Abstract
Stromal-cell-derived factor-1 (SDF-1) and its receptor CXC chemokine receptor 4 (CXCR4) play a well-established role during embryonic development of dentate gyrus granule cells. However, little is known about the regulation and function of CXCR4 in the postnatal dentate gyrus. Here, we identify a striking mismatch between intense CXCR4 mRNA and limited CXCR4 protein expression in adult rat subgranular layer (SGL) neurons. We demonstrate that CXCR4 protein expression in SGL neurons is progressively lost during postnatal day 15 (P15) to P21. This loss of CXCR4 protein expression was paralleled by a reduction in the number of SDF-1-responsive SGL neurons and a massive upregulation of SDF-1 mRNA in granule cells. Intraventricular infusion of the CXCR4-antagonist AMD3100 dramatically increased CXCR4 protein expression in SGL neurons, suggesting that CXCR4 is tonically activated and downregulated by endogenous SDF-1. Infusion of AMD3100 also facilitated detection of CXCR4 protein in bromodeoxyuridine-, nestin-, and doublecortin-labeled cells and showed that the vast majority of adult-born granule cells transiently expressed CXCR4. Chronic AMD3100 administration impaired formation of new granule cells as well as neurogenesis-dependent long-term recognition of novel objects. Therefore, our findings suggest that tonic activation of CXCR4 in newly formed granule cells by endogenous SDF-1 is essential for neurogenesis-dependent long-term memory in the adult hippocampus.
Keywords: CXCL12, internalization, Akt, desensitization, hippocampus, memory, neurogenesis
Introduction
Progenitor cells in the adult subgranular layer (SGL) produce thousands of granule cells (GCs) every day (Cameron and McKay, 2001; Seaberg and van der Kooy, 2002). Newly generated GCs are integrated into the granule cell layer (GCL) and become connected with the hippocampal network (van Praag et al., 2002). The immature GCs are more responsive to long-term potentiation and long-term depression stimuli than mature GCs. This unique form of bidirectional synaptic plasticity renders immature GCs particular well suited to process novel information (Schmidt-Hieber et al., 2004; Doetsch and Hen, 2005; Song et al., 2005; Leuner et al., 2006). Consistently, conditions of enhanced neurogenesis improve the performance of animals in memory tasks (e.g., long-term recognition of novel objects is improved when rats are housed in a neurogenesis-enhancing enriched environment and impaired when environmental enrichment is combined with application of antimitotic drugs) (Bruel-Jungerman et al., 2005).
The identification of factors controlling the generation, survival, and excitability of immature GCs will help to understand how neurogenesis and hippocampal plasticity are regulated. A good candidate is the stromal-cell-derived factor-1 (SDF-1)/CXC chemokine receptor 4 (CXCR4) system, which is indispensable for the embryonic development of numerous neuronal structures, including the dentate gyrus (Zou et al., 1998; Klein et al., 2001; Bagri et al., 2002; Lu et al., 2002; Chalasani et al., 2003; Stumm et al., 2003; Lieberam et al., 2005; Borrell and Marin, 2006; Stumm and Hollt, 2007). Given that the CXCR4 promoter is active in neuronal progenitors in the adult dentate gyrus as shown in CXCR4-enhanced green fluorescent protein (EGFP) bacterial artificial chromosome (BAC) transgenic mice (Tran et al., 2007), we examined CXCR4 protein expression in adult-born GCs, the influence of CXCR4 on adult hippocampal neurogenesis, and the role of CXCR4 in a neurogenesis-dependent long-term memory task. Moreover, based on neuromodulatory and neuroendocrine effects of SDF-1 on mature neurons (Guyon et al., 2005a,b, 2006; Callewaere et al., 2006), we studied whether SDF-1 affects the excitability of immature GCs.
Finally, we addressed the striking mismatch between intense CXCR4 mRNA expression (Stumm et al., 2002, 2003; Tissir et al., 2004; Tran et al., 2007) and weak CXCR4 protein expression in the SGL (Stumm et al., 2002). Studies in non-neuronal cell lines showed that CXCR4 is sequestered from the cell surface (internalized), sorted to lysosomes, and degraded after agonist stimulation (Orsini et al., 1999; Marchese and Benovic, 2001). Therefore, the mismatch between CXCR4 mRNA and CXCR4 protein in the SGL may reflect tonic activation and turnover of CXCR4 by endogenous SDF-1. Indeed, SDF-1 is abundantly expressed adjacent to the SGL by mature GCs (Tham et al., 2001). Therefore, we used the CXCR4 antagonist AMD3100, which prevents SDF-1-induced CXCR4 internalization, to study CXCR4 trafficking and downregulation in the SGL and in primary hippocampal cultures. Altogether, we provide the first study addressing the regulation and function of CXCR4 in adult-born GCs by histochemical, electrophysiological, and pharmacological approaches.
Materials and Methods
Animals.
For all animal procedures, ethical approval was sought according to the requirements of the German or French National Act on the Use of Experimental Animals. Unless indicated, experiments were performed with male Wistar rats (6–8 weeks of age) housed in groups of five in standard cages in a temperature-controlled animal facility with a 12 h light/dark cycle. Animals had free access to food and water. Analyses of postnatal SDF-1 and CXCR4 expression were done at postnatal day 10 (P10), P12, P14, P16, P18, and P21 by immunohistochemistry (n = 3, each) and in situ hybridization (n = 3, each).
In situ hybridization.
SDF-1 and CXCR4 mRNAs were detected by 35S-labeled riboprobes corresponding to the coding regions (Stumm et al., 2002, 2007b). Specificity of the hybridization conditions was controlled using SDF-1- and CXCR4-deficient embryos (Stumm et al., 2003). Quantitative analysis of hybridization signals in x-ray autoradiograms was performed by densitometry as described previously (Stumm et al., 2002). Dual in situ hybridization combining the 35S-CXCR4 probe with digoxigenin-labeled probes for glutamate decarboxylase, vesicular glutamate transporter 1 (VGLUT1), and somatostatin was performed as described previously (Stumm et al., 2007b).
Characterization of CXCR4 antibody.
Affinity-purified polyclonal rabbit antisera [2144, 1181] against the C-terminal sequence of mouse and rat CXCR4 (residues 338–359 in mouse) were used (Stumm et al., 2002). Antisera were characterized extensively by cytochemistry, Western blot, and immunoprecipitation assays, including preabsorption controls with CXCR4-transfected and nontransfected human embryonic kidney 293 (HEK293) cells (Stumm et al., 2002). Specificity of CXCR4-like immunostaining was controlled in embryonic day 16 (E16) CXCR4-deficient mice (supplemental Fig. 1, available at www.jneurosci.org as supplemental material).
Primary cultures.
Hippocampal cultures from E17 Sprague Dawley rat embryos (Charles River, Margate, Kent, UK) were prepared and transfected by using the Nucleofector device (Amaxa, Köln, Germany) as described previously (Schulz et al., 2004; Stumm et al., 2007a).
Immunocytochemistry.
Subcellular localization of endogenous CXCR4 or T7-epitope-tagged CXCR4 (T7-CXCR4) was studied after 1 d in vitro (DIV) by confocal microscopy (Stumm et al., 2002). Cells were treated with 10 nm SDF-1 (R&D Systems, Minneapolis, MN), 6 μm AMD3100 (Sigma-Aldrich, St. Louis, MO), or a combination of both as described in Results. After fixation and permeabilization, endogenous CXCR4 was labeled with anti-CXCR4 [1181] and T7-CXCR4 with affinity-purified anti-T7 antibody (Gramsch Laboratories, Schwabhausen, Germany) and detected with Cy3-labeled secondary antibody. For semiquantitative analysis of T7-CXCR4 internalization, primary cells were cotransfected with plasmids encoding for T7-CXCR4 and EGFP at a 10:1 molar ratio. Transfected cells were selected by their green fluorescence and categorized by an investigator ignorant of the treatment according to the subcellular distribution of T7-CXCR4 in the following: (1) cells with intensely labeled plasma membrane, (2) cells with sparse labeling at the plasma membrane and intense intracellular signal (internalized T7-CXCR4), and (3) cells with sparse or no T7-labeling. Results were averaged from three independent experiments with 20 categorized cells per treatment.
Internalization assay.
CXCR4 internalization was examined by surface labeling of the extracellular N-terminal T7-epitope of transfected T7-CXCR4 in living neuronal cells after 1 DIV or in HEK293 cells stably expressing T7-CXCR4. Receptor labeling was performed with 1 μg/ml T7 antibody before or after treatment. An ELISA was used for quantitative analyses of surface receptors (Pfeiffer et al., 2003). The labeled cells were fixed, incubated with peroxidase-conjugated secondary antibody (1:1000; GE Healthcare, Little Chalfont, Buckinghamshire, UK), and developed with ABTS solution (Roche Applied Science, Indianapolis, IN). After 15 min, 200 μl of the substrate solution was analyzed at 405 nm using a microplate reader (Bio-Rad, Hercules, CA). Cultures receiving no substances were fixed immediately after labeling and served as reference (100% surface receptors). For confocal analyses of receptors labeled before treatment, cells were fixed and permeabilized, and receptors were detected with Cy3-labeled secondary antibody.
Western blot analysis of CXCR4.
Treatment with SDF-1 (10 nm) and AMD3100 (6 μm) was performed in neurobasal medium after 1 DIV. Western blot analysis of CXCR4 was done as described using 15 × 106 primary cells per lane (Stumm et al., 2002). Blots were detected with anti-CXCR4 [1181], anti-rabbit horseradish peroxidase-linked IgG (NA934; GE Healthcare), and ECL Western Blotting kit (GE Healthcare). Blots were stripped and detected for transferrin receptor (TFR) as loading control using a mouse anti-human TFR antibody (1 μg/ml; #13–6800, Zymed Laboratories, South San Francisco, CA; Immunodetection, Invitrogen, Carlsbad, CA). The ratio of the CXCR4 and TFR signals was determined by densitometry and then normalized with the ratio of the control group. Normalized results were combined from three independent experiments.
Akt assay.
Culture medium was replaced by balanced salt solution (BSS) consisting of the following (in mm): 143 Na, 5.5 K, 1.8 Ca2, 1.8 Mg2, 125 Cl, 26 HCO3, 1 PO4, 0.8 SO4, pH 7.4, and 4.5 g/L glucose and treated with 10 nm SDF-1 as described in Results. Cells (0.5 × 106 per lane) were lysed in 500 μl of boiling SDS sample buffer. Lysates were separated by 10% SDS-PAGE and blotted. Ser-473-phosphorylated Akt (pAkt) was detected by rabbit monoclonal antibody (1:1000; #193H12; Cell Signaling Technology, Danvers, MA). After stripping, phosphorylated and nonphosphorylated Akt were detected using anti-Akt rabbit polyclonal IgG (0.125 μg/ml; #07–416; Biomol International, Hamburg, Germany) (Stumm et al., 2007a). The ratio of the pAkt and Akt signals was determined and normalized with the mean of the control group. Normalized results were combined from three independent experiments with three independent repeats each.
Antibodies used for immunohistochemistry.
Antibodies used for immunohistochemistry are as follows: rat monoclonal anti-bromodeoxyuridine (BrdU) (1:1000; BU1/75; #SM1667; Acris Antibodies, Hiddenhausen, Germany), guinea pig polyclonal anti-doublecortin (AB5910; 1:1000; Millipore Bioscience Research Reagents, Temecula, CA), mouse monoclonal anti-NeuN (neuronal-specific nuclear protein) (A60; 1:3000; MAB377; Millipore Bioscience Research Reagents), guinea pig polyclonal anti-GFAP (Gp52; 1:2000; Progen, Heidelberg, Germany), mouse monoclonal anti-nestin (Rat-401; 20 μg/ml; MAB353; Millipore Bioscience Research Reagents), mouse monoclonal anti-somatostatin (SOM-14; 1:25; Biomeda, Foster City, CA).
Immunohistochemistry.
Immunostainings were performed with free-floating sections as described previously (Stumm et al., 2002). For dual labeling with BrdU, proteins (CXCR4, doublecortin, and NeuN) were detected by primary antibody, biotinylated secondary antibody, peroxidase-conjugated avidin/biotin reagent (Vectastain Elite ABC kit), tyramine amplification, and Alexa Fluor 488 (MoBiTec, Göttingen, Germany). After completion of the biotin/tyramine reaction, sections were treated for 2 h with 50% formamide/2× SSC at 65°C, 5 min with 2× SSC at room temperature, 30 min with 2 m HCl at 37°C, and 15 min with 0.1 m boric acid, pH 8.5. After washing, anti-BrdU antibody was applied and detection completed with goat anti-rat Cy3 to visualize BrdU. For dual labeling of CXCR4 with nestin, doublecortin, GFAP, and somatostatin, CXCR4 was detected as described, and cell markers were visualized with Cy3-labeled secondary antibodies.
Intraventricular substance application.
Mini-osmotic pumps (models 1007D, 2002; flow rate, 0.5 μl/h; Alzet, Cupertino, CA) infusing either AMD3100 (5 mg/ml in saline) or saline (Thored et al., 2006) via brain infusion kit II (Alzet) were implanted subcutaneously in the neck. Rats were anesthetized with isoflurane [1.5% in a mixture of O2/N2O (30:70)] and fixed in a stereotaxic frame for trepanation and intraventricular insertion of the infusion cannula at 0.9 mm posterior and 1.9 mm lateral to bregma.
Quantitative and semiquantitative analyses of CXCR4 expression.
Whether AMD3100 affects hippocampal CXCR4 expression was quantified after 13 d intraventricular substance application by in situ hybridization (n = 6, saline; n = 7, AMD3100) and immunohistochemistry (n = 5, saline; n = 6, AMD3100). Additional qualitative analyses were performed 4 h, 1 d, 2 d, and 6 d after treatment with AMD3100 or saline. CXCR4 expression in adult-born SGL cells was examined 4 h, 30 h, 7 d, and 14 d (n = 3 each) after a single BrdU application (200 mg/kg). In each rat, 50 BrdU-labeled SGL cells were tested for CXCR4 protein expression by confocal immunohistochemistry, and the percentage of BrdU/CXCR4-labeled cells from all BrdU-labeled cells was averaged. To improve CXCR4 detection, rats received intraventricular AMD3100 for 24 h before they were killed.
Influence of AMD3100 on neurogenesis.
BrdU (100 mg/kg) was administered five times during a 48 h period before substances were applied as illustrated in Results. Briefly, substances were infused for 13 d starting on day 1, and animals were killed on day 13 (n = 5, saline; n = 6, AMD3100) and on day 23 (saline, AMD3100, n = 10 each). After immunostaining of BrdU, all labeled cells in the GCL and SGL were counted in every eighth frontal section from bregma −2.8 mm to bregma −4.4 mm on the side opposite to trepanation. The number of labeled cells per segment was calculated by multiplication by 8. Overlap of BrdU with doublecortin or NeuN was determined at bregma −3.44 and −3.76 using a 100×/1.4 objective and confocal microscopy. In each animal, 50 randomly selected BrdU-labeled cells were tested for colocalization of NeuN and doublecortin by recording a stack of five images along the z-axis. All evaluations were done in a blinded manner.
Enriched environment and intracerebroventricular treatment.
Rats were randomly assigned to an enriched environment (n = 20) or individual housing in standard cages (n = 20). Enriched environment was achieved by housing animals in groups of five in large boxes (1.5 × 0.8 × 0.8 m) containing various toys, tubes, climbing platforms, and hiding places as described previously (Bruel-Jungerman et al., 2005). In each group, 10 rats received saline intracerebroventricularly, and 10 rats received AMD3100 intracerebroventricularly for 13 d via osmotic minipumps. On day 13, minipumps were removed under isoflurane anesthesia, and memory tests were started on day 17. Before memory testing, animals were habituated to a 0.5 × 0.5 m open field as described previously (Bruel-Jungerman et al., 2005), and unchanged locomotor activity in AMD3100-treated rats was ascertained by a computerized activity meter (Moti Test; TSE Systems, Bad Homburg, Germany).
Novel object recognition test.
The test was performed as described using nine objects, which elicited a similar degree of spontaneous exploration in naive rats (Bruel-Jungerman et al., 2005) (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Exploration behavior was observed using a camera placed above the open field. Object exploration was scored when an animal faced an object at <1 cm distance. One animal with total exploration times below 20 s was excluded.
Electrophysiology.
Transverse 250-μm-thick hippocampal slices were cut at P11–P21 using a Microm vibroslicer (Guyon et al., 2006). Slices were placed under a microscope (Zeiss, Le Pecq, France) equipped with an infrared video camera (Axiocam; Zeiss) in a chamber superfused at 1 ml/min with oxygenated solution containing the following (in mm): 125 NaCl, 2.5 KCl, 0.4 CaCl2, 1 MgCl2, 25 glucose, 1.25 NaH2P04, 26 NaHC03, pH 7.4, when bubbled with 95% O2/5% CO2. Recordings were made at room temperature (23 ± 2°C) using an Axopatch 200B (Molecular Devices, Sunnyvale, CA). Patch-clamp pipettes made from borosilicate glass capillary (Hilgenberg, Masfeld, Germany) had a resistance of 6–12 MΩ when filled with internal solution containing the following (in mm): 135 K-gluconate, 0.3 CaCl2, 1 MgCl2, 10 HEPES, 1 EGTA, 4 Mg2ATP, 0.4 Na3GTP, pH adjusted to 7.3 with KOH. Access resistances ranging from 12 to 50 MΩ were compensated 60–80%. Cell capacitance and resistance were measured in V-clamp by applying 5 mV steps at a holding potential of −80 mV using pClamp software (Molecular Devices). Voltage signals were measured in the I-clamp fast mode. Membrane time constant was estimated by fitting a monoexponential function to the voltage decay after a 600 ms hyperpolarizing current pulse. SDF-1 was applied by a fast delivery system close to the cell recorded. Values of the analysis indicate mean ± SEM.
Results
Development of a mismatch between CXCR4 mRNA and CXCR4 protein expression in the subgranular layer coincides with SDF-1 upregulation in granule cells
A systematic comparison of the changes of CXCR4 mRNA and protein expression during postnatal development of the rat dentate gyrus (P10–P21) revealed a striking loss of CXCR4 protein in the SGL between P15 and P21 that was not accompanied by a similar reduction in CXCR4 mRNA. Between P10 and P15, CXCR4 protein was highly expressed in the molecular layer, GCL, and hilus (shown for P10 in Fig. 1A), which reflects the known expression of CXCR4 mRNA in Cajal-Retzius cells, immature GCs, and hilar GABAergic neurons (Stumm et al., 2003; Tran et al., 2007). Between P15 and P21, CXCR4 mRNA and protein decreased in all layers of the dentate gyrus. In the SGL and hilus, however, downregulation of CXCR4 protein was particularly intense and, by far, exceeded that of CXCR4 mRNA. Consequently, only very few CXCR4-immunoreactive (CXCR4-IR) cells were detectable in the SGL and hilus after P18 (Fig. 1B). In contrast, numerous cells with intense CXCR4 mRNA expression were detectable in the SGL and hilus at all postnatal stages (Fig. 1C) (Stumm et al., 2003; Tran et al., 2007). Therefore, there is a striking mismatch between CXCR4 mRNA and CXCR4 protein in the SGL and hilus of late postnatal and adult rats.
Figure 1.
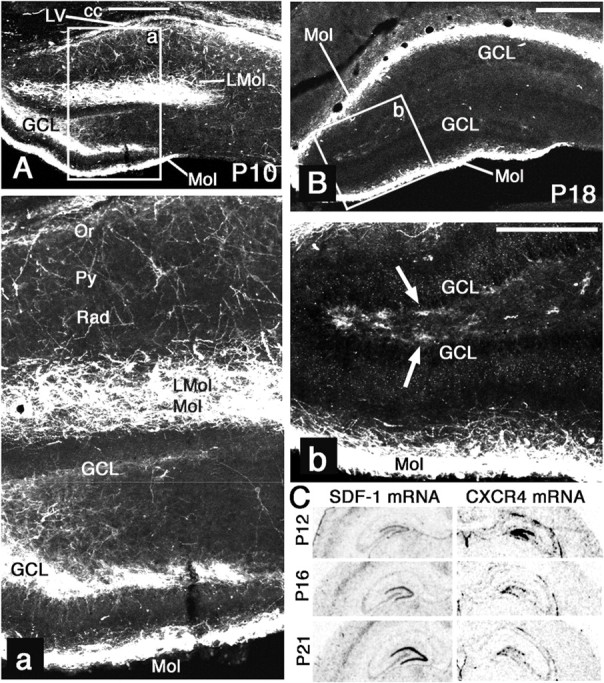
Postnatal downregulation of the CXCR4 protein in the SGL. A, B, Confocal images of coronal sections of the rat hippocampus at P10 (A) and P18 (B). A, Low- and high-power images show CXCR4 in the corpus callosum (cc) and wall of the lateral ventricle (LV). In the Ammon's horn, CXCR4 is detected in the oriens layer (Or), pyramidal cell layer (Py), and lacunosum moleculare layer (LMol). In the dentate gyrus, the molecular layer (Mol), the inner one-third of the GCL, and neurons in the hilus are stained. B, Low- and high-power images focusing on the dentate gyrus show intense CXCR4 immunostaining in the molecular layer. Only a few cells are stained in the SGL (arrows) and hilus. C, Autoradiograms comparing SDF-1 and CXCR4 mRNA expression in the dentate gyrus at P12, P16, and P21 after in situ hybridization with 35S-labeled probes. SDF-1 mRNA expression in the GCL starts at P12 and increases strongly until P21. CXCR4 mRNA expression in the GCL is strongly downregulated between P12 and P16 and shows only a small decrease between P16 and P21. Scale bars: A, B, 400 μm; a, b, 200 μm.
Analyses of SDF-1 mRNA expression showed that the mismatch between CXCR4 mRNA and CXCR4 protein in the SGL developed parallel to the pronounced postnatal upregulation of SDF-1 in GCs occurring between P15 and P21 (Fig. 1C). Notably, a mismatch between CXCR4 mRNA and CXCR4 protein was not observed in the molecular layer where local SDF-1 expression is low. Because the GCL belongs to the structures with the highest SDF-1 mRNA expression in the brain, CXCR4 in the SGL is likely to be exposed to an exceedingly high local SDF-1 concentration. Therefore, the loss of CXCR4 protein in the SGL during late postnatal development might be attributable to tonic activation and turnover of CXCR4.
Blocking CXCR4 activity abolishes the mismatch between CXCR4 mRNA and CXCR4 protein expression in the SGL
Based on our hypothesis that activation of CXCR4 by endogenous SDF-1 may cause the observed mismatch between CXCR4 mRNA and CXCR4 protein in the SGL, we administered the CXCR4 antagonist AMD3100 for 13 d into the lateral ventricle of adult rats via osmotic minipumps. We then analyzed CXCR4 expression in the dentate gyrus by in situ hybridization and immunohistochemistry. The results showed that CXCR4 mRNA expression was virtually unaffected by the antagonist (Fig. 2A,B). In contrast, CXCR4-like immunoreactivity (CXCR4-LIR) showed a dramatic increase in the SGL of AMD3100-treated rats (Fig. 2C,D). Based on the counting of strongly CXCR4-IR cells in the SGL from bregma −2.8 mm to bregma −4.4 mm (Fig. 2G), we estimated that 758 cells were labeled in saline-treated rats and 5512 cells in AMD3100-treated rats, which represents an increase by 627% (p < 0.0001; Student's t test). Similar results were obtained when AMD3100 was infused for 6 d, 2 d, or 1 d. Administration of AMD3100 for 4 h resulted in a slight increase of CXCR4-LIR in the SGL (data not shown).
Figure 2.
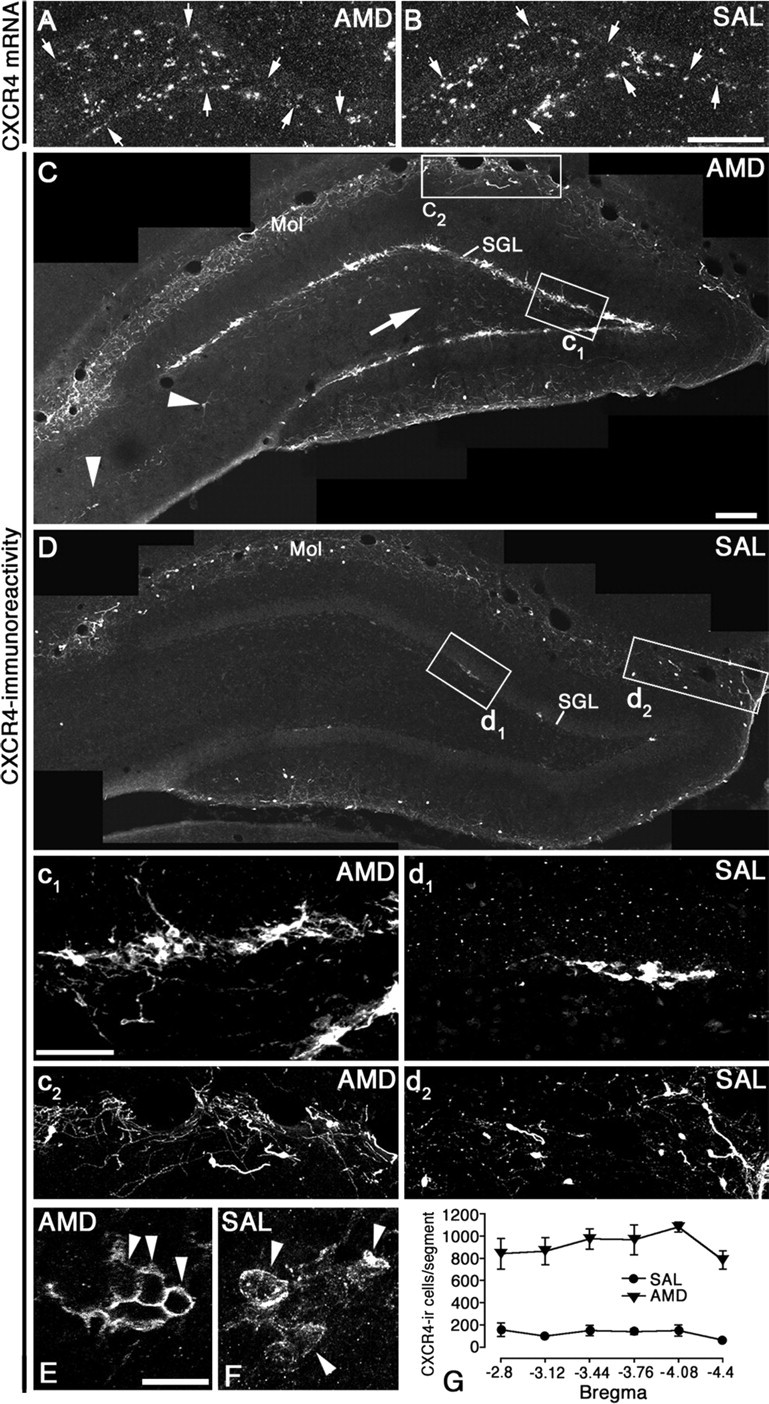
The CXCR4 antagonist AMD3100 prevents downregulation of the CXCR4 protein in the SGL and hilus. Rats received AMD3100 (A, C, E) or saline (B, D, F) intraventricularly for 13 d via osmotic minipumps. A, B, Dark-field micrographs of the dentate gyrus in hybridized sections showing that AMD3100 treatment has no effect on CXCR4 mRNA expression in the SGL (arrows). C, D, Composed confocal images showing CXCR4-LIR in the dentate gyrus. After AMD3100 treatment, CXCR4 is strongly increased in the SGL (c1, d1) and hilus (C, arrow). CXCR4 is slightly increased in the molecular layer (Mol; c2, d2) and CA3 (C, arrowheads). E, F, High-power images of single confocal planes showing the subcellular localization of CXCR4-LIR in cells of the SGL. In the saline-treated control, CXCR4 is found at the plasma membrane and intracellularly, which indicates partial CXCR4 internalization (F). CXCR4 is concentrated at the plasma membrane and virtually absent from intracellular compartments after AMD3100 treatment (E). G, The number of CXCR4-IR cells in the SGL per 320 μm hippocampal segment (bregma −2.8 mm to bregma −4.4 mm) is significantly increased after AMD3100 treatment compared with saline treatment (effect of AMD3100, p < 0.0001; effect of segment localization, p < 0.05; interaction, p > 0.05; two-way ANOVA with repeated measures). Data are presented as mean ± SEM. Scale bars: A, B, 300 μm; C, D, 125 μm; c1, c2, d1, d2, 50 μm; E, F, 10 μm.
The AMD3100 treatment enabled us to detect CXCR4 in the somata and in the proximal parts of dendritic and axonal processes of presumed immature GCs (Fig. 2c1). In addition, multipolar presumed GABAergic neurons were revealed in the hilus (Fig. 2C, arrow) and in the hilar part of the CA3 pyramidal cell layer (Fig. 2C, arrowheads). In saline-treated controls, CXCR4-LIR was found only in a few cells in the SGL (Fig. 2D,d1) and not seen in the hilus. In the molecular layer, where CXCR4-LIR is readily detectable in control rats, antagonist treatment slightly enhanced CXCR4-LIR (Fig. 2C,D,c2,d2). Analysis of single confocal planes of SGL neurons in saline-treated rats revealed CXCR4-LIR at the plasma membrane as well as in intracellular vesicle-like compartments (Fig. 2F), which may represent internalized receptors. After AMD3100 treatment, CXCR4-LIR was predominantly seen at the plasma membrane (Fig. 2E), suggesting that the antagonist prevented CXCR4 internalization. Together, these results show that inhibiting CXCR4 activity abrogates the mismatch between CXCR4 mRNA and protein expression in the SGL by strongly increasing CXCR4-LIR at the plasma membrane of SGL neurons.
SDF-1 causes internalization, downregulation, and desensitization of CXCR4
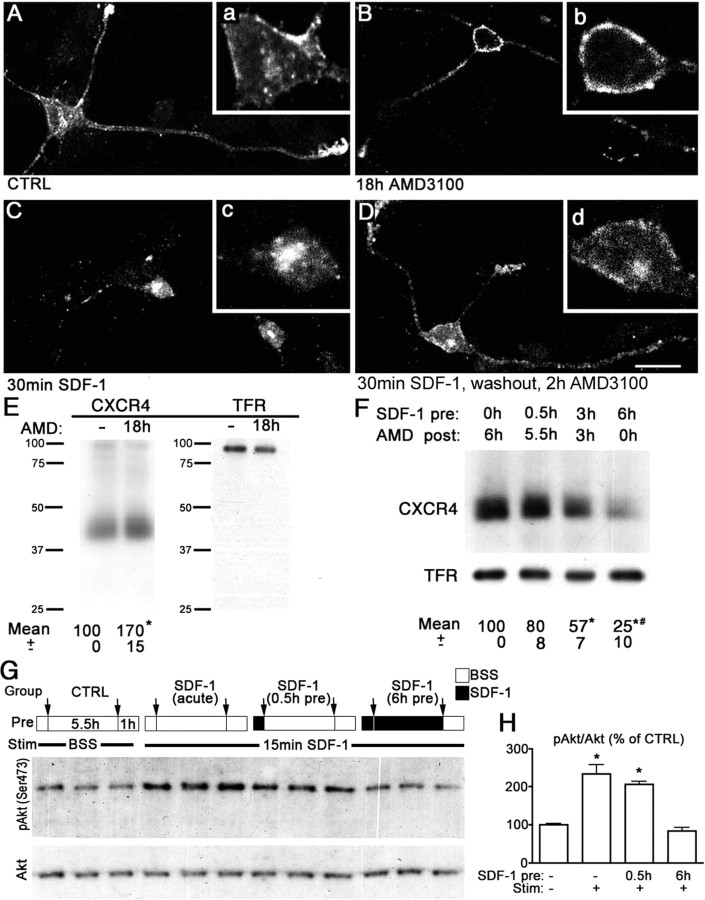
Although it is established that SDF-1 causes internalization and rapid downregulation of CXCR4 receptors heterologously expressed in cell lines (Marchese and Benovic, 2001), little is known about the regulation of endogenous CXCR4 receptors in immature neurons. We therefore studied desensitization, internalization, and downregulation of CXCR4 in dissociated cultures of the E17 rat hippocampus. The CXCR4 antagonist AMD3100 was used to block effects of endogenous and exogenous SDF-1. In untreated cultures, intense CXCR4-LIR decorated 23% of the cells at the somatic plasma membrane, leading tips of dendrites, and intracellular compartments (Fig. 3A). Overnight application of AMD3100 increased the proportion of CXCR4-IR cells to 35% and caused the receptor to accumulate at the plasma membrane (Fig. 3B), indicating constitutive turnover of CXCR4 by endogenous SDF-1 in control cultures. The assumption that SDF-1 is released from hippocampal cultures was corroborated by the finding that conditioned medium from control hippocampal cultures evoked partial CXCR4 internalization when transferred for 0.5 h to HEK293 cells stably expressing T7-epitope-tagged CXCR4 (supplemental Fig. 3A,B, available at www.jneurosci.org as supplemental material). Exogenous SDF-1 (10 nm for 0.5 h) caused complete plasma membrane depletion and intracellular clustering of CXCR4 in primary neurons (Fig. 3C) and HEK293 cells (supplemental Fig. 3C, available at www.jneurosci.org as supplemental material). The SDF-1-induced internalization was fully prevented in the presence of AMD3100 (data not shown). In another set of cultures, the SDF-1 pulse was terminated after 30 min by washout and subsequent AMD3100 supplementation. Within 2 h after the washout, CXCR4 was again detectable at the plasma membrane in 20% of the primary neurons (Fig. 3D), suggesting that a substantial proportion of the internalized CXCR4 receptors had been recycled to the cell surface.
Figure 3.
SDF-1 induced regulation of CXCR4 receptors in primary hippocampal neurons. A–D, Confocal images showing immunostained endogenous CXCR4. Insets (a–d) are magnifications of the somata in A–D. The characteristic subcellular distribution of CXCR4 is demonstrated for a control culture (CTRL; A), after overnight treatment with AMD3100 (B), after 30 min SDF-1 (C), and after 30 min SDF-1 followed by washout and 2 h treatment with AMD3100 (D). Note accumulation of CXCR4 at the plasma membrane after AMD3100 treatment (B), complete CXCR4 internalization after SDF-1 stimulation (C), and recycling of CXCR4 to the plasma membrane 2 h after the SDF-1 pulse (D). E, F, Western blots showing endogenous CXCR4 and TFR after stripping. Ratios of CXCR4 and TFR signals are given below the blots (means from 3 independent experiments after normalization with the respective control). E, Treatment for 18 h with AMD3100 (AMD) causes a 70% increase in CXCR4 (*p < 0.05; Student's t test). F, SDF-1 pretreatment (SDF-1 pre) was followed by washout and AMD3100 posttreatment (AMD post) as indicated. SDF-1 causes progressive downregulation of CXCR4 (*vs 0 h SDF-1, #vs 0.5 h and vs 3 h SDF-1; p < 0.05; ANOVA followed by Bonferroni's posttest). G, Desensitization of SDF-1-induced Akt-phosphorylation in primary neurons. Blots were detected for Ser473-pAkt and Akt after stripping. Pretreatment (Pre) involved 30 min BSS or SDF-1, washing (arrows), 5.5 h BSS or SDF-1, washing, and 1 h BSS. For stimulation (Stim), SDF-1 was applied for 15 min. H, The ratio of pAkt and Akt is expressed in percentage of the CTRL group receiving only BSS (*vs CTRL; p < 0.05; Student's t tests). E–H, All values are mean ± SD. Scale bar: A–D, 10 μm.
To exclude any misinterpretation of the SDF-1-induced CXCR4 trafficking resulting from low endogenous CXCR4 levels, we then studied overexpressed T7-epitope-tagged CXCR4 in primary hippocampal neurons after immunolabeling with T7 antibody. EGFP was cotransfected and used to randomly select transfected cells, in which the subcellular distribution of T7-CXCR4 was categorized into three groups by an investigator blinded to the pharmacological treatment: plasma membrane intensely labeled, sparse labeling at the plasma membrane and intense intracellular signal (internalized), and labeling faint to absent (nonlabeled). In untreated controls, 80% of the EGFP-labeled cells showed plasma membrane labeling, 8% internalization, and 12% no labeling (supplemental Fig. 4A, available at www.jneurosci.org as supplemental material). SDF-1 treatment for 6 h increased the proportion of cells with internalized CXCR4 to 83% (supplemental Fig. 4B, available at www.jneurosci.org as supplemental material). After 18 h of SDF-1 exposure, the proportion of nonlabeled cells increased to 77% (supplemental Fig. 4C, available at www.jneurosci.org as supplemental material). SDF-1-induced internalization and downregulation of T7-CXCR4 were fully prevented by AMD3100 (supplemental Fig. 4D, available at www.jneurosci.org as supplemental material).
We then pulse-labeled the extracellular T7-epitope of T7-CXCR4 by T7 antibody to selectively target surface receptors (supplemental Fig. 4E,F, available at www.jneurosci.org as supplemental material). As determined by a quantitative ELISA assay, 30 min exposure to SDF-1 caused a 21% internalization of prelabeled surface receptors (supplemental Fig. 4G, available at www.jneurosci.org as supplemental material). Constitutive internalization in saline-treated cultures and internalization by exogenous SDF-1 were fully prevented by AMD3100. Application of SDF-1 together with the recycling-inhibiting drug monensin (50 μm) increased SDF-1-induced internalization, suggesting that some internalized CXCR4 may recycle to the cell surface (supplemental Fig. 4G, available at www.jneurosci.org as supplemental material). We therefore examined the extent of T7-CXCR4 recycling. Surface receptors were labeled either immediately or 1 h after washout in cultures pretreated for 30 min with SDF-1. Using nontreated cultures as reference (100% surface receptors), we calculated that ∼36% of T7-CXCR4 that was internalized during the 30 min SDF-1 pulse was recycled to the cell surface during the 1 h washout interval. Recycling of T7-CXCR4 was fully prevented by monensin applied during the washout period (supplemental Fig. 4H, available at www.jneurosci.org as supplemental material).
We then addressed the question to what extent the CXCR4 protein expression level is regulated by endogenous and exogenous SDF-1 in primary neurons by immunoblotting of endogenous CXCR4. The TFR was detected as loading control. Cultures receiving AMD3100 overnight showed a 70% increase of the CXCR4/TFR ratio (Fig. 3E), which most likely reflects the prevention of constitutive CXCR4 turnover by endogenous SDF-1. The effect of exogenous SDF-1 was studied by combining various SDF-1 pretreatment periods (0, 0.5, 3, and 6 h) with complementary AMD3100 posttreatment periods so that a constant 6 h SDF-1/AMD3100 treatment time was achieved. Under these conditions, a 30 min SDF-1 pulse caused only a slight reduction of CXCR4. Extended stimulation for 3 or 6 h resulted in progressive CXCR4 downregulation (Fig. 3F). Therefore, CXCR4 downregulation requires a persistent SDF-1 stimulus. In contrast, a short SDF-1 pulse is able to trigger significant CXCR4 internalization but not sufficient to cause extensive CXCR4 downregulation.
We then examined whether CXCR4 downregulation was accompanied by CXCR4 desensitization using SDF-1-induced Akt phosphorylation (Fig. 3G,H), which plays a major role in neuronal survival (Datta et al., 1999). In control cultures, a 15 min SDF-1 stimulus increased the ratio of Ser473-pAkt to total Akt by 143%. When the stimulation was performed in cultures that had experienced a 6 h SDF-1 pretreatment and a 1 h agonist-free interval, SDF-1 was virtually ineffective. After a 30 min SDF-1 pretreatment and a 6.5 -h agonist-free interval, SDF-1 stimulation still increased pAkt by 105%. Therefore, extended SDF-1 exposure caused progressive desensitization of CXCR4-mediated Akt phosphorylation.
Detection of CXCR4-LIR in immature granule cells
We then addressed which cells express CXCR4 in the SGL/hilus of adult rats. Initially, we performed coexpression analyses at the mRNA level by dual in situ hybridization. This showed that most CXCR4 mRNA-positive cells in the hilus expressed markers for GABAergic neurons (glutamate decarboxylase/GAD mRNA and somatostatin mRNA). In contrast, most CXCR4 mRNA-expressing cells in the SGL were non-GABAergic. There was no overlap between CXCR4 mRNA and VGLUT1 mRNA in the SGL and hilus, indicating absence of CXCR4 from mature granule cells and mossy cells (supplemental Fig. 5, available at www.jneurosci.org as supplemental material).
Immunohistochemical detection of CXCR4 was improved by infusing AMD3100 for 24 h into the lateral ventricle before animals were killed. To substantiate the concept that adult-born SGL cells express CXCR4, we tested BrdU-labeled cells for CXCR4 expression in the SGL of rats that had received BrdU 4 h, 30 h, 7 d, or 14 d before the analysis. Semiquantitative evaluation of three rats per group showed that ∼60% of the BrdU-labeled SGL cells expressed CXCR4-LIR 4 and 30 h after BrdU incorporation. After 7 and 14 d, 36 and 11% of the BrdU-labeled SGL cells expressed CXCR4-LIR, respectively (CXCR4/BrdU-colabeling is demonstrated for SGL cells 30 h after BrdU-incorporation in Fig. 4A). These findings indicate that CXCR4 is transiently expressed in the majority of adult-born SGL cells. Colabeling with doublecortin identified CXCR4 in immature GCs. Approximately 30% of the doublecortin-IR cells showed intense and 30% weak CXCR4-like staining (Fig. 4B). CXCR4 was observed in the cell body, proximal dendrites, and in the initial part of the hilus-directed axons of the immature GCs. CXCR4 expression in immature GCs was further confirmed by colocalization with PSA-NCAM (data not shown). As expected, CXCR4-LIR was detected in hilar somatostatinergic neurons (Fig. 4C), which belong to the class of GABAergic neurons that are targeted by GCs and selectively innervate the molecular layer (Freund and Buzsaki, 1996). Identification of CXCR4-LIR in nestin-positive cells in the SGL points to the expression of CXCR4 in progenitor cells (supplemental Fig. 6A, available at www.jneurosci.org as supplemental material). A general overlap of CXCR4-LIR and multipolar GFAP-labeled astrocytes was not observed (supplemental Fig. 6B, available at www.jneurosci.org as supplemental material), which does not preclude that CXCR4 is colocalized in the population of nestin/GFAP-expressing progenitors as seen in CXCR4-EGFP BAC transgenic mice (Tran et al., 2007).
Figure 4.
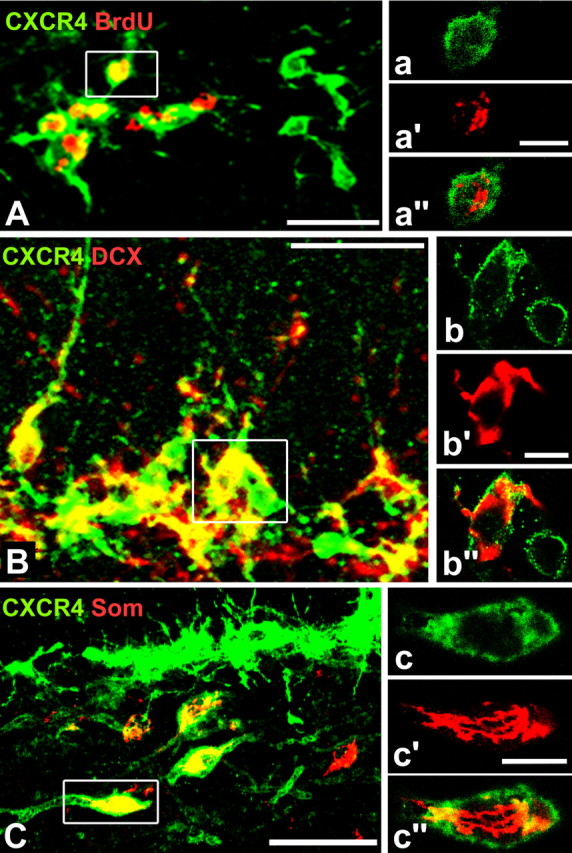
Cell types expressing CXCR4-LIR in the SGL and hilus of adult rats. A–C, Animals received AMD3100 intraventricularly to enhance the CXCR4 level. Images show superimposed confocal x/y-planes recorded along the z-axis with CXCR4-LIR in green and cell markers in red. A, There is an extensive overlap of CXCR4-LIR and BrdU (age of BrdU-labeled cells is 30 h). B, CXCR4 is expressed in doublecortin (DCX)-IR immature granule cells. C, The image demonstrates CXCR4 in somatostatin (Som)-expressing GABAergic neurons in the hilus. Note somatostatin-negative/CXCR4-positive neurons in the SGL. a–c, Single confocal x/y-planes of the cells highlighted in A–C demonstrate that CXCR4-LIR is localized predominantly at the cell surface. Scale bars: A, B, 25 μm; C, 50 μm; a, 6.5 μm; b, 5 μm; c, 10 μm.
SDF-1 enhances the excitability of immature GCs
It is established that SDF-1 exerts neuromodulatory effects on distinct populations of differentiated neurons (for review, see Guyon and Nahon, 2007). Here, we tested whether SDF-1 affects the excitability of immature GCs. Given the postnatal decline of CXCR4 expression in the SGL (Fig. 1), we analyzed effects of SDF-1 on GC excitability at various postnatal stages. Recorded GCs were classified as immature or mature according to their electrical activity (Schmidt-Hieber et al., 2004; Couillard-Despres et al., 2006). Among the 100 neurons recorded in the inner one-third of the GCL, 81 presented the characteristic features of immature GCs [i.e., high input resistance (1298 ± 119 MΩ; n = 81), slow membrane time constant (47.00 ± 3.81 ms; n = 81), and presence of a low threshold spike, which usually evoked a single action potential (67 of 81 neurons)] (Fig. 5A1). Thirteen neurons presented the electrophysiological properties of mature GCs [i.e., a significantly lower input resistance (424 ± 107 MΩ; n = 13; p < 0.01), faster membrane time constant (18.9 ± 2.4 ms; n = 13; p < 0.01), and a pattern of discharge characterized by trains of action potentials] (Fig. 5A2). Six cells presenting a very high input resistance (5400 ± 1720 MΩ), a very slow time constant (74.42 ± 25.23 ms), and a low threshold spike but no action potential were excluded from additional analyses. The pattern of discharge of all mature GCs (n = 13) was unaffected by SDF-1 (data not shown). Among the immature GCs tested (n = 72), 32% responded to SDF-1 by an increase in excitability (Fig. 5B), whereas the others were unaffected. The excitatory effect of SDF-1 was characterized by a reduced latency of the action potentials (Fig. 5B1) and an increase in the number of the action potentials that were induced by a given current pulse (Fig. 5B2–B3). Effects of SDF-1 were reversible after washout (Fig. 5B2) and prevented by the selective CXCR4 antagonist AMD3100 (200 nm; n = 5) (data not shown). The proportion of immature GCs responding to SDF-1 declined progressively during postnatal development: it was high during P12–P15 (61%; 14 of 23 neurons tested) and significantly lower during P17–P28 (18.4%; 9 of 49 neurons tested). After P15, the percentage of cells responding to SDF-1 remained stable at ∼18% (Fig. 5C).
Figure 5.
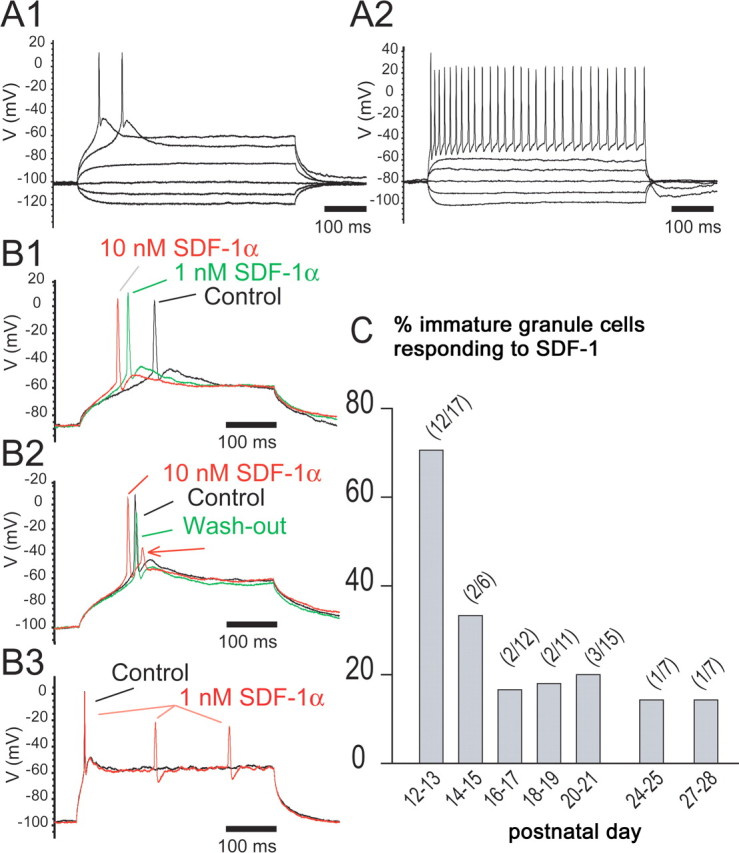
Immature granule cells respond to SDF-1. A, Distinct electrical properties of immature and mature GCs. A1, Low threshold spike in an immature GC with a single action potential. Voltage responses to current pulses of −10, −5, 0, 10, 20, and 25 pA. A2, Trains of action potentials in a mature GC. Voltage responses to current pulses with amplitudes of −40, −20, 0, 20, 40, and 100 pA. B, SDF-1 induces an increase in excitability in immature GCs. B1, Increasing concentrations of SDF-1 decrease the latency of the action potential. B2, SDF-1 induces the apparition of a second small action potential (arrow). The effect was reversible after washout. B3, Under SDF-1, two additional action potentials were evoked by a similar current pulse. B1–3, Voltage responses to current pulses of 10 pA (B1, B2) and 15 pA (B3). Current pulses were applied every 10 s. SDF-1 was applied for at least 1 min and washed out more than twice the time of application. No more than two applications per cell and per slice used were allowed to avoid desensitization. C, Percentage of immature GCs responding to 1–10 nm SDF-1 by an increase in excitability. The number of cells responding is given over the number of cells tested.
CXCR4 supports adult hippocampal neurogenesis
Because CXCR4 is required for the appropriate development of many neuronal structures during ontogenesis, it is likely that tonic CXCR4 activation in GC precursors plays a role in adult hippocampal neurogenesis. To test this hypothesis, we labeled mitotic cells during a 48 h period by five intraperitoneal BrdU applications. Thereafter, cerebral CXCR4 signaling was blocked for 13 d by administering AMD3100 into the lateral ventricle via an osmotic minipump (treatment is illustrated in Fig. 6D). The effect of the antagonist was evaluated relative to that of saline on day 13 by counting BrdU-labeled SGL cells in intervals of 320 μm in the hippocampal segment from bregma −2.8 mm to bregma −4.4 mm (Fig. 6B). This revealed a 31% reduction of BrdU-labeled cells in the AMD3100-treated group (calculated total amount of BrdU-labeled cells: saline, 2662 ± 420; AMD3100, 1851 ± 509; p < 0.05). We then examined whether the AMD3100-induced loss of newly formed SGL cells was caused by reduced neurogenesis by performing dual labeling of BrdU and doublecortin (Fig. 6A). Confocal analysis of 50 randomly selected BrdU-labeled SGL cells per animal showed that 78 and 65% of the BrdU-labeled cells expressed doublecortin in the saline-treated and AMD3100-treated groups, respectively (−13%; p < 0.05). In combination with the BrdU single-labeling study, this indicates that the AMD3100-treated group showed a 42% loss of BrdU-labeled immature GCs (Fig. 6C).
Figure 6.
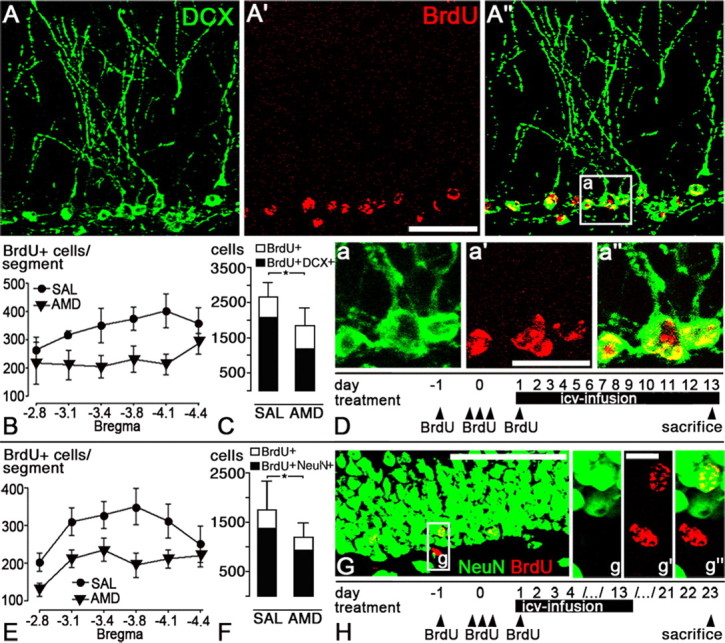
CXCR4 supports the formation of new granule cells. D, H, Adult rats received AMD3100 (AMD) or saline (SAL) intraventricularly via osmotic minipumps for 13 d (icv-infusion). Rats were administered BrdU on days −1, 0, and 1 and killed by transcardial perfusion on day 13 (A–D) or day 23 (E–H). A, G, Confocal images demonstrating dual labeling of BrdU with doublecortin (DCX; A) and NeuN (G) in the subgranular layer of saline-treated rats perfused on day 13 (A) and day 23 (G). B, E, Diagrams show the number of BrdU-labeled cells in the SGL per 320 μm hippocampal segment (bregma −2.8 mm to bregma −4.4 mm) in rats killed on day 13 (B) and day 23 (E). Two-way ANOVA with repeated measures showed a significant reduction of the amount of BrdU-labeled cells in B and E (p < 0.05). C, F, The proportion of BrdU-labeled cells expressing DCX (C) and NeuN (F) was determined in 50 randomly selected BrdU-labeled cells per animal. Diagrams show the calculated amount of BrdU single-labeled cells (white bars), BrdU/DCX-labeled cells (C, black bars), and BrdU/NeuN-labeled cells (F, black bars) in the hippocampal segment from bregma −2.8 mm to bregma −4.4 mm. Compared with the respective saline-treated group (SAL), AMD3100 treatment (AMD) reduced the amount of BrdU/DCX-labeled immature granule cells on day 13 by 42% and the amount of BrdU/NeuN-labeled mature granule cells on day 23 by 32% (p < 0.05; Student's t tests). Scale bars: A, 50 μm; a, 20 μm; G, 50 μm; g, 5 μm.
We then asked whether the AMD3100-induced loss of immature GCs leads to a reduction in the number of GCs reaching the mature developmental stage characterized by NeuN expression. To answer this question, we labeled mitotic cells by BrdU and administered saline or AMD3100 as in the previous experiment but performed evaluations after a 10 d drug-free interval to allow for maturation of the BrdU-labeled cells (treatment is illustrated in Fig. 6H; an example of BrdU/NeuN colabeling is shown in Fig. 6G). The AMD3100-treated group showed 32% less BrdU single-labeled cells in the SGL than the saline-treated group (Fig. 6E) (calculated total amount of BrdU-labeled cells: saline, 1748 ± 587; AMD3100, 1199 ± 287; p < 0.05). The proportion of BrdU-labeled SGL cells expressing NeuN was similar in the saline- and AMD3100-treated groups, indicating that the 32% reduction of BrdU single-labeled SGL cells observed in the AMD3100-treated group reflects a 32% loss of BrdU/NeuN-positive mature GCs (Fig. 6F). Together, AMD3100 treatment reduced the amount of BrdU-labeled cells that developed into immature and mature GCs by 42 and 32%, respectively. Our findings suggest that CXCR4 signaling during the first 2 weeks after cell genesis supports the survival/differentiation of new GCs in the adult dentate gyrus.
CXCR4 signaling is required for a neurogenesis-dependent form of long-term memory
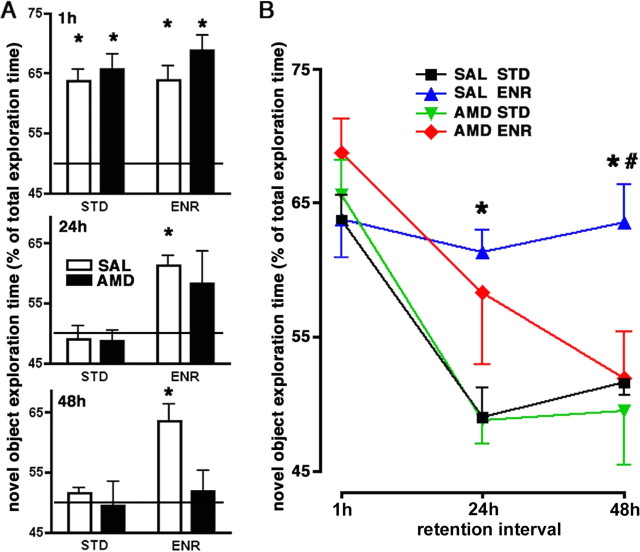
Given the fact that hippocampal neurogenesis plays a role in learning and memory and our finding that CXCR4 supports the generation of new GCs, we hypothesized that blocking CXCR4 signaling might impair neurogenesis-dependent forms of memory. To test this assumption, we used a recently described model in which improved long-term recognition of novel objects by rats housed in an enriched environment depends on hippocampal neurogenesis (Bruel-Jungerman et al., 2005). Rats receiving saline or AMD3100 intracerebroventricularly via minipumps for 13 d were housed in groups of five in an enriched environment or individually in standard cages. After removal of the minipumps, short- and long-term memory performance was evaluated by the novel object recognition task (Bruel-Jungerman et al., 2005). Rats were allowed to explore two novel objects for 4 min (acquisition phase). Short-term memory was tested after a 1 h retention interval by replacing one of the objects by a novel object. The time the animals spent exploring the known and the novel object was then recorded during a 4 min test phase. Longer exploration of the novel object indicates memory of the known object. Long-term memory was tested by repeating the procedure with 24 and 48 h retention intervals. After 1 h retention interval, animals in all groups spent significantly more time exploring the novel than the known objects (p < 0.05; Student's t test) (Fig. 7A). After the 24 and 48 h retention intervals, only saline-treated rats housed in the enriched environment preferred the novel objects (p < 0.05; Student's t test) (Fig. 7A). A two-way ANOVA with repeated measures performed with the four experimental groups confirmed that the time spent for novel object exploration depended on the retention interval (p < 0.01) as well as housing conditions (p < 0.01) and revealed that housing influenced the effect of the retention interval (p < 0.05). A Bonferroni's posttest comparing the four groups showed that saline-treated rats spent significantly more time exploring novel objects after the 24 and 48 h retention intervals when housed in the enriched environment (p < 0.05) (Fig. 7B). AMD3100 treatment of animals housed in the enriched environment significantly reduced novel object recognition after 48 h (p < 0.05) (Fig. 7B). Together, this experiment confirms that environmental enrichment enhances long-term recognition of novel objects and provides evidence that CXCR4 signaling is required for the promnesic effects of environmental enrichment.
Figure 7.
Effect of AMD3100 on long-term recognition of objects by rats housed in an enriched environment. A, B, Rats housed in standard cages (STD) or an enriched environment (ENR) received saline (SAL) or AMD3100 (AMD) intraventricularly for 13 d. Animals explored a novel object in the presence of an object that had been explored 1, 24, or 48 h before (known object). Novel object exploration time is expressed in percentage of the total exploration time. Values >50% (horizontal line) indicate recognition of the known object. A, Analysis of novel object preference. After the 1 h retention interval, all four groups prefer novel objects. After the 24 and 48 h retention intervals, only the SAL/ENR group prefers novel objects (*vs corresponding known object exploration time, p < 0.05; paired one-way Student's t test). B, Effect of the enriched environment and AMD3100 on novel object recognition. Comparison of the four experimental groups by a repeated-measures two-way ANOVA shows that novel object exploration depends on the retention interval (p < 0.01) and the environment (p < 0.01) with the effect caused by the retention interval being influenced by the environment (p < 0.05). Novel object exploration in the SAL/ENR group is larger than in the SAL/STD group at the 24 and 48 h retention intervals (*p < 0.01) and larger than in the AMD/ENR group at the 48 h retention interval (#p < 0.05) (*,#Bonferroni's posttest).
Discussion
Recent work has established that the CXCR4 promoter is active and CXCR4 mRNA is highly expressed in a large proportion of immature GCs in the postnatal and adult dentate gyrus (Stumm et al., 2003; Tran et al., 2007). Using immunohistochemical, electrophysiological, and pharmacological assays, we now show that CXCR4 receptors are functionally active in adult-born differentiating GCs. We provide evidence for tonic activation of CXCR4 in immature GCs and show that pharmacological inhibition of CXCR4 signaling impairs adult hippocampal neurogenesis and the formation of a neurogenesis-dependent form of long-term memory in animals housed in an enriched environment.
Tonic activation of CXCR4 in immature GCs
We show a striking mismatch between high CXCR4 mRNA and low CXCR4 protein expression in the SGL and hilus and provide several lines of evidence that this mismatch reflects tonic activation and turnover of CXCR4 in immature GCs and mature GABAergic neurons. First, chronic blockade of CXCR4 by intraventricular infusion of the CXCR4 antagonist AMD3100 dramatically increased the amount of CXCR4 protein in the SGL and hilus without affecting CXCR4 mRNA expression. Antagonist treatment revealed CXCR4-LIR in the majority of immature GCs, in hilar GABAergic neurons, and neuronal precursor cells, which is reminiscent of the established CXCR4 mRNA expression pattern in the dentate gyrus (Stumm et al., 2002, 2003; Tran et al., 2007). Second, GC precursors showed CXCR4-LIR almost exclusively at the plasma membrane after AMD3100 treatment, whereas a large proportion of CXCR4-LIR was clustered intracellularly in these cells in untreated animals. This effect is consistent with our own findings in primary hippocampal neurons showing that AMD3100 prevents SDF-1-induced internalization and downregulation of CXCR4. Third, CXCR4 protein expression in the dentate gyrus correlated reciprocally with the local SDF-1 expression level (i.e., the mismatch between CXCR4 mRNA and protein in the SGL/hilus and the decline of SDF-1-responsive immature GCs developed parallel to the upregulation of SDF-1 in GCs). In the molecular layer, where SDF-1 expression is low, the CXCR4 protein matched with CXCR4 mRNA and was increased only slightly by chronic AMD3100 treatment.
Recycling and degradation of CXCR4 in SDF-1-stimulated neurons
Internalization, recycling, and degradation of G-protein-coupled receptors (GPCRs) have a significant impact on the termination of receptor signaling and may be particularly relevant for chemokine receptors regulating migration, differentiation, and survival of neuronal precursors (Claing et al., 2002; Stumm and Hollt, 2007). Using primary hippocampal cultures as a model, we establish that the CXCR4 level in SDF-1-stimulated neurons is under dual control by recycling and degradation of internalized CXCR4. A brief SDF-1 pulse was sufficient for complete internalization of endogenous CXCR4. After stimulus, at least 30% of internalized CXCR4 recycled to the plasma membrane. When SDF-1 stimulation was prolonged to 6 h, 75% of CXCR4 receptors were degraded. We conclude that persistent SDF-1 exposure causes CXCR4 to undergo repeated internalization/recycling cycles with degradation of a small fraction of CXCR4 in each cycle. Therefore, regulation of CXCR4 in neurons is markedly distinct from that of other GPCRs, including the neuromodulatory somatostatin receptor 2, which shows virtually complete recycling after somatostatin-induced internalization (Tulipano et al., 2004).
Treatment of primary neurons with AMD3100 increased the amount of CXCR4 in Western blots and caused a dramatic increase of CXCR4 at the plasma membrane. This antagonist effect is attributable to endogenous SDF-1 and prevention of SDF-1-induced internalization by AMD3100 (i.e., recycled and newly generated CXCR4s are expected to accumulate at the plasma membrane of cells chronically exposed to the drug) (Hatse et al., 2002). Therefore, the dramatic increase of CXCR4 in the SGL and hilus of rats receiving chronic intraventricular AMD3100 infusion reflects persistent turnover of CXCR4 in these structures.
CXCR4 signaling activates survival pathways and supports the formation of new GCs
Adult hippocampal neurogenesis continuously generates an excess of new GCs (Cameron and McKay, 2001). Only a small fraction of newly formed cells survive to become mature neurons (Gould et al., 1999), which indicates a critical dependence of immature GCs on antiapoptotic signals by neurotrophic factors. Previously, a survival-supporting role of CXCR4 has been demonstrated in various neuronal populations, including cultured cortical and hippocampal neurons, dorsal root ganglion neurons, and retinal ganglion cells (Meucci et al., 1998; Chalasani et al., 2003; Odemis et al., 2005; Pritchett et al., 2007). Mature GCs, which abundantly express SDF-1 mRNA, may continuously provide SDF-1 to CXCR4-expressing immature neurons in the SGL. Hence, impaired neurogenesis after chronic intraventricular AMD3100 infusion may result from the inhibition of a tonic SDF-1/CXCR4-mediated survival signal. Previous work established that Erk1/2 plays a role in the CXCR4-mediated neurotrophic signal (Lazarini et al., 2000; Chalasani et al., 2003). Using hippocampal cultures, we now show that SDF-1 is a potent activator of Akt, which is a key component for neuronal survival (Datta et al., 1999). SDF-1-induced neurotrophic Erk and Akt signals are abrogated in the presence of opioids (Patel et al., 2006), which are potent inhibitors of hippocampal neurogenesis (Eisch et al., 2000; Harburg et al., 2007). This suggests that the survival of newly formed GCs might be oppositely modulated by the SDF-1 and opioid systems via the regulation of Erk and Akt signaling. Our experimental protocol by administering AMD3100 or saline for 13 d after the BrdU labeling of mitotic cells was designed to track whether the antagonist treatment affects survival/differentiation during the postmitotic stage. Therefore, our findings clearly favor the concept that SDF-1/CXCR4 signaling is neurotrophic. During the first days of the application period, however, the antagonist may have affected the proliferation rate of some BrdU-labeled transiently amplifying progenitor cells. Therefore, an influence on progenitor proliferation may add to the observed effect of the CXCR4 antagonist.
Using patch clamp, we demonstrated that CXCR4 facilitates the excitability of immature GCs. This suggests that CXCR4 may support the depolarization of immature GCs by ambient GABA and the associated increase of [Ca2+]i, which are important enhancers of hippocampal neurogenesis possibly via the induction of the proneuronal basic helix-loop-helix transcription factor NeuroD (Tozuka et al., 2005; Wang et al., 2005; Ge et al., 2006; Jagasia et al., 2006; Earnheart et al., 2007). Given that GABAergic neurons form the first synaptic contacts with immature GCs and that this GABAergic input is crucial for GC development (Overstreet Wadiche et al., 2005; Ge et al., 2007), SDF-1 might influence hippocampal neurogenesis indirectly by modulating axonal growth, synapse formation, and/or transmission of CXCR4-expressing GABAergic neurons targeting GC precursors.
A role of CXCR4 in the proliferation and migration of cultured astrocytes and oligodendrocytes has been demonstrated (Bajetto et al., 2001; Odemis et al., 2002; Dziembowska et al., 2005). Although our immunohistochemical analyses of the adult dentate gyrus provided no evidence for general expression of CXCR4 in quiescent astrocytes, astrocyte progenitors may contain CXCR4. During postnatal development, intense CXCR4-LIR was transiently detected in white matter, which supports the concept that CXCR4 plays a role in gliogenesis.
CXCR4 signaling is required for a neurogenesis-dependent form of long-term memory
Recent work has established that hippocampal neurogenesis plays a role in distinct learning and memory paradigms (Shors et al., 2001; Snyder et al., 2005; Winocur et al., 2006; Fan et al., 2007; Seigers et al., 2008). Rodents that are housed in an enriched environment show a strong increase in hippocampal neurogenesis and improved long-term memory of novel objects that depends on neurogenesis (Kempermann et al., 1997; van Praag et al., 2000; Bruel-Jungerman et al., 2005; Komitova et al., 2005). Here, we showed that chronic interference with CXCR4 signaling by intraventricular antagonist infusion abrogates this form of neurogenesis-dependent long-term memory, which is consistent with the neurogenesis-inhibiting effect of the CXCR4 antagonist. CXCR4 antagonism did not affect neurogenesis-independent short-term recognition of novel objects, suggesting a role of CXCR4 in memory consolidation. Although it is likely that impairment of neurogenesis-dependent memory by the CXCR4 antagonist is caused by the inhibition of tonic CXCR4 activation in immature GCs, inhibition of CXCR4 signaling in neurons other than GC precursors might be involved as well. However, most differentiated neuronal structures are CXCR4 negative in the adult brain (Stumm et al., 2002; Tissir et al., 2004). Neurons that continue to express CXCR4 after embryonic and postnatal brain development comprise GABAergic neurons in the hippocampus and pallium, neurons in the hippocampal molecular layer, amygdala, and hypothalamus, as well as neuronal precursors associated with the rostral migratory stream (Banisadr et al., 2002; Stumm et al., 2002, 2003, 2007b; Tissir et al., 2004; Tran et al., 2007). Therefore, cell-selective knock-out strategies will be required to test the cell autonomous role of CXCR4 signaling in GC precursors in neurogenesis and neurogenesis-dependent memory.
Conclusion
Our data show that activated CXCR4 receptors are rapidly internalized and efficiently recycled in neurons. Chronic agonist treatment causes CXCR4 downregulation. A mismatch between high CXCR4 mRNA and low CXCR4 protein expression in neurons of the SGL and hilus reflects a high degree of tonic CXCR4 activation, which is associated with CXCR4 internalization and downregulation. Chronic antagonist treatment causes CXCR4 to accumulate in the plasma membrane, allows the detection of CXCR4-LIR in immature GCs and hilar GABAergic neurons, and provides evidence that tonic CXCR4 signaling supports hippocampal neurogenesis and neurogenesis-dependent long-term memory.
Footnotes
This work was supported by Deutsche Forschungsgemeinschaft Grant STU295/3-1 (R.S.), the Centre National de la Recherche Scientifique, and the Association pour la Recherche sur le Cancer (subvention number 3375). We thank Dana Mayer, Karina Schäfer, and Anke Schmidt for excellent technical assistance.
References
- Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;129:4249–4260. doi: 10.1242/dev.129.18.4249. [DOI] [PubMed] [Google Scholar]
- Bajetto A, Barbero S, Bonavia R, Piccioli P, Pirani P, Florio T, Schettini G. Stromal cell-derived factor-1alpha induces astrocyte proliferation through the activation of extracellular signal-regulated kinases 1/2 pathway. J Neurochem. 2001;77:1226–1236. doi: 10.1046/j.1471-4159.2001.00350.x. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Fontanges P, Haour F, Kitabgi P, Rostene W, Melik Parsadaniantz S. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. Eur J Neurosci. 2002;16:1661–1671. doi: 10.1046/j.1460-9568.2002.02237.x. [DOI] [PubMed] [Google Scholar]
- Borrell V, Marin O. Meninges control tangential migration of hem-derived Cajal-Retzius cells via CXCL12/CXCR4 signaling. Nat Neurosci. 2006;9:1284–1293. doi: 10.1038/nn1764. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci. 2005;21:513–521. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- Callewaere C, Banisadr G, Desarmenien MG, Mechighel P, Kitabgi P, Rostene WH, Melik Parsadaniantz S. The chemokine SDF-1/CXCL12 modulates the firing pattern of vasopressin neurons and counteracts induced vasopressin release through CXCR4. Proc Natl Acad Sci USA. 2006;103:8221–8226. doi: 10.1073/pnas.0602620103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Chalasani SH, Baribaud F, Coughlan CM, Sunshine MJ, Lee VM, Doms RW, Littman DR, Raper JA. The chemokine stromal cell-derived factor-1 promotes the survival of embryonic retinal ganglion cells. J Neurosci. 2003;23:4601–4612. doi: 10.1523/JNEUROSCI.23-11-04601.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claing A, Laporte SA, Caron MG, Lefkowitz RJ. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog Neurobiol. 2002;66:61–79. doi: 10.1016/s0301-0082(01)00023-5. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Karl C, Lindemann G, Schmid P, Aigner R, Laemke J, Bogdahn U, Winkler J, Bischofberger J, Aigner L. Targeted transgene expression in neuronal precursors: watching young neurons in the old brain. Eur J Neurosci. 2006;24:1535–1545. doi: 10.1111/j.1460-9568.2006.05039.x. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Hen R. Young and excitable: the function of new neurons in the adult mammalian brain. Curr Opin Neurobiol. 2005;15:121–128. doi: 10.1016/j.conb.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Dziembowska M, Tham TN, Lau P, Vitry S, Lazarini F, Dubois-Dalcq M. A role for CXCR4 signaling in survival and migration of neural and oligodendrocyte precursors. Glia. 2005;50:258–269. doi: 10.1002/glia.20170. [DOI] [PubMed] [Google Scholar]
- Earnheart JC, Schweizer C, Crestani F, Iwasato T, Itohara S, Mohler H, Luscher B. GABAergic control of adult hippocampal neurogenesis in relation to behavior indicative of trait anxiety and depression states. J Neurosci. 2007;27:3845–3854. doi: 10.1523/JNEUROSCI.3609-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci USA. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Liu Z, Weinstein PR, Fike JR, Liu J. Environmental enrichment enhances neurogenesis and improves functional outcome after cranial irradiation. Eur J Neurosci. 2007;25:38–46. doi: 10.1111/j.1460-9568.2006.05269.x. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Guyon A, Nahon JL. Multiple actions of the chemokine stromal cell-derived factor-1{alpha} on neuronal activity. J Mol Endocrinol. 2007;38:365–376. doi: 10.1677/JME-06-0013. [DOI] [PubMed] [Google Scholar]
- Guyon A, Rovere C, Cervantes A, Allaeys I, Nahon JL. Stromal cell-derived factor-1alpha directly modulates voltage-dependent currents of the action potential in mammalian neuronal cells. J Neurochem. 2005a;93:963–973. doi: 10.1111/j.1471-4159.2005.03083.x. [DOI] [PubMed] [Google Scholar]
- Guyon A, Banisadr G, Rovere C, Cervantes A, Kitabgi P, Melik-Parsadaniantz S, Nahon JL. Complex effects of stromal cell-derived factor-1 alpha on melanin-concentrating hormone neuron excitability. Eur J Neurosci. 2005b;21:701–710. doi: 10.1111/j.1460-9568.2005.03890.x. [DOI] [PubMed] [Google Scholar]
- Guyon A, Skrzydelsi D, Rovere C, Rostene W, Parsadaniantz SM, Nahon JL. Stromal cell-derived factor-1alpha modulation of the excitability of rat substantia nigra dopaminergic neurones: presynaptic mechanisms. J Neurochem. 2006;96:1540–1550. doi: 10.1111/j.1471-4159.2006.03659.x. [DOI] [PubMed] [Google Scholar]
- Harburg GC, Hall FS, Harrist AV, Sora I, Uhl GR, Eisch AJ. Knockout of the mu opioid receptor enhances the survival of adult-generated hippocampal granule cell neurons. Neuroscience. 2007;144:77–87. doi: 10.1016/j.neuroscience.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatse S, Princen K, Bridger G, De Clercq E, Schols D. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 2002;527:255–262. doi: 10.1016/s0014-5793(02)03143-5. [DOI] [PubMed] [Google Scholar]
- Jagasia R, Song H, Gage FH, Lie DC. New regulators in adult neurogenesis and their potential role for repair. Trends Mol Med. 2006;12:400–405. doi: 10.1016/j.molmed.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Klein RS, Rubin JB, Gibson HD, DeHaan EN, Alvarez-Hernandez X, Segal RA, Luster AD. SDF-1 alpha induces chemotaxis and enhances Sonic hedgehog-induced proliferation of cerebellar granule cells. Development. 2001;128:1971–1981. doi: 10.1242/dev.128.11.1971. [DOI] [PubMed] [Google Scholar]
- Komitova M, Mattsson B, Johansson BB, Eriksson PS. Enriched environment increases neural stem/progenitor cell proliferation and neurogenesis in the subventricular zone of stroke-lesioned adult rats. Stroke. 2005;36:1278–1282. doi: 10.1161/01.STR.0000166197.94147.59. [DOI] [PubMed] [Google Scholar]
- Lazarini F, Casanova P, Tham TN, De Clercq E, Arenzana-Seisdedos F, Baleux F, Dubois-Dalcq M. Differential signalling of the chemokine receptor CXCR4 by stromal cell-derived factor 1 and the HIV glycoprotein in rat neurons and astrocytes. Eur J Neurosci. 2000;12:117–125. doi: 10.1046/j.1460-9568.2000.00894.x. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Lieberam I, Agalliu D, Nagasawa T, Ericson J, Jessell TM. A Cxcl12-CXCR4 chemokine signaling pathway defines the initial trajectory of mammalian motor axons. Neuron. 2005;47:667–679. doi: 10.1016/j.neuron.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci USA. 2002;99:7090–7095. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem. 2001;276:45509–45512. doi: 10.1074/jbc.C100527200. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci USA. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odemis V, Moepps B, Gierschik P, Engele J. Interleukin-6 and cAMP induce stromal cell-derived factor-1 chemotaxis in astroglia by up-regulating CXCR4 cell surface expression. Implications for brain inflammation. J Biol Chem. 2002;277:39801–39808. doi: 10.1074/jbc.M200472200. [DOI] [PubMed] [Google Scholar]
- Odemis V, Lamp E, Pezeshki G, Moepps B, Schilling K, Gierschik P, Littman DR, Engele J. Mice deficient in the chemokine receptor CXCR4 exhibit impaired limb innervation and myogenesis. Mol Cell Neurosci. 2005;30:494–505. doi: 10.1016/j.mcn.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Orsini MJ, Parent JL, Mundell SJ, Benovic JL, Marchese A. Trafficking of the HIV coreceptor CXCR4. Role of arrestins and identification of residues in the c-terminal tail that mediate receptor internalization. J Biol Chem. 1999;274:31076–31086. doi: 10.1074/jbc.274.43.31076. [DOI] [PubMed] [Google Scholar]
- Overstreet Wadiche L, Bromberg DA, Bensen AL, Westbrook GL. GABAergic signaling to newborn neurons in dentate gyrus. J Neurophysiol. 2005;94:4528–4532. doi: 10.1152/jn.00633.2005. [DOI] [PubMed] [Google Scholar]
- Patel JP, Sengupta R, Bardi G, Khan MZ, Mullen-Przeworski A, Meucci O. Modulation of neuronal CXCR4 by the micro-opioid agonist DAMGO. J Neurovirol. 2006;12:492–500. doi: 10.1080/13550280601064798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer M, Kirscht S, Stumm R, Koch T, Wu D, Laugsch M, Schroder H, Hollt V, Schulz S. Heterodimerization of substance P and mu-opioid receptors regulates receptor trafficking and resensitization. J Biol Chem. 2003;278:51630–51637. doi: 10.1074/jbc.M307095200. [DOI] [PubMed] [Google Scholar]
- Pritchett J, Wright C, Zeef L, Nadarajah B. Stromal derived factor-1 exerts differential regulation on distinct cortical cell populations in vitro. BMC Dev Biol. 2007;7:31. doi: 10.1186/1471-213X-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Schulz S, Mayer D, Pfeiffer M, Stumm R, Koch T, Hollt V. Morphine induces terminal micro-opioid receptor desensitization by sustained phosphorylation of serine-375. EMBO J. 2004;23:3282–3289. doi: 10.1038/sj.emboj.7600334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigers R, Schagen SB, Beerling W, Boogerd W, van Tellingen O, van Dam FS, Koolhaas JM, Buwalda B. Long-lasting suppression of hippocampal cell proliferation and impaired cognitive performance by methotrexate in the rat. Behav Brain Res. 2008;186:168–175. doi: 10.1016/j.bbr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Song H, Kempermann G, Overstreet Wadiche L, Zhao C, Schinder AF, Bischofberger J. New neurons in the adult mammalian brain: synaptogenesis and functional integration. J Neurosci. 2005;25:10366–10368. doi: 10.1523/JNEUROSCI.3452-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm R, Hollt V. CXC chemokine receptor 4 regulates neuronal migration and axonal pathfinding in the developing nervous system: implications for neuronal regeneration in the adult brain. J Mol Endocrinol. 2007;38:377–382. doi: 10.1677/JME-06-0032. [DOI] [PubMed] [Google Scholar]
- Stumm R, Kolodziej A, Prinz V, Endres M, Wu DF, Hollt V. Pituitary adenylate cyclase-activating polypeptide is up-regulated in cortical pyramidal cells after focal ischemia and protects neurons from mild hypoxic/ischemic damage. J Neurochem. 2007a;103:1666–1681. doi: 10.1111/j.1471-4159.2007.04895.x. [DOI] [PubMed] [Google Scholar]
- Stumm R, Kolodziej A, Schulz S, Kohtz JD, Hollt V. Patterns of SDF-1alpha and SDF-1gamma mRNAs, migration pathways, and phenotypes of CXCR4-expressing neurons in the developing rat telencephalon. J Comp Neurol. 2007b;502:382–399. doi: 10.1002/cne.21336. [DOI] [PubMed] [Google Scholar]
- Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, Hollt V, Schulz S. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci. 2002;22:5865–5878. doi: 10.1523/JNEUROSCI.22-14-05865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Hollt V, Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci. 2003;23:5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham TN, Lazarini F, Franceschini IA, Lachapelle F, Amara A, Dubois-Dalcq M. Developmental pattern of expression of the alpha chemokine stromal cell-derived factor 1 in the rat central nervous system. Eur J Neurosci. 2001;13:845–856. doi: 10.1046/j.0953-816x.2000.01451.x. [DOI] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- Tissir F, Wang CE, Goffinet AM. Expression of the chemokine receptor Cxcr4 mRNA during mouse brain development. Brain Res Dev Brain Res. 2004;149:63–71. doi: 10.1016/j.devbrainres.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol. 2007;500:1007–1033. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulipano G, Stumm R, Pfeiffer M, Kreienkamp HJ, Hollt V, Schulz S. Differential beta-arrestin trafficking and endosomal sorting of somatostatin receptor subtypes. J Biol Chem. 2004;279:21374–21382. doi: 10.1074/jbc.M313522200. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LP, Kempermann G, Kettenmann H. A subpopulation of precursor cells in the mouse dentate gyrus receives synaptic GABAergic input. Mol Cell Neurosci. 2005;29:181–189. doi: 10.1016/j.mcn.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]