt (2Me-SADP) (1 nmol/μl; Sigma), diluted in saline, MRS2395 (100 pmol/μl), and/or SB203580 (0.5 μg/μl), were administered into naive rats using the mini-osmotic pump. The selectivity of 2Me-SADP and MRS2395 was discussed previously (Möller et al., 2000; Xu et al., 2002; Abbracchio et al., 2006). Normal saline or 10% DMSO in saline was used as the vehicle control. Then, the pump was laid under the skin and the incision was closed.
Photomicrographs.
All emulsion-coated slides and DAB-stained slides were digitized with a Nikon (Tokyo, Japan) DIAPHOT-300 microscope connected to a Nikon DXM-1200F digital camera. We used Adobe Photoshop CS or Element 2.0 (Adobe Systems, Mountain View, CA) to optimize the images and to make all figures. The double-stained images were examined with a laser confocal microscope (Axiovert 200M; Zeiss, Oberkochen, Germany).
Western blotting.
The rats were killed by decapitation under deep ether anesthesia. The rats were transcardially perfused with PBS, and their spinal cords were removed, rapidly frozen with powdered dry ice, and stored at −80°C until used. Tissue samples were isolated by ISOGEN reagent according to the procedure of the manufacturer (Nippon Gene). Samples with equal amounts of protein were then separated by 5–20% PAGE, and the resolved proteins were electrotransferred to Immobilon-P transfer membrane (Millipore, Billerica. MA). Membranes were incubated with Blocking One P (Nakarai, Kyoto, Japan) in Tris buffer containing Tween 20 (TBS-T) (10 mm Tris-HCl, pH 8.0, 150 mm NaCl, and 0.2% Tween 20) for at least 20 min at room temperature and incubated with the polyclonal primary antibody for P2Y12 (1:200; Alomone Labs, Jerusalem, Israel), p38 (1:400; Cell Signaling Technology), p-p38 (1:400; Cell Signaling Technology), and β-actin (1:2000; Sigma) at 4°C overnight. Membranes were then washed twice with TBST and probed with goat anti-rabbit IgG conjugated with horseradish peroxidase (1:2000; Chemicon) at room temperature for 2 h. Membranes were finally washed several times with TBST to remove unbound secondary antibodies and visualized by chemiluminescence using CSPD ready-to-use reagent (Roche, Indianapolis, IN). Films were scanned and quantified using NIH Image version 1.61 and normalized against a loading control (β-actin). The protein level was expressed as a percentage of the protein level in the naive control. Each experiment was repeated at least twice, and, in all cases, the same results were obtained.
Data are expressed as mean ± SEM. Differences in changes of values over time of each group were tested using one-way ANOVA, followed by individual post hoc comparisons (Fisher's exact test) or pairwise comparisons (t test) to assess differences of values between the intrathecal groups. A difference was accepted as significant if p < 0.05.
Behavioral tests.
All PSNL rats were tested for mechanical allodynia and thermal hyperalgesia on the plantar surface of the hindpaw 1 d before surgery and 1, 3, 5, 7, 10, and 12 d after surgery. Mechanical allodynia was assessed with a dynamic plantar anesthesiometer (Ugo Basile, Comerio, Italy), which has an automated von Frey-type filament (0.5 mm diameter) (Kalmar et al., 2003; Lever et al., 2003). To measure mechanical thresholds of the hindpaw, rats were placed in a plastic cage with a wire mesh floor and allowed to acclimate for 15 min before each test session. A paw-flick response was elicited by applying an increasing force (measured in grams) directed on the middle of the plantar surface of the ipsilateral hindpaw. The force applied was initially below the detection threshold, then increased from 1 to 50 g in 0.5 g steps over 20 s, and was then held at 50 g for an additional 10 s. The rate of force increase was 2.5 g/s. The force to elicit a reflex removal of the ipsilateral hindpaw was monitored. This was defined as the mean of three measurements made at 5 min intervals.
Heat hyperalgesia was tested using the plantar test (7370; Ugo Basile). A radiant heat source beneath a glass floor was aimed at the planter surface of the hindpaw. Three measurements of latency were taken for each hindpaw in each test session. The hindpaws were tested alternately, with 5 min intervals between consecutive tests. The three measurements of latency per side were averaged. An assistant, who was unaware of the treatment group, performed all of the behavioral experiments. Data are expressed as mean ± SEM. Differences in changes of values over time of each group were tested using one-way ANOVA, followed by individual post hoc comparisons (Fisher's PLSD). Pairwise comparisons (Student's t test) were used to assess the effect of the P2Y12 antagonist or AS-ODN on the basal mechanical and thermal sensation. A difference was accepted as significant if p < 0.05.
Results
PSNL increases P2Y12 mRNA expression in ipsilateral spinal microglia
We used semiquantitative reverse transcription (RT)-PCR to examine the effect of PSNL on the expression of P2Y12 mRNA in the L4–L5 ipsilateral spinal cord (n = 4, each time point) (Fig. 1 A). The intensity of the amplified bands of P2Y12 mRNA significantly increased 3 d after injury (3.5-fold; p < 0.01). The increased P2Y12 mRNA returned to the normal level 30 d after PSNL. To examine the distribution of P2Y12 mRNA, we performed ISHH on sections of the L4–L5 spinal cord (Fig. 1 B–G,I,J). P2Y12 mRNA was detected sporadically in the spinal cord of naive rats (Fig. 1 C), and these labeled nuclei were small in size and rather densely stained by hematoxylin (Fig. 1 I). This finding is consistent with the idea that microglia constitutively expresses P2Y12 mRNA (Kobayashi et al., 2006). P2Y12 mRNA signals dramatically increased only in the ipsilateral dorsal horn and also ventral horn 3 d after PSNL (Fig. 1 B). A number of P2Y12 mRNA-expressing cells were found in the superficial dorsal horn (laminas I–III) 3 d after injury (Fig. 1 D) and maintained elevated 7–14 d after injury (Fig. 1 E,F). The expression of P2Y12 mRNA at day 30 after sciatic nerve transection was similar to the controls (Fig. 1 G). Quantification of the silver grains in the dorsal horn (laminas I–III) (n = 4 at each time point) revealed that the increase in P2Y12 mRNA levels peaked at 3 d after injury (4.3-fold; p < 0.001) and maintained significantly increased for at least 14 d after PNSL (Fig. 1 H). The mean intensity of ISHH signals on P2Y12 mRNA-positive cells was 1.4-fold higher in the ipsilateral dorsal horn 3 d after injury than that in the naive dorsal horn (p < 0.002) (Fig. 1 K). These data indicate that both the number of microglia expressing P2Y12 mRNA and the mRNA expression in each microglia increased after nerve injury.
Figure 1.
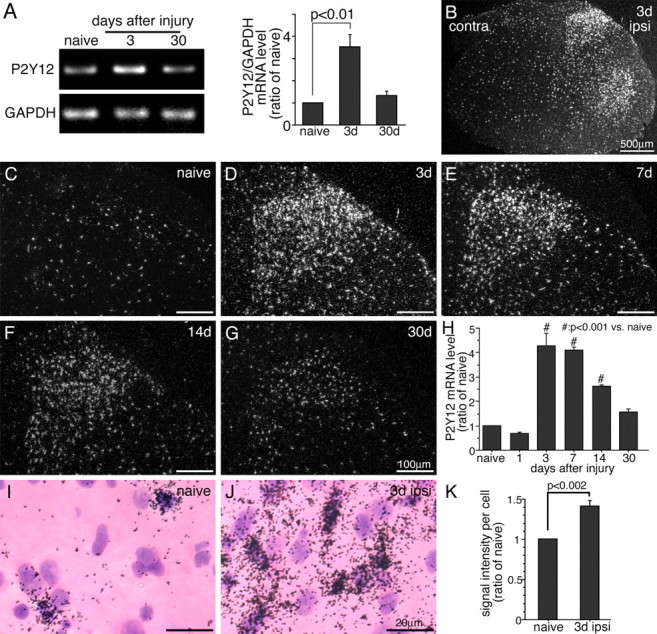
PSNL increases P2Y12 expression in the ipsilateral spinal cord. A, The levels of P2Y12 mRNA in the ipsilateral L4–L5 spinal cord were determined using the RT-PCR technique. Gel panels show PCR products from the L4–L5 spinal cord taken from naive and 3 and 30 d after surgery. Right graph shows quantification of the relative mRNA levels of P2Y12 in the spinal cord. P2Y12 mRNA levels were normalized against corresponding control (mean ± SEM) (p < 0.01 compared with naive). B–G, Dark-field images of ISHH show P2Y12 mRNA in the spinal cord of a naive rat (C), 3 (B, D), 7 (E), 14 (F), and 30 (G) days after PSNL. Scale bars: B, 500 μm; C–G, 100 μm. D–G show the dorsal horn ipsilateral to the surgery. H, Quantification of the silver grains in dorsal horn (laminas I–III) (n = 4, 10 sections at each time point). P2Y12 signals were normalized against naive rat. # p < 0.01 compared with naive control. I, J, Higher-magnification photographs of laminas II–III of the dorsal horn under bright-field illumination in the naive rat (I) and 3 d after PSNL (J). Scale bars, 20 μm. K, The mean intensity of P2Y12 mRNA signals per labeled cells in ISHH tissue sections of naive rats and 3 d after the surgery. p < 0.002 compared with naive control. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase; contra, contralateral; ipsi, ipsilateral.
To identify P2Y12 mRNA-expressing cells in the spinal cord after PSNL, we performed combined ISHH for P2Y12 mRNA with IHC for NeuN, GFAP, or Iba1. We found that the P2Y12 mRNA-positive cells did not colocalize with NeuN or GFAP (Fig. 2 A,B). Instead, the majority of the P2Y12 mRNA-positive cells were double labeled with Iba1 in the ipsilateral dorsal horn 3 d after nerve injury, indicating that the increase in P2Y12 mRNA occurred in microglia but not in neurons or astrocytes (Fig. 2 C).
Figure 2.
PSNL induced P2Y12 expression in microglia of the spinal dorsal horn 3 d after PSNL surgery. Bright-field photomicrographs of combined IHC for NeuN (A), GFAP (B), and Iba1 (C) with ISHH for P2Y12 mRNAs. Scale bars, 20 μm. Arrows indicate double-labeled cells. Arrowheads indicate single-labeled cells by ISHH (aggregation of silver grains), and open arrowheads indicate single immunostained cells (brown staining).
P2Y12 protein expression in microglia after PSNL
The expression of P2Y12 protein was examined by immunohistochemistry using anti- P2Y12 antibody. Consistent with the results of the ISH, PSNL induced substantial P2Y12- immunoreactive (IR) cells in the ipsilateral dorsal horn compared with the contralateral side (Fig. 3 A,B). The immunoreactive cells were distributed in dorsal horn, especially in superficial dorsal horn. Using double-labeling studies with P2Y12 protein and Iba1, we found most of the P2Y12-IR signals were colocalized with Iba1 (Fig. 3 C–H). It was noteworthy that microglia showed P2Y12 immunoreactivity only in their cell bodies, not in their projections. We did not detect P2Y12 immunoreactivity in nerve terminals in dorsal horn. Thus, P2Y12 protein was exclusively expressed in activated microglia after PSNL.
Figure 3.

Expression of P2Y12 protein in microglia in the dorsal horn 7d after PSNL with immunohistochemical analysis. P2Y12 immunoreactivity was increased on the side ipsilateral to the PSNL compared with the contralateral side (A, B). Double-immunofluorescent histochemistry (C–H) in both sides showed that P2Y12 protein (red; C, F) colocalized with Iba1-labeled cells (green; D, G). Merged images are E, H. Scale bars: A, B, 200 μm; C–H, 50 μm. contra, Contralateral; ipsi, ipsilateral.
Inhibition of P2Y12 function attenuates PNSL-induced mechanical allodynia and thermal hyperalgesia
Considering that P2Y12 increased in spinal microglia and the widely recognized role of microglia in pain, it is reasonable to expect that the P2Y12 increase in microglia has a role in neuropathic pain after nerve injury. To examine whether P2Y12 in microglia contributes to mechanical allodynia after nerve injury, the P2Y12 antagonist MRS2395 (10 and 100 pmol/h, respectively; n = 8 each model) was administrated intrathecally via osmotic pumps for 7 d through a cannula implanted at the lumber enlargement. The infusion of the drug started 2 d before the surgery of PNSL. The paw-withdrawal thresholds were measured every other day from 1 to 12 d after the surgery (Fig. 4 A–D). The basal mechanical thresholds were measured before the ligation, and two doses of MRS2395 were applied. When compared with the vehicle group, the attenuation of mechanical allodynia was observed in the MRS2395 administration groups (Fig. 4 A). Mechanical allodynia of the 100 pmol/h MRS2395 administration group was significantly reversed 3–10 d after PSNL (p < 0.05 vs vehicle). The group with the 10 pmol/h MRS2395 administration had inhibition of mechanical allodynia from 3 to 5 d after injury (p < 0.05 vs vehicle; ANOVA).
Figure 4.
Effects of the P2Y12 antagonist on neuropathic pain behaviors. Continuous intrathecal administration of the P2Y12 antagonist MRS2395 inhibited the mechanical allodynia (A, B) and thermal hyperalgesia (C, D) on the sides ipsilateral (A, C) and contralateral (B, D) to the PSNL surgery. The osmotic pump was set 2 d before PSNL surgery, and drug administration continued for 7 d. The higher concentration of MRS2395 (100 pmol/h) reversed the mechanical allodynia for up to 3–10 d after surgery. The lower concentration of MRS2395 (10 pmol/h) had a smaller effect. Injury-induced thermal hyperalgesia was also suppressed by the administration of MRS2395 in a dose-dependent manner. There was no effect of MRS2395 administrated on the mechanical allodynia and thermal hyperalgesia in the contralateral hindpaw. E, F, Posttreatment of MRS2395 (100 pmol/h) did not have effect on mechanical (E) and thermal (F) sensitivity. The osmotic pump was implanted 4 d after PSNL and worked for 7 d. In all graphs, values are represented as mean ± SEM (n = 8 in each group, *p < 0.05 vs vehicle; # p < 0.05 vs 10 pmol/h)
Thermal sensitivity was also examined in these animals with intrathecal administration of P2Y12 antagonist. The 100 pmol/h MRS2395 administration group had significantly reduced thermal hyperalgesia from 3 to 7 d (Fig. 4 C). Mechanical allodynia and thermal hyperalgesia on the contralateral side was unchanged by the MRS2395 administration (Fig. 4 B,D). To further examine whether the application of the P2Y12 antagonist would affect the established neuropathic pain, we infused MRS2395 (100 pmol/h) intrathecally via osmotic pumps 4 d after the injury when the pain behaviors were established. The delayed administration of MRS2395 from 4 d after injury did not suppress the pain behaviors (Fig. 4 E,F).
Next, we confirmed that the P2Y12 expression was involved in the generation of neuropathic pain, by means of intrathecal treatment with an AS-ODN (0.5 nmol/h; n = 8) targeting P2Y12 or with an MM-ODN (0.5 nmol/h; n = 8) as a control. The infusion of the ODN started 2 d before PNSL surgery. We found that P2Y12 AS-ODN significantly inhibited nerve injury-induced mechanical allodynia and thermal hyperalgesia from 3 to 5 d after surgery (p < 0.05) (Fig. 5 A,C). Mechanical withdrawal threshold and thermal latency in the MM-ODN group were not different from those of the vehicle group. Intrathecal administration of AS-ODN or MM-ODN produced no significant changes in both pain behaviors on the contralateral side (Fig. 5 B,D). We then confirmed the level of P2Y12 protein in the spinal cord after PSNL with AS-ODN or MM-ODN administration (n = 4 each group). As shown in Figure 5, E and F, PSNL induced an increase in P2Y12 protein in the spinal cord 3 d after injury. In the AS-ODN-treated rats, P2Y12 protein levels were significantly lower than in the MM-ODN-treated rats and vehicle-treated rats (p < 0.01).
Figure 5.
Knockdown of P2Y12 by AS-ODN alleviated neuropathic pain behaviors. The effects of continuous intrathecal administration of AS-ODN on mechanical allodynia (A, B) and thermal hyperalgesia (C, D) were examined on the sides ipsilateral (A, C) and contralateral (B, D) to the PSNL surgery. AS-ODN administration significantly reversed the mechanical allodynia on the ipsilateral side. Injury-induced thermal hyperalgesia was also suppressed by the administration of the AS-ODN. No effects of AS-ODN on mechanical allodynia (B) and thermal hyperalgesia (D) were observed on contralateral hindpaw after PSNL. In all graphs, values are represented as mean ± SEM (n = 8 in each group; p < 0.05 compared with MS control). E, Western blots from the spinal cord tissue showed immunoreactive bands at ∼50 kDa (15 μg of total protein loaded). Lower bands indicated β-actin. The increase of P2Y12 protein with missense and vehicle control was observed 3 d after PSNL. The inhibition of P2Y12 proteins with AS-ODN was confirmed. F, Quantification of Western blots showing P2Y12 protein, which was normalized to β-actin and expressed as a ratio of naive (n = 4 each groups; # p < 0.01).
P2Y12 is required for p38 MAPK activation in the spinal cord after PSNL
Previous reports have demonstrated that nerve injury activated the p38 MAP kinase cascade in microglia and the activation of p38 MAP kinase in microglia in the spinal cord contributes to the generation of neuropathic pain (Jin et al., 2003; Tsuda et al., 2004). We confirmed that infusion of the p38 MAPK inhibitor SB203580 reversed significantly the PSNL-induced neuropathic pain behaviors at 3 d after surgery (Fig. 6 A,B).
Figure 6.

Suppression of PSNL-induced pain behaviors and p38 MAPK phosphorylation by administration of P2Y12 antagonist and AS-ODN. A, B, Administration of the p38 MAPK inhibitor SB203580 reversed mechanical allodynia (A) and thermal hyperalgesia (B) induced by PSNL (# p < 0.01 compared with vehicle control). C, Western blot analyses with phospho-p38 MAPK, total p38 MAPK, and β-actin antibodies in ipsilateral spinal cord of naive rats and PSNL rats administered with MM-ODN, AS-ODN, vehicle, and MRS2395 (15 μg of total protein loaded). Pretreatment of P2Y12 AS-ODN and antagonist MRS2395 suppressed the activation of p-p38 MAPK but not MM-ODN and vehicle at day 3 after PSNL. D, E, Quantification of Western blot data. The graphs show the level of total p38 MAPK normalized to β-actin (D) and p-p38 MAPK normalized to total p38 MAPK and expressed as a ratio of naive (E). Data represent mean ± SEM (n = 4 each groups; # p < 0.01). F–J, IHC shows the expression of phospho-p38 immunoreactivity in the spinal dorsal horn. Tissue sections are from the L5 dorsal horn of a control naive rat (F), 3 d after PSNL administered with intrathecal MM-ODN (G), AS-ODN (H), vehicle (I), and MRS2395 (J). Scale bar, 100 μm. contra, Contralateral; ipsi, ipsilateral.
To examine whether p38 MAP kinase phosphorylation was regulated by P2Y12, we performed Western blot analysis (Fig. 6 C–E) and IHC (Fig. 6 F–J). The expression of phosphorylated p38 MAP kinase was examined in the spinal cord of PSNL rats that received administration of the P2Y12 antagonist MRS2395 or AS-ODNs or MM-ODNs. Western blot analysis revealed that intrathecal administration of the P2Y12 antagonist MRS2395 or AS-ODN decreased the level of phosphorylated p38 MAP kinase compared with the vehicle or MM-ODN treatment (p < 0.01) (Fig. 6 C,E). Total p38 expression after nerve injury were not significantly changed compared with those of naive control rats (Fig. 6 C,D). In addition, we found that the increase in p-p38 immunoreactivity after PSNL surgery was significantly suppressed by administration of MRS2395 or AS-ODN in the spinal dorsal horn (Fig. 6 H,J). The degree of inhibition of p-p38 correlated well with the changes in mechanical allodynia and thermal hyperalgesia 3 d after PSNL after the same treatment. The MM-ODN or vehicle control groups showed no decrease in p-p38-labeled neurons.
Spinal infusion of P2Y12 agonist activates p38 MAPK and induces pain behaviors
To determine whether signals through P2Y12 are involved in the pain behaviors via the activation of p-p38 MAPK, we administrated the potent P2Y12 agonist 2Me-SADP and examined the pain behavior and activation of p-p38 MAPK in spinal dorsal horn. We continuously infused 2Me-SADP (1 nmol/h) and/or MRS2395 (100 pmol/h) intrathecally for 3 d via an osmotic pump. We found that intrathecal administration of 2Me-SADP significantly induced both mechanical and thermal hyperalgesia. However, coadministration of 2Me-SADP and MRS2395 produced no changes in pain behaviors (Fig. 7 A,B). To further examine whether the effects of the p38 inhibitor SB203580 on 2Me-SADP-induced mechanical and thermal hypersensitivity, we coadministrated 2Me-SADP and SB203580 (0.5 μg/h) intrathecally to naive rats. The treatment of SB203580 diminished 2Me-SADP-induced mechanical hypersensitivity (Fig. 7 A,B). Moreover, we found that intrathecal administration of 2Me-SADP significantly increased phosphorylated p38 MAP kinase (Fig. 7 C,D,G,H). Coadministration of 2Me-SADP and MRS2395 produced a significant decrease in the number of labeled neurons for phosphorylated p38 MAP kinase (Fig. 7 E,G,H). MRS2395 administration itself had no effect (Fig. 7 F–H).
Figure 7.

Spinal infusion of the P2Y12 agonist 2Me-SADP induced pain behavior and phosphorylation of p38 MAPK. A, B, Spinal infusion of 2Me-SADP (1 nmol/h) induced mechanical allodynia (A) and thermal hyperalgesia (B). However, the P2Y12 antagonist MRS2395 (100 pmol/h) and p38 MAPK inhibitor SB203580 for 3 d reversed the effect of 2Me-SADP on pain behaviors. Values are represented as mean ± SEM (n = 8 in each group, *p < 0.05; # p < 0.001). C–F, IHC shows the expression of phospho-p38 immunoreactivity in the spinal dorsal horn. Rats received intrathecal administration of vehicle (C), the P2Y12 agonist 2Me-SADP (D), both 2Me-SADP and MRS2395 (E), and MRS2395 alone (F). Scale bar, 100 μm. G, Quantification of immunostaining intensity of phospho-p38 MAPK determined as the average pixel density in the ipsilateral dorsal horn 3 d after injury. H, Quantification of the numbers of p-p38-immunoreactive cells in the ipsilateral dorsal horn 3 d after injury. Data represent mean ± SEM; n = 4 per group. # p < 0.001.
Discussion
In the present study, we found the following new findings. (1) The peripheral nerve injury induced a dramatic increase in P2Y12 ATP receptor mRNA and protein expression in spinal microglia. The increase continued for at least 2 weeks after injury. (2) Two kinds of blockade of P2Y12 receptor, antagonist and antisense oligonucleotide, attenuated partial sciatic nerve transection-induced pain hypersensitivity. (3) Inhibition of P2Y12 signaling suppressed the activation of p38 MAPK in microglia. (4) The intrathecal infusion of the P2Y12 agonist 2Me-SADP into naive rats mimicked the nerve injury-induced activation of p38 in microglia and elevated pain behaviors.
The P2Y12 receptor was formerly called the “P2ADP” receptor, because the platelets have a P2 receptor that is activated by ADP, whereas ATP is a competitive antagonist (Daniel et al., 1998; Fagura et al., 1998). Later, this receptor was cloned from a rat platelet cDNA library and renamed “P2Y12” (Hollopeter et al., 2001). The constitutive expression of P2Y12 in spinal microglia has been reported previously (Sasaki et al., 2003; Kobayashi et al., 2006). The expression of P2Y12 in microglia was also reported around axotomized facial nerve nuclei in the rat (Sasaki et al., 2003). The present study extended these findings by showing the dynamic upregulation of P2Y12 mRNA and protein in spinal microglia after peripheral nerve injury.
Peripheral nerve injury induces the upregulation of a variety of molecules in spinal microglia; it includes the expression of microglial markers (CD11b, CD14, toll-like receptor 4, and others), the chemokine (C-C motif) receptor 2 and chemokine (C-X3-C) receptor 1 chemokine receptors, the CB2 cannabinoid receptor, and ATP receptor P2X4 ion channel (Abbadie et al., 2003; Tsuda et al., 2003; Zhang et al., 2003; DeLeo et al., 2004; Verge et al., 2004). P2X4 was reported to increase the expression in microglia after peripheral nerve injury (Tsuda et al., 2003) and after peripheral inflammation (Guo et al., 2005). The time course of upregulation of P2X4 in spinal microglia was different in these two models; it increased at 2 weeks after nerve injury but no information was available after that, and it peaked at 7 d after Formalin injection and returned to normal at 2 weeks. The time course of P2Y12 upregulation was different for P2X4 in microglia; it peaked at 3 d after nerve injury and returned to normal after 30 d, suggesting a different regulatory mechanism controlling the expression of ATP receptors in microglia.
The peripheral nerve injury could induce the P2Y receptor expression in other glial cells. Although there have been very few studies examining the expression of P2Y receptors in spinal cord in vivo, we reported that P2Y1 mRNA is expressed in spinal astrocytes (Kobayashi et al., 2006). In spinal cord astrocytes, the P2Y receptor mediates the rise in [Ca2+]i (Ho et al., 1995; Idestrup and Salter, 1998) and propagation of intercellular Ca2+ waves (Scemes et al., 2000), in which the P2Y1 subtype is especially involved in Ca2+ signaling and waves propagation (Fam et al., 2000). However, we could not detect any change in the expression of P2Y1 receptors in spinal astrocytes after peripheral nerve injury. The present finding that P2Y12 mRNA and protein exclusively increase in microglia is the first report of upregulation of P2Y receptors in spinal glial cells in response to the nerve injury.
It is believed that ATP is released from primary afferent terminals, dorsal horn neurons, and/or spinal astrocytes (Sawynok et al., 1993; Bardoni et al., 1997; Li et al., 1998; Jo and Schlichter, 1999; Fam et al., 2000; Nakatsuka and Gu, 2001). It is reasonable to suppose that peripheral nerve injury can induce the release or leakage of ATP from the primary afferent terminals in the spinal dorsal horn, but there has been neither direct evidence of this nor information about the quantity and time course of ATP release. One very important finding in the present study was that a selective P2Y12 agonist into naive rats could induce p38 MAPK activation in microglia and also elevated pain behaviors. P2Y receptors have different binding properties to extracellular nucleotides, such as ATP, ADP, UTP, or UDP (for review, see Burnstock, 2007). P2Y12, P2Y13, and P2Y1 receptors all preferentially bind to ADP (for review, see Communi et al., 2000), and in platelets ADP-specific P2Y12 and P2Y1 receptors are essential for normal aggregation responses to both exogenous and endogenous ADP in vivo and in vitro (Turner et al., 2001; Andre et al., 2003). In spinal cord tissues, among several P2Y receptor agonists, 2Me-SADP and, to a lesser extent, ADP are most potent at inducing a calcium response in mouse microglia, and P2Y6, P2Y12, and P2Y13 receptor activation mediates the rapid calcium responses (Light et al., 2006). Because the released ATP is rapidly hydrolyzed to ADP or AMP (for review, see Burnstock 2007), the upregulation and activation of microglia through the ADP-specific P2Y12 receptor in the dorsal horn could be important in the pathomechanism of the neuron–glia interaction after peripheral nerve injury. Of course, we cannot rule out the contribution of other P2Y receptors, such as the P2Y6, P2Y13, or P2X receptors.
P2Y12 action in microglia is believed to involve chemotaxis (Haynes et al., 2006; Ohsawa et al., 2007; Wu et al., 2007). Therefore, ATP from primary afferents and the degradation products, such as ADP, may work for the action and recruitment of microglia in the dorsal horn. Note that the number of microglia showing p38 upregulation in the dorsal horn after the peripheral nerve injury (Fig. 6 I) is greater than that after 2Me-SADP administration (Fig. 7 D). One possible reason for this is that microglial cell proliferation occurs in the dorsal horn after peripheral nerve injury (Echeverry et al., 2008), but agonist administration and activation of P2Y12 may have not induced the microglia proliferation. Another reason may be related to the efficacy and concentration of 2Me-SADP in the dorsal horn tissue intrathecally applied by osmotic pump.
There is no paper demonstrating direct evidences of possible role of P2Y12 in microglia in mechanisms responsible for neuropathic pain, although it has been suggested in reviews (Inoue, 2006; Trang et al., 2006). In terms of the mechanisms of neuropathic pain, studies of intracellular signaling cascades have collected much attention. Peripheral nerve injury induces a dramatic activation of intracellular signaling cascade proteins such as MAPK in neurons, astrocytes, and microglia (for review, see Ji and Strichartz, 2004; Obata and Noguchi, 2004). For example, we reported the activation of Src-family kinases and the downstream activation of extracellular signal-regulated kinase 1/2 in microglia, and these signaling pathways contribute to mechanical hypersensitivity after nerve injury (Katsura et al., 2006). Much more work has concentrated on p38 MAPK in microglia, which is activated or phosphorylated after nerve injury and is significantly involved in pain behaviors (Kim et al., 2002; Jin et al., 2003; Schafers et al., 2003; Tsuda et al., 2004). Activation of p38 in microglia is also reported in inflammatory pain models (Svensson et al., 2003; Sweitzer et al., 2004; Hua et al., 2005), suggesting an important role of p38 activation and the subsequent signaling cascade in microglia in pathological pain (Milligan et al., 2003; Obata et al., 2004; Ji et al., 2007). There are few studies examining the upstream mechanisms responsible for p38 MAPK activation in spinal microglia after nerve injury (Sung et al., 2005; Svensson et al., 2005; Zhuang et al., 2006). Moreover, there is no report demonstrating a possible pathway from Gi-coupled receptors to p38. Unknown mechanisms may work to activate p38 MAPK; other G-protein pathways may be activated under P2Y12 receptor or indirect mechanisms such as signaling under unknown factors associated with P2Y12 receptor may contribute to p38 activation. The signaling pathway to activate p38 MAPK in microglia should be further explored. The findings in the current study are the first report to suggest that the ATP receptor subtype in microglia regulates the activation of the p38 cascade resulting in elevated pain sensitivity.
Another important question is how p38 activation in microglia, downstream from the P2Y12 receptor, is involved in elevated pain sensitivity. The spinal glial activation is likely involved in the production and release of proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α, thus increasing pain hypersensitivity (DeLeo and Yezierski, 2001; Hanisch, 2002; Watkins and Maier, 2003). Glial cells also produce inflammatory enzymes such as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) (DeLeo and Yezierski, 2001; Watkins et al., 2001a,b; Koistinaho and Koistinaho, 2002). It is widely believed that p38 activation regulates the expression of these proinflammatory cytokines iNOS or COX-2 in microglia (Widmann et al., 1999; Ji and Woolf, 2001; Koistinaho and Koistinaho, 2002). These data suggest that the p38 MAPK is a key molecule that bridges from microglia to neurons, and this bridge is importantly a mechanism responsible for neuropathic pain.
In conclusion, our results have suggested that the P2Y12 receptor is increased in microglia after peripheral nerve injury, and the signals through this receptor activated by released ATP or the hydrolyzed products such as ADP activate downstream p38 MAPK. The activated microglia mediated by p38 signaling may produce proinflammatory cytokines or other effectors onto the nociceptive network in the spinal cord, resulting in the manifestation of neuropathic pain behaviors.
Footnotes
This work was supported in part by Grants-in-Aid for Scientific Research and an Open Research Center Grant, Hyogo College of Medicine, both from the Japanese Ministry of Education, Science, and Culture and Grants-in-Aid for Researchers Hyogo College of Medicine 2006. We thank Wadazumi and N. Kusumoto for technical assistance. We thank D. A. Thomas for correcting the English usage.
References
- Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci USA. 2003;100:7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre P, Delaney SM, LaRocca T, Vincent D, DeGuzman F, Jurek M, Koller B, Phillips DR, Conley PB. P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. J Clin Invest. 2003;112:398–406. doi: 10.1172/JCI17864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardoni R, Goldstein PA, Lee CJ, Gu JG, MacDermott AB. ATP P2X receptors mediate fast synaptic transmission in the dorsal horn of the rat spinal cord. J Neurosci. 1997;17:5297–5304. doi: 10.1523/JNEUROSCI.17-14-05297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borvendeg SJ, Gerevich Z, Gillen C, Illes P. P2Y receptor-mediated inhibition of voltage-dependent Ca2+ channels in rat dorsal root ganglion neurons. Synapse. 2003;47:159–161. doi: 10.1002/syn.10156. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CB, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Communi D, Janssens R, Suarez-Huerta N, Robaye B, Boeynaems JM. Advances in signalling by extracellular nucleotides. the role and transduction mechanisms of P2Y receptors. Cell Signal. 2000;12:351–360. doi: 10.1016/s0898-6568(00)00083-8. [DOI] [PubMed] [Google Scholar]
- Daniel JL, Dangelmaier C, Jin J, Ashby B, Smith JB, Kunapuli SP. Molecular basis for ADP-induced platelet activation. I. Evidence for three distinct ADP receptors on human platelets. J Biol Chem. 1998;273:2024–2029. doi: 10.1074/jbc.273.4.2024. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. The Neuroscientist. 2004;10:40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- Echeverry S, Shi XQ, Zhang J. Characterization of cell proliferation in rat spinal cord following peripheral nerve injury and the relationship with neuropathic pain. Pain. 2008;135:37–47. doi: 10.1016/j.pain.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Fagura MS, Dainty IA, McKay GD, Kirk IP, Humphries RG, Robertson MJ, Dougall IG, Leff P. P2Y1-receptors in human platelets which are pharmacologically distinct from P2Y(ADP)-receptors. Br J Pharmacol. 1998;124:157–164. doi: 10.1038/sj.bjp.0701827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fam SR, Gallagher CJ, Salter MW. P2Y1 purinoceptor-mediated Ca2+ signaling and Ca2+ wave propagation in dorsal spinal cord astrocytes. J Neurosci. 2000;20:2800–2808. doi: 10.1523/JNEUROSCI.20-08-02800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerevich Z, Borvendeg SJ, Schröder W, Franke H, Wirkner K, Nörenberg W, Fürst S, Gillen C, Illes P. Inhibition of N-type voltage-activated calcium channels in rat dorsal root ganglion neurons by P2Y receptors is a possible mechanism of ADP-induced analgesia. J Neurosci. 2004;24:797–807. doi: 10.1523/JNEUROSCI.4019-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo LH, Trautmann K, Schluesener HJ. Expression of P2X4 receptor by lesional activated microglia during formalin-induced inflammatory pain. J Neuroimmunol. 2005;163:120–127. doi: 10.1016/j.jneuroim.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- Hansson E, Ronnback L. Glial neuronal signaling in the central nervous system. FASEB J. 2003;17:341–348. doi: 10.1096/fj.02-0429rev. [DOI] [PubMed] [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- Ho C, Hicks J, Salter MW. A novel P2-purinoceptor expressed by a subpopulation of astrocytes from the dorsal spinal cord of the rat. Br J Pharmacol. 1995;116:2909–2918. doi: 10.1111/j.1476-5381.1995.tb15944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, Conley PB. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21:1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur J Neurosci. 2005;22:2431–2440. doi: 10.1111/j.1460-9568.2005.04451.x. [DOI] [PubMed] [Google Scholar]
- Idestrup CP, Salter MW. P2Y and P2U receptors differentially release intracellular Ca2+ via the phospholipase c/inositol 1,4,5-triphosphate pathway in astrocytes from the dorsal spinal cord. Neuroscience. 1998;86:913–923. doi: 10.1016/s0306-4522(98)00128-6. [DOI] [PubMed] [Google Scholar]
- Inoue K. The function of microglia through purinergicreceptors: neuropathic pain and cytokine release. Pharmacol Ther. 2006;109:210–226. doi: 10.1016/j.pharmthera.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Ji RR, Strichartz G. Cell signaling and the genesis of neuropathic pain. Sci STKE. 2004;2004 doi: 10.1126/stke.2522004re14. reE14. [DOI] [PubMed] [Google Scholar]
- Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8:1–10. doi: 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- Ji RR, Kawasaki Y, Zhuang ZY, Wen YR, Zhang YQ. Protein kinases as potential targets for the treatment of pathological pain. Handb Exp Pharmacol. 2007;177:359–389. doi: 10.1007/978-3-540-33823-9_13. [DOI] [PubMed] [Google Scholar]
- Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YH, Schlichter R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nat Neurosci. 1999;2:241–245. doi: 10.1038/6344. [DOI] [PubMed] [Google Scholar]
- Kalmar B, Greensmith L, Malcangio M, McMahon SB, Csermely P, Burnstock G. The effect of treatment with BRX-220, a co-inducer of heat shock proteins, on sensory fibers of the rat following peripheral nerve injury. Exp Neurol. 2003;184:636–647. doi: 10.1016/S0014-4886(03)00343-1. [DOI] [PubMed] [Google Scholar]
- Katsura H, Obata K, Mizushima T, Sakurai J, Kobayashi K, Yamanaka H, Dai Y, Fukuoka T, Sakagami M, Noguchi K. Activation of Src-family kinases in spinal microglia contributes to mechanical hypersensitivity after nerve injury. J Neurosci. 2006;26:8680–8690. doi: 10.1523/JNEUROSCI.1771-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- Kim SY, Bae JC, Kim JY, Lee HL, Lee KM, Kim DS, Cho HJ. Activation of p38 MAP kinase in the rat dorsal root ganglia and spinal cord following peripheral inflammation and nerve injury. NeuroReport. 2002;13:2483–2486. doi: 10.1097/00001756-200212200-00021. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Yamanaka H, Dai Y, Obata K, Tokunaga A, Noguchi K. Differential expression patterns of mRNAs for P2X receptor subunits in neurochemically characterized dorsal root ganglion neurons in the rat. J Comp Neurol. 2005;481:377–390. doi: 10.1002/cne.20393. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Yamanaka H, Dai Y, Obata K, Tokunaga A, Noguchi K. Neurons and glial cells differentially express P2Y receptor mRNAs in the rat dorsal root ganglion and spinal cord. J Comp Neurol. 2006;498:443–454. doi: 10.1002/cne.21066. [DOI] [PubMed] [Google Scholar]
- Koistinaho M, Koistinaho J. Role of p38 and p44/42 mitogen-activated protein kinases in microglia. Glia. 2002;40:175–183. doi: 10.1002/glia.10151. [DOI] [PubMed] [Google Scholar]
- Lakshmi S, Joshi PG. Co-activation of P2Y2 receptor and TRPV channel by ATP: implications for ATP induced pain. Cell Mol Neurobiol. 2005;25:819–832. doi: 10.1007/s10571-005-4936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever I, Cunningham J, Grist J, Yip PK, Malcangio M. Release of BDNF and GABA in the dorsal horn of neuropathic rats. Eur J Neurosci. 2003;18:1169–1174. doi: 10.1046/j.1460-9568.2003.02848.x. [DOI] [PubMed] [Google Scholar]
- Li P, Calejesan AA, Zhuo M. ATP P2x receptors and sensory synaptic transmission between primary afferent fibers and spinal dorsal horn neurons in rats. J Neurophysiol. 1998;80:3356–3360. doi: 10.1152/jn.1998.80.6.3356. [DOI] [PubMed] [Google Scholar]
- Light AR, Wu Y, Hughen RW, Guthrie PB. Purinergic receptors activating rapid intracellular Ca increases in microglia. Neuron Glia Biol. 2006;2:125–138. doi: 10.1017/S1740925X05000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Chu KL, Namovic MT, Donnelly-Roberts DL, Harris RR, Zhang XF, Shieh CC, Wismer CT, Zhu CZ, Gauvin DM, Fabiyi AC, Honore P, Gregg RJ, Kort ME, Nelson DW, Carroll WA, Marsh K, Faltynek CR, Jarvis MF. P2X7-related modulation of pathological nociception in rats. Neuroscience. 2007;146:1817–1828. doi: 10.1016/j.neuroscience.2007.03.035. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Twining C, Chacur M, Biedenkapp J, O'Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller T, Kann O, Verkhratsky A, Kettenmann H. Activation of mouse microglial cells affects P2 receptor signaling. Brain Res. 2000;853:49–59. doi: 10.1016/s0006-8993(99)02244-1. [DOI] [PubMed] [Google Scholar]
- Nakatsuka T, Gu JG. ATP P2X receptor-mediated enhancement of glutamate release and evoked EPSCs in dorsal horn neurons of the rat spinal cord. J Neurosci. 2001;21:6522–6531. doi: 10.1523/JNEUROSCI.21-17-06522.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K, Noguchi K. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci. 2004;74:2643–2653. doi: 10.1016/j.lfs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, Tokunaga A, Noguchi K. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J Neurosci. 2004;24:10211–10222. doi: 10.1523/JNEUROSCI.3388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa K, Irino Y, Nakamura Y, Akazawa C, Inoue K, Kohsaka S. Involvement of P2X4 and P2Y12 receptors in ATP-induced microglial chemotaxis. Glia. 2007;55:604–616. doi: 10.1002/glia.20489. [DOI] [PubMed] [Google Scholar]
- Okada M, Nakagawa T, Minami M, Satoh M. Analgesic effects of intrathecal administration of P2Y nucleotide receptor agonists UTP and UDP in normal and neuropathic pain model rats. J Pharmacol Exp Ther. 2002;303:66–73. doi: 10.1124/jpet.102.036079. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Hoshi M, Akazawa C, Nakamura Y, Tsuzuki H, Inoue K, Kohsaka S. Selective expression of Gi/o-coupled ATP receptor P2Y12 in microglia in rat brain. Glia. 2003;44:242–250. doi: 10.1002/glia.10293. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Downie JW, Reid AR, Cahill CM, White TD. ATP release from dorsal spinal cord synaptosomes: characterization and neuronal origin. Brain Res. 1993;610:32–38. doi: 10.1016/0006-8993(93)91213-c. [DOI] [PubMed] [Google Scholar]
- Scemes E, Suadicani SO, Spray DC. Intercellular communication in spinal cord astrocytes: fine tuning between gap junctions and P2 nucleotide receptors in calcium wave propagation. J Neurosci. 2000;20:1435–1445. doi: 10.1523/JNEUROSCI.20-04-01435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. Can we conquer pain? Nat Neurosci. 2002;5(Suppl):1062–1067. doi: 10.1038/nn942. [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Sung CS, Wen ZH, Chang WK, Chan KH, Ho ST, Tsai SK, Chang YC, Wong CS. Inhibition of p38 mitogen-activated protein kinase attenuates interleukin-1beta-induced thermal hyperalgesia and inducible nitric oxide synthase expression in the spinal cord. J Neurochem. 2005;94:742–752. doi: 10.1111/j.1471-4159.2005.03226.x. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86:1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Schafers M, Jones TL, Powell H, Sorkin LS. Spinal blockade of TNF blocks spinal nerve ligation-induced increases in spinal P-p38. Neurosci Lett. 2005;13:209–213. doi: 10.1016/j.neulet.2004.12.064. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Peters MC, Ma JY, Kerr I, Mangadu R, Chakravarty S, Dugar S, Medicherla S, Protter AA, Yeomans DC. Peripheral and central p38 MAPK mediates capsaicin-induced hyperalgesia. Pain. 2004;111:278–285. doi: 10.1016/j.pain.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang T, Beggs S, Salter MW. Purinoceptors in microglia and neuropathic pain. Pflügers Arch. 2006;452:645–652. doi: 10.1007/s00424-006-0074-5. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45:89–95. doi: 10.1002/glia.10308. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Turner NA, Moake JL, McIntire LV. Blockade of adenosine diphosphate receptors P2Y(12) and P2Y(1) is required to inhibit platelet aggregation in whole blood under flow. Blood. 2001;98:3340–3345. doi: 10.1182/blood.v98.12.3340. [DOI] [PubMed] [Google Scholar]
- Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci. 2004;20:1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001a;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Spinal cord glia: new players in pain. Pain. 2001b;93:201–205. doi: 10.1016/S0304-3959(01)00359-1. [DOI] [PubMed] [Google Scholar]
- Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Vadakkan KI, Zhuo M. ATP-induced chemotaxis of microglial processes requires P2Y receptor-activated initiation of outward potassium currents. Glia. 2007;55:810–821. doi: 10.1002/glia.20500. [DOI] [PubMed] [Google Scholar]
- Xu B, Stephens A, Kirschenheuter G, Greslin AF, Cheng X, Sennelo J, Cattaneo M, Zighetti ML, Chen A, Kim SA, Kim HS, Bischofberger N, Cook G, Jacobson KA. Acyclic analogues of adenosine bisphosphates as P2Y receptor antagonists: phosphate substitution leads to multiple pathways of inhibition of platelet aggregation. J Med Chem. 2002;45:5694–5709. doi: 10.1021/jm020173u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka H, Obata K, Fukuoka T, Dai Y, Kobayashi K, Tokunaga A, Noguchi K. Tissue plasminogen activator in primary afferents induces dorsal horn excitability and pain response after peripheral nerve injury. Eur J Neurosci. 2004;19:93–102. doi: 10.1046/j.1460-9568.2003.03080.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O'Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur J Neurosci. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, Decosterd I, Ji RR. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci. 2006;26:3551–3560. doi: 10.1523/JNEUROSCI.5290-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]





