Abstract
Considerable evidence implicates the mesolimbic dopamine (DA) system in the processing of nicotine's reinforcing properties, specifically the ventral tegmental area (VTA) and the terminal fields of VTA DAergic projections to the “core” (NAcore) and “shell” (NAshell) subdivisions of the nucleus accumbens (NAc). However, the specific roles of DA D1-like and D2-like receptor subtypes in nicotine reward processing within these NAc subregions have not been elucidated. We report that microinfusions of DA D1-like or D2-like receptor-specific antagonists into NAcore or NAshell double dissociate the rewarding and aversive properties of systemic or intra-VTA nicotine, and differentially regulate sensitivity to the rewarding properties as well as the motivational valence of either intra-VTA or systemic nicotine administration. Using a place conditioning procedure, NAshell infusions of a D2-like receptor antagonist switched the motivational valence of intra-VTA nicotine from aversive to rewarding and potentiated nicotine reward sensitivity to sub-reward threshold intra-VTA nicotine doses. In contrast, NAcore infusions of a D1-like receptor antagonist switched intra-VTA nicotine aversion to reward, and potentiated reward sensitivity to sub-reward threshold nicotine doses. Thus, D1-like versus D2-like receptors in NAcore versus NAshell subdivisions play functionally dissociable roles in modulating systemic or intra-VTA nicotine motivational processing.
Keywords: nicotine, addiction, dopamine, ventral tegmental area, receptors, nucleus accumbens
Introduction
Nicotine, the psychoactive component of tobacco smoke, produces a variety of motivational effects and has strong addictive properties. Several neural regions and neurotransmitter systems have been implicated as critical mediators of nicotine's dependence-producing effects. Notably, evidence from human- and animal-based research implicates the mesolimbic dopamine (DA) system, comprising the ventral tegmental area (VTA) and its projections to the nucleus accumbens (NAc) core (NAcore) and shell (NAshell) subregions, as important regulators of nicotine's motivational properties (Picciotto and Corrigall, 2002; Laviolette and van der Kooy, 2004). The importance of DA neurotransmission in nicotine's motivational effects is further underscored by evidence showing that DA release is strongly increased by nicotine, by directly exciting VTA DAergic neurons and/or increasing release of DA in the terminal fields of the mesolimbic DAergic projections (Nisell et al., 1994; Yin and French, 2000; Mansvelder and McGehee, 2002). Furthermore, nicotine can act directly within the VTA to produce motivational effects (Laviolette and van der Kooy, 2003; David et al., 2006; Ikemoto et al., 2006) through either DA-dependent or non-DAergic neural systems (Picciotto et al., 1998; Laviolette and van der Kooy, 2003, 2004). Like many drugs of abuse, nicotine possesses both reinforcing and aversive stimulus properties, and nicotine dose-dependently produces rewarding or aversive effects directly within the VTA when measured with associative conditioning procedures such as conditioned place preference (CPP) or conditioned taste aversion (CTA) (Iwamoto, 1990; Jorenby et al., 1990; Shoaib and Stolerman, 1995; Fenu et al., 2001; Laviolette et al., 2002a,b; Laviolette and van der Kooy, 2003).
One region of particular importance for the processing of nicotine's motivational properties is the NAcore and NAshell ventral striatal subregions. Clear differences between these regions have been demonstrated at the anatomical, neurochemical, and neuropharmacological levels (Brog et al., 1993; Kirouac and Ganguly, 1995); however, little is known about how specific DA receptor subtypes (D1 vs D2) within the NAshell versus NAcore may be involved differentially in nicotine motivational phenomena. An equally important question concerns how modulation of DA receptor transmission within specific striatal subregions may regulate how nicotine is processed as a motivational stimulus. Given the importance of mesolimbic DA transmission in the etiology of nicotine addiction, we performed a series of experiments to determine the functional role of mesolimbic D1-like versus D2-like receptors in the modulation of systemic or intra-VTA nicotine motivational processing and to examine whether the NAcore versus NAshell subdivisions may differentially modulate sensitivity to the rewarding properties of nicotine. Furthermore, chronic exposure to and withdrawal from nicotine has been demonstrated to induce neuroadaptations in striatal DA transmission (Nisell et al., 1997; Rada et al., 2001), suggesting that disturbances in striatal DA transmission may underlie the aversive effects of nicotine withdrawal. Therefore, we examined the potential role of NAshell versus NAcore D1-like versus D2-like receptor populations in the processing of the aversive effects of nicotine withdrawal. Our results demonstrate that separate DA D1-like versus D2-like receptor substrates within the NAshell versus NAcore can independently modulate both the motivational valence of nicotine and control sensitivity to the rewarding properties of systemic or intra-VTA nicotine exposure.
Materials and Methods
Surgical procedures.
Male Sprague Dawley rats (Charles River; 300–350 g at the start of the experiments) were anesthetized with a ketamine (80 mg/ml)/xylazine (6 mg/ml) mixture and placed in a stereotaxic device. Stainless-steel guide cannulae (22 gauge; Plastics One) were bilaterally implanted at a 10° angle, 1.5 mm dorsal to central injection sites. The following stereotaxic coordinates were used: for the VTA, from bregma, anteroposterior (AP) −5.0, lateral (L) ±2.3; from the dural surface, ventral (V) −8.0. These stereotaxic VTA coordinates have been shown previously to produce anatomically and pharmacologically specific, nicotine-mediated motivational effects, because control injector placements dorsal to the VTA or caudal to these coordinates in the interpeduncular nucleus produce no motivational effects as measured in our CPP procedure (Laviolette and van der Kooy, 2003, 2004). For “core” and “shell” microinfusion placements in the nucleus accumbens, we used the following stereotaxic coordinates: for “shell” placements, from bregma, at a 12° angle: AP +1.8, L ±2.6; from the dural surface, V −7.4; for “core” placements, from bregma, at an 8° angle: AP +1.8, L ±2.7; from the dural surface, V −6.4. These NAcore and NAshell coordinates have been reported previously to produce anatomically specific effects, relative to DA D1 versus D2 receptor blockade (Pattij et al., 2007). Furthermore, microinfusions of mixed D1/D2 receptor antagonists at locations dorsal to these NAc positions fail to influence intra-VTA nicotine motivational processing (Laviolette and van der Kooy, 2003). At the conclusion of experiments, animals were deeply anesthetized and transcardially perfused with isotonic saline followed by 10% formalin. Brain sections were stained with cresyl violet, and VTA or NAshell versus NAcore cannula placements were verified with light microscopy according to the anatomical boundaries for these structures determined by Paxinos and Watson (2005).
Drug treatments.
Nicotine-di-d-tartrate (Sigma), R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine (SCH 23390; Tocris), or eticlopride (Sigma) was dissolved in PBS (pH adjusted to 7.4). For intra-VTA, NAcore, or NAshell bilateral microinfusions (0.5 μl volume per infusion), injector cannulae were slowly lowered into the implanted guides, and drug or saline infusions were performed over 1 min. Injectors were left in place for an additional 1 min after injection, to ensure adequate diffusion from the tip. Based on previously reported intra-VTA nicotine dose–response curves, we selected five concentrations of nicotine to establish our initial dose–response curve for the motivational effects of intra-VTA nicotine (see Fig. 1C), representing aversive (0.008 nmol/0.5 μl), neutral (0.08–0.8 nmol/0.5 μl), and rewarding (8 nmol/0.5 μl) intra-VTA concentrations of nicotine [see also Laviolette et al. (2002a,b) and Laviolette and van der Kooy (2003)]. We chose dose ranges for intra-NAc SCH 23390 and eticlopride (0.1–1.0 μg/0.5 μl) that have been demonstrated previously to effectively block drug-related associative learning in rodents after intra-NAc microinfusions, including cocaine (Anderson et al., 2003), opiates (Bossert et al., 2007), and natural food rewards (Nowend et al., 2001), and to effectively block the locomotor stimulatory effects of systemic nicotine (Boye et al., 2001). To control for possible nonspecific motivational effects of SCH 23390 or eticlopride, rats received NAcore or NAshell microinfusions of these compounds immediately before both saline or nicotine intra-VTA microinfusions, thereby balancing out any possible motivational effects of either drug, across all conditioning environments. For all experiments, bilateral intra-NAc microinfusions of either SCH 23390 or eticlopride were administered immediately before bilateral microinfusions of intra-VTA nicotine. Systemic doses of nicotine (0.1–0.8 mg/kg) or the nicotinic ACh receptor (nAChR) antagonist mecamylamine (MEC; 1 mg/kg) were performed subcutaneously.
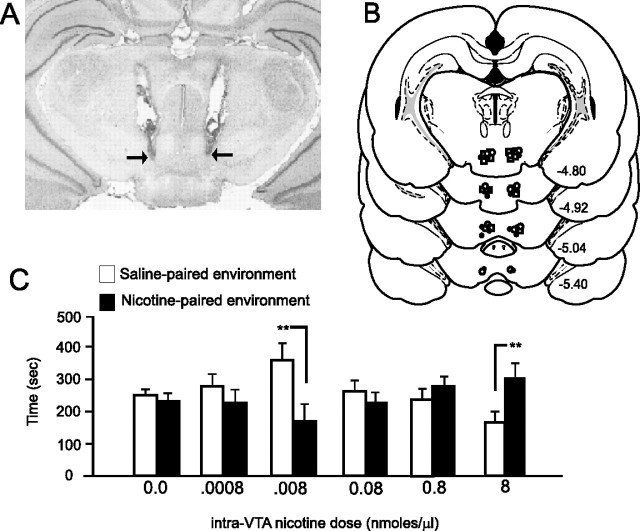
Figure 1.
A, Microphotograph demonstrating representative intra-VTA bilateral injector cannula tip placements (arrows). B, Schematic diagram showing representative locations for bilateral intra-VTA cannula injection sites for nicotine microinfusions, located in both the rostral and caudal regions of the VTA. C, Bilateral microinfusions of intra-VTA nicotine produce dose-dependent, bivalent motivational effects, as measured in an unbiased place conditioning paradigm over a wide dose range. Whereas a lower nicotine dose (0.008 nmol/0.5 μl) produces a significant CPA (**p < 0.01), middle-range concentrations produce no motivational effects (neither CPP nor CPA) (0.08–0.8 nmol/0.5 μl; p values > 0.05), and a higher dose (8 nmol/0.5 μl) produced significant CPP for the nicotine-paired environment (**p < 0.01). Error bars indicate SEM.
Chronic nicotine treatment.
For chronic nicotine exposure experiments, nicotine hydrogen tartrate salt (Sigma) was dissolved in sterile saline solution (0.9% NaCl). The pH was adjusted to 7.4 with NaOH. To achieve a nicotine dose of 9 mg/kg per day (nicotine free base), a stock solution of 600 mg/ml nicotine was used for subjects with a body weight of ∼300 g and adjusted accordingly (Malin et al., 1994; Suzuki et al., 1996). Control animals received saline vehicle osmotic pump implantations. For surgical osmotic pump implantation, animals were anesthetized with isoflurane. A sterile incision was made at the back of the shoulder, and osmotic minipumps (model 2002; Alzet) were implanted subdermally at the nape of the neck and infused a solution of nicotine tartrate salt dissolved in saline or saline alone (vehicle) at a rate of 0.5 ml/h for a total of 14 d. To determine nicotine dependence in chronically treated rats, a mecamylamine-induced withdrawal conditioned place aversion (CPA) procedure was performed with rats receiving 1 mg/kg (s.c.) MEC or saline injections immediately before being placed in one of the place conditioning environments, as described above. This dose of MEC has been reported previously to induce strong aversive and somatic nicotine withdrawal symptoms (Suzuki et al., 1996; Rada et al., 2001).
Place conditioning.
All animals were conditioned using a standard, unbiased and fully counterbalanced CPP procedure, as described in detail previously (Laviolette et al., 2002a,b; Laviolette and van der Kooy, 2003). Conditioning took place in one of two environments, which differed in color, texture, and smell. One environment was white with a wire mesh floor, covered in wood chips. The other environment was black with a smooth Plexiglas floor, wiped down with a 2% acetic acid solution before each conditioning session. Animals display no baseline preference for either of these two environments (Laviolette and van der Kooy, 2003). All conditioning sessions were 30 min in length. Animals received four drug-environment and four saline-environment conditioning sessions, and exposure to environments was fully counterbalanced in all experiments. All neurotransmitter-blocking drugs were administered before both drug and saline injections. At testing, 1 week after the end of conditioning (all animals were tested drug free), animals were placed on a narrow, neutral gray zone that separated the two test compartments. Times spent in each environment were scored separately for each animal over a 10 min test session. All data were analyzed with one- or two-way ANOVA or Student's t tests where appropriate. Post hoc analyses were performed with Newman–Keuls or Fisher's least significant difference tests where appropriate.
Results
Motivational effects of intra-VTA nicotine and histological analysis of intra-VTA and intra-NAc cannula placements
Using a previously described dose–response curve for intra-VTA nicotine's motivational effects (Laviolette et al., 2002a,b; Laviolette and van der Kooy, 2003), we first examined the motivational effects of intra-VTA nicotine using our unbiased CPP procedure (see Materials and Methods). We performed bilateral microinfusions of a wide concentration range of nicotine (0.0008–8.0 nmol/0.5 μl) directly to the VTA. In Figure 1A, a microphotograph shows representative bilateral injector tip placements within the VTA. For clarity, we present a representative schematic showing bilateral intra-VTA injection sites in Figure 1B from histological analysis of rats used in the initial intra-VTA nicotine dose–response analysis. As in previous reports (Laviolette et al., 2002a,b; Laviolette and van der Kooy, 2003), bilateral nicotine microinfusions were localized in both rostral and caudal regions of the VTA. Histological analysis revealed no correlation between rostral versus caudal VTA nicotine microinfusions in terms of the motivational properties revealed in the CPP procedure. Furthermore, nicotine microinfusions into these VTA regions are anatomically specific, because microinfusions of nicotine into regions dorsal to the VTA or in the caudal interpeduncular region produce no motivational effects whatsoever in the CPP procedure, as reported previously (Laviolette and van der Kooy, 2003). Bilateral intra-VTA microinfusions produce a biphasic dose–response curve when measured in our unbiased CPP procedure (n = 7 for all groups in Fig. 1C). Statistical analysis revealed a significant group × treatment interaction (F(6,125) = 9.82; p < 0001). Post hoc analysis showed that whereas a lower nicotine dose (0.008 nmol/0.5 μl) produced a significant CPA (p < 0.01), middle-range concentrations produced no motivational effects (neither CPP nor CPA) (0.08–0.8 nmol/0.5 μl; p values > 0.05), and a higher dose (8 nmol/0.5 μl) produced significant CPP for the nicotine-paired environment (p < 0.01), consistent with previous reports (Laviolette et al., 2002a,b; Laviolette and van der Kooy, 2003). In our next series of experiments, animals received quadruple cannulations with bilateral cannula implantations into NAcore or NAshell (see Materials and Methods) matched with bilateral intra-VTA cannula implants. Results of our histological analysis of NAcore versus NAshell cannula placements are summarized in Figure 2. Animals found to have cannula placements outside the anatomical boundaries of either NAcore or NAshell were removed from analysis. In Figure 2A, a representative microphotograph shows a typical intrashell injector tip location (for clarity, only one hemisphere is shown) within the “shell” subregion of the NAc. In Figure 2, B and C, we present schematic diagrams showing representative injector cannula tip placements for NAshell SCH 23390 microinfusions from rats receiving intra-VTA microinfusions of 0.008 nmol/0.5 μl of nicotine (n = 8, to be described presently) and representative injector tip locations for rats receiving NAshell eticlopride versus intra-VTA 0.008 nmol/0.5 μl of nicotine (n = 8, results to be described presently). In Figure 2D, a representative microphotograph shows a typical NAcore injector tip location (for clarity, only one hemisphere is shown). In Figure 2, E and F, we present schematic diagrams showing representative injector cannula tip placements for NAcore SCH 23390 microinfusions (Fig. 2E) from rats receiving intra-VTA microinfusions of 0.008 nmol/0.5 μl of nicotine (n = 8, to be described presently) and representative injector tip locations for NAcore eticlopride (Fig. 2F) injector tip locations from animals receiving intra-VTA 0.008 nmol/0.5 μl of nicotine (n = 8, results to be described presently).
Figure 2.
Histological analysis of NAcore versus NAshell microinfusion cannula tip placements. For clarity, placements are shown only for the intra-VTA nicotine 0.008 nmol/0.5 μl group experiments. A, Microphotograph showing representative cannula tip located within the shell subdivision of the NAc. B, C, Schematic diagram showing representative placements for bilateral NAshell microinfusion sites for SCH 23390 (1 μg/0.5 μl; B) or NAshell microinfusion placements for eticlopride microinjections (n = 8; C). D, Microphotograph showing representative cannula tip located within the NAcore. E, F, Schematic diagram showing representative placements for bilateral NAcore microinfusion sites for SCH 23390 (n = 8; E) or NAcore microinfusion placements for bilateral eticlopride microinjections (n = 7; F). Co, Core; Sh, shell.
Intra-Nac DA receptor blockade dose-dependently switches systemic nicotine aversion into reward
We next performed a series of experiments to determine (1) the effective dose range for NAcore and NAshell D1/D2 receptor blockade on modulating nicotine motivational signaling; (2) whether the behavioral effects of NAcore versus NAshell D1-like or D2-like receptor blockade would modulate the motivational properties of systemically administered nicotine; and (3) whether the behavioral effects of NAshell versus NAcore D1-like or D2-like receptor blockade are dose dependent. Accordingly, we first tested two separate doses of systemic nicotine (0.1 and 0.8 mg/kg, s.c.) in our CPP procedure (Fig. 3A). In contrast to intra-VTA nicotine microinfusions, higher versus lower systemic nicotine concentrations produce aversive motivational effects, as measured in unbiased CPP procedures (Jorenby et al., 1990; Laviolette and van der Kooy, 2003), possibly because of interactions with peripheral nAChR receptors as opposed to intra-VTA microinfusions, which would directly access the mesolimbic DA motivational system. Comparing difference scores between times spent in saline versus nicotine-paired environments revealed a significant difference between systemic nicotine doses (0.1 mg/kg vs 0.8 mg/kg; t(13) = 3.73; p < 0.01), with only the higher dose of nicotine (0.8 mg/kg, s.c.) producing a significant CPA, consistent with previous reports (Jorenby et al., 1990; Laviolette and van der Kooy, 2003). This dose regimen of subcutaneous nicotine (0.1–0.8 mg/kg, s.c.) given over four conditioning trials does not produce any observable motoric sensitization. We next compared the ability of NAcore versus NAshell blockade of either D1 or D2 receptors to dose-dependently switch the aversive properties of our effective systemic dose of nicotine (0.8 mg/kg, s.c.) into reward, by testing an order of magnitude dose range of either SCH 23390 (0.1–1.0 μg/0.5 μl) into the NAcore or eticlopride (0.1–1.0 μg/0.5 μl) into the NAshell. SCH 23390 microinfusions into NAcore dose-dependently reversed the aversive effects of nicotine (F(22,47) = 21.0; p < 0001; 0.8 mg/kg, s.c.), switching a CPA into a CPP at the higher dose of 1 μg/0.5 μl (n = 7; p < 0.01); however, animals receiving saline (n = 6; p < 0.05) or the lower dose of SCH 23390 (0.1 μg/0.5 μl; n = 6; p < 0.01) still displayed a significant nicotine CPA (Fig. 3B). To determine whether the effects of NAcore D1 or D2 receptor blockade were producing their behavioral effects through independent versus “additive” signaling effects, we ran a separate control group (n = 7) that received a mixture of subthreshold doses of both SCH 23390 (0.1 μg/ml) and eticlopride (0.1 mg/ml) into the NAcore, before administration of the aversive dose of systemic nicotine (0.8 mg/mg, s.c.). Combinations of the D1 and D2 antagonist did not produce any additive functional effects within the NAcore, because rats displayed a significant CPA to nicotine, similarly to saline controls (t(6) = 2.4; p < 05) (Fig. 3B). Similarly, NAshell microinfusions of the DA D2 antagonist, eticlopride (1 μg/0.5 μl; n = 7), reversed the aversive effects of systemic nicotine (F(2,41) = 17.9; p < 001); however, animals receiving either saline (n = 6) or a lower dose of eticlopride (0.1 μg/0.5 μl; n = 6) still displayed a significant nicotine CPA (p values < 0.05) (Fig. 3C). To determine whether the effects of NAshell D1-like versus D2-like receptor blockade were producing their behavioral effects through independent versus “additive” signaling effects, we ran a separate control group (n = 7) that received a mixture of subthreshold doses of both SCH 23390 (0.1 μg/μl) and eticlopride (0.1 μg/μl) into the NAshell, before administration of the aversive dose of systemic nicotine (0.8 mg/mg, s.c.). Similar to our observations in the NAcore, combinations of the D1-like and D2-like receptor antagonists did not produce any additive functional effects within the NAshell, as rats displayed a significant CPA to nicotine, similarly to saline controls (t(6) = 2.53; p < 05) (Fig. 3C).
Figure 3.
NAcore or NAshell D1-like versus D2-like receptor blockade dose-dependently switches systemic nicotine aversion into reward. A, Initial pilot studies show a dose-dependent, systemic nicotine (0.1–0.8 mg/kg, s.c.)-mediated significant CPA to environments paired with 0.8 mg/kg systemic nicotine administration (n = 8) versus a lower dose of 0.1 mg/kg (n = 8), consistent with previous reports (Jorenby et al., 1990; Laviolette and van der Kooy, 2003). B, SCH 23390 microinfusions into the core of the NAc dose-dependently reverse the aversive effects of nicotine (0.8 mg/kg, s.c.), switching a CPA into a CPP at the higher dose of 1 μg/0.5 μl (n = 7; **p < 0.01); however, neither saline controls (n = 6; *p < 0.05) nor a lower dose of SCH 23390 (0.1 μg/0.5 μl; n = 6; **p < 0.01) reversed the nicotine CPA. Combined NAcore administration of subthreshold doses of SCH 23390 (0.1 μg/0.5 μl) and eticlopride (0.1 μg/0.5 μl) did not reverse the aversive properties of nicotine, because animals still displayed a significant CPA to the nicotine-paired environment (*p < 0.05), demonstrating that the behavioral effects of these D1/D2 receptor manipulations in NAcore were not additive. C, Eticlopride microinfusions into NAshell dose-dependently reverse the aversive effects of nicotine, because these animals (n = 7) show significant CPP to nicotine-paired environments (**p < 0.01); however, both saline controls (n = 6) and a lower dose of eticlopride (0.1 μg/0.5 μl) still display a significant nicotine CPA (*p values < 0.05). Combined NAshell administration of subthreshold doses of SCH 23390 (0.1 μg/0.5 μl) and eticlopride (0.1 μg/0.5 μl) did not reverse the aversive properties of nicotine, because animals still displayed a significant CPA to the nicotine-paired environment (*p < 0.05), demonstrating that the behavioral effects of these D1/D2 receptor manipulations in NAshell were not additive. Error bars indicate SEM.
NAcore versus NAshell blockade of D1 or D2 receptors double dissociates nicotine motivational signaling in the VTA
After determining the effective dose range for NAshell and NAcore D1/D2 receptor blockade with SCH 23390 and eticlopride (Fig. 3), we next examined the roles of both D1-like and D2-like DA receptor subtypes within either the NAcore or NAshell striatal subregions in the modulation of nicotine motivational processing within the VTA. Because we have determined previously that global DA receptor blockade fails to modulate the acute rewarding properties of supra-reward threshold doses of intra-VTA nicotine (Laviolette and van der Kooy, 2003, 2004), we chose a lower, aversive dose of intra-VTA nicotine (0.008 nmol/0.5 μl) (Fig. 1C) and a neutral (sub-reward threshold) dose of intra-VTA nicotine (0.8 nmol/0.5 μl) for our NAcore versus NAshell D1 and D2 antagonist challenge experiments. Thus, we chose both an aversive (0.008 nmol) and sub-reward threshold “neutral” dose (0.8 nmol) of intra-VTA nicotine to examine specifically the role of DAergic intra-NAc receptor signaling in either potentiating intra-VTA nicotine reward signals or in the reversal of nicotine aversion signals into reward signals (Laviolette and van der Kooy, 2003, 2004). We first challenged the aversive motivational properties of a low dose of intra-VTA nicotine (0.008 nmol/0.5 μl) (Fig. 1C) with bilateral NAshell or NAcore microinfusions of either the DA D1-like receptor antagonist SCH 23390 (1 μg/0.5 μl) or the DA D2-like receptor antagonist, eticlopride (1 μg/0.5 μl) (Fig. 4A). Statistical analysis revealed a significant group × treatment interaction (F(2,47) = 14.6; p < 001). Post hoc analysis revealed that both saline control and animals receiving NAshell SCH 23390 displayed a significant CPA for nicotine-paired environments (p values < 0.01), whereas animals receiving NAshell eticlopride displayed a significant CPP for nicotine-paired environments (p < 0.01). Comparing NAcore microinfusions of either SCH 23390 or eticlopride (Fig. 4A, right) revealed a significant group × treatment interaction (F(2,47) = 43.4; p < 0001). Post hoc analysis revealed that saline control (n = 6) and animals receiving NAcore eticlopride (n = 8) displayed significant aversions to environments paired with an aversive dose of intra-VTA nicotine (0.008 nmol; p < 0.05 and p < 0.01, respectively), whereas animals receiving NAcore SCH 23390 demonstrated a robust CPP for this same dose of intra-VTA nicotine (p < 0.01). Thus, NAshell D2-like receptor blockade (but not D1 blockade) effectively switched an aversive dose of intra-VTA nicotine (CPA) into a rewarding effect (CPP), whereas NAcore D1-like receptor blockade (but not D2 blockade) effectively switched an aversive VTA nicotine effect (CPA) into a rewarding effect (CPP) (Fig. 4A). We next challenged the effects of a neutral, sub-reward threshold dose of intra-VTA nicotine (0.8 nmol/0.5 μl) with bilateral NAshell or NAcore infusions of either SCH 23390 (1 μg/0.5 μl) or eticlopride (1 μg/0.5 μl) (Fig. 4B). Statistical analysis revealed a significant group × treatment interaction (F(2,47) = 4.7; p < 0.05). Post hoc analysis revealed that both saline control and animals receiving NAshell SCH 23390 displayed neither CPA nor CPP for nicotine-paired environments (p values > 0.05), whereas animals receiving NAshell eticlopride displayed a significant CPP for the nicotine-paired environments (Fig. 4B, left) (p < 0.01). Comparing NAcore microinfusions of either SCH 23390 or eticlopride (Fig. 4B, right) revealed a significant group × treatment interaction (F(2,47) = 6.11; p < 01). Post hoc analysis revealed that saline control (n = 6) and animals receiving NAcore eticlopride (n = 8) displayed neither CPP nor CPA to nicotine-paired environments (p values > 0.05), whereas animals receiving NAcore SCH 23390 (n = 8) demonstrated a robust CPP for this same dose of intra-VTA nicotine (Fig. 4B, right) (p < 0.01). In Figure 4C, we summarize the results of this series of conditioning experiments, expressing place conditioning scores as difference scores (time expressed as drug minus saline-paired environments), which clearly demonstrates the double dissociation between NAshell versus NAcore DA D1-like versus D2-like receptor blockade on switching or potentiating the motivational effects of these doses of intra-VTA nicotine. Thus, whereas DA D1-like blockade in the NAshell has no effect on the aversive effects of intra-VTA nicotine and fails to potentiate nicotine reward signaling in the VTA, this same dose of SCH 23390 administered into the NAcore switches intra-VTA nicotine aversion to reward and potentiates a sub-reward threshold dose of intra-VTA nicotine into a reward stimulus. Conversely, DA D2-like receptor blockade with eticlopride (1 μg/0. 5 μl) in NAcore has no effect on the aversive effects of intra-VTA nicotine (0.008 nmol/0.5 μl) and fails to potentiate intra-VTA nicotine reward. However, this same dose of eticlopride, when administered into the NAshell region, switches intra-VTA nicotine aversion to reward and potentiates a sub-reward threshold dose of intra-VTA nicotine into a reward signal.
Figure 4.
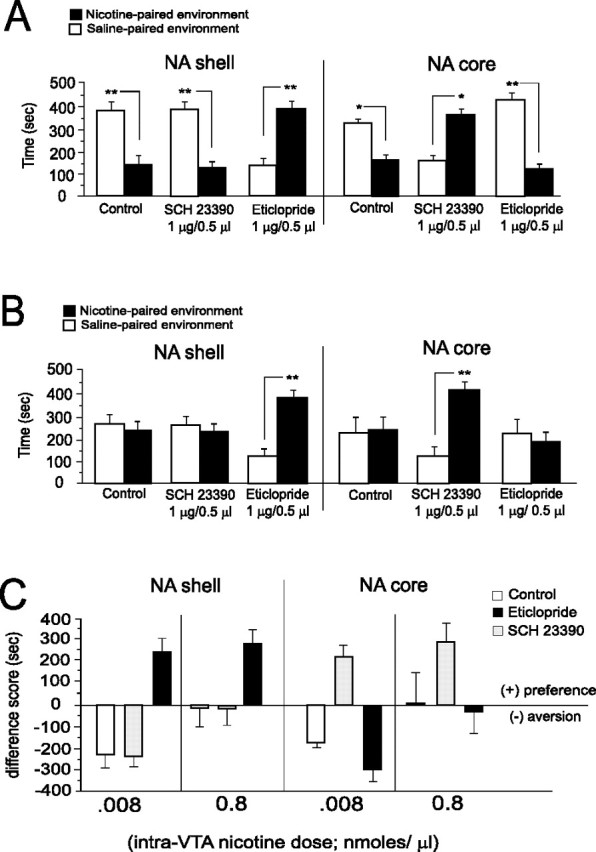
Effects of NAcore or NAshell D1-like versus D2-like receptor blockade on nicotine motivational signaling in the VTA. A, Saline control animals (n = 7) and animals pretreated with NAshell SCH 23390 (n = 8) displayed significant CPA to an aversive dose of intra-VTA nicotine (0.008 nmol/0.5 μl; **p values < 0.01). In contrast, in animals receiving NAshell eticlopride (1 μg/0.5 μl), the aversive motivational effects of intra-VTA nicotine were switched to a reward signal, with animals now displaying a robust CPP for nicotine-paired environments (**p < 0.01). Comparing NAcore microinfusions of either SCH 23390 or eticlopride revealed that whereas saline control (n = 6) or animals receiving NAcore eticlopride (n = 8) displayed significant aversions to environments paired with intra-VTA nicotine (0.008 nmol; *p < 0.05 and **p < 0.01, respectively), animals receiving NAcore SCH 23390 demonstrated a robust CPP for this same dose of intra-VTA nicotine (**p < 0.01). B, In saline control animals (n = 6) and animals pretreated with NAshell SCH 23390 (n = 8), a neutral dose of intra-VTA nicotine (0.8 μg/0.5 μl) produced no motivational effects (p values > 0.05). In contrast, in animals receiving NAshell eticlopride (1 μg/0.5 μl), the previously neutral dose of intra-VTA nicotine is potentiated into a robust reward signal, demonstrated by CPP to environments paired with this dose of intra-VTA nicotine (**p < 0.01). Comparing NAcore microinfusions of either SCH 23390 or eticlopride (right) revealed that saline control (n = 6) and animals receiving NAcore eticlopride (n = 8) displayed neither CPP nor CPA to nicotine-paired environments (p values > 0.05), whereas animals receiving intracore SCH 23390 (n = 8) demonstrated a robust CPP for this same dose of intra-VTA nicotine (**p < 0.01). C, Summary of conditioning effects on intra-VTA nicotine motivational signaling after NAshell versus NAcore microinfusions of SCH 23390 or eticlopride. CPP or CPA is expressed as difference scores, with negative values representing aversions to nicotine-paired environments and positive values representing preferences for nicotine-paired environments. Error bars indicate SEM.
Intra-Nac core versus shell D1 and D2 receptors differentially modulate the aversive effects of nicotine withdrawal
In addition to producing acute aversive effects, withdrawal from nicotine after chronic exposure is reported also to induce strong aversive effects (Epping-Jordan et al., 1998). Thus, either spontaneous withdrawal from chronic nicotine exposure or pharmacologically induced nicotine withdrawal via the administration of nAChR antagonists can produce aversive nicotine withdrawal (Suzuki et al., 1996; Epping-Jordan et al., 1998; Kenny and Markou, 2001). Given that NAcore or NAshell blockade of DA D1-like or D2-like receptors reverses the acute aversive effects of either intra-VTA nicotine (Fig. 4) or systemic nicotine (Fig. 3), we next examined whether blockade of D1 or D2 receptors in NAcore or NAshell subregions would modulate the aversive effects of nAChR antagonist-induced withdrawal aversions (see Materials and Methods). In Figure 5A, our chronic nicotine exposure and behavioral conditioning protocol is summarized. Bilateral NAcore versus NAshell microinfusion injector locations of suprathreshold doses of either SCH 23390 (1 μg/0.5 μl) or eticlopride (1 μg/0.5 μl) are schematically presented in Figure 5B (right and left, respectively). To determine the efficacy of our nicotine pump implantation protocol, we performed an initial pilot study to determine that systemic MEC administration (1 mg/kg, s.c.) would produce aversive withdrawal effects in chronic nicotine-treated rats (Fig. 5C), as previous studies have reported using this same MEC dose (Suzuki et al., 1996; Rada et al., 2001). We found that systemically administered MEC (1 mg/kg, s.c.) produced a significant CPA for MEC-paired environments in rats treated chronically with nicotine (t(6) = 5.0; p < 0.01; n = 7) versus saline controls (t(5) = 0.07; p > 05; n = 6). Comparing the effects of bilateral NAcore versus NAshell microinfusions of either SCH 23390 (1 μg/0.5 μl) or eticlopride(1 μg/0.5 μl) on the aversive effects of MEC-induced nicotine withdrawal aversions revealed that NAshell microinfusions of SCH 23390 did not block CPA to MEC-paired environments relative to control animals, whereas NAshell eticlopride completely blocked the aversive effects of nicotine withdrawal relative to saline controls (Fig. 6A) (F(1,41) = 21.9; p < 0.01). Post hoc analysis reveals that both NAshell saline controls (n = 6) and rats receiving SCH 23390 (n = 7) displayed significantly less time in environments paired previously with MEC (1 mg/kg, s.c.) (p values < 0.01), whereas this effect was blocked in rats receiving NAshell eticlopride (n = 7; p > 05). NAcore microinfusions of eticlopride did not block CPA to MEC-paired environments relative to control animals, whereas NAcore SCH 23390 completely blocked the aversive effects of nicotine withdrawal relative to saline controls (Fig. 6B) (F(2,41) = 6.55; p < 0.01). Post hoc analysis revealed that both NAcore saline controls (n = 6) and rats receiving eticlopride (n = 7) displayed significantly less time in environments paired previously with MEC (1 mg/kg, s.c.) (p values < 0.01), whereas this effect was blocked in rats receiving NAcore SCH 23390 (n = 7; p > 05).
Figure 5.
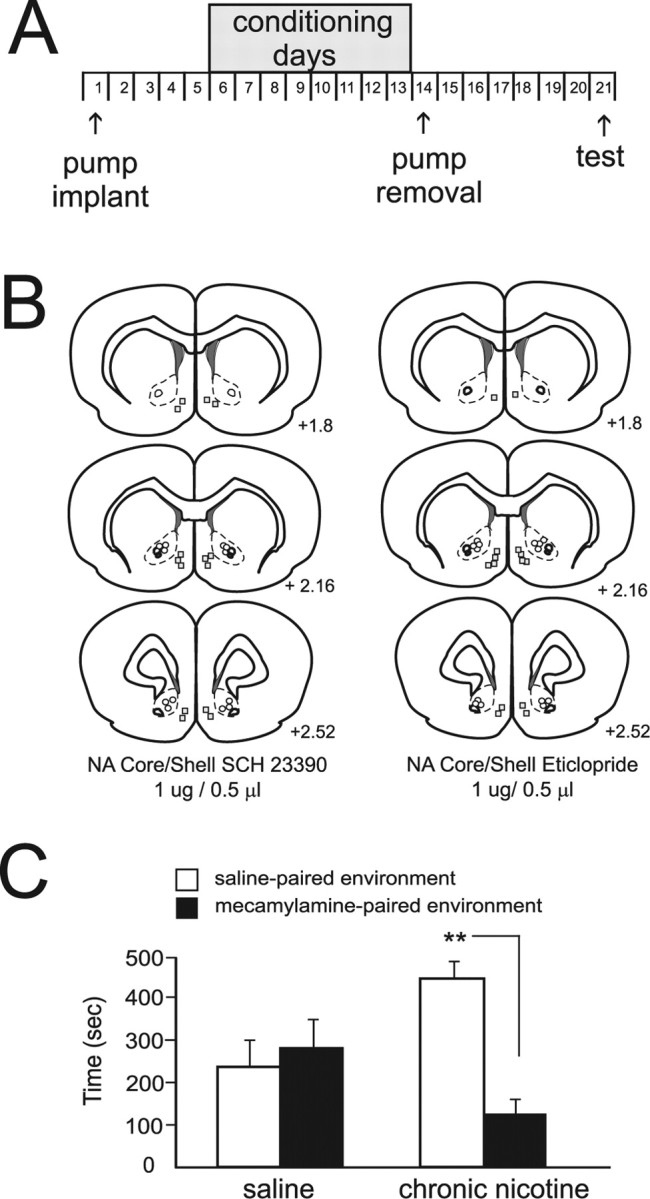
Chronic nicotine exposure conditioning procedure, nicotine withdrawal aversions, and intracore versus shell injection sites. A, Schematic illustration showing the experimental timeline for chronic nicotine exposure and withdrawal aversion conditioning and testing days. B, Schematic illustration showing NAcore versus NAshell injector tip placements for SCH 23390 core/shell placements (left column) or eticlopride core/shell placements (right column) in animals treated chronically with nicotine. C, In an initial pilot study, systemically administered MEC (1 mg/kg, s.c.) produced a significant CPA for MEC-paired environments in rats treated chronically with nicotine (n = 8; **p < 0.01) versus saline controls (p > 05; n = 6). Error bars indicate SEM.
Figure 6.

The effects of NAcore versus NAshell microinfusions of SCH 23390 or eticlopride on nicotine-withdrawal-induced CPA. A, NAshell SCH 23390 does not block CPA to MEC-paired environments relative to control animals, whereas intrashell eticlopride completely blocks the aversive effects of nicotine withdrawal, relative to saline controls. Both saline controls (n = 6) and rats receiving SCH 23390 (n = 7) displayed significantly less time in environments paired previously with MEC (1 mg/kg, s.c.) (**p values < 0.01), whereas this effect was blocked in rats receiving NAshell eticlopride (n = 7; p > 05). B, NAcore microinfusions of eticlopride do not block CPA to MEC-paired environments relative to control animals, whereas NAcore SCH 23390 completely blocks the aversive effects of nicotine withdrawal relative to saline controls. Both NAcore saline controls (n = 6) and rats receiving eticlopride (n = 7) displayed significantly less time in environments paired previously with MEC (1 mg/kg, s.c.; **p values < 0.01), whereas this effect was blocked in rats receiving NAcore SCH 23390 (n = 7; p > 05). Error bars indicate SEM.
Discussion
Given that only ∼50% or less of individuals that try tobacco go on to develop full nicotine dependence (McNeill, 1991; Pomerleau et al., 1998), understanding the neural systems that regulate sensitivity to nicotine's rewarding and addictive properties is of considerable importance. Several lines of evidence implicate the striatal DA system in the processing of nicotine's addictive properties. Animal studies report that nicotine elevates striatal DA levels (Nisell et al., 1994; Schilström et al., 1998) and amplifies reward-related “burst” firing of striatal DA release (Rice and Cragg, 2004). In human smokers, imaging studies report correlations between striatal DA receptor activity and subjective measures of nicotine-related motivational effects (Brody et al., 2006; Montgomery et al., 2007), suggesting that nicotine-induced DA release may be related to the positive effects of nicotine and nicotine craving. Alterations in striatal expression levels of DA D1 receptors have been reported in human smokers (Dagher et al., 2001); however, because of the correlative nature of such studies, it is difficult to determine whether these DAergic disturbances underlie the initial vulnerability to nicotine's addictive properties or are the consequence of long-term nicotine exposure. Animal studies report equally complex results. For example, intravenous self-administration paradigms show that blockade of D1/D2 receptors or mesolimbic neurotoxic lesions attenuate nicotine self-administration in rodents (Corrigall and Coen, 1991; Corrigall et al. 1992). However, global blockade of DA transmission in either NAshell or NAcore blocks the aversive properties of nicotine measured in CTA or CPP procedures (Laviolette and van der Kooy, 2003) and potentiates nicotine reward while reversing the motivational valence of nicotine from aversive to rewarding (Laviolette and van der Kooy, 2003, 2004). Considerable evidence suggests that blockade of DAergic neurotransmission may potentiate the motivational properties of nicotine in human subjects. For example, neuroleptic blockade of DA transmission strongly increases smoking rates in human smokers (McEvoy et al., 1995; Caskey et al., 1999, 2002). Furthermore, decreased levels of striatal DA receptors have been correlated with increased psychostimulant drug taking and craving in humans for cocaine (Volkow et al., 2001), heroin (Wang et al., 1997), amphetamine (Volkow et al., 2001), and nicotine (Dagher et al., 2001), all suggesting that disturbances in striatal DAergic transmission may increase vulnerability to the addictive properties of these drugs. Nevertheless, the abovementioned reports used anatomically nonspecific intra-NAc microinfusions or systemically administered D1/D2 receptor antagonists. Thus, until now, dissociable effects of D1 versus D2 DA receptor blockade within specific NAc subregions, on the motivational properties of either intra-VTA or systemic nicotine, have not been reported.
Functional dissociation between NAcore versus NAshell D1 and D2 receptor transmission in nicotine reward processing
The NAc comprises the medial shell region and a more laterally located core region, both of which possess distinct pharmacological characteristics and anatomical connections (Voorn et al., 1989; Brog et al., 1993). For example, whereas the NAshell receives a greater proportion of ventromedial and infralimbic cortical inputs, NAcore receives greater input from dorsal regions of the prefrontal cortex (Berendse et al., 1992). In addition, NAcore and NAshell neurons provide feedback to the VTA, primarily in the form of GABAergic projections that modulate the activity of VTA neuronal populations in a functional feedback loop (Kalivas, 1993; Rahman and McBride, 2001). Interestingly, only combined striatal D1 or D2 receptor activation appears to modulate VTA DA release via this pathway (Rahman and McBride, 2001). Although it is not presently understood how blockade of D1 versus D2 striatal receptors may influence this NAc–VTA feedback loop in terms of controlling VTA neuronal activity, we observed a functional dissociation between NAcore and NAshell D1/D2 receptors in the modulation of systemic or intra-VTA nicotine motivational processing, suggesting that separate feedback pathways from these NAc subregions may independently regulate the VTA neuronal circuit, which, in turn, may potentiate or alter the motivational valence of nicotine.
The NAcore and NAshell are both implicated in the processing of various associative reward stimuli, including natural rewards (Di Chiara et al., 2004) and drug-related effects, including cocaine (McFarland and Kalivas, 2001) and heroin (Bossert et al., 2007). Several theories suggest that striatal DA release resulting from phasic, “burst” firing of VTA DAergic inputs may be critical for motivationally salient information processing in the mesolimbic circuitry (Grace, 2000; Cooper, 2002). Given the ability of nicotine to amplify DA burst pattern signaling within the striatum (Rice and Cragg, 2004), one possibility is that D1 (NAcore) versus D2 (NAshell) receptor populations control either systemic or intra-VTA nicotine-mediated transmission of motivational signals to these separate striatal regions by independently regulating specific DA release patterns within the NAcore or NAshell, respectively. Our evidence demonstrates that pharmacological blockade of striatal DA D1-like versus D2-like receptors is capable of amplifying sub-reward threshold VTA nicotine signals and can reverse the aversive effects of either intra-VTA or systemic nicotine administration. Anatomical evidence suggests an approximately equivalent distribution of DA D1 and D2 receptors within the NAcore and NAshell subregions, localized both presynaptically and postsynaptically (Missale et al., 1998), and previous reports have suggested that striatal D1 and D2 receptor populations act synergistically in the processing of reward-related information. For example, rats will self-administer combinations of a D1 and D2 agonist directly into the NAshell (but not the NAcore), but neither agonist will support self-administration by themselves (Ikemoto et al., 1997). Other studies have found a preferential role for NAcore D1 receptors in the modulation of drug-related associative learning and memory such that D1 receptors in NAcore, but not NAshell, are essential for cue-induced heroin reinstatement in rats (Bossert et al., 2007). Furthermore, NAcore appears to be more sensitive to D1 receptor blockade in the attenuation of food-reward reinforcement (Nowend et al., 2001). The present findings are the first demonstration that different D1-like versus D2-like receptor populations localized to NAcore and NAshell regions may independently control nicotine reward sensitivity.
NAcore versus NAshell dopamine function in the acute versus withdrawal-related aversive effects of nicotine
Chronic nicotine exposure appears to dramatically alter the role of DA transmission in the processing of nicotine's physiological and motivational effects. Thus, nicotine exposure induces a complex array of neuroadaptations in multiple neural systems and has been shown to potentiate the activity of glutamate (Mansvelder and McGehee, 2002), dopamine (Nisell et al., 1997; Le Foll et al., 2003), and the expression of multiple nAChR subunits in various brain regions (Olale et al., 1997; Gentry and Lukas, 2002). Nisell et al. (1997) reported that acute nicotine caused strong increases in NAshell DA release, but less so in the core. However, after chronic nicotine exposure, DA release in both regions was attenuated (Nisell et al., 1997). Rada et al. (2001) reported that chronic nicotine exposure and withdrawal decreases endogenous extracellular DA levels in rodent striatum, suggesting that the aversive effects of nicotine withdrawal may be related to a dysregulation of striatal DA transmission after chronic nicotine exposure. Although the aversive effects of withdrawal from other drug classes such as opiates are dependent on DA transmission (Laviolette et al., 2002a,b), the precise role of D1 or D2 receptors within specific NAc subregions in the processing of nicotine withdrawal aversion has not been clarified. Blockade of either DA D1-like or D2-like receptors was sufficient to block the aversive effects of nicotine withdrawal; however, similar to our observations with the acute aversive effects of nicotine, the same NAshell versus NAcore functional dissociation was observed in the processing of nicotine withdrawal aversion: D1 blockade in NAcore but not NAshell, and D2 blockade in NAshell but not NAcore blocked nicotine withdrawal aversion. Interestingly, these results suggest that despite reported neuroadaptations in DA transmission after nicotine exposure, the same functional and pharmacological dissociation between NAshell and NAcore D1 versus D2 receptors is observable for both the acute effects of early nicotine exposure, as well as the aversive effects of nicotine withdrawal. One possibility is that dysregulation of DAergic transmission during nicotine withdrawal may be responsible for the aversive motivational state associated with withdrawal, in which case blocking this signal would alleviate the aversive effects of nicotine withdrawal, preventing the formation of the conditioned associative aversion. Although future studies are required to address these issues, our results demonstrate that both D1 and D2 NAc receptors are crucial for the processing of nicotine-withdrawal-related aversive effects, suggesting an important role for striatal DA transmission in both the acute and chronic stages of nicotine exposure.
Conclusions
Our results demonstrate dissociable roles for NAcore and NAshell DA D1-like versus D2-like receptor transmission in modulating the motivational properties of nicotine. We observed a functional segregation between DA D1-like receptors within the NAcore and D2-like receptors in the NAshell, indicative of specialized roles for these ventral striatal subregions in the nicotine addiction process. The modulatory roles for these different DA receptor subtypes within NAcore versus NAshell DA were nonadditive and were capable of regulating nicotine motivational signaling through anatomically and pharmacologically separate mechanisms within the ventral striatum. These results demonstrate a novel and important role for striatal DA receptor-subtype-specific transmission in mediating vulnerability to nicotine's addictive properties, as well as in the processing of the aversive effects of nicotine withdrawal.
Footnotes
This work was supported by the Canadian Psychiatric Research Foundation and the Canadian Institutes of Health Research.
References
- Anderson SM, Bari AA, Pierce RC. Administration of the D1-like receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology. 2003;168:132–138. doi: 10.1007/s00213-002-1298-5. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye SM, Grant RJ, Clarke PBS. Disruption of dopaminergic neurotransmission in nucleus accumbens core inhibits the locomotor stimulant effects of nicotine and d-amphetamine in rats. Neuropharmacology. 2001;40:792–805. doi: 10.1016/s0028-3908(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Scheibal D, Hahn E, Shiraga S, Zamora-Paja E, Farahi J, Saxena S, London ED, McCracken JT. Gene variants of brain dopamine pathways and smoking-induced dopamine release in the ventral caudate/nucleus accumbens. Arch Gen Psychiatry. 2006;63:808–816. doi: 10.1001/archpsyc.63.7.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The pattern of afferent innervation of the core and the shell in the accumbens part of the ventral striatum: immunohistochemical detection of retrogradely transported flour-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Caskey NH, Jarvik ME, Wirshing WC. The effects of dopaminergic D2 stimulation and blockade on smoking behavior. Exp Clin Psychopharmacol. 1999;7:72–78. doi: 10.1037//1064-1297.7.1.72. [DOI] [PubMed] [Google Scholar]
- Caskey NH, Jarvik ME, Wirshing WC, Madsen DC, Iwamoto-Schaap PN, Eisenberger NI, Huerta L, Terrace SM, Olmstead RE. Modulating tobacco smoking rates by dopaminergic stimulation and blockade. Nicotine Tob Res. 2002;4:259–266. doi: 10.1080/14622200210153830. [DOI] [PubMed] [Google Scholar]
- Cooper DC. The significance of action potential bursting in the brain reward circuit. Neurochem Int. 2002;41:333–340. doi: 10.1016/s0197-0186(02)00068-2. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology. 1991;104:171–176. doi: 10.1007/BF02244174. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL, Chow BL, Zhang J. Response of nicotine self-administration in the rat to manipulations of μ-opioid and γ-aminobutyric acid receptors in the ventral tegmental area. Psychopharmacology. 2000;149:107–114. doi: 10.1007/s002139900355. [DOI] [PubMed] [Google Scholar]
- Dagher A, Bleicher C, Aston JA, Gunn RN, Clarke PB, Cumming P. Reduced dopamine D1 receptor binding in the ventral striatum of cigarette smokers. Synapse. 2001;42:48–53. doi: 10.1002/syn.1098. [DOI] [PubMed] [Google Scholar]
- David V, Besson M, Changeux JP, Granon S, Cazala P. Reinforcing effects of nicotine microinjections into the ventral tegmental area of mice: dependence on cholinergic and dopaminergic D1 receptors. Neuropharmacology. 2006;50:1030–1040. doi: 10.1016/j.neuropharm.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47:227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Fenu S, Bassareo V, Di Chiara G. A role for dopamine D1 receptors of the nucleus accumbens shell in conditioned taste aversion learning. J Neurosci. 2001;21:6897–6904. doi: 10.1523/JNEUROSCI.21-17-06897.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry CL, Lukas RJ. Regulation of nicotinic acetylcholine receptor numbers and function by chronic nicotine exposure. Curr Drug Targets CNS Neurol Disord. 2002;1:359–385. doi: 10.2174/1568007023339184. [DOI] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000;95(Suppl 2):S119–S128. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci. 1997;17:8580–8587. doi: 10.1523/JNEUROSCI.17-21-08580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Qin M, Liu ZH. Primary reinforcing effects of nicotine are triggered from multiple regions both inside and outside the ventral tegmental area. J Neurosci. 2006;26:723–730. doi: 10.1523/JNEUROSCI.4542-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto ET. Nicotine conditions place preferences after intracerebral administration in rats. Psychopharmacology (Berl) 1990;100:251–257. doi: 10.1007/BF02244415. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Steinpreis RE, Sherman JE, Baker TB. Aversion instead of preference learning indicated by nicotine place conditioning in rats. Psychopharmacology. 1990;101:533–538. doi: 10.1007/BF02244233. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Brain Res Rev. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav. 2001;70:531–549. doi: 10.1016/s0091-3057(01)00651-7. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Ganguly PK. Topographical organization in the nucleus accumbens of afferents from the basolateral amygdala and efferents to the lateral hypothalamus. Neuroscience. 1995;67:625–630. doi: 10.1016/0306-4522(95)00013-9. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. Blockade of mesolimbic dopamine transmission dramatically increases sensitivity to the rewarding effects of nicotine in the ventral tegmental area. Mol Psychiatry. 2003;8:50–59. doi: 10.1038/sj.mp.4001197. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behavior. Nat Rev Neurosci. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Nader K, van der Kooy D. Motivational state determines the functional role of the mesolimbic dopamine system in the mediation of opiate reward processes. Behav Brain Res. 2002a;129:17–29. doi: 10.1016/s0166-4328(01)00327-8. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Alexson TO, van der Kooy D. Lesions of the tegmental pedunculopontine nucleus block the rewarding effects and reveal the aversive effects of nicotine in the ventral tegmental area. J Neurosci. 2002b;22:8653–8660. doi: 10.1523/JNEUROSCI.22-19-08653.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P. Increased dopamine D3 receptor expression accompanying behavioral sensitization to nicotine in rats. Synapse. 2003;47:176–183. doi: 10.1002/syn.10170. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Carter VA, Cunningham JS, Hebert KM, Conrad DL, Wilson OB. The nicotinic antagonist mecamylamine precipitates nicotine abstinence syndrome in the rat. Psychopharmacology. 1994;115:180–184. doi: 10.1007/BF02244770. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Freudenreich O, Levin ED, Rose JE. Haloperidol increases smoking in patients with schizophrenia. Psychopharmacology. 1995;119:124–126. doi: 10.1007/BF02246063. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;221:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill AD. The development of dependence on smoking in children. Br J Addiction. 1991;86:589–592. doi: 10.1111/j.1360-0443.1991.tb01813.x. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Montgomery AJ, Lingford-Hughes AR, Egerton A, Nutt DJ, Grasby PM. The effect of nicotine on striatal dopamine release in man: a [11C]raclopride PET study. Synapse. 2007;61:637–645. doi: 10.1002/syn.20419. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- Nisell M, Marcus M, Nomikos GG, Svensson TH. Differential effects of acute and chronic nicotine on dopamine output in the core and shell of the rat nucleus accumbens. J Neural Transm. 1997;104:1–10. doi: 10.1007/BF01271290. [DOI] [PubMed] [Google Scholar]
- Nowend KL, Arizzi M, Carlson BB, Salamone JD. D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav. 2001;69:373–382. doi: 10.1016/s0091-3057(01)00524-x. [DOI] [PubMed] [Google Scholar]
- Olale F, Gerzanich V, Kuryatov A, Wang F, Lindstrom J. Chronic nicotine exposure differentially affects the function of human α3, α4 and α7 neuronal nicotinic receptor subtypes. J Pharmacol Exp Ther. 1997;283:675–683. [PubMed] [Google Scholar]
- Pattij T, Janssen MCW, Vanderschuren LJ, Schoffelmeer ANM, van Gaalen MM. Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology. 2007;191:587–598. doi: 10.1007/s00213-006-0533-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 5. San Diego: Academic; 2005. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Corrigall WA. Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J Neurosci. 2002;22:3338–3341. doi: 10.1523/JNEUROSCI.22-09-03338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Namenek RJ. Early experiences with tobacco among women smokers, ex-smokers and never smokers. Addiction. 1998;93:595–599. doi: 10.1046/j.1360-0443.1998.93459515.x. [DOI] [PubMed] [Google Scholar]
- Rada P, Jensen K, Hoebel BG. Effects of nicotine and mecamylamine-induced withdrawal on extracellular dopamine and acetylcholine in the rat nucleus accumbens. Psychopharmacology. 2001;157:105–110. doi: 10.1007/s002130100781. [DOI] [PubMed] [Google Scholar]
- Rahman S, McBride WJ. D1-D2 dopamine receptor interaction within the nucleus accumbens mediates long-loop negative feedback to the ventral tegmental area (VTA) J Neurochem. 2001;77:1248–1255. doi: 10.1046/j.1471-4159.2001.00326.x. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Schilström B, Svensson HM, Svensson TH, Nomikos GG. Nicotine and food-induced dopamine release in the nucleus accumbens of the rat: putative role of α7 nicotinic receptors in the ventral tegmental area. Neuroscience. 1998;85:1005–1009. doi: 10.1016/s0306-4522(98)00114-6. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Stolerman IP. Conditioned taste aversion in rats after intracerebral administration of nicotine. Behav Pharmacol. 1995;6:375–385. [PubMed] [Google Scholar]
- Suzuki T, Ise Y, Tsuda M, Maeda J, Misawa M. Mecamylamine-precipitated nicotine withdrawal aversion in rats. Eur J Pharmacol. 1996;314:281–284. doi: 10.1016/s0014-2999(96)00723-6. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbital frontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Voorn P, Gerfen CR, Groenewegen HJ. Compartmental organization of the ventral striatum of the rat: immunohistochemical distribution of enkephalin, substance P, dopamine and calcium-binding protein. J Comp Neurol. 1989;289:189–201. doi: 10.1002/cne.902890202. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ, Pappas NS, Pascani K. Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology. 1997;16:174–182. doi: 10.1016/S0893-133X(96)00184-4. [DOI] [PubMed] [Google Scholar]
- Yin R, French ED. A comparison of the effects of nicotine on dopamine and non-dopamine neurons in the rat ventral tegmental area: an electrophysiological study. Brain Res Bull. 2000;51:507–514. doi: 10.1016/s0361-9230(00)00237-9. [DOI] [PubMed] [Google Scholar]