Abstract
Background
Urinary 20-hydroxyeicosatetraenoic acid (20-HETE) has been associated with hypertension in women with elevated urinary cadmium (Cd) excretion rates. The present study investigates the urinary Cd and 20-HETE levels in relation to the estimated glomerular filtration rate (eGFR) and albumin excretion in men and women.
Methods
A population-based, cross-sectional study, which included 225 women and 84 men aged 33–55 years, was conducted in a rural area known to be polluted with Cd.
Results
In all subjects, lower eGFR values were associated with higher urinary Cd excretion (P = 0.030), and tubulopathy markers N-acetyl-β-d-glucosaminidase (P < 0.001) and β2-microglobulin (β2-MG) (P < 0.001). On average, the hypertensive subjects with the highest quartile of urinary Cd had eGFR values of 12 and 17 mL/min/1.73 m2 lower than that in the hypertensive (P = 0.009) and normotensive subjects (P < 0.001) with the lowest quartile of urinary Cd, respectively. In men, urinary albumin was inversely associated with 20-HETE (β = −0.384, P < 0.001), while showing a moderately positive association with systolic blood pressure (SBP) (β = 0.302, P = 0.037). In women, urinary albumin was not associated with 20-HETE (P = 0.776), but was associated with tubulopathy, reflected by elevated urinary excretion of β2-MG (β = 0.231, P = 0.002).
Conclusions
Tubulopathy is a determinant of albumin excretion in women, while 20-HETE and SBP are determinants of urinary albumin excretion in men. Associations of chronic exposure to Cd with marked eGFR decline and renal tubular injury seen in both Cd-exposed men and women add to mounting research data that links Cd to the risk of developing chronic kidney disease.
Keywords: albuminuria, cadmium, estimated glomerular filtration rate, hypertension, 20-hydroxyeicosatetraenoic acid
INTRODUCTION
As cadmium (Cd) is a common food chain contaminant, a constituent of cigarette smoke and polluted air, chronic exposure to this insidious toxicant is a global health concern [1, 2]. The dietary Cd intake for the average consumer in various populations is estimated to be between 8 and 25 μg/day [1]. Of concern, long-term exposure to relatively low levels of Cd, such as is present in Asian and Western diets, is associated with an increased risk of developing chronic kidney disease (CKD), characterized by albuminuria and a fall in the estimated glomerular filtration rate (eGFR) to levels <60 mL/min/1.73 m2 [3–9]. Of further concern, Cd exposure has been implicated in the development of hypertension either as a direct effect or as a consequence of CKD [9–13]. We have previously reported an association between the prevalence of hypertension in Thai women with elevated urinary levels of Cd and the eicosanoid, 20-hydroxyeicosatetraenoic acid (20-HETE) [14–17]. In human kidneys, the majority of 20-HETE is derived from catalytic activity of enzymes of the cytochrome P450 (CYP) superfamily, notably CYP4A11 and CYP4F2 that are differentially expressed in the kidney glomeruli, proximal and distal tubules [15].
Experimental and clinical data suggest that an increase in 20-HETE synthesis in kidneys mediates androgen-dependent hypertension, thereby explaining the higher prevalence of hypertension in men compared with age-matched premenopausal women [18–20]. Of relevance, Cd exposure, assessed by urinary Cd levels, has been associated with altered serum levels of androgenic and estrogenic hormones [21–24]. Higher urinary Cd levels were associated with higher serum testosterone levels in postmenopausal women [21], whereas higher urinary Cd levels were associated with lower serum testosterone levels in premenopausal women [22]. However, none of these previous studies measured blood pressure, urinary 20-HETE levels or any markers of kidney injury often associated with Cd exposure. Therefore, the present study was undertaken to investigate potential associations of urinary excretion of Cd and 20-HETE with eGFR and albuminuria in men and women, respectively. In addition, evidence for kidney tubular injury and impairment was investigated with urinary excretion of N-acetyl-β-d-glucosaminidase (NAG) and β2-microglobulin (β2-MG) [2, 25, 26].
MATERIALS AND METHODS
Study subjects
Participants in the present study were drawn from rural communities in Mae Sot District, Tak Province, Thailand, where the prevalence of hypertension was higher than the Thai national average for a rural area [27]. The Human Research Ethics Committee of Thailand’s Ministry of Public Health approved the study protocol. All participants provided written informed consent prior to participation. Inclusion criteria were apparently healthy residents, who lived at their current address for at least 30 years and consumed as a staple mostly locally grown rice. Exclusion criteria were pregnancy, breastfeeding, history of metal work, a hospital record or diagnosis by physician of CKD, heart disease, diabetes, anemia or hyperlipidemia. Smoking, regular use of medications, level of education, occupation, family health history and anthropometric data were obtained from questionnaires. Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥90 mmHg, physician diagnosis or prescription of antihypertensive medications. After exclusion of subjects with an incomplete dataset, 309 persons (84 men and 225 women) formed the study subjects in the present study. All women (n = 225) were the same as in our previous report [14]. However, a coding error was rectified for one treated hypertensive woman who had previously been classified as normotensive.
Collection of blood and urine samples and their analysis
Second morning void urine samples were collected after overnight fasting. Whole blood samples were collected within 3 h after urine collection. Aliquots of whole blood, serum and urine samples were stored at −80°C for later analysis. Urine and blood Cd levels were quantified by atomic absorption spectrophotometry with a Zeeman effect background correction system (Unicam model 989, Thermo Elemental Corp., Franklin, MA, USA). Calibration, quality control and quality assurance for the Cd quantitation were accomplished by simultaneous analysis of blood and urine control samples (ClinChekTM, Germany). The limit of quantification was 0.5 µg/L for urinary Cd and 0.3 µg/L for blood Cd. The urinary albumin assay was based on a turbidimetric method (UniCel® DxC800 Synchron system, Beckman Coulter, Fullerton, CA, USA). The urinary 20-HETE assay was based on a competitive enzyme-linked immunoassay (Detroit R&D, Inc., USA). Urinary and serum creatinine assays were based on the Jaffe kinetic method (Siemens Healthcare Diagnostics, Newark, NJ, USA). The levels of β2-MG in urine samples were measured with a solid-phase microparticle enzyme immunoassay (AxSYM β2-MG, Abbott Diagnostics, Abbott Park, IL, USA), while the urinary levels of NAG were determined with a colorimetric assay (Diazyme laboratories, USA).
Measurement of kidney function, Cd excretion rates and toxicity
Kidney function was based on eGFR, calculated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [28]. We used urinary Cd excretion (ECd) rate to reflect Cd body burden, while using urinary excretion of β2-MG and NAG to reflect toxic effects of Cd in kidneys [2, 25, 26]. Urinary NAG excretion reflects damaged tubular cells as this enzyme originates exclusively from kidney tubules, especially the proximal tubular cells [2, 26], while an increased urinary β2-MG level reflects an increase in its production and/or a reduced tubular reabsorption capacity [26]. Excretion rates for Cd, 20-HETE, albumin, β2-MG and NAG were normalized to creatinine clearance, using the following equation: Ex/Ccr = [x]u [cr]p/[cr]u, where Ex/Ccr = excretion of × per volume of filtrate; [x]u = urine concentration of × (mass/volume); [cr]p = plasma creatinine concentration (mg/dL); [cr]u = urine creatinine concentration (mg/dL) [29]. ECd is commonly normalized to creatinine excretion (Ecr), which is determined by muscle mass. Excretion rate of Cd normalized to creatinine excretion as ECd/Ecr could vary at least 4-fold because of variations in human muscle mass. Excretion rate of Cd normalized to creatinine clearance as ECd/Ccr is not similarly susceptible to confounding, given that ECd is determined by filtered Cd (FCd) and the fractions of FCd that are excreted and sequestered in proximal tubules [2, 29]. Hence, ECd/Ccr conveys a reliable biologic message.
Statistical analysis
Data were analyzed with the SPSS statistical package 17.0 (SPSS Inc., Chicago, IL, USA). The Mann–Whitney U-test was used to compare mean differences between two groups of subjects. Distribution of the variables was examined for skewness and those showing right skewing were logarithmically transformed before analysis, as required. Departure from a normal distribution of variables was assessed by one sample Kolmogorov–Smirnov test. A multilinear regression model analysis was used to identify determinants of eGFR and albuminuria. A generalized linear model analysis was used to estimate effect sizes for Cd. P ≤ 0.05 for a two-tailed test were considered statistically significant.
RESULTS
Study subjects
Of 309 participants (Table 1), 164 (50 men, 114 women) were normotensive and 145 (34 men, 111 women) were hypertensive. The mean age of hypertensive men (48.1 years) and hypertensive women (47.1 years) did not differ from their normotensive counterparts. The mean body mass index (BMI) in men (24.5 kg/m2) and in women (25.3 kg/m2) with hypertension was higher than their normotensive counterparts. The majority of men (91.2%) and women (90.4%) with hypertension received antihypertensive medication. The mean SBP in men (135 mmHg) and women (130 mmHg) was 13% (P <0.001) and 9% (P <0.001) higher than their normotensive counterparts. The mean DBP and arterial blood pressure levels were also higher in men and women with hypertension compared with normotensive counterparts. The mean eGFR in hypertensive men was 10 mL/min/1.73 m2 lower than normotensive counterparts (P = 0.014), whereas the mean eGFR in hypertensive women was 4 mL/min/1.73 m2 lower than those without hypertension.
Table 1.
Study subjects
| Men (n = 84) |
Women (n = 225) |
|||||
|---|---|---|---|---|---|---|
| Descriptors/variables | Hypertension |
P-value | Hypertension |
P-value | ||
| No (n = 50) | Yes (n = 34) | No (n = 114) | Yes (n = 111) | |||
| Age (years) | 47.2 ± 5.3 | 48.1 ± 4.8 | 0.537 | 47.1 ± 4.8 | 47.1 ± 4.4 | 0.994 |
| BMI (kg/m2) | 22.7 ± 2.7 | 24.5 ± 3.4 | 0.007 | 24.4 ± 3.5 | 25.3 ± 3.7 | 0.054 |
| Smoking (%) | 74.0 | 82.4 | 0.369 | 33.3 | 20.7 | 0.033 |
| Hypertension (%) | − | 40.5 | − | − | 49.3 | − |
| Antihypertensive medication (%) | − | 91.2 | − | − | 90.4 | − |
| SBP (mmHg) | 117 ± 11 | 135 ± 18 | <0.001 | 118 ± 10 | 130 ± 15 | <0.001 |
| DBP (mmHg) | 76 ± 9 | 84 ± 11 | 0.002 | 77 ± 9 | 83 ± 11 | <0.001 |
| Mean arterial pressure (mmHg) | 90 ± 9 | 101 ± 12 | <0.001 | 91 ± 8 | 98 ± 11 | <0.001 |
| eGFR (mL/min/1.73 m2)a | 100 ± 11 | 90 ± 18.5 | 0.014 | 98 ± 16 | 94 ± 17 | 0.041 |
| Low eGFRb | 0 | 5.9 | 0.083 | 3.5 | 4.5 | 0.703 |
| Albuminuria (%)c | 8.0 | 11.8 | 0.564 | 7.8 | 11.7 | 0.335 |
| Low eGFR with albuminuria (%) | 0 | 25 | − | 11.1 | 7.7 | − |
| Kidney disease Stage 2d | 16 | 38 | − | 28.1 | 36.0 | − |
| Blood Cd (µg/L) | 4.52 ± 3.68 | 3.69 ± 3.21 | 0.185 | 4.05 ± 3.91 | 3.08 ± 2.56 | 0.073 |
| Serum creatinine (mg/dL) | 0.88 ± 0.13 | 1.01 ± 0.29 | 0.018 | 0.72 ± 0.16 | 0.76 ± 0.17 | 0.028 |
| Urine creatinine (mg/dL) | 138 ± 72 | 134 ± 81 | 0.659 | 106 ± 71 | 136 ± 85 | 0.013 |
| Urinary excretion rate normalized to creatinine clearanced | ||||||
| E20-HETE/Ccr (pg/mL) | 10.3 ± 11.1 | 11.0 ± 10.9 | 0.688 | 6.1 ± 8.0 | 12.8 ± 34.0 | 0.052 |
| ECd/Ccr × 100 (μg/L) | 3.66 ± 3.27 | 4.59 ± 4.04 | 0.489 | 4.35 ± 4.16 | 4.17 ± 3.62 | 0.280 |
| ENAG/Ccr (U/L) | 0.08 ± 0.04 | 0.11 ± 0.08 | 0.212 | 0.11 ± 0.16 | 0.11 ± 0.12 | 0.523 |
| Eβ2MG/Ccr (µg/L) | 2.76 ± 14.6 | 15.4 ± 68.7 | 0.455 | 5.80 ± 45.7 | 1.95 ± 6.16 | 0.171 |
| EALB/Ccr (mg/dL) | 0.14 ± 0.43 | 0.38 ± 1.08 | <0.001 | 0.07 ± 0.15 | 0.12 ± 0.26 | 0.049 |
Numbers are arithmetic mean ± SD. Mean arterial pressure = diastolic pressure + (pulse pressure)/3, where pulse pressure = systolic − diastolic.
eGFR is determined with the CKD-EPI equations [28].
Low eGFR is defined as eGFR ≤60 mL/min/1.73 m2.
Albuminuria is defined as albumin to creatinine ratio ≥30 mg/g. Kidney disease Stage 2 is defined as eGFR ranging between 60 and 89 mL/min/1.73 m2.
The urinary excretion rates of Cd, 20-HETE and albumin that are normalized to creatinine clearance, using the equation: Ex/Ccr = [x]u [cr]p/[cr]u, where Ex/Ccr = excretion of x per volume of filtrate; [x]u = urine concentration of x (mass/volume); [cr]p = plasma creatinine concentration (mg/dL); [cr]u = urine creatinine concentration (mg/dL) [29].
The prevalence of smoking was similarly high in men with hypertension and without (84% versus 74%, P = 0.369). In women, the prevalence of smoking was higher in those without hypertension (33.3% versus 20.7%, P = 0.033). The prevalence of low eGFR in men with hypertension (5.9%) tended to be higher than those without (P = 0.083), but the prevalence of low eGFR in hypertensive and normotensive women did not differ (3.5% versus 4.5%, P = 0.703). The prevalence of albuminuria (albumin to creatinine ratio ≥30 mg/g) in hypertensive (11.8%) and normotensive men (8%) was similar as was in hypertensive (11.7%) and normotensive women (7.8%). Low eGFR plus albuminuria was more prevalent in men with hypertension (25%) than in women with hypertension (7.7%). None of normotensive men had low eGFR plus albuminuria, while 11.1% of normotensive women did. The prevalence of Stage 2 CKD in hypertensive men (38%) was similar to hypertensive women (36%).
The mean urinary 20-HETE excretion rates in men with and without hypertension was similar (11 versus 10.3 pg/mL, P = 0.688). In sharp contrast, the mean 20-HETE excretion rate in women with hypertension was 2-fold higher than their normotensive counterparts (12.8 versus 6.1 pg/mL, P = 0.052). This mean 20-HETE excretion rate in women with hypertension (12.8 pg/mL) was similar to that of hypertensive men (11 pg/mL) (P = 0.146). The mean blood Cd in hypertensive women tended to be lower than their normotensive counterparts (3.08 versus 4.05 µg/L, P = 0.073) as did in hypertensive and normotensive men (3.69 versus 4.52 µg/L, P = 0.185). The means for urinary Cd, NAG and β-MG excretion rates in men and women with hypertension were similar to their normotensive counterparts. The mean albumin excretion rates in men with hypertension was 2.7-fold higher than those without (0.38 versus 0.14 mg/dL, P < 0.001). In women, the mean albumin excretion of those with hypertension of was 1.7-fold higher than those without (0.12 versus 0.07 mg/dL, P = 0.049).
Cd as a predictor of eGFR decline
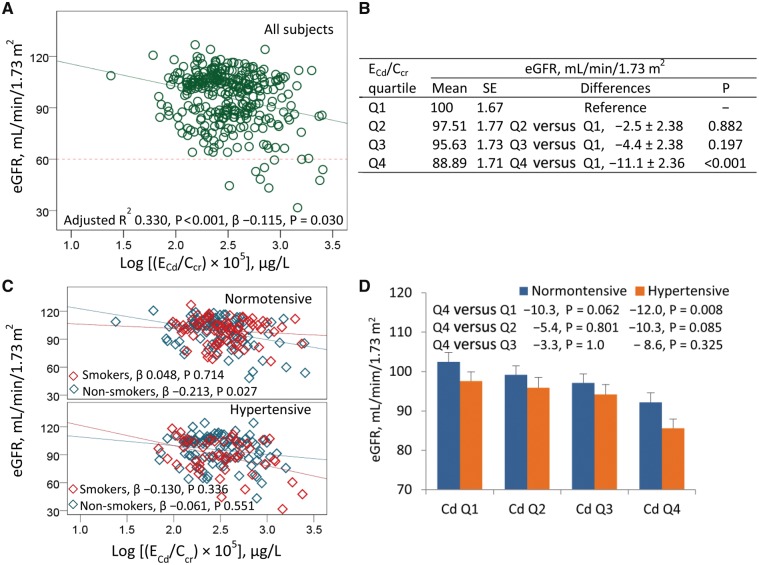
A set of independent variables (gender, smoking, age, BMI, SBP, DBP, log E20-HETE/Ccr, Log ECd/Ccr, log ENAG/Ccr and log Eβ2-MG/Ccr) accounted for 33, 31 and 34% of eGFR variability in all subjects, in women and in men, respectively (Table 2). In all subjects, eGFR values showed a moderately inverse association with age (β = −0.308, P < 0.001) and NAG (β = −0.310, P < 0.001), while showing a marginally inverse association with BMI (β = −0.130, P = 0.011), urinary β2-MG (β = −0.189, P < 0.001) and urinary Cd (β = −0.115, P = 0.030). A trend for an inverse association was indicated for eGFR and SBP (β = −0.109, P = 0.082). In women, eGFR values did not associate with Cd (β = −0.075, P = 0.232), but they showed a moderately inverse association with age (β = −0.295, P < 0.001), urinary NAG (β = −344, P < 0.001) and a marginally inverse association with urinary β2-MG (β = −0.166, P = 0.011). In men, eGFR values showed a moderately inverse association with urinary Cd (β = −0.226, P = 0.031), age (β = −0.316, P = 0.002), urinary NAG (β = −0.201, P = 0.051) and urinary β2-MG (β = −0.246, P = 0.016). An association of eGFR and urinary 20-HETE was insignificant in women (β = −0.071, P = 0.221) and men (β = −0.038, P = 0.697). A scatterplot of eGFR versus ECd/Ccr (Figure 1A) indicated an inverse association of these two parameters in all subjects (β = −0.115, P = 0.030). Results of a univariate analysis of eGFR indicated a Cd-dose dependent decline of eGFR (Figure 1B). The adjusted mean eGFR for subjects with ECd/Ccr quartile 4 was 11.1 mL/min/1.73 m2 lower than those with the lowest ECd/Ccr quartile (P < 0.001). In a subset analysis (Figure 1C), an inverse association of eGFR and urinary Cd was evident in normotensive non-smoking subjects (β = −0.213, P = 0.027).
Table 2.
Predictors of an eGFR
| Independent variables | eGFR, mL/min/1.73 m2 |
|||||
|---|---|---|---|---|---|---|
| All subjects (n = 309) |
Men (n = 84) |
Women (n = 225) |
||||
| β | P-value | β | P-value | β | P-value | |
| Gender | 0.033 | 0.546 | − | − | − | − |
| Smoking | 0.068 | 0.219 | 0.033 | 0.718 | 0.067 | 0.268 |
| Age (years) | −0.308 | <0.001 | −0.316 | 0.002 | −0.295 | <0.001 |
| BMI (kg/m2) | −0.130 | 0.011 | −0.086 | 0.393 | −0.128 | 0.034 |
| Systolic pressure (mmHg) | −0.109 | 0.082 | −0.197 | 0.137 | −0.091 | 0.219 |
| Diastolic pressure (mmHg) | 0.013 | 0.837 | 0.036 | 0.774 | 0.006 | 0.939 |
| Log [(EHETE/Ccr) × 103], pg/mL | −0.061 | 0.213 | −0.038 | 0.697 | −0.071 | 0.221 |
| Log [(ECd/Ccr) × 105], µg/L | −0.115 | 0.030 | −0.226 | 0.031 | −0.075 | 0.232 |
| Log [(ENAG/Ccr) × 103], U/L | −0.310 | <0.001 | −0.201 | 0.051 | −0.344 | <0.001 |
| Log [(Eβ2MG/Ccr) × 103], µg/L | −0.189 | <0.001 | −0.246 | 0.016 | −0.166 | 0.011 |
| Adjusted R2 | 0.330 | <0.001 | 0.340 | <0.001 | 0.311 | <0.001 |
eGFR was a continuous dependent variable, while variables listed in the first column were independent variables. Adjusted R2 indicates the total variation in eGFR explained by all independent variables. P ≤ 0.05 are considered to indicate statistically significant.
FIGURE 1.
E Cd rate as a predictor of eGFR decline. The eGFR versus log [(ECd/Ccr)×105] scatterplots compare eGFR to ECd rate in all subjects (A), and in normotensive and hypertensive subjects who smoked and did not smoke (C). The reference line in (A) is based on the CKD diagnosis, eGFR <60 mL/min/1.73 m2. (B) The mean eGFR±SE values for subjects in each quartile (Q) of ECd. (D) The bars represent mean eGFR±SE values in normotensive and hypertensive subjects in each quartile of urinary Cd. The numbers above the bars are mean differences of eGFR in urinary Cd quartile 4, compared with quartiles 1, 2 and 3. All mean eGFR values are adjusted for covariates (age at 47.2 years and BMI at 24.47 kg/m2) and interactions. The ECd/Ccr×100 (SD) values in urinary Cd quartiles 1, 2, 3 and 4 are 1.37 (0.35), 2.42 (0.31), 3.90 (0.61) and 9.22 (4.81) μg/L of filtrate, and the corresponding numbers of subjects are 77, 76, 77 and 79, respectively.
In hypertensive group (Figure 1D), eGFR decline was notable. The mean eGFR standard error (SE) in hypertensive subjects with urinary Cd quartile 4 was 16.87 (3.32) mL/min/1.73 m2 lower than normotensive subjects with the lowest quartile (P < 0.001) and 12 (3.29) mL/min/1.73 m2 lower than hypertensive subjects with the lowest quartile (P = 0.008). In contrast, the mean eGFR (SE) in normotensive subjects with Cd quartile 4 was 10.3 (3.36) mL/min/1.73 m2 lower than the lowest urinary Cd quartile, but this mean difference was not statistically significant (P = 0.062).
An association of eGFR and ECd was not evident in an equivalent analysis with excretion data normalized to creatinine excretion (Supplementary data, Table S1). There was an inverse association of eGFR with age (β = −0.330, P < 0.001), BMI (β = −0.134, P = 0.018) and urinary β2-MG (β = −0.225, P < 0.001), but there was only a tendency for an inverse association of eGFR and urinary NAG (β = −0.103, P = 0.074). The scatterplots of eGFR versus urinary Cd normalized to creatinine excretion (Supplementary data, Figure S1) indicated insignificant correlations between these two parameters.
Urinary β2-MG as a predictor of albumin excretion
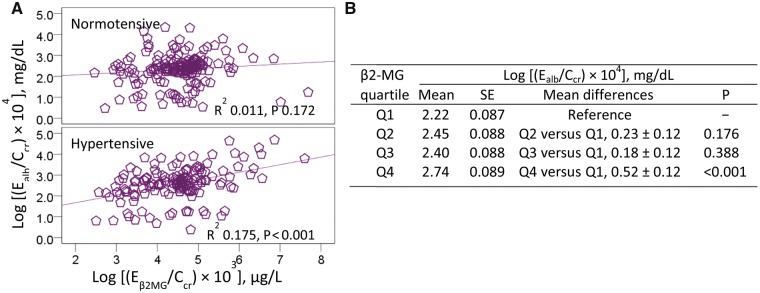
A much larger proportion of the variation in urinary albumin could be accounted for men (22.4%, P = 0.001) than for women (8.9%, P = 0.001) (Table 3). Of note, higher urinary albumin levels in men showed a moderate association with lower urinary 20-HETE levels (β = −0.384, P < 0.001), and higher SBP levels (β = 0.302, P = 0.037), while there was a trend for an association with younger age (β = −0.200, P = 0.071). In women, there was association between albumin and β2-MG (β = 0.231, P = 0.002) and BMI (β = 0.131, P = 0.060), but not 20-HETE (β = −0.019, P = 0.776). To examine urinary albumin versus β2-MG further, scatterplots comparing urinary albumin to β2-MG levels were constructed, and a linear relationship between β2-MG and albumin was evident in hypertensive subjects (Figure 2A). In an effect size analysis, the highest quartile of urinary β2-MG was associated with a 23.4% increase in urinary albumin excretion rate compared with the lowest quartile (P < 0.001) (Figure 2B).
Table 3.
Predictors of albumin excretion rate
| Independent variables | Log [(EALB/Ccr) × 104], mg/dL |
|||||
|---|---|---|---|---|---|---|
| All subjects (n = 309) |
Men (n = 84) |
Women (n = 225) |
||||
| β | P-value | β | P-value | β | P-value | |
| Gender | −0.002 | 0.974 | − | − | − | − |
| Smoking | 0.032 | 0.613 | −0.078 | 0.435 | 0.054 | 0.430 |
| Age (years) | −0.011 | 0.842 | −0.200 | 0.071 | 0.030 | 0.670 |
| BMI (kg/m2) | 0.129 | 0.027 | 0.131 | 0.232 | 0.131 | 0.060 |
| Systolic pressure (mmHg) | 0.196 | 0.007 | 0.302 | 0.037 | 0.159 | 0.062 |
| Diastolic pressure (mmHg) | 0.010 | 0.886 | 0.039 | 0.771 | 0.020 | 0.819 |
| Log [(EHETE/Ccr) × 103], pg/mL | −0.110 | 0.052 | −0.384 | <0.001 | −0.019 | 0.776 |
| Log [(ECd/Ccr) × 105], µg/L | 0.047 | 0.438 | 0.016 | 0.884 | 0.040 | 0.586 |
| Log [(ENAG/Ccr) × 103], U/L | 0.074 | 0.232 | 0.073 | 0.511 | 0.051 | 0.492 |
| Log [(Eβ2MG/Ccr) × 103], µg/L | 0.204 | 0.001 | 0.155 | 0.153 | 0.231 | 0.002 |
| Adjusted R2 | 0.117 | <0.001 | 0.224 | 0.001 | 0.089 | 0.001 |
Log EALB/Ccr was a continuous dependent variable, while variables listed in the first column were independent variables. Adjusted R2 indicates the total variation in EALB/Ccr explained by all independent variables. P ≤ 0.05 are considered to indicate statistically significant.
FIGURE 2.
Urinary β2-MG as a predictor of an increase in albumin excretion. (A) The scatterplots of log [(Ealb/Ccr) × 104] versus log [Eβ2MG/Ccr) × 103] compare albumin excretion to β2-MG excretion in normotensive and hypertensive subjects. (B) The mean [(Ealb/Ccr) × 104]±SE values for subjects in each quartile of urinary β2-MG excretion. The mean (SD) values for urinary β2-MG excretion as Eβ2MG/Ccr × 100 in urinary β2-MG quartiles (Q) 1, 2, 3 and 4 are 3.52 (2.48), 22.39 (8.47), 56.89 (13) and 1940 (7217) µg/L of filtrate, and the corresponding numbers of subjects are 76, 78, 77 and 76, respectively.
In an equivalent analysis with data normalized to creatinine excretion (Supplementary data, Table S2), a similarly inverse association between urinary albumin and 20-HETE (β = −0.399, P < 0.001) was seen in men together with a positive association of albumin and SBP (β = 0.284, P = 0.053). In women, higher urinary albumin levels were associated with higher urinary β2-MG (β = 0.213, P = 0.004). The scatterplots of urinary albumin versus urinary β2-MG normalized to creatinine excretion (Supplementary data, Figure S2) indicated a significant correlation of these two parameters especially in the hypertensive group (R2 = 0.143, P < 0.001).
DISCUSSION
An association of a reduced eGFR and an increase in urinary Cd levels, seen in both men and women after adjustment for covariates and interactions, lends a support to findings from large population-based studies, known as the US National Health and Nutrition Examination Surveys (the US NHANES), Korean NHANES and the Hortega Study in Spain, suggesting that long-term Cd exposure increase the risk of developing CKD [3–7]. Our observation of an association of a reduction in eGFR and ECd in non-smoking subjects without hypertension provides further evidence, linking chronic dietary Cd intake to a decline in eGFR. Our findings are in line with a report from China showing an association between Cd intake and an increase in the prevalence of CKD [9].
In an independent health survey of residents of areas with Cd pollution [30], the prevalence rates of tubular injury, proteinuria and eGFR < 60 mL/min/1.73 m2 (based on the Modification of Diet in Renal Disease equation) were 36.1, 24.1 and 16.2%, respectively. The corresponding prevalence rates in the control area were 28.3, 17.2 and 10%, respectively. Due to younger age, the prevalence of eGFR <60 mL/min/1.73 m2 in our study subjects was lower than the health survey report [30]. However, the prevalence of Stage 2 CKD among subjects in our study was notably high. Distinctively, data in the present study showed an association between eGFR decline and urinary Cd together with evidence for tubular damage and a reduced reabsorption capacity. Furthermore, an independent effect of hypertension on eGFR decline was evident; subjects with hypertension on average had 4.6 mL/min/1.73 m2 lower eGFR compared with the mean eGFR of normotensive subjects. These data were consistent with the US NHANES 1999–2006 data that showed higher CKD prevalence in those with hypertension; CKD prevalence rates in adult participants with normal blood pressure, prehypertension, undiagnosed hypertension and diagnosed hypertension were 13.4, 17.5, 22 and 27.5%, respectively [31].
We have herein observed, for the first time, differences between men and women in their urinary 20-HETE. Distinctively, the mean urinary 20-HETE in women with hypertension was 2-fold higher than their normotensive counterparts. Of further interest, women with hypertension excreted 20-HETE as much as men did. Conceivably, an increased 20-HETE excretion in Cd-exposed women may be due to an increase in serum testosterone levels and/or a fall in estrogen levels, reported elsewhere [21–24]. An increment of urinary Cd levels from <2–3 μg/g creatinine, found to be associated with a 28% rise in serum testosterone levels in postmenopausal Japanese women, supports our notion [21]. Furthermore, an inverse association between urine Cd and serum estradiol levels was seen in postmenopausal Japanese and Swedish women [22, 23]. In the Swiss Kidney Project on Genes in Hypertension [24], urinary Cd correlated with testosterone excretion in men, while there was a trend for an association in women.
Another gender-related difference was on urinary albumin excretion. In men only, urinary 20-HETE levels showed an inverse association with albumin levels. Intriguingly, the mean urinary β2-MG in hypertensive men was nearly 8-fold higher than the mean urinary β2-MG in hypertensive women, while urinary albumin excretion rate in subjects with the highest quartile of urinary β2-MG was 1.23-fold higher than those with the lowest quartile. The higher urinary albumin excretion seen in subjects with higher urinary β2-MG levels may have been secondary to Cd-induced tubular injury, leading to an impaired tubular reabsorptive function. A direct effect of Cd on albumin reabsorption has been evident in a study Lilly Laboratories Cell-Porcine Kidney1 (LLC-PK1) cell line [32], where Cd has been found to reduce the levels of megalin, the protein involved in albumin reabsorption.
In conclusion, a marked eGFR reduction was found to be associated with elevated urinary Cd levels in both men and women together with signs of tubular damage and dysfunction, evident from increases in urinary levels of NAG and β2-MG. A 2-fold increase in 20-HETE excretion in Cd-exposed women with hypertension, but not in men, may suggest a gender differential effect of Cd on renal cytochrome P450-mediated.
Strengths and limitations
The strengths of this study include an analysis of apparently healthy women and men, who were relatively young, the community-based recruitment of study subjects and the fact that excretion rates of Cd and 20-HETE were normalized to creatinine clearance. Furthermore, sources of Cd were relatively homogenous as none of the subjects had been exposed to metals in the workplace. The limitations of this study were its cross-sectional design, which limited a causal inference of Cd body burden on GFR reduction, and a small sample size. A lack of information on co-exposure to other metals, notably chromium and lead, was an additional limitation of our study.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Kenneth R. Phelps for reviewing our manuscript draft and for his advice on the rationale for data normalizing to creatinine clearance. This study was supported in part by the Royal Jubilee PhD scholarship (K.B.) and by the Reverse Brain Drain Award (S.S.) from the Commission for Higher Education, Thailand’s Ministry of Education.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Satarug S, Vesey DA, Gobe GC.. Current health risk assessment practice for dietary cadmium: data from different countries. Food Chem Toxicol 2017; 106: 430–445 [DOI] [PubMed] [Google Scholar]
- 2. Satarug S. Dietary cadmium intake and its effects on kidneys. Toxics 2018; 6: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferraro PM, Costanzi S, Naticchia A. et al. Low level exposure to cadmium increases the risk of chronic kidney disease: analysis of the NHANES 1999-2006. BMC Public Health 2010; 10: 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Navas-Acien A, Tellez-Plaza M, Guallar E. et al. Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am J Epidemiol 2009; 170: 1156–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin YS, Ho WC, Caffrey JL. et al. Low serum zinc is associated with elevated risk of cadmium nephrotoxicity. Environ Res 2014; 134: 133–138 [DOI] [PubMed] [Google Scholar]
- 6. Kim NH, Hyun YY, Lee KB. et al. Environmental heavy metal exposure and chronic kidney disease in the general population. J Korean Med Sci 2015; 30: 272–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grau-Perez M, Pichler G, Galan-Chilet I. et al. Urine cadmium levels and albuminuria in a general population from Spain: a gene-environment interaction analysis. Environ Int 2017; 106: 27–36 [DOI] [PubMed] [Google Scholar]
- 8. Satarug S, Ruangyuttikarn W, Nishijo M. et al. Urinary cadmium threshold to prevent kidney disease development. Toxics 2018; 6: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shi Z, Taylor AW, Riley M. et al. Association between dietary patterns, cadmium intake and chronic kidney disease among adults. Clin Nutr 2017; 5614: 31366–31368 [DOI] [PubMed] [Google Scholar]
- 10. Lee BK, Kim Y.. Association of blood cadmium with hypertension in the Korean general population: analysis of the 2008-2010 Korean National Health and Nutrition Examination Survey data. Am J Ind Med 2012; 55: 1060–1067 [DOI] [PubMed] [Google Scholar]
- 11. Garner RE, Levallois P.. Associations between cadmium levels in blood and urine, blood pressure and hypertension among Canadian adults. Environ Res 2017; 155: 64–72 [DOI] [PubMed] [Google Scholar]
- 12. Scinicariello F, Abadin HG, Murray HE.. Association of low-level blood lead and blood pressure in NHANES 1999–2006. Environ Res 2011; 111: 1249–1257 [DOI] [PubMed] [Google Scholar]
- 13. Oliver-Williams C, Howard AG, Navas-Acien A. et al. Cadmium body burden, hypertension, and changes in blood pressure over time: results from a prospective cohort study in American Indians. J Am Soc Hypertens 2018; 12: 426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boonprasert K, Vesey DV, Gobe GC. et al. Is renal tubular cadmium toxicity clinically relevant? Clin Kidney J 2018; 11: 681–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lasker JM, Chen WB, Wolf I. et al. Formation of 20-hydroxyeicosatetraenoic acid, a vasoactive and natriuretic eicosanoid, in human kidney. Role of Cyp4F2 and Cyp4A11. J Biol Chem 2000; 275: 4118–4126 [DOI] [PubMed] [Google Scholar]
- 16. Boonprasert K, Satarug S, Morais C. et al. The stress response of human proximal tubule cells to cadmium involves up-regulation of haemoxygenase 1 and metallothionein but not cytochrome P450 enzymes. Toxicol Lett 2016; 249: 5–14 [DOI] [PubMed] [Google Scholar]
- 17. Fan F, Muroya Y, Roman RJ.. Cytochrome P450 eicosanoids in hypertension and renal disease. Curr Opin Nephrol Hypertens 2015; 24: 37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu CC, Schwartzman ML.. The role of 20-HETE in androgen-mediated hypertension. Prostaglandins Other Lipid Mediat 2011; 96: 45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dalmasso C, Maranon R, Patil C. et al. 20-HETE and CYP4A2 ω-hydroxylase contribute to the elevated blood pressure in hyperandrogenemic female rats. Am J Physiol Renal Physiol 2016; 311: F71–F77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pandey V, Garcia V, Gilani A. et al. The blood pressure-lowering effect of 20-HETE blockade in cyp4a14(-/-) mice is associated with natriuresis. J Pharmacol Exp Ther 2017; 363: 412–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nagata C, Nagao Y, Shibuya C. et al. Urinary cadmium and serum levels of estrogens and androgens in postmenopausal Japanese women. Cancer Epidemiol Biomarkers Prev 2005; 14: 705–708 [DOI] [PubMed] [Google Scholar]
- 22. Nagata C, Konishi K, Goto Y. et al. Associations of urinary cadmium with circulating sex hormone levels in pre- and postmenopausal Japanese women. Environ Res 2016; 150: 82–87 [DOI] [PubMed] [Google Scholar]
- 23. Ali I, Engström A, Vahter M. et al. Associations between cadmium exposure and circulating levels of sex hormones in postmenopausal women. Environ Res 2014; 134: 265–269 [DOI] [PubMed] [Google Scholar]
- 24. Bochud M, Jenny-Burri J, Pruijm M. et al. Urinary cadmium excretion is associated with increased synthesis of cortico- and sex steroids in a population study. J Clin Endocrinol Metab 2018; 103: 748–758 [DOI] [PubMed] [Google Scholar]
- 25. Argyropoulos CP, Chen SS, Ng YH. et al. Rediscovering β-2 microglobulin as a biomarker across the spectrum of kidney diseases. Front Med 2017; 4: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tassi C, Abbritti G, Mancuso F. et al. Activity and isoenzyme profile of N-acetyl-β-D-glucosaminidase in urine from workers exposed to cadmium. Clin Chim Acta 2000; 299: 55–64 [DOI] [PubMed] [Google Scholar]
- 27. Boonprasert K, Ruengweerayut R, Satarug S. et al. Study on the association between environmental cadmium exposure, cytochrome P450-mediated 20-HETE, heme-oxygenase-1 polymorphism and hypertension in Thai population residing in a malaria endemic areas with cadmium pollution. Environ Toxicol Pharmacol 2011; 31: 416–426 [DOI] [PubMed] [Google Scholar]
- 28. Levey AS, Coresh J, Bolton K. et al. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis 2002; 39: S1–S266 [PubMed] [Google Scholar]
- 29. Phelps KR, Stote KS, Mason D.. Tubular calcium reabsorption and other aspects of calcium homeostasis in primary and secondary hyperparathyroidism. Clin Nephrol 2014; 82: 83–91 [DOI] [PubMed] [Google Scholar]
- 30. Swaddiwudhipong W, Nguntra P, Kaewnate Y. et al. Human health effects from cadmium exposure: comparison between persons living in cadmium-contaminated and non-contaminated areas in northwestern Thailand. Southeast Asian J Trop Med Publ Health 2015; 46: 133–142 [PubMed] [Google Scholar]
- 31. Crews DC, Plantinga LC, Miller ER. et al. Prevalence of chronic kidney disease in persons with undiagnosed or prehypertension in the United States. Hypertension 2010; 55: 1102–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gena P, Calamita G, Guggino WB.. Cadmium impairs albumin reabsorption by down-regulating megalin and ClC5 channels in renal proximal tubule cells. Environ Health Perspect 2010; 118: 1551–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.