Abstract
Axons and dendrites of developing neurons establish distributed innervation patterns enabling precise discrimination in sensory systems. We describe the role of the extracellular matrix molecule, laminin β2, interacting with the CaV2.2 calcium channel in establishing appropriate sensory innervation. In vivo, CaV2.2 is expressed on the growth cones of Xenopus laevis sensory neurites and laminin β2 is expressed in the skin. Culturing neurons on a laminin β2 substrate inhibits neurite outgrowth in a specific and calcium-dependent manner. Blocking signaling between laminin β2 and CaV2.2 leads to increased numbers of sensory terminals in vivo. These findings suggest that interactions between extracellular matrix molecules and calcium channels regulate connectivity in the developing nervous system.
Keywords: axon guidance, calcium channel, extracellular matrix, skin, growth cone, Xenopus
Introduction
During neuronal development, axonal and dendritic processes must distribute appropriately within target tissues. Neurite arbors efficiently innervate sensory surfaces while maintaining discrete spatial resolution in systems such as the mammalian retina (Wassle et al., 1981), the body wall of the leech and Drosophila (Grueber et al., 2003; Baker and Macagno, 2007), and zebrafish and Xenopus skin (Kitson and Roberts, 1983; Sagasti et al., 2005). In some cases, a tiled distribution is maintained by contact mediated repulsion (Emoto et al., 2004; Sagasti et al., 2005; Baker and Macagno, 2007; Hughes et al., 2007), whereas in other cases separate or additional mechanisms are involved (Grueber et al., 2003; Gallegos and Bargmann, 2004; Lin et al., 2004; Sagasti et al., 2005). These may include intrinsic limitation of neurite growth or instruction of neurite distribution by preexistent tiled patterns of extracellular matrix molecules within target tissue (Hari et al., 2004). Particularly, “stop signals” may direct termination or instruct neurites to avoid inappropriate regions.
Calcium signals play key roles in mediating growth cone extension, turning, collapse, and stopping (Spitzer, 2006). Elevations in intracellular calcium on growth cone contact with soluble guidance molecules or extracellular matrix molecules (Snow et al., 1994) lead to inhibition of growth or neurite avoidance. Calcium responses to guidance cues are mediated by a diverse repertoire of calcium channels within the growth cone that include transient receptor potential channels and voltage-gated channels (Lipscombe et al., 1988). We describe the expression and role of CaV2.2, a canonical voltage-gated calcium channel (Nowycky et al., 1985) in the growth cone of neurites innervating the skin. CaV2.2 is notable among voltage-gated calcium channels in that it is also mechanosensitive (Calabrese et al., 2002) and binds directly to the extracellular matrix molecule laminin β2 (Nishimune et al., 2004).
Laminins are heterotrimeric glycoproteins composed of an α, β, and γ chain. Currently five α, three β, and three γ chains have been identified in the mouse and human genomes. The laminin β2 chain is localized to the synaptic but not extrasynaptic regions of the basal laminas of the neuromuscular junctions (Patton et al., 1997), as well as the retina (Hunter et al., 1992a) and subplate and floorplate of the developing CNS (Hunter et al., 1992b). The laminin β2 chain is needed for proper synapse assembly (Noakes et al., 1995a) and organizes presynaptic nerve terminals through direct interactions with CaV2.1 and CaV2.2 (Nishimune et al., 2004). Furthermore, at least in vitro, laminin β2 acts as a stop signal for outgrowing motor axons (Porter et al., 1995).
We have tested whether the laminin β2 interactions with CaV2.2 are responsible for the laminin β2 stop signal. We show here that interactions between laminin β2 and CaV2.2 lead to inhibition of neurite outgrowth in vitro. This inhibition is accompanied by calcium transients in stalled growth cones. We describe a tiled expression pattern of laminin β2 in the developing skin and demonstrate that blocking the signal between CaV2.2 and laminin β2 in vivo leads to changes in sensory innervation of the skin.
Materials and Methods
Generation and staging of embryos.
Adult female Xenopus laevis were injected with human chorionic gonadotropin (Sigma, St. Louis, MO) and oocytes were fertilized in vitro. Embryos were staged according to Nieuwkoop and Faber (1967).
RT-PCR and in situ hybridization.
RNA was isolated from the dorsal region of stage 12–30 X. laevis embryos using RNAqueous-4-PCR (Ambion, Austin, TX). cDNA was generated using random hexamer primers and superscript II enzyme (Invitrogen, Carlsbad, CA). CaV2.2 primers were designed from X. tropicalis genomic sequence by aligning it with CaV2.2 sequence from other species to determine introns and exons. Sequence accession number and forward and reverse primers for PCR were as follows: CaV2.2 (JGI TKS329692.x1), 5′GAATTCTTCACTATGACAACGTACTGTGGGCGTTGC, and 5′GGATCCAAGGCTACACTCCGACATGACCTTATCTC, respectively. The reverse transcription (RT)-PCR product was cloned into pBluescript and sequenced (University of California, San Diego Center for AIDS Research). Antisense probes for in situ hybridization were generated from the PCR product of CaV2.2 using the digoxigenin RNA Labeling kit (Boehringer Mannheim, Indianapolis, IN). Sense probes were used as controls. In situ hybridization was performed as described previously (Harland, 1991) and modified (Burger and Ribera, 1996).
Culture.
Plastic culture dishes (Corning, Corning, NY) with 200 μl volume reduction rings or 10 μl microwells (Nunc, Naperville, IL) were incubated with 5 μg/ml laminin (Sigma) alone or with 200 μg/ml 20 kDa C terminus of laminin β2 leucine-arginine-glutamate (LRE) fragment solublized by linkage to maltose binding protein or a mutated version [(LRE→QRE (glutamine-arginine-glutamate)] (Nishimune et al., 2004) in PBS for 2 h. After rinsing with filtered PBS, dishes were blocked for 2 h with 30 mg/ml heat inactivated, filtered BSA. Dishes were rinsed at least five times with PBS and two times with modified Ringer's solution [MR; containing (in mm) 116 NaCl, 0.67 KCl, 1.31 MgSO4, 2 CaCl2, 4.6 Tris-base, pH adjusted to 7.8 with HCl]. For loop peptide experiments, cultures were incubated with 20 μm peptide corresponding to rat CaV2.1 or CaV1.2 11th extracellular loop (Nishimune et al., 2004) for 1 h before addition of cells. Stage 15–17 neural plates were dissected in MR containing 1 mg/ml collagenase-B (Boehringer Mannheim) and then dissociated in calcium-free medium for 1 h. Dissociated cells were cultured in MR for 14–18 h, fixed, and stained for β-tubulin as described below. For experiments with conotoxin, 5 μm ω-conotoxin-GVIA (Sigma) was added 1–2 h after plating. Tubulin-positive cells were scored for presence of a neurite longer than one cell body in diameter. There was no difference in percentage of neurons extending processes between 200 and 10 μl cultures (supplemental Fig. 1, available at www.jneurosci.org as supplemental material).
Imaging.
Culture dishes were coated with the laminin β2 LRE or QRE fragment. Interleaved bands of native and denatured laminin β2 were generated by placing three electron-microscopic section multislot support grids (RB-90-Ni; Electron Microscopy Sciences, Hatfield, PA) in each dish and exposing dishes to UV illumination (12–15 cm from an 8 W bulb) for 2 h (Porter et al., 1995). This procedure yields alternating 184-μm-wide bands of native and 90-μm-wide bands of denatured laminin; the boundary is clearly visualized with illumination at 442 nm. Neurons from stage 17–20 embryos plated across both native and denatured stripes rapidly extended neurites. Cells were loaded with Fluo-4 AM and imaged as growth cones approached the boundary between denatured and native laminin β2 fragments from the denatured side. Images were captured at 2 Hz on a Leica (Nussloch, Germany) SP5 confocal microscope and analyzed with Image J. An image of the grid taken at the beginning of acquisition was superimposed onto the supplemental Fluo-4 movie (available at www.jneurosci.org as supplemental material). Comparison with the grid at the end of acquisition confirmed that the dish had not moved.
Immunocytochemistry.
Whole or dissected Xenopus embryos were fixed with 4% paraformaldehyde (PFA), 0.1% glutaraldehyde in PBS for 30 min at 4°C. For open book dissections and HNK-1 antibody whole-embryo staining, embryos were incubated in 0.5% Triton X-100 PBS for 1–5 d before dissection or staining. Embryos were then dissected by making a ventral incision and removing the gut, notochord and myotomes to reveal the ventral surface of the neural tube and the internal surface of the skin. Cultured neurons were fixed in 4% PFA, 0.1% glutaraldehyde for 5 min. All preparations were blocked with 2% bovine serum albumin in 0.1% Triton X-100 in PBS before staining overnight at 4°C at the following concentrations: rabbit anti-CaV2.2 (Alomone, Jerusalem, Israel), 1:200; mouse IgG anti β-tubulin (Sigma), 1:500; mouse IgM anti-HNK-1 (Sigma), 1:100; mouse IgG anti-laminin β2 C4 (Hunter et al., 1989), 1:500; mouse IgM anti-laminin β2 D79, 1:10. HNK-1 whole-embryo staining was incubated at 4°C for 1–5 d. Alexa-Fluor secondary antibodies (Invitrogen) were used at 1:300 for 1–2 h at room temperature. Dissected embryos were mounted in 80% glycerol and whole-mount HNK-1-stained embryos were dehydrated in methanol and mounted in 2:1 benzyl benzoate:benzyl alcohol for clearing. Other preparations were visualized in PBS. Images of dissected embryos were acquired on a Bio-Rad (Hercules, CA) MRC-1024 confocal attachment on an Olympus (Tokyo, Japan) BX-50WI microscope. Whole-mount embryos and cultured cells were imaged with a Zeiss (Oberkochen, Germany) Axiocam MRm camera on an Axioskop 2 microscope using a 20× Achroplan water-immersion objective (whole mount and some cultures) or a Plan 10× objective (microwell cultures). Z-stacks of whole-mount HNK-1-stained embryos were deconvolved using the nearest neighbor algorithm on AxioVision.
Bead implants.
One to two hundred mesh Affigel Blue beads (Bio-Rad) were soaked overnight in 10 μm ω-conotoxin GVIA (Sigma) in bead buffer [20 nm Na citrate, 150 mm NaCl, 1 mm Mg(OAc)2, 20% glycerol, 0.02% CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonate]) or 1 mg/ml calcium channel loop peptides in PBS. Beads were implanted in stage 16–18 embryos. Embryos were fixed and stained at stages 34–36.
Analysis.
Cultured neurons stained with tubulin were scored for presence of a neurite longer than the diameter of the cell body. Length was measured using AxioVision software. Statistical analyses were ANOVAs followed by Dunnett's multiple comparison test against the laminin 111 control. In vivo images are through-series projections of the z-stacks of images that were used to capture the skin of the curved embryo. Quantification was done by examining slices of the z-stacks. A nerve terminal cluster was defined by the presence of two or more HNK-1-stained puncta in the plane of the skin and within three diameters of each other. Objectivity was assured because quantification was done blind to experimental condition. Analyses were ANOVAs followed by Tukey's multiple comparison test between all pairs of conditions. All tests were two sided. Descriptive statistics are reported as mean ± SEM.
Results
Expression and localization of CaV2.2
Calcium signaling is important for many aspects of early neuronal development, including neuronal proliferation and migration, specification of neurotransmitter phenotype, and axon guidance (Spitzer, 2006). These observations prompted us to ask what other neuronal phenotypes are regulated by early forms of calcium-dependent activity. We therefore screened candidate genes encoding calcium-permeable channels and receptors that could contribute to early excitability because of their expression patterns at early stages of development. From this screen we found that the Xenopus homolog of CaV2.2 was detected by RT-PCR beginning at the time of neural plate formation (stage 13) until the time of synapse formation (stage 30) (Fig. 1A). CaV2.2 mRNA continued to be detected until the swimming tadpole stage (stage 48) (data not shown). In situ hybridization with a 272 bp fragment of CaV2.2 revealed expression in the spinal cord and hindbrain beginning at the time of neural tube closure (Fig. 1B), leading us to pursue further investigation of this channel.
Figure 1.
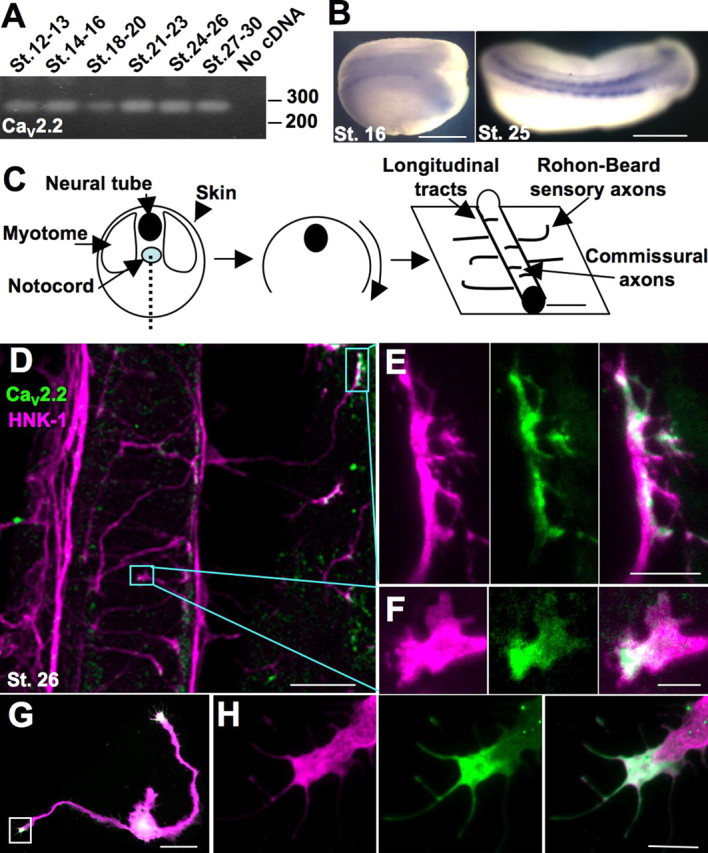
CaV2.2 is expressed in the developing Xenopus neural tube and is localized to the growth cone during axon outgrowth in vivo and in vitro. A, RT-PCR of a 272 base pair fragment of CaV2.2 using mRNA collected from dissections of dorsal halves of embryos from stages (St.) 12–30. B, In situ hybridization using the probe made from the same fragment reveals expression of CaV2.2 in the developing neural tube. Scale bar, 500 μm. C, Schematized dissection for immunostaining: a ventral incision was made to remove the myotomes and notochord and reveal the neural tube and skin. Staining with the neuronal marker HNK-1 reveals longitudinal tracts running anteroposteriorly, axons of sensory Rohon-Beard neurons innervating the skin, and commissural interneurons crossing the ventral midline. D, Ventral view of a spinal cord and skin of a dissected embryo stained for HNK-1 (purple) and CaV2.2 (green). Boxes outline the CaV2.2-positive growth cones pictured in E and F. Scale bar, 50 μm. E, Sensory Rohon-Beard growth cone expressing CaV2.2 (green). Scale bar, 10 μm. F, Commissural interneuron expressing CaV2.2 (green). Scale bar, 5 μm. G, Cultured neuron stained for β-tubulin (purple) and CaV2.2 (green) expressing CaV2.2 in the growth cone. Scale bar, 50 μm. H, Growth cone boxed in G. Scale bar, 10 μm.
To localize CaV2.2 we immunostained whole mount preparations (Fig. 1C). Tailbud (stage 26) Xenopus embryos were stained for the neuronal marker HNK-1, revealing longitudinal tracts running anteroposteriorly, commissural axons crossing the ventral midline, and sensory Rohon-Beard axons traversing the skin. Costaining with an antibody to CaV2.2 revealed expression in growth cones of extending commissural and Rohon-Beard sensory axons (Fig. 1D–F). Expression was also seen in the growth cones of the longitudinal tracts and growth cones initially emerging from the spinal cord (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Similarly, CaV2.2 is expressed in over 90% of growth cones of spinal neurons grown in vitro, specifically along the distal margin of the lamellipodia and in the filopodia (Fig. 1G,H). Expression of CaV2.2 in the growth cone suggested that it may participate in developmental growth decisions such as initiation, outgrowth, guidance, and stopping.
Expression and localization of laminin β2
Peripheral processes of sensory neurons in Xenopus and other organisms innervate the skin in a distributed, tiled manner (Kitson and Roberts, 1983; Emoto et al., 2004; Gallegos and Bargmann, 2004). In some cases, tiled patterns may be specified from external guidance cues, such as stop signals that limit neurite growth. Laminin β2 has been shown to act as a stop signal for neurites of developing motor neurons (Porter et al., 1995). Because laminin β2 interacts directly with CaV2.2 (Nishimune et al., 2004), we investigated its expression in Xenopus skin.
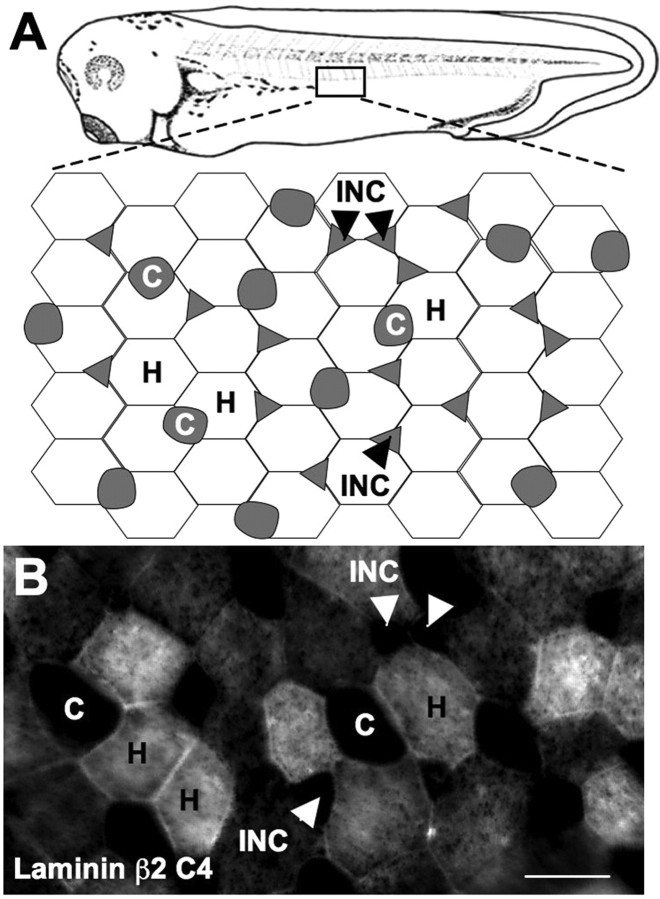
Xenopus skin is composed of two layers. The inner sensorial layer gives rise to precursor cells that intercalate into the superficial layer and differentiate into ciliated cells and intercalating nonciliated cells also termed conical cells. The superficial layer contains hexagonal mucus-secreting epidermal cells as well as the ciliated cells and intercalating nonciliated cells (Fig. 2A) (Somasekhar and Nordlander, 1997; Stubbs et al., 2006). Immunostaining 2-d-old larvae (stage 35) with monoclonal antibodies to laminin β2 revealed association with specific cell types. Regions defined by the hexagonal cells express laminin β2, whereas regions around ciliated cells and intercalating nonciliated (or conical) cells do not express laminin β2. Similar results were obtained with two monoclonal antibodies, C4 (Fig. 2B) and D79 (data not shown), which recognize distinct epitopes of laminin β2.
Figure 2.
Laminin β2 is expressed in a tiled pattern in the skin. A, Schematic of Xenopus skin. B, Whole-embryo staining with the C4 antibody to laminin β2 revealed expression coextensive with hexagonal epithelial (H) cells but not ciliated (C) or intercalating nonciliated cells (INC) in stage 34 larvae. Scale bar, 25 μm.
CaV2.2 interaction with laminin β2 in culture
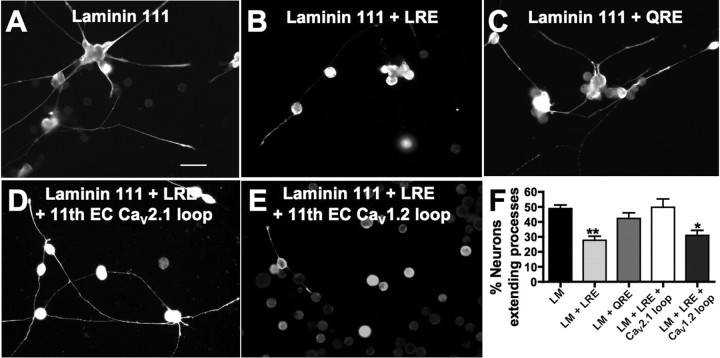
Because CaV2.2 and laminin β2 bind directly to each other (Nishimune et al., 2004) and are expressed where they may interact in vivo, we sought to determine whether this interaction affects neurite outgrowth. The eleventh extracellular loop of CaV2.2 binds an LRE motif on laminin β2 (Nishimune et al., 2004). We grew Xenopus neurons in vitro on a substrate of laminin-111 that does not have a β2 chain, on laminin-111 plus a solublized 20 kDa C-terminal fragment of the laminin β2 chain containing the LRE, or on laminin-111 plus a point mutated (LRE→QRE) laminin β2 fragment. Neurons, identified by immunostaining for neuron-specific β-tubulin, extended significantly fewer processes on substrates containing the LRE fragment (28 ± 3%) compared with QRE (42 ± 4%) or nonlaminin β2 substrates (49 ± 2%; n ≥ 6 embryos per condition) (Fig. 3A–C,F). These results demonstrate an effect of laminin β2 on neurite extension of Xenopus spinal neurons.
Figure 3.
Laminin β2 specifically inhibits spinal neurite outgrowth. Xenopus neurons from stage 17 embryos were cultured on the following substrates and analyzed at 14–18 h in vitro: A, laminin (LM) with a β1 but not a β2 chain; B, a solublized 20 kDa LRE containing C-terminal fragment of laminin β2; C, a point-mutated (LRE→QRE) laminin β2 fragment. Neurons identified by immunostaining for neuron-specific β-tubulin extended significantly fewer processes on substrates containing the LRE fragment compared with QRE or nonlaminin β2 substrates. Scale bar, 50 μm. D, Incubating neurons cultured on laminin plus the β2 LRE fragment with a competing peptide corresponding to the 11th extracellular LRE-binding loop of rat CaV2.1 (91% homologous to Xenopus CaV2.2) rescued neurite extension. E, Incubation with a control peptide corresponding to the 11th extracellular loop of rat CaV1.2 (61% homologous to Xenopus CaV2.2) did not prevent inhibition by laminin β2 LRE. F, Laminin β2 LRE significantly reduces the number of neurons extending processes in vitro. This effect is rescued by incubation with the competing CaV2.1 loop peptide (**p < 0.01, *p < 0.05, comparison to LM control; n ≥ 300 neurons from ≥6 cultures). Error bars indicate SEM.
To test whether the inhibition of neurite outgrowth is specific to interactions between laminin β2 and CaV2.2, we incubated neuronal cultures grown on laminin-111 plus the laminin β2 LRE fragment with a peptide corresponding to the eleventh extracellular loop of rat CaV2.1. This peptide binds the LRE motif of laminin β2 (Nishimune et al., 2004) and shares 91% homology with Xenopus CaV2.2. As a control, we incubated cultures with the 11th extracellular loop of rat CaV1.2 that shares only 61% homology with Xenopus CaV2.2 (supplemental Fig. 3, available at www.jneurosci.org as supplemental material) and does not bind to laminin β2. The peptide corresponding to the 11th extracellular loop of CaV2.1 effectively rescued the inhibition by the laminin β2 LRE fragment with 50 ± 6% neurons extending processes (Fig. 3D,F). The control peptide did not rescue the inhibition by laminin β2 LRE (31 ± 3%) (Fig. 3E,F). Thus, the laminin β2 LRE inhibition is specific. Laminin β2 LRE has no effect on survival of neurons in culture, as indicated by the lack of significant differences in number of neurons per culture across conditions (data not shown).
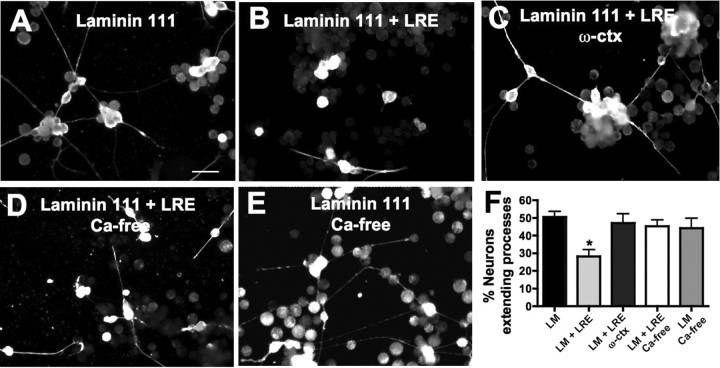
To test whether laminin β2 inhibition of spinal neurite outgrowth is calcium-dependent, we incubated cultures grown on laminin β2 LRE in calcium-free medium or with 5 μm ω-conotoxin GVIA in standard 2 mm calcium medium. Both conditions relieve inhibition by laminin β2 LRE (45 ± 4% and 47 ± 5%, respectively) (Fig. 4) indicating that the stop signal relies on calcium influx through CaV2.2. Incubating cultures grown on laminin-111 alone in calcium-free medium does not affect the number of neurons extending processes (44 ± 6%), indicating a specific recovery of inhibition in the absence of calcium rather than a nonspecific effect of growing cultures in calcium-free medium. In addition to demonstrating the calcium dependence of laminin β2 inhibition, these results reinforce the role of CaV2.2 in laminin β2 inhibition of neurite outgrowth, because ω-conotoxin GVIA blocks CaV2.2 (Takahashi and Momiyama, 1993).
Figure 4.
Laminin (LM) β2 inhibition is calcium dependent. Stage 16 dissociated neural tubes were cultured and neurons identified by staining for β-tubulin. A, B, Neurite outgrowth on LM is inhibited by addition of the laminin β2 fragment (LRE). Scale bar, 50 μm. C, D, Incubating neurons cultured on LM plus the β2 LRE fragment in 5 μm ω-conotoxin or in calcium-free medium prevents inhibition of neurite outgrowth indicating calcium dependence of LRE inhibition. E, Incubating neurons grown on LM alone in calcium-free medium does not lead to increases in neurite extension. F, Neurite extension on LRE is rescued by preventing calcium influx through CaV2.2 (n ≥ 250 neurons from ≥7 embryos; *p < 0.05). Error bars indicate SEM.
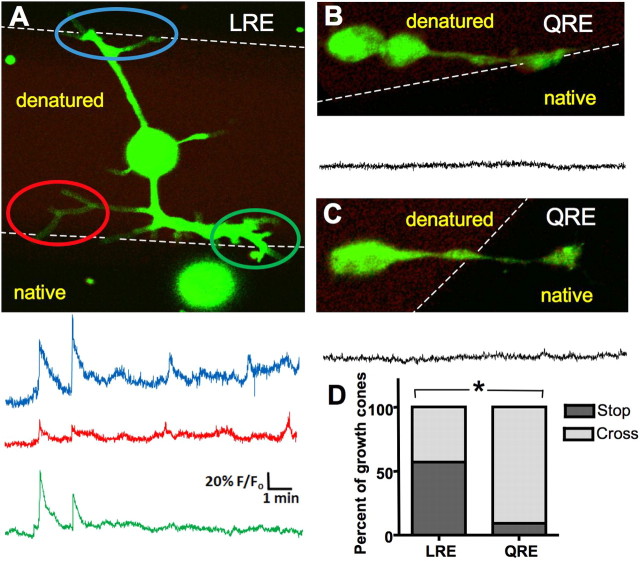
Whereas the laminin β2 LRE fragment inhibits the number of neurons that initiate processes, those neurites that have extended a process on laminin β2 are similar in length to control (supplemental Fig. 4, available at www.jneurosci.org as supplemental material) (Porter et al., 1995). We then determined whether neurites initially grown on a control substrate stall or stop when their growth cones encounter laminin β2. We generated 184-μm-wide stripes of native and 90-μm-wide stripes of UV-denatured laminin β2 LRE or QRE (Porter et al., 1995), plated neurons on them, and analyzed the behavior of growth cones migrating from denatured onto native laminin β2. We imaged intracellular calcium with Fluo-4 AM to assess calcium dynamics as growth cones palpate the border. Growth cones stalled at the LRE border generated calcium transients 10–70% above baseline fluorescence that were 1 s to 2 min in duration at half maximal amplitude and correlated with the contact of the border by one or more filopodia (Fig. 5A) (n = 9 of 9 growth cones at the border). These transients were observed at a significantly lower frequency at the QRE border (Fig. 5B) (n = 2 of 6 growth cones at the border; p < 0.05, Fisher's exact test). The calcium transients associated with LRE contact are distinct from the other classes of calcium transients described for these growth cones (Gu and Spitzer, 1995; Gomez et al., 2001; Conklin et al., 2005). Fifty-seven percent of growth cones stopped or turned back at the LRE border, whereas only 9% of growth cones stopped or turned at the QRE border (Fig. 5D) (p < 0.0001; n >80 growth cones; Fisher's exact test).
Figure 5.
Neurons at a border of laminin β2 generate calcium transients and stall. A, Neurons were cultured on stripes of native and UV-denatured laminin β2 LRE fragment and internal calcium concentrations were imaged using Fluo-4. Images were captured at 2 Hz. Fluo-4 is in green, denatured laminin β2 is in red, and native laminin β2 is in black. Three images were averaged to enhance visualization of the neuron. Traces show the calcium activity in each of three growth cones as they encounter the border. Axis scales apply throughout the figure. B, C, Less calcium transient activity is seen when a neuron approaches (B) or has crossed onto (C) activated point mutated laminin β2 (LRE→QRE). D, Significantly more neurons stop or turn back at an LRE border than a QRE border (*p < 0.001).
Cav2.2–laminin β2 interactions in vivo
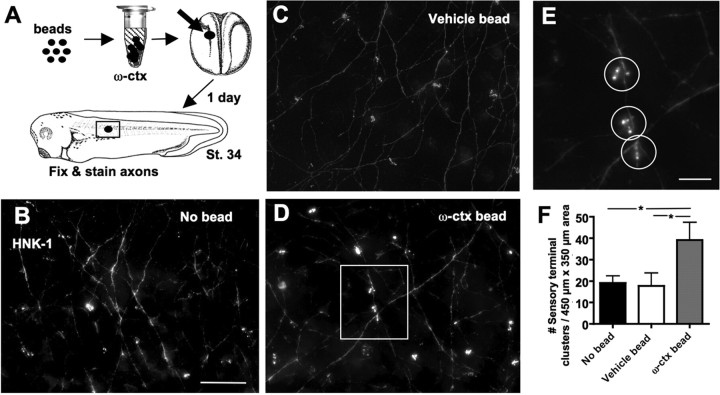
To examine the role that CaV2.2–laminin β2 interactions have in the skin in vivo, we first blocked signaling through CaV2.2 by implanting an agarose bead releasing ω-conotoxin GVIA into the developing embryo from the time of neural tube formation until a late larval stage (stages 16–35) when sensory terminals have been formed. This method is effective in delivering drugs that block early neuronal activity (Borodinsky et al., 2004). Using beads with fluorescently labeled ω-conotoxin, we found efficient diffusion at least 200 μm from the bead. Whole embryos were stained with HNK-1 to reveal spinal sensory neurites traversing the skin and sensory nerve terminal clusters innervating the skin. Rohon-Beard sensory axons initially run along the inner surface of the skin, continue for an undetermined length between the two layers of skin cells and form sensory varicosities in pits in the lateral or basal plasma membrane between superficial skin cells (Roberts and Hayes, 1977). The majority of these varicosities occur in clusters in an en passant manner, but some are located at nerve endings. The most heavily innervated skin cell type is the intercalating nonciliated cell (conical cell) (Somasekhar and Nordlander, 1997). Almost all nerve terminal clusters that we observe appear to form en passant as described previously by electron microscopy (Roberts and Hayes, 1977). Blocking signaling through CaV2.2 leads to a significant increase in numbers of sensory nerve terminal clusters in the skin (39 ± 8 clusters per 450 × 350 μm2 area) compared with embryos in which a vehicle bead (18 ± 6) or no bead (19 ± 3) was implanted (Fig. 6).
Figure 6.
Blocking signaling through CaV2.2 in vivo leads to increased innervation of the skin. A, Agarose beads soaked in 10 μm ω-conotoxin (ctx) were implanted in stage 16 embryos. After 1 d, stage 34 embryos were fixed and stained with anti-HNK-1 to reveal sensory processes and sensory nerve terminals innervating the skin. A 450 × 350 μm area centered around the bead was imaged and analyzed (box). B–D, Deconvolved and projected z-series through the skin of embryos in which no bead (B), a control bead (C), or an ω-conotoxin bead (D) were implanted. Scale bar, 50 μm. E, Enlarged image of the boxed region in D showing three sensory nerve terminal clusters, circles. Scale bar, 10 μm. F, Embryos exposed to ω-conotoxin exhibited significantly greater numbers of sensory nerve terminal clusters compared with embryos implanted with a vehicle bead or no bead (*p < 0.05). Error bars indicate SEM.
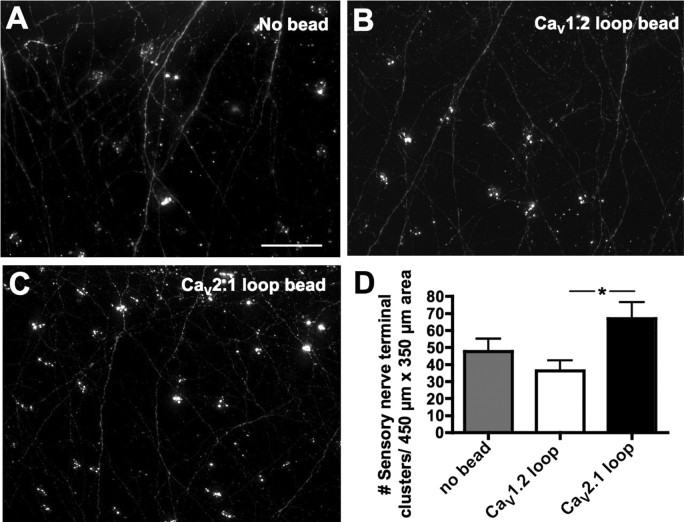
To ask whether the effect of CaV2.2 on sensory terminals was mediated by its interaction with laminin β2, we implanted beads that release the peptide corresponding to the eleventh extracellular loop of rat CaV2.1, which blocks this interaction. These beads resulted in significantly increased numbers of sensory nerve terminal clusters compared with embryos exposed to the control CaV1.2 loop peptide (67 ± 10 vs 36 ± 6, respectively) (Fig. 7). Although both ω-conotoxin and CaV2.1 loop-beads led to a doubling of the numbers of sensory nerve terminals in the skin, the absolute density varied in the two cases, possibly because the former were implanted during the summer and the latter during the winter; seasonal variation in amphibian development has been described previously (Wernig et al., 1980; O'Dowd et al., 1988).
Figure 7.
Blocking CaV2.2–laminin β2 interactions in vivo leads to increased innervation of the skin. A–C, Agarose beads soaked in 10 μm CaV2.1 or CaV1.2 11th extracellular loop peptide were implanted in stage 16 embryos. After 1 d, stage 34 embryos were fixed and stained with anti-HNK-1 to reveal sensory processes and sensory nerve terminals innervating the skin as in Figure 6. Scale bar, 50 μm. D, Embryos exposed to CaV2.1 loop peptide (C) exhibited significantly greater numbers of sensory nerve terminals than embryos exposed to CaV1.2 loop peptide (B) (*p < 0.05; n ≥ 3 embryos for each condition). Error bars indicate SEM.
Discussion
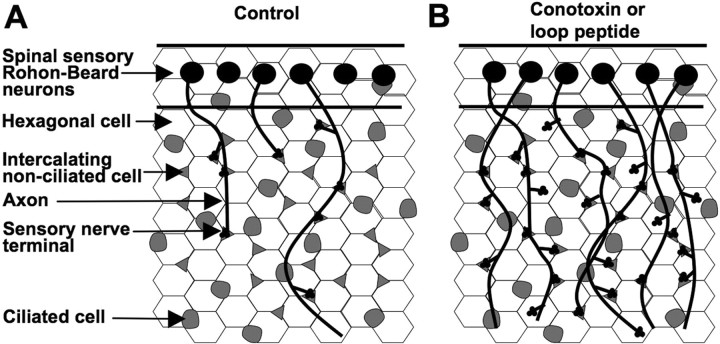
CaV2.2 is expressed in the growth cones of sensory Rohon-Beard neurons as they grow under skin cells, some of which express laminin β2. Either blocking CaV2.2 with ω-conotoxin GVIA or blocking CaV2.2–laminin β2 interactions with the CaV2.1 11th extracellular loop leads to generation of greater numbers of sensory terminals, potentially via more en passant varicosities, greater numbers of neurites, longer neurites, or less restricted branching. These results support a model in which neurite growth and subsequent innervation are normally restricted by a calcium-dependent stop signal generated by laminin β2–CaV2.2 interactions (Fig. 8).
Figure 8.
Model of laminin β2 and CaV2.2 interactions in development of skin innervation. A, In control conditions, outgrowing sensory terminals are inhibited or slowed by laminin β2-rich regions of the skin coextensive with hexagonal cells (white). B, The number of sensory nerve terminals increases when signaling interactions between laminin β2 and CaV2.2 are blocked.
Specificity of laminin β2–CaV2.2 inhibition of neurite outgrowth
Previous studies have demonstrated that laminin β2 binds directly to CaV2.2 via the 11th extracellular loop of CaV2.2 and the LRE motif of laminin β2 (Sunderland et al., 2000; Nishimune et al., 2004). We present three lines of evidence indicating that these specific interactions mediate the inhibition of spinal neuron outgrowth. First, inhibition is observed when neurons are grown on a laminin β2 fragment containing the LRE motif, but not one containing the point mutated QRE. Second, inhibition is rescued when cultures are incubated with the competing peptide corresponding to the eleventh extracellular loop of CaV2.2. Finally, ω-conotoxin GVIA, a specific inhibitor of CaV2.2, blocks laminin β2-mediated inhibition. These results indicate that laminin β2 acts via CaV2.2 to inhibit neurite outgrowth. Furthermore, growing neurons on stripes of native and denatured laminin β2 LRE demonstrates that neurites already growing on a denatured laminin β2 substrate are inhibited by initial contact with an active laminin β2 substrate. This initial contact appears to be signaled through the growth cone by a calcium transient.
Calcium dependence of laminin β2–CaV2.2 inhibition of neurite outgrowth
Inhibition of neurite outgrowth by laminin β2 is blocked by incubating cultures in calcium-free medium or ω-conotoxin GVIA, demonstrating calcium dependence. Additionally Fluo-4 calcium imaging of neurons approaching a laminin β2 border reveals calcium transients on growth cone contact with laminin β2. The reliance of laminin β2–CaV2.2 inhibition on calcium influx distinguishes this effect from the role that laminin β2 plays in clustering presynaptic calcium channels and other active zone proteins in the presynaptic nerve terminal, a calcium-independent effect (Nishimune et al., 2004). The calcium dependence of laminin β2–CaV2.2 neurite inhibition raises the intriguing possibility that laminin β2 may activate CaV2.2 or CaV2.1, the other presynaptic calcium channel to which it binds. However, channel properties of CaV2.1 heterologously expressed in human embryonic kidney (HEK) cells are not changed by presentation of the laminin β2 LRE fragment (Nishimune et al., 2004). There are several mechanisms by which laminin β2 may produce a change in the calcium signal through CaV2.2 that would affect neurite outgrowth but that would be undetected in recordings from HEK cells. First, CaV2.2 and CaV2.1 may require an additional molecule not present in HEK cells to be activated by laminin β2. Second, laminin β2 may serve to cluster CaV2.2 to a microdomain in growth cones at which it can effectively transduce a calcium signal. Indeed, laminin β2 clusters CaV2.1 at the mammalian neuromuscular junction (Nishimune et al., 2004). Similar changes in distribution of CaV2.2 in HEK cells might not lead to changes in the current recorded. Third, the involvement of CaV2.2 in generating a stop signal may rely on the mechanosensitivity of this channel (Calabrese et al., 2002). The interaction of the moving growth cone expressing CaV2.2 channels with the static LRE motif of laminin β2 may constitute an adequate stimulus for channel activation that is absent from CaV2.1 expressed in nonmotile HEK cells. Indeed, calcium influx through other mechanosensitive channels within the growth cone inhibits neurite outgrowth (Jacques-Fricke et al., 2006).
Regulation of sensory innervation by laminin β2–CaV2.2 interactions
Laminin β2 functions in synapse formation at neuromuscular junctions (Hunter et al., 1989; Noakes et al., 1995a; Porter et al., 1995; Nishimune et al., 2004) in glomerular filtration in the kidney (Noakes et al., 1995b), and in cell fate determination in the retina (Hunter et al., 1992a; Zenker et al., 2004). Here, we describe the expression of laminin β2 in the skin and its role in regulating sensory innervation. This expression is consistent with evidence for expression of human laminin β2 in embryonic dermis and epidermis (Iivanainen et al., 1995). Indeed, incorporating a mixture of laminins, including the laminin β2 chain, into skin grafts improves sensory perception achieved during regeneration (Caissie et al., 2006).
Growth of sensory neurites is mediated by neurotrophic factors such as NT-4 (Krimm et al., 2006) and guidance molecules such as ephrins (Moss et al., 2005). Branching and tiled patterning of sensory neurites relies on the NDR (nuclear Dbf2-related) kinase family in both like-repels-like tiling (Emoto et al., 2006) and noncontact-mediated distribution (Gallegos and Bargmann, 2004). We have identified a role for calcium signaling elicited by an extracellular matrix molecule in sensory neurite innervation. The activation of NDR kinases is calcium-dependent, providing a potential mechanism by which calcium influx through CaV2.2 may lead to changes in sensory innervation (Millward et al., 1998).
Classes of stop signals
Laminin β2 has been described as a stop signal for motor neurite outgrowth (Porter et al., 1995). A stop signal may function to indicate that neurites have reached their targets where they are to form synapses or terminal endings (Becker et al., 2000; Poskanzer et al., 2003; Dimitropoulou and Bixby, 2005). Alternatively, a stop signal may inhibit a neuron from growing into nontarget regions (Manzini et al., 2006). Our results are consistent with the latter function because inhibiting the signal through CaV2.2 leads to increased numbers of sensory nerve terminals. Behaviorally, this may lead to hypersensitivity to light touch and/or mislocalization of sensory signals. Pierson syndrome, a rare human disorder caused by mutations to the LAMB2 gene that encodes laminin β2, has been characterized by kidney failure, blindness, muscle dystonia, microcephaly, and neurological disorders (Zenker et al., 2004; Wuhl et al., 2007). It is likely that these infants would also have abnormalities in somatosensation.
Ziconotide, an inhibitor of CaV2.2 derived from conus snail venom, has recently begun to be used for pain treatment, delivered intrathecally (Lynch et al., 2006). Although the amount of this drug present in general circulation is quite low, with 56% of patients having <0.15 nm plasma levels, the IC50 for its inhibition of CaV2.2 heterologously expressed in Xenopus oocytes is 0.45 nm (Luchian, 2001). The role that we have demonstrated for CaV2.2 in axon guidance and sensory innervation during development suggests that the use of Ziconotide in breast feeding or pregnant women is ill advised. Laminin β2 is also expressed centrally in the mammalian developing cortex in the subplate (Hunter et al., 1992b), a transient population of neurons that is crucial to establishment of thalamocortical connections and maturation of inhibition (Kanold and Shatz, 2006). Ziconotide could disrupt normal innervation here as well.
Footnotes
This work was supported by National Institutes of Health Grant RO1MH074702 (N.C.S.) and National Science Foundation and Merck fellowships to S.B.S. We thank D. K. Berg, J. Isaacson, C. Kintner, L. N. Borodinsky, C. Root, L. W. Chang, N. A. Velasquez, B. Miller, K. Marek, G. Monsalve, W. Conroy, M. Blank, J. Stubbs, X. Nicol, and J. Y. Lin for helpful discussions and technical advice. S.B.S. and N.C.S. designed the experiments. S.B.S. and L.X. conducted the experiments. H.N. and J.R.S. provided reagents and technical advice. S.B.S., H.N., J.R.S., and N.C.S. wrote this manuscript.
The authors declare no competing financial interests.
References
- Baker MW, Macagno ER. In vivo imaging of growth cone and filopodial dynamics: evidence for contact-mediated retraction of filopodia leading to the tiling of sibling processes. J Comp Neurol. 2007;500:850–862. doi: 10.1002/cne.21228. [DOI] [PubMed] [Google Scholar]
- Becker CG, Meyer RL, Becker T. Gradients of ephrin-A2 and ephrin-A5b mRNA during retinotopic regeneration of the optic projection in adult zebrafish. J Comp Neurol. 2000;427:469–483. [PubMed] [Google Scholar]
- Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. doi: 10.1038/nature02518. [DOI] [PubMed] [Google Scholar]
- Burger C, Ribera AB. Xenopus spinal neurons express Kv2 potassium channel transcripts during embryonic development. J Neurosci. 1996;16:1412–1421. doi: 10.1523/JNEUROSCI.16-04-01412.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caissie R, Gingras M, Champigny MF, Berthod F. In vivo enhancement of sensory perception recovery in a tissue-engineered skin enriched with laminin. Biomaterials. 2006;27:2988–2993. doi: 10.1016/j.biomaterials.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Calabrese B, Tabarean IV, Juranka P, Morris CE. Mechanosensitivity of N-type calcium channel currents. Biophys J. 2002;83:2560–2574. doi: 10.1016/S0006-3495(02)75267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin MW, Lin MS, Spitzer NC. Local calcium transients contribute to disappearance of pFAK, focal complex removal, and deadhesion of neuronal growth cones and fibroblasts. Dev Biol. 2005;287:201–212. doi: 10.1016/j.ydbio.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Dimitropoulou A, Bixby JL. Motor neurite outgrowth is selectively inhibited by cell surface MuSK and agrin. Mol Cell Neurosci. 2005;28:292–302. doi: 10.1016/j.mcn.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Emoto K, He Y, Ye B, Grueber WB, Adler PN, Jan LY, Jan YN. Control of dendritic branching and tiling by the Tricornered-kinase/Furry signaling pathway in Drosophila sensory neurons. Cell. 2004;119:245–256. doi: 10.1016/j.cell.2004.09.036. [DOI] [PubMed] [Google Scholar]
- Emoto K, Parrish JZ, Jan LY, Jan YN. The tumour suppressor Hippo acts with the NDR kinases in dendritic tiling and maintenance. Nature. 2006;443:210–213. doi: 10.1038/nature05090. [DOI] [PubMed] [Google Scholar]
- Gallegos ME, Bargmann CI. Mechanosensory neurite termination and tiling depend on SAX-2 and the SAX-1 kinase. Neuron. 2004;44:239–249. doi: 10.1016/j.neuron.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Robles E, Poo M, Spitzer NC. Filopodial calcium transients promote substrate-dependent growth cone turning. Science. 2001;291:1983–1987. doi: 10.1126/science.1056490. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Ye B, Moore AW, Jan LY, Jan YN. Dendrites of distinct classes of Drosophila sensory neurons show different capacities for homotypic repulsion. Curr Biol. 2003;13:618–626. doi: 10.1016/s0960-9822(03)00207-0. [DOI] [PubMed] [Google Scholar]
- Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995;375:784–787. doi: 10.1038/375784a0. [DOI] [PubMed] [Google Scholar]
- Hari A, Djohar B, Skutella T, Montazeri S. Neurotrophins and extracellular matrix molecules modulate sensory axon outgrowth. Int J Dev Neurosci. 2004;22:113–117. doi: 10.1016/j.ijdevneu.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Hughes ME, Bortnick R, Tsubouchi A, Baumer P, Kondo M, Uemura T, Schmucker D. Homophilic dscam interactions control complex dendrite morphogenesis. Neuron. 2007;54:417–427. doi: 10.1016/j.neuron.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DD, Shah V, Merlie JP, Sanes JR. A laminin-like adhesive protein concentrated in the synaptic cleft of the neuromuscular junction. Nature. 1989;338:229–234. doi: 10.1038/338229a0. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Murphy MD, Olsson CV, Brunken WJ. S-laminin expression in adult and developing retinae: a potential cue for photoreceptor morphogenesis. Neuron. 1992a;8:399–413. doi: 10.1016/0896-6273(92)90269-j. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Llinas R, Ard M, Merlie JP, Sanes JR. Expression of s-laminin and laminin in the developing rat central nervous system. J Comp Neurol. 1992b;323:238–251. doi: 10.1002/cne.903230208. [DOI] [PubMed] [Google Scholar]
- Iivanainen A, Vuolteenaho R, Sainio K, Eddy R, Shows TB, Sariola H, Tryggvason K. The human laminin beta 2 chain (S-laminin): structure, expression in fetal tissues and chromosomal assignment of the LAMB2 gene. Matrix Biol. 1995;14:489–497. doi: 10.1016/0945-053x(95)90006-3. [DOI] [PubMed] [Google Scholar]
- Jacques-Fricke BT, Seow Y, Gottlieb PA, Sachs F, Gomez TM. Ca2+ influx through mechanosensitive channels inhibits neurite outgrowth in opposition to other influx pathways and release from intracellular stores. J Neurosci. 2006;26:5656–5664. doi: 10.1523/JNEUROSCI.0675-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Shatz CJ. Subplate neurons regulate maturation of cortical inhibition and outcome of ocular dominance plasticity. Neuron. 2006;51:627–638. doi: 10.1016/j.neuron.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Kitson DL, Roberts A. Competition during innervation of embryonic amphibian head skin. Proc R Soc Lond B Biol Sci. 1983;218:49–59. doi: 10.1098/rspb.1983.0025. [DOI] [PubMed] [Google Scholar]
- Krimm RF, Davis BM, Noel T, Albers KM. Overexpression of neurotrophin 4 in skin enhances myelinated sensory endings but does not influence sensory neuron number. J Comp Neurol. 2006;498:455–465. doi: 10.1002/cne.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Wang SW, Masland RH. Retinal ganglion cell type, size, and spacing can be specified independent of homotypic dendritic contacts. Neuron. 2004;43:475–485. doi: 10.1016/j.neuron.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Lipscombe D, Madison DV, Poenie M, Reuter H, Tsien RY, Tsien RW. Spatial distribution of calcium channels and cytosolic calcium transients in growth cones and cell bodies of sympathetic neurons. Proc Natl Acad Sci USA. 1988;85:2398–2402. doi: 10.1073/pnas.85.7.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchian T. The influence exerted by the beta(3) subunit on MVIIA omega-conotoxin binding to neuronal N-type calcium channels. Biochim Biophys Acta. 2001;1512:329–334. doi: 10.1016/s0005-2736(01)00336-4. [DOI] [PubMed] [Google Scholar]
- Lynch SS, Cheng CM, Yee JL. Intrathecal ziconotide for refractory chronic pain. Ann Pharmacother. 2006;40:1293–1300. doi: 10.1345/aph.1G584. [DOI] [PubMed] [Google Scholar]
- Manzini MC, Ward MS, Zhang Q, Lieberman MD, Mason CA. The stop signal revised: immature cerebellar granule neurons in the external germinal layer arrest pontine mossy fiber growth. J Neurosci. 2006;26:6040–6051. doi: 10.1523/JNEUROSCI.4815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward TA, Heizmann CW, Schafer BW, Hemmings BA. Calcium regulation of Ndr protein kinase mediated by S100 calcium-binding proteins. EMBO J. 1998;17:5913–5922. doi: 10.1093/emboj/17.20.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss A, Alvares D, Meredith-Middleton J, Robinson M, Slater R, Hunt SP, Fitzgerald M. Ephrin-A4 inhibits sensory neurite outgrowth and is regulated by neonatal skin wounding. Eur J Neurosci. 2005;22:2413–2421. doi: 10.1111/j.1460-9568.2005.04452.x. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin): a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. Ed 2. Amsterdam: North-Holland; 1967. [Google Scholar]
- Nishimune H, Sanes JR, Carlson SS. A synaptic laminin-calcium channel interaction organizes active zones in motor nerve terminals. Nature. 2004;432:580–587. doi: 10.1038/nature03112. [DOI] [PubMed] [Google Scholar]
- Noakes PG, Gautam M, Mudd J, Sanes JR, Merlie JP. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin beta 2. Nature. 1995a;374:258–262. doi: 10.1038/374258a0. [DOI] [PubMed] [Google Scholar]
- Noakes PG, Miner JH, Gautam M, Cunningham JM, Sanes JR, Merlie JP. The renal glomerulus of mice lacking s-laminin/laminin beta 2: nephrosis despite molecular compensation by laminin beta 1. Nat Genet. 1995b;10:400–406. doi: 10.1038/ng0895-400. [DOI] [PubMed] [Google Scholar]
- Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985;316:440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- O'Dowd DK, Ribera AB, Spitzer NC. Development of voltage-dependent calcium, sodium, and potassium currents in Xenopus spinal neurons. J Neurosci. 1988;8:792–805. doi: 10.1523/JNEUROSCI.08-03-00792.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton BL, Miner JH, Chiu AY, Sanes JR. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J Cell Biol. 1997;139:1507–1521. doi: 10.1083/jcb.139.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter BE, Weis J, Sanes JR. A motoneuron-selective stop signal in the synaptic protein S-laminin. Neuron. 1995;14:549–559. doi: 10.1016/0896-6273(95)90311-9. [DOI] [PubMed] [Google Scholar]
- Poskanzer K, Needleman LA, Bozdagi O, Huntley GW. N-cadherin regulates ingrowth and laminar targeting of thalamocortical axons. J Neurosci. 2003;23:2294–2305. doi: 10.1523/JNEUROSCI.23-06-02294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A, Hayes BP. The anatomy and function of “free” nerve endings in an amphibian skin sensory system. Proc R Soc Lond B Biol Sci. 1977;196:415–429. doi: 10.1098/rspb.1977.0048. [DOI] [PubMed] [Google Scholar]
- Sagasti A, Guido MR, Raible DW, Schier AF. Repulsive interactions shape the morphologies and functional arrangement of zebrafish peripheral sensory arbors. Curr Biol. 2005;15:804–814. doi: 10.1016/j.cub.2005.03.048. [DOI] [PubMed] [Google Scholar]
- Snow DM, Atkinson PB, Hassinger TD, Letourneau PC, Kater SB. Chondroitin sulfate proteoglycan elevates cytoplasmic calcium in DRG neurons. Dev Biol. 1994;166:87–100. doi: 10.1006/dbio.1994.1298. [DOI] [PubMed] [Google Scholar]
- Somasekhar T, Nordlander RH. Selective early innervation of a subset of epidermal cells in Xenopus may be mediated by chondroitin sulfate proteoglycans. Brain Res Dev Brain Res. 1997;99:208–215. doi: 10.1016/s0165-3806(97)00011-4. [DOI] [PubMed] [Google Scholar]
- Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444:707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- Stubbs JL, Davidson L, Keller R, Kintner C. Radial intercalation of ciliated cells during Xenopus skin development. Development. 2006;133:2507–2515. doi: 10.1242/dev.02417. [DOI] [PubMed] [Google Scholar]
- Sunderland WJ, Son YJ, Miner JH, Sanes JR, Carlson SS. The presynaptic calcium channel is part of a transmembrane complex linking a synaptic laminin (α4 β2 γ1) with non-erythroid spectrin. J Neurosci. 2000;20:1009–1019. doi: 10.1523/JNEUROSCI.20-03-01009.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993;366:156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- Wassle H, Peichl L, Boycott BB. Dendritic territories of cat retinal ganglion cells. Nature. 1981;292:344–345. doi: 10.1038/292344a0. [DOI] [PubMed] [Google Scholar]
- Wernig A, Pecot-Dechavassine M, Stover H. Sprouting and regression of the nerve at the frog neuromuscular junction in normal conditions and after prolonged paralysis with curare. J Neurocytol. 1980;9:278–303. doi: 10.1007/BF01181538. [DOI] [PubMed] [Google Scholar]
- Wuhl E, Kogan J, Zurowska A, Matejas V, Vandevoorde RG, Aigner T, Wendler O, Lesniewska I, Bouvier R, Reis A, Weis J, Cochat P, Zenker M. Neurodevelopmental deficits in Pierson (microcoria-congenital nephrosis) syndrome. Am J Med Genet A. 2007;143:311–319. doi: 10.1002/ajmg.a.31564. [DOI] [PubMed] [Google Scholar]
- Zenker M, Aigner T, Wendler O, Tralau T, Muntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wuhl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dotsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, et al. Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet. 2004;13:2625–2632. doi: 10.1093/hmg/ddh284. [DOI] [PubMed] [Google Scholar]