Abstract
Although the transient receptor potential vanilloid 4 (TRPV4) has been implicated in the process of osmomechanical transduction, it appears to make little contribution to the normal somatosensory detection of mechanical stimuli. However, evidence suggests that it may play an important role in mechanical hyperalgesia. In the present study, we examined the common requirement for TRPV4 in mechanical hyperalgesia associated with diverse pain models and investigated whether the very close association observed between TRPV4 and mechanical hyperalgesia, regardless of etiology, reflects a close functional connection of TRPV4 with other molecules implicated in mechanical transduction. In models of painful peripheral neuropathy associated with vincristine chemotherapy, alcoholism, diabetes, and human immunodeficiency virus/acquired immune deficiency syndrome therapy, mechanical hyperalgesia was markedly reduced by spinal intrathecal administration of oligodeoxynucleotides antisense to TRPV4. Similarly, mechanical hyperalgesia induced by paclitaxel, vincristine, or diabetes was strongly reduced in TRPV4 knock-out mice. We also show that α2β1 integrin and Src tyrosine kinase, which have been implicated in mechanical transduction, are important for the development of mechanical hyperalgesia, and that their contribution requires TRPV4. Furthermore, we establish a direct interaction between TRPV4, α2 integrin, and the Src tyrosine kinase Lyn in sensory neurons. We suggest that TRPV4 plays a role in mechanotransduction, as a component of a molecular complex that functions only in the setting of inflammation or nerve injury.
Keywords: sensory nerve, neuropathic pain, dorsal root ganglion, extracellular matrix, primary afferent nociceptors, inflammatory soup
Introduction
The contribution of transient receptor potential vanilloid 4 (TRPV4) to mechanical hyperalgesia associated with either paclitaxel chemotherapy-induced painful peripheral neuropathy or pronociceptive inflammatory mediators (Alessandri-Haber et al., 2004, 2006) suggests a potential role for TRPV4 in the transduction of mechanical stimuli in the sensitized primary afferent nociceptor. However, TRPV4 is unlikely to be directly gated by mechanical forces in the cell membrane because it does not respond to membrane distortion in vitro (Strotmann et al., 2000). Thus, we hypothesize that functional coupling between TRPV4 and other molecules underlies mechanical transduction in the setting of inflammation or nerve injury.
Integrins have been implicated in the transduction of osmotic stimuli and mechanical stretch (Wang et al., 1993; Wang and Ingber, 1994; Nebe et al., 1995; Shyy and Chien, 1997; Aplin et al., 1998; Mobasheri et al., 2002; Browe and Baumgarten, 2003; Ingber, 2003a; vom Dahl et al., 2003) and in mechanical hyperalgesia (Alessandri-Haber et al., 2004; Dina et al., 2004). Also, integrins can contribute to mechanical transduction through the modulation of ion channels, via a Src tyrosine signaling pathway (Aplin et al., 1998; Mobasheri et al., 2002; Waitkus-Edwards et al., 2002; Browe and Baumgarten, 2003). We recently demonstrated that TRPV4-mediated nociceptive behaviors in the presence of inflammatory mediators depend on Src tyrosine kinase (Alessandri-Haber et al., 2005) and that paclitaxel-induced hyperalgesia requires integrin, Src tyrosine kinase, and TRPV4 signaling pathways (Alessandri-Haber et al., 2004). In this study, we demonstrate that TRPV4 plays a basic role in mechanical hyperalgesia of diverse etiologies: painful peripheral neuropathy induced by chemotherapy (vincristine) (Aley et al., 1996), alcohol binge drinking (Dina et al., 2006), diabetes [streptozotocin (STZ)] (Aley and Levine, 2002), and nucleoside analog reverse transcriptase inhibitor acquired immune deficiency syndrome (AIDS) therapy [2′,3′-dideoxycytidine (ddC)] (Joseph et al., 2004). Furthermore, our findings suggest that TRPV4 is associated with α2β1 integrin and Src tyrosine kinase in a molecular complex in sensory neurons that mediates mechanotransduction in a diverse range of hyperalgesic states but that does not contribute to normal nociception.
Materials and Methods
Animals
Experiments were performed on 180–200 g adult male Sprague Dawley rats (Charles River, Hollister, CA) and on male C57BL/6 mice lacking a functional TRPV4 gene (TRPV4−/− mice) (Liedtke and Friedman, 2003) and male TRPV4 wild-type littermates (TRPV4+/+). Experimental protocols were approved by the University of California, San Francisco Committee on Animal Research and conformed to National Institutes of Health guidelines for the use of animals in research.
Drugs
Vincristine sulfate, ddC, STZ, paclitaxel, diisothiocyanatostilbene 2,2′-disulfonic acid (DIDS), prostaglandin E2 (PGE2), serotonin (5-HT), histamine, and substance P were purchased from Sigma (St. Louis, MO). Bradykinin was purchased from ICN Biomedicals (Aurora, OH). The Src family tyrosine kinase-specific inhibitor, PP1 (Hanke et al., 1996), the specific inhibitor of Syk, a related protein kinase not belonging to the Src family (Oliver et al., 1994), piceatannol (PCT), and the specific activator of Src tyrosine kinase, YEEIP, were purchased from Biomol (Plymouth Meeting, PA). The α2 integrin-blocking antibody was purchased from BD PharMingen (San Jose, CA).
For behavioral experiments, stock solutions of substance P, bradykinin, histamine, 5-HT, and YEEIP were made in saline. Stock solution of PGE2 was made in 10% ethanol, whereas stock solutions of PP1 and piceatannol were made in 10% DMSO. For all drugs, final experimental dilutions were made in saline on the day of the experiment (final concentrations of ethanol or DMSO were <1%). Stock solution and final experimental concentration of the α2 integrin-blocking antibody was made in distilled water.
For calcium imaging and immunoprecipitation experiments, stock solutions of PGE2, PP1, and piceatannol were made in 10% DMSO. Stock solutions of DIDS, YEEIP, and 5-HT were made in distilled water. Final experimental concentrations were made in isotonic or hypotonic solution on the day of the experiment.
Animal models of neuropathic pain in the rat and mouse
Vincristine-induced neuropathy (chemotherapy).
Vincristine sulfate was dissolved in saline and administered intravenously into the tail vein at a single dose of 200 μg/kg followed by 0.5 ml saline flush (Aley and Levine, 2002). Control rats received an equal volume of saline. Nociceptive testing was performed 5 d after vincristine injection, when mechanical hyperalgesia was maximal. TRPV4−/− and TRPV4+/+ mice received a single 200 μg/kg dose of vincristine intraperitoneally.
Paclitaxel-induced neuropathy (chemotherapy).
Paclitaxel was formulated at a concentration of 1 mg/ml in a vehicle composed of absolute ethanol and Cremophore EL; final paclitaxel concentration of 1 μg/2.5 μl was made in sterile saline at the time of injection (Dina et al., 2001; Alessandri-Haber et al., 2004). In rat, paclitaxel was injected intraperitoneally once a day for 10 d. In mice, it was injected intraperitoneally at a single dose of 6 mg/kg in TRPV4+/+ and TRPV4−/− mice.
Streptozotocin-induced neuropathy (diabetes).
Streptozotocin was dissolved in saline (50 mg/ml) and administered intravenously into a tail vein of the rat at a single dose of 50 mg/kg (Aley and Levine, 2001). Control rats received an equal volume of saline. Blood glucose concentration was determined daily using a glucometer (Life Scan, Milpitas, CA). Nociceptive testing was done 5 d after streptozotocin injection, when mechanical hyperalgesia was maximal. TRPV4+/+ and TRPV4−/− mice received a single intraperitoneal injection of streptozotocin (75 mg/kg).
ddC-induced neuropathy (AIDS therapy).
2′,3′-Dideoxycytidine was dissolved in saline to a concentration of 25 mg/ml and was administered intravenously into a tail vein at a single dose of 50 mg/kg (Joseph et al., 2004). Nociceptive testing was done 5 d after ddC injection, when mechanical hyperalgesia was maximal.
Alcohol withdrawal-induced neuropathy, a model of binge drinking.
Rats were fed Lieber-DeCarli liquid diet containing 6.5% v/v ethanol (Dyets, Bethlehem, PA) (Lieber and DeCarli, 1982, 1989; Lieber et al., 1989). Control rats were pair-fed (i.e., calorically matched to the ethanol-exposed rats) a diet in which equal calories of maltose-dextrin was consumed in place of ethanol (Lieber and DeCarli, 1989). The daily administration of ethanol or control diet (Dina et al., 2000) was modified in the present study to mimic binge drinking by administering the ethanol or control diet for four days of the week and then replacing it with standard lab chow for the remaining three days (Dina et al., 2006). Experiments involving Src inhibitors and α2 integrin and TRPV4 antisense and mismatch oligodeoxynucleotide (ODN) were performed 5 weeks after starting the alcohol diet, when mechanical hyperalgesia was maximal (Dina et al., 2006).
Mechanical nociceptive threshold in rat
Mechanical nociceptive thresholds were evaluated by the Randall-Sellito paw withdrawal test (Ugo-Basile algesimeter; Stoelting, Chicago, IL) as described previously (Aley and Levine, 2001; Aley et al., 2001). Baseline mechanical thresholds were recorded as the mean of three measurements (at 10 min intervals) before the injection of hypotonic solution or pharmacological reagents. As a negative control, we verified that the injection of 10% DMSO in rat hindpaw had no effect on paw-withdrawal threshold (E. K. Joseph and O. A. Dina, unpublished data). For experiments involving acute effects of PP1, piceatannol, YEEIP, inflammatory soup (PGE2, 5-HT, histamine, substance P, and bradykinin, 100 ng each), or the α2 integrin-blocking antibody, the agents were injected intradermally 30 min before behavioral testing.
Flinch test in rats
As described previously (Zheng and Chen, 2001; Zhang et al., 2003; Alessandri-Haber et al., 2004; Houck et al., 2004), rats were acclimated in a transparent observation chamber for 30 min. Then they were briefly restrained while 10 μl of hypotonic (deionized water, 17 mOsm) or isotonic (0.9% NaCl, 283 mOsm) solution was administered intradermally into the dorsum of the hindpaw via a 30-gauge needle connected to a 100 μl syringe by polyethylene tubing. Rats were observed immediately after the injection for a 5 min period, and the number of flinches observed was recorded as the flinch score. For experiments involving PP1, piceatannol, and α2 integrin-blocking antibody, these agents were injected intradermally at the site of the hypotonic solution injection, 45 min before behavioral testing. For the experiments testing the recovery from the acute effect of PP1 and piceatannol, flinching and mechanical thresholds were measured 24 h after the injection of the drugs.
Mechanical nociception in mouse
Mice were acclimated for 15–20 min in a transparent box with a metal mesh floor. A calibrated von Frey hair monofilament (Stoelting) was applied through the mesh floor to the plantar skin of the hindpaw. Mechanical nociception was measured as the total number of paw withdrawals in response to a series of 5 applications (at 3 min intervals) of a 0.17 mN von Frey hair (Alessandri-Haber et al., 2006).
ODN preparation and administration
The TRPV4 antisense ODN sequence 5′-CATCACCAGGATCTGCCATACTG-3′ and the α2 integrin antisense ODN sequence 5′-GCACCGAATAGCCAAACTGT-3′ (Invitrogen, Carlsbad, CA), were directed against unique regions of the rat TRPV4 channel and α2 integrin (GenBank accession numbers, NM_023970 and XM_345156.2, respectively). The mismatch ODN sequence was designed by mismatching 7 and 8 bases (denoted by bold face) of the TRPV4 (5′-CAACAGGAGGTTCAGGCAAACTG-3′) and α2 integrin (5′-GGTCCGTTTAGCGTAACTAC-3′) antisense sequence.
ODN was reconstituted in nuclease-free saline (10 μg/μl) and was administered into the spinal intrathecal space at a dose of 40 μg/20 μl, once a day for 3 d. For this procedure, rats were anesthetized with 2.5% isoflurane (97.5% O2), a 30-gauge needle was inserted into the subarachnoid space on the midline between the L4 and L5 vertebrae, and ODN was injected at 1 μl/s using a microsyringe (Alessandri-Haber et al., 2003).
We have demonstrated previously that TRPV4 is present in sensory nerve fibers, presumably being transported from the cell body toward the peripheral nerve endings, and that our antisense ODN treatment procedure induces a significant and specific decrease in TRPV4 protein expression level in ligated saphenous nerve (Alessandri-Haber et al., 2003, 2004).
For experiments investigating the effect of treatment with TRPV4 or α2 integrin antisense ODN on nociceptive thresholds, behavioral testing was performed 12 h after the last ODN injection. For experiments involving recovery from treatment with TRPV4 or α2 integrin antisense ODN, behavioral testing was performed 4 d after the last ODN injection to allow time for synthesis of new protein and its transport to the peripheral terminals.
Western blot
Saphenous nerves from anesthetized alcohol-fed rats were ligated with silk surgical suture (4-0) 1 cm above the knee-level bifurcation. A 5 mm section of saphenous nerve proximal to the ligation was removed 3 d after the ligation. Membrane protein preparation and Western blot analyses were performed as described previously (Alessandri-Haber et al., 2003). The membrane was probed with affinity-purified anti-TRPV4 (1:500) (Alessandri-Haber et al., 2003) or anti-α2 integrin antibody (1:100, SC-9089; Santa Cruz Biotechnology, Santa Cruz, CA) followed by incubation with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:5000; Pierce Biotechnology, Rockford, IL). To normalize the loaded samples, affinity-purified mouse monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (1:5000; Abcam, Cambridge, MA) was used followed by incubation with HRP-conjugated goat anti-mouse IgG (1:5000; Pierce Biotechnology). Membranes were incubated with enhanced chemiluminescence reagents (Pierce Biotechnology), and images of the membrane were acquired with Chemilmager chemiluminescence imaging system and analyzed with AlphaEaseFC software (Alpha Innotech, San Leandro, CA).
Dorsal root ganglion cell culture
L4–L6 dorsal root ganglia (DRGs) were harvested from TRPV4+/+ and TRPV4−/− mice or from rats treated with ethanol for 3 weeks, and dissociated and cultured as described previously (Alessandri-Haber et al., 2003). Briefly, dissociated cells were plated on coverslips treated with poly-dl-ornithine (0.1 mg/ml; Sigma) and laminin (5 μg/ml; Invitrogen), incubated at 37°C in 96.5% air and 3.5% CO2. Neurons were maintained in culture for 2 d in DMEM supplemented with 50–100 ng/ml nerve growth factor, 100 U/ml penicillin/streptomycin, MEM vitamins, and 10% heat-inactivated fetal calf serum (all from Invitrogen). Two days after plating, neurons were used for either calcium imaging or immunoprecipitation experiments.
Calcium imaging
Calcium imaging was performed using the fluorescent calcium indicator fura-2 AM applied to neurons between 24 and 72 h after DRG dissociation, as described previously (Alessandri-Haber et al., 2003). Briefly, neurons were loaded with 5 μm fura-2 AM for 20 min in isotonic solution (312 mOsm). Experiments were performed at 20–23°C with the perfusion at a flow rate of 1–2 ml/min. Cells were perfused with isotonic solution for 10 min before the beginning of the recording to allow complete removal of fura-ester that was not hydrolyzed.
Measurement of the concentration of free calcium ions ([Ca2+]i) was performed by ratiometric imaging with an intensified charge-coupled device camera. Fluorescence was excited at 340 and 380 nm, and emitted light was long filtered at 510 nm. The fluorescence ratio, F340/F380, was calculated with Metafluor software (Universal Imaging, Downingtown, PA). Calcium calibration was performed with a fura 2 calcium imaging calibration kit (Invitrogen), and apparent free [Ca2+]i was calculated from the equation [Ca2+]i = K d × [(R − R min)/(R max − R)] × (F max 380/F min 380), where R min is the ratio at zero free Ca2+, R max is the ratio at saturating Ca2+ (e.g., 39 μm), F max 380 is the fluorescence intensity exciting at 380 nm for zero Ca2+, and F min 380 is the fluorescence intensity at saturating free Ca2+.
Given the absence of specific pharmacological blockers of TRPV4, we minimized conductance via other ion channels by using a combination of room temperature, HEPES buffer, and variation of osmolarity by modifying only d-mannitol concentration. Thus, the standard isotonic solution (312 mOsm) contained (in mm) 88 NaCl, 5 KCl, 1 MgCl2, 2.4 CaCl2, 110 d-mannitol, and 10 HEPES, and was buffered to pH 7.38 with NaOH. The hypotonic solution was adjusted to 212 mOsm (30% hypotonic) by lowering the amount of d-mannitol to 10 mm. Osmolarity and pH were measured before each experiment. The vehicle for fura-2 AM, DMSO, at its final working dilution did not induce any response in DRG neurons.
To measure the calcium response of DRG neurons to hypotonicity, we averaged the value of the fluorescence ratio when the stimulus-induced increase in [Ca2+]i reached a maximum plateau. This fluorescence ratio was then normalized with respect to the baseline value: fluorescence ratio during hypotonic stimulation divided by resting fluorescence ratio in isotonic solution. The fluorescence ratio was then converted to apparent free [Ca2+]i. At the end of each experiment, a short exposure to solution containing 20 mm KCl was performed to confirm that all cells studied exhibited electrical excitability typical of healthy neurons.
For experiments testing the effect of the anti-α2 integrin antibody, neurons were challenged with 30% hypotonic solution containing PGE2 and 5-HT (10 μm each) for 3 min and rinsed with an isotonic solution containing PGE2 and 5-HT, until [Ca2+]i had recovered fully. Then neurons were perfused with isotonic solution containing PGE2, 5-HT, and anti-α2 integrin antibody (purified hamster anti-rat CD49b monoclonal antibody, catalog #554998; BD PharMingen, 5 μg/ml) for 20 min, and challenged with a 30% hypotonic solution containing PGE2, 5-HT, and anti-α2 integrin antibody for 3 min. The effect of the anti-α2 integrin antibody was calculated from the normalized fluorescence ratio during exposure to 30% hypotonic solution containing the antibody divided by the normalized fluorescence ratio in 30% hypotonic solution before the antibody. A similar protocol was used to test the effect of the inhibitors of Src tyrosine kinases, PP1 and piceatannol.
Immunoprecipitation
DRG neurons isolated from adult male rats and cultured for 2 d were incubated in ice-cold lysis buffer (50 mm Tris-HCl, 150 mm NaCl, 2 mm EDTA, 5 mm iodoacetamide, 1% NP-40, 0.25% sodium deoxycholate, pH 7.4, and complete mixture of protease inhibitors from Roche, Indianapolis, IN). TRPV4 was immunoprecipitated by incubating the supernatant with 1:500 of affinity-purified anti-TRPV4 antibody (Alessandri-Haber et al., 2003) for 3 h at 4°C, followed by overnight incubation with Protein A/G Plus-agarose (SC-2003; Santa Cruz Biotechnology) at 4°C. Immunoprecipitates were washed three times. Membrane lysates and immunoprecipitates were subjected to SDS gel electrophoresis. The membrane was then probed with anti-α2 integrin antibody (1:200, SC-9089; Santa Cruz Biotechnology) followed by incubation with HRP-conjugated goat anti-rabbit IgG or with the anti-Lyn antibody (1:300, SC-7274; Santa Cruz Biotechnology) followed by incubation with biotin-SP-conjugated affinipure goat anti-rabbit IgG (1:1000; Jackson ImmunoResearch, West Grove, PA) and a final incubation with streptavidin-peroxidase (1:500; Sigma). Membranes were incubated with enhanced chemiluminescence reagents (Pierce Biotechnology), and images of the membrane were acquired with Chemilmager chemiluminescence imaging system and analyzed with AlphaEaseFC software (Alpha Innotech).
Data analysis
Data are presented as mean ± SEM and comparisons between groups performed by Student's t test or ANOVA, which, if significant, was followed by Tukey's or Dunnett's post hoc multiple-comparisons test. Significance was defined as p ≤ 0.05.
To assess for significant differences between groups of rats receiving pretreatment with either TRPV4 ODN antisense or mismatch, in the onset of mechanical hyperalgesia, two-way repeated-measures ANOVAs with one within-subjects factor (days) and one between-subjects factor (ODN) was performed. If there was a significant two-way (days by ODN) interaction, follow-up one-way between-subjects ANOVAs with two levels (antisense and mismatch ODN) were performed for each time point. A Bonferroni-type adjustment was applied to the α level (α = 0.05/number of comparisons) for these analyses, to correct for effects of multiple comparisons.
Results
Mechanical and osmotic hyperalgesia depend on TRPV4
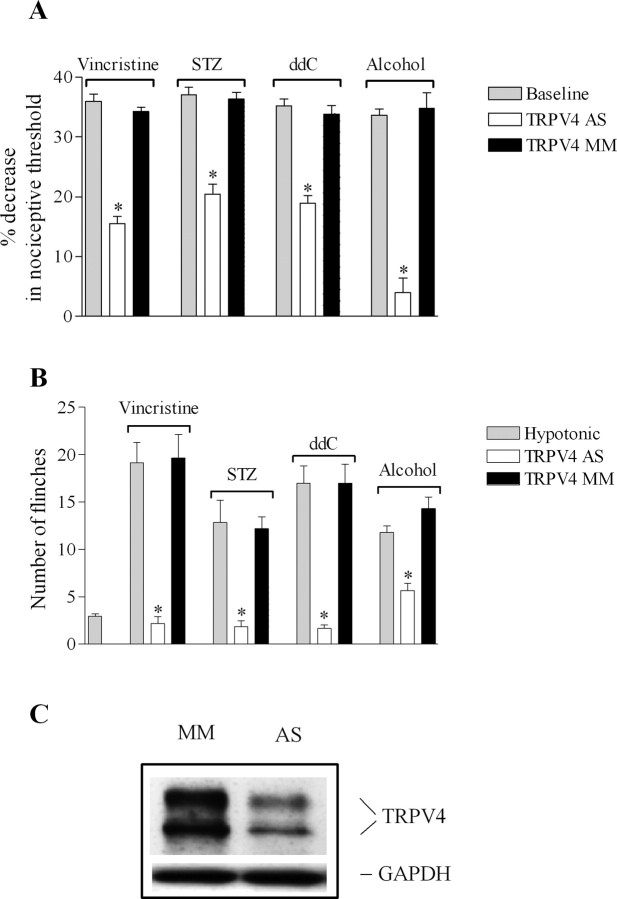
To assess the contribution of TRPV4 to the mechanical hyperalgesia associated with diverse models of neuropathic pain, rats experiencing hyperalgesia were treated with a spinal intrathecal administration of antisense or mismatch ODN for TRPV4, daily for 3 d. TRPV4 antisense ODN reversed the mechanical hyperalgesia in alcohol-fed rats (Fig. 1 A) (4 ± 2% decrease in mechanical nociceptive threshold for TRPV4 antisense- vs 35 ± 2% for mismatch-treated rats; n = 6 for each group; p < 0.0001, unpaired Student's t test), and reduced it by approximately one-half in the other three models (Fig. 1 A) (44% in both STZ- and ddC- and 53% in vincristine-treated rats; n = 8 for antisense- and n = 6 for mismatch-treated rats; all p < 0.05). Of note, this effect of antisense ODN on mechanical hyperalgesia was completely reversible; 4 d after the last ODN injection, the mechanical paw withdrawal threshold was no longer significantly different between antisense- and mismatch-treated rats (all p > 0.05, unpaired Student's t test).
Figure 1.
Contribution of TRPV4 to the hypersensitivity for mechanical and hypotonic stimuli in diverse models of painful peripheral neuropathy. A, Rats treated with vincristine, STZ, ddC, or alcohol showed a decrease in their mechanical nociceptive thresholds (gray bars). Spinal intrathecal treatment with TRPV4 antisense (TRPV4 AS, white bars), compared with mismatch oligodeoxynucleotides (TRPV4 MM, black bars), reversed the mechanical hyperalgesia in alcohol-fed rats and reduced it by 53% in vincristine- and 44% in STZ- and ddC-treated rats, respectively. B, Injection of 10 μl of hypotonic solution induced a significantly higher number of flinches in vincristine-, STZ-, ddC-, and alcohol-treated compared with control rats. TRPV4 antisense, compared with mismatch ODN, reduced the increase in the number of flinches induced by hypotonicity by 89% in vincristine-treated (2.2 ± 1.7 for antisense- vs 19.6 ± 2.4 for mismatch-treated rats; n = 6 for each ODN group; p < 0.0001, unpaired Student's t test), 83% in STZ-treated rats (1.8 ± 0.6 for antisense- vs 12.1 ± 1.2 for mismatch-treated rats; n = 6 for each ODN group; p < 0.0001, unpaired Student's t test), 88% in ddC-treated (1.7 ± 0.3 for antisense- vs 17 ± 2 for mismatch-treated; n = 6 for each ODN group; p < 0.0001, unpaired Student's t test), and 52% in alcohol-fed rats (5.6 ± 0.7 for antisense- vs 14.3 ± 1.2 for mismatch-treated; n = 6 for each ODN group; p < 0.0001, unpaired Student's t test). C, There was a significant decrease in the level of TRPV4 protein expression in saphenous nerve from alcoholic rats treated with antisense compared with mismatch ODN-treated rats (38 ± 8%; n = 6 for each ODN group; p < 0.05, unpaired Student's t test). The amount of protein loaded in each lane was normalized by probing the membrane with an anti-GAPDH antibody. *p < 0.05.
TRPV4 functions as an osmotransducer in nociceptors (Alessandri-Haber et al., 2003), and nociceptive flinching behavior in response to hypotonic stimulation depends on TRPV4 (Alessandri-Haber et al., 2003, 2005, 2006). Thus, we investigated whether TRPV4-mediated osmotransduction was enhanced in these pain models. Rats treated with vincristine, ddC, STZ, and alcohol showed a significant number of flinches in response to intradermal injection of hypotonic solution (10 μl of deionized water, 17 mOsm) in the hindpaw. As shown in Figure 1 B, rats treated with vincristine and ddC have, respectively, a 6.5- and a 5.9-fold increase in the number of flinches induced by hypotonicity, whereas rats treated with STZ and alcohol showed a fourfold increase compared with that in naive rats (2.9 ± 0.2, n = 46 for naive; 19 ± 2, n = 6 in vincristine-treated; 17 ± 2, n = 6 in ddC-treated; 12 ± 2, n = 6 in STZ-treated; and 12 ± 1, n = 24 in alcoholic-treated rats; p < 0.0001, Dunnett's multiple-comparison test). To test the contribution of TRPV4 to the hypotonicity-induced nociceptive behavior, rats received spinal intrathecal administrations of antisense or mismatch ODN for TRPV4, daily for 3 d. Compared with mismatch, antisense ODN for TRPV4 reduced the number of flinches induced by hypotonicity by 89% in vincristine-treated, 88% in ddC-treated, 83% in STZ-treated, and 52% in alcohol-fed rats (Fig. 1 B).
To determine whether the reduction in the nociceptive behaviors caused by TRPV4 antisense administration is associated with a decrease in the level of TRPV4 protein, a group of alcohol-fed rats was treated with TRPV4 ODN for 3 d, and saphenous nerves were harvested the following day and processed for Western blotting. As previously reported, a doublet band at 98 and 107 kDa was detected (Delany et al., 2001; Alessandri-Haber et al., 2003; Liedtke and Friedman, 2003; Xu et al., 2003). Antisense ODN treatment caused a 38 ± 8% decrease in the level of expression of TRPV4 protein in ligated nerve from antisense-treated vs mismatch-treated rats (Fig. 1 C) (n = 6 for each ODN group; p < 0.05, unpaired Student's t test). This value is similar to the decrease in TRPV4 protein reported previously in control or paclitaxel-treated rats after 3 d of antisense injection (Alessandri-Haber et al., 2003, 2004). To confirm the recovery of TRPV4 protein expression level after cessation of ODN injection, Western blots were performed on ligated saphenous nerve of alcoholic rats harvested 4 d after the last ODN injection; the level of TRPV4 protein expression was not significantly different between antisense- and mismatch-treated rats (n = 4 for each ODN group; p > 0.05, unpaired Student's t test).
Because TRPV4 antisense ODN reversed alcohol-induced mechanical hyperalgesia, we tested the possibility that the neuropathic hyperalgesia might be correlated with an increase in the level of TRPV4 protein expression, by performing Western blots on ligated saphenous nerves of rats fed alcohol or control diet. However, the level of TRPV4 protein in alcohol-fed rats was not significantly different from that in rats fed control diet (n = 8 for alcohol-fed and n = 6 for control rats; p > 0.05, unpaired Student's t test) suggesting that the alcohol-induced hyperalgesia is not related to an increase in TRPV4 protein expression. Of note, similarly, whereas TRPV4 antisense ODN reverses paclitaxel-induced mechanical hyperalgesia, the level of TRPV4 protein in ligated nerve from paclitaxel-treated rats is not significantly different from in control rats (Alessandri-Haber et al., 2004).
TRPV4 contributes to the development of mechanical hyperalgesia
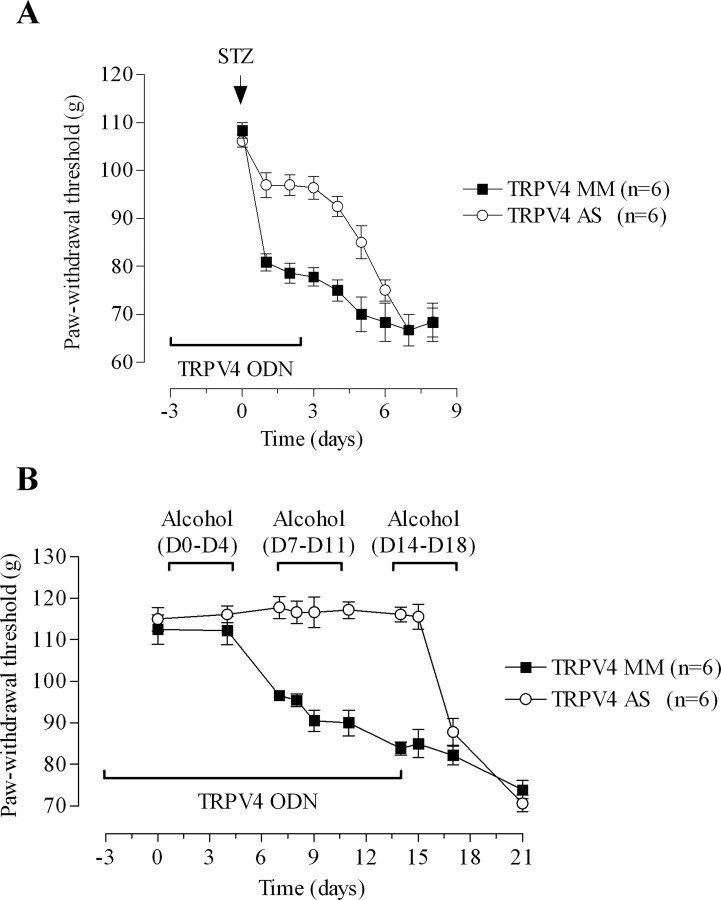
To determine whether TRPV4 is necessary for the development of neuropathy-induced mechanical hyperalgesia, we tested whether intrathecal treatment with TRPV4 antisense, initiated before rats were exposed to alcohol or STZ, would prevent the development of mechanical hyperalgesia. These two models were chosen because they represent models for which TRPV4 antisense ODN treatment is the most and the least effective at reversing mechanical hyperalgesia. Rats were treated with TRPV4 antisense for 3 d before and 3 d after STZ injection. On the day after the STZ injection, mechanical hyperalgesia was reduced by two-thirds in antisense- compared with mismatch-treated rats (Fig. 2 A) (n = 6 for each group; p < 0.001). After completion of the ODN treatment on day 3, the mechanical hyperalgesia was gradually unmasked, and by day 7 there was no significant difference in paw withdrawal threshold between the two ODN-treated groups (Fig. 2 A). Of note, day 7 corresponds to the fourth day after the last ODN injection, a time by which the level of TRPV4 protein expression in antisense- and mismatch-treated rats is not significantly different.
Figure 2.
Contribution of TRPV4 to neuropathic mechanical hyperalgesia. A, Rats were treated with either TRPV4 antisense (AS) or mismatch (MM) ODN for 6 d. Streptozotocin was administered on day 3. Two-way repeated-measures ANOVA showed a significant group by time interaction (F (4,88) = 22.511; p < 0.001) and a significant main effect of group (F (1,22) = 34.107; p < 0.001). Post hoc one-way ANOVAs (group with 2 levels, antisense and mismatch) revealed that paw withdrawal threshold differed significantly between the two groups from day 4 (the first day after receiving STZ) to day 8. B, TRPV4 antisense or mismatch ODN were administered daily for 14 d starting 3 d before the commencement of ethanol diet. Rats were fed the ethanol diet for four days of the week [day 0 (D0)–D4], and they were fed standard lab chow for the remaining three days (D4–D7). A repeated-measures ANOVA with one within-subjects factor (days with 10 levels) and one between-subjects factor (ODN with 2 levels, antisense and mismatch) demonstrated a significant ODN by days interaction (F (9,90) = 18.448; p < 0.001) and a significant main effect of ODN (F (1,10) = 50.744; p < 0.0001). Post hoc one-way between-subjects ANOVAs showed that the ODN groups differed significantly from day 7 (p < 0.001) to day 15 (p = 0.051).
A separate group of rats was treated with TRPV4 antisense ODN for 3 d before and 14 d after the first alcohol exposure. We chose 14 d after alcohol diet initiation because we recently demonstrated that two cycles of alcohol withdrawal (2 weeks) induces marked mechanical hyperalgesia (Dina et al., 2006). Alcohol-fed rats pretreated with TRPV4 antisense did not develop mechanical hyperalgesia compared with the mismatch-treated rats (Fig. 2 B) (n = 6 for each ODN group; p < 0.001). However, after completion of the ODN treatment on day 14, the mechanical hyperalgesia was gradually unmasked, and by day 18 the paw withdrawal threshold was not significantly different between the antisense and mismatch ODN groups (Fig. 2 B).
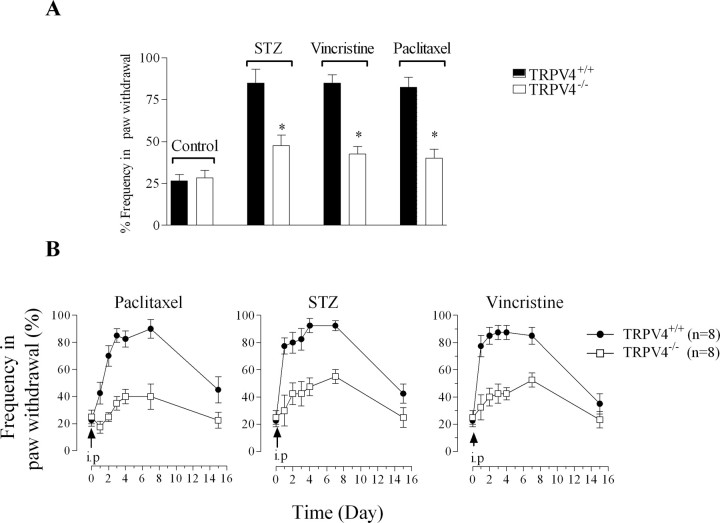
As an independent test of the importance of the contribution of TRPV4 in mechanical hyperalgesia associated with small-fiber neuropathy, C57BL/6 mice lacking a functional TRPV4 gene (TRPV4−/−) and wild-type littermates (TRPV4+/+) received an intraperitoneal injection of STZ (75 mg/kg), vincristine (200 μg/kg), or paclitaxel (6 mg/kg). All three drugs induced mechanical hyperalgesia that lasted at least 2 weeks in TRPV4+/+ mice. The baseline withdrawal response to von Frey hair stimulation was not significantly different between TRPV4−/− and TRPV4+/+ mice (n = 12 for each group; p > 0.05, unpaired Student's t test). However, 4 d after the injection of STZ, vincristine or paclitaxel, the withdrawal response frequency was markedly increased in TRPV4+/+ mice compared with that in TRPV4−/− mice (Fig. 3 A) (n = 8 for each group). To further investigate the role of TRPV4 in the development and maintenance of mechanical hyperalgesia, mice treated with STZ, vincristine, or paclitaxel were tested daily for a week. The mechanical hyperalgesia in the three groups was impaired in TRPV4−/− mice from the day after the drug injection (day 1), and by day 7 the withdrawal response frequency was decreased by 70% in TRPV4−/− mice treated with paclitaxel, whereas STZ- and vincristine-treated TRPV4−/− mice showed an ∼ 50% decrease in their response compared with TRPV4+/+ mice (Fig. 3 B).
Figure 3.
Paclitaxel-, STZ-, and vincristine-induced mechanical hyperalgesia is impaired in TRPV4−/− mice. A, The withdrawal response to von Frey hair mechanical stimuli is similar in naive TRPV4+/+ (solid bars; n = 12) and TRPV4−/− mice (clear bars; n = 12). Four days after a single intraperitoneal injection of STZ (75 mg/kg), vincristine (200 μg/kg), or paclitaxel (6 mg/kg), TRPV4−/− mice exhibit only an ∼1.5-fold increase in withdrawal frequency response compared with the approximately threefold increase present in TRPV4+/+ mice (n = 8 for each group of mice). *p < 0.05. B, Time course of mechanical hyperalgesia after a single intraperitoneal injection of paclitaxel, STZ, or vincristine in TRPV4+/+ (filled circles; n = 8) and TRPV4−/− mice (open squares; n = 8), expressed as mean hindpaw withdrawal frequency (%) ± SEM.
α2β1 integrin and Src tyrosine kinase contribute to TRPV4-dependent nociceptive behaviors
The demonstration of a role of TRPV4 in the mediation of mechanical hyperalgesia of various etiologies supports the suggestion that TRPV4 contributes to mechanotransduction in the setting of nociceptor sensitization. Given that TRPV4 does not respond to membrane deformation in vitro (Strotmann et al., 2000), we hypothesized that it requires interaction with other molecules to mediate mechanotransduction in sensitized primary afferents. Integrins are good candidates for such molecules because they are mechanosensors that can contribute to mechanical transduction through the modulation of ion channels via a Src tyrosine signaling pathway (Aplin et al., 1998; Mobasheri et al., 2002; Waitkus-Edwards et al., 2002; Browe and Baumgarten, 2003; Katsumi et al., 2004), and specific integrins are necessary for the development of mechanical hyperalgesia (Dina et al., 2004). In addition, we recently demonstrated that paclitaxel-induced mechanical hyperalgesia is dependent on TRPV4, integrins, and Src tyrosine kinase, suggesting the possibility of a required functional interaction between these molecules (Alessandri-Haber et al., 2004; Dina et al., 2004). We focused on α2β1 integrin because it is expressed in both DRG neurons and receptive endings of cutaneous mechanoreceptors (Andrew et al., 1992; Tomaselli et al., 1993; Khalsa et al., 2000) and it has been shown to modulate mechanotransduction in cutaneous mechanoreceptors (Khalsa et al., 2004). Thus, we investigated whether α2β1, Src tyrosine kinase, and TRPV4 closely interact to mediate mechanical hyperalgesia.
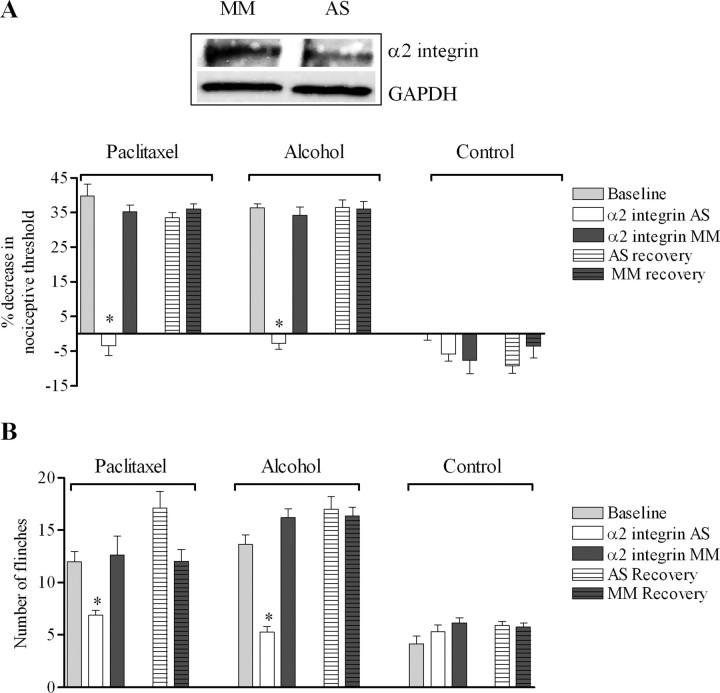
The first step to test this hypothesis was to assess the contribution of α2β1 integrin in mechanical hyperalgesia associated with paclitaxel and alcohol binge drinking, two very distinct models of small fiber neuropathies that are TRPV4 dependent. Rats were treated with α2 integrin antisense ODN daily for 3 d. This protocol reversed the mechanical hyperalgesia in both paclitaxel- and alcohol-treated rats (Fig. 4 A). In contrast, α2 integrin antisense ODN did not significantly affect the nociceptive mechanical threshold in control rats (Fig. 4 A), supporting the suggestion of a distinct role of α2β1 integrin in mechanotransduction in sensitized nociceptors. The effect of the α2 integrin antisense was reversible: 4 d after the last ODN injection, the mechanical hyperalgesia induced by alcohol or paclitaxel was not significantly different from the pre-ODN baseline (Fig. 4 A) (p > 0.05, Tukey's multiple-comparison test).
Figure 4.
Hyperalgesia to hypotonic and mechanical stimuli depend on α2 integrin. A, Treatment with α2 antisense (AS) ODN for 3 d reversed the mechanical hyperalgesia in alcohol-fed [n = 12 for antisense- and n = 10 for mismatch (MM)-treated; p < 0.0001, unpaired Student's t test] and paclitaxel-treated rats (n = 8 for antisense- and mismatch-treated rats; p < 0.0001, unpaired Student's t test). The effect of the antisense was reversible; 4 d after the last ODN injection, the mechanical hyperalgesia induced by alcohol or paclitaxel was not significantly different from the pre-ODN baseline. In contrast, α2 integrin antisense ODN did not affect mechanical thresholds in control-fed rats (n = 10 for antisense- and n = 8 for mismatch-treated; p > 0.05, unpaired Student's t test). Inset, There was a 38 ± 6% decrease in protein expression level in saphenous nerve of alcohol-fed rats treated with α2 integrin antisense- compared with mismatch-treated (n = 5 in each ODN group; p = 0.004, unpaired Student's t test). The amount of protein in both lanes was confirmed to be comparable by probing the membrane with an anti-GAPDH antibody. B, Treatment with α2 integrin antisense markedly reduced the number of flinches induced by intradermal injection of hypotonic solution in both alcohol-fed (n = 12 for antisense- and n = 10 for mismatch-treated rats; p < 0.0001, unpaired Student's t test) and paclitaxel-treated rats (n = 8 for each ODN group; p < 0.0001, unpaired Student's t test). In fact, the number of flinches induced by hypotonicity in alcohol-fed or paclitaxel-treated rats after treatment with α2 integrin antisense ODN was not significantly different from the number of flinches induced by hypotonicity in rats fed control diet (n = 8 for paclitaxel rats treated with α2 integrin AS; n = 12 for alcohol rats treated with α2 integrin AS; n = 10 for rats fed with control diet treated with α2 integrin AS; p > 0.05, ANOVA). *p < 0.05.
To further support a functional interaction between TRPV4 and α2β1 integrin, we investigated whether α2 integrin antisense could reduce nociceptive behaviors in response to hypotonicity. Treatment with α2 integrin antisense ODN reduced the number of flinches by 68 and 45% in alcohol-fed and paclitaxel-treated rats, respectively, compared with mismatch ODN (Fig. 4 B). In fact, α2 integrin antisense reduced the number of flinches in response to an intradermal hypotonic solution to the same extent as treatment with TRPV4 antisense in both alcohol-fed (Fig. 1 A) and paclitaxel-treated rats (Alessandri-Haber et al., 2004). Again, the effect of α2 integrin antisense was reversible and 4 d after the last ODN injection, nociceptive behaviors in response to hypotonicity were similar to the pre-ODN baseline. Of note, the antisense ODN did not affect the number of flinches in response to hypotonic stimulation in control rats.
To determine whether the reduction in nociceptive behaviors induced by α2 integrin antisense ODN treatment is associated with a reduction in the level of α2 integrin protein, we performed Western blot analyses on saphenous nerve harvested from alcohol-fed rats. As shown in the inset in Figure 4 A, the expected 150 kDa band was detected with a 38 ± 6% reduction in the α2 integrin protein expression level in ligated saphenous nerve of antisense- compared with mismatch-treated rats (n = 5 for each ODN group; p = 0.004, unpaired Student's t test).
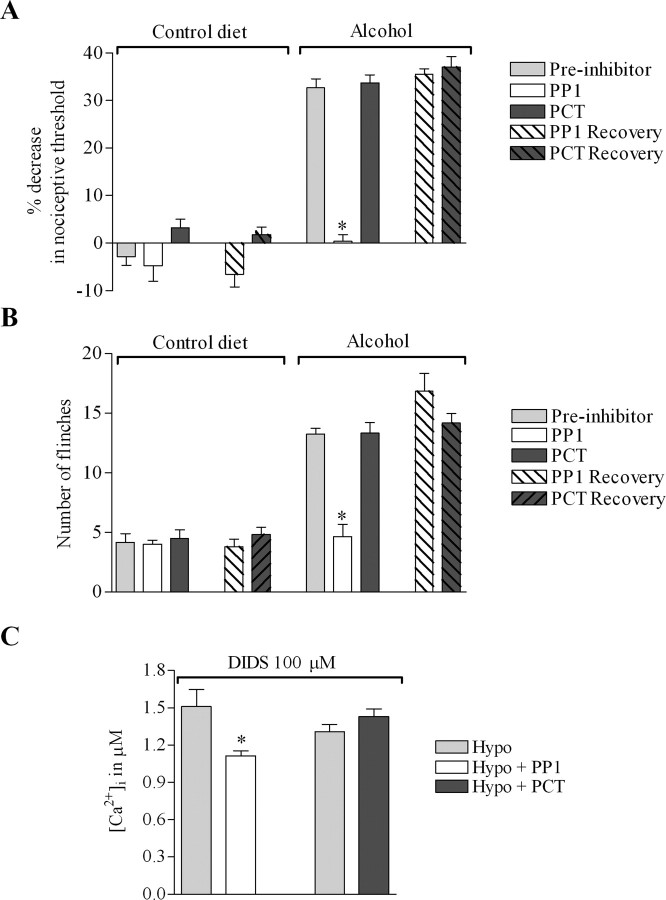
We next assessed the contribution of Src tyrosine kinase to mechanical hyperalgesia. We recently demonstrated that the enhanced responses to mechanical and osmotic stimuli in paclitaxel-treated rats as well as the hypotonicity-induced increase in [Ca2+]i in nociceptors in vitro depend on Src tyrosine kinase (Alessandri-Haber et al., 2004). Thus, we investigated the effect of PP1, a specific inhibitor of Src family tyrosine kinase (Hanke et al., 1996), on the enhanced nociceptive behaviors in alcohol-fed rats. As a control, we used piceatannol, a specific inhibitor of Syk, a related protein kinase not belonging to the Src family (Oliver et al., 1994).
Intradermal injection of PP1 (1 μg/2.5 μl) in the hindpaw 30 min before mechanical nociceptive threshold testing, inhibited mechanical hyperalgesia in alcohol-fed rats (Fig. 5 A) (32.7 ± 1.8%, n = 12 without vs 0.4 ± 1.4%, n = 6 after PP1; p < 0.001, unpaired Student's t test). In contrast, injection of PCT (1 μg/2.5 μl) did not have a significant effect (32.7 ± 1.8%, n = 12 without vs 33.7 ± 1.6%, n = 6 after piceatannol; p > 0.05, unpaired Student's t test). Twenty-four hours after the administration of PP1, its effect was no longer present and there was no significant difference between the two groups of rats (p > 0.05, unpaired Student's t test). Of note, PP1 did not affect the mechanical nociceptive threshold in rats fed control diet (Fig. 5 A).
Figure 5.
Enhanced response to hypotonic and mechanical stimuli in alcohol-fed rats depends on Src tyrosine kinase. A, Intradermal injection of PP1 (specific inhibitor of Src family tyrosine kinase) 30 min before measurement of nociceptive thresholds reversed the alcohol-induced mechanical hyperalgesia (mean ± SEM, 32.7 ± 1.8%, n = 12 before and 0.4 ± 1.4%, n = 6 after PP1; p < 0.001, unpaired Student's t test). In contrast, the injection of PCT had no effect on mechanical thresholds (n = 12 before and n = 6 after piceatannol; p > 0.05, unpaired Student's t test). There was no significant difference between the two groups of rats 24 h after the injection of the inhibitors (n = 6 after PP1 or piceatannol; p > 0.05, unpaired Student's t test). Of note, intradermal injection of PP1 did not affect mechanical thresholds in rats fed control diet. B, Intradermal injection of PP1 reduced the number of flinches induced by hypotonicity in alcohol-fed rats by 60% (13.2 ± 0.4, n = 12 before and 5 ± 1, n = 6 after PP1; p < 0.0001, unpaired Student's t test), whereas PCT had no effect. Of note, the number of flinches induced by hypotonicity after PP1 in alcohol-fed rats was not significantly different from the number of flinches induced by hypotonic solution in control diet rats. The number of flinches induced by hypotonicity was not significantly different between the rats treated with PP1 or piceatannol 24 h after the injection of the inhibitors (p > 0.05, unpaired Student's t test). C, Mean [Ca2+]i for neurons first challenged with a 30% hypotonic solution (212 mOsm) and then challenged with a 30% hypotonic solution containing either PP1 (10 μm) or piceatannol (30 μm). PP1 decreased the hypotonicity-induced increase in [Ca2+]i by 26% (1.51 ± 0.14 μm before and 1.11 ± 0.04 μm in presence of PP1; n = 14; p = 0.001, paired Student's t test), whereas piceatannol did not have a significant effect (n = 10; p > 0.05, paired Student's t test). All extracellular solutions contained DIDS (100 μm). *p < 0.05.
Next, we tested the effect of these inhibitors on hypotonicity-induced nociceptive behavior in alcohol-fed rats. Hypotonic solution was injected in the hindpaw 45 min after the injection of the inhibitors at the same site. As shown in Figure 5 B, PP1 reduced the number of flinches by 60% (13.2 ± 0.4, n = 12 without vs 5 ± 1, n = 6 after PP1; p < 0.0001, unpaired Student's t test), whereas piceatannol had no significant effect (13.2 ± 0.4, n = 12 without vs 13.3 ± 0.8, n = 6 after piceatannol; p > 0.05, unpaired Student's t test). Twenty-four hours after administration of the inhibitors, there was no significant difference in the number of flinches between the two groups of rats (p > 0.05, unpaired Student's t test). These inhibitors had no effect on the number of hypotonicity-induced flinches in rats fed control diet (Fig. 4 B) (n = 6 for each group of rats; p > 0.05, ANOVA). Also, the number of flinches induced by intradermal injection of hypotonic solution after pretreatment with PP1 in alcohol-fed rats was not significantly different from the number of flinches in rats fed control diet (n = 6 for control diet rat preinhibitor and n = 6 for alcohol-fed rats after PP1; p > 0.05, unpaired Student's t test). Of note, PP1 reduced the number of flinches induced by hypotonicity to the same extent as treatment with TRPV4 antisense (Fig. 1 A).
Finally, we investigated, in vitro, whether the Src kinase inhibitors modulated the response to hypotonic solution of DRG neurons from alcohol-fed rats. DRG neurons were first challenged with a 30% hypotonic solution and then challenged with a hypotonic solution containing either PP1 (10 μm) or piceatannol (30 μm). As shown in Figure 5 C, the presence of PP1 reduced the hypotonicity-induced increase in [Ca2+]i by 26% (n = 14; p = 0.001, paired Student's t test), whereas the presence of piceatannol had no significant effect (n = 10; p > 0.05, paired Student's t test); all extracellular solutions contained DIDS (100 μm) to inhibit swelling-activated chloride channels, which are known to interact with Src tyrosine kinase signaling pathways (Voets et al., 1998; Shi et al., 2002).
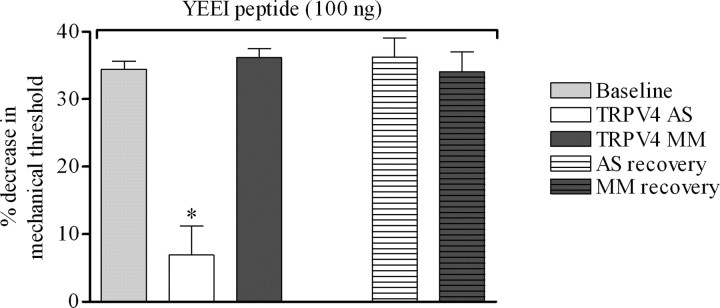
Our results demonstrate that a Src tyrosine kinase-dependent pathway and TRPV4 are essential to the development of mechanical hyperalgesia associated with distinct neuropathies. However, despite the similar reduction in the hyperalgesia to hypotonic and mechanical stimuli induced by PP1 and TRPV4 antisense ODN in alcohol-fed rats (Figs. 1 A,B, 5 A,B), these results do not demonstrate a direct interaction between Src tyrosine kinase and TRPV4. To begin to address this issue, we investigated whether intradermal injection of the Src tyrosine kinase activator peptide, YEEIP, could induce TRPV4-dependent mechanical hyperalgesia. Intradermal injection of YEEIP (100 ng) induced a significant decrease in mechanical nociceptive threshold in control rats (Fig. 6) (34.4 ± 1.2%; n = 10). To assess the contribution of TRPV4 to the mechanical hyperalgesia induced by YEEIP, rats were treated with TRPV4 antisense or mismatch ODN daily for 3 d. As shown in Figure 6, treatment with TRPV4 antisense markedly reduced the YEEIP-induced mechanical hyperalgesia (6.8 ± 4.3% decrease in mechanical nociceptive threshold for TRPV4 antisense- vs 36.2 ± 1.3% for TRPV4 mismatch-treated; n = 6 for each group; p < 0.0001 unpaired Student's t test). Four days after the last ODN injection, when TRPV4 protein expression level would have recovered, the decrease in nociceptive thresholds induced by intradermal injection of YEEIP was not significantly different between rats previously treated with TRPV4 antisense or mismatch (Fig. 6) (n = 6 for each ODN group; p > 0.05, unpaired Student's t test).
Figure 6.
Src tyrosine kinase-induced mechanical hyperalgesia is TRPV4 dependent. Intradermal injection of the specific activator of Src family tyrosine kinase, YEEIP peptide (100 ng), 30 min before measurement of nociceptive thresholds, induced a decrease in mechanical nociceptive thresholds in control rats (gray bar, 34.4 ± 1.2%; n = 10). Treatment with TRPV4 antisense (AS) markedly reduced the YEEIP-induced mechanical hyperalgesia (6.8 ± 4.3% decrease in mechanical nociceptive threshold for TRPV4 antisense- and 36.2 ± 1.3% for TRPV4 mismatch (MM)-treated rats; n = 6 for each group; p < 0.0001 unpaired Student's t test). Four days after ODN injection, the decrease in nociceptive thresholds induced by intradermal injection of YEEIP peptide was not significantly different between rats previously treated with antisense or mismatch (n = 6 for each group; p > 0.05, unpaired Student's t test). *p < 0.05.
Physical and functional interaction between TRPV4, the Src tyrosine kinase Lyn, and α2 integrin
Our results suggest that α2β1 integrin and Src tyrosine kinase might directly interact with TRPV4 in the production of mechanical hyperalgesia. Integrins have been shown to directly activate members of the Src tyrosine kinase family in focal adhesion kinase signaling (Shyy and Chien, 1997; Aplin et al., 1998; Wang et al., 2005), and Src tyrosine kinase Lyn has been demonstrated to phosphorylate TRPV4 in HEK 293 cells (Xu et al., 2003). Thus, we hypothesized that TRPV4 may contribute to mechanical hyperalgesia by direct interaction with α2 integrin and the Src tyrosine kinase, Lyn. To test this, we immunoprecipitated TRPV4 from cultured DRG neurons and used Western blots to detect α2 integrin and Lyn. Neurons were isolated from five alcohol-fed rats and immunoprecipitation with anti-TRPV4 antibody (1:500) was performed on the second day of culture. As shown in Figure 7 A, a specific 150 kDa band corresponding to α2 integrin and a 50 kDa band corresponding to Lyn were detected by Western blot. The coimmunoprecipitation of TRPV4, α2 integrin, and Lyn was reproducible in three independent experiments. To rule out the possibility of nonspecific binding to protein A/G, an immunoprecipitation without primary anti-TRPV4 antibody was run in parallel for each experiment, and no bands were detected at the molecular weight of α2 integrin or Lyn. These results suggest that α2β1, Lyn, and TRPV4 directly interact.
Figure 7.
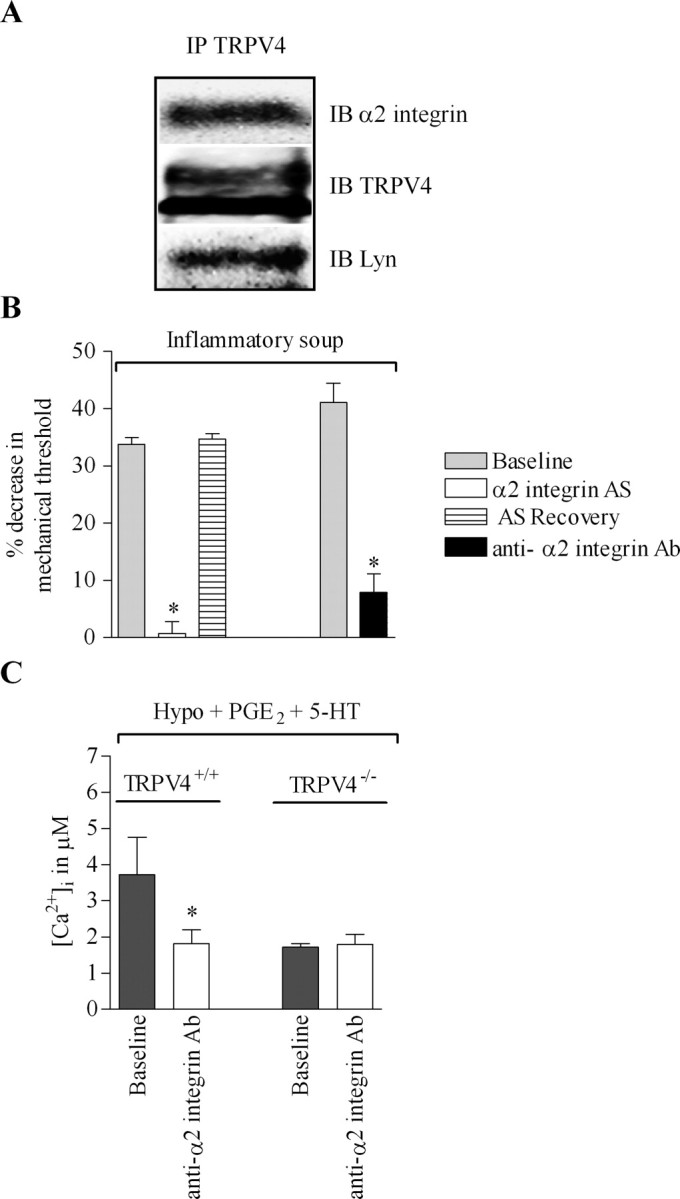
Direct interaction of TRPV4 with α2 integrin and Src tyrosine kinase. A, Proteins were extracted from DRG neurons isolated from alcohol-fed rats cultured for 2 d. TRPV4 was immunoprecipitated with anti-TRPV4 antibody (1:500) and immunoprecipitates were subjected to SDS gel electrophoresis. The membrane was then probed with anti-α2 integrin antibody (1:200) or with anti-Lyn antibody (1:300). IP, Immunoprecipitation; IB, immunoblot. B, Intradermal injection of a soup of inflammatory mediators in rat hindpaw induces a decrease in mechanical nociceptive thresholds in the rat. Spinal intrathecal injection of α2 integrin antisense (AS) ODN prevented the induced mechanical hyperalgesia (n = 10) and the effect of the antisense was reversible. Similarly, intradermal injection of anti-α2 integrin antibody (Ab; 100 ng/10 μl) before the intradermal injection of the inflammatory soup markedly reduced the mechanical hyperalgesia (n = 4). C, Small-diameter DRG neurons from TRPV4+/+ and TRPV4−/− mice were first challenged with a 30% hypotonic solution containing the inflammatory mediators PGE2 and 5-HT (10 μm each) for 3 min and then challenged with a 30% hypotonic solution containing PGE2, 5-HT, and the anti-α2 integrin antibody (5 μg/ml). The inflammatory mediator-induced increase in [Ca2+]i is reversed in the presence of anti-α2 integrin antibody in TRPV4+/+ mice (3.71 ± 1.03 before anti-α2 integrin antibody and 1.8 ± 0.3 after; n = 19; p < 0.0001, paired Student's t test), whereas it has no effect on [Ca2+]i in TRPV4−/− mice (1.8 ± 0.2 before and 1.7 ± 0.2 after anti-α2 integrin antibody; n = 30; p > 0.05, paired Student's t test). *p < 0.05.
To further demonstrate a functional contribution of α2β1/Src tyrosine kinase in TRPV4-mediated mechanical hyperalgesia, we investigated whether α2β1 integrin also contributes to the mechanical hyperalgesia induced by inflammatory mediators (Alessandri-Haber et al., 2006). We previously demonstrated that PP1 reversed the TRPV4-mediated nociceptive response to hypotonic stimuli in the presence of prostaglandin E2 (Alessandri-Haber et al., 2005). To assess the contribution of α2β1 integrin, rats were treated with α2 integrin antisense ODN for 3 d and were tested on the fourth day for a decrease in mechanical threshold after the injection of a soup of inflammatory mediators (bradykinin, substance P, PGE2, 5-HT, and histamine at 100 ng each). This inflammatory soup did not induce mechanical hyperalgesia in rats treated with α2 integrin antisense ODN (Fig. 7 B) (n = 14, 33.8 ± 1.1% decrease in mechanical nociceptive threshold for baseline vs 0.74 ± 2%, n = 10 for antisense-treated). The effect of the antisense ODN was reversible; 4 d after the last ODN treatment, the intradermal injection of inflammatory soup in rat hindpaw induced a mechanical hyperalgesia comparable in magnitude to that produced before antisense ODN treatment (n = 14 for baseline and n = 10 for α2 integrin antisense-treated; p > 0.05, unpaired Student's t test). As an independent test, we investigated whether intradermal injection of a function-blocking anti-α2 integrin antibody (100 ng) in rat hindpaw would also reduce mechanical hyperalgesia induced by inflammatory soup. As shown in Figure 7 B, anti-α2 integrin antibody reversed mechanical hyperalgesia (n = 4).
We recently demonstrated that inflammatory mediators enhance the increase in [Ca2+]i induced by 30% hypotonic solution in DRG neurons isolated from TRPV4+/+ mice but not from TRPV4−/− mice (Alessandri-Haber et al., 2006). Therefore, to further support direct functional interaction between α2β1 integrin and TRPV4, we tested whether α2 integrin-blocking antibody reduced the response of DRG neurons to hypotonic solution in the presence of inflammatory mediators in vitro. Small-diameter DRG neurons (≤25 μm) isolated from TRPV4+/+ and TRPV4−/− mice were challenged with a 30% hypotonic solution (212 mOsm) containing PGE2 and 5-HT (10 μm each) for 3 min and perfused with isotonic solution (312 mOsm) containing PGE2 and 5-HT until [Ca2+]i had fully recovered. Then the neurons were perfused with isotonic solution containing PGE2, 5-HT, and anti-α2 integrin antibody (5 μg/ml) for 20 min and challenged with a hypotonic solution containing PGE2, 5-HT, and anti-α2 integrin antibody for 3 min. The α2 integrin-blocking antibody reversed the increase in [Ca2+]i induced by inflammatory mediators in DRG neurons isolated from TRPV4+/+ mice (p < 0.05, paired Student's t test; n = 19).
Discussion
We recently demonstrated that mechanical hyperalgesia induced by inflammatory mediators and the chemotherapy drug paclitaxel both depend on TRPV4 (Alessandri-Haber et al., 2004, 2006). This finding was surprising because the etiologies of the mechanical hyperalgesia associated with these two conditions are very different. One explanation may be that TRPV4 plays a basic role in mechanical hyperalgesia and that it participates in the transduction of mechanical stimuli only in hyperalgesic states. Therefore, we investigated the role of TRPV4 in several additional pain models associated with very different cellular mechanisms. In every model tested, the mechanical hyperalgesia is markedly impaired in the absence of TRPV4 function. This finding suggests that drugs with very different mechanisms of action (vincristine, streptozotocin, ddC, paclitaxel, and alcohol) converge to activate a shared molecular mechanism to produce hypersensitivity to mechanical stimuli.
Moreover, after the recovery from TRPV4 antisense treatment and without additional drug administration, the level of mechanical hyperalgesia in antisense-treated rats returns to a value comparable with that in mismatch-treated rats, regardless of whether the antisense is given before or after the full development of the induced mechanical hyperalgesia (Figs. 1 A, 2 A). This observation suggests that although TRPV4 is required for expression of the hyperalgesia, additional mechanisms must maintain the latent hyperalgesic state induced by the neurotoxic drugs.
A potential role of TRPV4 in mechanotransduction has been controversial, with conflicting evidence reported in the literature. On one hand, TRPV4 contributes to both mechanical hypersensitivity and to the detection of intense noxious mechanical stimuli (Liedtke and Friedman, 2003; Suzuki et al., 2003; Alessandri-Haber et al., 2004, 2006), and transgenic expression of mammalian TRPV4 in ASH nociceptive neurons of Caenorhabditis elegans worms with a mutation of the osmosensing TRPV gene Osm9 restores osmotic and mechanical avoidance in these worms (Liedtke et al., 2003). On the other hand, TRPV4 does not contribute to the detection of threshold-level nociceptive mechanical stimuli (Liedtke and Friedman, 2003; Suzuki et al., 2003), and most importantly, membrane deformation in vitro does not increase TRPV4 current (Liedtke et al., 2000; Strotmann et al., 2000; Suzuki et al., 2003).
The fact that TRPV4 is not normally directly activated by membrane deformation does not, however, mean that it cannot participate in mechanotransduction, because different gating models of mechanotransduction are emerging in the literature (Lumpkin and Caterina, 2007). Based on the “tensegrity” model of mechanotransduction (Wang et al., 1993; Ingber, 1997), we hypothesized that TRPV4 may be coupled to other mechanically sensitive proteins in sensory neurons. In this model, mechanical forces applied to integrins either from the extracellular matrix or cytoskeleton are transduced into biochemical signals at focal adhesion sites (Ingber, 1997, 2003a,b; Wang et al., 2001; Alenghat and Ingber, 2002). Mechanotransduction would occur when forces are transmitted through integrins to modulate the function of associated ion channels (McPhee et al., 1998; Wang et al., 2000; Wu et al., 2001; Mobasheri et al., 2002; Wildering et al., 2002; Shakibaei and Mobasheri, 2003; Gui et al., 2006). Consistent with this model integrins have been implicated in the transduction of osmotic stimuli and mechanical stretch (Sadoshima and Izumo, 1997a,b; Voets et al., 1998; Browe and Baumgarten, 2003; vom Dahl et al., 2003) and in mechanical hyperalgesia (Dina et al., 2004). In the present study, we investigated the role of α2β1 integrin in particular, for several reasons: (1) paclitaxel-induced mechanical hyperalgesia is dependent on β1 integrin (Dina et al., 2004), (2) α2β1 colocalizes with peripherin in the receptive terminals of cutaneous neurons in rat hairy skin (Khalsa et al., 2000), and (3) α2β1 modulates mechanotransduction in slowly and rapidly adapting cutaneous mechanoreceptors (Khalsa et al., 2004).
We demonstrate that treatment with α2 integrin antisense ODN reverses mechanical hyperalgesia induced by peripheral administration of inflammatory mediators to the same extent as does TRPV4 antisense ODN (Alessandri-Haber et al., 2006). That α2β1 integrin and TRPV4 may act together to transduce mechanical stimuli in sensory neurons is suggested by our observation that anti-α2 integrin antibody markedly reduces the TRPV4-mediated response to hypotonicity in cultured dorsal root ganglion neurons (Fig. 7 C). Finally, the idea that TRPV4 and α2β1 integrin directly interact with each other as members of a molecular complex is supported by our observation that TRPV4 coimmunoprecipitates with α2 integrin in cultured DRG neurons.
Although we hypothesized that the interaction between TRPV4 and integrins occurs within the sensory endings of primary afferent nerve fibers, it is conceivable that an interaction between TRPV4 and α2β1 integrin in skin keratinocytes (Chung et al., 2003, 2004; Parks, 2007) may, indirectly, lead to nociceptor sensitization. However, because antisense ODN for TRPV4 and α2 integrin were administered by spinal intrathecal injection, among all the cells in the skin only sensory neurons are exposed to the ODN. In addition, we demonstrate that treatment with TRPV4 or α2 integrin antisense ODN significantly decreases expression of TRPV4 or α2 integrin protein in saphenous nerve. Because the nerves were ligated to dam transport of proteins from the cell body to the terminals, these results suggest that TRPV4 and α2 integrin are present in sensory nerve fibers, presumably being transported to the terminals in the skin. Although we cannot exclude the possibility of an additional effect of the intrathecal injection of antisense on spinal neurons or glia, our results strongly suggest that interaction between TRPV4 and α2β1 integrin within sensory nerve endings is required.
Our results do not indicate the exact nature of the interaction that occurs between TRPV4 and α2β1 integrin. However, integrins can contribute to mechanical transduction by the modulation of ion channels via a Src tyrosine pathway (Aplin et al., 1998; Mobasheri et al., 2002; Waitkus-Edwards et al., 2002; Browe and Baumgarten, 2003), and α2β1 integrin has been shown to activate Src tyrosine kinase in other cell types (Schmidt et al., 1998; Sanders and Basson, 2004). We demonstrate here that α2β1, an integrin implicated in mechanical transduction in sensory neurons (Khalsa et al., 2000, 2004), and Src tyrosine kinase are important for the development of mechanical hyperalgesia. Importantly, we also demonstrate that activation of Src tyrosine kinase, by YEEIP, induces a TRPV4-dependent mechanical hyperalgesia and that at least one member of the Src tyrosine kinase family, Lyn, coimmunoprecipitates with TRPV4 in DRG neurons. These findings support the idea that a direct interaction between Src tyrosine kinase and TRPV4 is responsible for the integrin-dependent activation of TRPV4 contribution to the mechanism of mechanical hyperalgesia. Tyrosine kinases are known to regulate trafficking of ion channels and receptors; recent reports demonstrate that Src tyrosine kinases participate in the modulation of TRP channel function (Xu et al., 2003; Odell et al., 2005; Yao et al., 2005; Zhang et al., 2005; Sternfeld et al., 2007) and can induce membrane insertion and activation of TRPC4, a canonical TRP channel (Odell et al., 2005).
One mechanism by which TRPV4 might come into play in hyperalgesic states would be by upregulation of its expression. Injury of the sciatic nerve can alter the expression of integrins in DRG neurons, including α2β1 (Wallquist et al., 2002, 2004). However, neither the present study nor previous investigations in rat models of diabetic neuropathy and traumatic nerve injury (Facer et al., 2007; Frederick et al., 2007) have detected a significant change in the expression of TRPV4. Alternatively, alterations of the extracellular matrix embedding peripheral primary afferent terminals do occur during neuropathy (Sango et al., 1995; Bradley et al., 2000; Dubovy et al., 2006; Yamanaka et al., 2007). Based on our results, we suggest that in the setting of inflammation or nerve injury, mechanical stimulation and swelling may activate a signaling cascade initiated by integrins that, via Src tyrosine kinase, induces membrane insertion and/or activation of the TRPV4 channel in sensory neurons. This may provide an explanation for why TRPV4 makes no apparent contribution to mechanical nociceptive thresholds although it plays a major role in mechanical hyperalgesia associated with both inflammation and painful peripheral neuropathies of diverse etiologies. Of note, integrins have been shown to also activate Src-dependent signaling cascades in response to both mechanical stretch and cell swelling in cardiac myocytes (Sadoshima and Izumo, 1997a,b; Browe and Baumgarten, 2003).
In conclusion, we propose that, in primary afferent nociceptors, TRPV4 contributes to mechanotransduction, as a component of a molecular complex including α2β1 integrin and at least one member of the Src tyrosine kinase family, whose function is restricted to the setting of inflammation or nerve injury.
Footnotes
This work was supported by National Institutes of Health Grant NS053880. We thank Dr. Robert W. Gear for his assistance with statistical analyses and Drs. Wolfgang Liedkte and Jeffrey Friedman for kindly providing the TRPV4 knock-out mice.
References
- Alenghat FJ, Ingber DE. Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Sci STKE. 2002;2002:PE6. doi: 10.1126/stke.2002.119.pe6. [DOI] [PubMed] [Google Scholar]
- Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. doi: 10.1016/s0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- Alessandri-Haber N, Dina OA, Yeh JJ, Parada CA, Reichling DB, Levine JD. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J Neurosci. 2004;24:4444–4452. doi: 10.1523/JNEUROSCI.0242-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandri-Haber N, Joseph E, Dina OA, Liedtke W, Levine JD. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain. 2005;118:70–79. doi: 10.1016/j.pain.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Alessandri-Haber N, Dina OA, Joseph EK, Reichling D, Levine JD. A transient receptor potential vanilloid 4-dependent mechanism of hyperalgesia is engaged by concerted action of inflammatory mediators. J Neurosci. 2006;26:3864–3874. doi: 10.1523/JNEUROSCI.5385-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley KO, Levine JD. Rapid onset pain induced by intravenous streptozotocin in the rat. J Pain. 2001;2:146–150. doi: 10.1054/jpai.2001.21592. [DOI] [PubMed] [Google Scholar]
- Aley KO, Levine JD. Different peripheral mechanisms mediate enhanced nociception in metabolic/toxic and traumatic painful peripheral neuropathies in the rat. Neuroscience. 2002;111:389–397. doi: 10.1016/s0306-4522(02)00009-x. [DOI] [PubMed] [Google Scholar]
- Aley KO, Reichling DB, Levine JD. Vincristine hyperalgesia in the rat: a model of painful vincristine neuropathy in humans. Neuroscience. 1996;73:259–265. doi: 10.1016/0306-4522(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Aley KO, Martin A, McMahon T, Mok J, Levine JD, Messing RO. Nociceptor sensitization by extracellular signal-regulated kinases. J Neurosci. 2001;21:6933–6939. doi: 10.1523/JNEUROSCI.21-17-06933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew SM, Edwards BD, Chalmers RJ, O'Driscoll JB. A quantitative immunohistochemical study of the expression of integrins by nerves in psoriatic and normal skin. Br J Dermatol. 1992;127:359–364. doi: 10.1111/j.1365-2133.1992.tb00454.x. [DOI] [PubMed] [Google Scholar]
- Aplin AE, Howe A, Alahari SK, Juliano RL. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev. 1998;50:197–263. [PubMed] [Google Scholar]
- Bradley JL, King RH, Muddle JR, Thomas PK. The extracellular matrix of peripheral nerve in diabetic polyneuropathy. Acta Neuropathol (Berl) 2000;99:539–546. doi: 10.1007/s004010051158. [DOI] [PubMed] [Google Scholar]
- Browe DM, Baumgarten CM. Stretch of beta 1 integrin activates an outwardly rectifying chloride current via FAK and Src in rabbit ventricular myocytes. J Gen Physiol. 2003;122:689–702. doi: 10.1085/jgp.200308899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MK, Lee H, Caterina MJ. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J Biol Chem. 2003;278:32037–32046. doi: 10.1074/jbc.M303251200. [DOI] [PubMed] [Google Scholar]
- Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J Biol Chem. 2004;279:21569–21575. doi: 10.1074/jbc.M401872200. [DOI] [PubMed] [Google Scholar]
- Delany NS, Hurle M, Facer P, Alnadaf T, Plumpton C, Kinghorn I, See CG, Costigan M, Anand P, Woolf CJ, Crowther D, Sanseau P, Tate SN. Identification and characterization of a novel human vanilloid receptor-like protein, VRL-2. Physiol Genomics. 2001;4:165–174. doi: 10.1152/physiolgenomics.2001.4.3.165. [DOI] [PubMed] [Google Scholar]
- Dina OA, Barletta J, Chen X, Mutero A, Martin A, Messing RO, Levine JD. Key role for the epsilon isoform of protein kinase C in painful alcoholic neuropathy in the rat. J Neurosci. 2000;20:8614–8619. doi: 10.1523/JNEUROSCI.20-22-08614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Chen X, Reichling D, Levine JD. Role of protein kinase Cepsilon and protein kinase A in a model of paclitaxel-induced painful peripheral neuropathy in the rat. Neuroscience. 2001;108:507–515. doi: 10.1016/s0306-4522(01)00425-0. [DOI] [PubMed] [Google Scholar]
- Dina OA, Parada CA, Yeh J, Chen X, McCarter GC, Levine JD. Integrin signaling in inflammatory and neuropathic pain in the rat. Eur J Neurosci. 2004;19:634–642. doi: 10.1111/j.1460-9568.2004.03169.x. [DOI] [PubMed] [Google Scholar]
- Dina OA, Messing RO, Levine JD. Ethanol withdrawal induces hyperalgesia mediated by PKCepsilon. Eur J Neurosci. 2006;24:197–204. doi: 10.1111/j.1460-9568.2006.04886.x. [DOI] [PubMed] [Google Scholar]
- Dubovy P, Jancalek R, Klusakova I. A heterogeneous immunofluorescence staining for laminin-1 and related basal lamina molecules in the dorsal root ganglia following constriction nerve injury. Histochem Cell Biol. 2006;125:671–680. doi: 10.1007/s00418-005-0115-8. [DOI] [PubMed] [Google Scholar]
- Facer P, Casula MA, Smith GD, Benham CD, Chessell IP, Bountra C, Sinisi M, Birch R, Anand P. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 2007;7:11. doi: 10.1186/1471-2377-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick J, Buck ME, Matson DJ, Cortright DN. Increased TRPA1, TRPM8, and TRPV2 expression in dorsal root ganglia by nerve injury. Biochem Biophys Res Commun. 2007;358:1058–1064. doi: 10.1016/j.bbrc.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Gui P, Wu X, Ling S, Stotz SC, Winkfein RJ, Wilson E, Davis GE, Braun AP, Zamponi GW, Davis MJ. Integrin receptor activation triggers converging regulation of Cav1.2 calcium channels by c-Src and protein kinase A pathways. J Biol Chem. 2006;281:14015–14025. doi: 10.1074/jbc.M600433200. [DOI] [PubMed] [Google Scholar]
- Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- Houck CS, Khodorova A, Reale AM, Strichartz GR, Davar G. Sensory fibers resistant to the actions of tetrodotoxin mediate nocifensive responses to local administration of endothelin-1 in rats. Pain. 2004;110:719–726. doi: 10.1016/j.pain.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Mechanosensation through integrins: cells act locally but think globally. Proc Natl Acad Sci USA. 2003a;100:1472–1474. doi: 10.1073/pnas.0530201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci. 2003b;116:1397–1408. doi: 10.1242/jcs.00360. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Chen X, Khasar SG, Levine JD. Novel mechanism of enhanced nociception in a model of AIDS therapy-induced painful peripheral neuropathy in the rat. Pain. 2004;107:147–158. doi: 10.1016/j.pain.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- Khalsa PS, Zhang C, Sommerfeldt D, Hadjiargyrou M. Expression of integrin alpha2beta1 in axons and receptive endings of neurons in rat, hairy skin. Neurosci Lett. 2000;293:13–16. doi: 10.1016/s0304-3940(00)01482-8. [DOI] [PubMed] [Google Scholar]
- Khalsa PS, Ge W, Uddin MZ, Hadjiargyrou M. Integrin alpha2beta1 affects mechano-transduction in slowly and rapidly adapting cutaneous mechanoreceptors in rat hairy skin. Neuroscience. 2004;129:447–459. doi: 10.1016/j.neuroscience.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol Clin Exp Res. 1982;6:523–531. doi: 10.1111/j.1530-0277.1982.tb05017.x. [DOI] [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol. 1989;24:197–211. [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM, Sorrell MF. Experimental methods of ethanol administration. Hepatology. 1989;10:501–510. doi: 10.1002/hep.1840100417. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci USA. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans . Proc Natl Acad Sci USA. 2003;100(Suppl 2):14531–14536. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445:858–865. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- McPhee JC, Dang YL, Davidson N, Lester HA. Evidence for a functional interaction between integrins and G protein-activated inward rectifier K+ channels. J Biol Chem. 1998;273:34696–34702. doi: 10.1074/jbc.273.52.34696. [DOI] [PubMed] [Google Scholar]
- Mobasheri A, Carter SD, Martin-Vasallo P, Shakibaei M. Integrins and stretch activated ion channels; putative components of functional cell surface mechanoreceptors in articular chondrocytes. Cell Biol Int. 2002;26:1–18. doi: 10.1006/cbir.2001.0826. [DOI] [PubMed] [Google Scholar]
- Nebe B, Rychly J, Knopp A, Bohn W. Mechanical induction of beta 1-integrin-mediated calcium signaling in a hepatocyte cell line. Exp Cell Res. 1995;218:479–484. doi: 10.1006/excr.1995.1181. [DOI] [PubMed] [Google Scholar]
- Odell AF, Scott JL, Van Helden DF. Epidermal growth factor induces tyrosine phosphorylation, membrane insertion, and activation of transient receptor potential channel 4. J Biol Chem. 2005;280:37974–37987. doi: 10.1074/jbc.M503646200. [DOI] [PubMed] [Google Scholar]
- Oliver JM, Burg DL, Wilson BS, McLaughlin JL, Geahlen RL. Inhibition of mast cell Fc epsilon R1-mediated signaling and effector function by the Syk-selective inhibitor, piceatannol. J Biol Chem. 1994;269:29697–29703. [PubMed] [Google Scholar]
- Parks WC. What is the alpha2beta1 integrin doing in the epidermis? J Invest Dermatol. 2007;127:264–266. doi: 10.1038/sj.jid.5700573. [DOI] [PubMed] [Google Scholar]
- Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol. 1997a;59:551–571. doi: 10.1146/annurev.physiol.59.1.551. [DOI] [PubMed] [Google Scholar]
- Sadoshima J, Izumo S. Tyrosine kinases mediation of c-fos expression by cell swelling in cardiac myocytes. Heart Vessels Suppl. 1997b;12:194–197. [PubMed] [Google Scholar]
- Sanders MA, Basson MD. Collagen IV regulates Caco-2 migration and ERK activation via alpha1beta1- and alpha2beta1-integrin-dependent Src kinase activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G547–G557. doi: 10.1152/ajpgi.00262.2003. [DOI] [PubMed] [Google Scholar]
- Sango K, Horie H, Okamura A, Inoue S, Takenaka T. Diabetes impairs DRG neuronal attachment to extracellular matrix proteins in vitro. Brain Res Bull. 1995;37:533–537. doi: 10.1016/0361-9230(95)00057-l. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Pommerenke H, Durr F, Nebe B, Rychly J. Mechanical stressing of integrin receptors induces enhanced tyrosine phosphorylation of cytoskeletally anchored proteins. J Biol Chem. 1998;273:5081–5085. doi: 10.1074/jbc.273.9.5081. [DOI] [PubMed] [Google Scholar]
- Shakibaei M, Mobasheri A. Beta1-integrins co-localize with Na, K-ATPase, epithelial sodium channels (ENaC) and voltage activated calcium channels (VACC) in mechanoreceptor complexes of mouse limb-bud chondrocytes. Histol Histopathol. 2003;18:343–351. doi: 10.14670/HH-18.343. [DOI] [PubMed] [Google Scholar]
- Shi C, Barnes S, Coca-Prados M, Kelly ME. Protein tyrosine kinase and protein phosphatase signaling pathways regulate volume-sensitive chloride currents in a nonpigmented ciliary epithelial cell line. Invest Ophthalmol Vis Sci. 2002;43:1525–1532. [PubMed] [Google Scholar]
- Shyy JY, Chien S. Role of integrins in cellular responses to mechanical stress and adhesion. Curr Opin Cell Biol. 1997;9:707–713. doi: 10.1016/s0955-0674(97)80125-1. [DOI] [PubMed] [Google Scholar]
- Sternfeld L, Anderie I, Schmid A, Al-Shaldi H, Krause E, Magg T, Schreiner D, Hofer HW, Schulz I. Identification of tyrosines in the putative regulatory site of the Ca2+ channel TRPV6. Cell Calcium. 2007;42:91–102. doi: 10.1016/j.ceca.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- Tomaselli KJ, Doherty P, Emmett CJ, Damsky CH, Walsh FS, Reichardt LF. Expression of beta 1 integrins in sensory neurons of the dorsal root ganglion and their functions in neurite outgrowth on two laminin isoforms. J Neurosci. 1993;13:4880–4888. doi: 10.1523/JNEUROSCI.13-11-04880.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Manolopoulos V, Eggermont J, Ellory C, Droogmans G, Nilius B. Regulation of a swelling-activated chloride current in bovine endothelium by protein tyrosine phosphorylation and G proteins. J Physiol (Lond) 1998;506:341–352. doi: 10.1111/j.1469-7793.1998.341bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Dahl S, Schliess F, Reissmann R, Gorg B, Weiergraber O, Kocalkova M, Dombrowski F, Haussinger D. Involvement of integrins in osmosensing and signaling toward autophagic proteolysis in rat liver. J Biol Chem. 2003;278:27088–27095. doi: 10.1074/jbc.M210699200. [DOI] [PubMed] [Google Scholar]
- Waitkus-Edwards KR, Martinez-Lemus LA, Wu X, Trzeciakowski JP, Davis MJ, Davis GE, Meininger GA. alpha(4)beta(1) Integrin activation of L-type calcium channels in vascular smooth muscle causes arteriole vasoconstriction. Circ Res. 2002;90:473–480. doi: 10.1161/hh0402.105899. [DOI] [PubMed] [Google Scholar]
- Wallquist W, Patarroyo M, Thams S, Carlstedt T, Stark B, Cullheim S, Hammarberg H. Laminin chains in rat and human peripheral nerve: distribution and regulation during development and after axonal injury. J Comp Neurol. 2002;454:284–293. doi: 10.1002/cne.10434. [DOI] [PubMed] [Google Scholar]
- Wallquist W, Zelano J, Plantman S, Kaufman SJ, Cullheim S, Hammarberg H. Dorsal root ganglion neurons up-regulate the expression of laminin-associated integrins after peripheral but not central axotomy. J Comp Neurol. 2004;480:162–169. doi: 10.1002/cne.20345. [DOI] [PubMed] [Google Scholar]
- Wang N, Ingber DE. Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophys J. 1994;66:2181–2189. doi: 10.1016/S0006-3495(94)81014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Wang N, Naruse K, Stamenovic D, Fredberg JJ, Mijailovich SM, Tolic-Norrelykke IM, Polte T, Mannix R, Ingber DE. Mechanical behavior in living cells consistent with the tensegrity model. Proc Natl Acad Sci USA. 2001;98:7765–7770. doi: 10.1073/pnas.141199598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, Chien S. Visualizing the mechanical activation of Src. Nature. 2005;434:1040–1045. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- Wang YG, Samarel AM, Lipsius SL. Laminin acts via beta 1 integrin signalling to alter cholinergic regulation of L-type Ca(2+) current in cat atrial myocytes. J Physiol 526 Pt. 2000;1:57–68. doi: 10.1111/j.1469-7793.2000.t01-1-00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildering WC, Hermann PM, Bulloch AG. Rapid neuromodulatory actions of integrin ligands. J Neurosci. 2002;22:2419–2426. doi: 10.1523/JNEUROSCI.22-07-02419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Davis GE, Meininger GA, Wilson E, Davis MJ. Regulation of the L-type calcium channel by alpha 5beta 1 integrin requires signaling between focal adhesion proteins. J Biol Chem. 2001;276:30285–30292. doi: 10.1074/jbc.M102436200. [DOI] [PubMed] [Google Scholar]
- Xu H, Zhao H, Tian W, Yoshida K, Roullet JB, Cohen DM. Regulation of a transient receptor potential (TRP) channel by tyrosine phosphorylation. SRC family kinase-dependent tyrosine phosphorylation of TRPV4 on TYR-253 mediates its response to hypotonic stress. J Biol Chem. 2003;278:11520–11527. doi: 10.1074/jbc.M211061200. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Obata K, Kobayashi K, Dai Y, Fukuoka T, Noguchi K. Alteration of the cell adhesion molecule L1 expression in a specific subset of primary afferent neurons contributes to neuropathic pain. Eur J Neurosci. 2007;25:1097–1111. doi: 10.1111/j.1460-9568.2007.05344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Kwan HY, Huang Y. Regulation of TRP channels by phosphorylation. Neurosignals. 2005;14:273–280. doi: 10.1159/000093042. [DOI] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Bai ZT, Chai ZF, Zhang JW, Liu Y, Ji YH. Suppressive effects of BmK IT2 on nociceptive behavior and c-Fos expression in spinal cord induced by formalin. J Neurosci Res. 2003;74:167–173. doi: 10.1002/jnr.10723. [DOI] [PubMed] [Google Scholar]
- Zheng JH, Chen J. Differential roles of spinal neurokinin 1/2 receptors in development of persistent spontaneous nociception and hyperalgesia induced by subcutaneous bee venom injection in the conscious rat. Neuropeptides. 2001;35:32–44. doi: 10.1054/npep.2000.0841. [DOI] [PubMed] [Google Scholar]