Abstract
Growing evidence shows that dysfunction of the limbic basal ganglia (BG) network is implicated in repetitive behaviors, such as obsessive compulsive disorder (OCD) and Tourette's syndrome (TS), in humans. Because deep brain stimulation (DBS) of the posterior subthalamic nucleus (STN), which modulates the sensorimotor BG network, is beneficial in movement disorders, stimulation of the anterior, limbic STN might improve intractable behavioral disorders. We therefore evaluated the effect of anterior STN stimulation on the repetitive behaviors induced in two monkeys after bicuculline-induced dysfunction of the limbic external globus pallidus. DBS in the anterior STN dramatically reduced the stereotypies, but had no effect on the performance of a simple food retrieval task. Stimulations outside the STN were less effective in reducing the stereotypies. Electrode trajectories, reconstructed postmortem, confirmed that the effective contacts were in the anterior STN. DBS in the limbic STN might therefore provide relief from the severe stereotyped behaviors observed in OCD and TS.
Keywords: basal ganglia, subthalamic nucleus, stimulation, globus pallidus, behavior, bicuculline, primate
Introduction
Deep brain stimulation (DBS) has proved to be a powerful tool for the treatment of Parkinson's disease (Limousin et al., 1995; Deuschl et al., 2006), essential tremor (Schuurman et al., 2000), and dystonia (Vidailhet et al., 2005; Kupsch et al., 2006). The optimal targets in these movement disorders, which included the cerebellar territory of the thalamus, the internal globus pallidus (GPi), and the subthalamic nucleus (STN), were selected on the basis of studies in primate models (Bergman et al., 1990, Benazzouz et al., 1993) or in accordance with past surgical experience with brain lesions. There is increasing evidence that a dysfunction within the limbic basal ganglia network is central to stereotyped behavioral disorders characterized by repetition of a set of behavioral patterns, such as Tourette syndrome (TS) (Vandewalle et al., 1999; Houeto et al., 2005) and obsessive compulsive disorder (OCD) (Aouizerate et al., 2004a). We showed previously that bicuculline microinjections in the limbic and associative parts of the external globus pallidus (GPe) in monkeys induced stereotypy and attention deficit or hyperactivity, respectively (Grabli et al., 2004), in accordance with the cognitive and behavioral functions attributed to the associative and limbic basal ganglia circuits (Alexander et al., 1986).
This primate model offers the opportunity to test whether modulation of the limbic STN by DBS can reduce repetitive behaviors. We have therefore investigated the effect of DBS in the anterior STN in this primate model of behavioral disorder. The anterior STN was chosen as the DBS target for three reasons. First, the anterior part of the STN is occupied by the limbic anatomofunctional territory (Karachi et al., 2005). Second, stimulation of this structure affects the neuronal activity of the GPi and SNr, the two outputs nuclei of the BG involved in the stereotypy (François et al., 2004). Third, the obsessive compulsive symptoms in two patients with Parkinson's disease and a severe OCD were improved by STN DBS (Mallet et al., 2002).
Materials and Methods
Experiments were conducted on two male African Green monkeys (Cercopithecus aethiops sabaeus) aged between 4 and 6 years. The monkeys were treated in accordance with National Institutes of Health guidelines (1996) and the recommendations of the EEC (86/609) and the French National Committee (87/848).
Surgical procedures.
The recording chamber was implanted during a first surgical procedure to allow bicuculline microinjections in the GPe as described previously (Grabli et al., 2004). During a second surgical procedure under general anesthesia, 1.2-mm-diameter quadripolar stimulating leads (DIXI Medical) with four cylindrical electrode contacts (1.0 mm of length spaced by 0.5 mm and numbered as contact 0, at the tip, to 3) were stereotaxically implanted into the left anterior STN of the monkeys using intraoperative microelectrode single unit recording (see Fig. 1A). The leads were connected to an external pulse generator (WPI) through a connector permanently fixed to the skull. Unipolar stimulation was applied with fixed frequency and pulse width (130 H z and 60 ms, respectively) but variable voltage (0–3 V).
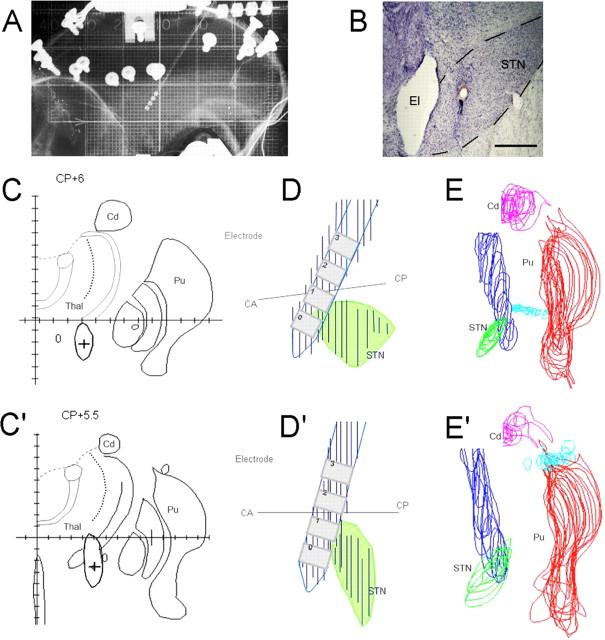
Figure 1.
A, Radiographic control of an electrode implanted in the STN. B, Section counterstained with cresyl violet showing the trace of the stimulation electrode in the anteromedial STN in monkey B. C, C′, Schematic representation of the brain section corresponding to the center of the stimulation contact 0, indicated by a cross (+) in monkey A and monkey B, respectively. The gray areas represent the electrode trace. D, D′, Reconstruction of the electrodes (blue) and the STN (green) from regularly spaced brain sections (50 μm thickness), viewed laterally in monkey A and monkey B, respectively. E, E′, The STN (green), putamen (Pu, red), caudate nucleus (Cd, pink), and the electrode (blue), as seen on regularly spaced brain sections. Thal, Thalamus. Scale bar, 1 mm.
Behavioral analysis.
Monkeys were acclimated to the experimental conditions and learned the simple food retrieval task during 3 months before the implantation of the recording chamber. After each surgical procedure, the animals were allowed to recover. One of the four following types of experimental sessions were conduced daily and randomly alternated: (1) control sessions without microinjections and without DBS, (2) sessions with microinjections of bicuculline in the limbic GPe, (3) sessions with stimulation alone, and (4) sessions with microinjections followed by anterior STN stimulation. Sessions with stimulation consisted in a random alternation of ON stimulation periods and OFF stimulation periods. Spontaneous behaviors and task execution were observed and recorded using a video system. The frequency and the duration of the behaviors or abnormal movements were quantified off-line from the after consensus of four experimenters (N.B., D.G., C.K., and S.M.) blinded to the condition. The duration of the stereotypies was evaluated by 3 min segments in each experimental session.
Simple food-retrieval task.
For the simple food-retrieval task (Grabli et al., 2004), the monkeys were conditioned to retrieve pieces of food from a two parts (left and right) board with the left and right hand respectively. The monkey had to perform six to eight blocks of this task in each experimental condition and the time needed to empty each part of the board was scored. The data were pooled for each monkey according to the type of session and to the condition (control periods, postinjection OFF DBS periods, and postinjection ON DBS periods).
Statistical analysis.
Behaviors were quantified by segment of 3 min over the entire experimental session. Each condition lasted 12–21 min corresponding to four to six periods of 3 min. For analysis of the effect of DBS on spontaneous behaviors (sessions without injection), the mean duration of behaviors during stimulation periods were compared alternately with the mean duration of behaviors during the previous or the succeeding periods without stimulation obtained the same day. For the analysis of DBS effect on stereotypies induced by bicuculline injection, the experimental sessions were divided as follows: (1) “control periods” corresponding to preinjection periods without induction of stereotypy, (2) “OFF stimulation periods” corresponding to postinjection periods with induction of stereotypies, and (3) “ON stimulation periods” corresponding to postinjection periods with induction of stereotypies and stimulation applied. The effect of injection was defined as the difference of the mean duration of stereotypies between the periods 1 and 2. The effect of stimulation on stereotypies was defined as the difference of the mean duration of stereotypies between the periods 2 and 3. The results were compared using the Mann–Whitney test. Finally, task data under the different conditions were compared with the Mann–Whitney test. Results of all the analyses were considered significant at p < 0.05.
Histological procedures.
After the experiments, the monkeys were killed with an overdose of anesthetic and perfused transcardially with 5 L of 4% paraformaldehyde (François et al., 2004). The electrode was removed and the brain was extracted and immersed in 20% sucrose in PBS for 2 d, then frozen and cut into 50-μm-thick frontal sections perpendicular to the intercommissural plane (François et al., 2004). The perimeter of the STN and the traces of the electrodes (see Fig. 1B) were drawn from serial sections (see Fig. 1C,C′), digitized with a dedicated software and reconstructed in a three dimensional space (see Fig. 1D–E′). The section corresponding to the center of each contact was determined by its distance from the tip of the electrode. The sensorimotor, associative and limbic territories of the GPe were defined by calbindin immunoreactivity (François et al., 1994). Microinjection sites and trajectories of the electrodes were reconstructed from cresyl violet-stained sections and compared with the borders of the functional territories of the GPe (see Fig. 2A,B).
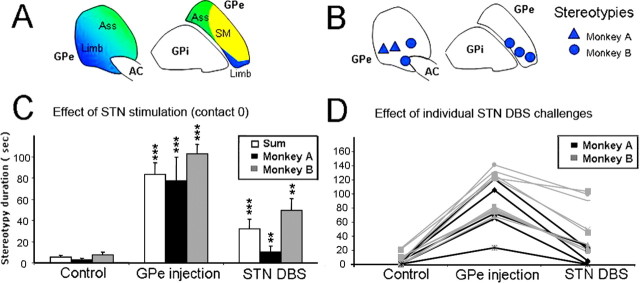
Figure 2.
A, Representation of the associative (Ass), limbic (Limb), and sensorimotor (SM) territories of the GPe illustrated in green, blue, and yellow, respectively. B, Localization of the injection sites in the anterior part of GPe in monkeys A (triangles) and B (circles). C, Mean total duration of behaviors by periods of 180 s for DBS challenges at contact 0. Values are given for monkey A (black bars), B (gray bars), and both (white bars). The error bars show the SEM. **p < 0.01; ***p < 0.001. D, Results of individual stimulation challenges at contact 0. Duration of stereotypies by periods of 180 s is given in three conditions: control, injection alone, and injection with stimulation for monkey A (black lines) and monkey B (gray lines).
Results
No increase in stereotypies has been found between the beginning and the end of the 3 month training period. No change in the spontaneous behavior (control sessions) and in task execution was observed after the implantation of the electrode in the monkeys. No lesions caused by electrode implantation were observed on histological sections. The most ventral contact (contact 0) of the quadripolar electrode was located in the anterior tip of the STN in both monkeys but was more anterior and ventral in monkey B than in monkey A (Fig. 1). Contact 1 was located more dorsally in the zona incerta just above the STN. Contacts 2 and 3 were located in the ventral and dorsal part of the motor thalamus.
The effect of DBS on movement, spontaneous behavior and task performance was first studied as a function of current intensity. For both monkeys, stimulation at all sites induced fixed dystonia in the contralateral limb, at current intensity >1.5 V for contact 1 and >3 V for contacts 0, 2, and 3. Subsequent experiments were therefore performed below a threshold of 2.5 V for contacts 0, 2 and 3. The particularly low threshold of contact 1 excluded this contact for the rest of the experiment for both monkeys. Under the thresholds for abnormal movements, the impact of the DBS was assessed on spontaneous behavior in the primate chair. The spontaneous behavior of monkey A mostly consisted in resting. No increase of behavioral activity was observed when stimulation was applied at contacts 0, 2, and 3. Conversely, monkey B displayed a wide range of behaviors including a spontaneous tendency to lick its fingers during control sessions. During stimulation at contact 0, this spontaneous behavior was reduced by nearly 100%, whereas there was no effect of stimulation through contacts 2 and 3 for monkey B. There was no additional effect on task execution and the retrieval time did not change.
We then analyzed the effects of anterior STN DBS on stereotypies induced by microinjections of bicuculline into the limbic GPe. All of the microinjections induced the same behavioral effect with slightly different intensities as described previously (Grabli et al., 2004). Stereotypies were induced by 15 microinjections of bicuculline (seven and eight injections in monkey A and B, respectively) into the limbic GPe at seven different sites. The localization of these injection sites, the behavioral effect induced and a schematic representation of the functional territories of the GPe are shown in Figure 2. Stereotypies obtained in both animals were comparable with those described previously (Grabli et al., 2004). They consisted of licking or biting the fingers or nails of both hands and began 3–12 min after the injection of bicuculline. This behavior was intense and repeated frequently during periods of 83.1 ± 11.3 s vs 5.5 ± 1.8 s during the control session, for a total observation time of 180 s (Fig. 2), and remained stable for >50 min. The type of behavior, its intensity, latency and duration were similar after each injection.
Stimulation applied at contact 0 significantly reduced stereotypies in a reproducible manner in both monkeys. The duration of the stereotypies was drastically reduced in monkey A, (decrease of 88.2 ± 15.4%) and slightly less in monkey B (decrease of 50.4 ± 18.5). In monkey B, the reduction was similar at 2.0 V (reduction of 55.3 ± 18.1%) and 2.5 V (reduction of 45.5 ± 18.9%). The effect of stimulation always resulted in a strong and significant decrease in the stereotypies (Mann–Whitney test, p < 0.05) in 90% of the trials in both monkeys (Fig. 2). Unilateral stimulation of the STN reduced stereotypies on both sides to the same extent. No apparent affective side effect (vocalization, fear) was observed at contact 0. When stimulation was applied through the upper contacts 2 and 3, thus outside the STN, it induced a significant reduction of stereotypies in only two of nine trials. No significant effect was observed in the majority of trials (seven of nine) with stimulation outside the STN. To exclude possible motor impairment induced by DBS in the reduction of stereotypies, task execution was closely monitored during each session. The stimulation of the contact 0 did not modify retrieval time or the capacity to perform the task.
Discussion
This study provides evidence that DBS of the anterior STN of monkeys can dramatically reduce stereotypies induced by an ipsilateral local dysfunction of the limbic GPe. The STN is therefore a promising target for DBS treatment of behavioral disorders as OCD and TS.
The significant reduction of induced stereotypies after DBS of the anterior part of the STN was consistent. This reduction exceeded 80% for monkey A and 50% for monkey B. The effects were highly reproducible in each monkey and between the monkeys, whatever the microinjection site in the anterior GPe. Importantly, there was no concomitant effect on the normal range of behaviors of the monkeys (ability to move, take attention or motivation to direct their actions to eat food). Electrode implantation did not induce motor side effects such as the hemiballism that was previously reported after STN lesion or dysfunction (Hammond et al., 1979; Crossman et al., 1984). This may be explained by the anteromedial location of the electrode in the limbic territory of the STN, rather than in the posterolateral sensorimotor part of the nucleus.
These results demonstrate in two normal monkeys that DBS of the anterior STN is safe and does not produce adverse effects. The efficacy of anterior STN simulation on pallidal-induced stereotypies is consistent with the finding that the pallidal sites where stereotypied behaviors were elicited are connected to the limbic territory of the anterior STN (François et al., 2004; Karachi et al., 2005). The transposition of these results to the surgical practice in human must however be cautious. First, even if our model reproduces symptoms that are similar to those of OCD or TS, there is no evidence in favor of a primary dysfunction in the limbic GPe in these disorders. Second, because the effect of bicuculline is acute, we tested stimulation over periods of 12–21 min, which is sufficient to demonstrate its efficacy for suppressing stereotypies but not to test it over periods of several months as it would be applied in human.
The choice of a limbic target in human is supported by previous studies that report successful surgical treatment of untreatable OCD and TS using DBS in various other brain areas, including the anterior limb of internal capsule (Abelson et al., 2005; Flaherty et al., 2005; Greenberg et al., 2006), the ventral caudate nucleus (Aouizerate et al., 2004b, 2005), the limbic GPi (Diederich et al., 2005; Houeto et al., 2005; Shahed et al., 2007) or the centromedian/parafascicularis nucleus (Visser-Vandewalle et al., 2003; Houeto et al., 2005; Ackermans et al., 2006; Servello et al., 2007). Our results in monkeys are also in line with recent surgical experience acquired in Parkinson's disease suggesting that DBS of the STN can produce unexpected behavioral processes (Krack et al., 2001; Houeto et al., 2006; Mallet et al., 2007) but also improve OCD symptoms associated with PD (Mallet et al., 2002). The advantage of the STN target is the small size of this nucleus which would allow to extend stimulation to the adjacent associative and motor territories that could also be involved in the motor and cognitive aspects of TS and OCD symptomatology.
Despite the limitations of the small number of monkeys and the acute DBS setting, this study provides a strong evidence of the efficacy of anterior STN-DBS for the treatment of abnormal behaviors in the monkey, and an additional argument in favor of its use in severe intractable TS or OCD in humans. Additional studies should now be performed to confirm these findings in additional monkeys and under chronic stimulation settings.
Footnotes
This work was supported by grants from the Tourette Syndrome Association and Fondation de l'Avenir, the Fondation pour la Recherche Médicale (N.B.), the Assistance Publique des Hôpitaux de Paris (D.G.), and the Association Française du Syndrome de Gilles de la Tourette (C.K., S.M.).
References
- Abelson JL, Curtis GC, Sagher O, Albucher RC, Harrigan M, Taylor SF, Martis B, Giordani B. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry. 2005;57:510–516. doi: 10.1016/j.biopsych.2004.11.042. [DOI] [PubMed] [Google Scholar]
- Ackermans L, Temel Y, Cath D, van der Linden C, Bruggeman R, Kleijer M, Nederveen P, Schruers K, Colle H, Tijssen MA, Visser-Vandewalle V. Deep brain stimulation in Tourette's syndrome: two targets? Mov Disord. 2006;21:709–713. doi: 10.1002/mds.20816. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Aouizerate B, Guehl D, Cuny E, Rougier A, Bioulac B, Tignol J, Burbaud P. Pathophysiology of obsessive-compulsive disorder: a necessary link between phenomenology, neuropsychology, imagery and physiology. Prog Neurobiol. 2004a;72:195–221. doi: 10.1016/j.pneurobio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Aouizerate B, Cuny E, Martin-Guehl C, Guehl D, Amieva H, Benazzouz A, Fabrigoule C, Allard M, Rougier A, Bioulac B, Tignol J, Burbaud P. Deep brain stimulation of the ventral caudate nucleus in the treatment of obsessive-compulsive disorder and major depression. Case report. J Neurosurg. 2004b;101:682–686. doi: 10.3171/jns.2004.101.4.0682. [DOI] [PubMed] [Google Scholar]
- Aouizerate B, Martin-Guehl C, Cuny E, Guehl D, Amieva H, Benazzouz A, Fabrigoule C, Bioulac B, Tignol J, Burbaud P. Deep brain stimulation for OCD and major depression. Am J Psychiatry. 2005;162:2192. doi: 10.1176/appi.ajp.162.11.2192. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Gross C, Féger J, Boraud T, Bioulac B. Reversal of rigidity and improvement in motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys. Eur J Neurosci. 1993;5:382–389. doi: 10.1111/j.1460-9568.1993.tb00505.x. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- Crossman AR, Sambrook MA, Jackson A. Experimental hemichorea/hemiballismus in the monkey. Studies on the intracerebral site of action in a drug-induced dyskinesia. Brain. 1984;107:579–596. doi: 10.1093/brain/107.2.579. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, Daniels C, Deutschländer A, Dillmann U, Eisner W, Gruber D, Hamel W, Herzog J, Hilker R, Klebe S, Kloss M, Koy J, Krause M, Kupsch A, Lorenz D, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- Diederich NJ, Kalteis K, Stamenkovic M, Pieri V, Alesch F. Efficient internal pallidal stimulation in Gilles de la Tourette syndrome: a case report. Mov Disord. 2005;20:1496–1499. doi: 10.1002/mds.20551. [DOI] [PubMed] [Google Scholar]
- Flaherty AW, Williams ZM, Amirnovin R, Kasper E, Rauch SL, Cosgrove GR, Eskandar EN. Deep brain stimulation of the anterior internal capsule for the treatment of Tourette syndrome: technical case report. Neurosurgery. 2005;57:E403. doi: 10.1227/01.neu.0000176854.24694.95. discussion E403. [DOI] [PubMed] [Google Scholar]
- François C, Yelnik J, Percheron G, Tandé D. Calbindin D-28k as a marker for the associative cortical territory of the striatum in macaque. Brain Res. 1994;633:331–336. doi: 10.1016/0006-8993(94)91557-1. [DOI] [PubMed] [Google Scholar]
- François C, Grabli D, McCairn K, Jan C, Karachi C, Hirsch EC, Féger J, Tremblay L. Behavioural disorders induced by external globus pallidus dysfunction in primates II. Anatomical study. Brain. 2004;127:2055–2070. doi: 10.1093/brain/awh239. [DOI] [PubMed] [Google Scholar]
- Grabli D, McCairn K, Hirsch EC, Agid Y, Féger J, François C, Tremblay L. Behavioural disorders induced by external globus pallidus dysfunction in primates: I. Behavioural study. Brain. 2004;127:2039–2054. doi: 10.1093/brain/awh220. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, Salloway SP, Okun MS, Goodman WK, Rasmussen SA. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31:2384–2393. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- Hammond C, Feger J, Bioulac B, Souteyrand JP. Experimental hemiballism in the monkey produced by unilateral kainic acid lesion in corpus Luysii. Brain Res. 1979;171:577–580. doi: 10.1016/0006-8993(79)91066-7. [DOI] [PubMed] [Google Scholar]
- Houeto JL, Karachi C, Mallet L, Pillon B, Yelnik J, Mesnage V, Welter ML, Navarro S, Pelissolo A, Damier P, Pidoux B, Dormont D, Cornu P, Agid Y. Tourette's syndrome and deep brain stimulation. J Neurol Neurosurg Psychiatry. 2005;76:992–995. doi: 10.1136/jnnp.2004.043273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houeto JL, Mallet L, Mesnage V, Tezenas du Montcel S, Béhar C, Gargiulo M, Torny F, Pelissolo A, Welter ML, Agid Y. Subthalamic stimulation in Parkinson disease: behavior and social adaptation. Arch Neurol. 2006;63:1090–1095. doi: 10.1001/archneur.63.8.1090. [DOI] [PubMed] [Google Scholar]
- Karachi C, Yelnik J, Tandé D, Tremblay L, Hirsch EC, François C. The pallidosubthalamic projection: an anatomical substrate for nonmotor functions of the subthalamic nucleus in primates. Mov Disord. 2005;20:172–180. doi: 10.1002/mds.20302. [DOI] [PubMed] [Google Scholar]
- Krack P, Kumar R, Ardouin C, Dowsey PL, McVicker JM, Benabid AL, Pollak P. Mirthful laughter induced by subthalamic nucleus stimulation. Mov Disord. 2001;16:867–875. doi: 10.1002/mds.1174. [DOI] [PubMed] [Google Scholar]
- Kupsch A, Benecke R, Müller J, Trottenberg T, Schneider GH, Poewe W, Eisner W, Wolters A, Müller JU, Deuschl G, Pinsker MO, Skogseid IM, Roeste GK, Vollmer-Haase J, Brentrup A, Krause M, Tronnier V, Schnitzler A, Voges J, Nikkhah G, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006;355:1978–1990. doi: 10.1056/NEJMoa063618. [DOI] [PubMed] [Google Scholar]
- Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas JF, Broussolle E, Perret JE, Benabid AL. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 1995;345:91–95. doi: 10.1016/s0140-6736(95)90062-4. [DOI] [PubMed] [Google Scholar]
- Mallet L, Mesnage V, Houeto JL, Pelissolo A, Yelnik J, Behar C, Gargiulo M, Welter ML, Bonnet AM, Pillon B, Cornu P, Dormont D, Pidoux B, Allilaire JF, Agid Y. Compulsions, Parkinson's disease, and stimulation. Lancet. 2002;360:1302–1304. doi: 10.1016/S0140-6736(02)11339-0. [DOI] [PubMed] [Google Scholar]
- Mallet L, Schüpbach M, N′Diaye K, Remy P, Bardinet E, Czernecki V, Welter ML, Pelissolo A, Ruberg M, Agid Y, Yelnik J. Stimulation of subterritories of the subthalamic nucleus reveals its role in the integration of the emotional and motor aspects of behavior. Proc Natl Acad Sci USA. 2007;104:10661–10666. doi: 10.1073/pnas.0610849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurman PR, Bosch DA, Bossuyt PM, Bonsel GJ, van Someren EJ, de Bie RM, Merkus MP, Speelman JD. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med. 2000;342:461–468. doi: 10.1056/NEJM200002173420703. [DOI] [PubMed] [Google Scholar]
- Servello D, Porta M, Sassi M, Brambilla A, Robertson MM. Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulation. J Neurol Neurosurg Psychiatry. 2007;79:136–142. doi: 10.1136/jnnp.2006.104067. [DOI] [PubMed] [Google Scholar]
- Shahed J, Poysky J, Kenney C, Simpson R, Jankovic J. GPi deep brain stimulation for Tourette syndrome improves tics and psychiatric comorbidities. Neurology. 2007;68:159–160. doi: 10.1212/01.wnl.0000250354.81556.90. [DOI] [PubMed] [Google Scholar]
- Vandewalle V, van der Linden C, Groenewegen HJ, Caemaert J. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet. 1999;353:724. doi: 10.1016/s0140-6736(98)05964-9. [DOI] [PubMed] [Google Scholar]
- Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Benabid AL, Cornu P, Lagrange C, Tézenas du Montcel S, Dormont D, Grand S, Blond S, Detante O, Pillon B, Ardouin C, Agid Y, Destée A, Pollak P. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med. 2005;352:459–467. doi: 10.1056/NEJMoa042187. [DOI] [PubMed] [Google Scholar]
- Visser-Vandewalle V, Temel Y, Boon P, Vreeling F, Colle H, Hoogland G, Groenewegen HJ, van der Linden C. Chronic bilateral thalamic stimulation: a new therapeutic approach in intractable Tourette syndrome. Report of three cases. J Neurosurg. 2003;99:1094–1100. doi: 10.3171/jns.2003.99.6.1094. [DOI] [PubMed] [Google Scholar]