Abstract
The congenital muscular dystrophies present in infancy with muscle weakness and are often associated with mental retardation. Many of these inherited disorders share a common etiology: defective O-glycosylation of α-dystroglycan, a component of the dystrophin complex. Protein-O-mannosyl transferase 1 (POMT1) is the first enzyme required for the glycosylation of α-dystroglycan, and mutations in the POMT1 gene can lead to both Walker-Warburg syndrome (WWS) and limb girdle muscular dystrophy type 2K (LGMD2K). WWS is associated with severe mental retardation and major structural abnormalities in the brain; however, LGMD2K patients display a more mild retardation with no obvious structural defects in the brain. In a screen for synaptic mutants in Drosophila, we identified mutations in the Drosophila ortholog of POMT1, dPOMT1. Because synaptic defects are a plausible cause of mental retardation, we investigated the molecular and physiological defects associated with loss of dPOMT1 in Drosophila. In dPOMT1 mutants, there is a decrease in the efficacy of synaptic transmission and a change in the subunit composition of the postsynaptic glutamate receptors at the neuromuscular junction. We demonstrate that dPOMT1 is required to glycosylate the Drosophila dystroglycan ortholog Dg in vivo, and that this is the likely cause of these synaptic defects because (1) mutations in Dg lead to similar synaptic defects and (2) genetic interaction studies suggest that dPOMT1 and Dg function in the same pathway. These results are consistent with the model that dPOMT1-dependent glycosylation of Dg is necessary for proper synaptic function and raise the possibility that similar synaptic defects occur in the congenital muscular dystrophies.
Keywords: Drosophila, NMJ, POMT1, dystroglycan, glutamate receptors, muscular dystrophy
Introduction
The congenital muscular dystrophies (CMDs) are a devastating group of inherited neuromuscular disorders that are characterized by the onset of muscle weakness early during infancy. A number of genetically distinct CMDs share an underlying molecular cause: defects in protein O-mannosylation (Michele et al., 2002; Grewal and Hewitt, 2003; Godfrey et al., 2007). Mutations in genes that encode putative glycosylation enzymes have been identified in a number of clinically distinct CMDs and are associated with hypoglycosylation of α-dystroglycan (α-DG), a component of the dystrophin complex that links the cytoskeleton to the extracellular matrix (Haliloglu and Topaloglu, 2004). Mutations in protein O-mannosyl transferase 1 (POMT1), the first enzyme necessary for O-mannosylation, are present in some patients with two CMD variants, Walker-Warburg syndrome (WWS) and limb girdle muscular dystrophy type 2K (LGMD2K) (Balci et al., 2005; D'Amico et al., 2006; van Reeuwijk et al., 2006). Both WWS and LGMD2 are associated with mental retardation. In WWS, the mental retardation is severe and is likely caused by structural abnormalities secondary to cell migration defects (Beltran-Valero de Bernabe et al., 2002). In LGMD2, the mental retardation can be mild and is not associated with structural abnormalities in the brain, suggesting that POMT1 deficiency may lead to more subtle defects in neuronal structure or function (Balci et al., 2005).
In a screen for synaptic abnormalities at the Drosophila neuromuscular junction (NMJ), we identified mutations in the Drosophila homolog of POMT1, rotated abdomen (rt), hereafter referred to as dPOMT1. dPOMT1 and its enzymatic partner dPOMT2 are highly homologous to the human hPOMT1 and hPOMT2, respectively, and both are required for proper glycosylation of Dg in SF21 cells (Ichimiya et al., 2004). It remains unclear, however, whether they are required for glycosylation of Drosophila Dg in vivo. Flies with mutations in dPOMT1 have been described previously as having a rotated or twisted abdomen that disrupts mating (Martin-Blanco and Garcia-Bellido, 1996; Ichimiya et al., 2004). Because mutations in human POMT1 can lead to mental retardation of unknown etiology, and because synaptic defects possibly result in mental retardation, we performed a detailed analysis of molecular and physiological synaptic defects associated with this Drosophila model of CMD.
The Drosophila NMJ is a well established system for studying the structure and function of glutamatergic synapses (Collins and DiAntonio, 2007). The postsynaptic glutamate receptor at the NMJ is comprised of three essential subunits and either of two nonessential subunits, DGluRIIA and DGluRIIB, which confer distinct physiological and pharmacological properties to the receptor (DiAntonio, 2006). In a large-scale screen, we identified dPOMT1 as a gene that, when mutated, leads to a selective reduction in the levels of DGluRIIB. In addition, synaptic strength is impaired in dPOMT1 mutants because of a decrease in the number of synaptic vesicles released from the presynaptic motoneuron. This synaptic requirement for POMT1 is likely a result of its role in glycosylating Dg, because we show that (1) dPOMT1 is required to glycosylate Dg in vivo, (2) loss-of-function mutations in Dg phenocopy dPOMT1 mutations, and (3) dPOMT1 and Dg genetically interact. These results are consistent with the model that POMT1-dependent glycosylation of Dg is necessary for proper synaptic function and development at the Drosophila NMJ and raise the possibility that similar synaptic defects occur in the human CMDs.
Materials and Methods
Fly stocks.
Flies were raised and maintained at 25°C on standard fly media. Wild-type (WT) flies were Canton S (CS) outcrossed to w−, elav-Gal4 (Yao and White, 1994), G7-Gal4 (Zhang et al., 2001), actin-Gal4 (Krasnow et al., 1989), or C142-Gal4 (de Jong et al., 2005) flies. The P-element collection (Bellen et al., 2004), including rtP line (the allele/gene is referred to as dPOMT1) (Martin-Blanco and Garcia-Bellido, 1996), deficiency lines for dPOMT1 [df(3L)ED4470] and dystroglycan [Df(2)JP6 (Deng et al., 2003) and Df(2R)ED2457], and a P-element that is inserted in the dystroglycan gene (referred to as dgP) (PBac{RB}Dge01554) were obtained from Bloomington Stock Center (Bloomington, IN). Dg323 and Dg248 were kindly provided by Dr. Hannele Ruohola-Baker (University of Washington, Seattle, WA). dPOMT1 transgenic lines were made by cloning the cDNA RE38203 (Ichimiya et al., 2004) into the pUAST vector (Brand and Perrimon, 1993).
Immunohistochemistry.
Larvae were dissected and stained as described (Marrus et al., 2004). In brief, wandering third instar larvae were dissected in cold PBS and fixed for 5–10 min in Bouin's fixative and washed three times with PBS + 0.1% Triton X-100. Primary antibodies were used in the following dilutions: mouse α-DGluRIIA (1:100); mouse α-BRP (1:250) (Wagh et al., 2006) obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the National Institute of Child Health and Human Development and maintained by the Department of Biological Sciences of the University of Iowa (Iowa City, IA); rabbit α-DGluRIII (1:2500); rabbit α-DGluRIIB (1:2500) (Marrus et al., 2004); α-dys-CO2H (1:2500) (van der Plas et al., 2006), and Cy3- or Cy5-conjugated goat α-HRP (Jackson ImmunoResearch, West Grove, PA). Goat Cy5-, Cy3-, and FITC-conjugated secondary antibodies against mouse and rabbit IgG were used at 1:1000 and were obtained from Jackson ImmunoResearch.
Western blots.
Wandering third instar larvae were dissected in ice-cold homogenization buffer (67 mm Tris-HCl, pH 8.0, 67 mm NaCl, 2 m urea, 1 mm EDTA, and 1.3% SDS) and stored immediately on dry ice. The samples were run on 6% SDS-PAGE gels according to standard procedures. Rabbit α-Dgpep was used at 1:1000 (Schneider et al., 2006); mouse α-β-tubulin antibody (E7), obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the National Institute of Child Health and Human Development and maintained by the Department of Biological Sciences of the University of Iowa, was used at 1:100. HRP-conjugated goat α-rabbit secondary antibody (Jackson ImmunoResearch) was used at 1:10,000.
Imaging and analysis.
Imaging and analysis was done as described previously (Viquez et al., 2006) with the following minor differences. The average intensity function in MetaMorph was used to measure the intensity of the NMJ at muscle 4. The experimenter was blinded to genotypes during both imaging and analysis. Statistical analysis was performed using one-way ANOVA for comparison of samples within an experimental group. n for each condition is described in the figures. All histograms and measurements are shown as mean ± SEM. Bruchpilot (BRP) puncta were manually counted at MN4b synapses on muscle 4. While counting, we made sure that each of the BRP puncta had a DGluRIII puncta apposing it. Images for α-dystrophin staining were acquired using a Zeiss (Thornwood, NY) digital camera attached to a Zeiss Axioplan microscope.
Electrophysiology.
Intracellular electrophysiological recordings were done as reported previously (Marrus and DiAntonio, 2004). Briefly, wandering third-instar larvae were dissected in 0 mm Ca2+ Stewart saline (HL3) (Stewart et al., 1994). Both spontaneous miniature excitatory junction potentials (mEJPs) and evoked potentials (EJPs) were then recorded 0.45 mm HL3. At least 60 consecutive miniature events were measured per cell using MiniAnalysis (Synaptosoft, Decatur, GA) and averaged to determine mean mEJP. Events with a slow rise time were rejected as likely artifacts arising from neighboring electrically coupled muscle cells. To record evoked EJPs, segmental nerves were cut and suctioned into a stimulating electrode, in which they received a brief depolarizing pulse. Quantal content was estimated by dividing the mean EJP by the mean mEJP (EJP/mEJP). Cells across all genotypes had similar mean input resistances and resting potentials. Quantal size was not significantly different among any of the genotypes compared with wild-type controls. Statistical analysis was performed using one-way ANOVA to compare samples in an experimental group. Paired-pulse facilitation of the EJPs was assayed in 0.4 mm Ca2+ with an interpulse interval of 50 ms. Mean EJPs of the first and the fifth pulses were used for calculating the facilitation index/ratio. For electrotonic stimulation, recordings were performed as described by Song et al. (2002). Briefly, the stimulation electrode was kept as near as possible to the muscle, and the recordings were performed in 1 μm TTX. EJCs were recorded as described by Marrus et al. (2004) in 2.0 mm Ca2+ (Marrus and DiAntonio, 2004). Only recordings with holding currents <1.0 nA were analyzed.
Results
Mutations in Drosophila POMT1 affect glutamate receptor subunit composition at the neuromuscular junction
Clustering of neurotransmitter receptors opposite active zones is essential for effective synaptic transmission. At the Drosophila NMJ, postsynaptic glutamate receptors are comprised of three essential subunits (DGluRIII, DGluRIID, and DGluRIIE), as well as either of two nonessential subunits (DGluRIIA or DGluRIIB) (Marrus et al., 2004; Featherstone et al., 2005; Qin et al., 2005). These two classes of receptors differ in their localization, physiological properties, and modulation by second messenger systems (Petersen et al., 1997; Davis et al., 1998; DiAntonio et al., 1999; Chen et al., 2005). To investigate molecular mechanisms that control the clustering and subunit composition of synaptic glutamate receptors, we have screened a large collection of Drosophila mutants for changes in glutamate receptor localization.
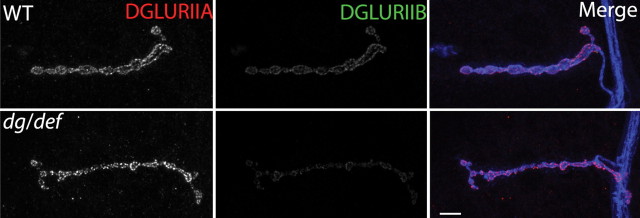
We stained for glutamate receptors at the NMJs from ∼1500 lines carrying unique insertions of P-element transposons in or near genes in the third chromosome (Bellen et al., 2004). Among this group, we identified a P-element insertion, rtP, which has a decrease in the levels of synaptic DGluRIIB with no detectable change in the levels of DGluRIIA. The rtP transposon insertion was previously identified as an allele of rt (referred to here as dPOMT1), the Drosophila ortholog of the congenital muscular dystrophy gene dPOMT1 (Martin-Blanco and Garcia-Bellido, 1996; Jurado et al., 1999). A second P-element insertion within the dPOMT1 locus, P{SUPor-P}rtKG04772, shows a similar phenotype (data not shown). We assayed glutamate receptor localization quantitatively in a transheterozygote between the rtP insertion and a genetically unrelated deficiency that deletes dPOMT1 and surrounding genes (Fig. 1A,B). This transheterozygote also displays a selective reduction in DGluRIIB levels at the synapse (Fig. 1A,B) (p < 0.001), demonstrating that this phenotype is caused by the loss of dPOMT1 and not by a second-site mutation elsewhere on the rtP chromosome. To investigate whether the rtP allele expresses dPOMT1 transcript, we performed reverse transcription-PCR from WT and rtP/df(3L)ED4470 (dPOMT1/def) flies. The dPOMT1 transcript is present in wild-type tissue, but is not detectable in the dPOMT1/def line (supplemental Fig. 1C, available at www.jneurosci.org as supplemental material), suggesting that the rtP (dPOMT1) mutant is either a severe hypomorph or a null. In this dPOMT1 mutant, other aspects of synapse morphology are unaffected. There is no significant difference in the levels or distribution of the synaptic vesicle protein DVGLUT (Daniels et al., 2004) or the postsynaptic scaffolding protein DLG (Lahey et al., 1994) (supplemental Fig. 1A, available at www.jneurosci.org as supplemental material). In addition, both the number and size of synaptic boutons are unchanged in the mutant (supplemental Fig. 1A,B, available at www.jneurosci.org as supplemental material). Hence, the selective reduction of synaptic DGluRIIB is the most prominent anatomical defect in the dPOMT1 mutant.
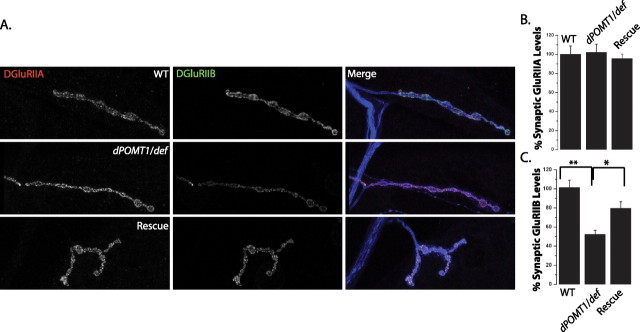
Figure 1.
Mutations in dPOMT1 alter glutamate receptor subunit composition at the neuromuscular junction. A, Confocal images of larval NMJs stained with antibodies against DGluRIIA (red), DGLURIIB (green), and HRP (blue). The genotypes of the larvae are as follows: WT (CS crossed with C142-Gal4), the dPOMT1 mutant (dPOMT1/def), and the dPOMT1 mutant rescued with a dPOMT1 transgene [C142-GAL4/UAS-dPOMT1; rtP/df(3L)ED4470]. B, C, Histograms show the mean staining intensity for DGluRIIA (B) and DGluRIIB (C) at the NMJ as a percentage of WT levels. Levels of DGluRIIB in the dPOMT1 mutant are significantly lower than in WT (*p < 0.01), and DGluRIIB levels increase at the NMJ after rescue (*p < 0.01). n = 10 for all genotypes. Error bars represent SEM.
To investigate where POMT1 functions, we used the Gal4/UAS system (Brand and Perrimon, 1993) to express transgenic dPOMT1 in the rtP (dPOMT1)/def mutant background. We generated a UAS-dPOMT1 transgene that rescues the rotated abdomen phenotype of rtP (dPOMT1) when ubiquitously expressed under the control of actin-Gal4 (Krasnow et al., 1989) (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Hence, our UAS-dPOMT1 transgene is functional, and the rotated abdomen phenotype is attributable to loss of dPOMT1. In an attempt to rescue the selective loss of DGluRIIB, we expressed dPOMT1 in the presynaptic neuron with elav-Gal4 (Yao and White, 1994), the postsynaptic muscle with G7-Gal4 (Zhang et al., 2001), or simultaneously on both sides of synapse using C142-Gal4 (de Jong et al., 2005). The selective expression of dPOMT1 in either nerve or muscle fails to rescue the DGluRIIB phenotype (supplemental Fig. 3A,B, available at www.jneurosci.org as supplemental material). However, when dPOMT1 is expressed in neurons and muscles with C142-Gal4, there is a significant increase in the levels of DGluRIIB at the synapse (Fig. 1C). The mutant is also rescued by the ubiquitous expression of dPOMT1 driven by actin-Gal4 (∼40%; p < 0.01; n = 10). The rescue of rtP (dPOMT1) by the transgenic expression of dPOMT1 demonstrates that the loss of dPOMT1 function is responsible for decrease in DGluRIIB at the synapse. Rescue of morphology was not complete, however, suggesting that the levels or timing of dPOMT1 expression may be critical for its full function. In addition, the failure to rescue with either presynaptic or postsynaptic expression suggests that dPOMT1 is required on both sides of the synapse.
dPOMT1 promotes neurotransmitter release by increasing probability of release
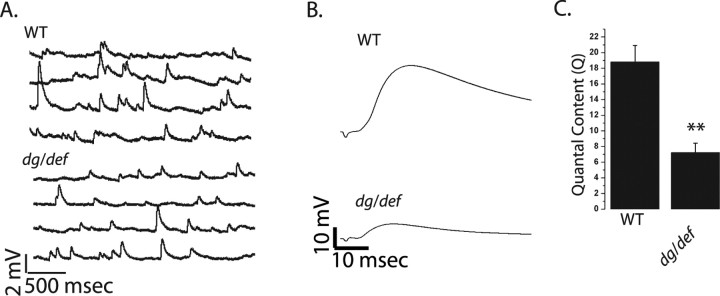
Changes in the subunit composition of the Drosophila glutamate receptor complex can change the function and structure of the NMJ (DiAntonio et al., 1999; Sigrist et al., 2002). DGluRIIA is the predominant subunit at a wild-type synapse, and the genetic deletion of DGluRIIB in the presence of wild-type levels of DGluRIIA does not lead to a change in quantal size, a measure of the postsynaptic response to a single vesicle (DiAntonio et al., 1999). As such, the selective reduction in DGluRIIB would not be predicted to decrease quantal size. To investigate synaptic function in the dPOMT1 mutant, we performed intracellular recordings from muscle 6 of segments A3 and A4 at the larval NMJs of WT and dPOMT1 mutants. We did not observe a significant decrease in the amplitude of miniature excitatory junctional potentials (mEJP: WT, 0.79 ± 0.02 mV; dPOMT1, 0.77 ± 0.03 mV; n = 9; p = 0.4) or frequency of mEJP (frequency: WT, 2.5 ± 0.9 Hz; dPOMT1, 3.1 ± 1.1 Hz; n = 9; p = 0.3) (Fig. 2A). There is, however, a dramatic decrease in evoked synaptic transmission. Indeed, the EJP amplitude is decreased nearly threefold in the dPOMT1 mutant (Fig. 2B). Because the response to a single vesicle is unchanged, the decrease in evoked release indicates that the nerve is releasing fewer synaptic vesicles. Indeed, estimates of quantal content based on the direct method (EJP/mEJP) show an approximately threefold reduction in the number of released vesicles (Fig. 2C). As with the change in DGluRIIB levels, expression of dPOMT1 in neither the nerve nor muscle was able to rescue the defect in synaptic release (data not shown). However, expression of dPOMT1 using C142-Gal4 restores transmitter release to wild-type levels (Fig. 2C). Expression of dPOMT1 from the ubiquitous actin-Gal4 driver also rescues quantal content (∼50% increase; p < 0.001; n = 9). Hence, the loss of dPOMT1 is responsible for the reduced quantal content in the rtP (dPOMT1) mutant, and therefore dPOMT1 is required for normal synaptic transmission at the Drosophila NMJ.
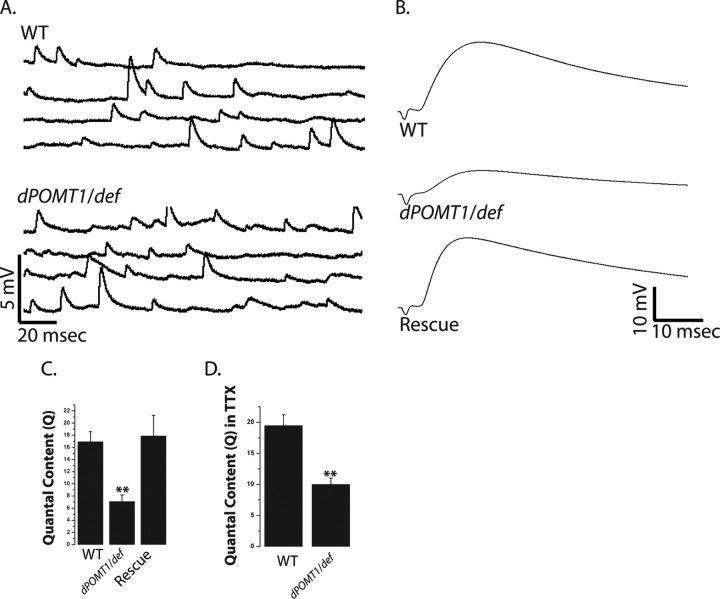
Figure 2.
dPOMT1 mutants have a defective synaptic transmission. A, Representative mEJP traces from WT (CS crossed with C142-Gal4) and dPOMT1 mutant (dPOMT1/def) larvae. B, Representative EJP traces for WT, dPOMT1/def, and dPOMT1 rescued [C142-Gal4/UAS-dPOMT1; rtP/df(3L)ED4470] larvae. C, Quantal content for all genotypes. n = 9 for all genotypes; **p < 0.001. Error bars represent SEM. D, Quantal content calculated by direct method from WT and mutant (dPOMT1/def) recorded in 1 μm TTX. n = 10; **p < 0.001.
Decreased transmitter release could be caused by defects in the generation or propagation of action potentials to the synapse. To test this hypothesis, we bypassed action potential-stimulated release by direct electrotonic stimulation of the nerve in 1μ m TTX, which suppresses action potentials (Song et al., 2002). If reduced transmitter release was a result of action potential defects, then direct stimulation should rescue the phenotype and lead to normal transmitter release. If the defect is downstream of the action potential, then electrotonic stimulation should still lead to defective transmitter release. With electrotonic stimulation in the presence of TTX, there is a significant decrease in evoked transmitter release in the rtP (dPOMT1)/def mutant when compared with wild type (Fig. 2D). This difference in quantal content is similar to that observed after action potential-stimulated release. Thus, defective action potentials do not explain the reduction in quantal content in dPOMT1 mutants.
Why is quantal content decreased in the dPOMT1 mutant? Reduced quantal content could be caused by a decrease in the number of release sites (n) or by a decrease in the probability that a synaptic vesicle will be released from a release site (p). To investigate the mechanism responsible for the decrease in quantal content in the dPOMT1 mutants, we have assessed both n and p. Active zones are the sites of neurotransmitter release, and in Drosophila individual release sites can be observed by staining for Bruchpilot, the Drosophila homolog of the active zone protein CAST (Wagh et al., 2006). Each active zone is apposed to a cluster of glutamate receptors (Marrus and DiAntonio, 2004), and a functional release site includes both elements of this synaptic dyad. We stained the NMJ of wild type and dPOMT1 mutants with α-Bruchpilot and an antibody to the essential glutamate receptor subunit DGluRIII and counted the number of release sites (Collins and DiAntonio, 2007; Pack-Chung et al., 2007; Dickman et al., 2008) (Fig. 3A). There is no significant difference in active zone number in the dPOMT1 mutant (WT, 254 ± 16; dPOMT1/def, 252 ± 18; n = 9). In addition, almost all active zones are apposed to glutamate receptors at both wild-type and mutant NMJs; thus, there is no evidence for postsynaptically silent synapses. The frequency of mEJPs in dPOMT1/def is also not significantly different from that of WT. Together, these data indicate that a change in the number of release sites is unlikely to explain the defects in transmitter release in the dPOMT1 mutant.
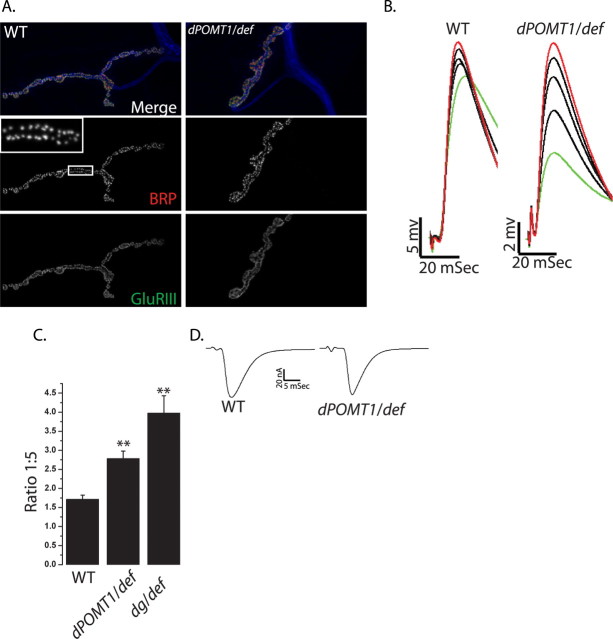
Figure 3.
Probability of release is decreased in dPOMT1 mutants. A, NMJs stained for active zones (α-BRP; red), glutamate receptor clusters (α-DGluRIII; green), and neuronal membrane (α-HRP; blue) from WT (CS crossed with w−) and dPOMT1 mutant (dPOMT1/def) larvae. Individual BRP puncta (A, inset) were counted from the entire NMJ (n = 9 for both; p = 0.8). B, First through fifth EJP sweeps in response to a train of stimuli in WT (CS crossed to w−) and the dPOMT1 mutant (dPOMT1/def) larvae. C, Quantification of the facilitation index (FI) for WT and dPOMT1/def. n for WT = 7 and for dPOMT1/def = 9; p < 0.001. The dystroglycan mutant (DgP/def) shows an increase in the FI similar to that of dPOMT1/def. n = 9; **p < 0.001. FI was calculated by dividing the fifth pulse (B, red) by the first pulse (B, green). Error bars represent SEM. D, Sample EJC traces from WT and mutant (dPOMT1/def) larvae. n = 10; p = 0.3.
The probability (p) that a synaptic vesicle will be released after an action potential is another key determinant of synaptic release. To investigate potential changes in release probability, we measured short-term facilitation, which varies inversely with p. When a nerve is repeatedly stimulated with a short interpulse interval, the subsequent evoked events are often larger because of residual calcium in the presynaptic terminal (Zucker and Regehr, 2002). When probability of release is low, such short-term facilitation is more pronounced. After a short train of stimuli (see Materials and Methods for details), we measured the amplitude of each EJP and calculated the facilitation index as the ratio of the fifth to the first EJP. Although the wild-type NMJ facilitates, the dPOMT1 mutant NMJ has greatly enhanced facilitation, leading to an approximately onefold increase in the degree of facilitation (Fig. 3B,C), consistent with a decrease release probability. To test this hypothesis directly, we recorded evoked junctional currents (EJCs) from WT and dPOMT1 mutants in presence of high external calcium (2.0 mm Ca2+). At this calcium concentration, the probability of release is very high, and transmitter release is near saturation. High calcium should rescue defects in probability of release, but would not rescue a reduction in the number of release sites. The dPOMT1/def mutants display wild-type EJC amplitudes when recorded in high calcium (WT, 66 ± 3.0 pA; dPOMT1/def, 57 ± 3.7 pA; n = 10; p = 0.3) (Fig. 3D), indicating that the mutant defect likely lies at the level of release probability. The rescue of transmitter release by high calcium, the large increase in facilitation at low calcium, and the lack of change in anatomically identifiable release sites in the mutant indicates that dPOMT1 promotes transmitter release by enhancing release probability.
dPOMT1 is required for the glycosylation of dystroglycan in vivo
Because POMT1 is required to O-glycosylate proteins, the lack of glycosylation of a target protein is a likely explanation for the phenotype of dPOMT1 mutants. In vertebrates, POMT1 glycosylates α-dystroglycan, and the loss of POMT1 result in hypoglycosylation, and in some cases loss, of dystroglycan (Jimenez-Mallebrera et al., 2003; Akasaka-Manya et al., 2004; Kim et al., 2004). Because glycosylation is important for dystroglycan function, the congenital muscular dystrophies associated with mutations in POMT1 likely are caused by loss of dystroglycan function. In Drosophila, dPOMT1 can promote the glycosylation of the dystroglycan ortholog Dg in vitro (Ichimiya et al., 2004), but it is not known whether it is required in vivo. To investigate whether dPOMT1 mutants affect the glycosylation state of Dg, we performed a Western blot with protein extract from larval brain and body walls of wild type and dPOMT1 mutants and probed for Dg with the previously characterized dgpep antibody (Schneider et al., 2006). This antibody recognizes two bands on a Western blot, one of ∼110 kDa, the predicted size for the unmodified protein, and another of >200 kDa, which is thought to be the glycosylated form (Schneider et al., 2006). From the immunoblot, levels of the 110 kDa Dg band are not significantly different between wild-type and dPOMT1 mutant larvae (Fig. 4B) (p = 0.3); however, the >200 kDa Dg band is undetectable in the dPOMT1 mutants (Fig. 4A,C). This loss of the putative glycosylated form of Dg is restored by the transgenic expression of dPOMT1. These data indicate that Dg is a likely target of dPOMT1 in the fly. In the absence of dPOMT1, we do not observe the high-molecular-weight glycosylated form of Dg; however, the levels of the nonglycosylated form are approximately normal. Hence, there are two changes to Dg protein in dPOMT1 mutants: total expression levels are reduced, and there is no apparent glycosylation. Either or both of these alterations may underlie the phenotypes we observe. If the nonglycosylated form of Dg can function, then overexpression of Dg should rescue defects caused by loss of dPOMT1. However, we find that whereas overexpressing Dg in a wild-type background leads to no gross defects, overexpression of Dg in the dPOMT1 mutant leads to lethality (data not shown). This suggests that glycosylation of Dg is essential for its function and that increased levels of the nonglycosylated Dg may be toxic.
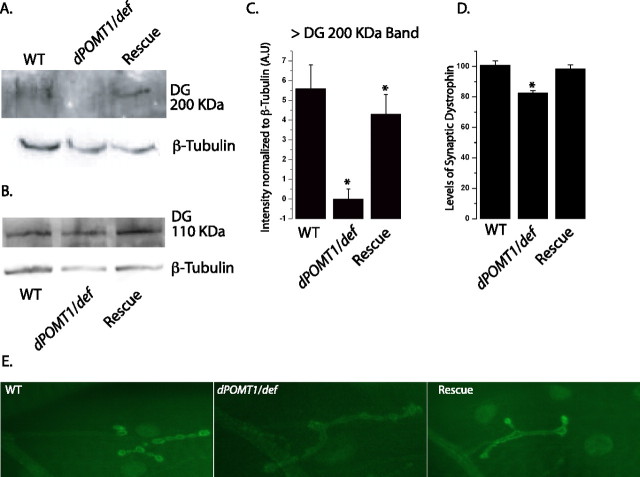
Figure 4.
dPOMT1 is necessary for the glycosylation of dystroglycan in vivo. A, Western blot showing the glycosylated >200 kDa dystroglycan protein in WT (CS crossed to w−), dPOMT1 mutant (dPOMT1/def), and dPOMT1 rescue [Act-GAL4/UAS-DPOMT1; rtP/df(3L)ED4470] larvae. β-Tubulin is shown as a loading control. B, Blot showing the nonglycosylated dystroglycan protein (∼110 kDa) in the same genotypes as in A. β-Tubulin is shown as a loading control. C, Levels of the glycosylated >200 kDa dystroglycan protein are significantly increased in larvae rescued with the dPOMT1 transgene (n = 2; p < 0.01). D, Quantification of synaptic dystrophin levels in the same genotypes as in A. n = 7 (WT); n = 15 (dPOMT1 mutant and rescue); *p < 0.01. E, Representative NMJs stained with α-dystrophin antibody. The genotypes are identical to those in A.
Dystroglycan is a component of the dystrophin glycoprotein complex (Ervasti et al., 1990). We wished to assess whether loss of glycosylated Dg affects the levels and localization of dystrophin. Drosophila dystrophin localizes to both the neuromuscular junction and the muscle sarcomeres (van der Plas et al., 2006, 2007). In the dPOMT1 mutant, dystrophin still localizes to these structures (Fig. 4D), so glycosylated Dg is not necessary for the localization of dystrophin, consistent with results from vertebrates (Cote et al., 2002). Although dystrophin is still present at the NMJ and sarcomeres, its levels are reduced. At the NMJ, there is an ∼20% reduction in dystrophin (p < 0.01), which is restored with transgenic dPOMT1 rescue (Fig. 4D,E). A similar reduction is observed for sarcomeric dystrophin (data not shown), suggesting that the glycosylation of Dg promotes the stability of dystrophin.
Dystroglycan and dPOMT1 mutants genetically interact
Because Dg is abnormally processed in the dPOMT1 mutant, the dPOMT1 phenotypes may be attributable to loss of Dg function. If this is the case, then Dg mutants may display phenotypes similar to those described above for dPOMT1. We obtained two Dg mutants and two genetically unrelated deficiencies that delete Dg and adjacent genes. For both Dg mutants in combination with either deficiency, there is a significant decrease in the levels of the DGluRIIB subunit at the larval NMJ [WT, 100% ± 6.8%; PBac{RB}Dge01554/Df(2R)ED2457 (DgP/def), 45% ± 4.8%; n = 10; p < 0.001], without a change in the levels of DGluRIIA (Fig. 5). Hence, loss of Dg, like loss of dPOMT1, leads to a change in glutamate receptor subunit composition. Electrophysiological studies also highlight the similarity in phenotype between Dg and dPOMT1 mutants (Fig. 6A,B). Both Dg alleles have a marked reduction in the amplitude of evoked EJPs with no change in the amplitude of spontaneous mEJPs (WT, 0.96 ± 0.05; DgP/def, 0.85 ± 0.05; n = 10; p = 0.4). Hence, quantal content is dramatically reduced when Dg function is impaired (Fig. 6C). As with dPOMT1 mutants, this decrease in transmitter release is accompanied by an increase in short-term facilitation (Fig. 3C), consistent with a lowered probability of release. In summary, the synaptic phenotypes observed in dPOMT1 mutants are also displayed by the Dg mutants.
Figure 5.
Mutants deficient in dystroglycan have altered glutamate receptor subunit composition. Sample larval NMJs from WT (CS crossed to w−) and the dystroglycan mutant (dgP/def) stained with α-DGluRIIA (red), α-DGluRIIB (green), and α-HRP (blue) antibodies. n = 10 for all genotypes; **p < 0.001.
Figure 6.
Quantal content is reduced in dystroglycan mutants. A, Sample mEJP traces from WT and DgP/def larvae. Genotypes are the same as in Figure 5. B, Sample EJP traces from WT and DgP/def larvae. C, Quantal content of WT and dystroglycan mutant (dgP/def) larvae. Quantal content was calculated by dividing the mean EJP amplitude by mean mEJP amplitude. n = 10; **p < 0.001.
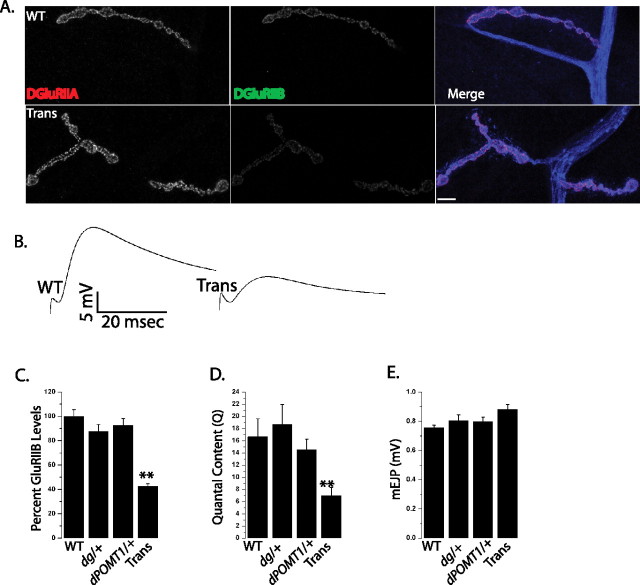
The similarity in phenotypes between dPOMT1 and Dg mutants is consistent with the model that dPOMT1-dependent glycosylation of Dg is necessary to promote synaptic vesicle release and regulate glutamate receptor subunit composition. However, it is formally possible that the two genes function in parallel pathways. A powerful approach for identifying genes in the same pathway is to test for transheterozygous interactions. Heterozygous mutations (loss of one of two alleles) in either dPOMT1 or Dg have no affect on either the levels of DGluRIIB or quantal content (Fig. 7C,D). If the genes function in parallel pathways, and neither pathway is impaired enough to show a phenotype, then we would predict that the transheterozygote, in which one allele of each gene is mutant, would also have no phenotype. However, if the genes function in the same pathway, then mutations in two steps of one pathway may enhance each other and lead to phenotype; this is what we observe. The transheterozygote between dPOMT1 and Dg exhibits both a decrease in the levels of DGluRIIB at the NMJ (Fig. 7A,C) and a marked reduction in quantal content (Fig. 7B,D) without a decrease in mEJP amplitudes (Fig. 7E). This experiment demonstrates that dPOMT1 and Dg genetically interact and, in conjunction with the biochemical data, supports the model that the glycosylation of Dg by dPOMT1 is required for normal synaptic development and function at the Drosophila NMJ.
Figure 7.
dPOMT1 interacts genetically with dystroglycan. A, Sample larval NMJs from WT (CS crossed to w−) and transheterozygotes of dPOMT1 and the dystroglycan mutant [Trans (dPOMT1/+; DgP/+)]. NMJs were stained with α-DGluRIIA in red α-DGluRIIB (green) and α-HRP (blue). B, Sample EJP traces from WT (CS crossed to w−) and dPOMT1 and dystroglycan Trans larvae. C, Quantification of DGluRIIB levels at larval NMJs from WT (CS crossed with w−), dystroglycan heterozygotes (DgP/+), POMT1 heterozygotes (dPOMT1/+), and dPOMT1 and dystroglycan Trans larvae (p < 0.001; n = 10). D, Quantal content for the same genotypes as in C (**p < 0.001; n = 6 for WT, dPOMT1/+, and DgP/+; n = 10 for dPOMT1/+; DgP/+ larvae). E, Quantification of mEJP amplitude in the same genotypes as in C. n = 10; p > 0.5.
Discussion
POMT1 is required for the O-glycosylation of dystroglycan, and mutations in POMT1 can lead to two variants of congenital muscular dystrophy, WWS and LGMD2K (Balci et al., 2005; D'Amico et al., 2006; van Reeuwijk et al., 2006). Both diseases are associated with mental retardation; however, for the milder LGMD2 no apparent structural abnormalities are present in the brain that would explain the onset of mental retardation. In this study, we characterize a Drosophila model of POMT1 deficiency. We find that, as in vertebrates, Drosophila POMT1 is required for glycosylation of dystroglycan. In Drosophila, the inability to glycosylate dystroglycan, or the genetic disruption of dystroglycan, does not lead to gross structural abnormalities at the neuromuscular junction, but rather disrupts presynaptic glutamate release and alters the subunit composition of postsynaptic glutamate receptors. Similar synaptic changes at vertebrate central synapses are a potential cause of mental retardation in CMD patients.
Glycosylation of dystroglycan by POMT1
The glycosylation of dystroglycan is affected in many forms of CMD (Grewal and Hewitt, 2003; Haliloglu and Topaloglu, 2004). Glycosylation of dystroglycan is required for its binding to components of the extracellular matrix (Martin, 2007). In addition, loss of glycosylation can lead to a decrease in the levels of dystroglycan (Jimenez-Mallebrera et al., 2003; van Reeuwijk et al., 2005). Hence, glycosylation enzymes such as POMT1 may be required for both the activity and stability of dystroglycan. In Drosophila, dPOMT1 promotes the glycosylation of Dg in vitro, and the loss of dPOMT1 in cultured Drosophila SF21 cells results in hypoglycosylation of Dg (Ichimiya et al., 2004). Here we demonstrate that dPOMT1 is required in vivo for the normal glycosylation of Dg. In the absence of dPOMT1, the total levels of dystroglycan are decreased, because the glycosylated band is lost with no commensurate increase in the nonglycosylated band. The failure to observe more nonglycosylated Dg suggests that glycosylation may be important for the stability of Dg.
Drosophila perlecan binds to Dg that lacks the mucin-rich O-glycosylation domain (Schneider et al., 2006), so it is plausible that the nonglycosylated Dg could retain some function. The ability to manipulate Dg and dPOMT1 independently allowed us to test whether the decrease in total Dg was the major cause of the dPOMT1 phenotypes. We find that increasing the levels of dystroglycan in a dPOMT1 mutant leads to lethality. This suggests that too much nonglycosylated Dg may be toxic, and is consistent with the model that glycosylation is required for Dg function.
dPOMT1 and Dg are required for efficient neurotransmitter release
Previous analysis of dPOMT1 mutants demonstrated that loss of dPOMT1 leads to a rotated abdomen phenotype and disrupted muscle structure (Ichimiya et al., 2004; Lyalin et al., 2006; Haines et al., 2007). Our analysis adds a second major phenotype: a severe impairment in the ability to release neurotransmitter. We investigated the mechanism underlying this synaptic phenotype. We detect no change in the number of anatomically defined neurotransmitter release sites (n), suggesting that probability of release (p) is impaired in the mutant. Consistent with this hypothesis, when transmitter is released in low calcium conditions, there is an increase in short-term facilitation, which usually varies inversely with release probability. In addition, high external calcium, which saturates release probability, rescues the defects in evoked transmitter release in the dPOMT1 mutant. These data demonstrate that the defect in synaptic transmission in the dPOMT1 mutant is attributable to a reduction in release probability rather than a reduction in the number of release sites.
What might be the molecular cause of this decrease in probability of release? Our data suggest that the proximate cause is probably the loss of glycosylated dystroglycan. We find that mutations in Dg also have a decrease in p, and the strong genetic interactions between dPOMT1 and Dg heterozygotes are consistent with the genes working in the same pathway to promote transmitter release. Why then would the loss of glycosylated dystroglycan impair transmitter release? We do not know the answer, but speculate that dystroglycan, via its interactions with the extracellular matrix, is an important part of a transsynaptic complex that plays a structural and/or functional role at the synapse to promote normal synaptic function. Indeed, components of the extracellular matrix and dystrophin regulate synaptic function at the Drosophila NMJ (Johnson et al., 2006; van der Plas et al., 2006). However, the reduced synaptic function in dPOMT1 and Dg mutants is unlikely to be caused by the reduction in levels of postsynaptic dystrophin, because mutations in dystrophin lead to an increase, rather than decrease, in evoked transmitter release (van der Plas et al., 2006).
In which cells does glycosylated dystroglycan function to promote transmitter release? We have generated a functional dPOMT1 transgene whose ubiquitous expression rescues the rotated abdomen phenotype. We investigated the spatial requirement for dPOMT1 in synaptic function by driving the transgene at the NMJ using neuronal, muscle, ubiquitous, and neuronal/muscle synaptic Gal4 driver lines. We find that the synaptic dPOMT1 phenotypes are rescued only when the transgene is driven by either the neuronal/muscle C142-Gal4 or ubiquitous actin-Gal4 driver and not when it is expressed exclusively in the presynaptic or postsynaptic cell. Therefore, dPOMT1 may be required for glycosylating dystroglycan both in neurons and muscles to maintain the normal function of the NMJ. Dystroglycan is expressed in both muscles and brain in Drosophila (Dekkers et al., 2004; Shcherbata et al., 2007), and our results are consistent with the model that it functions in both neurons and muscles at the NMJ.
Subunit-specific regulation of glutamate receptors by dPOMT1
One of the intriguing findings of this study is the specific reduction in the DGluRIIB subunit of the glutamate receptor in dPOMT1 and dg mutants. At the Drosophila NMJ, postsynaptic glutamate receptors are comprised of three essential subunits as well as either of two nonessential subunits, DGluRIIA and DGluRIIB (for review, see DiAntonio 2006). Receptors with these alternate subunits are differentially localized opposite the terminals of distinct motoneurons that synapse with the same muscle cell, leading to the suggestion that presynaptic activity may shape glutamate receptor subunit composition (Marrus et al., 2004). Although extensive studies have been done in vertebrate AMPA-type receptor subunit composition and trafficking (Barry and Ziff, 2002; Malinow and Malenka, 2002; McGee and Bredt, 2003), mechanisms that describe such subunit-specific regulation of glutamate receptors are not well understood at the Drosophila NMJ. Recently, the actin/spectrin-binding protein Coracle was shown to regulate the subunit composition of glutamate receptors at the Drosophila NMJ. Mutations in Coracle lead to a specific loss in the DGluRIIA subunit, demonstrating that distinct molecular pathways can control subunit composition (Chen et al., 2005). Our results indicate that dPOMT1 via Dg also regulates the subunit composition of glutamate receptors at the Drosophila NMJ. It is tempting to speculate that dystroglycan, which participates in clustering acetylcholine receptors in vertebrates (Cote et al., 1999; Grady et al., 2000), could be involved in the clustering of DGluRIIB subunit of glutamate receptors. However, it is also plausible that the changes in DGluRIIB levels are secondary to the changes in synaptic function and do not reflect a direct function of dystroglycan in receptor localization. These findings open a new path for understanding the molecular and/or activity-dependent cues that control the localization of specific glutamate receptor subunits at the Drosophila NMJ.
Footnotes
This work was supported by grants from the Muscular Dystrophy Association and National Institutes of Health (NS043171) to A.D. and by a Pioneer grant from the Netherlands Organization for Scientific Research to J.N.N. We thank Hannele Ruohola-Baker for the dystroglycan alleles and Martina Schneider for anti-dystroglycan (Dgpep) antibodies. We thank the Bloomington Stock Center (Bloomington, IN) for the flies used in this study. We thank James Skeath, E. J. Brace, Richard Daniels, Catherine Collins, and N. Rajalaxmi for their helpful comments on this manuscript. We also thank Xiaolu Sun and Anja de Jong for excellent technical assistance.
References
- Akasaka-Manya K, Manya H, Endo T. Mutations of the POMT1 gene found in patients with Walker-Warburg syndrome lead to a defect of protein O-mannosylation. Biochem Biophys Res Commun. 2004;325:75–79. doi: 10.1016/j.bbrc.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Balci B, Uyanik G, Dincer P, Gross C, Willer T, Talim B, Haliloglu G, Kale G, Hehr U, Winkler J, Topaloglu H. An autosomal recessive limb girdle muscular dystrophy (LGMD2) with mild mental retardation is allelic to Walker-Warburg syndrome (WWS) caused by a mutation in the POMT1 gene. Neuromuscul Disord. 2005;15:271–275. doi: 10.1016/j.nmd.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Barry MF, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol. 2002;12:279–286. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- Bellen HJ, Levis RW, Liao G, He Y, Carlson JW, Tsang G, Evans-Holm M, Hiesinger PR, Schulze KL, Rubin GM, Hoskins RA, Spradling AC. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167:761–781. doi: 10.1534/genetics.104.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran-Valero de Bernabe D, Currier S, Steinbrecher A, Celli J, van Beusekom E, van der Zwaag B, Kayserili H, Merlini L, Chitayat D, Dobyns WB, Cormand B, Lehesjoki AE, Cruces J, Voit T, Walsh CA, van Bokhoven H, Brunner HG. Mutations in the O-mannosyltransferase gene POMT1 give rise to the severe neuronal migration disorder Walker-Warburg syndrome. Am J Hum Genet. 2002;71:1033–1043. doi: 10.1086/342975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Chen K, Merino C, Sigrist SJ, Featherstone DE. The 4.1 protein coracle mediates subunit-selective anchoring of Drosophila glutamate receptors to the postsynaptic actin cytoskeleton. J Neurosci. 2005;25:6667–6675. doi: 10.1523/JNEUROSCI.1527-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, DiAntonio A. Synaptic development: insights from Drosophila. Curr Opin Neurobiol. 2007;17:35–42. doi: 10.1016/j.conb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Cote PD, Moukhles H, Lindenbaum M, Carbonetto S. Chimaeric mice deficient in dystroglycans develop muscular dystrophy and have disrupted myoneural synapses. Nat Genet. 1999;23:338–342. doi: 10.1038/15519. [DOI] [PubMed] [Google Scholar]
- Cote PD, Moukhles H, Carbonetto S. Dystroglycan is not required for localization of dystrophin, syntrophin, and neuronal nitric-oxide synthase at the sarcolemma but regulates integrin alpha 7B expression and caveolin-3 distribution. J Biol Chem. 2002;277:4672–4679. doi: 10.1074/jbc.M106879200. [DOI] [PubMed] [Google Scholar]
- D'Amico A, Tessa A, Bruno C, Petrini S, Biancheri R, Pane M, Pedemonte M, Ricci E, Falace A, Rossi A, Mercuri E, Santorelli FM, Bertini E. Expanding the clinical spectrum of POMT1 phenotype. Neurology. 2006;66:1564–1567. doi: 10.1212/01.wnl.0000216145.66476.36. discussion 1461. [DOI] [PubMed] [Google Scholar]
- Daniels RW, Collins CA, Gelfand MV, Dant J, Brooks ES, Krantz DE, DiAntonio A. Increased expression of the Drosophila vesicular glutamate transporter leads to excess glutamate release and a compensatory decrease in quantal content. J Neurosci. 2004;24:10466–10474. doi: 10.1523/JNEUROSCI.3001-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW, DiAntonio A, Petersen SA, Goodman CS. Postsynaptic PKA controls quantal size and reveals a retrograde signal that regulates presynaptic transmitter release in Drosophila. Neuron. 1998;20:305–315. doi: 10.1016/s0896-6273(00)80458-4. [DOI] [PubMed] [Google Scholar]
- de Jong S, Cavallo JA, Rios CD, Dworak HA, Sink H. Target recognition and synaptogenesis by motor axons: responses to the sidestep protein. Int J Dev Neurosci. 2005;23:397–410. doi: 10.1016/j.ijdevneu.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Dekkers LC, van der Plas MC, van Loenen PB, den Dunnen JT, van Ommen GJ, Fradkin LG, Noordermeer JN. Embryonic expression patterns of the Drosophila dystrophin-associated glycoprotein complex orthologs. Gene Expr Patterns. 2004;4:153–159. doi: 10.1016/j.modgep.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Deng WM, Schneider M, Frock R, Castillejo-Lopez C, Gaman EA, Baumgartner S, Ruohola-Baker H. Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development. 2003;130:173–184. doi: 10.1242/dev.00199. [DOI] [PubMed] [Google Scholar]
- DiAntonio A. Glutamate receptors at the Drosophila neuromuscular junction. Int Rev Neurobiol. 2006;75:165–179. doi: 10.1016/S0074-7742(06)75008-5. [DOI] [PubMed] [Google Scholar]
- DiAntonio A, Petersen SA, Heckmann M, Goodman CS. Glutamate receptor expression regulates quantal size and quantal content at the Drosophila neuromuscular junction. J Neurosci. 1999;19:3023–3032. doi: 10.1523/JNEUROSCI.19-08-03023.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman DK, Kurshan PT, Schwarz TL. Mutations in a Drosophila α2δ voltage-gated calcium channel subunit reveal a crucial synaptic function. J Neurosci. 2008;28:31–38. doi: 10.1523/JNEUROSCI.4498-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG, Campbell KP. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature. 1990;345:315–319. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- Featherstone DE, Rushton E, Rohrbough J, Liebl F, Karr J, Sheng Q, Rodesch CK, Broadie K. An essential Drosophila glutamate receptor subunit that functions in both central neuropil and neuromuscular junction. J Neurosci. 2005;25:3199–3208. doi: 10.1523/JNEUROSCI.4201-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey C, Clement E, Mein R, Brockington M, Smith J, Talim B, Straub V, Robb S, Quinlivan R, Feng L, Jimenez-Mallebrera C, Mercuri E, Manzur AY, Kinali M, Torelli S, Brown SC, Sewry CA, Bushby K, Topaloglu H, North K, et al. Refining genotype phenotype correlations in muscular dystrophies with defective glycosylation of dystroglycan. Brain. 2007;130:2725–2735. doi: 10.1093/brain/awm212. [DOI] [PubMed] [Google Scholar]
- Grady RM, Zhou H, Cunningham JM, Henry MD, Campbell KP, Sanes JR. Maturation and maintenance of the neuromuscular synapse: genetic evidence for roles of the dystrophin–glycoprotein complex. Neuron. 2000;25:279–293. doi: 10.1016/s0896-6273(00)80894-6. [DOI] [PubMed] [Google Scholar]
- Grewal PK, Hewitt JE. Glycosylation defects: a new mechanism for muscular dystrophy? Hum Mol Genet. 2003;12(Suppl 2):R259–R264. doi: 10.1093/hmg/ddg272. [DOI] [PubMed] [Google Scholar]
- Haines N, Seabrooke S, Stewart BA. Dystroglycan and protein O-mannosyltransferases 1 and 2 are required to maintain integrity of Drosophila larval muscles. Mol Biol Cell. 2007;18:4721–4730. doi: 10.1091/mbc.E07-01-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haliloglu G, Topaloglu H. Glycosylation defects in muscular dystrophies. Curr Opin Neurol. 2004;17:521–527. doi: 10.1097/00019052-200410000-00002. [DOI] [PubMed] [Google Scholar]
- Ichimiya T, Manya H, Ohmae Y, Yoshida H, Takahashi K, Ueda R, Endo T, Nishihara S. The twisted abdomen phenotype of Drosophila POMT1 and POMT2 mutants coincides with their heterophilic protein O-mannosyltransferase activity. J Biol Chem. 2004;279:42638–42647. doi: 10.1074/jbc.M404900200. [DOI] [PubMed] [Google Scholar]
- Jimenez-Mallebrera C, Torelli S, Brown SC, Feng L, Brockington M, Sewry CA, Beltran-Valero De Bernabe D, Muntoni F. Profound skeletal muscle depletion of alpha-dystroglycan in Walker-Warburg syndrome. Eur J Paediatr Neurol. 2003;7:129–137. doi: 10.1016/s1090-3798(03)00042-4. [DOI] [PubMed] [Google Scholar]
- Johnson KG, Tenney AP, Ghose A, Duckworth AM, Higashi ME, Parfitt K, Marcu O, Heslip TR, Marsh JL, Schwarz TL, Flanagan JG, Van Vactor D. The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron. 2006;49:517–531. doi: 10.1016/j.neuron.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Jurado LA, Coloma A, Cruces J. Identification of a human homolog of the Drosophila rotated abdomen gene (POMT1) encoding a putative protein O-mannosyl-transferase, and assignment to human chromosome 9q34.1. Genomics. 1999;58:171–180. doi: 10.1006/geno.1999.5819. [DOI] [PubMed] [Google Scholar]
- Kim DS, Hayashi YK, Matsumoto H, Ogawa M, Noguchi S, Murakami N, Sakuta R, Mochizuki M, Michele DE, Campbell KP, Nonaka I, Nishino I. POMT1 mutation results in defective glycosylation and loss of laminin-binding activity in alpha-DG. Neurology. 2004;62:1009–1011. doi: 10.1212/01.wnl.0000115386.28769.65. [DOI] [PubMed] [Google Scholar]
- Krasnow MA, Saffman EE, Kornfeld K, Hogness DS. Transcriptional activation and repression by Ultrabithorax proteins in cultured Drosophila cells. Cell. 1989;57:1031–1043. doi: 10.1016/0092-8674(89)90341-3. [DOI] [PubMed] [Google Scholar]
- Lahey T, Gorczyca M, Jia XX, Budnik V. The Drosophila tumor suppressor gene dlg is required for normal synaptic bouton structure. Neuron. 1994;13:823–835. doi: 10.1016/0896-6273(94)90249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyalin D, Koles K, Roosendaal SD, Repnikova E, Van Wechel L, Panin VM. The twisted gene encodes Drosophila protein O-mannosyltransferase 2 and genetically interacts with the rotated abdomen gene encoding Drosophila protein O-mannosyltransferase 1. Genetics. 2006;172:343–353. doi: 10.1534/genetics.105.049650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Marrus SB, DiAntonio A. Preferential localization of glutamate receptors opposite sites of high presynaptic release. Curr Biol. 2004;14:924–931. doi: 10.1016/j.cub.2004.05.047. [DOI] [PubMed] [Google Scholar]
- Marrus SB, Portman SL, Allen MJ, Moffat KG, DiAntonio A. Differential localization of glutamate receptor subunits at the Drosophila neuromuscular junction. J Neurosci. 2004;24:1406–1415. doi: 10.1523/JNEUROSCI.1575-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PT. Congenital muscular dystrophies involving the O-mannose pathway. Curr Mol Med. 2007;7:417–425. doi: 10.2174/156652407780831601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Blanco E, Garcia-Bellido A. Mutations in the rotated abdomen locus affect muscle development and reveal an intrinsic asymmetry in Drosophila. Proc Natl Acad Sci USA. 1996;93:6048–6052. doi: 10.1073/pnas.93.12.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee AW, Bredt DS. Assembly and plasticity of the glutamatergic postsynaptic specialization. Curr Opin Neurobiol. 2003;13:111–118. doi: 10.1016/s0959-4388(03)00008-4. [DOI] [PubMed] [Google Scholar]
- Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, Straub V, Mathews KD, Moore SA, Campbell KP. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- Pack-Chung E, Kurshan PT, Dickman DK, Schwarz TL. A Drosophila kinesin required for synaptic bouton formation and synaptic vesicle transport. Nat Neurosci. 2007;10:980–989. doi: 10.1038/nn1936. [DOI] [PubMed] [Google Scholar]
- Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron. 1997;19:1237–1248. doi: 10.1016/s0896-6273(00)80415-8. [DOI] [PubMed] [Google Scholar]
- Qin G, Schwarz T, Kittel RJ, Schmid A, Rasse TM, Kappei D, Ponimaskin E, Heckmann M, Sigrist SJ. Four different subunits are essential for expressing the synaptic glutamate receptor at neuromuscular junctions of Drosophila. J Neurosci. 2005;25:3209–3218. doi: 10.1523/JNEUROSCI.4194-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Khalil AA, Poulton J, Castillejo-Lopez C, Egger-Adam D, Wodarz A, Deng WM, Baumgartner S. Perlecan and Dystroglycan act at the basal side of the Drosophila follicular epithelium to maintain epithelial organization. Development. 2006;133:3805–3815. doi: 10.1242/dev.02549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcherbata HR, Yatsenko AS, Patterson L, Sood VD, Nudel U, Yaffe D, Baker D, Ruohola-Baker H. Dissecting muscle and neuronal disorders in a Drosophila model of muscular dystrophy. EMBO J. 2007;26:481–493. doi: 10.1038/sj.emboj.7601503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist SJ, Thiel PR, Reiff DF, Schuster CM. The postsynaptic glutamate receptor subunit DGluR-IIA mediates long-term plasticity in Drosophila. J Neurosci. 2002;22:7362–7372. doi: 10.1523/JNEUROSCI.22-17-07362.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Ranjan R, Dawson-Scully K, Bronk P, Marin L, Seroude L, Lin YJ, Nie Z, Atwood HL, Benzer S, Zinsmaier KE. Presynaptic regulation of neurotransmission in Drosophila by the g protein-coupled receptor methuselah. Neuron. 2002;36:105–119. doi: 10.1016/s0896-6273(02)00932-7. [DOI] [PubMed] [Google Scholar]
- Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1994;175:179–191. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- van der Plas MC, Pilgram GS, Plomp JJ, de Jong A, Fradkin LG, Noordermeer JN. Dystrophin is required for appropriate retrograde control of neurotransmitter release at the Drosophila neuromuscular junction. J Neurosci. 2006;26:333–344. doi: 10.1523/JNEUROSCI.4069-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plas MC, Pilgram GS, de Jong AW, Bansraj MR, Fradkin LG, Noordermeer JN. Drosophila Dystrophin is required for integrity of the musculature. Mech Dev. 2007;124:617–630. doi: 10.1016/j.mod.2007.04.003. [DOI] [PubMed] [Google Scholar]
- van Reeuwijk J, Janssen M, van den Elzen C, Beltran-Valero de Bernabe D, Sabatelli P, Merlini L, Boon M, Scheffer H, Brockington M, Muntoni F, Huynen MA, Verrips A, Walsh CA, Barth PG, Brunner HG, van Bokhoven H. POMT2 mutations cause alpha-dystroglycan hypoglycosylation and Walker-Warburg syndrome. J Med Genet. 2005;42:907–912. doi: 10.1136/jmg.2005.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reeuwijk J, Maugenre S, van den Elzen C, Verrips A, Bertini E, Muntoni F, Merlini L, Scheffer H, Brunner HG, Guicheney P, van Bokhoven H. The expanding phenotype of POMT1 mutations: from Walker-Warburg syndrome to congenital muscular dystrophy, microcephaly, and mental retardation. Hum Mutat. 2006;27:453–459. doi: 10.1002/humu.20313. [DOI] [PubMed] [Google Scholar]
- Viquez NM, Li CR, Wairkar YP, DiAntonio A. The B' protein phosphatase 2A regulatory subunit well-rounded regulates synaptic growth and cytoskeletal stability at the Drosophila neuromuscular junction. J Neurosci. 2006;26:9293–9303. doi: 10.1523/JNEUROSCI.1740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Durrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, Wichmann C, Kittel R, Sigrist SJ, Buchner E. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Yao KM, White K. Neural specificity of elav expression: defining a Drosophila promoter for directing expression to the nervous system. J Neurochem. 1994;63:41–51. doi: 10.1046/j.1471-4159.1994.63010041.x. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Bailey AM, Matthies HJ, Renden RB, Smith MA, Speese SD, Rubin GM, Broadie K. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell. 2001;107:591–603. doi: 10.1016/s0092-8674(01)00589-x. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]