Abstract
Modafinil is a wake-promoting compound with low abuse potential used in the treatment of narcolepsy. Although the compound is reported to affect multiple neurotransmitter systems such as catecholamines, serotonin, glutamate, GABA, orexin, and histamine, however, the molecular mechanism by which modafinil increases wakefulness is debated. Herein we used dopamine (DA) D2 receptor (D2R)-deficient mice combined with D1R- and D2R-specific antagonists to clarify the role of DA receptors in the arousal effects of modafinil. In wild-type mice, intraperitoneal modafinil induced wakefulness in a dose-dependent manner. Pretreatment with either D1R antagonist SCH23390 [R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine] at 30 μg/kg or D2R antagonist raclopride at 2 mg/kg blocked the arousal effects of low-dose modafinil at 22.5 and 45 mg/kg. When modafinil was given at 90 and 180 mg/kg, pretreatment of D1R antagonist did not affect the wakefulness at all, whereas D2R antagonist significantly attenuated the wakefulness to the half level compared with vehicle control. Similarly, D2R knock-out (KO) mice exhibited attenuated modafinil-induced wakefulness. However, pretreatment of D2R KO mice with D1R antagonist completely abolished arousal effects of modafinil. These findings strongly indicate that dopaminergic D1R and D2R are essential for the wakefulness induced by modafinil.
Keywords: dopamine receptor, EEG, knock-out mice, modafinil, sleep, wakefulness
Introduction
Modafinil [(diphenyl-methyl)-sulfinyl-2-acetamide; Modiodal, Provigil] is a novel wake-promoting agent first marketed in France in the early 1990s and currently approved by the United States Food and Drug Administration to treat excessive daytime sleepiness associated with narcolepsy, shift-work sleep disorder, and obstructive sleep apnea/hypopnea syndrome (Zeitzer et al., 2006; Minzenberg and Carter, 2008). Modafinil acts differently from established psychostimulants (Lin et al., 1992) and possesses only minimal potential for abuse (Jasinski, 2000; Deroche-Gamonet et al., 2002; Rao et al., 2007). However, the mechanism of modafinil-induced wakefulness is controversial.
Several hypotheses were suggested for modafinil's mechanism of action by in vivo and in vitro studies. Modafinil can increase catecholamine, serotonin, and glutamate release, and decrease GABA release in various brain regions (for review, see Minzenberg and Carter, 2008), as well as activate orexin-containing hypothalamic neurons (Chemelli et al., 1999) and the histaminergic tuberomammillary nucleus (TMN) (Scammell et al., 2000). To date, the only central neurotransmitter elements to which modafinil has been demonstrated to bind directly are the dopamine (DA) transporter (DAT) and norepinephrine (NE) transporter (NET), whereas no apparent specific binding to a range of other monoamine or neuropeptide receptors or transporters or nerve membrane ion channels has been reported (Mignot et al., 1994). The effects of modafinil on catecholamine are proposed to be primary, with those on other systems seemingly being secondary to the effects on DA and/or NE (Minzenberg and Carter, 2008).
Although some studies suggested that modafinil increases wakefulness by activating central noradrenergic transmission (Duteil et al., 1990; Lin et al., 1992; Stone et al., 2002), this hypothesis produced several unresolved questions about the role of noradrenergic systems in modafinil-induced wakefulness: mainly why modafinil does not affect the peripheral sympathetic system (Duteil et al., 1990) and why in narcoleptic patients and dogs modafinil effectively treats excessive daytime sleepiness but fails to prevent the loss of muscle tone (Billiard et al., 1994; Shelton et al., 1995; Nishino and Mignot, 1997; Nishino et al., 1998). In addition, pharmacological elimination of the NET-bearing forebrain projections in mice did not influence the efficacy of modafinil action (Wisor and Eriksson, 2005). On the other hand, modafinil enhances extracellular levels of DA in the nucleus accumbens and prefrontal cortex and increases wakefulness in rats (de Saint Hilaire et al., 2001; Murillo-Rodríguez et al., 2007). Inhibition of DAT increases extracellular levels of DA, which activates its receptors to regulate the sleep–wake cycle. Wisor et al. (2001) reported that modafinil does not induce wakefulness in DAT knock-out (KO) mice. However, Jones et al. (1999) and Fauchey et al. (2000) have found that DAT KO mice have downregulation of the D1 receptor (D1R) and D2R, opening up the question of whether the reduction in the response was attributable to reduction in receptor levels. Therefore, the role of dopaminergic systems and DA receptors in modafinil-induced wakefulness remains unclear.
D1R and D2R are the most widely and abundantly expressed receptors for DA in the brain (Kobayashi et al., 2004). Because double-KO mice for D1R and D2R do not survive to the age required for electroencephalogram (EEG) recording (Kobayashi et al., 2004), here we used D2R KO mice in conjunction with DA receptor antagonists and found that D1R and D2R are essential for the arousal effect of modafinil.
Materials and Methods
Animals.
D2R KO and wild-type (WT) mice of the inbred C57BL/6 strain were generated from heterozygotes (Yamaguchi et al., 1996) and maintained at Oriental Bioservice. Male D2R KO mice and their WT littermates, weighing 20–26 g (11–13 weeks old), were used in these experiments. The animals were housed in an insulated sound-proof recording room maintained at an ambient temperature of 22 ± 0.5°C with a relative humidity of 60 ± 2% on an automatically controlled 12 h light/12 h dark cycle (light on at 8:00 A.M.) and had ad libitum access to food and water. Experimental protocols were approved by the Animal Care Committee of Osaka Bioscience Institute. Every effort was made to minimize the number of animals used and any pain and discomfort experienced by them.
Pharmacological treatments.
Modafinil (Sigma-Aldrich) was dissolved in sterile PBS containing 10% DMSO and 2% (w/v) cremophor immediately before use and administered intraperitoneally at 10:00 A.M. on the experimental day at doses of 22.5, 45, 90, and 180 mg/kg. R-(+)-7-Chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine (SCH23390) (Sigma-Aldrich), a selective D1R antagonist, and raclopride (Sigma-Aldrich), a selective D2R antagonist, were dissolved in sterile saline and administered intraperitoneally 30 min before the injection of modafinil. For baseline data, mice were injected intraperitoneally with vehicle only at 9:30 and 10:00 A.M.
Polygraphic recordings and vigilance state analysis.
Under pentobarbital anesthesia (50 mg/kg, i.p.), mice were chronically implanted with EEG and electromyogram (EMG) electrodes for polysomnographic recordings. The implant consisted of two stainless-steel screws (1 mm diameter) inserted through the skull of the cortex (anteroposterior, +1.0 mm; left–right, −1.5 mm from bregma or lambda) according to the atlas of Franklin and Paxinos (1997) and served as EEG electrodes. Two insulated stainless-steel, Teflon-coated wires bilaterally placed into both trapezius muscles served as EMG electrodes. All electrodes were attached to a microconnector and fixed to the skull with dental cement.
The recordings of EEG and EMG were performed by means of a slip ring, designed so that the behavioral movement of the mice would not be restricted. After a 10 d recovery period, the mice were housed individually in transparent barrels and habituated to the recording cable for 3–4 d before polygraphic recording. Sleep–wakefulness states were monitored for a period of 48 h, which comprised baseline and experimental days. Baseline recordings were taken in each animal for 24 h, beginning at 8:00 A.M., which served as the control for the same animal.
Cortical EEG and EMG signals were amplified and filtered (EEG, 0.5–30 Hz; EMG, 20–200 Hz) and then digitized at a sampling rate of 128 Hz and recorded by using SleepSign (Kissei Comtec) as described previously (Huang et al., 2001, 2005; Qu et al., 2006). When complete, polygraphic recordings were automatically scored off-line by 10 s epochs as wakefulness, rapid eye movement (REM), and non-REM (NREM) sleep by SleepSign according to standard criteria (Huang et al., 2001, 2006; Qu et al., 2006; Kohtoh et al., 2008). As a final step, defined sleep–wake stages were examined visually and corrected if necessary.
Statistical analysis.
All results were expressed as means ± SEM. For the time course data, amounts of the different sleep–wake states were analyzed by the paired t test, with each animal serving as its own control. For the total amounts of each vigilance stage for 9 h after drug treatment, one- or two-way repeated-measures ANOVA followed by the Fisher's probable least-squares difference (PLSD) test was used to determine whether the difference among groups and genotypes was statistically significant. In all cases, p < 0.05 was taken as the level of significance.
Results
D1R antagonist blocked arousal effects of low-dose modafinil, but did not affect it when modafinil was given at 90 and 180 mg/kg
To investigate the contribution of D1R and D2R to the arousal effects of modafinil, we administered D1R antagonist SCH23390 (30 μg/kg) or D2R antagonist raclopride (2 mg/kg) at 9:30 A.M., followed by modafinil administration at 10:00 A.M. into C57BL/6 WT mice. The dosages for SCH23390 and raclopride were selected based on previous reports (Ongini et al., 1993; Simon et al., 1995).
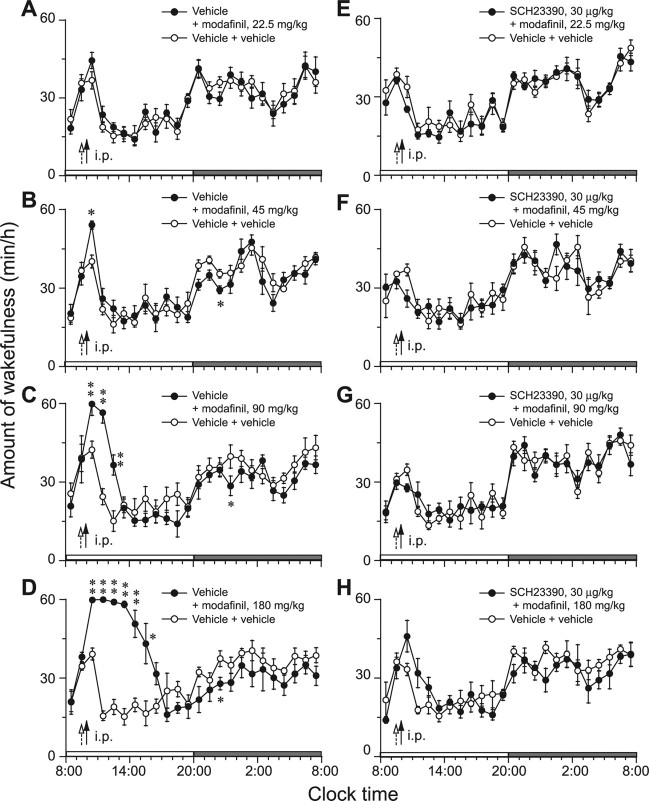
Time course changes in wakefulness showed that the arousal effects caused by intraperitoneal modafinil at doses of 22.5, 45, 90, and 180 mg/kg lasted for 2, 3, 5, and 9 h, respectively, compared with the wake time for the vehicle control (Fig. 1A–D). During the wakefulness, no NREM or REM sleep episodes appeared. This enhancement of wakefulness was concomitant with decreases in NREM and REM sleep (data not shown). There was no further disruption of sleep architecture during the subsequent light period, but a decrease in wakefulness during the subsequent dark periods was observed in the groups treated with modafinil at 45, 90, and 180 mg/kg.
Figure 1.
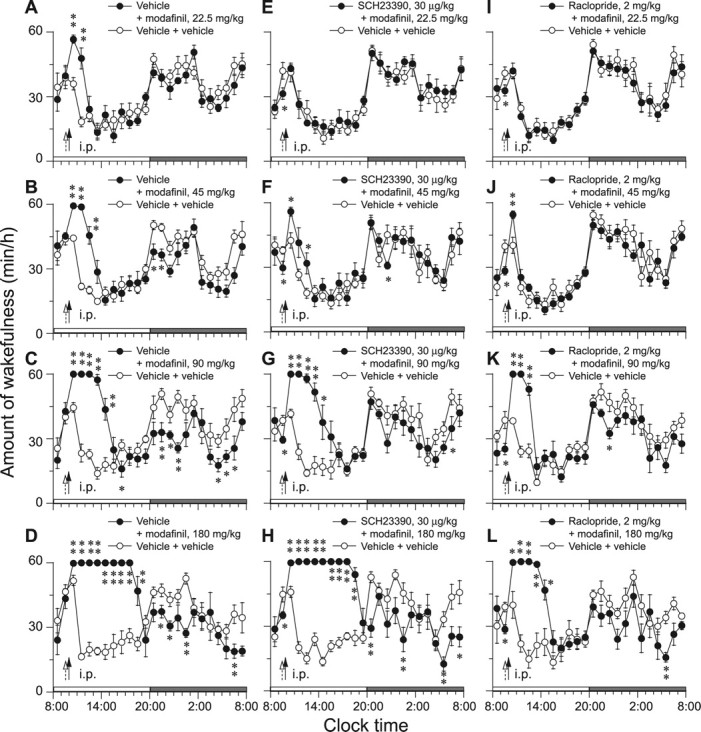
A–L, Time course changes in wakefulness of WT mice after intraperitoneal administration of modafinil at four doses (A–D) and pretreatment with D1R antagonist SCH23390 (E–H) or D2R antagonist raclopride (I–L). Each circle represents the hourly mean amounts of wakefulness. Open and closed circles stand for the baseline- and experimental-day profiles, respectively. On the experimental day, modafinil was given at 10:00 A.M., as indicated by the closed arrow, and D1R or D2R antagonist was given at 9:30 A.M., as indicated by the open arrow. The vehicle was used for the baseline day. The horizontal open and filled bars on the x-axes indicate the 12 h light and dark periods, respectively. Values are the means ± SEM (n = 6–8; 6 mice used in B, D, K, and L; 7 in A, E, F, H, and I; and 8 in C, G, and J). *p < 0.05, **p < 0.01 by the paired t test.
Pretreatment with the D1R antagonist SCH23390 at a dose of 30 μg/kg 30 min before administration of modafinil completely abolished the arousal effects of modafinil at 22.5 mg/kg and significantly attenuated them when modafinil was injected at 45 mg/kg (Fig. 1E,F). However, when given at 90 and 180 mg/kg, pretreatment with this antagonist did not lessen the wakefulness at all (Fig. 1G,H).
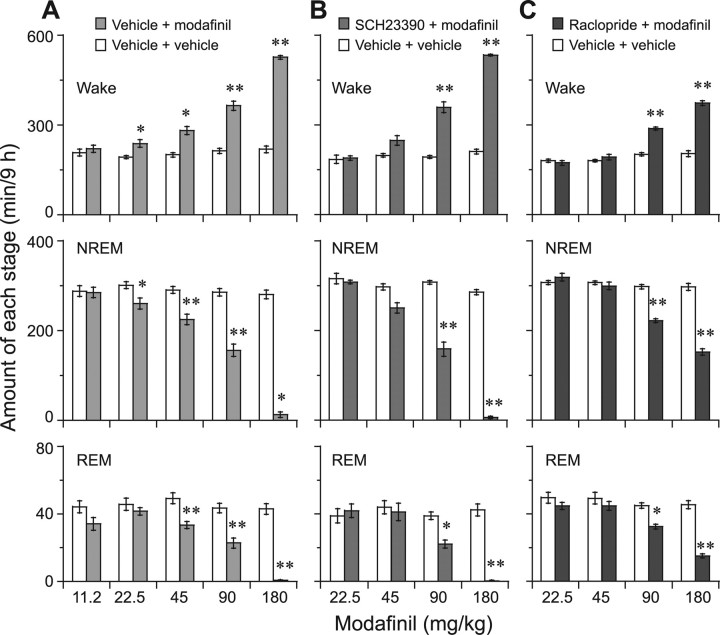
Because the highest dose of modafinil (180 mg/kg) induced wakefulness for 9 h, we calculated the total amount of wakefulness after modafinil administration. Compared with the vehicle control, modafinil at 22.5, 45, 90, and 180 mg/kg significantly increased wakefulness by 1.2-, 1.4-, 1.7-, and 2.4-fold, concomitant with a significant decrease in NREM sleep by 13, 22, 45, and 96%, respectively. As to REM sleep, modafinil at 22.5 mg/kg did not alter it for 9 h, whereas given at 45, 90, and 180 mg/kg, the drug did reduce REM sleep by 32, 51, and 98%, respectively. Modafinil at 11.25 mg/kg did not change the sleep–wake profiles (Fig. 2A). Pretreatment with the D1R antagonist abolished wakefulness caused by modafinil at 22.5 and 45 mg/kg. However, modafinil at doses of 90 and 180 mg/kg after D1R antagonist administration increased wakefulness by 1.2- and 2.5-fold, with a concomitant decrease in NREM by 48 and 98%, respectively, and one in REM sleep by 43 and 99%, respectively, compared with the vehicle control (Fig. 2B). These findings indicate that modafinil administered to WT mice rapidly and significantly increased the wake time in a dose-dependent manner and that the D1R antagonist could block arousal effects of modafinil at low dosages, but not at doses >90 mg/kg.
Figure 2.
A–C, Total time of WT mice spent in wakefulness and in NREM and REM sleep for 9 h after modafinil administration (A) or pretreatment with D1R antagonist SCH23390 (B) or D2R antagonist raclopride (C). Open and filled bars show the profiles for the baseline and experimental days, when the mice were treated with vehicle (for baseline day) or with modafinil or modafinil plus the indicated DA receptor antagonist (for experimental day). Modafinil was given at 10:00 A.M., and pretreatment with D1R or D2R antagonist was given at 9:30 A.M. on the experimental day. Values are the means ± SEM (n = 6–8). *p < 0.05, **p < 0.01 compared with its own control, by two-way ANOVA, followed by the PLSD test.
D2R antagonist blocked the arousal effect of low-dose modafinil and attenuated the effects of modafinil at 90 or 180 mg/kg
As shown in Figure 1, pretreatment of WT mice with the D2R antagonist raclopride at 2 mg/kg completely abolished the wakefulness caused by low-dose modafinil at 22.5 and 45 mg/kg (Fig. 1I,J) and decreased to 3 and 5 h the wakefulness caused by modafinil at 90 and 180 mg/kg, respectively (Fig. 1K,L). The antagonist pretreatment concomitantly decreased NREM sleep by 25 and 36%, respectively, and REM sleep by 32 and 61%, respectively, which are half the amounts of wakefulness obtained with modafinil administration only (Fig. 2A,C). These findings indicate that both D1R and D2R are involved in modafinil-induced wakefulness, with D2R having preferential importance.
D2R KO mice mimicked the effects of D2R antagonist on the wakefulness caused by modafinil
To clarify the importance of D2R in the arousal effects of modafinil, we used D2R KO mice. When modafinil was given at 22.5 mg/kg, D2R KO mice did not exhibit any significant increase in time spent in wakefulness, compared with the vehicle control (Fig. 3A). When administered at 45, 90, and 180 mg/kg, however, modafinil induced wakefulness for 3, 5, and 9 h, respectively, in WT mice (Fig. 1B–D), but it did so just for 1, 3, and 6 h, respectively, in the D2R KO mice (Fig. 3B–D).
Figure 3.
A–H, Time course changes in wakefulness of D2R KO mice after intraperitoneal administration of modafinil at four doses (A–D) or after pretreatment with D1R antagonist SCH23390 before modafinil (E–H). Each circle represents the hourly mean amount of wakefulness. Open and closed circles stand for the baseline- and experimental-day profiles, respectively. On the experimental day, modafinil was given at 10:00 A.M., as indicated by the closed arrow, and pretreatment with the D1R antagonist was done at 9:30 A.M., as indicated by the open arrow. The vehicle was used on the baseline day. The horizontal open and filled bars on the x-axes indicate the 12 h light and dark periods, respectively. Values are the means ± SEM (n = 6–8; 6 mice used in E and B; 7 in A, D, F, and H; and 8 in C and G). *p < 0.05, **p < 0.01 by the paired t test.
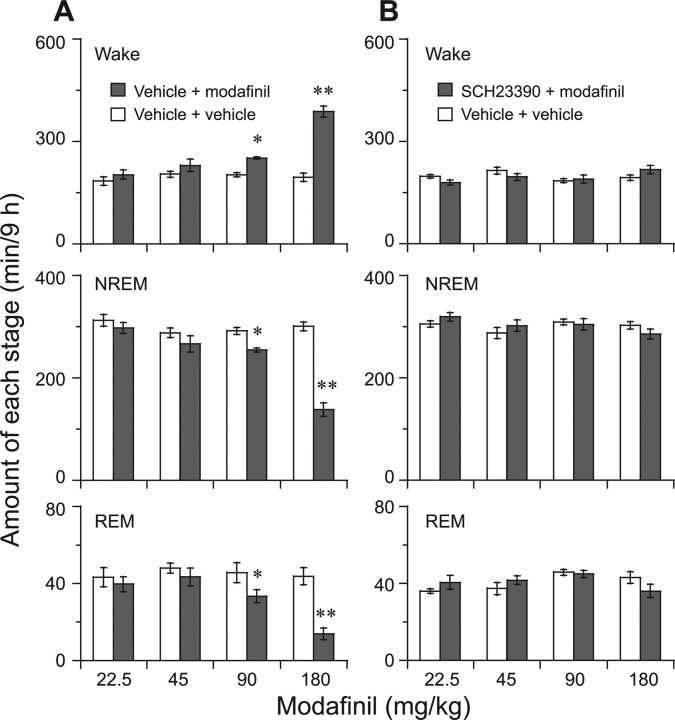
The total time spent in wakefulness and NREM and REM sleep for 9 h after administration was also determined. There was no essential difference in the amounts of wakefulness and NREM and REM sleep in response to vehicle versus modafinil administration at 22.5 and 45 mg/kg in D2R KO mice. Modafinil at 90 and 180 mg/kg increased wakefulness by 1.2 and twofold, respectively, during 9 h after administration, lower than the increase by 1.7- and 2.4-fold in WT mice (Fig. 4A). These findings, similar to those obtained from the WT mice pretreated with the D2R antagonist, indicate that the mice genetically deficient in D2R mimicked the attenuating effect of the drug on wakefulness in WT mice pretreated with the D2R antagonist.
Figure 4.
A, B, Total time spent in wakefulness and in NREM and REM sleep for 9 h after modafinil administration (A) and pretreatment with D1R antagonist before modafinil (B) to D2R KO mice. On the experimental day, modafinil was given at 10:00 A.M., and pretreatment with the D1R antagonist was given at 9:30 A.M. The vehicle was used on the baseline day. Each value is presented as the mean ± SEM (n = 6–8). *p < 0.05, **p < 0.01, significantly different from the control, as assessed by one-way ANOVA followed by the PLSD test.
Blockade of D1R in D2R KO mice completely abolished modafinil-induced wakefulness
There are no D1R and D2R double-KO mice available for sleep recording because these mutants do not survive to the second or third week after birth and are severely growth-retarded (Kobayashi et al., 2004). We pharmacologically blocked D1R and observed the arousal effects of modafinil in D2R KO mice. In WT mice, pretreatment with the D1R antagonist SCH23390 (30 μg/kg) did not affect the arousal effects of modafinil given at 90 and 180 mg/kg, because it increased the total amount of wakefulness during that 9 h period by 1.9- or 2.5-fold, respectively, the same effects as found for modafinil administration only. However, the arousal effect of modafinil at 22.5, 45, 90, or 180 mg/kg was completely antagonized (Fig. 3E–H), and also the amounts of NREM and REM sleep remained unchanged (Fig. 4B) in D2R KO mice pretreated with the D1R antagonist. These results clearly indicate that D1R and D2R act cooperatively or synergistically in the wakefulness-promoting effect of modafinil.
Effects of D1R and D2R on wake duration after modafinil administration
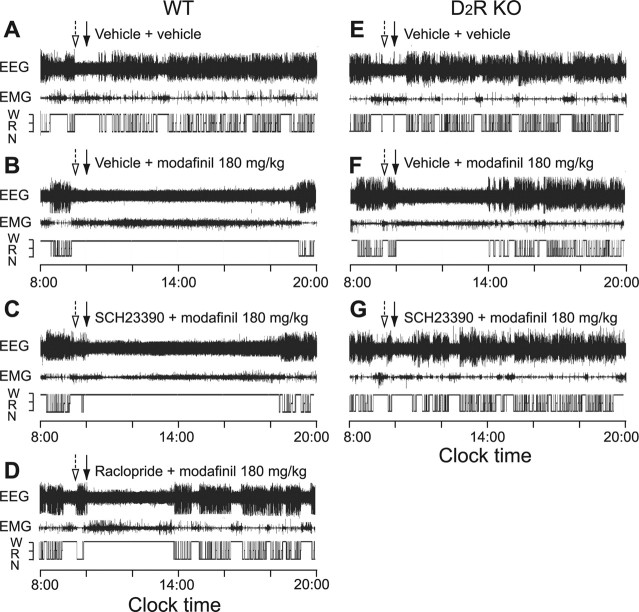
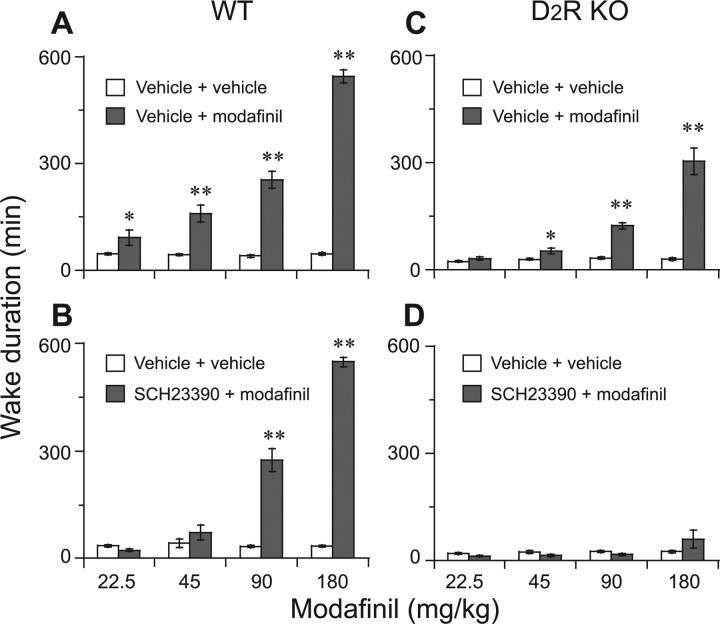
Wake duration is defined as the time from the injection of modafinil or vehicle to the appearance of the first NREM sleep episode lasting for at least 20 s (Huang et al., 2006). As shown in Figure 5, the modafinil-induced continuous wakefulness for 9 h at 180 mg/kg in WT mice (Fig. 5B) was shortened to 5 h in either D2R KO mice (Fig. 5F) or in WT mice pretreated with a D2R antagonist (Fig. 5D), and completely abolished by pretreatment of D2R KO mice with the D1R antagonist (Fig. 5G).
Figure 5.
Typical examples of EEG and EMG recordings and corresponding hypnograms in mice treated with vehicle or modafinil at 180 mg/kg. A–G, WT mice were treated with vehicle (A), modafinil only (B), SCH23390 + modafinil (C), or raclopride + modafinil (D), and D2R KO mice were treated with vehicle (E), modafinil only (F), and SCH23390 + modafinil (G). The open arrows indicate pretreatment with D1R or D2R antagonists or its vehicle, and closed arrows represent the time of modafinil injection or its vehicle. W, Wakefulness; R, REM sleep; N, NREM sleep.
Modafinil given at 22.5, 45, 90, and 180 mg/kg increased the duration of wakefulness relative to that of the vehicle alone by 2.6-, 4.1-, 6.3-, and 13.5-fold, respectively, in WT mice, and by 1.1-, 1.9-, 3.7-, and 10.1-fold, respectively, in D2R KO mice (Fig. 6A,C). Pretreatment of WT mice with the D1R antagonist blocked wakefulness by modafinil at 22.5 and 45 mg/kg, but modafinil at 90 and 180 mg/kg still increased wakefulness (Fig. 6B) in these mice; however, when pretreated with D1R antagonist in D2R KO mice, modafinil-induced wakefulness was completely abolished (Fig. 6D).
Figure 6.
Effects of pretreatment with SCH23390 on wake duration elicited by modafinil, which is defined as the time from the injection of modafinil or vehicle to the appearance of the first NREM sleep episode. A–D, WT mice were treated with modafinil only (A) or SCH23390 + modafinil (B), and D2R KO mice were treated with modafinil only (C) or SCH23390 + modafinil (D). Open and filled bars show the profiles for the vehicle and drug treatments, respectively. Each value represents the mean ± SEM (n = 6–8). *p < 0.05, **p < 0.01 compared with the vehicle injection and as assessed by ANOVA followed by the PLSD test.
Discussion
Genetic and pharmacological studies on the molecular mechanisms underlying modafinil-induced wakefulness revealed that D1R and D2R are essential to the mediation of the arousal effect of modafinil. Concomitant activation of D1R and D2R is also crucial in the control of other physiological functions regulated by DA (Aizman et al., 2000). For example, double-KO mice for D1R and D2R do not survive to the second or third week after birth. These mutants are severely growth-retarded with altered feeding behavior and dysfunction of the gastrointestinal system, suggesting the interrelationship between D1 and D2 receptor-mediated control of motor activity, food intake, and gastrointestinal functions (Kobayashi et al., 2004). Similarly, modafinil's effects on cognition is probably mediated by these two receptors (Minzenberg and Carter, 2008). These results indicate the presence of cooperative/synergistic functional interactions between D1R and D2R.
Pretreatment of D2R KO mice with D1R antagonist completely abolished modafinil-induced wakefulness, indicating that DA plays an important role in arousal effect of modafinil. Modafinil effects on wakefulness has been reported to be abolished in DAT KO mice (Wisor et al., 2001), in which D2 autoreceptor or function also appears severely impaired (Jones et al., 1999). mRNA levels of both the D1R and D2R are also demonstrated to be decreased in DAT KO mice (Fauchey et al., 2000). The sole role of DAT in the arousal effect of modafinil is not easily explained considering the fact that maintenance of the modafinil wake-promoting action occurs in cats treated with an inhibitor of catecholamine synthesis (Lin et al., 1992). These findings suggested that impairment of D1R and D2R may also contribute to the abolishment of modafinil wakefulness in DAT KO mice.
The D1R is a postsynaptic receptor and D2R has two variants, a short form and a long form, representing presynaptic autoreceptors and postsynaptic receptors, respectively (Usiello et al., 2000). Dopamine-containing cells of the substantia nigra pars compacta and the ventral tegmental area (VTA) do not express D1R mRNA (Weiner et al., 1991), whereas the D2R has been characterized on neuron cell bodies and dendrites in the substantia nigra pars compacta and the VTA, where it serves an autoreceptor function (Missale et al., 1998). D1R is coupled to adenylate cyclase, and its stimulation facilitates the activity of the enzyme. Dopamine receptors corresponding to the D2 subfamily are coupled to the inhibition of adenylate cyclase (Monti and Monti, 2007). An earlier study found no effects of modafinil on the activity of mesencephalic DA single units in rats (Akaoka et al., 1991), whereas a recent study found that in rat brain slices, modafinil (20 μm) inhibits the activity of VTA DA neurons, with this effect abolished by D2R antagonist sulpiride, blunted by DA reuptake blocker nomifensine and unaffected by α1-adrenoreceptor blocker prazosin (Korotkova et al., 2007). These latter findings are consistent with modafinil inhibition of DA reuptake, leading to increased activation of the DA cell body autoreceptor to diminish DA cell firing. However, the derived current–voltage relationships for modafinil-evoked versus nomifensine-evoked currents showed a very different reversal potential in response to these two agents, suggesting that modafinil may exert its action in this preparation at a site distinct from the DAT. These in vitro studies led the authors to propose a potential agonistic action of modafinil on the D2R (Korotkova et al., 2007).
The involvement of the D2R is an attractive hypothesis, because it could explain a number of findings that are not easily explained by the DAT inhibition hypothesis. Increased serotonin release would be expected because of the excitatory function of D2R on dorsal raphe serotonin neurons (Haj-Dahmane, 2001). Modafinil reduces the outflow of GABA in cortex, striatum, and posterior hypothalamus as measured by microdialysis (Ferraro et al., 1996, 1998). Inhibition of GABA release in the striatum (Ferraro et al., 1998) can be easily attributed to D2R activation. GABA release in the posterior hypothalamus may promote sleep via inhibition of the TMN (Hong et al., 2005), and a reduction in GABAergic activity could disinhibit these hypothalamic regions, resulting in increased wakefulness.
Modafinil does increase fos immunoreactivity in identified orexin cells in the perifornical area of mice and rats (Chemelli et al., 1999; Scammell et al., 2000). However, modafinil induces wakefulness more potently in orexin KO mice than in WT mice, with similar patterns of fos immunoreactivity (Willie et al., 2005). In addition, modafinil does not bind the orexin 1 receptor (Wieland et al., 2002) and retains effects on both extracellular striatal DA and wake-promoting activity in orexin 2 receptor-deficient narcoleptic dogs (Wisor et al., 2001). Histaminergic systems play an important role in orexin-induced wakefulness (Huang et al., 2001, 2007). Although modafinil also activates fos expression in the TMN, which contains wake-promoting histaminergic neurons (Scammell et al., 2000), and both intraperitoneal and intracerebroventricular administration of modafinil elevate extracellular histamine in the anterior hypothalamus, direct injection of modafinil into the TMN does not affect histamine release (Ishizuka et al., 2003), and arousal effects still appeared in the mice lacking the histamine synthesis enzyme histidine decarboxylase, and therefore lacking histamine in the CNS (Parmentier et al., 2007). Therefore, activation of orexin and histamine systems may be secondary effects to the wakefulness caused by modafinil.
D2R KO mice exhibit only minor changes in DA synthesis and metabolism, with normal extracellular DA levels (Dickinson et al., 1999). An immunocytochemistry study found an increased staining for DAT in the striatum of D2R KO mice (Parish et al., 2001). Autoradiography using [3H]SCH23390 revealed no qualitative regional differences in the distribution of D1R binding sites in the brains of D2R KO mice, including ventral striatum and frontal cortex (Kelly et al., 1998). Behaviorally, D2R KO mice exhibited a normal circadian profile of sleep–wake cycle (Fig. 3) but with an increase in NREM sleep during dark periods (data not shown). Although circadian rhythmicity was normal, D2R KO mice showed a markedly deficient light-masking response, indicating that D2R-mediated signaling is an essential component of the neuronal pathways leading to light masking of circadian rhythms (Doi et al., 2006).
In conclusion, we found that either D1R or D2R antagonist blocked the arousal effects of low-dose modafinil at 22.5 and 45 mg/kg. When modafinil given at 90 and 180 mg/kg, D1R antagonist did not effectively affect high-dose modafinil-induced wakefulness but D2R antagonist significantly attenuated it. D2R KO mice exhibited the similar effect of WT mice pretreated with a D2R antagonist. Pretreatment of D2R KO mice with a D1R antagonist completely abolished the arousal effects of modafinil. These findings strongly indicate that D1R and D2R are essential for the arousal effect of modafinil, with D2R being the receptor of primary importance.
Footnotes
This work was supported in part by Grants-in-Aid for scientific research from the Japan Society for the Promotion of Science (W.-M.Q.), the Genome Network Project from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (Y.U.), the National Natural Science Foundation of China (30625021), Shanghai Pujiang Program (06PJ14008), Shanghai Leading Academic Discipline Project (B119), Takeda Science Foundation, and Osaka City. We thank M. Katsuki for providing D2 receptor KO mice.
References
- Aizman O, Brismar H, Uhlén P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci. 2000;3:226–230. doi: 10.1038/72929. [DOI] [PubMed] [Google Scholar]
- Akaoka H, Roussel B, Lin JS, Chouvet G, Jouvet M. Effect of modafinil and amphetamine on the rat catecholaminergic neuron activity. Neurosci Lett. 1991;123:20–22. doi: 10.1016/0304-3940(91)90148-m. [DOI] [PubMed] [Google Scholar]
- Billiard M, Besset A, Montplaisir J, Laffont F, Goldenberg F, Weill JS, Lubin S. Modafinil: a double-blind multicentric study. Sleep. 1994;17:S107–S112. doi: 10.1093/sleep/17.suppl_8.s107. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Darnaudéry M, Bruins-Slot L, Piat F, Le Moal M, Piazza PV. Study of the addictive potential of modafinil in naive and cocaine-experienced rats. Psychopharmacology. 2002;161:387–395. doi: 10.1007/s00213-002-1080-8. [DOI] [PubMed] [Google Scholar]
- de Saint Hilaire Z, Orosco M, Rouch C, Blanc G, Nicolaidis S. Variations in extracellular monoamines in the prefrontal cortex and medial hypothalamus after modafinil administration: a microdialysis study in rats. Neuroreport. 2001;12:3533–3537. doi: 10.1097/00001756-200111160-00032. [DOI] [PubMed] [Google Scholar]
- Dickinson SD, Sabeti J, Larson GA, Giardina K, Rubinstein M, Kelly MA, Grandy DK, Low MJ, Gerhardt GA, Zahniser NR. Dopamine D2 receptor-deficient mice exhibit decreased dopamine transporter function but no changes in dopamine release in dorsal striatum. J Neurochem. 1999;72:148–156. doi: 10.1046/j.1471-4159.1999.0720148.x. [DOI] [PubMed] [Google Scholar]
- Doi M, Yujnovsky I, Hirayama J, Malerba M, Tirotta E, Sassone-Corsi P, Borrelli E. Impaired light masking in dopamine D2 receptor-null mice. Nat Neurosci. 2006;9:732–734. doi: 10.1038/nn1711. [DOI] [PubMed] [Google Scholar]
- Duteil J, Rambert FA, Pessonnier J, Hermant JF, Gombert R, Assous E. Central alpha 1-adrenergic stimulation in relation to the behaviour stimulating effect of modafinil; studies with experimental animals. Eur J Pharmacol. 1990;180:49–58. doi: 10.1016/0014-2999(90)90591-s. [DOI] [PubMed] [Google Scholar]
- Fauchey V, Jaber M, Caron MG, Bloch B, Le Moine C. Differential regulation of the dopamine D1, D2 and D3 receptor gene expression and changes in the phenotype of the striatal neurons in mice lacking the dopamine transporter. Eur J Neurosci. 2000;12:19–26. doi: 10.1046/j.1460-9568.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Tanganelli S, O'Connor WT, Antonelli T, Rambert F, Fuxe K. The vigilance promoting drug modafinil decreases GABA release in the medial preoptic area and in the posterior hypothalamus of the awake rat: possible involvement of the serotonergic 5-HT3 receptor. Neurosci Lett. 1996;220:5–8. doi: 10.1016/s0304-3940(96)13212-2. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, O'Connor WT, Tanganelli S, Rambert FA, Fuxe K. The effects of modafinil on striatal, pallidal and nigral GABA and glutamate release in the conscious rat: evidence for a preferential inhibition of striato-pallidal GABA transmission. Neurosci Lett. 1998;253:135–138. doi: 10.1016/s0304-3940(98)00629-6. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. San Diego: Academic; 1997. The mouse brain in stereotaxic coordinates. [Google Scholar]
- Haj-Dahmane S. D2-like dopamine receptor activation excites rat dorsal raphe 5-HT neurons in vitro. Eur J Neurosci. 2001;14:125–134. doi: 10.1046/j.0953-816x.2001.01616.x. [DOI] [PubMed] [Google Scholar]
- Hong ZY, Huang ZL, Qu WM, Eguchi N, Urade Y, Hayaishi O. An adenosine A2A receptor agonist induces sleep by increasing GABA release in the tuberomammillary nucleus to inhibit histaminergic systems in rats. J Neurochem. 2005;92:1542–1549. doi: 10.1111/j.1471-4159.2004.02991.x. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Qu WM, Li WD, Mochizuki T, Eguchi N, Watanabe T, Urade Y, Hayaishi O. Arousal effect of orexin A depends on activation of the histaminergic system. Proc Natl Acad Sci U S A. 2001;98:9965–9970. doi: 10.1073/pnas.181330998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Mochizuki T, Qu WM, Hong ZY, Watanabe T, Urade Y, Hayaishi O. Altered sleep-wake characteristics and lack of arousal response to H3 receptor antagonist in histamine H1 receptor knockout mice. Proc Natl Acad Sci U S A. 2006;103:4687–4692. doi: 10.1073/pnas.0600451103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZL, Urade Y, Hayaishi O. Prostaglandins and adenosine in the regulation of sleep and wakefulness. Curr Opin Pharmacol. 2007;7:33–38. doi: 10.1016/j.coph.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Ishizuka T, Sakamoto Y, Sakurai T, Yamatodani A. Modafinil increases histamine release in the anterior hypothalamus of rats. Neurosci Lett. 2003;339:143–146. doi: 10.1016/s0304-3940(03)00006-5. [DOI] [PubMed] [Google Scholar]
- Jasinski DR. An evaluation of the abuse potential of modafinil using methylphenidate as a reference. J Psychopharmacol. 2000;14:53–60. doi: 10.1177/026988110001400107. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Hu XT, Cooper DC, Wightman RM, White FJ, Caron MG. Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat Neurosci. 1999;2:649–655. doi: 10.1038/10204. [DOI] [PubMed] [Google Scholar]
- Kelly MA, Rubinstein M, Phillips TJ, Lessov CN, Burkhart-Kasch S, Zhang G, Bunzow JR, Fang Y, Gerhardt GA, Grandy DK, Low MJ. Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J Neurosci. 1998;18:3470–3479. doi: 10.1523/JNEUROSCI.18-09-03470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Iaccarino C, Saiardi A, Heidt V, Bozzi Y, Picetti R, Vitale C, Westphal H, Drago J, Borrelli E. Simultaneous absence of dopamine D1 and D2 receptor-mediated signaling is lethal in mice. Proc Natl Acad Sci U S A. 2004;101:11465–11470. doi: 10.1073/pnas.0402028101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohtoh S, Taguchi Y, Matsumoto N, Wada M, Huang ZL, Urade Y. Algorithm for sleep scoring in experimental animals based on fast Fourier transform power spectrum analysis of the electroencephalogram. Sleep Biol Rhythms. 2008;6:163–171. [Google Scholar]
- Korotkova TM, Klyuch BP, Ponomarenko AA, Lin JS, Haas HL, Sergeeva OA. Modafinil inhibits rat midbrain dopaminergic neurons through D2-like receptors. Neuropharmacology. 2007;52:626–633. doi: 10.1016/j.neuropharm.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Lin JS, Roussel B, Akaoka H, Fort P, Debilly G, Jouvet M. Role of catecholamines in the modafinil and amphetamine induced wakefulness, a comparative pharmacological study in the cat. Brain Res. 1992;591:319–326. doi: 10.1016/0006-8993(92)91713-o. [DOI] [PubMed] [Google Scholar]
- Mignot E, Nishino S, Guilleminault C, Dement WC. Modafinil binds to the dopamine uptake carrier site with low affinity. Sleep. 1994;17:436–437. doi: 10.1093/sleep/17.5.436. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33:1477–1502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Monti JM, Monti D. The involvement of dopamine in the modulation of sleep and waking. Sleep Med Rev. 2007;11:113–133. doi: 10.1016/j.smrv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Murillo-Rodríguez E, Haro R, Palomero-Rivero M, Millán-Aldaco D, Drucker-Colín R. Modafinil enhances extracellular levels of dopamine in the nucleus accumbens and increases wakefulness in rats. Behav Brain Res. 2007;176:353–357. doi: 10.1016/j.bbr.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Nishino S, Mignot E. Pharmacological aspects of human and canine narcolepsy. Prog Neurobiol. 1997;52:27–78. doi: 10.1016/s0301-0082(96)00070-6. [DOI] [PubMed] [Google Scholar]
- Nishino S, Mao J, Sampathkumaran R, Shelton J. Increased dopaminergic transmission mediates the wake-promoting effects of CNS stimulants. Sleep Res Online. 1998;1:49–61. [PubMed] [Google Scholar]
- Ongini E, Bonizzoni E, Ferri N, Milani S, Trampus M. Differential effects of dopamine D-1 and D-2 receptor antagonist antipsychotics on sleep-wake patterns in the rat. J Pharmacol Exp Ther. 1993;266:726–731. [PubMed] [Google Scholar]
- Parish CL, Finkelstein DI, Drago J, Borrelli E, Horne MK. The role of dopamine receptors in regulating the size of axonal arbors. J Neurosci. 2001;21:5147–5157. doi: 10.1523/JNEUROSCI.21-14-05147.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier R, Anaclet C, Guhennec C, Brousseau E, Bricout D, Giboulot T, Bozyczko-Coyne D, Spiegel K, Ohtsu H, Williams M, Lin JS. The brain H3-receptor as a novel therapeutic target for vigilance and sleep-wake disorders. Biochem Pharmacol. 2007;73:1157–1171. doi: 10.1016/j.bcp.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Qu WM, Huang ZL, Xu XH, Aritake K, Eguchi N, Nambu F, Narumiya S, Urade Y, Hayaishi O. Lipocalin-type prostaglandin D synthase produces prostaglandin D2 involved in regulation of physiological sleep. Proc Natl Acad Sci U S A. 2006;103:17949–17954. doi: 10.1073/pnas.0608581103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Y, Liu ZW, Borok E, Rabenstein RL, Shanabrough M, Lu M, Picciotto MR, Horvath TL, Gao XB. Prolonged wakefulness induces experience-dependent synaptic plasticity in mouse hypocretin/orexin neurons. J Clin Invest. 2007;117:4022–4033. doi: 10.1172/JCI32829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS, Saper CB. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20:8620–8628. doi: 10.1523/JNEUROSCI.20-22-08620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton J, Nishino S, Vaught J, Dement WC, Mignot E. Comparative effects of modafinil and amphetamine on daytime sleepiness and cataplexy of narcoleptic dogs. Sleep. 1995;18:817–826. [PubMed] [Google Scholar]
- Simon P, Hémet C, Ramassamy C, Costentin J. Non-amphetaminic mechanism of stimulant locomotor effect of modafinil in mice. Eur Neuropsychopharmacol. 1995;5:509–514. doi: 10.1016/0924-977x(95)00041-m. [DOI] [PubMed] [Google Scholar]
- Stone EA, Cotecchia S, Lin Y, Quartermain D. Role of brain alpha 1B-adrenoceptors in modafinil-induced behavioral activity. Synapse. 2002;46:269–270. doi: 10.1002/syn.10127. [DOI] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rougé-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Sunahara RK, Niznik HB, O'Dowd BF, Seeman P, Brann MR. D1 and D2 dopamine receptor mRNA in rat brain. Proc Natl Acad Sci U S A. 1991;88:1859–1863. doi: 10.1073/pnas.88.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland HA, Söll RM, Doods HN, Stenkamp D, Hurnaus R, Lämmle B, Beck-Sickinger AG. The SK-N-MC cell line expresses an orexin binding site different from recombinant orexin 1-type receptor. Eur J Biochem. 2002;269:1128–1135. doi: 10.1046/j.0014-2956.2001.02739.x. [DOI] [PubMed] [Google Scholar]
- Willie JT, Renthal W, Chemelli RM, Miller MS, Scammell TE, Yanagisawa M, Sinton CM. Modafinil more effectively induces wakefulness in orexin-null mice than in wild-type littermates. Neuroscience. 2005;130:983–995. doi: 10.1016/j.neuroscience.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Eriksson KS. Dopaminergic-adrenergic interactions in the wake promoting mechanism of modafinil. Neuroscience. 2005;132:1027–1034. doi: 10.1016/j.neuroscience.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21:1787–1794. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Aiba A, Nakamura K, Nakao K, Sakagami H, Goto K, Kondo H, Katsuki M. Dopamine D2 receptor plays a critical role in cell proliferation and proopiomelanocortin expression in the pituitary. Genes Cells. 1996;1:253–268. doi: 10.1046/j.1365-2443.1996.d01-238.x. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Nishino S, Mignot E. The neurobiology of hypocretins (orexins), narcolepsy and related therapeutic interventions. Trends Pharmacol Sci. 2006;27:368–374. doi: 10.1016/j.tips.2006.05.006. [DOI] [PubMed] [Google Scholar]