Abstract
Lbx1 is a transcription factor that determines neuronal cell fate and identity in the developing medulla and spinal cord. Newborn Lbx1 mutant mice die of respiratory distress during the early postnatal period. Using in vitro brainstem–spinal cord preparations we tested the hypothesis that Lbx1 is necessary for the inception, development and modulation of central respiratory rhythmogenesis. The inception of respiratory rhythmogenesis at embryonic day 15 (E15) was not perturbed in Lbx1 mutant mice. However, the typical age-dependent increase in respiratory frequency observed in wild-type from E15 to P0 was not observed in Lbx1 mutant mice. The slow respiratory rhythms in E18.5 Lbx1 mutant preparations were increased to wild-type frequencies by application of substance P, thyrotropin releasing hormone, serotonin, noradrenaline, or the ampakine drug 1-(1,4-benzodioxan-6-yl-carbonyl) piperidine. Those data suggest that respiratory rhythm generation within the pre-Bötzinger complex (preBötC) is presumably functional in Lbx1 mutant mice with additional neurochemical drive. This was supported by anatomical data showing that the gross structure of the preBötC was normal, although there were major defects in neuronal populations that provide important modulatory drive to the preBötC including the retrotrapezoid nucleus, catecholaminergic brainstem nuclei, nucleus of the solitary tract, and populations of inhibitory neurons in the ventrolateral and dorsomedial medullary nuclei. Finally, we determined that those defects were caused by abnormalities of neuronal specification early in development or subsequent neuronal migration.
Keywords: preBötzinger complex, transcription factor, apnea, medulla, fetal, Bötzinger
Introduction
Lbx1, the vertebrate homolog of the Drosophila ladybird gene (Jagla et al., 1995; Dietrich et al., 1998) is expressed in developing skeletal muscle and nervous system. This transcription factor is an important determinant of spinal dorsal neurons and somatosensory neurons in the hindbrain (Gross et al., 2002; Müller et al., 2002; Sieber et al., 2007). In the spinal cord, the inactivation of Lbx1 alters the developmental program resulting in Lbx1-deficient spinal neurons acquiring a more dorsally derived commissural interneuron phenotype (Gross et al., 2002; Müller et al., 2002). Furthermore, Lbx1 is expressed by both excitatory glutamatergic and inhibitory GABAergic/glycinergic neurons in the embryonic spinal cord (Cheng et al., 2005). The loss of Lbx1 selectively eliminates these inhibitory neurons. Sieber et al. (2007) recently described Lbx1-derived neurons as a major component of the spinal trigeminal tract (SpV) in the brainstem. Here, our analysis of the brainstem revealed a wider distribution of Lbx1-derived neurons in the medulla, including in major respiratory nuclei described in this study.
Lbx1 mutant mice die shortly after birth from apparent respiratory distress. In this study, we tested the hypothesis that the breathing abnormality arises from defective central respiratory drive. Specifically, we used in vitro preparations to determine whether Lbx1 expression is necessary for the inception and development of rhythmic respiratory activity within the pre-Bötzinger complex (preBötC). Furthermore, we assessed the importance of Lbx1 expression for the development of medullary networks that modulate central respiratory rhythmogenesis. Neurotransmitter receptor agonists, antagonists and modulators were administered to assess the ability of neuromodulatory systems to normalize respiratory rhythmogenesis in mutant mice.
Anatomical studies using a combination of markers (transcription factors, neurotransmitter synthesizing enzymes, and peptide receptors) were then performed to determine whether there are structural abnormalities within pontomedullary structures that could account for the respiratory phenotype identified by electrophysiological recordings. This includes the preBötC, retrotrapezoid nucleus (RTN), catecholaminergic brainstem nuclei, nucleus of the solitary tract (NTS), and populations of inhibitory neurons in the ventrolateral and dorsomedial medullary nuclei.
Materials and Methods
Generation and genotyping of mice.
The generation of Lbx1GFP(neo) has been described previously (Gross et al.,2002). Identification of mutant offspring was performed by PCR genotyping of snap frozen tissue with the specific primers for Lbx1 (MKG396, CAG CTG CAG AAG CCA GGA CTG; MKG321, CCG GAC ACG CTG AAC TTG TGG; MKG333, ATG ACT TCC AAG GAG GAC GGCA), green fluorescent protein (GFP)/yellow fluorescent protein (YFP) (GCA CGA CTT CTT CAA GTC CGC CAT GCC; GCG GAT CTT GAA GTT CAC CTT GAT GCC, 280 bp), Amplification of mutant and wild-type Lbx1 alleles generated diagnostic bands of 315 and 445 bp, respectively.
Brainstem–spinal cord preparations.
Newborn mouse pups (within 15 min of birth) were anesthetized with metofane. Prenatal mice at embryonic day 15.5 (E15.5)–E18.5 were delivered from timed-pregnant mice anesthetized with halothane and maintained at 37°C by radiant heat. All procedures used in this study were approved by the Animal Welfare Committee at the University of Alberta. Newborn pups and embryos were decerebrated and the brainstem–spinal cord with the diaphragm muscle attached was dissected following procedures similar to those established for perinatal rats (Smith et al., 1990; Greer et al., 1992). The neuraxis was continuously perfused at 27 ± 1°C (perfusion rate 5 ml/min, chamber volume of 1.5 ml) with Kreb's solution that contained (in mm): 128 NaCl, 3.0 KCl, 1.5 CaCl2, 1.0 MgSO4, 24 NaHCO3, 0.5 NaH2PO4, and 30 d-glucose equilibrated with 95%O2/5%CO2, pH 7.4.
Recording and analysis.
Recordings of C4 ventral roots and diaphragm electromyogram (EMG) were made with suction electrodes. Signals were amplified, rectified, low-pass filtered and recorded on computer using an analog–digital converter (Digidata 1200; Molecular Devices) and data acquisition software (Axoscope; Molecular Devices). Mean values relative to control for the period of respiratory motoneuron discharge were calculated from a minimum of 30 consecutive bursts. Values given are means and SDs. Statistical significance was tested using one way ANOVA with Holm-Sidak test; significance was accepted at p values lower than 0.05.
Plethysmographic measurements.
Whole-body plethysmographic measurements of the frequency and depth of breathing were made from unrestrained newborn mice using a pressure transducer (model DP 103; Validyne) and signal conditioner (CD-15; Validyne).
Pharmacological agents.
All drugs were purchased from Sigma. The ampakine CX546 was dissolved in dimethylsulfoxide to make a 50–200 mm stock solution.
Animal handling for anatomical studies.
Timed pregnant mice at embryonic ages (E)10 to E18 were anesthetized with halothane (1.5% delivered in 95% O2 and 5% CO2) and fetal mice were delivered and fixed by immersion in 4% paraformaldehyde in phosphate buffer (PB) at pH 7.2 (<E15). Postnatal mice were anesthetized by hyperthermia. Mice older than E15 were transcardially perfused with the same fixative solution. Brainstems were dissected and postfixed before sectioning with a vibratome (VT1000S; Leica) or cryoprotected and sectioned on a cryostat (Bright Instruments). All procedures used in this study were approved by the Animal Welfare Committee at the University of Alberta or Washington University in St. Louis.
Immunohistochemistry.
Mutant and wild-type mice within the same litter were processed together for comparisons. Immunohistochemical detection of GFP with a specific antibody improved the resolution for the detection of GFP-expressing (GFP+) cells (Gross et al., 2002). Detailed protocols for the immunohistochemistry experiments have been reported previously (Pagliardini et al., 2003). In brief, transverse vibratome (50 μm) or cryostat (20 μm) sections were incubated with 1.0% BSA or 10% normal horse serum and 0.2–0.3% Triton X-100 in PBS for 60 min and then incubated overnight with primary antibodies diluted in PBS, 0.1% BSA and 0.2–0.3% Triton X-100. Primary antibodies used for this study were as follows: goat anti-choline acetyl transferase (ChAT; 1:300, Millipore Bioscience Research Reagents), rabbit anti-somatostatin (SST; 1:1000, Immunostar), rabbit anti-green fluorescent protein (GFP; 1:500, Invitrogen), chicken anti-GFP (1:500, Aves Labs), rabbit anti-neurokinin 1 receptor (NK1R; 1:1000, Advance Targeting System), rabbit anti-paired homeobox domain transcription factor 2 (Pax2, 1:500, Zymed Labs/Invitrogen), rabbit anti-tyrosine hydroxylase (TH; 1:2000, Millipore Bioscience Research Reagents), rabbit anti-winged helix/Forkhead transcription factor P2 (FoxP2; 1:700, AbCam), guinea pig anti-LIM homeodomain transcription factor 1b (Lmx1b; 1:500, kindly provided by Dr. T. M. Jessell, Columbia University, New York, NY), and rabbit anti-paired-like homeobox 2b (Phox2b; 1:500, kindly provided by Dr. J. F. Brunet, CNRS, Paris, France).
The following day, sections were washed and incubated with specific secondary antibodies diluted in PBS and 0.1% BSA for 2 h (Cy3-, Cy5- or Cy2-conjugated donkey anti-rabbit, donkey anti-goat, donkey anti-guinea pig; 1:200; Jackson ImmunoResearch; or Alexa488-conjugated anti-rabbit, donkey anti-chicken; 1:1000, Invitrogen). Sections were further washed, mounted, and coverslipped.
In situ hybridization for GlyT2.
Slides are immersed in 4% PFA, permeablized with proteinase K, washed in 0.1 m triethanolamine-HCl with 0.25% acetic anhydride, blocked in hybridization buffer at 65°C, then placed into slide mailers containing hybridization buffer with DIG-labeled antisense RNA at 1 μg/ml overnight at 65°C. Slides are washed in SSC buffers at 62°C. Slides are washed and incubated in alkaline phosphatase conjugated anti-DIG antibody in 10% NHS and then incubated in NBT-BCIP until cellular labeling is clear. For combined immunohistochemistry and in situ hybridization, slides are stained for mRNA expression before immunohistochemical labeling.
Bright-field and confocal imaging.
Fluorescently immunostained sections were examined and processed using a Zeiss 100M microscope, LSM510 NLO laser, and LSM510 software (Zeiss) or an Improvision OptiGrid structured illumination confocal using a Photometrics HQ2 camera on a Nikon 90i microscope with Phylum Software (Improvision). Thin sections and multiple sectioning acquisitions along the z-plane were performed to obtain a suitable signal through the depth of the section. Combined alkaline phosphatase in situ hybridization and fluorescently immunostained sections were acquired in bright field and then fluorescence. Acquired images in JPEG format were then exported to Photoshop for figure composition and preparation.
Surface areas of vagus (X), nucleus ambiguus (NA) and hypoglossal (XII) nuclei were measured bilaterally for each section and an average area was calculated for each animal using LSM510 software. Noradrenergic cells immunolabeled for TH were counted in serial sections at E18 and an average and SD of cells/nucleus calculated. Paired t tests comparing Lbx1GFP/GFP mice to Lbx1GFP/+ litter mates were applied to determine statistical significance at p < 0.05.
Results
Respiratory rhythm generation by wild-type and Lbx1 mutant in vitro preparations
In preliminary experiments, newborn mouse pups were monitored immediately after delivery. All Lbx1GFP/GFP mice displayed both abnormal limb morphology and profound apnea; alternatively referred to as Lbx1 mutants in the paper. Previous studies have demonstrated that the expression of one copy of Lbx1 gene is sufficient to promote normal embryonic development (Gross et al., 2002; Müller et al., 2002). Consistent with this, we did not find any differences in the respiratory patterns generated by Lbx1+/+ and Lbx1GFP/+ mice. Lbx1GFP/+ mice were used as controls for this study and are also referred to as wild-type in the remainder of the paper. To determine whether the respiratory phenotype was caused by abnormal central respiratory drive, the brainstem–spinal cords with diaphragm muscle still attached were isolated from wild-type and mutant preparations. Figure 1A shows representative diaphragm EMG recordings obtained in the perinatal period, between E15.5 and postnatal day (P0). Both mutant and wild-type preparations commenced generating respiratory activity in vitro at E15. Furthermore, there were no differences in the frequency of respiratory discharge in vitro at that age. However, the frequency of respiratory rhythm was much slower in mutants relative to wild-type preparations after E15.5 (Fig. 1B). Population data show interburst intervals of 3.9 ± 1.2 s (n = 4) and 55 ± 25 s (n = 4) in wild-type and mutant P0 preparations. Figure 1C shows representative traces of plethysmographic recordings of newborn Lbx1+/+ and Lbx1GFP/+ mice.
Figure 1.
Respiratory rhythmic activity generated by perinatal wild-type and Lbx1 mutant mice. A, Rectified and integrated EMG recordings from the diaphragm in brainstem–spinal cord–diaphragm preparations isolated from Lbx1 mutant and wild-type mice of different perinatal stages. The frequency of rhythmic respiratory discharge increased with age in wild-type preparations but remained relatively slow and unstable in Lbx1 mutant preparations. Population data were summarized in B. Each data point was from 5, 6, 21, and 4 Lbx1 mutants, 5, 6, 16, and 4 wild-type mice for E15.5, E16.5, E18.5, and P0, respectively. *p < 0.05 between Lbx1 mutant and wild-type of the same age group. C, Plethysmographic recordings of newborn Lbx1+/+ and Lbx1GFP/+ mice.
All subsequent recordings were performed from in vitro preparations isolated from E18.5 mice delivered via caesarian section. This negated any potentially confounding problems associated with measurements from mutant newborn mouse preparations that may have had altered CNS function because of the hypoxia associated with postnatal hypoventilation.
A total of 45 Lbx1 mutant and 39 wild-type mice were subsequently selected for detailed analyses. For the first studies of E18.5 mice, the respiratory rhythm was monitored using brainstem–spinal cord preparations with the diaphragm muscle attached. Respiratory rhythmic bursting was apparent in population recordings of XII nerve roots and diaphragm EMG recordings in those preparations. The mean interval between inspiratory bursts was 4.2 ± 1.6 s (n = 16) in wild-type and 46 ± 18 s (n = 21) in Lbx1 mutant preparations. In addition to the respiratory activity, a second longer-duration (>3 s) bursting activity was prominent on XII nerve recordings in all E18.5 Lbx1 mutant preparations (Fig. 2). This type of nonrespiratory rhythm was seldom observed in wild-type preparations at the same stage of development. The longer-duration, nonrespiratory rhythm had the same characteristics described previously for fetal mice and rats (Greer et al., 1992a; Pagliardini et al., 2003; Ren and Greer, 2003; Thoby-Brisson et al., 2005). These spontaneously generated fetal rhythms are typically generated throughout the neuraxis at earlier stages of rodent development but cease occurring by E16 in mice (Thoby-Brisson et al., 2005).
Figure 2.
Lbx1 mutant in vitro brainstem–spinal cord preparations generated nonrespiratory rhythmic neural activity at E18.5. A, Rectified and integrated suction electrode recordings of diaphragm EMG and hypoglossal nerve root activity (XII) in brainstem–spinal cord–diaphragm preparations (BSSCD) isolated from E18.5 wild-type and Lbx1 mutant mice. Recordings were from preparations with (1) pons intact, (2) medulla intact and (3) without the rostral medulla. After removal of the pons, the rhythmic respiratory activity in wild-type preparations increased in frequency and irregularity. The rhythmic respiratory discharge in wild-type preparations was not significantly altered after removal the rostral medulla (RM). The motor discharge was markedly slower in all Lbx1 mutant preparations, without a significant change in frequency, amplitude, duration, or regularity upon transection of the pons and rostral medulla. Recordings from Lbx1 mutant hypoglossal nerve roots showed an additional longer-duration, nonrespiratory rhythm (#), that was not observed in wild-type preparations at E18.5. B, Population data showing the frequency of both respiratory and nonrespiratory rhythm for each type of brainstem–spinal cord–diaphragm preparation. Each data point was from four to five preparations tested. *p < 0.05, compared with wild-type.
These data demonstrate that Lbx1 mutant mouse preparations generate a very slow rate of inspiratory bursting, which in vivo would cause rapid hypoxia and inability to survive. Although, the basic inspiratory rhythm is thought to be generated within the preBötC, neurons in that region receive synaptic input and interact with respiratory nuclei in the pons, including the A5 noradrenergic group. The parafacial respiratory group (pFRG) is also thought to be coupled with and modulate the activity of the preBötC (Onimaru and Homma, 2003; Dutschmann et al., 2004). We therefore performed a series of experiments recording respiratory discharge in Lbx1 mutant preparations before and after removal of the pons and rostral medulla to test whether those structures were contributing to the abnormal respiratory rhythm in vitro.
Figure 2A shows examples of recordings from each type of preparation derived from wild-type and Lbx1 mutant preparations. Past reports state that E18.5 mouse brainstem–spinal cord preparations typically fail to generate a respiratory rhythm with the pons intact (Viemari and Gérard, 2002). However, all of our E18.5 wild-type brainstem–spinal cord–diaphragm preparations with the pons attached generated a robust stable rhythm with a frequency of 5.4 ± 3.4 bursts/min (n = 4). The removal of the pons in wild-type preparations resulted in the coefficient of variability increasing more than twofold (0.53 ± 0.10 with pons vs 1.22 ± 0.14 without pons, n = 5) and overall bursting frequency increasing to 16.9 ± 6.7 bursts/min (n = 5) (population data is shown in Fig. 2B). With the pons removed, the characteristic periodic pattern of motor discharge often seen in mouse, but only occasionally in rat preparations, emerged. Specifically, a train of shorter duration bursts was followed by a single burst of larger amplitude and longer duration which in turn was often followed by a short cessation of activity before the recommencement of shorter duration bursting (Fig. 2A, middle). We did not analyze relative changes in those various discharge burst patterns. The further removal of rostral medulla in wild-type preparations caused no significant changes in the frequency (12.1 ± 4.6 bursts/min without rostral medulla) of respiratory rhythm. All three types of preparations isolated from Lbx1 mutant mice generated much slower, yet regular, respiratory rhythms relative to corresponding wild-type preparations (n = 5). The removal of the pons and rostral medulla did not significantly affect the rhythmic discharge in mutant preparations. In addition, the longer duration, nonrespiratory rhythms were prominent in all three types of Lbx1 mutant preparations.
Neuromodulation of respiratory rhythm in E18.5 Lbx1 mutant mice
The slower respiratory rhythms generated by E18.5 Lbx1 mutant versus control could be attributable to an inability of the preBötC rhythm generating network to generate faster rhythms. Alternatively, it may reflect defects in the neuromodulatory systems that provide conditioning excitatory/inhibitory drive to the preBötC or a lack of sufficient synaptic drive among neurons within the preBötC network. To determine whether the respiratory frequency could be increased, we bath applied neuromodulators known to provide excitatory drive to the preBötC. This included substance P (SubP; 1 μm), thyrotropin releasing hormone (TRH; 1 μm), serotonin (5-HT; 25 μm), or noradrenaline (25 μm) (Al-Zubaidy et al., 1996; Greer et al., 1996; Viemari and Gérard, 2002; Pagliardini et al., 2003). We also applied the ampakine drug 1-(1-4-benzodioxan-6-yl-carbonyl) piperidine (CX546) (200 μm), a positive modulator of AMPA receptors, that has been shown to increase respiratory frequency by increasing the efficacy of endogenous glutamatergic synaptic drive (Ren et al., 2006). Diaphragm EMG recordings from brainstem–spinal cord–diaphragm preparations were used to monitor respiratory rhythm. Figure 3 shows that all of the excitatory agents caused a very pronounced increase in the frequency of respiratory rhythm in E18.5 Lbx1 mutant preparations. These data demonstrate that Lbx1 mutant mice at E18.5 are capable of generating robust respiratory rhythms similar to wild-type mice when provided with the necessary excitatory conditioning neurochemical drive.
Figure 3.
Effects of excitatory neuromodulators on respiratory rhythm generated by E18.5 Lbx1 mutant brainstem–spinal cord–diaphragm preparations. A, Examples of integrated, rectified diaphragm EMG recordings from the brainstem–spinal cord–diaphragm preparations of E18.5 Lbx1 mutant mice show excitatory actions of SubP 1(μm), TRH (1 μm), ampakine CX546 (200 μm), and noradrenaline (25 μm). B, Population data showing respiratory frequency relative to control after addition of each agent as well as 5-HT (25 μm). Each data point was from four to five preparations tested. *p < 0.05 relative to control.
We also tested whether the slow rhythms were in part caused by increased inhibitory drive via GABAergic or glycinergic mechanisms (Cheng et al., 2004). There were no significant changes in respiratory frequency or variability after bath application of the receptor antagonists bicuculline (free base, 10 μm; n = 3) or strychnine (1 μm; n = 3). The frequency and coefficient of variability in control, bicuculline, and strychnine conditions were 1.4 ± 0.56 bursts/min and 0.7 ± 0.16, 1.57 ± 0.9 bursts/min and 0.68 ± 0.21, and 1.47 ± 0.57 bursts/min and 0.60 ± 0.12, respectively.
Anatomical analyses of respiratory nuclei of wild-type and Lbx1 mutant mice
To investigate neuroanatomical abnormalities that may explain respiratory defects in Lbx1 mutant mice, we studied the expression of different neuronal markers within the caudal pons and medulla. For the purpose of this study, we primarily focused on the expression patterns and anatomical defects relevant to the key nuclei involved in modulating respiratory function and those data are discussed in detail below. However, for completion, we did determine Lbx1 expression patterns and several defects in Lbx1 mutants within other brainstem regions. Figure 4 shows the expression patterns of GFP in the caudal and rostral medulla of Lbx1GFP/+ mice and some of the major defects in the mutant Lbx1GFP/GFP mice. In heterozygote mice, GFP is expressed by cells in the spinal trigeminal subnuclei [caudalis (SpVc) and interpolaris (SpVi)], in the cuneate and gracile nuclei (Cn/Gr), in the area postrema (AP), in the NTS, in scattered cells of the ventral medulla below the NA, in the parvicellular reticular formation (PR), in the gigantocellular reticular formation (Gi), and in the vestibular nucleus (Ve).
Figure 4.

Lbx1 expression in wild-type and mutant brainstem. A–D, GFP expression in the caudal (A, B) and rostral (C, D) medulla of Lbx1GFP/+ and Lbx1GFP/GFP mice at E18, respectively. GFP is expressed in the spinal trigeminal subnuclei (caudalis, SpVc and interpolaris SpVi), in the Cn and Gr, in the AP, in the NTS, in scattered cells in the ventral medulla below the nucleus ambiguus, in the PR, and in the Ve in Lbx1GFP/+ mice. In Lbx1GFP/GFP mice, GFP is expressed in the putative region of SpV, Cn, Gr, AP where the cytoarchitecture of the sensory systems (SpV, Cn and Gr) is grossly abnormal. In addition, there is a reduction of cells in the ventral medulla (arrowhead in D) and a marked enlargement of the inferior olive (arrows in B, D). Scale bars, 400 μm.
The cytoarchitecture of the medulla of Lbx1GFP/GFP mice was severely disrupted at E18.5. In particular, the sensory nuclei located in the dorsolateral medulla (Cn/Gr, SpV, and NTS) were highly abnormal, as reported previously from Sieber et al. (2007). The SpV failed to form and neurons rather bundled around the NTS. In addition to the dorsal defects reported previously, we demonstrate a reduced distribution of GFP+ cell in the ventral medulla (Fig. 4, arrowhead) and a marked enlargement of the inferior olive (Fig. 4, arrows; supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Furthermore, in Lbx1GFP/GFP mice (E18.5), ascending and descending tracts were misrouted, and extensive longitudinally oriented fibers along the surface of the ventral medulla shifted ventral motoneuronal pools [NA and facial (VII) nuclei] to a more dorsolateral position relative to wild-type mice (data not shown). However, analysis of size extension and cell density of NA, VII, and XII showed no apparent defects in the respiratory related motoneuronal pools.
PreBötC
Neurokinin 1 receptor (NK1R) and SST immunoreactivity in the ventrolateral medulla identifies the region of the preBötC (Gray et al., 1999; Guyenet et al., 2002; Pagliardini et al., 2003). The NK1R+ and SST+ cells in the preBötC area are not Lbx1 derived as they did not express GFP in Lbx1GFP/+ or Lbx1GFP/GFP mice. Furthermore, immunolabeling for NK1R and SST showed that the gross structure of the preBötC formed normally in mutant mice. However, in mutant mice preBötC location was shifted in a more dorsolateral position (Fig. 5), further away from the ventral surface of the medulla because of the aberrant migration of other medullary and spinal neurons, fiber tracts, and the expansion of the inferior olive.
Figure 5.

A–F, GFP (green, A–F), NK1R (red, A, B, D, E), ChAT (blue, A, B, D, E), and SST (red, C, F) expression in the ventrolateral medulla of Lbx1GFP/+ and Lbx1GFP/GFP mice at E18. PreBötC neurons (NK1R+/ChAT− and SST+) are present in both Lbx1GFP/+ and Lbx1GFP/GFP mice. B, E, Details of preBötC neurons from A and D, respectively. There is a marked reduction in GFP+ neurons within the network of the ventrolateral medulla (asterisks). Scale bars: A, C, D, F, 200 μm; B, E, 100 μm.
RTN neurons
Data from recent studies suggests that a population of glutamatergic neurons ventral to the VII nucleus that express Phox2b directly sense the pH of the blood and provide a major chemosensory drive to the preBötC (Stornetta et al., 2006; Mulkey et al., 2007). This population corresponds to the classical location and phenotype described for chemosensitive neurons of the RTN (Feldman et al., 2003; Mulkey et al., 2004; Stornetta et al., 2006). Furthermore, mutant mice lacking Phox2b-positive neurons in the RTN die shortly after birth because of respiratory dysfunction (Dubreuil et al., 2008) similar to Lbx1 mutants. We found that the majority of Phox2b-expressing RTN neurons in Lbx1GFP/+ mice were GFP positive and also expressed the NK1R (Fig. 6A,C). Furthermore, Phox2b/NK1R-expressing neurons in the RTN were absent in Lbx1 mutant mice (Fig. 6B,D). Those data demonstrate that Lbx1 is necessary for the proper development of a subclass of Phox2b/NK1R RTN neurons in the caudal pons.
Figure 6.

Loss of NK1-Phox2b-Lbx1-positive neurons in the RTN. A, B, Parasagittal sections labeled for Phox2b (red) and GFP (green) expression in the RTN region of Lbx1GFP/+ and Lbx1GFP/GFP mice. Phox2b is expressed in facial motoneurons (VII) and in surrounding RTN neurons. Phox2b colocalizes with GFP in Lbx1GFP/+ mice. In Lbx1GFP/GFP mice, there is a strong reduction of GFP/Phox2b neurons in the RTN. C, D, Transverse sections immunostained for NK1R (red), ChAT (blue), and GFP (green) in the RTN region. NK1R+ RTN neurons also express Lbx1 in Lbx1GFP/+ mice and they are reduced in Lbx1GFP/GFP mice. Scale bars: A, B, 200 μm; C, 50 μm; D, 100 μm.
Catecholaminergic neurons
Respiratory function is strongly modulated by catecholaminergic neurons in pontine and medullary structures (Li et al., 2008). Many of these catecholaminergic neurons are also specified by Phox2b, however they originate from a distinct developmental population than glutamatergic RTN neurons (Pattyn et al., 2000; Qian et al., 2001; Brunet and Pattyn, 2002; Dauger et al., 2003) and they do not coexpress GFP in Lbx1GFP/+ mice (Fig. 7A–C). All of the catecholaminergic nuclei are present in Lbx1 null mice (Fig. 7D–F). However, loss of Lbx1 activation did cause a perturbation within these populations, as the numbers of TH-positive neurons were increased in the catecholaminergic groups A1/C1 (158.1 ± 15.9%; n = 6) and A5 (222.8 ± 63.7%; n = 3) nuclei in mutant mice (Fig. 7). A similar increase in TH-positive neurons was also observed dorsal to the nucleus ambiguus (246.5% ±95.2 SD; n = 5) and along the midline (288.0 ± 157.5% SD; n = 4). Increased staining for TH fibers and neurons, were also observed in the A2/C2 and C2/C3 catecholaminergic group. Collectively, the data suggest that loss of Lbx1 expression results in ectopic noradrenergic neuron expression.
Figure 7.

Ectopic expression of TH-positive neurons. A–F, Serial caudorostral transverse sections of an E18 medulla of Lbx1GFP/+ (A–C) and Lbx1GFP/GFP (D–F) mice labeled for TH (red) and GFP (green). Note the intense somatic and neuropilar staining in the noradrenergic structures of A1/C1, A2, C2/C3 of Lbx1GFP/GFP mice. In addition, there is ectopic localization of TH+ neurons along the midline and in the reticular formation. Scale bar: A–F, 500 μm.
We tested the hypothesis that the excess TH-positive neuronal populations resulted in increase noradrenergic input to the preBötC and influenced rhythmogenesis in mutant brainstem–spinal cord preparations. Bath application of α1 and α2 adrenoceptor antagonists prazosin (50 μm), and idazoxan (50 μm) had no significant effects on the respiratory frequency in mutant (n = 3) and wild-type (n = 3) preparations (data not shown).
GABAergic and glycinergic inhibitory inputs
Studies of spinal cord development have shown that Lbx1 is expressed in a subset of inhibitory neurons that express Pax2 (Burrill et al., 1997). Furthermore, mutant mouse models lacking substantial populations of GABAergic and glycinergic medullary neurons have a lethal respiratory phenotype (Kuwana et al., 2003; Fujii et al., 2007), consistent with what we observed with our electrophysiolgocial recordings of Lbx1 mutants. Thus, we focused on examining inhibitory neurons within the NTS and the ventrolateral medulla, given that these regions are involved in modulating respiratory rhythm.
Figure 8A–D shows the distribution of Lbx1-derived, Pax2-expressing inhibitory neurons in Lbx1GFP/+ or Lbx1GFP/GFP mice. A widespread reduction of Pax2/GFP-coexpressing neurons was observed in the sensory structures of Cn/Gr, in the SpV and in neurons of the ventral medulla and NTS. In mutant mice, Pax2+/GFP− neurons were apparently not affected by the absence of Lbx1 during development. Figure 8E–H shows Pax2 expression in the ventral medulla. Here, we observed in Lbx1GFP/+ mice, two distinct Pax2-expressing populations, one that is Lbx1 derived. In Lbx1GFP/GFP mice (Fig. 8F,H), the population of inhibitory GFP+/Pax2+ neurons is substantially decreased in this region.
Figure 8.
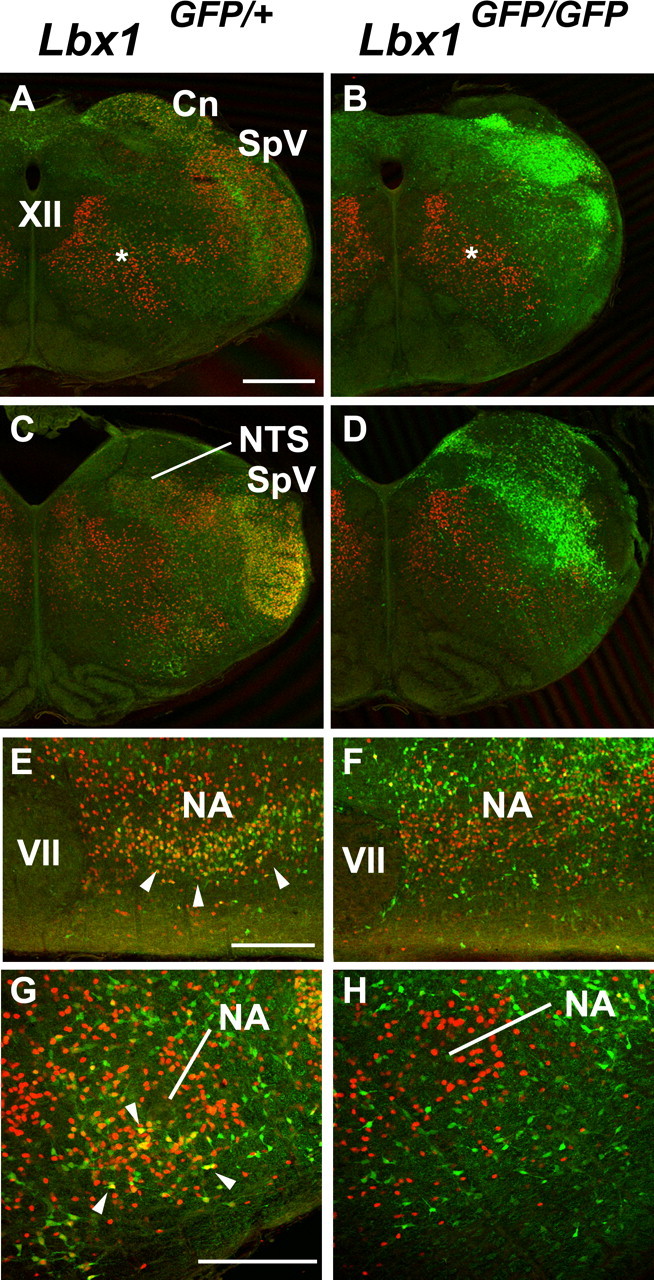
Pax2 (red) and GFP (green) expression in the medulla of Lbx1GFP/+ and Lbx1GFP/GFP mice at E18. A–D, Transverse section of caudal (A, B) and rostral medulla (C, D) labeled for Pax2 and GFP in Lbx1GFP/+ (A, C) and Lbx1GFP/GFP (B, D) mice. In Lbx1GFP/+ mice, Pax2 is expressed in cells located in reticular formation and lateral to the XII nucleus (asterisk, A), in the SpV, Gr/Cn, NTS nuclei and in the ventral medulla. Pax2 colocalizes with GFP in cells of Cn/Gr, SpV, and in a subpopulation of Pax2+ cells in the NTS and in the ventral medulla. In Lbx1GFP/GFP mice Pax2 is expressed in the medial reticular formation and lateral to XII nucleus (asterisks, B), only scattered cells are present in the Gr/Cn, SpV, NTS nuclei and in the ventral medulla. E–H, Parasagittal (E, F) and transverse (G, H) section details of the ventral medulla immunostained for Pax2 and GFP show the reduced number of neurons coexpressing Pax2 and GFP in the ventral medulla, below the NA, of Lbx1GFP/GFP mice compared with Lbx1GFP/+ mice (arrowheads, F, G). Pax2 cells that do not express GFP are not affected in Lbx1GFP/GFP mice. Scale bars: A–D, 500 μm; E, F, 200 μm; G, H, 100 μm.
The major inhibitory neurotransmitter in the respiratory column is glycine (Shao and Feldman, 1997). The best characterized inhibitory population in the ventrolateral medulla is the glycinergic expiratory neurons of the BötC. Figure 9, A and C, shows that Lbx1-derived neurons in the BötC express Pax2. Combined in situ hybridization for GlyT2(Slc6a5) (Fig. 9B,D) and immunohistochemistry for Pax2 (Fig. 9D) in the adjacent section demonstrates that BötC neurons are glycinergic and Lbx1 derived. Contrasting GlyT2 mRNA labeling in wild-type (Fig. 9B) and mutant (Fig. 9E) mice demonstrates the necessity of Lbx1 expression for development of glycinergic neurons in the ventral medulla. Furthermore, the loss of Lbx1 expression leads to severe migration abnormalities of GFP/Pax2-expressing neurons (Fig. 9F).
Figure 9.

BötC glycinergic neurons are Lbx1 derived. A, Lbx1-derived GFP+ (green) neurons in the BötC (arrow) express Pax2 (red). B, In situ hybridization for GlyT2 in the adjacent Lbx1GFP/+ section identifies BötC neurons. C, Expanded image from A showing GFP coexpression with Pax2 in the BötC. D, Pseudocolor image of coexpression of Pax2 protein (red) with GlyT2 mRNA (green) in Lbx1GFP/+ mice section adjacent to A. E, In situ labeling for GlyT2 in Lbx1GFP/GFP mice BötC. F, Lbx1-derived neurons show a migration deficit in Lbx1GFP/GFP mice BötC. Scale bar: A, B, 200 μm; C, D, 200 μm; E, F, 20 μm. D, Dorsal; M, medial.
Given the marked loss of glycinergic and GABAergic neurons in the medulla, we tested whether exogenous application of those neurotransmitters would normalize the slow rhythms in mutant brainstem–spinal cord preparations. However, bath application of the GABAA receptor agonist muscimol (0.1 μm) or glycine (30 μm) resulted in yet further slowing of the respiratory rhythm (∼50%; n = 3; data not shown).
Origins of anatomical defects in respiratory nuclei
The above data demonstrate that the absence of Lbx1 expression results in a marked reduction of Phox2b neuronal populations within the RTN and inhibitory neurons within multiple nuclei at E18.5. We examined the hindbrain of Lbx1GFP/+ and Lbx1GFP/GFP mice at E10.5–E12.5 to determine whether these defects were present early in development or were caused by some later stage developmental abnormality. A recent study has classified dorsally generated neuronal populations in the brainstem based on the expression of specific transcription factors (Sieber et al., 2007). This organization is summarized in Figure 10A. Briefly, most dorsal-generated neurons are classified into four subpopulations (dA1–dA4), and more ventrally generated neurons are classified in four subpopulations (dB1–dB4). Two additional subpopulations of neurons (dBLA–dBLB) are generated later in development. During the first wave of neurogenesis at E10.5–E11.5, Lbx1-expressing cells are present in dB1, dB2 (exclusively in the pons), dB3 and dB4 domains in the developing hindbrain. During the second wave of neurogenesis at E12.5, Lbx1 neurons develop in dBLA and dBLB populations (Sieber et al., 2007; Gray, 2008). We confirmed the classification of the Lbx1-expressing neurons outlined in Sieber et al., 2007 and further extended the analysis on the origin, identity and likely neurotransmitter phenotype of these neuronal populations in both wild-type and mutants.
Figure 10.

Developmental origins of anatomical defects in respiratory neuronal populations. A, Schematic expression of transcription factors in dorsally generated neurons of the hindbrain at E10–E12 of Lbx1GFP/+ and Lbx1GFP/GFP mice: data are summarized from this paper, Sieber et al. (2007) and Gray (2008). Transcription factor in gray are not expressed in Lbx1GFP/GFP mice. Transcription factor ectopically expressed in mutants are indicated as purple. B–I, Expression of GFP, Pax2 and Lmx1b in E11 and E12 in Lbx1GFP/+ and Lbx1GFP/GFP mice. In Lbx1GFP/+ mice at E11, GFP (green) is expressed in the dB1, dB3, dB4 dorsal interneurons. Lmx1b (red) is expressed in the dA3 (asterisks) and dB3 dorsal interneurons, whereas Pax2 (blue) is expressed in the dB1, dB4, and in ventral interneuronal populations (arrows in D, E). In Lbx1GFP/GFP mice, Pax2 is absent in dB1 (E, arrowheads), whereas Lmx1b is expressed in dA3 and dB3 dorsal interneurons. At E12, GFP is expressed in dBLA-B dorsal interneurons of both Lbx1GFP/+ and Lbx1GFP/GFP mice. Lmx1b is expressed in several interneurons (dBLB interneurons, G, I), whereas Pax2+/GFP+ cells are missing in the dBLA neurons of Lbx1GFP/GFP mice. Also note the absence of Pax2+/GFP+ neurons in the medial medulla (open arrows in F, G) and the extensive GFP+/Lmx1b+ colocalization in the ventral medulla (arrows in G, I). J, Q, In Lbx1GFP/+ mice at E11, Phox2b (red) is expressed solely in dA3 neurons and in a subset of ChAT+ (J, L, blue) motoneurons. In Lbx1GFP/GFP mice, ectopic expression of Phox2b is mainly present in dB3 neurons (arrowhead in M). At E12, in Lbx1GFP/+ mice, Phox2b+ cells of dA3 origin are intermingled with Lbx1/GFP-expressing neurons, and no colocalization with GFP is apparent. In Lbx1GFP/GFP mice, extensive ectopic expression of Phox2b is present, both in early generated dB3 neurons (arrows in O, Q) and in late generated dBL neurons (asterisk in Q). Scale bars: B, C, J, K, 200 μm; D, E, L, M, 100 μm; F, G, N, O, 200 μm; H, I, P, Q, 100 μm.
Inhibitory interneurons
When we studied the expression of Pax2 and Lmx1b in the early stages of development (Fig. 10B–E), we confirmed the absence of Pax2 from dB1 neurons as early as when the first dB neurons are generated (Fig. 10C,E) (data not shown). Furthermore, in late generated dLBA neurons, Pax2 expression is again absent (Fig. 10G,I). These results suggest that Pax2/Lbx1 inhibitory neurons in the medulla do not form and putative neurons are respecified into a more dorsally generated FoxP2 (dA2 neurons of inferior olive) (supplemental Fig. 1, available at www.jneurosci.org as supplemental material) and Phox2b-dependent neurons (dA3, that later in development settle in the dorsal medulla) (Sieber et al., 2007). Thus, the loss of glycinergic Pax2 positive neurons in the ventral medulla at E18 could be accounted for abnormal specification during the E10–E12 period of neurogenesis.
Phox2b RTN neurons
Because Lbx1/GFP is exclusively coexpressed with Phox2b in the dB2 population of Lbx1GFP/+ mice (Fig. 10, supplemental Fig. 2, available at www.jneurosci.org as supplemental material), we propose that RTN neurons originate solely from dB2 Lbx1/Phox2b-expressing neurons, in contrast with other cathecholaminergic and cholinergicneurons in the brainstem, which originate from dA3 neurons. In the early stages of development of Lbx1GFP/GFP mice we could not detect any abnormalities in the pontine origin of dB2 neurons (supplemental Fig. 2, available at www.jneurosci.org as supplemental material); therefore, it is likely that many of the putative Phox2b/Lbx1 RTN neurons originate from dB2 but fail to properly migrate to the ventral surface of the caudal pons. In addition, we observed a large increase in Phox2b-expressing neurons within the dB3 and dBl populations of Lbx1GFP/GFP mice medulla. Similar to what was reported by Sieber et al. (2007), we observed that the majority of the putative Lbx1-derived neurons switched their neuronal phenotype and started expressing Phox2b and Lmx1b, typical markers of the more dorsally derived dA3 neurons that give origin to NTS, AP, and several cathecolaminergic populations (Fig 10). Therefore, the identification of the exact location of the misrouted RTN neurons was masked by the ectopic presence of Phox2b/GFP-expressing neurons in the brainstem of Lbx1GFP/GFP mice.
Discussion
Abnormal respiratory rhythmogenesis caused by loss of Lbx1 expression
In vitro recordings demonstrated that mice lacking Lbx1 expression have a markedly depressed frequency of respiratory rhythm during the late stages of gestation and at birth compared with wild-type mice. However, in vitro, the inception of respiratory rhythmogenesis in Lbx1 mutants commenced at the same developmental stage as the wild-type mice. Indeed, at that early stage, there were no significant differences in respiratory frequency between wild-type and Lbx1 mutant preparations. By E18.5, the frequency of respiratory rhythm had increased approximately fivefold in the wild-type while not changing significantly from the E15.5 frequency in Lbx1 mutants. This raises the question of what is responsible for the typical increase in frequency of respiratory rhythm that occurs in vitro and in vivo during late fetal development. Potential mechanisms include: (1) age-dependent differences in neurons and/or the network underlying rhythmogenesis, (2) suppression of fetal network activity caused by endogenous inhibitory modulators, and (3) improper development of modulatory systems that provide excitatory drive to or within respiratory networks (Greer et al., 1992b; Kobayashi et al., 2001; Ren and Greer, 2003; Thoby-Brisson et al., 2005). In vitro, fetal rhythm-generating centers can oscillate at frequencies comparable to those of the neonate if agonists of excitatory modulators are administered (Greer et al., 2006). Similarly, in utero, the frequency of fetal breathing movements in rats is increased by administration of the respiratory stimulants doxapram and aminophylline (Kobayashi et al., 2001). Application of the excitatory neuromodulators TRH and SubP in vitro also produces markedly increases in the frequency of prenatal respiratory rhythm (Greer et al., 1996; Pagliardini et al., 2003). Here in this study, bath application of SubP, noradrenaline, 5-HT, and TRH revealed that the Lbx1 mutant E18.5 preparations could generate robust respiratory drive at frequencies similar to the wild-type if excitatory conditioning drive is added. Thus, the hypoventilation in Lbx1-deficient mice could be accounted for by a failure of the normal excitatory conditioning drive necessary to develop after E15.5.
Abnormal anatomical structures within pontomedullary structures that modulate respiratory rhythmogenesis in Lbx1 mutant mice.
There were no gross abnormalities of preBötC structure as defined by NK1R and SST labeling. The preBötC in Lbx1 mutants was positioned more dorsolaterally as a result of abnormal medullary nuclei and tract formation. This could result in perturbations in synaptic organization impinging onto the preBötC. However, the more substantial anatomical perturbations were with medullary and pontine structures that provide key modulatory drive to the preBötC. This included the following.
Significant loss of glutamatergic RTN neurons in the region of the facial nucleus
Our data suggest that Phox2b/Lbx1 RTN neurons likely originate from the dB2 pontine population in both wild-type and mutants but they fail to properly migrate in Lbx1 mutants. RTN neurons provide a major component of central chemosensitive drive to the preBötC. Thus, Lbx1 mutant mice lack a key element of tonic excitatory synaptic drive and reflex compensatory drive in response to hypoxia resulting from apneas. Replacement of excitatory drive with bath application of excitatory neuromodulators or enhancement of remaining glutamatergic drive with ampakines normalized the respiratory rhythm in Lbx1 mutants. Consistent with our data, another mutant mouse model lacking Phox2b derived glutamatergic neurons in the RTN region die of central apnea at birth because of abnormal respiratory rhythms (Dubreuil et al., 2008).
Reduction of GABA and glycinergic input from the dorsolateral medulla, NTS, and ventrolateral medulla attributable to failure of the initial formation of GABA/glycinergic precursors with dB1/dBLA populations at E10
There is typically very little tonic release of GABA or glycine that modulates respiratory rhythm in vitro (Zhang et al., 2002; Ren and Greer, 2006). One would not predict that a perturbation of those systems to significantly alter rhythmogenesis in vitro via alterations in membrane potential of preBötC neurons. Indeed, bath application of agonists to glycine and GABA receptors at E18 slowed the rhythm further and the addition of receptor antagonists did not alter rhythm. However, chloride-mediated conductances serve important roles in neuronal and network systems development. Specifically, at earlier stages (i.e., before E17) GABA and glycine act as excitatory neurotransmitters and promote growth factor expression and release (Gao and van den Pol, 2000; Ben-Ari, 2001; Gao and van den Pol, 2001; Gao et al., 2001). The loss of those actions may contribute to respiratory network and medullary structure abnormalities. Newborn mutant mice with deficiencies in GABA and glycine transmission have severely disrupted respiratory rhythm in vitro and in vivo (Kuwana et al., 2003; Fujii et al., 2007), similar to those generated by Lbx1 preparations lacking normal chloride-mediated neuromodulation during development.
Ctopic catecholaminergic neuron formation caused by alterations in neuronal phenotype
Catecholaminergic neurons are not essential for rhythmogenesis but they do modulate respiratory network function at multiple levels (for review, see Li et al., 2008). Noradrenaline induces an increase or decrease in respiratory frequency depending on the overall balance of α1 versus α2 receptor activation within the preBötC (Hilaire et al., 2004). We demonstrated that the abnormal respiratory rhythm generated in vitro did not result directly from abnormal activation of adrenergic receptors.
Persistence of nonrespiratory rhythms because of loss of Lbx1 expression
Wild-type and Lbx1 mutant brainstem spinal cord preparations at E15.5 generate robust rhythmic bursting that are large in amplitude, longer in duration and more diffuse in its spread within the neuraxis relative to respiratory rhythms. Episodes of spontaneous rhythmic activity are widespread in the developing vertebrate nervous system (Katz and Shatz, 1996; Milner and Landmesser, 1999; Nakayama et al., 1999; O'Donovan, 1999; Hanson and Landmesser, 2003; Ren and Greer, 2003; Yvert et al., 2004). However, they typically subside post E15 in the developing mouse (Thoby-Brisson et al., 2005). The nonrespiratory rhythms are dependent on glutamatergic synaptic drive, suppressed by gap junction blockers, and originate in the spinal cord and dorsal regions of the medulla (Ren and Greer, 2003; Thoby-Brisson et al., 2005; Ren et al., 2006). Functionally, the persistence of the rhythms in Lbx1 mutant E18.5 in vitro preparations will result in an additional perturbation and occlusion of respiratory rhythm (Ren and Greer, 2003; Thoby-Brisson et al., 2005). The marked deficiency in the development of dorsal medullary inhibitory neurons in Lbx1 mutant mice likely contributes to the persistence of nonrespiratory rhythms.
Functional implications for respiratory control
Lbx1 mutant mice die at birth because of defective respiratory rhythmogenesis. Specifically, the respiratory frequency is slow and robust nonrespiratory rhythms persist throughout gestation that would interfere with coordinated respiratory motor patterns. Pharmacological experiments demonstrated that respiratory rhythm could be normalized with additional excitatory neurochemical drive to the preBötC. The loss of excitatory drive from neurons in the RTN was likely a major contributor to abnormal rhythmogenesis. Furthermore, loss of chloride-mediated synaptic input from the NTS, BötC, and ventrolateral medulla could lead to developmental abnormalities of neuronal function and network development. Overall, these data demonstrate that a malformation of conditioning drive from these structures has profound implications for normal breathing and survival. Mouse models of Rett, Prader Willi and Congenital Central Hypoventilation Syndromes all have central respiratory drive dysfunction caused by specific genetic defects (for review, see Gaultier, 2004; Pagliardini et al., 2008). In each case, data from those studies are consistent with the respiratory phenotype arising from multiple perturbations of conditioning drive to the preBötC rather than a defect of the preBötC per se. Furthermore, the cause of respiratory depression associated with apnea of prematurity, obstructive sleep apnea and potentially a subset of Sudden Infant Death have been hypothesized to result from abnormalities of conditioning synaptic inputs necessary for adequate respiratory rhythmogenesis and transmission (for review, see Darnall et al., 2006; Horner and Bradley, 2006). Thus, an understanding of the transcriptional control of not only the preBötc, but also the associated respiratory nuclei will be necessary for understanding the ontogeny of respiratory neural control and pathologies. Results from this study demonstrate that Lbx1 is one of the critical “master genes” necessary for transcriptional control of key brainstem respiratory neural circuitry and function.
Footnotes
S.P. is a Fellow of the Alberta Heritage Foundation for Medical Research (AHFMR) and the Canadian Institute for Health Research. J.J.G. is a Scientist of the AHFMR. We thank Qiufu Ma for the Slc6a5 probe.
References
- Al-Zubaidy ZA, Erickson RL, Greer JJ. Serotonergic and noradrenergic effects on respiratory neural discharge in the medullary slice preparation of neonatal rats. Pflugers Arch. 1996;431:942–949. doi: 10.1007/s004240050089. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Developing networks play a similar melody. Trends Neurosci. 2001;24:353–360. doi: 10.1016/s0166-2236(00)01813-0. [DOI] [PubMed] [Google Scholar]
- Burrill JD, Moran L, Goulding MD, Saueressig H. PAX2 is expressed in multiple spinal cord interneurons, including a population of EN1+ interneurons that require PAX6 for their development. Development. 1997;124:4493–4503. doi: 10.1242/dev.124.22.4493. [DOI] [PubMed] [Google Scholar]
- Cheng L, Arata A, Mizuguchi R, Qian Y, Karunaratne A, Gray PA, Arata S, Shirasawa S, Bouchard M, Luo P, Chen CL, Busslinger M, Goulding M, Onimaru H, Ma Q. Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nat Neurosci. 2004;7:510–517. doi: 10.1038/nn1221. [DOI] [PubMed] [Google Scholar]
- Cheng L, Samad OA, Xu Y, Mizuguchi R, Luo P, Shirasawa S, Goulding M, Ma Q. Lbx1 and Tlx3 are opposing switches in determining GABAergic versus glutamatergic transmitter phenotypes. Nat Neurosci. 2005;8:1510–1515. doi: 10.1038/nn1569. [DOI] [PubMed] [Google Scholar]
- Dietrich S, Schubert FR, Healy C, Sharpe PT, Lumsden A. Specification of the hypaxial musculature. Development. 1998;125:2235–2249. doi: 10.1242/dev.125.12.2235. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Mörschel M, Kron M, Herbert H. Development of adaptive behaviour of the respiratory network: implications for the pontine Kolliker-Fuse nucleus. Respir Physiol Neurobiol. 2004;143:155–165. doi: 10.1016/j.resp.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M, Arata A, Kanbara-Kume N, Saito K, Yanagawa Y, Obata K. Respiratory activity in brainstem of fetal mice lacking glutamate decarboxylase 65/67 and vesicular GABA transporter. Neuroscience. 2007;146:1044–1052. doi: 10.1016/j.neuroscience.2007.02.050. [DOI] [PubMed] [Google Scholar]
- Gao XB, van den Pol AN. GABA release from mouse axonal growth cones. J Physiol. 2000;523:629–637. doi: 10.1111/j.1469-7793.2000.t01-1-00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XB, van den Pol AN. GABA, not glutamate, a primary transmitter driving action potentials in developing hypothalamic neurons. J Neurophysiol. 2001;85:425–434. doi: 10.1152/jn.2001.85.1.425. [DOI] [PubMed] [Google Scholar]
- Gao XB, Stricker C, Ziskind-Conhaim L. Transition from GABAergic to glycinergic synaptic transmission in newly formed spinal networks. J Neurophysiol. 2001;86:492–502. doi: 10.1152/jn.2001.86.1.492. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA. Transcription factors and the genetic organization of brainstem respiratory neurons. J Appl Physiol. 2008;104:1513–1521. doi: 10.1152/japplphysiol.01383.2007. [DOI] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nature Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JL. Respiratory and locomotor patterns generated in the fetal rat brain stem-spinal cord in vitro. J Neurophysiol. 1992a;67:996–999. doi: 10.1152/jn.1992.67.4.996. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JL. Glutamate release and presynaptic action of AP4 during inspiratory drive to phrenic motoneurons. Brain Res. 1992b;576:355–357. doi: 10.1016/0006-8993(92)90705-e. [DOI] [PubMed] [Google Scholar]
- Greer JJ, al-Zubaidy Z, Carter JE. Thyrotropin-releasing hormone stimulates perinatal rat respiration in vitro. Am J Physiol. 1996;271:R1160–1164. doi: 10.1152/ajpregu.1996.271.5.R1160. [DOI] [PubMed] [Google Scholar]
- Gross MK, Dottori M, Goulding M. Lbx1 specifies somatosensory association interneurons in the dorsal spinal cord. Neuron. 2002;34:535–549. doi: 10.1016/s0896-6273(02)00690-6. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Sevigny CP, Weston MC, Stornetta RL. Neurokinin-1 receptor-expressing cells of the ventral respiratory group are functionally heterogeneous and predominantly glutamatergic. J Neurosci. 2002;22:3806–3816. doi: 10.1523/JNEUROSCI.22-09-03806.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. Characterization of the circuits that generate spontaneous episodes of activity in the early embryonic mouse spinal cord. J Neurosci. 2003;23:587–600. doi: 10.1523/JNEUROSCI.23-02-00587.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagla K, Dolle P, Mattei MG, Jagla T, Schuhbaur B, Dretzen G, Bellard F, Bellard M. Mouse Lbx1 and human LBX1 define a novel mammalian homeobox gene family related to the Drosophila lady bird genes. Mech Dev. 1995;53:345–356. doi: 10.1016/0925-4773(95)00450-5. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Lemke RP, Greer JJ. Ultrasound measurements of fetal breathing movements in the rat. J Appl Physiol. 2001;91:316–320. doi: 10.1152/jappl.2001.91.1.316. [DOI] [PubMed] [Google Scholar]
- Kuwana S, Okada Y, Sugawara Y, Tsunekawa N, Obata K. Disturbance of neural respiratory control in neonatal mice lacking GABA synthesizing enzyme 67-kDa isoform of glutamic acid decarboxylase. Neuroscience. 2003;120:861–870. doi: 10.1016/s0306-4522(03)00338-5. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Müller T, Brohmann H, Pierani A, Heppenstall PA, Lewin GR, Jessell TM, Birchmeier C. The homeodomain factor lbx1 distinguishes two major programs of neuronal differentiation in the dorsal spinal cord. Neuron. 2002;34:551–562. doi: 10.1016/s0896-6273(02)00689-x. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Nishimaru H, Iizuka M, Ozaki S, Kudo N. Rostrocaudal progression in the development of periodic spontaneous activity in fetal rat spinal motor circuits in vitro. J Neurophysiol. 1999;81:2592–2595. doi: 10.1152/jn.1999.81.5.2592. [DOI] [PubMed] [Google Scholar]
- O'Donovan MJ. The origin of spontaneous activity in developing networks of the vertebrate nervous system. Curr Opin Neurobiol. 1999;9:94–104. doi: 10.1016/s0959-4388(99)80012-9. [DOI] [PubMed] [Google Scholar]
- Oliva AA, Jr, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci. 2000;20:3354–3368. doi: 10.1523/JNEUROSCI.20-09-03354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliardini S, Ren J, Greer JJ. Ontogeny of the pre-Botzinger complex in perinatal rats. J Neurosci. 2003;23:9575–9584. doi: 10.1523/JNEUROSCI.23-29-09575.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierani A, Moran-Rivard L, Sunshine MJ, Littman DR, Goulding M, Jessell TM. Control of interneuron fate in the developing spinal cord by the progenitor homeodomain protein Dbx1. Neuron. 2001;29:367–384. doi: 10.1016/s0896-6273(01)00212-4. [DOI] [PubMed] [Google Scholar]
- Ren J, Greer JJ. Ontogeny of rhythmic motor patterns generated in the embryonic rat spinal cord. J Neurophysiol. 2003;89:1187–1195. doi: 10.1152/jn.00539.2002. [DOI] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Botzinger complex: differential roles of glycinergic and GABAergic neural transmission. J Neurophysiol. 1997;77:1853–1860. doi: 10.1152/jn.1997.77.4.1853. [DOI] [PubMed] [Google Scholar]
- Sieber MA, Storm R, Martinez-de-la-Torre M, Müller T, Wende H, Reuter K, Vasyutina E, Birchmeier C. Lbx1 acts as a selector gene in the fate determination of somatosensory and viscerosensory relay neurons in the hindbrain. J Neurosci. 2007;27:4902–4909. doi: 10.1523/JNEUROSCI.0717-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Greer JJ, Liu G, Feldman JL. Neural mechanisms generating respiratory pattern in mammalian brainstem-spinal cord in vitro. I. Spatiotemporal patterns of motor and medullary neuron activity. J Neurophysiol. 1990;64:1149–1169. doi: 10.1152/jn.1990.64.4.1149. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. PreBötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Trinh JB, Champagnat J, Fortin G. Emergence of the pre-Botzinger respiratory rhythm generator in the mouse embryo. J Neurosci. 2005;25:4307–4318. doi: 10.1523/JNEUROSCI.0551-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvert B, Branchereau P, Meyrand P. Multiple spontaneous rhythmic activity patterns generated by the embryonic mouse spinal cord occur within a specific developmental time window. J Neurophysiol. 2004;91:2101–2109. doi: 10.1152/jn.01095.2003. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Gérard H. Noradrenergic receptors and in vitro respiratory rhythm: possible interspecies differences between mouse and rat neonates. Neurosci Lett. 2002;324:149–153. doi: 10.1016/s0304-3940(02)00191-x. [DOI] [PubMed] [Google Scholar]





