Abstract
Voltage-dependent calcium channels regulate many aspects of neuronal biology, including synaptic transmission. In addition to their α1 subunit, which encodes the essential voltage gate and selective pore, calcium channels also contain auxiliary α2δ, β, and γ subunits. Despite progress in understanding the biophysical properties of calcium channels, the in vivo functions of these auxiliary subunits remain unclear. We have isolated mutations in the gene encoding an α2δ calcium channel subunit (dα2δ-3) using a forward genetic screen in Drosophila. Null mutations in this gene are embryonic lethal and can be rescued by expression in the nervous system, demonstrating that the essential function of this subunit is neuronal. The photoreceptor phenotype of dα2δ-3 mutants resembles that of the calcium channel α1 mutant cacophony (cac), suggesting shared functions. We have examined in detail genotypes that survive to the third-instar stage. Electrophysiological recordings demonstrate that synaptic transmission is severely impaired in these mutants. Thus the α2δ calcium channel subunit is critical for calcium-dependent synaptic function. As such, this Drosophila isoform is the likely partner to the presynaptic calcium channel α1 subunit encoded by the cac locus. Consistent with this hypothesis, cacGFP fluorescence at the neuromuscular junction is reduced in dα2δ-3 mutants. This is the first characterization of an α2δ-3 mutant in any organism and indicates a necessary role for α2δ-3 in presynaptic vesicle release and calcium channel expression at active zones.
Keywords: calcium channel, synaptic transmission, bouton, active zone, neuromuscular junction, Drosophila
Introduction
Calcium channels have well established roles in synaptic transmission, cell excitability, intracellular signaling, and disease (Jen, 1999; Collin et al., 2005). Voltage-gated calcium channels have a unique responsibility for converting electrical changes across the plasma membrane into intracellular changes in calcium concentration. Molecularly, they contain a pore-forming α1 subunit that confers many of the basic properties of the ion channel, including its voltage-sensitive gating, selectivity for calcium, and pharmacological properties (De Waard et al., 1996). However, calcium channels also contain α2δ and β subunits that can have a substantial influence on the properties of calcium channels when expressed in heterologous systems (Arikkath and Campbell, 2003). Both α2δ and β subunits can markedly increase surface expression of the channels (Gurnett et al., 1996; Wiser et al., 1996; Bichet et al., 2000; Felix, 2005) and can also influence the gating properties of the channel (Arikkath and Campbell, 2003; Bernstein and Jones, 2007). The β subunit is entirely intracellular and is the target for several pathways that modulate calcium channel function (Dolphin, 2003). The α2δ subunit, in contrast, lacks an intracellular domain. This subunit consists of two polypeptides that are transcribed as a single transcript and posttranslationally cleaved into the α2 and δ chains, which remain linked by a disulfide bond (Klugbauer et al., 2003). The α2 portion is entirely extracellular and heavily glycosylated, whereas the δ chain also includes a C-terminal transmembrane domain (Gurnett et al., 1996). In addition, there is a γ subunit whose role is controversial and that need not assemble with the calcium channel complex (Kang and Campbell, 2003). Although we have learned much about the biophysical properties of calcium channels, the roles of the auxiliary subunits in regulating calcium channels in vivo is less clear.
Some insights into the role of these accessory subunits in vivo come from a series of spontaneously occurring mutations in mice. These include mutations in a β subunit in lethargic (Burgess et al., 1997), an α2δ subunit in ducky (Barclay et al., 2001; Brodbeck et al., 2002) and in a spontaneous variant of C57BL/10 strain mice (Wycisk et al., 2006), as well as a γ subunit in stargazer (Letts et al., 1998; Held et al., 2002; Moss et al., 2002). Interestingly, each of these mutants displays ataxia and some form of epilepsy. Moreover, the α2δ calcium channel subunit has been shown to be a target of the anti-epileptic drug gabapentin (Stahl, 2004), although the role of this subunit in the disease remains unclear.
One complication in the genetic analysis of these accessory subunits has been the presence of multiple isoforms in the genome. With regard to α2δ subunits, the number of genes in an organism's genome has remained relatively constant: there are three α2δ isoforms in worms and flies and four isoforms in mammals; these have been classed as α2δ-1,2,3, and 4 (Littleton and Ganetzky, 2000; Qin et al., 2002). In mammals, the α2δ-1 subunit is expressed ubiquitously, whereas the α2δ-2 subunit, the subunit mutated in ducky, is expressed in the brain, kidney, heart, and testes. The α2δ-3 subunit is expressed only in brain (Gurnett et al., 1996; Marais et al., 2001). In ducky mice, loss of the α2δ-2 subunit decreases the amplitude of calcium currents in Purkinje cells, in which it is highly expressed, but not in all neurons (Barclay et al., 2001; Brodbeck et al., 2002). Purkinje cells also have abnormal morphologies. At neuromuscular junctions, however, ducky mutations have little effect on transmitter release (Kaja et al., 2007). Loss of the α2δ-4 subunit causes abnormalities in the outer plexiform layer of the retina (Wycisk et al., 2006). At present, it is uncertain which α1 calcium channel assembles with which α2δ subunit in vivo. In heterologous systems, various combinations promote channel expression (Gurnett et al., 1996), but their associations may be less promiscuous in vivo. Thus, it has not been determined whether synaptic calcium channels also require an α2δ subunit and, if so, what significance that subunit would hold for the physiology of the synapse.
In a forward genetic screen for mutations affecting synaptic transmission, we have isolated mutations in the Drosophila α2δ-3 calcium channel subunit. This subunit (dα2δ-3) is essential for viability in Drosophila and shares many of the phenotypes described in mutations of the α1 calcium channel subunit, cacophony. We demonstrate a critical role for dα2δ-3 in synaptic function in both photoreceptors and motorneurons.
Materials and Methods
Drosophila stocks and genetics.
The y, w; FRT42D, GMR-hid, y+, cl 2R/CyO; EGUF/EGUF stock used in the mutagenesis was previously described (Stowers and Schwarz, 1999). The FRT42D chromosome (Xu and Rubin, 1993), elav-Gal4, mhc-Gal4, daughterless-Gal4, and all other stocks were obtained from the Bloomington Stock Center (Bloomington, IN) unless otherwise noted.
EGUF-hid screen.
Briefly, this screen engineered heterozygous flies whose eyes were homozygous for a mutagenized chromosome arm 2R (Stowers and Schwarz, 1999). We screened such flies for defects in photoreceptor synaptic transmission by behavioral and electrophysiological assays (phototaxis and electroretinogram, ERG). The results of this screen were previously described (Dickman et al., 2005). Eleven complementation groups were isolated with more than one allele that lacked light-evoked “on/off transients” in the ERG, a phenotype attributable to defects in transmission at the first synapse in the underlying lamina neuropil (Coombe, 1986). The photoreceptors themselves, however, could be activated by light, as indicated by the presence of a sustained component in the ERG.
Immunohistochemistry.
Larvae were dissected in HL-3, fixed in 4% paraformaldehyde or Bouin's fixative in ice-cold buffer (Dickman et al., 2006) and mounted in Vectashield (Vector Laboratories, Burlingame, CA). Larvae were imaged with a Zeiss (Oberkochen, Germany) LSM 510 laser scanning confocal microscope and 63× 1.4 NA objective using separate channels and processed using the LSM software or Adobe Photoshop. For bouton counting, larvae were grown on standard grape media at 25°C in uncrowded conditions (∼40 larvae per plate) until the late third instar (determined by age, wandering behavior, and lack of food in the gut). Third-instar larvae were dissected in HL3 media, fixed for 30 min in 4% PFA, and stained. These animals were then viewed using a Nikon (Tokyo, Japan) E800 fluorescent microscope. All bouton counts were done blind to the genotype of the larva, and only neuromuscular junctions on muscle 6/7 of segment A2 were analyzed. Bouton number was normalized to muscle 6/7 surface area. Cy5-, Cy3-, or FITC-conjugated anti-HRP (1:100) was obtained from Jackson ImmunoResearch (West Grove, PA). Mouse anti-nc82 (1:100) was obtained from Drs. E. Buchner and A. Hofbauer, Germany (Wagh et al., 2006). FITC- or Cy3-conjugated secondary antibodies (1:200) were obtained from Jackson ImmunoResearch.
Genetic rescue.
An expressed sequence tag construct (SD03196) containing the entire open reading frame of dα2δ-3 cDNA was obtained from Invitrogen (Carlsbad, CA). Two primers were generated against the dα2δ-3 cDNA: A BglII site was engineered into the forward primer and an HA tag in frame with the C-terminal end of dα2δ-3 and a KpnI site were engineered into the reverse primer. The PCR product containing the tagged open reading frame was cloned into pUAST (Brand and Perrimon, 1993). Transformant flies were generated by standard methods and one insertion was mapped to the second chromosome. This was recombined with the dα2δ-3DD106 and dα2δ-3DD196 chromosomes. The following genotypes could survive to adulthood: [dα2δ-3DD106, pUAS-dα2δ-3/dα2δ-3DD196; daGal4], [dα2δ-3DD106, pUAS-dα2δ-3/dα2δ-3DD196; elav-Gal4], [dα2δ-3DD106, pUAS-dα2δ-3/dα2δ-3DD196; daGal4/elavGal4], [dα2δ-3DD196, pUAS-dα2δ-3/dα2δ-3DD106; daGal4], [dα2δ-3DD196, pUAS-dα2δ-3/dα2δ-3DD106; elavGal4], [dα2δ-3DD196, pUAS-dα2δ-3/dα2δ-3DD106; daGal4/elavGal4].
Electrophysiology.
10–20 larvae were raised at 25°C, separated from heterozygous siblings as second-instar larvae and grown on agar apple plates with yeast coating (Loewen et al., 2001). Third-instar larvae were dissected in saline then bathed in modified HL-3 saline with the following ionic concentrations (in mm): NaCl 70, KCl 5, MgCl2 10, NaHCO3 10, sucrose 115, Trehalose 5, HEPES 5, pH7.2, and CaCl2 as indicated. Current clamp recordings were performed on muscle 6 or 7 in abdominal segments A2 or A3 with 10–20 MΩ electrodes. The nerve was cut and suction electrodes (fire polished to a diameter of ∼5 μm and filled with bath solution) were used for stimulation. Only muscles with resting potentials more hyperpolarized than −60 mV were considered for analysis. Data were collected using a Digidata and Axopatch 200B amplifier. pClamp 9 (Molecular Devices, Union City, CA), Excel (Microsoft, Seattle, WA), and miniAnalysis (Synaptosoft, Decatur, GA) were used for data analysis. Quantal content was determined by dividing the amplitude of the evoked junctional potentials (EJP) by the average mini amplitude (before stimulation) and correcting for nonlinear summation (Martin, 1955) in the case of high calcium conditions.
cacGFP quantification.
Neuronal expression of cacGFP (Kawasaki et al., 2004) was obtained using the following stocks: (1) UAScacGFP/elavGal4, 2) dα2δ-3k10814/dα2δ-3DD106; UAScacGFP/elavGal4, and (3) dα2δ-3DD196/Df(2R)7128; UAScacGFP/elavGal4. Larvae were dissected, fixed in 4% paraformaldehyde and mounted as described above. Wild-type (wt) and dα2δ-3 mutant larval synapses were immunostained with the neuronal membrane marker HRP and boutons were selected for imaging and analysis based on morphology and HRP staining alone. Larvae were imaged with a Zeiss LSM 510 laser-scanning confocal microscope and 63× 1.4 NA objective. A single optical slice was taken, with the focal plane chosen on the basis of anti-HRP staining of the neuronal boutons. Wild-type and mutant images were taken with the same settings within one experiment (although conditions and settings changed between experiments, and therefore results between experiments cannot be compared). Images were analyzed using MetaMorph Imaging software (Molecular Devices). Images were first filtered using a low-pass 10 × 10 pixel filter to get rid of noise. Local average background intensity measurements were taken, and subtracted from the image, after which the image was thresholded at 2× average background intensity. Puncta were defined as contiguous regions within boutons that were above threshold, and the average and integrated intensity of each punctum was measured.
Results
Isolation and identification of mutants in the dα2δ-3 calcium channel subunit
In the course of a forward genetic screen of chromosome 2R for mutants in synaptic transmission (Dickman et al., 2005), we isolated a complementation group displaying severe defects in the electroretinogram (ERG) response to light (Fig. 1A) when photoreceptors were made homozygous for the mutation (Stowers and Schwarz, 1999). In particular, the presence of sustained components but lack of “on/off transients” in ERG responses suggested an inability to properly activate second order neurons (Coombe, 1986). 5 lines were independently isolated that failed to complement each other for lethality. The ERGs of this complementation group differed from other synaptic mutants isolated in this and other screens (Stowers and Schwarz, 1999; Dickman et al., 2005) in that the wave form of the sustained components was abnormally slow in its onset and recovery (Fig. 1). A similar phenotype appears in mutants in the α1 calcium channel subunit cacophony (Smith et al., 1998b).
Figure 1.
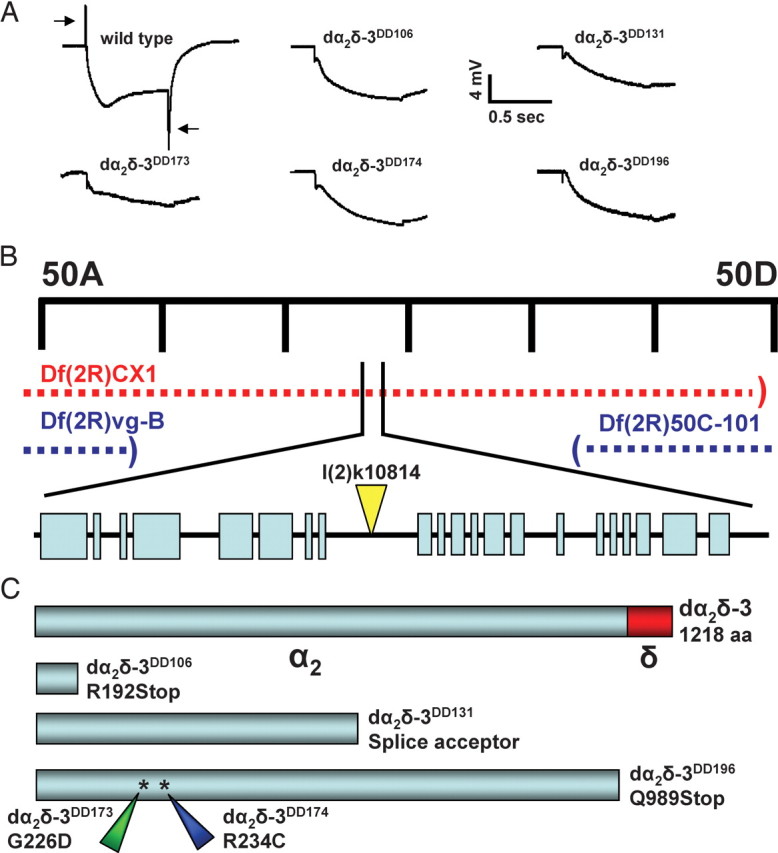
Identification of mutants in the dα2δ-3 calcium channel subunit. A, Representative ERG recordings of recombinant photoreceptors in control (y, w; FRT42/FRT42, GMR-hid, cl; EGUF/+) and five dα2δ-3 alleles (y, w; FRT42, dα2δ-3/FRT42, GMR-hid, cl; EGUF/+). EGUF is a chromosome carrying ey-GAL4 and UAS-FLP transgenes. The dα2δ-3 mutant eyes lack “on/off transients”. B, Mapping of dα2δ-3 mutants. A transposon insertion line (k10814) and a deficiency chromosome Df(2R)CX1 failed to complement the lethality of all five dα2δ-3 alleles, but those alleles were viable in combination with the deficiencies vg-B and 50C-101. C, Molecular analysis of dα2δ-3 mutations. dα2δ-3 open reading frames were sequenced in the mutants, and molecular lesions in all five were identified as shown.
This complementation group was mapped to the 49C-50D chromosomal region on the basis of the failure of chromosomal deficiency CX1 to complement the 5 alleles (Fig. 1B). A transposon insertion line in the region, k10814, also failed to complement these mutants for lethality. Inverse PCR and sequencing revealed the transposon to be inserted within an intron of an uncharacterized gene (CG12295) with homology to the dα2δ-3 subunit. The predicted transcription unit covers >14 kB of genomic DNA and 21 exons with a protein product of 1218 amino acids. The Drosophila genome contains three genes predicted to encode dα2δ subunits and these are homologous to three of the isoforms described in mammals (Littleton and Ganetzky, 2000). The gene containing the insert in k10814 corresponds to the dα2δ-3 isoform and predicts a protein with 31% overall sequence identity with rat α2δ-3. Expressed sequence tags from the Drosophila Genome Project show that two different transcripts arise from the locus, only differing in their 5′ untranslated regions. The open reading frame was sequenced in each mutant, revealing a molecular change in each of the alleles (Fig. 1C). The dα2δ-3DD106 allele encodes an early stop codon at the 192nd amino acid and is thus a likely null mutation. The DD196 allele encodes a late stop codon, creating a nonsense mutation at amino acid 989. Both DD173 and DD174 alleles are missense mutations in the α2 domain, and DD131 likely creates a splicing error midway through the open reading frame. Despite the variety of molecular lesions in the dα2δ-3 gene, all 5 of the dα2δ-3 mutants (DD106, DD131, DD173, DD174, and DD196) die as well formed, late-stage embryos when homozygous or when in combination with each other or either of two different deficiencies, Df(2)CX1 or Df(2)Ex7128. However, the k10814 insertion, when homozygous or in combination with either deficiency, survives to third-instar stages in standard conditions or to adulthood when raised apart from heterozygous siblings (Loewen et al., 2001); these adults are ataxic, unable to fly, and die a few days after eclosion. This allele is therefore likely to be a hypomorphic mutation, perhaps resulting in a reduction in wild-type levels of the protein.
To confirm that lethality was the specific result of a loss of dα2δ-3 function and to determine in what tissues dα2δ-3 function is required, we generated a rescue construct with a dα2δ-3 cDNA under the control of UAS elements (Brand and Perrimon, 1993). Adult viability could be restored with ubiquitous expression of dα2δ-3 using daughterless-Gal4 or with neuronal expression using elav-Gal4, but not with muscle expression using MHC-Gal4 (data not shown). Thus the only essential function of dα2δ-3 is in the nervous system.
Synaptic overgrowth in dα2δ-3 mutants
The similarity of the ERG responses to those of cacophony, an α1 subunit that is needed for triggering calcium-dependent neurotransmitter release at the neuromuscular junction (Kawasaki et al., 2000), raised the possibility that dα2δ-3 also functioned at the neuromuscular junction. We therefore examined the phenotype of dα2δ-3 at the well studied neuromuscular junctions on muscles 6 and 7 of third-instar larvae. cac hypomorphs that can survive to this stage have been reported to have decreases in synaptic strength at this synapse, and fewer synaptic boutons (Rieckhof et al., 2003). Because dα2δ-3k10814 mutants can also survive to third-instar stages, we examined their phenotype at this synapse.
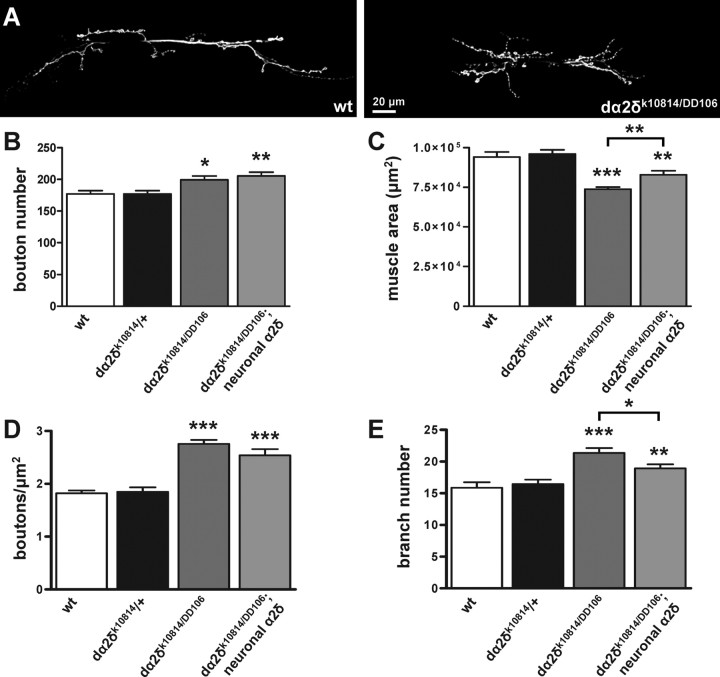
We found a 15% increase in bouton number in dα2δ-3k10814/DD106 mutants compared with controls (199 ± 6. compared with 175 ± 6 boutons per junction; mean ± SEM, p < 0.05, n = 25 and 22) (Fig. 2A,B). In addition, dα2δ-3 mutants exhibited a 23% decrease in muscle size compared with controls (74 × 103 ± 1 × 103 compared with 96 × 103 ± 3 × 103 μm2; p < 0.001) (Fig. 2C). In wild-type animals, muscle size and bouton number increase together during development, probably so that an increase in synaptic strength can match the decrease in input resistance of the muscle fiber as it grows (Lnenicka and Keshishian, 2000). Normalizing bouton number to muscle size may therefore be appropriate as a means to offset changes in boutons secondary to changes in muscle size. In the present study, this normalization yielded a 50% increase in bouton number per muscle area in dα2δ-3 mutants compared with controls (2.7 × 10−3 ± 0.1 × 10−3 compared with 1.8 × 10−3 ± 0.1 × 10−3 boutons per μm2; p < 0.001) (Fig. 2B). In contrast, cacNT27 mutants (Rieckhof et al., 2003) did not exhibit an increase in bouton number with or without normalizing to muscle size (data not shown). To control for the genetic background of the P-element containing chromosome, we recorded in heterozygous conditions (y, w/+; dα2δ-3k10814/FRT42D) and found no difference from y, w/+; Canton-S/FRT42D. We attempted to rescue the increase in bouton number by neuronally expressing dα2δ-3 or cacGFP in dα2δ-3k10814/DD106 mutants, however neither manipulation rescued the bouton number phenotype, although expression of dα2δ-3 in the nervous system did significantly improve muscle size.
Figure 2.
Synaptic overgrowth in dα2δ-3 neuromuscular junctions. A, Representative larval neuromuscular junctions at muscles 6 and 7 immunostained with the pan-neuronal marker anti-HRP in wild-type (y, w/+; +/FRT42D) and dα2δ-3 mutants (y, w/+; dα2δ-3 k10814/dα2δ-3DD106) showing additional boutons and more branching in dα2δ-3 mutant synapses. Both panels are at the same magnification. B–E, Quantification of bouton number (B), muscle size (C), bouton number normalized to muscle surface area (D), and branch number (E) in those genotypes along with the following additional genotypes: y, w/+; dα2δ-3 k10814/FRT42D and y, w/+; dα2δ-3 k10814/dα2δ-3DD106, UASdα2δ-3-HA; elavGal4/+ (neuronal dα2δ). For wild type, 20 neuromuscular junctions were analyzed from 10 larvae; in heterozygotes, 22 junctions from 11 larvae; in mutants, 25 junctions from 15 larvae; in neuronal dα2δ, 24 junctions from 12 larvae. p values are relative to wild type, unless otherwise indicated, and are unadjusted for multiple comparisons; however, significance was confirmed using one-way ANOVA and a Student's–Newman–Keuls post hoc test. *p < 0.05; **p < 0.01; ***p < 0.001.
In addition to the increase in bouton number, we found a 30% increase in branch number in dα2δ-3k10814/DD106 mutants (21 ± 0.8. compared with 16 ± 0.7 branches per junction; mean ± SEM, p < 0.001) (Fig. 2A,E). Neuronal expression of dα2δ-3 in dα2δ-3k10814/DD106 mutants significantly rescued this phenotype. Thus dα2δ-3 may play a modest role in determining synaptic bouton number and the extent of terminal branching.
Reduced transmitter release in dα2δ-3 synapses
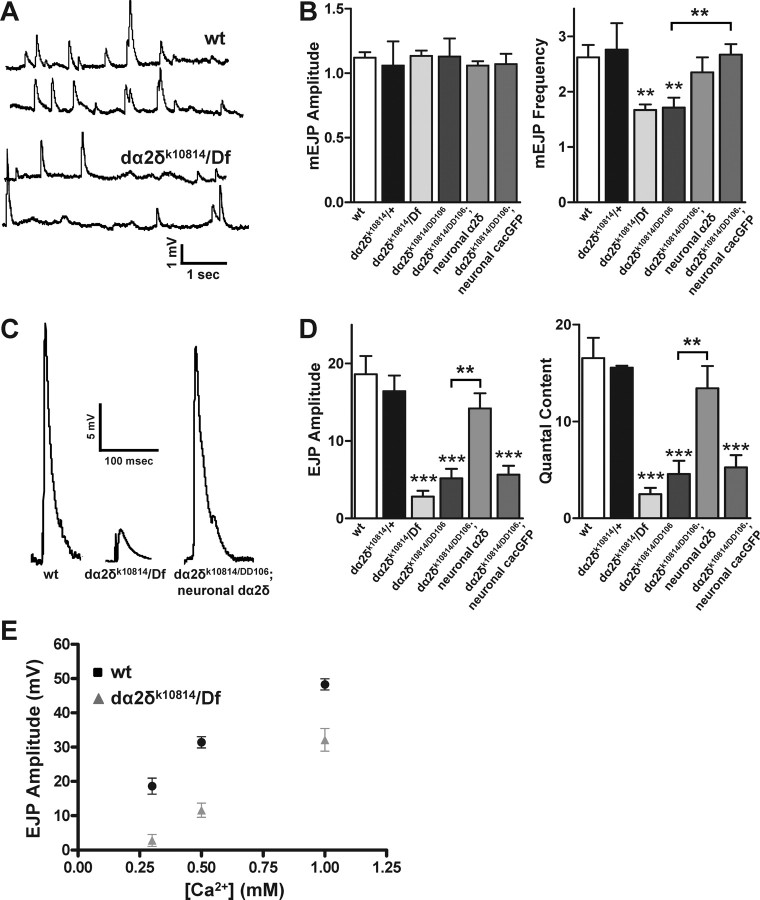
The anatomical alterations at dα2δ-3k10814 third-instar neuromuscular junctions were accompanied by substantial electrophysiological changes. Initial recordings were made in HL-3 saline with 0.3 mm Ca2+ and 10 mm Mg2+, conditions in which calcium influx and release are far from saturation and changes in synaptic strength are therefore readily apparent. Wild-type larvae (w1118) were compared with heterozygous mutants (y, w; dα2δ-3k10814/FRT42D) to control for changes in the genetic background and to two mutant allelic combinations (dα2δ-3k10814/dα2δ-3DD106 and dα2δ-3k10814/Df(2R)CX1). Miniature EJP (mEJP) amplitudes in the mutant genotypes (1.13 ± 0.04 mV, mean ± SEM, n = 47, in dα2δ-3k10814/Df(2R)CX1 and 1.13 ± 0.14 mV, n = 11, in dα2δ-3k10814/dα2δ-3DD106) were comparable with those in wild type (1.12 ± 0.43 mV. n = 35) or the heterozygotes (1.06 ± 0.19 mV, n = 7), but mEJP frequencies were reduced in dα2δ-3 mutants (1.67 ± 0.10 Hz in dα2δ-3k10814/Df(2R)CX1 and 1.71 ± 0.18 Hz in dα2δ-3k10814/dα2δ-3DD106, compared with 2.67 ± 0.23 Hz in w1118 or 2.76 ± 0.48 Hz in heterozygotes) (Fig. 3A,B). The amplitudes of EJPs in dα2δ-3 mutants were reduced by 85% (2.83 ± 0.74 mV, n = 22, in dα2δ-3k10814/Df(2R)CX1 and 5.18 ± 1.23 mV, n = 11, in dα2δ-3k10814/dα2δ-3DD106 compared with 18.61 ± 2.35 mV, n = 11, in w1118 or 16.43 ± 2.02 mV, n = 7, in heterozygous controls) (Fig. 3C,D). Given that quantal size, as determined from the amplitude of spontaneous minis, was unchanged, the quantal content of evoked responses must have been similarly reduced in dα2δ-3 mutant synapses (Fig. 3D). Consistent with a reduction in quantal content, nerve stimulation frequently failed to elicit an evoked response in dα2δ-3k10814/Df(2R)CX1 mutants: the synapses had a 48.2 ± 1.43% failure rate compared with 2.86 ± 0.61% in control larvae. We also found the reduction in quantal content to be apparent over a range of calcium concentrations (Fig. 3E).
Figure 3.
Reduced synaptic efficacy at dα2δ-3 neuromuscular junctions. Recordings were made from third-instar neuromuscular junctions from wild-type larvae (w1118), heterozygous and homozygous mutant larvae of the indicated genotypes, and homozygous mutant larvae with restored neuronal expression of dα2δ-3 (dα2δ-3k10814/dα2δ-3DD106, UASdα2δ-3-HA; elavGal4/+) or cac (dα2δ-3k10814/dα2δ-3DD106, UAScac-GFP; elavGal4/+). A, Representative mEJP events recorded from neuromuscular junctions in wild-type and mutant (dα2δ-3k10814/Df(2R)CX1) larval synapses. B, Quantification of mEJP amplitude and frequency. mEJP frequencies are slightly reduced at dα2δ-3 synapses, but quantal size is unchanged. C, Representative EJPs. D, Quantification of EJP amplitudes. Amplitudes are reduced by 85% in dα2δ-3 mutants but restored by neuronal expression of the UASdα2δ-3-HA transgene. E, EJP amplitude is reduced across several calcium concentrations in dα2δ-3 mutants. p values are relative to wild type unless otherwise indicated and were obtained using one-way ANOVA and a Tukey's post hoc test. **p < 0.01; ***p < 0.001.
To test whether the reduction in transmission was attributable to alterations in dα2δ-3 expression, we drove dα2δ-3 specifically in the nervous system in a mutant background (dα2δ-3k10814/dα2δ-3DD106, UAS-dα2δ-3; elavGAL4/+). This restored robust synaptic function to these synapses so that mEJP frequency, EJP amplitude, and quantal content were no longer significantly different from controls (Fig. 3) (mEJP amp = 1.06 ± 0.03 mV; mEJP freq = 2.35 ± 0.27 Hz; EJP amp = 14.21 ± 1.94 mV; n = 8).
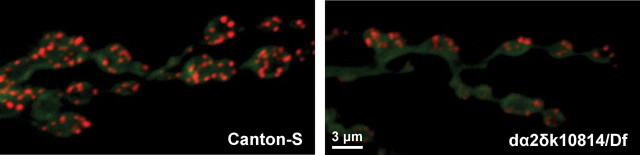
Together, these experiments demonstrate a critical requirement for the dα2δ-3 subunit in evoked synaptic release at the neuromuscular junction. The extent to which this allele alters the efficacy of release at a given active zone could be different from the change in quantal content if the number of active zones is altered in the mutant. As described above, the neuromuscular junctions of dα2δ-3 larvae have more boutons than control larvae. To determine whether this increase in bouton number represented a parallel increase in the number of active zones, neuromuscular synapses were immunolabeled with the monoclonal antibody nc82, a well characterized marker of active zones in Drosophila (Wagh et al., 2006). Despite the increase in bouton number, dα2δ-3 mutants exhibited a 20% decrease in nc82 puncta per neuromuscular junction compared with controls (932 ± 33 compared with 1165 ± 41; p < 0.001, n = 14 and 16) (Fig. 4). When normalized to the number of boutons per synapse, this translated to a 40% decrease in nc82 puncta per bouton in the mutants (3.76 ± 0.09 compared with 5.99 ± 0.22; p < 0.001) (Fig. 4). Counts are from 14 NMJs from 7 control larvae and 16 NMJs from 8 mutant larvae (p < 0.001). Assuming that this represents a true decrease in active zone number rather than just a mislocalization of the nc82 epitope, the 20% decrease in the total number of active zones is still insufficient to explain the 85% decrease in quanta released per stimulus. Thus the mutation of dα2δ-3 has caused not only a net decrease in transmitter release, but also a decrease in release per active zone.
Figure 4.
Active zones appear to be decreased in dα2δ-3 synaptic terminals. Wild-type and dα2δ-3 mutant (dα2δ-3k10814/Df(2R)CX1) larval synapses immunostained with the active zone marker nc82 (red) and the neuronal membrane marker anti-HRP (green). nc82 puncta per neuromuscular junction were reduced by 20% in the mutant (932 ± 33 compared with 1165 ± 41; p < 0.001; n = 14 and 16), and nc82 puncta per bouton were reduced by 40% (3.76 ± 0.09 compared with 5.99 ± 0.22; p < 0.001). Counts are from 14 NMJs from seven control larvae and 16 NMJs from eight mutant larvae. p < 0.001.
Overexpression of cacGFP rescues embryonic lethality in dα2δ mutants
The larval NMJ has been shown to use the α1 subunit encoded by cac for triggering synaptic transmission (Smith et al., 1998a; Dellinger et al., 2000; Kawasaki et al., 2000, 2002; Peng and Wu, 2007). If dα2δ-3 is necessary for α1 expression and/or stability, overexpression of this α1 subunit might compensate for loss of dα2δ-3. We tested this possibility by expressing a GFP-tagged cac transgene (Kawasaki et al., 2004). Neuronal overexpression of cacGFP in dα2δ-3DD196/Df(2)7128 mutants rescued the embryonic lethality of this genotype, resulting in progeny that survived to early pupal stages (data not shown). However, larvae and pupae were considerably smaller than wild type and extremely sluggish.
We similarly tested whether overexpression of the α1 calcium channel subunit cac in a mutant background could restore proper synaptic function to dα2δ-3k10814/dα2δ-3DD106 mutant synapses (Fig. 3). Although this rescued the change in mEJP frequency, it failed to increase EJP amplitude (mEJP amp = 1.07 ± 0.08 mV; mEJP freq = 2.67 ± 0.19 Hz; EJP amp = 5.65 ± 1.16 mV; n = 13). This finding may reflect a ceiling for α1 channel function at the synapse in the absence of dα2δ-3.
Synaptic cacGFP is reduced in dα2δ-3 mutants
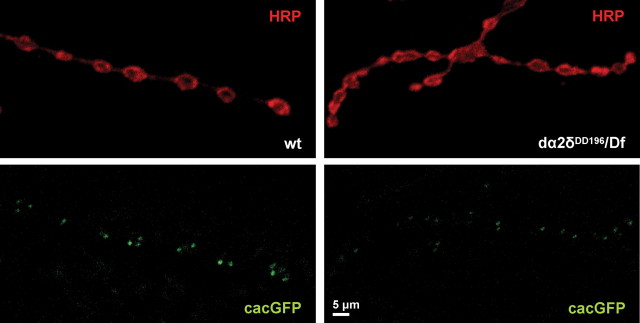
To examine the hypothesis that the dα2δ-3 subunit plays a role in ensuring the proper localization or expression level of the cac α1 subunit, we compared the fluorescent intensity of the GFP-tagged cac transgene at the third-instar larvae of wild-type and dα2δ-3k10814/dα2δ-3DD106 mutant neuromuscular junctions. In wild-type third-instar larvae, cacGFP has been shown to mark active zones with fluorescent puncta that represent small clusters of this channel subunit (Kawasaki et al., 2004) (Fig. 5). We analyzed two parameters of this fluorescence: their average fluorescent intensity, which reflects the density of the GFP-tagged channels at these sites, and the integrated fluorescent intensity, which reflects changes in both the density and size of the fluorescent puncta and may more accurately reflect the number of channels present. Although cacGFP puncta were observed at synaptic terminals of the mutant neuromuscular junctions, the average fluorescence intensity of these puncta was diminished by 25% (wt = 33 ± 0.6, n = 345 puncta from 4 larvae; mutant = 25 ± 0.3, n = 476 puncta from 3 larvae; mean ± SEM; p < 0.001), and the integrated intensity was diminished by 40% (wt = 13.3 × 103 ± 0.9 × 103; mutant = 7.9 × 103 ± 0.4 × 103; p < 0.001), indicating an apparent reduction in the number of α1 subunits present at these active zones. Furthermore, cacGFP puncta intensities were quantified for the rescued dα2δ-3DD196/Df(2)7128 mutants that survived to third instar during neuronal overexpression of cacGFP. These mutants also exhibited cacGFP puncta at the neuromuscular junction, however the fluorescence intensity was even lower than for the dα2δ-3k10814 mutant, consistent with this being a more severe allele. The average fluorescence intensity of cacGFP puncta was diminished by 28% (Fig. 5) (wt = 271 ± 7, n = 304 puncta from 3 larvae; mutant = 195 ± 4, n = 472 puncta from 4 larvae; p < 0.001), whereas the integrated intensity was diminished by 60% (Fig. 5) (wt = 130 × 103 ± 11 × 103; mutant = 53 × 103 ± 5 × 103; p < 0.001).
Figure 5.
cacGFP intensity is reduced in dα2δ-3 synaptic terminals. Neuronal expression of cacGFP was achieved at wild-type (UAScacGFP/elavGal4) and dα2δ-3 mutant larval synapses. cacGFP puncta (green) in wild-type and mutant synapses immunostained with the neuronal membrane marker anti-HRP (red) and imaged at the same gain settings. cacGFP fluorescence was determined as either the average intensity of pixels within puncta or the integrated intensity of pixels within puncta. The average fluorescence intensity of cacGFP puncta was diminished by 28% and the integrated fluorescence intensity by 60% in dα2δ-3DD106/Df(2R)7128; UAScacGFP/elavGal4 synaptic terminals compared with wild-type.
Discussion
Using an unbiased forward genetic approach, we have identified a calcium channel subunit essential for proper neurotransmission. This is the first description in any organism of an α2δ-3 mutant and its phenotype indicates that the α2δ-3 isoform of this subunit may preferentially be engaged in transmission. α2δ-2 mutant mice did not show physiological defects at synapses beyond what could be attributed to the small size of the animals (Barclay et al., 2001). α2δ-1 has not been studied genetically, but it is expressed in both neuronal and non-neuronal tissues and therefore is likely to have a more general function. Loss of the murine α2δ-4 subunit (Cacna2d4) results in a phenotype that comes closest to that which we have observed for loss of dα2δ-3: defects in the synaptic endings of photoreceptors, as revealed by electroretinograms and histology (Wycisk et al., 2006). The Drosophila genome also contains predicted isoforms of α2δ-1 and α2δ-2, but they do not appear to be functionally redundant with α2δ-3 as α2δ-3 null alleles are lethal and mutations in this isoform produce severe phenotypes in both photoreceptors and neuromuscular junctions. Thus, despite studies in heterologous expression systems that indicate that each α2δ isoform will promiscuously promote the surface expression of any α1 subunit, their functions in vivo are sufficiently distinct that loss of a single subunit can cause a severe phenotype.
Similarly, studies of mammalian channels have not resolved whether each α2δ isoform is associated in vivo with a particular α1 isoform, although there does appear to be some level of selective association. This pairing may derive primarily from the expression patterns of the α2δ and α1 subunits. However, from studies on gabapentin and on ducky mice, α2δ-2 and α2δ-3 appear to be the primary subunits in brain and preferentially associate with P- and N-type calcium channels (Klugbauer et al., 2003). In Drosophila, we find that loss of dα2δ-3 gives an electrophysiological phenotype similar to loss of the cac-encoded presynaptic α1 subunit in the ERG and neuromuscular junction. cac is the only Drosophila member of the Cav2 family, homologous to N-, P- and Q-type channels of mammals. Indeed, the cac channel has been established by both electrophysiological studies and cytochemical localization to be the major calcium channel in active zones for driving vesicle release (Smith et al., 1998a; Dellinger et al., 2000; Kawasaki et al., 2000, 2002; Peng and Wu, 2007). The present data indicate that the α2δ-3 subunit is its partner and necessary for its proper localization to the active zone. A similar pairing may occur in C. elegans in which unc-36, an α2δ subunit mutant, displays an identical phenotype to the α1 subunit mutant unc-2 (Schafer and Kenyon, 1995; Schafer et al., 1996). At murine photoreceptor synapses, L-type calcium channels mediate transmitter release and therefore, in a subset of mammalian synapses, α2δ-4 may play a similar role to α2δ-3, but partnering L-type rather than N-type channels (Wycisk et al., 2006), although this has not been investigated with direct recordings of synaptic properties. In the present study, transgenic rescue experiments demonstrated that the only essential function of α2δ-3 in Drosophila is in the nervous system. Other isoforms are thus likely to promote calcium channel expression in other cell types including muscle cells, which express the L-type α1 subunit Dmca1D (Ren et al., 1998).
The significance of the dα2δ-3 calcium channel subunit to synaptic function
How does the dα2δ-3 subunit contribute to presynaptic function? The leading hypothesis from mammalian work and studies in heterologous systems is that α2δ subunits promote robust plasma membrane expression of the α1 subunit (Gurnett et al., 1996; Wiser et al., 1996; Felix et al., 1997; Gurnett et al., 1997), at least in part by stabilizing them at the plasma membrane (Bernstein and Jones, 2007). The phenotype of dα2δ-3k10814 is consistent with the hypothesis that dα2δ-3 is similarly required for proper synaptic expression of the cacophony α1 subunit. Although we cannot record directly from these synaptic boutons to determine the amplitude of calcium currents, the reduction in quantal content per active zone and the decreased amplitude of the EJP are consistent with a decrease in calcium influx at the terminals attributable to decreased channel density. Because of the fourth-order dependence of release on calcium influx (Dodge and Rahamimoff, 1967), even a 33% reduction in channel density could account for the ∼5-fold observed reduction in vesicles released per active zone in the dα2δ-3k10814 allele. By fluorescent imaging of the cacGFP transgene, we observed a 25–60% reduction in the level of the cac α1 subunit in 2 different allellic combinations that, because they are not completely null, survive to the third-instar stage. This degree of reduction in α1 subunits at the active zone is consistent with the physiological findings and the hypothesis that the synaptic role of dα2δ-3 is to promote the expression, localization, or retention of the cac α1 subunit at active zones. Similarly, the ability of cac overexpression to extend the lifespan of dα2δ-3DD196/Df(2)7128 mutants suggests that the dα2δ-3 phenotype arises from an insufficiency of synaptic cac channels. In the context of the P-element insertion allele dα2δ-3k10814, however, in which substantial amounts of the α1 subunit likely were already present in the terminals, this overexpression was not adequate to significantly increase synaptic transmission.
The 20% decrease in active zones observed per neuromuscular junction, suggested by the decrease in nc82-immunoreactive puncta, is likely to account for a significant component of the 37% decrease in mEJP frequency, but a portion of the decrease may also arise from a change in calcium channel density. Similar decreases in mini frequency arise when neuromuscular junctions are placed in calcium-free salines (Sweeney et al., 1995; Okamoto et al., 2005), indicating that mEJPs are at least partially dependent on the entry of extracellular calcium. One possibility is that the ambient, resting calcium concentration in the synaptic cytosol depends on the amount of calcium that enters through spontaneous openings of calcium channels even at hyperpolarized potentials. Alternatively, sporadic, spontaneous calcium channel openings at the active zones of unstimulated terminals may cause brief, local increases in cytosolic calcium and trigger a portion of the observed minis.
Altered synaptic morphology in dα2δ-3
An unexpected feature of the dα2δ-3 mutants was their anatomical phenotype at the neuromuscular junction. In particular, mutants had slightly more boutons per junction, although the muscles themselves were smaller, and these boutons had a lower density of active zones, as scored by detectable puncta of nc82 immunoreactivity. This led to an overall 20% decrease in active zones per muscle.
Changes in the size and morphology of the Drosophila neuromuscular junction have been shown to occur as a result of perturbations in both presynaptic activity (Lnenicka et al., 2003; Mosca et al., 2005) and regulatory signaling (Packard et al., 2002; Keshishian and Kim, 2004; Ruiz-Canada and Budnik, 2006). However, many other perturbations of synaptic function do not have these effects, including viable mutations in synaptotagmin, syntaxin and SNAP-25 (DiAntonio and Schwarz, 1994; Schulze et al., 1995; Vilinsky et al., 2002). Mutations in the calcium channel α1 subunit, cac, did not cause an overgrowth of boutons in our studies, and others have reported fewer than normal boutons in cac alleles (Rieckhof et al., 2003; Xing et al., 2005). The changes in bouton number and active zone density in dα2δ-3 mutants therefore merit additional study. At present, these phenotypes may be direct consequences of the loss of this subunit or may include indirect consequences, possibly compensatory, in response to changes in the activation of the terminals, calcium influx, or transmitter release.
In summary, although much attention has been paid to the pore-forming α1 subunits, the calcium channel is a multi-subunit complex whose other subunits can also serve essential functions. The profound synaptic consequences of the loss of the dα2δ-3 subunit highlights the need to understand these subunits in their normal cellular milieu in which both physiological and developmental phenotypes may emerge that could not be appreciated in heterologous systems. These in vivo phenotypes should ultimately refine our understanding of the calcium channel complex in neuronal development, function, and disease.
Footnotes
This work was supported by National Institutes of Health Grant RO1 NS041062 (T.L.S.), a predoctoral fellowship from the Howard Hughes Medical Institute (D.K.D.), and National Defense Science and Engineering Graduate Fellowship (P.T.K.). We thank F. Kawasaki and R. Ordway for discussions on cacophony. We also thank J. T. Littleton, R. Ordway, and the Bloomington Stock Center for stocks, A. Hofbauer for antibodies, and L. Bu of the MRDDRC Imaging Core for assistance.
References
- Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol. 2003;13:298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- Barclay J, Balaguero N, Mione M, Ackerman SL, Letts VA, Brodbeck J, Canti C, Meir A, Page KM, Kusumi K, Perez-Reyes E, Lander ES, Frankel WN, Gardiner RM, Dolphin AC, Rees M. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J Neurosci. 2001;21:6095–6104. doi: 10.1523/JNEUROSCI.21-16-06095.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein GM, Jones OT. Kinetics of internalization and degradation of N-type voltage-gated calcium channels: role of the alpha2/delta subunit. Cell Calcium. 2007;41:27–40. doi: 10.1016/j.ceca.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Bichet D, Lecomte C, Sabatier JM, Felix R, De Waard M. Reversibility of the Ca2+ channel alpha(1)-beta subunit interaction. Biochem Biophys Res Commun. 2000;277:729–735. doi: 10.1006/bbrc.2000.3750. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brodbeck J, Davies A, Courtney JM, Meir A, Balaguero N, Canti C, Moss FJ, Page KM, Pratt WS, Hunt SP, Barclay J, Rees M, Dolphin AC. The ducky mutation in Cacna2d2 results in altered Purkinje cell morphology and is associated with the expression of a truncated alpha 2 delta-2 protein with abnormal function. J Biol Chem. 2002;277:7684–7693. doi: 10.1074/jbc.M109404200. [DOI] [PubMed] [Google Scholar]
- Burgess DL, Jones JM, Meisler MH, Noebels JL. Mutation of the Ca2+ channel beta subunit gene Cchb4 is associated with ataxia and seizures in the lethargic (lh) mouse. Cell. 1997;88:385–392. doi: 10.1016/s0092-8674(00)81877-2. [DOI] [PubMed] [Google Scholar]
- Collin T, Marty A, Llano I. Presynaptic calcium stores and synaptic transmission. Curr Opin Neurobiol. 2005;15:275–281. doi: 10.1016/j.conb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Coombe PE. The large monopolar cells L1 and L2 are responsible for ERG transients in Drosophila. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1986;159:655–665. [Google Scholar]
- De Waard M, Gurnett CA, Campbell KP. Structural and functional diversity of voltage-activated calcium channels. Ion Channels. 1996;4:41–87. doi: 10.1007/978-1-4899-1775-1_2. [DOI] [PubMed] [Google Scholar]
- Dellinger B, Felling R, Ordway RW. Genetic modifiers of the Drosophila NSF mutant, comatose, include a temperature-sensitive paralytic allele of the calcium channel alpha1-subunit gene, cacophony. Genetics. 2000;155:203–211. doi: 10.1093/genetics/155.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAntonio A, Schwarz TL. The effect on synaptic physiology of synaptotagmin mutations in Drosophila. Neuron. 1994;12:909–920. doi: 10.1016/0896-6273(94)90342-5. [DOI] [PubMed] [Google Scholar]
- Dickman DK, Horne JA, Meinertzhagen IA, Schwarz TL. A slowed classical pathway rather than kiss-and-run mediates endocytosis at synapses lacking synaptojanin and endophilin. Cell. 2005;123:521–533. doi: 10.1016/j.cell.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Dickman DK, Lu Z, Meinertzhagen IA, Schwarz TL. Altered synaptic development and active zone spacing in endocytosis mutants. Curr Biol. 2006;16:591–598. doi: 10.1016/j.cub.2006.02.058. [DOI] [PubMed] [Google Scholar]
- Dodge FA, Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol (Lond) 1967;193:419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin AC. Beta subunits of voltage-gated calcium channels. J Bioenerg Biomembr. 2003;35:599–620. doi: 10.1023/b:jobb.0000008026.37790.5a. [DOI] [PubMed] [Google Scholar]
- Felix R. Molecular regulation of voltage-gated Ca2+ channels. J Recept Signal Transduct Res. 2005;25:57–71. doi: 10.1081/rrs-200068102. [DOI] [PubMed] [Google Scholar]
- Felix R, Gurnett CA, De Waard M, Campbell KP. Dissection of functional domains of the voltage-dependent Ca2+ channel alpha2delta subunit. J Neurosci. 1997;17:6884–6891. doi: 10.1523/JNEUROSCI.17-18-06884.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnett CA, De Waard M, Campbell KP. Dual function of the voltage-dependent Ca2+ channel alpha 2 delta subunit in current stimulation and subunit interaction. Neuron. 1996;16:431–440. doi: 10.1016/s0896-6273(00)80061-6. [DOI] [PubMed] [Google Scholar]
- Gurnett CA, Felix R, Campbell KP. Extracellular interaction of the voltage-dependent Ca2+ channel alpha2delta and alpha1 subunits. J Biol Chem. 1997;272:18508–18512. doi: 10.1074/jbc.272.29.18508. [DOI] [PubMed] [Google Scholar]
- Held B, Freise D, Freichel M, Hoth M, Flockerzi V. Skeletal muscle L-type Ca2+ current modulation in gamma1-deficient and wildtype murine myotubes by the gamma1 subunit and cAMP. J Physiol (Lond) 2002;539:459–468. doi: 10.1113/jphysiol.2001.012745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen J. Calcium channelopathies in the central nervous system. Curr Opin Neurobiol. 1999;9:274–280. doi: 10.1016/s0959-4388(99)80040-3. [DOI] [PubMed] [Google Scholar]
- Kaja S, Todorov B, van de Ven RC, Ferrari MD, Frants RR, van den Maagdenberg AM, Plomp JJ. Redundancy of Ca(v) 2.1 channel accessory subunits in transmitter release at the mouse neuromuscular junction. Brain Res. 2007;1143:92–101. doi: 10.1016/j.brainres.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Kang MG, Campbell KP. Gamma subunit of voltage-activated calcium channels. J Biol Chem. 2003;278:21315–21318. doi: 10.1074/jbc.R300004200. [DOI] [PubMed] [Google Scholar]
- Kawasaki F, Felling R, Ordway RW. A temperature-sensitive paralytic mutant defines a primary synaptic calcium channel in Drosophila. J Neurosci. 2000;20:4885–4889. doi: 10.1523/JNEUROSCI.20-13-04885.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F, Collins SC, Ordway RW. Synaptic calcium-channel function in Drosophila: analysis and transformation rescue of temperature-sensitive paralytic and lethal mutations of cacophony. J Neurosci. 2002;22:5856–5864. doi: 10.1523/JNEUROSCI.22-14-05856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F, Zou B, Xu X, Ordway RW. Active zone localization of presynaptic calcium channels encoded by the cacophony locus of Drosophila. J Neurosci. 2004;24:282–285. doi: 10.1523/JNEUROSCI.3553-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshishian H, Kim YS. Orchestrating development and function: retrograde BMP signaling in the Drosophila nervous system. Trends Neurosci. 2004;27:143–147. doi: 10.1016/j.tins.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Klugbauer N, Marais E, Hofmann F. Calcium channel alpha2delta subunits: differential expression, function, and drug binding. J Bioenerg Biomembr. 2003;35:639–647. doi: 10.1023/b:jobb.0000008028.41056.58. [DOI] [PubMed] [Google Scholar]
- Letts VA, Felix R, Biddlecome GH, Arikkath J, Mahaffey CL, Valenzuela A, Bartlett FS, II, Mori Y, Campbell KP, Frankel WN. The mouse stargazer gene encodes a neuronal Ca2+-channel gamma subunit. Nat Genet. 1998;19:340–347. doi: 10.1038/1228. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Ganetzky B. Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron. 2000;26:35–43. doi: 10.1016/s0896-6273(00)81135-6. [DOI] [PubMed] [Google Scholar]
- Lnenicka GA, Keshishian H. Identified motor terminals in Drosophila larvae show distinct differences in morphology and physiology. J Neurobiol. 2000;43:186–197. [PubMed] [Google Scholar]
- Lnenicka GA, Spencer GM, Keshishian H. Effect of reduced impulse activity on the development of identified motor terminals in Drosophila larvae. J Neurobiol. 2003;54:337–345. doi: 10.1002/neu.10133. [DOI] [PubMed] [Google Scholar]
- Loewen CA, Mackler JM, Reist NE. Drosophila synaptotagmin I null mutants survive to early adulthood. Genesis. 2001;31:30–36. doi: 10.1002/gene.10002. [DOI] [PubMed] [Google Scholar]
- Marais E, Klugbauer N, Hofmann F. Calcium channel alpha(2)delta subunits-structure and Gabapentin binding. Mol Pharmacol. 2001;59:1243–1248. doi: 10.1124/mol.59.5.1243. [DOI] [PubMed] [Google Scholar]
- Martin AR. A further study of the statistical composition on the end-plate potential. J Physiol (Lond) 1955;130:114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca TJ, Carrillo RA, White BH, Keshishian H. Dissection of synaptic excitability phenotypes by using a dominant-negative Shaker K+ channel subunit. Proc Natl Acad Sci USA. 2005;102:3477–3482. doi: 10.1073/pnas.0406164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss FJ, Viard P, Davies A, Bertaso F, Page KM, Graham A, Canti C, Plumpton M, Plumpton C, Clare JJ, Dolphin AC. The novel product of a five-exon stargazin-related gene abolishes Ca(V)2.2 calcium channel expression. EMBO J. 2002;21:1514–1523. doi: 10.1093/emboj/21.7.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Tamura T, Suzuki K, Kidokoro Y. External Ca2+ dependency of synaptic transmission in Drosophila synaptotagmin I mutants. J Neurophysiol. 2005;94:1574–1586. doi: 10.1152/jn.00205.2005. [DOI] [PubMed] [Google Scholar]
- Packard M, Koo ES, Gorczyca M, Sharpe J, Cumberledge S, Budnik V. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002;111:319–330. doi: 10.1016/s0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng IF, Wu CF. Drosophila cacophony channels: a major mediator of neuronal Ca2+ currents and a trigger for K+ channel homeostatic regulation. J Neurosci. 2007;27:1072–1081. doi: 10.1523/JNEUROSCI.4746-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N, Yagel S, Momplaisir ML, Codd EE, D'Andrea MR. Molecular cloning and characterization of the human voltage-gated calcium channel alpha(2)delta-4 subunit. Mol Pharmacol. 2002;62:485–496. doi: 10.1124/mol.62.3.485. [DOI] [PubMed] [Google Scholar]
- Ren D, Xu H, Eberl DF, Chopra M, Hall LM. A mutation affecting dihydropyridine-sensitive current levels and activation kinetics in Drosophila muscle and mammalian heart calcium channels. J Neurosci. 1998;18:2335–2341. doi: 10.1523/JNEUROSCI.18-07-02335.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckhof GE, Yoshihara M, Guan Z, Littleton JT. Presynaptic N-type calcium channels regulate synaptic growth. J Biol Chem. 2003;278:41099–41108. doi: 10.1074/jbc.M306417200. [DOI] [PubMed] [Google Scholar]
- Ruiz-Canada C, Budnik V. Introduction on the use of the Drosophila embryonic/larval neuromuscular junction as a model system to study synapse development and function, and a brief summary of pathfinding and target recognition. Int Rev Neurobiol. 2006;75:1–31. doi: 10.1016/S0074-7742(06)75001-2. [DOI] [PubMed] [Google Scholar]
- Schafer WR, Kenyon CJ. A calcium-channel homologue required for adaptation to dopamine and serotonin in Caenorhabditis elegans. Nature. 1995;375:73–78. doi: 10.1038/375073a0. [DOI] [PubMed] [Google Scholar]
- Schafer WR, Sanchez BM, Kenyon CJ. Genes affecting sensitivity to serotonin in Caenorhabditis elegans. Genetics. 1996;143:1219–1230. doi: 10.1093/genetics/143.3.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze KL, Broadie K, Perin MS, Bellen HJ. Genetic and electrophysiological studies of Drosophila syntaxin-1A demonstrate its role in nonneuronal secretion and neurotransmission. Cell. 1995;80:311–320. doi: 10.1016/0092-8674(95)90414-x. [DOI] [PubMed] [Google Scholar]
- Smith LA, Peixoto AA, Hall JC. RNA editing in the Drosophila DMCA1A calcium-channel alpha 1 subunit transcript. J Neurogenet. 1998a;12:227–240. doi: 10.3109/01677069809108560. [DOI] [PubMed] [Google Scholar]
- Smith LA, Peixoto AA, Kramer EM, Villella A, Hall JC. Courtship and visual defects of cacophony mutants reveal functional complexity of a calcium-channel alpha1 subunit in Drosophila. Genetics. 1998b;149:1407–1426. doi: 10.1093/genetics/149.3.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM. Anticonvulsants and the relief of chronic pain: pregabalin and gabapentin as alpha(2)delta ligands at voltage-gated calcium channels. J Clin Psychiatry. 2004;65:596–597. doi: 10.4088/jcp.v65n0501. [DOI] [PubMed] [Google Scholar]
- Stowers RS, Schwarz TL. A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics. 1999;152:1631–1639. doi: 10.1093/genetics/152.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney ST, Broadie K, Keane J, Niemann H, O'Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Vilinsky I, Stewart BA, Drummond J, Robinson I, Deitcher DL. A Drosophila SNAP-25 null mutant reveals context-dependent redundancy with SNAP-24 in neurotransmission. Genetics. 2002;162:259–271. doi: 10.1093/genetics/162.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Durrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, Wichmann C, Kittel R, Sigrist SJ, Buchner E. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Wiser O, Trus M, Tobi D, Halevi S, Giladi E, Atlas D. The alpha 2/delta subunit of voltage sensitive Ca2+ channels is a single transmembrane extracellular protein which is involved in regulated secretion. FEBS Lett. 1996;379:15–20. doi: 10.1016/0014-5793(95)01475-6. [DOI] [PubMed] [Google Scholar]
- Wycisk KA, Budde B, Feil S, Skosyrski S, Buzzi F, Neidhardt J, Glaus E, Nurnberg P, Ruether K, Berger W. Structural and functional abnormalities of retinal ribbon synapses due to Cacna2d4 mutation. Invest Ophthalmol Vis Sci. 2006;47:3523–3530. doi: 10.1167/iovs.06-0271. [DOI] [PubMed] [Google Scholar]
- Xing B, Ashleigh Long A, Harrison DA, Cooper RL. Developmental consequences of neuromuscular junctions with reduced presynaptic calcium channel function. Synapse. 2005;57:132–147. doi: 10.1002/syn.20165. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]