Abstract
We investigated the influence of the bifunctional guidance molecule netrin-1 on axonal growth in the injured adult spinal cord. In the adult, netrin-1 is expressed on mature oligodendrocytes, cells of the central canal, and the meninges. Netrin-1 protein in white matter is selectively enriched adjacent to paranodal loops of myelin in nodes of Ranvier. The repulsion-mediating netrin-1 uncoordinated-5 (UNC5) receptors are expressed by neurons of the corticospinal and rubrospinal projections, and by intrinsic neurons of the spinal cord, both before and after spinal cord injury. Neutralization of netrin-1 in myelin prepared from adult rat spinal cord using UNC5 receptor bodies increases neurite outgrowth from UNC5-expressing spinal motor neurons in vitro. Furthermore, axon regeneration is inhibited in a netrin-1-enriched zone, devoid of other myelin-associated inhibitors, within spinal cord lesion sites in vivo. We conclude that netrin-1 is a novel oligodendrocyte-associated inhibitor that can contribute to axonal growth failure after adult spinal cord injury.
Keywords: spinal cord injury, axon regeneration, white matter inhibition, netrin-1, UNC5, DCC, nogo, MAG, OMgp, retrovirus, plasticity, corticospinal, rubrospinal, intraspinal
Introduction
Netrin-1 is a bifunctional ligand that can either attract or repel axons. The netrin-1 receptor Deleted in Colorectal Cancer (DCC) mediates axon attraction toward netrin-1 (Tessier-Lavigne et al., 1988; Hedgecock et al., 1990; Serafini et al., 1994; Keino-Masu et al., 1996; Serafini et al., 1996; de la Torre et al., 1997; Deiner et al., 1997; Finger et al., 2002), whereas UNC5 receptors are implicated in axon repulsion from netrin-1 (Hedgecock et al., 1990; Leung-Hagesteijn et al., 1992; Hamelin et al., 1993; Leonardo et al., 1997). DCC-expressing commissural axons are attracted toward the netrin-1-releasing floor plate at the embryonic spinal cord ventral midline (Tessier-Lavigne et al., 1988; Kennedy et al., 1994; Serafini et al., 1994, 1996). In contrast, axons of UNC5A-expressing developing motor neurons (Leonardo et al., 1997) and trochlear neurons are repelled by netrin-1-releasing floor plate explants or by aggregates of netrin-1 secreting COS cells in vitro (Colamarino and Tessier-Lavigne, 1995; Guthrie and Pini, 1995).
Repulsion by netrin-1 requires axonal expression of either UNC5 alone or of a complex of UNC5 and DCC receptors (Hedgecock et al., 1990; Colavita and Culotti, 1998; Hong et al., 1999). Studies in Drosophila suggest that expression of a combination of both UNC5 and DCC receptors is required to mediate long range repulsion of axons in the presence of low concentrations of netrin-1, whereas UNC5 alone is sufficient to mediate short range repulsion in the presence of high concentrations of netrin-1 (Keleman and Dickson, 2001). A developmental shift from the expression of the attraction-mediating netrin-1 receptor DCC during spinal cord development to the predominant expression of the repulsion-mediating UNC5 receptors in the adult rat spinal cord has been reported (Manitt et al., 2004). Netrin-1 is expressed by mature oligodendrocytes in the adult spinal cord (Manitt et al., 2001), and netrin-1 as well as UNC5 expression persist after midline myelotomy of the thoracic spinal cord (Manitt et al., 2006). Oligodendrocytes are also sources of the well known myelin-associated inhibitors nogo (Chen et al., 2000; GrandPre et al., 2000), myelin-associated glycoprotein (MAG) (McKerracher et al., 1994; Mukhopadhyay et al., 1994) and oligodendrocyte myelin glycoprotein (OMgp) (Wang et al., 2002). Oligodendrocytes also express the repulsive guidance molecules semaphorin4D (Moreau-Fauvarque et al., 2003) and ephrinB3 (Benson et al., 2005).
To date, the functional role of netrin-1 in the adult vertebrate CNS remains elusive. To evaluate whether netrin-1 influences axonal plasticity or regeneration in the adult spinal cord, we characterized netrin-1 receptor expression profiles in descending and intraspinal axonal populations before and after thoracic spinal cord complete transection lesions. We then evaluated spinal motor neurite outgrowth on extracts of adult myelin after neutralization of netrin, and motor axonal regeneration in an in vivo model of spinal cord injury in an environment enriched in netrin-1 and free of other myelin inhibitors. We now provide functional evidence that netrin-1 is an oligodendrocyte-associated inhibitor of axonal regeneration in the adult spinal cord.
Materials and Methods
Expression of netrin-1, UNC5, and DCC in the adult spinal cord.
To determine patterns and levels of netrin-1, UNC5A, UNC5C, and DCC expression in the adult intact and injured spinal cord, in situ hybridization and real-time (RT)-PCR were performed. In situ hybridization was performed on 35 μm paraformaldehyde (4%)-fixed sections as described previously (Lacroix et al., 1996). The following cDNA fragments were used as templates for in vitro transcription of radiolabeled antisense and sense cRNA probes (Riboprobe Combination system T3/T7; Promega, Madison, WI; 200 μCi α-35S-UTP per probe) from linearized Bluescript vectors. The following probes were used: mouse netrin-1 probe, 1315 bp BamHI (288)–BamHI (1603) fragment of the coding region excised from vector pMNET (mouse netrin-1 coding region in Bluescript vector) (Serafini et al., 1996); rat netrin-1 probe, 933 bp fragment ranging from nucleotide 882 of the coding region to the 3′ end of the open reading frame (Manitt et al., 2001) (a kind gift from Dr. T. Kennedy, McGill University, Montreal, Quebec, Canada); rat UNC5A probe, 800 bp PstI (2478)–XhoI (3′ UTR) fragment of the rat UNC5A cDNA (Leonardo et al., 1997); rat UNC5C probe, 1440 bp HindIII (209)–HindIII (1650) fragment within the UNC5C coding region (Leonardo et al., 1997); rat DCC probe, 609 bp fragment ranging from nucleotide position 3369–3981 within the coding region, retrieved from vector pCR2.1 (Leonardo et al., 1997). Emulsion-coated (NTB2; Kodak, Rochester, NY) slides were exposed for 3–5 weeks.
Expression of netrin-1 and its receptors UNC5A, UNC5C, and DCC in response to axotomy was analyzed by in situ hybridization 8 h (n = 4), 24 h (n = 4), 48 h (n = 4), 1 week (n = 4), and 2 weeks (n = 4) after a complete transection of the thoracic spinal cord and was compared with expression in sham-lesioned animals (n = 4) that had undergone a T7 laminectomy only (see below).
For real-time PCR analysis, the following intron-spanning primers were generated: rat netrin-1, forward 5′GCTTCCAAAGGAAAACTGAA 3′, reverse 5′CTTCCACCAGTCCCCTGCTT 3′ (104 bp product); rat UNC5A, forward 5′TCTCTCCATCCACGACGTGC 3′, reverse 5′ACTGCTGGGTGCCGTTCCAG 3′ (100 bp product); rat UNC5C, forward 5′ACAAGAGGCCATGACTGGAG 3′, reverse 5′TCCAGGATTACGCCAGTCGG 3′ (100 bp product); rat DCC, forward 5′ACAGCCCCTGAAGTGTCTGA 3′, reverse 5′AGCCCTTCCAAACTCGCCAT 3′ (103 bp product); rat Rplp1 household gene (ribosomal protein, large, P1), RT-PCR Primer Set Rplp1 (SuperArray Bioscience, Frederick MD). To obtain tissue for RT-PCR analysis, 1 cm spinal cord segments were isolated rostral and caudal to a thoracic spinal cord transection site (T7) 1 week (n = 4), 2 weeks (n = 4), and 4 weeks (n = 4) after injury or from sham-lesioned rats (n = 4) that had undergone laminectomy only (see below). Total RNA was isolated using the RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA). Genomic DNA was eliminated by on-column DNase digest. Five hundred nanograms of purified total RNA were reverse transcribed using the SuperScript III First-Strand synthesis system for RT-PCR (Invitrogen, Carlsbad, CA). PCR products were amplified in a 25 μl reaction from 2.5% of the cDNA synthesis reaction using RT2 Real-Time SYBR Green/Fluorescein master mix (SuperArray Bioscience) and 400 nm netrin-1, UNC5A, UNC5C, or DCC specific primer pairs. All real-time PCRs were performed in triplicate (real-time PCR iCycler iQ detection system in combination with the iCycler iQ software version 3.0A, Bio-Rad Laboratories, Hercules, CA) using the following protocol: 10 min 95°C (one cycle), 15 s 95°C, followed by 30 s 58°C (40 cycles). Data collection was performed during the last 95% of the annealing and extension step (58°C) of each cycle at 490 nm and data were PCR-baseline subtracted and curve fitted. Threshold cycles (Ct) were determined for all reactions at 1350 relative fluorescence units (RFUs) (≥10 SDs of the PCR baseline). Threshold cycles (Ct) of netrin-1 and its receptors were normalized to Cts of the household gene Rplp1 (ΔCt). The normalized expression levels of the genes of interest were then expressed relative to the mean corrected expression level of the corresponding gene in sham-lesioned animals by subtraction of the mean corrected threshold cycle in sham-lesioned animals from ΔCt at a certain time point after lesion (ΔΔCt). The fold change was calculated to be 10^ (ΔΔCt/mean value of slopes in gene of interest standard curve and household gene standard curve). Differences in expression levels were determined by ANOVA.
Preparation of UNC5 receptor bodies.
To test whether netrin-1 signaling can influence axonal outgrowth on myelin substrates, we generated functional-blocking UNC5B receptor bodies. UNC5B receptor bodies were engineered by fusion of the extracellular domain of the rat UNC5B receptor to the constant region heavy chain of human IgG1 in plasmid Pcep-4 (Invitrogen) and were purified from supernatants of stably transfected HEK 293-EBNA cells (Invitrogen) by protein A sepharose affinity chromatography (Leonardo et al., 1997). Netrin-1 binding by purified UNC5B receptor bodies was demonstrated in ELISA. Recombinant mouse netrin-1 was coated in PBS at 20 μg/ml for 2 h at room temperature, blocked with 1% (w/v) BSA (fraction V; Sigma, St. Louis, MO) in PBS and incubated with 1, 2, 4, 8, 16, 32, 64, and 128 μg/ml UNC5B receptor body in PBS, 1% BSA for 2 h at room temperature (all concentrations in triplicate). Bound UNC5B hu Fc was detected with peroxidase-coupled mouse anti-human IgG (H+L) (1:1000 in PBS, 1% BSA; Pierce, Rockford, IL) in an ABTS reaction [0.2 mg/ml 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (Sigma) in 25 mm citric acid and 51 mm Na2HPO4, 0.018% (v/v) H2O2,100 μl/well].
In vitro effects of adult myelin-associated netrin-1 on neurite outgrowth.
We tested whether netrin-1 in adult spinal cord myelin inhibits axon outgrowth from UNC5-expressing neuronal populations in vitro. Neurite outgrowth from UNC5A- and UNC5C-expressing spinal motor neurons [embryonic day 15 (E15)] was analyzed on myelin extracted from adult spinal cord, in the presence of UNC5B receptor body, or in controls with chrome pure human IgG, Fc fragment (009–000-008; Jackson ImmunoResearch, West Grove, PA). Myelin was purified from adult rat spinal cords by centrifugation over sucrose gradients according to Norton and Poduslo (1973). The presence of a major myelin inhibitory protein, MAG, was confirmed in this preparation by Western blot (33 μg of myelin protein/lane) using goat anti-MAG antibody (AF538; R&D Systems, Minneapolis, MN). For neurite outgrowth assays, plates were coated with 16 μg/ml poly-l-lysine for 1 h at room temperature, then washed with 0.1 m NaHCO3 and DMEM (Invitrogen 11965). Myelin containing the equivalent of 2 μg of myelin protein was coated per cm2 culture dish by evaporation of myelin suspension overnight. Coated myelin was washed twice with cell culture medium before preincubation with 50 μg/ml UNC5B hu Fc or 50 μg/ml hu Fc IgG in DMEM/F12 (Invitrogen 11330) for 8 h at 37°C. Spinal motor neurons were dissociated from E15 rat spinal cords after a 30 min digest with 0.05% trypsin EDTA (Invitrogen 25300) at 37°C. 2 × 104 cells per cm2 were seeded onto UNC5B receptor body/hu Fc IgG preincubated myelin in DMEM F12, containing B27 supplement (Invitrogen 0586), penicillin-streptomycin/glutamine and 50 μg/ml UNC5B receptor body or hu Fc IgG. After 48 h, cells were visualized with the live-cell stain Calcein AM (Invitrogen) and the length of the longest neurite was determined using NIH ImageJ software (version 1.37) in combination with the NeuronJ version 1.1.0 (C) plugin (developed by E. Meijering, Erasmus University Medical Center, Rotterdam, The Netherlands). At least 400 neurites were measured per condition and the experiment was replicated four times. Differences in neurite length were determined by unpaired t test. Axonal expression of UNC5A by spinal motor neurons on myelin was confirmed immunocytochemically using rabbit polyclonal antibodies against the intracellular domain of UNC5A (Ab 2026, kindly provided by Dr. Hinck, University of California, Santa Cruz, CA). Specificity of antibody 2026 for UNC5A has been well documented previously (Williams et al., 2003, 2006).
In vivo examination of netrin-1 effects on axonal regeneration after injury.
The presence of other myelin-associated inhibitors of axonal growth complicates the assessment of an independent role of netrin-1 on axonal regeneration in vivo. We therefore generated spinal cord lesions, and injected primary fibroblasts expressing netrin-1 or, in controls, the reporter gene enhanced green fluorescent protein (EGFP) into the injury site. This created a spinal cord injury site presumably free of other myelin associated inhibitors such as nogo, MAG, OMgp, semaphorin or ephrin, and permitted independent assessment of the role of netrin-1 on axon outgrowth after spinal cord injury. Thus, the netrin-1-expressing milieu differed from the control growth milieu by only a single gene.
Cloning of the netrin-1 cDNA into moloney leukemia virus-derived vectors.
To overexpress netrin-1, we cloned the mouse netrin-1 cDNA (Serafini et al., 1996) into the moloney leukemia virus (MLV) vector pLXIE (Lu et al., 2003). Included in the vector construct was an internal ribosomal entry site (IRES) expressing the reporter gene EGFP. We also generated a second netrin-1-expressing vector containing a hemagglutinin (HA) tag at the C terminus (netrin-1 HA IRES EGFP) to allow detection of netrin-1 expression. Control vectors expressed the reporter gene EGFP alone. These vectors were used to produce recombinant MLV and infect primary rat fibroblasts to overexpress the genes of interest, as described previously (Blesch and Tuszynski, 2001; Lu et al., 2003). Expression of netrin-1 in genetically modified fibroblasts was assessed by Northern and Western blots. For Northern blot, netrin-1 RNA was detected in 20 μg of fibroblast derived total RNA by hybridization (overnight, 65°C) with the α32P-dCTP labeled (3000 Ci/mmol) 1494 bp HincII-ScaI fragment of the mouse netrin-1 cDNA (Prime-It II Random Primer Labeling Kit; Stratagene, La Jolla, CA). For Western blot, fibroblast surface associated protein was extracted with ice-cold high salt buffer (1 m NaCl, 10 mm HEPES, 1 mm phenylmethylsulfonyl fluoride, 1× complete protease inhibitor mix; Roche, Mannheim, Germany) (Serafini et al., 1994), and methanol precipitation. HA-tagged netrin-1 was detected after separation on reducing 7% Tris-acetate polyacrylamide gels (Invitrogen) and transfer to nylon membranes (45 μm pore-size; Fisher Scientific, Pittsburgh, PA) with rabbit anti-HA-probe Y-11 IgG (400 ng/ml; Santa Cruz Biotechnology, Santa Cruz, CA) and peroxidase-conjugated goat anti-rabbit IgG (H+L), 1:100,000; Jackson ImmunoResearch) in a chemiluminescence reaction (SuperSignal West Pico chemiluminescent substrate; Pierce).
Neurite outgrowth assay from embryonic dorsal spinal cord explants.
To assess the bioactivity of netrin-1 released by genetically modified rat primary fibroblasts, its capacity to elicit axon outgrowth from DCC-expressing commissural axons from E13 rat dorsal spinal cord explants was tested. Primary rat fibroblasts expressing either EGFP, netrin-1 plus EGFP, or netrin-1HA plus EGFP were clustered as hanging drops for 14–16 h as described previously (Kennedy et al., 1994). Fibroblast aggregates were placed adjacent to E13 rat dorsal spinal cord explants (Serafini et al., 1994) in a collagen matrix, and axon outgrowth was assayed after 18 h of coculture.
Animal subjects and surgeries.
All surgery was performed using an anesthetic combination (2 ml/kg) of ketamine (25 mg/ml), rompun (1.3 g/ml) and acepromazine (0.25 mg/ml). National Institutes of Health guidelines for laboratory animal care and safety were strictly followed. To assess in vivo responses of injured axons to netrin-1, 50 adult female Fisher rats (160–200 g) underwent partial lesions of the cervical (C3) spinal cord. The lesion removed the dorsal columns and surrounding gray matter, using a tungsten wire knife inserted 0.8 mm lateral to the dorsal midline, 1.2 mm below the spinal cord surface, and extruded 2.5 mm toward and across the midline (Jones et al., 2003a). 30 of the 50 lesioned rats received netrin-1/EGFP-expressing fibroblast grafts, and 20 rats received EGFP-expressing control fibroblast grafts to the lesion site. 2 μl of a 105/μl cell suspension was pressure injected 0.8 mm below the intact dura using glass micropipettes and a picospritzer II (General Valve, Fairfield, NJ). Rats were killed either 5 weeks or 3 months postlesion for evaluation of axonal graft penetration using neurofilament immunocytochemistry: netrin-1/EGFP fibroblast grafted, n = 15 (5 weeks), n = 6 (3 months); EGFP control fibroblast grafted, n = 10 (5 weeks), n = 4 (3 months). Graft penetration by nociceptive calcitonin gene-related peptide (CGRP)-labeled axons was also examined in netrin-1/EGFP fibroblast grafted animals (n = 9 examined 5 weeks after injury) and EGFP control fibroblast grafted animals (n = 6 examined 5 weeks after injury).
Netrin-1 effects were further assessed on a supraspinal motor pathway, the rubrospinal projection, after cervical spinal cord injury. In an additional set of 16 rats, the lesion was unilaterally extended to include the left rubrospinal tract by rotation of the wire knife at its original insertion point by 180° and extrusion of the blade by 2.2 mm. Nine of the 16 rubrospinal tract-lesioned rats were grafted with netrin-1/neo-expressing fibroblasts, and seven rats received GFP/neo-expressing control fibroblasts. Two weeks before being killed, rubrospinal axons were labeled by injection of 300 nl of a 10% solution of biotinylated dextran amine (molecular weight, 10,000; Invitrogen) into the right red nucleus at the following coordinates: anteroposterior (AP) −0.57, mediolateral (ML) +0.08 (right hemisphere), dorsoventral (DV) −0.72; and AP −0.62, ML +0.07, DV −0.67 (Paxinos and Watson, 1998). Animals were killed 5 weeks after lesion.
In a separate set of 32 animals, to assess expression patterns of netrin-1 and its receptors using in situ hybridization and real-time PCR as described above, we performed complete T7 spinal cord transections (Blesch and Tuszynski, 2003). Subjects were killed at time points of 8, 24, and 48 h, and 1 and 2 weeks after the lesion for in situ hybridization (n = 4 subjects per time point) and at 1, 2, and 4 weeks after the lesion for real-time PCR (n = 4 subjects per time point). In six additional subjects, 5 μl of a 4% suspension of Fluorogold (FG) (Fluorochrome, Denver, CO) was injected into the thoracic spinal cord (T7) in several sites targeting the corticospinal and rubrospinal tracts, and in three of the six rats T7 complete transections were placed 1 week later. Animals were killed 2 weeks after Fluorogold injections and 1 week after T7 transections.
Immunocytochemistry.
Standard protocols for light-level and fluorescent immunohistochemistry (IHC) were used (Jones et al., 2003a). Primary antibody binding was detected with horse anti-goat, horse anti-mouse, or goat anti-rabbit biotin-conjugated IgG (1:200; Vector Laboratories, Burlingame, CA) followed by incubation with biotin–avidin-peroxidase complex (1:100 in TBS; Elite kit; Vector Laboratories) and Alexa Fluor 488 or Alexa Fluor 594-conjugated streptavidin (1:200 in TBS; Invitrogen), or by development in a diaminobenzidine reaction. The following antibodies were used: goat anti-netrin-1 (N18) IgG (4 μg/ml for light-level IHC, SC-9291; Santa Cruz Biotechnology); mouse anti-Neurofilament (200 kDa) IgG1, MAB5262, clone RT97 (25 ng/ml for light-level IHC; Millipore, Temecula, CA); mouse anti-NeuN (IgG), MAB 377 (5 μg/ml; Millipore); rabbit anti-CGRP, serum, AB1971 (1:8000 for light-level IHC, 1:2500 for fluorescent IHC; Millipore); mouse anti-adenomatous polyposis coli (APC) mature oligodendrocyte marker IgG, Ab-7 (2 μg/ml; Oncogene, San Diego, CA); rabbit anti-glial fibrillary acidic protein (GFAP), purified Ig fraction (1:1000 for light-level IHC, Z0334; Dako, High Wycombe, UK); goat anti-green fluorescent protein IgG (666 ng/ml for fluorescent IHC; Rockland Immunochemicals, Gilbertsville, PA). For netrin-1 immunolabeling, tissue sections were subjected to antigen retrieval consisting of a 5 h incubation in Tris-buffered saline (20 mm Tris, pH 9, 136 mm NaCl) at 60°C before antibody application. Specificity of the netrin-1 antibody was confirmed by (1) absence of labeling after primary antibody exclusion, (2) elimination of labeling after preincubation of sections with peptide against which antibody was raised, and (3) presence of selective labeling in adult hippocampal CA3 pyramidal neurons, which is also solely detected by netrin-1 in situ hybridization (data not shown). Double light-level immunocytochemistry was also performed with anti-netrin-1 and anti-contactin-associated protein 1 (CASPR; also known as neurexin IV or paranodin; rabbit anti-CASPR, H-66, IgG, 4 μg/ml; Santa Cruz Biotechnology), a marker that selectively labels perinodal segments of myelinated axons (Einheber et al., 1997; Huang et al., 2005). The CASPR signal was developed in a diaminobenzidine reaction omitting nickel chloride from the developing mix (yielding brown reaction product). Horseradish peroxidase was then quenched with 0.6% (v/v) H2O2 and tissue sections were subjected to antigen retrieval. Netrin-1 antibody binding was detected with biotin-conjugated donkey anti-goat IgG, followed by incubation with biotin-avidin-peroxidase complex and development with SG-substrate (blue reaction product, Vector SG, SK4700; Vector Laboratories).
Quantification of axonal density in netrin-1 and control grafts.
Every fourth 35-μm-thick neurofilament- or CGRP-labeled sagittal section was used for quantification of axon density. Graft area was outlined and pixel number occupied by neurofilament or CGRP label within the graft area was determined using NIH Image software and expressed relative to total pixel number comprising the graft area, as described previously (Grill et al., 1997). Threshold values were held constant between subjects. A mean value for neurofilament or CGRP density/section within the graft was determined for each animal and subsequently for each treatment group.
Quantification of number of lesioned rubrospinal axons.
The lesioned (left) rubrospinal tract was visualized by detection of the unilaterally injected anterograde tracer biotinylated dextran amine (BDA) in every fourth 35-μm-thick horizontal section of the spinal cord. The mean number of rubrospinal axons/section within the graft area was counted in a series of 1 in 4 horizontal sections in each animal. Mean axonal counts were corrected for BDA labeling efficiency in each animal by normalizing for the number of BDA-labeled axons in a coronal spinal cord section located 0.5 cm rostral to the lesion site.
Statistics.
Group differences were assessed by ANOVA or, when appropriate, unpaired t tests (when two groups were being compared), using a significance criterion of p < 0.05.
Results
Netrin-1 is expressed by mature oligodendrocytes in the adult spinal cord
Netrin-1 is expressed throughout the gray and white matter of the intact adult spinal cord, as demonstrated by in situ hybridization (Fig. 1a). The highest levels of expression are detected in adult white matter, meninges, and the central canal (Fig. 1). Specificity of the netrin-1 probe was verified by detection of the netrin-1-expressing floor plate in E15 rat spinal cord (Fig. 1e) (Serafini et al., 1996). Netrin-1 was also expressed in E15 cells of the central canal (Fig. 1e). The netrin-1 in situ hybridization signal colocalized with APC-immunolabeled oligodendrocytes in the adult cord, but not with GFAP-immunolabeled astrocytes or NeuN-labeled neurons (Fig. 1f–h). The netrin-1 sense probe (Fig. 1b) did not result in detectable signal. Neither netrin-3 nor netrin-4 were found in the adult spinal cord by in situ hybridization (data not shown).
Figure 1.
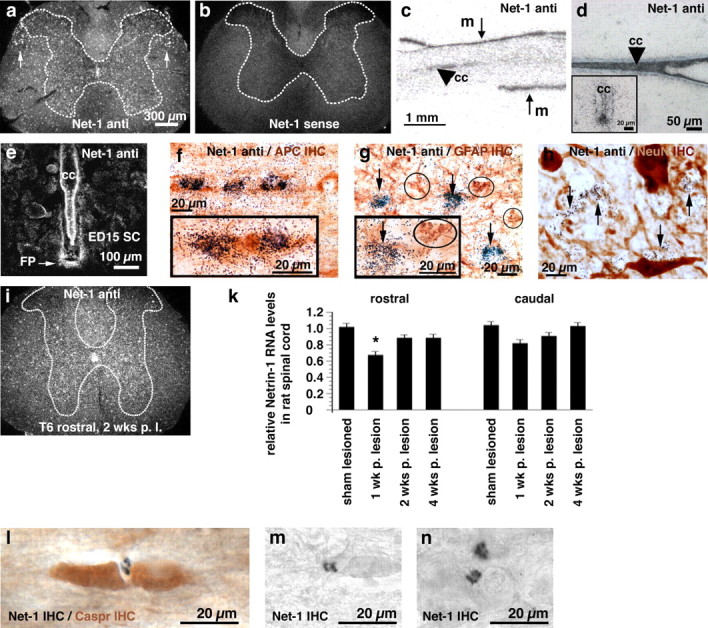
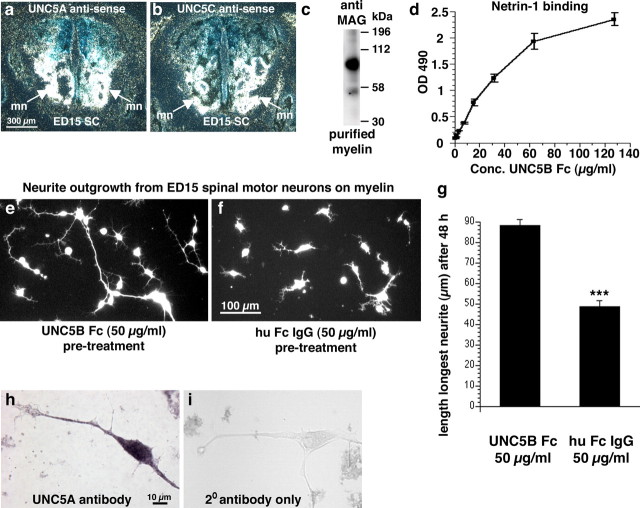
Netrin-1 expression in vivo. a–i, In situ hybridization was performed on rat spinal cord sections using rat netrin-1 antisense (Net-1 anti; a, c, d, e, f, g, h, i) or sense (b) α-35S-UTP labeled probes. a, b, Darkfield images of coronal sections through the adult intact cervical spinal cord. Note the netrin-1 signal in the central canal and throughout gray and white matter, with highest white matter expression levels in the dorsolateral fasciculus (white arrows). c, Autoradiography of sagittal section of adult spinal cord. Netrin-1 is expressed by meninges (m) surrounding the spinal cord and central canal (cc; arrowhead). d, Brightfield image of horizontal section of adult intact cervical spinal cord (Nissl counterstained). Netrin-1 is expressed by cells constituting the central canal. Inset, Coronal section of central canal. e, Coronal section of rat E15 spinal cord (darkfield). Netrin-1 is expressed in floor plate (FP) and by cells constituting the central canal as expected. f, Horizontal section through dorsolateral white matter of adult rat spinal cord. Combination of netrin-1 in situ hybridization with APC (mature oligodendrocyte marker) immunocytochemistry demonstrates netrin-1 expression by mature oligodendrocytes. Inset, Coronal section through dorsolateral white matter. g, h, Netrin-1 does not colocalize with GFAP (g, circled cells) or the neuronal marker NeuN (h). i, Detection of netrin-1 expression by in situ hybridization in the thoracic (T6) spinal cord adjacent to a T7 transection site, 2 weeks after lesion. k, RT-PCR of netrin-1 from spinal cord 1, 2, and 4 weeks after T7 transection, normalized to the housekeeping gene Rplp1. l, Immunolabeling for netrin-1 identifies its presence and enrichment in perinodal segments of adult white matter (black reaction product), using a counterstain for the perinodal marker Caspr (brown). m, n, Netrin-1 labeling in intact white matter of the dorsolateral funiculus (m), and persistent presence 3 months after spinal cord injury in the dorsolateral funiculus (n) located 500 μm rostral to injury site.
Patterns of netrin-1 expression were maintained 1, 2, and 4 weeks after T7 complete transection by both in situ hybridization and RT-PCR (Fig. 1i,k). Levels of netrin-1 expression differed little from the intact state, aside from a modest reduction in the rostral spinal cord segment 1 week after the lesion. Consistent with findings of in situ hybridization, netrin-1 was detected by immunolabeling in adult white matter (Fig. 1l–n). Notably, netrin-1 labeling was selectively enriched in perinodal regions, based on localization with the perinodal marker Caspr. Perinodal localization has been observed previously also with the myelin-associated inhibitors OMgp and MAG (Huang et al., 2005; Nie et al., 2006). Netrin-1 expression persisted 3 months after spinal cord injury (Fig. 1n). Also consistent with results of in situ hybridization, netrin-1 immunolabeling was detected on the meninges and around the central canal (data not shown).
UNC5 is expressed by motor systems projecting to, and intrinsic to, the spinal cord
Descending rubrospinal and corticospinal motor projections exhibit little spontaneous regeneration after injury. We find that both corticospinal and rubrospinal neurons express mRNA for the UNC5A receptor in the intact state, and this expression persists after spinal cord injury (Fig. 2). Neurons of layers II and III of the motor cortex also express an UNC5 receptor, but of the UNC5C type (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Within the adult spinal cord, UNC5A (Fig. 3a–d), UNC5C (Fig. 3f–i) and DCC (l–o) are expressed in gray matter by in situ hybridization. UNC5A is expressed throughout all laminas of the gray matter except laminas I/II (substantia gelatinosa) and lamina IX (ventral motor neuron pools) (Fig. 3a, c). This expression is maintained rostral and caudal to transection sites 8, 24, and 48 h, and 1 and 2 weeks later (two weeks shown in Fig. 3d). Real-time PCR confirms the expression of UNC5A 4 weeks after injury in spinal cord segments adjacent to the transection site (Fig. 3e); although levels diminish by approximately one-half compared with the sham-lesioned state, sufficient amounts remain to be readily detectable both by in situ hybridization and RT-PCR.
Figure 2.
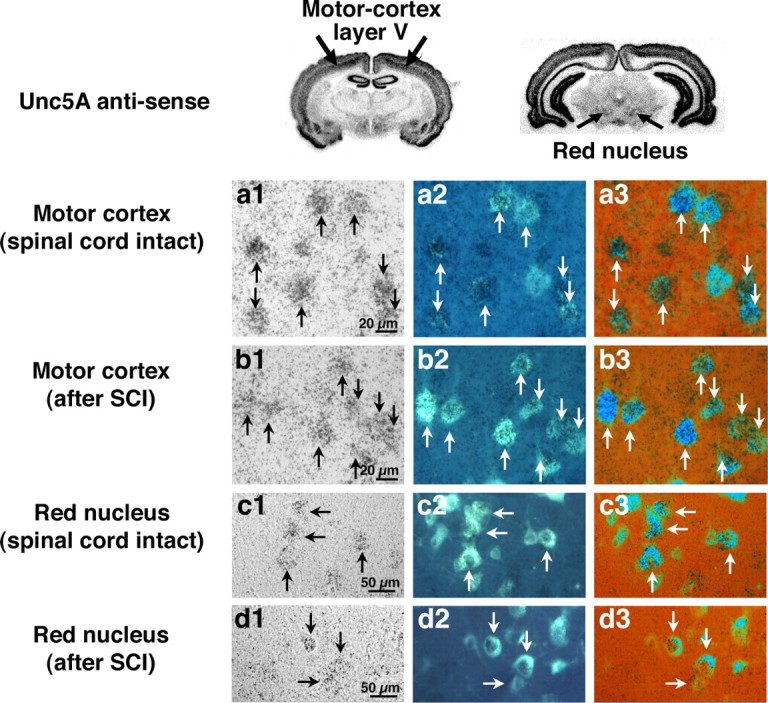
UNC5 receptor expression in corticospinal and rubrospinal neurons. a1–d1, UNC5A in situ signal in plane of photo emulsion (brightfield). a2–d2, Same section under UV excitation to visualize FG retrogradely labeled neurons. a3–d3, Overlay of columns 1 and 2 with color rendering. a1–b3, Motor cortex, layer V, corticospinal neurons. Intact (a1–a3) and lesioned (1 week; b1–b3) corticospinal neurons express UNC5A. c1–d3, Intact (c1–c3) and lesioned (1 week; d1–d3) rubrospinal neurons also express UNC5A.
Figure 3.
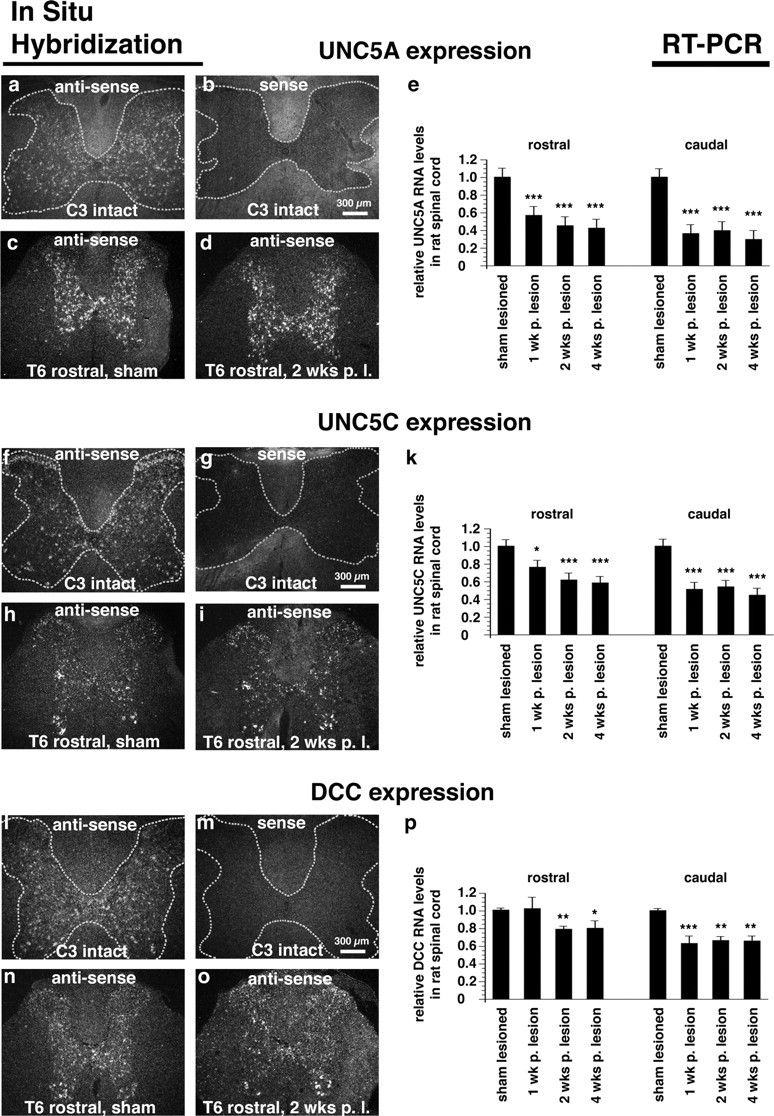
UNC5 expression in spinal cord. a, b, f, g, l, m, Coronal sections through adult intact cervical spinal cord (darkfield). c, d, h, i, n, o, Coronal sections through adult thoracic spinal cord (T6) after a sham lesion (c, h, n) and 2 weeks after a T7 full transection lesion (d, i, o). Expression of the netrin-receptors UNC5A (c, d), UNC5C (h, i) and DCC (n, o) is restricted to the spinal cord gray matter, and expression patterns are maintained 2 weeks after spinal cord transection. Compared with sham-lesioned animals, levels of UNC5A (e), UNC5C (k) and DCC (p) by real-time PCR are reduced to 42.8 ± 9.3% (SEM), 58.5 ± 8.6% (SEM) and 80.3 ± 0.85% (SEM) in the adjacent rostral spinal cord and to 29.8 ± 5.9% (SEM), 44.8 ± 6.3% (SEM) and 65.8 ± 5.7% (SEM) in the adjacent caudal spinal cord segments, respectively, 4 weeks after T7 complete transection (ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001).
UNC5C, in contrast to UNC5A, is predominantly expressed in lamina II (substantia gelatinosa) and in ventral and lateral motor neuron pools (lamina IX) of the spinal cord. UNC5C is also detected throughout other laminas of the gray matter, both in the cervical (Fig. 3f) and thoracic segments (Fig. 3h). Expression is reduced but maintained after thoracic transection by in situ hybridization and RT-PCR (Fig. 3i,k). DCC is also expressed in all laminas of intact and lesioned spinal cord gray matter, including substantia gelatinosa and ventral motor neuron pools (Fig. 3l,n,o). DCC RNA levels remain readily detectable up to 4 weeks after spinal cord transection (Fig. 3p).
Netrin-1 is an inhibitor to neurite outgrowth in vitro
To test whether expression levels of netrin-1 in the adult spinal cord influence axonal growth, we prepared myelin from the adult rat spinal cord and examined axon outgrowth from UNC5-expressing motor neurons in vitro with and without netrin-1 neutralization. Netrin-1 was neutralized using UNC5B receptor bodies, which prevent its association with UNC5 receptors (Leonardo et al., 1997). Purity and dimerization of UNC5B receptor body in our preparation were demonstrated in Coomassie-stained polyacrylamide gels (supplemental Fig. 3, available at www.jneurosci.org as supplemental material). Neurite extension from cultures of dissociated E15 motor neurons that express both UNC5A and UNC5C receptors was assessed after 48 h on myelin extracts derived from adult spinal cord, in the presence or absence of the UNC5B receptor body. Notably, receptor body-mediated netrin-1 neutralization significantly enhanced neurite outgrowth on myelin by 81% (p < 0.001), increasing mean neurite length to 88.2 ± 3 μm/neuron from 48.8 ± 2.6 μm/neuron in control Fc IgG-treated wells (Fig. 4). Thus, neutralizing netrin-1 in adult myelin extracts increases neurite outgrowth, indicating a potential role for netrin-1 as an inhibitor of axonal regeneration in the adult spinal cord.
Figure 4.
Netrin-1 neutralization enhances neurite outgrowth in vitro. a, b, UNC5A (a) and UNC5C (b) are expressed in motor neuron pools (mn) in ventral E15 rat spinal cord as expected, by in situ hybridization; thus, these neurons were used as a test system in vitro for effects of netrin-1 neutralization on axonal outgrowth. c, Detection of MAG in myelin purified from adult rat spinal cord by Western blot, indicating that the myelin isolate is of expected quality for in vitro studies. d, Netrin-1 ELISA demonstrating binding of UNC5B receptor body in a dose dependent, saturatable manner. Recombinant netrin-1 was coated at 20 μg/ml and incubated with increasing concentrations (1–128 μg/ml) of UNC5B receptor body. e, f, neurite outgrowth from UNC5-expressing E15 spinal motor neurons on adult rat spinal cord myelin is significantly enhanced when netrin-1 is neutralized by UNC5 receptor body (e), compared with control cultures containing IgG (f) (for details, see Materials and Methods). g, The length of the longest neurite of spinal motor neurons after 48 h on myelin was increased by 81% after netrin-1 neutralization with UNC5 receptor bodies (***p < 0.001). Thus, netrin-1 is present in adult rat spinal cord myelin in sufficient quantities to inhibit axonal outgrowth from UNC5-expressing neurons in vitro. h, Immunocytochemical verification of UNC5A expression by E15 spinal motor neurons. i, secondary antibody only control.
Netrin-1 inhibits axonal regeneration in vivo
We next tested the hypothesis that netrin-1 contributes to inhibition of axonal regeneration in vivo. As noted above, a number of myelin-associated molecules have been identified as potential (negative) regulators of axonal plasticity and regeneration in the adult spinal cord, including nogo, MAG, OMgp, semaphorin and ephrin. To determine whether netrin-1 can act independently of these molecules to influence axonal growth in vivo, we generated a region free of these other known myelin-associated inhibitors within spinal cord injury sites by overexpressing netrin-1 in primary fibroblasts. Netrin-1-overexpressing fibroblasts were grafted into C3 dorsal column lesion cavities, whereas control subjects received fibroblast grafts expressing the reporter gene EGFP. Thus, netrin-1-expressing implants differed from controls by a single gene, allowing detection of potential netrin-1 influences on axonal regeneration in vivo.
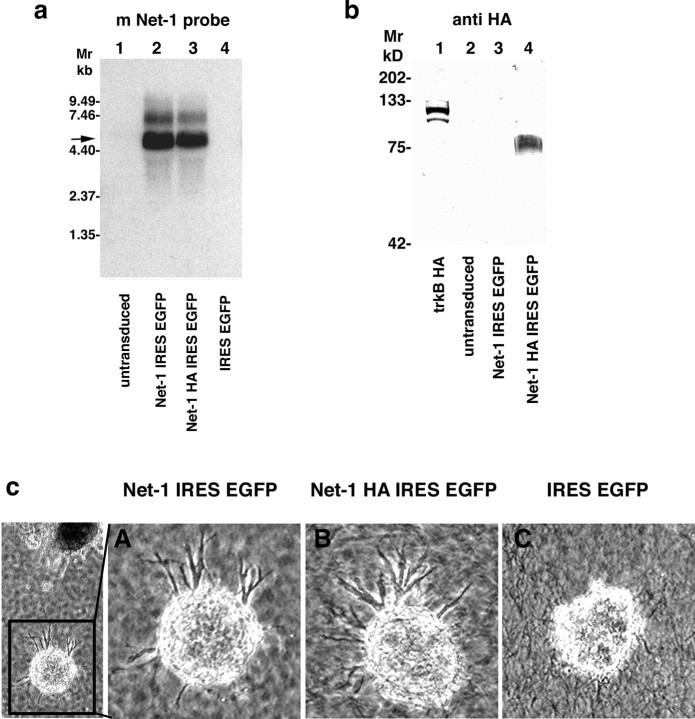
Netrin-1 expression from genetically modified fibroblasts in vitro was confirmed by Northern and Western blot (Fig. 5a,b). A single ∼85 kDa band was detected with an antibody against the HA-epitope corresponding to the expected size of HA-tagged netrin-1. Bioactivity of netrin-1 released from genetically engineered fibroblasts was confirmed by its capacity to elicit directed neurite outgrowth from E13 rat dorsal spinal cord explants containing DCC-expressing (and therefore netrin-1 responsive) commissural neurons (Fig. 5c) (Serafini et al., 1994; Keino-Masu et al., 1996). After 18 h of coculture, axons extended from dorsal spinal cord explants toward aggregates of netrin-1-expressing fibroblasts, but not toward control fibroblasts lacking the netrin-1 gene (Fig. 5c).
Figure 5.
In vitro characterization of engineered netrin-1-overexpressing fibroblasts. a, Northern blot to detect bicistronic netrin-1 (Net-1) IRES EGFP (5490 bp, lane 2) or netrin-1 HA IRES EGFP RNA (5530 bp, lane 3) in fibroblasts transduced with the respective netrin-1 IRES EGFP or netrin-1 HA IRES EGFP MLVs, using the 1494 bp HincII-ScaI fragment of the mouse netrin-1 cDNA as a probe. b, Confirmation of netrin-1 secretion from transduced fibroblasts by Western blot. A ∼85 kDa protein was specifically detected in high salt cell surface extracts of fibroblasts transduced with netrin-1 HA IRES EGFP MLV (lane 4) using a polyclonal antibody directed against the HA epitope (Y11; Santa Cruz Biotechnology; 200 ng/ml). HA-tagged trkB served as a positive control (lane 1). c, Axon outgrowth from E13 rat dorsal spinal cord explants was elicited in coculture with netrin-1 IRES EGFP MLV (A) and netrin-1 HA IRES EGFP MLV (B) transduced fibroblasts but not control fibroblasts infected with EGFP MLV (C) after 18 h in coculture.
Five weeks after grafting to C3 spinal cord dorsal column lesion sites, overall axonal penetration into the netrin-1-enriched environment in the spinal cord was significantly reduced compared with control, netrin-1-free grafts by neurofilament immunolabeling (p < 0.01) (Fig. 6a,b). Overall, 51% more axons penetrated the lesion milieu that was free of netrin-1, compared with grafts expressing netrin-1 in the absence of other known myelin-associated inhibitors (Fig. 6e). These observations were replicated at a time point 3 months postinjury: axon density was 57% greater in the netrin-1-free environment compared with netrin-1-secreting grafts (p < 0.01) (Fig. 6f).
Figure 6.
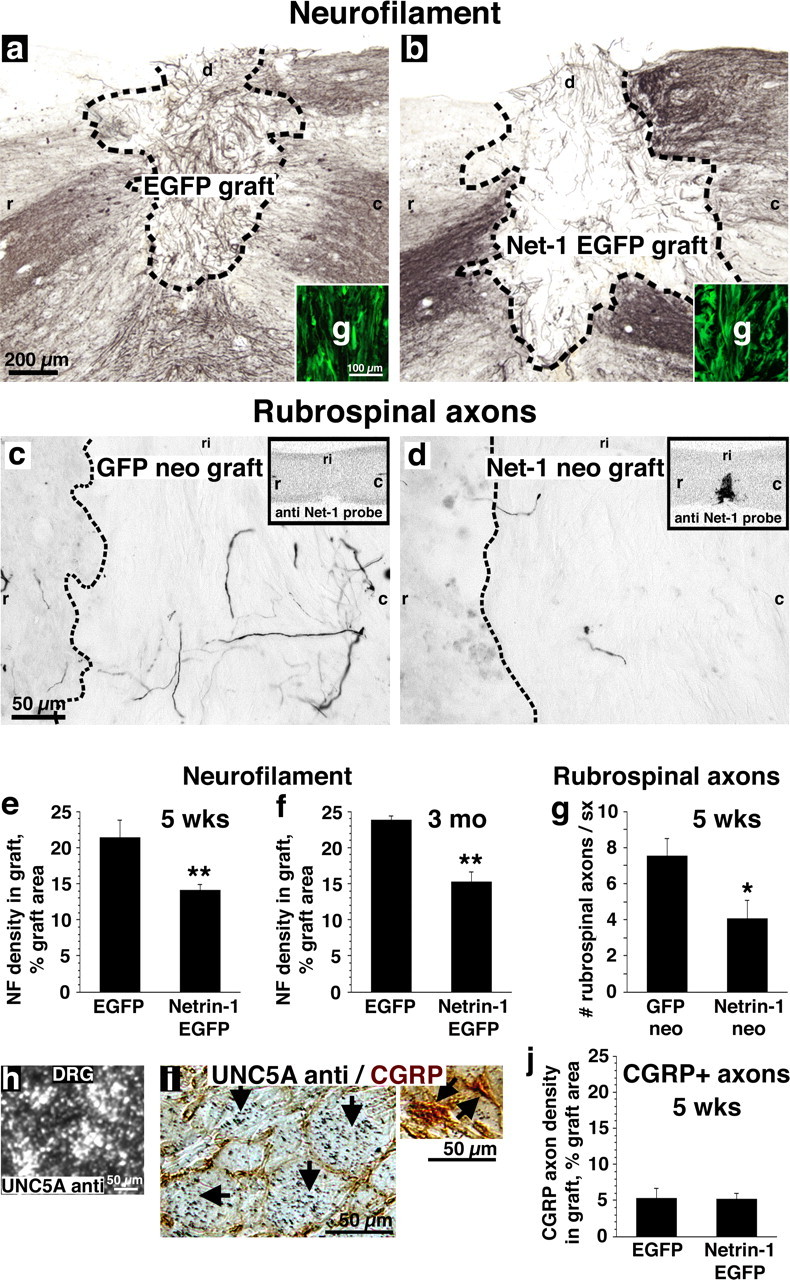
Netrin-1 inhibits axonal regeneration in vivo. Rats underwent cervical spinal cord lesions and were grafted with either netrin-1 secreting or control fibroblasts. a–d, Expression of netrin-1 within fibroblast grafts was monitored by expression of EGFP reporter (insets, a, b) or by in situ hybridization with netrin-1 antisense probe (insets, c, d). a, b, The density of neurofilament-labeled axons was 51% greater in control fibroblast grafts (a) than in netrin-1-secreting fibroblast grafts (b) 5 weeks after lesions and grafting (e; p < 0.01), and 57% greater by 3 months after lesion and grafting (f; p < 0.01). Thus, netrin-1 inhibits the growth of a general spinal cord population of axons. Shown in a and b are grafts 5 weeks after lesion. Similarly, the growth of UNC5A-expressing rubrospinal axons is 83% greater in (c) control fibroblast grafts than in (d) netrin-1-secreting grafts, 5 weeks after lesion (g; p < 0.05). h, i, UNC5A expression in DRGs is confined to large caliber neurons, which do not express CGRP (black arrows). j, CGRP-labeled sensory axons, which do not express UNC5 receptors, are not repelled from netrin-1-expressing fibroblast grafts. g, Graft; r, rostral; c, caudal; d, dorsal; ri, right.
To assess effects of netrin-1 expression on supraspinal motor projections to the lesioned spinal cord, rubrospinal axons whose cell bodies express UNC5A were examined. Consistent with the preceding findings, rubrospinal penetration of a spinal cord lesion site containing netrin-1 was significantly reduced compared with grafts lacking netrin-1 (Fig. 6c,d). Overall, 83% more rubrospinal axons were observed in the milieu free of netrin-1, compared with netrin-1-expressing grafts (p < 0.05) (Fig. 6g) five weeks postinjury. Values are corrected for BDA tracing efficiency. Thus, lesioned UNC5A-expressing rubrospinal axons that project to the spinal cord from the brainstem, and a general population of neurofilament-labeled axons within the spinal cord, exhibit growth inhibition in a netrin-1-bearing environment in a spinal cord lesion site.
To confirm that axonal inhibition in the above models was mediated through netrin-1-dependent mechanisms, rather than a secondary property induced by netrin-1 expression in a fibroblast graft milieu, we examined the growth of axons lacking netrin-1 receptors into netrin-1-producing cells placed in the lesion site. The CGRP-expressing subpopulation of nociceptive sensory neurons does not express repulsion-mediating UNC5 receptors, as shown by lack of colocalization of small CGRP-labeled neurons (Hall et al., 1997) with larger, UNC5A-expressing neurons in the dorsal root ganglion (DRG) (Fig. 6h,i). No other UNC5 receptors are expressed in DRG (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Notably, the growth of CGRP-expressing nociceptive axons was not reduced in fibroblast grafts expressing netrin-1 in the spinal cord injury site (Fig. 6j). Thus, observed reductions in axonal penetration of a netrin-1 milieu within the injured adult spinal cord is specific to axonal populations expressing netrin-1 receptors of the UNC5 family.
Netrin-1 expression by genetically modified fibroblasts in all studies above persisted through 3 months in vivo, confirmed both by in situ hybridization and by persistent expression of the reporter gene in the netrin-1 construct, EGFP (Fig. 6).
Discussion
Findings of this study identify netrin-1 as a novel, oligodendrocyte-associated inhibitor of axonal growth. Both netrin-1 and its receptors are expressed in the intact and lesioned adult CNS. Netrin-1 is expressed by oligodendrocytes, and is enriched in proximity to paranodal loops at nodes of Ranvier in adult white matter, in a location similar to that described for two other myelin-associated inhibitors, OMgp and MAG (Huang et al., 2005; Nie et al., 2006). The repulsion-mediating class of netrin UNC5 receptors is expressed by rubrospinal and corticospinal motor neurons, and by neurons in all laminas of spinal cord gray matter. Neutralization of netrin signaling in extracts of adult spinal cord myelin enhances neurite outgrowth in vitro, and axonal growth is inhibited in a zone containing netrin-1 that is devoid of other myelin-associated inhibitors in in vivo sites of spinal cord injury. The growth of sensory axonal populations lacking inhibitory (UNC5) receptors is not reduced by netrin-1 after in vivo spinal cord injury. We thus conclude that netrin-1 is an oligodendrocyte-associated inhibitor to regeneration in the adult spinal cord.
Unlike OMgp and MAG, which are transmembrane glycoproteins, netrin-1 is a secreted ligand. Because of an α-helical structure consisting of a cluster of positively charged basic amino acids at its C terminus, netrin-1 associates with membrane fractions (Serafini et al., 1994) and binds to heparin with high affinity (Kappler et al., 2000). Furthermore, netrin-1 has been identified in carbohydrate microarrays to interact with heparan sulfate and chondroitin sulfate (Shipp and Hsieh-Wilson, 2007). Proteomic dissection has identified the chondroitin sulfate proteoglycan versican as a component of the nodal axoglial apparatus (Huang et al., 2005); this and several other chondroitin sulfate proteoglycan family members are enriched at nodes of Ranvier as well as in regions surrounding sites of spinal cord injury (Davies et al., 1997; Wang et al., 1997; Bradbury et al., 2002; Jones et al., 2002, 2003b; Properzi et al., 2005). Thus, binding of netrin-1 to these proteoglycan species might lead to its enrichment both in perinodal regions and in regions of spinal cord injury.
The present findings are consistent with previous reports that netrin-1 is present on myelin of the adult CNS (Manitt et al., 2001), and that UNC5 receptors continue to be expressed after spinal cord injury (Manitt et al., 2006). In lamprey, it has also been noted that axonal regeneration capacity inversely correlates with UNC5 receptor expression (Shifman and Selzer, 2000). As noted in the Introduction, attractive or repulsive netrin signaling is mediated by the presence of DCC and UNC5 receptors in neurons: DCC expressed in isolation promotes attraction to netrin, whereas expression of UNC5 receptors, either solely or in combination with DCC, promotes repulsion. Our findings and those of Manitt et al. (2004) indicate that UNC5 expression predominates in several neural systems in adulthood, suggesting a primarily repulsive role for netrin-1 after development. Indeed, our findings confirm the inhibitory influences of netrin-1 on several motor systems in the adult CNS.
There are now six known myelin-associated inhibitors of axonal regeneration in the adult CNS: nogo, MAG, OMgp, semaphorins, ephrins, and netrin-1. The physiological function of myelin-associated inhibitors in the adult CNS remains unknown, although it has been speculated that these molecules act to retain the highly structured patterns of axonal projections throughout life by inhibiting aberrant sprouting. The presence of multiple, distinct classes of myelin-associated inhibitors in the adult CNS raises the possibility that effective neutralization of inhibition will require the simultaneous targeting of multiple proteins. Neutralization of one myelin-associated inhibitor, nogo, has been reported to enhance axonal plasticity and regeneration in the lesioned adult CNS in some studies (Li et al., 2004; Schwab, 2004), but not in others (Zheng et al., 2003; Steward et al., 2007). It seems likely that the targeting of multiple inhibitors may be necessary to unleash sufficient axonal growth to substantially improve axonal growth. Toward this end, immunization strategies targeting whole myelin extracts may be optimal (Huang et al., 1999; Sicotte et al., 2003), a hypothesis that remains to be fully tested.
A number of recent spinal cord injury studies report enhanced axonal growth after use of combination therapies (Azanchi et al., 2004; Lu et al., 2004; Pearse et al., 2004; Fouad et al., 2005; Houle et al., 2006). It is likely that strategies stimulating axonal growth with growth factors, favorable growth substrates, and activation of growth “master switches,” in combination with reduction of inhibition via myelin neutralization and degradation of the inhibitory extracellular matrix, will lead to summed improvements in axonal regeneration. Practical testing and implementation of these strategies is complex but important.
Footnotes
This work was supported by the National Institutes of Health, the Veterans Administration, and the Roman Reed Foundation. We thank Ervin Calvo for technical assistance, Dr. T. Kennedy for providing the rat netrin-1 probe, and Dr. Lindsay Hinck for providing the rabbit anti-UNC5A antibody 2026.
References
- Azanchi R, Bernal G, Gupta R, Keirstead HS. Combined demyelination plus Schwann cell transplantation therapy increases spread of cells and axonal regeneration following contusion injury. J Neurotrauma. 2004;21:775–788. doi: 10.1089/0897715041269696. [DOI] [PubMed] [Google Scholar]
- Benson MD, Romero MI, Lush ME, Lu QR, Henkemeyer M, Parada LF. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci USA. 2005;102:10694–10699. doi: 10.1073/pnas.0504021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesch A, Tuszynski MH. GDNF gene delivery to injured adult CNS motor neurons promotes axonal growth, expression of the trophic neuropeptide CGRP, and cellular protection. J Comp Neurol. 2001;436:399–410. doi: 10.1002/cne.1076. [DOI] [PubMed] [Google Scholar]
- Blesch A, Tuszynski MH. Cellular GDNF delivery promotes growth of motor and dorsal column sensory axons after partial and complete spinal cord transections and induces remyelination. J Comp Neurol. 2003;467:403–417. doi: 10.1002/cne.10934. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- Colamarino SA, Tessier-Lavigne M. The axonal chemoattractant netrin-1 is also a chemorepellent for trochlear motor axons. Cell. 1995;81:621–629. doi: 10.1016/0092-8674(95)90083-7. [DOI] [PubMed] [Google Scholar]
- Colavita A, Culotti JG. Suppressors of ectopic UNC-5 growth cone steering identify eight genes involved in axon guidance in Caenorhabditis elegans. Dev Biol. 1998;194:72–85. doi: 10.1006/dbio.1997.8790. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–683. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- de la Torre JR, Hopker VH, Ming GL, Poo MM, Tessier-Lavigne M, Hemmati-Brivanlou A, Holt CE. Turning of retinal growth cones in a netrin-1 gradient mediated by the netrin receptor DCC. Neuron. 1997;19:1211–1224. doi: 10.1016/s0896-6273(00)80413-4. [DOI] [PubMed] [Google Scholar]
- Deiner MS, Kennedy TE, Fazeli A, Serafini T, Tessier-Lavigne M, Sretavan DW. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron. 1997;19:575–589. doi: 10.1016/s0896-6273(00)80373-6. [DOI] [PubMed] [Google Scholar]
- Einheber S, Zanazzi G, Ching W, Scherer S, Milner TA, Peles E, Salzer JL. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J Cell Biol. 1997;139:1495–1506. doi: 10.1083/jcb.139.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger JH, Bronson RT, Harris B, Johnson K, Przyborski SA, Ackerman SL. The netrin 1 receptors Unc5h3 and Dcc are necessary at multiple choice points for the guidance of corticospinal tract axons. J Neurosci. 2002;22:10346–10356. doi: 10.1523/JNEUROSCI.22-23-10346.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- Grill R, Murai K, Blesch A, Gage FH, Tuszynski MH. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J Neurosci. 1997;17:5560–5572. doi: 10.1523/JNEUROSCI.17-14-05560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie S, Pini A. Chemorepulsion of developing motor axons by the floor plate. Neuron. 1995;14:1117–1130. doi: 10.1016/0896-6273(95)90260-0. [DOI] [PubMed] [Google Scholar]
- Hall AK, Ai X, Hickman GE, MacPhedran SE, Nduaguba CO, Robertson CP. The generation of neuronal heterogeneity in a rat sensory ganglion. J Neurosci. 1997;17:2775–2784. doi: 10.1523/JNEUROSCI.17-08-02775.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin M, Zhou Y, Su MW, Scott IM, Culotti JG. Expression of the UNC-5 guidance receptor in the touch neurons of C. elegans steers their axons dorsally. Nature. 1993;364:327–330. doi: 10.1038/364327a0. [DOI] [PubMed] [Google Scholar]
- Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990;4:61–85. doi: 10.1016/0896-6273(90)90444-k. [DOI] [PubMed] [Google Scholar]
- Hong K, Hinck L, Nishiyama M, Poo MM, Tessier-Lavigne M, Stein E. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–941. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, McKerracher L, Braun PE, David S. A therapeutic vaccine approach to stimulate axon regeneration in the adult mammalian spinal cord. Neuron. 1999;24:639–647. doi: 10.1016/s0896-6273(00)81118-6. [DOI] [PubMed] [Google Scholar]
- Huang JK, Phillips GR, Roth AD, Pedraza L, Shan W, Belkaid W, Mi S, Fex-Svenningsen A, Florens L, Yates JR, III, Colman DR. Glial membranes at the node of Ranvier prevent neurite outgrowth. Science. 2005;310:1813–1817. doi: 10.1126/science.1118313. [DOI] [PubMed] [Google Scholar]
- Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci. 2002;22:2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LL, Sajed D, Tuszynski MH. Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition. J Neurosci. 2003a;23:9276–9288. doi: 10.1523/JNEUROSCI.23-28-09276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003b;182:399–411. doi: 10.1016/s0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- Kappler J, Franken S, Junghans U, Hoffmann R, Linke T, Muller HW, Koch KW. Glycosaminoglycan-binding properties and secondary structure of the C-terminus of netrin-1. Biochem Biophys Res Commun. 2000;271:287–291. doi: 10.1006/bbrc.2000.2583. [DOI] [PubMed] [Google Scholar]
- Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, Tessier-Lavigne M. Deleted in colorectal cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- Keleman K, Dickson BJ. Short- and long-range repulsion by the Drosophila Unc5 netrin receptor. Neuron. 2001;32:605–617. doi: 10.1016/s0896-6273(01)00505-0. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Lacroix S, Vallieres L, Rivest S. C-fos mRNA pattern and corticotropin-releasing factor neuronal activity throughout the brain of rats injected centrally with a prostaglandin of E2 type. J Neuroimmunol. 1996;70:163–179. doi: 10.1016/s0165-5728(96)00114-2. [DOI] [PubMed] [Google Scholar]
- Leonardo ED, Hinck L, Masu M, Keino-Masu K, Ackerman SL, Tessier-Lavigne M. Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature. 1997;386:833–838. doi: 10.1038/386833a0. [DOI] [PubMed] [Google Scholar]
- Leung-Hagesteijn C, Spence AM, Stern BD, Zhou Y, Su MW, Hedgecock EM, Culotti JG. UNC-5, a transmembrane protein with immunoglobulin and thrombospondin type 1 domains, guides cell and pioneer axon migrations in C. elegans. Cell. 1992;71:289–299. doi: 10.1016/0092-8674(92)90357-i. [DOI] [PubMed] [Google Scholar]
- Li S, Liu BP, Budel S, Li M, Ji B, Walus L, Li W, Jirik A, Rabacchi S, Choi E, Worley D, Sah DW, Pepinsky B, Lee D, Relton J, Strittmatter SM. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J Neurosci. 2004;24:10511–10520. doi: 10.1523/JNEUROSCI.2828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- Lu P, Yang H, Jones LL, Filbin MT, Tuszynski MH. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J Neurosci. 2004;24:6402–6409. doi: 10.1523/JNEUROSCI.1492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manitt C, Colicos MA, Thompson KM, Rousselle E, Peterson AC, Kennedy TE. Widespread expression of netrin-1 by neurons and oligodendrocytes in the adult mammalian spinal cord. J Neurosci. 2001;21:3911–3922. doi: 10.1523/JNEUROSCI.21-11-03911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manitt C, Thompson KM, Kennedy TE. Developmental shift in expression of netrin receptors in the rat spinal cord: predominance of UNC-5 homologues in adulthood. J Neurosci Res. 2004;77:690–700. doi: 10.1002/jnr.20199. [DOI] [PubMed] [Google Scholar]
- Manitt C, Wang D, Kennedy TE, Howland DR. Positioned to inhibit: netrin-1 and netrin receptor expression after spinal cord injury. J Neurosci Res. 2006;84:1808–1820. doi: 10.1002/jnr.21070. [DOI] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Moreau-Fauvarque C, Kumanogoh A, Camand E, Jaillard C, Barbin G, Boquet I, Love C, Jones EY, Kikutani H, Lubetzki C, Dusart I, Chedotal A. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J Neurosci. 2003;23:9229–9239. doi: 10.1523/JNEUROSCI.23-27-09229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Nie DY, Ma QH, Law JW, Chia CP, Dhingra NK, Shimoda Y, Yang WL, Gong N, Chen QW, Xu G, Hu QD, Chow PK, Ng YK, Ling EA, Watanabe K, Xu TL, Habib AA, Schachner M, Xiao ZC. Oligodendrocytes regulate formation of nodes of Ranvier via the recognition molecule OMgp. Neuron Glia Biol. 2006;2:151–164. doi: 10.1017/S1740925X06000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton WT, Poduslo SE. Myelination in rat brain: method of myelin isolation. J Neurochem. 1973;21:749–757. doi: 10.1111/j.1471-4159.1973.tb07519.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. San Diego: Academic; 1998. The rat brain in stereotaxic coordinates, Ed 4. [Google Scholar]
- Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- Properzi F, Carulli D, Asher RA, Muir E, Camargo LM, van Kuppevelt TH, ten Dam GB, Furukawa Y, Mikami T, Sugahara K, Toida T, Geller HM, Fawcett JW. Chondroitin 6-sulphate synthesis is up-regulated in injured CNS, induced by injury-related cytokines and enhanced in axon-growth inhibitory glia. Eur J Neurosci. 2005;21:378–390. doi: 10.1111/j.1460-9568.2005.03876.x. [DOI] [PubMed] [Google Scholar]
- Schwab ME. Nogo and axon regeneration. Curr Opin Neurobiol. 2004;14:118–124. doi: 10.1016/j.conb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- Shifman MI, Selzer ME. Expression of the netrin receptor UNC-5 in lamprey brain: modulation by spinal cord transection. Neurorehabil Neural Repair. 2000;14:49–58. doi: 10.1177/154596830001400106. [DOI] [PubMed] [Google Scholar]
- Shipp EL, Hsieh-Wilson LC. Profiling the sulfation specificities of glycosaminoglycan interactions with growth factors and chemotactic proteins using microarrays. Chem Biol. 2007;14:195–208. doi: 10.1016/j.chembiol.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Sicotte M, Tsatas O, Jeong SY, Cai CQ, He Z, David S. Immunization with myelin or recombinant Nogo-66/MAG in alum promotes axon regeneration and sprouting after corticospinal tract lesions in the spinal cord. Mol Cell Neurosci. 2003;23:251–263. doi: 10.1016/s1044-7431(03)00053-8. [DOI] [PubMed] [Google Scholar]
- Steward O, Zheng B, Banos K, Yee KM. Response to: Kim et al., “axon regeneration in young adult mice lacking Nogo-A/B”. Neuron. 2007;38:187–199. doi: 10.1016/j.neuron.2007.04.004. Neuron 54:191–195. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Placzek M, Lumsden AG, Dodd J, Jessell TM. Chemotropic guidance of developing axons in the mammalian central nervous system. Nature. 1988;336:775–778. doi: 10.1038/336775a0. [DOI] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- Wang X, Messing A, David S. Axonal and nonneuronal cell responses to spinal cord injury in mice lacking glial fibrillary acidic protein. Exp Neurol. 1997;148:568–576. doi: 10.1006/exnr.1997.6702. [DOI] [PubMed] [Google Scholar]
- Williams ME, Wu SC, McKenna WL, Hinck L. Surface expression of the netrin receptor UNC5H1 is regulated through a protein kinase C-interacting protein/protein kinase-dependent mechanism. J Neurosci. 2003;23:11279–11288. doi: 10.1523/JNEUROSCI.23-36-11279.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ME, Lu X, McKenna WL, Washington R, Boyette A, Strickland P, Dillon A, Kaprielian Z, Tessier-Lavigne M, Hinck L. UNC5A promotes neuronal apoptosis during spinal cord development independent of netrin-1. Nat Neurosci. 2006;9:996–998. doi: 10.1038/nn1736. [DOI] [PubMed] [Google Scholar]
- Zheng B, Ho C, Li S, Keirstead H, Steward O, Tessier-Lavigne M. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron. 2003;38:213–224. doi: 10.1016/s0896-6273(03)00225-3. [DOI] [PubMed] [Google Scholar]