Abstract
SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins have a key role in membrane fusion. It is commonly assumed that pairing of SNARE proteins anchored in opposing membranes overcomes the repulsion energy between membranes, thereby catalyzing fusion. In this study, we have increased the distance between the coiled-coil SNARE motif and the transmembrane domain of the vesicular SNARE synaptobrevin-2 by insertion of a flexible linker to analyze how an increased intermembrane distance affects exocytosis. Synaptobrevin-2 lengthening did not change the frequency of exocytotic events measured at 1 μm free calcium but prevented the increase in the secretory activity triggered by higher calcium concentration. Exocytotic events monitored in adrenal chromaffin cells by means of carbon fiber amperometry were classified in two groups according to the rate and extent of fusion pore expansion. Lengthening the juxtamembrane region of synaptobrevin-2 severely reduced the occurrence of rapid single events, leaving slow ones unchanged. It also impaired the increase in the fast-fusion mode that normally follows elevation of intracellular Ca2+ levels. We conclude that mild stimuli trigger slow fusion events that do not rely on a short intermembrane distance. In contrast, a short intermembrane distance mediated by tight zippering of SNAREs is essential to a component of the secretory response elicited by robust stimuli and characterized by rapid dilation of the fusion pore.
Keywords: exocytosis, membrane fusion, neurosecretion, SNARE, amperometry, synaptic vesicle release
Introduction
Membrane fusion is essential to a host of biological processes such as exocytosis, intracellular trafficking, fertilization, or viral entry. This is not a spontaneous process because biological membranes are highly stable and repulsion forces oppose their approach. Energy must be supplied to approach membranes, to promote curvature deformations generated during the approach-step-and-stalk formation (the initial junction formed by the rearrangement of lipids in the two cis-leaflets), to create and to enlarge the pore (Chernomordik et al., 2006; Lee and Schick, 2007).
SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins are essential to intracellular membrane fusion events and may supply the force needed to surmount the free energy barriers (Jahn and Scheller, 2006). At the synapse and in neuroendocrine cells, the vesicle (v)-SNARE synaptobrevin-2 (Syb2) is inserted into the secretory vesicle membrane and can form a complex with plasma membrane-associated target-localized (t)-SNAREs (syntaxin-1 and SNAP-25). Assembly of the SNARE bundle proceeds from the membrane-distal to the membrane-proximal region of the proteins in a zipper-like manner (Hanson et al., 1997). In vitro assays of liposome fusion after reconstitution of recombinant proteins suggested that SNAREs constitute a minimal fusion machinery (Weber et al., 1998). Increasing the length of the juxtamembrane region of SNAREs reduced the efficacy of fusion, providing support to the “zipper model” according to which SNAREs catalyze fusion by reducing the intermembrane distance (McNew et al., 1999). However, liposome fusion is very slow unless SNARE-associated proteins are added (Martens et al., 2007; Shen et al., 2007). Therefore, it is not clear yet whether SNAREs catalyze fusion merely by reducing intermembrane distance. The energy released during SNARE assembly may also be used to stabilize the stalk or to dilate the pore. SNAREs could also be seen as scaffold proteins acting synergistically with other fusion proteins such as synaptotagmins (Sorensen et al., 2006; Zimmerberg et al., 2006; Martens et al., 2007).
Once initiated, the fusion process may proceed toward complete merging of the two membranes (full-collapse fusion). Fusion pore expansion may also arrest at some stage, followed by rapid reversal of the pore or conservation of vesicle shape during some time [a process known as “kiss-and-run” (KR) or “cavicapture”]. Full-collapse fusion and KR coexist in neurons and endocrine cells (Harata et al., 2006). These different modes of fusion are thought to condition vesicle reuse and transmitter efflux. In adrenal chromaffin cells, mild stimuli trigger the release of catecholamines by KR, whereas more robust stimuli evoke the discharge of the dense peptide core (Fulop et al., 2005) and increase the amount of catecholamines released per vesicle (Elhamdani et al., 2001). These changes in fusion pore dynamics are calcium dependent, but the underlying molecular mechanisms are not known.
In this study, we have analyzed the effect of a flexible linker inserted into the juxtamembrane region of Syb2 on exocytosis of secretory granules (SGs) of adrenal chromaffin cells to test the zipper model in living cells and to investigate the function of SNAREs in fusion pore dynamics. Catecholamine release from single SGs was monitored using carbon fiber amperometry, a highly sensitive technique that can resolve the dynamics of the fusion pore from its opening to its final dilation on membrane merging. We found that Syb2 lengthening has little effect on exocytosis triggered by moderate calcium elevation but impairs calcium-dependent increase in fusion probability and severely reduces the proportion of exocytotic events characterized by fast dilation of the fusion pore.
Materials and Methods
Materials.
Plasmids encoding rat Syb2R and Syb2 (in pcDNA3.1-mycHis vector; Invitrogen) were kindly provided by M. Seagar (Faculté de Médecine Secteur Nord, Inserm UMR 641, Marseille, France) and the one encoding bovine green fluorescent protein (GFP)-Syb2 [in the pEGFP (plasmid enhanced GFP) vector (Clontech)] by T. Galli (Institut Jacques Monod, CNRS UMR 7592, Paris, France). The plasmid pBN13-BoNT/B (botulinum neurotoxin type B) light chain (LC) encoding the light chain of BoNT/B was a gift from T. Binz (Institut für Biochemie, Medizinische Hochschule Hannover, Hannover, Germany). Collagenase A (used for dissociation of chromaffin cells) was from Roche Diagnostics. Carbon fiber electrodes were from ALA Scientific Intruments. Cell culture reagents were obtained from PAA Laboratories. DNA purifications were done using kits from Macherey-Nagel. Chemicals were from Sigma.
Plasmid constructs.
A BamHI site was added to the sequence of Syb2R using the QuickChange mutagenesis kit (Stratagene) according to manufacturer's instructions and the following primers: SybBamHI-f (forward), 5′-GGTGGAAAAACCTCGGATCCATGATCATCTTGGGAGTG, and SybBamHI-r (reverse), 5′-CACTCCCAAGATGATCATGGATCCGAGGTTTTTCCACC. The insertion generated the mutations K94G and M95S. The plasmid was then digested by BamHI and dephosphorylated and ligated to two prehybridized single-stranded phosphorylated oligonuleotides: GGS-BamHI-f, 5′GATCTGGTGGTTCCGGTGGTTCCGGAGGTTCCG and GGS-BamHI-r (5′-GATCCGGAACCTCCGGAACCACCGGAACCACCG. The double-stranded oligonucleotide codes for the 9 aa repeat sequence GGSGGSGGS. When this oligonucleotide is inserted into the parent vector in the correct orientation, the BamHI site is maintained. This annealed double-stranded oligonucleotide was ligated into the Syb2R parental vector digested by BamHI. The plasmid pcDNA3-Syb2R-i11-Myc-His encoding Syb2R-i11 was obtained. The sequence encoding BoNT/B LC was subcloned into pcDNA3.1-Myc-His vector. The Myc-His tag at the end of the BoNT/B sequence was then eliminated by PCR. The sequences were verified by automated sequencing.
Cell culture and transfection.
Primary dissociated chromaffin cells from bovine adrenal medulla were prepared by retrograde collagenase perfusion (Desnos et al., 2003) and cultured in DMEM/Ham's F12 medium supplemented with 10% fetal calf serum for 24 h and then with 1% serum. Cells were transfected by electroporation (Desnos et al., 2003). In brief, freshly prepared cells (1.5 × 106) were suspended in electrobuffer with 4.1 μg of vector DNAs (2 μg of Syb2R or Syb2R-i11, 2 μg of GFP-Syb2, and 100 ng of BoNT/B LC) and transfected with a single electric shock (700 V/cm, 24 ms). Experiments were generally performed 2–4 d after transfection. GFP-Syb2 was included to identify transfected cells. Cells with diffuse fluorescent staining, indicative of BoNT/B LC expression, were chosen.
Amperometry.
Amperometric recordings were made at room temperature (22–25°C), as described previously (Schonn et al., 2003), except that the data were sampled at 40 kHz. Acquisition and analysis were performed with Elphy software (G. Sadoc, Institut Alfred Fessard, CNRS UPR 2191, Gif sur Yvette, France) with locally written routines. Stimulations were performed with local perfusion of either high K+ saline containing (in mm): 55 KCl, 5.5 glucose, 108 NaCl, 5 CaCl2, 3 NaHCO3.6, 1.2 MgCl2, 15 HEPES-NaOH, pH 7.4, or a solution containing 20 μm digitonin and either 1 or 100 μm free Ca2+ (2 mm ATP, 0.4 mm GTP, 150 mm K-glutamate, 25 mm PIPES, pH 7.0, 2 mm EGTA, 2 mm nitrilo triacetic acid, 1.39 or 2.54 mm CaCl2, 3.53 or 3.34 mm MgCl2). Just before recording, cells were transferred to Locke's solution (Schonn et al., 2003), supplemented with 2.5 mm CaCl2 in the case of high K+ stimulation. Cells were stimulated at least 60 s. The detection threshold for frequency analysis was set to 3 pA. For single-events analysis, we selected spikes with Imax > 5 pA and Q > 500 fC. The maximal spike rise velocity was defined as the maximal slope of spike rising phase, computed on the basis of 1.5 ms (50 data points). Curve fitting was done using SigmaPlot (Systat Software).
Statistics. The significance of differences was analyzed using a nonparametric Mann–Whitney test.
Results
Increasing the length of Syb2 juxtamembrane region has mild effect on fusion probability
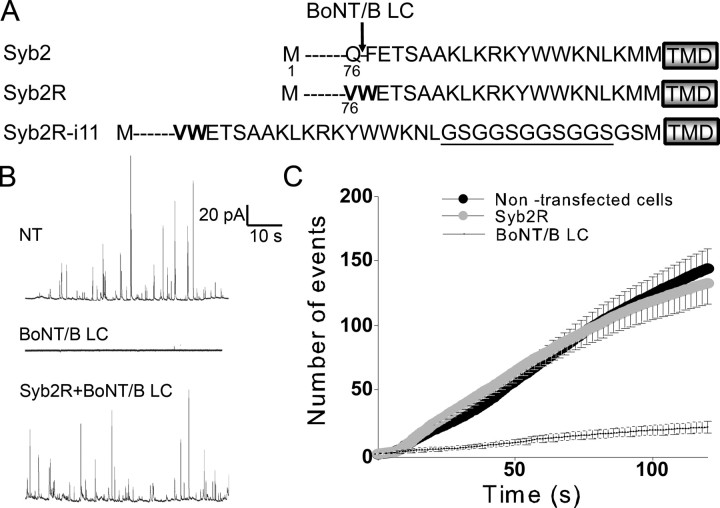
To investigate the function of the v-SNARE Syb2 in exocytosis, our strategy was to eliminate endogenous Syb2 by expressing the catalytic light chain of BoNT/B and to rescue the secretory activity by expressing toxin-insensitive Syb2 proteins (Quetglas et al., 2002). These modified Syb2 proteins (Syb2R) carry point mutations in the region targeted by the proteolytic activity of the toxin (Fig. 1A). Secretory responses were elicited by local application of high K+ saline (which induces calcium entry via voltage-dependent channels) and measured at the single-cell level by means of carbon fiber amperometry. Because of the high concentration of catecholamines in SGs, single exocytotic events can be detected in real time as transient oxidative currents (amperometric spikes). As illustrated in Figure 1B, the secretory activity of chromaffin cells was abolished by BoNT/B and restored by coexpressing Syb2R. The frequency of amperometric spikes observed in cells cotransfected with BoNT/B and Syb2R was almost identical to that observed in nontransfected cells (Fig. 1C).
Figure 1.
The secretory activity of chromaffin cells is abolished by BoNT/B light chain and restored by coexpression of Syb2R. A, Amino acid sequences of Syb2 and mutants. BoNT/B light chain cleaves Syb2 at Q76, as indicated. The transmembrane domain (TMD) is boxed. Point mutations that protect Syb2R from the proteolytic action of BoNT/B are in bold. Underlined amino acids were inserted to increase the distance between the SNARE motif and the transmembrane domain. Moreover, two amino acids were modified, K94G and M95S, to introduce the linkers (see Materials and Methods). The name of the construct (Syb2R-i11) refers to the number of inserted amino acids. B, Examples of amperometric recordings of a nontransfected chromaffin cell (top, NT), a chromaffin cell expressing BoNT/B light chain (middle, BoNT/B LC), and a chromaffin cell coexpressing Syb2R and BoNT/B (bottom, Syb2R+BoNT/B LC). C, Cumulated numbers of exocytic events (mean ± SE) as a function of time for nontransfected chromaffin cells (21 cells), cells expressing BoNT/B (20 cells), and cells coexpressing Syb2R and BoNT/B (50 cells).
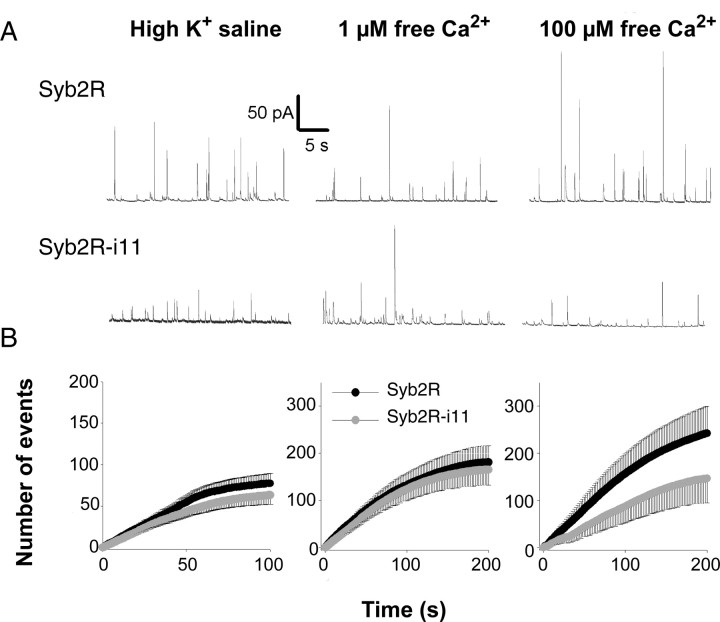
Using this approach, we investigated the functionality of modified Syb2R proteins (Syb2R-i11) carrying a flexible linker of 11 aa between the complex-forming SNARE motif and the membrane-spanning domain (Fig. 1A). High K+ depolarization induced similar secretory activity in cells expressing BoNT/B and either Syb2R or Syb2R-i11 (Fig. 2). To bypass Ca2+ channels and control the concentration of intracellular Ca2+ ([Ca2+]i), cells were permeabilized by local perfusion of a solution containing digitonin and either 1 or 100 μm free Ca2+. At 1 μm free Ca2+, Syb2R-i11 restored exocytosis of BoNT/B-treated cells with the same efficacy as Syb2R. Elevating the free calcium concentration from 1 to 100 μm increased the frequency of amperometric spikes in Syb2R-expressing cells. However, this extra component of release was not observed in Syb2R-i11-transfected cells (Fig. 2B, right). The data suggest that fusion probability depends on the completeness of SNAREs zippering at high but not at low [Ca2+]i.
Figure 2.
Inserting a linker (i11) prevents the occurrence of high-amplitude spikes and slightly affects the overall frequency of exocytotic events. A, B, Chromaffin cells coexpressing BoNT/B and either Syb2R or Syb2R-i11 were stimulated by high K+ saline (left panels) or permeabilized with digitonin in the presence of 1 (middle panels) or 100 (right panels) μm free Ca2+, as indicated. A, Representative amperometric recordings of Syb2R-expressing (top) or Syb2R-i11-expressing (bottom) cells. B, Cumulated numbers of exocytic events (mean ± SE) as a function of time. Spikes were obtained from 23 (high K+), 40 (1 μm), or 28 (100 μm) cells.
Adding linkers to Syb2 changes the dynamics of unitary fusion events
Increasing [Ca2+]i from 1 to 100 μm not only increased the overall frequency of exocytotic events but also markedly promoted the appearance of high-amplitude spikes (Fig. 2). The peak amplitude Imax, which corresponds to the maximal flux of catecholamines diffusing from the intravesicular matrix through the fusion pore, increases with the rate and extent of fusion pore dilation. The observed increase in Imax with [Ca2+]i agrees with previous reports (Elhamdani et al., 2001; Fulop et al., 2005; Grabner and Fox, 2006) indicating that the dynamics of the fusion pore depends on [Ca2+]i. However, amperometric spikes with an amplitude >50 pA were rare in Syb2R-i11-expressing cells, even at 100 μm free Ca2+ (Figs. 2, 3B). The rightward shift of the distribution of Imax values observed in control cells on calcium elevation from 1 to 100 μm was not found in Syb2R-i11-expressing cells (Fig. 3C,D).
Figure 3.
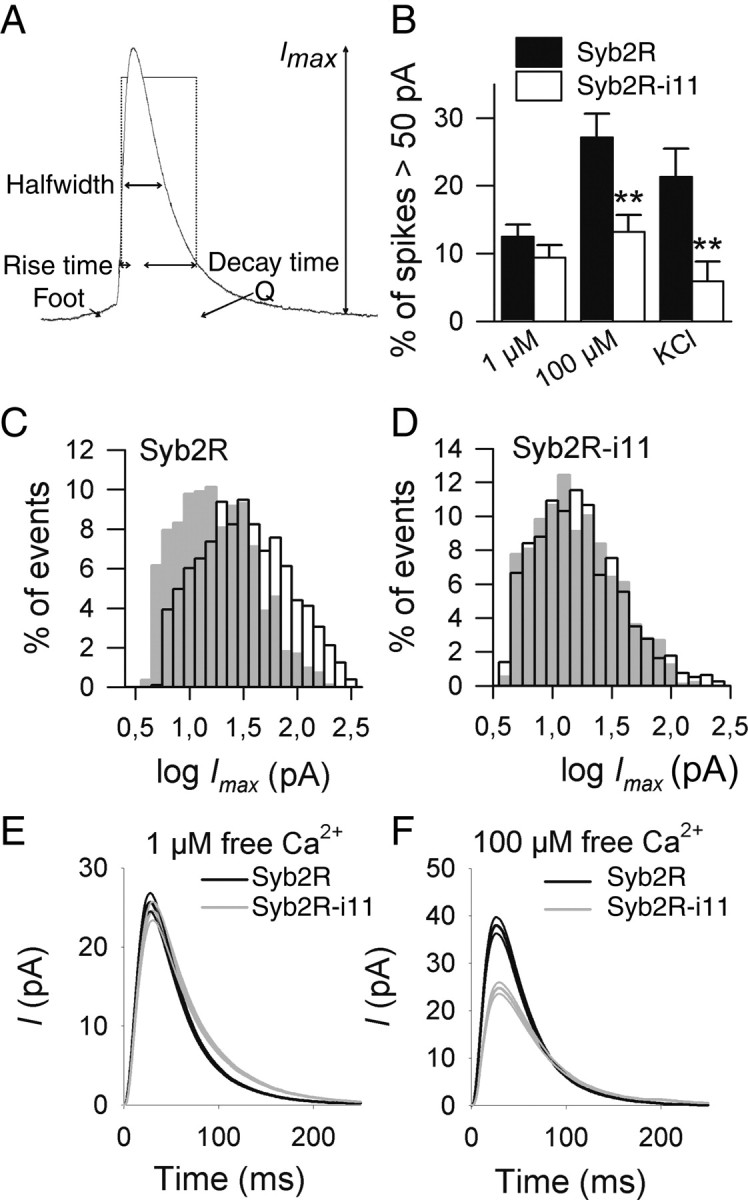
High calcium concentration increases Imax values in Syb2R but not in Syb2R-i11-expressing cells. A, Representation of an amperometric spike. Different parameters are depicted: amplitude (Imax), quantal charge (Q), halfwidth, 20–90% rise time, and 90–20% decay time. B, Shown is the mean (±SE) percentage of spikes with an amplitude >50 pA recorded in control cells (Syb2R, filled bars) or in cells expressing Syb2R-i11 (open bars) on stimulation with high K+ or on digitonin permeabilization at either 1 or 100 μm free Ca2+. Spikes were monitored from 11 to 15 cells in each condition. **p < 0.01. C, D, Raising the free Ca2+ concentration from 1 μm (gray bars) to 100 μm (open bars) shifted the distribution of log Imax values to the right in Syb2R-expressing (C) but not in Syb2R-i11-expressing (D) cells. Distributions were computed from 821 (Syb2R, 1 μm), 1045 (Syb2R, 100 μm), 965 (Syb2R-i11, 1 μm), and 797 (Syb2R-i11, 100 μm) spikes. E, The “mean” amperometric spike from Syb2R (black curve)- or Syb2R-i11 (gray curve)-expressing cells at 1 μm free calcium. All events were aligned at their onset (spikes with foot were omitted) and averaged. Shown is the mean (±SE) of >420 events. F, Averaged spike at 100 μm calcium.
These observations suggest that insertion of linkers in the juxtamembrane region of Syb2 affects fusion pore dynamics. We thus analyzed the shape of amperometric spikes in more detail. Mean values of rise time, decay time, and half-width (HW) were significantly increased in Syb2R-i11-expressing cells compared with control cells (Syb2R) at 100 μm free Ca2+ (Table 1). In contrast, the mean value of Imax and of the maximal spike rise velocity (smax), an index of the rate of fusion pore enlargement, were significantly decreased (by ∼50%) in Syb2R-i11 cells compared with control cells. Syb2 lengthening also reduced the quantal size (Q) (time integral of current). However, this effect was not significant (p = 0.096); it may be because of the fact that catecholamine collection by the electrode, especially during spike trail, is better for sharp spikes than for broad ones. Spike widening was confirmed by measuring shape parameters on single events. Compared with controls, HW/Imax was increased by ∼60%, whereas Imax/Q was reduced in Syb2R-i11 cells (Table 1). Spike widening was also observed in cells stimulated by high K+ depolarization and at 1 μm [Ca2+]i, but the effect was less pronounced under these conditions (Table 1). To illustrate these changes, the “mean spike” obtained by averaging all fusion events monitored under a given condition is shown in Figure 3 E,F. These results suggest that linker insertion slows the dilation of the fusion pore (increased rise time and reduced Imax) and reduced the diameter of the pore (slowed decay).
Table 1.
Summary of amperometric data
| Condition | High K+ saline |
1 μm free calcium |
100 μm free calcium |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Syb2R | Syb2R-i11 | p | Syb2R | Syb2R-i11 | p | Syb2R | Syb2R-i11 | p | |
| Number of cells | 15 | 14 | 13 | 11 | 11 | 11 | |||
| Imax (pA) | 29.8 ± 2.8 | 19.4 ± 2.3 | 0.007 | 27.2 ± 1.8 | 22.6 ± 1.9 | NS | 42.3 ± 4.2 | 25.9 ± 1.6 | 0.008 |
| Q (fC) | 1560 ± 130 | 1410 ± 100 | NS | 1700 ± 130 | 1800 ± 190 | NS | 2400 ± 230 | 1990 ± 120 | NS |
| HW (ms) | 46.6 ± 2.9 | 57.0 ± 4.0 | 0.013 | 54.8 ± 3.3 | 65.8 ± 3.1 | 0.030 | 47.6 ± 2.8 | 61.2 ± 2.7 | 0.004 |
| Slope (pA/ms) | 5.2 ± 0.7 | 3.0 ± 0.6 | 0.045 | 3.1 ± 0.3 | 2.2 ± 0.3 | 0.046 | 6.0 ± 0.8 | 3.0 ± 0.6 | 0.001 |
| Rise time (ms) | 12.8 ± 1.3 | 13.9 ± 1.0 | NS | 14.7 ± 1.2 | 17.3 ± 1.2 | NS | 12.2 ± 1.0 | 16.1 ± 0.9 | 0.005 |
| Decay time (ms) | 55.7 ± 4.2 | 79.8 ± 8.0 | 0.023 | 61.9 ± 3.9 | 76.2 ± 3.9 | 0.014 | 52.9 ± 3.0 | 67.8 ± 3.8 | 0.005 |
| Imax/Q(pA/pC) | 23.4 ± 2.1 | 17.8 ± 2.1 | 0.021 | 18.9 ± 1.0 | 15.6 ± 0.7 | 0.022 | 23.3 ± 1.4 | 17.8 ± 0.9 | 0.005 |
| HW/Imax (ms/pA) | 3.97 ± 0.38 | 5.56 ± 0.48 | 0.010 | 4.49 ± 0.55 | 5.58 ± 0.53 | NS | 3.07 ± 0.41 | 4.98 ± 0.30 | 0.005 |
Data are the mean ± SE values of samples created using mean spike parameter values from cells transfected with BoNT/B and either Syb2R or Syb2R-i11. Cells were stimulated with Locke's saline containing 50 mm KCl or by local application of a solution containing digitonin and either 1 or 100 μm free Ca2+, as indicated. For each condition, different parameters describing amperometric spikes are shown. Only cells from which at least 20 spikes were monitored were selected for analysis.
Linker insertion in Syb2 prevents rapid dilation of the fusion pore
The effect of linker insertion in Syb2 on the mean parameters of amperometric spikes may result either from a general modification of spike shape or from a more specific effect on a subset of events. To address this issue, we compared the distribution of different spike parameters measured at 1 or 100 μm [Ca2+]i. In control cells, rise time, decay time, HW, and smax display a broad distribution, but log-transformed data are almost normally distributed. However, the frequency plots are bimodal and better fitted with two normal density functions than with a single one, suggesting the existence of two categories of events (Fig. 4). Furthermore, rise time and decay time are correlated with smax and more loosely with Imax, but not with the quantal size (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). The data suggest that a population of rapid and large spikes coexists with a population of broader and smaller spikes. Raising the free calcium concentration from 1 to 100 μm increased the proportion of large and rapid spikes (Figs. 3, 4, supplemental Fig. 2, available at www.jneurosci.org as supplemental material).
Figure 4.
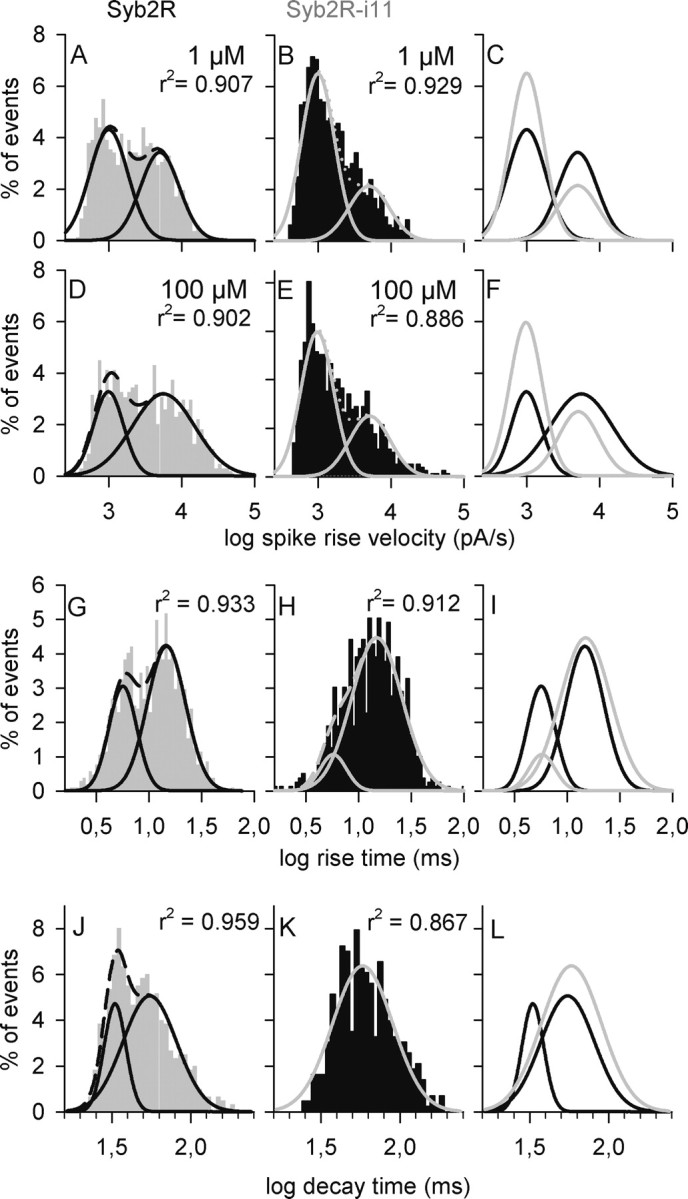
Syb2 lengthening reduces the proportion of rapid spikes. Chromaffin cells were transfected with Syb2R (A, D, G, J) or Syb2R-i11 (B, E, H, K) and stimulated by local perfusion of digitonin in the presence of 1 μm (A–C) or 100 μm (D–L) free Ca2+. A total of 821 (A, C) or 1045 (D–L) amperometric spikes from Syb2R-expressing cells and 965 (B, C) or 797 (D–L) spikes from Syb2R-i11-expressing cells were analyzed. A, D, Distribution of the values of the maximal spike rise velocity (smax) in control cells (Syb2R) at 1 μm (A) or 100 μm (D) free Ca2+. Frequency plots indicate the existence of two populations of spikes that can be well fitted with two Gaussian functions (black curves). The dotted line is the sum of the two normal density functions. Fitting with a single normal density function gives a lower r2 value (0.77). B, E, Distribution of smax values in cells expressing Syb2R-i11. This distribution is also better fitted by two normal density functions (gray curves) rather than by a single one (r2 = 0.76). C, F, Merge of the Gaussian fits indicating that the proportion of rapid spikes is higher in Syb2R- than in Syb2R-i11-expressing cells. G–I, Distribution of the values of log rise time is also bimodal in Syb2R (G)-expressing cells. This is less clear in Syb2R-i11 cells because the fit to a single Gaussian function is almost as good (r2 = 0.903). The proportion of spikes with a short rise time is strongly reduced in Syb2R-i11 cells compared with control ones (I). J–L, Frequency plots of the log-transformed values of 90–20% decay time. The distribution is clearly bimodal in Syb2R cells (J). In contrast, in Syb2R-i11 cells (K) it is well fitted with a single density function similar to the one that describes the slow spikes in Syb2R cells (L).
To determine whether the bimodal distribution of spike shape parameters observed in Syb2R-expressing cells resulted from the overexpression of Syb2, amperometric spikes from nontransfected cells were also analyzed. The distribution of spike rise velocity and rise time values was also consistent with the existence of two categories of fusion events (supplemental Fig. 3, available at www.jneurosci.org as supplemental material). Furthermore, no significant difference in any of the mean spike parameters was observed between Syb2R-expressing cells and nontransfected ones (data not shown).
In contrast, in Syb2R-i11-expressing cells, the proportion of rapid spikes was reduced, compared with control cells, or even suppressed (Fig. 4). A population of rapid events can still be seen in frequency histograms of smax. However, it is hardly detected in histograms of rise time or decay time values (Fig. 4, supplemental Fig. 2, available at www.jneurosci.org as supplemental material). In contrast to what was observed in control cells, elevating [Ca2+]i from 1 to 100 μm did not increase the proportion of large and rapid events in Syb2R-i11-expressing cells (Fig. 4A–F, supplemental Fig. 2, available at www.jneurosci.org as supplemental material). We conclude that Syb2 lengthening impairs one mode of fusion characterized by rapid dilation of the fusion pore.
The foot signal is reduced in Syb2R-i11-expressing cells
Amperometric spikes are preceded in ∼30% of the cases by a “foot” signal (also called a pre-spike signal) that reflects the diffusion of transmitters through a narrow, slowly expanding fusion pore (Albillos et al., 1997). The onset of the spike itself corresponds to the sudden enlargement of the fusion pore. At 1 μm [Ca2+]i, similar foot signals were observed in control cells (maximal amplitude = 3.8 ± 4.5 pA, n = 104) and in Syb2R-i11-expressing cells (3.9 ± 3.5 pA, n = 122). Raising [Ca2+]i to 100 μm increased the maximal amplitude of the foot current in Syb2R-transfected cells (7.5 ± 6.9 pA, n = 164) but not in Syb2R-i11-expressing cells (4.5 ± 4.5 pA, n = 118; p < 0.0001 on pooled spike values, p < 0.05 on mean values obtained in the different cells). The integral of the foot signal (data not shown) was also reduced in Syb2R-i11-expressing cells compared with Syb2R cells at 100 μm [Ca2+]i. In contrast, the duration of the foot signal was not changed.
Discussion
Two recent studies have addressed the effect of inserting a hydrophilic linker between the complex-forming SNARE motif of Syb2 and its transmembrane domain on exocytosis. Deák et al. (2006) found that lengthened Syb2 carrying a 12-aa-long linker could hardly rescue evoked neurotransmitter release in neurons from Syb2 KO mice but fully rescued spontaneous release (miniatures). Kesavan et al. (2007) found that linker insertion in Syb2 severely reduced the magnitude of the exocytotic burst in chromaffin cells (synchronous release) without changing the calcium dependence of the response and slowed fusion pore expansion.
Our data complement these findings. We found that (1) linker insertion in Syb2 does not reduce the occurrence of exocytotic events at low calcium, but prevents the increase in fusion probability normally observed at high calcium; and (2) fusion events can be categorized in two classes according to fusion pore dynamics. Linker insertion specifically abrogates “fast” fusion events, whereas “slow” fusion events remain unaffected. The effect on fusion pore dynamics is also more robust at high calcium because, schematically, the pore is slow at low calcium and fast at high calcium. Together, the data suggest that synchronous release and fast fusion are intimately linked because they are both inhibited by linker insertion. Moreover, both operate at high calcium levels and may rely on a calcium sensor with low affinity for calcium, or with high cooperativity, such as synaptotagmins (Sun et al., 2007).
Two modes of membrane fusion
Our categorization of fusion events is based on the finding that several parameters that describe amperometric spike shape (rise time, decay time, spike rise velocity) have a bimodal distribution in control cells. These parameters are correlated with each other and also, more loosely, with spike amplitude. Hence, a population of large and rapid spikes coexists with a second group of smaller and slower fusion events. The proportion of rapid events increases with calcium concentration. These two groups of fusion events might correspond to different-sized vesicles. However, rise and decay times are not correlated with quantal size. Furthermore, the size of dense core granules rather closely follows a Gaussian distribution (Plattner et al., 1997). Therefore, these two populations of amperometric spikes probably correspond to two modes of fusion with distinct rates of fusion pore dilation and not to two groups of vesicles. Two modes of exocytosis have been described previously. During full fusion, fusion pore dilation proceeds until completion of membrane merging, whereas it is restricted in KR/cavicapture (Harata et al., 2006). Evidence for both modes of fusion has been obtained in adrenal chromaffin cells and lactotrophs (Elhamdani et al., 2001, 2006; Taraska et al., 2003; Stenovec et al., 2004; Fulop et al., 2005; Vardjan et al., 2007). KR dominates at low [Ca2+]i, whereas full fusion increases with stimuli that generate high [Ca2+]i (Fulop et al., 2005; Elhamdani et al., 2006; Vardjan et al., 2007). Slow and rapid fusion events may thus correspond to KR/cavicapture and full fusion, respectively.
Intermembrane distance and membrane fusion
Fusion of giant unilamellar vesicles is steeply dependent on intermembrane distance (Heuvingh et al., 2004). Accordingly, theoretical models conclude that the free energy for the stalk intermediate increases with intermembrane distance (Chizmadzhev et al., 1995). Assuming an extended conformation of our 11-aa-long linker, the resulting augmentation of intermembrane distance (4.7 nm) is expected to increase the energy barrier for fusion by ∼100 kBT (kB, Boltzmann's constant; T, absolute temperature) (Lee and Schick, 2007). Such a large penalty should impair the exocytotic process. The lack of effect of Syb2 lengthening on the occurrence of fusion events at low calcium is therefore intriguing and suggests that SNAREs do not catalyze fusion merely by pulling membranes together. One possibility, however, would be that fusion is powered at low calcium by partially assembled SNAREs. The slow rate of fusion would result from the rather large intermembrane distance; the observed insensitivity of the process to linker insertion would be caused by the small relative increment in the intermembrane distance resulting from linker addition to partially assembled Syb2. Further zippering of the SNARE bundle could even compensate for this increment. Consistently, previous studies have indicated that SNARE complexes may exist in a partially zippered state in which they are still able to catalyze exocytosis (Xu et al., 1998, 1999; Sorensen et al., 2006). Implicit in this scheme is the fact that the energy released by SNARE assembly (Li et al., 2007) is not sufficient to overcome repulsion forces between apposing membranes.
High calcium levels increase the release probability, the temporal coupling between stimulus and response, and the kinetics of single fusion events. Linker insertion in Syb2 severely inhibits all of these effects that probably reflect the action of a calcium-dependent factor. A good candidate is synaptotagmin. At calyx-of-Held synapses, asynchronous release of neurotransmitters dominates at [Ca2+]i < 1 μm. Above this value, synchronous release operates and is dependent on synaptotagmin-2 (Sun et al., 2007). Synaptotagmins may thus contribute additional energy to assist SNAREpin zippering, leading to further reduction of the intermembrane distance and to increased fusion probability. Such a mechanism would be obviously blocked by linker insertion in Syb2.
Force transmission to the membrane
The intermembrane-distance paradigm may account for the observed data, but other possibilities should be considered. The membrane-proximal region of SNAREs is supposed to transmit force to the membrane-spanning domain. Interestingly, it interacts with phospholipids (Quetglas et al., 2002) and is even immersed in the bilayer (Chen et al., 2004). Recent molecular dynamics simulations have extended these observations by showing that the C-terminal end of the SNARE motif is also embedded in the membrane (M. Baaden, personal communication). On zippering of the complex, the membrane-proximal region of SNAREs could thus bend or destabilize the membrane. This may account for the severe reduction of membrane fusion that results from mutations or deletion of the C-terminal hydrophobic layers of SNAREs (Sorensen et al., 2006; Siddiqui et al., 2007). Insertion of a hydrophilic linker in Syb2 could perturb the insertion of the membrane-proximal region into the phospholipid bilayer and thus force-transmission to the membrane (Baaden, personal communication). This could be particularly important at high calcium when additional factors would add strain on SNAREs.
Fusion pore expansion
Linker insertion in Syb2 abrogates the fast-fusion mode and also reduces the initial expansion of the pore because the amplitude of the foot current was found to increase with Ca2+ levels in control cells but not in Syb2R-i11-expressing cells. What drives fusion pore enlargement? Membrane tension and pore lengthening were both proposed to lower energy barriers that prevent spontaneous fusion pore dilation (Chizmadzhev et al., 1995, 2000; Amatore et al., 2000). Membrane tension may result both from the interaction of synaptotagmins with SNAREs and phospholipids at the neck of the fusing vesicle (Zimmerberg et al., 2006) and from swelling of the granule matrix. Influx of Na+ through the fusion pore displaces matrix-bound Ca2+ and catecholamines, and leads to matrix swelling and increased vesicular membrane tension (Amatore et al., 2000; Gong et al., 2007). Energy would be stored in the membrane until the tension exceeds the cohesion of the fusion machinery and would then drive pore enlargement.
We propose that slow fusion is powered by partially zippered SNAREs. Activation of synaptotagmins at high calcium may promote fast fusion by adding strain on SNAREs or by strengthening ring assemblies at the neck of the fusing vesicle. Both mechanisms could be affected by linker insertion in Syb2. Reduced SNARE-to-membrane force transmission and weakened fusion machinery could account for its effects on the foot signal and on the main spike, respectively.
Footnotes
This work was supported by a grant from the Ministère de l'Enseignement Supérieur et de la Recherche. F.D. and C.A. are supported by Inserm. M.B. is the recipient of a Centre National de la Recherche Scientifique fellowship. We thank Drs. E. Karatekin and B. Gasnier for critical reading of this manuscript and K. Guillaumie for analysis routines.
References
- Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–512. doi: 10.1038/39081. [DOI] [PubMed] [Google Scholar]
- Amatore C, Bouret Y, Travis ER, Wightman RM. Interplay between membrane dynamics, diffusion and swelling pressure governs individual vesicular exocytotic events during release of adrenaline by chromaffin cells. Biochimie. 2000;82:481–496. doi: 10.1016/s0300-9084(00)00213-3. [DOI] [PubMed] [Google Scholar]
- Chen Y, Xu Y, Zhang F, Shin YK. Constitutive versus regulated SNARE assembly: a structural basis. EMBO J. 2004;23:681–689. doi: 10.1038/sj.emboj.7600083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik LV, Zimmerberg J, Kozlov MM. Membranes of the world unite! J Cell Biol. 2006;175:201–207. doi: 10.1083/jcb.200607083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizmadzhev YA, Cohen FS, Shcherbakov A, Zimmerberg J. Membrane mechanics can account for fusion pore dilation in stages. Biophys J. 1995;69:2489–2500. doi: 10.1016/S0006-3495(95)80119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizmadzhev YA, Kuzmin PI, Kumenko DA, Zimmerberg J, Cohen FS. Dynamics of fusion pores connecting membranes of different tensions. Biophys J. 2000;78:2241–2256. doi: 10.1016/S0006-3495(00)76771-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deák F, Shin OH, Karalali ET, Südhof TC. Structural determinants of synaptobrevin 2 function in synaptic vesicle fusion. J Neurosci. 2006;26:6668–6676. doi: 10.1523/JNEUROSCI.5272-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnos C, Schonn JS, Huet S, Tran VS, El-Amraoui A, Raposo G, Fanget I, Chapuis C, Ménasché G, de Saint Basile G, Petit C, Cribier S, Henry JP, Darchen F. Rab27A and its effector MyRIP link secretory granules to F-actin and control their motion towards release sites. J Cell Biol. 2003;163:559–570. doi: 10.1083/jcb.200302157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhamdani A, Palfrey HC, Artalejo CR. Quantal size is dependent on stimulation frequency and calcium entry in calf chromaffin cells. Neuron. 2001;31:819–830. doi: 10.1016/s0896-6273(01)00418-4. [DOI] [PubMed] [Google Scholar]
- Elhamdani A, Azizi F, Artalejo CR. Double patch clamp reveals that transient fusion (kiss-and-run) is a major mechanism of secretion in calf adrenal chromaffin cells: high calcium shifts the mechanism from kiss-and-run to complete fusion. J Neurosci. 2006;26:3030–3036. doi: 10.1523/JNEUROSCI.5275-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Radabaugh S, Smith C. Activity-dependent differential transmitter release in mouse adrenal chromaffin cells. J Neurosci. 2005;25:7324–7332. doi: 10.1523/JNEUROSCI.2042-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong LW, de Toledo GA, Lindau M. Exocytotic catecholamine release is not associated with cation flux through channels in the vesicle membrane but Na+ influx through the fusion pore. Nat Cell Biol. 2007;9:915–922. doi: 10.1038/ncb1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner CP, Fox AP. Stimulus-dependent alterations in quantal neurotransmitter release. J Neurophysiol. 2006;96:3082–3087. doi: 10.1152/jn.00017.2006. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Roth R, Morisaki H, Jahn R, Heuser JE. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell. 1997;90:523–535. doi: 10.1016/s0092-8674(00)80512-7. [DOI] [PubMed] [Google Scholar]
- Harata NC, Aravanis AM, Tsien RW. Kiss-and-run and full-collapse fusion as modes of exo-endocytosis in neurosecretion. J Neurochem. 2006;97:1546–1570. doi: 10.1111/j.1471-4159.2006.03987.x. [DOI] [PubMed] [Google Scholar]
- Heuvingh J, Pincet F, Cribier S. Hemifusion and fusion of giant vesicles induced by reduction of inter-membrane distance. Eur Phys J E Soft Matter. 2004;14:269–276. doi: 10.1140/epje/i2003-10151-2. [DOI] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Kesavan J, Borisovska M, Bruns D. v-SNARE actions during Ca(2+)-triggered exocytosis. Cell. 2007;131:351–363. doi: 10.1016/j.cell.2007.09.025. [DOI] [PubMed] [Google Scholar]
- Lee JY, Schick M. Dependence of the energies of fusion on the intermembrane separation: optimal and constrained. J Chem Phys. 2007;127 doi: 10.1063/1.2766945. 075102. [DOI] [PubMed] [Google Scholar]
- Li F, Pincet F, Perez E, Eng WS, Melia TJ, Rothman JE, Tareste D. Energetics and dynamics of SNAREpin folding across lipid bilayers. Nat Struct Mol Biol. 2007;14:890–896. doi: 10.1038/nsmb1310. [DOI] [PubMed] [Google Scholar]
- Martens S, Kozlov MM, McMahon HT. How synaptotagmin promotes membrane fusion. Science. 2007;316:1205–1208. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]
- McNew JA, Weber T, Engelman DM, Söllner TH, Rothman JE. The length of the flexible SNAREpin juxtamembrane region is a critical determinant of SNARE-dependent fusion. Mol Cell. 1999;4:415–421. doi: 10.1016/s1097-2765(00)80343-3. [DOI] [PubMed] [Google Scholar]
- Plattner H, Artalejo AR, Neher E. Ultrastructural organization of bovine chromaffin cell cortex-analysis by cryofixation and morphometry of aspects pertinent to exocytosis. J Cell Biol. 1997;139:1709–1717. doi: 10.1083/jcb.139.7.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quetglas S, Iborra C, Sasakawa N, De Haro L, Kumakura K, Sato K, Leveque C, Seagar M. Calmodulin and lipid binding to synaptobrevin regulates calcium-dependent exocytosis. EMBO J. 2002;21:3970–3979. doi: 10.1093/emboj/cdf404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonn JS, Desnos C, Henry JP, Darchen F. Transmitter uptake and release in PC12 cells overexpressing plasma membrane monoamine transporters. J Neurochem. 2003;84:669–677. doi: 10.1046/j.1471-4159.2003.01561.x. [DOI] [PubMed] [Google Scholar]
- Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell. 2007;128:183–195. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Siddiqui TJ, Vites O, Stein A, Heintzmann R, Jahn R, Fasshauer D. Determinants of synaptobrevin regulation in membranes. Mol Biol Cell. 2007;18:2037–2046. doi: 10.1091/mbc.E07-01-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen JB, Wiederhold K, Müller EM, Milosevic I, Nagy G, de Groot BL, Grubmüller H, Fasshauer D. Sequential N- to C-terminal SNARE complex assembly drives priming and fusion of secretory vesicles. EMBO J. 2006;25:955–966. doi: 10.1038/sj.emboj.7601003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenovec M, Kreft M, Poberaj I, Betz WJ, Zorec R. Slow spontaneous secretion from single large dense-core vesicles monitored in neuroendocrine cells. FASEB J. 2004;18:1270–1272. doi: 10.1096/fj.03-1397fje. [DOI] [PubMed] [Google Scholar]
- Sun J, Pang ZP, Qin D, Fahim AT, Adachi R, Südhof TC. A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature. 2007;450:676–682. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraska JW, Perrais D, Ohara-Imaizumi M, Nagamatsu S, Almers W. Secretory granules are recaptured largely intact after stimulated exocytosis in cultured endocrine cells. Proc Natl Acad Sci U S A. 2003;100:2070–2075. doi: 10.1073/pnas.0337526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardjan N, Stenovec M, Jorgacevski J, Kreft M, Zorec R. Subnanometer fusion pores in spontaneous exocytosis of peptidergic vesicles. J Neurosci. 2007;27:4737–4746. doi: 10.1523/JNEUROSCI.0351-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Söllner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Xu T, Binz T, Niemann H, Neher E. Multiple kinetic components of exocytosis distinguished by neurotoxin sensitivity. Nat Neurosci. 1998;1:192–200. doi: 10.1038/642. [DOI] [PubMed] [Google Scholar]
- Xu T, Rammner B, Margittai M, Artalejo AR, Neher E, Jahn R. Inhibition of SNARE complex assembly differentially affects kinetic components of exocytosis. Cell. 1999;99:713–722. doi: 10.1016/s0092-8674(00)81669-4. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J, Akimov SA, Frolov V. Synaptotagmin: fusogenic role for calcium sensor? Nat Struct Mol Biol. 2006;13:301–303. doi: 10.1038/nsmb0406-301. [DOI] [PubMed] [Google Scholar]