Abstract
Background
Patients who require acute initiation of dialysis have higher mortality rates when compared with patients with planned starts. Our primary objective was to explore the reasons and risk factors for acute initiation of renal replacement therapy (RRT) among patients with end-stage kidney disease (ESKD). Our secondary objective was to determine the difference in glomerular filtration rate (GFR) change in the year preceding RRT between elective and acute dialysis starts.
Methods
We conducted a single-centre retrospective observational study. ESKD patients either started dialysis electively (planned starters) or acutely and were known to renal services for >90 (unplanned starters) or <90 days (urgent starters).
Results
In all, 825 consecutive patients initiated dialysis between January 2013 and December 2015. Of these, 410 (49.7%) patients had a planned start. A total of 415 (50.3%) patients had an acute start on dialysis: 244 (58.8%) unplanned and 171 (41.2%) urgent. The reasons for acute dialysis initiation included acute illness (58%) and unexplained decline to ESKD (33%). Cardiovascular disease [n = 30 (22%)] and sepsis [n = 65 (48%)] accounted for the majority of acute systemic illness. Age and premorbid cardiovascular disease were independent risk factors for acute systemic illness among unplanned starts, whereas autoimmune disease accounted for the majority of urgent starts. The rate of decline in GFR was greater in the month preceding RRT among acute dialysis starters compared with planned starters (P < 0.001).
Conclusions
Cardiovascular disease and advancing age were independent risk factors for emergency dialysis initiation among patients known to renal services for >3 months. The rapid and often unpredictable loss of renal function in the context of acute systemic illness poses a challenge to averting emergency dialysis start.
Keywords: acute dialysis start, renal replacement therapy, unplanned
INTRODUCTION
Delayed referral to nephrology services and urgent initiation of dialysis are independent risk factors for morbidity and mortality among patients with end-stage kidney disease (ESKD) [1–7]. Urgent dialysis initiation among patients not known to renal services is associated with worse outcomes, including hospitalization, increased health care costs [8] and poorer quality of life [9]. Despite this, late nephrology referrals requiring unplanned dialysis initiation account for 20% of patients initiating dialysis [1, 3, 10].
Several studies have identified risk factors associated with delayed referral. These include patient-related factors such as older age [11, 12], comorbid illness [13, 14] and lower socio-economic status [15, 16]. Additional factors relate to the health system, such as the absence of communication between referring physicians and nephrologists and a lack of consensus among physicians regarding the appropriate timing of referral [17].
Even among patients known to renal services, inpatient ‘suboptimal’ dialysis starts occur in >40% of patients [18, 19]. The estimated glomerular filtration rate (eGFR) remains an important determinant for when to refer for dialysis, however, there is clearly a need for more frequent surveillance in pre-ESKD care to enable earlier recognition of those cases with rapidly progressive renal disease. A better understanding of the reasons for urgent dialysis initiation and being able to identify those patients at risk of a rapid decline in GFR may facilitate timely intervention and improve patient outcome.
Our primary objective was to explore the reasons for and risk factors associated with acute renal replacement therapy (RRT) initiation. Our secondary objective was to determine the difference in the rate of decline in eGFR in the year preceding dialysis initiation between elective and acute RRT starts.
MATERIALS AND METHODS
Setting
This study was conducted at the West London Renal and Transplant Centre, a regional kidney and transplant medicine centre. It serves a population of ∼1.6 million people, looking after >3500 patients on RRT.
Study population
We conducted a retrospective analysis of patients initiating chronic dialysis. All patients diagnosed with ESKD initiating dialysis between January 2013 and December 2015 were included in the analysis. Patients were followed from dialysis initiation until the last clinical encounter prior to 31 July 2017. Chronic RRT was defined by the need for dialysis for a minimum of 3 months in patients with Stage 5 chronic kidney disease (CKD). Patients transplanted pre-emptively were excluded from the analysis.
Data collection
A prospective database of patients presenting to renal services and initiating RRT is routinely maintained. Following the identification of cases, electronic patient records and laboratory records were reviewed and data collected retrospectively.
We categorized patients into planned, unplanned and urgent starters. Planned starters were those starting RRT electively (as an outpatient, with an arteriovenous fistula or planned central venous dialysis catheter/peritoneal dialysis catheter in place). Unplanned starters were patients known to renal services for >90 days who required acute initiation of RRT. Urgent starters were those patients known to renal services <90 days who required acute RRT. Although timing of referral to renal services prior to ESKD is a continuous variable, we selected 90 days as a ‘cut-off’ for late referrals, which allows our results to be interpreted in the context of other studies [2, 6, 7, 11].
We collected demographic data (age, gender, ethnicity, time known to renal services), clinical data (primary renal disease, comorbid illness, initial dialysis modality) and reasons why patients required acute initiation of dialysis. The eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) formula from serum creatinine values at the time of dialysis initiation and at 1, 3, 6 and 12 months prior to dialysis initiation. Creatinine measurements were made in clinical laboratories using the Jaffe method. Measurements made between different laboratories were not standardized. However, the results are deemed accurate for routine clinical use. In accordance with reporting guidelines, descriptive tables were not subjected to statistical testing [20].
Statistics
Multinomial logistic regression was used to assess risk factors associated with acute dialysis start. Planned starts on dialysis were used as the reference group. Candidate factors included ethnicity (reference group: Caucasian), gender (reference group: female), age range (reference group: age ≥75 years), primary renal diagnosis (reference group: diabetic nephropathy) and co-morbid illness. Data are presented as unadjusted and adjusted odds ratio (OR) with confidence interval (CI) and P-value.
We assessed the change in calculated eGFR over time in the year preceding dialysis initiation. We selected the time points of 1, 3, 6 and 12 months prior to dialysis initiation. If data were outside these time intervals by >15% (e.g. >5 days for the 1-month value or 55 days for the 1-year value), then we considered the data unavailable. Where patients had creatinine measurements more often, we selected the creatinine value closest to the predefined time point (1, 3, 6 or 12 months). Repeated measures analysis of variance with Greenhouse–Geisser correction was used to assess the interaction of planned status and eGFR change in the preceding 12 months prior to dialysis. Post hoc tests using the Bonferroni correction were used to assess differences in eGFR rate of change compared with planned starters. Data are presented as mean difference [standard error (95% CI); P-value]. Graphs were constructed and statistical analysis performed using Prism 5.0 (GraphPad Software, La Jolla, CA, USA) and SPSS version 22.0 (IBM, Armonk, NY, USA).
Ethics
The UK Renal Association has suggested that data on the ‘percentage of patients commencing RRT referred <3 months and <12 months before date of starting RRT’ are collected and analysed as part of clinical governance. As such, this study was deemed a quality improvement project and research ethics approval was not sought. Furthermore, this was a retrospective study and all treatment decisions were made prior to our assessment.
RESULTS
Demographics
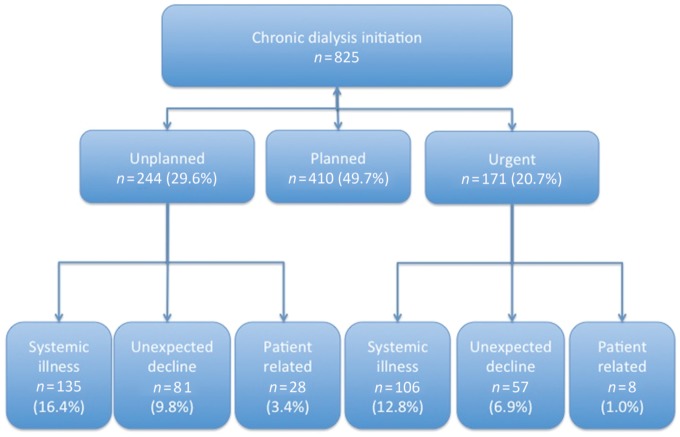
A total of 904 consecutive patients initiated RRT between January 2013 and December 2015 in our centre. In all, 79 patients (8.7%) were excluded from the analysis, as they had a planned start with a pre-emptive renal transplant. A total of 825 patients initiated on dialysis were included in the analysis (Table 1). Of these, 410 (49.7%) patients had a planned start. The remaining 415 (50.3%) patients had an acute start on dialysis, of which, 244 (58.8%) had an unplanned start and 171 (41.2%) had an urgent start (Table 1). Unplanned starters were known to renal services for significantly less time than planned starters (3.8 ± 4.2 years versus 5.0 ± 4.8 years; P < 0.001).
Table 1.
Baseline characteristics of patients with planned, unplanned or urgent starts to RRT categorized by the cause for emergency presentation
| Planned | Unplanned |
Urgent |
Total | |||||
|---|---|---|---|---|---|---|---|---|
| Systemic illness | Accelerated decline | Patient related | Systemic illness | Accelerated decline | Patient related | |||
| Patients, n | 410 | 135 | 81 | 28 | 106 | 57 | 8 | 825 |
| Age (years), mean (SD) | 61.9 (14.9) | 68.0 (11.4) | 63.3 (17.1) | 55.8 (16.8) | 63.9 (16.0) | 51.1 (18.3) | 68.9 (15.6) | 61.3 (15.8) |
| Time known to renal (years), mean (SD) | 5.0 (4.8) | 4.2 (4.2) | 3.0 (4.1) | 4.2 (4.5) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 3.6 (4.5) |
| Sex, n (%) | ||||||||
| Male | 256 (62.4) | 91 (67.4) | 49 (60.5) | 16 (57.1) | 69 (65.1) | 40 (70.2) | 2 (25.0) | 526 (63.2) |
| Female | 44 (32.6) | 32 (39.5) | 12 (42.9) | 37 (34.9) | 17 (29.8) | 6 (75.0) | ||
| Ethnicity, n (%) | ||||||||
| Asian | 188 (45.9) | 53 (38.5) | 33 (40.7) | 11 (39.3) | 32 (30.2) | 16 (28.1) | 4 (50.0) | 338 (40.6) |
| Black | 77 (18.8) | 24 (17.8) | 18 (22.2) | 7 (25.0) | 15 (14.2) | 19 (33.3) | 1 (12.5) | 163 (19.6) |
| Other | 21 (5.1) | 6 (4.4) | 5 (6.2) | 2 (7.1) | 2 (1.9) | 1 (1.8) | 0 (0.0) | 38 (4.6) |
| Caucasian | 124 (30.2) | 53 (39.9) | 25 (30.9) | 8 (28.6) | 57 (53.8) | 21 (36.8) | 3 (37.5) | 293 (35.2) |
| Primary renal diagnosis, n (%) | ||||||||
| Other | 62 (15.1) | 22 (16.3) | 8 (9.9) | 2 (7.1) | 35 (33.0) | 12 (21.1) | 3 (37.5) | 145 (17.4) |
| Unknown | 82 (20.0) | 19 (14.1) | 14 (17.3) | 6 (21.4) | 8 (7.5) | 22 (38.6) | 3 (37.5) | 155 (18.6) |
| Renovascular | 9 (2.2) | 7 (5.2) | 4 (4.9) | 0 (0.0) | 2 (1.9) | 2 (3.5) | 0 (0.0) | 24 (2.9) |
| Glomerulonephritis | 60 (14.6) | 15 (11.1) | 13 (16.0) | 4 (14.3) | 38 (35.8) | 2 (3.5) | 0 (0.0) | 134 (16.1) |
| ADPKD | 27 (6.6) | 3 (2.2) | 1 (1.2) | 2 (7.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 27 (6.6) |
| DM | 127 (50.4) | 69 (51.1) | 41 (50.6) | 14 (50.0) | 23 (21.7) | 19 (33.3) | 2 (25.0) | 170 (41.5) |
| Age range (years), n (%) | ||||||||
| <45 | 57 (13.9) | 5 (3.7) | 15 (18.5) | 7 (25.0) | 16 (15.1) | 24 (42.1) | 1 (12.5) | 127 (15.3) |
| 45–59 | 114 (27.8) | 23 (17.0) | 13 (16.0) | 10 (35.7) | 23 (21.7) | 12 (21.1) | 0 (0.0) | 197 (23.7) |
| 60–74 | 146 (25.6) | 65 (48.1) | 29 (35.8) | 7 (25.0) | 28 (26.4) | 13 (22.8) | 4 (50.0) | 294 (35.3) |
| >75 | 93 (22.7) | 42 (31.1) | 24 (29.6) | 4 (14.3) | 39 (36.8) | 8 (14.0) | 3 (37.5) | 214 (25.7) |
| DM, n (%) | 186 (45.4) | 91 (67.4) | 38 (59.3) | 15 (53.6) | 33 (31.1) | 22 (38.6) | 3 (37.5) | 402 (48.3) |
| Cardiac disease, n (%) | 86 (21.0) | 61 (45.2) | 23 (28.4) | 5 (17.9) | 31 (29.2) | 14 (24.6) | 2 (25.0) | 224 (26.9) |
| Cerebrovascular disease, n (%) | 30 (7.3) | 22 (16.3) | 6 (7.4) | 1 (3.6) | 9 (8.5) | 4 (7.0) | 1 (12.5) | 73 (8.8) |
| Peripheral vascular disease, n (%) | 34 (8.3) | 21 (15.6) | 13 (16.0) | 2 (7.1) | 10 (9.4) | 5 (8.8) | 1 (12.5) | 86 (10.3) |
| Viral disease, n (%) | 11 (2.7) | 6 (4.4) | 5 (6.2) | 2 (7.1) | 7 (6.6) | 6 (10.5) | 1 (12.5) | 38 (4.6) |
| Hypertension, n (%) | 103 (25.1) | 54 (40.0) | 35 (43.2) | 11 (39.3) | 48 (45.3) | 28 (49.1) | 6 (75.0) | 291 (35.0) |
| Haematological malignancy, n (%) | 10 (2.4) | 7 (5.2) | 3 (3.7) | 1 (3.6) | 8 (7.5) | 3 (5.3) | 1 (12.5) | 33 (4.0) |
| Biochemistry, n (%) | ||||||||
| Creatinine (μmol/L) | 672 (211) | 678 (240) | 767 (309) | 1001 (372) | 878 (482) | 1013 (589) | 790 (328) | 744 (335) |
| Modality, n (%) | ||||||||
| Peritoneal dialysis | 66 (16.1) | 1 (0.70) | 2 (2.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 69 (8.3) |
| Haemodialysis | 344 (83.9) | 134 (99.3) | 79 (97.5) | 28 (100.0) | 106 (100.0) | 57 (100.0) | 8 (100.0) | 763 (91.7) |
Data were complete for age, gender, planned status, mortality, initial RRT modality, primary renal diagnosis and reason for emergency RRT start. The following variables had missing data: ethnicity (n = 1), initial RRT access (n = 7), time known to renal services prior to RRT start (n = 13), creatinine (n = 1).
The mean age at RRT initiation was 61.3 (±15.8) years. The majority of our RRT cohort were of Asian ethnicity [n = 338 (40.6%)], followed by Caucasian [n = 293 (35.2%)], Black [n = 163 (19.6%)] and other ethnicities [n = 38 (4.6%)]. The leading cause of ESKD was diabetic nephropathy (41.5%), followed by autoimmune glomerulonephritis (GN; 16.1%), autosomal dominant polycystic kidney disease (ADPKD; 6.6%) and renovascular disease (2.9%). The aetiology of ESKD was unknown in 18.6% of patients.
Reasons for acute dialysis initiation
Reasons for acute initiation of dialysis were divided into patient-related factors, acute systemic illness or unexpected accelerated decline to ESKD (Table 2 and Figure 1). Health care behaviour–related factors consisted of non-attendance to clinic, previous refusal of RRT initiation despite advanced kidney disease, intentional consumption of drugs known to be nephrotoxic (e.g. herbal medications) and immigration (overseas patients presenting to UK health care with ESKD). Acute systemic illness consisted of cardiovascular events, sepsis, vascular disease, autoimmunity, major surgery, obstructive uropathy, drug toxicity and other systemic diseases (e.g. haematological malignancy). Patients who presented with ‘unexpected accelerated decline in renal function’ had no discrete systemic illness but presented with either volume overload, symptoms of uraemia or hyperkalaemia/significantly elevated serum creatinine on routine blood testing.
Table 2.
Reasons for initiation of RRT in patients with unplanned and urgent presentations to nephrology services
| Unplanned |
Urgent |
|||||
|---|---|---|---|---|---|---|
| Systemic illness | Unexpected decline | Patient related | Systemic illness | Unexpected decline | Patient related | |
| Patient related ,n (%) | ||||||
| Non-compliance/non-attendance | – | – | 11 (39) | – | – | 0 (0) |
| Previous refusal of haemodialysis/conservative care | – | – | 13 (46) | – | – | 1 (13) |
| Drug toxicity (illicit/intentional) | – | – | 3 (11) | – | – | 3 (38) |
| Immigration, n (%) | – | – | 1 (4) | – | – | 4 (50) |
| Systemic illness, n (%) | ||||||
| Sepsis | 65 (48) | – | – | 28 (26) | – | – |
| Cardiovascular | 30 (22) | – | – | 11 (10) | – | – |
| Peripheral vascular disease | 4 (3) | – | – | 1 (1) | – | – |
| Autoimmunity | 6 (4) | – | – | 38 (36) | – | – |
| Major surgery | 14 (10) | – | – | 5 (5) | – | – |
| Obstructive uropathy | 3 (2) | – | – | 5 (5) | – | – |
| Other systemic causes with secondary renal involvement | 10 (7) | – | – | 14 (13) | – | – |
| Iatrogenic drug toxicity | 3 (2) | – | – | 4 (4) | – | – |
| Accelerated decline, n (%) | ||||||
| Accelerated decline (unplanned) | – | 81 (100) | – | – | – | – |
| Accelerated decline (urgent) | – | – | – | – | 57 | – |
| Total, n (%) | 135 | 81 | 28 | 106 | 57 | 8 |
FIGURE 1.
Proportion of patients requiring planned, unplanned and urgent initiation of dialysis.
Cardiovascular disease (22%) and sepsis (48%) accounted for the majority of acute systemic illness presentations among unplanned starters. In contrast, autoimmune disease (36%) and sepsis (26%) accounted for most of the acute illness presentations among urgent starters (Table 2).
Risk factors for acute RRT initiation
Unplanned dialysis initiation from acute systemic illness
Patients <45 years of age [OR 0.28 (95% CI 0.10–0.78); P = 0.02] and 45–59 years of age [OR 0.47 (95% CI 0.25–0.88); P = 0.02] were significantly less likely to present needing unplanned dialysis initiation from acute systemic illness compared with patients >75 years of age. Cardiovascular comorbidities and risk factors were independently associated with unplanned dialysis initiation from acute systemic illness. These included renovascular disease [OR 4.94 (95% CI 1.12–21.89); P = 0.04], diabetes mellitus [DM; OR 5.46 (95% CI 2.46–11.76); P < 0.001], cardiac disease [OR 1.96 (95% CI 1.24–3.11); P < 0.001], previous cerebrovascular accident (CVA; OR 1.94 (95% CI 1.03–3.66); P = 0.04] and hypertension (HTN; OR 1.69 (95% CI 1.09–2.60); P = 0.02]. Other risk factors included ‘other’ renal diagnoses [OR 2.85 (95% CI 1.24–6.54); P = 0.01] (Table 3).
Table 3.
Outcomes from regression analysis identifying risk factors for emergency commencement of RRT, expressed as unadjusted OR and CI with P-values to delineate significance at the P < 0.05 level
| Unplanned with systemic illness |
Unplanned with unexpected decline |
Urgent with systemic illness |
Urgent with unexpected decline |
|||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Sig. | OR (95% CI) | Sig. | OR (95% CI) | Sig. | OR (95% CI) | Sig. | |
| Sex | ||||||||
| Male | 1.15 (0.74–1.78) | 0.54 | 0.89 (0.54–1.48) | 0.65 | 1.18 (0.72–1.92) | 0.52 | 1.61 (0.83–3.13) | 0.16 |
| Reference: female | ||||||||
| Ethnicity | ||||||||
| Asian | 0.66 (0.41–1.08) | 0.10 | 0.97 (0.53–1.76) | 0.92 | 0.55 (0.32–0.93) | 0.03 | 0.47 (0.22–1.00) | 0.05 |
| Black | 0.90 (0.49–1.65) | 0.73 | 1.46 (0.72–2.49) | 0.30 | 0.64 (0.32–1.28) | 0.21 | 1.43 (0.66–3.09) | 0.36 |
| Other | 1.16 0.40–3.37) | 0.79 | 1.83 (0.59–5.63) | 0.30 | 0.31 (0.07–1.42) | 0.13 | 0.27 (0.03–2.31) | 0.23 |
| Reference: Caucasian | ||||||||
| Primary renal diagnosis | ||||||||
| Other | 2.85 (1.24–6.54) | 0.01 | 1.19 (0.38–3.78) | 0.77 | 4.48 (1.61–12.43) | 0.00 | 1.71 (0.39–7.56) | 0.48 |
| Unknown | 2.40 (0.96–5.06) | 0.06 | 2.09 (0.66–6.56) | 0.21 | 0.79 (0.23–2.74) | 0.71 | 4.09 (0.89–18.94) | 0.07 |
| Renovascular disease | 4.95 (1.12–21.89) | 0.04 | 3.91 (0.69–22.34) | 0.13 | 1.17 (0.16–8.81) | 0.88 | 9.29 (0.79–108.7) | 0.08 |
| Glomerulonephritis | 5.93 (0.46–77.07) | 0.17 | 1.41 (0.19–10.67) | 0.74 | 6.07 (2.10–17.56) | 0.001 | 0.14 (0.01–2.91) | 0.20 |
| ADPKD | 0.77 (0.19–3.20) | 0.72 | 0.33 (0.04–3.11) | 0.33 | NA | 0.99 | NA | 0.99 |
| Reference: diabetic nephropathy | ||||||||
| Comorbid illness | ||||||||
| DM | 5.42 (2.46–11.76) | 0.00 | 3.83 (1.34–10.99) | 0.01 | 1.39 (0.55–3.55) | 0.48 | 2.13 (0.50–9.01) | 0.31 |
| Cardiac disease | 1.96 (1.24–3.11) | 0.00 | 1.18 (0.65–2.14) | 0.59 | 1.59 (0.91–2.78) | 0.10 | 1.66 (0.76–3.61) | 0.20 |
| Cerebrovascular disease | 1.94 (1.03–3.66) | 0.04 | 0.85 (0.32–2.23) | 0.74 | 1.12 (0.52–2.83) | 0.66 | 0.98 (0.30–3.17) | 0.97 |
| Peripheral vascular disease | 1.24 (0.59–2.62) | 0.57 | 1.92 (0.81–4.55) | 0.14 | 1.62 (0.64–4.12) | 0.31 | 0.78 (0.20–3.00) | 0.71 |
| Viral disease | 1.46 (0.49–4.31) | 0.49 | 3.03 (0.97–9.34) | 0.06 | 2.28 (0.80–6.54) | 0.12 | 6.02 (1.86–19.23) | 0.00 |
| Hypertension | 1.69 (1.09–2.60) | 0.02 | 2.19 (1.32–3.65) | 0.00 | 2.53 (1.56–4.10) | 0.00 | 3.16 (1.91–6.80) | 0.00 |
| Haematological malignancy | 1.46 (0.50–4.26) | 0.49 | 1.55 (0.39–6.12) | 0.54 | 1.79 (0.63–5.08) | 0.28 | 1.66 (0.76–3.61) | 0.20 |
| Reference: no comorbid illness | ||||||||
| Age range (years) | ||||||||
| <45 | 0.28 (0.10–0.78) | 0.02 | 1.20 (0.54–2.65) | 0.66 | 2.13 (1.00–4.55) | 0.05 | 10.11 (3.65–28.0) | 0.00 |
| 45–59 | 0.47 (0.25–0.88) | 0.02 | 0.39 (0.18–0.84) | 0.02 | 2.19 (1.13–4.24) | 0.02 | 1.34 (0.47–3.80) | 0.59 |
| 60–74 | 0.93 (0.56–1.55) | 0.78 | 0.66 (0.35–1.26) | 0.21 | 2.56 (1.40–4.69) | 0.00 | 1.17 (0.43–3.15) | 0.76 |
| Reference: >75 | . | . | . | |||||
Sig., significance.
Table 4.
Outcomes from regression analysis identifying risk factors for emergency commencement of RRT, expressed as adjusted OR and CI with P-values to delineate significance (sig.) at the P < 0.05 level
| Unplanned systemic illnessa |
Unplanned unexpected declinea |
Urgent systemic illnessa |
Urgent unexpected declinea |
|||||
|---|---|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | |
| Age | 1.02 (1.01–1.04) | <0.001 | 1.00 (0.99–1.02) | 0.67 | 1.01 (1.00–1.03) | 0.12 | 0.94 (0.92–0.96) | <0.01 |
| Comorbid illness | ||||||||
| DM | 5.40 (2.49–11.72) | <0.001 | 3.50 (1.25–9.87) | 0.02 | 1.32 (0.53–3.28) | 0.55 | 1.85 (0.43–7.86) | 0.41 |
| Cardiac | 2.11 (1.34–3.31) | <0.001 | 1.24 (0.69–2.22) | 0.47 | 1.80 (1.05–3.11) | 0.03 | 1.87 (0.87–4.01) | 0.11 |
| CVA | 1.83 (0.97–3.44) | 0.06 | 0.86 (0.34–2.21) | 0.76 | 1.17 (0.51–2.71) | 0.71 | 0.95 (0.30–3.07) | 0.94 |
| Vascular | 1.33 (0.64–2.75) | 0.45 | 1.83 (0.79–4.24) | 0.16 | 1.49 (0.60–3.68) | 0.39 | 0.77 (0.20–3.06) | 0.72 |
| Viral | 1.46 (0.50–4.24) | 0.49 | 2.40 (0.79–7.26) | 0.12 | 2.07 (0.75–5.75) | 0.16 | 4.85 (1.55–15.16) | 0.01 |
| HTN | 1.67 (1.09–2.56) | 0.02 | 2.02 (1.22–3.32) | 0.01 | 2.64 (1.54–3.95) | <0.01 | 3.26 (1.76–6.06) | <0.01 |
| Haematological malignancy | 1.24 (0.43–3.58) | 0.69 | 1.51 (0.38–5.97) | 0.56 | 1.62 (0.59–4.47) | 0.35 | 3.63 (0.81–16.32) | 0.09 |
| Reference: no comorbid illness | ||||||||
| Primary renal diagnosis | ||||||||
| Other | 2.98 (1.30–6.83) | 0.01 | 1.26 (0.40–3.92) | 0.69 | 4.73 (1.74–12.88) | <0.01 | 1.54 (0.34–6.93) | 0.57 |
| Unknown | 2.44 (0.99–6.01) | 0.05 | 2.16 (0.69–6.75) | 0.18 | 0.89 (0.26–3.02) | 0.86 | 3.96 (0.87–18.14) | 0.08 |
| Vascular | 4.53 (1.05–19.64) | 0.04 | 4.01 (0.71–22.52) | 0.12 | 1.34 (0.18–9.74) | 0.77 | 9.91 (0.81–120.77) | 0.07 |
| Autoimmune | 3.11 (1.23–7.87) | 0.02 | 2.63 (0.84–8.22) | 0.10 | 6.07 (2.13–17.27) | <0.01 | 0.28 (0.04–2.13) | 0.22 |
| ADPKD | 0.81 (0.20–3.33) | 0.77 | 0.32 (0.04–2.90) | 0.31 | NAb | 1.00 | NAb | . |
| Reference: DM | . | . | . | . | ||||
| Ethnicity | ||||||||
| Asian | 0.69 (0.43–1.12) | 0.13 | 0.89 (0.49–1.61) | 0.70 | 0.52 (0.31–0.89) | 0.02 | 0.40 (0.19–0.84) | 0.02 |
| Black | 0.89 (0.49–1.62) | 0.70 | 1.30 (0.55–2.60) | 0.46 | 0.62 (0.32–1.24) | 0.18 | 1.16 (0.55–2.45) | 0.70 |
| Other | 1.06 (0.37–3.05) | 0.92 | 1.65 (0.55–4.96) | 0.38 | 0.30 (0.07–1.36) | 0.12 | 0.23 (0.03–1.89) | 0.17 |
| Reference: White | . | . | . | . | ||||
| Gender | ||||||||
| Male | 1.07 (0.69–1.64) | 0.78 | 0.85 (0.52–1.41) | 0.54 | 1.12 (0.70–1.81) | 0.64 | 1.57 (0.82–3.02) | 0.18 |
| Reference: female | . | . | ||||||
Adjusted for age, sex, primary renal diagnosis, ethnicity and comorbid illness.
Reference category is planned.
No patients with ADPKD in this category.
Unplanned dialysis initiation from accelerated unexpected decline in renal function
Patients 45–59 years of age [OR 0.39 (95% CI 0.18–0.85); P = 0.02] were at lower risk of accelerated decline in renal function compared with patients >75 years of age, whereas patients <45 years of age were of similar risk. Risk factors for presenting with dialysis initiation from accelerated decline in renal function among unplanned starters included the presence of DM [OR 3.83 (95% CI 1.34–10.99); P = 0.01] and HTN [HR 2.19 (95% CI 1.32–3.65); P < 0.001] (Table 3).
Urgent dialysis initiation from acute systemic illness. Factors associated with urgent dialysis start from acute systemic illness included autoimmune GN [OR 6.07 (95% CI 2.10–17.56); P = 0.001], HTN [OR 2.53 (95% CI 1.56–4.10); P < 0.001)], ‘other’ diagnoses [OR 4.48 (95% CI 1.61–12.43); P < 0.001] and age. Younger patients were at greatest risk of presenting needing urgent dialysis initiation from acute systemic illness. Patients <45 years of age [OR 2.13 (95% CI 1.00–4.55); P = 0.05], 45–59 years [OR 2.19 (95% CI 1.13–4.24); P = 0.02] and 60–74 years [OR 2.56 (95% CI 1.40–4.69); P < 0.001] were more likely to present needing unplanned dialysis initiation from acute systemic illness compared with patients >75 years old. Compared with Caucasian patients, Asian patients were less likely to present with urgent RRT initiation from acute systemic illness [OR 0.55 (95% CI 0.32–0.95); P = 0.03] (Table 3).
Urgent dialysis initiation from accelerated unexpected decline in renal function
Risk factors for unexpected accelerated decline in renal function leading to ESKD included viral disease [OR (95% CI 1.47–15.38); P = 0.01] and HTN [OR 3.15 (95% CI 1.70–5.85); P < 0.001]. Patients were more likely to be <45 years of age compared with patients >75 years [OR (10.11 (95% CI 3.65–28.0); P < 0.001]. Compared with Caucasian patients, Asian patients were less likely to present with urgent RRT initiation from accelerated decline in renal function [OR 0.45 (95% CI 0.22–0.95); P = 0.04] (Table 3).
Acute dialysis initiation from patient-related factors
It was not possible to assess independent risk factors for acute RRT initiation associated with patient-related factors because of the relatively small sample size.
Rate of decline in GFR prior to dialysis initiation
Unplanned starters with an acute systemic illness (7.7 ± 3.4 mL/min/1.73 m2; P = 1.00), unplanned starters with an unexplained decline to ESKD (7.1 ± 4.7 mL/min/1.73 m2; P = 0.230) and urgent starters with an acute systemic illness (7.1 ± 4.7 mL/min/1.73 m2; P = 0.159) had similar eGFRs at the time of dialysis initiation compared with planned starters (7.6 ± 3.0 mL/min/1.73 m2). In contrast, urgent starters with an unexplained decline to ESKD (6.5 ± 4.1 mL/min/1.73 m2; P = 0.019) had a significantly lower eGFR at initiation of dialysis compared with planned starters.
The change in eGFR at 1, 3, 6 and 12 months prior to initiation of dialysis was ascertained where possible in order to provide an estimate of the eGFR decline over the 12 months preceding dialysis. Among patients known to renal services for >3 months, blood results were available for between 78 and 93% of patients at each time point. However, among urgent dialysis starters, blood results were available for between 28 and 59% of patients at each time point (Supplementary data, Table S1). Compared with planned starters, unplanned starters with systemic illness (−4.5 ± 0.9, P < 0.001), unplanned starters with accelerated unexpected decline in renal function (−3.6 ± 1.2, P = 0.029), urgent starters with systemic illness (−26 ± 1.5; P < 0.001) and urgent starters with unexpected accelerated decline in renal function (−13 ± 2.4, P < 0.001) had a greater rate of eGFR decline in the 12 months preceding dialysis (Figure 2; Supplementary data, Table S1).
FIGURE 2.
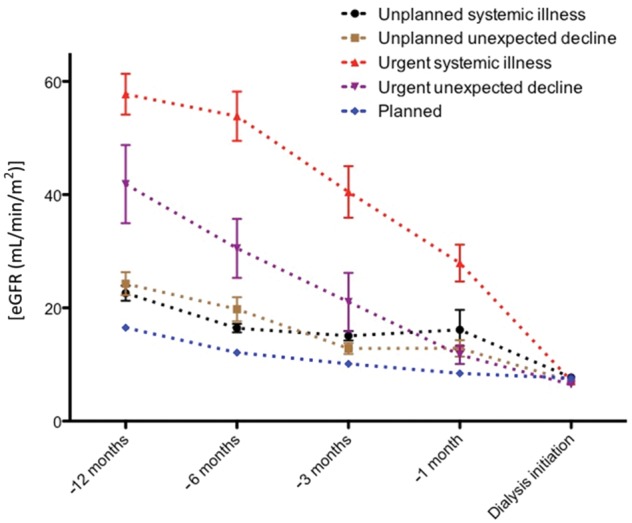
Rate of change of eGFR in the 12 months preceding dialysis initiation. Data represent mean and standard mean error. Compared with planned starters, unplanned starters with systemic illness (−4.5 ± 0.9, P < 0.001), unplanned starters with accelerated unexpected decline in renal function (−3.6 ± 1.2, P = 0.029), urgent starters with systemic illness (−26 ± 1.5, P < 0.001) and urgent starters with unexpected accelerated decline in renal function (−13 ± 2.4, P < 0.001) had a greater rate of eGFR decline in the 12 months preceding dialysis.
DISCUSSION
Acute initiation of dialysis is common, occurring in 46% of our ESKD cohort. This is consistent with other studies, reporting 40–63% inpatient initiation of dialysis [1, 3, 10]. Suboptimal dialysis initiation occurs in up to 56% of patients known to renal services for 1 year [18]. Reasons for unplanned initiation of RRT among patients known to renal services often include acute illness, unexpected decline in renal function, service-related and patient health behaviour–related factors [10, 21]. Understanding the reasons for urgent dialysis initiation, with a focus on risk stratification and identifying modifiable risk factors, is fundamental to improving patient care.
On initiation of dialysis, the eGFR of patients starting acute dialysis was similar to that of planned starters (apart from urgent starters with an unexplained decline to ESKD). The rate of eGFR decline 12 months prior to dialysis initiation of patients requiring urgent dialysis was greatest. The rapid and often unpredictable loss of renal function in the context of acute systemic illness may make any intervention to avert acute dialysis start difficult.
Urgent dialysis initiation
Delayed or late referral remains a negative aspect of renal care and is well known to be associated with adverse outcomes [22], including more frequent and longer hospitalization [23], suboptimal vascular access [24], poorer psychological well-being [25] and lower uptake of home dialysis therapies [26–28]. The reasons for late referrals need to be ascertained, as it may facilitate prevention of progression to ESKD. A shorter duration of pre-dialysis care is associated with increased morbidity and mortality [1, 4, 5, 7].
In all, 20.7% of our cohort had a late referral and urgent start on dialysis, consistent with the overall national UK rate of urgent dialysis initiation of 17% [29]. Similarly in the USA and Australia, ∼20% of patients requiring chronic RRT are referred to renal services within 3–4 months [2, 30].
Patients known to renal services for <3 months required acute dialysis because of acute systemic illness in 62% of cases. Unsurprisingly, the first presentation of autoimmune GN was a significant risk factor. This reflects the nature of our nephrology service, which is a tertiary referral centre for renal vasculitis. ‘Other’ renal diseases were also a significant risk factor and were comprised primarily of patients requiring nephrectomy for major surgery and haematological malignancy. This illustrates the important distinction between ‘late presentation’ and ‘late referral’. The same is true for patients at the opposite end of the spectrum, with slowly progressive but asymptomatic renal dysfunction over many years. In our cohort, 7% required dialysis within 3 months of presentation, without any obvious cause of ESKD. These patients often presented with small scarred kidneys on ultrasound scans and with negative immunology on serological tests.
The UK renal registry reports an overall national ‘late referral’ rate of 12.2%, with considerable variation in late presentation rates (5–40%) between UK renal centres [29]. We were able to ascertain historical serum eGFR values in half the patients referred to us within 3 months of requiring dialysis (urgent starters). A significant proportion of these patients had CKD up to 12 months prior to the initiation of dialysis, yet were referred within 3 months of requiring dialysis. Deficiencies in primary and secondary care recognition of CKD and delays in activating referral pathways for specialist nephrology input may be contributory. Laboratory eGFR reporting and prompts (e-alerts) to consider specialist referral are now routinely used by many centres. A recent UK-wide initiative (Assist-CKD) provides a graphical surveillance tool to monitor eGFR and identifies people with CKD who are at greatest risk of disease progression [31].
Unplanned dialysis initiation
One-third of patients requiring acute dialysis initiation had been known to renal services for >3 months, 55% of whom had an association with acute systemic illness. The cardiovascular disease burden and advancing age associated with acute systemic illness are a reflection of the vulnerability of this advanced CKD cohort to acute illness precipitating the need for emergency dialysis.
One-third of patients with unplanned starts presented with an accelerated unexpected decline in renal function precipitating the need for emergency dialysis. The cause of the accelerated decline in renal function compared with planned starters remains unclear. Clinical judgement among nephrology consultants has been shown to be sensitive but not specific in predicting the need for dialysis, and eGFR was the only independent correlate of predicted time to dialysis [32]. Diabetes is an independent risk factor for unplanned dialysis initiation. Diabetic patients may warrant more frequent eGFR monitoring, particularly those at higher risk of rapid decline, that is, with advanced albuminuria, ongoing poor glycaemic control, presence of retinopathy and neuropathy and those with dyslipidaemia [33].
A significant proportion of patients (11.5%) known to renal services for >3 months required acute dialysis associated with ‘health care behaviour–related factors’. This included non-compliance and patient denial. Although we do not have detailed information on health care education given to individual patients in this cohort, we routinely facilitate a patient education programme preparing patients (typically with eGFR <20 mL/min) for dialysis. Such programmes have been shown to improve patient mortality [34, 35]. Yet, for a small proportion of patients, additional measures may be required to achieve better patient engagement [36] and improve equity of dialysis access.
Strengths and limitations
The main limitation of our study is its retrospective nature. However, a record of all patients initiating dialysis has been prospectively collected to ensure no bias in patient selection. Pre-emptive renal transplant recipients were excluded from the analysis, as the primary objective of this analysis was to compare outcomes of patients undergoing elective or emergency dialysis initiation. One-year survival among pre-emptive renal transplant recipients was 99%, compared with 92% among planned dialysis initiation.
We do not have complete data on eGFR values in the 12 months preceding dialysis initiation, with less than half the values available for the cohort at some time points among urgent dialysis starters. While we run a comprehensive patient education programme available for all patients, we do not have details on the number of clinic attendances and educational sessions each patient has had.
Estimating the eGFR change by linear regression may potentially mask slow progression with acute chronic decline in the month prior to dialysis initiation. Despite this, there was a significant difference in eGFR change in the 12 months preceding dialysis between emergency and planned dialysis initiation.
Our findings may not reflect other populations of dialysis patients, given the ethnic diversity in our patient cohort. The high proportion of patients with diabetic nephropathy is likely to reflect the greater proportion of African and Asian patients in our population. The proportion of patients with hypertensive nephrosclerosis in our cohort is considerably lower than that reported in European data [37], although this may reflect the difficulty in defining patients with true ‘hypertensive nephrosclerosis’, as hypertension may be causal, an effect of or an epiphenomenon to CKD.
Despite these limitations, our study has a number of strengths. Compared with other studies with this level of detail, we describe a large patient cohort. We report differences in risk factors for initiating emergency dialysis based on the reasons behind emergency dialysis starts. We also demonstrate information about the rate of decline in eGFR in the 12 months preceding dialysis initiation in the context of reasons for acute dialysis starts. Future work needs to focus on ensuring timely referral of CKD patients to renal services.
CONCLUSIONS
Acute initiation of dialysis is common, even in patients known to renal services. Acute systemic illness and an unexplained decline in renal function are common reasons for requiring acute RRT initiation. A proportion of patients initiating dialysis were known to have CKD even 12 months prior to the initiation of RRT but were only referred to renal services within 3 months of needing dialysis. Cardiovascular comorbidities and risk factors and advancing age were significant risk factors for acute dialysis initiation among patients known to renal services for >3 months. The rapid and often unpredictable loss of renal function in the context of acute systemic illness may make any intervention to avert emergency dialysis start difficult. Patients with a rapid decline in GFR are at greatest risk of progression to ESKD and merit more frequent GFR monitoring to limit progression and facilitate earlier dialysis planning.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the pre-dialysis team at the West London Renal and Transplant Centre.
FUNDING
Funding was provided by the National Institute for Health Research Imperial College Biomedical Research Centre.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Mendelssohn DC, Curtis B, Yeates K. et al. Suboptimal initiation of dialysis with and without early referral to a nephrologist. Nephrol Dial Transplant 2011; 26: 2959–2965 [DOI] [PubMed] [Google Scholar]
- 2. Arora P, Obrador GT, Ruthazer R. et al. Prevalence, predictors, and consequences of late nephrology referral at a tertiary care center. J Am Soc Nephrol 1999; 10: 1281–1286 [DOI] [PubMed] [Google Scholar]
- 3. Crews DC, Jaar BG, Plantinga LC. et al. Inpatient hemodialysis initiation: reasons, risk factors and outcomes. Nephron Clin Pract 2010; 114: c19–c28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ifudu O, Dawood M, Homel P. et al. Excess morbidity in patients starting uremia therapy without prior care by a nephrologist. Am J Kidney Dis 1996; 28: 841–845 [DOI] [PubMed] [Google Scholar]
- 5. Jungers P, Zingraff J, Albouze G. et al. Late referral to maintenance dialysis: detrimental consequences. Nephrol Dial Transplant 1993; 8: 1089–1093 [PubMed] [Google Scholar]
- 6. Schmidt RJ, Domico JR, Sorkin MI. et al. Early referral and its impact on emergent first dialyses, health care costs, and outcome. Am J Kidney Dis 1998; 32: 278–283 [DOI] [PubMed] [Google Scholar]
- 7. Sesso R, Belasco AG.. Late diagnosis of chronic renal failure and mortality on maintenance dialysis. Nephrol Dial Transplant 1996; 11: 2417–2420 [DOI] [PubMed] [Google Scholar]
- 8. Levin A. Consequences of late referral on patient outcomes. Nephrol Dial Transplant 2000; 15(Suppl 3): 8–13 [DOI] [PubMed] [Google Scholar]
- 9. Caskey FJ, Wordsworth S, Ben T. et al. Early referral and planned initiation of dialysis: what impact on quality of life? Nephrol Dial Transplant 2003; 18: 1330–1338 [DOI] [PubMed] [Google Scholar]
- 10. Buck J, Baker R, Cannaby AM. et al. Why do patients known to renal services still undergo urgent dialysis initiation? A cross-sectional survey. Nephrol Dial Transplant 2007; 22: 3240–3245 [DOI] [PubMed] [Google Scholar]
- 11. Winkelmayer WC, Glynn RJ, Levin R. et al. Determinants of delayed nephrologist referral in patients with chronic kidney disease. Am J Kidney Dis 2001; 38: 1178–1184 [DOI] [PubMed] [Google Scholar]
- 12. Ifudu O, Dawood M, Iofel Y. et al. Delayed referral of black, Hispanic, and older patients with chronic renal failure. Am J Kidney Dis 1999; 33: 728–773 [DOI] [PubMed] [Google Scholar]
- 13. Khan IH, Catto GR, Edward N. et al. Chronic renal failure: factors influencing nephrology referral. QJM 1994; 87: 559–564 [PubMed] [Google Scholar]
- 14. Navaneethan SD, Nigwekar S, Sengodan M. et al. Referral to nephrologists for chronic kidney disease care: is non-diabetic kidney disease ignored? Nephron Clin Pract 2007; 106: c113–c118 [DOI] [PubMed] [Google Scholar]
- 15. Obialo CI, Ofili EO, Quarshie A. et al. Ultralate referral and presentation for renal replacement therapy: socioeconomic implications. Am J Kidney Dis 2005; 46: 881–886 [DOI] [PubMed] [Google Scholar]
- 16. Cass A, Cunningham J, Snelling P. et al. Urban disadvantage and delayed nephrology referral in Australia. Health Place 2003; 9: 175–182 [DOI] [PubMed] [Google Scholar]
- 17. Navaneethan SD, Aloudat S, Singh S.. A systematic review of patient and health system characteristics associated with late referral in chronic kidney disease. BMC Nephrol 2008; 9: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hughes SA, Mendelssohn JG, Tobe SW. et al. Factors associated with suboptimal initiation of dialysis despite early nephrologist referral. Nephrol Dial Transplant 2013; 28: 392–397 [DOI] [PubMed] [Google Scholar]
- 19. Brown PA, Akbari A, Molnar AO. et al. Factors associated with unplanned dialysis starts in patients followed by nephrologists: a retrospective cohort study. PLoS One 2015; 10: e0130080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vandenbroucke JP, von Elm E, Altman DG. et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology 2007; 18: 805–835 [DOI] [PubMed] [Google Scholar]
- 21. Tennankore KK, Soroka SD, Kiberd BA.. The impact of an “acute dialysis start” on the mortality attributed to the use of central venous catheters: a retrospective cohort study. BMC Nephrol 2012; 13: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baer G, Lameire N, Van Biesen W.. Late referral of patients with end-stage renal disease: an in-depth review and suggestions for further actions. NDT Plus 2010; 3: 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan MR, Dall AT, Fletcher KE. et al. Outcomes in patients with chronic kidney disease referred late to nephrologists: a meta-analysis. Am J Med 2007; 120: 1063–1070 [DOI] [PubMed] [Google Scholar]
- 24. Avorn Jerry, Winkelmayer Wolfgang C. et al. Delayed nephrologist referral and inadequate vascular access in patients with advanced chronic kidney failure. J Clin Epidemiol 2002; 55: 711–716 [DOI] [PubMed] [Google Scholar]
- 25. Wauters J-P, Lameire N, Davison A. et al. Why patients with progressing kidney disease are referred late to the nephrologist: on causes and proposals for improvement. Nephrol Dial Transplant 2005; 20: 490–496 [DOI] [PubMed] [Google Scholar]
- 26. Stack AG. Determinants of modality selection among incident US dialysis patients: results from a national study. J Am Soc Nephrol 2002; 13: 1279–1287 [DOI] [PubMed] [Google Scholar]
- 27. Lameire N, Van Biesen W, Dombros N.. The referral pattern of patients with ESRD is a determinant in the choice of dialysis modality. Perit Dial Int 1997; 17(Suppl 2): S161–S166 [PubMed] [Google Scholar]
- 28. Mehrotra R, Marsh D, Vonesh E. et al. Patient education and access of ESRD patients to renal replacement therapies beyond in-center hemodialysis. Kidney Int 2005; 68: 378–390 [DOI] [PubMed] [Google Scholar]
- 29. Gilg J, Caskey F, Fogarty D.. UK Renal Registry 18th Annual Report: chapter 1 UK renal replacement therapy incidence in 2014: national and centre-specific analyses. Nephron 2016; 132: 9–40 [DOI] [PubMed] [Google Scholar]
- 30. Foote C, Clayton PA, Johnson DW. et al. Impact of estimated GFR reporting on late referral rates and practice patterns for end-stage kidney disease patients: a multilevel logistic regression analysis using the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA). Am J Kidney Dis 2014; 64: 359–366 [DOI] [PubMed] [Google Scholar]
- 31. Kramer A, Pippias M, Noordzij M. et al. The European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) registry annual report 2015: a summary. Clin Kidney J 2018; 11: 108–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Green D, Ritchie JP, New DI. et al. How accurately do nephrologists predict the need for dialysis within one year? Nephron Clin Pract 2012; 122: 102–106 [DOI] [PubMed] [Google Scholar]
- 33. Zoppini G, Targher G, Chonchol M. et al. Predictors of estimated GFR decline in patients with type 2 diabetes and preserved kidney function. Clin J Am Soc Nephrol 2012; 7: 401–408 [DOI] [PubMed] [Google Scholar]
- 34. Goldstein M, Yassa T, Dacouris N. et al. Multidisciplinary predialysis care and morbidity and mortality of patients on dialysis. Am J Kidney Dis 2004; 44: 706–714 [PubMed] [Google Scholar]
- 35. Curtis BM, Ravani P, Malberti F. et al. The short- and long-term impact of multi-disciplinary clinics in addition to standard nephrology care on patient outcomes. Nephrol Dial Transplant 2005; 20: 147–154 [DOI] [PubMed] [Google Scholar]
- 36. Henry SL, Munoz-Plaza C, Garcia Delgadillo J. et al. Patient perspectives on the optimal start of renal replacement therapy. J Renal Care 2017; 43: 143–155 [DOI] [PubMed] [Google Scholar]
- 37.Kidney Research UK. Assist-CKD. https://assist-ckd.org
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.