Abstract
Background
Epidemiological data on paediatric acute kidney injury (AKI) in sub-Saharan Africa are limited and largely retrospective. We performed a prospective study of AKI among patients admitted through the emergency room.
Methods
Children admitted to the post-neonatal emergency room of the University College Hospital, Ibadan, Nigeria between February 2016 and January 2017 were studied. AKI was defined by Kidney Disease: Improving Global Outcomes serum creatinine criteria. AKI ascertainment relied on serum creatinine measurements carried out in routine care by post-admission Day 1. We compared in-hospital mortality by post-admission Day 7 for patients with and without AKI (no-AKI).
Results
Of the 1344 children admitted to the emergency room, 331 were included in the study. AKI occurred in 112 patients (33.8%) with a median age of 3.1 years [interquartile range (IQR) 0.9–9.4] and was Stage 3 in 50.5% of the cases. The no-AKI group had a median age of 1.8 (IQR 0.7–5.8) years. The underlying diagnoses in patients with AKI were sepsis (33.0%), malaria (12.5%) and primary renal disorders (13.4%). Twenty-four of the patients with AKI underwent dialysis: haemodialysis in 20 and peritoneal dialysis in 4. By Day 7 of admission, 7 of 98 (7.1%) patients in the AKI group had died compared with 5 of 175 (2.9%) patients in the no-AKI group [odds ratio 2.6 (95% confidence interval 0.8–8.5)]. Outcome data were not available for 58 (17.5%) patients.
Conclusions
AKI is common among paediatric emergency room admissions in a tertiary care hospital in sub-Saharan Africa. It is associated with high mortality risk that may be worse in settings without dialysis.
Keywords: acute kidney injury, children, dialysis, mortality, sub-Saharan Africa
INTRODUCTION
There is global interest in reducing mortality from acute kidney injury (AKI) in low-resource settings [1]. The International Society of Nephrology spearheads the 0 by 25 initiative with the goal of eliminating preventable deaths from AKI in low-resource countries by the year 2025 [1, 2]. Factors that contribute to high mortality from AKI in the region include late presentation to the hospital, delayed diagnosis and limited access to renal replacement therapy. Accurate data on the epidemiology of AKI in sub-Saharan Africa are crucial for the development and evaluation of interventional programmes, guidelines and clinical pathways for the management of AKI.
The available studies indicate that AKI accounts for 1–3% of paediatric hospital admissions in Nigeria and has an incidence of 10–13.5/million children [3–10]. A review of the AKI literature from sub-Saharan Africa indicated that 66% of children with AKI needed dialysis [11]. Furthermore, mortality associated with AKI in various studies from sub-Saharan Africa ranged from 20.9 to 46.8% [3, 4, 6, 9, 12–17].
Most data from sub-Saharan Africa on paediatric AKI are retrospective, predate the Kidney Disease: Improving Global Outcomes (KDIGOs) consensus definition of AKI, involve patient information extracted from a clinical dataset or focus only on specific disease conditions or patients who undergo dialysis [3, 4, 11, 12, 14, 15, 18]. Prospective cohort studies of all children presenting or admitted to acute care hospital settings are currently lacking. Such studies are needed because the incidence, aetiology and outcome of AKI are influenced by the definition of AKI, the study design and the study population.
The publication of the KDIGO consensus definition on AKI provides a unique opportunity to consistently document and stage AKI and to report outcomes in order to characterize and compare disease burden across geographical regions [19]. As an initial step towards addressing gaps in the literature regarding paediatric AKI in sub-Saharan Africa, we conducted a prospective study using AKI definitions based on the KDIGO criteria and within the first 2 days of admission to the emergency room in a tertiary paediatric health centre in Nigeria. We sought to describe the clinical characteristics of patients with and without AKI and compare in-hospital mortality on Day 7 of admission between patients diagnosed with AKI and those who did not have AKI.
MATERIALS AND METHODS
Setting
The University College Hospital, Ibadan is a tertiary care centre located in Ibadan, the capital city of Oyo State in South West Nigeria. Oyo State has a population of 5 580 894 and a population of 2 099 694 children <15 years of age (2 695 618 for children <20 years of age) [20]. More than half (54.4%) of the population earn less than the international poverty line of US$1.90/day [21]. Patients usually pay out of pocket for expenses related to medical treatment. The National Health Insurance Scheme is not yet in widespread use. Patients include those who are referred from other hospitals and those who are presenting to a health facility for the first time.
The University College Hospital, Ibadan is also a tertiary care referral centre for paediatrics, with 120 beds. Subspecialty care is provided and includes cardiology, pulmonology, endocrinology, infectious disease, haematology–oncology, gastroenterology, neonatology and nephrology. Neonatal emergencies are managed in the neonatal unit, whereas the other paediatric emergencies are managed in the Otunba Tunwase Children’s Emergency Ward.
Design and subjects
We conducted a prospective observational study. The study was approved by the institutional research ethics boards at the University of Ibadan/University College Hospital, Ibadan and the University of Calgary. The authors adhered to the Declaration of Helsinki in carrying out the work. Patients were recruited between February 2016 and January 2017. Children 28 days to <18 years of age who were admitted to the emergency room were included, while those on chronic haemodialysis were excluded. Eligible children whose parents provided consent to participate were recruited into the study. Participants were recruited between 9 a.m. and 5 p.m. on weekdays over the enrolment period except for November 2017, when the ethics certificate renewal was delayed. During study enrolment, patients who were admitted outside the hours of 9 a.m.–5 p.m. or on weekends were recruited between the hours of 9 a.m. and 5 p.m. on weekdays if they were still on admission in the hospital.
Data collection
Data were collected on study-specific case report forms. These forms were developed by the primary study investigator A.D.A, circulated and discussed at length electronically and via conference calls with the other investigators and finally modified to their final form after consensus. The case report form was developed with the goal of collecting detailed information relevant to AKI, balanced with the feasibility of data availability within clinical charts and data collection by study staff.
Information regarding baseline demographics, presenting symptoms, medication history, anthropometry, clinical examination findings such as the presence of oedema and blood pressure were extracted from medical charts on the day of admission. The results of serum creatinine performed on the day of admission or post-admission Day 1 were recorded, when available based on physicians’ orders during routine clinical care of patients. We extracted information from the charts regarding medications administered on the day of admission and post-admission Days 1 and 2. We obtained information regarding the patient’s underlying diagnosis from the medical charts. Primary renal diseases were defined as acute glomerulonephritis, nephrotic syndrome, acute on chronic kidney disease and posterior urethral valves.
AKI ascertainment
AKI ascertainment was made using the KDIGO serum creatinine criteria for the AKI definition based on the day of admission and/or post-admission Day 1 serum creatinine results. A patient was deemed to have AKI when the post-admission Day 1 serum creatinine was ≥0.3 mg/dL compared with the admission Day 1 serum creatinine. AKI was also defined when either the day of admission or post-admission Day 1 serum creatinine result was >1.5 times the estimated normal serum creatinine for height prior to illness. The estimated normal serum creatinine was back-calculated using the chronic kidney disease in children estimated glomerular filtration rate (eGFR) equation (baseline serum creatinine = 0.41 × height in centimetres/eGFR) [22, 23]. The estimated baseline serum creatinine corresponded to an eGFR of 120 mL/min/1.73 m2 for children >2 years of age. For children ≤2 years of age, estimated baseline serum creatinine corresponded to age-based normative values (Table 1) [23–25]. Serum creatinine measurements in our laboratory were performed using the modified Jaffe’s reaction [26] and the values are isotope dilution-mass spectrometry traceable.
Table 1.
| Age (years) | eGFR used (mL/min/1.73 m2) |
|---|---|
| ≤0.1 | 42 |
| 0.10–0.30 | 53 |
| 0.30–0.66 | 71 |
| 0.66–1.00 | 84 |
| 1.00–1.50 | 91 |
| 1.50–2.00 | 97 |
| >2.00 | 120 |
AKI staging was performed according to the magnitude of the increase in serum creatinine as stipulated by the KDIGO AKI definition [19]. Stage 1 AKI referred to serum creatinine 1.5–1.9 times baseline or a ≥0.3 mg/dL increase in baseline serum creatinine. Stage 2 AKI referred to serum creatinine 2.0–2.9 times baseline. Stage 3 AKI referred to serum creatinine 3.0 times baseline or increase in serum creatinine to ≥4.0 mg/dL or initiation of renal replacement therapy, or in patients <18 years of age, a decrease in eGFR to <35 mL/min/1.73 m2. Urine output criteria were not considered due to the feasibility of data collection (a large proportion of patients with missing data anticipated a priori).
Outcomes
The primary outcome was death, and the outcome ascertainment was made on post-admission Day 7 if death did not occur before that date. Patients who were discharged home before post-admission Day 7 were assumed to be alive on Day 7. In the cohort with AKI, we also recorded whether the patient received dialysis (either haemodialysis or peritoneal dialysis) by admission Day 7.
In our centre, parents who do not have enough funds for dialysis are usually able to get at least session of haemodialysis for their children through a waiver from the hospital, or they may be able to access funds for the management of indigent patients from the hospital or the Department of Paediatrics. Sometimes members of the medical team donate funds to provide dialysis for indigent patients. We may also be able to provide acute peritoneal dialysis using adaptations and donated peritoneal dialysis fluids, but we are unable to routinely offer chronic renal replacement therapy. Recently the federal government included six sessions of haemodialysis in the National Health Insurance Scheme. The scheme covers mainly federal government employees, and the general population is not yet widely covered.
Statistical analysis
All data were described using medians, interquartile ranges (IQRs) and proportions as appropriate. We determined the proportion of children with AKI by post-admission Day 1 among paediatric emergency room admissions. The age and gender distribution of patients with AKI, those without AKI and those in whom AKI status could not be determined were compared using the Kruskal–Wallis test. We determined the unadjusted odds ratio (OR; AKI versus non-AKI) for mortality within the first 7 days of admission using logistic regression. A P-value <0.05 indicated statistical significance. Statistical Analysis System (SAS) version 9.4 (SAS Institute, Cary, North Carolina, United States of America) was used to perform the data analysis.
RESULTS
Study population and subject characteristics
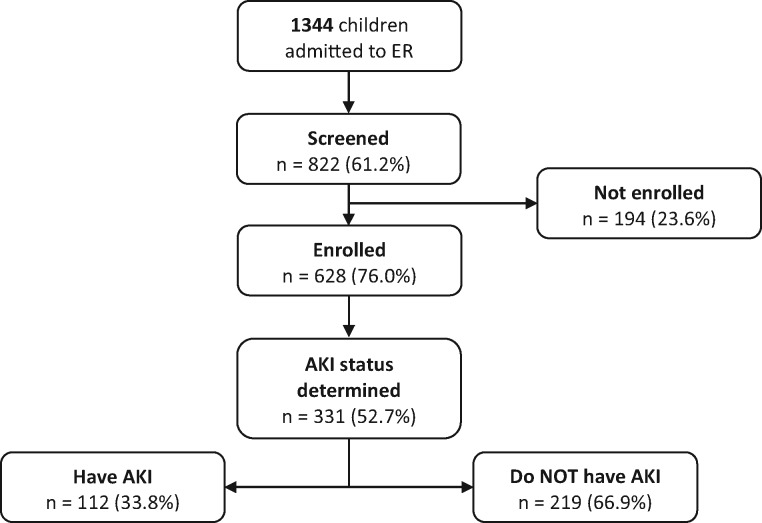
A total of 1344 children were admitted to the emergency room over the period. Of these, 822 (61.2%) were approached for recruitment into the study and 628 (76%) were enrolled in the study after obtaining informed consent. AKI status could be determined in 331 patients (52.7%); the AKI status could be determined as they had serum creatinine results by post-admission Day 1. Figure 1 shows participant recruitment, screening and selection. The 331 patients were 0.1–17 years of age and 54% were males. The patients in whom AKI status could not be determined (n = 297) had a median age of 2.1 years (IQR 0.8–6.0) and 58.1% were males. The patients with AKI, those without AKI and those in whom AKI status could not be determined were not significantly different in age (P = 0.06) or gender distribution (P = 0.6).
FIGURE 1.
Participant recruitment, screening and selection.
AKI prevalence and characteristics
Table 2 shows the demography, underlying diagnosis and medications by Day 7 of admission for patients with and without AKI. A total of 112 patients (33.8%) met the criteria for AKI [median age of 3.1 years (IQR 0.9–9.4) and 53.2% were males]. The patients without AKI had a median (IQR) age of 1.8 years (IQR 0.7–5.7) and 58.7% were males. The mother’s educational status was available for 291 (87.9%) of the included patients. Among those with AKI, 50 (44.6%) children had mothers who completed high school. In contrast, 119 (54.3%) of the children without AKI had mothers who completed high school (omnibus P = 0.02).
Table 2.
Comparing demographics, diagnosis and medications among patients with and without acute kidney injury (AKI) on Day 1 of admission for those with known AKI status
| Characteristics | AKI (n = 112) | No AKI (n = 219) |
|---|---|---|
| Demographics | ||
| Age, mean (SD), years | 3.1 (0.9–9.4) | 1.8 (0.7–5.7) |
| Male sex, n (%) | 59 (53.2) | 128 (58.7) |
| Mother’s education, n (%) | ||
| None/incomplete high school | 30 (26.7) | 30 (13.6) |
| Completed high school/degree | 50 (44.6) | 119 (54.3) |
| Trades/technical | 17 (15.1) | 45 (20.5) |
| Missing | 15 (13.3) | 25 (11.4) |
| Administered drugs, nephrotoxic agents, n (%) | ||
| Cefuroxime | 32 (28.6) | 58 (26.5) |
| Gentamicin | 29 (25.9) | 94 (42.9) |
| Amikacin | 4 (3.6) | 13 (5.9) |
| Ceftazidime | 2 (1.8) | 1 (0.5) |
| Ibuprofen | 2(1.8) | 12 (5.5) |
| Captopril | 2 (1.8) | 12 (5.5) |
| Administered drugs, diuretics, n (%) | ||
| Frusemide | 26 (23.2) | 20 (9.1) |
| Hydrochlorothiazide | 10 (8.9) | 18 (8.2) |
| Admitting diagnosis and/or presenting complaint, n (%) | ||
| Sepsis | 37 (33.0) | 41 (18.7) |
| Malaria | 14 (12.5) | 61 (27.9) |
| Haemoglobinuria (unknown cause) | 6 (5.4) | 0 (0.0) |
| Acute glomerulonephritis | 3 (2.7) | 0 (0.0) |
| Acute on chronic kidney disease | 4 (3.6) | 0 (0.0) |
| Sickle cell disease, (sickle haemoglobin) | 3 (2.7) | 23 (10.5) |
| Nephrotic syndrome | 3 (2.7) | 8 (3.7) |
| Posterior urethral valves | 5 (4.4) | 0 (0.0) |
| Gastroenteritis | 2 (1.8)a | 13 (5.9) |
| Malignancies | 4 (3.6) | 4 (1.8) |
Gastroenteritis was also documented in 10 of the patients with AKI and sepsis.
The patients with AKI were older than the patients without AKI [3.1 years (IQR 0.9–9.4) versus 1.8 (0.7–5.7); P = 0.06]. The majority of patients with AKI were in KDIGO Stage 3 [167 (50.5%)]. Stages 1 and 2 each occurred in 82 patients (24.8%).
Sepsis was the most frequent underlying diagnosis in the patients with AKI [37 (33.0%)]. The most common infections that were documented in patients with sepsis and AKI were pneumonia [16 (43.2%)], gastroenteritis [10 (27.0%)] and meningitis [7 (18.9%)]. Pneumonia was the most frequently documented infection in patients with sepsis who did not have AKI [13 (31.7%)]. Malaria was the underlying diagnosis in 14 (12.5%) patients with AKI, while primary renal disorders occurred in 15 (13.4%) AKI patients. Haemoglobinuria from an unknown cause was the underlying diagnosis in 6 (5.3%) of the patients with AKI. Among the patients without AKI, the most common disease was malaria, which occurred in 27.9% of the patients, followed by sepsis in 18.1%.
Outcomes
Vital status by post-admission Day 7 was known in 273 of 331 (82.4%) patients in whom AKI status could be determined. For the remaining 58 (17.5%) patients we could not determine whether death had occurred by Day 7 of admission because of missing data in the case report forms. Death occurred within the first 7 days of admission in 7 of 98 patients with AKI and in 5 of 175 patients without AKI. When excluding those with unknown vital status, the unadjusted OR for death in AKI versus non-AKI group was 2.6 (95% confidence interval 0.8–8.5). All the patients who needed dialysis received acute dialysis. Twenty-four (21.4%) of the patients with AKI underwent dialysis: 20 had haemodialysis, while 4 had peritoneal dialysis. There were three deaths among the patients who underwent dialysis.
DISCUSSION
We studied patients admitted from a paediatric emergency ward in South West Nigeria and determined the presence or absence of AKI based on available serum creatinine measurements within the first 2 days of admission. AKI was observed in 34% of patients with known AKI status, 17.8% of enrolled patients and at least 8% of all admitted patients during the period of the study. About 20% of patients with AKI received dialysis. In-hospital mortality on admission Day 7 was higher in the patients with AKI.
Findings in the present study indicate that the incidence of AKI among paediatric admissions in Nigeria, and possibly other parts of sub-Saharan Africa, may be higher than previously reported [3, 4, 6, 9, 15, 17, 27–29]. In a retrospective study from Ghana, AKI was found in ∼1.3% of paediatric admissions [17]. Our findings are similar to a prospective study from Uganda that noted an AKI prevalence of 13.5% among paediatric patients with gastroenteritis, malaria or pneumonia presenting to the emergency room [18]. The Uganda study did not include patients who presented to the emergency room with other diagnoses.
The diagnosis of AKI in our study was enhanced by the use of the KDIGO definition of AKI and the evaluation of all available serum creatinine measurements to determine the patients with AKI [19]. Many previous studies on AKI from sub-Saharan Africa were not based on consensus definitions for AKI and may have underestimated the prevalence of AKI [3, 7, 15, 16]. In addition, studies usually focused on patients who were managed in the paediatric nephrology unit and therefore may not report less severe AKI cases [3, 4, 10, 15–17]. In addition, the diagnosis of AKI may have been enhanced by comparing serum creatinine measurements with estimated baseline serum creatinine in most of our patients. Comparison of serum creatinine with estimated baseline serum creatinine has been noted to diagnose higher numbers of patients with AKI than when paired serum creatinine measurements are utilized [25, 30].
The prevalence of AKI observed in our study was similar to those reported in developed countries. Among hospitalized non-critically ill children in North America, the proportion with AKI was 30% among patients in whom AKI status could be determined and at least 5% among all patients [31].
The distribution of AKI stages in our study indicated that the majority had Stage 3 AKI. This is in contrast to paediatric AKI studies from developed countries that show the majority in Stages 1 and 2 AKI [31, 32]. In a study from Uganda that utilized serial creatinine measurements among patients with severe malaria, KDIGO Stage 1 AKI was documented in the majority (51.9%) of the patients with AKI. One possible explanation for the high proportion of patients with AKI Stage 3 in our study may be because we evaluated only serum creatinine measurements that were carried out in routine management of the patients, thus introducing a detection bias. In the study centre, serum creatinine measurements are more likely to be ordered in the more ill patients, and this may account for the finding that most patients with AKI were in KDIGO Stage 3. Late presentation to the health facility by the majority of patients may be an additional cause for the predominance of KDIGO AKI Stage 3.
We found that sepsis, malaria and primary renal disorders were the most common causes of AKI. Sepsis, malaria and primary renal disorders have been identified as important causes of AKI in various studies from sub-Saharan Africa [3, 4, 6, 10, 11, 15–17, 29, 33]. Gastroenteritis was associated with AKI in ∼10% of the cases and this is consistent with previous studies from sub-Saharan Africa, which indicate that gastroenteritis is a common aetiology or associated co-morbidity in patients with AKI [3, 8, 15, 18, 29]. Malignancies were not a common cause of AKI in our cohort study. This may be partly due to our study design in which we reported AKI within the first 2 days of emergency room admissions. Two studies from sub-Saharan Africa have noted malignancies to be common causes of AKI [15, 17].
Renal replacement therapy was required in about one-fifth of the patients with AKI. The proportion of patients with AKI who needed dialysis in our study, although high, is less than the pooled prevalence of 66% in a review of published papers on paediatric AKI in sub-Saharan Africa [11]. The lower proportion of patients who need dialysis may be due to differences in the definition of AKI. However, our study indicates that ∼20% of patients with AKI may need renal replacement therapy.
Mortality was higher in patients with AKI compared with patients without AKI. Our unit has the advantage that we were able to provide renal replacement therapy for patients with AKI either with adapted peritoneal dialysis catheters or haemodialysis, and AKI mortality is likely to be worse in settings without access to paediatric renal replacement therapy.
LIMITATIONS
Our study is susceptible to a case ascertainment bias where sicker patients who required blood work and/or patients whose family can afford to carry out blood tests were diagnosed more frequently with AKI and those without blood work or opportunity to perform blood work were missed. We did not measure serum creatinine at pre-specified time points in all consented patients due to a lack of funding to conduct these tests. Therefore some cases of AKI were missed. The true incidence among all admitted patients is probably between 8.3% and 34%. Furthermore, we did not include patients with AKI based on urine output criteria, due to a lack of strict recording of urine output during routine clinical care. We did not assess for hospital-acquired AKI, as AKI ascertainment was limited to the first 2 days of admission. The sample size and incomplete data reporting may have limited our ability to detect a statistically significant association of AKI with in-hospital mortality. Mortality outcome data were also limited to Day 7 of admission, and we conducted no further follow-up. Similarly, we did not have information on renal outcomes in those with AKI. Additionally, we conducted a single-centre study in a fee-paying university hospital and results may not be generalizable to other parts of sub-Saharan Africa, including more rural settings. Furthermore, our study did not include neonates, and these findings may not be generalizable to neonates in sub-Saharan Africa.
CONCLUSION
AKI is a common problem among children admitted from a paediatric emergency room in a tertiary centre in South West Nigeria and patients with AKI experience high mortality. Our study highlights the need to provide renal replacement therapy for AKI in low-resource settings and also to develop evidence-based clinical pathways for screening and management of AKI among hospitalized patients. We have also demonstrated the feasibility of large prospective studies of paediatric AKI in developing countries and the potential for reducing preventable deaths.
ACKNOWLEDGEMENTS
We thank Dr Paul Brown, who spent considerable time analysing the data.
FUNDING
The work was carried out with grant support from the International Society of Nephrology (ISN) Clinical Research Program (application code 04-047). The University of Calgary Section of Pediatric Nephrology and University College Hospital, Ibadan Division of Pediatric Nephrology have established an International Society of Nephrology (ISN) Sister Renal Centre partnership and have been provided funds by the to undertake educational activities. S.M.S. and A.D.A. are a mentor–mentee pair within the ISN mentorship programme. These relationships and funding made this work possible.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Mehta RL, Cerdá J, Burdmann EA. et al. International Society of Nephrology's 0 by 25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet 2015; 385: 2616–2643 [DOI] [PubMed] [Google Scholar]
- 2. Susantitaphong P, Cruz DN, Cerda J. et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 2013; 8: 1482–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anochie IC, Eke FU.. Acute renal failure in Nigerian children: Port Harcourt experience. Pediatr Nephrol 2005; 20: 1610–1614 [DOI] [PubMed] [Google Scholar]
- 4. Esezobor CI, Ladapo TA, Osinaike B. et al. Paediatric acute kidney injury in a tertiary hospital in Nigeria: prevalence, causes and mortality rate. PLoS One 2012; 7: e51229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Olowu WA. Renal failure in Nigerian children: factors limiting access to dialysis. Pediatr Nephrol 2003; 18: 1249–1254 [DOI] [PubMed] [Google Scholar]
- 6. Olowu WA, Adefehinti O, Bisiriyu AL.. Hospital-acquired acute kidney injury in critically ill children and adolescents. Saudi J Kidney Dis Transplant 2012; 23: 68–77 [PubMed] [Google Scholar]
- 7. Michael IO, Gabriel OE.. Pattern of renal diseases in children in midwestern zone of Nigeria. Saudi J Kidney Dis Transplant 2003; 14: 539–544 [PubMed] [Google Scholar]
- 8. Odetunde OI, Okafor HU, Uwaezuoke SN. et al. Renal replacement therapy in children in the developing world: challenges and outcome in a tertiary hospital in southeast Nigeria. ScientificWorldJournal 2014; 2014: 903151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ugwu GI, Nwajei G, Chinemelu U.. Pattern of renal diseases among children in the Niger Delta Region, Nigeria. Arab J Nephrol Transplant 2014; 7: 49–50 [PubMed] [Google Scholar]
- 10. Abdelraheem M, Ali E-T, Osman R. et al. Outcome of acute kidney injury in Sudanese children — an experience from a sub-Saharan African unit. Perit Dial Int 2014; 34: 526–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Olowu WA, Niang A, Osafo C. et al. Outcomes of acute kidney injury in children and adults in sub-Saharan Africa: a systematic review. Lancet Global Health 2016; 4: e242–e250 [DOI] [PubMed] [Google Scholar]
- 12. Ademola AD, Asinobi AO, Ogunkunle OO. et al. Peritoneal dialysis in childhood acute kidney injury: experience in southwest Nigeria. Perit Dial Int 2012; 32: 267–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anochie IC, Eke FU.. Paediatric acute peritoneal dialysis in southern Nigeria. Postgraduate Med J 2006; 82: 228–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Asinobi AO, Ademola AD, Alao MA.. Haemodialysis for paediatric acute kidney injury in a low resource setting: experience from a tertiary hospital in South West Nigeria. Clin Kidney J 2016; 9: 63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Olowu WA, Adelusola KA.. Pediatric acute renal failure in southwestern Nigeria. Kidney Int 2004; 66: 1541–1548 [DOI] [PubMed] [Google Scholar]
- 16. Aloni MN, Nsibu CN, Meeko-Mimaniye M. et al. Acute renal failure in Congolese children: a tertiary institution experience. Acta Paediatr 2012; 101: e514–e518 [DOI] [PubMed] [Google Scholar]
- 17. Antwi S, Sarfo A, Amoah A. et al. Acute kidney injury in children: 3-year data review from Ghana. Int J Pediatr Res 2015; 1: 2. [Google Scholar]
- 18. Imani PD, Odiit A, Hingorani SR. et al. Acute kidney injury and its association with in-hospital mortality among children with acute infections. Pediatr Nephrol 2013; 28: 2199–2206 [DOI] [PubMed] [Google Scholar]
- 19.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012; 2: 1–138 [Google Scholar]
- 20.National Population Commission. Priority table volume IV population distribution by age & sex (state and LGA), Federal Republic of Nigeria 2006 population and housing census 2010. http://www.ibenaija.org/uploads/1/0/1/2/10128027/priority_table_vol_4.pdf (22 November 2018, date last accessed)
- 21.United Nations International Children Emergency Fund. The state of the world's children 2016 statistical tables. https://data.unicef.org/resources/state-worlds-children-2016-statistical-tables/ (22 November 2018, date last accessed)
- 22. Schwartz GJ, Munoz A, Schneider MF. et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009; 20: 629–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hessey E, Ali R, Dorais M. et al. Evaluation of height-dependent and height-independent methods of estimating baseline serum creatinine in critically ill children. Pediatr Nephrol 2017; 32: 1953–1962 [DOI] [PubMed] [Google Scholar]
- 24. Piepsz A, Tondeur M, Ham H.. Revisiting normal (51)Cr-ethylenediaminetetraacetic acid clearance values in children. Eur J Nucl Med Mol Imaging 2006; 33: 1477–1482 [DOI] [PubMed] [Google Scholar]
- 25. Zappitelli M, Parikh CR, Akcan-Arikan A. et al. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol 2008; 3: 948–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Slot C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest 1965; 17: 381–387 [DOI] [PubMed] [Google Scholar]
- 27. Adedoyin OT, Bello OA, Anoba S. et al. Determinants of modality of management of acute kidney injury in children seen at a tertiary hospital in Nigeria. Nig J Paediatr 2013; 40: 395–399 [Google Scholar]
- 28. Adedoyin OT, Adesiyun OA, Mark F. et al. Childhood renal disorders in Ilorin, north central Nigeria. Nig Postgraduate Med J 2012; 19: 88–91 [PubMed] [Google Scholar]
- 29. Ladapo TA, Esezobor CI, Lesi FE.. Pediatric kidney diseases in an African country: prevalence, spectrum and outcome. Saudi J Kidney Dis Transplant 2014; 25: 1110–1116 [DOI] [PubMed] [Google Scholar]
- 30. Naik S, Sharma J, Yengkom R. et al. Acute kidney injury in critically ill children: risk factors and outcomes. Indian J Crit Care Med 2014; 18: 129–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McGregor TL, Jones DP, Wang L. et al. Acute kidney injury incidence in noncritically ill hospitalized children, adolescents, and young adults: a retrospective observational study. Am J Kidney Dis 2016; 67: 384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hursh BE, Ronsley R, Islam N. et al. Acute kidney injury in children with type 1 diabetes hospitalized for diabetic ketoacidosis. JAMA Pediatr 2017; 171: e170020. [DOI] [PubMed] [Google Scholar]
- 33. Esezobor CI, Ladapo TA, Lesi FE.. Clinical profile and hospital outcome of children with severe acute kidney injury in a developing country. J Trop Pediatr 2015; 61: 54–60 [DOI] [PubMed] [Google Scholar]