Abstract
Calciphylaxis, or calcific uraemic arteriolopathy (CUA), represents a life-threatening disease. Dialysis patients and those receiving warfarin are seen as populations at higher risk for CUA. Treatments for CUA are still uncertain despite the poor survival of the disease. Administration of intravenous sodium thiosulphate (STS) has been purposed to treat CUA in dialysis patients. Due to the poor tolerability of STS, characterized by nausea, hypocalcaemia, metabolic acidosis and QT-interval prolongation, its intralesional administration has been reported. We herein present the improvement of wounds in a haemodialysis patient affected by CUA, treated by multipronged intervention including both intravenous and intralesional STS.
Keywords: calciphic uraemic arteriolopathy, calciphylaxis, haemodialysis, intralesional, sodium thiosulphate
INTRODUCTION
Calciphylaxis, or calcific uraemic arteriolopathy (CUA), represents a life-threatening disease, characterized by microvascular occlusion in subcutaneous adipose tissue and dermis [1]. Clinical presentation consists of painful skin lesions, any potentially interesting district of cutis and internal organs. Lesions may evolve rapidly, starting with nodules, purpura and livedo, thereafter leading to jagged ulcers with black scars. To date, chronic dialysis patients and those receiving warfarin are those at greatest risk of CUA. Chronic kidney disease–mineral bone disorder syndrome, with deranged mineral metabolism and extraskeletal calcification, accounts for the major risk factor for CUA in end-stage renal disease (ESRD). Hyper–hypoparathyroidism together with calcium-based phosphate (P) binders, aluminium and vitamin D administration are additive triggers of CUA in dialysis. Warfarin may induce calciphylaxis, downregulating the matrix Gla protein, a constitutive inhibitor of vascular calcification, through vitamin K inhibition.
Despite the poor survival observed among CUA patients, knowledge of the mechanisms of and treatments for the disease are scanty. Multipronged intervention is suggested against CUA, based on a physiology-driven approach and clinical reports. The interdisciplinary management includes histological diagnosis, wound care, analgesia, nutritional support, correction of mineral parameters, intensive haemodialysis (HD), sodium thiosulphate (STS), calcimimetic bisphosphonate, hyperbaric oxygen therapy and withdrawal of iatrogenic factors such as warfarin, calcium-based binders and vitamin D [1].
Off-label intravenous (i.v.) infusion of STS has been reported to improve skin lesions in HD patients affected by CUA, at the dose of 25 g thrice weekly (TIW) after dialysis for 3 months. Dose reduction to 12.5 g TIW for body weight <60 kg has been suggested [2, 3]. However, doses and frequency of STS may be limited by side effects such as anion gap metabolic acidosis, hypocalcaemia, QT-interval prolongation and nausea. For these reasons, intralesional STS administration has been reported in isolated cases of CUA [4, 5].
We report the case of an HD patient affected by CUA, improved by multifactorial therapy including both i.v. and intralesional STS infusion.
CASE REPORT
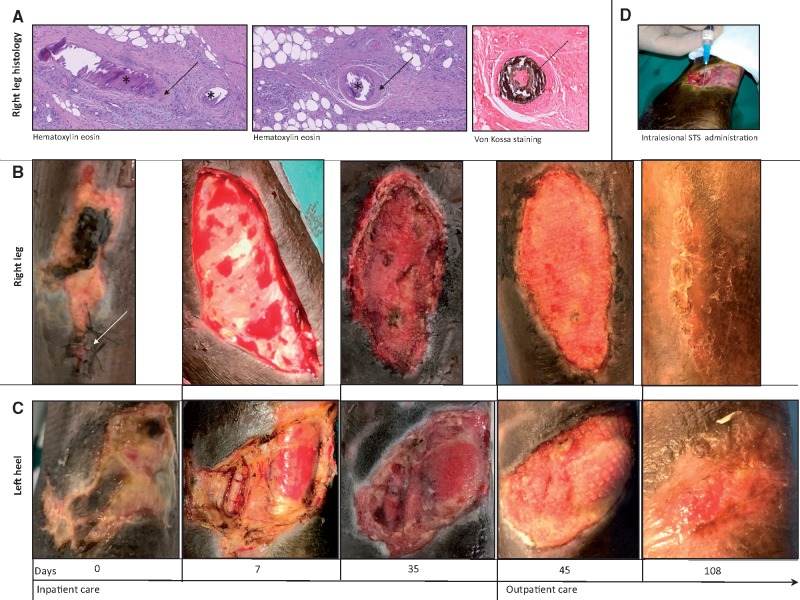
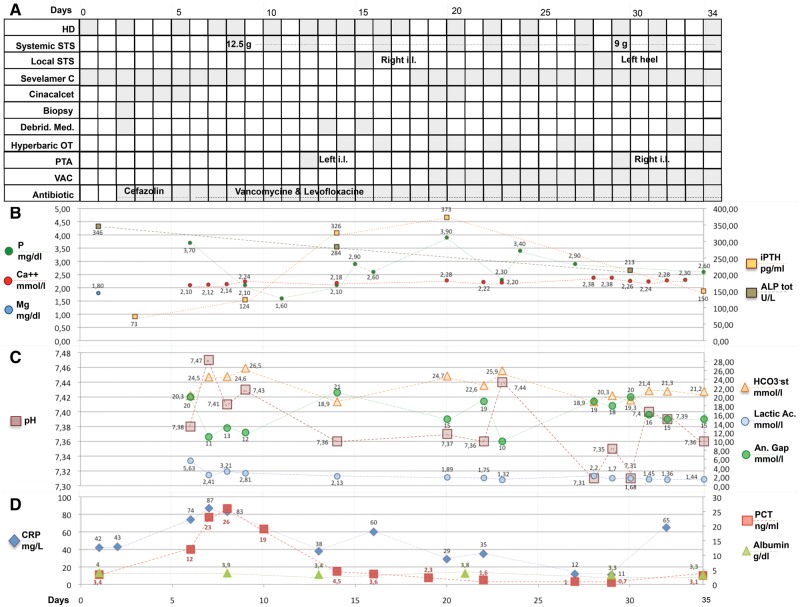
A 70-year-old African male was admitted to the renal unit due to painful ulcers at the middle third of right leg and left ankle. Clinical history was suggestive for ESRD secondary to vascular nephropathy, receiving TIW HD by native arterovenous fistula for 11 years, thrombosis of superior vena cava, secondary hyperparathyroidism with hyperphosphataemia, arterial hypertension, turbercular infection and gastric bleeding in the past. At admission, his medical therapies were acenocoumarol, i.v. paricalcitol, calcium carbonate, sevelamer carbonate, acetylsalicylic acid, nifedipine, bisoprolol, sodium polystyrene sulphonate, omeprazol and epoetin beta. CUA was suspected and shortly after was confirmed by histological examination at the right leg ulcer (Figure 1). The following multipronged intervention was established (Figure 2): (i) withdrawal of oral anticoagulant (complete resolution of central venous thrombosis was ruled out at computed tomography scan examination), calcium carbonate and vitamin D; (ii) increased dialysis dose up to five to six haemodiafiltration sessions per week with low calcium dialysate (1.25 mmol/L); (iii) hyperbaric therapy (10 sessions); (iv) i.v. STS at the dose of 12.5 g post-dialysis TIW according to body weight (nine doses) then tapered to 9 g TIW (due to persisting nausea and mild high anion gap metabolic acidosis, requiring daily administration of oral sodium bicarbonate) and shortly after reduced to 9 g weekly in a single dose with remission of nausea (still ongoing at the time of submission); (v) intralesional STS [prepared vials of STS (1 g/10 mL), of which 200 mg (2 mL) were aspirated purely (without diluent) into a 2.5 mL syringe, then the 200 mg were inoculated sterilely as small boluses in a circumferential direction along the edges of the lesion at the right leg on Day 14 and at the left heel on Day 27]; (vi) surgical debridments; (vii) vacuum-assisted closure therapy; (viii) successful percutaneous transluminal angioplasty (PTA) of the anterior and posterior right tibial arteries and partially successful PTA of the left interosseous and anterior tibial arteries; (ix) empiric antibiotic prophylaxis (vancomicine plus levofloxacine); (x) nutritional support; and (xi) low-dose buprenorphine transdermal patch. The association with oral cinacalcet was attempted, but immediately interrupted due to gastric intolerance. Intralesional infusions of STS were well tolerated with the exception of pain during infusion. No particular worsening of metabolic acidosis or hypocalcaemia was detected across the inoculation of STS. During 36 days of in-hospital observation, pain was quickly controlled and leg ulcers engaged a favourable healing process without infective complications or new-onset ulcers. Metabolic acidosis, calcaemia and QT interval were well controlled. Leg ulcers underwent rapid improvement during 10 days after discharge, with ambulatory wound care provided at a vulnological clinic (Figure 1). Wound care thereafter proceeded at an ambulatory setting without the need of further in-hospital admissions. At Day 108, restitutio ad integrum was observed at the right leg and considerable healing was even seen at the left heel.
FIGURE 1.
Iconographic report. (A) Histology (haematoxylin–eosin and Von Kossa staining) of cutaneous biopsy performed at the border of right leg. Asterisks indicate basophil intraluminal calcifications; solid arrows: fibrointimal hyperplasia; dashed arrow: intraluminal calcifications typical of calciphylaxis. (B) Pictures of the right leg ulcer. Arrow: site of biopsy. (C) Pictures of the left heel ulcer. (D) Intralesional administration of STS.
FIGURE 2.
Medical intervention and biochemical parameters. An. gap: anion gap; Ca++: ionized calcium; CRP: C-reactive protein; Debrid. Med.: debridment, medication; HCO3−: bicarbonate standard; iPTH: intact parathyroid hormone; Lactic Ac: lactic acid; Mg: serum magnesium; OT: oxygen therapy; P: serum phosphate; PCT: procalcitonin; Sevelamer C: sevelamer carbonate; VAC: vacuum-assisted closure therapy; ALP: alkaline phosphatase.
DISCUSSION
This is the second case of CUA treated with both i.v. and local STS administration to date [4]. Intralesional STS may represent a tolerable and adjuvant step to improve CUA healing, especially for patients with poor tolerance to i.v. STS at maximum doses. However, as multipronged therapy looks crucial against CUA, mutifactorial intervention weakens our understanding of the effect of single factors, such as local STS. In particular, it remains impossible to discriminate whether i.v. and intralesional STS had the strongest effect in improving the healing process, compared with the standard multipronged intervention. Furthermore, a longer follow-up is required to estimate the final benefits of this approach against life-threatening CUA. The effect of intralesional STS on acid–base balance requires further investigation.
NOTE
Basal levels of 25-hydroxyvitamin D were 7.16 ng/mL, which were not thereafter replenished in the presence of CUA. Only mild hypocalcaemia was observed, allowing adoption of 1.25 Ca dialysate, without the need of Ca infusion. Trends of inflammatory markers looked to be associated, at least partially, with surgical and endovascular interventions, while their basal values looked more compatible with chronic inflammation in the presence of CUA, rather than to overcome infections. Trend in parathyroid hormone values was attributed to the initial suspension of vitamin D activator and calcium carbonate, followed by the desired rapid reduction of serum P levels. At our institution, the target of circulating P levels was arbitrarily set to 2.5–3.0 mg/dL in the presence of CUA. Nutritional counselling was adopted for ensuring an adequate protein and caloric intake and, especially, for increasing dietary P intake whenever circulating P went <2.5 mg/dL. Calcium–aluminium-free P binder was tailored accordingly. Of note, only mild reduction of circulating albumin was observed during in-hospital stays.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Nigwekar SU, Thadhani R, Brandenburg VM.. Calciphylaxis. N Engl J Med 2018; 378: 1704–1714 [DOI] [PubMed] [Google Scholar]
- 2. Nigwekar SU, Brunelli SDM, Maede D.. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol 2011; 6: 1155–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nigwekar SU, Thaadhani R.. Calciphylaxis (calcific uremnic arteriolopathy) UpToDate. https://www.uptodate.com/contents/calciphylaxis-calcific-uremic-arteriolopathy (16 June 2018, date last accessed)
- 4. Strazzula L, Nigwekar SU, Steele D. et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol 2013; 149; 946–949 [DOI] [PubMed] [Google Scholar]
- 5. Ossorio-García L, Jiménez-Gallo D, Arjona-Aguilera C. et al. Intralesional sodium thiosulfate to treat calciphylaxis. Actas Dermosifiliogr 2016; 107: 359–362 [DOI] [PubMed] [Google Scholar]