Abstract
Despite their abundance, still little is known about the rather frequent, constantly proliferating progenitors spread throughout the adult mouse brain parenchyma. The majority of these progenitors express the basic-helix-loop-helix transcription factor Olig2, and their number further increases after injury. Here, we examine the progeny of this progenitor population by genetic fate mapping using tamoxifen-inducible Cre-recombination in the Olig2 locus to turn on permanent reporter gene expression in the adult brain. Consistent with Olig2 expression in proliferating NG2+ progenitors, most reporter+ cells seen shortly after initiating recombination at adult stages incorporated BrdU and contained the proteoglycan NG2 in both the gray (GM) and the white matter (WM) of the cerebral cortex. However, at longer time points after induction, we observed profound differences in the identity of reporter+ cells in the WM and GM. Whereas most of the Olig2+ progenitors had generated mature, myelinating oligodendrocytes in the WM, hardly any reporter+ cells showing mature oligodendrocyte characteristics were detectable even up to 6 months after recombination in the GM. In the GM, most reporter+ cells remained NG2+, even after injury, but stopped proliferating rather soon after recombination. Thus, our results demonstrate the continuous generation of mature, myelinating oligodendrocytes in the WM, whereas cells in the GM generated mostly postmitotic NG2+ glia.
Keywords: oligodendrocyte progenitors, NG2, myelination, stab wound injury, forebrain, lineage analysis
Introduction
Whereas progenitors in the adult neurogenic niches have attracted attention, still little is known about the fate of progenitors within the adult CNS parenchyma, despite their widespread nature (Horner and Gage, 2002). The vast majority of these dividing cells express markers found in oligodendrocyte precursors during development (NG2, PDGFRα, and/or Olig2) and have therefore been regarded as oligodendrocyte progenitors (Dawson et al., 2000; Miller, 2002; Ligon et al., 2006a). Indeed, after demyelination, remyelination occurs from dividing precursors (Gensert and Goldman, 1997; Chari and Blakemore, 2002), supporting the presence of oligodendrocyte progenitors in the adult white matter (WM). However, the progeny of these dividing cells in the absence of injury is not known. Indeed, few, if any, new oligodendrocytes were observed in the absence of a demyelinating injury after viral vector injection into the adult cortex WM (Gensert and Goldman, 1996; Menn et al., 2006). In addition, recent research has highlighted the distinct features of adult glial cells expressing the proteoglycan NG2. These cells possess unique physiological properties and are involved in neuron–glia communication (Butt et al., 2002; Matthias et al., 2003; Paukert and Bergles, 2006; Káradóttir et al., 2008). It is not known whether these represent a separate class of NG2+ cells that are different from the dividing NG2+ progenitors, and if so, whether their generation is restricted to developmental stages or continues into adulthood.
Although the progenitors characterized by Olig2 or NG2 are certainly involved in oligodendrogliogenesis during development, they have recently also been implicated in the generation of various other lineages during this time window. The transcription factor Olig2 is a necessary regulator of oligodendrocyte and motoneurons development, and Olig2-expressing cells also generate cholinergic neurons, ependymal cells, as well as some astrocytes during development (Takebayashi et al., 2002; Furusho et al., 2006; Ligon et al., 2006a; Masahira et al., 2006; Cai et al., 2007; Ono et al., 2008). Interestingly, Olig2 is also required for the specification of NG2+ cells (Ligon et al., 2006b). Similar to Olig2, NG2 is also expressed in progenitors of various lineages, including oligodendrocytes and astrocytes during development (Cai et al., 2007; Zhu et al., 2008; Ono et al., 2008), as well as multipotent progenitors in the postnatal brain (Belachew et al., 2003; Aguirre et al., 2004) and possibly even neuronal progenitors in the adult cerebral cortex (Dawson et al., 2000; Dayer et al., 2005; Tamura et al., 2007).
The multitude of lineages suggested for Olig2+ or NG2+ progenitors during development highlights the need for appropriate fate-mapping techniques to elucidate their lineage in the adult brain. This has now become possible by tamoxifen-inducible Cre-mediated recombination targeted to adult progenitors (Olig2::CreERTM) allowing to follow the progeny arising from Olig2+ progenitors in the adult brain.
Materials and Methods
Mice.
Heterozygous Olig2::CreERTM (Takebayashi et al., 2002) were crossed with the PLP-dsRed (Hirrlinger et al., 2005) or the reporter mice Z/EG (Novak et al., 2000) or R26R (Soriano, 1999) to obtain double-heterozygous animals. Double-heterozygous mice older than 2.5 months received tamoxifen orally (10 mg; dissolved at 40 mg/ml in a 1:9 ethanol/corn oil mixture) three times (once a day every second day for 5 d). Brains were collected at 8 (n = 8), 12 (n = 5), 35 (n = 10), 65 (n = 10) d or 6 months (n = 5) after the end of tamoxifen treatment. BrdU (Sigma-Aldrich) was supplied in the drinking water at a concentration of 1 mg/ml for 1 or 3 constitutive days, and animals were examined immediately after BrdU treatment. Five days after tamoxifen induction, some mice underwent a stab wound as described in Buffo et al. (2005). Briefly, mice received Rimadyl (4 mg/kg carprofen, s.c.) as analgesic treatment and were anesthetized by ketamine (100 mg/kg; ketavet; GE Healthcare) and xylacine (5 mg/kg; rompun; Bayer). After trepanation, a 1–1.5 mm long/1 mm deep stab wound was made using a curved scalpel in the right sensorimotor cortex. Brains were examined 3 (n = 8), 7 (n = 5), and 30 (n = 7) d after lesion. For BrdU/CC1 (n = 4) analysis, 3-month-old wild-type C57BL/6 animals were supplied for 2 weeks with BrdU in the drinking water and were killed 2 weeks after the treatment. For activated caspase3 (n = 9) analysis, 3- and 6-month-old C57BL/6 mice were used. All animal procedures were performed in accordance with the policies of the use of Animals and Humans in Neuroscience Research, revised and approved by the Society of Neuroscience and the state of Bavaria under license number 55.2-1-54-2531-23/04.
Histological analysis.
For histological analysis, animals were anesthetized and transcardially perfused with 4% paraformaldehyde. Brains were postfixed for 2–4 h, cryoprotected in 30% sucrose, and stained according to standard protocols. Immunohistochemistry was performed using antibodies described in Buffo et al. (2005) and Colak et al. (2008). For Olig2 immunostaining in Olig2::CreERTM mice, pretreatment with citrate buffer at 95°C and amplification with biotinylated secondary antibody was performed. The images were collected with an Olympus microscope (BX61TRF; Olympus) and confocal systems from Olympus (Fluoview F1000) or Zeiss (LSM710) and digital camera system (FluoviewII). For quantitative analysis, the number of reporter+ cells that were also immunopositive for a cell-type specific antigen was counted and expressed as a percentage of reporter+ cells. Quantification was focused on the sensorimotor cortex in 3–5 sections per animal, in at least 3 animals for each time point and area [gray matter (GM) vs WM or lesion]. Thus, >250 reporter+ cells per GM, >100 reporter+ cells per WM, and >50 cells in the lesion site were counted per animal. All results are presented as averages with SEM.
Results
Cells labeled by Olig2::CreERTM-mediated recombination are initially proliferating NG2+ progenitors
Before monitoring the progeny of Olig2-expressing progenitors, we determined the exact proportion of proliferating cells in the adult brain that express Olig2. Toward this aim, we labeled dividing cells by administering the DNA base analog on BrdU for 3 or 14 continuous days in the drinking water and doublestained the BrdU-incorporating cells for Olig2. In most parenchymal regions of the forebrain (neocortex, piriform cortex, corpus callosum, and putamen), virtually all BrdU-labeled cells were Olig2-immunoreactive (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). This was notably different in the regions of adult neurogenesis, such as the subgranular zone in the dentate gyrus, where only 13% of the proliferating cells were also Olig2+ (supplemental Fig. 1F, available at www.jneurosci.org as supplemental material). Thus, almost all adult progenitors outside the neurogenic niches express Olig2.
To follow the progeny of these Olig2+ dividing cells in the adult cerebral cortex, we used tamoxifen-inducible Cre-mediated recombination in the Olig2 locus (Olig2::CreERTM) of 2.5- to 6-month-old mice. Successful recombination was monitored by reporter gene expression in the brains of Olig2::CreERTM;Z/EG (GFP reporter) or Olig2::CreERTM;R26R (β-galactosidase reporter) mice 8, 12, 35, 65 d postrecombination (dpr) or 6 months postrecombination. As the results obtained with either reporter line were very similar, we pooled the data obtained with both lines. Although CreERTM has been knocked into the endogenous Olig2 locus, thereby reducing the endogenous levels of Olig2 to about half (data not shown), we attempted to monitor the expression of Olig2 in the reporter+ cells. Reporter+ cells were observed earliest 5 d after tamoxifen application, and the majority of these cells were also Olig2-immunoreactive (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). The reporter+ population amounted to ∼22% of the total Olig2-immunoreactive population with an average of 10.3 ± 1.4 recombined cells per mm2 in the GM and 9 ± 2 cells per mm2 in the WM (Table 1). Well consistent with the nature of Olig2-expresssing cells, the majority of these reporter+ cells at 8 dpr in the forebrain were NG2+ (Figs. 1A–C,J, 3A–C,J), whereas only a minor population was CC1+ oligodendrocytes (Figs. 1J, 3D–F,J; for Table 2, numbers of reporter+/CC1 double-positive cells). No reporter+ astrocytes were detectable in the cortical WM, whereas some were found in the GM (see Fig. 3G–I) as well as in other parenchymal forebrain regions (data not shown). Furthermore, consistent with the majority of recombination occurring in Olig2+/NG2+ double-positive progenitors, many reporter+ cells seen at 8 dpr had incorporated BrdU that was supplied for 3 d (59% in the GM and 46% in the WM) (Fig. 4) (see below). Notably, the proportion of reporter+ cells incorporating BrdU at 8 dpr appears to be higher than this proportion among the total Olig2-immunoreactive pool (data not shown), suggesting that the cells expressing the highest levels of CreERTM in the Olig2 locus, and hence mediating recombination most efficiently, are biased toward proliferation.
Table 1.
Absolute number of reporter cells per mm2 at different time points after recombination in the different analyzed areas
| Time after recombination (lesion) | GM | WM | Lesion |
|---|---|---|---|
| 8 d (3 d) | 10.3 ± 1.4 | 9 ± 2 | 22.5 ± 6 |
| 35 d (30 d) | 9.5 ± 0.9 | 13.8 ± 1.6 | 15 ± 2.7 |
| 65 d (60 d) | 9.9 ± 1.3 | 25.5 ± 1 | 14 |
| 6 months | 11.1 ± 1.5 | 24.5 ± 4.5 |
Time points in brackets refer to the time after lesion.
Figure 1.
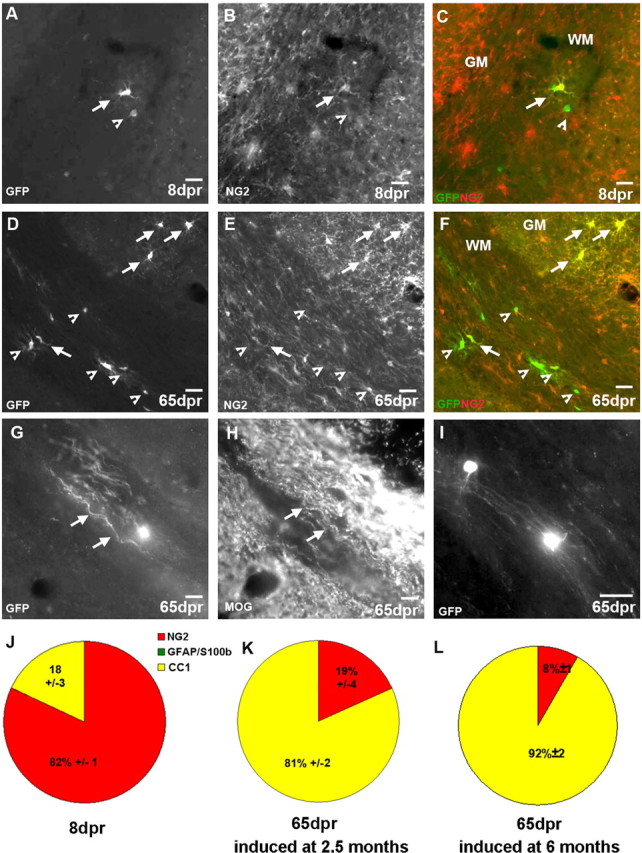
Identification of reporter+ cells in the WM. A–I, Micrographs of examples of GFP+ cells 8 (A–C) or 65 (D–I) dpr double-stained for cell type-specific antigens. The pies in J and K depict the quantitative cell type analysis among reporter+ cells 8 (J) or 65 (K) dpr recombined in 2.5-month-old animals. L, Cell type analysis at 65 dpr in mice recombined at 6 months of age. Arrows point to double-stained cells; arrowheads point to single positive cells. Scale bars, 20 μm.
Figure 3.
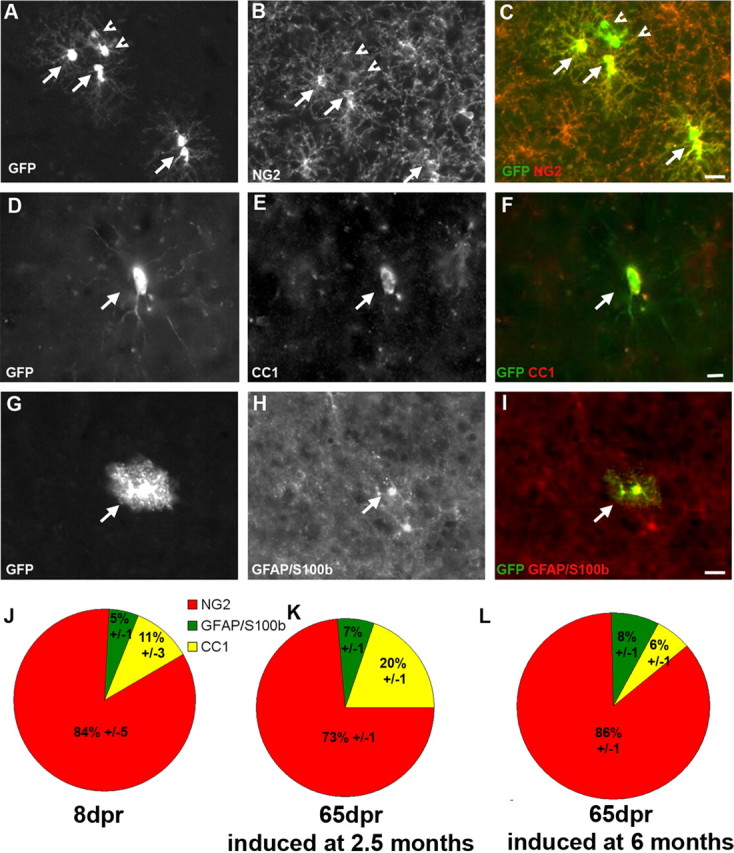
Identification of reporter+ cells in the GM. A–I, Micrographs of GFP+ cells at 8 dpr. Arrows point to colabeled cells, arrowheads to reporter+ cells negative for the cell type-specific antigen. Pies in J and K depict the quantitative cell type analysis of reporter+ cells (J, 8 dpr; K, 65 dpr) recombined in 2.5-month-old animals. L, Cell type analysis at 65 dpr in mice recombined at 6 months of age. Scale bars, 20 μm.
Table 2.
Absolute number of reporter+/CC1+ double-positive cells per mm2 (for GM and WM) and number of cells per lesion area (∼0.4 mm2 for lesion quantification) at different time points after recombination in the different analyzed areas
| Time after recombination (lesion) | GM | WM | Lesion |
|---|---|---|---|
| 8 d (3 d) | 1.1 ± 0.04 | 1.6 ± 0.06 | 3.2 ± 0.1 |
| 65 d (60 d) | 1.98 ± 0.01 | 20.7 ± 0.02 | 3.78 ± 0.2 |
| 6 months | 1.99 ± 0.06 | 20.3 ± 0.1 |
Time points in brackets refer to the time after lesion.
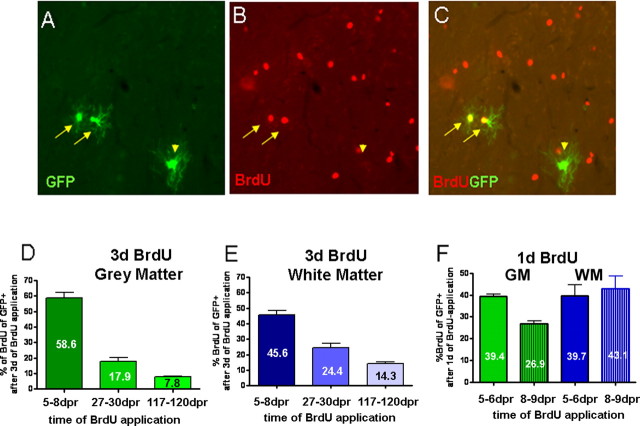
Figure 4.
A–C, Identification of proliferating reporter+ cells at 8 dpr. D–F, Graphs in D and E show the proportion of recombined cells that are proliferating at 8, 30, and 120 dpr in the gray (D) and white matter (E) and at 6 and 9 dpr in both areas (F). BrdU was applied for a period of 3 d (D, E) or 1 d (F) in the drinking water, directly before analysis of the animals.
Cells labeled by Olig2::CreERTM-mediated recombination generate mature oligodendrocytes in the WM of the adult brain
To determine the further fate of these progenitors, we examined reporter+ cells at later stages, from 35 d up to 6 months after recombination. In the WM, the composition of reporter+ cells had changed profoundly at these later stages (compare Fig. 1J,K). The proportion of NG2+ per reporter+ cells was reduced to 19%, whereas the majority of recombined cells at 65 dpr expressed the mature oligodendrocyte marker CC1 (Fig. 1D–F,K). Thus, the proportion of GFP+ per CC1+ cells increased by 4.5-fold from 18 to 82% (p < 0.001). Notably, also the total number of recombined cells increased (Table 1), suggesting that the relative increase in CC1+ cells is unlikely to result from selective cell death. Indeed, the total number of reporter+ cells double-labeled with CC1 also increased by almost 13-fold in the WM (Table 2).
Interestingly, the absolute number of reporter+ cells increased in the WM only during the first 65 dpr (from 9/mm2 at 8 dpr over 13.8/mm2 at 35 dpr to 25.5/mm2 at 65 dpr) and remained constant thereafter until 6 months after recombination (24.5/mm2) (Table 1). Indeed, this is also consistent with the decrease in the proportion of reporter+ cells expressing Olig2 or incorporating BrdU (supplemental Fig. 2B, available at www.jneurosci.org as supplemental material; see Fig. 4E), suggesting that most labeled dividing progenitors in the WM leave the cell cycle within a few weeks and are mostly differentiated by 2 months after recombination. Only a small proportion of reporter+ cells (14%), however, appears to continue to proliferate over the course of several months (see Fig. 4E). Nevertheless, no further increase in the number of reporter+/CC1+ double-positive cells or in the proportion of CC1+ cells could be observed within 2–6 months after recombination (Tables 1, 2; compare Fig. 1K with supplemental Fig. 4A, available at www.jneurosci.org as supplemental material). As the same number and high proportion of CC1+ cells among reporter+ cells was still seen at 6 months after recombination (supplemental Fig. 4A, available at www.jneurosci.org as supplemental material), these cells seem to survive for some time, implying that they may further differentiate into myelinating oligodendrocytes, a hypothesis prompted by the intriguing morphology of many reporter+ cells in the WM resembling myelinating oligodendrocytes (Fig. 1G,I).
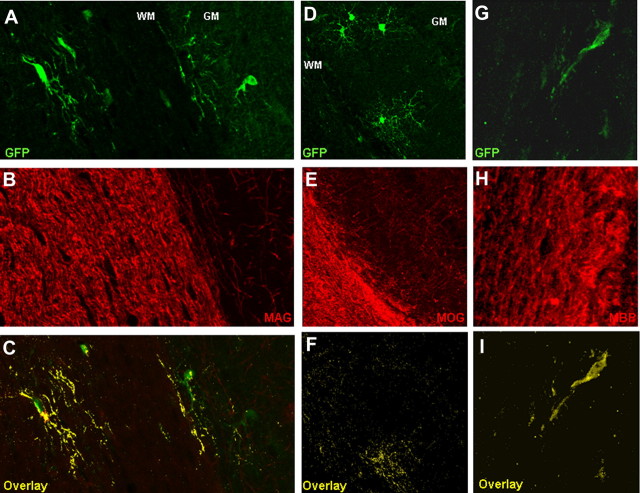
We therefore used several antibodies directed against different myelin proteins, such as the myelin oligodendrocyte glycoprotein (MOG) (Figs. 1G,H, 2D–F), the myelin associated glycoprotein (MAG) (Fig. 2A–C), and the myelin basic protein (MBP) (Fig. 2G–I) as these proteins are targeted into oligodendrocyte processes when myelin sheaths can be detected by electron microscopy (Bartsch et al., 1989; Lindner et al., 2008; Shen et al., 2008). Specifically, MAG localization to processes is correlated to the presence of at least one and a half myelin wrappings around the axons (Bartsch et al., 1989), and the reappearance of MOG after demyelination is detectable only after remyelination is apparent at the electron-microscopic level (Lindner et al., 2008). Notably, all of these proteins could be colocalized in the elongated processes of reporter+ cells in the WM, highly suggestive of their myelinating identity. Furthermore, we could observe spots immunoreactive for the paranodal marker Caspr in reporter+ processes, indicative for some degree of nodal specification in the newly generated oligodendrocytes (supplemental Fig. 3, available at www.jneurosci.org as supplemental material).
Figure 2.
A–I, Confocal microscope images showing the colabeling of reporter+ cells and myelin sheaths with the myelin markers MAG (A–C), MOG (D–F), and MBP (G–I) in the white matter (corpus callosum) at 65 dpr. C, F, I, Colocalizing pixels are in yellow.
As these proteins are mainly targeted to the processes of reporter+ cells, their colocalization cannot be used to quantify the number or proportion of myelinating oligodendrocytes among reporter+ cells. Toward this end, we crossed the Olig2::CreERTM;Z/EG mice to PLP-dsRed animals (Hirrlinger et al., 2005), visualizing the expression from a myelin protein promoter by cytoplasmic localization of fluorescent protein (supplemental Fig. 5A–C, available at www.jneurosci.org as supplemental material). Consistent with the predominant progenitor identity of initially recombined cells, only 6% of the reporter+ cells were expressing the PLP-transgene in the GM and the WM at 8 dpr (supplemental Fig. 5D, available at www.jneurosci.org as supplemental material). In pronounced contrast, however, 60% of reporter+ cells in the WM were colocalizing with the PLP-transgene at 65 dpr (supplemental Fig. 5D, available at www.jneurosci.org as supplemental material), strongly supporting the synthesis of myelin proteins by the progeny of Olig2+ progenitors in the WM. Thus, most (3 of 4) of the CC1+/reporter+ double-positive cells in the WM also express PLP, suggesting that more than half of the progeny of recombined Olig2+ cells in the adult WM differentiated into myelinating oligodendrocytes. These newly generated myelin-forming cells were not restricted to a specific region but rather wide spread throughout the corpus callosum at all time points analyzed. As a similar change in the composition of reporter+ cells was observed also when recombination was induced in 6-month-old mice (Fig. 1L), we conclude that Olig2+ progenitors in the WM generate mature, myelinating oligodendrocytes throughout this period.
Cells labeled by Olig2::CreERTM-mediated recombination generate mainly postmitotic NG2+ glia in the GM of the adult brain
Strikingly and in pronounced contrast to the WM, the identity of recombined cells in the GM hardly changed over time with the NG2+ cells still predominating at 65 dpr (Fig. 3K) and even 6 months after recombination (supplemental Fig. 4B, available at www.jneurosci.org as supplemental material). The same predominance of NG2+ cells was also observed when older animals (6 months) were induced with TM and examined 65 d later (Fig. 3L). In both cases, we observed little changes in the proportion of reporter+ astrocytes (5% initially, 7 or 6% at 65 dpr, and 11% at 6 months after recombination) (Fig. 3J–L; supplemental Fig. 4B, available at www.jneurosci.org as supplemental material). Similarly, only a small, nonsignificant (p = 0.123) increase in the proportion of reporter+ cells colabeling with CC1 was observed in the GM (11% initially, 20% at 65 dpr, and 18% at 6 months after recombination) (Fig. 3J,K; supplemental Fig. 4B, available at www.jneurosci.org as supplemental material). Consistently, a similarly low proportion of reporter+ cells in the GM became PLP+ (6% at 8 dpr increasing to 11% at 65 dpr) (supplemental Fig. 5, available at www.jneurosci.org as supplemental material). Moreover, reporter+ cells did not contain other myelin proteins (MOG, MAG, or MBP), and we could not observe recombined cells with a typical morphology of myelinating oligodendrocytes, even 6 months after TM application. Also, no reporter+ cells were double-labeled with neuron-specific antigens (doublecortin or neuronal-specific nuclear protein; n = 2125), even in the olfactory bulb and dentate gyrus (supplemental Fig. 6, available at www.jneurosci.org as supplemental material), suggesting that cells labeled by recombination mediated by CreERTM expressed in the Olig2 locus do not contribute to adult neurogenesis. Notably, in a different reporter line, we observed some reporter+ cells with neuronal morphology in the ventral thalamus already at 5 dpr, implying low-level Olig2 expression in some neurons of the adult brain.
As the composition of reporter+ cells in the lines used here (Z/EG, Rosa) did not change over time in the GM (even 6 months after recombination) (supplemental Fig. 4B, available at www.jneurosci.org as supplemental material), we examined whether the predominant NG2+ cells were still proliferating progenitors. When BrdU was given for 3 d at different time points after recombination, also in the GM, the proportion of BrdU-incorporating reporter+ cells decreased over time to 18% at 27–30 dpr and furthermore to 8% at 117–120 dpr (Fig. 4). Thus, the overall decrease in proliferation is similar between GM and WM, with initially a majority of BrdU-incorporating cells giving way to cells that no longer incorporate BrdU and hence are likely to have either left the cell cycle or divide too slowly to be labeled by BrdU application for 3 d. This analysis also highlights the different nature of the reporter+/NG2+ double-positive cells most of which are BrdU-incorporating at short times after recombination but gradually lose this property within a few weeks. Thus, the main progeny of Olig2+ progenitors in the GM is a slow or nonproliferating type of NG2+ glia.
The proliferation analysis of reporter+ cells in the GM also provided further insights. Similar to the observations in the WM, also in the GM, a small proportion of reporter+ cells (8%) continues to divide even up to 4 months after recombination, suggesting that Olig2+ cells also comprise a long-term self-renewing progenitor population. However, this population was much smaller, almost half of the one in the WM (8 vs 14%) (Fig. 4). Indeed, we noted a faster decrease in the proportion of reporter+ cells incorporating BrdU in the GM, compared with the WM (Fig. 4D–F), starting already shortly after recombination (8–9 dpr) (Fig. 4F). Comparing the proportion of reporter+ cells incorporating BrdU given for either 1 or 3 d also revealed an interesting difference in the speed of cell proliferation between GM and WM reporter+ cells. Although after 1 d BrdU administration at 5–6 dpr the proportion of BrdU+ per reporter+ cells was the same in the GM and WM, fewer reporter+ cells were labeled by 1 d BrdU application at 8–9 dpr in the GM compared with those in the WM (Fig. 4F). However, when BrdU was given for 3 d a larger proportion of reporter+ cells in the GM was labeled at 5–8 dpr, suggesting that GM Olig2+ progenitors slow their cell cycle speed rather faster (see also Fig. 4F, decrease in cells labeled with 1 d BrdU at 5–6 and 8–9 dpr) than WM reporter+ cells (Fig. 4F, no decrease in cells labeled with 1 d BrdU at 5–6 and 8–9 dpr). After further maturation, the proportion of cells labeled by 3 d BrdU application also declines faster among GM compared with WM reporter+ cells reaching approximately half at 120 dpr (Fig. 4).
At least partially consistent with a slower proliferation of GM progenitors, the reporter+ GM population hardly increased at all (∼10% increase over 6 months) (Table 1). As such a profile could also be caused by cell death, we performed stainings for activated Caspase3 at 35 and 65 dpr, i.e., in the time interval when the number of reporter+ cells increased most in the WM but failed to do so in the GM (Table 1). However, we could not detect any activated Caspase3+/reporter+ double-positive cells, either in the GM or in the WM. Conversely, many activated Caspase3-immunoreactive reporter+ cells could be observed after stab wound injury (see below), indicating that the staining works reliably. As reporter+ cells only label a small fraction of all Olig2+ cells, we quantified the total number of activated Caspase3+ cells in the GM and the WM based on the rationale that selective cell death in the GM should be detectable in these quantifications. However, quantification in nine animals at the age of 2.5 and 6 months revealed no differences in the number of activated Caspase3+ cells between the GM (0.015 ± 0.005/mm2) and the WM (0.034 ± 0.023/mm2). Thus, the intriguing difference in the progeny of Olig2+ progenitors in the GM and WM seems to be rather caused by a different fate and proliferation behavior than by selective cell death.
Cells labeled by Olig2::CreERTM-mediated recombination still generate mostly NG2+ glia after stab wound injury in the GM
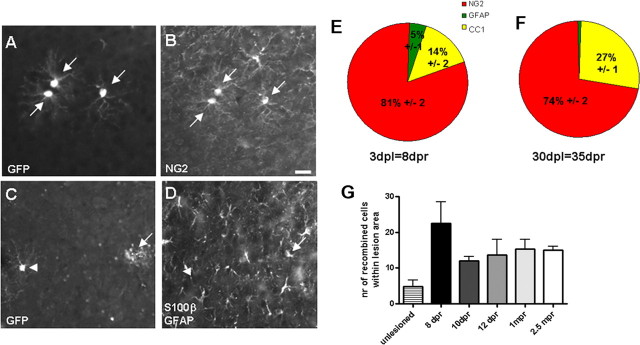
To unravel whether GM progenitors have the potential to generate new oligodendrocytes after injury, when some of the previously existing oligodendrocytes degenerate, we performed stab wound lesions as described previously (Buffo et al., 2005) at 5 dpr and analyzed the recombined cells at 3, 5, 7, 30, and 60 d postlesion (dpl), corresponding to 8, 10, 12, 35, and 65 dpr, respectively (Fig. 5). Consistent with our previous data that Olig2+ cells increase in number after injury (Buffo et al., 2005), here we also observed an increase in the number of reporter+ cells around the lesion site to an average of 22.5 ± 6 cells at 3 dpl (Fig. 5G, 4.7× increase compared with contralateral side or control cortices). However, some of these cells are still subject to cell death, as the number of reporter+ cells decreased between 3 and 5 dpl (Table 1) and plenty of activated Caspase3+/reporter+ double-positive cells were observed in the lesion site at this stage (6% ±3 reporter+ cells per lesion area). Despite these dynamic changes, however, the cell type composition of reporter+ cells at 3 or 30 dpl (Fig. 5E,F) did not change and closely resembled the composition in the noninjured cortex (Fig. 3J,K) with most reporter+ cells colabeling with NG2+ (Fig. 5A,B), some with CC1, few with S100β/GFAP (Fig. 5C,D), and none with neuroblast or neuronal antigens (n = 569). Similar to the unlesioned GM, the proportion and number of CC1+ per reporter+ cells did not significantly increase (Fig. 5, Table 2) (p = 0.5476), and no signs of reporter+ cells expressing myelin proteins or exhibiting a morphology indicative of myelinating oligodendrocytes could be observed after stab wound injury. Thus, even after injury, the main progeny of Olig2-expressing progenitors remained NG2+ glia in the GM, profoundly different from the maturating oligodendrocytes observed in the WM.
Figure 5.
Identification of reporter+ cells after stab wound injury in the GM. A–D, Micrographs of GFP+ cells in the lesion site at 3 dpl (8 dpr) double-stained for cell type-specific antigens as indicated in the panels. Pies in E and F depict the quantitative analysis of reporter+ cells with regard to cell identity (E, 3 dpl; F, 30 dpl). Quantification of absolute numbers of recombined cells at different time points after the stab wound within the lesion area localized 150 μm away from the lesion track is shown in G. Scale bar, 20 μm.
Progeny of BrdU-labeled cells in GM and WM
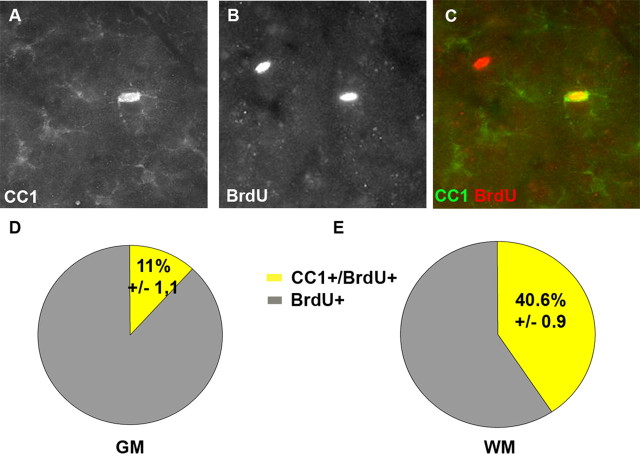
Given the surprising difference observed in the progeny of Olig2+ cells in the GM and WM, we aimed to confirm this finding with an independent technique. In particular, it has to be kept in mind that the fate mapping experiments are performed on a heterozygous Olig2 background as one allele does not express Olig2 but rather CreERTM (knock-in into the Olig2-locus) (Takebayashi et al., 2002). It is therefore conceivable that the lower dose of Olig2 may impair the full oligodendrocyte differentiation in the GM. Moreover, the recombination labels only a certain proportion of Olig2+ cells (as mentioned above) that may perform differently from the total population of Olig2+ cells. To ensure that the differences in progeny observed between the WM and GM may not be because of the low endogenous Olig2 levels or the nature of the labeled subtypes, we took advantage of the fact that virtually all proliferating, BrdU-incorporating cells express Olig2 in the adult brain parenchyma. We thus analyzed the progeny of all BrdU-labeled cells in wild-type animals 4 weeks after the onset of BrdU treatment. Whereas only 12% of the BrdU+ cells acquired CC1-immunoreactivity in the GM, this proportion was profoundly higher in the WM with 41% (Fig. 6). Thus, within a month we could identify a 3.4× higher proportion of reporter+ cells differentiating into CC1+ oligodendrocytes in the WM compared with the GM, thereby confirming the results obtained by fate mapping.
Figure 6.
A–C, Colabeling of BrdU with CC1 in the GM. Pies in D and E show the proportion of BrdU+ cells colabeling with CC1 (yellow) in the GM (D) and in the WM (E) after application of BrdU in adult wild-type mice.
Discussion
Progeny of Olig2-expressing cells in the adult versus developing brain
Consistent with the concept of so-called “adult oligodendrocyte progenitors” (Goldman, 2003), the Olig2+ progenitors labeled by Cre-mediated recombination in the adult brain generated mainly NG2+ glia or mature oligodendrocytes, but hardly any astrocytes and no neurons. This is in pronounced contrast to the multitude of lineages observed to arise from this progenitor pool during development using even the very same mouse line (Furusho et al., 2006; Masahira et al., 2006; Miyoshi et al., 2007; Ono et al., 2008) or the NG2::CreERTMT2 line (Zhu et al., 2008). Importantly, during development, the different cell types arise from distinct sets of Olig2+ progenitors (e.g., Olig2+ cells that generate motoneurons do not share their lineage with those that generate oligodendrocytes) (Mukouyama et al., 2006; Wu et al., 2006). Similarly, Mash1-expressing progenitors that generate neurons in the embryonic spinal cord do not share their lineage with those generating oligodendrocytes at later stages (Battiste et al., 2007). Similar to the Olig2-derived lineage, Mash1+ progenitors in the adult also generate virtually exclusively cells of the oligodendrocyte fate with the exception that they continue to contribute to adult olfactory bulb and dentate gyrus interneuron generation (Kim et al., 2007). In contrast, Olig2-CreERTM-labeled progenitors contribute to this lineage only during development (Miyoshi et al., 2007) but no longer in the adulthood. Thus, the change in progeny of both Mash1- and Olig2-expressing progenitors over time seems to be caused by the selective persistence of only some of the lineages present at previous stages into adulthood.
Mechanisms mediating distinct progeny of Olig2+ progenitors
This raises the suggestion that also the different progeny in the WM and GM may be attributable to distinct lineages persisting into adulthood. The difference in the progeny of proliferating Olig2+ cells in the GM and WM of the adult cerebral cortex has been shown by both BrdU-labeling as well as Olig2::CreERTM-mediated fate mapping. Also in this regard, our data are further corroborated by Mash1-CreERT2-mediated fate mapping showing also a higher proportion of CC1+ oligodendrocytes in the WM compared with the GM (Kim et al., 2007). In addition to the possibility of intrinsic lineage differences of these progenitors in the GM and WM, differences in the local environment may be responsible. The previous suggestion that the local GM environment arrests oligodendrocyte differentiation at an immature state (Dawson et al., 2000; Levine et al., 2001) is indeed consistent with several of our observations. First, the GM reporter+ cells mostly maintain Olig2 (and NG2) expression, in contrast to those in the WM that downregulate Olig2 (and NG2) during differentiation into oligodendrocytes (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Second, Olig2+ progenitors proliferate slower in the GM compared with the WM and may even be subject to cell cycle arrest (see below). Third, even the few oligodendrocytes generated in the GM fail to acquire any further mature properties resembling myelinating oligodendrocytes in their morphology or expressing myelin proteins. It is therefore well conceivable that the Olig2-derived cells are arrested in an “immature,” although postmitotic state. These NG2+ glial cells could then perform specific physiological functions (Butt et al., 2002; Greenwood and Butt, 2003; Paukert and Bergles, 2006; Nishiyama, 2007; Káradóttir et al., 2008), although it is not clear whether NG2+ progenitors also exhibit these properties. If it is the environment that inhibits the differentiation of mature oligodendrocytes in the GM, this inhibition is also not overcome by stab wound injury. It will be important, however, to further examine the fate of Olig2+ progenitors after demyelinating injuries where different local signaling pathways may be active.
A further difference between the progeny of Olig2+ cells in the GM and WM was their population size, with reporter+ cells increasing during the first 2 months after recombination in the WM, but remaining rather constant in the GM. Only after injury the total number of reporter+ cells in the GM also increased, despite a transient phase of cell death. The differences in the number of reporter+ cells in GM and WM without injury may result from differences in cell death, cell proliferation, or cell migration. Although staining for activated Caspase3 in the intact cortex provided no evidence for selective cell death in the GM compared with WM, it is possible that we missed a short window of cell death. However, such a mechanism would not explain the difference in “surviving” NG2+ cells in the GM that also change their proliferation mode over time. In contrast, we could obtain direct evidence for different cell cycle kinetics that may contribute to the increase in number of reporter+ cells in the WM as opposed to the GM. A third possibility to explain the difference in the number and identity of reporter+ cells (in the WM and GM) may also be that cells recombined in the GM and differentiating into oligodendrocytes migrate into the WM. Whichever the exact mechanism may be, it may also be relevant for the impaired remyelination observed in the GM after demyelinating injuries (Levine et al., 2001).
Adult generation of mature oligodendrocytes in the WM
In pronounced difference to the predominance of an NG2+ progeny of Olig2+ progenitors in the GM, mature oligodendrocytes with the myelin proteins MAG, MOG, and MBP in their reporter+ processes were seen in the WM. Although the generation of new myelinating oligodendrocytes has been frequently observed after demyelination (Gensert and Goldman, 1997; Chari and Blakemore, 2002), few newly generated oligodendrocytes were seen after viral vector injections into the intact WM (Gensert and Goldman, 1996; Menn et al., 2006). Our results now suggest that a considerable population of dividing Olig2-expressing progenitors in the intact WM give rise to mature oligodendrocytes. Viral vector tropism missing this progenitor population or partial silencing of virally driven reporters in specific cell types (Gaiano et al., 1999) may explain why this population has been missed in previous work. Moreover, previous studies have been performed in rat (Gensert and Goldman, 1996, 1997) or different strains of inbred mice (Menn et al., 2006). Given that different species and strains of mice show a high number of differentially expressed genes involved in myelination (Dimou et al., 2006), some of these differences may also be relevant with regard to the differentiation of oligodendrocytes. A third explanation for this discrepancy between previous data and our results is the rather focal labeling performed by viral vector injections as opposed to the more widespread labeling by Olig2::CreERTM-mediated recombination or BrdU incorporation. If differentiating oligodendrocytes migrate from other regions toward the WM as discussed above, this may explain why fewer cells are labeled by focal injection into the WM. In addition to the suggestion proposed above that oligodendrocyte progenitors may migrate from the GM into the WM, it has been shown that a small proportion of progenitors originating in the adult subependymal zone give rise to oligodendrocytes in the cortical WM (Menn et al., 2006; Colak et al., 2008).
Whatever their origin might be, the observation of continuous generation of myelinating oligodendrocytes in the adult cortical WM raises intriguing questions about the identity of the axons that are newly myelinated. One possibility may be a turnover of adult oligodendrocytes such that the newly generated oligodendrocytes replace older ones. Indeed, this is the case for some specific neuronal populations, such as those in the olfactory bulb and dentate gyrus that are subject to constant turnover (Lagace et al., 2007; Ninkovic et al., 2007), whereas most neurons in other brain regions are not turned over. Alternatively or additionally, new oligodendrocytes may be added (rather than replacing preexisting oligodendrocytes). However, an increase in oligodendroglia was mostly found during the first months in rodents (Ling and Leblond, 1973; Levison et al., 1999) or years in humans (Peters et al., 1991), whereas the generation of myelinating oligodendrocytes observed by Olig2::CreERTM-mediated fate mapping continues into 6 month old mice, an age when the growth of the brain should have come to an end. These data therefore propose that the generation of new oligodendrocytes is not limited to the early life but continues throughout.
The new findings are rather intriguing with regard to the continuous plasticity occurring in the adult cerebral cortex (Chang et al., 2005; Hofer et al., 2006) that may require changes in myelination also (Fields, 2008). It has been suggested that the end of the critical period coincides with the completion of myelin formation (Fields, 2008). However, our results now show a continuation of myelination into adult stages in line with the continued neuronal plasticity. Thus, our fate-mapping experiments identifying the adult generation of NG2 glia in the GM and mature oligodendrocytes in the WM prompts profound questions with regard to their function and why it is important to generate these cell types continuously in the adult brain. The new tools that allow inducing genetic recombination in the adult will help us to elucidate the function of this adult gliogenesis widespread in the brain parenchyma as opposed to the highly restricted adult neurogenesis.
Note added in proof.
Similar results were obtained using a PDGFRα-CreERT2 line shown in a study by Rivers et al. (2008).
Footnotes
This work was supported by grants of the Deutsche Forschungsgemeinschaft, including the excellence cluster Center for Integrated Protein Science Munich, the European Union, Bundesministerium für Bildung und Forschung, the Bavarian State Ministry of the Sciences, and Research and the Arts (M.G.). We thank Annalisa Buffo and Gwendolyn Behrendt for very helpful discussions and comments on this project and, together with Inmaculada Rite, for help with initiating the mouse colony. We are grateful to Peter Brophy and Matt Rassband for antibodies, to Leanne Godinho and Stefanie Robel for careful correction of this manuscript, to Simone Bauer and Gabriele Jäger for excellent technical assistance, and to Susanne Schickle and Petra Peter for maintenance of the mouse colony. We are also particularly grateful to Bill Richardson for sharing unpublished data.
References
- Aguirre AA, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165:575–589. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch U, Kirchhoff F, Schachner M. Immunohistological localization of the adhesion molecules L1, N-CAM, and MAG in the developing and adult optic nerve of mice. J Comp Neurol. 1989;284:451–462. doi: 10.1002/cne.902840310. [DOI] [PubMed] [Google Scholar]
- Battiste J, Helms AW, Kim EJ, Savage TK, Lagace DC, Mandyam CD, Eisch AJ, Miyoshi G, Johnson JE. Ascl1 defines sequentially generated lineage-restricted neuronal and oligodendrocyte precursor cells in the spinal cord. Development. 2007;134:285–293. doi: 10.1242/dev.02727. [DOI] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffo A, Vosko MR, Ertürk D, Hamann GF, Jucker M, Rowitch D, Götz M. Expression pattern of the transcription factor Olig2 in response to brain injuries: implications for neuronal repair. Proc Natl Acad Sci U S A. 2005;102:18183–18188. doi: 10.1073/pnas.0506535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AM, Kiff J, Hubbard P, Berry M. Synantocytes: new functions for novel NG2 expressing glia. J Neurocytol. 2002;31:551–565. doi: 10.1023/a:1025751900356. [DOI] [PubMed] [Google Scholar]
- Cai J, Chen Y, Cai WH, Hurlock EC, Wu H, Kernie SG, Parada LF, Lu QR. A crucial role for Olig2 in white matter astrocyte development. Development. 2007;134:1887–1899. doi: 10.1242/dev.02847. [DOI] [PubMed] [Google Scholar]
- Chang EF, Bao S, Imaizumi K, Schreiner CE, Merzenich MM. Development of spectral and temporal response selectivity in the auditory cortex. Proc Natl Acad Sci U S A. 2005;102:16460–16465. doi: 10.1073/pnas.0508239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chari DM, Blakemore WF. New insights into remyelination failure in multiple sclerosis: implications for glial cell transplantation. Mult Scler. 2002;8:271–277. doi: 10.1191/1352458502ms842oa. [DOI] [PubMed] [Google Scholar]
- Colak D, Mori T, Brill MS, Pfeifer A, Falk S, Deng C, Monteiro R, Mummery C, Somer L, Götz M. Adult neurogenesis requires Smad4-mediated bone morphogenic protein signaling in stem cells. J Neurosci. 2008;28:434–446. doi: 10.1523/JNEUROSCI.4374-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MR, Levine JM, Reynolds R. NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J Neurosci Res. 2000;61:471–479. doi: 10.1002/1097-4547(20000901)61:5<471::AID-JNR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou L, Schnell L, Montani L, Duncan C, Simonen M, Schneider R, Liebscher T, Gullo M, Schwab ME. Nogo-A-deficient mice reveal strain-dependent differences in axonal regeneration. J Neurosci. 2006;26:5591–5603. doi: 10.1523/JNEUROSCI.1103-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusho M, Ono K, Takebayashi H, Masahira N, Kagawa T, Ikeda K, Ikenaka K. Involvement of the Olig2 transcription factor in cholinergic neuron development of the basal forebrain. Dev Biol. 2006;293:348–357. doi: 10.1016/j.ydbio.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Gaiano N, Kohtz JD, Turnbull DH, Fishell G. A method for rapid gain-of-function studies in the mouse embryonic nervous system. Nat Neurosci. 1999;2:812–819. doi: 10.1038/12186. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. In vivo characterization of endogenous proliferating cells in adult rat subcortical white matter. Glia. 1996;17:39–51. doi: 10.1002/(SICI)1098-1136(199605)17:1<39::AID-GLIA4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Goldman S. Glia as neural progenitor cells. Trends Neurosci. 2003;26:590–596. doi: 10.1016/j.tins.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Greenwood K, Butt AM. Evidence that perinatal and adult NG2-glia are not conventional oligodendrocyte progenitors and do not depend on axons for their survival. Mol Cell Neurosci. 2003;23:544–558. doi: 10.1016/s1044-7431(03)00176-3. [DOI] [PubMed] [Google Scholar]
- Hirrlinger PG, Scheller A, Braun C, Quintela-Schneider M, Fuss B, Hirrlinger J, Kirchhoff F. Expression of reef coral fluorescent proteins in the central nervous system of transgenic mice. Mol Cell Neurosci. 2005;30:291–303. doi: 10.1016/j.mcn.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hübener M. Prior experience enhances plasticity in adult visual cortex. Nat Neurosci. 2006;9:127–132. doi: 10.1038/nn1610. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Gage FH. Regeneration in the adult and aging brain. Arch Neurol. 2002;59:1717–1720. doi: 10.1001/archneur.59.11.1717. [DOI] [PubMed] [Google Scholar]
- Káradóttir R R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11:450–456. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Leung CT, Reed RR, Johnson JE. In vivo analysis of Ascl1 defined progenitors reveals distinct developmental dynamics during adult neurogenesis and gliogenesis. J Neurosci. 2007;27:12764–12774. doi: 10.1523/JNEUROSCI.3178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, Arguello AA, Donovan MH, Fischer SJ, Farnbauch LA, Beech RD, DiLeone RJ, Greer CA, Mandyam CD, Eisch AJ. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27:12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- Levison SW, Young GM, Goldman JE. Cycling cells in the adult rat neocortex preferentially generate oligodendroglia. J Neurosci Res. 1999;57:435–446. [PubMed] [Google Scholar]
- Ligon KL, Fancy SP, Franklin RJ, Rowitch DH. Olig gene function in CNS development and disease. Glia. 2006a;54:1–10. doi: 10.1002/glia.20273. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Kesari S, Kitada M, Sun T, Arnett HA, Alberta JA, Anderson DJ, Stiles CD, Rowitch DH. Development of NG2 neural progenitor cells requires Olig gene function. Proc Natl Acad Sci U S A. 2006b;103:7853–7858. doi: 10.1073/pnas.0511001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner M, Heine S, Haastert K, Garde N, Fokuhl J, Linsmeier F, Grothe C, Baumgärtner W, Stangel M. Sequential myelin protein expression during remyelination reveals fast and efficient repair after central nervous system demyelination. Neuropathol Appl Neurobiol. 2008;34:105–114. doi: 10.1111/j.1365-2990.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- Ling EA, Leblond CP. Investigation of glial cells in semithin sections. II. Variation with age in the numbers of the various glial cell types in rat cortex and corpus callosum. J Comp Neurol. 1973;149:73–81. doi: 10.1002/cne.901490105. [DOI] [PubMed] [Google Scholar]
- Masahira N, Takebayashi H, Ono K, Watanabe K, Ding L, Furusho M, Ogawa Y, Nabeshima Y, Alvarez-Buylla A, Shimizu K, Ikenaka K. Olig2-positive progenitors in the embryonic spinal cord give rise not only to motoneurons and oligodendrocytes, but also to a subset of astrocytes and ependymal cells. Dev Biol. 2006;293:358–369. doi: 10.1016/j.ydbio.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Matthias K, Kirchhoff F, Seifert G, Hüttmann K, Matyash M, Kettenmann H, Steinhäuser C. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23:1750–1758. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;67:451–467. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- Miyoshi G, Butt SJ, Takebayashi H, Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci. 2007;27:7786–7798. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukouyama YS, Deneen B, Lukaszewicz A, Novitch BG, Wichterle H, Jessell TM, Anderson DJ. Olig2+ neuroepithelial motoneuron progenitors are not multipotent stem cells in vivo. Proc Natl Acad Sci U S A. 2006;103:1551–1556. doi: 10.1073/pnas.0510658103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkovic J, Mori T, Götz M. Distinct modes of neuron addition in adult mouse neurogenesis. J Neurosci. 2007;27:10906–10911. doi: 10.1523/JNEUROSCI.2572-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A. Polydendrocytes: NG2 cells with many roles in development and repair of the CNS. Neuroscientist. 2007;13:62–76. doi: 10.1177/1073858406295586. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- Ono K, Takebayashi H, Ikeda K, Furusho M, Nishizawa T, Watanabe K, Ikenaka K. Regional- and temporal-dependent changes in the differentiation of Olig2 progenitors in the forebrain, and the impact on astrocyte development in the dorsal pallium. Dev Biol. 2008;320:456–468. doi: 10.1016/j.ydbio.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Paukert M, Bergles DE. Synaptic communication between neurons and NG2+ cells. Curr Opin Neurobiol. 2006;16:515–521. doi: 10.1016/j.conb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Peters A, Josephson K, Vincent SL. Effects of aging on the neuroglial cells and pericytes within area 17 of the rhesus monkey cerebral cortex. Anat Rec. 1991;229:384–398. doi: 10.1002/ar.1092290311. [DOI] [PubMed] [Google Scholar]
- Shen S, Sandoval J, Swiss VA, Li J, Dupree J, Franklin RJ, Casaccia-Bonnefil P. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat Neurosci. 2008;11:1024–1034. doi: 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Nabeshima Y, Yoshida S, Chisaka O, Ikenaka K, Nabeshima Y. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr Biol. 2002;12:1157–1163. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Kataoka Y, Cui Y, Takamori Y, Watanabe Y, Yamada H. Multi-directional differentiation of doublecortin- and NG2-immunopositive progenitor cells in the adult rat neocortex in vivo. Eur J Neurosci. 2007;25:3489–3498. doi: 10.1111/j.1460-9568.2007.05617.x. [DOI] [PubMed] [Google Scholar]
- Wu S, Wu Y, Capecchi MR. Motoneurons and oligodendrocytes are sequentially generated from neural stem cells but do not appear to share common lineage-restricted progenitors in vivo. Development. 2006;133:581–590. doi: 10.1242/dev.02236. [DOI] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]