Abstract
Taurine is one of the most abundant free amino acids in the brain. In a number of studies, taurine has been reported to activate glycine receptors (Gly-Rs) at moderate concentrations (≥100 μm), and to be a weak agonist at GABAA receptors (GABAA-Rs), which are usually activated at high concentrations (≥1 mm). In this study, we show that taurine reduced the excitability of thalamocortical relay neurons and activated both extrasynaptic GABAA-Rs and Gly-Rs in neurons in the mouse ventrobasal (VB) thalamus. Low concentrations of taurine (10–100 μm) decreased neuronal input resistance and firing frequency, and elicited a steady outward current under voltage clamp, but had no effects on fast inhibitory synaptic currents. Currents elicited by 50 μm taurine were abolished by gabazine, insensitive to midazolam, and partially blocked by 20 μm Zn2+, consistent with the pharmacological properties of extrasynaptic GABAA-Rs (α4β2δ subtype) involved in tonic inhibition in the thalamus. Tonic inhibition was enhanced by an inhibitor of taurine transport, suggesting that taurine can act as an endogenous activator of these receptors. Taurine-evoked currents were absent in relay neurons from GABAA-R α4 subunit knock-out mice. The amplitude of the taurine current was larger in neurons from adult mice than juvenile mice. Taurine was a more potent agonist at recombinant α4β2δ GABAA-Rs than at α1β2γ2 GABAA-Rs. We conclude that physiological concentrations of taurine can inhibit VB neurons via activation of extrasynaptic GABAA-Rs and that taurine may function as an endogenous regulator of excitability and network activity in the thalamus.
Keywords: α4 subunit, tonic inhibition, synaptic transmission, VB, ion channel, glycine receptor
Introduction
Taurine (2-aminoethane sulfonic acid) is one of the most abundant free amino acids in the brain and is required for the normal development of the nervous system (Neuringer and Sturman, 1987; Neuringer et al., 1987). It has a variety of physiological effects (Huxtable, 1992) that have attracted the attention of pharmacologists (Gupta et al., 2005), yet not all of its effects have been satisfactorily explained to date. The local application of taurine to central neurons typically inhibits firing (Curtis and Watkins, 1960) via activation of a Cl− conductance. Taurine is a structural analog of the inhibitory neurotransmitters glycine and GABA, and activates both glycine and GABAA receptors. For example, low to moderate concentrations (200 μm to 1 mm) of taurine have been shown to activate glycine receptors in the basolateral amygdala (McCool and Botting, 2000), hippocampus (Wu and Xu, 2003), nucleus accumbens (Jiang et al., 2004), supraoptic nucleus (Hussy et al., 1997), and inferior colliculus (Xu et al., 2004), whereas high concentrations of taurine (1–10 mm) activate GABAA receptors in these brain regions.
In addition to classical fast synaptic inhibition that is mediated by GABA and glycine in the CNS, a novel form of tonic inhibition has been described, first in the cerebellum (Brickley et al., 1996), and then in the dentate gyrus (Nusser and Mody, 2002) and recently in the relay neurons of the thalamus (Porcello et al., 2003; Belelli et al., 2005; Cope et al., 2005; Jia et al., 2005; Chandra et al., 2006). Tonic inhibition results from the continuous activation of extrasynaptic GABAA receptors containing δ-subunits, in response to low concentrations of ambient GABA. In the thalamus, extrasynaptic GABAA receptors have distinct pharmacological properties that differentiate them from synaptic GABAA receptors (consisting mainly of α1, β2, and γ2 subunits). GABA-mediated tonic inhibition in thalamus also requires expression of the GABAA receptor α4 subunit, because tonic currents are absent in thalamic relay neurons from GABAA receptor α4 subunit knock-out (Gabra4−/−) mice (Chandra et al., 2006).
In this study, we investigated the actions of taurine in the mouse ventrobasal (VB) thalamus, and found that taurine, at low concentrations in the physiological range (10–100 μm) (Lerma et al., 1986; Albrecht and Schousboe, 2005), is a potent inhibitor of VB neurons, where it elicits a conductance increase and tonic current that is blocked by gabazine, a selective GABAA receptor antagonist. In addition, we studied the actions of taurine at α4β2δ GABAA receptors heterologously expressed in human embryonic kidney 293 (HEK293) cells, where taurine showed greater efficacy than GABA. Taurine was also more potent at α4β2δ receptors than at “synaptic” α1β2γ2s GABAA receptors. The taurine-activated current was absent in VB neurons from Gabra4−/− mice, which lack extrasynaptic GABAA receptors containing the α4 subunit (Chandra et al., 2006). Our data suggest that taurine may act as an important endogenous modulator of thalamic activity in vivo, via the activation of extrasynaptic GABAA receptors in VB neurons.
Materials and Methods
Electrophysiological recordings in brain slices.
Experiments were performed in accordance with institutional and federal guidelines, using mice (C57BL/6, Gabra4+/+, and Gabra4−/−) between 23 and 50 d old (except for the experiments in Figure 5, which were done with 8- to 10-d-old C57BL/6 mice). Animals were anesthetized with halothane and brains were removed and placed in ice-cold slicing solution, which contained the following (in mm): 2.5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 220 sucrose, 11 glucose, 10 MgSO4, and 0.5 CaCl2, before cutting horizontal slices (300 μm thick) on a microslicer (VT1000S; Leica, Wetzlar, Germany).
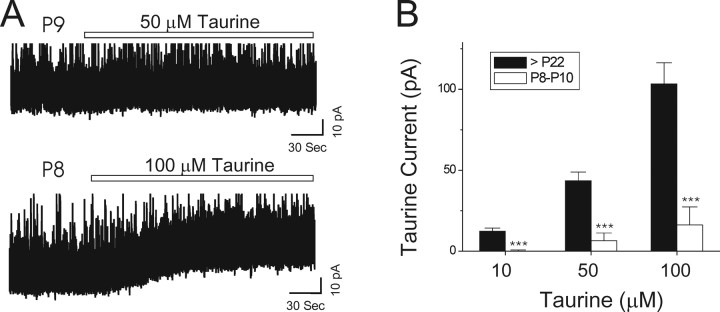
Figure 5.
Expression of taurine-evoked currents is age dependent. A, Taurine (50 μm) failed to evoke a current shift in a VB neuron from a P9 mouse. Taurine (100 μm) produced small outward current (∼20 pA) in a VB neuron from a P8 mouse. B, Bar graph demonstrates that taurine (10, 50, and 100 μm) induced much smaller currents in VB neurons from young mice (10 μm, 0.1 ± 0.7 pA, n = 4; 50 μm, 6.5 ± 4.7 pA, n = 9; 100 μm, 16.2 ± 11.1 pA, n = 8; ***p < 0.001 vs >P22 group at all three concentrations). Data for age >P22 group are from Figure 2C. Error bars represent SE.
Slices were perfused with carbongenated artificial CSF (aCSF), which contained the following (in mm): 124 NaCl, 2.5 KCl, 2 MgSO4, 2 CaCl2, 26 NaHCO3, 1.25 NaH2PO4, and 10 glucose. Whole-cell patch-clamp recordings from visually identified thalamic neurons were performed using an Axopatch 200A amplifier (Molecular Devices, Sunnyvale, CA) as previously described (Jia et al., 2005). Intracellular solution for voltage-clamp recordings contained the following (in mm): 130 CsCH3SO3, 8.3 NaCH3SO3, 1.7 NaCl, 1 CaCl2, 10 EGTA, 2 ATP-Mg2+, 0.3 GTP-Na+, 10 HEPES; pH was adjusted to 7.2 with CsOH. Intracellular solution for current-clamp recordings contained the following (in mm): 130 K+-gluconate, 5 NaCl, 2 MgCl2, 10 HEPES, 0.5 EGTA, 2 ATP-K+, and 0.3 GTP-Na+, pH adjusted to 7.25 with KOH. During voltage-clamp recordings, spontaneous IPSCs (sIPSCs) were recorded at 0 mV and isolated by bath application of 3–5 mm kynurenic acid (Jia et al., 2005). Access resistance was monitored throughout the recording period; cells were included for analysis only if the series resistance was <20 MΩ, and change of series resistance was <20% over the course of the experiment. Off-line analysis was performed using MiniAnalysis 5.5 (Synaptosoft, Decatur, GA), SigmaPlot 6.0 (SPSS, Chicago, IL), and Excel 2000 (Microsoft, Redmond, WA). The holding current shift was measured as the difference between the holding current in the presence and absence of drug. IPSCs were detected and analyzed using MiniAnalysis as previously described (Jia et al., 2005). Unless otherwise indicated, all numeric data are expressed as mean ± SEM. The statistical significance of results was assessed using Student's t test, and a level of p < 0.05 was considered significant. Drugs were dissolved in aCSF and applied by perfusion. All compounds were obtained from Sigma (St. Louis, MO), except guanidinoethyl sulfonate (GES), which was purchased from Toronto Research Chemicals (North York, Ontario, Canada).
Recordings from HEK293 cells expressing recombinant GABAA receptors.
The cDNAs encoding the human α1, mouse α4, rat β2, human γ2s, and human δ subunits were subcloned into the pcDNA3.1 expression vector and transiently expressed in HEK293 cells (American Type Culture Collection, Manassas, VA), and recorded as described by Jia et al. (2005). Ligand-gated currents were recorded at room temperature (voltage clamped at −60 mV) using an Axopatch 200 amplifier (Molecular Devices). The extracellular solution contained the following (in mm): 145 NaCl, 3 KCl, 1.5 CaCl2, 1 MgCl2, 6 d-glucose, 10 HEPES, and pH adjusted to 7.4 with NaOH. Patch pipettes were filled with the intracellular solution, which contained the following (in mm): 145 N-methyl-d-glucamine hydrochloride, 0.1 CaCl2, 5 ATP-K+, 1.1 EGTA, 2 MgCl2, 5 HEPES, and pH adjusted to 7.2 with KOH. GABA or taurine was rapidly applied (∼50 ms exchange time) to the cell via a multichannel motor-driven solution exchange device (Rapid Solution Changer RSC-100; Molecular Kinetics, Pullman, WA). Concentration–response data for each individual cell was fitted (using a sum of least squares method) to a Hill equation of the form: I = Imax × [agonist]nH/([agonist]nH + EC50nH), where I is the peak current, Imax is the maximum whole-cell current amplitude, [agonist] is the agonist concentration, EC50 is the agonist concentration eliciting a half-maximal current response, and nH is the Hill coefficient (Jia et al., 2005). Averaged data are expressed as mean ± SEM. Statistical significance was assessed using Student's t test, and a level of p < 0.05 was considered significantly different.
Generation and use of α4 subunit knock-out mice.
α4 subunit gene knock-out mice were generated as described previously (Chandra et al., 2006). All knock-out (Gabra4−/−) and wild-type (Gabra4+/+) littermates used were age-matched and were on the same genetic background (129X1/S1 × C57BL/6J hybrid; F2–F4 generations). Experimenters were blind to genotype in all studies.
Results
Taurine decreases the excitability of thalamic neurons in the ventrobasal complex
We began by asking whether taurine (50 μm) could alter the excitability of thalamic relay neurons. At resting membrane potentials (about −75 mV), most VB neurons in our recordings displayed “burst” firing in response to depolarizing current steps (Jahnsen and Llinãs, 1984; Huguenard, 1996). To facilitate measurements of firing rate, we depolarized their membrane potentials to about −60 mV by constant current injection. At this membrane potential, VB neurons were generally silent but displayed sustained action potential (AP) firing in response to depolarizing current steps. The amplitude of the current step (500 ms duration) was adjusted to induce ∼10 APs (Fig. 1A), corresponding to a firing frequency of ∼20 Hz. We then compared the numbers of APs evoked by depolarizing current steps before and after taurine application. Taurine (50 μm) significantly reduced the number of evoked APs, from 9.8 ± 1.0 to 7.2 ± 1.1 (p < 0.01; n = 5), and this effect was fully reversible on removal of taurine (Fig. 1A,B). In addition to the effect on spike firing, taurine (50 μm) hyperpolarized the neurons by 2.0 ± 0.8 mV (n = 5). Taurine (50 μm) also decreased the membrane input resistance (Rm) to 90 ± 3% of control (p < 0.01; n = 5), and this effect was also fully reversible (Fig. 1C). In the presence of TTX (0.5 μm), taurine (50 μm) still reduced Rm to 91 ± 2% of control (p < 0.05; n = 4), which suggests that the inhibitory effect of taurine on Rm is independent of fast voltage-gated Na+ channels, and therefore also independent of intact synaptic transmission.
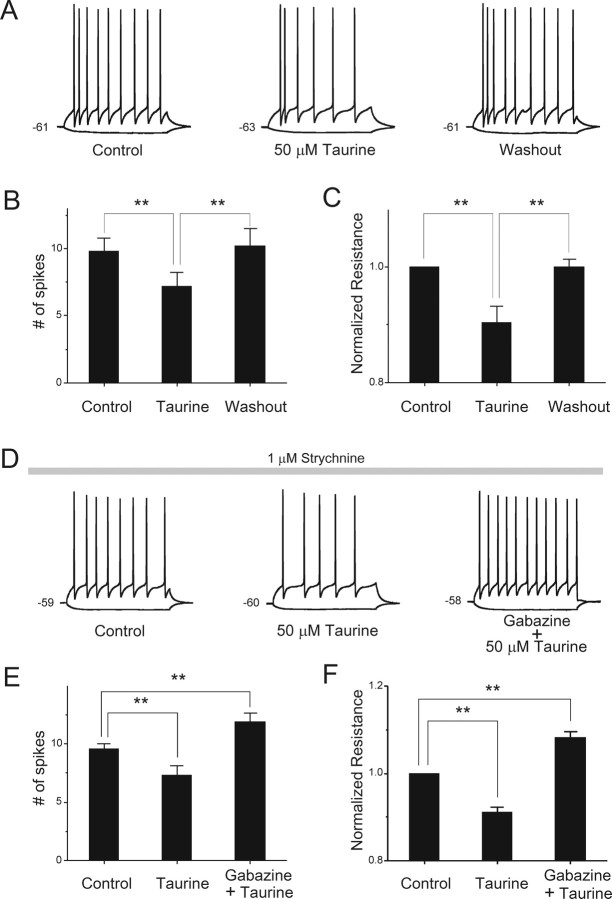
Figure 1.
Taurine decreases the excitability of VB neurons through GABAA receptors. A, Representative current-clamp traces demonstrate AP firing evoked by a 0.1 nA current step (duration, 500 ms) in a VB neuron. Membrane resistance (Rm) was measured by injecting hyperpolarizing current (−0.02 nA). After taurine (50 μm) perfusion, AP firing decreased. The inhibitory effects of taurine were completely reversible on washing out taurine. B, Pooled data show that taurine reduces the excitability of VB neurons (**p < 0.01; n = 5). C, Bar graph illustrates the normalized Rm from the same (as in B) VB neurons (**p < 0.01). Under control conditions, Rm was 267 ± 11 MΩ, whereas in the presence of taurine and after the washout of taurine, Rm values were 242 ± 15 and 267 ± 8 MΩ, respectively. D, Representative current-clamp trace demonstrating AP firing evoked by a 0.1 nA current step (duration, 500 ms) in another VB neuron; after taurine (50 μm) perfusion, AP firing decreased. Subsequent gabazine application increased the number of evoked APs. Strychnine (1 μm) was present throughout the recordings to prevent the activation of glycine receptors. E, Pooled data show that taurine reduces the excitability of VB neurons (**p < 0.01 vs control; n = 6). Gabazine was able to block the inhibition produced by taurine (**p < 0.01 vs control; n = 6). F, Bar graph illustrates parallel changes (as in E) in membrane resistance (Rm) produced by taurine and gabazine (**p < 0.01 vs control; n = 6). Under control conditions, Rm was 234 ± 19 MΩ, whereas in the presence of taurine and taurine plus gabazine, Rm was 213 ± 17 and 253 ± 20 MΩ, respectively. Error bars represent SE.
We investigated the contribution of glycine receptors to the inhibitory effects of taurine in an additional set of experiments by adding strychnine (1 μm), a selective glycine receptor antagonist (Fig. 1D). In the presence of strychnine, taurine (50 μm) still reduced the number of evoked APs (control, 9.6 ± 0.5; taurine, 7.3 ± 0.8; n = 6; p < 0.01), and hyperpolarized the neurons by 2.2 ± 0.8 mV (n = 6). The subsequent application of gabazine (10 μm) increased the number of APs to 11.9 ± 0.8 (p < 0.01; n = 6) and blocked the effects of taurine. Taurine also decreased Rm to 91 ± 1% of control (p < 0.01; n = 6) in the presence of strychnine, but the subsequent application of gabazine blocked this effect of taurine and increased Rm to 108 ± 1% of control (p < 0.01; n = 6) (Fig. 1F). We also used depolarizing current steps to evoke burst firing in VB neurons held near −70 mV. The amplitude of the injected current pulse was adjusted to be just above threshold level for burst firing in the control condition. In all five neurons tested, the evoked burst firing was reversibly inhibited by taurine (50 μm); a typical recording is shown in supplemental Figure 1 (available at www.jneurosci.org as supplemental material). Together, these data suggest that 50 μm taurine reduced the excitability of VB neurons mainly via an action on GABAA receptors.
Taurine evokes outward currents in VB neurons under voltage clamp
Voltage-clamp recordings were obtained from VB neurons (in the presence of kynurenic acid to block ionotropic glutamate receptors; holding potential, 0 mV) to explore the pharmacological properties of the ionic currents elicited by taurine. In every VB neuron from mice older than postnatal day 22 (P22), we found that taurine (50 μm) elicited a steady and sustained outward current (Fig. 2A); the mean amplitude of the taurine-activated currents was 67 ± 13 pA (n = 6). When glycine receptors were blocked by 1 μm strychnine, taurine currents were slightly smaller in amplitude (44 ± 5 pA; n = 16; p > 0.05 vs control). When we used gabazine (10 μm), a selective GABAA receptor antagonist, much smaller current responses were observed in response to 50 μm taurine (11 ± 3 pA; n = 8; p < 0.001 vs control), which suggests that a small component of the taurine-evoked current was likely mediated by glycine receptors. The dominant contribution of GABAA receptors to the currents elicited by taurine (Fig. 2A) is consistent with the results of the current-clamp experiments (Fig. 1).
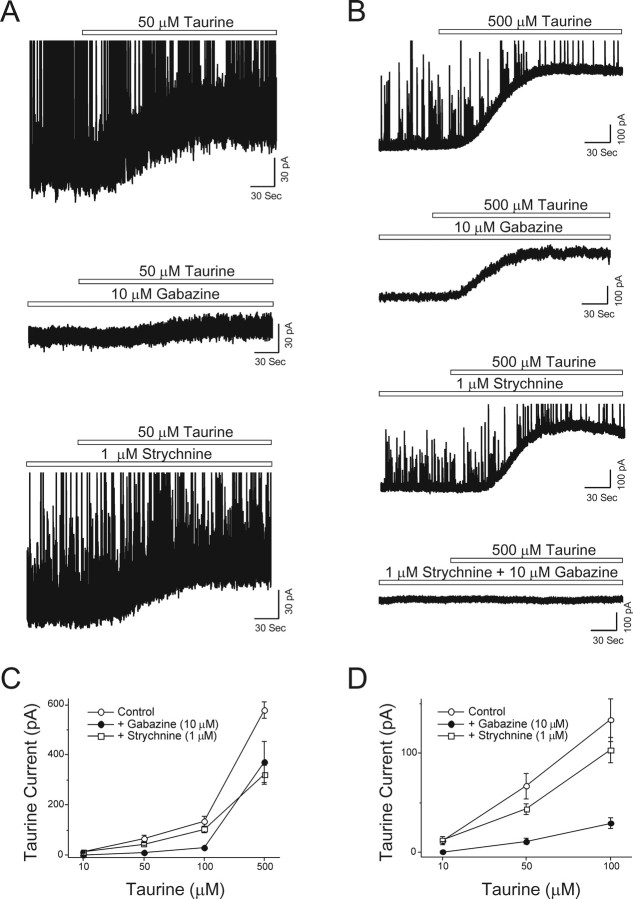
Figure 2.
Low concentrations of taurine preferentially activate GABAA receptors. A, Exemplar traces of taurine-induced currents in a VB neuron voltage clamped at 0 mV (here and in subsequent figures). Application of 50 μm taurine evoked a modest (∼50 pA) outward current in a VB neuron, whereas in the presence of the GABAA receptor antagonist gabazine, 50 μm taurine produced very little current shift in a different VB neuron. In another VB neuron, 50 μm taurine produced a modest (∼50 pA) current shift in the presence of the glycine receptor antagonist strychnine. The bars above the traces indicate the period of drug application at the indicated concentration in this and subsequent figures. B, Exemplar current trace demonstrating that application of 500 μm taurine evoked large outward currents in VB relay neurons (the scale bars here are different from those in A). In the presence of GABAA or glycine receptors antagonist, 500 μm taurine still elicited current shifts in each instance, but had no effect on the holding current when both GABAA and glycine receptors are blocked. All traces are from different VB neurons. C, Concentration–response curves of taurine-activated currents in the absence or presence of GABAA and glycine receptors antagonist applied individually. Taurine-activated currents mediated by GABAA receptors are larger than currents mediated by glycine receptors. Error bars represent SE. D, Concentration–response curves in C were enlarged to illustrate the differences in current amplitude evoked by taurine of low concentrations (10–100 μm). Taurine-activated currents mediated by GABAA receptors are significantly larger than currents mediated by glycine receptors. When glycine receptors were blocked by strychnine, taurine evoked concentration-dependent outward currents in thalamic relay neurons. Control group: 10 μm, 11.8 ± 4.3 pA, n = 6; 50 μm, 66.8 ± 12.7 pA, n = 6; 100 μm, 133.8 ± 21.5 pA, n = 5; strychnine group: 10 μm, 12.4 ± 1.8 pA, n = 12; 50 μm, 43.6 ± 5.3 pA, n = 16; 100 μm, 103.4 ± 13.0 pA, n = 8; gabazine group: 10 μm, 0.4 ± 0.8 pA, n = 8; 50 μm, 11.1 ± 3.0 pA, n = 8; 100 μm, 29.4 ± 5.0 pA, n = 5. Error bars represent SE.
At high concentrations (e.g., 500 μm taurine), we recorded large outward currents (580 ± 33 pA; n = 8) (Fig. 2B). These large taurine-induced currents were reduced in amplitude in the presence of either strychnine (321 ± 38 pA; n = 7; p < 0.05) or gabazine (371 ± 81 pA; n = 7; p < 0.05), and when strychnine (1 μm) and gabazine (10 μm) were applied in combination, the response to taurine was abolished (2 ± 3 pA; n = 9) (Fig. 2B), indicating that currents activated by high concentrations of taurine are mediated, equally but exclusively, by a combination of glycine and GABAA receptors. We then constructed a series of concentration-response curves for taurine in the presence and absence of the two antagonists (Fig. 2C). Closer examination of these curves at lower concentrations of taurine (≤100 μm) clearly indicates that taurine preferentially activated GABAA receptors rather than glycine receptors (Fig. 2D). In fact, at 10 μm taurine, the responses were completely blocked by gabazine (control, 12 ± 2 pA, n = 12; gabazine, 0 ± 1 pA, n = 8), although taurine has been described as a more potent agonist at glycine receptors.
To investigate the possibility of synergistic effects between taurine and GABA, we also coapplied taurine and GABA in the presence of strychnine (1 μm). GABA itself (1 μm) evoked a modest outward current (18 ± 5 pA; n = 7). When taurine was applied in the presence of GABA (1 μm), the taurine-induced currents were smaller than under control condition at all concentrations tested, although this difference was not significant (10 μm, 9 ± 4 pA, n = 4; 50 μm, 39 ± 13 pA, n = 7; 100 μm, 69 ± 12 pA, n = 5; p > 0.05 vs control group at all three concentrations). There is, therefore, no evidence for synergistic interactions between GABA and taurine, which is consistent with the idea that both taurine and GABA are activating the same population of GABAA receptors in the thalamus.
IPSCs are insensitive to taurine
There have been no previous reports indicating that taurine activates native GABAA receptors in this low concentration range (10–100 μm), and we set out to establish the molecular identity of the GABAA receptors responsible for generating these currents. All of the experiments described below were therefore performed in the presence of strychnine (1 μm) to isolate taurine-evoked currents mediated by GABAA receptors. In the presence of 1 μm strychnine, glycine (100 μm) failed to evoke an outward current, and thus any currents activated by taurine in the presence of strychnine were mediated only by GABAA receptors.
In thalamic VB neurons, synaptically localized GABAA receptors consist primarily of α1, β2, and γ2 subunits, and sIPSCs are readily observed in VB neurons. All sIPSCs that we recorded could be blocked by gabazine (Fig. 2), indicating that they were mediated by GABAA receptors. Taurine (50 μm) had no effect on either IPSC amplitude or decay time (Fig. 3A). The pooled data from this set of experiments (Fig. 3B) confirm that the properties of these sIPSCs were not significantly affected by 50 μm taurine (amplitude: control, 55 ± 5 pA, taurine, 53 ± 5 pA, p > 0.05; decay time constant: control, 14.5 ± 1.3 ms, taurine, 14.8 ± 1.4 ms, p > 0.05; frequency: control, 7.8 ± 1.1 Hz, taurine, 7.8 ± 1.1 Hz, p > 0.05; n = 11). Thus, taurine (50 μm) does not appear to modulate the function of synaptic GABAA receptors in VB neurons.
Figure 3.
The effects of taurine on synaptic versus extrasynaptic GABAA receptors. A, A typical recording of sIPSCs in a VB neuron in the presence of 50 μm taurine. Strychnine (1 μm) was present throughout so as to exclude the contribution of glycine receptors (as well as recordings in Figs. 4 and 6). Averaged sIPSC traces (>100 events per ensemble trace) before (black) and after (gray) taurine application; superimposed traces illustrate the similarity in amplitude and decay time. B, The normalized amplitude (0.97 ± 0.06), decay time constant (1.03 ± 0.03), and frequency (1.03 ± 0.09) of sIPSCs after 50 μm taurine perfusion. These parameters are not significantly different from those obtained under control conditions (p > 0.05; n = 11). C, A representative current trace demonstrating that in the presence of 50 μm taurine, 500 nm midazolam did not shift the baseline current. D, In a different neuron than in C, ZnCl2 (20 μm) partially blocked the taurine-induced outward current as evidenced by the small (∼20 pA) shift in the current baseline. E, In a different neuron than in C and D, gabazine (10 μm) effectively blocked the taurine-induced outward current as well as all sIPSCs. The shift in the current baseline is ∼60 pA. F, Averaged current shifts produced by gabazine (n = 7), Zn2+ (n = 6), and midazolam (n = 7) after taurine application. Error bars represent SE.
Pharmacology of taurine-evoked currents in VB
We have previously shown that Zn2+ partially blocks the tonic gabazine-sensitive current in VB neurons, whereas the benzodiazepine midazolam has no effect on tonic inhibition (Jia et al., 2005). We investigated the pharmacology of taurine responses using midazolam and Zn2+. Midazolam (500 nm) had no effect on the amplitude of the outward current induced by taurine (current reduction: 1 ± 1 pA, n = 7, p > 0.05) (Fig. 3C,F), whereas Zn2+ (20 μm) partially blocked the outward current (current reduction: 23 ± 4 pA, n = 6, p < 0.01) (Fig. 3D,F), and gabazine completely blocked the taurine-activated currents (current shift: 61 ± 6 pA, n = 7) (Fig. 3E,F). We also tested 1 μm Zn2+ so as to distinguish between αβδ/αβγ and αβ GABAA receptors (Draguhn et al., 1990; Smart et al., 1991; Storustovu and Ebert, 2006). In the presence of 50 μm taurine, 1 μm Zn2+ failed to induce an appreciable change in the holding current (0.7 ± 0.9 pA; n = 4); thus, it is unlikely that GABAA receptors consisting only of α and β subunits contribute to taurine-evoked currents.
Taurine can act as a direct endogenous agonist at thalamic extrasynaptic GABAA receptors
Taurine has been shown to inhibit GABA uptake mechanisms in the CNS via GABA transporters 2 (GAT-2) and 3 (GAT-3) (Liu et al., 1993; Takanaga et al., 2001). To examine whether taurine might be indirectly activating GABAA receptors by increasing ambient GABA, we studied the effect of 1-[2-[tris(4-methoxy-phenyl)methoxy]ethyl]-(S)-3-piperidinecarboxylic acid (SNAP-5114) (50 μm), which inhibits both GAT-2 and GAT-3 (Borden et al., 1994a; Borden, 1996). We found that SNAP-5114 alone had no effect on membrane current in VB neurons (0.3 ± 2.3 pA; n = 8) (Fig. 4A,C) and that taurine (50 μm) evoked a current shift in the presence of SNAP-5114 (47 ± 7 pA; n = 5), which was comparable with that reported above for taurine alone, suggesting that taurine does not increase ambient GABA levels via GAT-2/3-dependent heteroexchange mechanisms in our preparation. These data argue against a significant contribution of an indirect effect of taurine on extrasynaptic GABAA receptors.
Figure 4.
Endogenous taurine directly evokes GABAergic currents in VB neurons. A, An exemplar current trace (top) demonstrating that SNAP-5114 (50 μm), a GAT-2 and GAT-3 inhibitor, failed to induce a current shift in a VB neuron. An appreciable shift in the baseline current was observed after coapplication of SNAP-5114 and a second reuptake inhibitor, NO-711 (10 μm; bottom trace). B, Exemplar current trace showing that GES (10 μm), a taurine uptake inhibitor, evoked current shift (∼20 pA) in a different VB neuron than in A. GES (50 μm) induced a greater outward current (∼100 pA) in a different VB neuron (note the different vertical scale bar here from that for 10 μm GES trace). C, Bar graph demonstrates that GES induced significant outward currents in VB neurons (10 μm: 21.5 ± 3.9 pA, n = 8; 50 μm: 105.5 ± 18.9 pA, n = 5; 100 μm: 215.3 ± 31.2 pA, n = 7), whereas SNAP-5114 induced little outward current (50 μm: 0.3 ± 2.3 pA, n = 8). Coapplication of NO-711 (10 μm) and SNAP-4114 (50 μm) also produced a significant outward current (193.4 ± 37.9; n = 4). D, Sample current traces demonstrating the effect of taurine (50 μm) on the outward current in the presence of SNAP-4114 (50 μm; top trace) or SNAP-4114 plus NO-711 (10 μm; bottom trace). E, Bar graph summarizing the effect of SNAP-5114 (50 μm), alone and in combination with NO-711 (10 μm), on taurine (50 μm)-evoked currents. Control taurine currents were from Figure 2. In the presence of SNAP-5114 alone, taurine produced a 46.6 ± 7.1 pA current shift (n = 5), whereas taurine alone produced a 67 ± 13 pA current shift (n = 6; same data as in Fig. 2); taurine again produced a somewhat smaller outward current (26.8 ± 6.6 pA; n = 4) in the presence of SNAP-5114 and NO-711. Error bars represent SE.
We also studied the effects of coapplied 1-[2-[[(diphenylmethylene)imino]oxy]ethyl]-1,2,5,6-tetrahydro-3-pyridinecarboxylic acid hydrochloride (NO-711) (10 μm; a GAT-1 inhibitor) (Borden et al., 1994b) and SNAP-5114 (50 μm) (Fig. 4A,C), and recorded a current shift of 193 ± 38 pA in response to these two inhibitors in combination (n = 4); this presumably reflects activation of receptors by endogenous GABA. In the presence of NO-711 and SNAP-5114, the sIPSC decay time was also increased, from 14.5 ± 1.0 to 27.2 ± 2.5 ms. In the presence of NO-711 and SNAP-5114, taurine (50 μm) application evoked an outward current (27 ± 7 pA; n = 4), which was not dissimilar from that recorded under control conditions (Fig. 4D,E). The small decrease in the taurine-evoked current under these conditions suggests that extrasynaptic GABAA receptors may have been partly desensitized by the sustained increase in ambient GABA induced by extensive GAT blockade with SNAP-5114 and NO-711.
We next investigated the taurine reuptake inhibitor GES to determine whether reducing taurine uptake could indirectly activate extrasynaptic GABAA receptors in the thalamus. In the presence of 1 μm strychnine, concentrations of GES as low as 10 μm were able to induce detectable currents in VB neurons (Fig. 4B). Higher concentrations of GES evoked bigger currents (10 μm: 22 ± 4 pA, n = 8; 50 μm: 106 ± 19 pA, n = 5; 100 μm: 215 ± 31 pA, n = 7) (Fig. 4C). GES-induced (100 μm) currents were totally blocked by gabazine (n = 3). These experiments suggest that taurine could act as an endogenous agonist for thalamic extrasynaptic GABAA receptors.
Taurine evokes smaller currents in VB neurons of young mice (P8–P10)
In previous work, 500 μm taurine was reported to reduce membrane resistance of thalamic relay neurons from young rats, mostly via activation of glycine receptors (Ghavanini et al., 2005), whereas our data in adult mice suggest that there is a significant contribution of GABAA receptors (Fig. 2). To resolve this apparent discrepancy, we tested taurine in VB neurons of young mice (P8–P10) in the presence of strychnine. Taurine elicited much smaller currents in these neurons than in recordings from mature mice (Fig. 5). The mean amplitude of the taurine-evoked currents were as follows: 10 μm, 0.1 ± 0.7 pA (n = 4); 50 μm, 6.5 ± 4.7 pA (n = 9); 100 μm, 16.2 ± 11.1 pA (n = 8); p < 0.001 versus >P22 group at all three concentrations. It therefore appears that thalamic extrasynaptic GABAA receptors are not fully expressed in these young animals and continue to increase during adolescence.
Taurine-evoked currents in HEK cells expressing GABAA α4β2δ and α1β2γ2s receptors
The synaptic GABAA receptors present in VB neurons are thought to be the α1β2γ2 subtype (Zhang et al., 1997; Okada et al., 2000), whereas the extrasynaptic receptors are thought to represent the α4β2δ subtype (Jia et al., 2005). To study the taurine sensitivity of “synaptic” and “extrasynaptic” GABAA receptor subtypes in a controlled setting, we studied the effects of taurine on two populations of recombinant GABAA receptors, α1β2γ2s and α4β2δ, using heterologous expression in HEK293 cells. Taurine was more potent in activating α4β2δ receptors than at α1β2γ2s (Fig. 6), with a threshold concentration of ≤300 μm taurine and an EC50 (7.5 ± 1.3 mm; n = 12) that was lower than that observed for the α1β2γ2s receptors (21.2 ± 7.3 mm; n = 8; p < 0.05). Taurine threshold concentrations in HEK cells were somewhat higher than those in slice recordings; the disparity may result from the differences in the technique for agonist application, or from the effects of unknown intracellular modulator(s) of extrasynaptic GABAA receptor function in VB neurons. We compared the efficacy of taurine and GABA at both GABAA receptor subtypes. Itaurinemax (maximum taurine current) was larger than IGABAmax in α4β2δ receptors (145 ± 12%; n = 12), whereas Itaurinemax was smaller than IGABAmax in α1β2γ2s receptors (70 ± 9%; n = 8). The relative efficacy of taurine at α4β2δ receptors with respect to GABA [i.e., (Itaurinemax/IGABAmax)] is significantly larger than was the case at α1β2γ2s receptors (p < 0.001). In this set of experiments, the relative maximal currents quoted above represent the mean ± SEM of the maxima estimated from the curve fits for each individual experiment.
Figure 6.

Comparison of taurine-evoked Cl− currents recorded in HEK293 cells expressing α1β2γ2s and α4β2δ GABAA receptors. A, Typical concentration-dependent taurine-activated and maximum GABA-activated currents recorded from α4β2δ GABAA receptors expressed in HEK293 cells. B, Typical concentration-dependent taurine-activated and maximum GABA-activated currents recorded from α1β2δ2s GABAA receptors. C, Averaged relative concentration–response curves (Itaurine/IGABA-max) for α1β2γ2s (n = 8) and α4β2δ (n = 12) GABAA receptors. The averaged concentration–response data in the figure were fitted using a sum of least-squares method to a Hill equation of the form: I = Imax × [agonist]nH/([agonist]nH + EC50nH), where I is the peak current, Imax is the maximum whole-cell current amplitude, [agonist] is the agonist concentration, EC50 is the agonist concentration eliciting a half-maximal current response, and nH is the Hill coefficient. Error bars represent SE.
Taurine-evoked currents are absent in VB neurons from Gabra4−/− mice
We also examined neurons in the reticular thalamic nucleus (RTN) for the presence of taurine-evoked currents, but no significant current shifts were detected (10 μm taurine: 0.1 ± 1.6 pA, n = 7; 50 μm taurine: 1.8 ± 0.8 pA, n = 6). We have previously demonstrated that extrasynaptic GABAA receptors are absent in the thalamus of Gabra4−/− mice (Chandra et al., 2006), and we therefore investigated the actions of taurine in VB neurons from Gabra4−/− mice and their wild-type littermates (Fig. 7). No significant taurine-evoked currents were detected in VB neurons from α4 knock-out mice (10 μm: 1 ± 1 pA, n = 6; 50 μm: 2 ± 1 pA, n = 6). In contrast, wild-type neurons showed measurable taurine-activated outward currents (10 μm: 14 ± 4 pA, n = 8; 50 μm: 54 ± 13 pA, n = 7), which were comparable with taurine-induced currents recorded from standard C57BL/6 mice. This difference between the genotypes was highly significant (p < 0.001 at both concentrations). These results are consistent with the idea that low concentrations of taurine selectively activate extrasynaptic GABAA receptors that contain α4/δ subunits (Jia et al., 2005), which are present in VB neurons of wild-type mice, but not in RTN neurons or in VB neurons from the knock-out animals (Chandra et al., 2006).
Figure 7.
Taurine-mediated currents are absent in VB neurons from mice lacking the GABAA receptor α4 subunit. A, Taurine (50 μm) evoked a marked current (∼50 pA) in a VB neuron from a wild-type mouse. In contrast, taurine produced no current shift in a VB neuron from a Gabra4−/− mouse. B, Bar graph demonstrates that taurine (10 and 50 μm) induced current shifts in wild-type, but not α4 knock-out, VB neurons (***p < 0.001; n = 6–8). Error bars represent SE. WT, Wild type; KO, knock-out.
Discussion
Taurine is known to act as an agonist at glycine and GABAA-Rs in the brain (Albrecht and Schousboe, 2005). Most previous studies have found that taurine preferentially activates strychnine-sensitive glycine receptors, whereas very high concentrations (1–10 mm) of taurine also activate GABAA-Rs (Hussy et al., 1997; del Olmo et al., 2000; McCool and Botting, 2000; Wu and Xu, 2003; Jiang et al., 2004; Xu et al., 2004). This study represents the first report that low micromolar concentrations (10–100 μm) of taurine can activate GABAA-Rs in the brain. It is likely that these levels of taurine are achieved in the environment of these receptors, because levels of extracellular taurine measured by microdialysis range from ∼1 to 10 μm in the resting state to 120 μm after depolarization or other activating stimuli (Lerma et al., 1986; Albrecht and Schousboe, 2005).
Previous electrophysiological studies using thalamic brain slices have demonstrated that tonic inhibition is sensitive to Zn2+, but not benzodiazepines (Belelli et al., 2005; Cope et al., 2005; Jia et al., 2005), consistent with the idea that it is generated via extrasynaptic GABAA-Rs (consisting of α4, β2, and δ subunits). We observed that taurine-sensitive GABAA-Rs displayed remarkably similar pharmacological properties to those of thalamic extrasynaptic GABAA-Rs in being partially blocked by Zn2+ (20 μm) but unaffected by midazolam (500 nm). Furthermore, taurine had no effect on inhibitory synaptic currents in VB neurons, which are mainly mediated by α1β2γ2 GABAA-Rs (Zhang et al., 1997; Huntsman and Huguenard, 2000), again supporting the idea that taurine activates extrasynaptic GABAA-Rs preferentially.
A selective action of taurine on extrasynaptic GABAA-Rs was also supported by the experiments in RTN neurons. The main GABAA receptor subunits found in the RTN are α3, β3, and γ2 (Pirker et al., 2000), and tonic inhibition is notably absent in RTN neurons (Belelli et al., 2005; Cope et al., 2005; Jia et al., 2005), consistent with a lack of expression of extrasynaptic GABAA-Rs; accordingly, we did not observe taurine-activated currents in RTN neurons.
Additional supporting evidence was provided by recordings made using neurons from GABAA-R α4 subunit knock-out mice. We demonstrated previously that gabazine has little effect on the holding current in thalamic VB neurons from Gabra4−/− mice (Chandra et al., 2006). GABAA-Rs containing α4/δ subunits are located extrasynaptically (Jia et al., 2005), so the absence of the tonic current in Gabra4−/− VB neurons is consistent with a loss of these extrasynaptic receptors. Likewise, taurine (10 and 50 μm) failed to evoke significant current shifts in VB neurons from Gabra4−/− mice, which indicates that extrasynaptic GABAA-Rs are the major target of taurine in the thalamus.
Although taurine has been reported to inhibit GABA uptake through effects on GAT-2 and GAT-3 (Liu et al., 1993; Takanaga et al., 2001), it seems unlikely from our experiments that taurine achieves its effects in the thalamus purely via an increase in ambient GABA concentrations, because a combination of SNAP-5114, a GAT-2 and GAT-3 inhibitor, and NO 711, a GAT-1 inhibitor, could mimic, but not occlude, the effects of taurine in VB neurons. These observations argue against an indirect action of taurine on GABAA-Rs. Consistent with our conclusion that taurine acts directly on extrasynaptic GABAA-Rs, it is known that there are high levels of 3H-taurine binding in the dentate gyrus, cerebellum, and thalamus (Bureau and Olsen, 1991), and it is of interest that these brain regions also express high levels of the GABAA-R δ subunit (Pirker et al., 2000; Farrant and Nusser, 2005).
Mammalian GABAA-Rs are pentameric structures consisting of distinct subunits, and different subunit compositions result in receptors with distinct pharmacological profiles (Sieghart and Sperk, 2002). We examined the taurine sensitivity of heterologously expressed GABAA-Rs configured to resemble the native extrasynaptic (α4β2δ) and synaptic (α1β2γ2s) receptors. We found that taurine was a more potent agonist at α4β2δ receptors expressed in HEK293 cells than at α1β2γ2 receptors. We also observed that taurine, like THIP (gaboxadol), has a high efficacy at α4β2δ GABAA-Rs relative to GABA itself, which is a partial agonist at these receptors.
These findings are in agreement with those of other studies in δ subunit-containing GABAA-Rs; for example, it has been shown that low concentrations of taurine activate currents in heterologously expressed α6βδ GABAA-Rs (Bianchi and Macdonald, 2003; Hadley and Amin, 2007). Together, all of these findings in recombinant GABAA-Rs suggest that taurine is selective for GABAA-Rs containing the δ subunit over those containing the γ subunit.
In previous work in the rat thalamus, taurine was reported to activate glycine receptors selectively in thalamic relay neurons (Ghavanini et al., 2005, 2006), whereas the present data suggest that there is a significant contribution of GABAA-Rs to the taurine response in the mouse thalamus (Fig. 2). One possible explanation for this difference in results is that Ghavanini and colleagues used young (P13–P15) rats, whereas we used older (P23–P50) mice. In young rats, there was no effect of bicuculline (50 μm) on the membrane potential or input resistance of thalamic relay neurons (Ghavanini et al., 2005), which suggests a low level of extrasynaptic GABAA-R expression in the thalamus of the immature rat. In fact, our data in younger mice (P8–P10) support the idea that there is a delayed development of thalamic extrasynaptic GABAA-Rs, because taurine (10–100 μm) evoked much smaller currents in neurons from young mice than those from older animals. A similar developmental increase of tonic inhibition has been reported in rat cerebellar granule cells (Brickley et al., 1996), which parallels the developmental time course of δ subunit expression.
Extrasynaptic GABAA-Rs play an important role in regulating excitability at the level of individual neurons and within neuronal networks (Semyanov et al., 2004). Additionally, an increasing body of evidence from diverse studies suggests that tonic inhibition mediated by extrasynaptic GABAA-Rs can play a role in the regulation of seizure susceptibility (Brooks-Kayal et al., 1998; Dibbens et al., 2004; Peng et al., 2004; Maguire et al., 2005). Extrasynaptic GABAA-Rs have been proposed as an important novel target for therapeutic development (Richerson, 2004; Semyanov et al., 2004). It has been known for some time that taurine has antiseizure activity against tonic-clonic seizures (Gupta et al., 2005). Exogenously administered taurine may also have a beneficial effect during withdrawal from chronic ingestion of alcohol (Gupta et al., 2005), a hyperexcitable state that is also accompanied by changes in the expression of the GABAA-R δ subunit (Cagetti et al., 2003; Liang et al., 2006). GABAA-Rs containing the δ subunit are reported to be sensitive to low concentrations of ethanol (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003; Wei et al., 2004; Hanchar et al., 2005; Glykys et al., 2007). In light of the present data, it seems likely that taurine might exert antiseizure effects, at least in part, by virtue of its agonist activity at extrasynaptic GABAA-Rs containing the δ subunit, resulting in an increase in conductance that reduces network excitability. However, one should keep in mind that the etiology of absence seizures is quite different from that of generalized seizures, and that under the right conditions, hyperpolarization of thalamic relay neurons by taurine can potentially promote burst firing, as reported for gaboxadol (Cope et al., 2005), and might therefore actually predispose the brain to the generation of absence seizures. Clearly, the overall contribution of taurine to epileptogenesis remains to be elucidated.
In conclusion, we demonstrate that low concentrations of taurine can activate extrasynaptic GABAA-Rs and reduce the excitability of thalamic relay neurons in vitro. In the brain, taurine has been reported to be released in response to hypo-osmotic stimulation, energy deprivation, and membrane depolarization (Oja and Saransaari, 2000). Taurine can be released from glia (Holopainen et al., 1989; Philibert et al., 1989; Koyama et al., 1994; Bres et al., 2000) and neurons (Holopainen et al., 1989; Schousboe and Pasantes-Morales, 1989; Van Vliet et al., 1989), and the release mechanisms appear to involve volume-sensitive anion channels (Hussy et al., 2001) and taurine transporters (Saransaari and Oja, 1999). Several lines of evidence indicate that extrasynaptic GABAA-Rs are the principal site of action for taurine in the thalamus. The total taurine concentrations measured in brain homogenates range from 6 to 20 mm (Palkovits et al., 1986), whereas the levels of free extracellular taurine, as measured by microdialysis in vivo, range from ∼1 to 10 μm in the resting state to 120 μm after depolarization or other stimuli (Lerma et al., 1986; Albrecht and Schousboe, 2005). Given these observations, it seems entirely plausible that taurine acts as an endogenous ligand at extrasynaptic GABAA-Rs, because we observed significant inhibitory responses within this physiologically relevant concentration range (10–100 μm) (Fig. 2), and the results of our experiments using the taurine transporter inhibitor, GES, seem to support this idea. The activation of extrasynaptic GABAA-Rs by taurine may therefore have important physiological and pathophysiological effects: to modulate thalamic network activity under a variety of physiological conditions, and perhaps also to protect neurons from toxicity under pathological conditions (Louzada et al., 2004).
Footnotes
This work was supported by National Institutes of Health Grants AA 16393 (N.L.H.), AA 13004 and GM 47818 (G.E.H.), and GM 066840 (P.A.G.). We thank Carolyn Ferguson for expert assistance. We also thank Felix Wolf [Research Animal Resource Center (RARC), Weill Medical College, Cornell University, New York, NY] and the RARC staff for their assistance.
References
- Albrecht J, Schousboe A. Taurine interaction with neurotransmitter receptors in the CNS: an update. Neurochem Res. 2005;30:1615–1621. doi: 10.1007/s11064-005-8986-6. [DOI] [PubMed] [Google Scholar]
- Belelli D, Peden DR, Rosahl TW, Wafford KA, Lambert JJ. Extrasynaptic GABAA receptors of thalamocortical neurons: a molecular target for hypnotics. J Neurosci. 2005;25:11513–11520. doi: 10.1523/JNEUROSCI.2679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABAA receptor channels from low- to high-efficacy gating patterns. J Neurosci. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden LA. GABA transporter heterogeneity: pharmacology and cellular localization. Neurochem Int. 1996;29:335–356. doi: 10.1016/0197-0186(95)00158-1. [DOI] [PubMed] [Google Scholar]
- Borden LA, Dhar TG, Smith KE, Branchek TA, Gluchowski C, Weinshank RL. Cloning of the human homologue of the GABA transporter GAT-3 and identification of a novel inhibitor with selectivity for this site. Receptors Channels. 1994a;2:207–213. [PubMed] [Google Scholar]
- Borden LA, Murali Dhar TG, Smith KE, Weinshank RL, Branchek TA, Gluchowski C. Tiagabine, SK&F 89976-A, CI-966, and NNC-711 are selective for the cloned GABA transporter GAT-1. Eur J Pharmacol. 1994b;269:219–224. doi: 10.1016/0922-4106(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Bres V, Hurbin A, Duvoid A, Orcel H, Moos FC, Rabie A, Hussy N. Pharmacological characterization of volume-sensitive, taurine permeable anion channels in rat supraoptic glial cells. Br J Pharmacol. 2000;130:1976–1982. doi: 10.1038/sj.bjp.0703492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol (Lond) 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Bureau MH, Olsen RW. Taurine acts on a subclass of GABAA receptors in mammalian brain in vitro. Eur J Pharmacol. 1991;207:9–16. doi: 10.1016/s0922-4106(05)80031-8. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, Homanics GE. GABAA receptor α4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci USA. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DR, Watkins JC. The excitation and depression of spinal neurones by structurally related amino acids. J Neurochem. 1960;6:117–141. doi: 10.1111/j.1471-4159.1960.tb13458.x. [DOI] [PubMed] [Google Scholar]
- del Olmo N, Bustamante J, del Rio RM, Solis JM. Taurine activates GABAA but not GABAB receptors in rat hippocampal CA1 area. Brain Res. 2000;864:298–307. doi: 10.1016/s0006-8993(00)02211-3. [DOI] [PubMed] [Google Scholar]
- Dibbens LM, Feng HJ, Richards MC, Harkin LA, Hodgson BL, Scott D, Jenkins M, Petrou S, Sutherland GR, Scheffer IE, Berkovic SF, Macdonald RL, Mulley JC. GABRD encoding a protein for extra- or peri-synaptic GABAA receptors is a susceptibility locus for generalized epilepsies. Hum Mol Genet. 2004;13:1315–1319. doi: 10.1093/hmg/ddh146. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Verdorn TA, Ewert M, Seeburg PH, Sakmann B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+ Neuron. 1990;5:781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Ghavanini AA, Mathers DA, Puil E. Glycinergic inhibition in thalamus revealed by synaptic receptor blockade. Neuropharmacology. 2005;49:338–349. doi: 10.1016/j.neuropharm.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Ghavanini AA, Mathers DA, Kim HS, Puil E. Distinctive glycinergic currents with fast and slow kinetics in thalamus. J Neurophysiol. 2006;95:3438–3448. doi: 10.1152/jn.01218.2005. [DOI] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Win T, Bittner S. Taurine analogues; a new class of therapeutics: retrospect and prospects. Curr Med Chem. 2005;12:2021–2039. doi: 10.2174/0929867054546582. [DOI] [PubMed] [Google Scholar]
- Hadley SH, Amin J. Rat α6β2δ GABAA receptors exhibit two distinct and separable agonist affinities. J Physiol (Lond) 2007;581:1001–1018. doi: 10.1113/jphysiol.2007.132886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABAA receptor activity. Nat Neurosci. 2005;8:339–345. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holopainen I, Kontro P, Oja SS. Release of taurine from cultured cerebellar granule cells and astrocytes: co-release with glutamate. Neuroscience. 1989;29:425–432. doi: 10.1016/0306-4522(89)90069-9. [DOI] [PubMed] [Google Scholar]
- Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- Huntsman MM, Huguenard JR. Nucleus-specific differences in GABAA-receptor-mediated inhibition are enhanced during thalamic development. J Neurophysiol. 2000;83:350–358. doi: 10.1152/jn.2000.83.1.350. [DOI] [PubMed] [Google Scholar]
- Hussy N, Deleuze C, Pantaloni A, Desarmenien MG, Moos F. Agonist action of taurine on glycine receptors in rat supraoptic magnocellular neurones: possible role in osmoregulation. J Physiol (Lond) 1997;502:609–621. doi: 10.1111/j.1469-7793.1997.609bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussy N, Bres V, Rochette M, Duvoid A, Alonso G, Dayanithi G, Moos FC. Osmoregulation of vasopressin secretion via activation of neurohypophysial nerve terminals glycine receptors by glial taurine. J Neurosci. 2001;21:7110–7116. doi: 10.1523/JNEUROSCI.21-18-07110.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- Jahnsen H, Llinãs R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol (Lond) 1984;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Krnjevic K, Wang F, Ye JH. Taurine activates strychnine-sensitive glycine receptors in neurons freshly isolated from nucleus accumbens of young rats. J Neurophysiol. 2004;91:248–257. doi: 10.1152/jn.00106.2003. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Ishibashi T, Tanaka K, Baba A. l-Glutamate-stimulated taurine release from rat cerebral cultured astrocytes. J Neurosci Res. 1994;38:75–80. doi: 10.1002/jnr.490380110. [DOI] [PubMed] [Google Scholar]
- Lerma J, Herranz AS, Herreras O, Abraira V, Martin del Rio R. In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res. 1986;384:145–155. doi: 10.1016/0006-8993(86)91230-8. [DOI] [PubMed] [Google Scholar]
- Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QR, Lopez-Corcuera B, Mandiyan S, Nelson H, Nelson N. Molecular characterization of four pharmacologically distinct γ-aminobutyric acid transporters in mouse brain. J Biol Chem. 1993;268:2106–2112. [PubMed] [Google Scholar]
- Louzada PR, Lima AC, Mendonca-Silva DL, Noel F, De Mello FG, Ferreira ST. Taurine prevents the neurotoxicity of beta-amyloid and glutamate receptor agonists: activation of GABA receptors and possible implications for Alzheimer's disease and other neurological disorders. FASEB J. 2004;18:511–518. doi: 10.1096/fj.03-0739com. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- McCool BA, Botting SK. Characterization of strychnine-sensitive glycine receptors in acutely isolated adult rat basolateral amygdala neurons. Brain Res. 2000;859:341–351. doi: 10.1016/s0006-8993(00)02026-6. [DOI] [PubMed] [Google Scholar]
- Neuringer M, Sturman J. Visual acuity loss in rhesus monkey infants fed a taurine-free human infant formula. J Neurosci Res. 1987;18:597–601. doi: 10.1002/jnr.490180413. [DOI] [PubMed] [Google Scholar]
- Neuringer M, Imaki H, Sturman JA, Moretz R, Wisniewski HM. Abnormal visual acuity and retinal morphology in rhesus monkeys fed a taurine-free diet during the first three postnatal months. Adv Exp Med Biol. 1987;217:125–134. doi: 10.1007/978-1-4899-0405-8_12. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Oja SS, Saransaari P. Modulation of taurine release by glutamate receptors and nitric oxide. Prog Neurobiol. 2000;62:407–425. doi: 10.1016/s0301-0082(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Okada M, Onodera K, Van Renterghem C, Sieghart W, Takahashi T. Functional correlation of GABAA receptor α subunits expression with the properties of IPSCs in the developing thalamus. J Neurosci. 2000;20:2202–2208. doi: 10.1523/JNEUROSCI.20-06-02202.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovits M, Elekes I, Lang T, Patthy A. Taurine levels in discrete brain nuclei of rats. J Neurochem. 1986;47:1333–1335. doi: 10.1111/j.1471-4159.1986.tb00761.x. [DOI] [PubMed] [Google Scholar]
- Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the δ subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibert RA, Rogers KL, Dutton GR. K+-evoked taurine efflux from cerebellar astrocytes: on the roles of Ca2+ and Na+ Neurochem Res. 1989;14:43–48. doi: 10.1007/BF00969756. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Porcello DM, Huntsman MM, Mihalek RM, Homanics GE, Huguenard JR. Intact synaptic GABAergic inhibition and altered neurosteroid modulation of thalamic relay neurons in mice lacking δ subunit. J Neurophysiol. 2003;89:1378–1386. doi: 10.1152/jn.00899.2002. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Looking for GABA in all the wrong places: the relevance of extrasynaptic GABAA receptors to epilepsy. Epilepsy Curr. 2004;4:239–242. doi: 10.1111/j.1535-7597.2004.46008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saransaari P, Oja SS. Characteristics of ischemia-induced taurine release in the developing mouse hippocampus. Neuroscience. 1999;94:949–954. doi: 10.1016/s0306-4522(99)00384-x. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Pasantes-Morales H. Potassium-stimulated release of [3H]taurine from cultured GABAergic and glutamatergic neurons. J Neurochem. 1989;53:1309–1315. doi: 10.1111/j.1471-4159.1989.tb07429.x. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Smart TG, Moss SJ, Xie X, Huganir RL. GABAA receptors are differentially sensitive to zinc: dependence on subunit composition. Br J Pharmacol. 1991;103:1837–1839. doi: 10.1111/j.1476-5381.1991.tb12337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storustovu SI, Ebert B. Pharmacological characterization of agonists at δ-containing GABAA receptors: functional selectivity for extrasynaptic receptors is dependent on the absence of γ2. J Pharmacol Exp Ther. 2006;316:1351–1359. doi: 10.1124/jpet.105.092403. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated a4b2d GABAA receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanaga H, Ohtsuki S, Hosoya K, Terasaki T. GAT2/BGT-1 as a system responsible for the transport of γ-aminobutyric acid at the mouse blood-brain barrier. J Cereb Blood Flow Metab. 2001;21:1232–1239. doi: 10.1097/00004647-200110000-00012. [DOI] [PubMed] [Google Scholar]
- Van Vliet BJ, Sebben M, Dumuis A, Gabrion J, Bockaert J, Pin JP. Endogenous amino acid release from cultured cerebellar neuronal cells: effect of tetanus toxin on glutamate release. J Neurochem. 1989;52:1229–1239. doi: 10.1111/j.1471-4159.1989.tb01870.x. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances a4b3d and a6b3d γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by δ subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZY, Xu TL. Taurine-evoked chloride current and its potentiation by intracellular Ca2+ in immature rat hippocampal CA1 neurons. Amino Acids. 2003;24:155–161. doi: 10.1007/s00726-002-0314-8. [DOI] [PubMed] [Google Scholar]
- Xu H, Zhou KQ, Huang YN, Chen L, Xu TL. Taurine activates strychnine-sensitive glycine receptors in neurons of the rat inferior colliculus. Brain Res. 2004;1021:232–240. doi: 10.1016/j.brainres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Huguenard JR, Prince DA. GABAA receptor-mediated Cl− currents in rat thalamic reticular and relay neurons. J Neurophysiol. 1997;78:2280–2286. doi: 10.1152/jn.1997.78.5.2280. [DOI] [PubMed] [Google Scholar]