Abstract
Establishment of the neuromuscular synapse requires bidirectional signaling between the nerve and muscle. Although much is known on nerve-released signals onto the muscle, less is known of signals important for presynaptic maturation of the nerve terminal. Our results suggest that the Ret tyrosine kinase receptor transmits a signal in motor neuron synapses that contribute to motor neuron survival and synapse maturation at postnatal stages. Ret is localized specifically to the presynaptic membrane with its ligands, GDNF (glial cell line-derived neurotrophic factor)/NTN (neurturin), expressed in skeletal muscle tissue. Lack of Ret conditionally in cranial motor neurons results in a developmental deficit of maturation and specialization of presynaptic neuromuscular terminals. Regeneration of Ret-deficient adult hypoglossal motor neurons is unperturbed, but despite contact with the unaffected postsynaptic specializations, presynaptic axon terminal maturation is severely compromised in the absence of Ret signaling. Thus, Ret transmits a signal in motor nerve terminals that participate in the organization and maturation of presynaptic specializations during development and during regeneration in the adult.
Keywords: neuromuscular junction, neurotrophic factors, development, nerve terminal, synaptic vesicles, Ret
Introduction
Synaptogenesis requires the coordination between presynaptic and postsynaptic elements through which the structures assemble and mature. This is achieved by sets of signals passing between the cells and which initiate and maintain the synaptic apparatus leading postsynaptically to the organization of scaffolding proteins, signaling receptors, and neurotransmitter receptors. One of the best studied synapses is the vertebrate skeletal neuromuscular synapse. Assembly of the postsynaptic neuromuscular synapse depends on Agrin released from motor nerve terminals (Godfrey et al., 1984; McMahan, 1990; Sanes and Lichtman, 2001; Burden, 2002) that via interactions with its receptor the muscle-specific receptor tyrosine kinase (MuSK) (DeChiara et al., 1996) organize postsynaptic differentiation by clustering acetylcholine receptors (AChRs) and by increasing AChR expression only in the myonuclei closely aligned to the nerve–muscle contact (Sanes and Lichtman, 2001; Burden, 2002).
Mice lacking Agrin, MuSK, and Rapsyn, which couples MuSK activation to AChR clustering, have deficits also in presynaptic specialization (Gautam et al., 1995), suggesting a signal also onto axon terminals that is necessary for presynaptic maturation. In agreement with this, recent in vivo data show that fibroblast growth factors (FGFs) participate in the concentration of synaptic vesicles transiently during embryonic stages, and together with a postnatal requirement of extracellular matrix (ECM) proteins participate in presynaptic motor nerve terminal maturation (Fox et al., 2007). However, the considerable differentiation even in the absence of both FGF signaling and ECM components predict the existence of other presynaptic organizers (Fox et al., 2007). Such candidate target-derived molecules could be neurotrophic factors that not only provide necessary trophic signals for neuronal survival during development but also promote cell differentiation, axonal growth, and maturation of many central and peripheral neuronal cell types (Bibel and Barde, 2000; Ernfors, 2001). Glial cell line-derived neurotrophic factor (GDNF) is one of the most potent survival factors for motor neurons in culture (Henderson et al., 1994; Oppenheim et al., 1995) and regulates motor neuron survival also in vivo during embryogenesis (Moore et al., 1996; Sanchez et al., 1996; Garces et al., 2000; Mikaels et al., 2000; Oppenheim et al., 2000). In transgenic mouse overexpression experiments, GDNF affects axonal branching of motor neurons (Nguyen et al., 1998; Keller-Peck et al., 2001) and increases the size or amplitude and the frequency of spontaneous synaptic currents (Wang et al., 2002; Lu and Je, 2003). These results place GDNF as a candidate signaling molecule at the neuromuscular synapse.
Because mouse mutants lacking Ret signaling die at birth as a result of the failure of kidney development, little is known of the physiological role of GDNF and Ret activation for motor neuron survival and for development of the neuromuscular synapse. Our finding that Ret is concentrated at the presynaptic nerve terminal opened for its direct participation in shaping the neuromuscular synapse. We report that Ret signaling promotes motor neuron survival and maturation of neuromuscular synapses throughout postnatal and adult life.
Materials and Methods
Animals.
Generation of the ret conditional knock-out mice (Retflox) was as follows: A 129/Sv mouse genomic BAC clone containing the ret gene was obtained from Research Genetics (Huntsville, AL). Two overlapping fragments covering a 12 kb portion of the ret gene (protein coding exons 7–17) have been identified by enzymatic restriction and Southern analysis. To target the ret locus, we used the pFlrt1 vector, generated by Orban PC (University of British Columbia, Vancouver, British Columbia, Canada) in which we have inserted a 1.2 kb fragment containing exons 12 and 13 between the two lox-P sites, upstream of the frt-flanked neomycin resistance cassette (neo). 129/Ola-derived E14 embryonic stem (ES) cells were then screened by Southern blotting for homologous recombination of the targeting construct after electroporation, using a 1.6 kb external probe and a 1.3 kb internal probe. Two independent ES cell clones were used to generate the Retflox-neo/+ mice. Germline transmission of the floxed ret allele was verified by PCR analysis. Crossing Retflox-neo/+ mice with ACTB:Flpe transgenic mice (Rodriguez et al., 2000) allowed us to remove the neo-cassette and generate the Retflox/+ animals. Mice homozygous for the modified allele were born at Mendelian frequency and survived through adulthood. Ret kinase dead mutants (RetΔ/Δ) were then obtained by breeding the offspring born from the crossing of Retflox/+ animals with PGK:Cre mice (Lallemand et al., 1998). Mice homozygous for the mutant allele were born at Mendelian frequency and die at birth. Nkx6.2+/lz mice have been described previously (Vallstedt et al., 2001). Nkx6.2+/cre mice were generated following the same strategy inserting a Cre/PGKneo cassette in place of the lacZ/PGKneo cassette. Genotyping was performed by PCR; primers and protocols are available on request. The mice used in this study were kept on a C57BL/6 genetic background. Retflox/flox and RetΔ/Δ mice are available through the European Mouse Mutant Archive (www.emmanet.org) as Retflox EM:02080 and RetKO EM:02081.
Tissue preparation.
Embryos were collected at different stages [from embryonic day 10 (E10) to E18], directly fixed in 4% paraformaldehyde (PFA) at 4°C (from 2 to 18 h depending on their age), rinsed with PBS, and preserved at 4°C in 30% sucrose–PBS until further use. Also, after overnight fixation of E18 embryos, kidneys and urogenital system were dissected out to be photographed; tongues were dissected out and maintained in 30% sucrose–PBS for additional analysis. Brainstems, spinal cords, and tongues were dissected out from adult (3–4 months of age) or aged (>12 months of age) female mice after perfusion with 4% PFA, postfixed overnight in 4% PFA at 4°C, rinsed with PBS, and stored at 4°C in 30% sucrose–PBS before cryosectioning. Brainstems were also dissected out from E11.5, E13.5, and E15.5 embryos, and they were fixed overnight in 4% PFA at 4°C in an “open-book” configuration, rinsed with PBS, and stored at 4°C in 30% sucrose–PBS until additional use. For whole-mount in situ hybridization, the embryos were dehydrated in methanol, rehydrated, treated with proteinase K, and refixed. For in situ hybridization and immunohistochemistry on cryosections, the embryos (up to E18) and adult tissues were mounted onto a mold with OCT, snap-frozen on dry ice, coronally (CNS tissue) or transversally (muscle) sectioned (16 μm), and collected on alternating slides in four or six series, respectively. Before use, the sections were air-dried 1–2 h at room temperature. For whole-mount immunochemistry, embryos were postfixed overnight in cold Dent's fixative (one part DMSO, four parts methanol), bleached, and rehydrated. For real-time PCR, segments of the brainstem dissected out from E11.5 embryos were snap frozen on dry ice before use.
Probes.
The extracellular domain (ECD) of the mouse ret, the exons 12 and 13 of the mouse ret (ex12–13), the full-length mouse GDNF, and mouse Islet 1 were amplified by reverse transcription (RT)-PCR and either subcloned using a TOPO TA cloning kit from Invitrogen (Carlsbad, CA) or ligated into the PCDNA3 vector. Specific digoxigenin-labeled antisense probes were synthesized according to supplier's instructions (Roche, Indianapolis, IN) to perform nonradioactive in situ hybridization.
Nonradioactive in situ hybridization procedures.
Sections were hybridized overnight at 70°C with a solution containing 0.19 m NaCl, 10 mm Tris, pH 7.2, 5 mm NaH2PO42H2O/Na2HPO4, pH 6.8, 50 mm EDTA, 50% formamide, 10% dextran sulfate, 1 mg/ml yeast tRNA, 1× Denhardt solution, and 100–200 ng/ml probe. Sections were then washed four times 20 min at 65°C in 0.4× SSC, pH 7.5, 50% formamide, 0.1% Tween 20, and three times 20 min at room temperature in MABT (0.1 m maleic acid, 0.15 m NaCl, and 0.1% Tween 20, pH 7.5). Sections were blocked for 1 h at room temperature in presence of 20% goat serum and 2% blocking agent (Roche) before an overnight incubation with alkaline phosphatase (AP)-conjugated antibody (Roche; 1:2000). After extensive washes with MABT, revelation was performed using nitroblue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolyl phosphate (BCIP) (Roche) in 100 mm Tris-HCl, pH 9.5, 100 mm NaCl, 50 mm MgCl2, and 0.1% Tween 20 (NTMT). As for the whole embryos and brainstems, they were prehybridized for 1 h at 70°C in 1.3× SSC, 50% formamide, 2% Tween 20, 0.5% CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate), 5 mm EDTA, and 50 μg/ml yeast tRNA. Hybridization was performed overnight with digoxigenin-labeled riboprobes in the same buffer. Washes with hybridization buffer were followed by RNase treatment and subsequent washes with hybridization buffer at 65°C. Samples were then blocked in MABT containing 20% goat serum and incubated overnight at 4°C with AP-conjugated antibody (Roche; 1:2000 in MABT with 2% goat serum). After extensive washes with MABT, revelation was performed using NBT and BCIP (Roche; in NTMT).
Immunostaining and histology.
Primary antibodies were used at the following dilutions: Ret (1:500; Santa Cruz, Santa Cruz, CA), Peripherin (1:500; Chemicon, Temecula, CA), Islet1 (1:100; Developmental Studies Hybridoma Bank, Iowa City, IA), Neurofilaments (1:500; Sigma, St. Louis, MO), Synapsin I (1:1000; Biogenesis, Bournemouth, UK), Synaptophysin (1:150; Zymed, San Francisco, CA), and Snap-25 (1:2000; Biosite, San Diego, CA). They were incubated at room temperature for 1–2 h or overnight at 4°C and detected using either species-specific fluorescent antibodies (donkey Cy2-, Cy3-, Cy5-, or Alexa-conjugated antibodies, 1:500 (Jackson ImmunoResearch, West Grove, PA; Invitrogen) or using a Vectastain ABC kit (Vector Labs, Burlingame, CA) with DAB for detection. α-Bungarotoxin, Alexa Fluor 488 conjugated (1:1000; Invitrogen) was used to visualized endplates in the tongue, buccinator, and oculomotor muscles. X-Gal staining of whole embryos was performed overnight at room temperature in phosphate buffer containing 3.1 mm FeK3(CN)6, 3.1 mm FeK4(CN)6, and 0.4 mg/ml X-gal.
Cresyl violet staining was performed on one series of sections through the brainstem for total neuronal count. Pictures were taken through a Zeiss (Oberkochen, Germany) Axioplan2, a Zeiss Axiovert 100M, a Zeiss LSM 510 confocal, or a Stemi 2000-C microscope.
Neuron quantification on cryosections.
Neuronal numbers in E18 and adult nuclei were established by counting neurons with a clear nucleus in every fourth or sixth section, respectively. The number of neurons was estimated using the following equation: N = nT/(T + D), where n is the number of neurons counted multiplied by section separation, T is the thickness of the section, and D is the average diameter of a nucleus, estimated to ∼10 μm at E18 and ∼15 μm in the adult (Abercrombie, 1946).
Primary cell and organotypic cultures.
Neural tubes from E11.5 embryos were dissected out. For primary culture purpose, rhombomeres 4–6 (containing the migrating facial motoneurons) and rhombomere 8 (containing the condensing hypoglossal motoneurons) were separated. Cells were dissociated and cultured at a high density for 6 h following a previously described protocol (Baudet et al., 2000). For organotypic culture, the intact neural tube was maintained in an open-book configuration and cultured also for 6 h in a defined medium described previously (Pozas and Ibanez, 2005). GDNF and neurturin (NTN) (Promega, Madison, WI) were used at 50 ng/ml.
Axonal length.
Primary culture were fixed with 4% PFA after 6 h, washed three times with PBS, permeabilized in PBT [1% bovine serum albumin (BSA), 0.3% Triton X-100 in PBS] for 20 min, and stained to reveal Islet1 and Peripherin expression. Cells expressing both proteins are recognized as motoneurons, and their axon is measured. Fifty to 100 motoneurons were randomly photographed for each culture condition, and the measurements were made using the NeuronJ plugin of NIH Image/J software.
Neurofilament quantification.
After neurofilament immunostaining, 20 evenly spaced images per animal through the extent of the lesioned one-half of the tongue were acquired by a LSM 5.1 scanning microscope (Zeiss). Every image consisted of the scanning of a volume of 643 × 643 × 20 μm that was projected into a bidimensional image. The fraction of the area occupied by the staining was measured after threshold normalization with the NIH Image/J software. At least three animals were analyzed per genotype.
Endplate quantification and neuromuscular junction analysis.
After α-bungarotoxin, Alexa Fluor 488-conjugated staining, the total number of the endplates was counted in every fourth section at E18 and every sixth section in adult and multiply by the number of sections to get the total number in the whole tongue. Endplates were randomly photographed using a Zeiss microscope with the same image capture settings in normal and lesioned animals. The area of the endplate was delimited and the total intensity of synapsin I, synaptophysin, and bungarotoxin staining per synapse was measured using the NIH Image/J software. The data were normalized to the average background in each image by the same software. At least three different animals were analyzed per genotype and between 70 and 100 endplates per animal.
Quantitative real-time PCR.
Total RNA were isolated from each sample of the organotypic culture using RNeasy (Qiagen, Hilden, Germany). Single-stranded cDNA were synthesized using SuperScript (Invitrogen) and oligo-d(T)15 primers (Promega). A 64 bp amplicon was obtained using the following primers: for ret forward, 5′-CTTGGCAGAAATGAAGCTTGTACA-3′, and reverse, 5′-GTCCCTCAGCCACCAAGATGT-3′; GDNF forward, TCC TGA CCA GTT TGA TGA CG, and reverse, AAC ATG CCT GGC CTA CTT TG; NTN forward, GCT CCC TGC TAT CTG TCT GG, and reverse, GTC TCA TCC GAC GTG TAG CC; artemin (ART) forward, ATT TGT GCA GCG AAA GAA CC, and reverse, ATG AAG GAG ACG GCC TCA TA; and persephin (PSP) forward, ATC CTG TGT CTG CTG CTC CT, and reverse, CAA GGA AGG TCA CAT CAG CA. Real-time PCR was performed on a PerkinElmer (Wellesley, MA) ABI Prism 5700 using SYBR Green Master Mix (Applied Biosystems, Foster City, CA) with the following thermal profile. HPRT (hypoxanthine phosphoribosyltransferase) was used as an external control (5′-GAATCTGCAAATACGAGGAGTCCT-3′ and 5′-CTTTACTAGGCAGATGGCCACA-3′).
Lesion.
Surgical procedures were performed on 3- to 4-month-old female mice under anesthesia with intraperitoneal injection of ketamine (75 mg/kg body weight) and medetomidine (1 mg/kg body weight). After surgery, atipamezol (∼60 mg/kg body weight) was injected subcutaneously to reverse the effects of medetomidine. In one group of animals, the main trunk of the left facial nerve main trunk was exposed caudally to the external auditory meatus. The nerve was transected using a pair of scissors, and the wound was immediately closed. In another group of animals, the left hypoglossal nerve was carefully exposed proximal to its bifurcation at the hyoid bone and the nerve was transected. In all experiments, the unoperated contralateral side served as a control. After 21 d, the animals were killed and perfused (n = 3 control mice and N = 9 RetNkx6.2-cre mice for each lesion paradigm).
Ethical approval of animal studies.
Animal experiments conformed to the European Communities Council Directive (86/609/EEC) and were approved by the Stockholm North Ethical Board on Laboratory Animals (N17/03, N293/05, and N385/04).
Results
Concentration of Ret receptors at the neuromuscular synapse
In order for Ret to transmit a retrograde signal, the protein would be expected to be concentrated to the neuromuscular synapse. Immunostainings of the intrinsic muscles of the tongue, a target of hypoglossal motor neurons, were performed from E18 and adult mice with antibodies against Ret combined with the localization of AChRs using fluorescently conjugated α-bungarotoxin. Ret immunoreactivity was revealed at E18 by a punctuate staining overlying forming postsynaptic AChR clusters. The staining appeared to be concentrated to the neuromuscular synapse, because only weak and occasional staining was seen in axons within the muscle. Adult neuromuscular synapses displayed a strong Ret immunoreactivity matching closely the elaborate postsynaptic specialization of the neuromuscular synapse (Fig. 1A). We next determined whether Ret is located presynaptically or postsynaptically. Thick sections were stained for Ret and AChRs, which are localized postsynaptically, or for Ret and synaptosome-associated protein of 25 kDa (SNAP25), which is a presynaptic protein, and thin optical sections through the thickness of the sections were acquired using a confocal microscopy. The reconstructed neuromuscular synapses revealed Ret in the nerve terminals overlaying the AChR containing postsynaptic membrane. Consistently, Ret also colocalized with the presynaptic protein SNAP25 (Fig. 1B). This finding shows that Ret receptors are concentrated at the presynaptic terminal of the synapse. We examined which ligand(s) were expressed in the muscle tissue and therefore could be acting on Ret. mRNA expression of the Ret ligands GDNF, NTN, ART, and PSP was examined in the muscle by RT-PCR. Strong expression of GDNF and lower levels of NTN were detected, whereas neither ART nor PSP was found expressed in the muscle (Fig. 1C).
Figure 1.
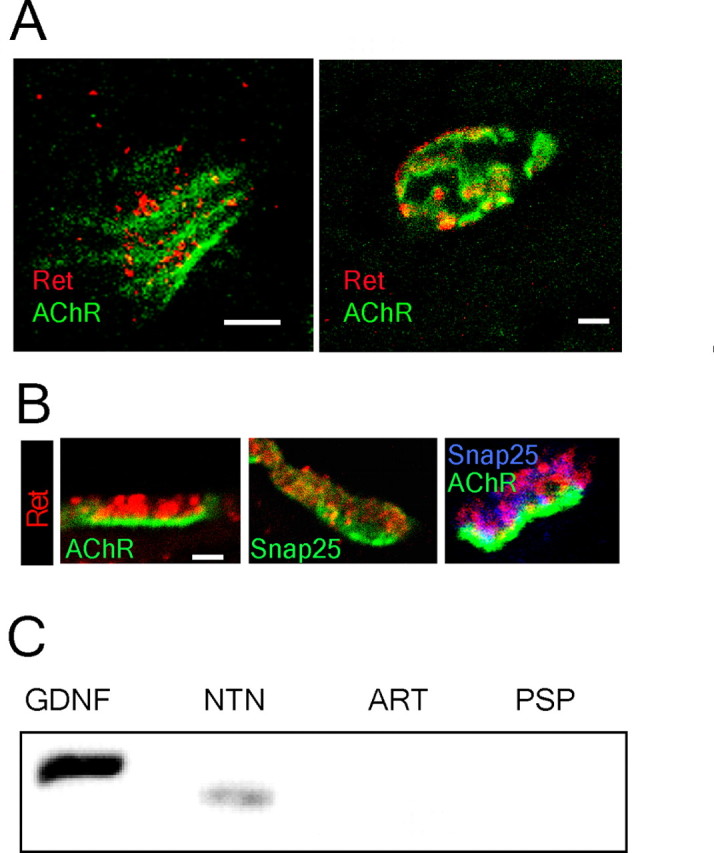
Concentration of Ret receptors presynaptically in the neuromuscular synapse and expression of GDNF family ligands in the target tissue. A, Double labeling for Ret and AChRs (bungarotoxin binding) on the internal muscle of the tongue shows the presence of Ret in the neuromuscular synapse of E18 and adult mice. B, Confocal images show the specific presence of Ret receptors in the presynaptic area (labeled by Snap-25 immunohistochemistry) but not in the postsynaptic membrane (stained by bungarotoxin binding to AChRs). C, RT-PCR for all GDNF family ligands shows the expression of GDNF and NTN in the neonatal tongue. Scale bars, 4 μm.
Conditional inactivation of Ret in cranial motor neurons
We addressed whether the expression of Ret in motor neuron nerve terminals reflect a function for cell survival, axonal growth, and/or for differentiation and maturation of the neuromuscular synapse. Ret-null mutant mice die at birth and can therefore not be used for determining its roles at later developmental stages. Because of this, and to address whether Ret plays a cell-autonomous function for motor neurons, mice carrying inactivated Ret specifically in cranial motor neurons were established. Using the Cre-LoxP recombination system, mice carrying a conditional allele of Ret with exon 12 and 13 flanked by LoxP sites were generated (Fig. 2A,B). The PGKneo cassette was eliminated in these mice by crossing with a deleter FLP mouse strain to generate Retflox/flox mice. To specifically delete Ret in cranial motor neurons, mice expressing Cre under the Nkx6.2 promoter were generated. These mice were produced by inserting a Cre/PGKneo cassette in place of the lacZ/PGKneo cassette into a previously described targeting vector (Vallstedt et al., 2001). The resulting Nkx6.2+/cre mice were crossed to Retflox/flox mice to produce animals carrying the Nkx6.2+/cre alleles and homozygous for the floxed Ret allele, producing a mouse strain with a specific deletion of Ret in cranial motor neurons (RetNkx6.2-cre mice). RetNkx6.2-cre mice survived into adulthood with an expected frequency, were fertile, and did not show any gross phenotype. Ret-null mutant mice (RetΔ/Δ), generated by breeding the Retflox/flox alleles to a deleter Cre mouse strain (PGK:cre), died at birth similar to previously described Ret-null mutant mice (Schuchardt et al., 1994). RetNkx6.2-cre mice, similar to wild-type mice, developed normal kidneys, whereas kidney development failed in RetΔ/Δ mice (Fig. 2C). Ret mRNA expression was unperturbed in spinal cord motor neurons and dorsal root ganglia of RetNkx6.2-cre mice (Fig. 2C), but was mostly eliminated from cranial motor neurons of the facial (VII) and hypoglossal (XII) motor nuclei in E18 embryos as seen with an in situ hybridization probe against exons 12 and 13 (“ex 12–13” probe) of the Ret gene (Fig. 2D). The successful elimination of Ret expression was confirmed in the adult with the ex 12–13 probe (Fig. 2E). However, when using a probe directed against the Ret ECD, Ret transcripts could be detected in the hypoglossal motor nucleus both at E18 and in the adult (Fig. 2E). Cell count showing expression with the ECD probe is presented in Figure 2F. Such a transcript lacks the ATP binding domain, which is located in the deleted exon and is expected to produce a nonfunctional truncated (kinase dead) receptor because the resulting transcript is out of frame when splicing exon 11 to exon 14. The ECD probe can therefore be used to monitor Ret expression in the absence of Ret signaling. Noteworthy, unlike the hypoglossal motor nucleus, expression from the Ret locus failed in the facial motor nucleus both at E18 and in the adult in the absence of Ret signaling (Fig. 2E,F). This suggests that Ret expression is induced by Ret signaling in facial but not hypoglossal motor neurons. A successful elimination of Ret protein at the neuromuscular synapse was confirmed. Whereas wild-type mice contained abundant Ret protein, RetNkx6.2-cre mice displayed a complete loss of Ret immunoreactivity at the neuromuscular synapse (Fig. 2G).
Figure 2.
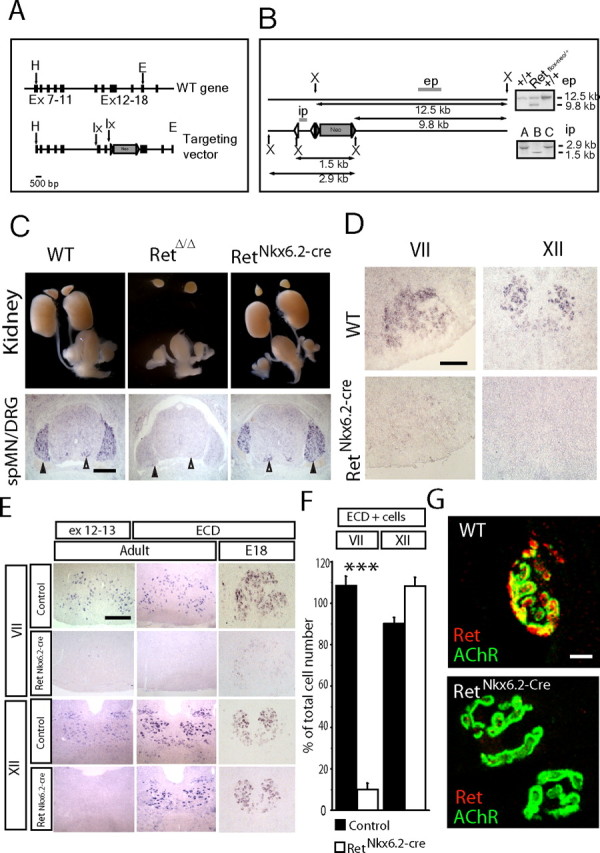
Conditional inactivation of Ret in cranial motor neurons. A, Strategy for producing a conditional Ret allele with schematic representation of the wild-type Ret locus and the targeting vector. The black rectangles represent exons, and the gray triangles represent Frt sites. LoxP (lx) sites are indicated by arrows. The targeting vector, a 12 kb HindIII(H)/EcoRI(E) fragment from the mouse Ret gene contains exons 7–18. One loxP site was introduced in intron 11. The second loxP, inserted in intron 13, was directly followed by the selection cassette (Neo) flanked by Frt sites. B, Schematic representation of the strategy used to analyze homologous recombination by Southern blot and select the ES cell clones (Retflox-neo/+) for blastocyst injection. Digestion with XbaI (X) generated a 12.5 kb band for the wild-type allele and a 9.8 kb band for the floxed allele using the external probe (ep) probe. The internal probe (ip) was used to discriminate ES cell clones in which the homologous recombination had occurred between the LoxP sites; expected sizes are indicated. Flp- and Cre-mediated excision was used to produce RetNkx6.2-Cre and RetΔ/Δ animals. RetΔ/Δ mice carry a deletion of exons 12 and 13 in all cells (germline mutation), whereas RetNkx6.2-Cre mice carry this deletion only in cells expressing Nkx6.2. C, Genetic excision of Ret exons 12 and 13 analyzed in E18 embryos. RetΔ/Δ mice carrying a deletion of Ret in the germline displayed kidney agenesis and absence of Ret exons 12 and 13 expression in spinal cord motor neurons and dorsal root ganglion by in situ hybridization. RetNkx6.2-Cre mice show an intact urogenital system and kidney, and Ret expression can be detected in spinal cord motor neurons (spMNs) (white arrowheads) and in the dorsal root ganglia (DRG) (black arrowheads). D, RetNkx6.2-Cre mice show a specific loss of Ret exons 12 and 13 expression in the facial (VII) and hypoglossal (XII) MNs. E, F, Motor neuron-specific regulation of Ret expression in cranial nuclei VII and XII. E, In situ hybridization using the ex 12–13 and ECD probes on coronal section of the brainstem for the indicated motor nuclei, ages, and genotypes. F, Ret ECD expression in motor neurons as percentage of total cell number in adult animals. Note the dramatic and specific loss of Ret transcript expression in the facial (VII) but not hypoglossal (XII) nucleus. G, Complete loss of Ret protein at the neuromuscular synapse of RetNkx6.2-Cre mice (n = 4–6 nuclei; ***p < 0.0001). Scale bars: C, 250 μm; D, E, 100 μm; G, 5 μm.
Early role of Ret for hindbrain motor neurons
The Ret-induced Ret expression in facial motor neurons suggested that onset of expression during development is induced by a Ret ligand. To directly address whether GDNF can induce Ret expression in the facial motor nucleus, E11.5 organotypic hindbrain cultures were established from wild-type mice and treated with GDNF for 6 h. The explants were either immunohistochemically stained for Ret in an open-book preparation or rhombomeres 4–6 were dissected out for RNA purification and quantitative PCR (Q-PCR). In control cultures receiving BSA instead of GDNF, Ret was expressed in migratory and postmigratory facial motor neurons (Fig. 3A,C), consistent with our previous results (Mikaels et al., 2000). GDNF treatment led to a rapid induction of Ret immunoreactivity in the migrating facial motor neurons as well as a precocious expression of Ret in premigratory facial motor neurons that normally do not express Ret (Fig. 3A). Q-PCR confirmed a markedly increased Ret mRNA expression in rhombomere 4–6 explants after GDNF stimulation for 6 h (Fig. 3B). Facial motor neurons emerge at rhombomere 4 and migrate rostrocaudally and dorsoventrally to form the facial motor nucleus with Ret expression initiating at the time of migration (Fig. 3C). Motor neuron migration was not affected in RetNkx6.2-cre mice (Fig. 3D).
Figure 3.
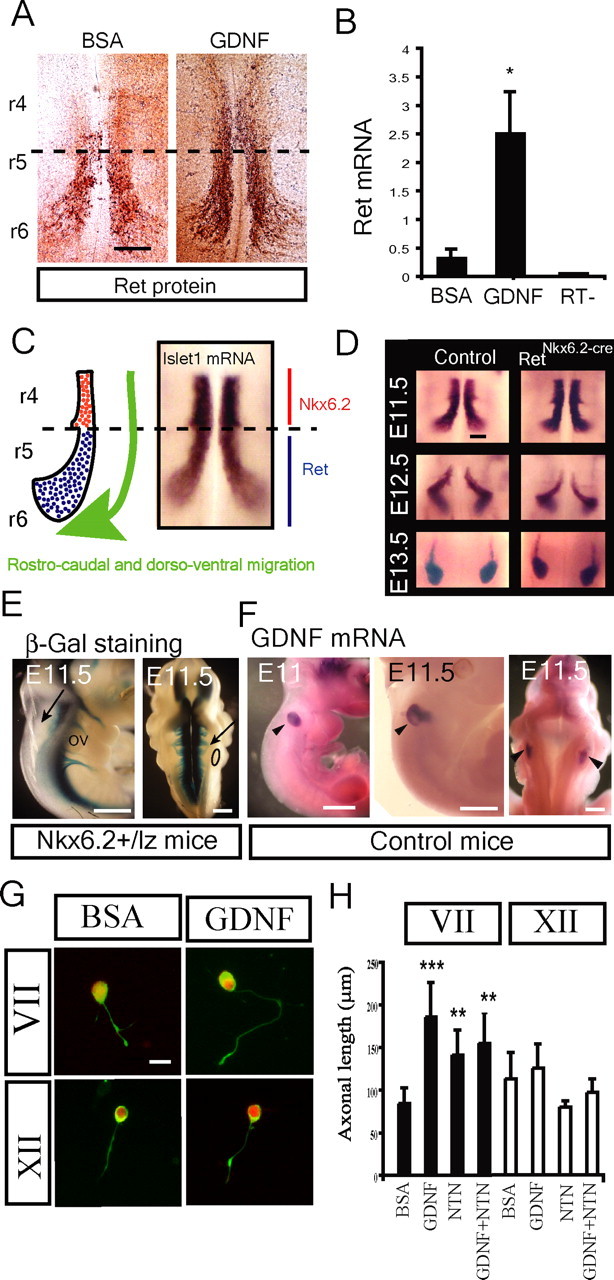
Ret activated Ret expression and Ret induced axonal growth in facial but not hypoglossal motor neurons. A, GDNF (50 ng/ml) upregulated Ret protein expression in organotypic culture of E11.5 hindbrains [rhombomere 4–6 (r4–r6)] after 6 h compared with BSA-treated control. Whole-mount immunostaining with Ret antibody on the control samples shows Ret expression restricted to migratory and postmigratory motoneurons (r5–r6, limited by dashed lines). GDNF induces increased expression of Ret in migrating motor neurons (r5–r6) as well as a precocious expression by premigatory motoneurons (r4). B, GDNF upregulated Ret mRNA expression in organotypic culture of E11.5 hindbrains [rhombomere 4–6 (r4–r6)] after 6 h compared with BSA-treated control (RT−, reaction without reverse transcriptase). Quantitative real-time PCR was conducted for ret mRNA (mean ± SEM of 3 independent experiments). C, Schematic illustration and islet1 in situ hybridization of an open-book preparation of hindbrain showing the path of facial motor neuron migration. Premigratory (red) cells express Nkx6.2, whereas migrating and postmigratory cells express Ret (blue) (Vallstedt et al., 2001). D, Islet1 staining revealing that elimination of Ret as detected in RetNkx6.2-Cre mice did not affect cell migration. E–H, GDNF is expressed along the axonal pathway of the facial nerve and promotes axonal growth of facial but not hypoglossal motoneurons in primary culture. E, The facial nerve exits the hindbrain at the level of the otic vesicle (arrows) as shown by the lateral (left) and dorsal (right) view of E11.5 Nkx6.2+/lz embryos after β-gal staining. F, Specific expression of GDNF mRNA in the otic vesicle of wild-type E11 and E11.5 embryos (black arrowheads) as shown by in situ hybridization. G, H, Axonal growth of facial but not hypoglossal motor neurons is affected by GDNF and NTN. G, Facial and hypoglossal motoneurons from E11.5 wild-type embryos were cultured in the presence of BSA or 50 ng/ml GDNF and stained for peripherin (green) and Islet1 (red) and the length of the axons measured. H, Quantification of axonal length in the presence of BSA or 50 ng/ml neurotrophic factors (GDNF, NTN, and GDNF plus NTN) in primary culture of facial and hypoglossal motor neurons (n = 50–100). Graphs represent the mean ± SEM. *p ≤ 0.05; **p ≤ 0.005; ***p ≤ 0.0005. Scale bars: A, 100 μm; E, F, 250 μm; H, 20 μm.
Axonal growth and nerve exit from the neural tube occur concomitant with the migration of the cell somata (Garel et al., 2000). At E11.5, the facial nerve projections with exit points rostral to the otic vesicle were seen in Nkx6.2+/lz mice (Fig. 3E). β-Galactosidase staining of these mice detects specifically cranial motor neurons and their axonal projections (Vallstedt et al., 2001). In situ hybridization for GDNF at E11.5 revealed GDNF expression in the otic vesicle, closely associated with the exit point and initial trajectory of the facial nerve (Fig. 3F), and could, thus, represent the endogenous source of Ret ligand regulating Ret expression in early facial motor neurons. GDNF was not expressed at the exit point or along the path of the hypoglossal nerve (Fig. 3F). Neurite outgrowth of dissociated E11.5 facial and hypoglossal motor neurons in response to GDNF for 6 h was studied in hindbrain cultures. Facial but not hypoglossal motor neurons showed significantly increased axonal length in response to GDNF and NTN, and the effects were not additive when GDNF and NTN were administered together (Fig. 3G,H). The GDNF-induced axonal growth of facial motor neurons was dependent on Ret, because the effect was missing in facial motor neurons from RetΔ/Δ mice (supplemental Fig. 1, available at www.jneurosci.org as supplemental material).
Ret-dependent survival of motor neurons during development and regeneration
We next examined the role of Ret for motor neuron survival. Facial and hypoglossal motor neuron numbers were quantified at E18 and in the adult RetNkx6.2-cre mice. Facial motor nucleus displayed a 22% decrease at E18 and 37% in the adult, whereas the hypoglossal motor nucleus showed 20% reduction at E18 and 42% in the adult (Fig. 4A). The survival of adult regenerating axotomized facial motor neurons was examined in wild-type and RetNkx6.2-cre mice. Almost twice as many regenerating motor neurons were lost in the absence of Ret in the facial nucleus (Fig. 4B,D). In contrast, no additional loss was seen in hypoglossal motor nucleus after axotomy (Fig. 4C,D). Thus, Ret is necessary for motor neuron survival both during embryogenesis as well as postnatally in both the facial and the hypoglossal motor nuclei. Ret is also supporting motor neuron survival of regenerating facial but not hypoglossal motor neurons.
Figure 4.
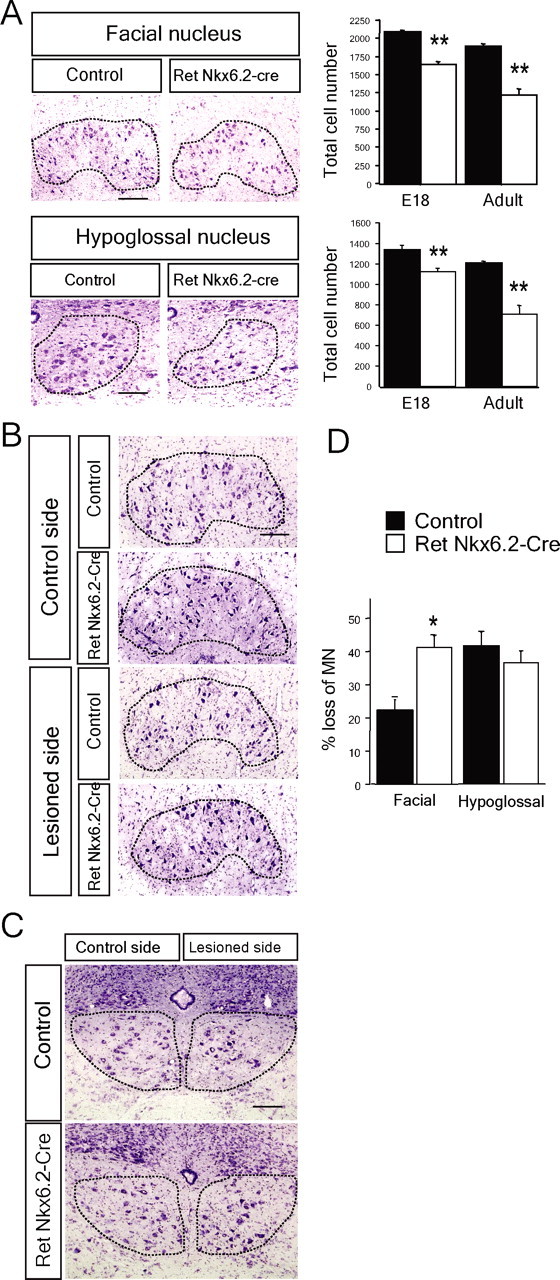
Postnatal motor neuron death in the absence of Ret. A, Motor neuron loss in facial and hypoglossal motor nuclei of adult Ret-deficient mice. Cresyl violet staining of coronal sections of facial and hypoglossal nuclei and graph showing the total number of motoneurons in control (black bars; n = 5–12 nuclei) and RetNkx6.2-Cre mice (white bars; n = 4–14 nuclei) at E18 and 3–4 months (adult). Nuclei are outlined by the dashed lines. Graphs represent the mean ± SEM. All the groups were statistically significant from controls; **p ≤ 0.005. B–D, Ret inactivation decreases the survival of facial but not hypoglossal adult motor neurons 21 d after nerve lesion. B, Photomicrographs of cresyl violet staining of the right facial nucleus (control side) and the left facial nucleus (lesioned side) from control and RetNkx6.2-Cre mice, as indicated. The dashed line represents the limit of the nucleus. C, Photomicrographs of sections showing the right hypoglossal nucleus (control side) and the left hypoglossal nucleus (lesioned side) from control and RetNkx6.2-Cre mice, as indicated. The dashed lines delimit the nucleus. D, Quantification of facial and hypoglossal motor neuron loss by the lesion as percentage of the control side in control mice (control, n = 3 mice; RetNkx6.2-Cre, n = 9 mice). Note the significant loss of facial but not hypoglossal MN as a consequence of the Ret genomic inactivation (*p < 0.015). Scale bars, 100 μm.
Ret signaling and development of the neuromuscular synapse
We addressed whether Ret, localized to the neuromuscular synapse, is acting as a retrograde signal for neuromuscular synapse maturation. Deficits in presynaptic maturation are manifested in a reduction of synaptic vesicle density and a decrease of the postsynaptic membrane covered by nerve terminals, which can be determined by synaptophysin immunohistochemistry (Fox et al., 2007). Neuromuscular synapses in the tongue were analyzed by several markers of the postsynaptic and presynaptic elements. Postsynaptic aggregation of AChR was visualized by the binding of fluorescently conjugated α-bungarotoxin. Synapsin I and synaptophysin immunohistochemistry were used to detect components of the synaptic vesicles of the presynaptic specialization. Some sections were also stained for neurofilament to detect motor nerve projections to the muscle (Fig. 5A). At early postnatal stages AChR distribution at junctions are oval “plaque”-like receptor insertions with less clear boundaries that lack distinct outlines and any segregation of the synaptic zone into AChR-rich and AChR-poor regions. Maturation involves first a reduction in synaptic size and the emergence of streaks of more intense labeling within the synapse, which during the following weeks expand and develop into the highly branched localization of receptors in “pretzel-like” specialization (Slater, 1982a; Balice-Gordon and Lichtman, 1990, 1993). In E18 wild-type animals, postsynaptic specialization was seen by AChRs aggregated into streaks with clear receptor-rich and receptor-poor areas (Fig. 5A, arrowheads). In RetΔ/Δ mice, AChR clustering was markedly reduced with a lack of streaks of more intense specializations (Fig. 5A), and the overall size as measured by the area of AChRs was significantly larger than in wild-type mice (928 ± 195 μm2 in wild-type mice and 1396 ± 184 um2 in RetΔ/Δ mice). However, quantification revealed that the overall amount of AchRs was similar between the different strains (Fig. 5C). Thus, a deficit of AChR clustering into rich and poor regions and the absence of synaptic area reduction show that postsynaptic maturation of neuromuscular synapses is impaired in the absence of Ret. Analysis of the presynaptic region of the neuromuscular junction indicated that the overall quantity of both synapsin I and synaptophysin immunoreactivity was significantly reduced in the RetΔ/Δ compared with the control mice (Fig. 5A,C). In addition, unlike wild-type neuromuscular synapses, synapses of RetΔ/Δ mice often showed nerve terminal sprouting (Fig. 5A, arrows). Similar terminal sprouts from intact nerve terminals have been observed in the adult partially denervated muscle (Brown et al., 1981).
Figure 5.
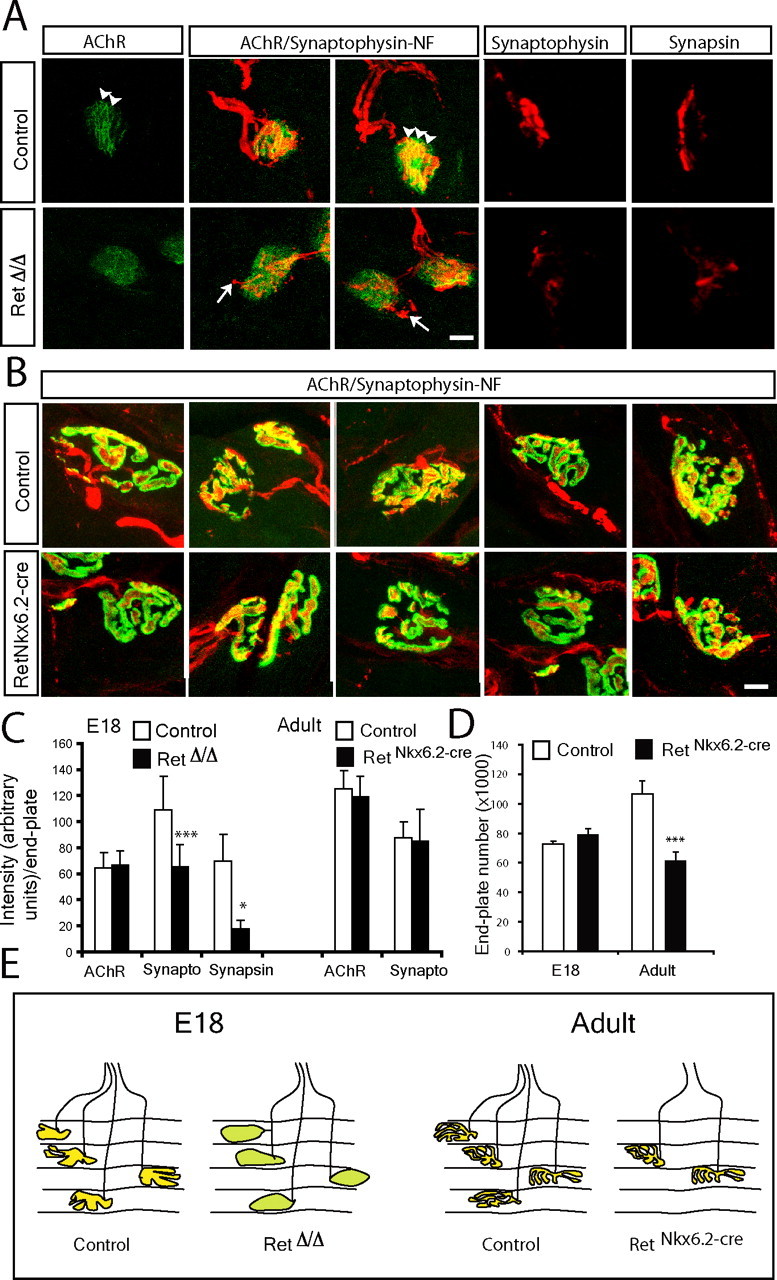
Deficits of synapse maturation in the absence of Ret signaling. A, B, Photomicrographs of neuromuscular synapses in the E18 (A) and adult (B) tongue of control and the Ret-deficient mice (RetΔ/Δ in A and RetNkx6.2-Cre in B). The presynaptic region was stained by synapsin I or synaptophysin immunohistochemistry (red, as indicated). AChRs in the postsynaptic membrane were labeled by fluorescent coupled bungarotoxin binding (green). Neuromuscular junctions visualized by a triple staining that combined synaptophysin-neurofilament (synaptophysin-NF; red) with bungarotoxin binding (AChR; green) are indicated. In A, note the reduction in the immunostaining of presynaptic components (synapsin I and synaptophysin) in Ret-deficient mice. The postsynaptic AChRs are more aggregated in control than RetΔ/Δ mice at E18 (arrowheads). Also note terminal sprouts in RetΔ/Δ mice (arrows in A), whereas general morphology of both presynaptic and postsynaptic elements of the neuromuscular junction is similar between adult control and RetNkx6.2-Cre mice (B). C, Quantification of synaptophysin (synapto) and synapsin immunoreactivity as a measure of synapse maturations at the neuromuscular synapse and AChRs staining intensity at E18 and in adult mice. Note the significant loss of synaptophysin and synapsin but not postsynaptic AChRs in the absence of Ret signaling at E18. No significant difference was found in adult mice. D, Quantification of the total number of AChR containing endplates in serial sections through the entire tongue at E18 and in the adult of both genotypes. Note a significant reduction of the total number of endplates in adult RetNkx6.2-Cre mice. E, Schematic illustration summarizing our interpretation of the results from the above studies depicting vesicle containing presynaptic terminals overlaying the postsynaptic AChR membrane in control mice (yellow). In E18 RetΔ/Δ mice, synapses are less developed (green) but in similar number as in control embryos. Adult RetNkx6.2-Cre mice display a reduced number of synapses, which otherwise appear normal. Graphs represent the mean ± SEM. ***p ≤ 0.005; *p ≤ 0.05. Scale bars, 10 μm.
The number of endplates was quantified in bungarotoxin-labeled serial sections of the E18 and adult tongues. At E18, a similar number was present in RetNkx6.2-cre and in control mice (72,927 ± 1370 in control and 78,670 ± 4200 in RetNkx6.2-cre mice) (Fig. 5D). We next analyzed synapses in the adult where the number in control animals is expected to be similar as at E18 because synapse elimination is believed to be nearly complete already at the time of birth in tongue muscles (Yamane et al., 2001). Adult RetNkx6.2-cre mice had significantly fewer endplates than control mice (Fig. 5D) (62,206 ± 625 synapses in adult RetNkx6.2-cre mice and 106,770 ± 1053 in control mice). Because the tongue muscle is larger in adult than at E18, the sampling method introduces a common bias that prevents direct comparison between age groups. In the adult, the AChRs and synaptic vesicles as well as the branching and maturity of the synapses were similar between control and RetNkx6.2-cre mice and no significant differences were observed in the quantification of the presynaptic and postsynaptic compartments (Fig. 5B–D) (and summarizing illustration in Fig. 5E). We found that all AChR-positive endplates also contained synaptophysin immunoreactivity at both stages in control and RetNkx6.2-cre mice (n = 60–100 synapses analyzed per genotype and age condition), suggesting that all remaining endplates in adult Ret-deficient mice are innervated.
Because there seems to be different requirements of Ret activation for Ret expression between facial and hypoglossal motor neurons, we were interested to examine whether the presynaptic role of Ret for maturation was also seen in other cranial motor neuron muscle targets (Fig. 6). The synapses of the oculomotor extraocular medial rectus muscle were less mature than synapses in the tongue of E18 control mice, with less synaptic vesicles as revealed by weaker synaptophysin staining. In RetΔ/Δ mice, the synaptophysin intensity was significantly reduced (18.6% reduction compared with control; p = 0.022) (Fig. 6) and the synapse area was significantly larger (2417 ± 597 vs 3167 ± 821 μm2, control and RetNkx6.2-cre, respectively; p = 0.008). In the buccinator muscle receiving innervation from the bucal branch of the facial nerve, the reduction of synaptophysin immunoreactivity was even more pronounced than in oculomotor and hypoglossal synapses (65.9% reduction vs control; p ≤ 0.0005). Furthermore, facial motor neuron synapses displayed a loss of AChRs postsynaptically (22.2% of loss; p ≤ 0.005). The size of the facial nerve synapse area was significantly reduced compared with control mice (2245 ± 564 vs 1764 ± 171 μm2, control and RetNkx6.2-cre, respectively; p = 0.040). These results show that there is a requirement of Ret for presynaptic maturation in several muscles innervated by different motor neuron nuclei, consistent with the expression of Ret in all cranial motor nuclei (Mikaels et al., 2000). However, the most affected synapses were those from the facial nerve, which also displayed a Ret-dependent Ret expression and required Ret for cell survival in the adult during regeneration.
Figure 6.
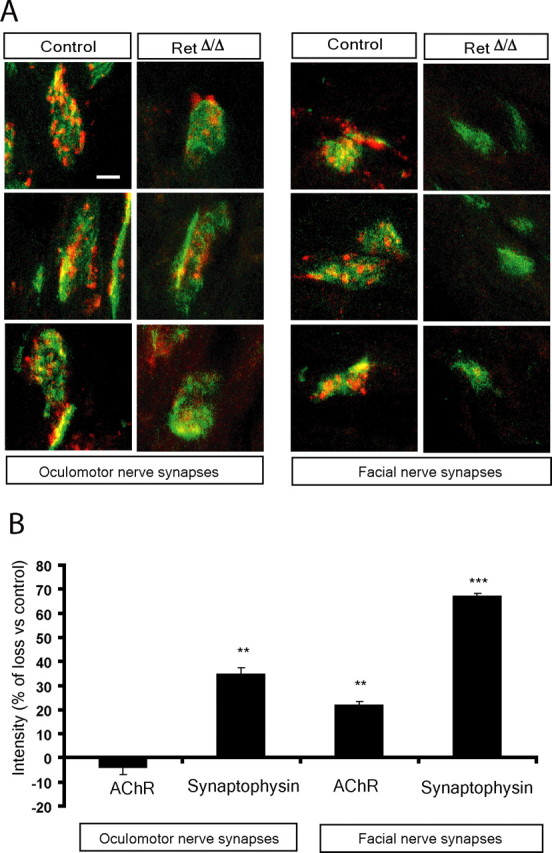
Developmental deficit of synapse maturation in the absence of Ret signaling in facial and oculomotor nerve synapses. A, Photomicrographs of neuromuscular synapses in the E18 facial and oculomotor and muscles. The nerve and presynaptic region was stained by synaptophysin and neurofilament immunohistochemistry (red) and AChRs in the postsynaptic membrane by fluorescent-coupled bungarotoxin binding (green). Note similar deficits of synapse maturation in these muscles as in the tongue of RetΔ/Δ mice. B, Quantification of AChR and synaptophysin immunoreactivity at the neuromuscular synapse. Note deficits in synaptophysin immunoreactivity in RetΔ/Δ mice of both muscles, with a more pronounced deficit in the facial nerve synapses. Also note loss of AChR in the buccinator but not oculomotor muscle. Graphs represent the mean ± SEM. *p ≤ 0.05; **p ≤ 0.005; ***p ≤ 0.0005. Scale bar, 10 μm.
Ret signaling in the adult regenerating neuromuscular synapse
The previous data established a role for Ret signaling in the maturation of the neuromuscular synapse during development. We examined whether Ret signaling also is essential for the formation of new synapses in the adult after nerve lesion. The hypoglossal nerve was transected and reinnervation as well as neuromuscular synapses was analyzed 3 weeks later. In lesioned control mice, neurofilament immunostaining showed the presence of regenerated nerve fibers (Fig. 7A), similar to unlesioned controls (Fig. 7A), and an establishment of new synaptic contacts as a result of reinnervation (Fig. 7A). The size of the neuromuscular synapses appeared significantly smaller than in the contralateral unlesioned side (Fig. 7A) correlating with AchR distribution conforming precisely to the shape of the reinnervating nerve terminal, similar to what was described previously (Rich and Lichtman, 1989). Aggregates of postsynaptic AchRs are maintained for long periods of time in the absence of nerve innervation (Slater, 1982b; Rich and Lichtman, 1989) and are reoccupied by reinnervating nerves (Letinsky et al., 1976). In RetNkx6.2-cre mice, neurofilament-positive nerve fibers reinnervating the muscle on the lesioned side were similar to control lesioned mice (Fig. 7A). Quantification of the volume occupied by the regrowing neurofilament-positive nerve fibers showed no difference between control mice and RetNkx6.2-cre, suggesting an equal reinnervation in both genotypes (Fig. 7C). The size of the AChR postsynaptic membrane was larger in the reinnervated synapses of the RetNkx6.2-cre mice compared with wild-type mice (1890 ± 235 μm2 in wild-type vs 2263 ± 78.8 μm2 in RetNkx6.2-cre mice; p ≤ 0.05) with more diffuse AChR localizations within the specialization. Quantification of the synaptic markers showed a reduction of 20% of AChRs, 71.6% of synapsin 1, and 65.5% synaptophysin in lesioned RetNkx6.2-cre mice compared with the lesioned side of control mice (Fig. 7A,B). The marked reduction of both synapsin I and synaptophysin represents an overall loss in most or all synapses (Fig. 7A,B), because measurement of the number of endplates containing any staining for presynaptic markers was similar between lesioned control and RetNkx6.2-cre mice (percent endplates containing any synaptophysin staining: control, 62.5 ± 6.5%; RetNkx6.2-cre mice, 50.1 ± 7.8%; n = 81 and 89, respectively; similar results were obtained for synapsin I staining: control, 58.3 ± 7.6%; RetNkx6.2-cre mice, 48.0 ± 2.6%; n = 97 and 106, respectively). This suggests that Ret is important for presynaptic maturation but not for axonal regeneration. Another presynaptic marker, anchored in the cytosolic face of the membrane, Snap-25, confirmed an impairment of mature nerve terminals at the site of AChR specializations in the RetNkx6.2-cre mice after regeneration (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Combined, our data show that Ret is important for presynaptic maturation but appears not to be essential for regeneration and initial axon contact with the postsynaptic membrane.
Figure 7.
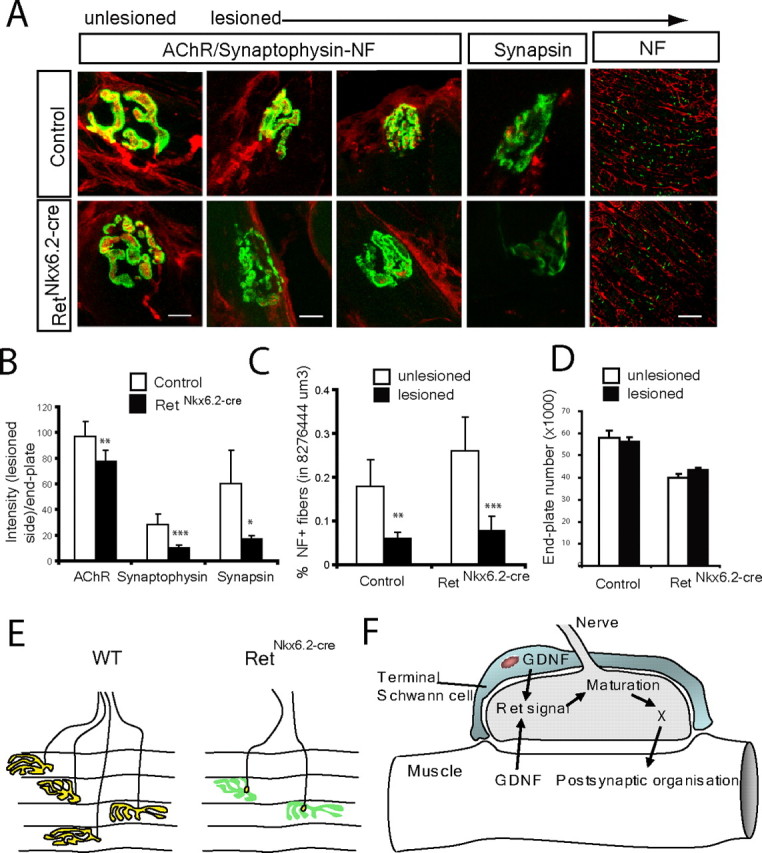
Requirement of Ret for maturation of regenerating neuromuscular synapses in the adult mouse. A–D, The hypoglossal nerve was lesioned and reinnervation of synaptic sites at the muscle examined 3 weeks later in the muscle. A, Immunohistochemistry for neurofilament (NF) and synaptophysin immunohistochemistry (red) or synapsin I (red, where indicated) and AChRs (green), comparing tongue sections from the side where the hypoglossal nerve was lesioned and the contralateral unlesioned side, as indicated. After lesion, the neurofilament immunostaining shows that reinnervation occurs in the tongue of both control and mutant mice, but the establishment of the mature synaptic contact after reinnervation detected in control is absent in RetNkx6.2-Cre mice. B, Quantification of AChRs, synaptophysin, and synapsin I intensity in control and reinnerverated muscles of RetNkx6.2-Cre mice versus control mice shows a strong reduction of all measured intensities. C, Quantification neurofilament staining in a defined volume of each side of the tongue (unlesioned vs lesioned) for control and mutant mice. Note the reduction of NF staining on the lesioned site of the tongue both in control and RetNkx6.2-Cre mice. There was no statistical significance between control and RetNkx6.2-Cre mice. D, The number of postsynaptic endplates in one-half of the tongue (lesioned and unlesioned sides, as indicated) of control and RetNkx6.2-Cre mice. Note that the number of postsynaptic structures were not affected by Ret deletion. E, Schematic illustration summarizing the results from the above studies. Although we find mature presynaptic terminals localizing with AChR-rich regions (yellow) in control animals 3 weeks after lesion, RetNkx6.2-Cre mice show only punctuate presynaptic contact in all endplates (yellow) overlaying AChR postsynaptic organizations (green), but displaying severe deficits of presynaptic maturation. F, Proposed model for Ret signaling in the establishment and maturation of the neuromuscular synapse. The solid arrows indicate processes with direct or indirect supporting data, and the dashed arrow indicates a suggestion that postsynaptic maturation may affect GDNF production/release and thereby also affect presynaptic maturation. **p ≤ 0.005; ***p ≤ 0.0005. Scale bars: NF, 100 μm; all other images, 10 μm.
Hypoglossal nerve lesion did not cause any excessive cell death in the hypoglossal nucleus as a consequence of an absence of Ret (Fig. 4C,D). The number of AChR-positive localizations in the control and RetNkx6.2-cre mice after nerve lesion was counted. Because lesions were performed unilaterally, numbers were established in the experimental one-half of the tongue. In control mice, the number of AChR specializations was not reduced at 3 weeks after lesion compared with control, unlesioned muscles (Fig. 7D). In agreement with our previous results, the unlesioned side of the RetNkx6.2-cre mice displayed a significantly reduced number compared with control mice but lesioning did not reduce this further (Fig. 7D). Thus, an absence of Ret does not have any effect on endplate numbers during reinnervation. Figure 7E summarizes the phenotype of RetNkx6.2-cre mice during reinnervation in the adult.
Discussion
The present study was conducted to elucidate whether Ret signaling is required for maturation of the neuromuscular synapse. We report that Ret receptors are concentrated in nerve endings at the presynaptic compartment of the synapses, whereas Ret ligands are expressed in the target tissue. In early development, Ret signaling reinforces additional Ret expression in facial but not hypoglossal neurons, whereas motor neuron survival embryonically as well as postnatally is compromised in both facial and hypoglossal motor nuclei in Ret-null and conditionally null mutant mice. Our findings also show that Ret-deficient motor neurons display deficits in presynaptic maturation during development. A large number of endplates are lost postnatally in the absence of Ret, but those that form appear normal in the adult mouse. Maturation of the adult reinnervated nerve endings is severely compromised when Ret signaling is abolished. This set of results provides evidence that Ret is physiologically important postnatally for both motor neuron survival and establishment of the neuromuscular synapse. Based on our results, we propose a model in which Ret could play a function in the presynaptic and postsynaptic coordination of the developing neuromuscular synapse. Ret activation by ligands released from the muscle and/or terminal Schwann cells promotes presynaptic maturation, which indirectly also can have effects on the postsynaptic organization (Fig. 7F). Albeit Ret clearly is important, the development of many synapses in its absence underscores the presence of other synaptic organizers during development, such as FGFs (Fox et al., 2007).
Early roles of Ret
Our data provide evidence for a selective role of Ret for axonal growth of facial but not hypoglossal motor neurons at early embryonic stages. It is intriguing that the differential biological effect of Ret activation between these classes of motor neurons corresponded with a Ret-dependent Ret expression in facial but not hypoglossal motor neurons. In our experiments, addition of Ret ligand even resulted in a precocious Ret expression in premigratory facial motor neurons. We also identified a source of GDNF expression in the otic vesicle at the time of Ret induction in the migrating facial motor neurons, which coincides with facial nerve exit [Fig. 3E,F (Garel et al., 2000)], whereas GDNF expression was not seen at the exit zone of the hypoglossal nerve. The temporal coincidence of facial nerve exit with GDNF expression at the exit position indicates this ligand as the endogenous source inducing Ret expression in migratory facial motor neurons. The identification of a ligand-induced expression of its own receptor suggests an autoregulatory loop in which the growing nerve fibers receiving ligand acquires an increased response. Our data are interesting in light of a recent study showing a nerve growth factor (NGF)-dependent expression of Ret and its coreceptors in sensory neurons during development. This dependency for onset of expression was evident only in one sensory subpopulation expressing Ret [i.e., Ret expressing TrkA+ small size nociceptive neurons but not in the large size Ret+ neurons (Luo et al., 2007)]. Although initial expression is NGF dependent, maintenance of coreceptor expression at postnatal stages was found to be autoregulated by Ret (Luo et al., 2007). It is conceivable that Ret itself also is autoregulated in sensory neurons, similar to our findings in facial motor neurons. Because Ret expression underlies several aspects of the diversification of nociceptive neurons into two different classes, the peptidergic and nonpeptidergic nociceptors shortly after birth (Molliver et al., 1997) by regulating expression of channels determining the receptor properties of these cells (Luo et al., 2007), a putative autoregulation of Ret suggests a role in reinforcing plasticity of cellular function during normal somatosensory perception, as well as during chronic pain. As we learn more about the diversity between motor neuron subtypes, our results on the facial and hypoglossal motor neurons, which raise parallels to the sensory nervous system, encourage future studies on Ret functions in motor neuron subtypes.
Motor neuron survival by GDNF family ligand signaling via Ret
A large proportion of motor neurons initially generated during development are lost by naturally occurring cell death (Hamburger, 1975). Despite that GDNF is the most potent motor neurotrophic factor (Henderson et al., 1994; Oppenheim et al., 1995), the role of GDNF and the other GDNF family members for motor neuron survival postnatally and in adulthood has not been addressed in vivo. Ret-null mutant mice die at birth, but the conditional elimination of Ret specifically in cranial motor neurons allowed the mice to survive into adulthood in our experiments. Because of this, we could determine its role for motor neuron survival in the postnatal animal.
Mice lacking GDNF and GDNF family receptor α1 (GFRα1) display a loss of close to 20% of trigeminal, hypoglossal, facial, and spinal lumbar motor neurons at birth (Moore et al., 1996; Sanchez et al., 1996; Cacalano et al., 1998; Mikaels et al., 2000; Oppenheim et al., 2000). Mice lacking NTN or GFRα2, the NTN binding receptor, do not have any clear loss of motor neurons (Heuckeroth et al., 1999; Rossi et al., 1999; Garces et al., 2000), and a third coreceptor, GFRα3, is not expressed in embryonic motor neurons (Mikaels et al., 2000). Although GDNF can signal independently of Ret, via GFRα1 as well as by the neural cell adhesion molecule NCAM (Sariola and Saarma, 2003), the present study suggests that the effects on embryonic motor neuron survival determined in loss- and gain-of-function studies of GDNF in vivo are attributable to a Ret-dependent signaling.
Considerable evidence supports the idea that motor neurons depend on multiple neurotrophic factors produced by different cell types reflecting their location and peripheral targets for their survival. For instance, only limb innervating motor neurons can be rescued from cell death during development by hepatocyte growth factor (Yamamoto et al., 1997; Novak et al., 2000) and in the facial motor nucleus, GFRα1 and GFRα2 are expressed in distinct subpopulations that discriminate motor neuron pools during development (Mikaels et al., 2000). However, Ret is expressed in most or all neurons of the facial nucleus during development and is present in many cells also in the adult (Fig. 2) (Mikaels et al., 2000). This opens for the possibility that GDNF family ligands via Ret could be important for the survival of motor neurons by acting also after birth. Indeed, conditionally deleting Ret in cranial motor neurons led to an additional loss after birth in facial and hypoglossal motor neurons. Thus, we established that Ret signaling is required for motor neuron survival also postnatally, but this requirement may be unrelated to its importance for establishment of functional synapses, because mice without synaptogenesis do not show any excessive death of motor neurons (Terrado et al., 2001). Our data also conclusively show that not all motor neurons depend on Ret signaling for survival, because large numbers survived independently of Ret into adulthood.
Exogenous GDNF has a robust survival effect on axotomized facial motor neurons (Henderson et al., 1994; Zurn et al., 1994; Oppenheim et al., 1995; Yan et al., 1995) that most likely is a reflection of a physiological role of Ret activation for the survival of lesioned motor neurons, because our data show an excessive cell death in the axotomized RetNkx6.2-cre mice. This survival role of Ret signaling for damaged motor neurons was specific for this nucleus, because lesioned hypoglossal motor neurons were unaffected by the lack of Ret signaling. Ret (Fig. 2E) and GFRα1 (Glazner et al., 1998; Mikaels et al., 2000) are abundantly expressed in most neurons of both these nuclei, and Ret-deficient mice no longer express any active Ret receptors, so the differences in dependency does not reflect distinct expression and/or localization of the receptor as a consequence of the lesion. GDNF expressed in Schwann cells is rapidly upregulated after sciatic nerve lesion with peak levels at 7 d and soluble GFRα1 released from these cells may act on regenerating motor axons (Naveilhan et al., 1997; Fundin et al., 1999; Paratcha et al., 2001). It is conceivable that the additional neuronal loss in facial but not hypoglossal nuclei of RetNkx6.2-cre mice after lesion is a result of a greater supply of GDNF and cofactors in facial than hypoglossal regenerating nerves. It is intriguing that only ∼20% of motor neurons were lost in facial nucleus, whereas the hypoglossal nucleus displayed >40% motor neuron loss in control animals. Hence, the conjecture of a differential supply of Ret ligands in different nerves would also explain the greater loss in the hypoglossal nucleus than facial nucleus of control animals. In all, our data support a view that Ret plays essential roles for motor neuron survival during embryogenesis, during postnatal development, and during regeneration in the adult but also that different motor nuclei and subpopulations of motor neurons display different requirements of Ret signaling for their survival.
Retrograde GDNF/Ret signaling and synapse development
There is good evidence that Ret activation by GDNF can increase axonal branching within the muscle and thereby influence motor neuron axons at the neuromuscular synapses. However, there are no previous data demonstrating that GDNF family members act as retrograde signals at the neuromuscular synapse and are necessary for the maturation of the presynaptic nerve terminal. Overexpression of GDNF in muscles of transgenic mice results in a marked increase of the number of motor axons innervating each muscle fiber at the developmental time when these are normally eliminated (Nguyen et al., 1998). Recent work suggests that this is attributable to an increased axonal branching within the muscle rather than an increase of motor neuron numbers or stability of synapses, which would prevent the rearrangements that normally occur (Keller-Peck et al., 2001). However, the physiological role of Ret signaling for motor synapse development and maintenance could not be addressed because of the early postnatal lethality of the Ret mutant mice. Our data show that Ret signaling plays a function by shaping structurally and molecularly the presynaptic terminals during embryonic development and adulthood, suggesting that GDNF family ligands not only affect motor axon branching but also synapse maturation. In the present study, a deficit of presynaptic maturation is defined by a reduction in the synaptic vesicle density and postsynaptic membrane covered by nerve terminals as determined using immunohistochemistry for presynaptic specialization and vesicle components (Fox et al., 2007).
Previous data suggest that synapse elimination is nearly complete in the tongue at birth (Yamane et al., 2001), which is consistent with the acquisition of new suckling and swallowing functions. Muscle endplate numbers were normal at birth in Ret-deficient mice despite fewer motor neurons, whereas in the adult Ret-deficient mice, close to one-half of the neuromuscular synapses were missing. Because similar numbers of synapses were formed at E18 in Ret-deficient and control mice, growth, branching, and initial establishment of nerve muscle contact do not appear to be defective in the absence of Ret signaling. On the contrary, terminal sprouts resulting in innervation of endplates lacking motor nerve innervation may occur because there are fewer neurons generating an equal number of neuromuscular synapses in Ret-deficient mice at E18 (schematically illustrated in Fig. 5E). Consistently, terminal sprouts were often associated with motor nerve terminals in E18 RetΔ/Δ but not control mice. A similar mechanism has been described in the adult in which partial denervation results in terminal sprouts from innervated nearby neuromuscular junctions leading to reinnervation of the postsynaptic organizations lacking motor nerve terminals (Son et al., 1996). Nearly one-half of the synapses were missing in adult Ret-deficient mice. Thus, there is a greater proportional loss of synapses than motor neurons between E18 and adult, implying that synapse loss is not secondary to motor neuron loss. Consistent with this conclusion are our data showing an important role of Ret for synapse maturation in the reinnervated adult hypoglossal target, where no additional motor neuron loss takes place. In fact, the deficits were even more pronounced in the adult with a marked deficiency of maturing axon terminals within the reinnervated muscle in the absence of Ret. It is conceivable that this deficit reflects a deferred maturation of neuromuscular synapses and, similarly the deficit of presynaptic maturation during development in the absence of Ret, could eventually catch up at later time points. Although the survival role of Ret for motor neurons is subtype- and stage-specific, the role of Ret signaling for maturation of the neuromuscular synapse might be a more general mechanism, because we found similar deficiencies in all neuromuscular synapses from various targets muscles of different motor neuron nuclei.
The phenotype on neuromuscular synapse maturation in the absence of Ret involved mostly presynaptic but also some postsynaptic deficits. We conclude that the primary deficit is in presynaptic maturation because (1) GDNF is expressed in the target tissue, (2) Ret mRNA is expressed in the motor neurons and its protein is localized presynaptically in the neuromuscular synapse, and (3) the most severe deficits were presynaptically, including the adult reinnervated synapse in which the postsynaptic organization was not much affected. Thus, the less pronounced postsynaptic alterations in these mice are likely secondary to deficits of presynaptic maturation. Our data suggest that synapse maturation is the result of a continuous cross talk between the nerve and the muscle, and GDNF ligands via Ret activation participate in this process by acting on the synaptic nerve terminal.
Footnotes
This work was supported by the Swedish Research Council (P.E., C.B.), The Swedish Cancer Society (P.E.), Karolinska Foundation (E.P.), and the Swedish Foundation for Strategic Research (Center of Excellence in Developmental Biology Grant to P.E.). We thank the personnel at Karolinska Center for Transgene Technologies, mainly Dr. Johannes Wilbertz for giving us E14 ES cells and performing blastocysts injections of ES clones, the personnel of the Scheele Animal House (Evis A. and Veronica P.), and Claudia Tello and Johnny Soderlund for technical support. We are grateful to Sebastian Thams for instructions on nerve lesions.
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Lichtman JW. In vivo visualization of the growth of pre- and postsynaptic elements of neuromuscular junctions in the mouse. J Neurosci. 1990;10:894–908. doi: 10.1523/JNEUROSCI.10-03-00894.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Lichtman JW. In vivo observations of pre- and postsynaptic changes during the transition from multiple to single innervation at developing neuromuscular junctions. J Neurosci. 1993;13:834–855. doi: 10.1523/JNEUROSCI.13-02-00834.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudet C, Mikaels A, Westphal H, Johansen J, Johansen TE, Ernfors P. Positive and negative interactions of GDNF, NTN and ART in developing sensory neuron subpopulations, and their collaboration with neurotrophins. Development. 2000;127:4335–4344. doi: 10.1242/dev.127.20.4335. [DOI] [PubMed] [Google Scholar]
- Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- Brown MC, Holland RL, Hopkins WG. Motor nerve sprouting. Annu Rev Neurosci. 1981;4:17–42. doi: 10.1146/annurev.ne.04.030181.000313. [DOI] [PubMed] [Google Scholar]
- Burden SJ. Building the vertebrate neuromuscular synapse. J Neurobiol. 2002;53:501–511. doi: 10.1002/neu.10137. [DOI] [PubMed] [Google Scholar]
- Cacalano G, Farinas I, Wang LC, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF, Hynes M, Davies A, Rosenthal A. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChiara TM, Bowen DC, Valenzuela DM, Simmons MV, Poueymirou WT, Thomas S, Kinetz E, Compton DL, Rojas E, Park JS, Smith C, DiStefano PS, Glass DJ, Burden SJ, Yancopoulos GD. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85:501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- Ernfors P. Local and target-derived actions of neurotrophins during peripheral nervous system development. Cell Mol Life Sci. 2001;58:1036–1044. doi: 10.1007/PL00000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MA, Sanes JR, Borza DB, Eswarakumar VP, Fassler R, Hudson BG, John SW, Ninomiya Y, Pedchenko V, Pfaff SL, Rheault MN, Sado Y, Segal Y, Werle MJ, Umemori H. Distinct target-derived signals organize formation, maturation, and maintenance of motor nerve terminals. Cell. 2007;129:179–193. doi: 10.1016/j.cell.2007.02.035. [DOI] [PubMed] [Google Scholar]
- Fundin BT, Mikaels A, Westphal H, Ernfors P. A rapid and dynamic regulation of GDNF-family ligands and receptors correlate with the developmental dependency of cutaneous sensory innervation. Development. 1999;126:2597–2610. doi: 10.1242/dev.126.12.2597. [DOI] [PubMed] [Google Scholar]
- Garces A, Haase G, Airaksinen MS, Livet J, Filippi P, deLapeyriere O. GFRα1 is required for development of distinct subpopulations of motoneuron. J Neurosci. 2000;20:4992–5000. doi: 10.1523/JNEUROSCI.20-13-04992.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel S, Garcia-Dominguez M, Charnay P. Control of the migratory pathway of facial branchiomotor neurones. Development. 2000;127:5297–5307. doi: 10.1242/dev.127.24.5297. [DOI] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Mudd J, Nichol M, Chu GC, Sanes JR, Merlie JP. Failure of postsynaptic specialization to develop at neuromuscular junctions of rapsyn-deficient mice. Nature. 1995;377:232–236. doi: 10.1038/377232a0. [DOI] [PubMed] [Google Scholar]
- Glazner GW, Mu X, Springer JE. Localization of glial cell line-derived neurotrophic factor receptor alpha and c-ret mRNA in rat central nervous system. J Comp Neurol. 1998;391:42–49. doi: 10.1002/(sici)1096-9861(19980202)391:1<42::aid-cne4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Godfrey EW, Nitkin RM, Wallace BG, Rubin LL, McMahan UJ. Components of Torpedo electric organ and muscle that cause aggregation of acetylcholine receptors on cultured muscle cells. J Cell Biol. 1984;99:615–627. doi: 10.1083/jcb.99.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V. Cell death in the development of the lateral motor column of the chick embryo. J Comp Neurol. 1975;160:535–546. doi: 10.1002/cne.901600408. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Simpson LC, Koliatsos VE, Rosenthal A. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- Heuckeroth RO, Enomoto H, Grider JR, Golden JP, Hanke JA, Jackman A, Molliver DC, Bardgett ME, Snider WD, Johnson EM, Jr, Milbrandt J. Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron. 1999;22:253–263. doi: 10.1016/s0896-6273(00)81087-9. [DOI] [PubMed] [Google Scholar]
- Keller-Peck CR, Feng G, Sanes JR, Yan Q, Lichtman JW, Snider WD. Glial cell line-derived neurotrophic factor administration in postnatal life results in motor unit enlargement and continuous synaptic remodeling at the neuromuscular junction. J Neurosci. 2001;21:6136–6146. doi: 10.1523/JNEUROSCI.21-16-06136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand Y, Luria V, Haffner-Krausz R, Lonai P. Maternally expressed PGK-Cre transgene as a tool for early and uniform activation of the Cre site-specific recombinase. Transgenic Res. 1998;7:105–112. doi: 10.1023/a:1008868325009. [DOI] [PubMed] [Google Scholar]
- Letinsky MS, Fischbeck KH, McMahan UJ. Precision of reinnervation of original postsynaptic sites in frog muscle after a nerve crush. J Neurocytol. 1976;5:691–718. doi: 10.1007/BF01181582. [DOI] [PubMed] [Google Scholar]
- Lu B, Je HS. Neurotrophic regulation of the development and function of the neuromuscular synapses. J Neurocytol. 2003;32:931–941. doi: 10.1023/B:NEUR.0000020633.93430.db. [DOI] [PubMed] [Google Scholar]
- Luo W, Wickramasinghe SR, Savitt JM, Griffin JW, Dawson TM, Ginty DD. A hierarchical NGF signaling cascade controls Ret-dependent and Ret-independent events during development of nonpeptidergic DRG neurons. Neuron. 2007;54:739–754. doi: 10.1016/j.neuron.2007.04.027. [DOI] [PubMed] [Google Scholar]
- McMahan UJ. The agrin hypothesis. Cold Spring Harb Symp Quant Biol. 1990;55:407–418. doi: 10.1101/sqb.1990.055.01.041. [DOI] [PubMed] [Google Scholar]
- Mikaels A, Livet J, Westphal H, De Lapeyriere O, Ernfors P. A dynamic regulation of GDNF-family receptors correlates with a specific trophic dependency of cranial motor neuron subpopulations during development. Eur J Neurosci. 2000;12:446–456. doi: 10.1046/j.1460-9568.2000.00924.x. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, ElShamy WM, Ernfors P. Differential regulation of mRNAs for GDNF and its receptors Ret and GDNFR alpha after sciatic nerve lesion in the mouse. Eur J Neurosci. 1997;9:1450–1460. doi: 10.1111/j.1460-9568.1997.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Nguyen QT, Parsadanian AS, Snider WD, Lichtman JW. Hyperinnervation of neuromuscular junctions caused by GDNF overexpression in muscle. Science. 1998;279:1725–1729. doi: 10.1126/science.279.5357.1725. [DOI] [PubMed] [Google Scholar]
- Novak KD, Prevette D, Wang S, Gould TW, Oppenheim RW. Hepatocyte growth factor/scatter factor is a neurotrophic survival factor for lumbar but not for other somatic motoneurons in the chick embryo. J Neurosci. 2000;20:326–337. doi: 10.1523/JNEUROSCI.20-01-00326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim RW, Houenou LJ, Johnson JE, Lin LF, Li L, Lo AC, Newsome AL, Prevette DM, Wang S. Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature. 1995;373:344–346. doi: 10.1038/373344a0. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW, Houenou LJ, Parsadanian AS, Prevette D, Snider WD, Shen L. Glial cell line-derived neurotrophic factor and developing mammalian motoneurons: regulation of programmed cell death among motoneuron subtypes. J Neurosci. 2000;20:5001–5011. doi: 10.1523/JNEUROSCI.20-13-05001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paratcha G, Ledda F, Baars L, Coulpier M, Besset V, Anders J, Scott R, Ibanez CF. Released GFRalpha1 potentiates downstream signaling, neuronal survival, and differentiation via a novel mechanism of recruitment of c-Ret to lipid rafts. Neuron. 2001;29:171–184. doi: 10.1016/s0896-6273(01)00188-x. [DOI] [PubMed] [Google Scholar]
- Pozas E, Ibanez CF. GDNF and GFRalpha1 promote differentiation and tangential migration of cortical GABAergic neurons. Neuron. 2005;45:701–713. doi: 10.1016/j.neuron.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Rich M, Lichtman JW. Motor nerve terminal loss from degenerating muscle fibers. Neuron. 1989;3:677–688. doi: 10.1016/0896-6273(89)90236-5. [DOI] [PubMed] [Google Scholar]
- Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- Rossi J, Luukko K, Poteryaev D, Laurikainen A, Sun YF, Laakso T, Eerikainen S, Tuominen R, Lakso M, Rauvala H, Arumae U, Pasternack M, Saarma M, Airaksinen MS. Retarded growth and deficits in the enteric and parasympathetic nervous system in mice lacking GFR alpha2, a functional neurturin receptor. Neuron. 1999;22:243–252. doi: 10.1016/s0896-6273(00)81086-7. [DOI] [PubMed] [Google Scholar]
- Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- Sariola H, Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci. 2003;116:3855–3862. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- Schuchardt A, D'Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Slater CR. Postnatal maturation of nerve-muscle junctions in hindlimb muscles of the mouse. Dev Biol. 1982a;94:11–22. doi: 10.1016/0012-1606(82)90063-x. [DOI] [PubMed] [Google Scholar]
- Slater CR. Neural influence on the postnatal changes in acetylcholine receptor distribution at nerve-muscle junctions in the mouse. Dev Biol. 1982b;94:23–30. doi: 10.1016/0012-1606(82)90064-1. [DOI] [PubMed] [Google Scholar]
- Son YJ, Trachtenberg JT, Thompson WJ. Schwann cells induce and guide sprouting and reinnervation of neuromuscular junctions. Trends Neurosci. 1996;19:280–285. doi: 10.1016/S0166-2236(96)10032-1. [DOI] [PubMed] [Google Scholar]
- Terrado J, Burgess RW, DeChiara T, Yancopoulos G, Sanes JR, Kato AC. Motoneuron survival is enhanced in the absence of neuromuscular junction formation in embryos. J Neurosci. 2001;21:3144–3150. doi: 10.1523/JNEUROSCI.21-09-03144.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallstedt A, Muhr J, Pattyn A, Pierani A, Mendelsohn M, Sander M, Jessell TM, Ericson J. Different levels of repressor activity assign redundant and specific roles to Nkx6 genes in motor neuron and interneuron specification. Neuron. 2001;31:743–755. doi: 10.1016/s0896-6273(01)00412-3. [DOI] [PubMed] [Google Scholar]
- Wang CY, Yang F, He XP, Je HS, Zhou JZ, Eckermann K, Kawamura D, Feng L, Shen L, Lu B. Regulation of neuromuscular synapse development by glial cell line-derived neurotrophic factor and neurturin. J Biol Chem. 2002;277:10614–10625. doi: 10.1074/jbc.M106116200. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Livet J, Pollock RA, Garces A, Arce V, deLapeyriere O, Henderson CE. Hepatocyte growth factor (HGF/SF) is a muscle-derived survival factor for a subpopulation of embryonic motoneurons. Development. 1997;124:2903–2913. doi: 10.1242/dev.124.15.2903. [DOI] [PubMed] [Google Scholar]
- Yamane A, Ohnuki Y, Saeki Y. Developmental changes in the nicotinic acetylcholine receptor in mouse tongue striated muscle. J Dent Res. 2001;80:1840–1844. doi: 10.1177/00220345010800091301. [DOI] [PubMed] [Google Scholar]
- Yan Q, Matheson C, Lopez OT. In vivo neurotrophic effects of GDNF on neonatal and adult facial motor neurons. Nature. 1995;373:341–344. doi: 10.1038/373341a0. [DOI] [PubMed] [Google Scholar]
- Zurn AD, Baetge EE, Hammang JP, Tan SA, Aebischer P. Glial cell line-derived neurotrophic factor (GDNF), a new neurotrophic factor for motoneurones. NeuroReport. 1994;6:113–118. doi: 10.1097/00001756-199412300-00030. [DOI] [PubMed] [Google Scholar]


