Abstract
Changes in axon outgrowth patterns are often associated with synaptogenesis. Members of the conserved Pam/Highwire/RPM-1 protein family have essential functions in presynaptic differentiation. Here, we show that Caenorhabditis elegans RPM-1 negatively regulates axon outgrowth mediated by the guidance receptors SAX-3/robo and UNC-5/UNC5. Loss-of-function rpm-1 mutations cause a failure to terminate axon outgrowth, resulting in an overextension of the longitudinal PLM axon. We observe that PLM overextension in rpm-1 mutants is suppressed by sax-3 and unc-5 loss-of-function mutations. PLM axon overextension is also induced by SAX-3 overexpression, and the length of extension is enhanced by loss of rpm-1 function or suppressed by loss of unc-5 function. We also observe that loss of rpm-1 function in genetic backgrounds sensitized for guidance defects disrupts ventral AVM axon guidance in a SAX-3-dependent manner and enhances dorsal guidance of DA and DB motor axons in an UNC-5-dependent manner. Loss of rpm-1 function alters expression of the green fluorescent protein (GFP)-tagged proteins, SAX-3::GFP and UNC-5::GFP. RPM-1 is known to regulate axon termination through two parallel genetic pathways; one involves the Rab GEF (guanine nucleotide exchange factor) GLO-4, which regulates vesicular trafficking, and another that involves the F-box protein FSN-1, which mediates RPM-1 ubiquitin ligase activity. We show that glo-4 but not fsn-1 mutations affect axon guidance in a manner similar to loss of rpm-1 function. Together, the results suggest that RPM-1 regulates axon outgrowth affecting axon guidance and termination by controlling the trafficking of the UNC-5 and SAX-3 receptors to cell membranes.
Keywords: axon guidance, Caenorhabditis elegans, development, genetics, growth cone, guidance, receptor
Introduction
During development, axons migrate along specific pathways to reach their targets and make functional connections. As axons migrate, they are directed by extracellular guidance cues, including members of the UNC-6/netrin and Slt-1/slit protein families (Tessier-Lavigne and Goodman, 1996; Dickson, 2002). These guidance cues are detected by receptors on the surface of the migrating axons; in Caenorhabditis elegans, UNC-40/DCC and UNC-5/UNC5 associate with UNC-6/netrin, and the SAX-3/robo associates with SLT-1/slit (Hedgecock et al., 1990; Leung-Hagesteijn et al., 1992; Chan et al., 1996; Zallen et al., 1998; Hao et al., 2001). One view of axon guidance is that the cues orient outgrowth-promoting activity through the asymmetric localization of molecules that control protrusion activity (Adler et al., 2006; Quinn et al., 2006) (C. C. Quinn, D. S. Pfeil, and W. G. Wadsworth, unpublished observations).
Axons reaching their targets and assembling synapses may undergo changes that alter axon outgrowth responses to guidance cues. For example, some axons terminate additional axon outgrowth once synaptic connections are made. There are many unanswered questions regarding the regulation of these morphological changes. Do the signals that promote presynaptic assembly also induce new axon outgrowth patterns or are the morphological changes the result of the formation of stable synapses? Candidate molecules for regulating such changes include members of the Pam/Highwire/RPM-1 protein family, which regulate presynaptic differentiation and function in different organisms (Schaefer et al., 2000; Wan et al., 2000; Zhen et al., 2000; Burgess et al., 2004; D'Souza et al., 2005). In C. elegans, loss of rpm-1 function causes a range of defects affecting neuronal morphology and synaptic organization (Schaefer et al., 2000; Zhen et al., 2000). For example, mechanosensory neurons in the mutants fail to form stable synaptic branches and overextend their axons (Schaefer et al., 2000). It is difficult from these observations alone to distinguish whether the inability to form stable synapses is what triggers the axon overextension.
RPM-1 is a large protein with several conserved domains that may mediate different functions. RPM-1 functions cell autonomously in the mechanosensory neurons to regulate axon termination and synaptogenesis (Schaefer et al., 2000). RPM-1 binds FSN-1 to negatively regulate a DLK-1 MAP (mitogen-activated protein) kinase cascade through ubiquitin-mediated protein degradation (Liao et al., 2004; Nakata et al., 2005). RPM-1 also binds GLO-4 and may promote vesicular trafficking through a Rab GTPase pathway (Grill et al., 2007). Components of these pathways appear to be expressed throughout the nervous system (Liao et al., 2004; Nakata et al., 2005; Grill et al., 2007). In the mature nervous system, RPM-1 is localized to the presynaptic periactive zone (Nakata et al., 2005).
Here, we report that RPM-1 regulates SAX-3/robo- and UNC-5/UNC5-mediated axon outgrowth. Loss of rpm-1 function promotes SAX-3/robo- and UNC-5/UNC5-mediated axon outgrowth and influences both the longitudinal axon extension of mechanosensory neurons as well as the dorsal and ventral guidance of other axons in sensitized genetic backgrounds. The regulation of axon termination by RPM-1 is proposed to function through the GLO-4 and FSN-1 pathways (Grill et al., 2007). For the axon outgrowth affecting guidance, our results suggest that RPM-1 could function through the GLO-4 pathway, rather than the FSN-1 pathway. Also, because axon guidance defects prevent the axons from reaching their targets in the sensitized backgrounds, the results suggest that RPM-1-mediated regulation of the guidance receptors through the GLO-4 pathway is not triggered by the formation of stable synapses. Based on these observations, we propose that RPM-1 responses to the signals that promote synaptogenesis and negatively regulates SAX-3/robo- and UNC-5/UNC5-mediated axon outgrowth by controlling vesicular trafficking via GLO-4.
Materials and Methods
Strains.
Bristol strain N2 was used as the standard wild-type strain. Worms were manipulated according to standard protocols and maintained at 20°C (Brenner, 1974). All mutations used for this study are strong loss-of-function or null alleles unless otherwise indicated.
Strains constructed and used for this study were as follows: IM661: unc-6(rh46)X; evIs82aIV, IM662: unc-6(e78)X; evIs82aIV, IM207: unc-6(ev400)X; evIs82aIV, IM729: unc-5(e152) IV; evIs82aIV, IM731: rpm-1(ur299)V; unc-5(e152) IV; evIs82aIV, IM832: unc-40(e1430)I; evIs82aIV, IM838: unc-6(rh46)X; zdIs5I, IM650: unc-6(ev400)X; zdIs5I, IM647: slt-1(eh15)X; zdIs5I, IM649: unc-6(ev400)X; slt-1(eh15)X; zdIs5I, IM712: sax-3(ky123)X; zdIs5I, IM739: unc-5(e53)IV; zdIs5I, IM648: unc-40(e1430)I; zdIs5I, IM843: rpm-1(ur299)V, IM844: rpm-1(ur299)V; evIs82aIV, IM805: rpm-1(ur299)V; unc-6(rh46)X; evIs82aIV, IM856: rpm-1(ur299)V; unc-6(e78)X; evIs82aIV, IM848: rpm-1(ur299)V; unc-6(ev400)X; evIs82aIV, IM850: rpm-1(ur299)V; unc-40(e1430)I; evIs82aIV, IM870: rpm-1(ur299)V;unc-6(rh46)X; unc-40(e1430)I; evIs82aIV, IM845: rpm-1(ur299)V; zdIs5I, IM847: rpm-1(ur299)V; unc-6(rh46)X; zdIs5I, IM849: rpm-1(ur299)V; unc-6(ev400)X; zdIs5I, IM817: rpm-1(ur299)V; slt-1(eh15)X; zdIs5I, IM804: rpm-1(ur299)V; sax-3(ky123)X; zdIs5I, IM851: rpm-1(ur299)V; unc40(e1430)I; zdIs5I, IM736: rpm-1(ur299)V; unc-5(e53)IV; zdIs5I, IM885: rpm-1(ur299)V; slt-1(eh15)X; sax-3(ky123)X; zdIs5I, IM883: rpm-1(ur299)V; evIs98V, IM884: rpm-1(ur299)V; kyEx253, IM886: fsn-1(hp1); zdIs5I, IM890: rpm-1(ur299); glo-4(ok6230;zdIs5I, IM891: rpm-1(ur299); glo-1(zu391); zdIs5I, IM913: gmIs28; zdIs5I, IM965: rpm-1(ur299)V; gmIs28, IM966: rpm-1(ur299) V; gmIs28; zdIs5I, IM970: juIs58; zdIs5I, IM971: unc-5(e53)IV; gmIs28; zdIs5I, IM975: unc-40(e1430)I;gmIs28; zdIs5I, IM976: rpm-1(ur299); unc-5(e152) IV; unc-6(rh46)X; evIs82aIV, IM1039: slt-1(eh15); glo-4 (ok623); zdIs5, IM1040: slt-1(eh15); fsn-1(hp1); zdIs5I, IM1037: glo-4(ok623); zdIs5I, IM1038: glo-1(zu391)zdIs5I.
Strains that were not derived in the Wadsworth Laboratory were kindly provided by Joe Culotti [evIs82a(unc-129::gfp)], Scott Clark [zdIs-5(mec-4::gfp)], or Theresa Stiernagle (CB4856 and other strains used for mapping) of the Caenorhabditis Genetics Center (Minneapolis, MN). Strains of various double mutants or triple mutants were constructed using standard genetic procedures and confirmed by either complementation tests or PCR genotyping.
Molecular characterization of rpm-1(ur299).
The ur299 allele was recovered from a genetic screen for suppressors of dorsal axon guidance defect caused by the unc-6(rh46) mutation (our unpublished data). The ur299 worms show Egl and small in body size (dumpy). The ur299 allele was first mapped to chromosome V using traditional two factors mapping strategy. For fine mapping, we used single-nucleotide polymorphisms to map ur299 to the area shown (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Cosmids containing sequences for this area were obtained from the Sanger Center (Cambridge, UK) and were injected into the mutant animals at concentrations of 2–10 ng/μl, along with a coinjection marker pIM175(unc-119::gfp) at 100 ng/μl. The animals were examined for rescue of the mild dumpy body phenotype caused by ur299 mutation. Germline transformation with a mixture of cosmids that included CO1B7, which contains the rpm-1 sequence, rescued the ur299 phenotypes. In a complement assay, the ur299 mutation failed to complement rpm-1(js317) (Schaefer et al., 2000) and the rpm-1(js317);unc-6(rh46) double mutant phenotyped ur299;unc-6(rh46) in both morphological and axonal phenotype. The molecular lesions in rpm-1(ur299) were identified and confirmed by sequencing genomic PCR products from mutant animals with unique DNA primers for rpm-1 and aligning with the reported genomic sequence from the C. elegans Genome Sequencing Consortium.
Axon migration assays.
To visualize the DA/DB motorneurons in living animals, we use the integrated unc-129::gfp transgene evIs82a. To analyze the axon guidance in mutants, worms were mounted on 5% agar pad in M9 buffer containing 10 mm levamisole and observed with a 40× objective, using fluorescence optics on a Carl Zeiss (Oberkochen, Germany) Axio-Imager Z1 microscope. Dorsal guidance of the DA and DB axons was scored as defective if none of the axons from the neuron cell bodies situated along the ventral nerve cord in the region between the pharynx and vulva reach the dorsal cord.
To visualize the mechanosensory neurons, we used the mec-4::gfp transgene zdIs5. These neurons have well defined cell body position and axon extensions. The AVM is localized at the lateral side of the body wall and extends an axon ventrally to the ventral nerve cord and then extends anteriorly. Worms were scored as having an AVM ventral guidance defect if the axon failed to reach the ventral cord. The paired PLM neurons are located posteriorly on the lateral body wall. In wild-type young adult animals, the PLM axon extends anteriorly and terminates in the middle of the animal near the vulva. PLM axon was scored as overextended if it migrated to or past the position of the ALM cell body, or was scored as displaying a short extension if it failed to reach the position of the vulva. A two-tailed z test was used to determine whether the phenotypes were significantly different between two strains.
Because the AVM cell body migrates before extending an axon, we determined whether the anterior–posterior position of the AVM cell body was affected in different mutant strains. We determined the ratio of the distance between the posterior edge of the pharyngeal terminal bulb and the AVM cell body to the distance between the posterior edge of the pharyngeal terminal bulb and the center of the vulva. This ratio was 0.49 ± 0.01 (SEM) (n = 25) for wild-type animals, and we found no significant differences (two-tailed z test) in mutant strains of rpm-1(ur299), rpm-1(ur299);gmIs28, rpm-1(ur299);glo-4(ok623), rpm-1(ur299);glo-1(zu391), or rpm-1(ur299);fsn-1(hp1).
Transgenic animals and green fluorescent protein analysis.
evIs98[unc-5::GFP; dpy-20(+)] and evEx66[unc-40::GFP;rol-6)] were provided by Joe Culotti (University of Toronto, Toronto, Ontario, Canada). kyEx253[sax-3::GFP;lin-15(+)] was obtained from Cori Bargmann (The Rockefeller University, New York, NY); gmIs28 [mec-7::SAX-3::GFP; ttx-3::gfp] was provided by Gian Garriga (University of California, Berkeley, Berkeley, CA) and juIs58[rpm-1:GFP; rol-6] was obtained from Yishi Jin (University of California, San Diego, La Jolla, CA). Green fluorescent protein (GFP) analysis in living worms was performed using 40 or 63× objective on a Carl Zeiss Axio-Imager Z1 microscope equipped with an apotome imager. Image analysis was performed using Axio-Vision LE 4.5 software. SAX-3::GFP localization in the ALM and PLM neurons were examined in L4 or young adults. Using a 63× objective, images of the neurons were collected under identical exposure conditions. Neurons were scored as having a punctate pattern if in any focal plane five or more puncta could be counted.
Results
rpm-1 alleles
We isolated an allele of rpm-1 from a genetic screen for mutations that could suppress dorsal guidance defects caused by the unc-6(rh46) mutation. The unc-6(rh46) mutation is a partial loss-of-function allele (Hedgecock et al., 1990; Wadsworth et al., 1996), and mutations were isolated that could improve the ability of DA and DB motor axons to reach the dorsal cord in the unc-6(rh46) background. The ur299 mutation was mapped to a region near the center of chromosome V. We observed that ur299 causes a mild dumpy phenotype and subtle egg laying defects that were similar to those reported for mutations in rpm-1, a gene located in the same region (Schaefer et al., 2000; Zhen et al., 2000). We found that the ur299 mutation fails to complement rpm-1(js317), suggesting that ur299 is a rpm-1 mutation. The js317 mutation generates an early stop codon and likely causes complete loss of function (Schaefer et al., 2000). Sequencing revealed that the ur299 mutation causes a nonsense mutation (a CAG to TAG change) at nucleotide 3589 (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). RPM-1 is a putative RING finger/E3 ubiquitin ligase of 3766 aa. Loss of rpm-1 function disrupts presynaptic architecture and causes abnormal axon morphology (Schaefer et al., 2000; Zhen et al., 2000). The expression and analysis of rpm-1 functions indicates that RPM-1 acts cell autonomously (Schaefer et al., 2000; Zhen et al., 2000); we observe RPM-1::GFP expression in the neurons that we assay in this study (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). RPM-1 is a member of a conserved family of proteins that includes Drosophila Highwire (Hiw), zebrafish Esrom, and mammalian Phr1 and Pam, proteins that have also been shown to influence neuronal development (Karlstrom et al., 1996; Guo et al., 1998; Schaefer et al., 2000; Wan et al., 2000; Zhen et al., 2000; D'Souza et al., 2005).
RPM-1 regulates dorsal axon guidance mediated by UNC-5
We examined the ability of the rpm-1(ur299) mutation to improve the dorsal migration of DA and DB motor neuron axons in different unc-6 mutant backgrounds. The DA and DB ventral cord motor neurons send axons dorsally that travel circumferentially along the body wall away from ventral sources of the UNC-6 guidance cue (Fig. 1A–C) (Hedgecock et al., 1990; Wadsworth et al., 1996). We found that rpm-1 mutation improve the dorsal guidance in unc-6(rh46) and unc-6(e78) mutants, but not in unc-6(ev400) mutants (Fig. 1D–F). The unc-6(rh46) and unc-6(e78) are reduction-of-function missense mutations that are predicted to encode altered forms of UNC-6, whereas unc-6(ev400) causes an early stop mutation and is considered a null allele (Hedgecock et al., 1990; Wadsworth et al., 1996). These results suggest that the improved guidance provided by the loss of rpm-1 function requires the information provided by UNC-6.
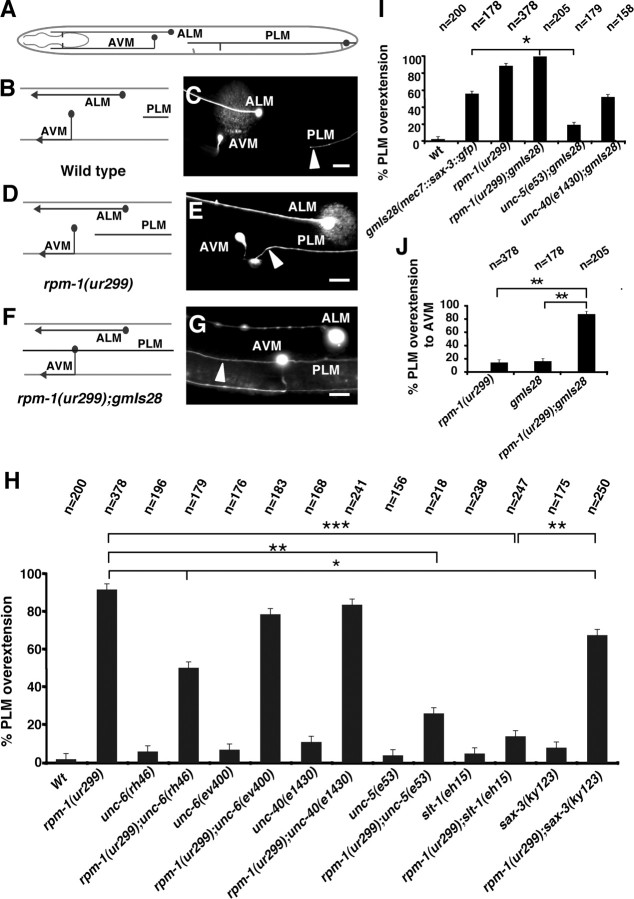
Figure 1.
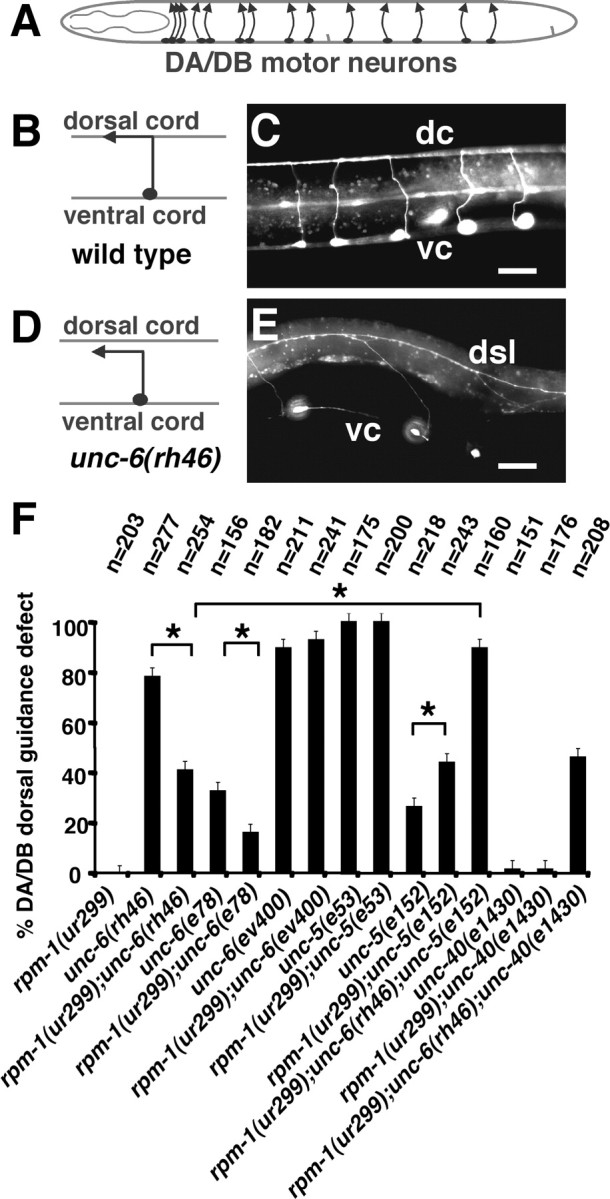
rpm-1(ur299) affects guidance receptor activities for dorsal axon guidance. A, Schematic diagram of the migration of DA/DB motorneurons. These neurons extend axons dorsally away from ventral UNC-6 sources. B–E, The phenotypes of DA/DB axon guidance in wild-type (B, C) and unc-6(rh46) mutant larva (D, E). DA and DB motor axons in L4-stage animals were visualized with evIs82a [unc-129::gfp]. In the image of the unc-6(rh46) mutant larva, some axons have extended and joined the dorsal sublateral nerve. F, Quantification of DA/DB dorsal guidance defect in various mutants. rpm-1(ur299) suppresses the dorsal guidance defects caused by the unc-6(rh46) and unc-6(e78) hypomorphic alleles but not the null allele, unc-6(ev400). Triple-mutant analysis suggests that suppression by rpm-1(ur299) is dependent on unc-5 function. Asterisks indicate statistically significant difference (*p < 0.05). Anterior is left and dorsal is up; vc, ventral cord; dc, dorsal cord; dsl, dorsal–sublateral cord. Error bars represent SEs of proportions. Scale bars, 20 μm.
The UNC-6 dorsal guidance of DA and DB motor neurons is mediated by UNC-5 and UNC-40 receptors (Leung-Hagesteijn et al., 1992; Chan et al., 1996). To determine whether these receptors are involved in the suppression mediated by loss of rpm-1 function, we examined rpm-1;unc-40;unc-6(rh46) and rpm-1;unc-5;unc-6(rh46) triple mutants (Fig. 1F). The results show that the rpm-1 mutation can suppress the unc-6(rh46) dorsal guidance phenotype in the absence of UNC-40. Because the suppression by loss of rpm-1 function is not mediated through UNC-40, we suggest that loss of rpm-1 function increases the guidance activity of other UNC-6 receptor, UNC-5. However, a triple mutant experiment using the strong loss-of-function allele unc-5(e53) would not be as informative because dorsal guidance is already severely impaired by the loss of unc-5 function alone. Furthermore, our attempts to make such a strain were not successful; we believe that the triple combination might not produce viable animals. However, using a weaker partial loss-of-function allele, unc-5(e152), we find that the suppression of the unc-6(rh46) dorsal axon migration defects by rpm-1(ur299) is reduced in the unc-5(e152) background, which is consistent with the requirement of UNC-5 activity.
RPM-1 regulates ventral guidance mediated by SAX-3 and UNC-40
The influence of UNC-6 and SLT-1, and their respective UNC-40 and SAX-3 receptors, has been well studied using the ventral migration of the AVM axon (Hedgecock et al., 1990; Hao et al., 2001; Yu et al., 2002; Gitai et al., 2003). Relative to the AVM neuron, SLT-1 is secreted from dorsal sources, whereas UNC-6 is secreted from ventral sources (Fig. 2). Loss of both cues results in almost complete failure of AVM axon ventral guidance, whereas the loss of either slt-1 or unc-6 function results in partial failure. The AVM responses to UNC-6 and SLT-1 are mediated by UNC-40 and SAX-3, respectively. We find that rpm-1;slt-1 double mutants (Fig. 2F) have AVM guidance defects as severe as double mutants of unc-40;slt-1 (Hao et al., 2001) or slt-1;unc-6. This suggests that, in the rpm-1;slt-1 double mutants, the signaling by UNC-6 and UNC-40 is inhibited by the loss of rpm-1 function.
Figure 2.
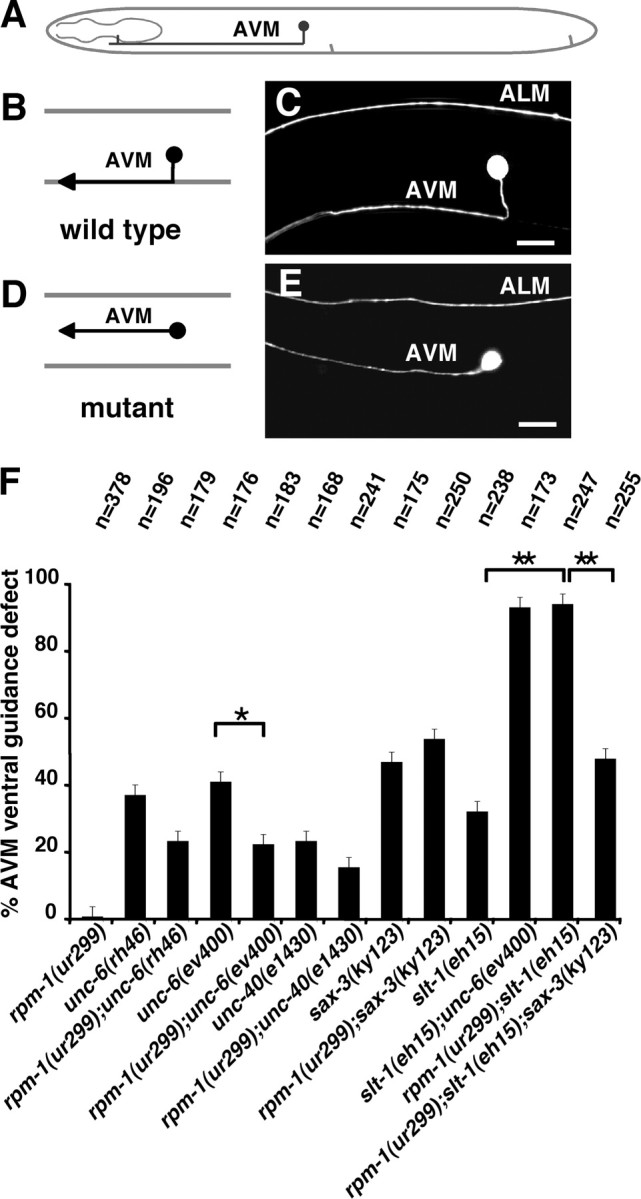
rpm-1(ur299) affects guidance receptor activities for ventral axon migration. A, Schematic diagram of the AVM axon. The AVM axon is repelled from dorsal SLT-1 sources and is attracted toward ventral UNC-6 sources. B–E, AVM axon guidance in wild-type (C) and mutant larva (E). The AVM axons in L4 stage animals were visualized with zdIs5 [mec-4::gfp]. F, Quantification of the AVM guidance defects in different mutant backgrounds. The rpm-1;slt-1 double mutants are as severe as slt-1;unc-6 mutants suggesting that loss of rpm-1 function prevents UNC-6 signaling, likely by inhibiting UNC-40 signaling. This effect is not observed in rpm-1;sax-3 double mutants indicating that the ability of the rpm-1 mutation to inhibit UNC-6 signaling is dependent on the SAX-3 receptor. This is consistent with biochemical evidence indicating that an interaction between UNC-40/DCC and SAX-3/robo silences the guidance effects of UNC-6/netrin (Stein and Tessier-Lavigne, 2001). The silencing effect occurs in rpm-1;slt-1 mutants, indicating that the SLT-1 ligand is not required. The silencing effect observed in rpm-1;slt-1 mutants is reversed in the triple, rpm-1;slt-1;sax-3 showing that the silencing effect is mediated by SAX-3 receptor. Also, the AVM defects caused by unc-6 or unc-40 mutants are suppressed by loss of rpm-1 function suggesting the upregulation of SAX-3 improves SLT-1 signaling. Asterisks indicate statistically significant difference (*p < 0.05; **p < 0.005). Anterior is left and dorsal is up. Error bars represent SEs of proportions. Scale bars, 20 μm.
We also find that rpm-1;sax-3 double mutants are not as severe as rpm-1;slt-1 or slt-1;unc-6. Nor are they as severe as unc-40;slt-1 double mutants (Hao et al., 2001). This suggests that the ability of the rpm-1(ur299) to inhibit UNC-40 signaling is dependent on SAX-3. Indeed, the penetrance of the AVM guidance defect in triple rpm-1;slt-1;sax-3 mutants is similar to that of rpm-1;sax-3 mutants, rather than to the severe condition observed in rpm-1;slt-1 mutants (Fig. 2F). Together, the AVM results are consistent with a hypothesis that SAX-3 in the rpm-1;slt-1 mutant can silence UNC-40-mediated axon outgrowth activity.
RPM-1 regulates longitudinal axon outgrowth mediated by UNC-5 and SAX-3
In rpm-1 mutants, the PLM anterior process often extends beyond the normal termination point (Fig. 3) (Schaefer et al., 2000). We examined whether genetic interactions between rpm-1 and unc-6, slt-1, and the receptor genes affect PLM axon extension. We found that the overextension in the rpm-1 mutant depends on UNC-5 and SAX-3 because the overextension phenotype is reduced in rpm-1;unc-5 and rpm-1;sax-3 double mutants (Fig. 3H). However, the overextension phenotype remains unaffected in unc-40;rpm-1 double mutants, indicating that the overextension does not require UNC-40. We note that the position of the PLM cell body is not affected by the rpm-1 mutation, although in some mutant backgrounds, such as sax-3 and slt-1, there is a low penetrance of a mispostioned cell body. However, we did not observe a clear correlation between the position of the cell body and axon overextension.
Figure 3.
rpm-1(ur299) affects guidance receptor activities for longitudinal axon migration. A, Schematic diagram of the PLM longitudinal axon. B, C, In wild-type animals, PLM axons extend to a region near the vulva (arrowhead). D–G, In rpm-1(ur299) (D, E) and rpm-1(ur299);gmIs28 (F, G) animals, the PLM axon overextends, passing the ALM cell body or AVM cell body (arrowhead). H–J, Quantification of the PLM overextension in various mutant backgrounds. The PLM axons were visualized with zdIs5[mec-4::gfp]. Loss of rpm-1 function causes overextension of PLM axon. H, The penetrance of the overextension phenotype of rpm-1 is reduced by loss of unc-5, slt-1, or sax-3 function suggesting the same pathways that control directed dorsal and ventral axon outgrowth also control the longitudinal PLM axon extension. I, J, Overexpression of SAX-3 in mechanosensory neurons, which includes PLM, causes the PLM axon overextension phenotype. SAX-3 overexpression was obtained using a transgenic line, which carried the integrated Pmec7::SAX-3::GFP transgene (gmIs28) (Watari-Goshima et al., 2007). The overextension phenotype caused by overexpression of SAX-3 is suppressed by loss of unc-5 function, but not by the loss of unc-40 function. Overexpression of SAX-3 in rpm-1(ur299) mutant causes even longer extensions than the rpm-1 mutation alone, suggesting rpm-1 influences the SAX-3 function that regulates PLM axon extension. Asterisks indicate statistically significant difference (*p < 0.05; **p < 0.005; ***p < 0.0005). Anterior is left and dorsal is up. Error bars represent SEs of proportions. Scale bars, 20 μm.
SAX-3 influences PLM axon extension. In sax-3;rpm-1 double mutants, the penetrance of the overextension is reduced compared with animals with the rpm-1 mutation alone (Fig. 3H). Overexpression of SAX-3 causes the PLM axon overextension phenotype (Fig. 3I). This was observed in transgenic animals using the Pmec-7::sax-3::gfp integrated transgene (gmIs28) (Watari-Goshima et al., 2007). Expression of this transgene was shown to cause the rerouting of longitudinal axons by overexpression of SAX-3 (Watari-Goshima et al., 2007). We observe that the overextension caused by SAX-3 overexpression is suppressed by loss of unc-5 function, but not by the loss of unc-40 function (Fig. 3I). Because overextension caused by loss of rpm-1 function is also suppressed by the loss of unc-5 function, but not by the loss of unc-40 function, the same mechanisms might be causing the overextensions. In addition, we find that SAX-3 overexpression in combination with the rpm-1(ur299) mutation cause the process to extend farther (Fig. 3J). Finally, we find that the loss of the SAX-3 ligand, Slt-1, strongly suppresses the axon overextension caused by loss of rpm-1 function (Fig. 3H). Together, these results suggest an association among SAX-3, SLT-1, and RPM-1 that influences PLM axon extension.
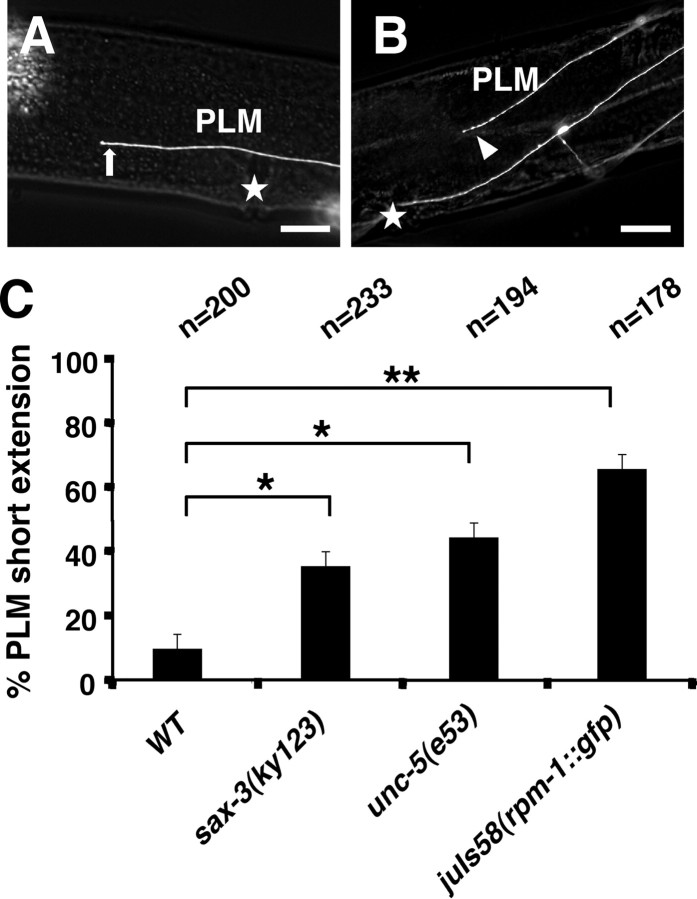
We also observe that overexpression of RPM-1 has an opposite effect; whereas loss of rpm-1 function results in PLM axon overextension, when a rpm-1::gfp transgene is expressed in the PLM neuron, 59% (n = 178) of the axon extensions are shorter (Fig. 4). The extension is also shorter in 32% (n = 233) and 40% (n = 194) of sax-3 and unc-5 mutants, respectively (Fig. 4C). These results are consistent with the idea that RPM-1 can negatively regulate SAX-3 and UNC-5 activity to affect PLM extension.
Figure 4.
Overexpression of RPM-1 causes short PLM axon extension. The PLM axons were visualized with zdIs5 [mec-4::gfp]. RPM-1 overexpression was obtained using a transgenic line, which carried the integrated rpm-1::GFP transgene (juIs58). A, In wild-type animals, PLM axons stop their anterior extension near the vulva (arrow). B, In juIs58 worms, PLM displays short extension (arrowhead). C, Quantification of the PLM short extension in different mutant backgrounds. The PLM is also shorter in sax-3 and unc-5 mutants, suggesting that RPM-1 might negatively regulate SAX-3 and UNC-5 activity to affect PLM extension. Asterisks indicate statistically significant difference (*p < 0.05; **p < 0.005). Anterior is left and dorsal is up; stars indicate the position of vulva. Error bars represent SEs of proportions. Scale bars, 20 μm.
RPM-1 affects UNC-5 and SAX-3 expression/localization
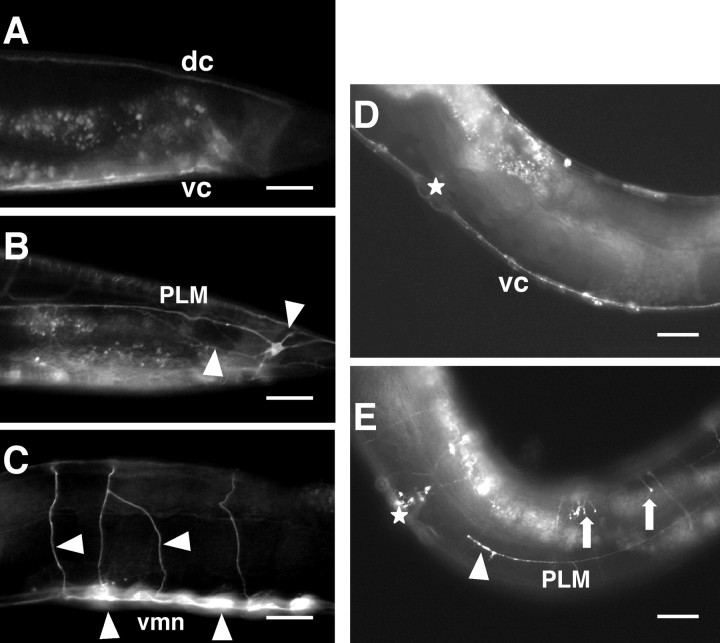
The SAX-3::GFP is expressed transiently in most neurons and in some epidermal and muscle cells (Zallen et al., 1998). We observe that in rpm-1 loss-of-function mutants, the SAX-3::GFP signal is more widely detected. In rpm-1(+) L4 larvae, SAX-3::GFP is detected in 25% (n = 60) of the PLM neurons, whereas in 57% (n = 60) of rpm-1(ur299) L4 larvae SAX-3::GFP signal is observed (Fig. 5A,B). In 23% (n = 60) of rpm-1 loss-of-function mutants, there are abnormal multiple processes from the PLM cell body. Whereas in ventral cord motor neurons the SAX-3::GFP signal is observed in 19% (n = 60) of rpm-1(+) larvae, 76% (n = 60) of rpm-1 loss-of-function mutants show strong expression (Fig. 5C).
Figure 5.
RPM-1 affects SAX-3 and UNC-5 expression. SAX-3 and UNC-5 overexpression were obtained using transgenic animals kyEx253 [sax-3::GFP] (Zallen et al., 1998) and evIs98 [unc-5::GFP] (Killeen et al., 2002), respectively. A, Example of the posterior lateral region in kyEx253 [sax-3::GFP] larvae. No expression is detected in PLM neurons. B, Compared with A, in kyEx253 [sax-3::GFP] larvae with the rpm-1(ur299) mutation a strong GFP signal is observed in PLM neurons. Multiple processes are often present (arrowheads). C, Compared with A, in kyEx253 [sax-3::GFP] larvae with the rpm-1(ur299) mutation a strong GFP signal is observed in ventral cord motor neurons and their circumferential axons (arrowheads). D, Example of the lateral midbody region in evIs98 [unc-5::GFP] animals. E, Compared with D, in evIs98 [unc-5::GFP] larvae with the rpm-1(ur299) mutation the GFP signal is detected in PLM (arrowhead). Axons often show multiple short branches; shown are abnormal branches from the dorsal nerve cord (arrows). Anterior is left; dorsal is up; dc, dorsal cord; vc, ventral cord; vmn, ventral motor neurons. Stars indicate the position of vulva. Scale bars, 20 μm.
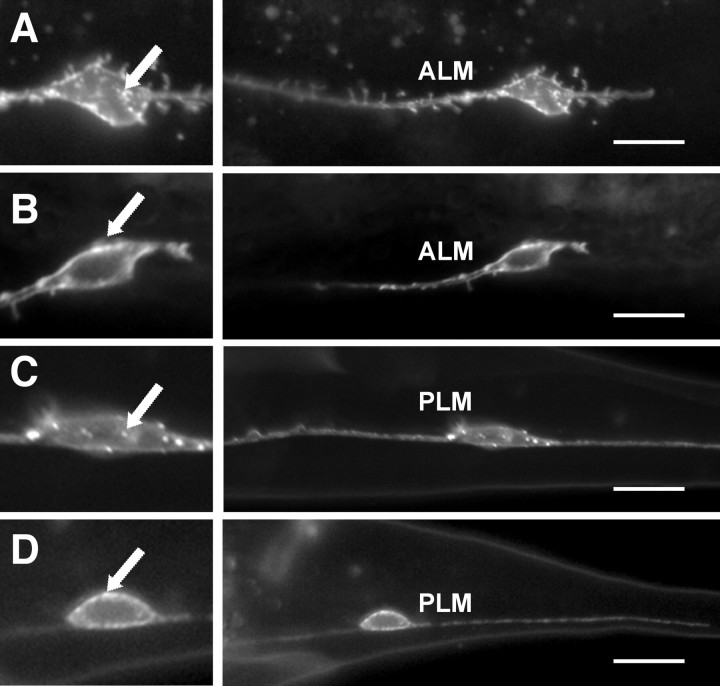
We also observe a change in the subcellular localization of SAX-3::GFP in the rpm-1 loss-of-function mutants (Fig. 6). In the rpm-1(+) background, SAX::GFP is observed in the ALM and PLM neurons as a punctate pattern in 88 and 84%, respectively, of the animals (n = 103). However in rpm-1(−) mutants (n = 105), the pattern appears more uniform and the punctate pattern was observed in only 39 and 37% of the ALM and PLM neurons.
Figure 6.
RPM-1 affects the localization of SAX-3::GFP in ALM and PLM neurons. SAX-3 overexpression was observed in transgenic animals carrying an integrated Pmec7::SAX-3::GFP transgene (gmIs28) (Watari-Goshima et al., 2007). A, C, In wild-type animals, SAX-3::GFP is localized in the AVM and PLM cell bodies and axons in a punctate pattern. Cell bodies are magnified in the left image in each panel. B, D, In rpm-1 mutants, the number of SAX-3::GFP puncta are reduced and they appear more localized to the cell surface rather than to the cytoplasm. Anterior is left and dorsal is up. Arrows indicate the SAX-3::GFP puncta. Scale bars, 20 μm.
A functional UNC-5::GFP is expressed in a punctate pattern in neurons and cells, including those that require unc-5 cell autonomously for guiding migrations (Killeen et al., 2002). We note some subtle changes in the UNC-5::GFP expression pattern in rpm-1 mutants. Whereas in rpm-1(+) animals we did not detect UNC-5::GFP in the PLM neurons, in 7% (n = 150) of rpm-1 loss-of-function mutants expression in PLM is observed (Fig. 5D,E). Also, in the rpm-1 loss-of-function mutants the axons often have multiple short branches (Fig. 5E).
The expression of each transgene was scored in L4 stage or young adult animals. We found that it is more difficult to reliably score pattern changes at earlier stages because the fluorescence signals from the tagged proteins tend to be weaker. Although the results indicate that loss of rpm-1 function can affect the expression patterns, we cannot be sure whether the changes we observe are present during the earlier stages when axon morphology is perturbed in the mutants. In rpm-1 mutants, we did not observe changes in the expression pattern of UNC-40::GFP from two different transgenes, evEX66[unc-40::GFP] and kyEx1212[punc-86::UNC-40::GFP] (Chan et al., 1996; Adler et al., 2006). Furthermore, in rpm-1 mutant strains we used reporters expressing GFP to observe axon positions and we did not observe the morphological changes of neurons as in the strains expressing SAX-3::GFP or UNC-5::GFP. Together, these observations suggest that the differences described above are attributable mainly to changes in SAX-3 or UNC-5 expression, rather than effects caused by GFP expression.
GLO-4 and RPM-1 have similar effects on ventral AVM and dorsal motor neuron axon guidance
RPM-1 functions through at least two parallel pathways to regulate axon termination and synaptogenesis; one pathway involves signaling through GLO-4 and GLO-1 and the other involves signaling through FSN-1 (Liao et al., 2004; Nakata et al., 2005; Grill et al., 2007). Using the strong loss-of-function alleles, we tested double rpm-1;glo-4, rpm-1;glo-1, and rpm-1;fsn-1 mutants for the PLM overextension phenotypes (Fig. 3, Table 1). As previously observed, the combinations could significantly enhance the overextension phenotype relative to the single mutants, which is consistent with the interpretation that GLO-4 and FSN-1 pathways promote RPM-1-mediated axon termination (Grill et al., 2007). To test whether RPM-1 might act through either GLO-4 or FSN-1 to influence ventral and dorsal axon guidance, we asked whether the loss of either glo-4 or fsn-1 function could affect axon guidance in a manner similar to loss of rpm-1 function. Like rpm-1;slt-1 double mutants (Fig. 2), the glo-4;slt-1 double mutants have AVM defects that are significantly enhanced relative to the single mutants; however, fsn-1;slt-1 double mutants do not show an enhanced penetrance (Table 1). We also observe that, similar to loss of rpm-1 function, loss of glo-4 function can suppress the dorsal guidance defect of the DA and DB motor neurons in unc-6(rh46) mutants. In glo-4(ok623);unc-6(rh46) mutants, 46% (n = 205) of DA/DB motor neuron axons migrate dorsally compared with 21% (n = 157) in the unc-6(rh46) mutants. Together, these results suggest that the FSN-1 pathway may play distinct roles in the different neurons and that the glo-4 pathway could mediate the rpm-1 function that affects axon outgrowth in circumferentially migrating axons as well as longitudinally migrating axons.
Table 1.
Axon guidance defects and overextension phenotypes in mutant strains
| Genotype | Ventral guidance AVM axon (% defective) | Overextension PLM axon (%) | Overextension to AVM PLM axon (%) | n |
|---|---|---|---|---|
| glo-4(ok623) | 0 | 23 | 3 | 131 |
| glo-1(zu391) | 0 | 21 | 3 | 194 |
| fsn-1(hp1) | 0 | 28 | 5 | 257 |
| rpm-1(ur299) | 0 | 90 | 15 | 270 |
| rpm-1(ur299);glo-4(ok623) | 0 | 100 | 42* | 119 |
| rpm-1(ur299);glo-1(zu391) | 0 | 100 | 87** | 146 |
| rpm-1(ur299);fsn-1(hp1) | 0 | 100 | 93** | 213 |
| slt-1(eh15) | 32 | 0 | 0 | 238 |
| slt-1(eh15);glo-4(ok623) | 63* | 7 | 0 | 208 |
| slt-1(eh15);fsn-1(hp1) | 34 | 28 | 2 | 181 |
Strains were analyzed in the zdIs5(mec-4::GFP) background at 20° C. n, Total number of animals scored. Asterisks indicate differences between rpm-1 or slt-1 single- or double-mutant strains determined by a two-tailed Z test (*p < 0.005; **p < 0.0005).
We observe that in glo-4(ok623) mutants, the SAX-3::GFP signal is more widely detected. In glo-4(+) L4 larvae, SAX-3::GFP is detected in 25% (n = 60) of the PLM neurons, whereas in 48% (n = 60) of glo-4(ok623) L4 larvae SAX-3::GFP signal is observed. In 15% (n = 60) of glo-4(ok623) mutants, there are abnormal multiple processes from the PLM cell body. Whereas in ventral cord motor neurons the SAX-3::GFP signal is observed in 19% (n = 60) of glo-4(+) larvae, 50% (n = 60) of glo-4(ok623) mutants show strong expression. The phenotypes are similar to the changes observed in rpm-1(ur299) mutants, which is consistent with the idea that RPM-1 may regulate SAX-3 activity through the GLO-4 pathway.
Discussion
Axon termination and axon guidance both involve the control of axon outgrowth. Axon termination is the cessation of axon outgrowth, whereas axon guidance involves the orientation of axon outgrowth-promoting activity by guidance cues (Quinn et al., 2006). In fact, observations using the HSN neuron show that physically the UNC-40 receptor and other proteins that may mediate protrusion activity are localized to the site of axon formation in response to UNC-6 (Adler et al., 2006; Chang et al., 2006; Quinn et al., 2006) (Quinn, Pfeil, and Wadsworth, unpublished observations). This model predicts that the association of the guidance cue and its receptor triggers orientation (i.e., neuronal polarity and the asymmetric trafficking of the receptors to specific cell membranes). At these membranes, the receptors promote the assembly of complexes that promote outgrowth (Quinn, Pfeil, and Wadsworth, unpublished observations). In fact, there are several examples in which guidance receptor-mediated axon outgrowth appears independent of the known ligands for the receptor (Kim et al., 1999; Honigberg and Kenyon, 2000; Yu et al., 2002). In this study, for example, UNC-5 is required to mediate PLM axon extension; however, the UNC-6 ligand is not. In summary, we infer that the axon guidance process is composed of two separate mechanisms mediated by the receptors, one that orients the outgrowth-promoting activity in response to guidance cues and a second that promotes axon outgrowth.
Mutations in VAB-8, UNC-73, and MIG-2 have also been shown to regulate the UNC-40 and SAX-3 receptors and to affect longitudinal axon outgrowth (Levy-Strumpf and Culotti, 2007; Watari-Goshima et al., 2007). These genes are thought to be important for the appropriate spatial localization of the receptors; perturbing their function affects neuronal polarity. In our model, these genes might be considered as positively regulating the orientation of the receptor outgrowth-activity. Perturbing the orientation would, in turn, cause the guidance receptor-mediated outgrowth activity to be misdirected.
In contrast to positively regulating orientation, RPM-1 negatively regulates UNC-5- and SAX-3-mediated axon outgrowth-promoting activity. We hypothesize that an increase in UNC-5 axon outgrowth activity in rpm-1 loss-of-function mutants produces the PLM axon overextension phenotype and improves the dorsal DA and DB motor neuron axon migrations. It is worth noting that, in the DA and DB motor neuron experiments, we used a reduction-of-function allele, unc-5(e152). Although the results from the rpm-1(ur299);unc-6(rh46);unc-5(e152) mutants suggest that the unc-5(e152) allele reduces the ability of rpm-1(ur299) to suppress the guidance defects caused by unc-6(rh46), it is interesting that the guidance defects in the rpm-1(ur299);unc-5(e152) double mutants are more severe than in either the unc-5(e152) or rpm-1(ur299) mutants (Fig. 1F). A possibility is that altered forms of UNC-5 could become detrimental to the guidance process when not regulated by RPM-1. We do not yet know whether this effect is specific to the unc-5(e152) allele.
The results also suggest that RPM-1 helps control SAX-3 interactions with SLT-1 and UNC-40. Although both slt-1 and sax-3 loss-of-function mutations cause a similar penetrance of the mutant phenotype for the AVM and PLM axons, the slt-1 mutation in combination with the rpm-1 loss-of-function mutation causes the greatest change to the mutant phenotype. We hypothesize that this because SAX-3, when not associated with SLT-1, is free to silence UNC-40-mediated signaling. The phenotype of triple rpm-1;slt-1;sax-3 mutants resembles the less severe phenotype caused by rpm-1;sax-3 rather than the severe defects caused by rpm-1;slt-1, which is consistent with the predicted role of SAX-3.
The idea that SAX-3 might inhibit UNC-40-mediated guidance of the AVM axon was also suggested by Fujisawa et al. (2007) while this study was under review. In addition, the interactions of guidance receptors in vitro suggest that a guidance receptor can directly influence the signaling by another. In cultured Xenopus spinal cord neurons, an association between the DCC and Robo receptors is thought to silence the ability of DCC to mediate turning toward netrin (Stein and Tessier-Lavigne, 2001) and an association between DCC and UNC5 is thought to convert an attractive response to netrin to a repulsive response (Hong et al., 1999). In C. elegans, unc-40 mutations have been shown to suppress SLT-1 gain-of-function phenotypes and enhance weak sax-3 loss-of-function phenotypes, whereas biochemical experiments indicate UNC-40 and SAX-3 directly interact (Yu et al., 2002). These observations suggest that SAX-3 signaling is potentiated by an association between UNC-40 and SAX-3 (Yu et al., 2002).
RPM-1 could regulate axon outgrowth that affects axon guidance and termination by controlling the trafficking of the UNC-5 and SAX-3 receptors to cell membranes (Fig. 7). RPM-1 is proposed to positively regulate the Rab guanine nucleotide exchange factor (GEF) GLO-4 pathway to promote vesicle trafficking for presynaptic development (Grill et al., 2007). By positively regulating vesicle trafficking to presynaptic sites, RPM-1 might restrict the trafficking of the receptors to the sites where they would promote axon outgrowth. Our observations suggest that RPM-1 can regulate axon outgrowth in migrating axons that have not formed stable presynaptic complexes. Moreover, RPM-1 activity in the migrating axons does not appear to play a major role in axon guidance. In fact, in our study, axon guidance defects are not observed in the rpm-1 mutants unless there is a genetic background sensitized for axon guidance defects. An attractive idea is that RPM-1 may be triggered to negatively regulate axon outgrowth by signals within local target environments that promote synaptogenesis. In this manner, RPM-1 could help coordinate UNC-5- and SAX-3-mediated axon outgrowth with presynaptic development.
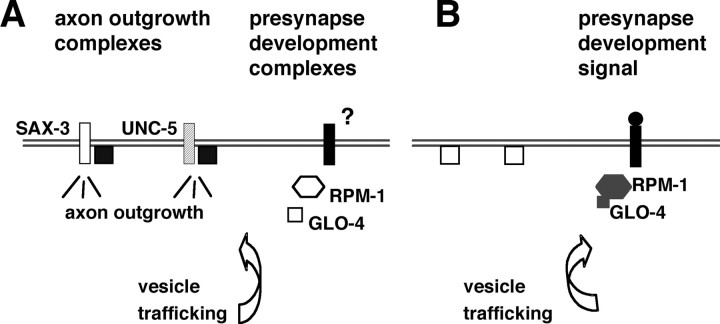
Figure 7.
A model for RPM-1 regulation of guidance receptor functions. A, We hypothesize that RPM-1 negatively regulates SAX-3 and UNC-5 activity at complexes on the cell surface membrane that promote outgrowth. Lack of RPM-1 or GLO-4 activity increases receptor axon outgrowth-promoting activity. B, RPM-1 is proposed to bind GLO-4 and positively regulate a Rab GEF GLO-4 pathway to promote vesicular trafficking for synaptogenesis (Grill et al., 2007). This RPM-1 activity may be activated by signals that promote synaptogenesis. The subsequent regulation of vesicular trafficking for presynaptic development might inhibit the trafficking of SAX-3 and UNC-5 receptors to the locations where complexes could form to promote axon outgrowth.
Footnotes
This work was supported by National Institutes of Health Grant R01 NS033156 and grants from the New Jersey Commission on Spinal Cord Research. We thank S. Clark, J. Culotti, C. Bargmann, G. Garriga, Y. Jin, and the Caenorhabditis Genetics Center for kindly providing strains and constructs; we also thank members of the Wadsworth Laboratory for constructive input and Sunita Kramer, Christopher Quinn, and Martha Soto for critical discussion and comments on this manuscript.
References
- Adler CE, Fetter RD, Bargmann CI. UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat Neurosci. 2006;9:511–518. doi: 10.1038/nn1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess RW, Peterson KA, Johnson MJ, Roix JJ, Welsh IC, O'Brien TP. Evidence for a conserved function in synapse formation reveals Phr1 as a candidate gene for respiratory failure in newborn mice. Mol Cell Biol. 2004;24:1096–1105. doi: 10.1128/MCB.24.3.1096-1105.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SS, Zheng H, Su MW, Wilk R, Killeen MT, Hedgecock EM, Culotti JG. UNC-40, a C. elegans homolog of DCC (deleted in colorectal cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996;87:187–195. doi: 10.1016/s0092-8674(00)81337-9. [DOI] [PubMed] [Google Scholar]
- Chang C, Adler CE, Krause M, Clark SG, Gertler FB, Tessier-Lavigne M, Bargmann CI. MIG-10/Lamellipodin and AGE-1/PI3K promote axon guidance and outgrowth in response to slit and netrin. Curr Biol. 2006;16:854–862. doi: 10.1016/j.cub.2006.03.083. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- D'Souza J, Hendricks M, Le Guyader S, Subburaju S, Grunewald B, Scholich K, Jesuthasan S. Formation of the retinotectal projection requires Esrom, an ortholog of PAM (protein associated with Myc) Development. 2005;132:247–256. doi: 10.1242/dev.01578. [DOI] [PubMed] [Google Scholar]
- Fujisawa K, Wrana JL, Culotti JG. The slit receptor EVA-1 coactivates a SAX-3/Robo mediated guidance signal in C. elegans. Science. 2007;317:1934–1938. doi: 10.1126/science.1144874. [DOI] [PubMed] [Google Scholar]
- Gitai Z, Yu TW, Lundquist EA, Tessier-Lavigne M, Bargmann CI. The netrin receptor UNC-40/DCC stimulates axon attraction and outgrowth through enabled and, in parallel, Rac and UNC-115/AbLIM. Neuron. 2003;37:53–65. doi: 10.1016/s0896-6273(02)01149-2. [DOI] [PubMed] [Google Scholar]
- Grill B, Bienvenut WV, Brown HM, Ackley BD, Quadroni M, Jin Y. C. elegans RPM-1 regulates axon termination and synaptogenesis through the Rab GEF GLO-4 and the Rab GTPase GLO-1. Neuron. 2007;55:587–601. doi: 10.1016/j.neuron.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Guo Q, Xie J, Dang CV, Liu ET, Bishop JM. Identification of a large Myc-binding protein that contains RCC1-like repeats. Proc Natl Acad Sci USA. 1998;95:9172–9177. doi: 10.1073/pnas.95.16.9172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao JC, Yu TW, Fujisawa K, Culotti JG, Gengyo-Ando K, Mitani S, Moulder G, Barstead R, Tessier-Lavigne M, Bargmann CI. C. elegans slit acts in midline, dorsal-ventral, and anterior-posterior guidance via the SAX-3/Robo receptor. Neuron. 2001;32:25–38. doi: 10.1016/s0896-6273(01)00448-2. [DOI] [PubMed] [Google Scholar]
- Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990;4:61–85. doi: 10.1016/0896-6273(90)90444-k. [DOI] [PubMed] [Google Scholar]
- Hong K, Hinck L, Nishiyama M, Poo MM, Tessier-Lavigne M, Stein E. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–941. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Honigberg L, Kenyon C. Establishment of left/right asymmetry in neuroblast migration by UNC-40/DCC, UNC-73/Trio and DPY-19 proteins in C. elegans. Development. 2000;127:4655–4668. doi: 10.1242/dev.127.21.4655. [DOI] [PubMed] [Google Scholar]
- Karlstrom RO, Trowe T, Klostermann S, Baier H, Brand M, Crawford AD, Grunewald B, Haffter P, Hoffmann H, Meyer SU, Muller BK, Richter S, van Eeden FJ, Nusslein-Volhard C, Bonhoeffer F. Zebrafish mutations affecting retinotectal axon pathfinding. Development. 1996;123:427–438. doi: 10.1242/dev.123.1.427. [DOI] [PubMed] [Google Scholar]
- Killeen M, Tong J, Krizus A, Steven R, Scott I, Pawson T, Culotti J. UNC-5 function requires phosphorylation of cytoplasmic tyrosine 482, but its UNC-40-independent functions also require a region between the ZU-5 and death domains. Dev Biol. 2002;251:348–366. doi: 10.1006/dbio.2002.0825. [DOI] [PubMed] [Google Scholar]
- Kim S, Ren XC, Fox E, Wadsworth WG. SDQR migrations in Caenorhabditis elegans are controlled by multiple guidance cues and changing responses to netrin UNC-6. Development. 1999;126:3881–3890. doi: 10.1242/dev.126.17.3881. [DOI] [PubMed] [Google Scholar]
- Leung-Hagesteijn C, Spence AM, Stern BD, Zhou Y, Su MW, Hedgecock EM, Culotti JG. UNC-5, a transmembrane protein with immunoglobulin and thrombospondin type 1 domains, guides cell and pioneer axon migrations in C. elegans. Cell. 1992;71:289–299. doi: 10.1016/0092-8674(92)90357-i. [DOI] [PubMed] [Google Scholar]
- Levy-Strumpf N, Culotti JG. VAB-8, UNC-73 and MIG-2 regulate axon polarity and cell migration functions of UNC-40 in C. elegans. Nat Neurosci. 2007;10:161–168. doi: 10.1038/nn1835. [DOI] [PubMed] [Google Scholar]
- Liao EH, Hung W, Abrams B, Zhen M. An SCF-like ubiquitin ligase complex that controls presynaptic differentiation. Nature. 2004;430:345–350. doi: 10.1038/nature02647. [DOI] [PubMed] [Google Scholar]
- Nakata K, Abrams B, Grill B, Goncharov A, Huang X, Chisholm AD, Jin Y. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell. 2005;120:407–420. doi: 10.1016/j.cell.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Quinn CC, Pfeil DS, Chen E, Stovall EL, Harden MV, Gavin MK, Forrester WC, Ryder EF, Soto MC, Wadsworth WG. UNC-6/Netrin and SLT-1/Slit guidance cues orient axon outgrowth mediated by MIG-10/RIAM/lamellipodin. Curr Biol. 2006;16:845–853. doi: 10.1016/j.cub.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Schaefer AM, Hadwiger GD, Nonet ML. rpm-1, a conserved neuronal gene that regulates targeting and synaptogenesis in C. elegans. Neuron. 2000;26:345–356. doi: 10.1016/s0896-6273(00)81168-x. [DOI] [PubMed] [Google Scholar]
- Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291:1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Wadsworth WG, Bhatt H, Hedgecock EM. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron. 1996;16:35–46. doi: 10.1016/s0896-6273(00)80021-5. [DOI] [PubMed] [Google Scholar]
- Wan HI, DiAntonio A, Fetter RD, Bergstrom K, Strauss R, Goodman CS. Highwire regulates synaptic growth in Drosophila. Neuron. 2000;26:313–329. doi: 10.1016/s0896-6273(00)81166-6. [DOI] [PubMed] [Google Scholar]
- Watari-Goshima N, Ogura K, Wolf FW, Goshima Y, Garriga G. C. elegans VAB-8 and UNC-73 regulate the SAX-3 receptor to direct cell and growth-cone migrations. Nat Neurosci. 2007;10:169–176. doi: 10.1038/nn1834. [DOI] [PubMed] [Google Scholar]
- Yu TW, Hao JC, Lim W, Tessier-Lavigne M, Bargmann CI. Shared receptors in axon guidance: SAX-3/Robo signals via UNC-34/Enabled and a Netrin-independent UNC-40/DCC function. Nat Neurosci. 2002;5:1147–1154. doi: 10.1038/nn956. [DOI] [PubMed] [Google Scholar]
- Zallen JA, Yi BA, Bargmann CI. The conserved immunoglobulin superfamily member SAX-3/Robo directs multiple aspects of axon guidance in C. elegans. Cell. 1998;92:217–227. doi: 10.1016/s0092-8674(00)80916-2. [DOI] [PubMed] [Google Scholar]
- Zhen M, Huang X, Bamber B, Jin Y. Regulation of presynaptic terminal organization by C. elegans RPM-1, a putative guanine nucleotide exchanger with a RING-H2 finger domain. Neuron. 2000;26:331–343. doi: 10.1016/s0896-6273(00)81167-8. [DOI] [PubMed] [Google Scholar]