Abstract
We extended the mortality follow-up of a cohort of 25,460 workers employed at 8 acrylonitrile (AN)-producing facilities in the United States by 21 years. Using 8,124 deaths and 1,023,922 person-years of follow-up, we evaluated the relationship between occupational AN exposure and death. Standardized mortality ratios (SMRs) based on deaths through December 31, 2011, were calculated. Work histories and monitoring data were used to develop quantitative estimates of AN exposure. Hazard ratios were estimated by Cox proportional hazards regression. All-cause mortality and death from total cancer were less than expected compared with the US population. We observed an excess of death due to mesothelioma (SMR = 2.24, 95% confidence interval (CI): 1.39, 3.42); no other SMRs were elevated overall. Cox regression analyses revealed an elevated risk of lung and bronchial cancer (n = 808 deaths; for >12.1 ppm-year vs. unexposed, hazard ratio (HR) = 1.43, 95% CI: 1.13, 1.81; P for trend = 0.05), lagged 10 years, that was robust in sensitivity analyses adjusted for smoking and co-exposures including asbestos. Death resulting from bladder cancer (for >2.56 ppm vs. unexposed, lagged 10-year HR = 2.96, 95% CI: 1.38, 6.34; P for trend = 0.02) and pneumonitis (for >3.12 ppm-year vs. unexposed, HR = 4.73, 95% CI: 1.42, 15.76; P for trend = 0.007) was also associated with AN exposure. We provide additional evidence of an association between AN exposure and lung cancer, as well as possible increased risk for death due to bladder cancer and pneumonitis.
Keywords: acrylonitrile, bladder cancer, lung cancer, mortality, occupational cohort
Acrylonitrile (AN) is used in the production of acrylic fibers, resins, and chemical intermediates (1), with end-use products ranging from clothing and upholstery to automotive parts and various plastics (2). Each year, more than 10 billion pounds of AN are produced globally (3). Exposure to AN has been characterized in the occupational setting (2, 4, 5); the current permissible exposure limit is 2 ppm as an 8-hour time-weighted average (6). Exposure to the general population may occur from tobacco smoke; AN concentrations in cigarette smoke are 24.1–85.6 μg/cigarette (7).
Experimental data show that AN is mutagenic and causes chromosomal aberrations, cell transformation, and unscheduled DNA synthesis in human cells (8). Results of animal bioassay studies suggest that AN may cause development of tumors of the brain, spinal cord, Zymbal gland, forestomach, and mammary glands (2, 8). Results from early epidemiologic studies suggested slightly elevated death rates from lung (9–13), prostate (11, 14), bladder (12, 15, 16), brain/central nervous system (17), and lymphohematopoietic (12, 17) cancers, but evidence is not consistent. On the basis of these data, in 1999, the International Agency for Research on Cancer classified AN as possibly carcinogenic to humans (group 2B). Recent re-analyses of previous cohorts have shown no clear association between AN exposure and death from any cancer (18–20).
The largest study of AN-exposed workers in the world involved a cohort of 25,460 workers employed at 8 US facilities and was first investigated by Blair et al. (10) in 1998. The first report evaluated the association between AN and disease-specific mortality among 1,919 deaths ascertained from date of hire (the earliest of which was in 1942) through 1989 (10). This analysis exhibited no clear evidence of association between AN and any cause of death, but the results did suggest increased risk of lung cancer in some subgroups. Here, we extended the death follow-up of this cohort by 21 years (ending on December 31, 2011) to further evaluate associations between mortality and AN exposure for an additional 1,023,922 person-years of follow-up and 8,124 total deaths.
METHODS
Study population, exposure assessment
Characteristics of the plants in the study and details of the exposure assessment have been reported previously (Web Appendix, available at https://academic.oup.com/aje) (4, 10, 21–23). Briefly, the cohort included all workers employed at least 1 day (n = 25,460) at any of the 8 participating plants after the start of AN operations (1952–1965). Work histories covered the date of hire (i.e., 1942–1983) through date of record abstraction at the plant (1983). For this extended follow-up, we matched individuals who were alive or who had an unknown vital status in 1989 to the National Death Index (NDI)-Plus service and the Social Security Administration death files through December 31, 2011. Causes of death were coded according to the International Statistical Classification of Diseases (ICD) revision in effect at the time of death (ICD-8, ICD-9, and ICD-10). We used entire work histories, plant records, and monitoring data to develop 8-hour time-weighted average estimates of AN exposure for each job/department/plant combination by time period from first AN exposure through 1983 (Web Tables 1 and 2). Potential exposure to 340 other substances (yes/no) was also assessed. For asbestos, an expert (P.A.S.), blinded to outcome status, assigned a probability score of exposure for each job, department, and plant combination on the basis of job titles and tasks. The asbestos exposure scores were as follows: 0, unlikely to be exposed; 1–3, low likelihood; and 4–5, high likelihood; analyses were based on a worker’s maximum score. No asbestos measurements were available. In the original study, investigators collected tobacco use information on all deaths due to lung and brain cancer and from a 10% systematic sample of workers, using a case-cohort design to evaluate the potential confounding of lung cancer results from cigarette smoking (Web Appendix) (10).
Statistical analysis
We calculated overall and exposure-specific standardized mortality ratios (SMRs) for underlying causes of death using 5-year age and calendar time, race, and sex-specific mortality rates from the US population (24, 25) using the Surveillance, Epidemiology, and End Results*Stat Multiple Primary Standardized Incidence Ratio session. Person-years of follow-up for SMRs were characterized from time of hire, the earliest of which was 1942, through 2011. Cancer and noncancer causes of death included major groups as outlined in the Surveillance, Epidemiology, and End Results Program Cause of Death Recode (26) (Web Table 3). In addition, we calculated hazard ratios and 95% confidence intervals for AN exposure and each outcome with at least 10 AN-exposed cases, using Cox proportional hazard models with age as the time metric and adjustment for race, sex, birth year, and salary-wage classification using SAS, version 9.3 (SAS Institute Inc., Cary, North Carolina). We also explored stratified Cox regression by sex and categories of birth year to allow for distinct background hazard function; however, this did not materially affect the hazard ratios. Cumulative exposure (ppm-years), average intensity (ppm), and duration of exposure (years) were modeled as time-dependent variables. For cancer outcomes, cumulative and average intensity of exposure were lagged 10 years. For Cox models, person-year accumulation began at first AN use (plant-specific, 1952–1960) through 2011. The proportional hazards assumption was not violated. Additional adjustments based on operation type (fiber vs. nonfiber), plant, and possible co-exposures were also evaluated. Cutpoints for exposure categories were based on outcome-specific distribution of deaths (median, tertiles (T), or quintiles (Q)) (Web Table 4) for outcomes with at least 10 exposed deaths. Tests for linear trend were calculated using the median value for each exposure category as continuous. We also evaluated work-related metrics, including age at first exposure (≤25, 26–32, 33–39, >39 years), year of first exposure (<1955, 1956–1960, 1961–1967, ≥1968), years since last exposure (<15, 15–29, ≥30), years since first exposure (≤10, 11–19, ≥20), and operation type. Likelihood ratio tests were used to evaluate effect modification by age, sex, wage, and work-related metrics.
For lung cancer, we conducted sensitivity analyses to explore the absence of exposure information beyond December 31, 1983. There were 7,260 workers (28.5%) actively employed at the end of 1983. In the main analyses, we assumed zero AN exposure after 1983 for these active workers and assumed that nonactive workers were not re-exposed to AN after 1983. We also ran the following additional Cox models for lung cancer: 1) censoring all active workers in 1983 (n = 7,260 censored) and 2) excluding workers still active in 1983 (leaving 18,200 remaining). To assess the cumulative exposure-response curve for lung cancer, we used an expanded number of ppm-years categories: 0 to <1, 1 to <2, 2 to <4, 4 to <8, 8 to <16, 16 to <32, and ≥32. We fitted various functional forms in continuous cumulative AN and determined the 2-parameter log-linear-quadratic model provided the best fit based on the Akaike Information Criteria.
We evaluated potential confounding by cigarette smoking on the association between AN and lung cancer, using data from the case-cohort substudy (10). Because information on smoking was missing for some individuals within the subcohort sample (20.7%), we multiply imputed smoking status (never vs. ever) and category of duration smoked (1–20 years, 21–40 years, ≥41 years) using PROC MI in SAS, version 14.3 (SAS Institute) to create 25 imputations (see Web Appendix). Cox regression using PROC SURVEYPHREG in SAS was used to obtain adjusted hazard ratios. Statistical tests were 2-sided and based on a χ2 Wald test, with P < 0.05 considered statistically significant.
RESULTS
Table 1 shows the characteristics of the cohort by vital status. Extended follow-up of the cohort included 6,205 newly identified deaths for a total of 8,124 deaths, with 68% of the cohort still living at the end of follow-up. Of the total cohort, 66.4%was exposed to AN (66.2% still alive and 66.8% deceased).
Table 1.
Characteristics (Overall and by Vital Status at the End of Extended Follow-up) of Workers Employed at 8 Acrylonitrile-Producing Facilities, United States, 1942–2011
| Characteristic | All Subjects (n = 25,460; Person-Years = 1,023,922) | Alive (n = 17,336; Person-Years = 739,199) | Deceased (n = 8,124; Person-Years = 284,723) | |||
|---|---|---|---|---|---|---|
| No. | % Person-Time | No. | % Person-Time | No. | % Person-Time | |
| Year of hire | ||||||
| ≤1955 | 3,761 | 16.8 | 1,173 | 9.2 | 2,588 | 36.5 |
| 1956 through 1960 | 3,275 | 14.8 | 1,648 | 12.0 | 1,627 | 22.0 |
| 1961 through 1965 | 4,697 | 20.0 | 3,189 | 20.7 | 1,508 | 18.3 |
| 1966 through 1970 | 5,204 | 20.5 | 3,948 | 23.3 | 1,256 | 13.4 |
| 1971 through 1983 | 8,523 | 27.9 | 7,378 | 34.8 | 1,145 | 9.8 |
| Year left work | ||||||
| ≤1960 | 2,308 | 9.1 | 1,073 | 6.2 | 1,235 | 15.2 |
| 1961 through 1970 | 6,236 | 24.5 | 3,962 | 22.9 | 2,271 | 27.9 |
| 1971 through 1983 | 9,656 | 37.9 | 6,818 | 39.3 | 2,841 | 35.0 |
| Active at the end of 1983 | 7,260 | 28.5 | 5,483 | 31.6 | 1,777 | 21.9 |
| Age at end of follow-up, years | ||||||
| <60 | 6,336 | 18.3 | 3,958 | 18.4 | 2,379 | 18.2 |
| 60–69 | 8,868 | 34.5 | 6,812 | 38.0 | 2,060 | 25.3 |
| 70–79 | 6,857 | 30.6 | 4,606 | 29.7 | 2,246 | 32.7 |
| ≥80 | 3,399 | 16.6 | 1,960 | 13.9 | 1,439 | 23.8 |
| Sex | ||||||
| Male | 20,270 | 79.7 | 13,237 | 77.1 | 7,033 | 86.4 |
| Female | 5,190 | 20.3 | 4,099 | 22.9 | 1,091 | 13.6 |
| Race | ||||||
| White | 22,372 | 89.2 | 14,963 | 88.0 | 7,409 | 92.3 |
| Other | 3,088 | 10.8 | 2,373 | 12.0 | 715 | 7.7 |
| Plant no. | ||||||
| 1 | 1,896 | 7.4 | 1,181 | 7.0 | 715 | 8.5 |
| 2 | 1,989 | 7.4 | 1,585 | 8.5 | 404 | 4.4 |
| 3 | 1,545 | 5.9 | 1,093 | 6.0 | 452 | 5.4 |
| 4 | 3,379 | 12.2 | 2,471 | 13.3 | 908 | 9.6 |
| 5 | 7,321 | 30.4 | 5,138 | 31.4 | 2,183 | 27.7 |
| 6 | 2,653 | 9.9 | 1,778 | 9.6 | 875 | 10.7 |
| 7 | 2,339 | 9.2 | 1,750 | 10.1 | 589 | 7.1 |
| 8 | 4,338 | 17.6 | 2,340 | 14.1 | 1,998 | 26.6 |
| Acrylonitrile exposure | ||||||
| Unexposed | 8,571 | 33.6 | 5,840 | 33.8 | 2,731 | 33.2 |
| Exposed | 16,889 | 66.4 | 11,496 | 66.2 | 5,393 | 66.8 |
Standardized mortality ratios
Deaths from all causes were less than expected compared with the general population (overall SMR = 0.79, 95% CI: 0.78, 0.81) (Table 2; Web Table 5). For cancer, there were deficits for all malignant cancers combined, as well as for cancers of the digestive system (n = 555), lung and bronchus (n = 808), and lymphoma (n = 89); SMRs for these cancers were less than 1 in AN-unexposed and AN-exposed workers for these outcomes (Table 2). Since 1990 (the start of ICD-10 coding for mesothelioma), there were 21 deaths, with excesses overall (SMR = 2.24, 95% CI: 1.39, 3.42) and in AN-unexposed and AN-exposed workers (SMR = 1.93, 95% CI: 0.63, 4.50; and SMR = 2.36, 95% CI: 1.35, 3.83, respectively). There were several deficits in observed deaths in noncancer causes of death, including diabetes mellitus, cerebrovascular disease, and other outcomes. There was an excess of death from Alzheimer disease (ICD-9 and 10 only), a subset of the nervous system deaths, overall (n = 115, SMR = 1.17, 95% CI: 0.96, 1.40), and for AN-unexposed deaths (SMR = 1.47, 95% CI: 1.11, 1.91) (Table 2), but no excess was apparent among the AN-exposed.
Table 2.
Selected Standardized Mortality Ratios Overall and by Acrylonitrile Exposure for Workers Employed at 8 Acrylonitrile-Producing Facilities, United States, 1960–2011
| Cause of Death | Overall | AN Unexposed | AN Exposed | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Observed | O/E | 95% CI | Observed | O/E | 95% CI | Observed | O/E | 95% CI | |
| All causes of death | 8,124 | 0.79 | 0.78, 0.81 | 2,731 | 0.82 | 0.79, 0.85 | 5,393 | 0.79 | 0.77, 0.81 |
| All malignant cancers | 2,440 | 0.87 | 0.84, 0.91 | 843 | 0.90 | 0.84, 0.97 | 1,597 | 0.86 | 0.82, 0.90 |
| Digestive system | 555 | 0.83 | 0.76, 0.90 | 186 | 0.87 | 0.75, 1.00 | 369 | 0.81 | 0.73, 0.90 |
| Esophagus | 81 | 0.95 | 0.76, 1.18 | 24 | 1.01 | 0.65, 1.50 | 57 | 0.93 | 0.71, 1.21 |
| Respiratory system | 832 | 0.86 | 0.80, 0.92 | 260 | 0.85 | 0.75, 0.96 | 572 | 0.87 | 0.80, 0.94 |
| Lung and bronchus | 808 | 0.87 | 0.81, 0.93 | 249 | 0.84 | 0.74, 0.95 | 559 | 0.88 | 0.81, 0.96 |
| Mesothelioma (ICD-10 only) | 21 | 2.24 | 1.39, 3.42 | 5 | 1.93 | 0.63, 4.50 | 16 | 2.36 | 1.35, 3.83 |
| Breast | 73 | 0.86 | 0.68, 1.08 | 57 | 1.00 | 0.76, 1.29 | 16 | 0.59 | 0.34, 0.95 |
| Urinary system | 123 | 0.89 | 0.74, 1.06 | 38 | 0.90 | 0.63, 1.23 | 85 | 0.88 | 0.71, 1.09 |
| Urinary bladder | 55 | 0.84 | 0.63, 1.10 | 16 | 0.81 | 0.46, 1.31 | 39 | 0.86 | 0.61, 1.17 |
| Brain and other nervous system | 80 | 1.05 | 0.83, 1.31 | 32 | 1.30 | 0.89, 1.83 | 48 | 0.94 | 0.69, 1.25 |
| Lymphoma | 89 | 0.75 | 0.60, 0.93 | 30 | 0.78 | 0.53, 1.11 | 59 | 0.75 | 0.57, 0.96 |
| Noncancer causes of death | |||||||||
| Diabetes mellitus | 170 | 0.64 | 0.55, 0.75 | 62 | 0.71 | 0.54, 0.91 | 108 | 0.61 | 0.50, 0.74 |
| Diseases of the nervous system | 239 | 0.87 | 0.77, 0.99 | 102 | 1.07 | 0.87, 1.30 | 137 | 0.77 | 0.65, 0.91 |
| Alzheimer disease (ICD-9 and 10 only) | 115 | 1.17 | 0.96, 1.40 | 56 | 1.47 | 1.11, 1.91 | 59 | 0.98 | 0.74, 1.26 |
| Heart diseases | 2,381 | 0.79 | 0.76, 0.82 | 725 | 0.75 | 0.70, 0.81 | 1,656 | 0.81 | 0.78, 0.85 |
| Cerebrovascular diseases | 359 | 0.76 | 0.68, 0.84 | 118 | 0.71 | 0.59, 0.85 | 241 | 0.78 | 0.69, 0.89 |
| All nonmalignant respiratory conditions | 566 | 0.67 | 0.62, 0.73 | 181 | 0.64 | 0.55, 0.74 | 385 | 0.69 | 0.63, 0.77 |
| COPD and allied conditions (includes emphysema and chronic bronchitis) | 322 | 0.66 | 0.59, 0.74 | 106 | 0.64 | 0.52, 0.77 | 216 | 0.68 | 0.59, 0.77 |
| Respiratory due to external causes | 35 | 0.72 | 0.50, 1.00 | 5 | 0.32 | 0.10, 0.75 | 30 | 0.90 | 0.61, 1.29 |
| Pneumonitis due to solids/liquids | 27 | 0.66 | 0.43, 0.96 | 4 | 0.30 | 0.08, 0.77 | 23 | 0.83 | 0.53, 1.24 |
Abbreviations: AN, acrylonitrile; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ICD-9, International Statistical Classification of Diseases, Ninth Revision; ICD-10, International Statistical Classification of Diseases, 10th Revision; O/E, observed/expected.
Hazard ratios
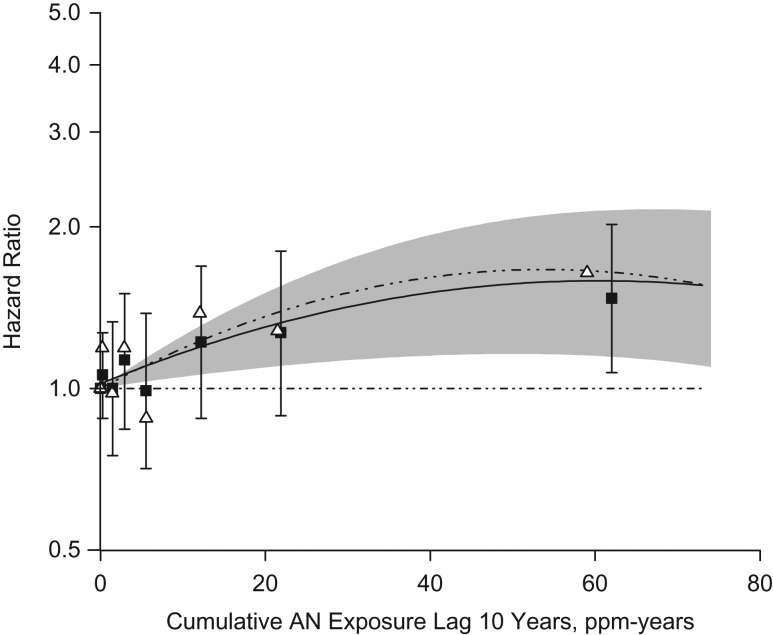
The hazard ratio for lung cancer was significantly elevated in the highest quintile of cumulative exposure (>12.1 ppm-years (Q5); for Q5 vs. unexposed, HR = 1.43, 95% CI: 1.13, 1.81; P for trend = 0.05), but not for average intensity or duration of exposure (Table 3; see Web Table 4 for cutpoints). Results were similar when excluding lung cancer deaths diagnosed within the first 5 years of follow-up (for Q5 vs. unexposed, HR = 1.47, 95% CI: 1.16, 1.87; P for trend = 0.03) (data not shown) or when short-term workers (<1 year) were excluded from the analysis (for Q5 vs. unexposed, HR = 1.46, 95% CI: 1.14, 1.87) among 19,621 workers. There was a consistent trend in risk among hazard ratios across the full range of cumulative exposures (Figure 1; Web Table 6) showing a significant exposure-response (for ≥32 ppm-years vs. unexposed, HR = 1.47, 95% CI: 1.07, 2.02; P for trend = 0.02). Results for lung cancer using unlagged cumulative exposure were similar (Web Table 7). No significant interactions were observed.
Table 3.
Hazard Ratios for Selected Causes of Death by Cumulative, Average, and Duration of Acrylonitrile Exposurea for Workers Employed at 8 Acrylonitrile-Producing Facilities, United States, 1952–2011
| Cause of Death | No. of Deaths | Cumulative Exposure Lagged 10 Years | No. of Deaths | Average Exposure Lagged 10 Years | No. of Deaths | Duration of Exposure | |||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | ||||
| Lung and bronchus, quintile | |||||||||
| Unexposed | 263 | 1.00 | Referent | 263 | 1.00 | Referent | 249 | 1.00 | Referent |
| 1 | 109 | 1.15 | 0.92, 1.45 | 110 | 1.00 | 0.79, 1.25 | 112 | 1.08 | 0.85, 1.36 |
| 2 | 109 | 0.96 | 0.77, 1.21 | 108 | 1.21 | 0.96, 1.53 | 112 | 1.00 | 0.79, 1.25 |
| 3 | 109 | 1.03 | 0.81, 1.29 | 109 | 1.07 | 0.85, 1.35 | 112 | 1.01 | 0.80, 1.27 |
| 4 | 109 | 1.06 | 0.84, 1.33 | 109 | 1.05 | 0.84, 1.32 | 112 | 1.27 | 1.01, 1.60 |
| 5 | 109 | 1.43 | 1.13, 1.81 | 109 | 1.22 | 0.97, 1.54 | 111 | 1.16 | 0.92, 1.46 |
| P for trend | 0.05 | 0.14 | 0.10 | ||||||
| Urinary bladder, tertile | |||||||||
| Unexposed | 16 | 1.00 | Referent | 16 | 1.00 | Referent | 16 | 1.00 | Referent |
| 1 | 13 | 0.94 | 0.45, 1.99 | 13 | 0.63 | 0.30, 1.32 | 13 | 1.29 | 0.61, 2.74 |
| 2 | 13 | 0.78 | 0.37, 1.65 | 13 | 0.94 | 0.45, 1.99 | 13 | 0.96 | 0.46, 2.02 |
| 3 | 13 | 1.45 | 0.69, 3.08 | 13 | 2.96 | 1.38, 6.34 | 13 | 0.73 | 0.35, 1.54 |
| P for trend | 0.56 | 0.02 | 0.33 | ||||||
| Pneumonitis, solids/liquidsb | |||||||||
| Unexposed | 4 | 1.00 | Referent | 4 | 1.00 | Referent | 4 | 1.00 | Referent |
| ≤ Median | 12 | 2.23 | 0.69, 7.27 | 12 | 2.78 | 0.85, 9.10 | 12 | 2.30 | 0.71, 7.49 |
| > Median | 11 | 4.73 | 1.42, 15.76 | 11 | 3.17 | 0.97, 10.37 | 11 | 4.53 | 1.35, 15.21 |
| P for trend | 0.007 | 0.07 | 0.01 | ||||||
| Mesothelioma (ICD-10 only) | |||||||||
| Unexposed | 5 | 1.00 | Referent | 5 | 1.00 | Referent | 5 | 1.00 | Referent |
| ≤ Median | 8 | 1.27 | 0.40, 4.01 | 8 | 1.67 | 0.53, 5.23 | 8 | 0.87 | 0.27, 2.74 |
| > Median | 8 | 1.15 | 0.37, 3.66 | 8 | 0.94 | 0.30, 2.99 | 8 | 2.01 | 0.63, 6.44 |
| P for trend | 0.84 | 0.79 | 0.20 | ||||||
Abbreviations: CI, confidence interval; HR, hazard ratio; ICD-10, International Statistical Classification of Diseases, 10th Revision.
a Internal comparisons use age as the time metric and are adjusted for race, sex, birth year, and salary-wage classification. Cutpoint values for all categories are provided in Web Table 5.
b HRs are all unlagged.
Figure 1.
Hazard ratios for lung cancer by expanded categories of cumulative acrylonitrile (AN) lagged 10 years (ppm-years) among all subjects assuming no additional exposure and for all subjects and censoring active workers after 1983. Squares represent all subjects and triangles represent all subjects censoring AN-exposed workers at the end of December 31, 1983. Lines indicate log of the hazard ratio fitted using continuous cumulative exposure and its square. See Web Table 6 for hazard ratios.
Workers in the top tertile (T3) of average exposure (>2.56 ppm) had a significantly elevated risk of bladder cancer (for T3 vs. unexposed, HR = 2.96, 95% CI: 1.38, 6.34; P for trend = 0.02), and those with a cumulative exposure of more than 6.69 ppm-years had a nonsignificantly increased risk (for T3 vs. unexposed, HR = 1.45, 95% CI: 0.69, 3.08). Because individuals with bladder cancer frequently die of other causes, we added 16 bladder deaths where bladder cancer was listed as a contributing cause from the NDI linkage. The hazard ratio was significantly increased (for T3 vs. unexposed, HR = 2.62, 95% CI: 1.33, 5.15) (data not shown); results for cumulative and duration of exposure were still nonsignificant with these 16 deaths.
Compared with unexposed workers, the hazard ratio for pneumonitis was significantly elevated in the upper category of the 3 exposure metrics (for >3.12 ppm-years, HR = 4.73, 95% CI: 1.42, 15.76; P for trend = 0.007; for >0.23 ppm, HR = 3.17, 95% CI: 0.97, 10.37; P for trend = 0.07; and for >14.5 years, HR = 4.53, 95% CI: 1.35, 15.21; P for trend = 0.01). These hazard ratios were unchanged with additional adjustment for acetic anhydride and phthalic anhydride, which have been linked to this outcome (27) (data not shown). We found no significant association between AN exposure and mesothelioma (Table 3). Hazard ratios for internal comparisons of other outcomes with at least 10 exposed cases are presented in Web Table 7 (unlagged cumulative exposure) and Web Table 8 (cumulative exposure lagged 10 years for cancer outcomes).
Sensitivity analyses
Cox models that censored active workers after 1983 resulted in hazard ratios that were higher in magnitude for the relationship between cumulative AN lagged 10 years and lung cancer death (with censoring, for ≥32 ppm-years vs. unexposed, HR = 1.64 (95% CI: 1.11, 2.42), P for trend = 0.04; compared with 32 ppm-years overall: for ≥32 ppm-years vs. unexposed, HR = 1.47 (95% CI: 1.07, 2.02), P for trend = 0.02) (Figure 1; Web Table 6). When we restricted models to only workers who had left employment by the end of 1983, we also observed hazard ratios of higher magnitude between cumulative AN lagged 10 years and lung cancer death (P for trend = 0.009) (Web Table 6).
We observed no significant differences for the association between cumulative AN and lung cancer by categories of age at first exposure, year of first exposure, or time since first exposure (P for interaction > 0.05). The risk of lung cancer death was significantly elevated in some of these groups, however, including for workers first exposed between 1956 and 1960 (for Q5 vs. unexposed, HR = 1.84, 95% CI: 1.13, 2.98; P for trend = 0.07) (Web Table 9) and among workers for whom it had been 20 or more years since first exposure to AN (for Q5 vs. unexposed, HR = 1.49, 95% CI: 1.16, 1.90; P for trend = 0.02) (Table 4). Compared with those for whom it had been less than 15 years since last exposure, the risk of death due to lung cancer was significantly decreased for those with 15–29 years and 30 or more years since last exposure (for 15–29 vs. <15 years, HR = 0.77, 95% CI: 0.58, 1.01; for ≥30 vs. <15 years, HR = 0.62, 95% CI: 0.46, 0.84; P for trend = 0.02) (Web Table 10). Analyses that adjusted for plant or distinguished fiber versus nonfiber plant showed similar associations to the overall AN–lung cancer relationship, with consistently increased risks for lung cancer death in the top quintile of AN exposure (Table 4).
Table 4.
Hazard Ratios for Cumulative Acrylonitrile Exposure Lagged 10 Years and Risk of Lung Cancer by Various Indicators of Exposurea for Workers Employed at 8 Acrylonitrile-Producing Facilities, United States, 1952–2011
| Cumulative Exposure | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P for Trend | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | HR | 95% CI | No. | HR | 95% CI | No. | HR | 95% CI | No. | HR | 95% CI | No. | HR | 95% CI | ||
| Full cohort | 109 | 1.16 | 0.92, 1.46 | 109 | 0.96 | 0.77, 1.21 | 109 | 1.03 | 0.81, 1.29 | 109 | 1.06 | 0.84, 1.33 | 109 | 1.43 | 1.13, 1.81 | 0.05 |
| Adjusted for plant | 109 | 1.08 | 0.86, 1.37 | 109 | 0.94 | 0.74, 1.19 | 109 | 1.01 | 0.80, 1.27 | 109 | 1.04 | 0.82, 1.31 | 109 | 1.40 | 1.10, 1.79 | 0.08 |
| Adjusted for asbestos | 109 | 1.15 | 0.91, 1.45 | 109 | 0.96 | 0.76, 1.21 | 109 | 1.00 | 0.79, 1.26 | 109 | 1.03 | 0.81, 1.30 | 109 | 1.32 | 1.03, 1.69 | 0.23 |
| Adjusted for fiber plants | 66 | 1.13 | 0.81, 1.58 | 44 | 0.72 | 0.50, 1.04 | 46 | 0.92 | 0.64, 1.33 | 47 | 0.80 | 0.55, 1.15 | 80 | 1.28 | 0.93, 1.78 | 0.61 |
| Adjusted for nonfiber plants | 43 | 1.03 | 0.73, 1.45 | 65 | 1.17 | 0.87, 1.58 | 63 | 1.08 | 0.80, 1.46 | 62 | 1.32 | 0.98, 1.78 | 29 | 1.54 | 1.03, 2.32 | 0.02 |
| ≥20 years since first exposure | 93 | 1.19 | 0.93, 1.53 | 90 | 0.93 | 0.72, 1.20 | 98 | 1.03 | 0.81, 1.32 | 105 | 1.10 | 0.87, 1.40 | 106 | 1.49 | 1.16, 1.90 | 0.02 |
| Adjusted for plant | 93 | 1.12 | 0.86, 1.45 | 90 | 0.90 | 0.70, 1.17 | 98 | 1.01 | 0.79, 1.30 | 105 | 1.08 | 0.84, 1.38 | 106 | 1.47 | 1.14, 1.90 | 0.04 |
| Adjusted for asbestos | 93 | 1.19 | 0.92, 1.53 | 90 | 0.92 | 0.72, 1.19 | 98 | 1.00 | 0.78, 1.29 | 105 | 1.07 | 0.84, 1.37 | 106 | 1.38 | 1.06, 1.79 | 0.12 |
| Adjusted for fiber plants | 58 | 1.34 | 0.93, 1.94 | 41 | 0.86 | 0.58, 1.28 | 41 | 1.04 | 0.70, 1.55 | 44 | 0.92 | 0.62, 1.36 | 79 | 1.55 | 1.09, 2.20 | 0.16 |
| Adjusted for nonfiber plants | 35 | 0.96 | 0.66, 1.41 | 49 | 0.99 | 0.71, 1.39 | 57 | 1.03 | 0.75, 1.41 | 61 | 1.31 | 0.96, 1.79 | 27 | 1.44 | 0.95, 2.21 | 0.05 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Internal comparisons use age as the time metric and are adjusted for race, sex, birth year, and salary-wage classification.
The lung cancer hazard ratios by exposure to asbestos for 1–3 versus 0 and 4–5 versus 0, respectively, were HR = 1.01 (95% CI: 0.80, 1.27) and 1.32 (95% CI: 1.10, 1.58). Among workers who had zero score probability of being exposed to asbestos, the hazard ratio in the top quintile of cumulative AN exposure was 1.63 (95% CI: 1.17, 2.26); no evidence of statistical heterogeneity in the hazard ratios across strata was apparent (P for interaction = 0.65) (Table 5). The association between cumulative exposure to AN and lung cancer was also statistically significant in the top quintile of exposure after adjustment for asbestos exposure probability (HR = 1.32, 95% CI: 1.03, 1.69) (Table 4). Adjustment for other chemical exposures did not change the observed lung cancer hazard ratios (data not shown).
Table 5.
Hazard Ratios for Acrylonitrile Exposure Lagged 10 Years and Risk of Lung Cancer for Workers by Strata of Asbestos Exposure Probability Scorea,b for Workers Employed at 8 Acrylonitrile-Producing Facilities, United States, 1952–2011
| Cumulative AN Exposure, Lagged 10 Years, ppm-year | Asbestos Exposure Probability Scorec | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1–3 | 4–5 | |||||||
| No. of Deaths | HR | 95% CI | No. of Deaths | HR | 95% CI | No. of Deaths | HR | 95% CI | |
| Unexposed | 228 | 1.00 | Referent | 12 | 0.91 | 0.50, 1.63 | 20 | 1.13 | 0.71, 1.80 |
| 0–0.09 | 90 | 1.20 | 0.93, 1.54 | 5 | 0.51 | 0.21, 1.25 | 14 | 1.73 | 1.00, 2.98 |
| >0.09 but ≤0.64 | 74 | 0.91 | 0.70, 1.19 | 17 | 0.97 | 0.59, 1.61 | 18 | 1.42 | 0.87, 2.30 |
| >0.64 but ≤2.30 | 52 | 0.85 | 0.63, 1.16 | 26 | 1.35 | 0.89, 2.05 | 31 | 1.32 | 0.90, 1.95 |
| >2.30 but ≤12.10 | 60 | 0.98 | 0.73, 1.31 | 16 | 0.99 | 0.60, 1.66 | 33 | 1.39 | 0.95, 2.02 |
| >12.10 | 45 | 1.63 | 1.17, 2.26 | 13 | 1.31 | 0.74, 2.30 | 51 | 1.39 | 1.01, 1.90 |
Abbreviations: AN, acrylonitrile; CI, confidence interval; HR, hazard ratio.
a Internal comparisons use age as the time metric and are adjusted for race, sex, birth year, and salary-wage classification.
b There were 549 lung cancer deaths with probability score of 0, 89 with a probability score of 1–3, 167 with a probability score of 4–5, and 3 lung deaths assigned as unknown.
cP for interaction between cumulative AN and asbestos exposure probability is based on likelihood ratio test = 0.65.
With extended follow-up, 53 additional deaths occurred from lung cancer within the original 10% systematic sample of workers for whom we collected smoking information (n = 47 smokers; n = 6 missing information). Among lung cancer deaths in the subcohort with nonmissing smoking information, 104 of 111 lung cancer deaths occurred in ever smokers (93.7%). The correlation between cigarette smoking and AN levels was small (correlation coefficient of 0.07). The updated hazard ratios for smoking (status and duration) and lung cancer were as follows: for ever smoking versus never smoking, HR = 19.1 (95% CI: 5.3, 68.9); for 1–20 years of smoking versus never smoking, HR = 9.7 (95% CI: 2.7, 35.1); for 21–40 years of smoking versus never HR = 18.3 (95% CI: 5.3, 63.3); and for ≥41 years of smoking versus never HR = 32.5 (95% CI: 8.4, 125.5). Among AN-exposed workers, the hazard ratio for more than 12.1 ppm-years and risk of lung cancer death, adjusted for smoking status, was 1.32 (95% CI: 0.55, 3.17), and adjusted for duration smoked was 1.46 (95% CI: 0.62, 3.46). The hazard ratio for more than 12.1 ppm-years and risk of lung cancer in the subcohort, unadjusted for smoking, was 1.36 (95% CI: 0.57, 3.22) among those who were exposed to AN.
DISCUSSION
Our analysis of the largest cohort of AN-exposed workers indicated an increased risk of lung cancer death among workers. This excess, observed in the initial study (10), was sustained and became statistically significant with the addition of 615 lung cancer deaths during 21 years of extended follow-up. We also observed an increased risk of deaths from bladder cancer and pneumonitis with AN exposure.
For lung cancer, we found a 43% significant increased risk in the top quintile of cumulative AN exposure. The observed exposure-response relationship occurred across the full range of cumulative AN exposure (Figure 1). In addition, there was a significant decrease in lung cancer death with increasing time since last exposure, indicating that cessation of AN exposure eventually resulted in fewer lung cancer deaths. These findings provide evidence of an association between exposure to AN and lung cancer death. Previous AN studies showed no significant excess of lung cancer deaths when compared with an external population (9, 11, 18–20). However, use of the general population is often associated with a healthy-worker effect, which is known to depress SMRs for many causes relative to the general population (28). It is also possible that the healthy-worker survivor effect could influence results if employment status is a time-varying confounder affected by prior exposure (29, 30); this has been shown to result in a bias (likely downward) of effect estimates of cumulative exposure even in studies of lung cancer (31, 32). In studies in which internal analyses of workers were conducted, no association was found between AN exposure and lung cancer risk (18–20). These studies, however, only followed a small number of workers (maximum size of 2,842 (19)) and identified few deaths among exposed workers (the maximum total lung cancer deaths reported was 88 (20)).
We evaluated several potential confounding factors. We observed an excess of mesothelioma deaths among the AN-exposed and AN-unexposed workers, which indicates the presence of asbestos. Because asbestos causes lung cancer (33), we evaluated asbestos as a potential confounder of the AN–lung cancer relationship. There was a small attenuation of the hazard ratio for cumulative AN exposure and lung cancer after adjustment for asbestos, but the hazard ratio for lung cancer death in the upper quintile for cumulative exposure remained significantly elevated. Importantly, the hazard ratio in the top quintile of cumulative AN exposure remained significantly elevated among workers who were unlikely to have been exposed to asbestos, suggesting minimal confounding from asbestos. Adjustment for co-exposures to other chemicals also had little or no impact on the observed hazard ratios for cumulative AN and lung cancer. A limitation of these results, however, includes the qualitative nature of asbestos and other co-exposure assessment.
Confounding by smoking is also a concern in estimating lung cancer risk. Although smoking information was limited to the subcohort sample, our results indicated that smoking-adjusted hazard ratios for AN exposure and lung cancer are similar to the hazard ratios unadjusted for smoking (for the top quintile: ever-smoking adjusted HR = 1.33 and the duration-adjusted HR = 1.46, compared with 1.43 in the total cohort and 1.36 in the smoking subcohort unadjusted for smoking). Furthermore, rates of death for several smoking-related causes, such as esophageal cancer, chronic obstructive pulmonary disease, diseases of the heart, and cerebrovascular disease, were not elevated among AN-exposed workers, as would be expected if smoking were responsible for the increased risk of lung cancer.
A new finding from the updated analysis is a significantly increased risk for death due to bladder cancer with average AN exposure (>2.56 ppm), based on only 13 deaths. Inclusion of 16 deaths from bladder cancer listed as a contributing cause of death from the NDI linkage retained the statistical significance of the hazard ratio for average exposure. Adjustment for smoking was not possible; however, the adjustment for smoking had little impact on the lung cancer risk, so it is also unlikely to influence the bladder cancer risk. Excesses of bladder cancer death had been noted in earlier mortality analyses (12, 15, 16), but more recent follow-up reports have not shown a link (19, 20). Marsh et al., in an analysis of 1 of the facilities included in our cohort, reported an excess of death from bladder and other urinary organ cancers (grouped together) (18, 34). The elevated bladder cancer hazard ratio in the current study, however, is not limited to a single facility. These findings require additional exploration, particularly a characterization of both fatal and incident bladder cancer, because the favorable survival rate from bladder cancer yields a small number of bladder cancer deaths relative to incident cases (35).
With the extended follow-up, we observed 35 deaths from nonmalignant respiratory disease with a strong monotonic exposure response; the majority (n = 27) were coded as unspecified pneumonitis due to solids and liquids (ICD-10 J69 and ICD-9 507). These deaths likely include a heterogeneous group of interstitial lung diseases, defined by either acute or chronic inflammation of the lung (36). The etiology of this group of nonmalignant respiratory conditions has not been identified specifically but includes exposure to chemicals in the workplace (27, 37). It is possible that exposures other than AN may have contributed. The hazard ratio for pneumonitis, however, was unchanged after adjustment for other chemical exposures, and risk was elevated for workers with low levels of AN exposure and for those with high levels of exposure. More work, including additional medical record information for disease characterization, would be valuable for this rare outcome.
In previous studies, other outcomes have been identified with links to AN exposure, including breast, prostate, and lymphohematopoietic cancers and death from central nervous system cancers (2, 8). Our findings do not support associations between AN and these cancers (Web Table 6). Internal analyses also showed no association between AN exposure and brain and nervous system cancers and inverse associations for nonmalignant nervous system diseases (Web Table 7). Interestingly, for Alzheimer disease, we observed a significant excess among AN-unexposed workers (SMR = 1.47, 95% CI: 1.11, 1.91) but not among AN-exposed workers. This finding may be due to chance or an unknown etiology.
To our knowledge, our study represents the largest cohort of AN-exposed workers based on detailed historical, quantitative exposure assessment with information on potential confounders such as smoking and asbestos exposure. Despite these strengths, certain limitations exist. Nondifferential misclassification of AN inevitably occurs, which tends to dilute observed hazard ratios. Work histories, and thus exposure estimates, were only available through 1983. Despite this missing exposure information, it is known that 71.5% of the cohort had left employment by the end of 1983. For active workers, however, AN exposure was likely underestimated. Because exposure decreased over the study period, the contribution of additional exposure for workers actively employed after 1983 is likely to be small. Indeed, when we censored workers who were actively employed in 1983, hazard ratios were slightly larger in magnitude, perhaps due to the elimination of misclassified exposure in the group of active workers for which exposure was truncated in 1983. Numbers of deaths for many outcomes are small, which limited analyses for rarer outcomes. We also made several comparisons, which increases the likelihood of a chance finding; however, significant exposure-response relationships are unlikely to be due to chance. Various sensitivity analyses and the lack of excess deaths for many tobacco-related causes suggest that confounding from smoking was unlikely to significantly affect our findings.
Our analysis of this cohort of AN-exposed workers provides evidence of an association between exposure to AN and lung cancer death, as well as a possible link between AN and death from bladder cancer and pneumonitis. Additional work on the impact of AN exposure on other cancer outcomes, particularly bladder cancer, and occupational interstitial lung diseases is warranted given AN’s continued global production.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Occupational and Environmental Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute (NCI), National Institutes of Health (NIH), Department of Health and Human Services (DHHS), Bethesda, Maryland (Stella Koutros, Aaron Blair, Laura E. Beane Freeman, Debra T. Silverman); Biostatistics Branch, Division of Cancer Epidemiology and Genetics, NCI, NIH, DHHS, Bethesda, Maryland (Jay H. Lubin, Barry I. Graubard); and Stewart Exposure Assessments, LLC, Arlington, Virginia (Patricia A. Stewart).
This work was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health (Z01 CP010120-22).
We thank Drs. Kyle Steenland of Emory University and Richard W. Hornung of the University of Cincinnati for the input they provided. We also thank Marianne Hyer of Information Management Services, Inc., for her analytic support for the study.
Conflicts of interest: none declared.
Abbreviations
- AN
acrylonitrile
- CI
confidence interval
- HR
hazard ratio
- ICD
International Classification of Diseases
- NDI
National Death Index
- SMR
standardized mortality ratio
REFERENCES
- 1. National Toxicology Program (NTP) Report on Carcinogens. 12th ed Research Triangle Park, NC: National Toxicology Program, Public Health Service, US Department of Health and Human Services; 2011. [Google Scholar]
- 2. IARC Working Group on the Evaluation of Carcinogenic Risk to Humans Acrylonitrile In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 71. Re-evaluation of Some Organic Chemicals, Hydrazine, and Hydrogen Peroxide. Lyon, France: International Agency for Research on Cancer; 1999:43–108. [Google Scholar]
- 3. AN Group Inc About acrylonitrile. Acrylonitrile production. http://angroup.org/about/production.php. Accessed July 19, 2012.
- 4. Stewart PA, Zaebst D, Zey JN, et al. Exposure assessment for a study of workers exposed to acrylonitrile. Scand J Work Environ Health. 1998;24(suppl 2):42–53. [PubMed] [Google Scholar]
- 5. Wood SM, Buffler PA, Burau K, et al. Mortality and morbidity of workers exposed to acrylonitrile in fiber production. Scand J Work Environ Health. 1998;24(Suppl 2):54–62. [PubMed] [Google Scholar]
- 6. Occupational Safety and Health Administration, Department of Labor Acrylonitrile Fed Regist 2017;Z(1045):331–349. To be codified at 29 CFR §1910.
- 7. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 8. US Environmental Protection Agency IRIS Toxicological Review of Acrylonitrile (External Review Draft). 2011. https://cfpub.epa.gov/si/si_public_record_report.cfm?dirEntryId=198583&Lab=NCEA. Accessed July 19, 2015.
- 9. Benn T, Osborne K. Mortality of United Kingdon acrylonitrile workers–an extended and updated study. Scand J Work Environ Health. 1998;24(suppl 2):17–24. [PubMed] [Google Scholar]
- 10. Blair A, Stewart PA, Zaebst DD, et al. Mortality of industrial workers exposed to acrylonitrile. Scand J Work Environ Health. 1998;24(suppl 2):25–41. [PubMed] [Google Scholar]
- 11. O’Berg MT, Chen JL, Burke CA, et al. Epidemiologic study of workers exposed to acrylonitrile: an update. J Occup Med. 1985;27(11):835–840. [DOI] [PubMed] [Google Scholar]
- 12. Thiess AM, Frentzel-Beyme R, Link R, et al. Mortality study in chemical personnel of various industries exposed to acrylonitrile [in German]. Zentralbl Arbeitsmed Arbeitsschutz Prophyl Ergonomie. 1980;30(7):259–267. [PubMed] [Google Scholar]
- 13. Scélo G, Constantinescu V, Csiki I, et al. Occupational exposure to vinyl chloride, acrylonitrile and styrene and lung cancer risk (Europe). Cancer Causes Control. 2004;15(5):445–452. [DOI] [PubMed] [Google Scholar]
- 14. Chen JL, Fayerweather WE, Pell S. Mortality study of workers exposed to dimethylformamide and/or acrylonitrile. J Occup Med. 1988;30(10):819–821. [DOI] [PubMed] [Google Scholar]
- 15. Delzell E, Monson RR. Mortality among rubber workers: VI. Men with potential exposure to acrylonitrile. J Occup Med. 1982;24(10):767–769. [PubMed] [Google Scholar]
- 16. Kiesselbach N, Korallus U, Lange HJ, et al. Acrylonitrile–epidemiological study–Bayer 1977: a report on a prospective epidemiological study with a past beginning of coworkers at the Leverkusen plant of Bayer AG with acrylonitrile (ACN) exposure [in German]. Zentralbl Arbeitsmed Arbeitsschutz Prophyl. 1979;29(10):256–259. [PubMed] [Google Scholar]
- 17. Swaen GM, Bloeman LJ, Twisk J, et al. Mortality update of workers exposed to acrylonitrile in The Netherlands. Scand J Work Environ Health. 1998;24(suppl 2):10–16. [PubMed] [Google Scholar]
- 18. Marsh GM, Zimmerman SD. Mortality among chemical plant workers exposed to acrylonitrile: 2011 follow-up. J Occup Environ Med. 2015;57(2):134–145. [DOI] [PubMed] [Google Scholar]
- 19. Swaen GM, Bloemen LJ, Twisk J, et al. Mortality update of workers exposed to acrylonitrile in The Netherlands. J Occup Environ Med. 2004;46(7):691–698. [DOI] [PubMed] [Google Scholar]
- 20. Symons JM, Kreckmann KH, Sakr CJ, et al. Mortality among workers exposed to acrylonitrile in fiber production: an update. J Occup Environ Med. 2008;50(5):550–560. [DOI] [PubMed] [Google Scholar]
- 21. Stewart PA, Lemanski D, White D, et al. Exposure assessment for a study of workers exposed to acrylonitrile. I. Job exposure profiles: a computerized data management system. Appl Occup Environ Hyg. 1992;7(12):820–825. [Google Scholar]
- 22. Stewart PA, Triolo H, Zey J, et al. Exposure assessment for a study of workers exposed to acrylonitrile. II. A computerized exposure assessment program. Appl Occup Environ Hyg. 1995;10(8):698–705. [Google Scholar]
- 23. Stewart PA, Zey JN, Hornung R, et al. Exposure assessment for a study of workers exposed to acrylonitrile. III: Evaluation of exposure assessment methods. Appl Occup Environ Hyg. 1996;11(11):1312–1321. [Google Scholar]
- 24. Surveillance, Epidemiology, and End Results (SEER) U.S. mortality data, 1969-2016. https://seer.cancer.gov/mortality/. Accessed July 19, 2015.
- 25. National Center for Health Statistics Mortality Multiple Cause-of-Death. 2012 Mortality https://www.cdc.gov/nchs/nvss/mortality_public_use_data.htm. Accessed July 19, 2015.
- 26. Surveillance, Epidemiology, and End Results (SEER), U.S. National Cancer Institute SEER cause of death recode. https://seer.cancer.gov/codrecode/. Accessed July 19, 2015.
- 27. Riario Sforza GG, Marinou A. Hypersensitivity pneumonitis: a complex lung disease. Clin Mol Allergy. 2017;15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steenland K, Stayner L. The importance of employment status in occupational cohort mortality studies. Epidemiology. 1991;2(6):418–423. [DOI] [PubMed] [Google Scholar]
- 29. Buckley JP, Keil AP, McGrath LJ, et al. Evolving methods for inference in the presence of healthy worker survivor bias. Epidemiology. 2015;26(2):204–212. [DOI] [PubMed] [Google Scholar]
- 30. Garcia E, Picciotto S, Costello S, et al. Assessment of the healthy worker survivor effect in cancer studies of the United Autoworkers-General Motors cohort. Occup Environ Med. 2017;74(4):294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Keil AP, Richardson DB, Troester MA. Healthy worker survivor bias in the Colorado Plateau uranium miners cohort. Am J Epidemiol. 2015;181(10):762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Naimi AI, Cole SR, Hudgens MG, et al. Estimating the effect of cumulative occupational asbestos exposure on time to lung cancer mortality: using structural nested failure-time models to account for healthy-worker survivor bias. Epidemiology. 2014;25(2):246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Steenland K, Hoar Zham S, Blair A. Occupational cancer In: Thun MJ, Linet MS, Cerhan C, et al. eds. Schottenfeld and Fraumeni Cancer Epidemiology and Prevention. New York, NY: Oxford University Press; 2018:275–290. [Google Scholar]
- 34. Marsh GM, Gula MJ, Youk AO, et al. Bladder cancer among chemical workers exposed to nitrogen products and other substances. Am J Ind Med. 2002;42(4):286–295. [DOI] [PubMed] [Google Scholar]
- 35. Howlander N, Noone AM, Krapcho M, et al. Previous Version: SEER Cancer Statistics Review, 1975–2012 http://seer.cancer.gov/csr/1975_2012/. Updated November 2015. Accessed July 19, 2016.
- 36. Litow FK, Petsonk EL, Bohnker BK, et al. Occupational interstitial lung diseases. J Occup Environ Med. 2015;57(11):1250–1254. [DOI] [PubMed] [Google Scholar]
- 37. Cullinan P, Munoz X, Suojalehto H, et al. Occupational lung diseases: from old and novel exposures to effective preventive strategies. Lancet Respir Med. 2017;5(5):445–455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.