Abstract
Hippocampal early long-term potentiation (LTP) elicited by a weak (one or two) tetanic stimulus normally fades away within 90 min. Late LTP elicited by strong (four) stimuli lasts >180 min and requires new protein synthesis to persist. If a strong tetanus is injected once into a synapse, even a weak tetanus injected into another synapse can evoke persistent LTP. It was hypothesized that a synaptic tag enables capture of newly synthesized synaptic molecules. Here, we found two synaptic capture mechanisms for a weakly stimulated synapse to acquire persistency (i.e., neuropsin dependent and independent). The single tetanus evokes a neuropsin-dependent form that follows downstream signaling into integrin/actin signal and L-type voltage-dependent Ca2+ channel (LVDCC) pathway. Additionally, a neuropsin-independent form of synaptic capture is evoked by a stronger (two) tetanus than the former. Both forms converging on LVDCC might serve different associative memories depending on their input strength. Our study strongly supports the hypothesis of synaptic tagging and demonstrates that neuropsin-dependent late associativity is particularly important in nonstressful associative memory.
Keywords: kallikrein-related peptidase 8, extracellular proteolysis, LTP (long-term potentiation), synaptic capture, synaptic plasticity, integrin
Introduction
Long-term potentiation (LTP) induced by brief repetitive stimulation brings about activity-dependent and long-lasting enhancement of synaptic efficacy that may explain short-term memory (Bliss and Lømo, 1973; Bliss and Collingridge, 1993; Malenka and Nicoll, 1999). Among the numerous synaptic contacts received by a single neuron, a subset undergoes highly selective modifications when it is repetitively stimulated. Such LTP consists of two temporal phases, early (E-) and late (L-) LTP, occurring independently to establish hyperefficacy in synaptic transmission and regulated by different signaling systems (Krug et al., 1984). Particular temporal and frequency patterns of electric stimuli can evoke E- and L-LTP individually [i.e., weak (one or two trains) stimulation evokes E-LTP, whereas strong (four trains) stimulation evokes L-LTP]. L-LTP requires the activation of a molecular cascade that transfers signals into the nucleus, alters gene expression, and synthesizes new gene products to stabilize LTP in activated synapses (Kandel, 2001). With E-LTP, the synapse does not achieve persistency until the delivery of new proteins, but paradoxically, weak stimulation itself does not induce protein synthesis; therefore, a weakly stimulated synapse produces only a tag (a synaptic mark) to capture new proteins generated by other L-LTP-evoked synaptic signaling. Accordingly, accepting and/or addressing mechanisms for the delivery of new proteins from the site of translation were hypothesized (tagging and mailing hypotheses) (Frey and Morris, 1997, 1998; Barco et al., 2002, 2005; Sajikumar and Frey, 2004; Sajikumar et al., 2005; Young and Nguyen, 2005). The candidate molecules relating to the mechanism(s) might be involved in cell-to-cell adhesion, local proteolysis related to synaptic modulation, the formation and degradation of actin networks, the regulation of kinases and ion channels, and local translation (for review, see Martin and Kosik, 2002).
There are possible interactions between the extracellular matrix (ECM) and synaptic membrane modulating LTP-relevant signaling. LTP is interfered with by peptides or antibodies that block the extracellular interactions of integrins and adhesion molecules (Luthl et al., 1994; Ronn et al., 1995; Bahr et al., 1997; Staubli et al., 1998) and that inhibit the activity of ECM proteases: neuropsin [KLK8 (kallikrein-related peptidase 8)] (Komai et al., 2000; Tamura et al., 2006), tissue plasminogen activator (Frey et al., 1996; Huang et al., 1996a; Baranes et al., 1998), and matrix metalloproteases (for review, see Lendeckel and Hooper, 2005; Nagy et al., 2006). The proteases that interact with matrix/adhesion molecules might modify synaptic adhesion to modulate synaptic plasticity without any interference with basal synaptic transmission (Frey et al., 1996; Huang et al., 1996a; Baranes et al., 1998; Komai et al., 2000). We found that neuropsin is a significant modulator of Schaffer-collateral E-LTP and long-term depression acting in a dose-dependent manner (Komai et al., 2000; Tamura et al., 2006) and, in unbalanced conditions, causes abnormal plasticity and kindling epileptogenesis (Chen et al., 1995). The modulator function is attributable to (1) cleavage of a presynaptic cell adhesion molecule (CAM)-L1 in an NMDA receptor-dependent manner (Matsumoto-Miyai et al., 2003) and (2) the activity-based changes of neuropsin in postsynaptic signal transduction (Tamura et al., 2006). Considering these findings, neuropsin might be important in the cell biological process for the acquisition of memory depending on input strength. The potentials of extracellular modulation by neuropsin raise a question whether neuropsin induces an interaction between two independent synapses. Therefore, we investigated this issue by using neuropsin-deficient mouse and phase-specific stimulation in two separate inputs.
Materials and Methods
Hippocampal slice preparation.
All experiments were conducted with male C57BL/6J mice and neuropsin-deficient mice (age, 4–6 weeks). The production of neuropsin-deficient mice was described by Hirata et al. (2001). Mice were maintained according to the guidelines of the Nara Institute of Science and Technology, and the study was approved by the institutional animal care and use committee. Anesthetized animals were cardiac perfused with ice-cold artificial CSF (ACSF) for deblooding and cooling of the brain. They were decapitated, and their brains were removed and immersed in ice-cold (4°C) ACSF bubbled with a mixture of 95% O2 and 5% CO2. ACSF consisted of the following (in mm): 125 NaCl, 2.6 KCl, 1.3 MgSO47H2O, 1.24 KH2PO4, 26 NaHCO3, 2.4 CaCl2, and 10 d-glucose. The hippocampi were dissected free, and transverse slices (400 μm thickness) were cut on a slicer (LinearSlicer Pro7; Dosaka, Kyoto, Japan). Slices were allowed to recover at 28°C for at least 120 min before experiments commenced.
Electrophysiology.
Extracellular field EPSPs (fEPSPs) were recorded in the stratum radiatum of area CA1 with a glass microelectrode (Narishige, Tokyo, Japan) filled with ACSF (electrical resistances, 2–4 MΩ). Extracellular stimulation of the Schaffer-collateral pathway was accomplished with two nickel–chromium (Unique Medical, Tokyo, Japan) bipolar stimulating electrodes (diameter, 40 μm) placed on either side of a single recording electrode in the stratum radiatum. Evoked fEPSPs were amplified (ER-1; Cygnus, Delaware Water Gap, PA), digitized (DigiData 1200 Interface; Molecular Devices, Palo Alto, CA), and analyzed using the LTP program (Anderson and Collingridge, 2001). The test stimulus intensity was adjusted to produce “baseline” fEPSP sizes that were 40% of the maximal evoked fEPSP slope (SIU-91; Cygnus). Test stimuli were delivered once per minute (0.1 ms stimulus duration) to the Schaffer collaterals with 30 s separation between stimulations through the two electrodes (stimulating electrodes S0 and S1). To ensure that fEPSPs evoked through each stimulating electrode resulted from the activation of two independent synaptic pathways, we positioned the electrodes so that no paired-pulse facilitation (PPF) was evident after sequential activation of S0 and S1. The interpathway PPF was assessed at various time intervals (50 and 500 ms) before baseline acquisition. Basal transmission (input–output and PPF) was not significantly different between wild-type (WT) and knock-out (KO) mice (supplemental Fig. 1, available at www.jneurosci.org as supplemental material).
LTP was elicited by delivering the phase-specific potentiation protocol; weak stimulation (single or two tetanic trains; 1 s duration at 100 Hz; intertrain interval, 5 min) induced E-LTP; strong stimulation (four tetanic trains) induced L-LTP (Huang and Kandel, 1994; Huang et al., 1996b; Roberson et al., 1996).
Drugs.
Anisomycin (Sigma, Tokyo, Japan), a translation inhibitor, was added to ACSF to a final concentration of 20 μm. GRGDSP (H-Gly-Arg-Gly-Asp-Ser-Pro-OH; Calbiochem, La Jolla, CA) was added to a final concentration of 100 μm. Cytochalasin D (Sigma) was added to a final concentration of 0.5 μm. Nitrendipine (Sigma) was added to a final concentration of 5 μm. All drugs were bath applied. The final concentration of applied DMSO was 0.01%. At this concentration, baseline fEPSP slopes were not significantly affected (supplemental Fig. 2, available at www.jneurosci.org as supplemental material).
Statistical analysis.
The initial fEPSP slope was measured and expressed as a percentage of the averaged baseline. The latter was obtained by averaging 30 min of fEPSPs measured during baseline acquisition. Data are plotted as the mean ± SEM. Data sets were analyzed with the Tukey–Kramer multiple-comparisons test if ANOVA indicated a significant difference between groups (p < 0.05; denoted on graphs with an asterisk).
Results
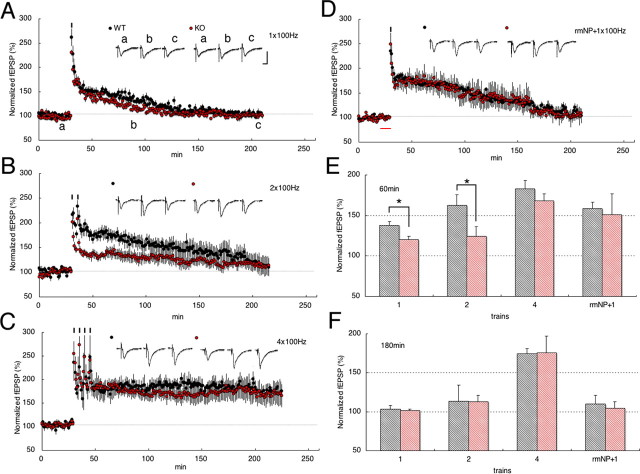
Previously, using behavioral and in vivo electrophysiological studies, we found that neuropsin KO mice were significantly impaired in hippocampus-dependent behavioral memory as analyzed with the Morris water maze and Y-maze and exhibit a significant decrease in E-LTP induced by a single titanic stimulus (Tamura et al., 2006). To confirm the result and to further analyze stimulation protocols in detail, we used in vitro electrophysiology of hippocampal slice sections, in which one, two, or four high-frequency tetanic stimuli were delivered into the Schaffer-collateral pathway (Fig. 1A–C). One or two stimuli evoked E-LTP significantly lower in KO (red circles) than WT (black circles) mouse slices (WT vs KO; 1 × 100 Hz, 137 ± 4%, n = 8 vs 119 ± 4%, n = 5; 2 × 100 Hz, 162 ± 12%, n = 6 vs 124 ± 11%, n = 7) (Fig. 1A,B,E). L-LTP was never induced by these weak stimuli both in WT and KO mouse slices (Fig. 1F) or by weak stimuli on slices with bath application of preactivated recombinant mouse (pro)neuropsin (rmNP) in slices (see below). In contrast, an equal level of E- and L-LTP was found in the strong tetanus (4 × 100 Hz) in slices from WT and KO mouse slices (WT vs KO; 174 ± 6%, n = 6 vs 175 ± 21%, n = 9) (Fig. 1C,F). Thus, neuropsin might be a potent modulator of E-LTP elicited by a weak (one or two) tetanic stimulus but not of L-LTP elicited by a strong (four) tetanic stimulus.
Figure 1.
E-LTP was decreased by neuropsin deficiency and reversed by application of rmNP. A, B, One 100 Hz train of stimulus (A) and two 100 Hz trains of stimulus (B) induced E-LTP in WT mice. E-LTP expression was significantly decreased in mice deficient in neuropsin (KO). C, Four 100 Hz trains of stimulus (strong) induced stable L-LTP both in WT and in KO mice. Note that L-LTP expression was not declined in KO. D, rmNP administration before a single train of stimulus (rmNP plus single100 Hz) in KO mice reversed E-LTP. The red bar indicates rmNP administration. E, Summary histogram showing E-LTP at 60 min after induction. E-LTP expression was significantly decreased by one 100 Hz and two 100 Hz trains in KO. F, Summary histogram showing L-LTP at 180 min after induction. Calibration: 1 mV, 10 ms. Asterisks indicate statistical significance (*p < 0.05). Error bars indicate SEM. Inset traces show sample fEPSPs recorded during the baseline (a) period and at 60 min (b) and 180 min (c) after stimulation. Black short vertical bars indicate tetanic trains.
Because endogenous proneuropsin (inactive) was converted into neuropsin (active) only a few minutes after tetanization, as shown in our previous study (Matsumoto-Miyai et al., 2003), we next checked whether a brief (10 min) bath application of rmNP (0.3 mU/ml) with a weak tetanic stimulus potentiates E-LTP in KO as well as in WT mouse slices. The rmNP with a 1 × 100 Hz tetanus in KO mouse slices was successfully reversed into equal levels of E-LTP in WT [KO+rmNP vs WT+rmNP; 150 ± 25%, n = 5 vs 158 ± 8%, n = 5 (Fig. 1D–F); compare with KO, 119 ± 4%, n = 5 (Fig. 1A)]. Because a brief bath application of rmNP reversed the altered E-LTP without any potentiation of L-LTP (Fig. 1E,F), we further analyzed the timing for the injection of rmNP and tetanus. Intriguingly, there was an efficacious time window for the brief application of rmNP and the stimulation. The rmNP washed out with ACSF was fully effective at potentiating E-LTP for 30 min, but the effect was eliminated when a single stimulus was injected after 60 min (data not shown). Thus, tissue slices, once exposed to rmNP (<30 min before the tetanus), might maintain the effect to potentiate E-LTP.
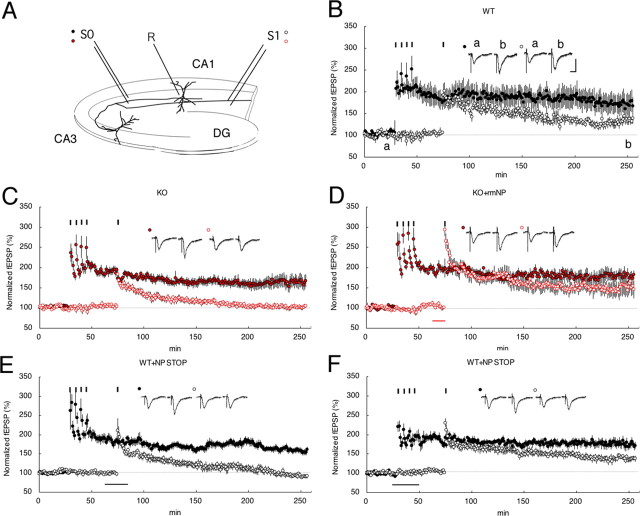
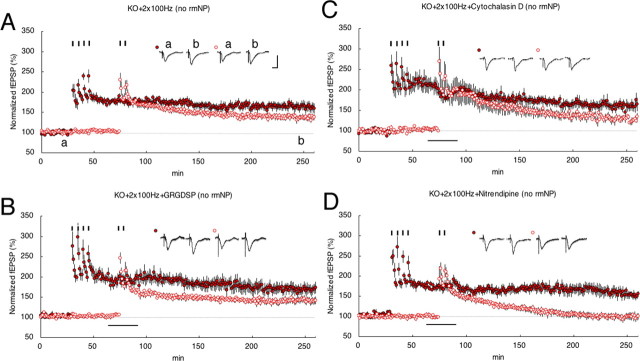
Phase-specific stimulation can evoke E- or L-LTP independently in two separate inputs (S0 and S1) for a CA1 pyramidal neuron (Fig. 2A) and was originally used to analyze the associativity of LTPs (Dunwiddie and Lynch, 1978; Frey and Morris, 1997). Using this protocol and KO mouse slices, we analyzed a neuropsin-dependent interaction between two separate synapses. When one pathway was given four tetanic stimuli (S0), S1 with a single tetanic stimulus, which normally produces only E-LTP, resulted in synaptic persistency, representing the association of two synapses (180 min after the tetanus: 136 ± 6% at S1, n = 7) (Fig. 2B). This phenomenon is referred to as “late associativity”; however, it completely disappeared in the KO mouse (180 min after the tetanus: 101 ± 4% at S1, n = 10) (Fig. 2, compare C, B). This impairment of late associativity was reversed by a brief (10 min) bath application of rmNP (180 min after the tetanus: 154 ± 22% at S1, n = 7) (Fig. 2D); ANOVA (WT vs KO vs KO+rmNP), F(2,10.29) = 11.07, p < 0.003) (see Fig. 5). To further confirm neuropsin-dependent late associativity, we examined it by bath application of NP STOP, a neuropsin-specific inhibitor, into the hippocampal slices from the WT mouse (Fig. 2E,F). Single stimulus in S1 during the application of NP STOP (1 μg/ml, 10 min) completely eliminated the late associativity between S0 and S1 synapses (Fig. 2E). In contrast, when a strong (four) stimulus in S0 was followed by the application of NP STOP (1 μg/ml), the late associativity between S0 and S1 synapses was not eliminated (Fig. 2F) (ANOVA, F(2,12.3) = 23.68, p < 0.0001). Thus, enzymatically active neuropsin might not participate in late associativity between S0 and S1 at the S0 where strong tetanus was injected, but might participate at the S1 site where single tetanus was injected.
Figure 2.
Synaptic late associativity impaired by neuropsin deficiency. A, Two independent inputs (S0 and S1) into the same population of postsynaptic neurons were alternatively stimulated. R, Recording; DG, dentate gyrus. B, A single train of 100 Hz tetanus to S1 after four trains of 100 Hz tetanus in S0 (filled circles) expressed stable L-LTP in S1 (open circles). C, In contrast, in KO, fEPSP values were impaired in S1 (red open circles) with 180 min posttetanus after four trains of 100 Hz tetanus in S0 (red filled circles). D, rmNP administration in KO reversed the induction of L-LTP in weak stimulated S1. The red bar indicates rmNP administration. E, F, The application of NP STOP was ineffective during strong stimuli (four trains of 100 Hz tetanus) at S0 (E) but effective during a single 100 Hz tetanus at S1 (F) in WT mice. The black bar indicates NP STOP administration. Calibration: 1 mV, 10 ms. Inset traces show sample fEPSPs recorded during the baseline (a) period and 180 min (b) after stimulation at S1. Black short vertical bars indicate tetanic trains.
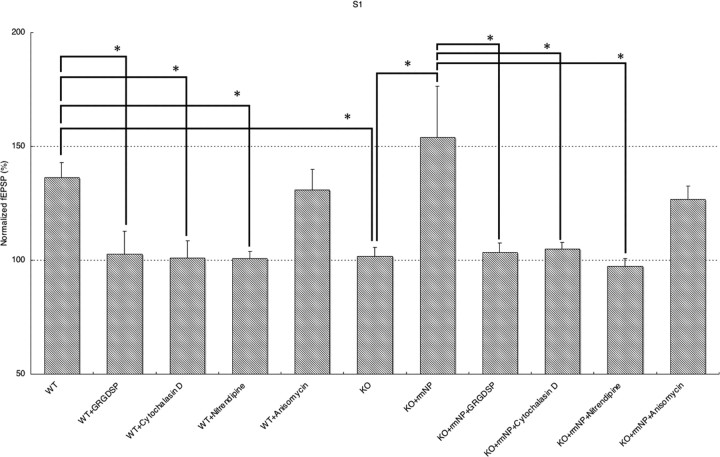
Figure 5.
Summary of L-LTP in stimulated S1 synapse after strong stimulation in S0 synapse. Mean fEPSP slopes 180 min after weak tetanus were presented. Single tetanus was paired with four trains of 100 Hz tetanus in S0 in WT, WT+GRGDSP, WT+cytochalasin D, WT+nitrendipine, and WT+anisomycin. Using KO mice coadministered with rmNP, single tetanus was paired with four trains of 100 Hz tetanus in S0 (KO, KO+rmNP, KO+rmNP+GRGDSP, KO+rmNP+cytochalasin D, KO+rmNP+nitrendipine, and KO+rmNP+anisomycin). KO mouse slices impaired single tetanus-induced synaptic association, which was reversed with rmNP administration. Late-association LTP was impaired by bath application of GRGDSP, cytochalasin D, and nitrendipine but not by bath application of anisomycin. Asterisks indicate statistical significance (*p < 0.05). Error bars indicate SEM.
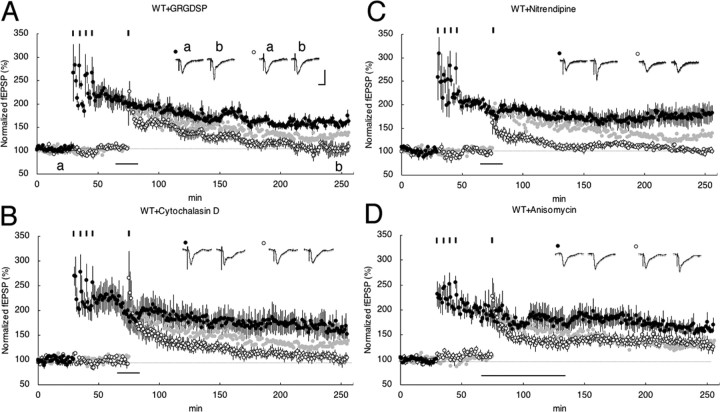
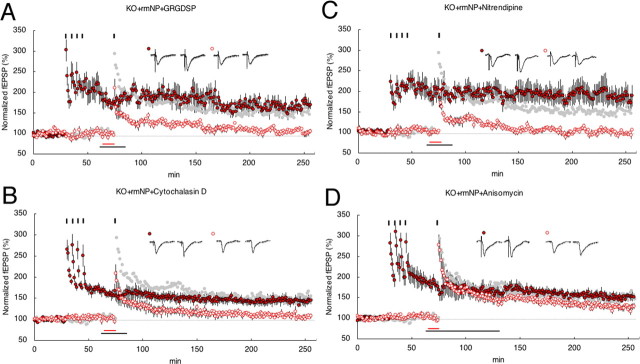
Neuropsin is an extracellular protease secreted from axon terminals (Oka et al., 2002b) as a potent endoprotease for fibronectin (Shimizu et al., 1998) and presynaptic CAM-L1 (Matsumoto-Miyai et al., 2003). Neuropsin might be important in the process of outside-in signaling of neurons (Tamura et al., 2006); however, the details of molecules involved in this process are not yet known. Therefore, we investigated one of the plausible candidates, integrin/actin polymerization, as a process of outside-in signaling for neuropsin-dependent synaptic late associativity. GRGDSP peptide is known to block the extracellular interactions of adhesion receptors belonging to a subclass of the integrin family, and cytochalasin D is known to prevent actin polymerization, and both drugs had a marked detrimental effect on the induction of E-LTP (Bahr et al., 1997; Kramar et al., 2002; Chan et al., 2003). To investigate whether integrin/actin signaling was involved in late associativity first, we examined by bath application of GRGDSP and cytochalasin D on WT mouse slices. Single tetanus on WT slices normally induced evident L-LTP in S1 (1 × 100 Hz) after four tetanus in S0 (4 × 100 Hz). Bath application of GRGDSP or cytochalasin D, followed by single tetanus in S1, completely impaired late associativity [180 min after single tetanus: WT+GRGDSP, 102 ± 10% at S1, n = 7 (Figs. 3A, 5); WT+cytochalasin D, 101 ± 7% at S1, n = 5 (Figs. 3B, 5)]. Considering the analogous profile of these experiments on impaired late associativity in KO mouse slices, neuropsin-dependent late associativity and integrin/actin signaling might share a common signaling pathway; therefore, we next investigated whether KO mouse slices supplemented with rmNP and tetanized S1 (1 × 100 Hz) after S0 (4 × 100 Hz) lose late associativity by the bath application of drugs. Here, if late associativity is not eliminated by drugs, outside-in signaling by neuropsin or integrin/actin is separated. Interestingly, we found that integrin/actin-dependent late associativity shares the neuropsin-dependent late associativity [180 min after single tetanus: KO+rmNP+GRGDSP, 103 ± 4% at S1, n = 6 (Figs. 4A, 5); KO+rmNP+cytochalasin D, 105 ± 3% at S1, n = 5 (Fig. 4B, 5)]; therefore, both neuropsin and integrin/actin signaling are part of the pathway leading to late associativity.
Figure 3.
Synaptic late-associativity signaling through integrin/actin into LVDCC. A, GRGDSP (100 μm) bath applied in WT slices inhibited the expression of stable L-LTP in S1 (open circles) after four trains of 100 Hz tetanus in S0 (filled circles). B, Cytochalasin D (0.5 μm) bath applied in WT slices inhibited the expression of stable L-LTP in S1 (open circles) after four trains of 100 Hz tetanus in S0 (filled circles). C, Nitrendipine (5 μm) bath applied in WT slices inhibited the expression of stable L-LTP in S1 (open circles) after four trains of 100 Hz tetanus in S0 (filled circles). D, Anisomycin (20 μm) in WT slices. The black bar indicates periods of drug administration. An fEPSP in the S0 pathway is shown by filled circles, and an fEPSP in the S1 pathway is shown by open circles. Calibration: 1 mV, 10 ms. The 100 Hz-captured LTP in the absence of inhibitors (taken from Fig. 2B) is depicted in light gray for comparison. Inset traces show sample fEPSPs recorded during the baseline (a) period and 180 min (b) after stimulation at S1. Black short vertical bars indicate tetanic trains, respectively.
Figure 4.
Neuropsin-dependent late associativity and its downstream signaling through integrin/actin into LVDCC. A, GRGDSP (100 μm) coadministration with rmNP in KO slices inhibited the expression of stable L-LTP in S1 (red open circles) after four trains of 100 Hz tetanus in S0 (red filled circles). B, Cytochalasin D (0.5 μm) coadministration with rmNP in KO slices inhibited the expression of stable L-LTP in S1 (red open circles) after four trains of 100 Hz tetanus in S0 (red filled circles). C, Nitrendipine (5 μm) coadministration with rmNP in KO slices inhibited the expression of stable L-LTP in S1 (red open circles) after four trains of 100 Hz tetanus in S0 (red filled circles). D, Anisomycin (20 μm) rmNP coadministration in KO slices. The black bar indicates periods of drug administration. The red bar indicates periods of rmNP administration. An fEPSP in the S0 pathway is shown by filled circles, and an fEPSP in the S1 pathway is shown by open circles. Calibration: 1 mV, 10 ms. The 100 Hz-captured LTP in the absence of inhibitors (taken from Fig. 2D) is depicted in light gray for comparison. Inset traces show sample fEPSPs recorded during the baseline (a) period and 180 min (b) after stimulation at S1. Black short vertical bars indicate tetanic trains, respectively.
Because LTP is known to be regulated by L-type voltage-dependent Ca2+ channel (LVDCC) (Grover and Teyler, 1990; Cavus and Teyler, 1996) activation as well as integrin/actin signaling, we therefore examined whether the LVDCC inhibitor (nitrendipine) prevents late associativity of LTPs in a similar manner as described above. Bath application of nitrendipine followed by a single tetanus treatment with nitrendipine, as shown in Figures 3C and 4C, effectively blocked synaptic late associativity both in WT and KO mouse slices supplemented with rmNP [180 min after tetanus: WT+nitrendipine, 100 ± 3% at S1, n = 6 (Figs. 3C, 5); 180 min after tetanus: KO+rmNP+nitrendipine, 97 ± 3% at S1, n = 6 (Fig. 4C, 5)].
These observations using drugs, GRGDSP peptide, actin polymerization inhibitor, and calcium channel blocker suggest that signals from neuropsin-dependent association pass through integrin/actin signaling and also converge into LVDCC signaling. Because both drugs completely canceled the late associativity of S0 and S1 provided by rmNP on KO slices, integrin/actin signaling and LVDCC are located downstream of the neuropsin-dependent signaling pathway (ANOVA (intergroup of WT), F(4,11.21) = 6.58, p < 0.0057; ANOVA(intergroup of KO), F(5,14.28) = 4.12, p < 0.016) (Fig. 5).
Because neuropsin, integrin/actin signaling, and LVDCC might contribute to late associativity, we examined whether persistency in a single titanic-stimulated S1 synapse is blocked by a protein synthesis inhibitor, anisomycin. After four stimuli were delivered to S0, S1 paired with a single tetanic stimulus in KO slices in the presence of rmNP did not accompany novel protein synthesis; therefore, in accord with the synaptic tagging hypothesis, it is interpreted that S1 synapses might not induce signaling for protein synthesis but capture synthesized and delivered proteins to S1 synapse [180 min after the tetanus: WT+anisomycin, 131 ± 9% at S1 (open circle), n = 5 (Figs. 3D, 5); 180 min after the tetanus: KO+rmNP+anisomycin, 127 ± 6% at S1 (red open circle), n = 5 (Figs. 4D, 5) (supplemental Fig. 3, available at www.jneurosci.org as supplemental material)]. The results also show that a weakly stimulated S1 synapse is labeled with tag to capture newly synthesized proteins that are essential for persistency. The tag formation might be controlled by virtue of neuropsin-dependent synaptic association signals.
Stronger (2 × 100 Hz) stimuli also evoke E-LTP similar to a single tetanus (Fig. 1B). When two tetanus were injected in S1 after tetanizing four in S0, late associativity was observed even in KO mouse slices (180 min after tetanus: KO+2 × 100 Hz, 137 ± 5% at S1, n = 11) (Fig. 6A). The results might represent the neuropsin-independent form of association mechanisms; therefore, we further analyzed the RGD peptide, actin polymerization inhibitor, and LVDCC blocker using KO mouse slices without applying rmNP. If drugs impair late associativity produced by two tetanus, neuropsin-independent late associativity also passes through the same signaling. Persistent LTP in KO slices in S1 appeared after two tetanus were not blocked by bath application of GRGDSP (180 min after tetanus: KO+2 × 100 Hz+GRGDSP, 140 ± 7% at S1, n = 7) (Fig. 6B) and cytochalasin D (180 min after tetanus: KO+2 × 100 Hz+cytochalasin D, 128 ± 5% at S1, n = 6) (Fig. 6C), and the result shows that signals from neuropsin-independent late associativity are not included in integrin/actin signaling. However, nitrendipine clearly blocked two tetanus-evoked late associativity (180 min after tetanus: KO+2 × 100 Hz+nitrendipine, 99 ± 7% at S1, n = 6; ANOVA, F(3,13.086) = 5.69, p < 0.02) (Fig. 6D); therefore, we concluded that two independent signaling pathways, neuropsin (and integrin/actin signaling)-dependent and neuropsin-independent late associativity, are present and both systems converge into the same signaling pathway of LVDCC.
Figure 6.
Neuropsin-independent late associativity was blocked by an LVDCC inhibitor. A, Two tetanus-induced tagging occurred after four trains of 100 Hz tetanus in S0 in KO slices. An fEPSP in S0 to which strong tetanus was applied (red filled circles) evoked stable L-LTP in S1-stimulated two tetanus (red open circles). B–D, Late associativity was not blocked by bath application of GRGDSP (B) and cytochalasin D (C) but was clearly blocked by nitrendipine (D). The black bar indicates the period of drug administration. Calibration: 1 mV, 10 ms. Inset traces show sample fEPSPs recorded during the baseline (a) period and 180 min (b) after stimulation at S1. Black short vertical bars indicate tetanic trains, respectively.
Discussion
Two distinct forms of tagging systems
Synaptic tagging is a concept that explains how a weakly stimulated synapse that normally never induces de novo protein synthesis can exhibit persistency in association with a separate strongly stimulated pathway. Because local protein synthesis is not observed in a weakly stimulated synapse, the proteins necessary for persistency should be transported from transcription and translation sites into all synaptic areas. This hypothesis was first introduced by Frey and Morris (1997), who thought that the synapse marked by a weak tetanic stimulus could capture newly synthesized synaptic proteins delivered to the same synapse. Also, a neuropsin-dependent signaling system that is specific for late associativity is engaged in local synaptic capture. This neuropsin-dependent late associativity is followed by integrin/actin polymerization and LVDCC signaling in which memory signaling presumably crosses over subcellular signaling.
The neuropsin-independent form induced by two high-frequency tetanus drives LVDCC. It is known that LVDCC-dependent LTP is evoked by high-frequency stimuli (100–200 Hz) as NMDA-independent LTP (Grover and Teyler, 1990; Cavus and Teyler, 1996) and is involved in the formation of longer-lasting memories, particularly based on stress-driven memory tasks such as food exploration in the radial maze under severe starvation (Borroni et al., 2000) and fear conditioning (Moosmang et al., 2005; McKinney and Murphy, 2006). Neuropsin KO mice were significantly impaired in spatial working memory without any deficit in spatial reference memory in the three-trial Y-maze task under nonstressful conditions of the hippocampus (Tamura et al. 2006) (Y. Ishikawa; Y. Horii, S. Shiosaka, unpublished observation). Spontaneous alternation behavior based on nonstressful or low-stressful Y-maze behavioral tasks is regarded as a measure of spatial working memory. Therefore, the neuropsin-dependent form of tagging might be critical for spatial working memory of nonaversive action of mice. In contrast, the neuropsin-independent form of tagging might be responsible for the stronger stimuli, such as acquisition and retention of spatial reference memory under severe stress-associated conditions, although additional studies are needed to further define this issue.
Neuropsin is related to synaptic morphological change and signaling late associativity
Perisynaptic proteolysis of the ECM and signal transduction triggered by a complex of neuropsin and serine protease inhibitors may be involved in synaptic morphological changes and synaptogenesis/maturation (Oka et al., 2002a; Nakamura et al., 2006). Matsuzaki et al. (2004) have shown, using two-photon photolysis of caged glutamate at single spines, that glutamate release induces rapid and selective enlargement of the stimulated spine. They argued that small spines are preferential sites for the induction of LTP, whereas larger spines represent physical traces of long-term memory (Matsuzaki et al., 2004). In parallel, neuropsin allows a maturational change of small CAM-L1-immunoreactive boutons, and this step may be important in synaptic plasticity based on activity-dependent structural change (Nakamura et al., 2006). Such dynamic synaptic changes might be involved in the early stages of LTP, including synaptic tagging, and might send outside-in signals to induce the activation of integrin and LVDCC as shown in the present study. The signals might further lead to the activation of c-Src and cAMP-dependent kinase, which are known to be involved in the regulation of LTP (Matsumoto-Miyai et al., 2003; Gui et al., 2006; Isiegas et al., 2006).
In conclusion, the present study revealed neuropsin-dependent and -independent late associativity. Neuropsin-dependent late associativity might serve in the acquisition of nonaversive memory (e.g., inquisitive behavior). Consistently, our unpublished analyses of human SNPs revealed a significant association of memory and verbal intelligence quotient with the human neuropsin gene in healthy subjects (Izumi et al., 2008).
Footnotes
This work was supported in part by a Grant-in-Aid for KAKENHI (17300118) from the Ministry of Education, Culture, Sports, Science, and Technology (Japan).
References
- Anderson WW, Collingridge GL. The LTP program: a data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J Neurosci Methods. 2001;108:71–83. doi: 10.1016/s0165-0270(01)00374-0. [DOI] [PubMed] [Google Scholar]
- Bahr BA, Staubli U, Xiao P, Chun D, Ji ZX, Esteban ET, Lynch G. Arg-Gly-Asp-Ser-selective adhesion and the stabilization of long-term potentiation: pharmacological studies and the characterization of a candidate matrix receptor. J Neurosci. 1997;17:1320–1329. doi: 10.1523/JNEUROSCI.17-04-01320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranes D, Lederfein D, Huang YY, Chen M, Bailey CH, Kandel ER. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998;21:813–825. doi: 10.1016/s0896-6273(00)80597-8. [DOI] [PubMed] [Google Scholar]
- Barco A, Alarcon JM, Kandel ER. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell. 2002;108:689–703. doi: 10.1016/s0092-8674(02)00657-8. [DOI] [PubMed] [Google Scholar]
- Barco A, Patterson S, Alarcon JM, Gromova P, Mata-Roig M, Morozov A, Kandel ER. Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron. 2005;48:123–137. doi: 10.1016/j.neuron.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol (Lond) 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni AM, Fichtenholtz H, Woodside BL, Teyler TJ. Role of voltage-dependent calcium channel long-term potentiation (LTP) and NMDA LTP in spatial memory. J Neurosci. 2000;20:9272–9276. doi: 10.1523/JNEUROSCI.20-24-09272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavus I, Teyler T. Two forms of long-term potentiation in area CA1 activate different signal transduction cascades. J Neurophysiol. 1996;76:3038–3047. doi: 10.1152/jn.1996.76.5.3038. [DOI] [PubMed] [Google Scholar]
- Chan CS, Weeber EJ, Kurup S, Sweatt JD, Davis RL. Integrin requirement for hippocampal synaptic plasticity and spatial memory. J Neurosci. 2003;23:7107–7116. doi: 10.1523/JNEUROSCI.23-18-07107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZL, Yoshida S, Kato K, Momota Y, Suzuki J, Tanaka T, Ito J, Nishino H, Aimoto S, Kiyama H, Shiosaka S. Expression and activity-dependent changes of a novel limbic-serine protease gene in the hippocampus. J Neurosci. 1995;15:5088–5097. doi: 10.1523/JNEUROSCI.15-07-05088.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T, Lynch G. Long-term potentiation and depression of synaptic responses in the rat hippocampus: localization and frequency dependency. J Physiol (Lond) 1978;276:353–367. doi: 10.1113/jphysiol.1978.sp012239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Weak before strong: dissociating synaptic tagging and plasticity-factor accounts of late-LTP. Neuropharmacology. 1998;37:545–552. doi: 10.1016/s0028-3908(98)00040-9. [DOI] [PubMed] [Google Scholar]
- Frey U, Muller M, Kuhl D. A different form of long-lasting potentiation revealed in tissue plasminogen activator mutant mice. J Neurosci. 1996;16:2057–2063. doi: 10.1523/JNEUROSCI.16-06-02057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover LM, Teyler TJ. Two components of long-term potentiation induced by different patterns of afferent activation. Nature. 1990;347:477–479. doi: 10.1038/347477a0. [DOI] [PubMed] [Google Scholar]
- Gui P, Wu X, Ling S, Stotz SC, Winkfein RJ, Wilson E, Davis GE, Braun AP, Zamponi GW, Davis MJ. Integrin receptor activation triggers converging regulation of Cav1.2 calcium channels by c-Src and protein kinase A pathways. J Biol Chem. 2006;281:14015–14025. doi: 10.1074/jbc.M600433200. [DOI] [PubMed] [Google Scholar]
- Hirata A, Yoshida S, Inoue N, Matsumoto-Miyai K, Ninomiya A, Taniguchi M, Matsuyama T, Kato K, Iizasa H, Kataoka Y, Yoshida N, Shiosaka S. Abnormalities of synapses and neurons in the hippocampus of neuropsin-deficient mice. Mol Cell Neurosci. 2001;17:600–610. doi: 10.1006/mcne.2000.0945. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learn Mem. 1994;1:74–82. [PubMed] [Google Scholar]
- Huang YY, Bach ME, Lipp HP, Zhuo M, Wolfer DP, Hawkins RD, Schoonjans L, Kandel ER, Godfraind JM, Mulligan R, Collen D, Carmeliet P. Mice lacking the gene encoding tissue-type plasminogen activator show a selective interference with late-phase long-term potentiation in both Schaffer collateral and mossy fiber pathways. Proc Natl Acad Sci USA. 1996a;93:8699–8704. doi: 10.1073/pnas.93.16.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Nguyen PV, Abel T, Kandel ER. Long-lasting forms of synaptic potentiation in the mammalian hippocampus. Learn Mem. 1996b;3:74–85. doi: 10.1101/lm.3.2-3.74. [DOI] [PubMed] [Google Scholar]
- Isiegas C, Park A, Kandel ER, Abel T. Transgenic inhibition of neuronal protein kinase A activity facilitates fear extinction. J Neurosci. 2006;26:12700–12707. doi: 10.1523/JNEUROSCI.2743-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi A, Iijima Y, Noguchi H, Numakawa T, Okada T, Hori H, Kato T, Tatsumi M, Kosuga A, Kamijima K, Asada T, Arima K, Saitoh O, Shiosaka S, Kunugi H. Genetic variations of the neuopsin gene and psychiatric disorders: polymorphism screening and possible association with bipolar disorder and cognitive functions. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.29. in press. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialog between genes and synapses. Biosci Rep. 2001;21:565–611. doi: 10.1023/a:1014775008533. [DOI] [PubMed] [Google Scholar]
- Komai S, Matsuyama T, Matsumoto K, Kato K, Kobayashi M, Imamura K, Yoshida S, Ugawa S, Shiosaka S. Neuropsin regulates an early phase of Schaffer-collateral long-term potentiation in the murine hippocampus. Eur J Neurosci. 2000;12:1479–1486. doi: 10.1046/j.1460-9568.2000.00035.x. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Bernard JA, Gall CM, Lynch G. Alpha3 integrin receptors contribute to the consolidation of long-term potentiation. Neuroscience. 2002;110:29–39. doi: 10.1016/s0306-4522(01)00540-1. [DOI] [PubMed] [Google Scholar]
- Krug M, Lossner B, Ott T. Anisomycin blocks the late phase of long-term potentiation in the dentate gyrus of freely moving rats. Brain Res Bull. 1984;13:39–42. doi: 10.1016/0361-9230(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Lendeckel U, Hooper N. New York: Springer Science and Business; 2005. Proteases in the brain. [Google Scholar]
- Luthl A, Laurent JP, Figurov A, Muller D, Schachner M. Hippocampal long-term potentiation and neural cell adhesion molecules L1 and NCAM. Nature. 1994;372:777–779. doi: 10.1038/372777a0. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation—a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Martin KC, Kosik KS. Synaptic tagging—who's it? Nat Rev Neurosci. 2002;3:813–820. doi: 10.1038/nrn942. [DOI] [PubMed] [Google Scholar]
- Matsumoto-Miyai K, Ninomiya A, Yamasaki H, Tamura H, Nakamura Y, Shiosaka S. NMDA-dependent proteolysis of presynaptic adhesion molecule L1 in the hippocampus by neuropsin. J Neurosci. 2003;23:7727–7736. doi: 10.1523/JNEUROSCI.23-21-07727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney BC, Murphy GG. The L-Type voltage-gated calcium channel Cav1.3 mediates consolidation, but not extinction, of contextually conditioned fear in mice. Learn Mem. 2006;13:584–589. doi: 10.1101/lm.279006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmang S, Haider N, Klugbauer N, Adelsberger H, Langwieser N, Muller J, Stiess M, Marais E, Schulla V, Lacinova L, Goebbels S, Nave KA, Storm DR, Hofmann F, Kleppisch T. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci. 2005;25:9883–9892. doi: 10.1523/JNEUROSCI.1531-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, Costa RM, Silva AJ, Kaczmarek L, Huntley GW. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26:1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Tamura H, Horinouchi K, Shiosaka S. Role of neuropsin in formation and maturation of Schaffer-collateral L1cam-immunoreactive synaptic boutons. J Cell Sci. 2006;119:1341–1349. doi: 10.1242/jcs.02862. [DOI] [PubMed] [Google Scholar]
- Oka T, Akisada M, Okabe A, Sakurai K, Shiosaka S, Kato K. Extracellular serine protease neuropsin (KLK8) modulates neurite outgrowth and fasciculation of mouse hippocampal neurons in culture. Neurosci Lett. 2002a;321:141–144. doi: 10.1016/s0304-3940(01)02470-3. [DOI] [PubMed] [Google Scholar]
- Oka T, Hakoshima T, Itakura M, Yamamori S, Takahashi M, Hashimoto Y, Shiosaka S, Kato K. Role of loop structures of neuropsin in the activity of serine protease and regulated secretion. J Biol Chem. 2002b;277:14724–14730. doi: 10.1074/jbc.M110725200. [DOI] [PubMed] [Google Scholar]
- Roberson ED, English JD, Sweatt JD. A biochemist's view of long-term potentiation. Learn Mem. 1996;3:1–24. doi: 10.1101/lm.3.1.1. [DOI] [PubMed] [Google Scholar]
- Ronn LC, Bock E, Linnemann D, Jahnsen H. NCAM-antibodies modulate induction of long-term potentiation in rat hippocampal CA1. Brain Res. 1995;677:145–151. doi: 10.1016/0006-8993(95)00147-i. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, Frey JU. Late-associativity, synaptic tagging, and the role of dopamine during LTP and LTD. Neurobiol Learn Mem. 2004;82:12–25. doi: 10.1016/j.nlm.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, Navakkode S, Sacktor TC, Frey JU. Synaptic tagging and cross-tagging: the role of protein kinase Mzeta in maintaining long-term potentiation but not long-term depression. J Neurosci. 2005;25:5750–5756. doi: 10.1523/JNEUROSCI.1104-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu C, Yoshida S, Shibata M, Kato K, Momota Y, Matsumoto K, Shiosaka T, Midorikawa R, Kamachi T, Kawabe A, Shiosaka S. Characterization of recombinant and brain neuropsin, a plasticity-related serine protease. J Biol Chem. 1998;273:11189–11196. doi: 10.1074/jbc.273.18.11189. [DOI] [PubMed] [Google Scholar]
- Staubli U, Chun D, Lynch G. Time-dependent reversal of long-term potentiation by an integrin antagonist. J Neurosci. 1998;18:3460–3469. doi: 10.1523/JNEUROSCI.18-09-03460.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura H, Ishikawa Y, Hino N, Maeda M, Yoshida S, Kaku S, Shiosaka S. Neuropsin is essential for early processes of memory acquisition and Schaffer collateral long-term potentiation in adult mouse hippocampus in vivo. J Physiol (Lond) 2006;570:541–551. doi: 10.1113/jphysiol.2005.098715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JZ, Nguyen PV. Homosynaptic and heterosynaptic inhibition of synaptic tagging and capture of long-term potentiation by previous synaptic activity. J Neurosci. 2005;25:7221–7231. doi: 10.1523/JNEUROSCI.0909-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]