Abstract
Thermal changes activate some members of the transient receptor potential (TRP) ion channel super family. They are primary sensors for detecting environmental temperatures. The Drosophila TRP channel Painless is believed responsible for avoidance of noxious heat because painless mutant flies display defects in heat sensing. However, no studies have proven its heat responsiveness. We show that Painless expressed in human embryonic kidney-derived 293 (HEK293) cells is a noxious heat-activated, Ca2+-permeable channel, and the function is mostly dependent on Ca2+. In Ca2+-imaging, Painless mediated a robust intracellular Ca2+ (Ca2+i) increase during heating, and it showed heat-evoked inward currents in whole-cell patch-clamp mode. Ca2+ permeability was much higher than that of other cations. Heat-evoked currents were negligible in the absence of extracellular Ca2+ (Ca2+o) and Ca2+i, whereas 200 nm Ca2+i enabled heat activation of Painless. Activation kinetics were significantly accelerated in the presence of Ca2+i. The temperature threshold for Painless activation was 42.6°C in the presence of Ca2+i, whereas the threshold was significantly increased to 44.1°C when only Ca2+o was present. Temperature thresholds were further reduced after repetitive heating in a Ca2+-dependent manner. Ca2+-dependent heat activation of Painless was observed at the single-channel level in excised membranes. We found that a Ca2+-regulatory site is located in the N-terminal region of Painless. Painless-expressing HEK293 cells were insensitive to various thermosensitive TRP channel activators including allyl isothiocyanate, whereas mammalian TRPA1 inhibitors, ruthenium red, and camphor, reversibly blocked heat activation of Painless. Our results demonstrate that Painless is a direct sensor for noxious heat in Drosophila.
Keywords: Drosophila, Painless, TRP channel, Ca2+ ion, temperature sensation, noxious heat
Introduction
Animals need to be able to distinguish between permissive habitats and those that could cause injury from excessive temperatures. Thus, the ability to sense environmental temperatures is a critical and fundamental trait. Recent studies have revealed that several ion channels belonging to the transient receptor potential (TRP) ion channel super family have an ability to sense temperature changes. In mammals, nine TRP channels have been reported to respond to a physiological range of temperatures, from cold to hot (Dhaka et al., 2006; Caterina, 2007; Tominaga, 2007). Some of these thermosensitive TRP channels are strongly expressed in nociceptive neurons, and a number of reports claim that they have critical roles in detection of temperatures in the noxious range. TRPV1 expressed in C-fibers is activated by heat (>43°C) (Caterina et al., 1997), and TRPV1-null mice are defective in sensing noxious heat (Caterina et al., 2000). TRPM8, which senses cold temperatures (<27°C) (McKemy et al., 2002; Peier et al., 2002), is expressed in nociceptive and non-nociceptive neurons and its loss causes deficiencies in cold sensation (Bautista et al., 2007; Colburn et al., 2007; Dhaka et al., 2007, 2008; Takashima et al., 2007). TRPA1 is expressed in C-fibers, mostly accompanying TRPV1 (Story et al., 2003), and acts as a sensor for harmful stimuli, although its responsiveness to noxious cold is controversial (Jordt et al., 2004; Bautista et al., 2006; Kwan et al., 2006; Sawada et al., 2007). Thus, the thermosensitive TRP channels are implicated in the detection of harmful temperatures that might otherwise cause serious damage to the organism.
Detecting ambient temperatures is particularly important for small insects. Body temperature and locomotive activity of small insects predominantly depend on environmental temperatures because their large volume-to-weight ratios allow rapid heat exchange with the environment. In Drosophila, three ankyrin-repeat TRP (TRPA) channels are involved in temperature sensation. Temperatures >27°C activate dTRPA1 (Viswanath et al., 2003) and its knockdown by RNA interference revealed the importance of the channel for thermotaxis (Rosenzweig et al., 2005). Pyrexia is activated by heat (∼40°C) and is proposed to protect flies from high-temperature stress (Lee et al., 2005). Another TRPA subtype, dubbed Painless, was reported to be responsible for avoidance of noxious heat >42°C in Drosophila larvae (Tracey et al., 2003) and adults (Xu et al., 2006). Wild-type larvae displayed distinct rolling behavior within 1 s when touched with a heated probe, whereas painless mutant larvae required more than 10 s to show such behavior. Painless appears to be distributed in a subset of sensory neurons of the peripheral nervous system, which resemble sensory neurons in mammals. Because the other two members of the TRPA subfamily possess the properties of temperature sensitivity, Painless has been regarded as a heat-activated channel. However, in contrast to dTRPA1 and pyrexia, no studies have proven that Painless itself responds to heat. In this study, we examined the heat sensitivity and activation properties of Painless in a heterologous expression system to assess whether Painless truly functions as a thermosensitive TRP channel.
Materials and Methods
Cloning of painless and construction of the expression vector.
The full length of painless cDNA was isolated by RT-PCR with adult fly head RNA and two primers, 5′-TTTTTGCGGCCGCACCATGGACTTTAACAACTGCGGCTTCATTGATCCG-3′ and 5′-TTTCTAGACTCTTCCGGTCCTGGACCAGCTGTATTAATTGCTCCA-3′. The PCR product was digested with NotI and XbaI, and then cloned in a pAc5.1/V5-His B vector (Invitrogen) in which the Drosophila actin 5C promoter was replaced with a cytomegalovirus (CMV) promoter. The resulting construct (painless/V5 expression vector) was sequenced, and two base substitutions were found in the cDNA which did not cause amino acid substitutions. The vector containing full-length painless cDNA with stop codon (painless expression vector) or N356A, S357A, N363A, or D366A mutants were constructed by single amino acid substitutions using a modified Quikchange site-directed mutagenesis method (Stratagene). Briefly, PCR was performed using a painless/V5 expression vector as a template, two synthetic oligonucleotide primers containing a stop codon or specific mutations (painless expression vector-S and AS, 5′-ACGCAAATGGGCGGTAGG-3′ and 5′-CCTCTAGACGTCACTTCCGGTCCTGGACCAGCTGTATTAATTGCTCCA-3′; N356A-S and AS, 5′-GCTTTGAATTGCTCATTgcCAGCGATCGCGTAGAC-3′ and 5′-GTCTACGCGATCGCTGgcAATGAGCAATTCAAAGC-3′; or S357A-S and AS, 5′-GAATTGCTCATTAACgcCGATCGCGTAGACATCAAC-3′ and 5′-GTTGATGTCTACGCGATCGgcGTTAATGAGCAATTC-3′; or N363A-S and AS, 5′-GATCGCGTAGACATCgcCGAAGCTGATTCCGG-3′ and 5′-CCGGAATCAGCTTCGgcGATGTCTACGCGATC-3′; or D366A-S and AS, 5′-CATCAACGAAGCTGcTTCCGGACGCCTG-3′ and 5′-CAGGCGTCCGGAAgCAGCTTCGTTGATG-3′), and Primestar HS DNA polymerase (TaKaRa). The PCR products were digested with DpnI (New England BioLabs) at 37°C for 1 h, and transformed into DH5α competent cells. The entire sequence including desired substitution in the mutants was confirmed. The painless/V5 expression vector was used for immunostaining. The painless expression vector and the four mutant vectors were used for Ca2+-imaging and/or patch-clamp methods.
Cell culture, transfection, and immunostaining.
Human embryonic kidney-derived 293 (HEK293) cells were maintained in DMEM containing 4500 mg/L glucose (Wako Pure Chemicals), 10% FBS (Biological Industries) and penicillin/streptomycin (Invitrogen) at 37°C in 5% CO2. For Ca2+-imaging, 1 μg of painless expression vector and 0.1 μg of pCMV-DsRed-express cDNAs in OPTI-MEM medium (Invitrogen) were transfected to HEK293 cells cultured on 35 mm dishes using Lipofectamine Plus reagents (Invitrogen). For patch-clamp recording, 0.1 μg of pGreen Lantern 1 cDNAs were used instead of DsRed cDNAs. After incubating for 3 to 4 h, cells were reseeded on cover glasses and further incubated at 33°C in 5% CO2. Cells were used for the experiments 20–40 h after transfection. For immunostaining, HEK293 cells were inoculated in eight-well chamber slides (Nunc), and painless/V5 expression vectors were transfected using Effectene reagent (Qiagen). Two days after transfection, the cells were fixed with 4% paraformaldehyde/PBS, and then permeabilized with 0.5% Triton X-100/PBS for 5 min followed by blocking with 5% normal donkey serum/PBS containing 0.1% Triton X-100. The cells were incubated with rabbit anti-V5 epitope antibody (Sigma) at a 1000-fold dilution for 2 h at room temperature, after which FITC-conjugated anti-rabbit IgG antibody (Millipore) was added at a 300-fold dilution as above.
Ca2+ imaging.
Transfected HEK293 cells on cover glasses were incubated in culture medium containing 5 μm fura-2 AM (Invitrogen) at 33°C for 1 to 2 h. The cover glasses were washed with a standard bath solution containing (in mm) 140 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose, pH 7.4, adjusted with NaOH, and fura-2 fluorescence was measured in a standard bath solution. The cover glasses were mounted in a chamber (RC-26G; Warner Instruments) connected to a gravity flow system to deliver various stimuli and heated bath solutions. Thermal stimulation was applied by increasing the bath temperature with a preheated solution through an inline heater (SH-27B; Warner Instruments). A xenon lamp was used as an illumination source. Fura-2 loaded in the cells was excited with 340 and 380 nm wavelengths and emission was monitored at a wavelength of 510 nm with a CCD camera (CoolSnap ES; Roper Scientific/Photometrics) to obtain fluorescent intensities of Ca2+-bound and Ca2+-free fura-2, respectively. Data were acquired and analyzed by IPlab software (Scanalytics). Chamber temperature was monitored with a thermocouple (TA-29; Warner Instruments) and sampled with an analog-to-digital converter with pClamp software (Molecular Devices).
Electrophysiology.
The standard bath solution containing 2 mm CaCl2 for whole-cell patch-clamp experiments was the same as that used in Ca2+-imaging experiments. The same solution was used as the standard pipette solution for inside-out patch-clamp recording. The Ca2+(−) bath solution for whole-cell patch-clamp and the Ca2+(−) pipette solution for inside-out patch-clamp contained (in mm) 140 NaCl, 5 KCl, 2 MgCl2, 5 EGTA, 10 HEPES, and 10 glucose, pH 7.4, adjusted with NaOH. The Cs-Asp/Ca2+(−) pipette solution for whole-cell patch-clamp and the Cs-Asp/Ca2+(−) bath solution for the inside-out patch-clamp contained (in mm) 120 aspartate, 10 CsCl, 1 MgCl2, 5 EGTA, 17 mannitol, and 10 HEPES, pH 7.4, adjusted with CsOH. CaBuf (http://www.kuleuven.be/fysio/trp/cabuf) was used to calculate the free Ca2+ concentration and CaCl2 was added to the Ca2+(−) pipette solution. The final concentrations of free Ca2+ in CaCl2 solution were as follows (in mm): 0.077 CaCl2 for 1 nm free Ca2+, 0.675 CaCl2 for 10 nm free Ca2+, 3.032 CaCl2 for 100 nm free Ca2+, 3.72 CaCl2 for 200 nm free Ca2+, 4.105 CaCl2 for 300 nm free Ca2+, 4.68 CaCl2 for 1000 nm free Ca2+, 4.977 CaCl2 for 10,000 nm free Ca2+, and 5.099 nm CaCl2 for 100,000 nm free Ca2+. The Cs-Asp/Ca2+ 200 nm bath solution for inside-out patch-clamp was the same as the Cs-Asp/Ca2+ 200 nm pipette solution for whole-cell patch-clamp recording. For cation permeability experiments, the bath solutions contained (in mm) 140 NaCl (or 140 CsCl), 10 glucose, and 10 HEPES, pH 7.4, adjusted with NaOH (or CsOH) (for monovalent cations), or 110 MgCl2 (or 110 CaCl2), 2 Mg(OH)2 [or 2 Ca(OH)2], 10 glucose, and 10 HEPES, pH 7.4, adjusted with HCl (for divalent cations). The KCl pipette solution used for the ion permeability assay contained (in mm) 140 KCl, 5 EGTA, and 10 HEPES, pH 7.4, adjusted with KOH. The KCl/200 nm Ca2+ pipette solution contained 3.868 mm CaCl2 in the KCl pipette solution. Ca2+o and Ca2+i indicate 2 mm extracellular Ca2+ or 200 nm intracellular Ca2+, respectively, unless specified otherwise. Whole-cell recording data were sampled at 10 kHz and filtered at 5 kHz for analysis (Axopatch 200B amplifier with pClamp software; Molecular Devices). Inside-out patch recording data were sampled at 10 kHz and filtered at 2 kHz for analysis.
Membrane potential was clamped at −60 mV, unless specified. Shifts in liquid junction potentials during heating (ΔJPH) were measured directly in separate experiments, and significant changes during heating (>5 mV) were observed. Therefore, membrane potentials and reversal potentials were corrected by subtracting the average ΔJPH from data. All of the patch-clamp experiments were performed at room temperature except during heat stimulation. Thermal stimulation was applied by increasing the bath temperature with a preheated solution through the inline heater (1°C/s around thresholds, with a maximum of 46°C for fast heat application, a maximum of 51°C for high heat application) or by heating stage (slow heat application, 0.2°C/s around thresholds). After whole-cell configuration was achieved, the cell was raised 50 μm and placed in the center of the chamber. Temperature was monitored with a thermocouple (TA-30; Warner Instruments) placed within 100 μm of the patch-clamped cell. The current–voltage (I–V) relationship during heating was obtained by using voltage ramps (−100 to +100 mV in 100 ms). Permeability ratios for cations were calculated as described previously (Adams et al., 1980; Caterina et al., 1997). In brief, the reversal potential was measured using voltage ramps (−100 to +100 mV in 100 ms). The permeability ratio for monovalent cations to K (PX/PK) was calculated as follows: PX/PK = exp(ΔVrevF/RT), where Vrev is the reversal potential, F is Faraday's constant, R is the universal gas constant, and T is absolute temperature. For measurement of divalent cation permeability, PY/PK was calculated as follows: PY/PK = 3[K+]i exp(ΔVrevF/RT) (1 + exp(ΔVrevF/RT))/4[Y2+]o, where bracketed terms are activities. Assumed activity coefficients are 0.75 for monovalent ions and 0.25 for divalent ions. Temperature profiles and Arrhenius plots were calculated using Origin software (Microcal). The temperature coefficient Q10 was used to characterize the temperature dependence of the membrane current. The absolute current values were plotted on a log scale against the reciprocal of the absolute temperature (T) (Arrhenius plot). Q10 values were calculated from QΔT = (Q10)ΔT/10 for an arbitrary temperature ΔT.
Statistical analysis.
Multiple comparisons were performed using Tukey's method. Values are shown as mean ± SEM, unless indicated. A value of p < 0.05 was considered significant.
Results
Painless is a heat-activated, Ca2+-permeable channel
We cloned painless cDNA from adult Drosophila heads and constructed expression vectors for mammalian cells. Abundant expression of Painless in the plasma membrane was observed when the plasmid was transiently transfected into HEK293 cells (Fig. 1A). Because the majority of the thermosensitive TRP channels show high conductance for Ca2+, we first performed Ca2+-imaging experiments using fura-2 to test whether Painless could raise intracellular Ca2+ concentration ([Ca2+]i) with heat stimulation. A robust [Ca2+]i increase was observed in Painless-expressing cells when the temperature was raised >40°C (Fig. 1B,C, left). The [Ca2+]i increase was completely abolished in the absence of extracellular Ca2+ (Ca2+o) (Fig. 1C, right), indicating that it was caused by an influx from extracellular space through Painless.
Figure 1.
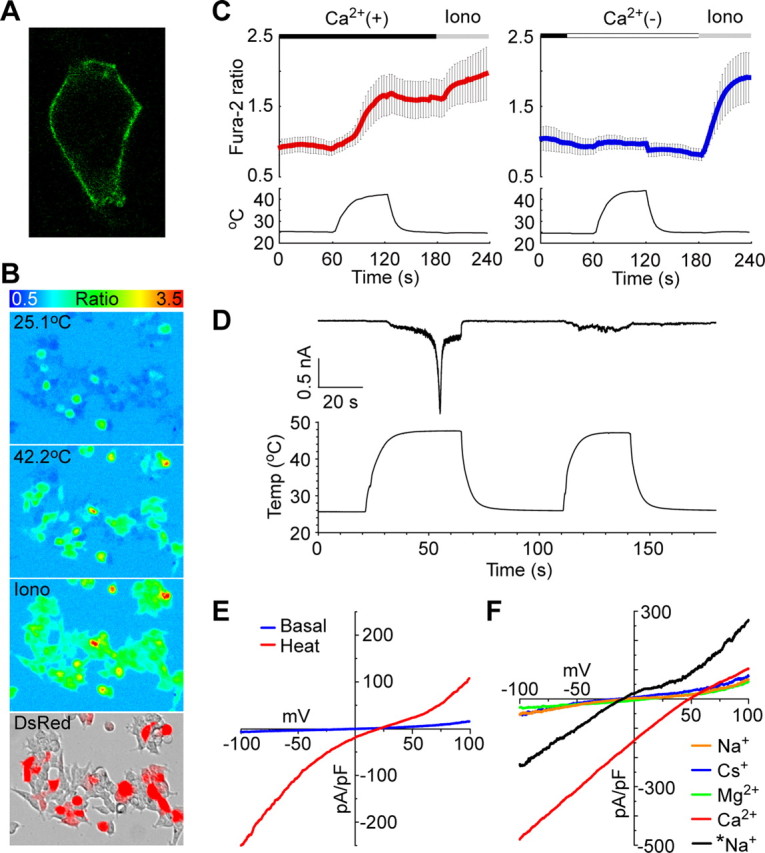
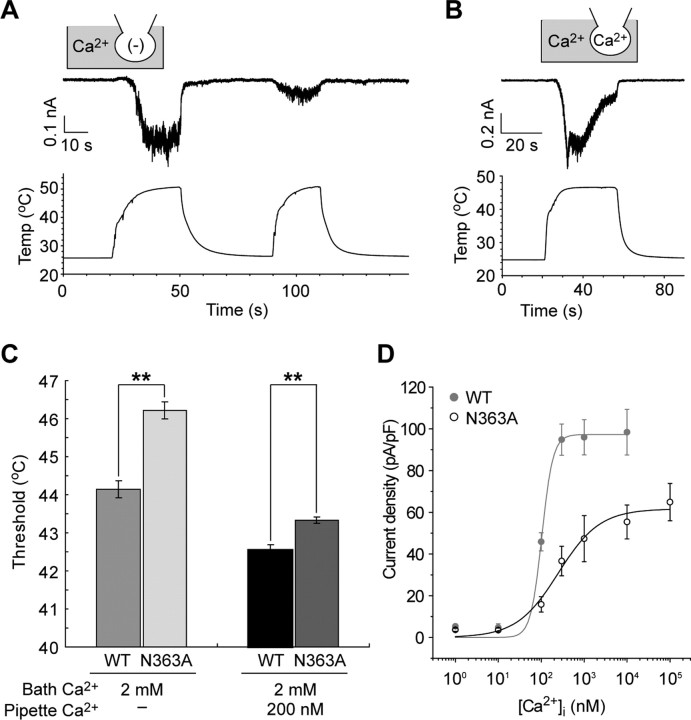
Heat-evoked activation and ion selectivity of Painless. A, A representative image of a Painless-expressing HEK293 cell immunostained with anti-V5-tag antibody. B, Representative fura-2 Ca2+ imaging shows [Ca2+]i increase in Painless-expressing HEK293 cells during heating (∼42°C). Pseudocolor indicates intensity of the fluorescence ratio of 340/380 nm. Ionomycin (5 μm) with 10 mm CaCl2 (Iono) was applied to confirm cell viability. DsRed (red color) indicates Painless-expressing cells. C, Quantitative changes in the fura-2 ratio in Painless-expressing cells during heating. A clear [Ca2+]i increase was observed in the presence [Ca2+(+), red bar], but not in the absence [Ca2+(−), blue bar] of 2 mm extracellular CaCl2. Average traces from 111 cells (left) or 73 cells (right) ±SD are shown in the top panels. Bottom panels show temperature changes. D, Heat elicits inward current activation in a Painless-expressing HEK293 cell at −60 mV holding potential in a whole-cell patch-clamp mode (n = 12). A standard bath solution and Cs-Asp/Ca2+(−) pipette solution were used. E, Current–voltage relationship of heat-evoked current exhibits dual-rectification with positive reversal potential. Heat-dependent shift of the liquid junctional potentials (ΔJPH) were not corrected in the plot. The reversal potential was 28.8 ± 1.0 mV after compensation of ΔJPH (n = 8). A standard bath solution and Cs-Asp/Ca2+(−) pipette solution were used. F, Heat-evoked currents exhibit high Ca2+ permeability (n = 6–7). NaCl, CsCl, MgCl2, or CaCl2 bath solutions and KCl pipette solution were used. Note that *Na+ (black trace) was obtained using NaCl bath and KCl/200 nm Ca2+ pipette solution. Basal traces were subtracted but ΔJPH values were not compensated in the plot. After compensation of ΔJPH values of the reversal potentials, permeability ratios were calculated (PNa:PCs:PK:PMg:PCa = 1:0.84:1.13:4.87:41.66).
Next, we conducted patch-clamp experiments to clarify the electrophysiological properties of Painless. In the whole-cell configuration, Painless appeared to show transient inward currents during heating at a holding potential of −60 mV (Fig. 1D). The currents developed gradually when temperature was raised to >45°C, followed by fast activation and inactivation. The activation temperature was similar to that observed in heat-sensitive neurons in Drosophila larva (Tracey et al., 2003). After the activated currents were almost desensitized, the second heat stimulation could no longer elicit large currents and the inactivation state lasted for >20 min (data not shown). The I–V relationship of Painless showed dual rectification with an apparent positive reversal potential of 28.8 ± 1.0 mV (Fig. 1E), suggesting that Painless has high permeability to either of the cations included in the bath solution. We measured the reversal potentials in various extracellular monovalent or divalent cation solutions with the KCl pipette solution (Fig. 1F). The reversal potentials obtained with extracellular NaCl, CsCl, or MgCl2 shifted modestly from zero, whereas that obtained with extracellular CaCl2 showed a distinct positive shift (−3.37 ± 0.84 mV for NaCl, −7.94 ± 1.56 mV for CsCl, 14.34 ± 1.64 mV for MgCl2, and 47.43 ± 1.31 mV for CaCl2). Calculated relative permeability ratios indicated that Painless is a cation channel with extremely high Ca2+ permeability (PNa:PCs:PK:PMg:PCa = 1:0.84:1.13:4.87:41.66). The PCa/PNa value was similar to that for the TRP channel of Drosophila (Liu et al., 2007). We did not observe large currents when CaCl2 was absent in extracellular and intracellular solutions, but larger currents were obtained when physiological intracellular Ca2+ (Ca2+i) was present in the pipette solution (Fig. 1F) (see below, Heat-evoked Painless activation requires Ca2+ ions). The reversal potential and the relative permeability ratio obtained with extracellular NaCl and intracellular KCl/200 nm free-Ca2+i were similar to those obtained without Ca2+i (−6.15 ± 0.33 mV and PNa:PK = 1:1.25). From these results, we conclude that Painless is a heat-sensitive TRP channel with high Ca2+ permeability.
Heat-evoked Painless activation requires Ca2+ ions
The activation properties of the thermosensitive TRP channels are modified by Ca2+ in various ways (Mohapatra et al., 2003; Strotmann et al., 2003; Chuang et al., 2004; Nagata et al., 2005; Talavera et al., 2005; Nilius et al., 2006; Tong et al., 2006; Doerner et al., 2007; Zurborg et al., 2007; Xiao et al., 2008). Because Painless displayed high Ca2+ permeability, we tested heat responsiveness under different combinations of Ca2+o and Ca2+i. In the absence of Ca2+o and Ca2+i, heat-evoked currents were almost negligible (<50 pA at −60 mV) (Fig. 2A). Conversely, as already shown in Figure 1, large currents were observed in the presence of Ca2+o in the same cell. Therefore, we applied higher heat (∼50°C) to test whether the heat responsiveness of Painless decreased without Ca2+. Activation currents were slightly increased with higher heat in the absence of Ca2+o and Ca2+i; however, they were still much smaller than those in the presence of Ca2+o (supplemental Fig. 1A, available at www.jneurosci.org as supplemental material). Painless was also activated by heat in the presence of physiological levels of Ca2+o and Ca2+i (Fig. 2B). Interestingly, the presence of Ca2+i was sufficient to elicit heat activation without Ca2+o (Figs. 1F, 2C). The inactivation occurred regardless of Ca2+o in the presence of Ca2+i, followed by small currents in the second heat application (Fig. 2B,C). These results suggest that Painless requires Ca2+i to generate large currents during heating, and desensitization of the channel is not caused by Ca2+i increase.
Figure 2.
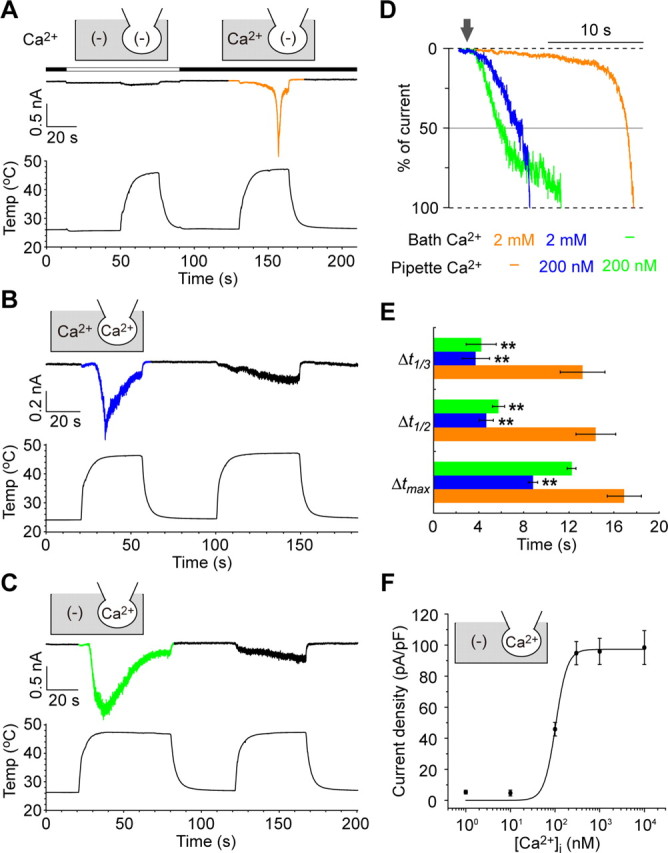
Ca2+-requiring activation of Painless. A, Painless exhibits only faint currents during heating in the absence of Ca2+o and Ca2+i. Cs-Asp/Ca2+(−) pipette solution [(−) in the pipette] and Ca2+(−) bath solution [(−) in gray area] were used. Functional Painless expression was confirmed by heat application in standard bath solution (Ca2+ in gray area). B, Heat elicits inward currents in the presence of Ca2+o and Ca2+i. After inactivation, Painless shows small currents after a second heating. Cs-Asp/200 nm Ca2+ pipette solution (Ca2+ in the pipette) and standard bath solution (Ca2+ in gray area) were used. C, Heat elicits inward currents in the absence of Ca2+o and the presence of Ca2+i. After inactivation, Painless shows small currents after a second heating. Cs-Asp/200 nm Ca2+ pipette solution (Ca2+ in the pipette) and Ca2+(−) bath solution [(−) in gray area] were used. Currents in A–C are typical examples in a whole-cell patch-clamp mode. D, Ca2+i facilitates activation kinetics of heat-evoked currents. Colored traces correspond to the ones shown in A–C. The arrow indicates the initial points of the currents (0%). One hundred percent of the current means maximal activation. Half of the maximal current is indicated as a gray line. E, Quantification of the time required for 33% (Δt1/3), 50% (Δt1/2), and 100% (Δtmax) of maximal activation of Painless by heat. Colored bars correspond to the ones shown in A–C. Data represent mean ± SEM. **p < 0.01 (n = 9–14). F, Heat responsiveness of Painless depends on Ca2+i concentration. Cs-Asp pipette solution including 1–104 nm Ca2+ (Ca2+ in the pipette) and Ca2+(−) bath solution [(−) in the gray area] were used. Maximal values of current density were obtained and fitted to Hill plots. The Ca2+i concentration required for eliciting half of the maximal current was 103.4 ± 6.2 nm (Hill coefficient of 3.4 ± 1.8). Data represent mean ± SEM (n = 8–18). The background currents were taken in each [Ca2+]i by applying heat to mock-transfected HEK293 cells and were subtracted from each point.
We also found that the activation kinetics varied depending on different Ca2+ conditions. In the presence of Ca2+o alone, heat-evoked currents developed gradually (Fig. 2A,D, orange), whereas Ca2+i elicited rapidly developing currents (Fig. 2B–D, blue, green). Indeed, the time required to generate 1/3 (Δt1/3) or one-half (Δt1/2) of the maximal activation of Painless by heat was significantly shorter in the presence of Ca2+i than that in the presence of Ca2+o alone (Fig. 2E). The inactivation rate during heat application also seemed to differ between the absence and presence of Ca2+i (Fig. 2A–C). However, the time required for full inactivation varied and sometimes inactivation never occurred in the presence of Ca2+i. Therefore, we did not pursue the difference in inactivation kinetics. We next evaluated the Ca2+i requirement for Painless during heating by changing Ca2+i concentration ([Ca2+]i) (Fig. 2F). The [Ca2+]i required for half activation by heat was 103.4 ± 6.2 nm; a Hill coefficient of ∼3.4. Ca2+ alone was not sufficient to activate Painless because we never observed visible currents in the presence of 2 mm Ca2+o and 1 mm Ca2+i (supplemental Fig. 1B, available at www.jneurosci.org as supplemental material). Together, Painless activity is coordinated by physiological levels of Ca2+i, permitting it to function as a heat sensor.
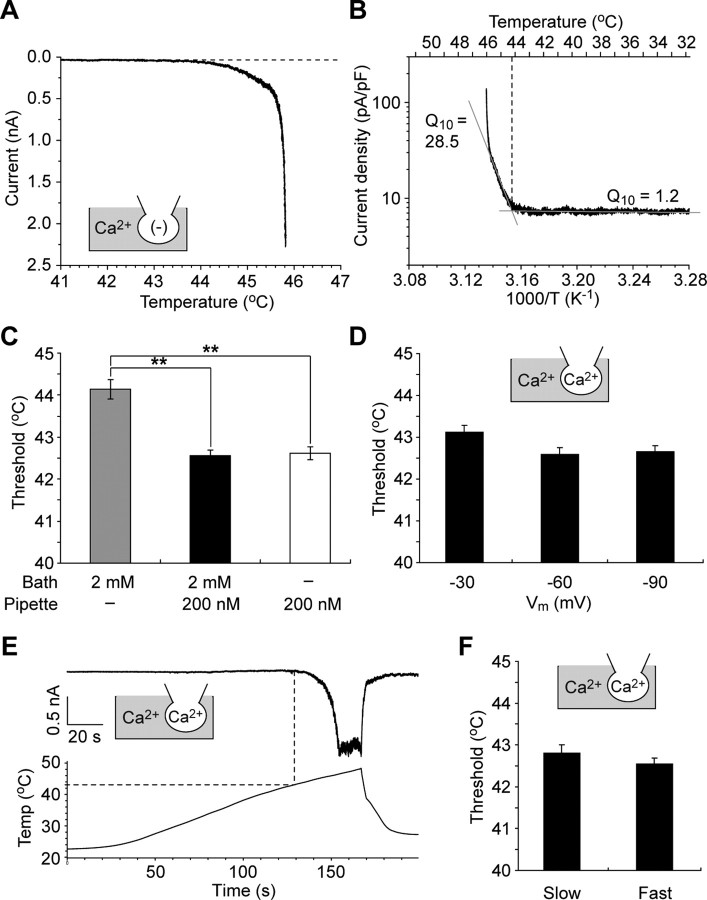
Temperature threshold for Painless activation is Ca2+ dependent
We determined the temperature threshold for Painless activation under different combinations of Ca2+o and Ca2+i. The temperature-response profile of Painless showed that currents were activated over ∼44°C in the presence of Ca2+o alone (Fig. 3A). To determine the temperature thresholds more precisely, we generated Arrhenius plots, which displayed an explicit flex point during heating (Fig. 3B). The basal Q10 value was 1.2, but it rapidly increased to 28.5 above the flex point. Because thermosensitive TRP channels are known to have Q10 values >10 (regarded as a thermosensitive reaction), we defined the flex point as a Q10 value sufficiently >10 as the temperature threshold for Painless activation, which was 44.1 ± 0.2°C when only Ca2+o was present (Fig. 3C, left). The temperature threshold in the presence of Ca2+o and Ca2+i was 42.6 ± 0.1°C, and the threshold in the presence of Ca2+i alone was 42.6 ± 0.2°C (Fig. 3C, middle, right). These values were significantly lower than the temperature threshold in the presence of Ca2+o alone. Thus, physiological levels of Ca2+i reduce the temperature threshold for Painless activation.
Figure 3.
Temperature thresholds of Painless activation. A, A representative temperature-response profile for heat-evoked Painless current in the presence of Ca2+o alone at −60 mV holding potential. The dotted line indicates basal level. B, An Arrhenius plot for heat-evoked Painless current shows a clear flex point on temperature dependency (data in A were converted). The crossing point of the two linear-fitted lines (a flex point) was defined as a temperature threshold for Painless activation. The Q10 value was calculated for each line (see Materials and Methods). C, Temperature thresholds for Painless activation are significantly lower in the presence of Ca2+i. Data represent mean ± SEM. **p < 0.01 (n = 9–14). All of the values were obtained from the same cells analyzed in Figure 2E. D, Temperature thresholds do not depend on membrane potential in the presence of Ca2+o and Ca2+i. Data represent mean ± SEM (n = 9–11). E, A representative trace shows activation currents of Painless elicited by slow heat application (0.2°C/s) in the presence of Ca2+o and Ca2+i. The dotted line indicates a temperature threshold. F, Temperature thresholds do not depend on heat application rate in the presence of Ca2+o and Ca2+i. Painless was stimulated with slow (0.2°C/s) or fast (1°C/s) heat application. Data represent mean ± SEM (n = 12–14).
It has been shown that mammalian thermosensitive TRP channels such as TRPV1 and TRPM8 have voltage dependency, and their temperature thresholds strongly depend on voltage (Voets et al., 2004). To test whether the temperature threshold for Painless has such a voltage dependency, the thresholds were determined under different membrane potentials (Fig. 3D). There was no significant difference in the thresholds among holding potentials of −30, −60, and −90 mV (thresholds were 43.1 ± 0.2°C at −30 mV, 42.6 ± 0.2°C at −60 mV, and 42.7 ± 0.1°C at −90 mV, respectively). The current sizes varied among samples probably because of different expression levels of Painless protein, but there was no correlation between current sizes and the temperature thresholds defined by our method in each membrane potential (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Additionally, we examined whether heat application rate affected the temperature threshold of Painless, because some thermosensitive TRP channels have been known to show such dependency, and, especially, some neurons in Drosophila have been reported to display increasing neural activity at temperatures as low as 28°C after slow increases in temperature (Tracey et al., 2003). Interestingly, however, slow heat application (∼0.2°C/s) activated Painless at 42.8 ± 0.2°C, which was not significantly different from that of fast heat application (42.6 ± 0.1°C, 1°C/s) (Fig. 3E,F). Together, it is likely that our method to determine the thresholds is suitable for this analysis, and the temperature thresholds for Painless activation are dependent on Ca2+i, but not on either membrane potential or heating rate.
Ca2+ sensitizes Painless with repeated heat stimulation
As described above, second heat application failed to activate Painless when the channel was fully inactivated during the first heat stimulation (Figs. 1C, 2B,C). However, multiple heat-evoked currents were attainable when heat stimulation was terminated at the early state of current development (Fig. 4A). Thermal stimulation elicited current activation more than three times in this repeated heating protocol. Utilizing such a protocol, we next asked whether Painless was sensitized after repetitive exposure to heat. In the presence of Ca2+o and Ca2+i, the temperature thresholds for activation on the second and third heat application were significantly reduced compared with the first one in the same cell (thresholds in the first, second, and third heat applications were 42.5 ± 0.1°C, 41.3 ± 0.3°C, and 41.2 ± 0.3°C, respectively) (Fig. 4B,D,F). In contrast, in the presence of Ca2+i alone, the temperature thresholds were not changed through the repeated heat application (thresholds in the first, second, and third heat applications were 42.7 ± 0.2°C, 42.5 ± 0.1°C, and 42.5 ± 0.2°C, respectively) (Fig. 4C,E,G). This result indicates that Painless is sensitized after repeated heat exposure under physiological extracellular and intracellular Ca2+ conditions.
Figure 4.
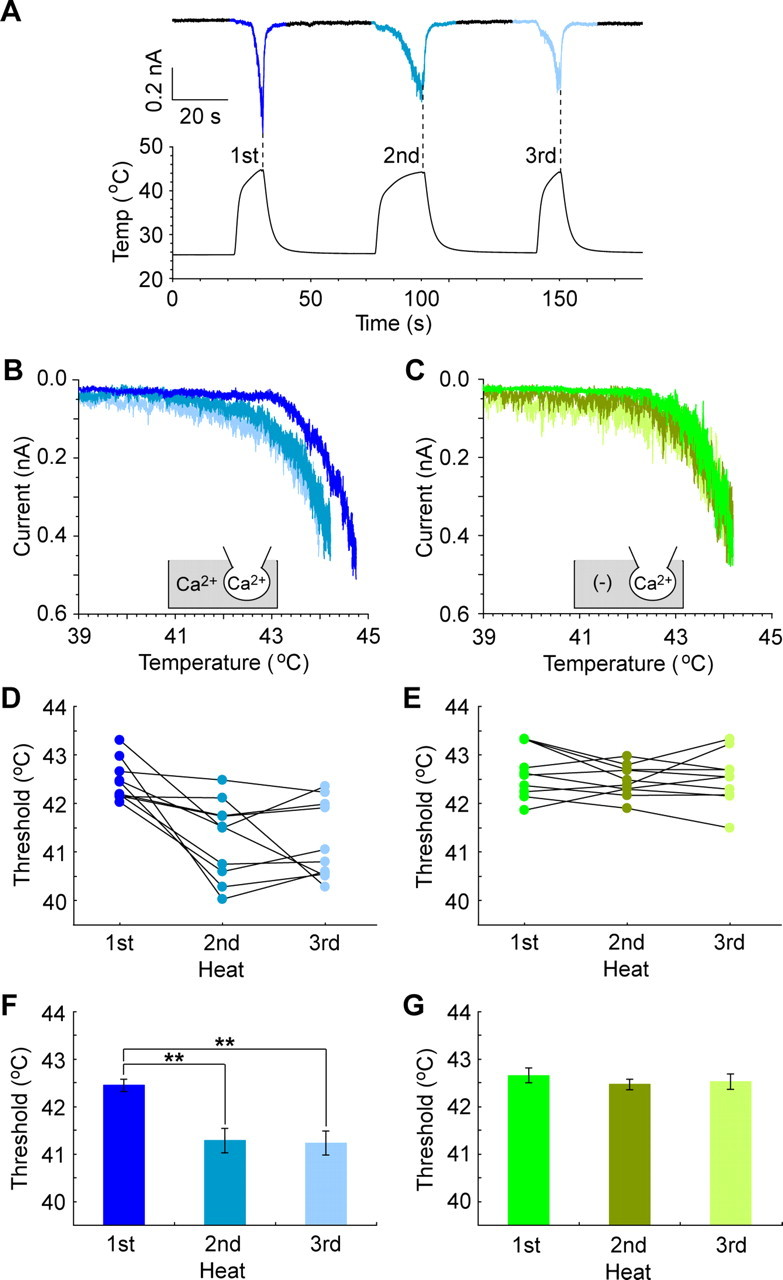
Temperature thresholds in repeated exposure to heat. A, Multiple currents are observed in the repeated heating protocol (see Results). Representative heat-evoked currents in the presence of Ca2+o and Ca2+i are shown. Heat application was terminated when the current reached ∼0.5 nA (dotted lines). More than three responses at equivalent currents could be observed without desensitization. B, C, Temperature thresholds are reduced after repeated heat with (B), but not without (C), Ca2+o. Standard (Ca2+ in gray area) or Ca2+(−) bath solution [(−) in gray area] and Cs-Asp/200 nm Ca2+ pipette solution (Ca2+ in the pipette) were used. D, E, Raw data of temperature thresholds for the first to third heating cycle are shown in individual cells (n = 10). Standard (D) or Ca2+ (−) bath solution (E) and Cs-Asp/Ca2+ 200 nm pipette solution were used. Thresholds were calculated as in Figure 2C. F, G, The temperature thresholds are significantly reduced after repeated heating in the presence of Ca2+o and Ca2+i (F), but not in the presence of Ca2+i alone (G). Data represent mean ± SEM. **p < 0.01 (n = 10). All of the colored traces, dots, and bars correspond to the first, second, and third heat applications indicated in F and G.
Painless is activated by heat in a membrane-delimited manner
To clarify whether Painless is directly activated by heat, we tried to observe the heat activation currents at the single-channel level. We chose a slow heat application protocol (∼0.2°C/s) (Fig. 3E), because the basal line fluctuated during fast heat application (∼1°C/s). In an inside-out patch-clamp configuration, heat-evoked single-channel currents were observed during heating with an apparent threshold of 42°C in Painless-expressing cells, and its unitary conductance was 49.6 ± 3.5 pS (Fig. 5A). Furthermore, Painless was rarely activated during heating in the absence of Ca2+o and Ca2+i, whereas Painless responded to heat when Ca2+ was applied to the cytoplasmic side in the same membrane (Fig. 5B). Interestingly, we sometimes observed Painless-mediated single-channel currents at innocuous temperatures (<30°C) (Fig. 5B, arrowhead). These results indicate that Painless is activated by heat in a membrane-delimited manner, and intracellular Ca2+ is required for its activation.
Figure 5.
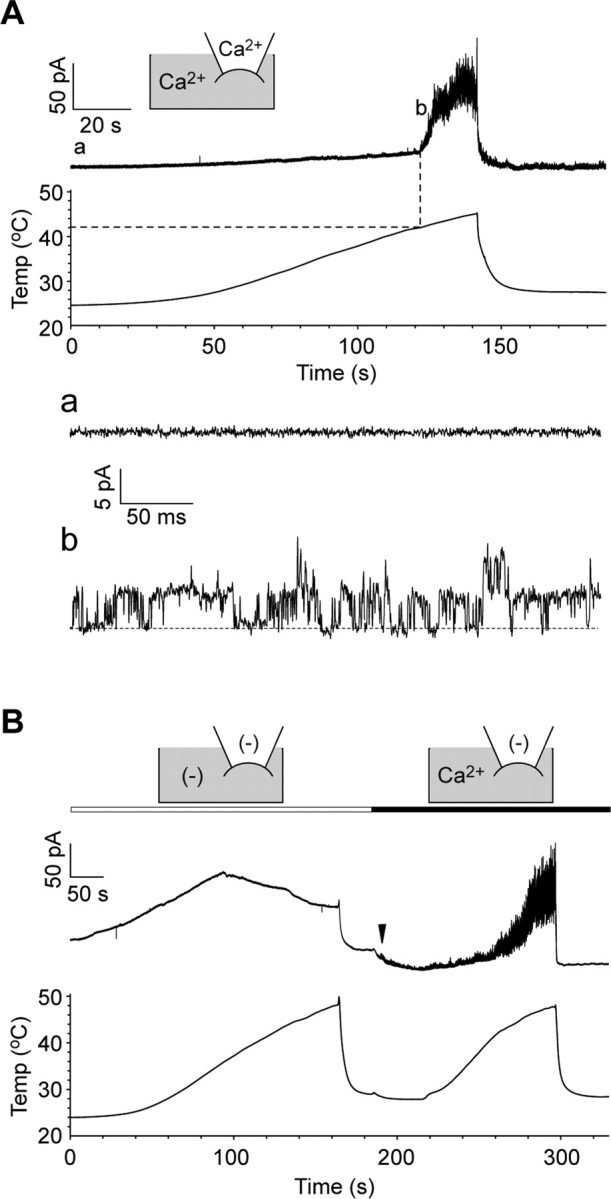
Single-channel activation of Painless during heating. A, The top trace shows activation currents in Painless-expressing excised membrane during slow heating (0.2°C/s) in an inside-out patch-clamp mode at +60 mV holding potential (n = 4). The dotted line indicates an initiation point of the currents. Standard pipette solution (Ca2+ in the pipette) and Cs-Asp/200 nm Ca2+ bath solution (Ca2+ in gray area) were used. The basal trace in a and single-channel currents in b are magnified from corresponding lines in the top trace. The dotted line in b indicates the closed-channel level. B, Painless is robustly activated by heat in the presence of Ca2+ on the cytoplasmic side (n = 6). Ca2+(−) pipette solution [(−) in the pipette] and Cs-Asp/Ca2+(−) bath solution [(−) in gray area] or Cs-Asp/200 nm Ca2+ bath solution (Ca2+ in gray area) were used. Note that movements in basal lines including leak always occurred in the absence of Ca2+o and Ca2+i, but those are clearly different from single-channel currents observed in the presence of Ca2+i. Moreover, small but apparent single-channel currents were evoked as soon as Ca2+ was applied to the cytoplasmic side before heat application (arrowhead).
Cytoplasmic regulatory region for Ca2+ in Painless
The results shown above demonstrate that intracellular Ca2+ is critical for the responsiveness of Painless to heat and it seems to directly regulate the activity of the channel. Intracellular Ca2+ directly activates mammalian TRPA1 via binding to its putative EF-hand-like motif in the cytoplasmic N-terminal region (Doerner et al., 2007; Zurborg et al., 2007). We compared the amino acid sequences of the N-terminal cytoplasmic region between Painless and mammalian TRPA1 and focused on a region in Painless that corresponds to the EF-hand-like motif in the mammalian TRPA1 (supplemental Fig. 3A, available at www.jneurosci.org as supplemental material). This region is located in the ankyrin repeat domain in Painless. We then investigated the possibility that this region is a Ca2+-regulatory region for Painless. Painless mutants bearing N356, S357, N363, or D366 substitutions to alanine were constructed, because each amino acid is functionally important in EF-hand-like motif in TRPA1 (Doerner et al., 2007; Zurborg et al., 2007). Heat was applied to HEK293 cells expressing the mutant Painless with or without Ca2+i, and the temperature thresholds were evaluated. Interestingly, in the presence of Ca2+o alone, the N363A mutant elicited small currents, whereas the other three mutants showed large currents during heating (Fig. 6A) (data not shown). All of the mutants including N363A exhibited heat-evoked currents similar to wild-type Painless in the presence of Ca2+o and Ca2+i (Fig. 6B, supplemental Fig. 3B–D, available at www.jneurosci.org as supplemental material). Additionally, the temperature threshold for activation of Painless N363A was 46.2 ± 0.2°C in the presence of Ca2+o alone, and 43.3 ± 0.1°C in the presence of Ca2+o and Ca2+i, both of which were significantly higher than those of wild-type Painless (44.1 ± 0.2°C in the presence of Ca2+o alone, 42.6 ± 0.1°C in the presence of Ca2+o and Ca2+i) (Fig. 6C). In contrast, temperature thresholds for activation of Painless N356A, S357A, and D366A were not different from those of wild-type Painless in the presence or the absence of Ca2+i (supplemental Fig. 3E, available at www.jneurosci.org as supplemental material). The [Ca2+]i required for half activation of Painless N363A by heat was 249.3 ± 59.8 nm, with a Hill coefficient of ∼0.9 (Fig. 6D), both of which were different from those of wild-type Painless (Fig. 2). Thus, this region may play a role as a Ca2+-regulatory site for heat activation of Painless.
Figure 6.
Effects of N-terminal mutation on heat responsiveness of Painless. A, B, Mutant Painless N363A shows small currents in the presence of Ca2+o alone (A), but exhibits large currents in the presence of Ca2+o and Ca2+i (B). Standard bath solution (Ca2+ in gray area) and Cs-Asp/Ca2+(−) pipette solution [(−) in the pipette] (A) or Cs-Asp/Ca2+ 200 nm pipette solution (Ca2+ in the pipette) (B) were used. Current traces are typical examples in a whole-cell patch-clamp mode (n = 10–14). C, Temperature thresholds for mutant Painless N363A are significantly higher than those for wild-type Painless in the presence of Ca2+o alone or in the presence of Ca2+o and Ca2+i. Data represent mean ± SEM. **p < 0.01 (n = 9–14). D, [Ca2+]i sensitivity of mutant Painless N363A was decreased. Cs-Asp pipette solution including 1–105 nm Ca2+ and Ca2+(−) bath solution were used. Maximal values of current density were obtained and fitted to Hill plots. Gray points and the fitted line indicate wild-type Painless (see also Fig. 2F). [Ca2+]i required for eliciting half of the maximal current was 249.3 ± 59.8 nm (Hill coefficient of 0.9 ± 0.2). Data represent mean ± SEM (n = 9–15). The background currents were taken in each [Ca2+]i by applying heat to mock-transfected HEK293 cells and were subtracted from each point.
Painless is insensitive to thermosensitive TRP channel activators in HEK293 cells
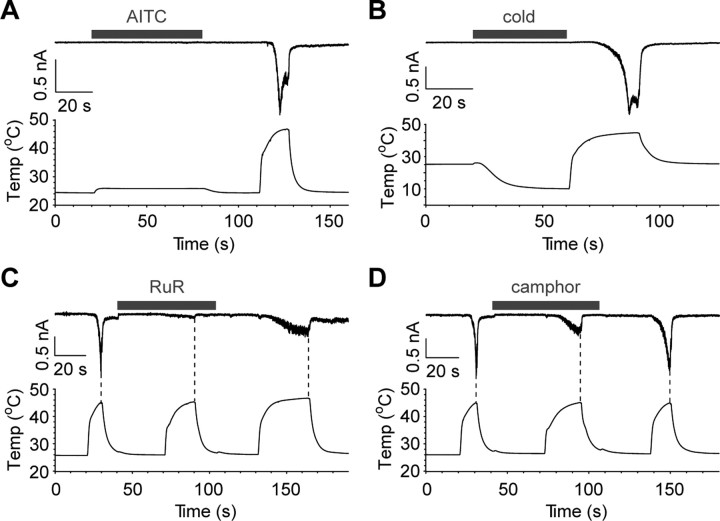
Painless was reported to be important for avoidance of wasabi as well as noxious heat (Al-Anzi et al., 2006). Wild-type flies tended to avoid food containing allyl isothiocyanate (AITC), which is the main ingredient of wasabi and mustard, in a dose-dependent manner. In contrast, painless mutant flies were defective in such avoidance behavior. The fact that Painless is most closely related to mammalian TRPA1, which is activated by AITC, implies that Painless might be a direct sensor for AITC. Surprisingly, however, data from Ca2+-imaging and patch-clamp experiments indicated that Painless-expressing HEK293 cells were completely insensitive to AITC (Fig. 7A, supplemental Fig. 4, available at www.jneurosci.org as supplemental material). We did not observe a positive response in Painless-expressing HEK293 cells up to 2 mm AITC. We also examined other TRPA1 activators, such as cold stimulus (∼10°C), cinnamaldehyde, allicin, icilin, acrolein, formalin, methyl paraben, menthol, and 2-APB. All failed to activate Painless expressed in HEK293 cells (Fig. 7B, supplemental Fig. 4, available at www.jneurosci.org as supplemental material) (data not shown). Typical thermosensitive TRP channel activators such as capsaicin, camphor and 4α-phorbol-12,13-didecanoate also failed to activate Painless (supplemental Fig. 5, available at www.jneurosci.org as supplemental material) (data not shown).
Figure 7.
The effects of mammalian TRPA1 agonists and antagonists on Painless. A, B, Painless-expressing cells are not activated by AITC (2 mm; A) or cold stimulation (∼10°C; B) (n = 3–5). Standard bath solution and Cs-Asp/200 nm Ca2+ pipette solution were used. Functional Painless expression was confirmed by heat application. C, D, Heat activation of Painless was reversibly blocked by ruthenium red (RuR, 10 μm; C) or camphor (3 mm; D) treatment (n = 6–8). Standard bath solution and Cs-Asp/200 nm Ca2+ pipette solution were used. The repeated heating protocol (Fig. 4) was used to validate the function of Painless before and after the treatment with antagonists. Heat termination points are indicated as dotted lines. Cells were treated with each antagonist, followed by application of heated bath solution containing antagonist. The inhibitory effects of ruthenium red were incompletely reversed. Higher concentrations of camphor could not be applied because camphor severely damaged the gigaohm seal.
Painless mutant flies reportedly display an increased threshold for mechanical stimulation compared with wild-type (Tracey et al., 2003). However, we failed to detect any intracellular Ca2+ increase or current activation in Painless-expressing HEK293 cells in response to super hypotonic (160 mOsm) or hypertonic (432 mOsm) stimulus, or by direct touch with a glass pipette tip (supplemental Fig. 5, available at www.jneurosci.org as supplemental material) (data not shown). To determine whether Painless responds to other hazardous stimuli or bitter substances, acidic/alkaline solution (pH 5 or 10) or caffeine were applied. None of them elicited activation of Painless (supplemental Fig. 5, available at www.jneurosci.org as supplemental material). Together, the only activator thus far identified for Painless is heat.
We next tested whether mammalian TRPA1 channel blockers affected heat-evoked Painless activation. Ruthenium red, a broad blocker for some TRP channels including TRPA1, reversibly inhibited heat-evoked activation of Painless (Fig. 7C). Camphor and menthol, which are well known agonists for TRPV3 and TRPM8, respectively, have been reported to inhibit mammalian TRPA1 activation (Xu et al., 2005; Macpherson et al., 2006; Karashima et al., 2007). Camphor (3 mm) could partially block heat-evoked currents of Painless, whereas 2 mm camphor could not suppress the heat activation of Painless (Fig. 7D) (data not shown). Heated solutions containing >3 mm camphor consistently broke the gigaohm seal so that we did not evaluate the effects of higher concentrations of camphor. However, menthol (2 mm) failed to block heat activation of Painless (data not shown). Problems similar to those experienced with heated camphor were encountered with heated menthol. Thus, we were unable to determine whether higher concentrations of menthol inhibited Painless activation. These results indicate that Painless shares some properties with the mammalian TRPA1.
Discussion
Painless is a direct heat sensor
The present study provides direct evidence that the Drosophila TRP channel Painless is a heat sensor. Although the biophysical properties of Painless have yet to be elucidated, members of the Drosophila TRPA subfamily, dTRPA1 and pyrexia, show temperature sensitivity both in vitro and in vivo, from which it has been inferred that Painless might also be a heat-sensitive channel (Montell, 2005). Indeed, a robust Ca2+ influx and current activation via Painless was observed during heating in our heterologous expression system (Fig. 1). The temperature threshold for Painless activation (∼42.6°C) was consistent with the temperature that causes avoidance behavior in vivo (Tracey et al., 2003). Painless was activated by heat in a membrane-delimited and Ca2+-dependent manner (Fig. 5), indicating that Painless can detect heat directly by using Ca2+i, but not intracellular signaling pathways. Thus, Painless itself may act as a primary heat detector to facilitate neural activity in vivo.
In vivo and in vitro analyses revealed different temperature thresholds. Previous work reported that there were two types of Painless-expressing neurons with low (∼28°C) and high (∼39°C) temperature thresholds (Tracey et al., 2003). However, the temperature thresholds of Painless in our system were 41 ∼ 44°C, rather than <40°C, in various heating conditions such as Ca2+o and Ca2+i concentrations, membrane potentials, or heat application rates. These differences might be due, in part, to the methods determining the thresholds. We defined each threshold as a reflex point with an increasing Q10 value >10 in Arrhenius plots. In this case, minuscule current development (Q10 < 10) was not regarded as the onset of Painless activation. However, magnitude of the background current does not affect the thresholds, because there was no correlation between current sizes and thresholds (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). We sometimes observed single-channel currents of Painless initiated at ∼28°C, a temperature close to the value at which neural activity differed between wild-type and the painless mutant (Tracey et al., 2003), during slow heat application (0.2°C/s) (Fig. 6). This result suggests that Painless has an ability to respond to lower temperature in vitro, which might contribute to neural excitability. Alternatively, there could be biological differences between in vitro and in vivo. The lipid composition of the mammalian plasma membrane may affect Painless activity differently than it would in its native environment. In fact, the functions of TRP channels, including thermosensitive ones, are regulated by a series of lipids (Hardie, 2007). Temperature threshold for activation of mammalian TRPV1, which is activated by noxious heat (>43°C) like Painless, has been reported to be reduced with phosphorylation by PKC (Numazaki et al., 2002), and it is also known that TRPV1 function is regulated through binding with specific accessory proteins (Kim et al., 2006, 2008). Such physiological regulation may also exist to alter Painless property in vivo.
Painless activation is regulated by extracellular and intracellular Ca2+
We determined four essential roles of Ca2+. First, Ca2+ enables Painless to respond to heat. Whereas higher heat (∼50°C) elicited only faint currents in the absence of Ca2+o and Ca2+i, 200 nm Ca2+i was sufficient for heat activation (Fig. 2), suggesting that Painless requires Ca2+i for functionality. Thermosensitive TRP channels such as TRPM4, TRPM5, and TRPA1 are activated by Ca2+i (Talavera et al., 2005; Nilius et al., 2006; Doerner et al., 2007; Zurborg et al., 2007), whereas Painless demands Ca2+ as a coagonist for heat. A similar concept has been reported in TRPM8, in that Ca2+i supports robust icilin-evoked responses (Chuang et al., 2004). [Ca2+]i required for half activation of Painless was ∼103 nm (Fig. 2), a concentration close to the reported value in the terminal and dorsal organ of the larval head (Liu et al., 2003).
Second, Ca2+ accelerates the activation kinetics of Painless. The longer time required for activation in the presence of Ca2+o alone (Fig. 2) is probably explained by a necessity for Ca2+o to first enter the cytoplasm until [Ca2+]i reaches the threshold for maximal activation, which results in gradual current development, followed by accelerated activation. In the presence of Ca2+i, Painless is activated quickly because sufficient Ca2+i for full activation exists. Indeed, fly larvae moved away from a heated probe within 0.4 s (Tracey et al., 2003), supporting this idea. Moreover, inactivation rates of Painless seemed to be affected by Ca2+ conditions (Fig. 2). Ca2+i might delay the inactivation; however, Ca2+i should increase during activation also in the presence of Ca2+o alone, which shows rapid inactivation. Nevertheless, Ca2+ is apparently important for regulating the activation kinetics.
Third, Ca2+ reduces temperature thresholds. Painless is “ready for activation” in the presence of Ca2+i, so that it may respond to heat at its reduced temperature threshold of ∼42.6°C (Fig. 3). However, the temperature threshold was ∼44.1°C in the presence of Ca2+o alone, where heat should initiate the gating of Painless in a Ca2+i-independent manner. Quick avoidance from a heated probe occurred at ∼42°C in fly larvae (Tracey et al., 2003); therefore, the in vivo temperature threshold is close to that obtained in the presence of Ca2+i. Thermosensitive TRP channels such as TRPV1 and TRPM8 have strong voltage dependencies in their temperature thresholds, whereas Painless did not (Fig. 3). This is not surprising because dual rectified I–V relationship of Painless is apparently different from outward rectified I–V relationship of TRPV1 and TRPM8.
Finally, Ca2+ contributes to sensitization of Painless during repetitive heating (Fig. 4). Significant reduction in the temperature thresholds during repeated heating was observed in the presence of Ca2+o and Ca2+i, but not in the presence of Ca2+i alone (Fig. 4), suggesting that Ca2+o and/or Ca2+ influx may be involved in the sensitization. This would be physiologically important, because flies are able to escape from a hazardous heat source in less time after second exposure. Sensitization during repetitive heating is a common feature in TRPV1, TRPV2, and TRPV3 (Caterina et al., 1999; Xu et al., 2002), although the underlying mechanism is still unknown including the requirement for Ca2+.
N-terminal region of Painless is involved in Ca2+-dependent heat activation
Recently, mammalian TRPA1 was reported to be activated by Ca2+ (Doerner et al., 2007; Zurborg et al., 2007). Ca2+i-dependent activation significantly deteriorated when the EF-hand-like motif in the N-terminal region was mutated. We compared amino acid sequences between Painless and TRPA1 and determined the candidate region for Ca2+i regulation in Painless, which was located in the ankyrin repeat domain (Fig. 6, supplemental Fig. 3, available at www.jneurosci.org as supplemental material). The Painless N363A mutant displayed small heat-evoked currents, increased temperature thresholds, and higher [Ca2+]i requirement for half activation with a reduced Hill coefficient (Fig. 6). These features could be explained if the “Ca2+-regulatory region” in Painless included N363, and its affinity to Ca2+i was reduced by mutation. Thus, the mutant channel requires increasing [Ca2+]i to be fully activated, which results in a higher temperature threshold and small size in currents. These results suggest that N363 is a key residue in Ca2+ sensitivity of Painless, although the function is different from the EF-hand-like motif in mammalian TRPA1. Painless is not activated by high [Ca2+]i alone (supplemental Fig. 1, available at www.jneurosci.org as supplemental material) and requires much less [Ca2+]i for its regulation, whereas TRPA1 requires Ca2+ at a micromolar level for activation. Furthermore, mutation of N356, S357, or D366 did not affect the heat responsiveness of Painless, whereas the corresponding amino acids in the EF-hand-like motif are necessary for Ca2+i-dependent TRPA1 activation.
Painless is insensitive to thermosensitive TRP channel activators in HEK293 cells
Thermosensitive TRP channels can be activated by various stimuli and one stimulant sometimes activates multiple thermosensitive TRP channels (Dhaka et al., 2006; Ramsey et al., 2006; Tominaga, 2007). Accordingly, Painless could mediate several stimuli such as AITC or a mechanical stimulus (Tracey et al., 2003; Al-Anzi et al., 2006). However, Painless-expressing HEK293 cells did not respond to a range of possible activators (Fig. 7, supplemental Figs. 4, 5, available at www.jneurosci.org as supplemental material). Recently, AITC was reported to activate mammalian TRPA1 through covalent modification (Hinman et al., 2006; Macpherson et al., 2007). However, several cysteines important for covalent modification were not present in Painless (supplemental Fig. 3, available at www.jneurosci.org as supplemental material), which might explain its insensitivity to AITC, cinnamaldehyde, allicin, acrolein, and formalin. Thus, Painless is not likely to be a direct receptor for these cysteine modifiers. Alternatively, interaction with accessory protein(s), formation of heteromeric channel, and/or splice variants might alter properties of Painless in vivo. These possibilities remain to be addressed. Painless is expressed in the CNS as well as peripheral sensory neurons (Tracey et al., 2003; Al-Anzi et al., 2006), where Painless could be activated by endogenous ligands. Heat activation of Painless was inhibited by ruthenium red and camphor, indicating that Painless shares some properties with mammalian TRPA1. Camphor, a wood derivative from camphor laurel, has been used as a repellent for pests and proved to be effective for mosquitoes (Gillij et al., 2007). The repellent may inhibit the noxious heat sensor, perhaps interfering with the normal sensing ability of flies. It would therefore be intriguing to test the effects of camphor at the behavioral level.
Footnotes
This work was supported by a grant from the Japan Society for the Promotion of Science in Japan (T.S.). We thank N. Fukuta for technical assistance and Drs. K. Shibasaki and H. Inada for discussion.
References
- Adams DJ, Dwyer TM, Hille B. The permeability of endplate channels to monovalent and divalent metal cations. J Gen Physiol. 1980;75:493–510. doi: 10.1085/jgp.75.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Anzi B, Tracey WD, Jr, Benzer S. Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr Biol. 2006;16:1034–1040. doi: 10.1016/j.cub.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R64–R76. doi: 10.1152/ajpregu.00446.2006. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Chuang HH, Neuhausser WM, Julius D. The super-cooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel. Neuron. 2004;43:859–869. doi: 10.1016/j.neuron.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D'Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Dhaka A, Viswanath V, Patapoutian A. TRP ion channels and temperature sensation. Annu Rev Neurosci. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Dhaka A, Earley TJ, Watson J, Patapoutian A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci. 2008;28:566–575. doi: 10.1523/JNEUROSCI.3976-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007;282:13180–13189. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- Gillij YG, Gleiser RM, Zygadlo JA. Mosquito repellent activity of essential oils of aromatic plants growing in Argentina. Bioresour Technol. 2007;99:2507–2515. doi: 10.1016/j.biortech.2007.04.066. [DOI] [PubMed] [Google Scholar]
- Hardie RC. TRP channels and lipids: from Drosophila to mammalian physiology. J Physiol. 2007;578:9–24. doi: 10.1113/jphysiol.2006.118372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Högestätt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, Nilius B. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci. 2007;27:9874–9884. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AY, Tang Z, Liu Q, Patel KN, Maag D, Geng Y, Dong X. Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell. 2008;133:475–485. doi: 10.1016/j.cell.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kang C, Shin CY, Hwang SW, Yang YD, Shim WS, Park MY, Kim E, Kim M, Kim BM, Cho H, Shin Y, Oh U. TRPV1 recapitulates native capsaicin receptor in sensory neurons in association with Fas-associated factor 1. J Neurosci. 2006;26:2403–2412. doi: 10.1523/JNEUROSCI.4691-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Lee Y, Lee Y, Lee J, Bang S, Hyun S, Kang J, Hong ST, Bae E, Kaang BK, Kim J. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat Genet. 2005;37:305–310. doi: 10.1038/ng1513. [DOI] [PubMed] [Google Scholar]
- Liu CH, Wang T, Postma M, Obukhov AG, Montell C, Hardie RC. In vivo identification and manipulation of the Ca2+ selectivity filter in the Drosophila transient receptor potential channel. J Neurosci. 2007;27:604–615. doi: 10.1523/JNEUROSCI.4099-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Yermolaieva O, Johnson WA, Abboud FM, Welsh MJ. Identification and function of thermosensory neurons in Drosophila larvae. Nat Neurosci. 2003;6:267–273. doi: 10.1038/nn1009. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci. 2006;32:335–343. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Mohapatra DP, Wang SY, Wang GK, Nau C. A tyrosine residue in TM6 of the Vanilloid Receptor TRPV1 involved in desensitization and calcium permeability of capsaicin-activated currents. Mol Cell Neurosci. 2003;23:314–324. doi: 10.1016/s1044-7431(03)00054-x. [DOI] [PubMed] [Google Scholar]
- Montell C. Drosophila TRP channels. Pflugers Arch. 2005;451:19–28. doi: 10.1007/s00424-005-1426-2. [DOI] [PubMed] [Google Scholar]
- Nagata K, Duggan A, Kumar G, García-Añoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, Vennekens R, Voets T. The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J. 2006;25:467–478. doi: 10.1038/sj.emboj.7600963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y, Hosokawa H, Hori A, Matsumura K, Kobayashi S. Cold sensitivity of recombinant TRPA1 channels. Brain Res. 2007;1160:39–46. doi: 10.1016/j.brainres.2007.05.047. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Strotmann R, Schultz G, Plant TD. Ca2+-dependent potentiation of the nonselective cation channel TRPV4 is mediated by a C-terminal calmodulin binding site. J Biol Chem. 2003;278:26541–26549. doi: 10.1074/jbc.M302590200. [DOI] [PubMed] [Google Scholar]
- Takashima Y, Daniels RL, Knowlton W, Teng J, Liman ER, McKemy DD. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J Neurosci. 2007;27:14147–14157. doi: 10.1523/JNEUROSCI.4578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 2005;438:1022–1025. doi: 10.1038/nature04248. [DOI] [PubMed] [Google Scholar]
- Tominaga M. The role of TRP channels in thermosensation. In: Liedtke WB, editor. TRP ion channel function in sensory transduction and cellular signaling cascades. New York: CRC; 2007. pp. 271–286. [PubMed] [Google Scholar]
- Tong Q, Zhang W, Conrad K, Mostoller K, Cheung JY, Peterson BZ, Miller BA. Regulation of the transient receptor potential channel TRPM2 by the Ca2+ sensor calmodulin. J Biol Chem. 2006;281:9076–9085. doi: 10.1074/jbc.M510422200. [DOI] [PubMed] [Google Scholar]
- Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, Hwang SW, Patapoutian A, Jegla T. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- Xiao R, Tang J, Wang C, Colton CK, Tian J, Zhu MX. Calcium plays a central role in the sensitization of TRPV3 channel to repetitive stimulations. J Biol Chem. 2008;283:6162–6174. doi: 10.1074/jbc.M706535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- Xu H, Blair NT, Clapham DE. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J Neurosci. 2005;25:8924–8937. doi: 10.1523/JNEUROSCI.2574-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SY, Cang CL, Liu XF, Peng YQ, Ye YZ, Zhao ZQ, Guo AK. Thermal nociception in adult Drosophila: behavioral characterization and the role of the painless gene. Genes Brain Behav. 2006;5:602–613. doi: 10.1111/j.1601-183X.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nat Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]