Abstract
Background
MYH9-related diseases (MYH9-RD) are autosomal dominant disorders caused by mutations of the MYH9 gene encoding the non-muscle myosin heavy chain IIA. They are characterized by congenital thrombocytopenia, giant platelets and leucocyte inclusions. Hearing impairment, pre-senile cataract and nephropathy can also occur. We aimed to evaluate renal involvement and outcome in MYH9-RD patients followed-up by nephrologists.
Methods
We conducted a retrospective multicentre observational study of 13 patients among 9 families with MYH9 mutation diagnosed by genetic testing and immunofluorescence assay referred to nephrologists.
Results
At initial referral, median age was 30 (range 14–76) years. Median estimated glomerular filtration rate was 66 mL/min/1.73 m2 (0–141) and two patients had already end-stage renal disease (ESRD). Renal presentation associated proteinuria (n = 12), haematuria (n = 6) and hypertension (n = 6). Three patients developed a rapid onset ESRD whereas five others had a relatively stable kidney function over a 3-year median follow-up (1–34). Extra-renal features varied widely, with hearing impairment in six patients, cataract in two and mild liver dysfunction in seven. Thrombocytopenia existed at referral in 11 patients. Time to diagnosis varied from 0 to 29 years (median 3 years). Initial diagnoses such as idiopathic thrombocytopenic purpura (n = 4) and focal segmental glomerulosclerosis (n = 1) led to corticosteroid administration (n = 4), intravenous immunoglobulins (n = 3), cyclophosphamide (n = 1) and splenectomy (n = 1).
Conclusions
Renal involvement and outcome in MYH9-RD are heterogeneous. The diagnosis is often delayed and misdiagnoses can lead to unnecessary treatments. MYH9-RD should be considered in any patient with glomerular involvement associated with a low or slightly decreased platelet count and/or hearing loss and liver dysfunction.
Keywords: deafness, FSGS, hereditary nephropathy, MYH9, thrombocytopenia
INTRODUCTION
MYH9-related diseases (MYH9-RD) are a spectrum of autosomal dominant (AD) disorders characterized by congenital thrombocytopenia, giant platelets and leucocyte inclusions named Döhle bodies. Additional extra-haematological manifestations can also occur, which are mainly hearing impairment, pre-senile cataract and nephropathy [1].
May et al. in 1909 and Hegglin et al. in 1945 described, respectively, the leucocytic inclusions and their co-existence with giant platelets [2, 3]. In 1972, Epstein et al. [4] reported two families with hereditary nephritis, deafness and macrothrombocytopenia. The description differed from Alport’s syndrome by the severity of the disease in affected females and by the existence of macrothrombocytopenia. However, the patients presented with similar initial manifestations such as albuminuria and microhaematuria, which were followed by progressive renal deterioration. Thereafter, in 1985, one family with four generations was reported with nephritis, deafness, macrothrombocytopenia and cataract, associated with leucocytic inclusions that were not previously described by Esptein et al. The Fechtner syndrome was, therefore, defined as a novel variant of hereditary nephritis [5]. Finally, the Sebastian syndrome was characterized in 1990 upon one family without any feature of Alport syndrome, but with hereditary macrothrombocytopenia and neutrophilic inclusions differing morphologically from those found in patients with the May–Hegglin disease [6].
All these disorders appeared to be caused by mutations in the MYH9 gene, which is present on chromosome 22q11–13 and encodes the non-muscle myosin heavy chain IIA (NMMHC-IIA) [7, 8]. The Class IIA non-muscle myosin is an ubiquitous hexameric motor protein consisting of two heavy chains and two pairs of light chains. It is implicated in spreading, cell motility, maintenance of cell morphological states and cytokinesis [7].
Several studies suggest a genotype–phenotype correlation, with some of them displaying more aggressive features of the disease [9–13]. These reports also reflect the highly inconstant existence and severity of kidney involvement [1]. However, precise data concerning renal involvement in MYH9-RD are scarce with few renal series especially in Europe, and poor follow-up and data regarding the evolution of the disease.
This study focuses on the disease’s evolution, renal and extra-renal presentation with documented genetic data of 13 patients among 9 families with a MYH9-RD and followed-up by nephrologists. The aim of the study was to demonstrate that renal involvement is greatly heterogeneous even between pedigrees and patients harbouring the same mutation.
MATERIALS AND METHODS
The study was approved by an independent protection committee of the North-West of France and conducted according to the declaration of Helsinki.
Patient selection
We conducted a retrospective multicentre observational study and identified 13 patients among 9 families with MYH9 mutation referred to nephrologists, before or after the diagnosis was confirmed by genetic testing (Table 1).
Table 1.
Renal characteristics at referral to the nephrologist and at last follow-up
| Case | Age (years) | Proteinuria (g/day) | SCr (mg/dL) | eGFR (mL/min/1.73 m2) | Annual eGFR slope (mL/min/1.73 m2) | Hypertension | Haematuria |
|---|---|---|---|---|---|---|---|
| 1 | 26–54 | 0.95–0.5 | 1.03–1.35 | 69–43 | −0.9 | − + | −− |
| 2 | 76–78 | 3.1–0.5 | 1.11–5.7 | 51–8 | −21.5 | + + | + + |
| 3 | 18–52 | >3, anuria | 1.3–HD | 76–0 | −12.7 | − + | − Anuria |
| 4 | 17–20 | 0.22–0.32 | 1.1–0.9 | 94–114 | +6.7 | −− | −− |
| 5 | 30–33 | 1.16–0.2 | 0.7–0.7 | 141–138 | −1 | − + | −− |
| 6 | 32–50 | >3, anuria | 1–HD, deceased | 90–0 | −5 | − + | − Anuria |
| 7 | 44–47 | 1.79–3 | 1.9–4, deceased | 41–19 | −7.3 | + + | + + |
| 8 | 14–15 | 6.24–0.09 | 14–transplanted | 4–0 | + + | + − | |
| 9 | 21–23 | 3–1 | 1.1–1.06 | 90–92 | +1 | − − | −− |
| 10 | 64–69 | 0–0 | 1.2–1.3 | 44–41 | −1 | + + | + + |
| 11 | 44–47 | 1.5–0 | 1.5–transplanted | 38–0 | −12.7 | + + | −− |
| 12 | 23–59 | 2.68–0 | 1.1–transplanted | 66–0 | −1.8 | + − | + − |
| 13 | 35–39 | >3 (anuria)–0 | 5.6–transplanted | 0 (anuria)–0 | − | + − |
The first number in each column corresponds to what was found at the initial presentation, the second number to that of the last evaluation.
eGFR, estimated glomerular filtration rate; HD, haemodialysis.
Data collection
A standardized data collection protocol was subsequently formulated recording age, sex, mode of presentation, clinical characteristics, follow-up and genotype. The characteristic features of the disease were collected. Leucocytic inclusions and giant platelets were identified by May–Grünwald staining on a blood smear. Thrombocytopenia was defined as a platelet count <150 × 109/l. The glomerular filtration rate (GFR) was estimated by the Modification of Diet in Renal Disease equation. The renal biopsy was undergone according to standard technics. We specifically noted whether patients had developed end-stage renal disease (ESRD) and evaluated the progression of kidney disease. Hearing impairment was evaluated by audiogram and eye involvement by ophthalmologist assessment.
MYH9 disease diagnosis
Abnormal NMMHC-IIA inclusions in the cytoplasm of neutrophils were assessed by immunofluorescent staining [14] in the Department for platelet disorders (provided by N.S.) as well as the mutational screening on the MYH9 gene.
Statistical analysis
Data were described as counts and percent for categorical variables, and as mean and SD for continuous variables.
Results
Family A
Patient 1
This patient was referred to a nephrologist at the age of 26 years with a 0.95 g/24 h proteinuria, whereas she was considered to have orthostatic proteinuria since the age of 14 years. Blood pressure was normal, urinary sediment was bland; estimated GFR (eGFR) was 69 mL/min/1.73 m2 with mild thrombocytopenia (102 000/mm3). She refused kidney biopsy. Since bone marrow aspiration was normal, a diagnosis of idiopathic thrombocytopenic purpura (ITP) associated with glomerulonephritis was made. Steroids were initiated without conclusive results. Renal function declined progressively, and nephrotic range proteinuria at the age of 45 years led to renin–angiotensin system (RAS) inhibitors initiation. After a follow-up of 28 years, eGFR was 43 mL/min/1.73 m2, proteinuria level was 0.95 g/24 h and blood pressure was normal on treatment.
Patient 2
Patient 2 is the proband’s mother. Chronic kidney disease (CKD) was diagnosed at 76 years old with eGFR and proteinuria at 51 mL/min/1.73 m2 and 3.1 g/24 h, respectively, microscopic haematuria and hypertension. Platelet count was low at 62 000/mm3. Two years later, she underwent a bone marrow aspirate because of pancytopenia, revealing a type 2 myelodysplastic syndrome. Shortly after the diagnosis of myelodysplasia, a neutropenic sepsis accelerated the decline of renal function towards ESRD.
Patient 3
Patient 3 is the proband’s brother. At the age of 18 years, he was hospitalized for a nephrotic syndrome with mild renal impairment (serum creatinine (SCr) level of 1.3 mg/dL). Kidney biopsy findings were consistent with focal segmental glomerulosclerosis (FSGS). The immunofluorescence evaluation was not available. Chronic vascular lesions were also reported with massive intimal fibrosis of arteries and arteriolar hyalinosis. The nephrotic syndrome proved to be resistant to steroids and then to a combination of steroids and cyclophosphamide (CPA). ESRD occurred 6 years later leading to haemodialysis at 24 and transplantation at 26 years of age. Of note, thrombocytopenia was intermittent with a normal platelet count (160 000/mm3) at initial presentation.
Patient 4
Patient 4 is the proband’s son. During a pre-operative check-up at 17 years old, peripheral thrombocytopenia at 50 000/mm3 was evidenced, without prior bleeding event or known thrombocytopenia at birth. A diagnosis of ITP led to steroids and intravenous immunoglobulins (IVIg) administration, which proved to be ineffective. At that time, kidney involvement was noticed, with proteinuria ranging from 0.2 to 0.5 g/24 h, SCr at 1.1 mg/dL and blood pressure was 144/82 mmHg. At the latest evaluation 3 years later proteinuria level was 0.32 g/24h, SCr 0.9 mg/dL and blood pressure remained normal without medication.
MYH9-RD diagnosis
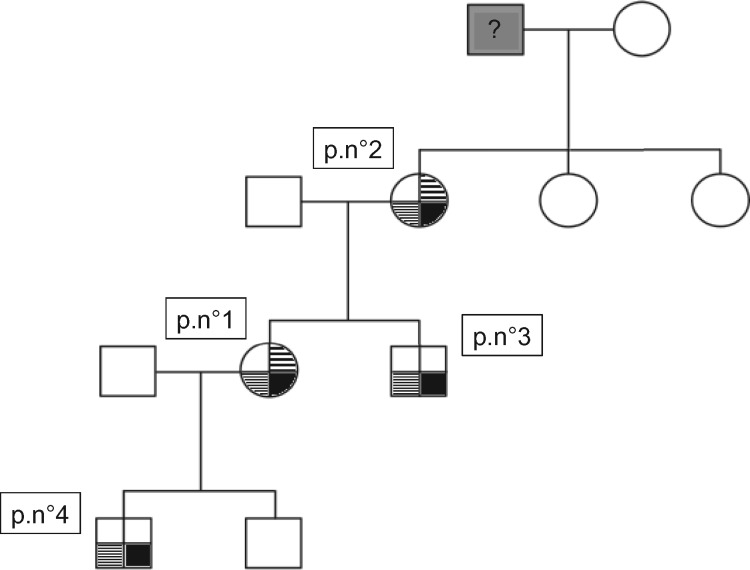
In 2009, when Patient 4 was diagnosed with thrombocytopenia and mild proteinuria, a hereditary syndrome was suspected. The pedigree chart (Figure 1) revealed an AD transmission and that the proband’s grandfather had died of ‘uraemia’ at the age of 82 years. The association of glomerular nephropathy, thrombocytopenia and the family history led to suspicion of an MYH9-RD. Blood smears was performed in all members and revealed the presence of 3–5% of giant platelets but no Döhle bodies; neutrophilic inclusions were disclosed by immunofluorescence assay. Audiogram and ophthalmological tests were performed in all the members except Patient 3 and evidenced neurosensory hearing loss for the proband and her mother (Patients 1 and 2), but no cataract. Mutational screening of all the members of the family revealed a c.2152C>T mutation on exon 16 predicting a p.R718W substitution on the mature protein.
FIGURE 1.
Pedigree chart of the Family A. Proband’s (Patient 1) grandfather died at the age of 82 years of so-called ‘uraemia’. The bottom right part of each box (in plain black) corresponds to renal involvement; the bottom left part (in fine stripes) to thrombocytopenia and the upper right part (in large stripes) to hearing disability.
Characteristics of the rest of the cohort: one family, nine patients
At initial referral to the nephrologist
Median age was 32 years (range 14–64). Proteinuria was present in all patients but one (Patient 10), and Patient 6 was nephrotic at presentation. Haematuria and hypertension were both present in five patients out of nine. eGFR was <60 mL/min/1.73 m2 in five patients (Table 1). Patients 8 and 13 were referred to nephrologists without known urine abnormalities or CKD prior to admission. At initial evaluation, renal function was normal in Patient 8, and ESRD occurred 6 years later at the age of 15 years, without kidney function monitoring during this period. Acute renal failure secondary to cortical necrosis following post-partum haemorrhage did not recover for Patient 13. Eight out of nine patients had thrombocytopenia at presentation ranging from 4000 to 80 000/mm3, whereas patient 13 had a normal platelet count of 350 000/mm3 (Table 2). Patients 11 and 13 had serious haemorrhagic events related to a post-partum haemorrhage and a ruptured spleen following a serious road accident, respectively. None of the other patients displayed even mild haemorrhagic or thrombotic events. Patients 11 and 12 had a history of cataract. Patient 11 was diagnosed with a Fuchs syndrome in childhood, and Patient 12 received surgery at the age of 47 years. Hearing loss was already diagnosed in three patients, and Patient 10 had a cochlear implant (Table 3). Interestingly, seven patients had persistent but slight liver function abnormalities.
Table 2.
Haematological characteristics
| Case | Family | Pl count | Intermittent normal Pl count | Bleeding event | Leucocytes inclusions | Giant platelets |
|---|---|---|---|---|---|---|
| 1 | A probant | 77 000 | − | − | + | + |
| 2 | A mother | 103 000 | − | − | − | + |
| 3 | A brother | 160 000 | + | − | + | + |
| 4 | A son | 76 000 | − | − | + | + |
| 5 | B probant | 40 000 | − | − | + | + |
| 6 | B father | 80 000 | + | − | ? | ? |
| 7 | − | 9000 | − | − | + | + |
| 8 | − | 53 000 | − | − | + | + |
| 9 | − | 4000 | − | − | + | + |
| 10 | − | 19 000 | − | − | + | + |
| 11 | − | 60 000 | + | + | + | + |
| 12 | − | 80 000 | − | − | + | + |
| 13 | − | 350 000 | + | + | + | + |
The platelet count corresponds to that of the first referral to the nephrologist. Pl, platelet. Patient 11’s bleeding event: road accident. Patient 13’s bleeding event: post-partum haemorrhage. Pl Count per mm3.
Table 3.
General characteristics
| Case | Family | Gender | Mutation | Inheritance | Hearing impairment | Cataract | Liver enzymes increase |
|---|---|---|---|---|---|---|---|
| 1 | A probant | F | p.R718W | AD | + | − | − |
| 2 | A mother | F | p.R718W | AD | + | − | + |
| 3 | A brother | M | p.R718W | AD | ? | ? | + |
| 4 | A son | M | p.R718W | AD | − | − | + |
| 5 | B probant | M | p.R1165C | AD | − | − | − |
| 6 | B father | M | p.R1165C | AD | ? | ? | + |
| 7 | − | M | p.K74del | AD | + | − | + |
| 8 | − | F | p.D1424N | AD | − | − | − |
| 9 | − | M | p.S96L | Sporadic | + | − | + |
| 10 | − | F | Missing data | AD | + | − | − |
| 11 | − | F | p.E894K | Sporadic | − | + | − |
| 12 | − | F | p.Q1070_A1087dup | AD | + | + | + |
| 13 | − | F | p.C2113T | Sporadic | − | − | − |
Predicted change in the mature protein based on genetic assessment of the mutations.
M, male; F, female; AD, autosomal dominant transmission.
Patients 3 and 6 did not undergo audiometry and eye examinations but neither of them had patent clinical symptoms consistent with hear or eye involvements. Ear and eye evaluations were carried out systematically.
At the time of genetic testing
Genetic tests were performed following the occurrence of either new cases in relatives (Patients 5, 6 and 10) or of new symptoms (Patient 11 was initially followed for thrombocytopenia, and then presented with proteinuria and hypertension), or blood smears analysis revealing giant platelets and leucocyte inclusions (Cases 7, 8, 9, 12 and 13). Three patients (Cases 9, 11 and 13) were likely to have a de novo mutation since their relatives (parents and siblings) were tested for the mutation and were found negative. Mutations and mode of inheritance are listed in Table 3.
Five out of nine patients were referred to the nephrologist without the diagnosis of MYH9-RD. Initial diagnosis, time to diagnosis and treatment are summarized in Table 4. Time to MYH9-RD diagnosis was highly variable ranging from 0 to 19 years. The most common initial misdiagnoses were ITP (Patients 8 and 9) and other hereditary nephropathies, including Alport syndrome (Patients 5 and 12).
Table 4.
Initial diagnosis, treatment and time to diagnosis
| Case | Initial diagnosis | Treatment | Time to diagnosis (years) |
|---|---|---|---|
| 1 | ITP and glomerulonephritis | CS | 23 |
| 2 | Hereditary nephropathy | 0 | 0 |
| 3 | FSGS | CS, CPA | 29 |
| 4 | ITP | CS, IVIg | 1 |
| 5 | Hereditary nephropathy | 0 | 2 |
| 6 | Glomerulonephritis | 0 | 19 |
| 7 | May–Hegglin anomaly | 0 | 0 |
| 8 | ITP | IVIg | 5 |
| 9 | ITP | CS, IVIg, splenectomy | 10 |
| 10 | May–Hegglin anomaly | 0 | 0 |
| 11 | Thrombocytopenia | 0 | 10 |
| 12 | Alport syndrome | 0 | 3 |
| 13 | Cortical necrosis | Antifibrinolytics | 1 |
CPA, cyclophosphamide; CS, corticosteroids; FSGS, focal segmental glomerulosclerosis; ITP, idiopathicthrombocytopenic purpura; IVIg, intravenous immunoglobulins.
Seven mutations were identified, two located in the head domain (Patient 7: p.K74del and Patient 9: p.S96L) and five affecting the tail domain (Family B: p.R1165C, Patient 8: p.D1424N, Patient 11: p.E894K, Patient 12: p.Q1070_A1087dup and Patient 13: p.C2113T).
At last evaluation
Renal characteristics are listed in Table 1. Median follow-up by nephrologists was 3 years (range 1–34). Median annual eGFR change was −1.8 mL/min/1.73 m2 ranging from ±6.7 to −21.5 mL/min/1.73 m2. At the last evaluation of the four patients without ESRD, three of them had hypertension and were treated with RAS inhibitors, with variable proteinuria, ranging from 0 to 3 g/day. eGFR ranged from 19 to 138 mL/min/1.73 m2.
Five out of nine patients developed ESRD. Patients 8 and 13 were already in ESRD at the first evaluation by nephrologists. Patients 6, 11 and 12 developed ESRD, respectively, in 18, 3 and 36 years.
Patients 8, 11, 12 and 13 were transplanted at ages 15–46 years, with no haemorrhagic peri-operative complications. Patients 5 and 7 died at age 50 years from arrhythmia due to hyperkalaemia, and at 48 years old from a massive myocardial infarction, respectively.
DISCUSSION
This series focuses on the characteristics of 13 individuals belonging to 9 families with genetically proven MYH9-RD. When first evaluated by a nephrologist, a majority of patients (10/13) presented with the association of thrombocytopenia and proteinuria, from mild proteinuria to nephrotic syndrome. Extra-renal features varied widely, with hearing impairment in six patients, cataract in two and mild liver dysfunction in seven. eGFR <60 mL/min/1.73 m2, hypertension and haematuria were each present in six patients. Renal outcome was also highly unpredictable among patients and among families, with three patients developing a rapid onset ESRD whereas others, especially Patient 1, had a relatively stable kidney function over a long follow-up.
The prevalence of MYH9-RD is probably underestimated because of frequent misdiagnosis and underreporting [1]. In particular, the knowledge of MYH9-RD among the nephrologist community is poor and mild thrombocytopenia could go unnoticed. Moreover, eye involvement could incorrectly be labelled as pre-senile cataract. These reasons could explain the frequent delay in diagnosis in our series. These misdiagnoses can lead to unnecessary treatments including aggressive therapies. In our study, ITP was an initial misdiagnosis in four patients, preceding from 1 to 23 years before MYH9 diagnosis, and leading to the administration of corticosteroids (CS), IVIgs and even a splenectomy in one patient, as in other published series [12, 13, 15]. One of our patients was diagnosed as FSGS, consistently with literature [16] (Table 5), leading to potentially deleterious and inefficient treatments including CS and CPA.
Table 5.
Pathological findings in MYH9 syndrome
| References | Age (years) | Sex | MYH9 mutation | SCr (mg/dL) | Proteinuria | Light microscopy | Electron microscopy |
|---|---|---|---|---|---|---|---|
| Epstein et al. [4] | 13 | F | R702C | 0.6 | 0.2 g/day | Segmental and global glomerulosclerosis, mesangial proliferation | Not performed |
| Clare et al. [17] | 15 | M | ND | 1.2 | 14 g/day | Endocapillary proliferation, mesangial expansion, adhesions to Bowman's capsule, and moderate interstitial fibrosis | GBM thickening, lamellation |
| Peterson et al. [5] | 23 | M | ND | ESRD | + | Preserved glomeruli with increased mesangial cell number and matrix | GBM thickening, focal areas of attenuation; focal podocyte foot process effacement |
| Túri et al. [18] | 14 | M | ND | 0.8 | 1.6 g/day | Segmental glomerulosclerosis and diffuse mesangial proliferation | Focal GBM thickening and splitting, foot process effacement |
| Iyori et al. [19] | 14 | F | ND | NS | + | Mild tubular atrophy | Mesangial interposition, GBM splitting |
| Moxey-Mims et al. [20] | 7 | M | ND | 0.6 | 1.6 g/day | Mild mesangial expansion | Mesangial cell proliferation and matrix expansion, GBM with variable thickening and basket-weave splitting |
| Naito et al. [21] | 16 | F | ND | NS | + | Normal | GBM thickening and reticulation of the lamina densa |
| Naito et al. [21] | 12 | F | ND | Normal | 2 g/day | Mesangial proliferation | Partial splitting of GBM lamina densa |
| Naito et al. [21] | 15 | M | ND | NS | 4+ | Segmental glomerulosclerosis | GBM thinning |
| Ghiggeri et al. [22] | 49 | M | D1424H | 5 | + | Glomerulosclerosis | Non-specific |
| Ghiggeri et al. [22] | 24 | F | D1424H | NS | + | Normal | Focal segmental foot process effacement |
| Alhindawi et al. [23] | 10 | M | ND | eGFR 65 mL/min/1.73 m2 | + | Segmental and global glomerulosclerosis | Not performed |
| Yap et al. [24] | 17 | M | R702H | 2.3 | 4.5 g/day | Global glomerulosclerosis | Not performed |
| Sekine et al. [12] | 9 | F | R702C | 0.4 | 1+ | Mild mesangial cell proliferation and expansion | Mesangial cell proliferation and matrix expansion, focal foot process effacement, focal GBM thickening |
| Han et al. [13] | 28 | M | D1424N | 0.8 | + | FSGS | Not performed |
| Han et al. [13] | 22 | M | S96L | 3 | + | FSGS | Focal GBM thickening |
| Han et al. [13] | 1.2 | M | S96L | 0.3 | + | Mild mesangial expansion | Focal GBM thickening |
| Hao et al. [25] | 58 | M | E1841K | 1.3 | 1.56 g/day | FSGS, IgM deposits | Not performed |
| Sun et al. [26] | 43 | M | E1945X | Abnormal | NS | FSGS, mesangial hyperplasia, matrix proliferation | Foot process fusion, microvillus hyperplasia |
| Min et al. [27] | 11 | F | C2104 | Normal | + | Diffuse proliferative mesangial glomerulonephritis | Not performed |
| Min et al. [27] | 13 | M | C287T | NS | + | Diffuse proliferative mesangial glomerulonephritis | Not performed |
| Oh et al. [28] | 14 | F | E1841K | 0.7 | 0.96 g/day | 4 glomeruli with global sclerosis out of 21, others normal | Foot process effacement |
| Present study | 18 | M | R718W | 1.3 | >3 g/day | FSGS, intimal fibrosis of arteries, arteriolar hyalinosis | Not performed |
Pathological features of all the reported kidney biopsies, in our knowledge, of patients with an MYH9-RD diagnosis. Partly based on the review from Kopp [16]. Predicted change in the mature protein based on genetic assessment of the mutations.
M, male; F, female; NS, not stated.
The spectrum of renal differential diagnosis is wide, depending on the detected symptoms. Proteinuria and pathological findings can lead to hereditary FSGS diagnosis. The main differential diagnosis is Alport syndrome, sharing the features of nephritis, cataract and sensorineural deafness, suspected in one of the patients of our study. Of note, microscopic haematuria consistent with Alport syndrome’s renal features was present in five of our patients. Even though normal platelet counts do not exclude MYH9-RD [1], the two syndromes can be differentiated by the most frequently X-linked inheritance of Alport syndrome, whereas MYH9-RD inheritance is AD. However, AD Alport disease is probably more frequent than first assumed and a non-negligible cause of hereditary FSGS [29]. Its presentation can be misleading, with various but probably less severe phenotypes than X-linked Alport disease [30]. Of note, previous ultrastructural analyses of kidney samples of MYH9-RD patients reported in Table 5 may reveal glomerular basement membrane (GBM) abnormalities suggestive of Alport syndrome, such as irregular thinning and thickening GBM with lamellated and basket-weave appearance [12, 16, 22]. Extra-renal manifestations of MYH9-RD should thus be widely screened as well as family history. Even a moderate decrease in platelet count should lead to blood smears analysis. The diagnosis relies on the presence of immunofluorescent-positive NMMHC-IIA aggregates in neutrophils [14] and genetic testing [31].
Secondly, this study demonstrates that the severity of kidney involvement is heterogeneous, even within individuals harbouring the same mutation. Indeed, nephropathy occurs in 25–37% of patients with MYH9-RD according to the largest reported series [9–11], with a variable age at onset. Pecci et al. have reported an overall rate of nephropathy per 100 person-year of 0.77 (95% CI 0.56–1.08). It usually presents with proteinuria, sometimes in the nephrotic range, with or without microhaematuria, leading to renal failure and eventually ESRD requiring renal replacement therapy in 43% of these patients according to the latter authors [10].
In our study, the renal outcome was poorly predictable, whereas some patients presented with only mild proteinuria and stable eGFR during an overall median follow-up of 3 years, others rapidly evolved towards ESRD, even without nephrotic syndrome. Moreover, the onset of kidney disease may be late as in Patient 2, who was diagnosed at the age of 76 years. Noteworthy, the majority of family members of patients with a proven MYH9-RD had no kidney involvement. This incomplete penetrance underscores the need for regular renal examination including proteinuria, urinalysis and SCr in MYH9-RD patients and all family members.
The exact renal prognosis is thus largely unknown, and probably highly variable among patients with MYH9 mutations, even though genotype–phenotype correlations have been described [10]. In previous reports as well as in our study, renal outcome was very heterogeneous and unpredictable [4, 5, 12, 13, 15, 19–21, 24, 27, 28, 32–35]. As shown in Table 6 listing available data on renal outcome from reported cases of MYH9 nephropathy, we cannot assert that usual prognostic factors of renal outcome (such as baseline eGFR, the degree of proteinuria and hypertension) are applicable in MYH9-RD.
Table 6.
Renal prognosis in reported cases of MYH9 nephropathy
| MYH9 nephropathy | First evaluation |
Follow-up duration (years) | eGFR slope (mL/min/1.73 m2/year) | Last evaluation |
||||
|---|---|---|---|---|---|---|---|---|
| Age (years) | Proteinuria | Hypertension | CKD stage | CKD stage | Age at ESRD (years) | |||
| Non-ESRD (n =14) | 22 (2–64) | 86% (12/14) | 21% (3/14) | 1 (1–4) | 3 (2–28) | −1 (−20.3 to 6.7) | 3 (1–5) | – |
| ESRD (n =29) | 16 (1–76) | 79% (23/29) | 35% (10/29) | 2 (1–5) | 5 (0–23) | −12.7 (−21.5 to 1.8) | 5 | 21 (7–78) |
Data reported, when available, from all the reported cases of MYH9 nephropathy in our knowledge and our case series [4, 5, 12, 13, 15, 20–22, 24, 27, 32–35]. Age, CKD stages, follow-up duration, eGFR slope are expressed in median (range) values. Proteinuria and hypertension are expressed in percentages (N/n total).
However, according to previous studies, it must be emphasized that renal pathological findings may have prognostic significance [36, 37]. For instance, Sekine et al. reported an MYH9-RD patient who underwent a kidney biopsy showing interstitial fibrosis and tubular atrophy, whereas eGFR was still normal, and subsequently developed ESRD 4 years later [12].
Besides case reports, only two series have previously focused on renal involvement in MYH9-RD patients. Sekine et al. [12] in the first one included nine unrelated patients with R702 mutations in the motor domain that all developed early proteinuria and four adult patients reached ESRD at the last evaluation [12]. Han and colleagues reported seven unrelated patients with various mutations. Three patients demonstrating the most severe renal involvement were sporadic cases and had mutations in the motor domain, including one patient with the p.R718W substitution, like our study’s Family A [13].
According to previous studies, there was no overt bleeding diathesis, even in patients with the lowest level of platelets and in patients who received kidney biopsy or transplantation [27]. This finding is consistent with earlier studies showing first, that the automated blood counters underestimate platelet rate since the large platelets of these patients are not taken into account [38], and second, that these platelets display normal aggregation [39, 40]. Nonetheless, haemorrhage occurred in two patients following high bleeding risk events (road accident and post-partum bleeding). Transfusions of platelet concentrates and other therapies like desmopressin or eltrombopag [41–43] can be used in the prevention and treatment of bleeding.
Cataract was a less frequent non-congenital feature of the disease, as has been reported in previous studies [9–11]. It should be noted that hearing impairment was asymptomatic in our patients, and therefore should be systematically screened by a complete evaluation including audiogram. Noteworthy, altered liver profile was the most frequent extra-renal manifestation in our series in accordance with literature [15, 44]. This defect is not assumed to lead to liver disease but should rather provide an additional argument leading to MYH9-RD diagnosis.
Pathogenesis of nephropathy caused by MYH9 mutations is poorly known. NMII-A is expressed in podocytes and exerts an important regulation of cytoskeleton and cell migration [45, 46]. Consistently, it was reported that its expression was decreased in the glomerulus of patients with MYH9-RD and ultrastructural analysis of renal biopsies showed podocyte foot process effacement (Table 5) [12, 16]. In experimental models, MYH9 podocyte-specific knockout in mice resulted in spontaneous glomerulosclerosis with foot process effacement and loss of the filtration slit membranes leading to proteinuria and eventually kidney failure [45]. Furthermore, in Patient 3, FSGS was associated with unexpected severe intrarenal arterial and arteriolar lesions while the onset of the symptoms was recent and the patient was young without vascular history. These findings could be related to MYH9 expression in endothelial cells [47], but to what extent MYH9 mutation could alter renal vasculature will need further research. Indeed, renal microvascular lesions have never been reported in humans and in mouse models of MYH9-RD [48].
The position of mutations was initially thought to predict the severity of the phenotype. Mutations in the motor domain, allowing the mutant protein to copolymerize with wild-type NMMHC-IIA, might therefore have a dominant-negative effect, that is, a more severe phenotype [11]. In contrast to this interpretation, Pecci et al. [10] recently collected 255 MYH9-RD patients from 121 families and developed a model allowing prediction of the phenotype on the basis of the identified mutations. They found that all the motor domain mutations did not confer a severe prognosis. In our cohort, none of the patients harboured the mutations expected to be associated with the worse renal outcome, namely the one hitting the R702 residue and the p.D1424H substitution. Moreover, one of our patients with the most severe renal outcome (Patient 8) carried the p.D1424N substitution, known as a low-risk mutation [10, 11]. Hence, this study confirms the incomplete penetrance of nephropathy in MYH9-RD.
Our study has several limitations. First, it is a retrospective study with various follow-up durations. Secondly, only one patient underwent a kidney biopsy. Finally, two patients did not undergo systematic ear and eye evaluation during their follow-up. Despite these limitations, our study provides additional information on renal involvement in MYH9-RD with a long-term follow-up, pointing out inter- and intra-familial heterogeneity of the disease.
In conclusion, our study gathers 13 cases of MYH9-RD with kidney involvement and confirms the great heterogeneity in renal and extra-renal manifestations, even within the same family. The diagnosis of MYH9-RD is often delayed. It should be considered in any patient with a variable degree of glomerular involvement associated with a low or slightly decreased platelet count and/or hearing loss, cataract and liver dysfunction. Genetic testing will confirm the diagnosis after careful analysis of blood smear and immunofluorescence.
ACKNOWLEDGEMENTS
We thank all the team members and nursing staff for providing our patients with high-quality care. We are also grateful to Drs Laura Moussalieh, Charlotte Samaille, Thomas Guincestre, Corinne Lemoine and Louis Terriou who helped in our research collecting and providing data for their patients.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Althaus K, Greinacher A.. MYH9-related platelet disorders. Semin Thromb Hemost 2009; 35: 189–203 [DOI] [PubMed] [Google Scholar]
- 2. Hegglin R. Gleichzeitige konstitutionelle Veranderungen an Neutrophilen und Thrombozyten. Helv Med Acta 1945; 12: 439. [PubMed] [Google Scholar]
- 3. Lusher JM, Schneider J, Mizukami I. et al. The May-Hegglin anomaly: platelet function, ultrastructure and chromosome studies. Blood 1968; 32: 950–961 [PubMed] [Google Scholar]
- 4. Epstein CJ, Sahud MA, Piel CF. et al. Hereditary macrothrombocytopathia, nephritis and deafness. Am J Med 1972; 52: 299–310 [DOI] [PubMed] [Google Scholar]
- 5. Peterson LC, Rao KV, Crosson JT. et al. Fechtner syndrome–a variant of Alport’s syndrome with leukocyte inclusions and macrothrombocytopenia. Blood 1985; 65: 397–406 [PubMed] [Google Scholar]
- 6. Greinacher A, Nieuwenhuis HK, White JG.. Sebastian platelet syndrome: a new variant of hereditary macrothrombocytopenia with leukocyte inclusions. Blut 1990; 61: 282–288 [DOI] [PubMed] [Google Scholar]
- 7. Seri M, Cusano R, Gangarossa S. et al. Mutations in MYH9 result in the May-Hegglin anomaly, and Fechtner and Sebastian syndromes. The May-Heggllin/Fechtner Syndrome Consortium. Nat Genet 2000; 26: 103–105 [DOI] [PubMed] [Google Scholar]
- 8. Heath KE, Campos-Barros A, Toren A. et al. Nonmuscle myosin heavy chain IIA mutations define a spectrum of autosomal dominant macrothrombocytopenias: May-Hegglin anomaly and Fechtner, Sebastian, Epstein, and Alport-like syndromes. Am J Hum Genet 2001; 69: 1033–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saposnik B, Binard S, Fenneteau O. et al. Mutation spectrum and genotype-phenotype correlations in a large French cohort of MYH9-Related Disorders. Mol Genet Genomic Med 2014; 2: 297–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pecci A, Klersy C, Gresele P. et al. MYH9-related disease: a novel prognostic model to predict the clinical evolution of the disease based on genotype-phenotype correlations. Hum Mutat 2014; 35: 236–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pecci A, Panza E, Pujol-Moix N. et al. Position of nonmuscle myosin heavy chain IIA (NMMHC-IIA) mutations predicts the natural history of MYH9-related disease. Hum Mutat 2008; 29: 409–417 [DOI] [PubMed] [Google Scholar]
- 12. Sekine T, Konno M, Sasaki S. et al. Patients with Epstein-Fechtner syndromes owing to MYH9 R702 mutations develop progressive proteinuric renal disease. Kidney Int 2010; 78: 207–214 [DOI] [PubMed] [Google Scholar]
- 13. Han KH, Lee HK, Kang HG. et al. Renal manifestations of patients with MYH9-related disorders. Pediatr Nephrol Berl Ger 2011; 26: 549–555 [DOI] [PubMed] [Google Scholar]
- 14. Kunishima S, Kojima T, Matsushita T. et al. Mutations in the NMMHC-A gene cause autosomal dominant macrothrombocytopenia with leukocyte inclusions (May-Hegglin anomaly/Sebastian syndrome). Blood 2001; 97: 1147–1149 [DOI] [PubMed] [Google Scholar]
- 15. Vassallo D, Erekosima I, Kanigicherla D. et al. Myosin heavy chain-9-related disorders (MYH9-RD): a case report. Clin Kidney J 2013; 6: 516–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kopp JB. Glomerular pathology in autosomal dominant MYH9 spectrum disorders: what are the clues telling us about disease mechanism? Kidney Int 2010; 78: 130–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clare NM, Montiel MM, Lifschitz MD. et al. Alport’s syndrome associated with macrothrombopathic thrombocytopenia. Am J Clin Pathol 1979; 72: 111–117 [DOI] [PubMed] [Google Scholar]
- 18. Túri S, Kóbor J, Erdős A. et al. Hereditary nephritis, platelet disorders and deafness-Epstein’s syndrome. Pediatr Nephrol 1992; 6: 38–43 [DOI] [PubMed] [Google Scholar]
- 19. Iyori H, Tokushige A, Ishitoya N. et al. [A case report of Epstein syndrome]. Nihon Jinzo Gakkai Shi 1995; 37: 62–68 [PubMed] [Google Scholar]
- 20. Moxey-Mims MM, Young G, Silverman A. et al. End-stage renal disease in two pediatric patients with Fechtner syndrome. Pediatr Nephrol 1999; 13: 782–786 [DOI] [PubMed] [Google Scholar]
- 21. Naito I, Nomura S, Inoue S. et al. Normal distribution of collagen IV in renal basement membranes in Epstein’s syndrome. J Clin Pathol 1997; 50: 919–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ghiggeri GM, Caridi G, Magrini U. et al. Genetics, clinical and pathological features of glomerulonephritis associated with mutations of nonmuscle myosin IIA (Fechtner syndrome). Am J Kidney Dis 2003; 41: 95–104 [DOI] [PubMed] [Google Scholar]
- 23. Alhindawi E, Al-Jbour S.. Epstein syndrome with rapid progression to end stage renal disease. Saudi J Kidney Dis Transplant 2009; 20: 1076–1078 [PubMed] [Google Scholar]
- 24. Yap DYH, Tse KC, Chan TM. et al. Epstein syndrome presenting as renal failure in young patients. Ren Fail 2009; 31: 582–585 [DOI] [PubMed] [Google Scholar]
- 25. Hao J, Kunishima S, Guo X. et al. A large family with MYH9 disorder caused by E1841K mutation suffering from serious kidney and hearing impairment and cataracts. Ann Hematol 2012; 91: 1147–1148 [DOI] [PubMed] [Google Scholar]
- 26. Sun X-H, Wang Z-Y, Yang H-Y. et al. Clinical, pathological, and genetic analysis of ten patients with MYH9-related disease. Acta Haematol 2013; 129: 106–113 [DOI] [PubMed] [Google Scholar]
- 27. Min SY, Ahn HJ, Park WS. et al. Successful renal transplantation in MYH9-related disorder with severe macrothrombocytopenia: first report in Korea. Transplant Proc 2014; 46: 654–656 [DOI] [PubMed] [Google Scholar]
- 28. Oh T, Jung Seo H, Taek Lee K. et al. MYH9 nephropathy. Kidney Res Clin Pract 2015; 34: 53–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gast C, Pengelly RJ, Lyon M. et al. Collagen (COL4A) mutations are the most frequent mutations underlying adult focal segmental glomerulosclerosis. Nephrol Dial Transplant 2016; 31: 961–970 [DOI] [PubMed] [Google Scholar]
- 30. Pescucci C, Mari F, Longo I. et al. Autosomal-dominant Alport syndrome: natural history of a disease due to COL4A3 or COL4A4 gene. Kidney Int 2004; 65: 1598–1603 [DOI] [PubMed] [Google Scholar]
- 31. Seri M, Pecci A, Di Bari F. et al. MYH9-related disease: May-Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome are not distinct entities but represent a variable expression of a single illness. Medicine (Baltimore) 2003; 82: 203–215 [DOI] [PubMed] [Google Scholar]
- 32. Fabbian F, Ricci F, De Giorgi A. et al. The May-Hegglin anomaly in a kidney transplant recipient. NDT Plus 2010; 3: 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nel N, van Rensburg BW, du Plessis L. et al. Coincidental finding of May-Hegglin anomaly in a patient with end-stage renal failure. Am J Hematol 1992; 40: 216–221 [DOI] [PubMed] [Google Scholar]
- 34. Demeter J, Lelkes G, Nemes L. et al. Familial occurrence of the May-Hegglin anomaly: is the accompanying renal failure part of a new subentity? Ann Hematol 2001; 80: 368–371 [DOI] [PubMed] [Google Scholar]
- 35. Sevignani G, Pavanelli GM, Milano SS. et al. Macrothrombocytopenia, renal dysfunction and nephrotic syndrome in a young male patient: a case report of MYH9-related disease. J Bras Nefrol 2018; 40: 198–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 1992; 20: 1–17 [DOI] [PubMed] [Google Scholar]
- 37. Srivastava A, Palsson R, Kaze AD. et al. The prognostic value of histopathologic lesions in native kidney biopsy specimens: results from the Boston Kidney Biopsy Cohort Study. J Am Soc Nephrol 2018; 29: 2213–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Drachman JG. Inherited thrombocytopenia: when a low platelet count does not mean ITP. Blood 2004; 103: 390–398 [DOI] [PubMed] [Google Scholar]
- 39. Shivdasani RA. My, oh Myh9 … and platelets, too. Blood 2007; 110: 3093 [Google Scholar]
- 40. Léon C, Eckly A, Hechler B. et al. Megakaryocyte-restricted MYH9 inactivation dramatically affects hemostasis while preserving platelet aggregation and secretion. Blood 2007; 110: 3183–3191 [DOI] [PubMed] [Google Scholar]
- 41. Pecci A, Gresele P, Klersy C. et al. Eltrombopag for the treatment of the inherited thrombocytopenia deriving from MYH9 mutations. Blood 2010; 116: 5832–5837 [DOI] [PubMed] [Google Scholar]
- 42. Balduini CL, Noris P, Belletti S. et al. In vitro and in vivo effects of desmopressin on platelet function. Haematologica 1999; 84: 891–896 [PubMed] [Google Scholar]
- 43. Léon C, Evert K, Dombrowski F. et al. Romiplostim administration shows reduced megakaryocyte response-capacity and increased myelofibrosis in a mouse model of MYH9-RD. Blood 2012; 119: 3333–3341 [DOI] [PubMed] [Google Scholar]
- 44. Pecci A, Biino G, Fierro T. et al. Alteration of liver enzymes is a feature of the MYH9-related disease syndrome. PloS One 2012; 7: e35986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Johnstone DB, Zhang J, George B. et al. Podocyte-specific deletion of Myh9 encoding nonmuscle myosin heavy chain 2A predisposes mice to glomerulopathy. Mol Cell Biol 2011; 31: 2162–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Arrondel C, Vodovar N, Knebelmann B. et al. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J Am Soc Nephrol 2002; 13: 65–74 [DOI] [PubMed] [Google Scholar]
- 47. Huang Y, Shi H, Zhou H. et al. The angiogenic function of nucleolin is mediated by vascular endothelial growth factor and nonmuscle myosin. Blood 2006; 107: 3564–3571 [DOI] [PubMed] [Google Scholar]
- 48. Zhang Y, Conti MA, Malide D. et al. Mouse models of MYH9-related disease: mutations in nonmuscle myosin II-A. Blood 2012; 119: 238–250 [DOI] [PMC free article] [PubMed] [Google Scholar]