Abstract
Extracellular nucleotides have been implicated as signaling molecules used by microglia to sense adverse physiological conditions, such as neuronal damage. They act through purinoceptors, especially the G-protein-coupled P2Y receptor P2Y12R. Emerging evidence has indicated that activated spinal microglia responding to nerve injury are key cellular intermediaries in the resulting highly debilitating chronic pain state, namely neuropathic pain. However, the role of microglial P2Y12Rs in neuropathic pain remains unknown. Here, we show that the level of P2Y12R mRNA expression was markedly increased in the spinal cord ipsilateral to the nerve injury and that this expression was highly restricted to ionized binding calcium adapter molecule 1-positive microglia. An increase in the immunofluorescence of P2Y12R protein in the ipsilateral spinal cord was also observed after nerve injury, and P2Y12R-positive cells were double labeled with the microglial marker OX-42. Blocking spinal P2Y12R by the intrathecal administration of its antagonist AR-C69931MX prevented the development of tactile allodynia (pain hypersensitivity to innocuous stimuli), a hallmark of neuropathic pain syndrome. Furthermore, mice lacking P2ry12 (P2ry12−/−) displayed impaired tactile allodynia after nerve injury without any change in basal mechanical sensitivity. Moreover, a single intrathecal administration of AR-C69931MX or oral administration of clopidogrel (a P2Y12R blocker clinically in use) to nerve-injured rats produced a striking alleviation of existing tactile allodynia. Together, our findings indicate that activation of P2Y12Rs in spinal microglia may be a critical event in the pathogenesis of neuropathic pain and suggest that blocking microglial P2Y12R might be a viable therapeutic strategy for treating neuropathic pain.
Keywords: P2Y12 receptor, microglia, neuropathic pain, purinergic, spinal cord, analgesia
Introduction
Peripheral nerve injury leads to a persistent neuropathic pain state in which innocuous stimulation elicits pain behavior (tactile allodynia). Recent studies have revealed that microglia, the immune effector cells in the CNS, critically contribute to the pathogenesis of neuropathic pain (Watkins and Maier, 2003; Marchand et al., 2005; Tsuda et al., 2005). Therefore, glial cells, especially microglia, have received much attention as a new therapeutic target for the treatment of neuropathic pain.
Extracellular nucleotides are known to be potent stimulators of microglia (Inoue, 2006); they play roles in various physiological and pathophysiological functions by activating purinergic receptors, which are classified into the ionotropic P2X receptors (P2XR) and the metabotropic P2Y receptors (P2YR) (Burnstock, 2006). Microglia predominantly express P2X4R, P2X7R, P2Y6R, and P2Y12R purinoceptors (Koizumi et al., 2007). We have shown previously that activation of P2X4R, which is upregulated in spinal microglia after peripheral nerve injury, contributes to neuropathic pain through a release of brain-derived neurotrophic factor (Tsuda et al., 2003; Coull et al., 2005). Our recent study revealed that activating P2Y6Rs induces phagocytosis of brain microglia through the Gq/phospholipase C/IP3 pathway (Koizumi et al., 2007). Furthermore, P2Y12R, which is coupled to Gi signaling in microglia, is implicated in ATP-induced membrane ruffling and chemotaxis toward a source of ATP (Honda et al., 2001; Haynes et al., 2006; Ohsawa et al., 2007). A recent study has demonstrated that P2Y12R is required for the extension of microglial processes to engulf injured cells in vivo (Davalos et al., 2005; Haynes et al., 2006; Kurpius et al., 2007). Moreover, whereas the microglial ATP receptors (for example, P2X4R, P2X7R, and P2Y6R) are expressed in both microglia and peripheral macrophages (Di Virgilio et al., 2001), the P2Y12R subtype is unique in that its expression is restricted to microglia in CNS parenchyma (Sasaki et al., 2003; Haynes et al., 2006). These observations suggest that microglia are key sensors of adverse conditions in the CNS, detecting nucleotides via P2Y12Rs. Despite rapid progress in elucidating the physiological functions of microglia mediated by P2Y12R, relatively little insight has been gained concerning the in vivo role of P2Y12Rs in pathophysiological conditions in the CNS.
In the present study, we sought to investigate the role of microglial P2Y12Rs in the spinal cord in neuropathic pain and discovered that activation of P2Y12Rs in spinal microglia is a critical step in the pathogenesis of neuropathic pain, using selective antagonists for P2Y12R and mice lacking P2Y12R. Our present data suggest that blocking microglial P2Y12Rs might be a viable therapeutic strategy for treating neuropathic pain.
Materials and Methods
All experimental procedures were performed under the guidelines of Kyushu University.
Animals.
Male Wistar rats (Japan SLC, Hamamatsu, Japan) and 10-week-old wild-type and P2ry12−/− mice, both of which were derived from C57BL/6J × 129S1 mice (Hashimoto et al., 2007) and were kindly provided by Dr. Kazuo Umemura (Hamamatsu University School of Medicine, Hamamatsu, Japan), were used in this study. Animals were housed with a 12 h light/dark cycle (lights on at 8:00 A.M., lights off at 8:00 P.M.) at a constant room temperature of 23 ± 2°C and humidity of 45–65%.
Neuropathic pain model.
The left L5 spinal nerve of rats was tightly ligated with silk and cut just distal to the ligature under isoflurane (2%) anesthesia. For the experiments using mice, the left L5 spinal nerve was transected. To assess the tactile allodynia, calibrated von Frey filaments (0.4–15 g for rats, 0.02–2.0 g for mice; North Coast Medical, Morgan Hill, CA) were applied to the plantar surface of the hindpaw. The 50% paw-withdrawal threshold (PWT) was determined by the up–down method (Chaplan et al., 1994).
Real-time reverse transcription-PCR.
Rats were deeply anesthetized with pentobarbital, perfused transcardially with PBS, and the L5 spinal cord was removed immediately. The tissues were vertically separated by median, and hemisections of the spinal cord were subjected to total RNA extraction using Trisure (Bioline, Danwon-Gu, South Korea) according to the protocol of the manufacturer and purified with RNeasy mini plus kit (Qiagen, Valencia, CA). The amount of total RNA was quantified by measuring OD260 using a Nanodrop spectrophotometer (Nanodrop, Wilmington, DE). For reverse transcription with random 6-mer primers, 400 ng of total RNA was transferred to the reaction with Prime Script reverse transcriptase (Takara, Kyoto, Japan). Quantitative PCR was performed with Premix Ex Taq (Takara) using a 7500 real-time PCR system (Applied Biosystems, Foster City, CA) according to protocol of the manufacturer, and the data were analyzed by 7500 System SDS Software 1.3.1 (Applied Biosystems) using the standard curve method. Expression levels were normalized to the values for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The TaqMan probe for P2RY12 (5′-FAM-CACCAGACCATTTAAAACTTCCAGCCCC-TAMRA-3′), the forward primer for P2RY12 (5′-TAACCATTGACCGATACCTGAAGA-3′), and the reverse primer for P2RY12 (5′-TTCGCACCCAAAAGATTGC-3′), as well as the primers and probe for GAPDH, were obtained from Applied Biosystems.
In situ hybridization.
Digoxigenin (DIG)-labeled RNA probes were designed having complementary sequence of rat P2ry12 mRNA (GenBank accession number NM_022800) positioned at 26–616 bases. Animals were anesthetized and perfused transcardially with 4% paraformaldehyde/PBS, pH 7.4, 7 d after nerve injury. The L5 spinal cord was removed and again fixed with Tissue Fixative (Genostaff, Tokyo, Japan). Paraffin-embedded tissues (6 μm) were dewaxed with xylene and rehydrated. After proteinase K treatment (7 mg/ml, 30 min, 37°C) and acetylation by acetic anhydride (0.25%), hybridization was performed with probes at concentrations of 300 ng/ml at 60°C for 16 h. After hybridization, a series of washing was performed, followed by RNase treatment (50 mg/ml, 30 min, 37°C). The sections were blocked with 0.5% blocking reagent (Roche, Indianapolis, IN) in Tris-buffered saline containing Tween 20 and incubated with anti-DIG alkaline phosphatase conjugate (1:1000; Roche) for 2 h at room temperature. Coloring reactions were performed with nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate solution (NBT/BCIP) (Sigma, St. Louis, MO) overnight. The sections were counterstained with Kernechtrot stain solution (Mutoh, Tokyo, Japan) and then mounted with Crystal/Mount (BioMeda, Foster City, CA). For immunohistochemistry as a second staining after NBT/BCIP treatment, the sections were treated 0.3% hydrogen peroxide and Protein Block (Dako, High Wycombe, UK) for 10 min and then incubated with the anti-ionized binding calcium adapter molecule 1 (Iba1) rabbit polyclonal antibody (0.1 μg/ml; Wako Pure Chemicals, Osaka, Japan) at 4°C overnight. The sections were treated with Histofine Simplestain rat MAX-PO (MULTI) (Nichirei, Tokyo, Japan) for 30 min, incubated with DAB, and then counterstained with Kernechtrot stain solution. Colocalization was assessed in 12 nonoverlapping regions of the spinal cord (88 cells in total).
Immunohistochemistry.
Animals were anesthetized and perfused transcardially with 4% paraformaldehyde/PBS, pH 7.4, on day 14 after nerve injury. The L5 spinal cord was removed and postfixed at 4°C for 5 h and then transferred to 30% sucrose/PBS for 24 h. Floating transverse sections (30 μm) were blocked in solution containing 3% normal goat serum and 0.1% Triton X-100 for 3 h at room temperature. Then, the sections were incubated 48 h at 4°C with primary antibodies against P2Y12R (rabbit polyclonal anti-P2Y12R, 1:500; kindly provided by Dr. David Julius, University of California, San Francisco, San Francisco, CA), OX-42 (rat or mouse monoclonal anti-OX-42, 1:2000; Serotec, Oxford, UK), Iba1 (rabbit polyclonal anti-Iba1, 1:2000; Wako Pure Chemicals), glial fibrillary acidic protein (GFAP) (mouse monoclonal anti-GFAP, 1:1000; Millipore Bioscience Research Reagents, Temecula, CA), and microtubule-associated protein-2 (MAP2) (mouse monoclonal anti-MAP2, 1:500; Millipore Bioscience Research Reagents). After washing, the sections were then incubated with fluorescent-conjugated secondary antibody (Alexa 488 and Alexa 543, 1:1000; Invitrogen, Carlsbad, CA) for 3 h at room temperature. The sections were mounted with Vectashield (Vector Laboratories, Burlingame, CA). Fluorescent images were obtained with a confocal microscope (LSM 5 Pascal; Carl Zeiss, Jena, Germany) and analyzed with Zeiss LSM Image Brower (Carl Zeiss).
Drug administration.
For intrathecal drug administration, under isoflurane (2%) anesthesia, rats were implanted with a 32-gauge intrathecal catheter (ReCathCo, Allison Park, PA) in the lumbar enlargement (close to L4–L5 segments) of the spinal cord (Tsuda et al., 2003). After 4 d recovery, the catheter placement was verified by the observation of transient hindpaw paralysis induced by intrathecal injection of lidocaine (2%, 5 μl). Animals that failed to show any paralysis were not used in experiments. After peripheral nerve injury, rats were administered intrathecally PBS (5 μl, as a vehicle control) or AR-C69931MX (0.5, 5.0, and 50 nmol in 5 μl) twice a day (9:00 A.M. and 7:00 P.M.) from day 0 (just after nerve injury) to day 7. Measurement of PWT was performed just before evening drug administration (at least 9 h after the first of daily administration). To test the effects of AR-C69931MX (0.5, 5.0, and 50 nmol in 5 μl of PBS), the orally active P2Y12R antagonist (Emmons and Taylor, 2007) clopidogrel (1, 10, and 25 mg/kg, suspended in 0.5% calboxymethyl cellulose; LKT Laboratories, St. Paul, MN), MRS2211(500 pmol in 5 μl PBS; Tocris Bioscience, Bristol, UK), and MRS2179 (50 pmol in 5 μl PBS; Sigma), on existing tactile allodynia, drugs were acutely administered intrathecally or orally to nerve-injured rats on day 7 after nerve injury. Drug administration was performed just after premeasurement, and PWT was measured at each time point.
Statistical analysis.
All data are presented as means ± SEM. The statistical significance of differences between the values was determined by paired t tests or ANONVA with a post hoc test (Tukey's test). A p value of <0.05 was considered to be statistically significant.
Results
Upregulation of P2Y12 receptor in the dorsal horn after peripheral nerve injury
Using real-time PCR, we examined the level of P2Y12R mRNA in total RNA extracts from the spinal cord ipsilateral and contralateral to an injury to the L5 spinal nerve. We found that the expression of P2Y12R mRNA in the ipsilateral spinal cord was markedly increased after nerve injury (p < 0.05) (Fig. 1A). A significant increase was observed from day 3 (3.23 ± 0.48-fold increase to uninjured spinal cord) after injury; P2Y12R mRNA levels peaked on day 7 (3.92 ± 0.69-fold) and decreased afterward. We also performed in situ hybridization and observed strong P2Y12R mRNA signals dotted throughout the ipsilateral dorsal horn 7 d after nerve injury. The number of P2Y12R mRNA-positive cells in the ipsilateral dorsal horn was ∼3.3-fold higher than that in the contralateral side (Fig. 1B). To identify the cell type positive for P2Y12R mRNA signals, we combined in situ hybridization for P2Y12R mRNA with immunohistochemistry for Iba1, a marker of microglia, and found that almost all of P2Y12R mRNA-expressing cells were labeled with Iba1 (94.5 ± 3.1% of P2Y12R-positive cells) in both the ipsilateral and contralateral dorsal horns (Fig. 1 C–E). We also performed immunolabeling for P2Y12R using a specific antibody (Haynes et al., 2006) and found that the P2Y12R immunofluorescence was markedly enhanced in the ipsilateral dorsal horn on days 7 and 14 after nerve injury (Fig. 2 A–D). Using cell-type-specific markers (MAP2 to label neurons, GFAP to label astrocytes, and OX-42 to label microglia), we found that almost all of the P2Y12R-positive cells were double labeled with OX-42 (Fig. 2E,F) but not with the other markers (Fig. 2 G–J). From these results, we conclude that, in the dorsal horn after nerve injury, the level of P2Y12R expression is dramatically increased and that this expression is highly restricted to microglia. P2Y12R expression was not observed in neurons or astrocytes, although periphery platelets also expressed P2Y12R.
Figure 1.
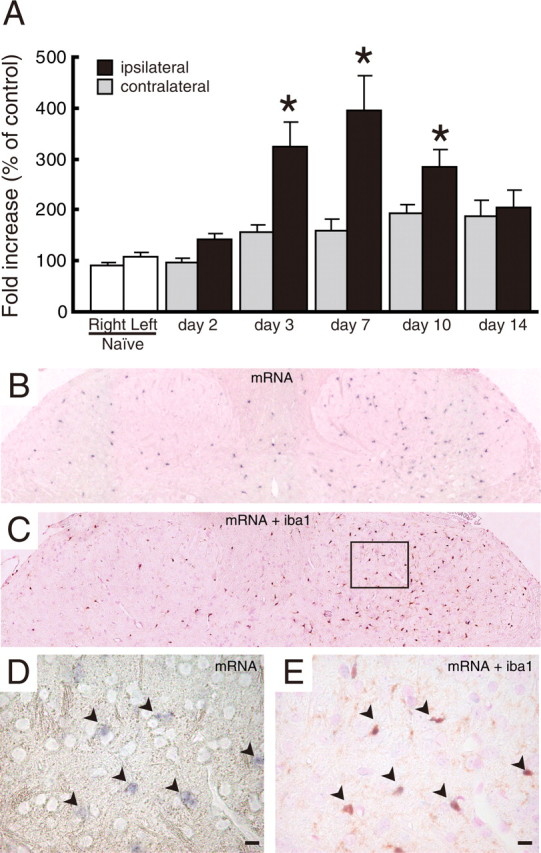
Upregulation of P2Y12R mRNA after peripheral nerve injury. Quantitative and histological analyses of P2Y12R mRNA expression in the spinal cord are shown. A, Total RNA extracted from rat spinal cord was subjected to quantitative analysis of P2Y12 mRNA expression after peripheral nerve injury by real-time PCR. Bar graphs show the average fold increase in the level of P2Y12 mRNA expression in spinal cord hemisections compared with the mean expression level of P2Y12 mRNA in naive animals. Each measurement was normalized to GAPDH content. Data are means ± SEM of five individual animals (*p < 0.05 vs the naive spinal cord, one-way ANOVA post hoc Tukey's test). B, A DIG-labeled RNA probe specific for P2Y12 mRNA was visualized by in situ hybridization in rat spinal cord, 7 d after the nerve injury. C, An RNA-probed slice was subsequently stained with anti-Iba1 antibody and visualized with DAB staining. D, E, Magnifications of the squared area show the sections before and after Iba1 staining. Arrowheads indicate colocalization of P2Y12 mRNA-positive cells and Iba1 signals. Scale bars, 10 μm.
Figure 2.
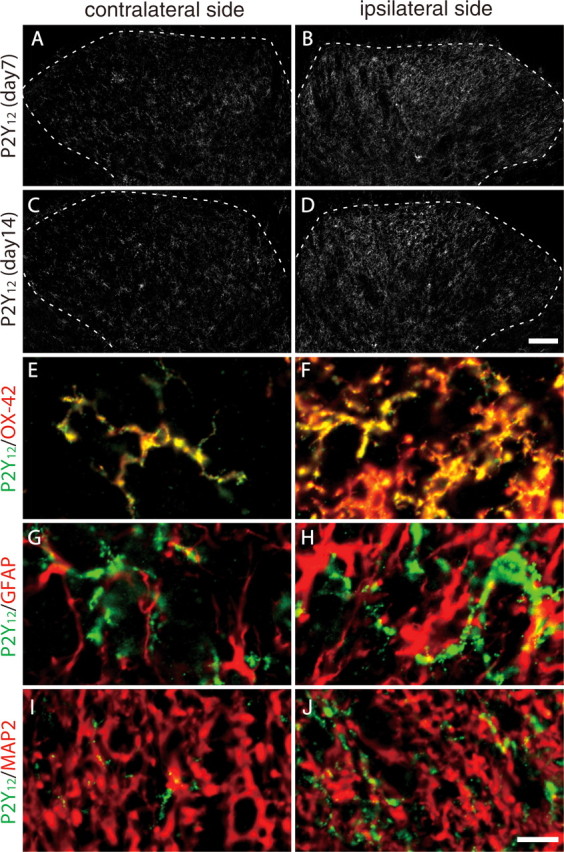
P2Y12R immunoreactivity localized to rat spinal microglia. A–D, Immunoreactivity of P2Y12R protein was detected by a specific antibody for P2Y12R in the dorsal spinal cord 7 (A, B) and 14 (C, D) days after nerve injury. Scale bar, 100 μm. E–J, Double-immunofluorescence labeling of P2Y12R with OX-42 (a marker of microglia), GFAP (a marker of astrocytes), and MAP2 (a marker of neurons). Scale bar, 10 μm.
Inhibition of tactile allodynia development by blocking P2Y12R
To investigate the role of P2Y12Rs expressed in spinal microglia in neuropathic pain, we intrathecally administered AR-C69931MX, an antagonist for P2Y12R (Ingall et al., 1999), to rats twice a day for 7 d, through a catheter whose tip was positioned near the L4/5 spinal cord. In vehicle (PBS)-treated rats, the PWT was decreased after injury to the L5 spinal nerve (p < 0.05) (Fig. 3A). In contrast, repeated intrathecal administration of AR-C69931MX markedly suppressed this decrease in PWT after nerve injury, in a dose-dependent manner (p < 0.05) (Fig. 3A). In rats that had received 50 pmol of AR-C69931MX, the PWT on every testing day was not significantly different from that before nerve injury. However, AR-C69931MX, at any dose we tested, did not affect PWT on the side contralateral to the nerve injury (Fig. 3B). In addition, neither motor abnormality nor sedative effects were observed in PBS- or AR-C69931MX-treated rats throughout the experiments. These results indicate that AR-C69931MX prevents the development of tactile allodynia after peripheral nerve injury.
Figure 3.
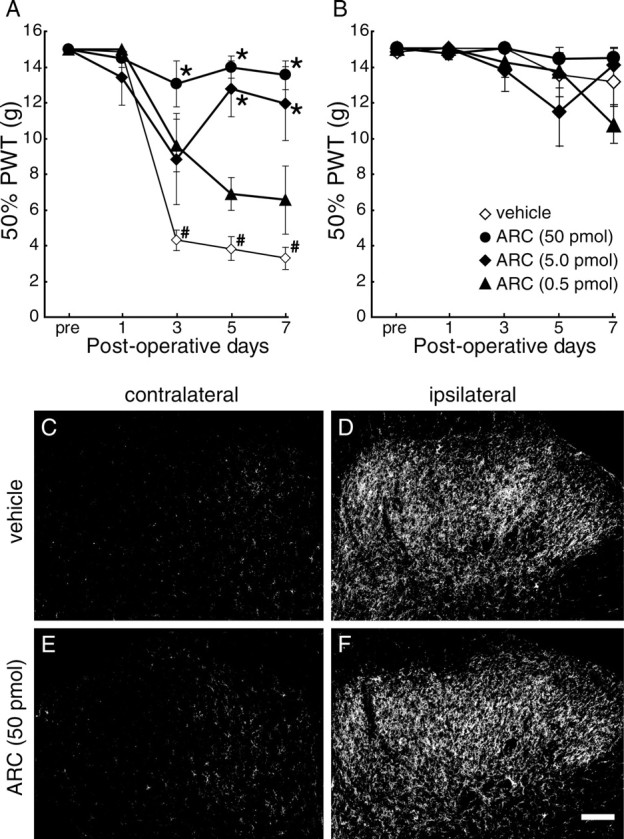
Inhibition of P2Y12R prevented the development of tactile allodynia. A, The PWT of the ipsilateral hindpaw in response to tactile stimulation in rats were examined before nerve injury and 1, 3, 5, and 7 d after nerve injury. Rats were subjected to intrathecal administration of AR-C69931MX (ARC) at several doses (n = 5 each) or vehicle (n = 7), twice a day, after peripheral nerve injury. B, PWTs were measured in the contralateral hindpaws. Each data point represents the mean ± SEM of PWT (*p < 0.05 vs vehicle group; #p < 0.05 vs premeasurement by multiple comparison by Tukey's test after a repeated-measure two-way ANOVA). C–F, L5 spinal cord segment from 7 d postoperative rats that received intrathecal injections of vehicle (PBS; C, D) or AR-C69931MX (50 pmol; E, F) twice daily were subjected to immunohistochemistry using anti-OX-42 antibody. Scale bar, 100 μm.
To examine whether AR-C69931MX affects activation of spinal microglia, we performed immunolabeling of spinal sections from AR-C69931MX-treated nerve-injured rats with the microglial marker OX-42. As reported previously (Tsuda et al., 2003), a marked increase in OX-42 immunoreactivity was observed in the ipsilateral dorsal spinal cords of vehicle-treated rats 7 d after nerve injury (Fig. 3C,D). In contrast to the effect of tactile allodynia, repeated intrathecal administration of AR-C69931MX (50 pmol) did not suppress this increase in OX-42 labeling (Fig. 3E,F).
P2ry12 deficiency attenuates development of tactile allodynia
To clearly determine the functional relevance of P2Y12R in neuropathic pain, we used P2Y12R-deficient mice (P2ry12−/− mice). P2ry12−/− mice showed no alteration in basal mechanical sensitivity compared with wild-type mice (wild-type, 1.64 ± 0.17 g; P2ry12−/−, 1.81 ± 0.18 g). In wild-type mice with an injury to the L5 spinal nerve, a progressive decrease in PWT was observed after nerve injury (p < 0.05) (Fig. 4A). In contrast, this decrease in PWT was not seen in P2ry12−/− mice. A significant difference in PWT between P2ry12−/− mice and wild-type mice was observed from day 5 to day 14 (p < 0.05). However, the loss of P2ry12 did not change the PWT in the contralateral hindpaw after nerve injury (Fig. 4A) and did not result in a deficit in motor function. In addition, we confirmed that the level of P2Y12 mRNA in the ipsilateral spinal cord was increased after injury in wild-type mice (data not shown), as observed in the rat spinal cord.
Figure 4.
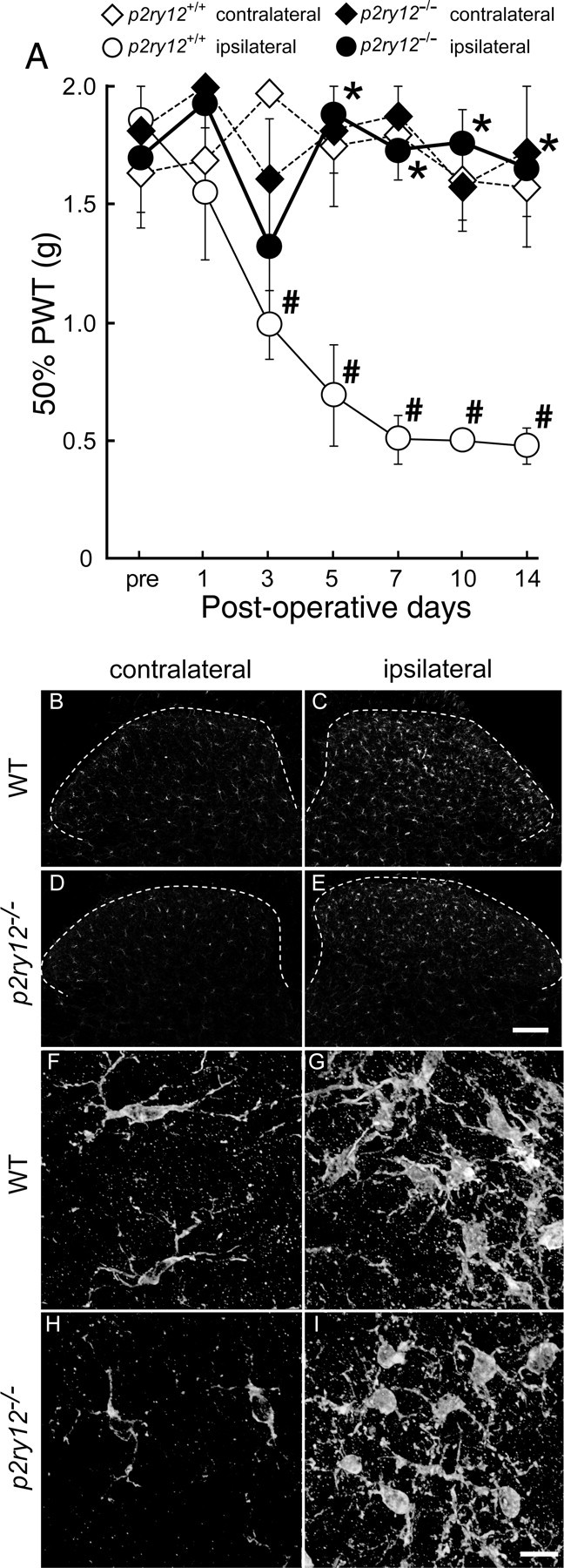
Deletion of P2ry12 attenuated the development of tactile allodynia. A, PWT was measured in wild-type and P2ry12−/− mice before and after L5 spinal nerve transection. Each data point represents the mean ± SEM of PWT (in grams) of four animals from each group (*p < 0.05 vs p2ry12+/+ group; #p < 0.05 vs premeasurement by multiple comparison by Tukey's test after a repeated-measure two-way ANOVA). B–E, L5 spinal cord segments from 14 d postoperative mice were subjected to immunohistochemistry using anti-Iba1 antibody. Dotted lines trace the outline of the dorsal horn. Scale bar, 100 μm. F–I, High-magnification images constructed from a series of z-stack images using the deconvolution technique. Scale bar, 10 μm.
Because several studies have revealed that P2Y12Rs on microglia contribute to their chemotaxis toward sites of injury, we performed immunohistochemical analysis to observe how the localization of spinal microglia is altered by P2ry12 deficiency. Immunofluorescence for Iba1 in the dorsal horns of wild-type mice was enhanced in the ipsilateral dorsal horn compared with the contralateral dorsal horn, 14 d after nerve injury (Fig. 4B,C). In P2ry12−/− mice, Iba1 immunofluorescence was also increased in the ipsilateral side (Fig. 4D,E). In wild-type mice, the number of Iba1-positive microglia in the dorsal horn was increased by ∼2.6 ± 0.2-fold compared with contralateral side (181 ± 41 cells in the ipsilateral side, 68 ± 7 cells in the contralateral side). In contrast, the increase in P2ry12−/− mice was ∼2.0 ± 0.2-fold (136 ± 22 cells on the ipsilateral side, 69 ± 8 cells on the contralateral side) increase to the contralateral side. Statistical analysis indicated a significant increase of microglial number in the ipsilateral dorsal horn in each genotypes, but in the ipsilateral dorsal horn between these two genotypes (p = 0.11 in comparisons between ipsilateral numbers, p = 0.09 in comparisons between fold increases). Microglia in the ipsilateral dorsal horns of wild-type mice displayed hypertrophy in their somata and had short, thick processes (Fig. 4F,G), which are known to be morphological features of activated microglia. Similar morphological changes in microglia were observed in the dorsal horn microglia of P2ry12−/− mice after nerve injury (Fig. 4H,I).
P2Y12R blocking agents induced a relieving effect on existing tactile allodynia
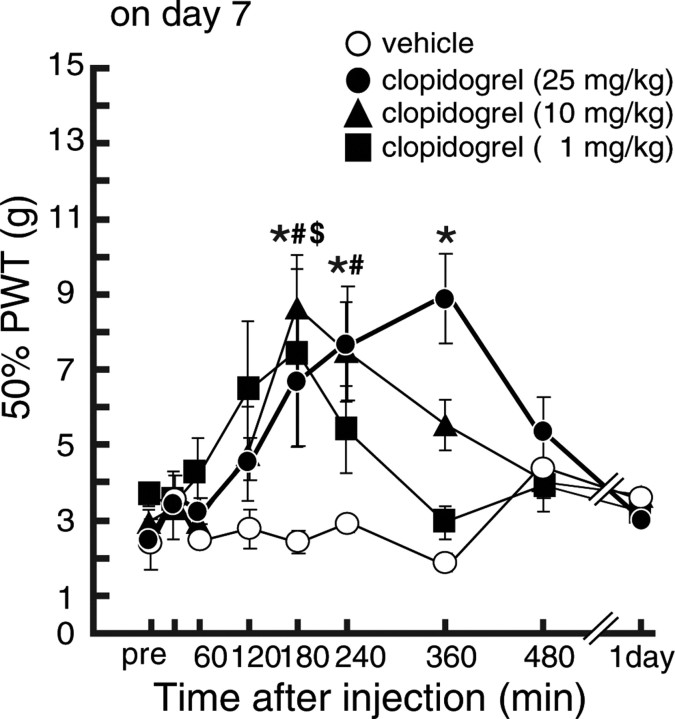
We then examined whether pharmacological blockade of P2Y12R could also be effective in treating existing tactile allodynia. On day 7 after nerve injury, the decreased PWT (p < 0.05; 12.25 ± 0.73 g on the contralateral side, 3.61 ± 0.26 g on the ipsilateral side) was not changed by intrathecal vehicle administration over a 6 h period. In contrast, nerve-injured rats intrathecally administered AR-C69931MX on day 7 showed a significant recovery of this decreased PWT from 2 h after the injection, in a dose-dependent manner (p < 0.05) (Fig. 5). AR-C69931MX also produced a recovery of this decreased PWT (p < 0.05; 13.46 ± 0.68 g on the contralateral side, 3.34 ± 0.27 g on the ipsilateral side) on day 14 (p < 0.05) (Fig. 5C). To test for the involvement of other receptors that are antagonized by AR-C69931MX, P2Y13, and G-protein-coupled receptor 17 (GPR17) (Abbracchio et al., 2006; Ciana et al., 2006; von Kugelgen, 2006), we also tested a selective P2Y13 receptor antagonist, MRS2211 (500 pmol), and MRS2179 (50 pmol), which works as an antagonist for GPR17 (also as P2Y1 but not for P2Y12) with high affinity (IC50 of 0.18 pm to rat GPR17) (Ciana et al., 2006). These two antagonists failed to produce any effect on the decrease in PWT (Fig. 5B). To further investigate the relieving effect resulting from P2Y12R inhibition in a more clinically relevant paradigm, we examined the effect of the peripherally active P2Y12R blocker clopidogrel. After a single oral administration of clopidogrel (1, 10, or 25 mg/kg) to nerve-injured rats on day 7, the PWT (p < 0.05; 12.24 ± 0.59 g on the contralateral side, 2.92 ± 0.21 g on the ipsilateral side) was increased (Fig. 6), and statistically significant differences were observed 3 h (1, 10, or 25 mg/kg), 4 h (25 mg/kg), and 6 h (25 mg/kg) after the administration of clopidogrel (p < 0.05, ipsilateral PWT compared with the vehicle-treated group), whereas vehicle-treatment did not produce any effect.
Figure 5.
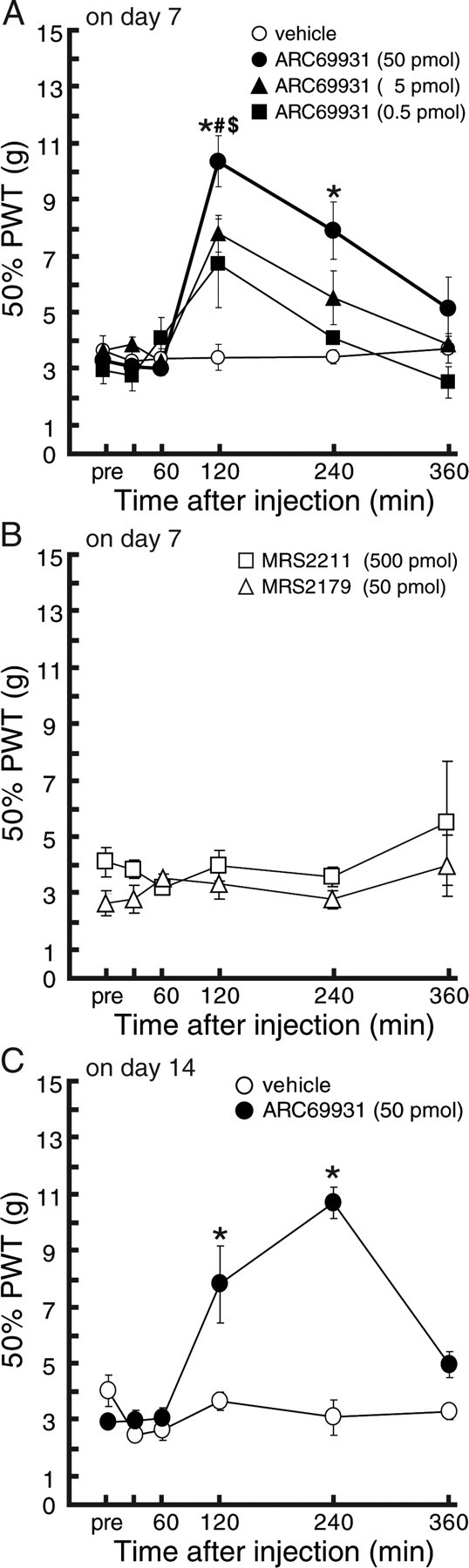
Relieving effects of AR-C69931MX in rats with tactile allodynia. A, B, Seven day postoperative rats showing strong tactile allodynia were subjected to verification of the analgesic effect induced by intrathecal administration of drugs. Vehicle (n = 6), AR-C69931MX (0.5, 5, and 50 pmol; n = 5–7), MRS2211 (500 pmol; n = 4), or MRS2179 (50 pmol; n = 3) was injected intrathecally, and PWT was measured. C, Fourteen day postoperative rats showing strong tactile allodynia were subjected to verification of the analgesic effect induced by P2Y12 blocking agents. Vehicle (n = 3) or AR-C69931MX (50 pmol; n = 5) was injected intrathecally, and PWT was measured. Each data point represents the mean ± SEM of PWT ($,#,*p < 0.05; 0.5, 5, and 50 pmol-treated groups vs the vehicle group by multiple comparison by Tukey's test after a repeated-measure two-way ANOVA).
Figure 6.
Relieving effects of systemically administered clopidogrel in rats with tactile allodynia. Seven day postoperative rats showing strong tactile allodynia were subjected to verification of the analgesic effect induced by clopidgrel. Systemically administered clopidogrel (1, 10, and 25 mg/kg; n = 4, 5, and 6), but not vehicle (n = 3), also showed an analgesic effect in rats with tactile allodynia 7 d after peripheral nerve injury. Each data point represents the mean ± SEM of PWT ($,#,*p < 0.05; 1, 10, and 25 mg/kg-treated groups vs vehicle group by multiple comparison by Tukey's test after a repeated-measure two-way ANOVA).
Discussion
In the present study, we provide the first evidence that activation of P2Y12Rs in spinal microglia is critical for the pathogenesis of neuropathic allodynia, a major behavioral consequence of nerve injury, using molecular, immunohistochemical, pharmacological, and genetic approaches. Our data from real-time PCR and in situ hybridization analyses show that P2Y12R gene expression is increased in the ipsilateral spinal cord after nerve injury, expression of which in the dorsal horn is highly restricted to microglia. The numbers of microglia were also increased in the ipsilateral spinal cord. Because there are some consistencies between the fold increase in the amount of mRNA and the number of microglia, the main reason for the P2Y12R mRNA upregulation in the ipsilateral spinal cord tissue is considered to result from the increased numbers of microglia. However, the possibility of transcriptional upregulation of P2Y12R in individual activated microglia may also be expected, because our results showed that the fold increase of mRNA was higher than the increase in the number of P2Y12R mRNA-positive cells. The increased expression of spinal P2Y12R after nerve injury is strongly supported by our data showing an increase in the immunofluorescence for P2Y12R protein after nerve injury and its specific localization in spinal microglia. The P2Y12R immunofluorescence in activated microglia persisted for at least 14 d after nerve injury, although, after day 7, the level of P2Y12R mRNA began to gradually return to the basal level. However, a recent study reported a rapid downregulation of P2Y12R protein expression in microglia in hippocampal slice cultures after tissue damage and in the striatum in vivo after treatment with lipopolysaccharide (LPS), a major constituent of the outer membrane of Gram-negative bacteria (Haynes et al., 2006). The exact reason for the inconsistency in the regulation of P2Y12R expression in activated microglia remains unclear, but it might be explained by differences between different regions of the CNS (spinal cord vs hippocampus and striatum) and/or in the experimental methods for activation of microglia (peripheral nerve injury vs LPS and slicing the brain tissue). Consistently with our results, microglial P2Y12R upregulation is also observed in the facial nucleus after injury to the peripheral facial motor nerve (Sasaki et al., 2003). Therefore, the results of the present study suggest that activated microglia may upregulate the expression of P2Y12R, at both mRNA and protein levels, in response to peripheral nerve injury.
Our present behavioral study demonstrated that blocking spinal P2Y12R chronically, by means of repeated intrathecal administration of AR-C69931MX, prevented the development of nerve injury-induced tactile allodynia. The functional relevance of P2Y12R in neuropathic allodynia is substantially supported by our data obtained from a behavioral analysis of P2Y12R-deficient mice; P2ry12−/− mice failed to show tactile allodynia after nerve injury and showed no deficit in basal sensitivity to mechanical stimuli and motor function. These results, together with the highly restricted localization of P2Y12R to microglia, indicate that the P2Y12R activation in spinal cord microglia may be responsible for the expression of neuropathic allodynia after nerve injury. Thus, P2Y12R-mediated microglial functions (for example, morphological changes, chemotaxis, and process movement) are considered to be required for the development of neuropathic allodynia. However, P2ry12−/− mice were indistinguishable from wild-type mice with regard to the number and morphology of activated microglia in the ipsilateral spinal dorsal horn. Therefore, P2Y12Rs might not have a major role in the morphological changes of spinal microglia triggered by peripheral nerve injury.
We thus considered the possibility that P2Y12Rs may participate in the functions of already activated microglia. This notion is supported by our data showing that (1) neither the pharmacological blockade of P2Y12Rs nor the genetic ablation of P2Y12R affected the mechanical sensitivity on the contralateral side in nerve-injured animals or that in non-injured animals whose dorsal horns have normal resting type microglia, and (2) the upregulation of P2Y12R occurs in activated microglia in the dorsal horn after nerve injury. In the present study, we demonstrated that nerve injury-induced tactile allodynia was reversed by a single intrathecal administration of AR-C69931MX, implying that an ongoing activity of P2Y12R in spinal microglia also contributes to emerging tactile allodynia after nerve injury. Notably, we also demonstrated a reversing effect of a orally administered clopidogrel (Emmons and Taylor, 2007), a well known antithrombotic compound targeting P2Y12R in platelets with safety profiles from an extensive clinical program (Savi and Herbert, 2005), on existing tactile allodynia. It has been reported that the antagonistic effect of clopidogrel on P2Y12R is dependent on its active metabolite through hepatic metabolism, and 10 mg/kg oral clopidogrel induced ∼90% inhibition of ex vivo platelet aggregation, and a transfer of the clopidogrel or its metabolite across the blood–brain barrier was also observed (Herbert et al., 1993). Because we observed a significant effect of clopidogrel with 10 mg/kg oral administration and a higher dose (25 mg/kg) achieved a rather longer-lasting relieving effect on existing tactile allodynia, transfer of active metabolite of clopidogrel from plasma to the spinal cord parenchyma might be limited. Furthermore, the slow onset of the anti-allodynic effects may be attributable in part to such pharmacokinetics. It should be noted that this analgesia may be an off-target effect of clopidogrel, because the clinical target of this compound is P2Y12R in platelets. These behavioral data thus suggest that activation of P2Y12R, which is upregulated in spinal microglia, may be crucial for the maintenance of neuropathic pain after nerve injury, in addition to the role in its development.
The mechanism(s) underlying microglial P2Y12R-mediated neuropathic pain regulation remains to be determined. Accumulating evidence has indicated that the development and maintenance of neuropathic pain are also regulated by activities of other microglial molecules [for example, P2X4R (Tsuda et al., 2003), chemokine receptors (Zhang et al., 2007; Zhuang et al., 2007), Toll-like receptors (Tanga et al., 2005; Kim et al., 2007), mitogen-activated protein kinases (Jin et al., 2003; Zhuang et al., 2005), Src-family kinases (Katsura et al., 2006), and cathepsin S (Clark et al., 2007)]. It is possible that P2Y12R may interact with these molecules in activated spinal microglia and that this interaction may cause neuropathic pain. Indeed, we recently demonstrated that ATP-induced chemotaxis of microglial cells requires both P2Y12R and P2X4R, an important microglial molecule for neuropathic pain (Ohsawa et al., 2007), although its role in neuropathic pain remains unknown. Several lines of evidence have indicated that P2Y12R is implicated in the motility of microglial cell bodies and processes (Honda et al., 2001; Davalos et al., 2005; Haynes et al., 2006; Wu et al., 2007). It is thus possible that P2Y12R activity in microglia may influence the abilities of microglia to extend the tips of their branched processes toward neighboring pain transmission neurons, which may in turn affect microglia–neuron communications. Future studies focusing on the physical contact between microglial processes and other cells, using electron microscopy, will be important to advance our understanding of the mechanisms by which spinal microglia control nerve injury-induced neuropathic pain.
In summary, the present study demonstrated that P2Y12R expression is upregulated at both mRNA and protein levels in the ipsilateral spinal cord after nerve injury and that this expression is highly restricted to microglia. Intrathecal administration of the P2Y12R antagonist AR-C69931MX prevented the development of tactile allodynia, and P2ry12−/− mice displayed impaired tactile allodynia after nerve injury. We also found that a single intrathecal administration of AR-C69931MX or oral administration of clopidogrel to nerve-injured rats produced a striking alleviation of existing tactile allodynia. These results suggest that activation of P2Y12Rs in spinal microglia may be a critical event in the development and maintenance of neuropathic pain and that blocking microglial P2Y12Rs might represent a therapeutic strategy for treating neuropathic pain.
Footnotes
This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (H.S.-T., M.T., K.I.). We are grateful to Dr. Kazuo Umemura for providing P2ry12 knock-out mice and to Dr. David Julius for providing polyclonal anti-P2Y12 antibody.
References
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic P2 receptors as targets for novel analgesics. Pharmacol Ther. 2006;110:433–454. doi: 10.1016/j.pharmthera.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, Ferrario S, Parravicini C, Capra V, Gelosa P, Guerrini U, Belcredito S, Cimino M, Sironi L, Tremoli E, Rovati GE, Martini C, Abbracchio MP. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 2006;25:4615–4627. doi: 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AK, Gentry C, Bradbury EJ, McMahon SB, Malcangio M. Role of spinal microglia in rat models of peripheral nerve injury and inflammation. Eur J Pain. 2007;11:223–230. doi: 10.1016/j.ejpain.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- Emmons KL, Taylor NR. Contemporary issues in clopidogrel therapy: new evidence shaping clinical practice. Pharmacotherapy. 2007;27:553–563. doi: 10.1592/phco.27.4.553. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Sugidachi A, Isobe T, Niitsu Y, Ogawa T, Jakubowski JA, Asai F. The influence of P2Y12 receptor deficiency on the platelet inhibitory activities of prasugrel in a mouse model: evidence for specific inhibition of P2Y12 receptors by prasugrel. Biochem Pharmacol. 2007;74:1003–1009. doi: 10.1016/j.bcp.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- Herbert JM, Frehel D, Vallee E, Kieffer G, Gouy D, Berger Y, Necciari J, Defreyn G, Maffrand JP. Clopidogrel, a novel antiplatelet and antithrombotic agent. Cardiovasc Drug Rev. 1993;11:180–198. [Google Scholar]
- Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21:1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingall AH, Dixon J, Bailey A, Coombs ME, Cox D, McInally JI, Hunt SF, Kindon ND, Teobald BJ, Willis PA, Humphries RG, Leff P, Clegg JA, Smith JA, Tomlinson W. Antagonists of the platelet P2T receptor: a novel approach to antithrombotic therapy. J Med Chem. 1999;42:213–220. doi: 10.1021/jm981072s. [DOI] [PubMed] [Google Scholar]
- Inoue K. The function of microglia through purinergic receptors: neuropathic pain and cytokine release. Pharmacol Ther. 2006;109:210–226. doi: 10.1016/j.pharmthera.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura H, Obata K, Mizushima T, Sakurai J, Kobayashi K, Yamanaka H, Dai Y, Fukuoka T, Sakagami M, Noguchi K. Activation of Src-family kinases in spinal microglia contributes to mechanical hypersensitivity after nerve injury. J Neurosci. 2006;26:8680–8690. doi: 10.1523/JNEUROSCI.1771-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kim MA, Cho IH, Kim MS, Lee S, Jo EK, Choi SY, Park K, Kim JS, Akira S, Na HS, Oh SB, Lee SJ. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J Biol Chem. 2007;282:14975–14983. doi: 10.1074/jbc.M607277200. [DOI] [PubMed] [Google Scholar]
- Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446:1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurpius D, Nolley EP, Dailey ME. Purines induce directed migration and rapid homing of microglia to injured pyramidal neurons in developing hippocampus. Glia. 2007;55:873–884. doi: 10.1002/glia.20509. [DOI] [PubMed] [Google Scholar]
- Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- Ohsawa K, Irino Y, Nakamura Y, Akazawa C, Inoue K, Kohsaka S. Involvement of P2X4 and P2Y12 receptors in ATP-induced microglial chemotaxis. Glia. 2007;55:604–616. doi: 10.1002/glia.20489. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Hoshi M, Akazawa C, Nakamura Y, Tsuzuki H, Inoue K, Kohsaka S. Selective expression of Gi/o-coupled ATP receptor P2Y12 in microglia in rat brain. Glia. 2003;44:242–250. doi: 10.1002/glia.10293. [DOI] [PubMed] [Google Scholar]
- Savi P, Herbert JM. Clopidogrel and ticlopidine: P2Y12 adenosine diphosphate-receptor antagonists for the prevention of atherothrombosis. Semin Thromb Hemost. 2005;31:174–183. doi: 10.1055/s-2005-869523. [DOI] [PubMed] [Google Scholar]
- Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci USA. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- von Kugelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Vadakkan KI, Zhuo M. ATP-induced chemotaxis of microglial processes requires P2Y receptor-activated initiation of outward potassium currents. Glia. 2007;55:810–821. doi: 10.1002/glia.20500. [DOI] [PubMed] [Google Scholar]
- Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci. 2007;27:12396–12406. doi: 10.1523/JNEUROSCI.3016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Kawasaki Y, Tan PH, Wen YR, Huang J, Ji RR. Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain Behav Immun. 2007;21:642–651. doi: 10.1016/j.bbi.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]