Abstract
Resilience to mental and physical stress is a key determinant for the survival and functioning of mammals. Although the importance of stress resilience has been recognized, the underlying neural mediators have not yet been identified. Neuropeptide Y (NPY) is a peptide known for its anti-anxiety-like effects mediated via the amygdala. The results of our current study demonstrate, for the first time that repeated administration of NPY directly into the basolateral nucleus of the amygdala (BLA) produces selective stress-resilient behavioral responses to an acute restraint challenge as measured in the social interaction test, but has no effect on hypothalamic–adrenal–pituitary axis activity or stress-induced hyperthermia. More importantly, the resilient behaviors observed in the NPY-treated animals were present for up to 8 weeks. Antagonizing the activity of calcineurin, a protein phosphatase involved in neuronal remodeling and present in NPY receptor containing neurons within the BLA, blocked the development of long-term, but not the acute increases in social interaction responses induced by NPY administration. This suggests that the NPY-induced long-term behavioral resilience to restraint stress may occur via mechanisms involving neuronal plasticity. These studies suggest one putative physiologic mechanism underlying stress resilience and could identify novel targets for development of therapies that can augment the ability to cope with stress.
Keywords: NPY, BNST, amygdala, anxiety, anxiolytic, autonomic, CRF, CRH, hypothalamus, neuropeptide, RAPHE, septum
Introduction
A common approach to studying the effects of stress and anxiety involves identification of risk factors that make individuals especially vulnerable to stress-related disorders. This approach is useful in many respects; however, little is learned about subjects that either do not demonstrate similar stress responses (i.e., stress resistant) or whose stress response is of shorter duration and does not lead to stress responses (i.e., stress resilient) despite being subjected to similar stress challenges. Therefore, identifying mechanisms that make individuals less vulnerable to stressful stimuli is an important alternative approach.
Many central and peripheral stress-induced responses are initiated by the neuropeptide corticotrophin-releasing factor (CRF) (for review, see Bale and Vale, 2004). In previous studies, we demonstrated that administration of a subthreshold dose of urocortin (Ucn), a CRF agonist, into the basolateral nucleus of the amygdala (BLA) daily for 5 d induces a persistent state of anxiety-like behavior without any acute effects on behavior (Rainnie et al., 2004). More importantly, we found that pretreatment with neuropeptide Y (NPY) 30 min before CRF blocked the long-term anxiety (Sajdyk et al., 2006). Additional studies from our laboratory have shown that NPY administered directly into the BLA significantly decreases anxiety-like behavior of rats (Sajdyk et al., 1999a, 2002). Similarly, other investigators have also shown that the amygdalar NPY system is critically involved in the stress response (for review, see Kask et al., 2002; Heilig, 2004).
The BLA expresses bidirectional synaptic plasticity consisting of both long-term potentiation (LTP) and long-term depression (LTD). LTP is associated with fear conditioning and memories (Davis, 1994; McGaugh, 2002) and often involves the activation of kinases [e.g., calcium calmodulin kinase II (CaMKII)] (Shekhar et al., 2003), whereas LTD, involves phosphatases (e.g., calcineurin) and is associated with fear extinction (Lin et al., 2003a,b). Given that we observed CRF receptor-mediated long-term anxiety-like responses in rats by activation of CaMKII (Shekhar et al., 2003) pathway which could be blocked with NPY pretreatment (Sajdyk et al., 2004, 2006), we hypothesized that repeated NPY may elicit long-term anxiolytic-like responses through a calcineurin-mediated pathway.
To address our hypotheses, we assessed animals in the social interaction (SI) test after repeated intra-BLA NPY and vehicle for up to 8 weeks from the initial injection. In addition, we measured the effect of a stress challenge (30 min restraint stress) in the NPY and vehicle-treated animals to determine stress resiliency. Along with anxiety-like behaviors we also measured body temperature, plasma corticosterone and adrenocorticotrophin hormone (ACTH) levels. We next conducted similar NPY injections with or without a calcineurin inhibitor. In the final study, we used c-Fos immunohistochemistry to assess patterns of neural responses associated with NPY-mediated stress resilience specifically focusing on the paraventricular nucleus of the hypothalamus (PVN), bed nucleus of the stria terminalis (BNST)/lateral septum (LS), and the raphe pallidus (RPa) because these regions are associated with corticosterone secretion, social interaction, and thermal regulation, respectively.
Materials and Methods
Animals
All animals used for the behavior and immunohistochemical experiments were male Wistar rats (250–275 g) obtained from Harlan Laboratories (Indianapolis, IN). They were individually housed throughout the experiment (4–10 weeks, depending on experiment) and given food ad libitum. The housing room was maintained on a 12 h light/dark cycle (7:00 A.M./7:00 P.M.) and maintained at 72°F. Animal care procedures were conducted in accordance with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals and the guidelines of the Indiana University-Purdue University Indianapolis Institutional Animal Care and Use Committee.
Stereotaxic surgery procedures
Rats were anesthetized with isoflurane (2.5%) and placed in a stereotaxic apparatus. Bilateral injection cannulas (26 gauge; Plastics One, Roanoke, VA) were implanted into the BLA [anteroposterior (AP): −2.1; mediolateral (ML): +5.0; dorsoventral (DV): −8.0; incisor bar: −3.3 mm] according to a standard stereotaxic atlas of the rat brain (Paxinos and Watson, 1986). The cannulas were secured to the skull with three stainless steel screws (2.8 mm; Plastics One) and locktite adhesive (Applied Industrial Technologies, Indianapolis, IN). After completion of surgery, all animals received buprenex (Sigma, St. Louis, MO; 1 mg/kg, s.c.) and were placed on a warming pad until they had fully recovered from the anesthetic.
Intracranial injection procedures
Acute microinjections of all compounds were delivered via injection cannulas (33 gauge; Plastics One) that fit into, and extended 1 mm beyond, the guide cannulas. A 10 μl Hamilton syringe was placed on an infusion pump (Harvard Apparatus, Holliston, MA; model PHD 2000) and connected to the injection cannulas via polyethylene (PE 50) tubing (Fisher Scientific, Pittsburgh, PA). The pump was then turned on and set to deliver 100 nl of solution per site over 30 s. The injection cannulas remained in place for an additional minute to ensure complete delivery of the solution through the guide cannulas. After removal from the injection site, the pump was turned back on and the cannulas were tested to ensure that there was proper flow and injection cannulas had not become clogged during the administration of the compound. The sham injection procedure was identical to the treatment protocol except nothing was injected into the BLA.
Pharmacological agents
The peptides used in the study are as follows: NPY (C189H285N55O57S1, Tyr-Pro-Ser-Lys-Pro-Asp-Asn-Pro-Gly-Glu-Asp-la-Pro-Ala-Glu-Asp-Met-Ala-Arg-Tyr-Tyr-Ser-Ala-Leu-DArg-His-Tyr-Ile-Asn-Leu-Ile-Thr-Arg-Gln-Arg-Tyr-NH2), calcineurin autoinhibitory peptide (C124H205N39O39S2, Ile-Thr-Ser-Phe-Glu-Glu-Ala-Lys-Gly-Leu-Asp-Arg-Ile-Asn-Glu-Arg-Met-Pro-Pro-Arg-Arg-Asp-Ala-Met-Pro), and NPY free acid (deamidated NPY; C189H284N54 O58S1, Tyr-Pro-Ser-Lys-Pro-Asp-Asn-Pro-Gly-Glu-Asp-Ala-Pro-Ala-Glu-Asp-Met-Ala-Arg-Tyr-Tyr-Ser-Ala-Leu-DArg-His-Tyr-Ile-Asn-Leu-Ile-Thr-Arg-Gln-Arg-Tyr), and were all obtained from American Peptide Company (Sunnyvale, CA).
Behavioral paradigms
The SI test.
The modified SI test is a paradigm that we have used extensively in our laboratory to measure anxiety-like behaviors in rats (Sajdyk et al., 2003). The protocol involved placing the treated animal simultaneously into the SI box (91.44 cm long × 91.44 cm wide × 30.48 cm high box with an open top) with a partner rat. All partner rats were of same sex and similar weight, housed under identical conditions and had no previous contact with the treated animal. The 5 min test was performed under low-light (40 W red lighting) familiar conditions. All testing was video taped via a camera mounted on the ceiling directly above the SI box and scored at a later time. The total amount of time the experimental animal spent initiating social interaction (i.e., sniffing, grooming, and crawling) with the partner rat was scored from the video tape by two blinded raters with an inter-rater reliability Pearson's r coefficient of 0.87. All testing was conducted between the h of 8:00 A.M. and 1:00 P.M. to compare the current results with our previous studies.
Locomotor activity.
The locomotor activity chamber was purchased from Med Associates (St. Albans, VT). The arena measured 43.18 × 43.18 cm with three 16 infrared beam arrays connected to a desktop computer with Activity Monitor 5.0 software. The animals were placed into the center of the chamber and allowed to explore for a total of 1 h. The total distance traveled during the test session was used for analysis.
EPM.
The elevated plus maze (EPM) used for this study was obtained from Hamilton-Kinder (San Diego, CA). The maze is 111.76 cm wide × 111.76 cm deep × 85.1 cm tall and each arm is 10.79 cm wide and 50.16 cm long; intersection is 10.79 cm by 10.79 cm; closed walls are 40.00 cm high. The animals were placed in the center area of the maze and allowed to explore the open and closed arms for 5 min. The total amount of time spent in each arm was recorded directly to the computer from the infrared beam breaks and analyzed later.
Restraint stress.
The animals were placed in a decapicone (Brain Tree Scientific, Braintree, MA) with the end securely closed and the space directly above the cannulas cut open to prevent undue pressure and subsequent breaking away from the skull. The animals remained restrained for 30 min and were then removed for behavioral assessment in the SI test.
Corticosterone and ACTH assays
Trunk blood was collected into EDTA-coated tubes (Brinkman Instruments, Westbury, NY) on ice (4°C). Blood samples were centrifuged at 4000 relative centrifugal force; plasma was collected and stored at −80°C. Plasma corticosterone and ACTH were measured with radioimmunoassay kits (MP Biomedicals, Solon, OH; corticosterone #07-120102; ACTH #07-106101). Trunk blood was collected between 10:00 and 11:30 A.M. for all animals.
Perfusions
Sixty minutes after restraint, rats were anesthetized with pentobarbitol and perfused with 250 ml of 0.05 m sodium phosphate buffer and 4% paraformaldehyde plus 1.5% sucrose and prepared for immunohistochemistry as previously described (Johnson et al., 2005) To maintain a consistent plane for coronal sections, brains were placed in a rat brain matrix and cut with a razor at the caudal border of the mammillary bodies. Serial coronal sections (30 μm) were cut using a cryostat to yield six alternate sets of sections [two of the six alternative sets of sections were stained, one set each for neutral red/c-Fos and c-Fos/tryptophan hydroxylase (TPH)].
Immunohistochemistry protocols
c-Fos.
Immunostaining for c-Fos protein was accomplished using primary antiserum directed against c-Fos (rabbit anti-c-Fos-polyclonal antiserum, catalog number PC38, Ab-5; Calbiochem, La Jolla, CA; diluted 1:10,000) as described previously (Johnson et al., 2005). The chromogen reaction for c-Fos used was done using a Vector SG kit as described by the vendor (catalog number SK-4700; Vector Laboratories, Burlingame, CA; 20 min). Sections were then mounted onto slides and allowed to dry overnight. The following day, the slides were dehydrated with ethanol, placed in a 1:1 concentration of ethanol-chloroform solution for 1 h, rehydrated, stained with neutral red (catalog number 198080; Sigma) for 5 min, and then dehydrated before application of coverslips.
Double immunostaining for c-Fos protein and TPH was done using antibodies directed against c-Fos (rabbit anti-c-Fos-polyclonal, affinity purified antibody, catalog number PC38, Ab-5; Calbiochem; diluted 1:10,000) and then either neutral red for the forebrain or antibodies directed against the TPH protein (sheep anti-TPH-polyclonal, affinity-purified antibody catalog number 9260-2505; Biogenesis; diluted 1:10,000) for the brainstem as described previously (Johnson et al., 2005). Briefly, sections were washed in PBS and then incubated in 1% H2O2 in PBS for 20 min. Sections were washed in PBS then incubated 12–16 h in PBS plus 0.3% Triton X-100 detergent (PBST) with primary antibody. The following day, sections were incubated for 2 h in the appropriate secondary antibody: biotinylated swine anti-rabbit IgG (c-Fos, catalog number E0353; DakoCytomation, Carpinteria, CA; diluted 1:200) or biotinylated rabbit anti-sheep IgG (TPH; catalog number BA6000; Vector Laboratories; diluted 1:500). Sections were washed again for 30 min in PBST then incubated 1.5 h in an avidin-biotin complex (catalog number PK-6100; Vector Laboratories; diluted 1:200). Substrates for chromogen reactions were SG (c-Fos, SK-4700; Vector Laboratories) or 0.01% 3,3′-diaminobenzidine tetrahydrochloride (D-5637; Sigma) in PBS containing 0.003% H2O2, pH 7.4. Substrate reactions were run for 20 min for c-Fos and TPH.
Photomicrographs were obtained using a Leica (Nussloch, Germany) brightfield microscope using N plan 5×, 10×, 20× and 40× objective lenses (model DMLB; Leica), an Insight digital camera (Diagnostics Instruments, Sterling Heights, MI) and SPOT 3.5.5 for Windows digital imaging software (Silicon Graphics, Mountain View, CA). Photographic plates were prepared in CorelDraw 11.633 for Windows (Viglen, London, UK).
Calcineurin and NPY1r.
Animals were anesthetized with pentobarbital (50 mg/kg, i.p.) and transcardially perfused with warm (37°C) PBS containing 0.1% procaine and heparin, followed by cold 4% paraformaldehyde in PBS. After perfusion, the brains were removed and placed in fixative at 4°C overnight. Tissues were sectioned on a vibratome (40 μm) and placed in PBS.
Double-label immunohistochemistry was performed on free floating sections. In brief, sections were rinsed in PBS, pH 7.4, treated with 1% H2O2 for 15 min and further rinsed in three 5 min PBS washes. The sections were blocked for 1 h in 10% normal donkey serum (NDS) in PBS-gelatin and then incubated for 72 h in 1° antibody (NPY1r, 1:1500; ImmunoStar, Hudson, WI) containing 4% NDS. This antibody has been characterized previously by our laboratory (Wolak et al., 2003) and specificity of staining was further assessed in three wild-type and three NPY1r knock-out mice (Deltagen, San Mateo, CA). After incubation, the sections were rinsed in PBS-gelatin and incubated in biotinylated donkey anti-rabbit antibody (1:2000) for 1 h. After washes in PBS-gelatin, the tissues were incubated in avidin-biotin complex (2 μl/ml, ABC reagent; Vector Laboratories) for 30 min and rinsed in PBS-gelatin. For amplification, the tissues were incubated in biotinylated tyramide in 0.01% H2O2/PBS for 10 min, rinsed and incubated with FITC-streptavidin (1:250; Jackson ImmunoResearch, West Grove, PA) for 3 h. After four 5 min washes in Tris buffered saline, pH 7.4, sections were incubated with calcineurin antibody (1:250; BD Transduction Laboratories, Franklin Lakes, NJ; 610260) in 4% NDS for 72 h and rinsed in PBS-gelatin. Tissues were then incubated in Cy3 donkey anti-mouse (1:250; Jackson ImmunoResearch) containing 4% NDS for 3 h, rinsed in Tris buffered saline, and mounted on gelatin-subbed slides and air-dried. Coverslips were applied using PVA-DABCO (polyvinyl alcohol-1, 4 diazabicyclo[2.2.2.] octane).
Immunoreactive staining patterns within sections of the BLA were visualized using confocal microscopy (Olympus, Tokyo, Japan), captured using a digital camera and analyzed using Fluoview (Olympus) and Stereo Investigator software (MicroBrightField, Williston, VT). Immunopositive cells were counted manually through three atlas-matched sections of the BLA (2.12–3.3 mm caudal to bregma). Z-series with 1 μm increments were captured for three randomly selected BLA fields of view per section using a 60× objective lens. A criterion was set so that cells had to have immunoreactive staining in multiple steps of the z-series to be considered immunopositive. Data was obtained from five animals. To represent confocal images of immunoreactive staining patterns in the BLA, images were captured using the Fluoview software (Olympus) and imported into Adobe (San Jose, CA) Photoshop. Brightness and contrast, as well as superimposition of the images were done using Adobe Photoshop.
Histology.
Cannula tip placement was verified on forebrain brains sections (stained with c-Fos/neutral red) at 400× magnification using a Leica DME binocular microscope by an investigator (P.L.J.) who was blinded to the experimental treatment of each rat.
Data management and statistical analyses
For single and double immunostaining each dependent variable [number of single c-Fos, number of c-Fos/TPH double labeled neurons, and total number of TPH-immunoreactive (ir) neurons] was analyzed using a two-way ANOVA with NPY and restraint as main factors. In the presence of significant main effects or interactions between main effects, post hoc tests were conducted using a Fisher's least significant difference (LSD) test.
Brain regions analyzed for the number of c-Fos-ir nuclei were chosen based on their roles in central anxiety, autonomic, and/or neuroendocrine regulation (Singewald and Sharp, 2000; Singewald et al., 2003; Nakamura et al., 2004). Selection of anatomical levels for analysis and identification of subnuclei was conducted with reference to illustrations from a standard stereotaxic rat brain atlas (Paxinos and Watson, 1986) and landmarks such as the location and morphology of white matter tracts (e.g., fornix, mammillothalamic tracts, and optic tracts) and ventricles. All statistical analyses were performed using SPSS 14.0 for Windows (SPSS, Chicago, IL), and all graphs were generated using SigmaPlot 2001 for Windows (SPSS).
Quantification of the number of c-Fos-ir cells in the ventral subdivision of the LS (LSV) and intermediate subdivision of the LS (LSI; 1.00 mm bregma) was performed by taking high-resolution photographs (200× magnification) of the LSV and LSI and importing them into a graphic illustration program (CorelDraw 11.633 for Windows; Viglen). The photograph was divided in half with the LSV adjacent to the lateral ventricle and the LSI medial to that. A dot was placed over each c-Fos-ir nuclei to prevent over or under counting. Out-of-focus c-Fos-ir nuclei were verified at 400× magnification using a binocular brightfield microscope. The area of each region or subregion was calculated with an image analysis program (SPOT 3.5.5 for Windows digital imaging software; Silicon Graphics). The area counted for the LSV and LSI was 0.7 mm2 area for each side of brain.
Because of the irregular shape of some brain regions, high-resolution photographs were taken at 200× magnification and placed in a graphic illustration program (CorelDraw). For the rostral (+0.20 mm bregma) and caudal (−0.26 mm bregma) BNST an outline was drawn around the oval nucleus (i.e., BSTL) and the BSTMA which were defined by neutral red staining (see Fig. 2). An image analysis program (SPOT 3.5.5 for Windows digital imaging software; Silicon Graphics) was used to calculate the areas. A two-way ANOVA was used to verify that these areas did not differ between treatment groups.
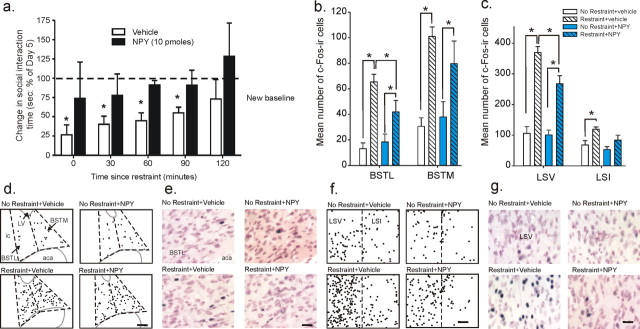
Figure 2.
Effect of NPY treatment on stress-induced alterations in SI and c-Fos immunoreactivity in subdivisions of the BNST and LS. a, Stress-induced alterations in SI time over a 2 h time course after a 30 min restraint session in vehicle and NPY-treated rats. b, c, Mean number of c-Fos-immunoreactive cells in the BNST (b) and LS (c) subdivisions. c-Fos immunostaining was measured 60 min after a 30 min restraint session or cage control in rats injected daily for 5 d into the BLA with either vehicle (1% bovine serum albumin) or NPY (10 pmol/100 nl). Data are represented as mean ± SEM. *Significantly different from the new SI baseline (post-treatment baseline), p < 0.05 in graph. d–g, Illustration of c-Fos-immunoreactive cells in the BNST (d, e) and LS (f, g), respectively. In all four treatment groups where each black dot equals 1 c-Fos-ir cell, gray solid lines indicate outline of lateral ventricle (LV) and anterior commissure (aca), and black dashed lines indicated BNST and LS subdivisions. e, High-magnification photomicrographs of lateral BNST (BSTL) and ventral LS (LSV; g), with neutral red staining of cell membrane/cytoplasm and dark blue chromogen immunostain of nuclear c-Fos. Scale bars: d, f, 100 μm; e, g, 25 μm.
Quantification of the number of c-Fos-ir cells in the RPa (at −11.30, −11.60 and −11.90 mm bregma) was performed at 400× magnification by centering the field of view over the ventromedial medulla and counting only the c-Fos-ir cells in this field of view which included the entire RPa and part of the raphe magnus (RMg) and represented a 0.25 mm2 area (see Fig. 4). TPH counterstaining was done to identify c-Fos induction in serotonergic neurons and to assist in locating consistent rostrocaudal sections containing the RPa.
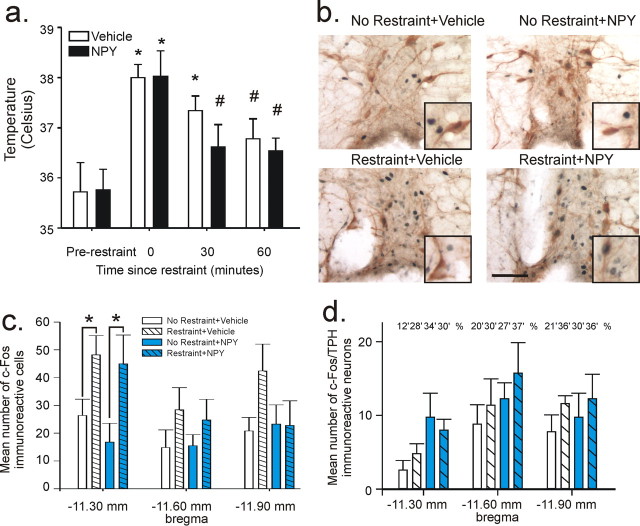
Figure 4.
Effect of NPY treatment on stress-induced alterations in body temperature and c-Fos responses in the RPa/RMg complex. a, b, Changes in temperature over a 60 min time course after a 30 min restraint session [a; *p < 0.05 (versus prerestraint); #p < 0.05 (vs time 0 min)] and photomicrographs of c-Fos (dark blue nuclear immunostain) plus TPH (orange/brown cytoplasmic immunostain) immunostaining of the RPa/RMg complex 60 min after a 30 min restraint session or cage control in rats injected daily for 5 d into the BLA with either vehicle (1% bovine serum albumin) or NPY (10 pmol/100 nl) (b). Black boxes (insets) at bottom right of photomicrographs in b show representative cells at higher magnification. Scale bar: b, 50 μm; for insets, 30 μm. c, d, Mean number of single c-Fos-immunoreactive cells (c) and c-Fos/TPH double-immunostained neurons in the RPa/RMg complex at three coronal sections (d; distance from bregma below bars). Data are represented as mean ± SEM (*p < 0.05). Numbers above bars in d represent the percentage of total TPH-ir neurons which were c-Fos immunoreactive.
The PVN (−1.80 mm bregma) was identified with the aid of neutral red staining which differentiates between magnocellular (pm) and parvocellular divisions of the PVN. The parvocellular division of the PVN was divided into two subdivisions [dorsomedial parvocellular PVN (mpd) and ventromedial parvocellular PVN (mpv)] because the mpd contains the majority of CRF producing neurons (Herman et al., 2002) (see Fig. 5). High-resolution photographs were taken at 200× magnification and placed in a graphic illustration program (CorelDraw). A dot was placed over each c-Fos-ir nuclei to prevent over or under counting. Out-of-focus c-Fos-ir nuclei were verified at 400× magnification using a binocular brightfield microscope. The areas of the subdivisions were 0.014, 0.013, and 0.009 mm2 for the pm, mpd and mpv, respectively.
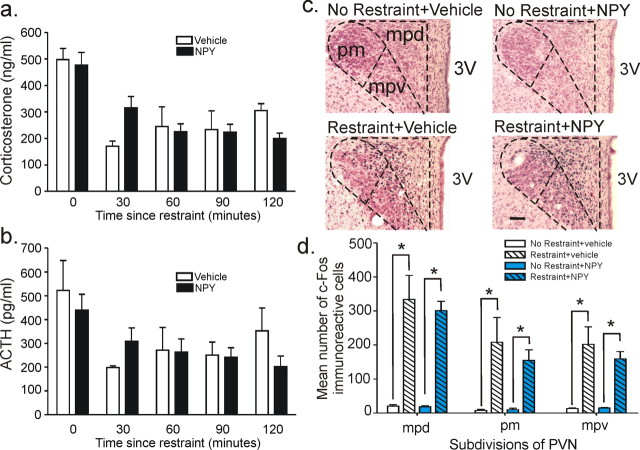
Figure 5.
Effect of NPY treatment on stress-induced alterations in plasma stress hormones and c-Fos responses in subdivisions of the PVN. a, b, Changes in corticosterone (a) and ACTH levels over a 2 h time course after a 30 min restraint session (b). c, d, c-Fos expression in the PVN 60 min after a 30 min restraint session or cage control in rats injected daily for 5 d into the BLA with either vehicle (1% bovine serum albumin) or NPY (10 pmol/100 nl). Scale bar, 100 μm.
Analysis of the behavioral studies was performed using SPSS 14.0 for Windows (SPSS), and all graphs were generated using GraphPad Prism 4.0. A two-way ANOVA was conducted for all sets of experiments. In the presence of significant main effects or interactions between main effects, post hoc tests were conducted using a Fisher's LSD test. Because of sample sizes <10, a Levene's homogeneity test was conducted to assure normal distribution of data.
Experimental protocols
For the assessment of baseline SI time, male Wistar rats were implanted with bilateral cannulas into the BLA. After recovery, ∼72 h later, all animals were subjected to a 5 min habituation session to the SI box, then 48 h later they were assigned to their appropriate test group.
Time course of anxiolytic-like and stress-resilient effects on SI in rats treated for 5 d with NPY.
On experimental day 1, the animals were divided into three groups (n = 6/group) and assigned one of three treatments: NPY (10 pmol), deamidated NPY (free acid 10 pmol), or vehicle [1% bovine serum albumin (BSA)]. Each animal received their designated treatment and 30 min later placed in the SI box. Animals were injected with their treatment again on experimental days 2–5, but only reassessed in the SI test 30 min after injection on day 5. Three days later, on day 8, all animals were subjected to restraint stress for 30 min and then 60 min after restraint were placed in the SI test for assessment of anxiety-related behavior. Forty-eight hours later on day 10, all rats received a sham injection and were reassessed in the SI test. All rats were retested weekly for an additional 7 weeks. On experimental day 59, all animals were placed in restraint for 30 min and then 60 min later reassessed in the SI test. After completion of the experiment all animals were killed and their brains removed for histological verification of cannulas placement.
Time course of SI after restraint stress in 5 d NPY-treated rats.
On experimental day 1, the rats were assigned to one of two treatments (vehicle or NPY; n = 30/group). Then each group was further separated into five subgroups (n = 6/group) and assigned to a specific time point (0, 30, 60, 90 or 120 min). On experimental day 1, animals in both groups received a sham injection and then 30 min later assessed in the SI test. The animals received their assigned treatment daily on days 2–6. On day 6, 30 min after the injection, all rats were reassessed in the SI test. On experimental day 8, all animals were placed in restraint stress for 30 min. Immediately after the restraint session the animals assigned to the 0 time point were assessed in the SI test. Then each subsequent group was placed in the SI box in accordance with their assigned time point. After completion of the experiment, all animals were killed and their brains removed for histological verification of the site of cannulas implantation.
Time course of temperature, corticosterone, and ACTH after restraint stress in 5 d NPY-treated rats.
On experimental day 1, the rats were divided in half (n = 25/group) and assigned to one of two treatments (vehicle or NPY). Then each group was further separated into five subgroups (n = 5/group) and assigned to a specific time point (0, 30, 60, 90, or 120 min). On experimental day 1, animals in both groups received a sham injection and then 30 min later assessed in the SI test. The animals received their assigned treatment daily on days 2–6. On day 6, 30 min after the injection, all rats were reassessed in the SI test. On experimental day 8, the temperature of all animals was taken using a digital rectal thermoprobe (Becton Dickinson, Franklin Lankes, NJ) immediately before being placed in restraint. After 30 min of restraint, each animal had their temperature retaken at their assigned time point. Immediately after temperature assessment, the animals were killed and their trunk blood collected in EDTA-coated tubes, centrifuged for plasma extraction and stored in a −80°C freezer until assayed for corticosterone and ACTH. In addition to trunk blood, all animals had their brains removed for histological verification of the cannulas placement.
c-Fos study in 5 d NPY and vehicle-treated rats after restraint.
Forty-eight hours after the baseline SI test, the animals were divided into two groups (NPY or vehicle; n = 10). Each group received their assigned treatment then 30 min later they were assessed in the SI test. Additional injections were given daily on days 2–5 and 30 min after the last injection, the animals were reassessed in the SI test. Seventy-two hours later, on experimental day 8, each treatment group was divided in half and assigned to one of two conditions (restraint or cage control). Ninety min after the onset of the restraint session, the animals were perfused with 4% paraformaldehyde to fix the brains. The cage controls were also perfused. All brains were removed and processed for immunohistochemical analysis during which histological verification of the injection site was confirmed.
Blockade of NPY treatment-mediated long-term SI increases with calcineurin inhibitory peptide.
On experimental day 1, the animals were divided into three treatment groups [vehicle plus NPY (n = 3), calcineurin inhibitory peptide plus NPY (n = 5), or calcineurin inhibitory peptide plus vehicle (n = 7)]. Each animal was then injected with their assigned treatment (30 min between compounds) and 30 min later assessed in the SI test. Additional daily injections were given on days 2–5, but animals were only reassessed in the SI test 30 min after injection five. Seventy-two h later, on experimental day 8, all animals were administered a sham injection and 30 min later tested in SI. After completion of the experiment, all animals were killed and their brains removed for histological verification of cannulas placement.
The effect of 5 d NPY treatment in rats on the elevated plus maze.
Twenty male Wistar rats had bilateral cannulas implanted into the BLA. Approximately 1 week after surgery, the animals were divided into two treatment groups (NPY and vehicle). On experimental days 1–5, each animal received their assigned treatment. On experimental day 8, all animals were placed on the automated elevated plus maze for a 5 min test session.
The effect of 5 d NPY treatment in rats on locomotor activity.
Twelve male Wistar rats had bilateral cannulas implanted into the BLA. Approximately 1 week after surgery, the animals were divided into two treatment groups (NPY and vehicle). On experimental days 1–5, each animal received their assigned treatment. On experimental day 8, all animals were placed in the automated locomotor activity chambers for a 1 h session.
Results
Repeated NPY administration into the BLA results in long-term anxiolytic-like effect in SI
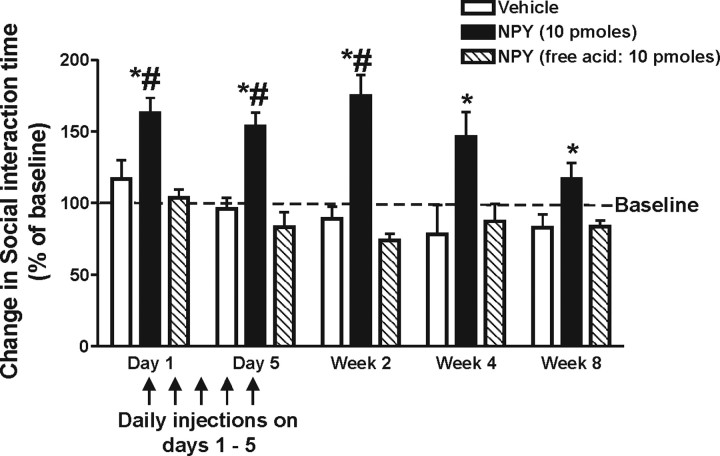
To determine the duration of the anxiolytic-like effects of NPY, animals received either an anxiolytic dose of NPY (10 pmol/100 nl/side), the inactive free acid form of NPY (10 pmol/100 nl/side) or vehicle (1% bovine serum albumin in deionized water; BSA/100 nl/side) directly into the BLA once daily for 5 consecutive days (n = 5). Animals were assessed in the SI test for anxiety-like behavior 30 min after sham injection (baseline) or injection of their assigned treatment. Data in Figure 1 show that only the group receiving the active form of NPY had a significant increase in SI time on injection days 1 and 5. More importantly, analysis with a two-way ANOVA indicated these same animals exhibited an increase in SI with significant effects of treatment (F(2,68) = 62.74, p < 0.0001) and time (F(4,68) = 2.71, p = 0.0373) for up to 8 weeks after the last NPY injection. A second experiment assessed behavior in the elevated plus maze and locomotor tests. NPY-treated animals showed no changes in their responses in either test compared with the vehicle group (supplemental Fig. 1, available at www.jneurosci.org as supplemental material).
Figure 1.
Time course of changes in SI time in animals receiving daily injections of either vehicle (1% BSA), NPY (10 pmol/100 nl), or free acid NPY (10 pmol/100 nl) into the BLA for 5 d. #Significantly different from baseline; *significantly different from vehicle and free acid NPY p < 0.05 (n = 5). Baseline values represent the pretreatment SI time. Error bars indicate SEM.
Repeated NPY injections result in long-term resilience to stress-induced reductions in SI
Given this long-term reduction in anxiety-like behavior in the NPY-treated animals, we hypothesized that these rats might also be less vulnerable to a stress challenge. In the next set of experiments, we first characterized the effects of an acute 30 min restraint session on SI response in naive animals and then tested the effects of this challenge in animals treated for 5 d with either vehicle or NPY. The time course for restraint-induced anxiety-like effects in SI of naive male Wistar rats immediately after the restraint session show that SI times are significantly decreased and do not return back to baseline levels until 90 min post challenge (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Similar to naive animals, rats treated with vehicle daily for 5 d exhibit a decrease in SI time until the 90 min time point (F(5,76) = 11.45, p < 0.0001, n = 7–12), whereas animals treated with five daily injections of NPY (10 pmol/100 nl/side) showed no change in SI time at any of the time points after 30 min restraint (Fig. 2a). Additionally, in animals subjected to restraint, the number of c-Fos-ir cells was increased in all subdivisions of the LS and BNST, two key areas associated with anxiety-like responses (Davis et al., 1997; Koob, 1999; Singewald et al., 2003). Restraint stress increased cFos expression in the lateral and medial subdivisions of rostral BNST and caudal BNST (i.e., BSTL, F(1,15) = 40.0, p < 0.001, n = 5) and BSTM (F(1,15) = 35.0, p < 0.001, n = 5) (Fig. 2b), along with the ventral and intermediate divisions of the LS (i.e., LSV, F(1,15) = 106.2, p < 0.001, n = 5) and LSI (F(1,15) = 11.9, p = 0.004, n = 5) (Fig. 2c,f,g). NPY treatment attenuated restraint-induced c-Fos immunoreactivity in both the LSV (NPY, F(1,15) = 6.5, p = 0.022; NPY by restraint effect, F(1,15) = 5.3, p = 0.036) and the rostral BSTL (NPY by restraint effect, F(1,15) = 5.1, p = 0.039), as shown in graphs in Figure 2, b and c, and photomicrographs in d–g. In summary, NPY treatment significantly decreased c-Fos expression specifically in the LSV and BSTL and did not alter c-Fos in other brain regions and the decrease in c-Fos in these areas correlated with stress resilience in the animals assessed in the SI test (Fig. 2a).
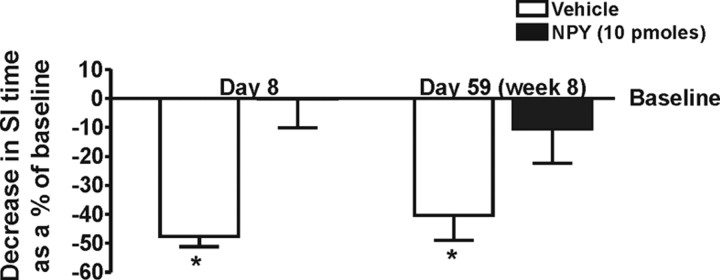
Resilience to restraint-induced reductions in SI observed on experimental day 8 was still present when the NPY-treated animals were rechallenged with restraint stress during experimental week 8 (Fig. 3) (two-way ANOVA for treatment effect, F(1,17) = 27.58, p < 0.0001, n = 5–6) and post hoc analysis showing significant change from baseline for vehicle-treated animals on day 8 and day 59 (F(3,15) = 30.8, p < 0.0001, n = 5–6).
Figure 3.
Decrease in SI time after 30 min of restraint stress on days 8 and 59 (week 8) in animals treated daily for 5 d with either vehicle (1% bovine serum albumin) or NPY (10 pmol/100 nl). *Significantly different from baseline and NPY-treated animals, p < 0.05 (n = 5–6). Baseline is day 5 for the first session and 56 for the second session.
Repeated NPY-induced resilience differentially modulates specific components of the stress response
In addition to SI behavior, animals were also assessed for changes in hyperthermia (Fig. 4a), corticosterone, and ACTH levels (Fig. 5a,b) after restraint stress. Both NPY and vehicle-treated rats showed a significant increase in core temperature immediately after restraint and a similar time course for recovery back to baseline, thus there was a significant effect across time for both groups, but no interaction or treatment effect (Fig. 4a) (F(3,24) = 8.78, p < 0.001, n = 5/time point). Post hoc analysis indicated that core temperature in the NPY-treated animals were not different from baseline values at 30 min and vehicle-treated rats at 60 min. Assessment of c-Fos expression showed a significant increase in the number of c-Fos-ir cells in the RPa and RMg complex, areas well known to regulate stress-induced hyperthermia (Nakamura et al., 2004). Within the RPa/RMg, restraint increased the mean number of c-Fos-ir nonserotonergic cells (not positive for tryptophan hydroxylase, TPH) in the RPa/RMg at −11.30 mm bregma (Fig. 4b–d) (F(1,14) = 11.2, p = 0.005, n = 4–5/group). Basal and stress-induced c-Fos expression in this region was not different between vehicle or NPY-treated animals.
Although there was a significant increase in ACTH (time effect, F(4,16) = 4.7, p < 0.02) and corticosterone (time effect, F(4,16) = 8.6, p < 0.002) levels after restraint, no difference were detected between NPY and vehicle-treated groups (no NPY by time interaction was detected for ACTH, F(4,18) = 1.0, p = 0.424; or CORT, F(4,18) = 1.7, p = 0.200) (Fig. 5a,b). Consistent with this neuroendocrine profile, restrained rats had significant increases in the mean number of c-Fos-ir cells in all subdivisions of the PVN (i.e., mpd, F(1,15) = 57.1, p < 0.001, n = 5; mpv, F(1,15) = 33.5, p < 0.001, n = 5; pm, F(1,15) = 17.9, p = 0.001, n = 5) regardless of treatment (Fig. 5c,d). The restraint induced increases in c-Fos induction in the pm division occurred in the magnocellular and smaller parvocellular cells as evidenced by neutral red counter-staining.
Because the central amygdala (CeA) is an important relay site of the BLA and also is known to be involved in stress-related behavior, autonomic and endocrine activity (Davis, 1993; Petrov et al., 1995; Sullivan et al., 2004), c-Fos-ir cells were counted in the entire CeA. A significant increase in the number of c-Fos-ir cells was observed in the CeA after restraint regardless of NPY treatment (mean number of c-Fos-ir cells ± SEM were as follows: no restraint plus vehicle group, 28 ± 2; restraint plus vehicle, 171 ± 54; no restraint plus NPY, 23 ± 7; restraint plus NPY, 145 ± 42; F(1,15) = 5.3, p < 0.02].
NPY-induced plasticity is calcineurin mediated
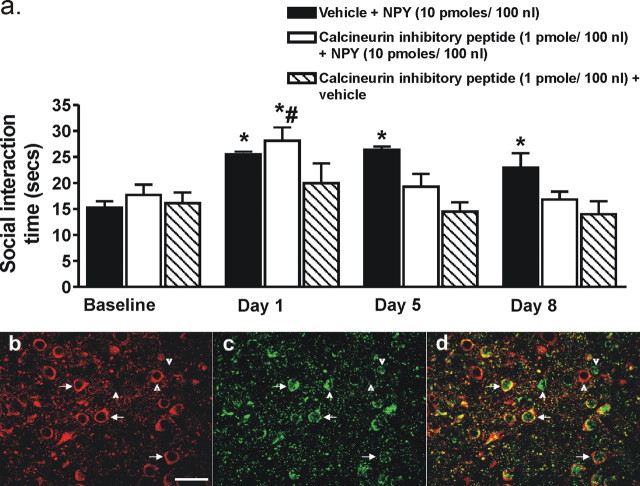
The long-term persistence of increased baseline SI in the NPY-treated animals led us to hypothesize that neuronal plasticity, specifically, some form of LTD may be occurring within the BLA. The protein phosphatase calcineurin has been implicated as having an important role in LTD-like plasticity (Lin et al., 2003a,b). Therefore, to test this hypothesis, we administered a calcineurin inhibitory peptide 30 min before vehicle or NPY (10 pmol/100 nl/side) into the BLA. Figure 6a shows that the calcineurin inhibitory peptide did not block the acute behavioral effects of NPY treatment observed on day 1, but it did prevent the long-term anxiolytic-like effects in SI as can be seen for days 5 and 8 (F(3,7) = 16.78, p < 0.0001, n = 8). Animals that received vehicle plus NPY showed a significant increase in SI on all days compared with baseline (F(4,2) = 5.345, p = 0.0215, n = 3), whereas those receiving the calcineurin inhibitory peptide plus vehicle showed no difference throughout the treatment.
Figure 6.
a, Changes in SI time for animals administered either vehicle plus NPY (10 pmol/100 nl; n = 3), calcineurin inhibitory peptide (1 pmol/100 nl) plus NPY (10 pmol/100 nl; n = 5), or calcineurin inhibitory peptide (1 pmol/100 nl; n = 7) plus vehicle into the BLA daily for 1 and 5 d. SI was assessed on days 1, 5, and 8. *Significantly different from baseline (p < 0.05) and #significantly different from days 5 and 8 (p < 0.05). Representative photomicrograph of calcineurin- (b) and NPY1r-immunoreactive (c) cells in the BLA (−3.30 mm bregma) of a naive male rat. d, Merged image of b and c demonstrating colocalization of calcineurin and NPY1r immunoreactivities as indicated by arrows. Arrowheads indicate single-labeled cells. Scale bar, 40 μm.
Colocalization of NPY1 receptors and calcineurin in the BLA neurons
Both calcineurin and NPY1 receptor (NPY1r) immunoreactivity were present within cells distributed throughout the rostral-caudal extent of the BLA (Fig. 6b–d). There was a high degree of colocalization of calcineurin- and NPY1r- throughout the BLA with ∼89% of calcineurin cells expressing NPY1r. Of the NPY1R immunopositive cells, 77% also coexpressed calcineurin whereas 23% were single labeled. In general, these double labeled NPY1r-/calcineurin immunopositive cells have the appearance that is typical for glutamatergic projection neurons (pyramidal, 15–20 μm diameter).
Histological verification of cannulas placement
The target area of injections was the basolateral amygdaloid nucleus (BL), including its anterior and posterior parts (Fig. 7). The basolateral amygdaloid complex (BLC) is made up of three major nuclei, including the BL, which can be recognized on the basis of cytoarchitectural and neurochemical criteria (McDonald, 2003).
Figure 7.
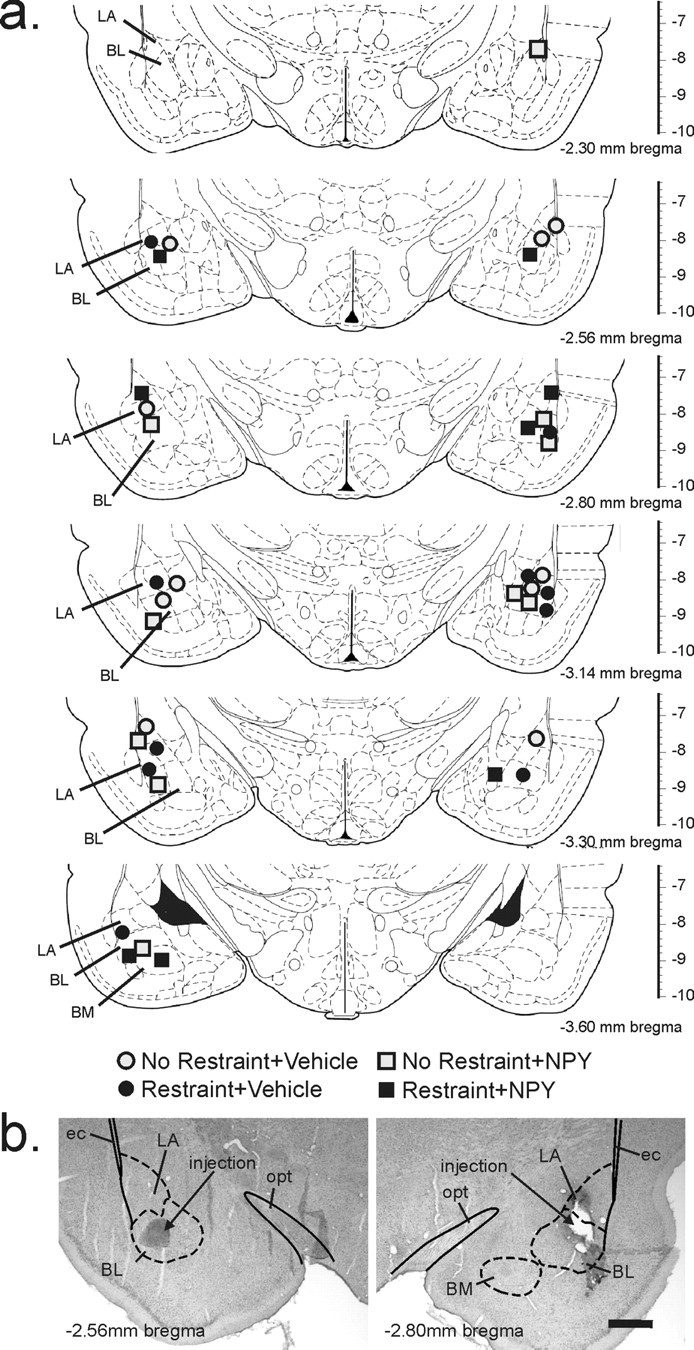
a, Schematic representation of the bilateral injection sites as determined by histology. Infusion cannulas placements are illustrated as symbols for each treatment (gray circle, no restraint plus vehicle injection; black circle, restraint plus vehicle; gray square, no restraint plus NPY; black square, restraint plus NPY). Illustrations of coronal brain sections are based on the rat brain atlas of Paxinos and Watson (1986). Numbers below each section indicate the distance posterior from Bregma; the scale on the right of each section represents the distance ventral from Bregma (in millimeters). b, Photomicrographs illustrate an example of histological verification of the injector tip placements for microinjections of NPY or vehicle targeting the BL. Photomicrographs show the injection sites on the left and right sides of a single rat through the BLC, which is made up of the lateral amygdaloid nucleus (LA), BL, and basomedial amygdaloid nucleus (BM) at approximately −2.56 (left) and −2.80 (right) mm Bregma. Solid lines represent white matter tracts and dashed lines illustrate subdivisions of the BLC. ec, External capsule; opt, optic tract. Scale bar, 800 μm.
For each rat, injection sites were all located within the BLC (Fig. 7). The rostrocaudal distribution of injections were located −2.30 to −3.60 mm from Bregma. Injection sites were verified using illustrations from a standard stereotaxic atlas of the rat brain (Paxinos and Watson, 1986) to determine the anatomical position of each injection site.
Discussion
Although the underlying mechanisms that determine the overall impact of stress on an organism are unclear, it is likely a balance between at least two opposing mechanisms: one makes organisms more vulnerable, and the other makes them more resilient or resistant. Our previous findings support the hypothesis that NPY release in the BLA opposes the effects of stress (Sajdyk et al., 2004, 2006) and current findings demonstrate that repeated NPY receptor activation induces stress resilience. Here, repeated injections of NPY in the BLA decreased restraint induced anxiety-like behavior, without altering other physiological measures (e.g., hyperthermia, or corticosterone/ACTH responses), and increased resilience to restraint stress induced anxiety for 8 weeks after the initial treatment. Furthermore, the dose of NPY that was effective in this study (10 pmol/100 nl) is not too far out of the “physiological range” based on what is known from the few studies measuring extra-cellular fluid levels of NPY in the CNS (Gamber et al., 2005; Kozak et al., 2005), suggesting a true biological significance of the results. Additionally, NPY-mediated behavioral resilience was prevented with pretreatment of a calcineurin inhibitor, suggesting an LTD-like plasticity may underlie this mechanism, which is opposite to the behavior and cellular changes produced by administering CRF into the BLA of rats (Sajdyk et al., 1999b; Rainnie et al., 2004).
The NPY dose used in the current study is in-line with electrophysiological studies conducted within our collaborative group. Both Dr. Rainnie (Emory University, Atlanta, GA) and Dr. Colmers (University of Alberta, Edmonton, Alberta, CA) conducted studies where they recorded from BLA projection neurons during NPY perfusion and determined that the effective doses of NPY within the BLA ranged between 200 nm to low μm levels.
The current results also demonstrate that five daily injections of NPY into the BLA induce an anxiolytic-like response in the SI test for up to 8 weeks without altering either locomotor activity or EPM behavior. Although other studies have indicated that centrally administered NPY has anxiolytic-like effects in the EPM (Broqua et al., 1995; Heilig, 1995; Kask et al., 1998), it appears that the doses of NPY were either 10-fold greater than what was used in the current study or administered into other brain areas. Alternatively, it could be the case that the SI test is a more sensitive measure for the effects of NPY in the BLA and had we injected a higher dose, we may have observed an effect on the EPM. Additionally, it may be that under baseline conditions, NPY treatment in the BLA may not affect behavior as determined by EPM and that the animals would need to have a stress challenge to see behavioral changes. Therefore, it may be informative to repeat the EPM test using a wider dose range of NPY and a stress challenge.
In addition to showing that chronic NPY induces long-term changes in SI, our results also showed that NPY treatment blocked the development of anxiety-like responses in SI after an acute stressor such as restraint stress and protected from the anxiogenic-like effects of restraint stress in the SI test for almost two full months past the last NPY injection. Previous work showed that NPY in the BLA also plays a role in fear responses (Gutman et al., 2006). These studies found that administration of NPY into the BLA can block the expression as well as enhance the extinction of fear-potentiated startle, another instance where NPY has an opposite effect to that mediated by CRF (Lee et al., 1994). Together, we propose that NPY in the BLA plays a critical role in reducing social anxiety-like behaviors and opposes the deleterious behavioral effects of an acute stressor. These findings supports the potential for developing novel NPY mediated intervention strategies to either treat chronic preexisting social anxiety disorders, or prevent the emergence of post-traumatic stress disorder (PTSD)-like anxiety disorders as consequences of trauma by treating subjects soon after the traumatic event.
The LS and BNST are two key brain regions implicated in both social behaviors and PTSD (Insel, 1992; Grillon et al., 1996; Koolhaas et al., 1998; Gilmor et al., 2003). Here, we show that NPY, but not vehicle, attenuated restraint induced cellular responses in the LS and BNST, which is consistent with previous findings. For example, NPY in the LS is anxiolytic and antagonizes CRF-mediated anxiety responses (Kask et al., 2001), and CRF1 receptor knock-out mice have attenuated stressor induced anxiety-like behavioral responses which coincides with blunted c-Fos responses in the LS (Nguyen et al., 2006). Additionally, results from intracellular recordings in the BNST indicate that CRF and NPY have opposing effects (Kash and Winder, 2006). Thus, it appears that the behavioral effects of NPY observed in this study are consistent with changes in key sites implicated in stress-related and social behaviors.
Although NPY has been shown to be involved in temperature regulation (Currie and Coscina, 1995; Felies et al., 2004), NPY injections into the BLA failed to alter restraint-induced hyperthermia. Additional investigation of c-Fos responses in the RPa, a key nucleus involved in mediating hyperthermia (Nakamura et al., 2004), revealed that local c-Fos induction was unaffected by NPY treatment. Overall, it appears that application of NPY in the BLA does not alter restraint induced hyperthermia or alter cellular responses in thermoregulatory brain regions.
Although anxiety-like behavior in the SI test was prevented in NPY-treated animals, NPY had no effect on restraint stress-induced hypothalamic–adrenal–pituitary (HPA) axis activity. This finding was consistent with the finding that c-Fos expression in the PVN was increased after restraint in both NPY and vehicle-treated groups. The CeA, another important nucleus in fear and anxiety showed an increase in c-Fos activity; however, this increase was observed regardless of NPY or vehicle treatment. This suggests that the PVN and CeA may be more important in the restraint-induced endocrine (i.e., increases in plasma CORT and ACTH) and autonomic responses (see also Davis, 1993; Petrov et al., 1995; Sullivan et al., 2004). Overall, these results show a striking dichotomy in the NPY regulation of behavioral and physiological responses during stress, suggesting that different facets of the stress response are not only elicited through a wide range of pathways but are also differentially regulated.
Unlike the BNST, the c-Fos expression in the CeA showed no correlation with NPY treatment. Although the BLA projects indirectly to the BNST via the CeA (Sun et al., 1991), the BLA also has direct projections to the BNST (Pitkanen and Amaral, 1991; Savander et al., 1995) which may explain the differences between cellular responses in the CeA and BNST.
Previous studies in our laboratory have shown that long-term anxiety-like responses are elicited by repeated injections of Ucn into the BLA, and this long term plasticity is mediated via activation of CaMKII (Shekhar et al., 2003). Conversely, in this study we demonstrated that intra-BLA injections of the neuropeptide NPY induced a long-term anxiolytic-like response. Because the BLA is known to expresses bidirectional synaptic plasticity, we hypothesized that NPY may be mediating the behavioral effects via an LTD-like pathway. Because calcineurin is a key phosphatase within the LTD pathway that is associated with the extinction of fear-related behaviors (Lin et al., 2003a,b), we injected calcineurin inhibitory peptide with NPY into the BLA and prevented the development of the long-term anxiolytic-like response in the SI, without altering the acute anxiolytic effects of NPY administration into the BLA. These exciting results support the notion that the acute and long-term effects of NPY on stress-related behaviors are mediated via different pathways. Only repeated injections of NPY appear to initiate these intracellular mechanisms associated with neuronal plasticity, and calcineurin is critical for this effect.
Previous studies show that the anxiolytic-like actions of NPY in the amygdala are mediated through the NPY1r subtype (Heilig et al., 1993; Sajdyk et al., 1999a). The precise cell type mediating these anxiolytic effects is unknown. Immunoreactivity for NPY1r-ir in cells of the BLA extends throughout the rostral-caudal BLA and exhibits a moderate to low intensity of immunoreactive signal (Fig. 6) (Wolak et al., 2003). The presence of NPY1r-ir within the BLA has also been identified in fibers and few scattered cell bodies using a similar NPY1r antibody (Kopp et al., 2002). Kishi et al., (2005) reported lightly labeled cells expressing NPY1r mRNA also distributed throughout the rostral-caudal extent of the nucleus. Although there are slight discrepancies regarding the relative degree of NPY1r expression in the BLA, the reports are consistent with the presence the receptor, which is supported by behavioral pharmacology using NPY1r selective ligands (Sajdyk et al., 1999a). Using immunohistochemistry we demonstrated that calcineurin positive cells in the BLA express NPY1r-ir, suggesting that NPY could directly activate a calcineurin-mediated intracellular mechanism. Although we have shown that 75% of the NPY1r-immunoreactive cells coexpressed CaMKII (Rostkowski and Urban, 2006), a marker for glutamatergic projection neurons in the BLA, it has not been determined whether calcineurin is expressed within glutamatergic projection neurons. It is likely that a large portion of these calcineurin-NPY1r immunopositive cells are glutamatergic projection neurons because the persistent anxiety-like behavior in animals after repeated administration of CRF into the BLA is blocked by pretreatment with either a CaMKII inhibitor (Shekhar et al., 2003) or NPY (Sajdyk et al., 2004, 2006); and preliminary evidence from two different electrophysiological studies show that application of an NPY1r agonists (F7, P34 NPY, and Leu-Pro NPY) hyperpolarizes glutamatergic cells within the BLA which would result in an overall decrease in BLA output (W. F. Colmers and D. G. Rainnie, unpublished observations). Thus, we hypothesize that CRF and NPY receptors are on the same subset of glutamatergic projection cells within the BLA and act in direct opposition to each other to modulate plasticity.
Together, these data strongly support the concept that neuropeptides modulate plasticity in the CNS to bidirectionally alter synaptic activity such that the neurons can become more or less resistant to the effects of stress. If we consider our findings in the context of the human studies showing a direct relationship between NPY and coping (Yehuda et al., 2006a,b), the data suggest a potential strategy of novel peptidergic therapeutics for the prevention of disorders arising from stress or traumatic events, including PTSD, depression, and drug abuse.
Footnotes
This work was supported by National Institutes of Health Grants K01 MH01869 (T.J.S.), MH62621 (J.H.U.), and R01 MH065702 and MH52691 (A.S.). We thank Pam Kelley for her assistance in scoring the SI test and Drs. William Truitt and Younglim Lee for scientific discussion. All authors contributed to the writing and editing of this manuscript. Primary authorship, experimental design and behavioral analysis were performed by T.J.S. Coauthorship, experimental design, and counting and analysis of c-Fos experiments were conducted by P.L.J. Secondary authorship and experimental design were contributed by A.S. Secondary authorship and fluorescence immunohistochemistry studies were conducted by J.H.U. and R.J.L. Proofreading of this manuscript and corticosterone and ACTH assays were completed by D.R.G. and M.M. All surgical procedures and behavior experiments were conducted by S.D.F. Brain processing for histological verification of cannulas placement and c-Fos immunostaining was done by A.D.
The authors declare no competing financial interests.
References
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Broqua P, Wettstein JG, Rocher MN, Gauthier-Martin B, Junien JL. Behavioral effects of neuropeptide Y receptor agonists in the elevated plus-maze and fear-potentiated startle procedures. Behav Pharmacol. 1995;6:215–222. [PubMed] [Google Scholar]
- Currie PJ, Coscina DV. Dissociated feeding and hypothermic effects of neuropeptide Y in the paraventricular and perifornical hypothalamus. Peptides. 1995;16:599–604. doi: 10.1016/0196-9781(95)00020-k. [DOI] [PubMed] [Google Scholar]
- Davis M. Pharmacological analysis of fear-potentiated startle. Braz J Med Biol Res. 1993;26:235–260. [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in emotional learning. Int Rev Neurobiol. 1994;36:225–266. doi: 10.1016/s0074-7742(08)60305-0. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Possible relevance to PTSD. Ann NY Acad Sci. 1997;821:305–331. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- Felies M, von Horsten S, Pabst R, Nave H. Neuropeptide Y stabilizes body temperature and prevents hypotension in endotoxaemic rats. J Physiol (Lond) 2004;561:245–252. doi: 10.1113/jphysiol.2004.073635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamber KM, Macarthur H, Westfall TC. Cannabinoids augment the release of neuropeptide Y in the rat hypothalamus. Neuropharmacology. 2005;49:646–652. doi: 10.1016/j.neuropharm.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Gilmor ML, Skelton KH, Nemeroff CB, Owens MJ. The effects of chronic treatment with the mood stabilizers valporic acid and lithium on corticotropin-releasing factor neuronal systems. J Phamacol Exp Ther. 2003;305:434–439. doi: 10.1124/jpet.102.045419. [DOI] [PubMed] [Google Scholar]
- Grillon C, Southwick SM, Charney DS. The psychobiological basis of posttraumatic stress disorder. Mol Psychiatry. 1996;1:278–297. [PubMed] [Google Scholar]
- Gutman AR, Chatwal JP, Davis M. Neuropeptide Y modulation of startle. Soc Neurosci Abstr. 2006;32:67–9. [Google Scholar]
- Heilig M. Antisense inhibition of neuropeptide Y (NPY)-Y1 receptor expression blocks the anxiolytic-like action of NPY in amygdala and paradoxically increases feeding. Regul Pept. 1995;59:201–205. doi: 10.1016/0167-0115(95)00103-i. [DOI] [PubMed] [Google Scholar]
- Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Brot M, Heinrichs SC, Menzaghi F, Koob GF, Britton KT. Anxiolytic-like action of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacology. 1993;8:357–363. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Ziegler DR, Tasker JG. Role of the paraventricular nucleus microenvironment in stress integration. Eur J Neurosci. 2002;16:381–385. doi: 10.1046/j.1460-9568.2002.02133.x. [DOI] [PubMed] [Google Scholar]
- Insel TR. Oxytocin–a neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology. 1992;17:3–35. doi: 10.1016/0306-4530(92)90073-g. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Hollis JH, Moratalla R, Lightman SL, Lowry CA. Acute hypercarbic gas exposure reveals functionally distinct subpopulations of serotonergic neurons in rats. J Psychopharmacol. 2005;19:327–341. doi: 10.1177/0269881105053281. [DOI] [PubMed] [Google Scholar]
- Kash TL, Winder DG. Neuropeptide Y and corticotropin-releasing factor bi-directionally modulate inhibitory synaptic transmission in the bed nucleus of the stria terminalis. Neuropharmacology. 2006;51:1013–1022. doi: 10.1016/j.neuropharm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Kask A, Rago L, Harro J. Anxiolytic-like effect of neuropeptide Y (NPY) and NPY13–36 microinjected into vicinity of locus coeruleus in rats. Brain Res. 1998;788:345–348. doi: 10.1016/s0006-8993(98)00076-6. [DOI] [PubMed] [Google Scholar]
- Kask A, Nguyen HP, Pabst R, Von Horsten S. Neuropeptide Y Y1 receptor-mediated anxiolysis in the dorsocaudal lateral septum: functional antagonism of corticotropin-releasing hormone-induced anxiety. Neuroscience. 2001;104:799–806. doi: 10.1016/s0306-4522(01)00116-6. [DOI] [PubMed] [Google Scholar]
- Kask A, Harro J, von Horsten S, Redrobe JP, Dumont Y, Quirion R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci Biobehav Rev. 2002;26:259–283. doi: 10.1016/s0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Choi BJ, Lopez ME, Lee CE, Liu H, Hollenberg AN, Friedman JM, Elmquist JK. Neuropeptide Y Y1 receptor mRNA in rodent brain: distribution and colocalization with melanocortin-4 receptor. J Comp Neurol. 2005;482:217–243. doi: 10.1002/cne.20432. [DOI] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Everts H, de Ruiter AJ, de Boer SF, Bohus B. Coping with stress in rats and mice: differential peptidergic modulation of the amygdala-lateral septum complex. Prog Brain Res. 1998;119:437–448. doi: 10.1016/s0079-6123(08)61586-1. [DOI] [PubMed] [Google Scholar]
- Kopp J, Xu ZQ, Zhang X, Pedrazzini T, Herzog H, Kresse A, Wong H, Walsh JH, Hokfelt T. Expression of the neuropeptide Y Y1 receptor in the CNS of rat and of wild-type and Y1 receptor knock-out mice. Focus on immunohistochemical localization. Neuroscience. 2002;111:443–532. doi: 10.1016/s0306-4522(01)00463-8. [DOI] [PubMed] [Google Scholar]
- Kozak R, Richy S, Beck B. Persistent alterations in neuropeptide Y release in the paraventricular nucleus of rats subjected to dietary manipulation during early life. Eur J Neurosci. 2005;21:2887–2892. doi: 10.1111/j.1460-9568.2005.04101.x. [DOI] [PubMed] [Google Scholar]
- Lee Y, Schulkin J, Davis M. Effect of corticosterone on the enhancement of the acoustic startle reflex by corticotropin releasing factor (CRF) Brain Res. 1994;666:93–98. doi: 10.1016/0006-8993(94)90286-0. [DOI] [PubMed] [Google Scholar]
- Lin CH, Lee CC, Gean PW. Involvement of a calcineurin cascade in amygdala depotentiation and quenching of fear memory. Mol Pharmacol. 2003a;63:44–52. doi: 10.1124/mol.63.1.44. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Leu TH, Chang WC, Wang ST, Gean PW. Identification of calcineurin as a key signal in the extinction of fear memory. J Neurosci. 2003b;23:1574–1579. doi: 10.1523/JNEUROSCI.23-05-01574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Is there an amygdala and how far does it extend? An anatomical perspective. Ann NY Acad Sci. 2003;985:1–21. doi: 10.1111/j.1749-6632.2003.tb07067.x. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory consolidation and the amygdala: a systems perspective. Trends Neurosci. 2002;25:456. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Hubschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogkoi Z, Konig M, Thiel HJ, Gerstberger R, Kobayashi S, Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci. 2004;24:5370–5380. doi: 10.1523/JNEUROSCI.1219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen NK, Keck ME, Hetzenauer A, Thoeringer CK, Wurst W, Deussing JM, Holsboer F, Muller MB, Singewald N. Conditional CRF receptor 1 knock-out mice show altered neuronal activation pattern to mild anxiogenic challenge. Psychopharmacology (Berl) 2006;188:374–385. doi: 10.1007/s00213-006-0513-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 2. New York: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- Petrov T, Krukoff TL, Jhamandas JH. Convergent influence of the central nucleus of the amygdala and the paraventricular hypothalamic nucleus upon brainstem autonomic neurons as revealed by c-fos expression and anatomical tracing. J Neurosci Res. 1995;42:835–845. doi: 10.1002/jnr.490420612. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Amaral DG. Demonstration of projections from the lateral nucleus to the basal nucleus of the amygdala: a PHA-L study in the monkey. Experimental Brain Res. 1991;83:465–470. doi: 10.1007/BF00229822. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostkowski AB, Urban JH. Annual Society for Neuroscience Meeting. Atlanta, GA: 2006. Neuropeptide Y (NPY) Y1 receptor (Y1r) is expressed on both interneurons and projection neurons in the rat basolateral amygdala (BLA) [Google Scholar]
- Sajdyk TJ, Vandergriff MG, Gehlert DR. Amygdalar neuropeptide Y Y1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. Eur J Pharmacol. 1999a;368:143–147. doi: 10.1016/s0014-2999(99)00018-7. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behavioural Brain Res. 1999b;100:207–215. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR. Neuropeptide Y receptor subtypes in the basolateral nucleus of the amygdala modulate anxiogenic responses in rats. Neuropharmacology. 2002;43:1165–1172. doi: 10.1016/s0028-3908(02)00234-4. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Keim SR, Thielen SK, Fitz SD, Shekhar A. Measurements of anxiety-like behavior following intravenous infusion of sodium lactate in primed rats. Current Protocols in Neuroscience. 2003;(Supplement 24) doi: 10.1002/0471142301.ns0917s24. Unit 9.17. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A, Gehlert DR. Interactions between NPY and CRF in the amygdala regulate emotionality. Neuropeptides. 2004;38:225–234. doi: 10.1016/j.npep.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Fitz SD, Shekhar A. The role of neuropeptide Y in the amygdala on corticotropin-releasing factor receptor-mediated behavioral stress responses in the rat. Stress. 2006;9:21–28. doi: 10.1080/10253890600557315. [DOI] [PubMed] [Google Scholar]
- Savander V, Go CG, LeDoux JE, Pitkanen A. Intrinsic connections of the rat amygdaloid complex: projections originating in the basal nucleus. J Comp Neurol. 1995;361:345–368. doi: 10.1002/cne.903610211. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Sajdyk TJ, Gehlert DR, Rainnie DG. The amygdala, panic disorder, and cardiovascular responses. Ann NY Acad Sci. 2003;985:308–325. doi: 10.1111/j.1749-6632.2003.tb07090.x. [DOI] [PubMed] [Google Scholar]
- Singewald N, Sharp T. Neuroanatomical targets of anxiogenic drugs in the hindbrain as revealed by Fos immunocytochemistry. Neuroscience. 2000;98:759–770. doi: 10.1016/s0306-4522(00)00177-9. [DOI] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Sun N, Roberts L, Cassell MD. Rat central amygdaloid nucleus projections to the bed nucleus of the stria terminalis. Brain Res Bull. 1991;27:651–662. doi: 10.1016/0361-9230(91)90041-h. [DOI] [PubMed] [Google Scholar]
- Wolak ML, deJoseph MR, Cator AD, Mokashi AS, Brownfield MS, Urban JH. Comparative distribution of neuropeptide Y Y1 and Y5 receptors in the rat brain by using immunohistochemistry. J Comp Neurol. 2003;464:285–311. doi: 10.1002/cne.10823. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006a;59:660–663. doi: 10.1016/j.biopsych.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Flory JD, Southwick S, Charney DS. Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Ann NY Acad Sci. 2006b;1071:379–396. doi: 10.1196/annals.1364.028. [DOI] [PubMed] [Google Scholar]